Abstract
Cymbopogon genus is a member of the family of Gramineae which are herbs known worldwide for their high essential oil content. They are widely distributed across all continents where they are used for various purposes. The commercial and medicinal uses of the various species of Cymbopogon are well documented. Ethnopharmacology evidence shows that they possess a wide array of properties that justifies their use for pest control, in cosmetics and as anti-inflammation agents. These plants may also hold promise as potent anti-tumor and chemopreventive drugs. The chemo-types from this genus have been used as biomarkers for their identification and classification. Pharmacological applications of Cymbopogon citratus are well exploited, though studies show that other species may also useful pharmaceutically. Hence this literature review intends to discuss these species and explore their potential economic importance.
Keywords: Cymbopogon, ethnopharmacology, secondary metabolites, terpenes, chemo-types
1. Introduction
The presence of secondary metabolites in plants is characterized by their ability to provide defenses against biotic and abiotic stress [1]. The mechanism of defense varies from plant to plant, their environmental conditions and climatic variations. However, the presence of these metabolites in plant are usually in minimum amounts though several molecular techniques are available to either increase or decrease the quantity of a particular metabolite by blocking competitive pathways and enriching metabolites of choice [2]. Terpenes, alkaloids (N-containing compounds) and phenolics constitute the largest groups of secondary metabolites. The shikimic acid pathway is the basis of the biosynthesis of phenolics while the terpenes which are comprised of isoprene units arise from the mevalonate pathway [3]. Aspirin (1) from white willow, quinine (2) from the cinchona plant and artemisinin (3) from Artemisia annum are all plant secondary metabolites. The biological application of these metabolites as therapeutic agents for a broad spectrum of ailments and the microbial infections has been salutary in human history.
The genus Cymbopogon is widely distributed in the tropical and subtropical regions of Africa, Asia and America. Comprised of 144 species, this genus is famous for its high content of essential oils which have been used for cosmetics, pharmaceuticals, and perfumery applications [4]. Two main species, C. flexuosus and C. citratus (lemongrass) are commercially cultivated in the Democratic Republic of Congo (DRC), Madagascar, and the Comoros Island. However, the leading exporter of these plants is Guatemala, trading about 250,000 kg per year and while the USSR sells about 70,000 kg per year [5].
The commercial value of some Cymbopogon species is further enhanced by their ability to grow in moderate and extremely harsh climatic conditions [6]. In environments where they are not used for cosmetics, drug or perfumery, such as in the Eastern Cape Province of South Africa, these plants have found a good application as roof thatches and grass brooms [7].
2. Ethnopharmacology of Cymbopogon Species
Traditional applications of Cymbopogon genus in different countries shows high applicability as a common tea, medicinal supplement, insect repellant, insecticide, in flu control, and as anti-inflammatory and analgesic. Table 1 shows the common names of some species, their relevance and how they are applied. C. citratus is ranked as one of the most widely distributed of the genus which is used in every part of the world. Its applications in Nigeria include cures for upset stomach, malaria therapy, insect repellent and as an antioxidant (tea) [8]. C. citratus and C. flexuosus are the prevailing species in Eastern and Western India and have been used locally in cosmetics, insecticides, and for the treatment of digestive disorders and fevers [9,10].
Table 1.
Several Cymbopogon species, common name, regions, plant part used and the uses.
| Species | Region | Common Name | Parts | Medicinal Uses | References |
|---|---|---|---|---|---|
| C. nardus (L.) Rendle | India | Citronella oil | Leaves | Insect repellent and as perfumes | [11] |
| C. parkeri Stapf | Pakistan | Lemon grass | Aerial | Antiseptic and stomachic treatment | [12] |
| C. excavatus Hoscht | South Africa | Bread-leavened Turpentine grass | Sheaths | Used as insecticides | [13] |
| C. olivieri(Boss) | Pakistan | Pputar | Aerial | Pyretic, vomit, diuretic, rheumatism, and as anti-malaria condiment. | [14,15] |
| C. validus(Stapf) | Eastern and Southern Africa | African bluegrass | Essential oils | skin toner, anti-ageing in men, fumigant and for rodent control | [16] |
| C. winterianus(Jowitt) | Brazil | Java grass | Fleshy leaves | Treatment of epilepsy and anxiety | [17] |
| C. marginatus(Steud.) | South Africa | Lemon-Scented grass | Root | They are used as moth repellent | [18] |
| C. citratus Stapf | India | Lemon grass | Aerial | Fever, digestive disorders | [9] |
| Nigeria | Lemon grass | Leaves | Diabetes, inflammation and nerve disorders | [8] | |
| Argentina | Limonaria | Leaves | Against cold and flu, and digestive complaints, stomach upsets and as decoction with other plants for malaria | [19] | |
| Cuba | Cana Santa | Leaves | [20] | ||
| Costa Rica | Grass tea | Leaves | To relieve cough, carminative, expectorant and depurative | [21] | |
| Colombia | Limonaria | Rhizome | It is chewed and used as toothbrush and for pest control. | [22,23] | |
| Brazil | Capimsanto | Leaves | Anxiolytic and anti-hypertensive | [24] | |
| Trinidad & Tobago | “fever grass” | Grass and rhizomes | The teas from it are used to treat cold, flu, fever and diabetes | [24] | |
| C. giganteus(Hochst.) Chiov. | Cameroon | Tsauri grass | decoctions of leaves and flowers | Cough and arterial hypertension | [25] |
| C. ambiguous (Hack.) A. Camus. | Australia | Native Lemon Grass | Leaves and stems | Headache remedy, chest infections, muscle cramp and Scabies | [26,27] |
| C. procerus (R.Br.) Domin | Australia | Scent grass | Leaves and stems | Leaves and stem are pounded and used as medicinal body wash used for headache | [28] |
| C. flexuosus (Nees ex Steud.) Wats. | India | Lemon grass | Leaves | Cosmetics, antiseptic and for treatment of fever | [10] |
| C. pendulus (Nees ex Steud.) Wats. | India | Jammu Lemongrass | Leaves | Antiseptic and for perfumery | [29] |
| C. scheonanthus (L.) Spreng | Saudi Arabia | Ethkher | Leaves | Antidiarrheal, to treat fever, treatment of jaundice and tonic | [30] |
| C. obtectus (S.T. Blake) | Central Australia | Silky-heads | Mixture | Cold and flu, headaches, fever and sore throat | [27] |
| C. proximus (Stapf.) | Egypt | Halfabar | Leaves | Expulsion of renal and ureteric calculi | [31] |
| Cymbopogon refractus (R.Brown) A. Camus. | Australia | Barbed wire grass | Leaves | Feed for animals | [32] |
| C. densiflorus (Steud.) Stapf | Congo | Lemongrass | Leaves and rhizome | Employed against asthma, epilepsy, abdominal cramps and pains and also for interpreting dreams by witch doctors. | [33,34] |
| C. jwarancusa(Jones) Schult. | Egypt | Thé Limon | The whole plant | Condiment and for medicinal purpose | [35] |
In the Middle East, C. olivierri and C. parkeri are more predominant, and they are used as antiseptics, anti-malarial condiments, diuretics and also to cure rheumatism [12,14,15]. The high amounts of volatile compounds from these species are responsible for their diverse uses.
