Abstract
Continued interest in the metabolites of Wedelia trilobata (L.) Hitchc, a notoriously invasive weed in South China, led to the isolation of twenty-six ent-kaurane diterpenoids, including seven new ones 1–7. Their structures and relative configuration were elucidated on the basis of extensive spectroscopic analysis, including 1D- and 2D-NMR experiments. The antimicrobial activities of all isolated diterpenoids were evaluated against a panel of bacteria and fungi.
Keywords: invasive weed, Wedelia trilobata, ent-kaurane diterpenoids, antimicrobial
1. Introduction
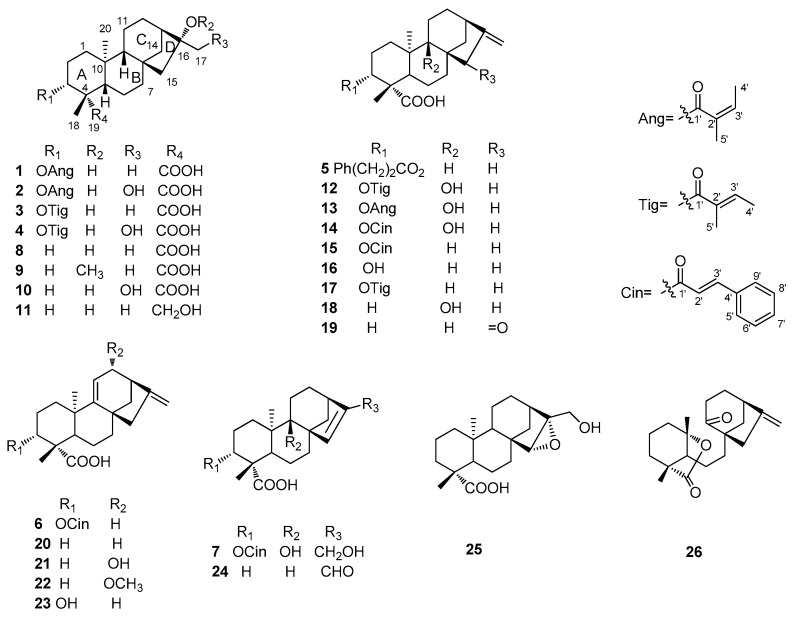
Wedelia trilobata is a notoriously invasive weed in a wide range of tropical and subtropical areas [1]. In southern China, this creeping, matforming perennial herb has caused significant damage to farmlands, forests, and orchards [2,3]. Studies have shown that W. trilobata has a strong allelopathic potential on neighboring native plants [4,5]. The major chemical constituents of W. trilobata are ent-kaurane diterpenes, sesquiterpene lactones, and triterpenes with a variety of biological activities, such as antibacterial, antitumor, hepatoprotective, and central nervous system depressant properties [6]. We previously reported ten eudesmanolides isolated from this plant as potential inducers of plant systemic acquired resistance [7]. As continuation of that work, twenty-six ent-kaurane diterpenoids including seven new ones 1–7 were obtained from the whole plant W. trilobata (Figure 1). All diterpenoids were evaluated against a panel of bacteria and fungi, and compounds 2, 4, 7, 10, 12, and 13 showed weak inhibitory activities against Monilia albicans with MICs of ca. 125 μg/mL. Herein, we report the isolation and structural elucidation of these compounds, as well as their antimicrobial properties.
Figure 1.
Chemical structures of 1–26 from Wedelia trilobata.
2. Results and Discussion
2.1. Structure Elucidation of Compounds
Compound 1 was obtained as a white amorphous powder, with a molecular formula determined as C25H38O5 on the basis of HREIMS which indicated a molecular ion peak at m/z 418.2722 M+ (calcd. for C25H38O5, 418,2719). The IR spectrum revealed absorption bands of hydroxyl (3431 cm−1) and carbonyl (1711 cm−1) groups. In the 1H-NMR spectrum (Table 1), the downfield olefinic proton at δH 6.02 (1H, q, J = 7.0 Hz) and two methyl signals at δH 1.82 (3H, s) and 1.92 (3H, d, J = 7.0 Hz), indicated the presence of an angeloyloxy group in 1 [8].
Table 1.
1H-NMR Data for Compounds 1–7.
