Abstract
Reactions of ethylidenethiocarbohydrazide with hydrazonoyl halides gave 1,3-thiazole or 1,3,4-thiadiazole derivatives according to the type of hydrazonoyl halides. Treatment of ethylidenethiosemicarbazide with hydrazonoyl halides and dimethylacetylene dicarboxylate (DMAD) afforded the corresponding arylazothiazoles and 1,3-thiazolidin-4-one derivatives, respectively. The structures of the synthesized products were confirmed by IR, 1H-NMR, 13C-NMR and mass spectral techniques. The cytotoxic activity of the selected products against the Hepatic carcinoma cell line (Hepg-2) was determined by MTT assay indicating a concentration dependent cellular growth inhibitory effect, especially for compounds 14c and 14e. The dose response curves indicated the IC50 (the concentration of test compounds required to kill 50% of cell population) were 0.54 μM and 0.50 μM, respectively. Confocal laser scanning imaging of the treated cells stained by Rhodamin 123 and Acridine orange dyes confirmed that the selected compounds inhibit the mitochondrial lactate dehydrogenase enzymes. The binding mode of the active compounds was interpreted by a molecular docking study. The obtained results revealed promising cytotoxic activity.
Keywords: ethylidenethiocarbohydrazide; ethylidenethiosemicarbazide; hydrazonoyl halides; 1,3-thiazole; 1,3,4-thiadiazole; cytotoxic activity; molecular docking
1. Introduction
Hepatocellular Carcinoma (HCC) is considered the fifth most common cancer type worldwide and the third most common cause of cancer mortality. Globally, over 560,000 people develop liver cancer each year and an almost equal number, 550,000, die of it [1].
At stage I, surgical resection or transplantation is considered a potentially curative modality for HCC; patients with localized unrespectable disease are usually treated with some form of localized therapy. Local therapeutic modalities include targeted chemotherapy through hepatic artery combined with embolization, percutaneous ethanol ablation, radio embolization, radiofrequency ablation, and cryosurgery [2]. Thus, novel approaches for the treatment of unrespectable advanced or metastatic HCC represents a high-unmet medical need. In this work, novel thiazole derivatives were used as a module for management of HCC. Thiazoles are a familiar group of heterocyclic compounds possessing a wide variety of biological activities, and their usefulness as medicines are well established. Thiazole derivatives are reported to exhibit diverse biological activities as antimicrobial [3,4,5], antioxidant [6], antitubercular [7], and anticonvulsant [8], and anticonvulsant, anticancer [9,10] agents. Moreover, many derivatives of thiazoles are used as selective Cyclooxygenase-2 Inhibitors [11], in addition to their use as 5-HT3 receptor antagonists [12] and as potent and selective acetyl Co-A carboxylase-2 inhibitors [13]. Furthermore, the interesting properties of thiazole derivatives [14,15] in relation to the various changes in the structures of these compounds is worth studying for the synthesis of some less toxic and more potent drugs. Thus, the introduction of other heterocyclic moieties (as the thiazole and thiadiazole ring) should certainly help to fulfill this objective. The aforementioned biological, pharmacological, and industrial importance of these derivatives prompted our interest for the synthesis of some new examples of this class of compounds.
As a part of our research interest towards developing new routes for the synthesis of a variety of heterocyclic systems with promising antitumor activities [9,10,16,17,18,19,20,21,22], we report in the present work the synthesis of a new series of thiazolyl-thiazoles as promising cytotoxic antitumor drugs.
2. Results and Discussion
2.1. Chemistry
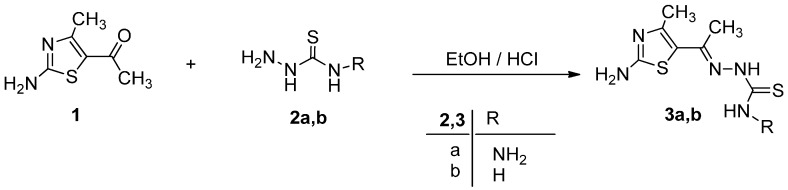
Ethylidenethiocarbohydrazide (3a) and ethylidenethiosemicarbazide (3b) were prepared via condensation of 5-acetyl-2-amino-4-methylthiazole (1) [23] with thiocarbohydrazide (2a) and thiosemicarbazide (2b) in absolute ethanol in the presence of a catalytic amount of concentrated HCl as depicted in Scheme 1.
Scheme 1.
Synthesis of ethylidenethiocarbohydrazide derivative 3a and ethylidenethiosemicarbazide derivative 3b.
The structure elucidation of the products 3a and 3b were substantiated through spectral data. The mass spectrum of isolated product (m/z 245) was consistent with the expected product 3a. The 1H-NMR spectrum of 3a showed broad singlet bands for NH2 and NH groups of the thiocarbohydrazide moiety at 4.92, 9.62 and 14.12 ppm, respectively, and the band for NH2 group of the thiazole ring at 7.42 ppm as well as the two singlet bands for methyl groups at 2.42 and 2.85 ppm.
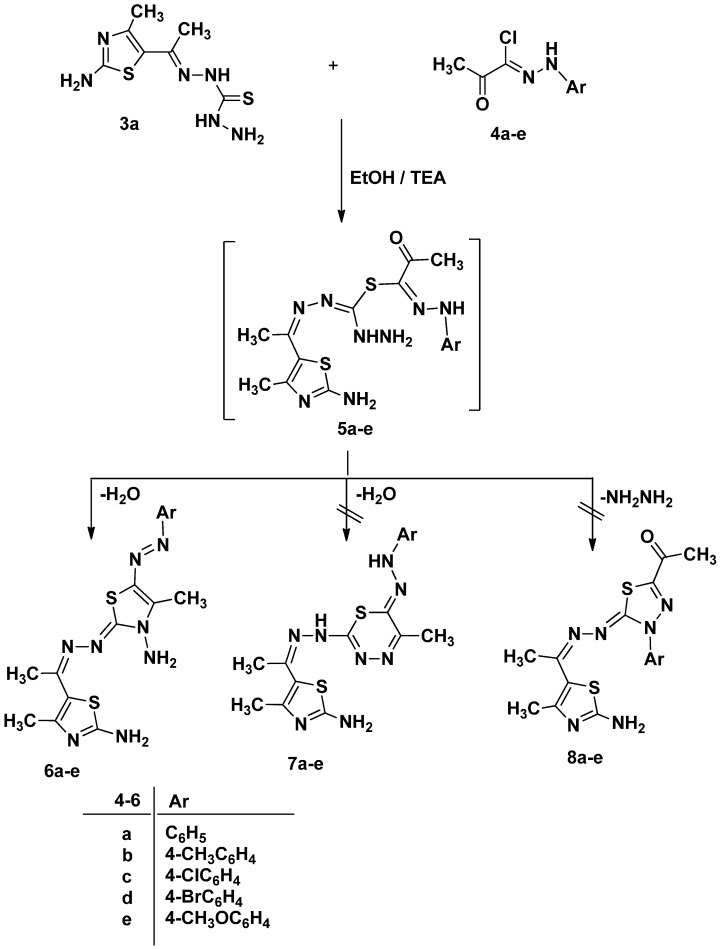
We commenced our study on the reactivity of ethylidenethiocarbohydrazide 3a toward different types of hydrazonoyl halides 4a–e and 9 to investigate the effect of the presence of carbonyl group on the course of the reaction. Initially, ethylidenethiocarbohydrazide 3a reacted with α-keto-hydrazonoyl halide 4a–e in refluxing ethanol in presence of triethylamine to give dark red color products that proved we are isolated azo-thiazole derivatives 6a–e and not the hydrazo-thiadiazine derivatives 7a–e via elimination of hydrochloric acid and water as shown in Scheme 2. Spectroscopic analyses (IR, MS, 1H- and 13C-NMR) confirmed the structure of 1,3-thiazole derivatives 6a–e and not 7a–e was isolated. The 1H-NMR spectrum showed two broad singlet signal for two NH2 groups at ~2.5 and ~7.11 ppm.
