How the prefrontal cortex and subthalamic nucleus coordinate their activity during decision-making is unclear. By recording simultaneously from the two structures, Zavala et al. show that within-trial cognitive control involves theta oscillations, whereas across-trial adaptations involve beta oscillations. The two structures participate in separate, complementary stages of the decision-making process.
Keywords: cognitive control, STN, mPFC, oscillations, deep brain stimulation (DBS)
Abstract
There is increasing evidence that the medial prefrontal cortex participates in conflict and feedback monitoring while the subthalamic nucleus adjusts actions. Yet how these two structures coordinate their activity during cognitive control remains poorly understood. We recorded from the human prefrontal cortex and the subthalamic nucleus simultaneously while participants (n = 22) performed a novel task involving high conflict trials, complete response inhibition trials, and trial-to-trial behavioural adaptations to conflict and errors. Overall, we found that within-trial adaptions to both conflict and complete response inhibition involved changes in the theta band while across-trial behavioural adaptations to both conflict and errors involved changes in the beta band (P < 0.05). Yet the role each region’s theta and beta oscillations played during the task differed significantly between the two sites. Trials that involved either within-trial conflict or complete response inhibition were associated with increased theta phase synchrony between the medial prefrontal cortex and the subthalamic nucleus (P < 0.05). Despite increased synchrony, however, increases in prefrontal theta power were associated with response inhibition, while increases in subthalamic theta power were associated with response execution (P < 0.05). In the beta band, post-response increases in prefrontal beta power were suppressed when the completed trial contained either conflict or an erroneous response (P < 0.05). Subthalamic beta power, on the other hand, was only modified during the subsequent trial that followed a conflict or error trial. Notably, these adaptation trials exhibited slower response times (P < 0.05), suggesting that both brain regions contribute to across-trial adaptations but do so at different stages of the adaptation process. Taken together, our data shed light on the mechanisms underlying within-trial and across-trial cognitive control and how disruption of this network can negatively impact cognition. More broadly, however, our data also demonstrate that the specific role of a brain region, rather than the frequency being utilized, governs the behavioural correlates of oscillatory activity.
Introduction
To successfully navigate a complex and rapidly changing world, we must continually monitor and adjust our actions due to conflicts, mistakes, and feedback. This ability is referred to as cognitive control, and involves several separate yet related processes that together optimize goal-directed behaviour (Miller and Cohen, 2001; Ridderinkhof et al., 2004; Cohen, 2014). Cognitive control is necessary, for instance, in situations that require overcoming a habitual movement when selecting the most appropriate action among multiple conflicting choices (Miller and Cohen, 2001). For example, when crossing the street, individuals from most countries will habitually first look for cars coming from the left, and then the right, before committing to crossing the street. However, when these individuals are crossing a street in the UK for the first time, they may abruptly find that they actually need to first look to the right. Cognitive control involves not only monitoring for such instances that require altering a pre-potent action, but also adapting to the current environment so as to appropriately adjust future actions. When approaching the next street, the same individuals may now consciously make an effort to look to the right first so as not to repeat the same mistake.
Cognitive control has been largely thought to involve structures of the medial prefrontal cortex (mPFC) (Miller and Cohen, 2001; Cavanagh et al., 2011, 2012; Cohen and Cavanagh, 2011). During conflict, complete response inhibition, and error trials, neural activity within the mPFC increases, and these increases correlate with slower response times on the current trial and on subsequent trials (Kerns et al., 2004; Cavanagh et al., 2009; Danielmeier et al., 2011; Nigbur et al., 2011; Sheth et al., 2012; Cohen and van Gaal, 2014; Chinn et al., 2018). Accordingly, the ability to adjust response times following error or conflict is impaired in both humans and rodents with lesions of the mPFC (Modirrousta and Fellows, 2008; Sheth et al., 2012; Narayanan et al., 2013).
Recent evidence, however, has suggested that the subthalamic nucleus (STN) may also participate in cognitive control (Zavala et al., 2015). The STN receives afferent connections from cortical regions involved in cognitive control (Alexander et al., 1986; Aron et al., 2007; Kelley et al., 2018), and projects efferent connections to other basal ganglia structures involved in movement inhibition (Albin et al., 1989; DeLong, 1990). Beta oscillations (10–30 Hz) within the STN regulate both motor and non-motor actions and increase when individuals must cancel or slow down a pre-planned action (Kühn et al., 2004; Brittain et al., 2012; Leventhal et al., 2012; Ray et al., 2012; Alegre et al., 2013; Bastin et al., 2014; Benis et al., 2014; Wessel et al., 2016; Herz et al., 2017; Zavala et al., 2017b). Theta oscillations (2–8 Hz) and spiking activity in the STN also increase when individuals are making decisions in the presence of conflict (Zaghloul et al., 2012; Zavala et al., 2013), and importantly, conflict-related increases in theta oscillations are coherent with theta activity in the mPFC (Zavala et al., 2014, 2016). Furthermore, high frequency deep brain stimulation (DBS) of the STN appears to disrupt this mPFC-STN network, consequently leading to more conflict-related errors (Frank et al., 2007; Cavanagh et al., 2011). Conversely, low frequency DBS to the STN in the theta band seems to enhance mPFC theta activity and improve response inhibition (Kelley et al., 2018). These anatomical and functional links between the mPFC and the STN therefore suggest that the two structures may intimately interact to mediate cognitive control. Yet in what ways the roles of these two brain regions may or may not be complementary remains unclear.
One possibility is that the STN and mPFC form a hierarchical relationship in the context of cognitive processing. The mPFC has been implicated in determining which actions and strategies are most appropriate in a changing environment (Miller and Cohen, 2001; Rushworth et al., 2004; Schuck et al., 2015). Monitoring for errors and adjusting to changing task rules would require the higher order level of cognitive control afforded by the mPFC, focused in this case on continuously selecting the optimal strategy for the behavioural task at hand. Conversely, the STN may serve as a downstream target for implementing the strategy identified by the mPFC, in this case directly involved in the execution of actions rather than selecting which strategy is most appropriate for the task at hand (Jenkinson and Brown, 2011; Goldberg et al., 2013). The STN would therefore participate in cognitive control by adjusting the timing of individual actions and deferring a particular response so that the most appropriate action may be achieved among competing choices.
It seems reasonable therefore to ascribe different, albeit overlapping and complementary, roles to these two coupled structures. But how are these roles subserved by changes in neuronal activity? Specifically, can these roles be mapped on to the classical distinction between cognitive-theta and motoric-beta activities, or is the coordination of processing more nuanced? We explore these questions here by simultaneously recording from the human STN and mPFC during DBS surgery while participants perform a novel response inhibition task. We were specifically interested in how complementary activity within the STN and mPFC reflects cognitive control related to both the execution of a current action and the adjustments that are made for future actions. We hypothesized that whereas the STN may be directly involved in the execution of actions, activity in the mPFC may mediate adjustments and strategies that are used for selecting the appropriate action. Our recordings revealed a temporally sequenced, spatio-spectral segregation in the pattern of neural activity between the two sites. This activity reflects the specific actions involved in making or inhibiting a motor movement, and the adjustments that are necessary to select the appropriate movement during conflict and for subsequent trials. Taken together, our results evidence the basic principle that the precise behavioural associations of oscillatory activity are determined not by frequency band per se, but by the circuits in which they are expressed.
Materials and methods
We made intraoperative recordings in 22 participants (n = 22) undergoing DBS surgery of the STN for Parkinson’s disease. As per routine DBS surgery, we simultaneously advanced three targeting electrodes during each recording session (Fig. 1C). Each targeting electrode consisted of a microelectrode contact and a macroelectrode contact positioned 3 mm dorsal to the microelectrode tip (Alpha Omega). During the operative procedure, we also acquired simultaneous intracranial EEG recordings from a subdural strip electrode temporarily placed through the DBS burr hole (PMT Corporation). The electrodes were placed over the superior frontal gyrus and therefore over the superior portion of the medial prefrontal cortex. Thus, we refer to the area from which we recorded as the mPFC.