| NO. | 1 a | 2 b | 3 a | 4 b | 5 a | 6 c | 7 d |
|---|---|---|---|---|---|---|---|
| 1a | 0.97 (1H, *) | 1.05 (1H, s) | 0.97 (1H, *) | 1.04 (1H, m) | 1.03 (1H, d, 9.6) | 1.55 (1H, m) | 1.56 (1H, m) |
| 1b | 1.88 (1H, br d, 13.5) | 1.96 (1H, m) | 1.87 (1H, br d, 13.7) | 1.95 (1H, m) | 1.94 (1H, br d) | 2.01 (1H, *) | 2.18 (1H, td, 13.7, 4.3) |
| 2a | 1.72 (1H, m) | 1.70 (1H, m) | 1.69 (1H, m) | 1.65 (1H, m) | 1.68 (1H, m) | 1.87 (1H, m) | 1.71 (1H, m) |
| 2b | 2.30 (1H, m) | 2.46 (1H, m) | 2.27 (1H, m) | 2.42 (1H, m) | 2.33 (1H, m) | 2.55 (1H, m) | 2.57 (1H, m) |
| 3 | 4.50 (1H, dd, 12.2, 4.7) | 4.56 (1H, dd, 12.1, 4.6) | 4.50 (1H, dd, 12.2, 4.7) | 4.50 (1H, dd, 12.1, 4.6) | 4.52 (1H, dd, 12.2, 4.6) | 4.77 (1H, dd, 12.0, 4.8) | 4.61 (1H, dd, 12.5, 4.5) |
| 5 | 1.04 (1H, br d, 11.9) | 1.12 (1H, d, 6.4) | 1.04 (1H, br d, 11.9) | 1.11 (1H, m) | 1.08 (1H, m) | 1.85 (1H, m) | 1.93 (1H, dd, 12.5, 2.2) |
| 6a | 1.61 (1H, *) | 1.67 (1H, m) | 1.61 (1H, *) | 1.68 (1H, m) | 1.63 (1H, m) | 2.01 (1H, *) | 1.66 (1H, m) |
| 6b | 1.80 (1H, *) | 1.87 (1H, m) | 1.79 (1H, *) | 1.86 (1H, m) | 1.84 (1H, m) | 2.01 (1H, *) | 1.85 (1H, dd, 13.9, 2.4) |
| 7a | 1.37 (1H, *) | 1.49 (1H, m) | 1.37 (1H, *) | 1.47 (1H, dd, 10.3, 3.3) | 1.45 (1H, m) | 1.56 (1H, m) | 1.29 (1H, t, 3.2) |
| 7b | 1.59 (1H, *) | 1.65 (1H, m) | 1.59 (1H, *) | 1.64 (1H, m) | 1.53 (1H, *) | 2.10 (1H, m) | 2.11 (1H, m) |
| 9 | 0.90 (1H, br s) | 1.04 (1H, br d) | 0.91 (1H, br s) | 1.03 (1H, br s) | 1.04 (1H, br s) | ||
| 11a | 1.49 (1H, *) | 1.63 (2H, *) | 1.49 (1H, *) | 1.62 (2H, m) | 1.53 (1H, *) | 5.28 (1H, s) | 1.47 (1H, m) |
| 11b | 1.49 (1H, *) | 1.49 (1H, *) | 1.65 (1H, d, 4.9) | 1.62 (1H, m) | |||
| 12a | 1.44 (1H, m) | 1.51 (1H, m) | 1.43 (1H, m) | 1.51 (1H, d, 3.8) | 1.47 (1H, m) | 2.03 (1H, m) | 1.42 (1H, dd, 12.9, 5.3) |
| 12b | 1.51 (1H, *) | 1.63 (1H, *) | 1.50 (1H, *) | 1.62 (1H, m) | 1.56 (1H, m) | 2.44 (1H, m) | 2.01 (1H, m) |
| 13 | 1.77 (1H, *) | 2.03 (1H, br s) | 1.77 (1H, *) | 2.02 (1H, br s) | 2.62 (1H, s) | 2.80 (1H, s) | 2.53 (1H, m) |
| 14a | 1.55 (1H, m) | 1.63 (1H, *) | 1.55 (1H, m) | 1.63 (1H, m) | 1.12 (1H, m) | 1.52 (1H, m) | 1.48 (1H, dd, 5.0, 2.0) |
| 14b | 1.81 (1H, *) | 1.89 (1H, m) | 1.80 (1H, *) | 1.89 (1H, d, 11.3) | 1.91 (1H, d, 11.0) | 1.65 (1H, m) | 2.24 (1H, d, 10.5) |
| 15a | 1.50 (1H, *) | 1.41 (1H, d, 14.4) | 1.50 (1H, *) | 1.40 (1H, d, 14.2) | 2.05 (1H, br s) | 2.24 (1H, d, 15.6) | 5.58 (1H, br s) |
| 15b | 1.50 (1H, *) | 1.53 (1H, d, 14.4) | 1.50 (1H, *) | 1.54 (1H, d, 14.2) | 2.05 (1H, br s) | 2.64 (1H, d, 15.6) | |
| 17a | 1.30 (3H, s) | 3.60 (1H, d, 11.4) | 1.30 (3H, s) | 3.60 (1H, d, 11.3) | 4.74 (1H, s) | 4.84 (1H, s) | 4.10 (1H, d, 14.3) |
| 17b | 3.70 (1H, d, 11.4) | 3.70 (1H, d, 11.3) | 4.80 (1H, s) | 4.95 (1H, s) | 4.14 (1H, d, 14.3) | ||
| 18 | 1.22 (3H, s) | 1.23 (3H, s) | 1.20 (3H, s) | 1.20 (3H, s) | 1.15 (3H, s) | 1.35 (3H, s) | 1.26 (3H, s) |
| 20 | 0.97 (3H, s) | 1.07 (3H, s) | 0.97 (3H, s) | 1.07 (3H, s) | 1.01 (3H, s) | 1.19 (3H, s) | 1.18 (3H, s) |
| 3-ester | 6.02 (1H, q, 7.0) | 6.11 (1H, dq, 7.0, 1.4) | 6.80 (1H, q, 7.1) | 6.89 (1H, dq, 7.0, 1.2) | 2.68 (2H, dd, 15.0, 7.2) | 6.46 (1H, d, 16.2) | 6.53 (1H, d, 15.8) |
| 1.92 (3H, d, 7.0) | 1.96 (3H, dd, 7.2, 1.5) | 1.72 (3H, d, 7.1) | 1.79 (3H, d, 7.2) | 2.95 (2H, m) | 7.70 (1H, d, 16.2) | 7.68 (1H, d, 15.8) | |
| 1.82 (3H, s) | 1.86 (3H, s) | 1.76 (3H, s) | 1.81 (3H, s) | 7.26 (2H, m) | 7.53 (2H, m) | 7.67 (2H, m) | |
| 7.19 (2H, *) | 7.40 (2H, *) | 7.43 (2H, *) | |||||
| 7.19 (1H, *) | 7.40 (1H, *) | 7.43 (1H, *) |
a Recorded in CDCl3 at 400 MHz; b Recorded in CD3OD at 400 MHz; c Recorded in CDCl3 at 600 MHz; d Recorded in acetone-d6 at 400 MHz. * Overlapped.
