Abstract
A new pseudoguaianolide 1 and two new guaiane-type sesquiterpene glucosides 2 and 3, were isolated from the aerial parts of Ambrosia artemisiifolia L together with two known sesquiterpene dilactones 4 and 5. The new compounds were determined on the basis of spectroscopic and chemical methods to be 3β-acetoxy-4β-hydroxy-1α,7α, 10β,11αH-pseudoguaia-12,8β-olide (1), 1β,7β,9β,10β,13αH-guaia-4(5)-en-12,6β-olide 9-O-β-d-glucoside (2) and 4β-hydroxy-1α,5α,7α,9αH-guaia-10(14),11(13)-dien-12-acid 9-O-β-d-glucoside (3). The isolated compounds were evaluated for cytotoxicity against human promyelocytic leukemia HL-60 cell lines in vitro, but were all inactive.
Keywords: Ambrosia artemisiifolia L., sesquiterpenoid, sesquiterpene glucoside
1. Introduction
Ambrosia artemisiifolia L. (Asteraceae), an invasive alien plant species in China, is nowadays widespread in an area ranging from Guangdong Province in the south to Heilongjiang Province in the north [1]. This species is considered as a harmful weed capable of quickly colonizing both agricultural and urban areas with competitive seeds, and its pollen can induce serious allergic disorders during a certain period of its development [2]. However, many investigations into the composition and properties of A. artemisiifolia indicate that this species can serve a valuable source of biologically active substances. The extracts of this plant showed various bioactivities, such as molluscicidal [3], plant growth inhibitory [4], anti-inflammatory [5], antithrombin [6], antibacterial [7], insecticidal [8], hepatoprotective and hypolipemic activities [9]. On the other hand, the main chemical components of this species were identified as sesquiterpene lactones, which account for the antihelminthic, cardiotonic, antiinflammatory, analgesic, sedative (calming), antimalarial, anti-tumor, and other types of pharmacological activity [10,11]. As part of an ongoing search for novel bioactive compounds, we studied Ambrosia artemisiifolia L. growing in Hunan Province of China. Herein, this paper reports the isolation and identification of three new sesquiterpenoids from the aerial parts of the plant.
2. Results and Discussion
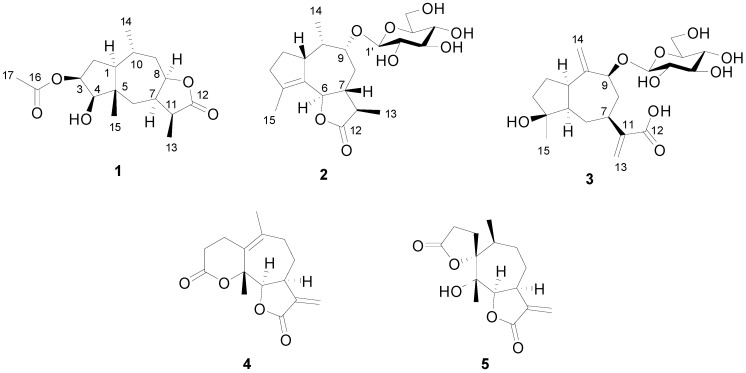
The dried aerial parts of A. artemisiifolia were extracted three times with MeOH at room temperature. The MeOH extract residue was suspended in water and then partitioned successively with petroleum ether and EtOAc. Column chromatography of the EtOAc-soluble fraction yielded three new compounds 1–3 and two known compounds, psilostachyin B (4) [12] and psilostachyin (5) [13] (Figure 1).
Figure 1.
Structures of compounds 1–5 isolated from A. artemisiifolia.