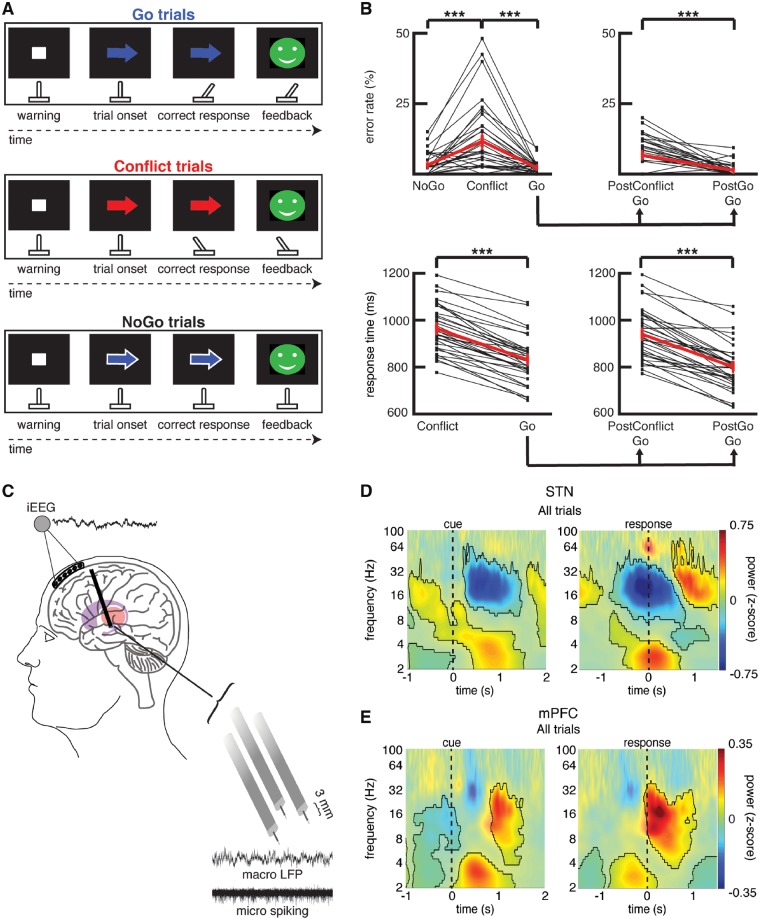
Figure 1.
Arrow task during recordings captured from the human STN and mPFC. (A) Task. A warning cue appears on the screen to prepare the participant for the upcoming trial. During each trial, a coloured arrow appears on the screen to indicate the appropriate action. If the arrow is blue, the participant must move a handheld joystick in the direction the arrow is pointing (Go trials). If the arrow is red, the movement must be in the opposite direction from where the arrow is pointing (Conflict trials). If the arrow has a white border around it (regardless of arrow colour), the participant must withhold all movements (NoGo trials). (B) Error rates during the intraoperative sessions, shown for each session (thin black lines) as well as averaged across all sessions [thick red line, mean ± standard error of the mean (SEM), n = 34], are shown in the upper panel. Average response times during the correct trials are shown in the lower panel. Error rates and response times for all correct Go trials are also plotted separately for Go trials that followed a previous Go trial and Go trials that followed a Conflict trial. ***P < 0.001. Overall, subjects showed higher error rates and slower response times during the Conflict trials. Go trials that followed a Conflict trial (PostConflict Go) also showed slower response times and higher error rates. (C) Six-contact electrode strips are placed over the mPFC to capture intracranial EEG activity during the task. Simultaneously, three macro/microelectrode pairs capture local field potential (macro) and action potential spiking activity (micro) from the STN. A 5-s sample recording is shown for each. (D) Normalized oscillatory power averaged across all STN electrodes and across all correct trials. Data are aligned to arrow onset (left) and to the motor response (right, t = 0); mask indicates time-frequency regions exhibiting significant differences from baseline (P < 0.05, corrected for multiple comparisons, permutation test). NoGo trials were excluded from the response aligned analysis. (E) Same as in D but for the mPFC. Both brain regions showed a pre-response increase in theta power, although this is earlier and greatest averaged to the cue in the case of mPFC. In the beta band, the STN showed a pre-response decrease in power while the mPFC showed a post-response increase in beta power. iEEG = intracranial EEG.
Participants performed a novel response inhibition task (Fig. 1A) in the intraoperative environment on a testing laptop running PsychoPy (Peirce, 2007). Each trial began with the subjects staring at a blank black screen. A white fixation dot then appeared in the centre of the screen for 500 ms. We subsequently displayed an arrow pointing in either the leftward or rightward direction. The colour of the arrow could be either blue or red. If the arrow was blue (Go trials), then the participant was required to move a digital joystick in the direction the arrow was pointing. If the arrow was red (Conflict trials), then the participant was required to move the joystick in the opposite direction of the arrow. If, however, the arrow had a white border around it (NoGo trials), the participant was instructed to withhold all movements until the arrow disappeared from the screen. Of the 200 trials in each session, 120 were blue arrow Go trials, 40 were red arrow Conflict trials, and 40 were white border NoGo trials. The colour of the arrow on the NoGo trials was blue on 30 of the trials and red on 10 of the trials (consistent with the overall 3:1 ratio of blue:red arrows), although this information was irrelevant given that the participants were withholding a response on these trials. One hundred and fifty milliseconds after each trial, participants were given visual feedback for that trial and then the feedback was replaced by the blank screen for the next trial.
To obtain magnitude and instantaneous phase information in the frequency domain, we convolved each trial’s local field potential signals captured from the STN and intracranial EEG signals captured from the subdural contacts with complex valued Morlet wavelets. All time-frequency points were then normalized to a 500 ms baseline period recorded during the blank-screen intertrial intervals. For each recording, we then averaged the normalized power across all macroelectrodes that were within the STN and across the five intracranial EEG bipolar channels. This procedure resulted in one spectrogram for the STN and one for the mPFC during each trial. We then calculated the trial-averaged normalized power for all three conditions in each region in each experimental session. To assess differences in spectral power between conditions across recording sessions, we used a non-parametric permutation procedure (Maris and Oostenveld, 2007) that compared the real difference to an empiric distribution and used the empiric distribution to control for multiple comparisons (Supplementary material). To estimate the time-varying inter-site phase coherence between the STN and the mPFC, we used the same continuous-time estimate methods we have previously described (Zavala et al., 2017b), which are based on the techniques developed by Lachaux et al. (2002) (Supplementary material).
We also extracted spiking activity from the micro unit recordings. Given the difficulty of isolating single unit activity in the STN (Weinberger et al., 2006; Sharott et al., 2014), we used a previously outlined technique (Stark and Abeles, 2007) to identify STN multi-unit activity (Supplementary material). It is important to note, however, that multi-unit activity is thought to reflect a composite of the highly focal firing rate of neurons, bursting, recruitment, and synchronization effects (Moran et al., 2008). Though most of our analysis of STN spiking involved the multi-unit activity data, repeating the analysis using the more traditional voltage threshold method produced very similar patterns of activity for task responsive recordings (Supplementary Figs 4 and 5). To determine if the STN multi-unit activities exhibited a significant difference between trial types across STN recording sessions, we used the same non-parametric clustering-based procedure described above. Finally, we analysed the consistency of the relationship between macro-electrode local field potential phase and micro-electrode spiking to quantify the phase-multi-unit activity coupling (PMAUC; Supplementary material) during the task.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Behaviour
Twenty-two participants undergoing DBS surgery for Parkinson’s disease performed a novel response inhibition task involving three different trial types: Go, Conflict, and NoGo trials (Fig. 1A). The NoGo trials served as an important control that dissociated movement from difficulty by making two critical distinctions: a distinction between difficult (Conflict) and easy (Go and NoGo) trials independent of movement, and a distinction between movement (Go and Conflict) and no-movement (NoGo) trials independent of difficulty. These distinctions were justified by the behavioural responses of the participants (Fig. 1B). Overall, participants committed significantly more errors on the Conflict trials than the Go and the NoGo trials [ANOVA, within subjects repeated measures, F(2,33) = 21.35, P < 0.001]. Correct Conflict trials also had significantly longer response times than correct Go trials [t(33) = 15.18, P < 0.001, paired t-test]. Notably, participants adjusted their behaviour following correct trials that involved Conflict and following error trials. Response times during correct Go trials that followed a correct Conflict trial were 139.0 ± 11.8 ms slower than response times during correct Go trials that followed a previous correct Go trial [Fig. 1B; t(33) = 11.75, P < 0.001, paired t-test]. Similarly, response times were 51.2 ± 19.2 ms slower during correct Go trials that followed errors than during correct Go trials that followed correct trials [t(14) = 2.66, P < 0.05, paired t-test].