3. Phytochemistry
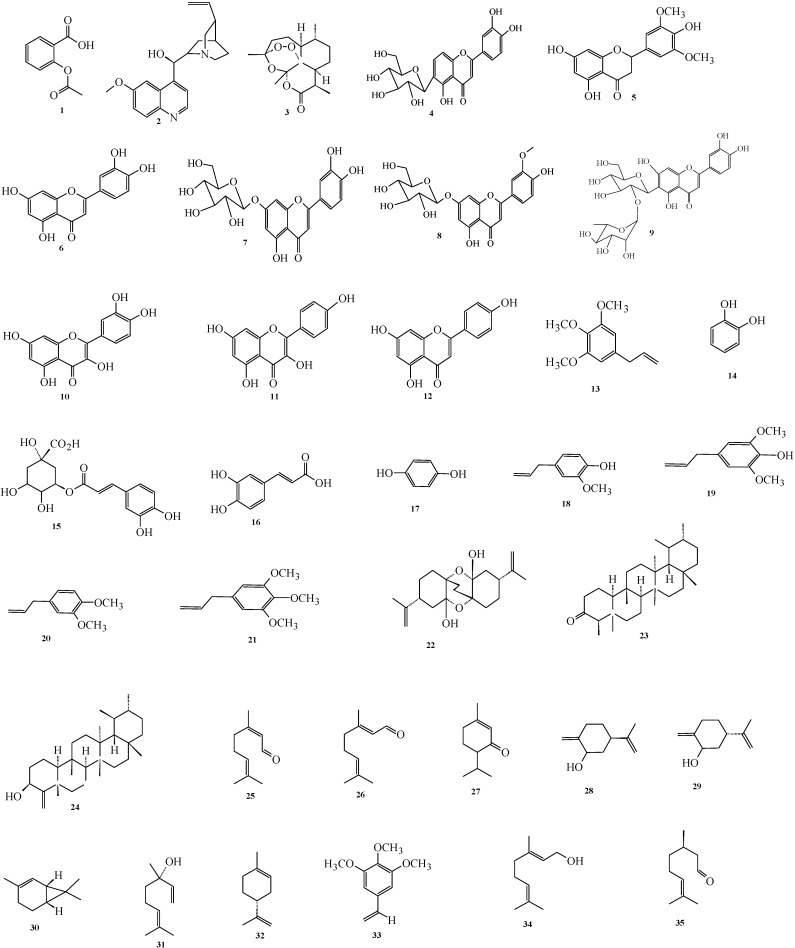
The enormous information gathered from the ethno-pharmacological applications of Cymbopogons begged the investigation of its chemical constituents. These studies have led to the isolation of alkaloids, volatile and non-volatile terpenoids, flavonoids, carotenoids and tannins from every part of these plants. Figure 1 displays some of the compounds isolated from Cymbopogon species.
Figure 1.
Flavonoids and triterpenoids from Cymbopogon species.
3.1. Alkaloids
The rhizome of C. citratus from Nigeria was reported to contain about 0.52% alkaloids from 300 g plant material [36].
3.2. Flavonoids
This class of compounds has potent antioxidant properties. Some of the flavonoids isolated from Cymbopogon species are presented in Figure 1. Isoorientin (4) and tricin (5) were isolated from the dichloromethane extract of C. parkeri [37], evaluation of these two compounds revealed their muscle relaxation activity [38]. Isolation of luteolin (6), luteolin 7-O-glucoside (cynaroside) (7), isoscoparin (8) and 2''-O-rhamnosyl isoorientin (9) from the leaves and rhizomes of C. citratus has been reported. Other flavonoid compounds isolated from the aerial parts of C. citratus are quercetin (10), kaempferol (11) and apigenin (12) [39], isolated elimicin (13), catechol (14), chlorogenic acid (15), caffeic acid (16) and hydroquinone (17) from the aerial parts of the same species. Isolation of 4-phenylpropanoids from Australian species of C. ambiguus has been reported. These compounds are eugenol (4-allyl-2-methoxyphenol) (18); elemicin (5-allyl-1,2,3-trimethoxybenzene) (19); eugenol methylether (4-allyl-1,2-dimethoxybenzene) (20) and trans-iso-elemicin (1,2,3-trimethoxy-5-(1-propenyl) benzene) (21) and all these isolates exhibited good inhibition activity against ADP-induced human platelet serotonin release which is associated with headaches [26].
3.3. Cymbopogon Terpenoids
3.3.1. Non-Volatile Terpenoids
Plants in the Cymbopogon genus contain large amounts of volatile terpenoids though a few species from this genus are reported to contain non-volatile terpenoids as well. Bottini et al. [40] isolated a novel bis-monoterpenoid named cymbodiacetal (22) from C. martinii. The triterpenoids cymbopogone (23) and cymbopogonol (24) (Figure 1) were also reported from the leaves of C. citratus [41].