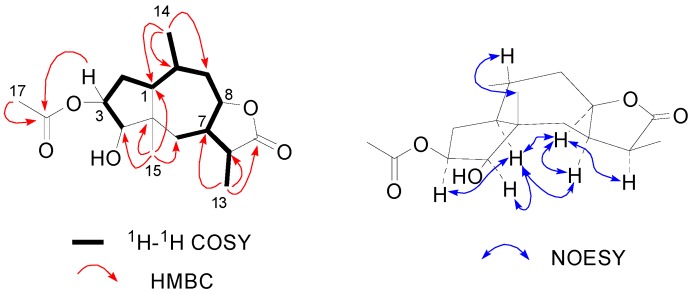
Compound 1 was obtained as white amorphous powder, and the molecular formula was assigned as C17H26O5 from its HRESIMS (m/z 311.1852 [M+H]+) and NMR data. The 1H-NMR spectrum displayed readily recognizable signals for four methyl groups [δH 1.18 (3H, s, H-15), 2.04 (3H, s, H-17), 0.90 (3H, d, J = 7.0 Hz, H-14) and 1.02 (3H, d, J = 7.4 Hz, H-13)] (Table 1). The 13C-NMR and DEPT spectra of 1 exhibited 17 carbons corresponding to four methyls, three methylenes, seven methines (including three oxy-methine carbons), a quaternary carbon, and two quaternary carbonyl groups (Table 1). The structure of 1 was deduced based on 1H-1H COSY, HSQC, and HMBC techniques using those methyl groups as starting points. The HMBC correlations from the H-atoms of the four methyl groups to corresponding C-atoms [H3-17 (δH 2.04) to C-16; H3-14 (δH 0.90) to C-1, C-9 and C-10; H3-13 (δH 1.02) to C-7, C-11 and C-12; H3-15 (δH 1.18) to C-1, C-4, C-5 and C-6) (Table 1)] established a typical acetyl moiety (δH 2.04, δC 169.8 and 20.3) and three fragments as 14CH3-10CH(1CH)-9CH-, 13CH3-11CH (7CH)-12COOH, and 15CH3-5C(4CH/1CH)-6CH2- (Figure 2). Those fragments were further linked to form a 5/7-membered fused-ring structure based on the analysis of the 1H-1H-COSY correlations (Figure 2). Considering the molecular formula C17H26O5 and the presence of two C=O bonds, 1 was suggested to be an acetylated derivative of pseudoguaianolide [14]. The lactone ring was formed between C-12 and C-8, which deduced from the low-field chemical shift of C-12 (δC 177.8) and C-8 (δH 4.58 and δC 79.7) [15]. While the acetyl moiety was found to be attached to C-3 via an ester linkage from long-range correlation of H-3 [δ 5.45 (ddd, J = 8.1, 7.5, 5.0 Hz)] with C-16 (δC 169.8) observed in the HMBC spectrum (Figure 2). Thus the planar structure of 1 was deduced as 3-acetoxy-4-hydroxy-pseudoguaia-12,8-olide. The relative stereochemistry of 1 was assigned by analyses of the NOESY spectrum and the proton coupling patterns (Table 1). The key NOE correlation observed between H-7 and H-8, as well as the large coupling constant measured for H-8 (8.6 Hz), indicating the presence of a cis-fused lactone ring [16]. While the NOE correlation was absent between H-1 and H3-15 suggesting a typical trans-fused 5/7-membered ring, a model of this molecule indicated that H-1 was α-orientation [14]. Meanwhile, the correlations of H-1 with H-3, H-4 and H-7, and H-7 with H-8 and H-11 revealed that all of these protons (H-1, H-3, H-4, H-7 and H-11) were cofacial and were arbitrarily assigned α-oriented. The β-orientation of H-10 was revealed by the coupling constant measured H-1 (JH-1/H-10 = 13.6 Hz), and corroborated by an NOE between H-10 and H3-15. Finally, compound 1 was established as 3β-acetoxy-4β-hydroxy-1α,7α,10β,11αH-pseudoguaia-12,8β-olide.
Table 1.
NMR assignments of 1 by DEPT, HSQC, HMBC, and NOESY experiments in C5D5N a.