Within-trial conflict and movement signals
First, we investigated the overall changes in power in the STN and the mPFC during all correct trials compared to a baseline period (Fig. 1D and E). Overall, 2–5 Hz activity increased following the presentation of each arrow in both the STN and mPFC (Supplementary Fig. 2). A within-contact analysis revealed that the changes in mPFC spectral power were focal in nature (Supplementary Fig. 3). In both the mPFC and the STN, the 2–5 Hz power increases occurred prior to the response; however, the 2–5 Hz power peaked significantly earlier in the mPFC than in the STN [593 ± 76 ms versus 876 ± 86 ms; t(61) = 2.46, P < 0.01, unpaired t-test]. Conversely, the changes over the broad 8–30 Hz range were different in the STN and the mPFC. Overall, 8–30 Hz power decreased prior to the response in the STN, but increased in the mPFC and peaked after the movements were made (Fig. 1D and E). We focused the rest of our analyses on the trial-type related differences in the two frequencies bands (2–5 Hz and 8–30 Hz) that showed changes relative to baseline. We subsequently refer to the 2–5 Hz band as the theta band and the 8–30 Hz region as the beta band, although we recognize that these frequency ranges include frequencies often labelled as alpha and delta. We chose these frequencies because they demonstrated the most prominent task-related changes when all trials were compared to baseline, but we found similar results for our main analyses when we used more conventional frequency ranges for theta and beta (2–8 Hz and 12–30 Hz; Supplementary Fig. 8).
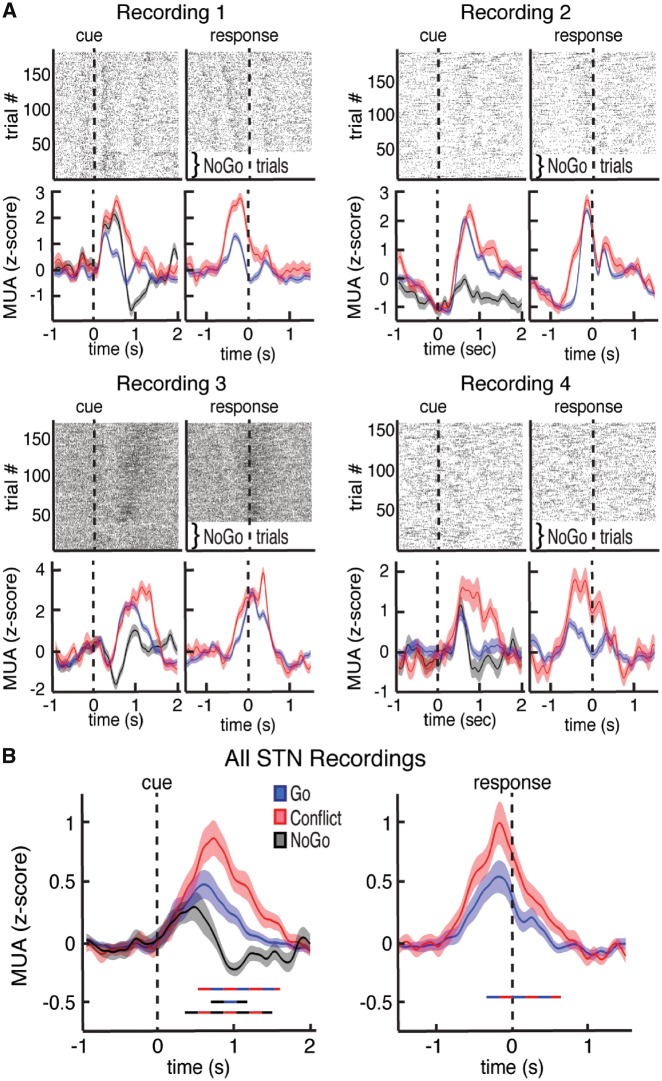
Within each trial, we first used the microelectrode recordings to examine how the output multi-unit spiking activity of the STN is modulated by the task. The majority of recordings (39 of 49) individually demonstrated significantly higher multi-unit activity during the task compared to baseline (P < 0.05, permutation test). However, even within these recordings, spiking activity was heterogeneous (Fig. 2A and Supplementary Fig. 4). Some recordings demonstrated higher multi-unit activity during Conflict and NoGo trials, whereas others demonstrated higher multi-unit activity during trials with movement (Go and Conflict trials). Overall, when we examined differences in multi-unit activity across the three trial types in all recordings, we found that Conflict trials exhibited the highest level of multi-unit activity, followed by Go trials, and then NoGo trials (P < 0.05, permutation test; Fig. 2B).
Figure 2.
Task-related changes in multi-unit activity in the STN. (A) Top: Raster plot for four neuronal clusters exhibiting changes in voltage thresholded spiking activity during the task. Bottom: Multi-unit activity (MUA) was extracted from each recording and plotted separately for Go, Conflict, and NoGo trials. Data are aligned to arrow onset (left) and to the motor response (right, t = 0) for four separate recordings. (B) Average continuous-time multi-unit activity for all STN microelectrode recordings that showed spiking activity. Time points exhibiting a significant difference between trial types (P < 0.05, corrected for multiple comparisons, permutation test) are denoted by coloured horizontal bars denoting which comparison was made. Conflict trials showed significantly higher multi-unit activity than Go trials. Notably, the trials in which participants inhibited all movements (NoGo trials) showed the lowest average multi-unit activity levels.
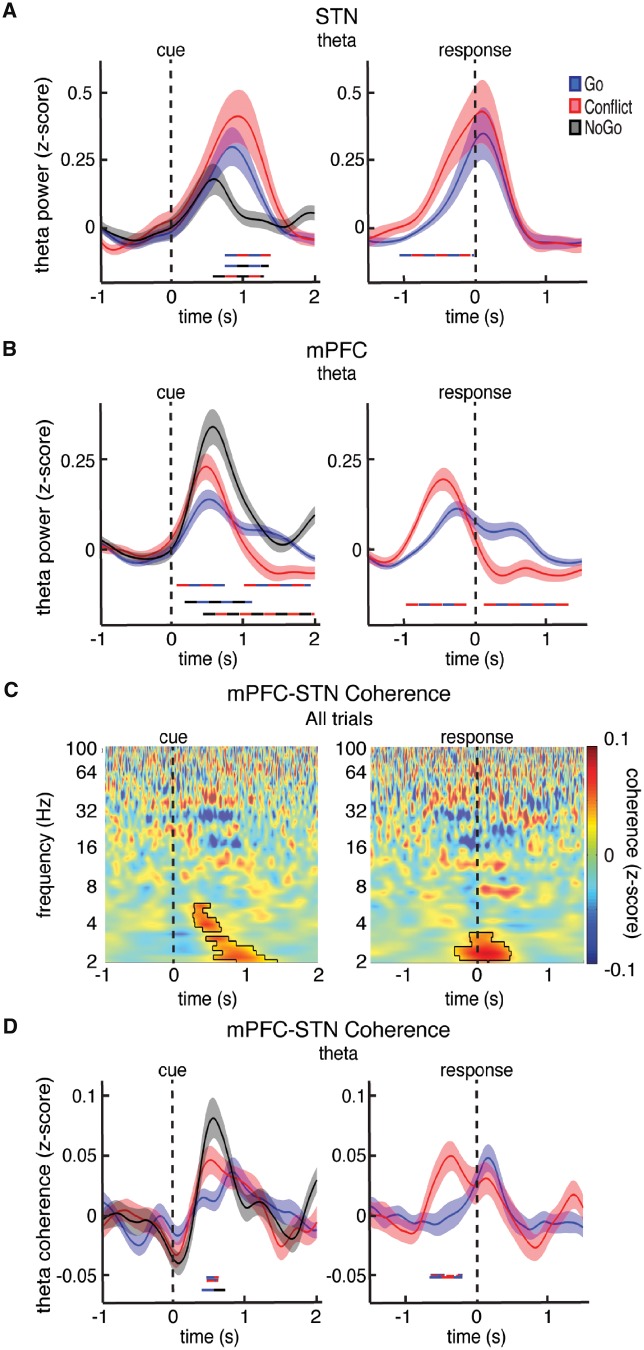
We next examined how the within-trial changes in theta power observed in both the STN and mPFC differ between trial types. The STN and the mPFC both demonstrated significantly higher pre-response theta power during the more difficult Conflict trials compared to the easier Go trials (P < 0.05, permutation test; Fig. 3A; post-response differences between Go and Conflict trials are most likely an extension of the post-response beta band differences between trials types discussed below). Across all trials, the increases in both mPFC and STN theta power were weakly correlated with individual trial response times (Supplementary Fig. 9). The NoGo trials, however, during which no movements were made, showed the largest theta power increases in the mPFC and the smallest increases in the STN.