3.3.2. Volatile Terpenoids of Cymbopogon Species
Different chemotypes of Cymbopogon species contain varying major compounds such as citral, geraniol, citronellol, piperitone and elemin (Table 2). In the literature, the majority of the C. citratus analysed showed a remarkably high percentage of neral (25) and geranial (26). Analysis of C. citratus species from Brazil [42], India [43], West and Eastern Africa [43,44,45,46,47,48,49] and Asia [50] showed the high value of neral and geranial chemotypes. A special distinguishing feature between C. citratus of African origin is the high amount of myrcene observed in them [44,45,46,47,48,49]. High occurance of piperitone (27) characterizes the oils of C. parkeri and C. olivieri from Iran. Jiroveltz et al. [25] reported a significant presence of cis-p-mentha-1(7),8-dien-2-ol (28) and its isomer trans-p-mentha-1(7),8-dien-2-ol (29) in the oils of C. giganteus from Cameroon [25]. Predominant components observed in other Cymbopogon species essential oils from around the world include δ-2-carene (30) in C. proximus from Cameroon [51], linalool (31) from Malaysia’s C. nardus [52], limonene (32) in C. schoenanthus (Tunisia) and C. giganteus (Burkina Faso) [46] and elemicin (33) from the oils of C. pendulus from India [53]. Observation of the oil of C. winterianus from different parts of Brazil showed two major chemotypes based on the amount of geraniol (34) and citronellal (35) [17,54,55,56].
Table 2.
Major components observed in some Cymbopogon species.
| Compound | Species | Country/Region | Major % | References |
|---|---|---|---|---|
| cis-p-mentha-1(7),8-dien-2-ol (C10H16O) | C. giganteus(F) | Cameroon | 22.8 | [25] |
| Burkina Faso | 12.0 | [46] | ||
| Madagascar | 19.0 | [57] | ||
| trans-p-mentha-1(7),8-dien-2-ol | C. giganteus | Cameroon | 26.5 | [25] |
| C. giganteus | Burkina Faso | 14.2 | [46] | |
| C. densiflorus | Zambia | 11.1 | [57] | |
| C. giganteus | Madagascar | 22.4 | [56] | |
| Limonene (C10H16) | C. giganteus | Cameroon | 7.4 | [25] |
| C.giganteus | Burkina Faso | 42.0 | [46] | |
| C. proximus | Burkina Faso | 3.9 | [51] | |
| C. schoenanthus | Tunisia | 24.2 | [58] | |
| Elemicin (C12H16O3) | C. pendulus | India | 53.7 | [53] |
| α-Pinene (C10H16) | C. pendulus | India | 6.1 | [53] |
| Camphene (C10H16) | C. pendulus | India | 9.1 | [53] |
| C.winterianus | India | 8.0 | [59] | |
| Geranial (C10H16O) | C. flexuosus | India (Kumauon region) | 33.1 | [60] |
| India (Bilhar) | 42.4 | [43] | ||
| C. citratus | Burkina Faso | 48.1 | [46] | |
| Brazil | 50.0 | [42] | ||
| Egypt | 40.72 | [61] | ||
| Zambia | 39.0 | [47] | ||
| Kenya | 39.53 | [57] | ||
| Benin republic | 27.04 | [62] | ||
| Nigeria | 33.7 | [44] | ||
| Angola | 40.55 | [63] | ||
| Congo Brazaville | 48.88 | [45] | ||
| Ivory Coast | 34.0 | [45] | ||
| Mali | 45.3 | [45] | ||
| Iran | 39.16 | [50] | ||
| C. winterianus | S.E. Brazil | 8.05 | [55] | |
| Neral (C10H16O) | C. flexuosus | India | 30.0 | [60] |
| Burkina Faso | 34.6 | [46] | ||
| India (Bilhar) | 29.8 | [43] | ||
| Brazil (North) | 30.1 | [42] | ||
| Egypt | 34.98 | [61] | ||
| Zambia | 29.4 | [47] | ||
| Kenya | 33.31 | [48] | ||
| C. giganteus | Benin republic | 19.93 | [62] | |
| Nigeria | 26.5 | [44] | ||
| C. citratus | Angola | 28.26 | ||
| Malaysia | 50.81 | [64] | ||
| Congo Brazzaville | 36.24 | [49] | ||
| Brazil | 4.53 | [17] | ||
| Ivory Coast | 32.5 | [45] | ||
| Mali | 26.3 | [45] | ||
| Iran | 30.95 | [50] | ||
| Geranyl acetate (C12H20O2) | C. flexuosus | India | 12.0 | [60] |
| Linalool (C10H18O) | C. flexuosus | India | 2.6 | [60] |
| C.winterianus | India | 1.5 | [59] | |
| C. martini | India | 2.0 | [65] | |
| C. nardus | Malaysia | 11.0 | [52] | |
| Geraniol (C10H18O) | C. winterianus | India | 23.9 | [59] |
| C. martinii | India | 84.16 | [65] | |
| C. winterianus | Brazil | 32.82 | [17] | |
| Brazil (para state) | 16.2 | [54] | ||
| C. winterianus | S.E Brazil | 40.06 | [55] | |
| Citronellal (C10H18O) | C.winterianus | India | 32.7 | [59] |
| C. nardus | Malaysia | 29.6 | [52] | |
| C. winterianus | Brazil | 36.19 | [17] | |
| C. winterianus | Brazil (para state) | 26.5 | [54] | |
| C. winterianus | S.E. Brazil | 27.44 | [55] | |
| Citronellol (C10H20O) | C. winterianus | India | 15.9 | [59] |
| C. winterianus | Brazil | 11.34 | [17] | |
| C. winterianus | Brazil (Para state) | 7.3 | [54] | |
| C. winterianus | S.E. Brazil | 10.45 | [55] | |
| Myrcene (C10H16) | C. citratus C. citratus C. citratus | Burkina Faso | 11.0 | [46] |
| Egypt | 15.69 | [61] | ||
| Zambia | 18.0 | [47] | ||
| Benin republic | 27.83 | [62] | ||
| Nigeria | 25.3 | [44] | ||
| Angola | 10.57 | [63] | ||
| Ivory Coast | 18.1 | [45] | ||
| Mali | 9.1 | [45] | ||
| Selina-6-en-4-ol (C15H26O) | C. citratus | Brazil | 27.8 | [42] |
| α-Cadinol (C15H26O) | C. citratus | Brazil | 8.2 | [42] |
| Piperitone (C10H16O) | C. olivieri | Iran | 72.8 | [14] |
| C. parkeri | Iran | 80.8 | [12] | |
| C. proximus | Burkina Faso | 59.1 | [51] | |
| 4-Carene (C10H16) | C. olivieri | Iran | 11.8 | [12] |
| Germacrene-D (C15H24) | C. parkeri | Iran | 5.1 | [11] |
| δ-2-Carene (C10H16) | C. proximus | Burkina Faso | 22.3 | [51] |
| β-Phellandrene (C10H16) | C. schoenanthus | Tunisia | 13.4 | [58] |
3.4. Tannins
A literature search on the phytochemical screening of C. citratus also reveals the presence of tannins, however, very little effort has been made in the isolation of these compounds despite the appreciable amounts reported through quantitative phytochemical tests. Figueirinha et al. fractionated extracts of the species collected from Portugal and reported about 10 mg dry weight of hydrolysable tannins (prothocyanidins) [66] while C. citratus from Nigeria showed about 0.6% of tannins [36]. C. citratus is the single species of Cymbopogon which is most exploited for its tannin content.
