Abstract
The stems of Dendrobium officinale Kimura et Migo, named Tie-pi-shi-hu, is one of the most endangered and precious species in China. Because of its various pharmacodynamic effects, D. officinale is widely recognized as a high-quality health food in China and other countries in south and south-east Asia. With the rising interest of D. officinale, its products have a high price due to a limited supply. This high price has led to the proliferation of adulterants in the market. To ensure the safe use of D. officinale, a fast and convenient method combining normal and fluorescence microscopy was applied in the present study to distinguish D. officinale from three commonly used adulterants including Zi-pi-shi-hu (D. devonianum), Shui-cao-shi-hu (D. aphyllum), Guang-jie-shi-hu (D. gratiosissimum). The result demonstrated that D. officinale could be identified by the characteristic “two hat-shaped” vascular bundle sheath observed under the fluorescence microscopy and the distribution of raphides under normal light microscopy. The other three adulterants could be discriminated by the vascular bundle differences and the distribution of raphides under normal light microscopy. This work indicated that combination of normal light and fluorescence microscopy is a fast and efficient technique to scientifically distinguish D. officinale from the commonly confused species.
Keywords: discrimination, Dendrobium officinale, adulterants, normal light microscopy, fluorescence microscopy
1. Introduction
The genus Dendrobium, containing of 1,100 species or more all over the world, is one of the largest groups of the family Orchidaceae. There are 76 species of Dendrobium in China, including 74 species and two varieties [1], of which the dried stem of D. officinale is listed in the Chinese pharmacopoeia under the name of Dendrobii Officinalis Caulis (Tie-pi-shi-hu) as an individual entry [2]. According to Traditional Chinese Medicine theory, the main function of D. officinale is to nourish yin and clear away heat-evil, tonifying the stomach and promoting fluid [2]. It was used for maintaining stomach tonicity, promoting body fluid production, and relieving symptoms such as throat dryness and dry eyes with blurred vision in the clinic, and was found to possess immunostimulating [3,4,5] and antitumor effects [6].
Because of its various pharmacodynamic effects, D. officinale is widely recognized as a high-quality health food in China and other countries in south and south-east Asia. It was reported that in Zhejiang Province the industrial output value of D. officinale was up to 2 billion yuan in 2011, and expected to increase to 4 billion yuan in 2015. However, as one of the most endangered and precious Traditional Chinese Medicines, the limited supply and the huge demand for D. officinale has triggered a dramatic rise in price. In 2013, the price of dried stems of D. officinale was around ¥ 1000–80,000/kg, while other Dendrobium species which possess similar morphological and anatomical characteristics to D. officinale, such as D. devonianum, D. aphyllum and D. gratiosissimum, have much lower prices (less than one-fourth of the price of D. officinale). Consequently, adulterants, confused species, and counterfeits have proliferated in the market due to the great disparity in price between them [7,8], making identification of authenticity important and essential to the quality control of D. officinale and its related products.
Up to now, the authentication and discrimination of D. officinale have already been carried out by determination of chemical data [9,10], infrared spectrometry analysis [11], or using molecular biology techniques, such as DNA sequencing [12,13]. However, the previous methods are inadequate because they are either lack a well-accepted marker compound or are complicated to perform, costly, unstable, and time-consuming.
In contrast, as a facile and inexpensive technique, microscopy has been proposed to authenticate D. officinale [14,15,16], but the conventional microscopic authentication only provided simple descriptions, and the hand-drawn pictures of some microscopic features could not effectively differentiate the plant of Dendrobium species. Nowadays, fluorescence microscopy has earned a special place in the area of scientific research. Fluorescence microscopy is a fast and simply method, which allows less-experienced personnel to identify botanical raw materials. The combination of normal light and fluorescence microscopic technique, which enhanced the accuracy and convenience of identification, has been successfully applied in authentication and quality evaluation of Chinese herbal medicines [17,18,19,20]. The present study focuses on the microscopy technique, combining normal light and fluorescence microscopy, for discrimination of D. officinale and its commonly adulterants, including D. devonianum, D. aphyllum and D. gratiosissimum. The color figures of their microscopic characters and related descriptions are presented in detail and compared to distinguish them from each other.
2. Results and Discussion
2.1. Macroscopic Characters
2.1.1. Stems of D. officinale
Tie-pi-feng-dou: A commonly trade form of dried stem of D. officinale. Twist or spring-like, usually 2–6 spiral striation. When stretched, stem of 3.5–8 cm in length, 0.2–0.4 cm in diameter. Outer surface yellowish-green or golden-yellow, marked with fine longitudinal grooves. Node obvious, rudimental grayish-white leaf sheath sometimes can be found in node. Solid, breaks easily; broken surface is flat, grayish-white to grayish green, and slightly horny. Odour: faint; taste: mild; sticky when chewed (Figure 1A1).
Figure 1.
Photographs of four species of Dendrobium stems and forms. (A) D. officinale (B) D. devonianum (C) D. aphyllum (D) D. gratiosissimum. 1 Feng-dou (dried), 2 Dried samples, 3 Fresh samples.