| Position | δH (J in Hz) | δC, Mult. | HMBC | NOESY |
|---|---|---|---|---|
| 1 | 1.51, ddd (13.6, 5.6, 5.6) | 39.0 CH | C-2, C-4, C-5, C-10, C-14,C-15 | H-3, H-4, H-7 |
| 2 | Hα: 1.82, m | 31.5 CH2 | C-1, C-3 | |
| Hβ: 2.01, m | C-1, C-3 | |||
| 3 | 5.45, ddd (8.1, 7.5, 5.0) | 71.0 CH | C-1, C-4, C-5, C-16 | Hα-2, Hβ-2, H-4 |
| 4 | 3.64, d (7.5) | 75.9 CH | C-3, C-5, C-6, C-15 | H-1, H-3, H-7 |
| 5 | – | 43.3 qC | ||
| 6 | Hα: 1.94, br d (14.3) | 30.5 CH2 | C-5, C-7, C-15 | H-4, H-7 |
| Hβ: 1.16, overlapped | Hβ-9 | |||
| 7 | 2.51, m | 36.7 CH | C-5, C-6, C-11, C12 | H-1, H-4, H-8, H-11 |
| 8 | 4.58, ddd (13.2, 8.6, 2.8) | 79.7 CH | C-7 | H-7, Hα-9, Hβ-9, H-11 |
| 9 | Hα: 2.07, m | 36.4 CH2 | C-1, C-7, C-8, C-10, C-14 | |
| Hβ: 1.66, br d (12.7) | C-1, C-7, C-8, C-10 | Hβ-6, H-14, H-15, | ||
| 10 | 1.80, m | 28.5 CH | C-1, C-5, C-14 | |
| 11 | 2.94, m | 37.5 CH | C-6, C-7, C-12, C-13 | H-7, H-8, H-13 |
| 12 | – | 177.8 qC | ||
| 13 | 1.02, d (7.4) | 9.7 CH3 | C-7, C-11, C-12 | Hβ-6, H-7, H-11 |
| 14 | 0.90, d (7.0) | 16.4 CH3 | C-1, C9, C-10 | Hα-9 |
| 15 | 1.18, s | 17.5 CH3 | C-1, C-4, C-5, C-6 | Hβ-9, H-10 |
| 16 | – | 169.8 qC | ||
| 17 | 2.04, s | 20.3 CH3 | C-16 | H-3 |
Note: a 1H (400 MHz) and 13C (100 MHz) NMR; δ in ppm.
Figure 2.
Key 1H-1H COSY, HMBC and NOESY correlations of 1.
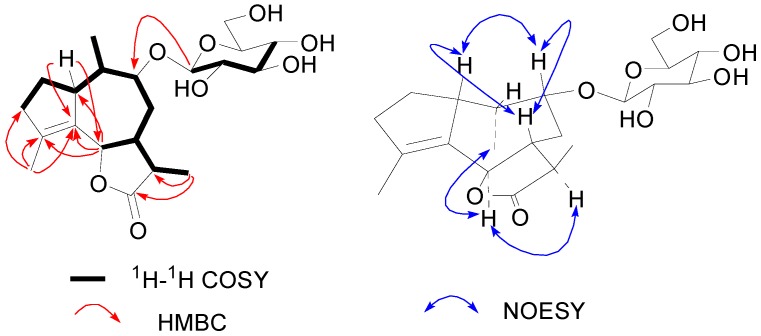
Compound 2 has the molecular formula C21H32O8 according to its HRESIMS and 13C-NMR data. 1H-, 13C-NMR and DEPT spectra revealed that 2 contained a typical β-d-glucopyranosyl [δH 4.38 (d, J = 7.8, H-1'); δC 102.5, 75.2, 77.9, 71.8, 78.2, 62.8], which was confirmed by acid hydrolysis and then co-chromatography with an authentic sample. Besides, the remaining 15 carbon signals, which belong to the aglycone, were attributable to three methyls, three methylenes, six methines (including two oxy-methines), two olefinic quaternary carbons, and a quaternary carbonyl group (Table 2). To deduce the structure of the aglycone and the glycosidic connection, a complete 1H and 13C-NMR spectral assignment was carried out using a combination of DEPT, HSQC, 1H-1H COSY, HMBC and NOESY experiments. The 1H-1H COSY spectrum of 2 revealed a fragment with eleven carbons [-3CH2-2CH2-1CH-10CH(14CH3)-9CH(OR)-8CH2-7CH(6CHOR)-11CH-13CH3] which was corroborated by HMBC correlations (Figure 3). The fragment was further connected by HMBC spectrum, the key HMBC correlations from H-1 to C-4, C-5 and C-6, from H-6 to C-1, C-4 and C-5, from H-13 to C-11, C-12 and C-7, and from H-15 to C-3, C-4 and C-5 indicated a sesquiterpene skeleton with a 5/7-membered fused-ring, which belong to that kind of guaiane-type sesquiterpenoid (Figure 