4. Pharmacology
Several bioassays have confirmed the potency of Cymbopogon species for their several uses (Table 3). C. citratus was found to have chemoprotective activity by preventing of diethylnitrosamine (DEN)-initiated hepatocellular lesions in rats [67]. In South Africa, extract from C. citratus was applied for treatment of oral thrush in patients who tested positive to HIV/AIDS and proved effective [68].
Table 3.
Pharmacological evidence of some Cymbopogon species.
| Cymbopogon Species | Pharmacology | Activity | References |
|---|---|---|---|
| C. citratus | Cytotoxicity | Shows high toxicity against Chinese Hamster Ovary (CHO) cells (IC50 = 10.63 μg/mL) and moderately toxic against human fibroblast cell line 138 (W138) cells (IC50 = 39.77 μg/mL). | [72] |
| Insecticidal | LC50 of 48.6 μL/L against housefly larvae | [43] | |
| Neurobehavioral effects | Ability to be active as sedative, anxiolytic and anticonvulsant agent | [73] | |
| Antitrypanosomal | Modest activity against Trypanasoma brucei IC50 = 1.837 ± 0.13 μg/mL | [72] | |
| Anti-diabetic | Shows activity against poloxamer-407 induced type 2 diabetic (T2D) in Wistar rats | [43] | |
| HIV/AIDS | As a highly effective control for oral thrush in HIV/AIDS victims in South Africa | [68] | |
| Larvicidal activity | It shows high inhibition and mortality rate against larva of A. aegypti | [74] | |
| Chemopreventive activity | Inhibits the early phase of hepatocarcinogenesis in rats | [67] | |
| Anti-inflammations | Hexane extract inhibited iNOS (inducible nitric oxide synthase)expression, NO (nitric oxide) production and various LPS (lipopolysaccharide)-induced pathways | [75] | |
| C. schoenanthus | Antioxidant(DPPH) | 36%–73.8% activity per 2 μL of oil | [58] |
| Acetylcholinesterase inhibitory | IC50 = 0.26 ± 0.03 mg mL−1 | [58] | |
| Insecticidal activity | 2.7 μL/L obtained for LC50 against Callosobruchus maculatus | [76] | |
| C. winterianus | Moluscidal | LC90 = 97.0mg/L and LC50 = 54.0 mg/L | [54] |
| Larvicidal | LC 50 = 181.0mg/L | [54] | |
| Anti-fungal | Inhibited the growth of 15 strains of Candida albicans at concentrations of 625 μg/mL and 1250 μg/mL | [77] | |
| C. giganteus | Antimicrobial | High activity against gram +ve and gram −ve bacteria | [25] |
| Cytotoxicity | Low cytotoxicity against CHO cells and the human non cancer fibroblast cell line (W138) | [72] | |
| Anti-trypanosomal | IC50 = 0.25 ± 0.11 μg/mL against Trypanasoma brucei | [72] | |
| Antiplasmodial | High activity with an IC50 ≤ 20 μg/mL | [72] | |
| C. pendulus | Antifungal | Strong activity against Microsporum audouinii, Trichophyton rubrum and Epidermophyton floccosum at 100% for all the species | [78] |
| C. flexuosus | Chemopreventive | Potent in vivo activity against Ehrlich and Sarcoma-180 tumors. | [71] |
| C. densiflorus Stapf | Antibacterial | Gram-negative bacteria. MICs were found to be between 250 and 500 ppm for the Gram-positive and between 500 and 1000 ppm for the Gram-negative bacteria | [79] |
| C. ambiguus | Inflammatory | Inhibition of ADP-induced human platelet serotonin release in the cell. | [26] |
| C. nardus | Antibacterial | MIC values ranged from 0.244 µg/mL to 0.977 µg/mL when tested against the bacterial isolates | [52] |
| C. nervatus | Molluscidal activity | It inhibits Biomphalaria pfeifferi at LD50 of 213.099 ppm dose dependent | [80] |
| C. olivieri | Antimicrobial activity | Exhibited excellent antimicrobial activity against gram ±ve organisms | [14] |
Insecticidal activity is one of the biological effects of most plant of the Cymbopogon genus; it is either applied as pest control for stored crops or as mosquito repellent/ insecticide. The essential oils of C. martinii have been studied and found to display high anthelmintic activity against Caenorhabditis elegans at ED50 value of 125.4 µg/mL, C schoenanthus, C. giganteus and C. citratus essential oils from Benin Republic in West Africa all displayed about 100% mortality rate against adult Anopheles gambiae [69]. The essential oil from C. winterianus caused a dose dependent mortality of Culex quinquefasciatus with LC50 of 0.9% [70].
The anticancer properties of Cymbopogon species have also been studied. The essential oils of C. flexuosus was effective in inhibiting the growth and killing of Ehrlich and Sarcoma-180 tumors cells. In this study, it was discovered that at a dose of 200 mg/kg, Ehrlich solid tumor inhibition was about 57.83% compared to the 45.23% inhibition observed with 5-fluorouracil (22 mg/kg) [71]. Inhibition of early phase of hepatocarcinogenesis was also observed in C. citratus [67]. Positive results in several other bioassays such as antiprotozoal, anti-inflammatory, antimicrobial, anti-bacterial, anti-diabetic, anticholinesterase, molluscidal, antifungal and larvicidal activity are also prominent with Cymbopogon species as outlined in Table 3.