Figure 3.
Trial type related differences in theta power and mPFC-STN phase coherence. (A and B) Cue and response aligned time evolving theta power changes averaged over all STN (A) and mPFC (B) electrodes during the Go, Conflict, and NoGo trials. Time points exhibiting a significant difference between trial types (P < 0.05, corrected for multiple comparisons, permutation test) are denoted by coloured horizontal bars denoting which comparison was made. (C) Normalized mPFC-STN phase coherence averaged across all mPFC-STN electrode pairs and across all correct trials. Mask indicates time-frequency regions exhibiting significant differences from baseline at P < 0.05, corrected for multiple comparisons, permutation test. NoGo trials were excluded from the response aligned analysis. (D) Same data as in C but averaged across the theta band and plotted separately for the Go, Conflict, and NoGo trials. Time points exhibiting a significant difference between trial types are denoted as in A. In general, conflict and response inhibition involved higher levels of theta power and mPFC-STN phase coherence prior to the response.
Because of the similar increases in theta power we observed in both brain regions, we also investigated whether there were any changes in phase coherence during the task. Across all trials, we found significantly greater theta band phase coherence during the task compared to the baseline period (P < 0.05, permutation test; Fig. 3C). When we compared the three trial types, we found significantly higher levels of theta phase coherence during Conflict and NoGo trials compared to Go trials (P < 0.05, permutation test; Fig. 3D).
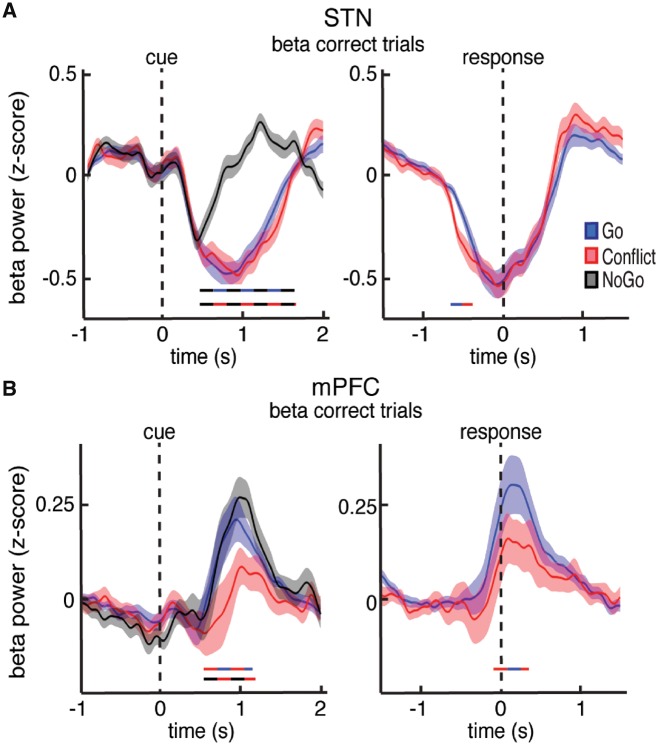
We also examined the changes in beta band power in the STN and found a significant decrease during all three trial types (Fig. 4A). The decrease in beta power was accompanied by a significant decrease in coupling between STN beta oscillatory phase and multi-unit activity amplitude (P < 0.05, permutation test; Supplementary Fig. 10). Notably, the STN beta power decrease was aborted earlier during NoGo trials, resulting in a significant difference in beta power between trials that involved movement and those that did not (P < 0.05, permutation test; Fig. 4A).
Figure 4.
Trial type related differences in beta power. (A and B) Cue and response aligned time evolving beta power changes averaged over all STN (A) and mPFC (B) electrodes during the Go, Conflict, and NoGo trials. Time points exhibiting a significant difference between trial types (P < 0.05, corrected for multiple comparisons, permutation test) are denoted by coloured horizontal bars denoting which comparison was made. Whereas the STN showed a pre-response decrease in beta power that was suppressed when movement had to be completely withheld, the mPFC showed a post-response increase in beta power that was suppressed during the more difficult Conflict trials.
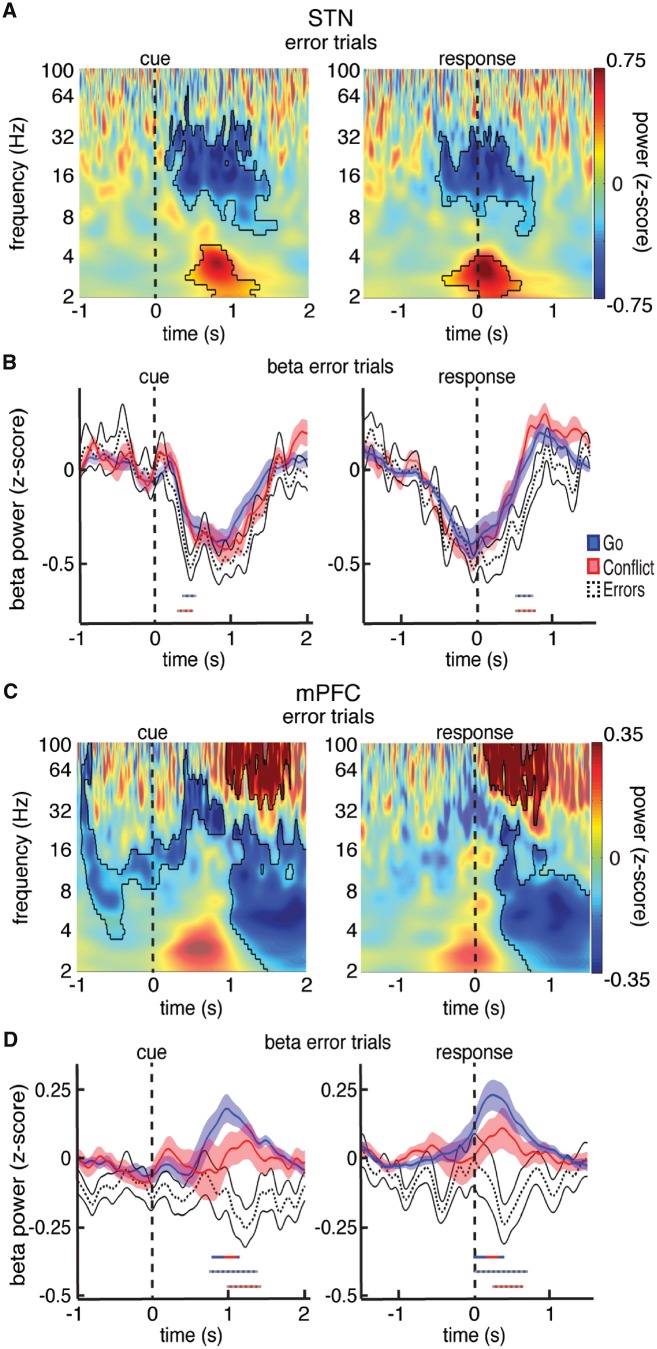
A prior study has suggested that premature decreases in beta power may be associated with the erroneous execution of prepotent actions (Brittain et al., 2012). We therefore analysed the relatively few errors that participants made (Fig. 5A). We found a significantly lower level of STN beta power within the first 500 ms of error trials compared to either Go or Conflict trials (P < 0.05, permutation test; Fig. 5B; see Supplementary Fig. 11 for STN spiking data during errors). These differences were locked to the cue and could not be due to the differences in movement onset as there was no significant difference in response times between error trials and correct Go trials [811.7 ± 26.6 ms versus 831.5 ± 17.2 ms; t(19) = 0.76, P > 0.05, paired t-test].