Scheme 2.
Synthesis of 1,3-thiazole derivatives 6a–e.
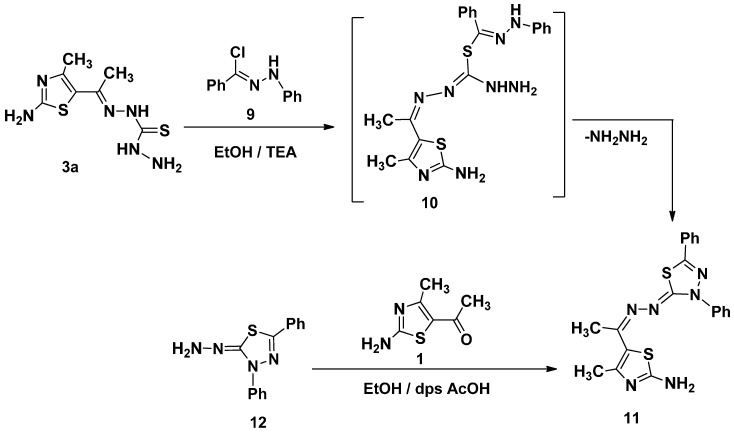
The reaction of N′-phenylbenzohydrazonoyl chloride 9 not containing α-haloketone with 3a in the same condition gave only one isolable product as examined by thin layer chromatography (TLC) The molecular formula of 11, C20H18N6S2, was consistent with elimination of HCl and NH2NH2. The structure of 11 was elucidated by elemental analysis and spectroscopic data (see Experimental Section) (Scheme 3). Compound 11 was also obtained via the reaction of 5-acetyl-2-amino-4-methylthiazole (1) with 2-hydrazono-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (12) [24] in ethanol in presence of drops of acetic acid afford product identical in all respects with that obtained from reaction of the 9 with of ethylidenethiocarbohydrazide 3a (Scheme 3).
Scheme 3.
Synthesis of 1,3,4-thiadiazole 11.
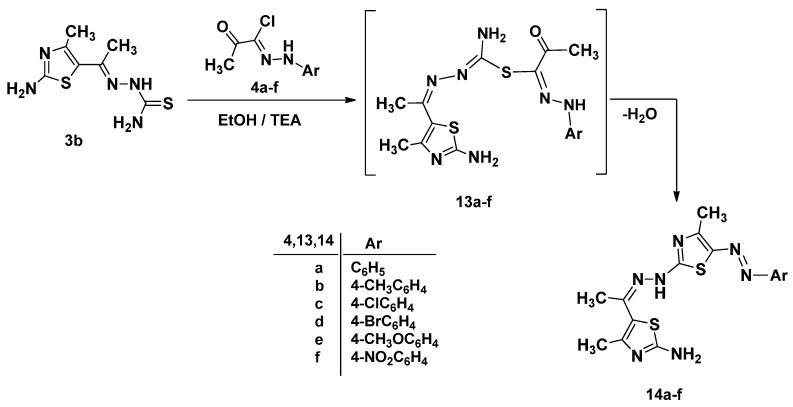
Next, the reaction of 1-[1-(2-amino-4-methylthiazol-5-yl)ethylidene]thiosemicarbazide 3b with α-keto-hydrazonoyl halides 4a–f was performed under similar reaction conditions, and afforded the final product 5-hydrazono-thiazole derivatives 14a–f via same route of elimination HCl and H2O (Scheme 4) [25]. The 1H-NMR of 14a exhibited three singlet signals for methyl groups and a multiplet resonance at δ 6.95–7.35 for aromatic group (see Experimental Section & Figure S3).
Scheme 4.
Synthesis of 5-hydrazono-thiazole derivatives 14a–f.
Finally, refluxing an equimolecular mixture of 3b and dimethylacetylenedicarboxylate 15 in methanol yielded methyl 4-oxo-thiazolidin-5-ylidene acetate 17 (Scheme 5). The structure of 17 was established on the basis of analytical and spectral data. Thus, the 1H-NMR spectrum showed singlet signal at δ 12.77 ppm (D2O-exchangeable), assignable to NH group. In addition, the presence of a signal at δ 6.60 assigned to the =CH, and singlet at δ 3.29 for ester methyl protons [9,26,27,28].
Scheme 5.
Synthesis of thiazolidin-4-one 17.
2.2. Cytotoxiclogical Activity against HEP G2 Cell Line
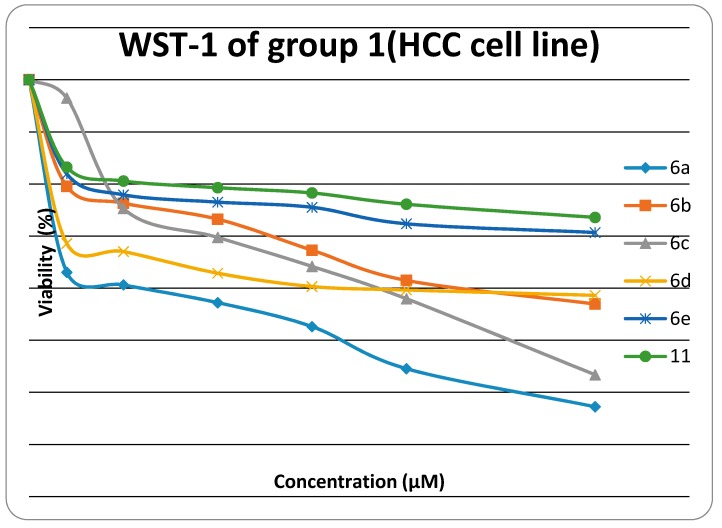
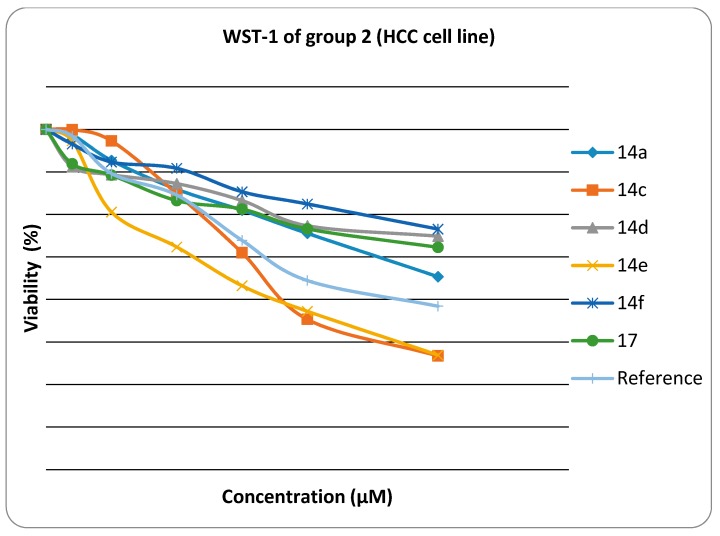
The cytotoxiclogical activity of synthesized products Group 1 (6a, 6b, 6c, 6d, 6e, 6f, 11) and Group 2 (14a, 14c, 14d, 14e, 14f, 17) were evaluated against HEP G2) using the WST-1 cell proliferation assay as a fast and sensitive quantification of cell proliferation and viability. In brief, the assay is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases. Expansion in the number of viable cells results in an increase in the overall activity of the mitochondrial dehydrogenases in the sample. The augmentation in enzyme activity leads to the increase in the amount of formazan dye formed. The formazan dye produced by viable cells can be quantified by a multiwell spectrophotometer (microplate reader) by measuring the absorbance of the dye solution at 450 nm. The viability of the tested compounds in response to their concentration is illustrated in Figure 1 and Figure 2. Data generated were used to plot a dose response curve to determine the concentration of test compounds required to kill 50% of cell population (IC50) was estimated exponentially.