Tie-pi-shi-hu: dried stem of D. officinale, slender and cylindrical, various in length (Figure 1A2).
Fresh Tie-pi-shi-hu: cylindrical or flat cylindrical, about 15 cm in length, 0.3–0.8 cm in diameter. Outer surface grayish-green, marked with longitudinal grooves. Texture soft and flexible, broken surface slightly fibrous. Odour: faint; taste: succulent; sticky when chewed (Figure 1A3).
2.1.2. Stems of D. devonianum
Zi-pi-shi-hu (or Zi-pi-feng-dou): Trade form of dried stem of D. devonianum. Macroscopic characters are very similar to Tie-pi-feng-dou (Figure 1B). Twist or spring-like, usually 2–7 spiral striation. Stem 0.2–0.7 cm in diameter. Outer surface dark greenish-yellow, sometimes with purple spots, marked with fine longitudinal grooves. Node obvious, rudimental grayish-white leaf sheath sometimes can be found in node. Fibrous, difficult to break, broken surface uneven. Odour: faint; taste: mild; sticky when chewed.
2.1.3. Stems of D. aphyllum
Shui-cao-shi-hu (or Shui-cao-feng-dou): Trade form of dried stem of D. aphyllum. Appearance is very similar to Tie-pi-feng-dou (Figure 1C). Twist or spring-like, usually 2–5 spiral striations. Stems 0.2–1.1 cm in diameter. Outer surface dark yellowish-green, marked with fine longitudinal grooves. Node obvious, rudimental grayish-white leaf sheath sometimes can be found in node. Fibrous, difficult to break, broken surface uneven. Odour: faint; taste: slightly bitter, and not sticky when chewed.
2.1.4. Stems of D. gratiosissimum
Guang-jie-shi-hu (or Guang-jie-feng-dou): Trade form of dried stem of D. gratiosissimum. Appearance is very similar to Tie-pi-feng-dou (Figure 1D). Twist or spring-like, usually with 2–5 spiral striations. Stem 0.2–0.8 cm in diameter. Outer surface dark greenish-yellow, marked with fine longitudinal grooves. Nodes obvious. Fibrous, difficult to break, broken surface uneven. Odour: faint; taste: slightly bitter, and not sticky when chewed.
2.2. Microscopic Characters
Characteristic microscopic differences of the four Dendrobium species in transverse section of stems are summarized in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6.
Table 1.
Comparison of key microscopic characters of four species of Dendrobium (under normal light microscopy).
| 40× * | 100× * | 200× * | |
|---|---|---|---|
| D. officinale | 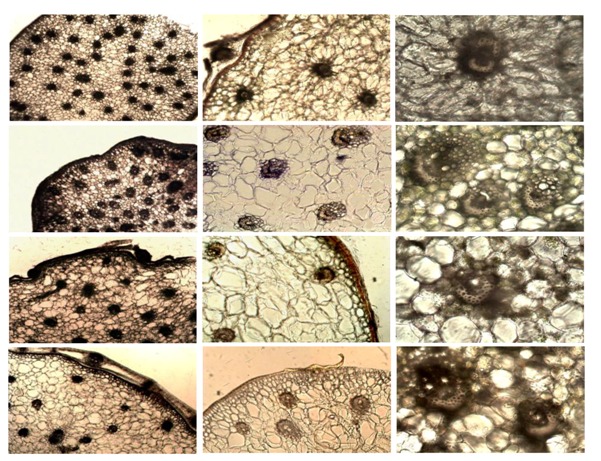 |
||
| D. devonianu | |||
| D . aphyllum | |||
| D. gratiosissimum | |||
| 500 µm | 250 µm | 100 µm | |
* “×” stands for the amplification of the microscope.
Table 2.
Comparison of microscopic features of Dendrobium officinale.