5. Conclusions
Cymbopogon species have been used as traditional medicine in many countries. Of all the species reviewed, C. citratus and C. flexuosus are the most widely used in traditional and in conventional medicine due to the pharmacological potential of their phytochemicals. The majority of these species contain a voluminous amount of essential oils which have shown several biological activities such as insecticidal, anti-protozoan, anticancer, anti-HIV, anti-inflammatory and anti-diabetes effects.
Acknowledgments
The authors are grateful to Govan Mbeki Research office, UFH, Directorates of Research and Development, WSU and NRF for financial support.
Author Contributions
Opeyemi Avoseh carry out the literature survey and wrote part of first draft of the manuscript. Pamela Rungqu investigated the essential oil composition of Cymbopogon species found in the Eastern Cape and wrote part of the first draft of the manuscript. Opeoluwa Oyedeji, Benedicta Nkeh-Chungag and Adebola Oyedeji are supervisors to the above authors on the chemistry and inflammatory studies of the essential oils. They also contributed editorial to the writing and editing of the final manuscript
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ballhorn D.J., Kautz S., Heil M, Hegeman A.D. Analyzing plant defenses in nature. Plant Signal. Behav. 2009;4:743–745. doi: 10.4161/psb.4.8.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verpoorte R., Memelink J. Engineering secondary metabolite production in plants. Curr. Opin. Biotechnol. 2002;13:181–187. doi: 10.1016/S0958-1669(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 3.Bourgaud F., Gravot A., Milesi S., Gontier E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001;161:839–851. doi: 10.1016/S0168-9452(01)00490-3. [DOI] [Google Scholar]
- 4.Khanuja S.P.S., Shasany A.K., Pawar A., Lal R.K., Darokar M.P., Naqvi A.A., Rajkumar S., Sundaresan V., Lal N., Kumar S. Essential oil constituents and RAPD markers to establish species relationship in Cymbopogon Spreng. (Poaceae) Biochem. Syst. Ecol. 2005;33:171–186. doi: 10.1016/j.bse.2004.06.011. [DOI] [Google Scholar]
- 5.Lemongrass Production: In Essential Oil Crops, Production Guideslenes for Lemongrass. Directorate Communication Services, Department of Agriculture, Forestry and Fisheries Pretoria; Pretoria, South Africa: 2012. pp. 1–26. A Publication of the Department of Agriculture, Forestry and Fisheries. [Google Scholar]
- 6.Padalia R.C., Verma R.S., Chanotiya C.S., Yadav A. Chemical fingerprinting of the fragrant volatiles of nineteen indian cultivars of Cymbopogon Spreng (Poaceae) Rec. Nat. Prod. 2011;5:290–299. [Google Scholar]
- 7.Shackleton C.M., Timmermans H.G., Nongwe N., Hamer N., Palmer N.R. Direct-use values of non-timber forest products from two areas on the Transkei Wild Coast. Agrekon. 2007;46:113–134. doi: 10.1080/03031853.2007.9523764. [DOI] [Google Scholar]
- 8.Aibinu I., Adenipekun T., Adelowowtan T., Ogunsanya T., Ogungbemi T. Evaluation of the antimicrobial properties of different parts of Citrus aurantifolia (lime fruit) as used locally. Afr. J. Biotechnol. 2007;2:185–190. [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong M.-R., Park P.B., Kim D.-H., Jang Y.-S., Jeong H.S., Choi S.-H. Essential oil prepared from Cymbopogon citrates exerted an antimicrobial activity against plant pathogenic and medical microorganisms. Mycobiology. 2009;37:48–52. doi: 10.4489/MYCO.2009.37.1.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai M.A., Parikh J. Microwave assisted extraction of essential oil from Cymbopogon flexuosus (Steud.) wats: A parametric and comparative study. Sep. Sci. Technol. 2012;47:1963–1970. doi: 10.1080/01496395.2012.659785. [DOI] [Google Scholar]
- 11.Noor S., Latip H., Lakim M.Z., Syahirah A., Bakar A. The Potential of Citronella Grass, Cymbopogon Nardus as Biopesticide Against Plutella Xylostella Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA, 40450 Shah Alam; Proceedings of the UMT 11th International Annual Symposium on Sustainability Science and Management; Kuala Terengganu, Malaysia. 9–11 July 2012; pp. 190–193. [Google Scholar]
- 12.Bagheri R., Mohamadi S., Abkar A., Fazlollahi A. Essential oil components of Cymbopogon parkeri STAPF from Iran. Pak. J. Biol. Sci. 2007;10:3485–3486. doi: 10.3923/pjbs.2007.810.813. [DOI] [PubMed] [Google Scholar]
- 13.Govere J., Durrheim D.N., Baker L., Hunt R., Coetzee M. Efficacy of three insect repellents against the malaria vector Anopheles arabiensis. Med. Vet. Entomol. 2000;14:441–444. doi: 10.1046/j.1365-2915.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 14.Mahboubi M., Kazempour N. Biochemical activities of Iranian Cymbopogon olivieri (Boiss) Bor. essential oil. Indian J. Pharm. Sci. 2012;74:356–360. doi: 10.4103/0250-474X.107071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas H., Hassan V.A. Chemical constituents and efficacy of Cymbopogon olivieri (BOISS.) BAR essential oil against Malaria. DARU. 2003;11:125–128. [Google Scholar]
- 16.Kepe T. Land restitution and biodiversity conservation in South Africa: The case of Mkambati, eastern cape province. Can. J. Afr. Stud. 2004;38:688–704. doi: 10.2307/4107262. [DOI] [Google Scholar]