Figure 1.
Viability chart of tested Group 1 compounds against HEP G2 cell line.
Figure 2.
Viability chart of tested Group 2 compounds against HEP G2 cell line.
Cytotoxic activity was expressed as the mean IC50 of three independent experiments. The results are represented in Table 1.
Table 1.
IC50 values of tested compounds ± standard deviation against HEP G2.
| Compound No. | IC50 (μM) | Compound No. | IC50 (μM) |
|---|---|---|---|
| Doxorubicin | 0.68 ± 0.03 | 14a | 0.84 ± 0.04 |
| 6a | 1.00 ± 0.08 | 14c | 0.52 ± 0.03 |
| 6b | 1.49 ± 0.1 | 14d | 1.19 ± 0.09 |
| 6c | 1.04 ± 0.07 | 14e | 0.50 ± 0.02 |
| 6d | 1.73 ± 0.12 | 14f | 1.28 ± 0.08 |
| 6e | 2.17 ± 0.13 | 17 | 1.07 ± 0.06 |
| 11 | 2.91 ± 0.15 |
The results presented in Table 1 showed that:
The in vitro inhibitory activities of tested compounds against the hepatocellular carcinoma cell line (HEP G2) have the descending order as follow: 14e > 14c > 14a > 6a > 6c > 17 >14d >14f > 6b > 6d > 6e > 11. The thiazole rings 6a–e and 14a–f have better in vitro inhibitory activity than the thiadiazole ring 11. The thiazolone ring 17 has better in vitro inhibitory activity than the thiadiazole ring 11. The introduction of a amino group on N3 of thiazole ring increases the activity when fixing the substituent at 4-position of phenyl group at position 5 in the thiazole ring (thiazole 14a is more active than thiazole 6a, thiazole 14c is more active than thiazole 6c, thiazole 14d is more active than thiazole 6d, and thiazole 14e is more active than thiazole 6e).
The results revealed that compounds 14e, 14c and 14a (IC50 were 0.5 ± 0.02 µM, 0.52 ± 0.03 µM and 0.84 ± 0.04 µM, respectively) have promising antitumor activity against hepatocellular carcinoma cell line (HEP G2) when compared to doxorubicin as a reference drug (IC50 value of doxorubicin = 0.68 ± 0.03 µM), while 6a, 6c, 17, 14d and 14f have moderate activity (IC50 were 1.04 ± 0.07 µM, 1.07 ± 0.06 µM, 1.19 ± 0.09 µM, and 1.28 ± 0.08 µM, respectively). On the other hand, 6b, 6d, 6e and 11 have lower inhibitory activity against (HEP G2) (IC50 = 1.49 ± 0.1 µM, 1.73 ± 0.12 µM, 2.17 ± 0.13 µM and 2.91 ± 0.15 µM, respectively). The small values of IC50 for the selected compounds indicate that, for more anticancer effect, higher concentrations can be used.
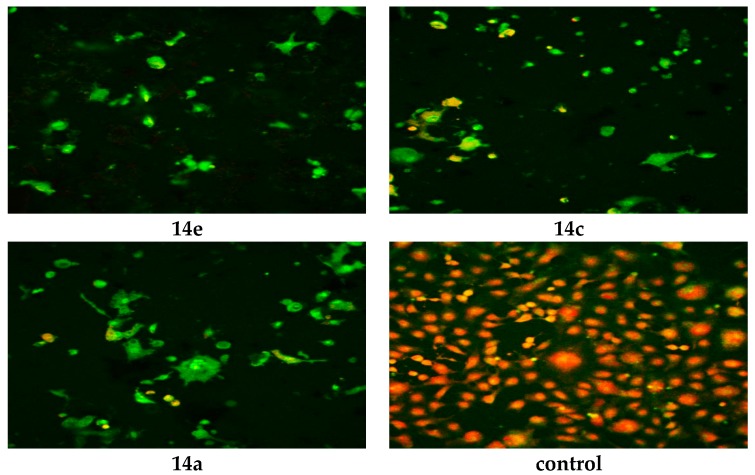
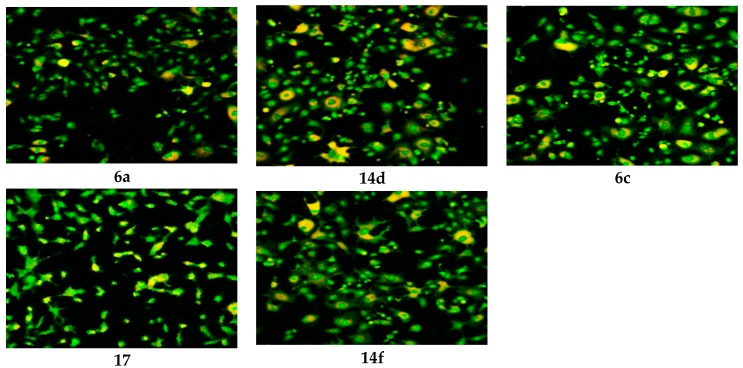
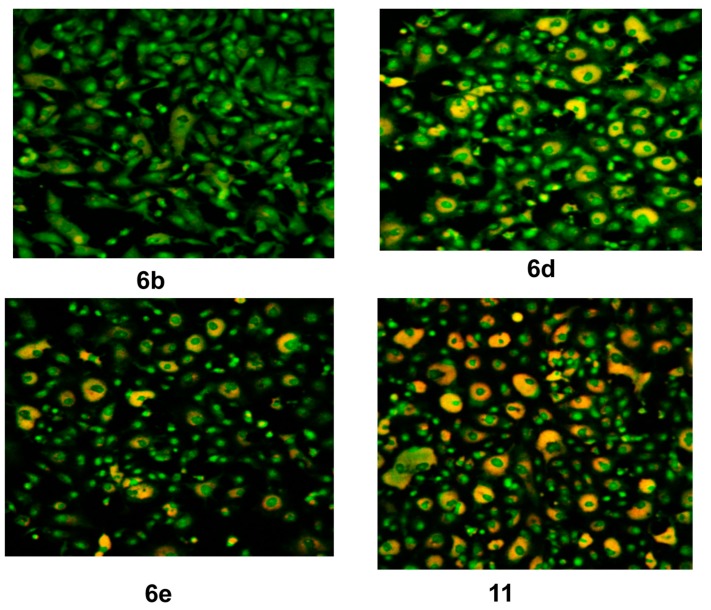
The WST-1 assay results revealed a significant decrease in the mitochondrial dehydrogenase activity as a function of the growth rate of the tumor cells but did not explain the mode of action of the compounds. Confocal laser scanning microscopic (CLSM) imaging of HEP G2 cell line stained with acridine orange dye for nucleic acids (green stain) and Rodamine 123 (Orange stain) for inner mitochondrial membrane, where the dehydrogenases located, reflects the activity of mitochondrial dehydrogenase enzyme activity. It was obvious that the activity of dehydrogenases significantly decreased with cells treated with 14e, 14c and 14a compared to the untreated control (Figure 3), while moderate decrease for 6a, 6c, 17, 14d and 14f (Figure 4), and mild decrease for the rest of the compounds (Figure 5). The higher amount of orange color reflects the higher activity of mitochondrial dehydrogenase enzyme, which means higher viability and vice versa.