3) [17,18]. In addition, the oxy-methine carbon δC 82.6 (C-9) showed a long-range correlation with the anomeric proton of glucose at δH 4.38 (d, J = 7.8 Hz, H-1'), indicating that the hydroxyl group at C-9 is glucosylated. The low-field chemical shift of δC 181.2 (C-12) and δC 83.3 (C-6), as well as the molecular formula C21H32O8 revealed that a lactone ring was formed between C-12 and C-6 [18,19]. Therefore, the planar structure of compound 2 was determined as guaia-4(5)-en-12,6-olide 9-O-β-d-glucoside. In the NOESY spectrum, there were no NOE correlations between H-6 and H-7, as well as the large values of the constants of J6,7 and J7,11 (10–11 Hz), suggesting the presence of a trans-fused lactone ring [19]. Moreover, the correlations of H-7 with H-1 and H-9, and H-6 with H-11 and H3-14 revealed that protons H-7, H-1 and H-9 were cofacial, while H-6, H-11 and H3-14 were on the opposite side. Assume the usual α-configuration for the isopropyl at C-7 [19], the stereochemistry of 2 was established as 1β,7β,9β,10β,13αH-guaia-4(5)-en-12,6β-olide 9-O-β-d-glucoside.
Table 2.
1H- (400 MHz) and 13C- (100 MHz) NMR spectral data of compounds 2 and 3.
| Position | 2 a | 3 b | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, Mult. | δH (J in Hz) | δC, Mult. | |
| 1 | 3.09, m | 49.9 CH | 3.24, m | 45.4 CH |
| 2 | Hα: 1.56, m; Hβ: 2.10, overlapped |
29.4 CH2 | 1.96, m | 27.0 CH2 |
| 3 | 2.36, m | 39.4 CH2 | Hβ: 1.77, m; Hα: 1.98, m | 40.9 CH2 |
| 4 | – | 131.6 qC | – | 80.7 qC |
| 5 | – | 142.5 qC | 2.38, br t (10.8) | 54.2 CH |
| 6 | 4.80, d (11.5) | 83.3 CH | Hβ: 1.39, ddd (12.2, 11.0, 10.8); Hα: 2.01, br d (12.2) |
33.4 CH2 |
| 7 | 1.89, ddd (11.5, 11.6, 11.6) | 46.8 CH | 2.92, br t (11.0) | 39.8 CH |
| 8 | Hα: 1.67, m; Hβ: 2.14, overlapped |
32.2 CH | Hα: 1.88, m; Hβ: 2.62, br d (11.8) |
45.3 CH2 |
| 9 | 3.88, m | 82.6 CH | 4.79, dd (11.0, 3.0) | 79.5 CH |
| 10 | 2.11, overlapped | 41.3 CH | – | 152.5 qC |
| 11 | 2.34, m | 42.5 CH | – | 149.0 qC |
| 12 | – | 181.2 qC | – | 169.8 qC |
| 13 | 1.18, d (6.9) | 12.5 CH3 | 5.55, br s; 6.42, br s | 121.9 CH2 |
| 14 | 0.80, d (7.0) | 8.1 CH3 | 5.27, br s; 6.18, br s | 110.1 CH2 |
| 15 | 1.81, s | 15.5 CH3 | 1.32, s | 24.9 CH3 |
| Glc-1' | 4.38, d (7.8) | 102.5 CH | 5.06, d (7.7) | 101.1 CH |
| 2' | 3.15, dd (8.0, 7.8) | 75.2 CH | 4.11, dd (8.2, 7.7) | 75.8 CH |
| 3' | 3.35, m | 77.9 CH | 4.23, m | 78.7 CH |
| 4' | 3.31, m | 71.8 CH | 4.26, m | 71.9 CH |
| 5' | 3.27, m | 78.2 CH | 3.88, m | 78.8 CH |
| 6' | 3.66, dd (11.6, 5.2); 3.88, br d (11.6) |
62.8 CH2 | 4.36, dd (11.9, 5.2); 4.51, dd (11.9, 2.2) |
62.9 CH2 |
Notes: a measured in CD3OD, b measured in C5D5N; δ in ppm.
Figure 3.