Figure 5.
Trial type related differences in beta power during errors. (A) Normalized oscillatory power averaged across all STN electrodes and across all incorrect trials. Mask indicates time-frequency regions exhibiting significant differences from baseline (P < 0.05, corrected for multiple comparisons, permutation test). (B) Same as in A but averaged across the entire beta band. The beta power time series are also shown for the Go and Conflict trials for comparison. Time points exhibiting a significant difference between trial types (P < 0.05, corrected for multiple comparisons, permutation test) are denoted by coloured horizontal bars denoting which comparison was made. (C and D) Same as in A and B but for the mPFC. Whereas the STN showed an early decrease in beta power that was more pronounced during error trials, the mPFC showed a post-response increase in beta power that was attenuated or even reversed during trials that involved either conflict or errors.
Post-response adaptations in the mPFC
Increases in overall mPFC beta activity occurred following the motor response (Fig. 1E). We found significantly higher beta power in the mPFC during Go trials compared to Conflict trials (P < 0.05, permutation test; Fig. 4B). Notably, these differences occurred after the participants executed the motor response. Furthermore, we found no significant differences between the Go and NoGo trials, as both showed a similar increase during the task. This suggests that the observed differences in mPFC beta activity between the Conflict trials and the Go and NoGo trials were strictly related to the difficulty of the completed trial, and not to movement.
The attenuated increases in mPFC beta power following Conflict trials suggest that beta oscillations may be involved in the recruitment of additional resources used for cognitive control following Conflict. Errors often recruit similar resources (Yeung et al., 2004). Therefore, we analysed the relatively few errors that participants made (Fig. 5C). Unlike the increases in mPFC beta activity observed following correct trials, we found that error trials exhibited a significant decrease in mPFC beta activity following the response. The post-response changes in beta power were significantly different between the error trials and the correct Go and correct Conflict trials (P < 0.05, permutation test; Fig. 5D). Moreover, error trials also demonstrated a significant increase in mPFC gamma power following the response (P < 0.05, permutation test; Fig. 5C and Supplementary Fig. 12). Subsequent analyses further revealed that Conflict trials were also associated with a moderate increase in gamma power relative to Go trials (P < 0.05, permutation test; Supplementary Fig. 12).
Across-trial adaptations
We observed slower response times in correct Go trials that followed a previous correct Conflict trial (Fig. 1B). As such, we investigated whether beta activity in the mPFC or STN was different during correct Go trials that followed either a previous correct Conflict trial or a previous correct Go trial. We only included in this analysis trials during which participants responded correctly on both adjacent trials. Importantly, the stimuli presented in the subsequent Go trials were identical, and the primary difference between the trial types of comparison here was what had occurred during the previous correct trial.
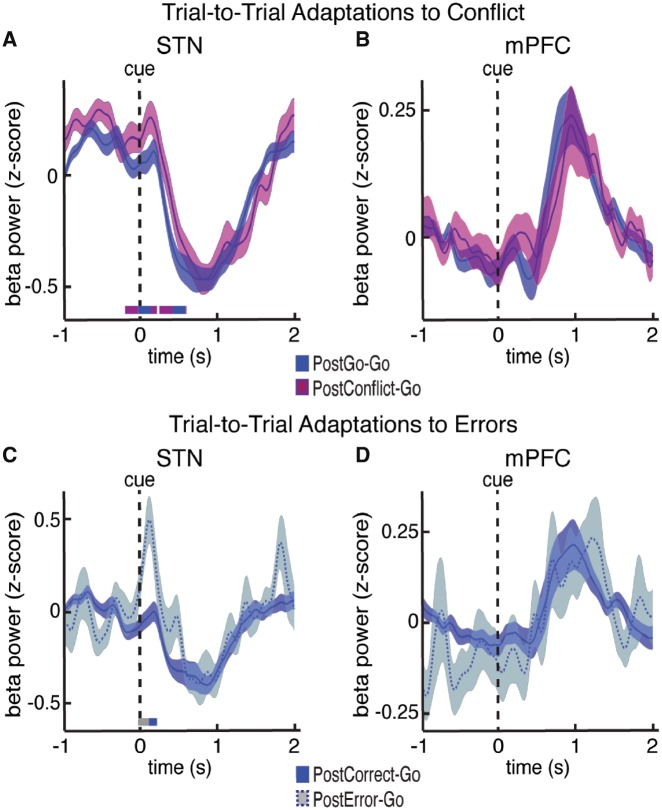
We found significantly higher levels of STN beta power during Go trials following a previous Conflict trials compared to Go trials following previous Go trials (P < 0.05, permutation test; Fig. 6A). The higher levels of STN beta power occurred in the first 200 ms of the subsequent Go trial. This was a time period that did not show any significant differences when we compared the Go, NoGo, and Conflict trials (Fig. 4A), but did show significantly lower beta power during the error trials (Fig. 5B). These data suggest that the level of STN beta power early in a trial may significantly influence the response time during that trial. Indeed, across participants we found a significant, albeit weak, positive correlation between STN beta power and response time early in the task (Supplementary Fig. 9). Of note, these differences were not simply due to the rule switching that occurred when participants observed a Conflict cue after having seen a Go cue on the previous trial (Supplementary Fig. 13). In contrast, we found no significant differences in mPFC beta power during the execution phase of the second trial (Fig. 6B), suggesting that if mPFC beta activity is involved in across-trial adaptations to conflict, this involvement is only reflected in the time period immediately following the previously completed trial.
Figure 6.
Changes in oscillatory power related to across-trial adaptations to conflict and errors. (A and B) Cue aligned time evolving beta power changes averaged over all STN (A) and mPFC (B) electrodes during the correct Go trials that followed a correct Go trial (postGo-Go) and during the correct Go trials that followed a correct Conflict trial (postConflict-Go). Time points exhibiting a significant difference between trial types (P < 0.05, corrected for multiple comparisons, permutation test) are denoted by coloured horizontal bars. (C and D) Same as in A and B but plotted for the correct Go trials that followed any correct trial (postCorrect-Go) and for the correct Go trials that followed any error trial (postError-Go). Only the STN showed a post-Conflict and post-error related increase in beta power right after the arrow presentation of the second trial.
Because correct Go trials also showed slower response times when they followed an error trial, we also examined whether the commission of an error on the previous trial affected mPFC and STN beta power on subsequent correct Go trials. In this analysis we only included trials during which a participant incorrectly responded to the stimulus on the previous trial, but then correctly responded to stimulus on the subsequent Go trial. Like the response to previous Conflict, STN beta power during Go trials following errors was significantly higher than STN beta power during Go trials following correct trials (P < 0.05, permutation test; Fig. 6C). These differences also occurred within the first 200 ms of the subsequent Go trial. Also in line with the response to previous Conflict, we found no significant differences in mPFC beta power during the execution phase of the second trial (Fig. 6D), further suggesting that the mPFC’s involvement in across-trial adaptations is restricted to the time period immediately following the error. Analysing STN theta power and multi-unit activity amplitude during across-trial adaptations did not show any significant differences between trial types (data not shown).