Figure 3.
Confocal Laser Scanning Microscopy (CLSM) image of HEP G2 cell line treated with 0.6 μM tested compounds (14e, 14c, 14a and untreated control). Stained by Acridine orange (green) and Rodamine 123 (Orange).
Figure 4.
CLSM image of HEP G2 cell line treated with 0.6 μM tested compounds (6a, 6c, 17, 14d and 14f). Stained by Acridine orange (green) and Rodamine 123 (Orange).
Figure 5.
CLSM image of HEP G2 cell line treated with 0.6 μM tested compounds (6b, 6d, 6e and 11). Stained by Acridine orange (green) and Rodamine 123 (Orange).
2.3. Molecular Docking Study
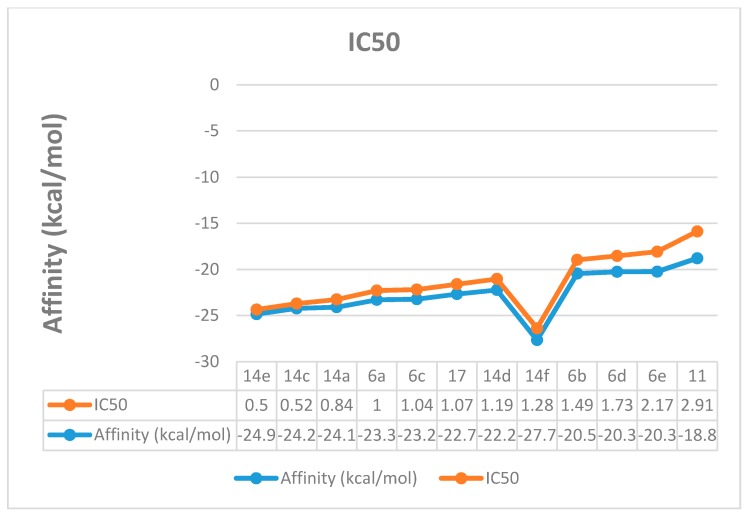
In cancer cells, the increased glucose uptake results in high glycolytic activity, which, in turn, causes elevated levels of lactate production [29]. The lactate production is controlled by lactate dehydrogenase-5 (LDH-5), an isoenzyme from the lactate dehydrogenase that is mainly found in the liver cells [30]. It has been reported that LDH-5 has an important role in tumor maintenance in many human cancers like the hepatic cancer [31]. Recently, LDH-5 inhibitors have been reported to show potential anticancer activity [32], which confirms that LDH-5 inhibition is a good target for developing anticancer agents. Molecular docking study was conducted to interpret the LDH inhibitory activity for the synthesized compounds that were confirmed biologically. The docking was performed using Leadit 2.1.5 software to calculate and analyze all parameters that may have a direct relationship to the LDH inhibition (Table 2). A direct correlation between the activity and the binding affinity was observed (Figure 6). For example, compound 14e showed the best affinity (–24.85 kcal/mol) and the best IC50 as well. The entropy ligand conformation score for the highly active group was a neglected value (0.00), which is favored for best fitting.
Table 2.
Docking Results of the active compounds using Leadit 2.1.5 software (software license was purchased from BioSolveIT GmbH, Germany).
| Compounds | Affinity Score kcal/mol | Lipophilic Contribution Score | Clash Score | Ligand Entropy Conformation Score |
|---|---|---|---|---|
| 14e | −24.85 | −8.50 | 4.32 | 0.00 |
| 14c | −24.23 | −8.56 | 4.54 | 0.00 |
| 14a | −24.10 | −12.27 | 4.12 | 0.00 |
| 6a | −23.50 | −8.31 | 5.82 | 1.40 |
| 6c | −23.23 | −8.28 | 5.88 | 1.40 |
| 17 | −22.68 | −6.36 | 3.85 | 1.40 |
| 14d | −22.23 | −13.86 | 7.55 | 0.00 |
| 14f | −21.66 | −6.91 | 8.23 | 0.00 |
| 6b | −20.46 | −8.52 | 5.89 | 1.40 |
| 6d | −20.27 | −8.31 | 5.88 | 1.40 |
| 6e | −20.25 | −8.39 | 4.54 | 2.80 |
| 11 | −18.80 | −9.23 | 5.11 | 0.00 |
Figure 6.
Correlation between the docking affinity and the IC50.
There is no doubt that all the tested compounds showed promising cytotoxic activity against HEP G2 cell line in low micromolar range ranged from 0.50 μM (compound 14e) to 2.91 μM (compound 11). All compounds shared the same 5-(substituted)-ethylidene-hydrazono-2-amine-4-methyl-1,3-thiazole scaffold. This scaffold showed the ability to form some interactions with the main residues in the active site of LDH-5 that was clear in the NH-N= moiety hydrogen bond formation with Arg 99, and the 2-amino group of the 1,3-thizole ring and its hydrogen bond formation with Gly 29, Gly 27, and Thr 95. The compounds were divided into three groups according to their activity: highly active group (14e, 14c, and 14f), moderately active group (6a, 6c, 17, 14d, and 14f) and low active group (6b, 6d, 6e and 11).
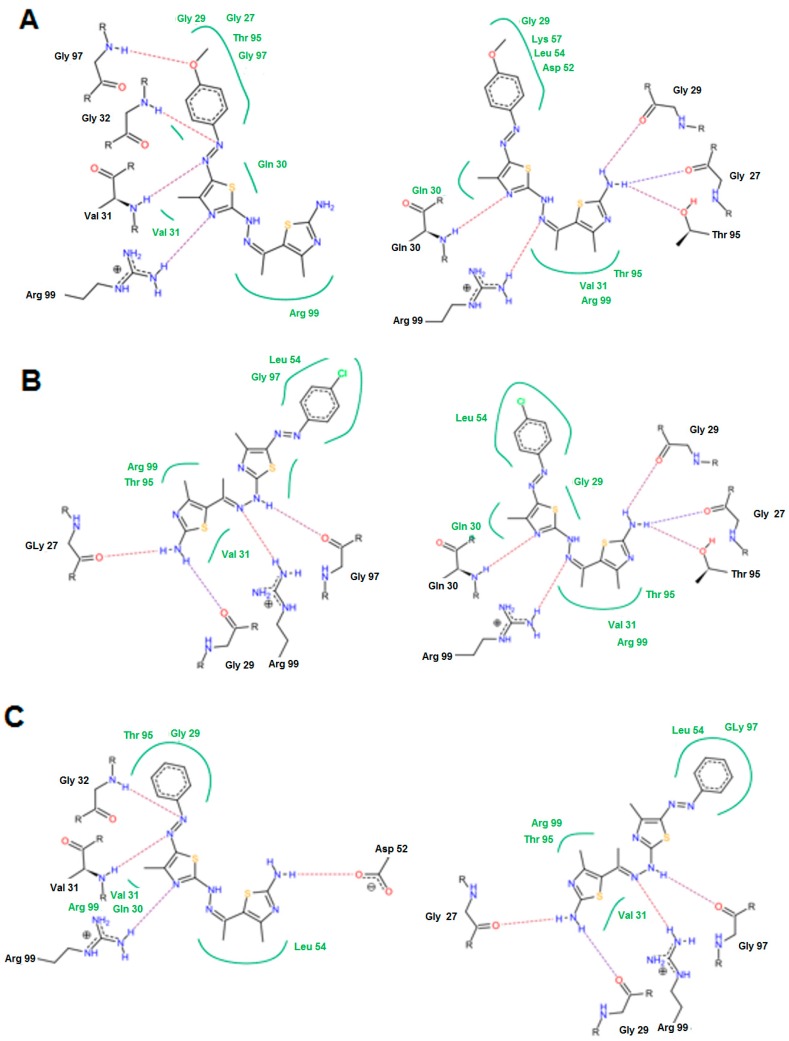
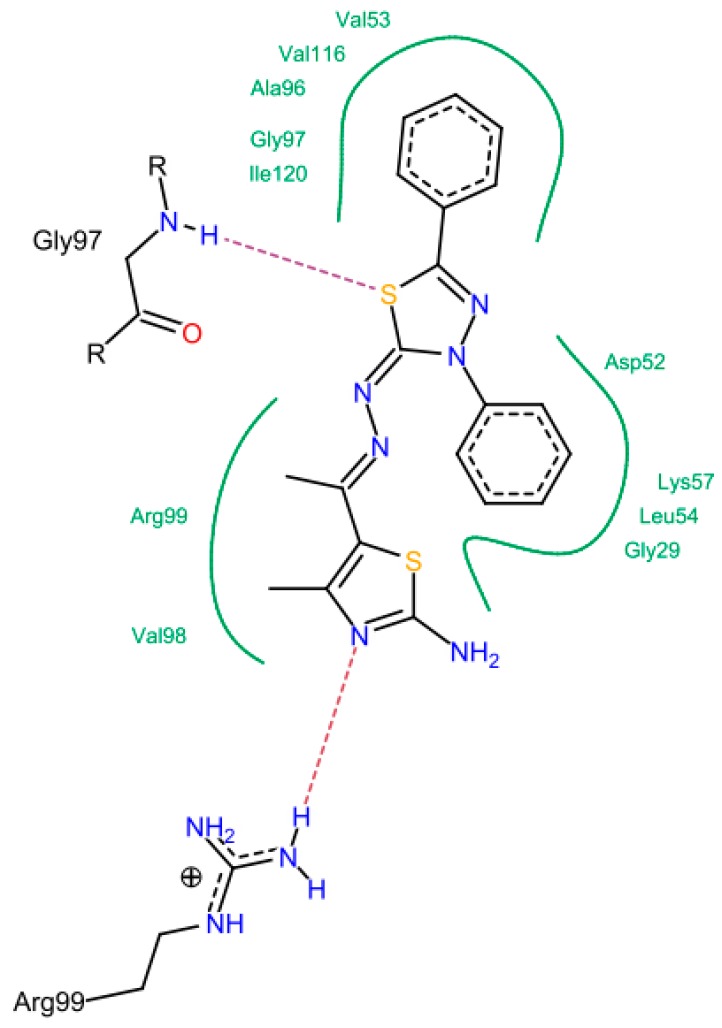
For the highly active group, a common binding mode was observed in which the Gly 97, Gly 32, Val 31, Arg 99, Gln 30 and Thr 95 were the most common involved residues. The N=N, C=N, NH2, and OCH3 groups were the most important groups for formation of the hydrogen bonds (Figure 7). The oxygen atom of the OCH3 group in case of 14e interacted with Gly 97 to form a hydrogen bond. This feature was absent in compound 14c with the p-chloro phenyl and compound 14a with the non-substituted phenyl ring.
Figure 7.