- 17.Leite B.L., Souza T.T., Antoniolli A.R., Guimarães A.G., Rosana S.Q., Jullyana S.S., Bonjardim L.R., Alves P.B., Arie F.B., Marco A.A., et al. Volatile constituents and behavioral change induced by Cymbopogon winterianus leaf essential oil in rodents. Afr. J. Biotechnol. 2011;10:8312–8319. [Google Scholar]
- 18.Secoy D.M., Smith A.E. Use of plants in control of agricultural and domestic pests. Econ. Bot. 1983;37:28–57. doi: 10.1007/BF02859304. [DOI] [Google Scholar]
- 19.Hilgert N.I. Plants used in home medicine in the Zenta River basin, Northwest Argentina. J. Ethnopharmacol. 2001;76:11–34. doi: 10.1016/S0378-8741(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 20.Valdés A.F., Martínez J.M., Lizama R.S., Gaitén Y.G., Rodríguez D.A., Payrol J.A. In vitro antimalarial activity and cytotoxicity of some selected Cuban medicinal plants. Rev. Inst. Med. Trop. Sao Paulo. 2010;52:197–201. doi: 10.1590/S0036-46652010000400006. [DOI] [PubMed] [Google Scholar]
- 21.Morton J.F. Atlas of Medicinal Plants of Middle America, Bahamas to Yucatan. Charles C. Thomas; Springfield, IL, USA: 1981. [Google Scholar]
- 22.Olivero-Verbel J., Nerio L.S., Stashenko E.E. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag. Sci. 2010;66:664–668. doi: 10.1002/ps.1927. [DOI] [PubMed] [Google Scholar]
- 23.Moreira F.V., Bastos J.F., Blank A.F., Alves P.B., Santos M.R. Chemical composition and cardiovascular effects induced by the essential oil of Cymbopogon citratus DC. Stapf, Poaceae, in rats. Rev. Bras. Farmacogn. 2010;20:904–909. doi: 10.1590/S0102-695X2010005000012. [DOI] [Google Scholar]
- 24.Mahabir D., Gulliford M.L. Use of medicinal plants for diabetes in Trinidad and Tobago. Rev. Panam. Salud Publica. 1997;3:174–179. doi: 10.1590/S1020-49891997000300002. [DOI] [PubMed] [Google Scholar]
- 25.Jirovetz L., Buchbauer G., Eller G., Ngassoum M.B., Maponmetsem P.M. Composition and antimicrobial activity of Cymbopogon giganteus (Hochst.) Chiov. essential flower, leaf and stem oils from Cameroon. J. Essent. Oil Res. 2007;19:485–489. doi: 10.1080/10412905.2007.9699959. [DOI] [Google Scholar]
- 26.Grice I.D., Rogers K.L., Griffiths L.R. Isolation of bioactive compounds that relate to the anti-platelet activity of Cymbopogon ambiguus. Evid. Based Complement. Alternat. Med. 2011;2011:467134. doi: 10.1093/ecam/nep213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dayalan A.M. Traditional Aboriginal Medicine Practice in the Northern Territory; Proceedings of the International Symposium on Traditional Medicine; Awaji Island, Japan. 11–13 September 2000. [Google Scholar]
- 28.Smith N.M. Ethnobotanical Field Notes From The Northern Territory, Australia. J. Adelaide Bot. Gard. 1991;14:1–65. [Google Scholar]
- 29.Jayasinha P. Medicinal and Aromatic Plant Series. Industrial Technology Institute; Colombo, Sri Lanka: 1999. pp. 1–32. [Google Scholar]
- 30.Al-Ghamdi S.S., Al-Ghamdi A.A., Shamman A.A. Inhibition of calcium oxalate nephrotoxicity with cymbopogon schoenanthus (al-ethhkher) Drug Metab. Lett. 2007;1:241–244. doi: 10.2174/187231207783221420. [DOI] [PubMed] [Google Scholar]
- 31.El-askary H.I., Meselhy M.R., Galal A.M. Sesquiterpenes from Cymbopogon proximus. Molecules. 2003;8:670–677. doi: 10.3390/80900670. [DOI] [Google Scholar]
- 32.Taking Stock of Your Future. Volume 37. Queensland Government; Queensland, Australia: 2011. [(accessed on 24 January 2015)]. Beeftalk; pp. 1–24. Available online: http:www.futurebeef.com.au. [Google Scholar]
- 33.Takaisi-Kikuni N.B., Krüger D., Gnann W., Wecke J. Microcalorimetric and electron microscopic investigation on the effects of essential oil from Cymbopogon densiflorus on Staphylococcus aureus. Microbios. 1996;88:55–62. [PubMed] [Google Scholar]
- 34.De-Smet P.A. Some ethnopharmacological notes on African hallucinogens. J. Ethnopharmacol. 1996;50:141–146. doi: 10.1016/0378-8741(95)01337-7. [DOI] [PubMed] [Google Scholar]
- 35.El-bakry A.A., Abdel-salam A.M. Regeneration from embryogenic callus and suspension cultures of the wild medicinal plant Cymbopogon schoenanthus. Afr. J. Biotechnol. 2012;11:10098–10107. [Google Scholar]
- 36.Asaolu M.F., Oyeyemi O.A., Olanloku J.O. Chemical compositions, Phytochemical Constituents and in vitro Biological Activity of Various Extracts of Cymbopogon citratus. Pakistan J. Nutr. 2009;8:1920–1922. doi: 10.3923/pjn.2009.1920.1922. [DOI] [Google Scholar]
- 37.Rizk A.M., Hammouda F.M., Ismail S.I., Kame A.S., Rimpler H. Constituents of Plants Growing in Qatar Part XXV11: Flavonoids of Cymbopogon Parkerii. Qatar Univ. Sci. J. 1995;15:33–35. [Google Scholar]
- 38.Rizk A.M., Rimpler H., Ghaleb H., Heiba H.I. The antispasmodic components of Cymbopogon parkeri Stapf. Int. J. Crude Drug Res. 1986;24:69–74. [Google Scholar]
- 39.Cheel J., Theoduloz C., Rodriaguez J., Schmeda-Hirschmann G. Free Radical Scavengers and Antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.) J. Agric. Food Chem. 2005;53:2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 40.Bottini A.T., Dev V., Garfagnoli D.J., Hope H., Joshi P., Lohani H., Mathela C.S., Nelson T.E. Isolation and crystal structure of a novel dihemiacetal bis-monoterpenoid from Cymbopogon martinii. Phytochemistry. 1987;26:2301–2302. doi: 10.1016/S0031-9422(00)84706-8. [DOI] [Google Scholar]