| Sample No. | Cuticle | Epidermis | Fiber | Vascular bundles | Raphide | |||
|---|---|---|---|---|---|---|---|---|
| Thickness | Diameter | Number | Diameter | Number | Diameter | Length | ||
| Tangential | radial | |||||||
| TP-1 | 9–12 | 14–34 | 17–29 | 8–19 | 78–90–102 | 73–125 | 85–229 | 65–68 |
| TP-2 | 10–15 | 16–45 | 15–26 | 12–27 | 52–61–77 | 55–83 | 92–173 | 100–112 |
| TP-3 | 8–13 | 12–29 | 16–37 | 10–28 | 84–92–108 | 61–118 | 80–187 | 75–79 |
| TP-4 | 8–10 | 17–33 | 21–26 | 6–23 | 87–98–111 | 59–100 | 72–183 | 65–71 |
| TP-5 | 8–10 | 13–26 | 20–58 | 8–24 | 84–88–108 | 67–104 | 90–236 | 100–103 |
| TP-6 | 10–11 | 14–39 | 12–25 | 9–25 | 83–92–101 | 46–101 | 60–158 | 76–82 |
| TP-7 | 9–11 | 12–37 | 14–22 | 11–29 | 54–69–86 | 42–140 | 51–147 | 59–96 |
| TP-8 | 10–13 | 23–37 | 10–35 | 8–19 | 84–96–118 | 45–102 | 67–168 | 68–72 |
| TP-9 | 9–10 | 17–27 | 20–30 | 5–18 | 85–92–103 | 45–89 | 50–135 | 53–92 |
| TP-10 | 10–12 | 15–30 | 18–46 | 6–25 | 89–93–108 | 67–103 | 98–159 | 10–15 |
| TP-11 | 10–18 | 13–38 | 18–33 | 6–20 | 79–93–99 | 50–102 | 63–169 | 146–194 |
| TP-12 | 8–12 | 12–28 | 12–26 | 7–24 | 85–97–103 | 48–83 | 62–129 | 62–93 |
| TP-13 | 10––12 | 12–36 | 11–46 | 7–31 | 61–65–97 | 56–119 | 73–196 | 62–156 |
| TP-14 | 8–15 | 13–30 | 12–34 | 6–34 | 89–94–110 | 49–127 | 72–197 | 48–81 |
| TP-15 | 9–21 | 11–39 | 9–31 | 8–37 | 93–109–134 | 54–145 | 71–237 | 52–190 |
| Total | 8–21 | 11–45 | 9–58 | 5–37 | 78(52) –134 | 42–145 | 50–237 | 10–194 |
Table 3.
Comparison of microscopic features of Dendrobium devonianum.
| Sample No. | Cuticle | Epidermis | Fiber | Vascular bundles | Raphide | |||
|---|---|---|---|---|---|---|---|---|
| Thickness | Diameter | Number | Diameter | Number | Diameter | Length | ||
| Tangential | Radial | |||||||
| ZP-1 | 10–11 | 17–26 | 14–29 | 5–19 | 58–72–84 | 74–134 | 135–208 | 74–90 |
| ZP-2 | 11–12 | 32–85 | 31–37 | 11–45 | 72–76–91 | 172–283 | 296–487 | 51–132 |
| ZP-3 | 13–20 | 50–72 | 7–43 | 7–25 | 57–64–80 | 62–171 | 82–265 | 52–63 |
| Total | 11–20 | 17–85 | 7–43 | 7–45 | 57–91 | 62–283 | 82–487 | 51–132 |
Table 4.
Comparison of microscopic features of Dendrobium aphyllum.
| Sample No. | Cuticle | Epidermis | Fiber | Vascular bundles | Raphide | |||
|---|---|---|---|---|---|---|---|---|
| Thickness | Diameter | Number | Diameter | Number | Diameter | Length | ||
| Tangential | Radial | |||||||
| SC-1 | 9–14 | 17–37 | 11–36 | 8–25 | 56–67–84 | 84–174 | 82–251 | 93–115 |
| SC-2 | 14–16 | 13–30 | 20–47 | 5–33 | 64–72–85 | 88–127 | 99–281 | 65–182 |
| SC-3 | 10–12 | 19–38 | 8–22 | 7–20 | 53–66–79 | 73–166 | 81–207 | 143–202 |
| Total | 9–16 | 13–38 | 8–47 | 5–33 | 53–85 | 73–174 | 81–281 | 65–202 |
Table 5.
Comparison of microscopic features of Dendrobium gratiosissimum.
| Sample No. | Cuticle | Epidermis | Fiber | Vascular bundles | Raphide | |||
|---|---|---|---|---|---|---|---|---|
| Thickness | Diameter | Number | Diameter | Number | Diameter | Length | ||
| Tangential | Radial | |||||||
| GJ-1 | 8–10 | 11–32 | 20–66 | 6–31 | 57–65–124 | 104–346 | 89–218 | 53–124 |
| GJ-2 | 17–18 | 15–33 | 23–52 | 6–29 | 59–72–81 | 108–293 | 92–173 | 39–73 |
| GJ-3 | 16–17 | 17–35 | 19–31 | 7–24 | 68–71–84 | 127–278 | 102–225 | 51–71 |
| Total | 8–18 | 11–35 | 19–66 | 6–31 | 57–84(124) | 104–346 | 89–225 | 39–124 |
Table 6.
Comparison of key microscopic characters of four species of Dendrobium (under fluorescence microscopy).
| 40× * | 200× * | |||
|---|---|---|---|---|
| B-1 | G-1 | B-1 | G-1 | |
| D. officinale | 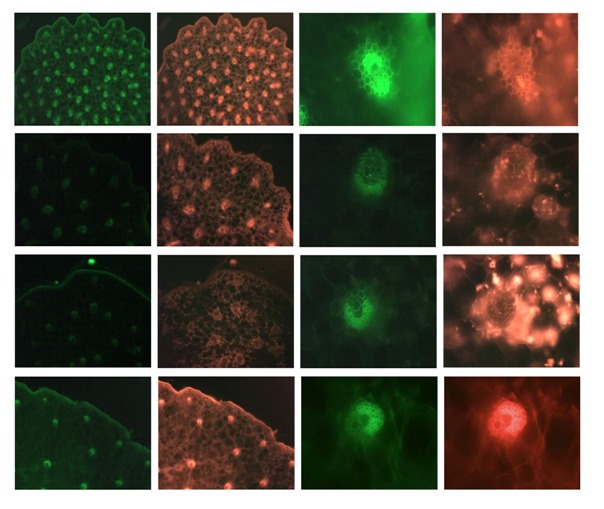 |
|||
| D. devonianum | ||||
| D. aphyllum | ||||
| D. gratiosissimum | ||||
| 500 µm | 100 µm | |||
B-1, emission filter of EF 450–500 nm; G-1, emission filter of EF 530–590 nm. * “×” stands for the amplification of the microscope.