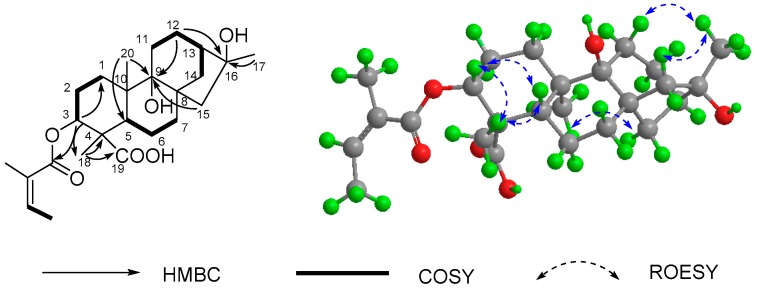
Apart from five carbon signals assigned to the angeloyloxy group (δC 167.9, 128.1, 138.6, 20.9, and 16.0), the 13C-NMR (DEPT) spectrum of 1 (Table 1) also exhibited 20 carbons composed of three methyls, eight methylenes, four methines (one oxygenated), and five quaternary carbons, which were consistent with a skeleton of an ent-kauranoid [9]. In particular, the NMR spectroscopic features of 1 are similar to those of 8 (16α-hydroxy-ent-kauran-19-oic acid), and only differed in the appearance of an angeloyloxy group at C-3 in 1. It was also confirmed by the chemical shift value of C-3 (δC 78.9, CH), C-9 (δC 56.1, CH) and the HMBC correlations (Figure 2) from H-3 (δH 4.50, dd, J = 12.2, 4.7 Hz) to C-1′ (δC 167.9, C), C-1 (δC 38.9, CH2), and C-18 (δC 24.7, CH3) as well as the correlations from Me-20, H-12, and H-15 to C-9, and from the methyl at C-4 (Me-18) to a downfield quaternary carbon (C-19) at δC 178.1. The ROESY correlations of H-3 with H-5 and H3-18 suggested that the angeloyloxy was α-orientated, and the hydroxy at C-16 was also assigned as α-orientated by the ROESY correlations of H3-17 with H2-11 and H-14β along with the ROESY correlations of H3-20 with H2-15. Consequently, the structure of 1 was finally determined as 3α-angeloyloxy-16α-hydroxy-ent-kauran-19-oic acid.
Figure 2.
Key 2D-NMR data of 1.
Compound 2 had the molecular formula C25H38O6 as determined by the HREIMS, with 16 mass units more than 1. The 1H- and 13C-NMR data similarities between 2 and 1 (Table 1 and Table 2) suggested that they were structural analogues. As compared with compound 1, the main differences were due to the presence of a hydroxymethyl group (δC 66.8) and the absence of a methyl group in 2. The hydroxymethyl group was assigned to C-17 by the HMBC correlations of H2-17 to C-14, C-15, and C-16. Therefore, the structure of 2 was established as shown.
Table 2.
13C-NMR Data for Compounds 1–7.
| No. | 1 a | 2 b | 3 a | 4 b | 5 a | 6 c | 7 d |
|---|---|---|---|---|---|---|---|
| 1 | 38.9 t | 40.0 t | 38.9 t | 40.0 t | 38.7 t | 39.5 t | 30.7 t |
| 2 | 24.3 t | 25.3 t | 24.2 t | 25.1 t | 25.3 t | 25.0 t | 24.7 t |
| 3 | 78.9 d | 80.7 t | 79.0 d | 80.8 t | 79.0 d | 79.5 d | 79.9 d |
| 4 | 48.0 s | 48.8 s | 48.1 s | 49.0 s | 47.8 s | 49.6 s | 48.3 s |
| 5 | 56.4 d | 57.3 d | 56.4 d | 57.3 d | 56.3 d | 46.0 d | 49.6 d |
| 6 | 21.9 t | 23.0 t | 22.0 t | 23.0 t | 21.4 t | 18.9 t | 21.5 t |
| 7 | 41.9 t | 43.0 t | 41.9 t | 43.0 t | 40.9 t | 38.1 t | 34.8 t |
| 8 | 45.2 s | 45.5 s | 45.2 s | 45.5 s | 43.8 s | 42.3 s | 54.2 s |
| 9 | 56.1 d | 57.3 d | 56.0 d | 57.3 d | 55.1 d | 155.6 s | 75.6 s |
| 10 | 39.5 s | 40.5 s | 39.5 s | 40.5 s | 39.3 s | 38.4 s | 44.4 s |
| 11 | 18.5 t | 19.7 t | 18.5 t | 19.7 t | 18.5 t | 115.2 t | 26.6 t |
| 12 | 26.9 t | 27.2 t | 26.9 t | 27.2 t | 33.0 t | 38.0 t | 31.5 t |
| 13 | 49.0 d | 46.2 d | 48.9 d | 46.2 d | 43.7 d | 41.2 d | 41.1 d |
| 14 | 37.6 t | 38.0 t | 37.5 t | 38.0 t | 39.4 t | 44.9 t | 45.1 t |
| 15 | 57.5 t | 53.5 t | 57.5 t | 53.5 t | 48.7 t | 51.0 d | 134.2 d |
| 16 | 79.6 s | 82.8 s | 79.7 s | 82.8 s | 155.0 s | 158.2 s | 149.2 s |
| 17 | 24.1 q | 66.8 t | 24.1 q | 66.8 t | 103.0 t | 106.0 t | 60.9 t |
| 18 | 24.7 q | 24.5 q | 24.7 q | 24.5 q | 23.6 q | 24.3 q | 24.4 q |
| 19 | 178.1 s | 177.9 s | 178.3 s | 178.1 s | 180.0 s | 178.1 s | 176.0 s |
| 20 | 15.7 q | 16.1 q | 15.7 q | 16.1 q | 15.3 q | 23.3 q | 17.4 q |
| 3-ester | 167.9 s | 169.4 s | 167.9 s | 169.4 s | 173.0 s | 166.8 s | 166.9 s |
| 128.1 s | 129.3 s | 128.9 s | 129.5 s | 36.0 t | 118.4 d | 119.5 d | |
| 138.6 d | 139.0 d | 137.8 d | 138.8 d | 30.9 t | 145.3 d | 145.2 d | |
| 16.0 q | 16.0 q | 14.7 q | 14.4 q | 140.0 s | 134.5 s | 135.4 s | |
| 20.9 q | 20.9 q | 12.3 q | 12.1 q | 128.0 d | 128.4 d | 129.0 d | |
| 128.0 d | 129.1 d | 131.1 d | |||||
| 126.0 d | 130.5 d | 129.8 d |
a Recorded in CDCl3 at 100 MHz; b Recorded in CD3OD at 100 MHz; c Recorded in CDCl3 at 150 MHz; d Recorded in acetone-d6 at 100 MHz.