Possible binding modes of compounds (A) 14e; (B) 14c; and (C) 14a.
In the moderately active group (6a, 6c, 17, 14d, and 14f), the presence of 3-amino group in the thiazol-2-(3H) moiety was important to form a hydrogen bond with Thr 99, Asn 113 and Ala 96. The N=N and C=N groups were involved in the interactions. An overall good fitting and placement was observed for the docked compounds inside the active site where the nicotinamide-adenine dinucleotide was bound. Regarding the low active group (6b, 6d, 6e, and 11), the most obvious result was in compound 11 that has a 3-phenyl thiadiazole moiety. This phenyl ring restricts the interactions and the flexibility of the compound resulting in a low binding affinity. The steric hindrance of compound 11 resulted from the 3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole ring system that was the main reason for bad fitting and low affinity (Figure 8).
Figure 8.
The possible binding mode of compound 11.
Generally, in the binding site area with mild hydrophobic amino acids, like Ala 96, Gly 97 and Gly 32, the activity was achieved by non-substituted phenyl ring, -OCH3 substitution, and/or chloro substitution that do not affect strongly the electron cloud on the phenyl ring. The main interactions in this area are not hydrophobic but, hydrogen bonding so, any substitution by bromo atom or nitro group affected the electron cloud and the π-π system resulting in shifting in the activity.
3. Experimental Section
3.1. Chemistry
Melting points were measured on an Electrothermal IA 9000 series digital melting point apparatus (Bibby Sci. Lim. Stone, Staffordshire, UK). IR spectra were recorded in potassium bromide discs on Shimadzu FTIR 8101 PC infrared spectrophotometer (Shimadzu, Tokyo, Japan). NMR spectra were recorded on a Varian Mercury VX-300 NMR spectrometer (Varian, Inc., Karlsruhe, Germany) operating at 300 MHz (1H-NMR) or 75 MHz (13C-NMR) and run in deuterated dimethylsulfoxide (DMSO-d6). Chemical shifts were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer at 70 eV (Tokyo, Japan). Elemental analyzes were measured by using a German made Elementar vario LIII CHNS analyzer (GmbH & Co. KG, Hanau, Germany). Antitumor activity was evaluated by the Nanotechnology & Advanced materials central lab, Agricultural Research Center, Giza, Egypt. Hydrazonoyl halides were prepared as previously reported in the respective literature [33,34].
3.1.1. Synthesis of 3a and 3b
5-Acetyl-2-amino-4-methylthiazole 1 (7.8 g, 50 mmol) was dissolved in 100 mL of absolute ethanol and stirred with an equimolar quantity of thiocarbohydrazide (2a) or thiosemicarbazide (2b) for 24 h at room temperature with catalytic amounts of concentrated HCl. The desired products precipitated from reaction mixture were filtered, washed with ethanol and recrystallized from acetic acid to give pure product of compound 3a and 3b.
1-[1-(2-Amino-4-methylthiazol-5-yl)ethylidene]thiocarbohydrazide (3a). White crystals (84%); mp 234–236 °C; IR (KBr): v 3436, 3231 (NH2), 3182 (NH), 1631(C=N), 1258 (C=S) cm−1; 1H-NMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 2.85(s, 3H, CH3-thiazole), 4.92 (s, br, 2H, D2O-exchangeable, NH2, CSNHNH2), 7.42 (s, br, 2H, D2O-exchangeable, NH2-thiazole), 9.64 (s, br, 1H, D2O-exchangeable, NH, CSNHNH2), 14.12 (s, br, 1H, D2O-exchangeable, NH, C=N-NH-CS); MS m/z (%): 245 (M+, 19), 244 (M+, 21), 219 (27), 122 (29), 79 (100). Anal. Calcd. for C7H12N6S2 (244.34): C, 34.41; H, 4.95; N, 34.39. Found C, 34.48; H, 4.86; N, 34.24%.
1-[1-(2-Amino-4-methylthiazol-5-yl)ethylidene]thiosemicarbazide (3b). White crystals (76%); mp 245–247 °C; IR (KBr): ν 3406, 3275 (NH2), 3217 (NH), 1639 (C=N), 1242 (C=S) cm−1; 1H-NMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 2.86 (s, 3H, CH3-thiazole), 4.08 (s, br, D2O-exchangeable, 2H, NH2), 7.45(s, br, 2H, D2O-exchangeable, NH2-thiazole), 9.78 (s, br, 1H, D2O-exchangeable, NH); MS m/z (%): 229 (M+, 6), 212 (7), 128 (19), 42 (100). Anal. Calcd. for C7H11N5S2 (229.33): C, 36.66; H, 4.83; N, 30.54. Found C, 36.60; H, 4.69; N, 30.41%.