2.2.1. Transverse Section of Stems (Observed Under Normal Light Microscope)
2.2.1.1. Stems of D. officinale–Outline is nearly circular (Table 1 and Table 2)
-
(1).
Epidermis: a row of cells, thin and flat, 17–45 μm in diameter, lateral walls were slightly lignified, covered with yellow to orange cuticles. A layer of pericladium consisting of parenchymatous cells and vascular bundles can be observed outside the epidermis sometimes.
-
(2).
Parenchyma: Parenchymatous cells similar in size, usually smaller near the vascular bundles. Parenchymatous cells containing raphides, starch granules or silica masses can be observed.
-
(3).
Vascular bundles: Closed collateral vascular bundles, 78 (52)–134, arranged in 4–5 whorls in parenchyma, with similar size.
-
(4).
Fiber groups: outside vascular bundles, two hat-shaped, consisting of 9–58 fiber cells, 5–37 μm in diameter. Occasionally, a hat-shaped or a ring-shaped fiber group can be observed.
-
(5).
Raphides: occurring in parenchymatous cells near the epidermis, (10) 52–194 μm in length.
-
(6).
Silica masses: occurring in parenchymatous cells outside the vascular bundles.
2.2.1.2. Stems of D. devonianum—Outline is nearly circular (Table 1 and Table 3)
-
(1).
Epidermis: a row of cells, thin and flat, 17–85 μm in diameter, lateral walls were slightly lignified, covered with yellow to orange cuticles. A layer of pericladium consisting of parenchymatous cells and vascular bundles can sometimes be observed outside the epidermis.
-
(2).
Parenchyma: Parenchymatous cells various in size, usually small near the vascular bundles. Parenchymatous cells containing raphides, starch granules or silica masses can be observed.
-
(3).
Vascular bundles: closed collateral vascular bundles, 57–91, various in size.
-
(4).
Fiber groups: outside vascular bundles, hat-shaped, consisting of 7–43 of fiber cells, 7–45 μm in diameter.
-
(5).
Raphides: occurring in parenchymatous cells near the epidermis and vascular bundles, 51–132 μm in length.
-
(6).
Silica masses: occurring in parenchymatous cells outside the vascular bundles.
2.2.1.3. Stems of D. aphyllum—Outline is nearly circular (Table 1 and Table 4)
-
(1).
Epidermis: a row of cells, thin and flat, 13–38 μm in diameter, lateral walls were slightly lignified, covered with yellow to orange cuticles. A layer of pericladium consisting of parenchymatous cells and vascular bundles can be observed outside the epidermis sometimes.
-
(2).
Parenchyma: Parenchymatous cells similar in size, usually smaller near the vascular bundles. Parenchymatous cells containing raphides, starch granules or silica masses can be observed.
-
(3).
Vascular bundles: closed collateral vascular bundles, 53–85, with similar size.
-
(4).
Fiber groups: outside vascular bundles, hat-shaped, consisting of 8–47 fiber cells, 5–33 μm in diameter.
-
(5).
Raphides: scattered or in bundles, non-specific raphide distribution, 65–202 μm in length.
-
(6).
Silica masses: occurring in parenchymatous cells outside the vascular bundles.
2.2.1.4. Stems of D. gratiosissimum—Outline is nearly circular (Table 1 and Table 5)
-
(1).
Epidermis: a row of cells, thin and flat, 11–35 μm in diameter, lateral walls were slightly lignified, covered with yellow to orange cuticles.
-
(2).
Parenchyma: Parenchymatous cells various in size, usually small near the vascular bundles. Parenchymatous cells containing raphides, starch granules or silica masses can be observed.
-
(3).
Vascular bundles: closed collateral vascular bundles, 57–84 (124), various in size.
-
(4).
Fiber groups: outside vascular bundles, hat-shaped, consisting of 19–66 fiber cells, 6–31 μm in diameter.
-
(5).
Raphides: occurring in parenchymatous cells near vascular bundles, 39–124 μm in length.
-
(6).
Silica masses: occurring in parenchymatous cells outside the vascular bundles.