Key 1H-1H COSY, HMBC and NOESY correlations of 2.
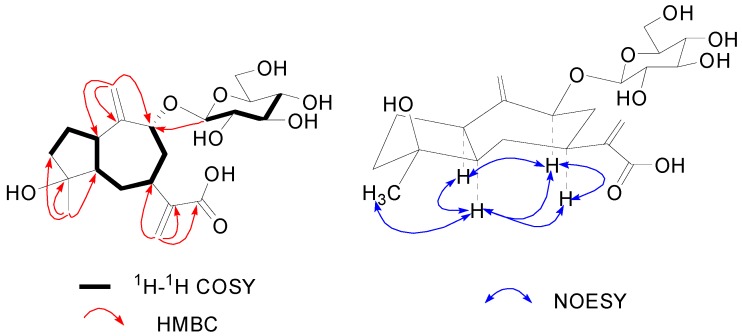
Compound 3 was obtained as a colorless gum. The molecular formula was shown to be C21H32O9 by its HRESIMS and 13C-NMR data. The structure of 3 contained a β-d-glucopyranosyl, which was confirmed by methods previously described in compound 2. Moreover, two pair of exomethylene singlets at δH 5.55 /6.42 (br s, H2-13) and 5.27 /6.18 (br s, H2-14), along with a methyl singlets δH 1.32 (s, H3-15) was clearly observed in 1H-NMR spectrum. The 13C-NMR and DEPT spectra (Table 2) showed a total of 21 carbon signals, among which, 15 were ascribable to the aglycone. The structure of the aglycone and the glycosidic connection were carried out by 2D-NMR methods (HSQC, 1H-1H COSY, HMBC and NOESY experiments). The HMBC correlations from the H-atoms of the exomethylenes and methyl groups to corresponding C-atoms revealed three C4 units as 13CH2=11C (7CH)-12COOH, 14CH2=10C(1CH)-9CH-, and 15CH3-4C(3CH2)-5CH-, respectively. Those C4 units in combination with a C8 unit -3CH2-2CH2-1CH-5CH-6CH2-7CH-8CH2-9CHOR deduced from 1H-1H COSY correlations (Figure 4), established the structure of the aglycone as 4,9-dihydroxyguaia-10(14),11(13)-dien-12-acid, which was good agree with the formula C21H32O9. Moreover, an informative correlation was also observed between the anomeric proton signal at δH 5.06 (d, J = 7.7 Hz, H-1') and a methine carbon signal at δC 79.5 (C-9) in the HMBC spectrum (Figure 4), implying that the sugar moiety was linked at the C-9 position. Therefore, the planar structure of 3 was established as 4-hydroxyguaia-10(14),11(13)-dien-12-acid 9-O-β-d-glucoside. The key NOESY correlations of H-1 with H-5 and H-9, and H-5 with H-7, H-9 and H3-15 showed that all of those protons (H-1, H-5, H-7, H-9 and H3-15) were cofacial. Meanwhile, the coupling constants of H-9 (δH 4.79, dd, J = 11.0, 3.0 Hz) revealed its α-oriented position. Thus compound 3 was determined to be 4β-hydroxy-1α,5α,7α,9αH-guaia-10(14),11(13)-dien-12-acid 9-O-β-d-glucoside.
Figure 4.
Key 1H-1H COSY, HMBC and NOESY correlations of 3.
The cytotoxicity of compounds 1–5 were tested against human promyelocytic leukemia HL-60 cell by the MTT method in vitro. The results revealed that all compounds were inactive (LC50 > 100 μM).
3. Experimental Section
3.1. General
Optical rotations were measured on WZZ2B automatic polarimeter (Precision Instrument Co., Shanghai, China); melting points were obtained on a SGW X-4 micromelting point apparatus (INESA Physico Optical Instrument Co., Ltd, Shanghai, China). The 1D- and 2D-NMR spectra were measured with a Bruker DRX-400 instrument (Bruker BioS-pin GmbH Company, Rheinstetten, Germany) with TMS as internal standard. ESIMS data were recorded on an API QSTAR mass spectrometer (Applied Biosystem/MSD Sciex, Concord, ON, Canada). Column chromatography was performed on silica gel 60 (200–300 mesh, Qingdao Marine Chemical Ltd, Qingdao, China), Sephadex LH-20 (GE Healthcare, Uppsala, Sweden) and Develosil ODS (50 μm, Nomura Chemical Co. Ltd., Osaka, Japan). Preparative HPLC was performed on a Waters 1525 Binary HPLC pump and a Waters 2414 refractive index detector (Waters Corp, Millipore, Milford, MA, USA) using a YMC-Pack ODS-A column (250 mm × 10 mm I.D.; S-5 μm, 12 nm).