3.1.2. Synthesis of 5-[1-((3-Amino-4-methyl-5-(substitutedphenyldiazenyl)thiazol-2(3H)-ethylidene) hydrazono)]-2-amine-4-methyl-1,3-thiazole (6a–e)
A mixture of 1-[1-(2-amino-4-methylthiazol-5-yl)ethylidene]thiocarbohydrazide 3a (0.244 g, 1 mmol) and appropriate hydrazonoyl halides 4a–e (1 mmol) in dioxan (30 mL) containing triethylamine (0.1 g, 1 mmol) was refluxed for 6–8 h (monitored by TLC). The formed precipitate after cooling at room temperature was isolated by filtration, washed with methanol, dried and recrystallized from appropriate solvent to give products 6a–e.
5-[1-((3-Amino-4-methyl-5-(phenyldiazenyl)thiazol-2(3H)-ethylidene)hydrazono)]-2-amine-4-methyl-1,3-thiazol (6a). Red solid (67%); mp 192–194 °C; IR (KBr): ν 3406, 3298, 3121 (2NH2), 1639 (C=N), 1552 (N=N) cm−1; 1H-NMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 2.52 (s, br., 2H, D2O-exchangeable, NH2), 2.85 (s, 3H, CH3), 3.43 (s, 3H, CH3), 7.15(s, br., 2H, D2O-exchangeable, NH2), 7.31–7.73 (m, 5H, Ar-H); 13C-NMR (DMSO-d6): δ 16.21 (CH3), 17.10 (CH3), 19.89 (CH3), 114.67, 120.32, 121.56, 128.61, 139.54, 143.14 (Ar-C), 154.15, 161.67, 167.45, 169.78, 176.75 (C=N); MS m/z (%): 387 (M+ + 1, 6), 387 (M+, 8), 163 (18), 110 (67), 77 (100). Anal. Calcd. for C16H18N8S2 (386.50): C, 49.72; H, 4.69; N, 28.99. Found C, 49.65; H, 4.60; N, 28.78%.
5-[1-((3-Amino-4-methyl-5-(4-methylphenyldiazenyl)thiazol-2(3H)-ethylidene)hydrazono)]-2-amine-4-methyl-1,3-thiazol (6b). Red solid (68%); mp 216–218°C; IR (KBr): ν 3407, 3317, 3113 (2NH2), 1636 (C=N), 1556 (N=N) cm−1; 1H-NMR (DMSO-d6): δ 2.23 (s, 3H, CH3), 2.42 (s, 3H, CH3), 2.50 (s, br., 2H, D2O-exchangeable, NH2), 2.84(s, 3H, CH3), 3.47 (s, 3H, CH3), 7.11 (s, br., 2H, D2O-exchangeable, NH2), 7.32–7.75 (m, 4H, Ar-H); MS m/z (%): 402 (M+ + 1, 11), 401 (M+ + 1, 18), 400 (M+, 29), 233 (19), 153 (69), 105 (100). Anal. Calcd. for C17H20N8S2 (400.52): C, 50.98; H, 5.03; N, 27.98. Found C, 50.68; H, 5.00; N, 27.69%.
5-[1-((3-Amino-5-((4-chlorophenyl)diazenyl)-4-methylthiazol-2(3H)-ethylidene)hydrazono)]-2-amine-4-methyl-1,3-thiazole (6c). Red solid (65%); mp 243–245 °C; IR (KBr): ν 3409, 3312, 3151 (2NH2), 1637 (C=N), 1553 (N=N) cm−1; 1H-NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.52 (s, br., 2H, D2O-exchangeable, NH2), 2.86 (s, 3H, CH3), 3.49 (s, 3H, CH3), 7.35 (s, br., 2H, D2O-exchangeable, NH2), 7.38-7.83 (m, 4H, Ar-H); MS m/z (%): 422 (M+ + 2, 2), 421 (M+ + 1, 4), 420 (M+, 10), 299 (17), 244 (43), 212 (46), 155 (52), 113 (85), 72 (100). Anal. Calcd. for C16H17ClN8S2 (420.94): C, 45.65; H, 4.07; N, 26.62. Found C, 45.60; H, 4.14; N, 26.38%.
5-[1-((3-Amino-5-(4-bromophenyl)diazenyl)-4-methylthiazol-2(3H)-ethylidene)hydrazono)]-2-amine-4-methyl-1,3-thiazol (6d). Red solid (66%); mp 218–219 °C; IR (KBr): ν 3409, 3294, 3113 (2NH2), 1632 (C=N), 1557 (N=N) cm−1; 1H-NMR (DMSO-d6): δ 2.46 (s, 3H, CH3), 2.51 (s, br., 2H, D2O-exchangeable, NH2), 2.85 (s, 3H, CH3), 3.47 (s, 3H, CH3), 7.26 (s, br., 2H, D2O-exchangeable, NH2), 7.30–7.81 (m, 4H, Ar-H); MS m/z (%): 467 (M+ + 2, 15), 466 (M+ + 1, 19), 465 (M+, 21), 405 (14), 299 (19), 153 (86), 113 (62), 65 (100). Anal. Calcd. for C16H17BrN8S2 (465.39): C, 41.29; H, 3.68; N, 24.08. Found C, 41.11; H, 3.57; N, 24.01%.
5-[1-((3-Amino-5-(4-methoxyphenyl)diazenyl)-4-methylthiazol-2(3H)-ethylidene)hydrazono)]-2-amine-4-methyl-1,3-thiazol (6e). Red solid (68%); mp 212–214 °C; IR (KBr): ν 3403, 3298, 3117 (2NH2), 1635 (C=N), 1555 (N=N) cm−1; 1H-NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.50 (s, br., 2H, D2O-exchangeable, NH2), 2.81(s, 3H, CH3), 3.41 (s, 3H, CH3), 3.64 (s, 3H, CH3), 7.12 (s, br., 2H, D2O-exchangeable, NH2), 7.24-7.72 (m, 4H, Ar-H); MS m/z (%): 416 (M+, 4), 322 (13), 273 (12), 182 (61), 124 (100), 109 (99). Anal. Calcd. for C17H20N8OS2 (416.52): C, 49.02; H, 4.84; N, 26.90. Found C, 48.89; H, 4.76; N, 26.72%.
3.1.3. Synthesis of 2-[(1-(2-Amino-4-methylthiazol-5-yl)ethylidene)hydrazono]-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (11)
A mixture of 1-[1-(2-amino-4-methylthiazol-5-yl)ethylidene]thiocarbohydrazide 3a (0.244 g, 1 mmol) and N′-phenylbenzohydrazonoyl chloride 9 (0.230 g, 1 mmol) in dioxane (20 mL) containing triethylamine (0.1 g, 1 mmol) was refluxed for 6 h. The formed precipitate was isolated by filtration, washed with methanol, dried and recrystallized from DMF to give product 11.
Yellow solid (76%); mp = 240–242 °C; IR (KBr): ν 3407, 3213 (NH2), 1634 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.39 (s, 3H, CH3), 2.54 (s, 3H, CH3), 3.37 (s, br., 2H, D2O-exchangeable, NH2), 7.30–8.14 (m, 10H, Ar-H); MS m/z (%): 407 (M+ + 1, 67), 406 (M+, 52), 361 (59), 304 (64), 233 (91), 172 (64), 138 (67), 90 (79), 63 (100). Anal. Calcd. for C20H18N6S2 (406.53): C, 59.09; H, 4.46; N, 20.67. Found C, 59.01; H, 4.54; N, 20.45%.