2.2.2. Transverse Section of Stems (Observed Under Fluorescence Microscope)
Stems of D. officinale—Light intensity of dried samples is more strongly than fresh ones. Observed with excitation filter ex 450–500 nm and DM 505 nm emission filters, the cuticle, wall of epidermal cells and vascular bundles emitted green fluorescence. In vascular bundles, xylem vessels and fiber groups outside emitted green fluorescence. Especially, the “two hat-shaped” fiber groups can be easily observed. Some parenchymatous cells emitted green fluorescence (Table 6). The light intensity is various in different samples. Observed with excitation filter ex 530–590 nm and DM 595 nm emission filters, the cuticle and wall of epidermal cells emitted red fluorescence. The fluorescence of vascular bundles is characteristic, sometimes xylem vessels and fiber groups outside emitted strong red fluorescence, sometimes the content of parenchymatous cell around vascular bundles emit red fluorescence, while xylem vessels and fiber groups outside emit no fluorescence. In addition, some parenchymatous cells emitted red fluorescence (Table 6).
Stems of D. devonianum-Light intensity of dried samples is more strong than for fresh ones. Observed with excitation filter ex 450–500 nm and DM 505 nm emission filters, only “hat-shaped” fiber groups can be easily observed. The other fluorescence characteristics of D. devonianum are similar to those of D. officinale (Table 6). Observed with excitation filter ex 530–590 nm and DM 595 nm emission filters, the fluorescence characteristics of D. devonianum are similar to those of D. officinale (Table 6).
Stems of D. aphyllum-Light intensity of dried samples is more strongly than fresh ones. Observed with excitation filter ex 450–500 nm and DM 505 nm emission filters, the fluorescence characteristics of D. aphyllum are nearly the same as those of D. devonianum (Table 6). Observed with excitation filter ex 530–590 nm and DM 595 nm emission filters, the fluorescence characteristics of D. aphyllum are similar to those of D. officinale (Table 6).
Stems of D. gratiosissimum-Light intensity of dried samples is more strongly than fresh ones. Observed with excitation filter ex 450–500 nm and DM 505 nm emission filters, the fluorescence characteristics of D. gratiosissimum are nearly the same as those of D. devonianum (Table 6). Observed with excitation filter ex 530–590 nm and DM 595 nm emission filters, the fluorescence characteristics of D. gratiosissimum are similar to those of D. officinale (Table 6).
2.3. Discussion
By combining normal light and fluorescence microscopy, D. officinale and the common adulterants can be easily differentiated from each other based on the characteristics of their transverse sections. Both types of microscopy have their own merits in the authentication study. Normal light microscopy can provide the basic morphological characteristics. As a supplemental tool, fluorescence microscopy can exhibit specific auto-fluorescence from different plant tissues by virtue of their varied chemical constituents, and it excels in manifestation of the shape (as shown in Table 6), which makes the authentication work more easy and convenient. The comparison of D. officinale and its adulterants can be summarized as follows:
-
(1)
Cuticle: color and thickness of cuticle of four studied Dendrobium species are similar.
-
(2)
Epidermis: size of epidermal cells of D. officinale, D. aphyllum and D. gratiosissimum are similar, epidermal cells of D. devonianum is bigger than the other three species, up to 85 μm in diameter.
-
(3)
Vascular bundle: Vascular bundles of D. officinale, usually more than 90, are more abundant than in the three adulterants. The size of vascular bundles in D. officinale is similar apart from those near the epidermis which are slightly smaller. Vascular bundles of D. devonianum are about 70 in number, various in size, and much bigger in the centre than at the margins of the stem. Vascular bundles of D. aphyllum are about 65, with similar size, apart from those near to theepidermis that are slightly smaller. Vascular bundles of D. gratiosissimum are about 70, althoughmore than 100 can be observed occasionally. Various in size with no apparent distribution rules.
-
(4)
Fiber groups: D. officinale with “two hat-shaped” fiber groups, while the other three species only have “one hat-shaped” fiber groups.
-
(5)
Raphides: D. officinale scattered in parenchmatous cells near epidermis. D. devonianum scatteredin parenchmatous cells near epidermis and vascular bundles. D. aphyllum scattered in parenchmatous cells throughout. D. gratiosissimum scattered in parenchmatous cells near vascular bundles.
We can therefore put forth the following key to identifying D. officinale and its common adulterants:
| (1). “Two hat-shaped” fiber groups emitted green fluorescence can be easily observed, Raphides distribute near the epidermis | D. officinale | |
| (1). “One hat-shaped” fiber groups emitting green fluorescence can be easily observed | ||
| (2). Vascular bundles similar in size, non-specific raphide distribution | D. aphyllum | |
| (2). Vascular bundles various in size | ||
| (3). Vascular bundles are much bigger in the centre than in the margin of the stem. Raphides distribute near the epidermis and vascular bundles | D. devonianum | |
| (3). Vascular bundles of different size distribute with no obvious rule. Raphides distribute near the vascular bundle | D. gratiosissimum | |
When fluorescence microscopy used, observed with excitation filter ex 450–500 nm and DM 505 nm emission filters, the cuticle, wall of epidermal cells and xylem vessels together with fiber groups outside vascular bundles emitted green fluorescence. Among them, the “two hat-shaped” fiber groups can be easily observed as the diagnostic feature of D. officinale. However, the other three Dendrobium species only possess “one hat-shaped” fiber groups. When observed with excitation filter ex 530–590 nm and DM 595 nm emission filters, though the red fluorescence can be observed in vascular bundles, it is not identical. Generally, xylem vessels in vascular bundles and fiber groups outside the vascular bundles emit red fluorescence, otherwise, the content of parenchymatous cells around the vascular bundles emit red fluorescence. Interestingly, the intensity and distribution of fluorescence are various in different samples, even of the same species, which may due to their different production areas, collection time and processing methods. Therefore, identification of the three adulterants only by fluorescence microscopy seems insufficient. The non-fluorescence microscopic features such as the distribution of rapheids, the size and amount of vascular bundles provided by the normal light microscopy were proposed to differentiate the three adulterants species.