Compounds 3 and 4 showed the same mass units as those of 1 and 2, respectively, on the basis of the HREIMS. The 1D NMR data of 3 and 4 (Table 1 and Table 2) also closely resembled those of 1 and 2, respectively, except for the presence of the tigloyloxy group at C-3 of 3 and 4 instead of the angeloyloxy group. These conclusions were verified by the HMBC correlations from H-3′ (δH 6.80 in 3, δH 6.89 in 4) and H-3 (δH 4.50 in 3, δH 4.50 in 4) to C-1′ (δC 167.9 in 3, δC 169.4 in 4). The NMR data suggested that compounds 3 and 4 possessed the same relative configuration as those of 1 and 2, respectively. Thus, compounds 3 and 4 were determined as 3α-tigloyloxy-16α-hydroxy-ent-kauran-19-oic acid and 3α-tigloyloxy-16α, 17-dihydroxy-ent-kauran-19-oic acid, respectively.
Compound 5, a white powder, possessed the molecular formula C29H38O4, as determined by the HREIMS, 13C-NMR (Table 2) and DEPT data. Comparison of the 1D- and 2D-NMR spectroscopic data of 5 with those of 3α-cinnamoyloxy-ent-kaur-16-en-19-oic acid (15) revealed that their structures were closely similar to each other. The only difference between them was that the double bond of the cinnamoyloxy group at C-3 in 15 was reduced in 5, which was supported by the molecular weights of 5, showing two mass units more than those of 15. This was further confirmed by the HMBC cross-peaks of H-2′ and H-3′ with C-1′ and C-4′. The α-orientation of the 3-dihydrocinnamoyloxy group was apparent from the ROESY correlations of H-3β with H-5β and H3-18β. Thus, compound 5 was determined as 3α-dihydrocinnamoyloxy-ent-kaur-16-en-19-oic acid.
The molecular formula of compound 6 was deduced as C29H34O4 on the basis of the positive HREIMS at m/z 446, 2463 [M]+ (calcd. for 446,2457). The 1H- and 13C-NMR data of 6 (Table 1 and Table 2) showed many similarities to those of 20, indicating that they were structural analogues as ent-kaura-9(11),16-dien-19-oic acid. As compared with compound 20, the obvious difference was due to the presence of one more cinnamoyloxy group at C-3 in 6. HMBC correlations from H-3, H-2′, and H-3′ to C-1′ further validated the conclusion above. The ROESY correlations of H-3 with H-5 and H3-18 suggested that the cinnamoyloxy was α-orientated. Consequently, the structure of 6 was determined as 3α-cinnamoyloxy-ent-kaura-9(11), 16-dien-19-oic acid.
Compound 7 was isolated as a white powder, and its molecular formula was determined as C29H36O6 by HREIMS based on m/z 480.2508 [M]+ (calcd. for 480,2512). The IR spectrum showed absorptions at 3441 (OH), 1701 (C = O), and 1639 and 1449 cm−1 (aromatic C = C). The presence of a cinnamoyloxy moiety was deduced by comparison with the NMR data (Table 1 and Table 2) of compound 6. Besides this cinnamoyloxy moiety, the remaining twenty C-atoms included a trisubstituted double bond (δH 5.58 (br. s); δC 134.2 and 149.2), a carboxyl group (δC 176.0), an O-bearing methylene group (δH 4.10 (d, J = 14.3 Hz) and 4.14 (d, J = 14.3 Hz); δC 60.9), and an O-bearing quaternary carbon (δC 75.6). Further analyses demonstrated that compound 7 showed a closely similar NMR pattern to that of 6, indicating that compound 7 was a structural analogue of ent-kaurane-19-oic acid. The double bond was located between C-15 and C-16 by the HMBC cross-peaks of H-15 with C-8, C-14, C-16 and C-17. Meanwhile, the O-bearing methylene group was only connected to C-17 by the HMBC correlations from H2-17 to C-14, C-15, and C-16. At last, the O-bearing quaternary carbon could be attributed to C-9 due to the HMBC correlations of H2-7, H2-11, and H3-20 to C-9. The relative configuration of 7 was shown to be identical with that of 6 by NMR analysis. Thus, compound 7 was determined as 3α-cinnamoyloxy-9β, 17-dihydroxy-ent-kaur-15-en-19-oic acid.