Discussion
Our data demonstrate that the STN and mPFC play complementary roles as individuals monitor for conflicts and errors and adjust subsequent actions accordingly. Theta oscillations are coordinated between the mPFC and the STN when detecting conflict within an ongoing trial. Actions within that trial may then be facilitated by decreased beta activity only in the STN, and these decreases exhibit an early return to baseline when individuals inhibit a response. Following a trial, however, changes in beta activity in the mPFC, but not the STN, reflect the difficulty of the completed trial or whether an error occurred. This information is then associated with an elevation of STN beta power early in the subsequent trial, possibly contributing to the longer response times seen following conflict and error trials. The trial type related differences are summarized in schematic form in Fig. 7 (see Supplementary Figs 6 and 7 for spectragrams showing trial-type related differences for the STN and mPFC).
Figure 7.
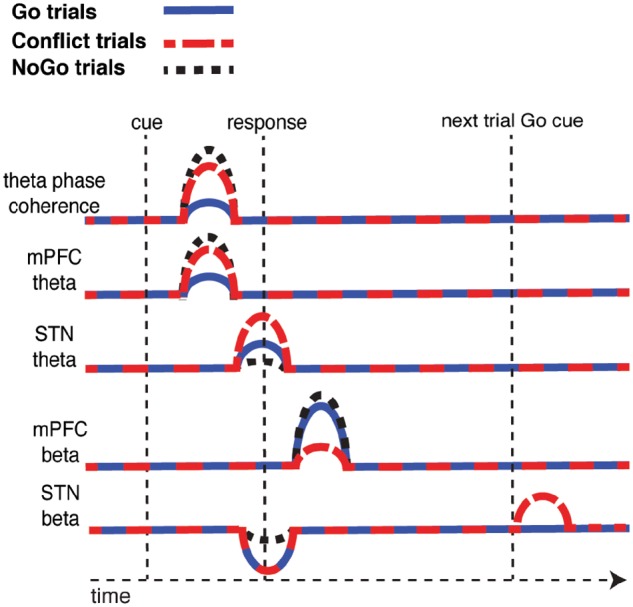
Summary schematic of trial type related differences. Following the presentation of the cue, mPFC-STN coherence and mPFC theta power increases, and this increase is significantly higher during Conflict and NoGo trials. Around the time of the response, STN theta power also increases, but this increase is only significantly higher during the Conflict trials and not the NoGo trials. The motor response is also associated with a decrease in STN beta power, and this decrease is attenuated during NoGo trials. Following each trial, there is an increase in mPFC beta power and this increase is attenuated during Conflict trials and during error trials (not shown). On a subsequent Go trial that follows a correct Conflict trial (or an error trial, not shown), there is an increase in STN beta power when the Go stimulus is presented.
The novel response inhibition task used here requires movement while monitoring for conflict and errors, processes that collectively support cognitive control (Miller and Cohen, 2001; Ridderinkhof et al., 2004; Cohen, 2014). In several respects, the Go and NoGo trials are relatively easy, and require a simple translation from a cue on the screen directly to a movement (or withholding thereof). In contrast, Conflict trials are more difficult, as suggested by the slower response times and higher error rates. They require greater cognitive control in order to overcome a prepotent response in the direction of the arrow and move in the opposite direction. Once completed, these trials then elicit an appropriate adjustment for the next trial, which manifests itself as a slower response time. Overall, the response times we observed in our task were quite slow relative to prior response inhibition studies. This was most likely due to the intraoperative environment from which the recordings were acquired (see Zavala et al., 2017a for another intraoperative study with similarly slow response times), but may have also been due to patients slowing down their response times in case the current trial was a NoGo trial.
Contrasting within-trial effects of theta oscillations in the mPFC and STN
Within a given trial, a participant must identify the goal indicated by the visual stimulus, decide whether and in what direction a movement must occur, and delay actions until any perceptual and response conflicts are resolved (Nigbur et al., 2012). Here, the mPFC and STN seem to coordinate their activity. Relative to Go trials, Conflict results in higher theta power in both the mPFC and the STN and higher theta phase coherence between the two brain regions. These results are consistent with previous studies demonstrating mPFC-STN theta communication during conflict (Zavala et al., 2014, 2016). Notably, the NoGo trials exhibit the highest levels of mPFC theta power and mPFC-STN theta phase synchrony. To our knowledge, no prior work has shown increased synchrony between these two brain regions when participants are instructed to completely withhold an action. These data therefore lend the strongest support yet to the hypothesis that mPFC-STN theta synchrony conveys an anti-kinetic signal that can either cancel a movement entirely or slow down a response in the presence of conflict (Cavanagh et al., 2011; Cohen and Cavanagh, 2011; Nigbur et al., 2011; Rae et al., 2015; Kelley et al., 2018).
Theta power in the STN, however, diverges from that of the mPFC during the NoGo trials, as these trials evoke the smallest, rather than largest, increases in STN theta power. Intriguingly, multi-unit spiking activity seems to follow a very similar pattern of trial type related differences. STN theta power and multi-unit activity are both lowest during the NoGo trials that involve no movement and peak at the time of the response during Go and Conflict trials, suggesting an involvement in the execution of the movement. Together, these data would appear inconsistent with classical models of basal ganglia circuitry in which the STN is presumably involved in activating the inhibitory indirect pathway (Albin et al., 1989; DeLong, 1990). However, more recent evidence has suggested that indeed most STN neurons increase, rather than decrease, spiking activity during movement (Nambu et al., 2002; Goldberg et al., 2013; Zavala et al., 2017a). Furthermore, increased STN theta activity has been implicated in pathologies that involve excess movement such as dystonia, dyskinesia, impulsivity, and OCD (Alonso-Frech et al., 2006; Rodriguez-Oroz et al., 2011; Neumann et al., 2012, 2017; Rappel et al., 2018).
This, then, presents a paradox; why should elevated levels of mPFC theta power and mPFC-STN phase synchrony be anti-kinetic while STN theta power facilitates movement? One possibility may be that the differences in theta oscillatory activity are related to the specific neural circuits in which they are expressed. The increases in mPFC theta power and in mPFC-STN phase synchronization occur early and are separable in time from the increases in STN theta activity both in our current paradigm and in prior studies (Zavala et al., 2014, 2016; Pearson et al., 2017). Thus, the relative timing of increases in theta power may be consistent with the sequential engagement of two circuits converging on the STN, one conveying information regarding conflict early in a trial, and a second that is responsible for a more explicit motor drive. Another possibility, however, is that both theta and spiking activity are indeed inhibitory in nature as originally proposed in the classical models (Albin et al., 1989; DeLong, 1990). Early in the trial, they may globally inhibit all movements while the appropriate action is being selected. During the response, however, they use a centre-surround architecture (Nambu et al., 2002) to selectively inhibit only undesired movements that would otherwise interfere with the correct action. In this scenario, NoGo trials demonstrate an abrupt termination of theta power and multi-unit activity because no movements are executed, making any inhibition of irrelevant movements unnecessary. Our data cannot distinguish these possibilities, and the precise role of STN theta activity remains unresolved.
Contrasting within-trial and across-trial adaptation effects of beta oscillations in the mPFC and STN
The contrasting roles of beta activity in the STN and the mPFC during this task provide an even clearer exposition of the principle that the behavioural associations of oscillatory activity reflect the complementary roles of these structures. Not surprisingly, STN beta activity was directly related to movement itself, decreasing following the Go and Conflict cues and remaining attenuated until the response. These decreases prematurely return to resting levels during NoGo trials that require complete response inhibition. Though it is unclear if the return to baseline observed during the NoGo trials is due to an increase in an anti-kinetic signal or an attenuation of a pro-kinetic signal, the relative power differences between movement trials and NoGo trials as well as the timing of the STN beta power changes are consistent with previous studies investigating STN beta activity when motor and non-motor actions are stopped (Kühn et al., 2004; Ray et al., 2012; Alegre et al., 2013; Bastin et al., 2014; Benis et al., 2014; Wessel et al., 2016; Zavala et al., 2017b). Of note, the reductions in STN beta band power during each movement trial were accompanied by a significant reduction in beta band spiking entrainment. Given that movement also results in an overall increase in spiking activity, these changes suggest that STN beta may serve as a gate for action. Resting levels of beta power and spike entrainment may impede processing related to movement, while reduced beta power levels free the STN neurons to encode a diverse variety of task-relevant spiking patterns, as we observe in our data (Supplementary Fig. 4).