- 41.Hanson S.W., Crawford M., Koker M.E., Menezes F.A. Cymbopogonol, a new triterpenoid from Cymbopogon citratus. Phytochemistry. 1976;15:1074–1075. doi: 10.1016/S0031-9422(00)84411-8. [DOI] [Google Scholar]
- 42.Andrade E.H., Zoghbi M.D., Lima M.D. Chemical composition of the essential oils of Cymbopogon citratus (DC.) Stapf cultivated in north of Brazil. J. Essent. Oil Bear. Plants. 2009;12:41–45. doi: 10.1080/0972060X.2009.10643689. [DOI] [Google Scholar]
- 43.Kumar B.S. Essential oil of Cymbopogon citratus against diabetes: Validation by in vivo experiments and computational studies. J. Bioanal. Biomed. 2013;5:194–203. [Google Scholar]
- 44.Kasali A.A., Oyedeji A.O., Ashilokun A.O. Volatile leaf oil constituents of Cymbopogon citratus (DC) Stapf. Flavour Fragr. J. 2001;16:377–378. doi: 10.1002/ffj.1019. [DOI] [Google Scholar]
- 45.Sidibé L., Chalchat J.-C., Garry R.-P., Lacombe L., Harama M. Aromatic plants of Mali (IV): chemical composition of essential oils of Cymbopogon citratus (DC) Stapf and C. giganteus (Hochst.) Chiov. J. Essent. Oil Res. 2001;13:110–112. doi: 10.1080/10412905.2001.9699629. [DOI] [Google Scholar]
- 46.Bassolé I.H., Lamien-Meda A., Bayala B., Obame L.C., Ilboudo A.J., Franz C., Novak J., Nebié R.C., Dicko M.H. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine. 2011;18:1070–1074. doi: 10.1016/j.phymed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Chisowa E.H., Hall D.R., Farman D.I. Volatile constituents of the essential oil of Cymbopogon citratus Stapf grown in Zambia. Flavour Fragr. J. 1998;13:29–30. doi: 10.1002/(SICI)1099-1026(199801/02)13:1<29::AID-FFJ682>3.0.CO;2-S. [DOI] [Google Scholar]
- 48.Matasyoh J.C., Wagara I.N., Nakavuma J.L., Kiburai A.M. Chemical composition of Cymbopogon citratus essential oil and its effect on mycotoxigenic Aspergillus species. Afr. J. Food Sci. 2011;5:138–142. [Google Scholar]
- 49.Loumouamou A.N., Biassala E., Silou T., Ntondele-Nsansi P., Diamouangana J., Nzikou J.M., Chalchat J.C., Figueredo G. Characterisation of a Giant Lemon Grass Acclimatised in the Congo-Brazzaville. Adv. J. Food Sci. Technol. 2010;2:312–317. [Google Scholar]
- 50.Farhang V., Amini J., Javadi T., Nazemi J., Ebadollahi A. Chemical composition and antifungal activity of essential oil of Cymbopogon citratus (DC.) Stapf. against three Phytophthora species. Greener J. Biol. Sci. 2012;3:292–298. [Google Scholar]
- 51.Menut C., Bessiére J.M., Samaté D., Djibo A.K. Aromatic plants of tropical west Africa. XI. chemical composition, antioxidant and antiradical properties of the essential oils of three Cymbopogon species from Burkina Faso. J. Essent. Oil Res. 2011;12:37–41. [Google Scholar]
- 52.Wei L.S., Wee W. Chemical composition and antimicrobial activity of Cymbopogon nardus citronella essential oil against systemic bacteria of aquatic animals. Iran. J. Microbiol. 2013;5:147–152. [PMC free article] [PubMed] [Google Scholar]
- 53.Shahi A.K., Sharma S.N., Tava A. Composition of Cymbopogon pendulus (Nees ex Steud) wats, an elemicin-rich oil grass grown in Jammu region of India. J. Essent. Oil Res. 1997;9:561–563. doi: 10.1080/10412905.1997.9700777. [DOI] [Google Scholar]
- 54.Rodrigues K.A., Dias C.N., Moraes D.F., Filho V.M., Andrade E.H., Mala J.G. Molluscicidal and larvicidal activities and essential oil composition of Cymbopogon winterianus. Pharm. Biol. 2013;51:1293–1297. doi: 10.3109/13880209.2013.789536. [DOI] [PubMed] [Google Scholar]
- 55.Quintans-Júnior L.J., Souza T.T., Leite B.S., Lessa M.N., Bonjardim L.R., Santos M.R., Alves P.B., Blank A.F., Antoniolli A.R. Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine. 2008;15:619–624. doi: 10.1016/j.phymed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Rabehaja D.J., Raoelison G., Ihandriharison H., Ramanoelina P.A., Casanova J., Tomi F. Volatile components from Cymbopogon giganteus (Hochst) Chiov var. madagascariensis (A. Camus) J. Essent. Oil Bear. Plants. 2010;13:522–527. doi: 10.1080/0972060X.2010.10643857. [DOI] [Google Scholar]
- 57.Chisowa E.H. Chemical composition of flower and leaf oils of Cymbopogon densiflorus Stapf from Zambia. J. Essent. Oil Res. 1997;9:469–470. doi: 10.1080/10412905.1997.9700752. [DOI] [Google Scholar]
- 58.Khadri A., Serralheiro M.L., Nogueira J.M., Neffati M., Smiti S., Araújo M.E. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC-mass spectrometry and 13C NMR. Food Chem. 2008;109:630–637. doi: 10.1016/j.foodchem.2007.12.070. [DOI] [Google Scholar]
- 59.Wany A., Jha S., Nigam V.K., Pandey D.V. Chemical analysis and therapeutic uses of citronella oil from Cymbopogon winterianus: A short review. Int. J. Adv. Res. 2013;1:504–521. [Google Scholar]
- 60.Chowdhury S.R., Tandon P.K., Chowdhury A.R. Chemical composition of the essential oil of Cymbopogon flexuosus (Steud) Wats. growing in Kumaon Region. J. Essent. Oil Bear. Plants. 2010;13:588–593. doi: 10.1080/0972060X.2010.10643867. [DOI] [Google Scholar]
- 61.Mohamed H.R., Sallam Y.I., el-Leithy A.S., Aly S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012;57:113–116. [Google Scholar]