Nineteen known ent-kaurane derivatives, namely 16α-hydroxy-ent-kauran-19-oic acid (8) [10], 16α-methoxy-17-hydroxy-ent-kauran-19-oic acid (9) [11], 16α-17-dihydroxy-ent-kauran-19-oic acid (10) [12], 16α, 18-dihydroxy-ent-kaurane (11) [13], 3α-tigloyloxypterokaurene L3 (12) [14], 3α-angeloyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (13) [15], 3α-cinnamoyloxy-9β-hydroxy-ent-kaur-16-en-19-oic acid (14) [15], 3α-cinnamoyloxy-ent-kaur-16-en-19-oic acid (15) [12], 3α-hydroxy-ent-kaur-16-en-19-oic acid (16) [16], 3α-tiglinoyloxy-ent-kaur-16-en-19-oic acid ent-kaura-9 (17) [8], ent-9α-hydroxy-16-kauren-19-oic acid (18) [17], ent-15-oxokaur-16-en-19-oic acid (19) [18], (11),16-dien-19-oic acid (20) [19], 12α-hydroxy-ent-kaur-9(11),16-dien-19-oic acid (21) [20], 12α-methoxy-ent-kaur-9(11),16-dien-19-oic acid (22) [21], 3α-hydroxy-ent-kaura-9(11), 16-dien-19-oic acid (23) [21], ent-17-oxokaur-15-en-19-oic acid (24) [22], 15α,16α-epoxy-17-hydroxy-ent-kauran-19-oic acid (25) [12], and wedeliaseccokaurenolide (seco) (26) [14], were also isolated. Their structures were identified on the basis of spectroscopic analysis and comparison with reported data.
The in vitro antimicrobial activities of all ent-kaurane derivatives isolated were tested against Pseudomonas aeruginosa (ATCC 27853), Staphyloccocus aureus (ATCC 25923), Monilia albicans (ATCC Y0109) and Escherichia coli (ATCC 25922) using an agar well diffusion method [23]. Compounds 2, 4, 7, 10, 12, and 13 showed weak activities against M. albicans (zone of inhibition > 10 mm at 1 mg/mL). The minimum inhibitory concentrations (MICs) of compounds above against M. albicans, 1R (R: methicillin-resistant M. albicans), 2R, 3R, 4R, 5R, and 535R were determined by the 2-fold dilution method (Table 3). Fluconazole was used as standard drug for comparison.
Table 3.
Antimicrobial activities of compounds 2, 4, 7, 10, 12 and 13.
| Compounds | Antimicrobial Activities (MIC in μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| M. albicans | 1R b | 2R | 3R | 4R | 5R | 535R | |
| 2 | 125 | >250 | >250 | >250 | >250 | >250 | >250 |
| 4 | 125 | 125 | >250 | >250 | >250 | >250 | >250 |
| 7 | 125 | >250 | >250 | >250 | >250 | >250 | >250 |
| 10 | 125 | >250 | >250 | >250 | >250 | >250 | >250 |
| 12 | 125 | >250 | >250 | >250 | >250 | >250 | >250 |
| 13 | 125 | >250 | >250 | >250 | >250 | >250 | >250 |
| Fluconazole a | 125 | >250 | >250 | >250 | >250 | >250 | >250 |
a Positive control; b R means methicillin-resistant M. albicans.
2.2. Evaluation of Anti-Micobial Activity
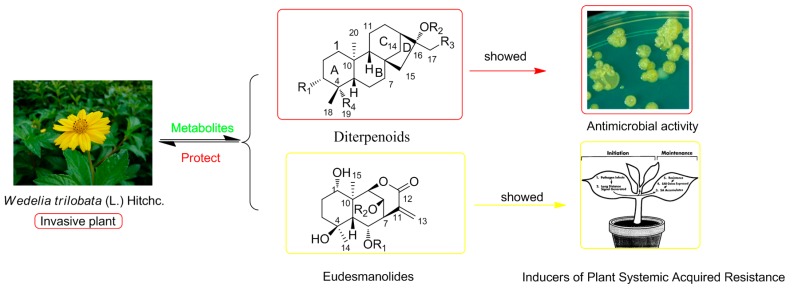
In summary, seven new and nineteen known ent-kaurane diterpenoid metabolites were obtained from whole plant W. trilobata, and some compounds exhibited weak antimicrobial activities. Moreover, we previously reported ten eudesmanolides as potential inducers of plant systemic acquired resistance isolated from this species [7]. Above all, a conclusion that can be drawn is that diterpenes and sesquiterpenes are the main metabolites of W. trilobata and they may be significant as chemical defenses allowing this notoriously invasive weed to adapt to varying surroundings rapidly and effectively (Figure 3).
Figure 3.
The correlations between Wedelia trilobata and its metabolites.
3. Experimental Section
3.1. General Procedures
1D- and 2D-NMR spectra were recorder on either an AM-400 or a DRX-500 or an Avance III-600 spectrometer (Bruker, Karlsruhe, Germany) with TMS as an internal standard. Unless otherwise specified, chemical shifts (δ) were expressed in ppm. MS were measured on a HPLC-Thermo Finnigan LCQ Advantage ion trap mass spectrometer (Waters, Milford, PA, USA). Optical rotation was determined on a SEPA-300 polarimeter (Horiba, Tokyo, Japan). UV spectroscopic data were measured on a 210A double-beam spectrophotometer (Shimadzu, Kyoto, Japan). IR spectra of samples in KBr discs were recorded on a Tensor-27 spectrometer with KBr pellets (Bruker, Rheinstetten, Germany). Column chromatography (CC) was carried out on silica gel G (100−200 mesh, 200−300 mesh, Qingdao Haiyang Chemical Co., Qingdao, China), silica gel H (10−40 μm, Qingdao Haiyang Chemical Co.), Sephadex LH-20 (40−70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), and Lichroprep RP-18 gel (20−45 μm, Merck, Darmstadt, Germany). Semi-preparative HPLC was performed on an Agilent 1200 series instrument equipped with a quaternary pump, a vacuum degasser, an auto-sampler, a thermos-tatted column compartment with a Zorbax SB-C18 (10 μm; Agilent Co. Ltd, St. Louis, MO, USA) column (i.d. 9.4 mm × 250 mm), and a diode array detector. Thin-layer chromatography (TLC) was conducted on precoated silica gel plates GF 254 (Qingdao Haiyang Chemical Co.). TLC spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH.