The trials that follow conflicts or errors further support the hypothesis that STN beta activity is anti-kinetic in nature. Both Conflict and error trials evoke longer response times in the subsequent trial. Importantly, STN beta band power is significantly elevated at the beginning of these subsequent trials. To our knowledge, this is the first study to provide evidence that STN beta band activity increases in response to higher levels of cognitive control required by a previous trial, and that these increases are associated with slower response times. It is possible that the higher levels of beta power we observed on Go trials that followed a Conflict trial are due to the rule change that occurred across trials (Bissonette and Roesch, 2017). However, this is unlikely as an analysis of the trials that exhibited a rule change in the opposite direction (Conflict trials that followed a Go trial) showed no significant differences. A rule change effect would also not explain why the error trials were associated with higher levels of beta power on the subsequent trial, although it should be noted that the post-error results were obtained from a relatively few number of trials. Our findings are therefore consistent with the suggestion that elevated beta band activity in both the STN and cortex (Swann et al., 2009, 2012; Wessel et al., 2013) suppresses automatic pre-potent responses in favour of more goal-oriented behaviour (Frank et al., 2007). This framework also provides insight into our observation that trials with errors of commission exhibit significant early decreases in beta band activity. These decreases could cause the inappropriate early release of more automatic pre-potent responses. In the context of drift diffusion models, this would reflect a decreased decision threshold that would predispose an individual to automatic, non-goal oriented actions and lead to premature erroneous responses (Herz et al., 2017). Following these erroneous responses, an optimal behavioural strategy would likely involve increasing the decision threshold for subsequent trials to avoid repeating the same mistake. We did not find a significant difference between correct and error trials when examining the post-response beta rebound in the STN, which has previously been implicated in across-trial adaptations (Tan et al., 2014, 2016). Instead, our data suggest that such adaptations may be mediated by other brain regions such as the mPFC, which then may lead to differences within the STN on subsequent trials.
Indeed, beta band activity in the mPFC changes very little within each trial but increases immediately following the response. Importantly, unlike in the STN, the mPFC beta increases following each trial are related to the difficulty of the trial itself, and not to whether a movement occurs. These increases were significantly attenuated following Conflict trials, and even reversed following errors. Few studies have analysed mPFC activity during a Go-NoGo task (Yamanaka and Yamamoto, 2009; Nigbur et al., 2011, 2012), and most studies of mPFC oscillatory activity during response inhibition have focused on surface EEG changes in the theta band (Cohen, 2014). Nevertheless, several lines of evidence suggest that decreased levels of mPFC beta activity after Conflict and error trials may be related to across-trial cognitive control. First, these changes in mPFC beta activity only occur following the completion of the trial, and any differences in mPFC beta activity are related to difficulty rather than movement. Moreover, as in our data, conflicts and errors are thought to recruit similar mPFC resources when higher levels of cognitive control are needed to adjust behaviour or to prevent subsequent errors (Kerns et al., 2004; Yeung et al., 2004; Cavanagh et al., 2009, 2012; Compton et al., 2011). Finally, errors necessarily imply some uncertainty regarding what the predicted outcome of an action was, or will be. Recent evidence has demonstrated that both internally generated and externally induced errors during sensorimotor adaptation tasks result in attenuations of the post-response increases in mPFC beta power, thereby linking these changes in mPFC beta power to this aspect of cognitive control (Tan et al., 2016).
Beta oscillations and predictive coding during cognitive control
Indeed, the observed changes in mPFC and STN beta activity may in fact be consistent with models of predictive coding as they relate to the various cognitive and motor aspects of this task (Friston et al., 2015). This task generally requires following a simple rule, to move in the direction of an arrow. On occasion, however, and therefore unexpectedly, there is a change in the rule, and participants must move in the opposite direction. During these instances, there is both sensorimotor conflict regarding which way to move, and a prediction error related to which rule the participant was expecting to follow. Increasing evidence suggests that cortical processing is optimized for predictive coding, and that such top-down predictions are conveyed through beta oscillations (Engel et al., 2001; Buschman and Miller, 2007; van Ede et al., 2011; Brown et al., 2013). The neural mechanisms for predicting a set of task rules should be analogous, and in this case would involve areas like the mPFC that have been implicated in setting goals and rules (Miller and Cohen, 2001; Ridderinkhof et al., 2004; Cohen, 2014; Bissonette and Roesch, 2017). The reductions in mPFC power following Conflict or errors would, according to this paradigm, reflect a switch from a top-down state in which predictions are weighed more heavily to a bottom-up state in which there is greater uncertainty regarding previously held expectations (van Ede et al., 2011; Brown et al., 2013). The observed increases in high gamma activity following both Conflict and error trials support this possibility as gamma oscillations have been linked to bottom-up sensory driven processes (Engel et al., 2001; Oakes et al., 2004; Buschman and Miller, 2007).
A similar framework linking beta activity to predictive coding may also be relevant for interpreting the changes observed in STN beta activity with movement (Friston et al., 2015). Although such a link between STN beta activity and prediction has yet to be firmly established, these models posit that beta oscillations reflect predictions related to the expected state of sensory inputs and how those inputs may or may not change with movement. When making a movement, one must actively discard predictions related to the current sensory state, as those inputs will surely change with movement. This is thought to result in decreased beta activity. Conversely, if beta remains elevated, it is difficult to make a movement because it is difficult to change those predictions and anticipate a new sensory state. In these cases, higher beta activity maintains the status quo (Gilbertson et al., 2005; Engel and Fries, 2010; Friston et al., 2015). Hence, it is possible that the observed changes in beta activity in both the mPFC and the STN are related to the extent to which predictions regarding the task are met or violated, and that the distinction between the two structures reflects the distinction in the types of predictions that are relevant. On the one hand, STN beta signals are strictly related to the action, whereas mPFC beta signals are related to the task rules.
Taken together, our results evidence the broad principle that oscillatory activity at the same frequency in different circuits may be associated with different behavioural effects (Fries, 2015). This is achieved by the coordinated sequencing of these neural activities. Specifically, we have provided evidence that although beta activity may be considered anti-kinetic when expressed in the STN prior to movement, in the mPFC it is associated with the modulation of cognitive control necessary following response evaluation. Similarly, although early increases in mPFC theta power and mPFC-STN phase synchrony may be considered anti-kinetic, later theta power increases in the STN may actually promote movement. Our inferences are drawn from data collected in patients with Parkinson’s disease, and so should be interpreted with caution as dopamine depletion and DBS surgery are known to alter the basal ganglia activity (Hammond et al., 2007; Singh et al., 2018). Nonetheless, we find that patterns of theta and beta band activity in the STN and mPFC play complementary, temporally sequenced roles in supporting different processes that together contribute to cognitive control.
Supplementary Material
Acknowledgements
We are indebted to all patients who selflessly volunteered to participate in this study.
Glossary
Abbreviations
- DBS
deep brain stimulation
- mPFC
medial prefrontal cortex
- STN
subthalamic nucleus
Funding
This work was supported by the Intramural Research Program of the National Institute for Neurological Disorders and Stroke. P.B. was supported by the Medical Research Council (MC UU 12024/1).