3.1.4. Alternative Synthesis of 11
A mixture of 5-acetyl-2-amino-4-methylthiazole (1) (0.156 g, 1 mmol) and 2-hydrazono-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (12) (0.268 g, 1 mmol) in 10 mL of ethanol with catalytic amounts of glacial acetic acid were refluxed for 4h. The solid precipitated after cooling was filtered, washed with ethanol and recrystallized from acetic acid to give pure 11 which identical in all respects (m.p., mixed m.p. and IR spectra) with those obtained from reaction of 3a with 9 but in 66% yield.
3.1.5. Synthesis of 2-Amino-4-methyl-5-(1-(2-(4-methyl-5- (substitutedphenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14a–f)
A mixture of 1-[1-(2-amino-4-methylthiazol-5-yl)ethylidene]thiosemicarbazide 3b (0.229 g, 1 mmol) and appropriate hydrazonoyl halides 4a–f (1 mmol) in dioxane (30 mL) containing triethylamine (0.1 g, 1 mmol) was refluxed for 6–8 h. (monitored by TLC). The formed precipitate after cooling was isolated by filtration, washed with methanol, dried and recrystallized from appropriate solvent to give products 14a–f.
2-Amino-4-methyl-5-(1-(2-(4-methyl-5-(phenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14a). Red solid (80%); mp 168–169 °C; IR (KBr): ν 3438, 3283 (NH2, NH), 1597 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.56 (s, 3H, CH3), 3.56 (s, 3H, CH3), 6.95–7.35 (m, 5H, Ar-H), 7.57 (s, 2H, D2O-exchangeable, NH2), 10.51 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 16.87 (CH3), 17.29 (CH3), 19.39 (CH3), 114.58, 119.46, 122.31, 129.69, 138.97, 144.14, 153.95 (Ar-C), 161.24, 168.59, 169.31, 176.75 (C=N); MS m/z (%): 372 (M+ + 1, 4), 371 (M+, 21), 218 (29), 153 (100), 78 (90), 42 (79). Anal. Calcd. for C16H17N7S2 (371.48): C, 51.73; H, 4.61; N, 26.39. Found C, 51.70; H, 4.46; N, 26.27%.
2-Amino-4-methyl-5-(1-(2-(4-methyl-5-(methylphenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14b). Red solid (82%); mp 247–249 °C; IR (KBr): ν 3391, 3278 (NH2, NH), 1632 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 2.43 (s, 3H, CH3), 2.53 (s, 3H, CH3), 3.56 (s, 3H, CH3), 7.13 (d, 2H, J = 6.9 Hz, Ar-H), 7.26 (d, 2H, J = 6.9 Hz, Ar-H), 7.55 (s, 2H, D2O-exchangeable, NH2), 10.44 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 16.85 (CH3), 17.28 (CH3), 19.39 (CH3), 20.83 (CH3), 114.59, 119.48, 130.12, 131.28, 138.34, 141.83, 153.86 (Ar-C), 161.04, 168.69, 169.26, 176.58 (C=N); MS m/z (%): 386 (M+ + 1, 12), 385 (M+, 36), 232 (53), 153 (85), 113 (100). Anal. Calcd. for C17H19N7S2 (385.51): C, 52.96; H, 4.97; N, 25.43. Found C, 52.91; H, 4.86; N, 25.33%.
2-Amino-4-methyl-5-(1-(2-(4-methyl-5-(chlorophenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14c). Red solid (75%); mp 178–180 °C; IR (KBr): ν 3996, 3283 (NH2, NH), 1632, 1606 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.57 (s, 3H, CH3), 3.56 (s, 3H, CH3), 7.34 (s, 4H, Ar-H), 7.57 (s, 2H, D2O-exchangeable, NH2), 10.57 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 16.87 (CH3), 17.29 (CH3), 19.43 (CH3), 116.04, 119.41, 125.79, 129.55, 139.81, 143.13, 154.26 (Ar-C), 161.53, 168.31, 169.38, 176.77 (C=N); MS m/z (%): 407 (M+ + 1, 5), 406 (M+, 18), 252 (18), 153 (99). 112 (95), 72 (100). Anal. Calcd. for C16H16ClN7S2 (405.93): C, 47.34; H, 3.97; N, 24.15. Found C, 47.39; H, 3.90; N, 24.06%.
2-Amino-4-methyl-5-(1-(2-(4-methyl-5-(bromophenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14d). Red solid (75%); mp 176–178 °C; IR (KBr): ν 3368, 3191 (NH2, NH), 1632 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 2.53 (s, 3H, CH3), 3.59 (s, 3H, CH3), 7.28 (d, 2H, J = 4.5 Hz, Ar-H), 7.48(d, 2H, J = 4.5 Hz, Ar-H), 7.58 (s, 2H, D2O-exchangeable, NH2), 10.58 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 16.87 (CH3), 17.30 (CH3), 19.43 (CH3), 113.65, 116.48, 119.42, 132.55, 139.91, 143.53, 154.25 (Ar-C), 161.53, 168.29, 169.38, 176.75 (C=N); MS m/z (%): 452 (M+ + 1, 2), 451 (M+, 15), 296 (14), 153 (100), 113 (86), 72 (98). Anal. Calcd. for C16H16BrN7S2 (450.38): C, 42.67; H, 3.58; N, 21.77. Found C, 42.55; H, 3.67; N, 21.65%.
2-Amino-4-methyl-5-(1-(2-(4-methyl-5-(methoxylphenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14e). Red solid (78%); mp 187–189 °C; IR (KBr): ν 3414, 3202 (NH2, NH), 1624 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.43 (s, 3H, CH3), 2.53 (s, 3H, CH3), 3.56 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 6.91–7.30 (m, 4H, Ar-H), 7.87 (s, 2H, D2O-exchangeable, NH2), 10.48 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 8.93 (CH3), 16.85 (CH3), 17.19 (CH3), 55.73 (OCH3), 115.07, 115.87, 119.38, 137.60, 137.74, 155.30, 160.59 (Ar-C), 169.08, 169.21, 169.26, 176.60 (C=N); MS m/z (%): 402 (M+ + 1, 2), 401 (M+, 13), 248 (17), 153 (38), 113 (42), 44 (100). Anal. Calcd. for C17H19N7OS2 (401.51): C, 50.85; H, 4.77; N, 24.42. Found C, 50.82; H, 4.65; N, 24.23%.
2-Amino-4-methyl-5-(1-(2-(4-methyl-5-(nitrophenyldiazenyl)thiazol-2-yl)hydrazono)ethyl)thiazole (14f). Red solid (78%); mp 183–185°C; IR (KBr): v 3375, 3194 (NH2, NH), 1643 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 2.54 (s, 3H, CH3), 3.57 (s, 3H, CH3), 6.91–7.38 (m, 4H, Ar-H), 7.59 (s, 2H, D2O-exchangeable, NH2), 10.53 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 16.45 (CH3), 17.29 (CH3), 19.76 (CH3), 114.54, 119.53, 122.31, 129.60, 138.91, 144.23, 153.92 (Ar-C), 161.34, 168.55, 169.36, 176.75 (C=N); MS m/z (%): 417 (M+ + 1, 3), 416 (M+, 3), 153 (33), 113 (24), 43 (100). Anal. Calcd. for C16H16N8O2S2 (416.48): C, 46.14; H, 3.87; N, 26.90. Found C, 46.11; H, 3.76; N, 26.64%.