3.2. Plant Material
The aerial parts of A. artemisiifolia were collected from Miluo, Hunan Province, P. R. China, in August 2012, and identified by Prof. Zhongshi Zhou, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The voucher specimen (No. 20120821) was deposited in Hunan Agricultural University.
3.3. Extraction and Isolation
Dried aerial parts of A. artemisiifolia (10.0 kg) were powdered and extracted three times with MeOH (95% v/v) at room temperature, then concentrated under reduced pressure to obtain a crude residue (0.5 kg). The residue was further suspended in H2O (2 L) and extracted with petroleum ether (PE) and EtOAc successively, to yield a PE-soluble fraction (90.0 g), an EtOAc-soluble fraction (90.0 g). The EtOAc-soluble fraction was subjected to silica gel column chromatography (CC) (100–200 mesh) with elution of CHCl3–MeOH (100:0 → 60:40, v/v) to give six fractions (Fr. C1–C6). The Fr. C2 was further separated by ODS-C18 CC (MeOH–H2O 30:70 → 70:30, v/v) to produce seven sub-fractions (C2-1–C2-7), then sub-fraction C2-3 was further purified by Sephadex LH-20 column (MeOH) and normal silica gel CC (petroleum ether–EtOAc, 9:1) to yield colourless crystal 4 (100.0 mg) and 5 (30.0 mg). Similarly, Fractions C3 (6.0 g) and C5 (8.3 g) were fractionated by an ODS-C18 column with elution of MeOH–H2O (30:70 → 70:30, v/v), respectively. Compound 1 (60.0 mg) was obtained from sub-fraction C3-5 by Sephadex LH-20 column (MeOH) and semi-preparative HPLC chromatography (MeOH-H2O 48%, v/v, flow rate 3 mL/min, tR = 23.6 min). Compounds 2 (8.0 mg) and 3 (6.0 mg) were obtained from sub-fraction C5-2 which purified successively on Sephadex LH-20 column (MeOH) and semi-preparative HPLC chromatography (MeOH-H2O 38%, v/v, flow rate 3 mL/min; tR-3 = 37.3 min, tR-2 = 50 min).
3.4. Characterization of Compounds 1–3
Compound 1: white powder, mp 290–293 °C, −44.8 (c 0.9, MeOH); IR (KBr) υmax 3342, 2951, 1769, 1740, 1727, 1704, 1660, 1176, 1035 cm−1; 1H-NMR (400 MHz, C5D5N) and 13C-NMR (100 MHz, C5D5N) spectroscopic data, see Table 1; positive ion ESIMS m/z: 311 [M+H]+, 333 [M+Na]+; negative ESIMS m/z: 655 [2M+Cl]−; HRESIMS m/z: 311.1852 [M+H]+ (calcd for C17H27O5, 311.1853).
Compound 2: white powder, mp 305–307 °C, +33.2 (c 0.5, MeOH); IR (KBr) υmax 3392, 2921, 2850, 1754, 1688, 1458, 1384, 1229, 1176 cm−1; 1H-NMR (400 MHz, MeOD) and 13C-NMR (100 MHz, MeOD) spectroscopic data, see Table 2; positive ion ESIMS m/z: 413 [M+H]+, 435 [M+Na]+; negative ESIMS m/z: 411 [M−H]−, 859 [2M+Cl]−; HRESIMS m/z: 435.1971 [M+Na]+ (calcd for C21H32O8Na, 435.1989).
Compound 3: yellowish syrup, −37.7 (c 1.1, MeOH); IR (KBr) υmax 3400, 2923, 2870, 1702, 1458, 1380, 1221 cm−1; 1H-NMR (400 MHz, C5D5N) and 13C-NMR (100 MHz, C5D5N) spectroscopic data, see Table 2; positive ion ESIMS m/z: 451 [M+Na]+, 879 [2M+Na]+; negative ESIMS m/z: 427 [M−H]−, 463 [M+Cl]−; HRESIMS m/z: 451.1935 [M+Na] + (calcd for C21H32O9Na, 451.1939).