Competing interests
The authors report no competing interest.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989; 12: 366–75. [DOI] [PubMed] [Google Scholar]
- Alegre M, Lopez-Azcarate J, Obeso I, Wilkinson L, Rodriguez-Oroz MC, Valencia M, et al. . The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson’s disease. Exp Neurol 2013; 239: 1–12. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357–81. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, Zamarbide I, Alegre M, Rodríguez-Oroz MC, Guridi J, Manrique M, et al. . Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 2006; 129: 1748–57. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 2007; 27: 3743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J, Polosan M, Benis D, Goetz L, Bhattacharjee M, Piallat B, et al. . Inhibitory control and error monitoring by human subthalamic neurons. Transl Psychiatry 2014; 4: e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benis D, David O, Lachaux J-P, Seigneuret E, Krack P, Fraix V, et al. . Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson’s disease. Neuroimage 2014; 91: 273–81. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Roesch MR. Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience 2017; 345: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain J-S, Watkins KE, Joundi RA, Ray NJ, Holland P, Green AL, et al. . A role for the subthalamic nucleus in response inhibition during conflict. J Neurosci 2012; 32: 13396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Adams RA, Parees I, Edwards M, Friston K. Active inference, sensory attenuation and illusions. Cogn Process 2013; 14: 411–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 2007; 315: 1860–2. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci 2009; 29: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, et al. . Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 2011; 14: 1462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology 2012; 49: 220–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn LK, Pauker CS, Golob EJ. Cognitive control and midline theta adjust across multiple timescales. Neuropsychologia 2018; 111: 216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci 2014; 37: 480–90. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front Percept Sci 2011; 2: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, van Gaal S. Subthreshold muscle twitches dissociate oscillatory neural signatures of conflicts from errors. Neuroimage 2014; 86: 503–13. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A. Cognitive control in the intertrial interval: evidence from EEG alpha power. Psychophysiology 2011; 48: 583–90. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci 2011; 31: 1780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13: 281–5. [DOI] [PubMed] [Google Scholar]
- van Ede F, Lange F de, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J Neurosci 2011; 31: 2016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 2010; 20: 156–65. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat Rev Neurosci 2001; 2: 704–16. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in Parkinsonism. Science 2007; 318: 1309–12. [DOI] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: communication through coherence. Neuron 2015; 88: 220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Bastos AM, Pinotsis D, Litvak V. LFP and oscillations—what do they tell us? Curr Opin Neurobiol 2015; 31: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Lazzaro VD, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13-35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci 2005; 25: 7771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Farries MA, Fee MS. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci 2013; 36: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 2007; 30: 357–64. [DOI] [PubMed] [Google Scholar]
- Herz DM, Tan H, Brittain J-S, Fischer P, Cheeran B, Green AL, et al. . Distinct mechanisms mediate speed-accuracy adjustments in cortico-subthalamic networks. Elife 2017; 6: e21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 2011; 34: 611–18. [DOI] [PubMed] [Google Scholar]
- Kelley R, Flouty O, Emmons EB, Kim Y, Kingyon J, Wessel JR, et al. . A human prefrontal-subthalamic circuit for cognitive control. Brain 2018; 141: 205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303: 1023–6. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider G-H, et al. . Event‐related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 2004; 127: 735–46. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Lutz A, Rudrauf D, Cosmelli D, Le Van Quyen M, Martinerie J, et al. . Estimating the time-course of coherence between single-trial brain signals: an introduction to wavelet coherence. Neurophysiol Clin 2002; 32: 157–74. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron 2012; 73: 523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007; 164: 177–90. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci 2008; 28: 14000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Bergman H, Israel Z, Bar-Gad I. Subthalamic nucleus functional organization revealed by parkinsonian neuronal oscillations and synchrony. Brain 2008; 131: 3395–409. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’ pathway. Neurosci Res 2002; 43: 111–17. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci 2013; 16: 1888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann W-J, Horn A, Ewert S, Huebl J, Brücke C, Slentz C, et al. . A localized pallidal physiomarker in cervical dystonia. Ann Neurol 2017; 82: 912–24. [DOI] [PubMed] [Google Scholar]
- Neumann W-J, Huebl J, Brücke C, Ruiz MH, Kupsch A, Schneider G-H, et al. . Enhanced low-frequency oscillatory activity of the subthalamic nucleus in a patient with dystonia. Mov Disord 2012; 27: 1063–6. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Cohen MX, Ridderinkhof KR, Stürmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J Cogn Neurosci 2012; 24: 1264–74. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Ivanova G, Stürmer B. Theta power as a marker for cognitive interference. Clin Neurophysiol 2011; 122: 2185–94. [DOI] [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, et al. . Functional coupling of simultaneous electrical and metabolic activity in the human brain. Hum Brain Mapp 2004; 21: 257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Hickey PT, Lad SP, Platt ML, Turner DA. Local fields in human subthalamic nucleus track the lead-up to impulsive choices [Internet]. Front Neurosci 2017; 11 Available from: https://www.frontiersin.org/articles/10.3389/fnins.2017.00646/full(3 January 2018, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy—Psychophysics software in Python. J Neurosci Methods 2007; 162: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci 2015; 35: 786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappel P, Marmor O, Bick AS, Arkadir D, Linetsky E, Castrioto A, et al. . Subthalamic theta activity: a novel human subcortical biomarker for obsessive compulsive disorder. Transl Psychiatry 2018; 8: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Brittain J-S, Holland P, Joundi RA, Stein JF, Aziz TZ, et al. . The role of the subthalamic nucleus in response inhibition: evidence from local field potential recordings in the human subthalamic nucleus. Neuroimage 2012; 60: 271–8. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science 2004; 306: 443–7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, López-Azcárate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, et al. . Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 2011; 134: 36–49. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 2004; 8: 410–17. [DOI] [PubMed] [Google Scholar]
- Schuck NW, Gaschler R, Wenke D, Heinzle J, Frensch PA, Haynes J-D, et al. . Medial prefrontal cortex predicts internally driven strategy shifts. Neuron 2015; 86: 331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Gulberti A, Zittel S, Jones AAT, Fickel U, Münchau A, et al. . Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in Parkinson’s disease. J Neurosci 2014; 34: 6273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, et al. . Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 2012; 488: 218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Richardson SP, Narayanan N, Cavanagh JF. Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia 2018; 117: 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Abeles M. Predicting movement from multiunit activity. J Neurosci 2007; 27: 8387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, et al. . Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 2009; 29: 12675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. . Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 2012; 59: 2860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Wade C, Brown P. Post-movement beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. J Neurosci 2016; 36: 1516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Zavala B, Pogosyan A, Ashkan K, Zrinzo L, Foltynie T, et al. . Human subthalamic nucleus in movement error detection and its evaluation during visuomotor adaptation. J Neurosci 2014; 34: 16744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, et al. . Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol 2006; 96: 3248–56. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Conner CR, Aron AR, Tandon N. Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. J Neurosci 2013; 33: 19611–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Ghahremani A, Udupa K, Saha U, Kalia SK, Hodaie M, et al. . Stop-related subthalamic beta activity indexes global motor suppression in Parkinson’s disease. Mov Disord 2016; 31: 1846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Yamamoto Y. Single-trial EEG power and phase dynamics associated with voluntary response inhibition. J Cogn Neurosci 2009; 22: 714–27. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 2004; 111: 931–59. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Weidemann CT, Lega BC, Jaggi JL, Baltuch GH, Kahana MJ. Neuronal activity in the human subthalamic nucleus encodes decision conflict during action selection. J Neurosci 2012; 32: 2453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Brittain J-S, Jenkinson N, Ashkan K, Foltynie T, Limousin P, et al. . Subthalamic nucleus local field potential activity during the eriksen flanker task reveals a novel role for theta phase during conflict monitoring. J Neurosci 2013; 33: 14758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Damera S, Dong JW, Lungu C, Brown P, Zaghloul KA. Human subthalamic nucleus theta and beta oscillations entrain neuronal firing during sensorimotor conflict. Cereb Cortex 2017a; 27: 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Jang AI, Zaghloul KA. Human subthalamic nucleus activity during non-motor decision making. Elife 2017b; 6: e31007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Tan H, Ashkan K, Foltynie T, Limousin P, Zrinzo L, et al. . Human subthalamic nucleus–medial frontal cortex theta phase coherence is involved in conflict and error related cortical monitoring. Neuroimage 2016; 137: 178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Zaghloul K, Brown P. The subthalamic nucleus, oscillations, and conflict. Mov Disord 2015; 30: 328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala BA, Tan H, Little S, Ashkan K, Hariz M, Foltynie T, et al. . Midline frontal cortex low-frequency activity drives subthalamic nucleus oscillations during conflict. J Neurosci 2014; 34: 7322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.