3.2. Plant Material
The whole plant of Wedelia trilobata (L.) Hitchc was collected in Simao, Yunnan Province, China, in August 2011. The specimen was identified by Yu Chen of Kunming Institute of Botany (KIB), Chinese Academy of Sciences (CAS). A voucher specimen (H20110805) has been deposited in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany.
3.3. Extraction and Isolation
Dried powder of the whole plant of W. trilobata (9 kg) was extracted under reflux with MeOH (70 L, three times for 4, 4, and 3 h). The solvent was removed under reduced pressure to give a residue (1020.0 g, 11.3%), which was suspended with water and then extracted with petroleum ether, chloroform, EtOAc, and n-BuOH successively. The extracts were evaporated under vacuum to afford the corresponding extracts of petroleum ether (200.0 g), chloroform (90.0 g), EtOAc (90.0 g), and n-BuOH (380.0 g). The EtOAc (90.0 g) extract was separated with a silica gel G column (100−200 mesh, 10 cm × 120 cm, 450.0 g), eluted with petroleum ether/acetone (v/v = 9:1, 7:3, 6:4, 1:1, 0:1, each 10 L), to give five fractions (1−5). Fraction 3 (12.0 g) was extensively chromatographed over column of silica gel (3.6 × 100 cm, 36.0 g) and Sephadex LH-20 (CHCl3−MeOH, 1:1, 3.2 × 140 cm) to afford compounds 1 (1.2 mg), 2 (10.1 mg), 3 (0.4 mg), 4 (20.0 mg), 11 (2.3 mg), 12 (3.0 mg), 13 (4.5 mg), 14 (4.8 mg) and 15 (4.0 mg). Fraction 4 (7.0 g) was subjected to a column of reversed-phase silica gel (5 cm × 50 cm, 100 g) eluted with a MeOH/H2O (50/50 to 100/0) gradient to yield four sub-fractions (A−D). Sub-fraction B (200 mg) was purified by semi-preparative HPLC with 70% MeOH in H2O as the mobile phase to yield 5 (6.7 mg, tR = 13.54 min), 6 (1.8 mg, tR = 14.30 min), and 7 (13.0 mg, tR = 15.75 min). Sub-fraction C (2.2 g) was applied to silica gel (5 cm × 80 cm, 30 g) eluted with DCM (dichloromethanemethylene chloride)/MeOH (100/1 to 10/1) to yield four sub-fractions (C1 to C4), respectively. Sub-fraction C2 (320.0 mg) was purified by semi-preparative HPLC with 72% MeOH in H2O as the mobile phase to yield 16 (26.8 mg, tR = 18.23 min), 17 (17.2 mg, tR = 20.40 min), and 18 (1.7 mg, tR = 21.62 min), and in a similar procedure, sub-fraction C3 (200.0 mg) yielded 19 (2.3 mg, tR = 16.52 min), 20 (7.0 mg, tR = 17.24 min), 21 (8.0 mg, tR = 19.93 min), and 25 (4.0 mg, tR = 20.92 min). By using the same purification procedures, sub-fraction C4 yielded 22 (6.5 mg), 23 (5.0 mg), and 24 (14.0 mg). Sub-fraction D was subjected to a column of Sephadex LH-20 gel (3.2 cm × 140 cm) eluted with MeOH to obtain six major sub-fractions, each of which was then purified by semi-preparative HPLC with the mobile phase MeOH/H2O (65/35) to produce compounds 8 (10.0 mg, tR = 12.44 min), 9 (9.0 mg, tR = 14.93 min), 10 (5.0 mg, tR = 15.45 min), and 26 (37.1 mg, tR = 16.75 min).
3.4. Data for 1–7
3α-Angeloyloxy-16α-hydroxy-ent-kauran-19-oic acid (1): white powder, : −57.0 (c 0.07, MeOH); UV (MeOH) λmax (log ε) 211.8 (3.6) nm; IR (KBr) νmax 3431, 2927, 2854, 1711, 1626, 1461, 1382, 1235, 1163, 1122, 1044 cm−1; positive-ion ESI-MS m/z 441 [M + Na]+, 859 [2M + Na]+, HR-EIMS m/z 418.2722 M+ (calcd. for C25H38O5, 418.2719); 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 150 MHz), see Table 1 and Table 2.
3α-Angeloyloxy-16α,17-dihydroxy-ent-kauran-19-oic acid (2): white powder, : −50.37 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 216 (3.79) nm; IR (KBr) νmax 3432, 2931, 2869, 2853, 1707, 1641, 1454, 1356, 1236, 1021, 988 cm−1; ESIMS m/z 457 [M + Na]+; HREIMS m/z 434.2672 [M]+ (calcd. for C25H38O6, 434.2668); 1H- and 13C-NMR data see Table 1 and Table 2.
3α-Tigloyloxy-16α-hydroxy-ent-kauran-19-oic acid (3): white powder, : −42.8 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 210.2 (3.6) nm; IR (KBr) νmax 3442, 2927, 2854, 1705, 1624, 1446, 1383, 1122, 1080, 1007 cm−1; positive-ion ESI-MS m/z 441 [M + Na]+, 859 [2M + Na]+, HR-EIMS m/z 418.2723 M+ (calcd. for C25H38O5, 418.2719); 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 150 MHz), see Table 1 and Table 2.
3α-Tigloyloxy-16α,17-dihydroxy-ent-kauran-19-oic acid (4): white powder, : −41.00 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 216 (3.84) nm; IR (KBr) νmax 3431, 2929, 2868, 2851, 1706, 1649, 1464, 1384, 1270, 1151, 1126, 1083, 1053 cm−1; ESIMS m/z 457 [M + Na]+; HREIMS m/z 434.2677 [M]+ (calcd. for C25H38O6, 434.2668); 1H- and 13C-NMR data see Table 1 and Table 2.