3.5. Determination of the Configurations of Sugar Unit in 2 and 3
Compounds 2 (2.0 mg) in 1 N HCl (5 mL, 1,4-dioxane–H2O, 1:1) was heated under reflux for 8 h. After removal of the solvent, the residue was partitioned between Et2O and H2O. The water layer was neutralized with 5% NaOH and desalted (Sephadex LH-20, MeOH) to afford the sugar residue (1.0 mg). The sugar residue was derivatized with Sigma Sil-A for 35 min at 70 °C and analyzed by GC-MS [HP-5MS column (30 m × 0.25 mm, 0.25 mm); injection temperature: 250.0 °C; column flow: 1.33 mL/min; ion source temperature: 200.0 °C; interface temperature: 220.0 °C]. In the acid hydrolysate of 2, d-Glucose was confirmed by comparison of the retention times of the aforementioned derivative with the authentic d-glucose derivative prepared in a similar way, both of which showed identical retention time of 12.17 min. By the same method, the sugar moiety of 3 was identified as d-glucose.
3.6. Cytotoxicity Assays
The cytotoxic activity of each compound against human promyelocytic leukemia HL-60 cell was examined in vitro at the Second Xiangya Hospital of Central South University, and was determined by the MTT assay [20,21].
4. Conclusions
The phytochemical investigation of the aerial parts of A. artemisiifolia afforded a new pseudoguaianolide (1), two new guaiane-type sesquiterpene glucosides (2 and 3), as well as two known sesquiterpene dilactones psilostachyin B (4) and psilostachyin (5). Although all the isolated compounds were inactive against human promyelocytic leukemia HL-60 cell, the discovery of these new compounds further expands our knowledge of the structural diversity of the sesquiterpenoids produced by the plant A. artemisiifolia.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (No. 31400308, 31171908) and Special Fund for Agro-scientific Research in the Public Interest (No. 201303021).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/03/4450/s1.
Author Contributions
In this paper, the extraction and isolation were accomplished by Rui Huang; Zhongshi Zhou was in charge of the plant material identification; Wenbing Ding carried out the structural elucidations and the manuscript writing; as corresponding author Youzhi Li organized the study and analyzed the results.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1, 4 and 5 are available from the authors.
References
- 1.Lu D.W., Liu J., Qu H. Spread of ragweed and advances of preventive measures in China. Chin. J. Allergy Clin. Immunol. 2012;6:60–63. [Google Scholar]
- 2.Taglialatela-Scafati O., Pollastro F., Minassi A., Chianese G., de Petrocellis L., Di Marzo V., Appendino G. Sesquiterpenoids from common ragweed (Ambrosia artemisiifolia L.), an invasive biological polluter. Eur. J. Org. Chem. 2012;27:5162–5170. doi: 10.1002/ejoc.201200650. [DOI] [Google Scholar]
- 3.El-Sawy M.F., Bassiouny H.K., El-Magdoub A.I. Biological combat of schistosomiasis Ambrosia maritima (Damsissa) for snail control. J. Egypt. Soc. Parasitol. 1981;11:99–117. [PubMed] [Google Scholar]
- 4.Bradow J.M. Germination regulation by Amaranthus palmeri and Ambrosia artemisiifolia. In: Thompson A.C., editor. The Chemistry of Allelopathy. American Chemical Society; Washington, DC, USA: 1985. pp. 285–299. Chapter 19. [Google Scholar]
- 5.Pérez-G R.M. Anti-inflammatory activity of Ambrosia artemisiaefolia and Rhoeo spathacea. Phytomedicine. 1996;3:163–167. doi: 10.1016/S0944-7113(96)80030-4. [DOI] [PubMed] [Google Scholar]