3. Experimental Section
3.1. Materials
3.1.1. Samples
Twenty-four samples comprising four species of Dendrobium stems were collected from different main production areas in China and Myanmar, and authenticated by Associate Prof. Hua-wei Zhang (Zhejiang University of Technology, Hangzhou, People’s Republic of China) and Prof. Zeng-xi Guo (Zhejiang Institute for Food and Drug Control, Hangzhou, People’s Republic of China). The details of each crude drug are given in Table 7. The voucher specimens were deposited in the Herbarium of Traditional Chinese Medicine, Zhejiang University of Technology.
Table 7.
Crude drugs collected in markets and the main production areas.
| Sample No. | Origin | Collection area/market | Collection date | Trade name |
|---|---|---|---|---|
| TP-1 | D. officinale | GAP bases, Pu’er, Yunnan Province | September 2011 | Tie-pi-shi-hu |
| TP-2 | D. officinale | GAP bases, Tiantai, Zhejiang Province | October 2011 | Tie-pi-shi-hu |
| TP-3 | D. officinale | GAP bases, Tiantai, Zhejiang Province | September 2011 | Tie-pi-shi-hu |
| TP-4 | D. officinale | GAP bases, Tiantai, Zhejiang Province | August 2011 | Tie-pi-shi-hu |
| TP-5 | D. officinale | GAP bases, Tiantai, Zhejiang Province | April 2013 | Tie-pi-shi-hu |
| TP-6 | D. officinale | GAP bases, Tiantai, Zhejiang Province | May 2013 | Tie-pi-shi-hu |
| TP-7 | D. officinale | GAP bases, Tiantai, Zhejiang Province | Marrch 2013 | Tie-pi-shi-hu |
| TP-8 | D. officinale | Pan’an market, Zhejiang Province | September 2013 | Tie-pi-shi-hu |
| TP-9 | D. officinale | Pan’an market, Zhejiang Province (fresh) | September 2013 | Tie-pi-shi-hu |
| TP-10 | D. officinale | Pan’an market, Zhejiang Province (fresh) | September 2013 | Tie-pi-shi-hu |
| TP-11 | D. officinale | Wuyi, Zhejiang Province (fresh) | July 2013 | Tie-pi-shi-hu |
| TP-12 | D. officinale | Wuyi, Zhejiang Province (fresh) | July 2013 | Tie-pi-shi-hu |
| TP-13 | D. officinale | Pu’er, Yunnan Province (fresh) | July 2013 | Tie-pi-shi-hu |
| TP-14 | D. officinale | Pu’er, Yunnan Province (fresh) | July 2013 | Tie-pi-shi-hu |
| TP-15 | D. officinale | Pu’er, Yunnan Province (fresh) | July 2013 | Tie-pi-shi-hu |
| ZP-1 | D. devonianum | Myanmar (fresh) | January 2013 | Zi-pi-shi-hu |
| ZP-2 | D. devonianum | Pan’an market, Zhejiang Province | September 2013 | Zi-pi-shi-hu |
| ZP-3 | D. devonianum | Pan’an market, Zhejiang Province | September 2013 | Zi-pi-shi-hu |
| SC-1 | D. aphyllum | Myanmar (fresh) | January 2013 | Shui-cao-shi-hu |
| SC-2 | D. aphyllum | Pan’an market, Zhejiang Province | September 2013 | Shui-cao-shi-hu |
| SC-3 | D. aphyllum | Pan’an market, Zhejiang Province | September 2013 | Shui-cao-shi-hu |
| GJ-1 | D. gratiosissimum | Myanmar (fresh) | January 2013 | Guang-jie-shi-hu |
| GJ-2 | D. gratiosissimum | Pan’an market, Zhejiang Province | September 2013 | Guang-jie-shi-hu |
| GJ-3 | D. gratiosissimum | Pan’an market, Zhejiang Province | September 2013 | Guang-jie-shi-hu |
3.1.2. Apparatus
-
(a)
Optika digital camera DS-Fi1
-
(b)
Optika Microscope equipped with CCD from Photometrics Coolsnap to capture photos.
-
(c)
Optika Fluorescence Microscope B-600TiFL
-
(d)
Canon digital camera 550D
3.1.3. Software
Optika Vision Pro, TWAIN interface, SDK were used.
3.2. Method
3.2.1. Morphological Characteristics of four Dendrobium Stems
The gross exterior characters of each sample were examined by observing, measuring, touching, smelling, and tasting. The color digital photographs were taken by Canon digital camera 550D.