- 62.Gbenou J.D., Ahounou J.F., Akakpo H.B., Laleye A., Yayi E., Gbaguidi F., Baba-Moussa L., Darboux R., Dansou P., Moudachirou M., et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep. 2013;40:1127–1134. doi: 10.1007/s11033-012-2155-1. [DOI] [PubMed] [Google Scholar]
- 63.Soares M.O., Vinha A.F., Barreira S.V., Coutinho F., Aires-Goncalves S., Oliveira M.B., Pires P.C., Castro A. Cymbopogon citratus EO antimicrobial activity against multi-drug resistant Gram-positive strains and non- albicans-Candida species. FORMATEX. 2013:1081–1086. [Google Scholar]
- 64.Ranitha M., Nour A.H., Sulaiman A.Z., Nour A.H., Thani R.S. A Comparative study of Lemongrass (Cymbopogon citratus) essential oil extracted by microwave-assisted hydrodistillation (MAHD) and conventional hydrodistillation (HD) method. Int. J. Chem. Eng. Appl. 2014;5:104–108. [Google Scholar]
- 65.Dubey V.S., Mallavarapu G.R., Luthra R. Changes in the essential oil content and its composition during palmarosa (Cymbopogon martinii (Roxb.) Wats. var. motia) inflorescence development. Flavour Fragr. J. 1999;15:309–314. doi: 10.1002/1099-1026(200009/10)15:5<309::AID-FFJ914>3.0.CO;2-F. [DOI] [Google Scholar]
- 66.Figueirinha A., Paranhos A., Perez-Alonso J., Santos-buelga C., Batista M. Cymbopogon citratus leaves: Characterisation of flavonoids by HPLC-PDA-ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008;110:718–728. doi: 10.1016/j.foodchem.2008.02.045. [DOI] [Google Scholar]
- 67.Puatanachokchai R., Kishida H., Denda A., Murata N. Inhibitory effects of lemon grass (Cymbopogon citratus, Stapf) extract on the early phase of hepatocarcinogenesis after initiation with diethylnitrosamine in male Fischer 344 rats. Cancer Lett. 2002;183:9–15. doi: 10.1016/S0304-3835(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 68.Wright S.C., Maree J.E., Sibanyoni M. Treatment of oral thrush in HIV/AIDS patients with lemon juice and lemon grass (Cymbopogon citratus) and gentian violet. Phytomedicine. 2009;16:118–124. doi: 10.1016/j.phymed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Nonviho G., Wotto V.D., Noudogbessi J., Avlessi F., Akogbeto M., Sohounhloué D.C. Original research paper insecticidal activities of essential ils extracted from three species of poaceae On Anopheles Gambiae Spp, major vector Of Malaria. Sci. Study Res. 2010;11:411–420. [Google Scholar]
- 70.Makhaik M., Naik S.N., Tewary D.K. Evaluation of anti-mosquito properties of essential oils. J. Sci. Ind. Res. 2005;64:129–133. [Google Scholar]
- 71.Sharma P.R., Mondhe D.M., Muthiah S., Pal H.C., Shahi A.K., Saxena A.K., Qazi G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. Biol. Interact. 2009;179:160–168. doi: 10.1016/j.cbi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Kpoviessi S., Bero J., Agbani P., Gbaguidi F., Kpadonu-Kpoviessi B., Sinsin B., Accrombessi G., Frederich M., Moudachirou M., Quetin-Leclercq J. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J. Ethnopharmacol. 2014;151:652–659. doi: 10.1016/j.jep.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 73.Blanco M.M., Costa C.R., Freire O., Santos J.G., Costa M. Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine. 2009;16:265–270. doi: 10.1016/j.phymed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 74.Barreira C.E., Morais S.M., Lima M.A., William E. Larvicidal activity of essential oils from Brazilian Plants against. Mem. Inst. Oswaldo Cruz Rio Janeiro. 2004;99:541–544. doi: 10.1590/S0074-02762004000500015. [DOI] [PubMed] [Google Scholar]
- 75.Francisco V., Figueirinha A., Neves B.M., García-Rodríguez C.L., Maria C.C., Maria T.B. Cymbopogon citratus as source of new and safe anti-inflammatory drugs: Bio-guided assay using lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2011;133:818–827. doi: 10.1016/j.jep.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 76.Ketoh G.K., Koumaglo H.K., Glitho I.A., Huignard J. Comparative effects of Cymbopogon schoenanthus essential oil and piperitone on Callosobruchus maculatus development. Fitoterapia. 2006;77:506–510. doi: 10.1016/j.fitote.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 77.Oliveira W.A., Pereira F.O., de Luna G.C., Lima I.O., Wanderley P.A., de Lima R.B., Lima E.O. Antifungal activity of Cymbopogon winterianus Jowitt Ex Bor against Candida Albicans. Braz. J. Microbiol. 2011;42:433–441. doi: 10.1590/S1517-83822011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pandey M.C., Sharma J.R., Dikshits A. Antifungal evaluation of the essential oil of Cymbopogon pendulus (Nees ex Steud.) Wats. cv. Praman. Flavour Fragr. J. 1996;11:257–260. doi: 10.1002/(SICI)1099-1026(199607)11:4<257::AID-FFJ576>3.0.CO;2-5. [DOI] [Google Scholar]
- 79.Takaisi-Kikuni N.B., Tshilanda D., Babady B. Antibacterial activity of the essential oil of Cymbopogon densiflorus. Fitoterapia. 2000;71:69–71. doi: 10.1016/S0367-326X(99)00097-0. [DOI] [PubMed] [Google Scholar]
- 80.El-kamali H.H., Om R., Khalid A. Molluscicidal Activity of the Essential Oils of Cymbopogon nervatus Leaves and Boswellia papyrifera Resins. Curr. Res. J. Biol. Sci. 2010;2:139–142. [Google Scholar]