- 6.Chistokhodova N., Nguyen C., Calvino T., Kachirskaia I., Cunningham G., Howard-Miles D. Antithrombin activity of medicinal plants from central Florida. J. Ethnopharmacol. 2002;81:277–280. doi: 10.1016/S0378-8741(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 7.Solujić S., Sukdolak S., Vuković N., Nićiforović N., Stanić S. Chemical composition and biological activity of the acetone extract of Ambrosia artemisiifolia L. pollen. J. Serb. Chem. Soc. 2008;73:1039–1049. doi: 10.2298/JSC0811039S. [DOI] [Google Scholar]
- 8.Zhang G.C., Zhao Y., Ma L., Bi B., Huang Y., Zhuang C., Zhang X., Meng S. Toxicity testing of insecticidal active substances from Ambrosia artemisiifolia and its security. J. Northeast For. Univ. 2010;38:94–96. [Google Scholar]
- 9.Parkhomenko A.Y., Oganesyan E.T., Andreeva O.A., Dorkina E.G., Paukova E.O., Agadzhanyan Z.S. Pharmacologically active substances from Ambrosia artemisiifolia. Part 2. Pharm. Chem. J. 2006;40:627–632. doi: 10.1007/s11094-006-0208-2. [DOI] [Google Scholar]
- 10.David J.P., de O. Santos A.J., da S. Guedes M.L., David J.M., Chai H.B., John M., Pezzuto J.M., Angerhofer C.K., Cordell G.A. Sesquiterpene lactones from Ambrosia artemisiaefolia (Asteraceae) Pharm. Biol. 1999;37:165–168. doi: 10.1076/phbi.37.2.165.6077. [DOI] [Google Scholar]
- 11.Parkhomenko A.Y., Andreeva O.A., Oganesyan E.T., Ivashev M.N. Ambrosia artemisiifolia as a source of biologically active substances. Pharm. Chem. J. 2005;39:149–153. doi: 10.1007/s11094-005-0106-z. [DOI] [Google Scholar]
- 12.Mabry T.J., Kagan H.B., Miller H.E. Psilostachyin B, a new sesquiterpene dilactone from Ambrosia psilostachya DC. Tetrahedron. 1996;22:1943–1948. doi: 10.1016/S0040-4020(01)82268-7. [DOI] [Google Scholar]
- 13.Borges-del-Castillo J., Manrese-Ferrero M.T., Rodriguez-Luis F., Vazquez-Bueno P., Nathan J.P. Carbon-13 NMR study of psilostachynolides from some Ambrosia species. Org. Magn. Res. 1981;17:232–234. doi: 10.1002/mrc.1270170322. [DOI] [Google Scholar]
- 14.Bohlmann F., Zdero C., King R.M., Robinson H. Pseudoguaianolides and other sesquiterpene lactones from Gaillardia species. Phytochemistry. 1984;23:1979–1988. doi: 10.1016/S0031-9422(00)84954-7. [DOI] [Google Scholar]
- 15.Vasquez M., Quijano L., Urbatsch L.E., Fischer N.H. Sesquiterpene lactones and other constituents from Rudbeckia mollis. Phytochemistry. 1992;31:2051–2054. doi: 10.1016/0031-9422(92)80361-H. [DOI] [Google Scholar]
- 16.Stavri M., Mathew K.T., Gordon A., Shnyder S.D., Falconer R.A., Gibbons S. Guaianolide sesquiterpenes from Pulicaria crispa (Forssk.) Oliv. Phytochemistry. 2008;69:1915–1918. doi: 10.1016/j.phytochem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Konovalova O.A., Sheichenko V.I. Chemical composition of Artemisia frigida. Chem. Nat. Compd. 1991;27:127–128. doi: 10.1007/BF00629853. [DOI] [Google Scholar]
- 18.Barrero A.F., Herrador M.M., Arteaga P. Sesquiterpene lactones and other constituents of Seseli vayredanum. Phytochemistry. 1994;37:1351–1358. doi: 10.1016/S0031-9422(00)90411-4. [DOI] [Google Scholar]
- 19.Huneck S., Zdero C., Bohlmann F. Seco-guaianolides and other constituents from Artemisia species. Phytochemistry. 1986;25:883–889. doi: 10.1016/0031-9422(86)80021-8. [DOI] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Himeji M., Ohtsuki T., Fukazawa H., Tanaka M., Yazaki S., Ui S., Nishio K., Yamamoto H., Tasaka K., Mimura A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007;245:269–274. doi: 10.1016/j.canlet.2006.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.