3.2.2. Transverse Section of Four Dendrobium Stems
The dried samples were moderately moistened firstly. Then, transverse section of samples (fresh sample or moistened dried sample) is made by cutting bare-handed. General procedures are as follows: three left fingers were used to fix the material at first. Then, put the blade which is held with the right hand against the material and slice smoothly from the left outward to the right inward. The tree-hand sections were investigated for possible auto fluorescence after sealing the mounted specimen along with purified water obtained from a Mili-Q water purification system (Millipore, Bedford, MA, USA).
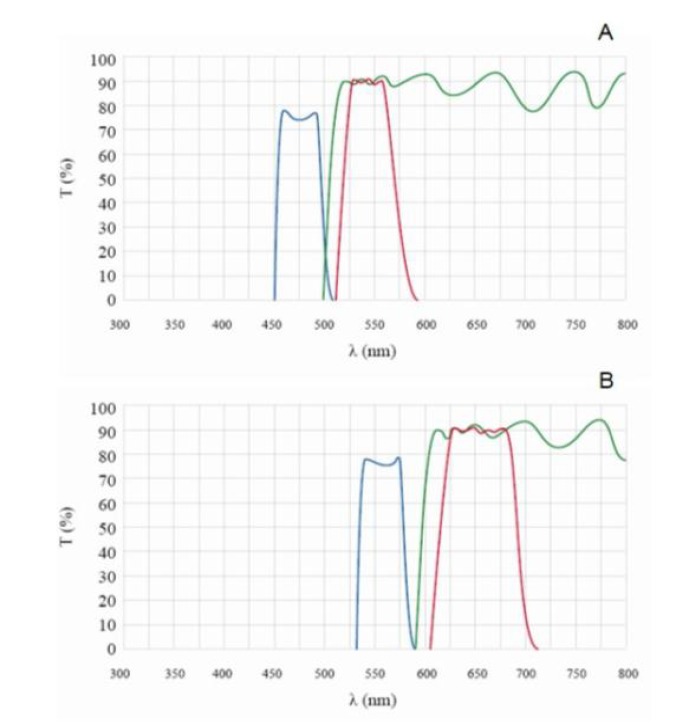
The images observed with normal light and fluorescence microscope were recorded digitally. The transmittance spectra of two emission filters are shown in Figure 2. Excitation from HBO 100 light source through the excitation filter (EF) 450–500 nm and emission through dichroic mirror (DM) 505 nm (blue light), as well as excitation through filter BP 530–590 nm and emission through dichroic mirror DM 595 nm (green light) were used for observing fluorescence.
Figure 2.
The transmittance spectra of fluorescence. (A) with EF 450–500 nm and DM 505 nm; (B) with EF 530–590 nm and DM 595 nm.
The blue line is the excitation wavelength; the red line stands for the barrier filter wavelength; the green line stands for the dichroic mirror cut-off wavelength.
4. Conclusions
This is the first research combining normal light and fluorescence microscopy to thoroughly identify Dendrobium officinale and its counterfeits sold in the market through an investigation of the transverse sections of crude drug stems. Fluorescence microscopy, as a supplementary tool for routine microscopic identification, provides in vivo pictures and needs no professional researchers. According to the fluorescence characteristics, a further histochemical investigation on the chemical distribution in different tissues of D. officinale will be carried out. It is expected to be of great use in the identification and quality evaluation of Dendrobium species.
Acknowledgments
This work was supported by grants from Qianjiang talent Project of Zhejiang Province (Project No. 2012R10053).
Author Contributions
All listed authors contributed to this work. Chu Chu, Li Xia and Jizhong Yan contributed to the conception of the study. Chu Chu, Huimin Yin and Dongping Cheng performed all of the experiments in this study and contributed to the data. Li Xia did edition of pictures. Chu Chu and Jizhong Yan obtained financial support. Lin Zhu helps to revise and polish up the manuscript. All authors participated in the drafting of this manuscript. All authors read and approved the final manuscript.