3α-Diydrocinnamoyloxy-ent-kaur-16-en-19-oic acid (5): white powder, : −64.00 (c 0.14, MeOH); UV (MeOH) λmax (log ε) 204 (3.96), 261 (2.57) nm; IR (KBr) νmax 3441, 2927, 2855, 1732, 1703, 1631, 1453, 1181 cm−1; ESIMS m/z 473 [M + Na]+; HREIMS m/z 450.2781 [M]+ (calcd. for C29H38O4, 450.2770); 1H- and 13C-NMR data see Table 1 and Table 2.
3α-Cinnamoyloxy-ent-kaura-9(11),16-dien-19-oic acid (6): white powder, : −6.32 (c 0.14, MeOH); UV (MeOH) λmax (log ε) 203 (3.74), 277 (3.67) nm; IR (KBr) νmax 3450, 2931, 2866, 1636, 1387, 1173 cm−1; ESIMS m/z 469 [M + Na]+; HREIMS m/z 446.2463 [M]+ (calcd. for C29H34O4, 446.2457); 1H- and 13C-NMR data see Table 1 and Table 2.
3α-Cinnamoyloxy-9β,17-dihydroxy-ent-kaur-15-en-19-oic acid (7): white powder, : −55.76 (c 0.11, MeOH); UV (MeOH) λmax (log ε) 205 (4.21), 215 (4.20), 277 (4.28) nm; IR (KBr) νmax 3441, 2925, 2863, 1701, 1639, 1449, 1311, 1281, 1204, 1185 cm−1; ES−–MS m/z 479 [M − H]−; HREIMS m/z 480.2508 [M]+ (calcd. for C29H36O6, 480.2512); 1H- and 13C-NMR data see Table 1 and Table 2.
3.5. Antimicrobial Assays
The strains used in antimicrobial tests were obtained from the Research Center of Natural Medicine, Clinical School of Kunming General Hospital of Chengdu Military Command. The test organisms in this bioassay were the bacteria Pseudomonas aeruginosa (ATCC 27853), Staphyloccocus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) (all grown on MH medium) and the fungus Monilia albicans (ATCC Y0109) (grown on Sabauraud′s medium). For the agar plate punch assay [23], all tested compounds were dissolved in DMSO at a concentration of 1 mg/mL. Then, 50 μL of the solution was added onto a well (6 mm in diameter) that had been punched in the appropriate agar growth medium smeared with a suspension of the test organism (1.0 × 109 cfu/mL; cfu = colony forming unit). All active compounds with a diameter of inhibition greater than 10 mm were submitted to minimum inhibitory concentration testing. The MICs of compounds 2, 4, 7, 10, 12, and 13 against M. albicans, 1R (R: methicillin-resistant M. albicans), 2R, 3R, 4R, 5R, and 535R were determined using a 2-fold dilution method [23]. The 2-fold serially diluted compounds in MH broth were dispensed into 96-well microtiter plates (100 μL/well), and then an aliquot of 5 × 104 cfu/mL of bacterial culture was added to each well (100 μL/well) to final concentrations in a range of 1.95−250 μg/mL. After incubating at 37 °C for 18 h, the lowest concentration without any colony growth was recorded as the MIC value. The resulting values were compared with the value for a positive control (fluconazole, range 125–250 μg/mL) under the same conditions.
4. Conclusions
A systematic chemical search was performed and resulted in the separation of seven new ent-kaurane diterpenoids together with nineteen known ent-kaurane derivatives from Wedelia trilobata (L.) Hitchc, a notoriously invasive weed in South China. The structures of the new compounds were identified based on detailed spectroscopic analysis and comparison with the published data of analogues. All ent-kaurane derivatives isolated were tested against Pseudomonas aeruginosa, Staphyloccocus aureus, Monilia albicans and Escherichia coli, and compounds 2, 4, 7, 10, 12, and 13 showed weak activities against M. albicans. Taken together with data from prior research [7], the conclusion that diterpenes and sesquiterpenes are main metabolites of W. trilobata can be drawn and they may be significant as chemical defenses allowing this notoriously invasive weed to adapt to varying surroundings rapidly and effectively.
Acknowledgments
This project was supported financially by grants from the National Natural Science Foundation of China (No. 31470427, 31270404).
Author Contributions
Shi-Fei Li conceived and designed the main ideas of this paper, analyzed the experimental results, and wrote the paper. Jia-Yin Ding carried out the experiments. Ya-Ting Li participated in the experiments. Xiao-Jiang Hao and Shun-Lin Li revised this paper and guided the writing of this paper. The authors read and approved the final manuscript.
Conflicts of Interest
There is no conflict of interest among all authors of this manuscript.
Footnotes
Sample Availability: Samples of the compounds 4, 7, 16, 17, 24, and 26 are available from the authors.