Conflictts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Editorial Board of Flora of China . Angiospermae, Monocotyledoneae. Flora Reipublicae Popularis Sinica. Volume 19. Science Press; Beijing, China: 1999. pp. 67–146. (in Chinese) [Google Scholar]
- 2.China Pharmacopoeia Committee . Pharmacopoeia of China. Volume I. Medical Science Press; Beijing, China: 2010. pp. 265–266. [Google Scholar]
- 3.Lin X., Shaw P.C., Sze S.C., Tong Y., Zhang Y. Dendrobium officinale polysaccharides ameliorate the abnormality of aquaporin 5, pro-inflammatory cytokines and inhibit apoptosis in the experimental Sjogren’s syndrome mice. Int. Immunopharmacol. 2011;11:2025–2032. doi: 10.1016/j.intimp.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Xiang L., Stephen Sze C.W., Ng T.B., Tong Y., Shaw P.C., Sydney Tang C.W., Kalin Zhang Y.B. Polysaccharides of Dendrobium officinale inhibit TNF-alpha-induced apoptosis in A-253 cell line. Inflamm. Res. 2013;62:313–324. doi: 10.1007/s00011-012-0584-x. [DOI] [PubMed] [Google Scholar]
- 5.Xiao L., Ng T.B., Feng Y.B., Yao T., Wong J.H., Yao R.M., Li L., Mo F.Z., Xiao Y., Shaw P.C., et al. Dendrobium candidum extract increases the expression of aquaporin-5 in labial glands from patients with Sjogren’s syndrome. Phytomedicine. 2011;18:194–198. doi: 10.1016/j.phymed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kowitdamrong A., Chanvorachote P., Sritularak B., Pongrakhananon V. Moscatilin inhibits lung cancer cell motility and invasion via suppression of endogenous reactive oxygen species. Biomed. Res. Int. 2013;2013:765894. doi: 10.1155/2013/765894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Y.Y., Wen J.Y, Fan J.S. Pharmacognostic identification of Dendrobium officinale and Dendrobium devonianum. Strait Pharm. J. 2011;23:52–53. [Google Scholar]
- 8.Li J., Li S.X., Huang D. Advances in the resources, constituents and pharmacological effects of Dendrobium officinale. Sci. Technol. Rev. 2011;29:74–79. [Google Scholar]
- 9.Chen X., Wang F., Wang Y., Li X., Wang A., Wang C., Guo S. Discrimination of the rare medicinal plant Dendrobium officinale based on naringenin, bibenzyl, and polysaccharides. Science China. Life Sci. 2012;55:1092–1099. doi: 10.1007/s11427-012-4419-3. [DOI] [PubMed] [Google Scholar]
- 10.Yan M.Q., Chen S.H., Lv G.Y., Zhou G.F., Liu X. HPLC specific chromatogram of Dendrobium officinale. China J. Chin. Mater. Med. 2013;38:516–519. [PubMed] [Google Scholar]
- 11.Lv X.K., Cheng C.G., Yang G.P., Jin Y., Ye H., Xu D.W. Appl ication of FTIR spectroscopy to the analysis of eleven kinds of Dendrobium. China J. Chin. Mater. Med. 2005;30:738–740. [PubMed] [Google Scholar]
- 12.Zhang W., Ding X., Xie M., Feng Z., Lu S., Li X., Zhang F., Ding G. Authentication of three valuable Dendrobium species by adapter ligation-mediated allele-specific amplification. Eur. Food Res. Technol. 2009;229:1–7. doi: 10.1007/s00217-009-1019-y. [DOI] [Google Scholar]
- 13.Xu H., Ying Y., Wang Z.T., Cheng K.T. Identification of Dendrobium species by dot blot hybridization assay. Biol. Pharm. Bull. 2010;33:665–668. doi: 10.1248/bpb.33.665. [DOI] [PubMed] [Google Scholar]
- 14.Xu L.S., Xu G.J., Sha W.L., Luo J.Y. Studies on the microscopic identification of Chinese drug Shi-Hu. J. China Pharm. Univ. 1980;2:1–7. [Google Scholar]
- 15.Guan Y.H., Li H.T., Wang Y.Q., Li X.L. Comparison of Microscopical characters between Dendrobium officinale and Dendrobium devonianum. J. Chin. Med. Mater. 2010;33:1869–1871. [Google Scholar]
- 16.Liu X.P., Tang M.H., Dai Y., Liu H.J., Xu G.J., Xu L.S. Microscopic identification of the powder of Chinese drug Shihu. J. China Pharm. Univ. 1992;22:148–151. [Google Scholar]
- 17.Liang Z.T., Jiang Z.H., Leung K.S., Peng Y., Zhao Z.Z. Distinguishing the medicinal herb Oldenlandia diffusa from similar species of the same genus using fluorescence microscopy. Microsc. Res. Tech. 2006;69:277–282. doi: 10.1002/jemt.20312. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y.Q., Liang Z.T., Li Q., Yang H., Chen H.B., Zhao Z.Z., Li P. Identification of powdered Chinese herbal medicines by fluorescence microscopy, Part 1: Fluorescentcharacteristics of mechanical tissues, conducting tissues, and ergastic substances. Microsc. Res. Tech. 2011;74:269–280. doi: 10.1002/jemt.20901. [DOI] [PubMed] [Google Scholar]
- 19.Wan X.J., Liang Z.T., Chen H.B., Zhao Z., Li P. Identification of Daqingye and Banlangen including crude drugs and decoction dregs from three plant species by normal light and fluorescence microscopy. Microsc. Res. Tech. 2013;76:774–782. doi: 10.1002/jemt.22228. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y.N., He X.C., Chen Q.L., Fan L.L., Zhang J.Y., Zhao Z.Z., Dong L.S., Liang Z.T., Yi T., Chen H.B. A mixed microscopic method for differentiating seven species of “Bixie”-related Chinese Materia Medica. Microsc. Res. Tech. 2014;77:57–70. doi: 10.1002/jemt.22313. [DOI] [PubMed] [Google Scholar]