References
- 1.IUCN . 100 of the World’s Worst Invasive Alien Species. Invasive Species Specialist Group; Auckland, New Zealand: 2001. [Google Scholar]
- 2.Xie L.J., Zeng R.S., Bi H.H., Song Y.Y., Wang R.L., Su Y.J., Chen M., Chen S., Liu Y.H. Allelochemical mediated invasion of exotic plants in China. Allelopath. J. 2010;25:31–50. [Google Scholar]
- 3.Wu J.R., Peng S.L., Zhao H.B., Xiao H.L. Allelopathic effects of Wedelia trilobata residues on lettuce germination and seedling growth. Allelopath. J. 2008;22:197–204. [Google Scholar]
- 4.Zhang Y.H., Liu M.F., Lin T.J., Wei X.Y. Allelopathic sesquiterpene lactones from Wedelia trilobata. J. Trop. Subtrop. Bot. 2004;12:533–537. [Google Scholar]
- 5.Nie C.R., Zeng R.S., Luo S.M., Li H.S., Hong M.Q., Cheng L.Q. Allelopathic potentials of Wedelia trilobata L. on rice. Acta Agron. Sin. 2004;30:942–946. [Google Scholar]
- 6.Wu M.L., Zhang D.Z. Progress of researches on the invasive plant Wedelia trilabata. Pharm. Today. 2008;6:21–23. [Google Scholar]
- 7.Li Y.T., Hao X.J., Li S.F., He H.P., Yan X.H., Chen Y.D., Dong J.H., Zhang Z.K., Li S.L. Eudesmanolides from Wedelia trilobata (L.) Hitchc. as potential inducers of plant systemic acquired resistance. J. Agric. Food Chem. 2013;61:3884–3890. doi: 10.1021/jf400390e. [DOI] [PubMed] [Google Scholar]
- 8.Ragasa C.Y., Padolina W.G., Bowden B.F., Li S.X., Tapiolas D.M., Coll J.C. New eudesmanolide sesquiterpenes from a Philippines collection of Wedelia prostata. J. Nat. Prod. 1993;56:386–393. doi: 10.1021/np50093a011. [DOI] [Google Scholar]
- 9.Hutchison M., Lewer P., MacMillan J. Carbon-13 nuclear magnetic resonance spectra of eighteen derivatives of ent-kaur-16-en-19-oic acid. J. Chem. Soc. Perkin Trans. 1. 1984;10:2363–2366. doi: 10.1039/p19840002363. [DOI] [Google Scholar]
- 10.Duan H., Takaishi Y., Momota H., Ohmoto Y., Taki T., Jia Y., Li D. Immunosuppressive diterpenoids from Tripterygium wilfordii. J. Nat. Prod. 1999;62:1522–1525. doi: 10.1021/np9902183. [DOI] [PubMed] [Google Scholar]
- 11.Jung H.A., Lee E.J., Kim J.S., Kang S.S., Lee J.H., Min B.S., Choi J.S. Cholinesterase and BACE1 inhibitory diterpenoids from Aralia cordata. Arch. Pharm. Res. 2009;32:1399–1408. doi: 10.1007/s12272-009-2009-0. [DOI] [PubMed] [Google Scholar]
- 12.Qiang Y., Du D.L., Chen Y.J., Gao K. Ent-Kaurane diterpenes and further constituents from Wedelia trilobata. Hel. Chim. Acta. 2011;94:817–823. doi: 10.1002/hlca.201000301. [DOI] [Google Scholar]
- 13.Pacheco A.G., Machado de Oliveira P., Piló-Veloso D., Flávio de Carvalho Alcântara A. 13C-NMR data of diterpenes isolated from Aristolochia species. Molecules. 2009;14:1245–1262. doi: 10.3390/molecules14031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma B.J., Wen C.N., Gao Y., Ren F.C., Wang F., Liu J.K. ent-Kaurane diterpenoids from the plant Wedelia trilobata. Nat. Prod. Bioprospect. 2013;3:107–111. doi: 10.1007/s13659-013-0029-4. [DOI] [Google Scholar]
- 15.Bohlmann F., Ziesche J., King R.M., Robinson H. Naturally occurring terpene derivatives. Part 300. Eudesmanolides and diterpenes from Wedelia trilobata and an ent-kaurenic acid derivative from Aspilia parvifolia. Phytochemistry. 1981;20:751–756. [Google Scholar]
- 16.Zhang D.Z., Li X., Zhu T.R. Spectroscopic study of kaurane type diterpene compounds. Bopuxue Zazhi. 1990;7:349–350. [Google Scholar]
- 17.Murakami T., Iida H., Tanaka N., Saiki Y., Chen C.M., Iitaka Y. Chemical and chemotaxonomic studies of ferns. XXXIII. Chemical studies on the constituents of Pteris longipes Don. Chem. Pharm. Bull. 1981;29:657–662. [Google Scholar]
- 18.De Oliveira A.B., Hanson J.R., Takahashi J.A. The biotransformation of ent-15-oxokaur-16-en-19-oic acid and its methyl ester by Cephalosporium aphidicola. Phytochemistry. 1995;40:439–442. doi: 10.1016/0031-9422(95)00289-J. [DOI] [Google Scholar]
- 19.Enriquez R.G., Barajas J., Ortiz B., Lough A.J., Reynolds W.F., Yu M., Leon I., Gnecco D. Comparison of crystal and solution structures and 1H and 13C chemical shifts for grandiflorenic acid, kaurenoic acid, and monogynoic acid. Can. J. Chem. 1997;75:342–347. doi: 10.1139/v97-039. [DOI] [Google Scholar]
- 20.Ahmed M., Jakupovic J., Castro V. Kaurene derivatives from Lasianthea fruticosa, revision of stereochemistry of related compounds. Phytochemistry. 1991;30:1712–1714. doi: 10.1016/0031-9422(91)84242-K. [DOI] [Google Scholar]
- 21.Beattie K.D., Bhadbhade M.M., Craig D.C., Leach D.N. 13C-methoxy-1,2,3,13c-tetrahydrodibenzo[a,kl]xanthan-1-one. Acta Cryst. Sect. E Struct. Rep. Online. 2012;68:526–527. doi: 10.1107/S1600536812002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monte F.J., Dantas E.M., Braz-Filho R. New diterpenoids from Croton argyrophylloides. Phytochemistry. 1988;27:3209–3212. doi: 10.1016/0031-9422(88)80027-X. [DOI] [Google Scholar]
- 23.Xu S.Y., Bian R.L., Chen X. Pharmacological Experiment Methodology. 3rd ed. People’s Medical Publishing House; Beijing, China: 2002. pp. 1647–1719. [Google Scholar]