3.1.6. Synthesis of Methyl 2-(2-((1-(2-amino-4-methylthiazol-5-yl)ethylidene)hydrazono)-4-oxothiazolidin-5-ylidene)acetate (17)
To a solution of 1-[1-(2-amino-4-methylthiazol-5-yl)ethylidene]thiosemicarbazide 3b (0.229 g, 1 mmol) in dry methanol (20 mL) was added dimethylacetylenedicarboxylate 15 (0.142 g, 1 mmol). The solution was refluxed for 2 h. The precipitated product after cooling was filtered, washed with methanol, and recrystallized from ethanol to give product 17.
Canary yellow solid (75%); mp 352–354 °C; IR (KBr): v 3306, 3167 (NH2, NH), 1708, 1689 (2C=O), 1609 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 2.39 (s, 3H, CH3), 2.50 (s, 3H, CH3), 3.76 (s, 3H, OCH3), 6.60 (s, 1H, =CHCOOCH3), 9.43 (s, 2H, D2O-exchangeable, NH2), 12.88 (s, 1H, D2O-exchangeable, NH); 13C-NMR (DMSO-d6): δ 15.03 (CH3), 16.01 (CH3), 52.92 (OCH3), 114.64, 118.82, 139.60 143.31 (Ar-C), 157.67, 160.07, 165.97 (C=N), 168.37, 168.37 (C=O); MS m/z (%): 339 (M+, 68), 153 (53), 113 (38), 85 (100), 43 (57). Anal. Calcd. for C12H13N5O3S2 (339.39): C, 42.47; H, 3.86; N, 20.63. Found C, 42.40; H, 3.89; N, 20.43%.
3.2. Biological Assay
3.2.1. WST-1 Assay
The human hepatocellular carcinoma cell line were cultured and tested at Nanotechnology & Advanced materials central lab, Giza, Egypt. The culture was maintained in DMEM with 10% FBS at 37 °C humidified with 5% CO2. Various concentrations of the compound being test, as well as doxorubicin as a reference drug (0.0, 0.04, 0.1, 0.2, 0.3, 0.4, and 0.6 μg/mL), were added to the cell monolayer in triplicate wells; then, the individual doses and their cytotoxicity were tested using a standard WST-1 cell proliferation assay as a fast and sensitive quantification of cell proliferation and viability in a 96-well microtiter plate for 24 h, measuring the absorbance of the dye solution at 450 nm [35].
3.2.2. Confocal Laser Scanning Microscopy (CLSM)
The Mode of Potential cytotoxicity action was evaluated using confocal laser scanning microscopic (Carrl Zeiss CLSM 710, diverse net ventures, Boston, MA, USA) imaging of Hep G2 treated cell lines at 0.6 μg/mL concentration of the tested compounds. Cells were plated in 96-multiwill plates (104 cells/well) for 24 h before treatment with the tested compound to allow attachment of cells to the wall of the plate. A selected concentration of the compounds being tested (0.6 μM) was added to the cell monolayer in triplicate wells for each individual dose; monolayer cells were incubated with the compounds for 24 h at 37 °C and in atmosphere of 5% CO2. After 24 h, cells were stained by Rhodamine 132 and Acridine orange stains (Sigma-Aldrich, Boston, MA, USA); after further waiting for five minutes, microscopic examination was done using excitation laser lines at 588 nm and 633 nm by two channel detection.
3.3. Molecular Docking Using Leadit 2.1.5
All compounds were built and saved as Mol2. The crystal structure of human LDH-5B in complex with nicotinamide adenine dinucleotide was downloaded from protein data bank (pdb code = 1T2F). The protein was loaded into Leadit 2.1.5 and the receptor components were chosen by selection of chain A as a main chain. Binding site was defined by choosing NAD+ as a reference ligand to which all coordinates were computed. Amino acids within radius 6.5 A° were selected in the binding site. All chemical ambiguities of residues were left as default. Ligand binding was driven by enthalpy (classic Triangle matching). For scoring, all default settings were restored. Intra-ligand clashes were computed using clash factor = 0.6. Maximum number of solutions per iteration = 200. Maximum number of solutions per fragmentation = 200. The base placement method was used as a docking strategy.
4. Conclusions
New thiazole derivatives have been synthesized using ethylidenethiosemicarbazide and ethylidenethiocarbohydrazide as starting materials under thermal conditions. Compounds 14e, 14c and 14a may have significant and promising anticancer efficiency for hepatocellular carcinoma with low IC50, 0.5 ± 0.02, 0.52 ± 0.03, and 0.84 ± 0.04 μM, respectively. The cytotoxic effect was due to its inhibitory effect to the inner mitochondrial membrane Lactate dehydrogenase enzyme, which, in turn, decreases cellular activity, including the rate of cell division. The molecular docking study confirmed high binding affinities of –24.85, –24.23, and –24.10 kcal/mol for 14e, 14c and 14a, respectively. A direct correlation between the computed affinity and the IC50 was observed.
Acknowledgments
The support from Chemistry Department, Faculty of Science, Cairo University, is gratefully acknowledged.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/01/0003/s1.
Author Contributions
S.M.G., H.M.E.H. and H.M.A. designed research, performed research, analyzed the data, wrote and read the paper. T.A.S. designed the pharmacological part. M.A.K. designed the molecular modeling part. S.M.G. and M.A.K. approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the synthesized compounds are available from the authors.
References
- 1.Ahmad J., Rabinovitz M. Etiology and Epidemiology of Hepatocellular Carcinoma. In: Carr B.I., editor. Hepatocellular Cancer: Diagnosis and Treatment. Humana Press Inc.; Totowa, NJ, USA: 2010. pp. 1–22. [Google Scholar]
- 2.Bruix J., Sherman M., Llovet J.M. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-200 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001;35:421–430. doi: 10.1016/S0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 3.Karegoudar P., Karthikeyan M.S., Prasad D.J., Mahalinga M., Holla B.S., Kumari N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008;43:261–267. doi: 10.1016/j.ejmech.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Cukurovali A., Yilmaz I., Gur S., Kazaz C. Synthesis antibacterial and antifungal activity of some new thiazolylhydrazone derivatives containing 3-substituted cyclobutane ring. Eur. J. Med. Chem. 2006;41:201–207. doi: 10.1016/j.ejmech.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Wahab B.F., Abdel-Aziz H.A., Ahmed E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009;44:2632–2635. doi: 10.1016/j.ejmech.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Shih M.H., Ying K.F. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004;12:4633–4643. doi: 10.1016/j.bmc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Shiradkar M., Kumar G.V.S., Dasari V., Tatikonda S., Akula K.C., Shah R. Clubbed triazoles: A novel approach to antitubercular drugs. Eur. J. Med. Chem. 2007;42:807–816. doi: 10.1016/j.ejmech.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Amin K.M., Rahman A.D.E., Al-Eryani Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008;16:5377–5388. doi: 10.1016/j.bmc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Gomha S.M., Riyadh S.M., Abbas I.M., Bauomi M.A. Synthetic utility of ethylidene-thiosemicarbazide: Synthesis and anti-cancer activity of 1,3-thiazines and thiazoles with imidazole moiety. Heterocycles. 2013;87:341–356. [Google Scholar]
- 10.Gomha S.M., Salah T.A., Abdelhamid A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015;146:149–158. doi: 10.1007/s00706-014-1303-9. [DOI] [Google Scholar]
- 11.Carter J.S., Kramer S., Talley J.J., Penning T., Collins P., Graneto M.J., Seibert K., Koboldt C., Masferrer J., Zweifel B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999;9:1171–1174. doi: 10.1016/S0960-894X(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L.P., Ye D.Y., Tang Y. Structure-based 3D-QSAR studies on thiazoles as 5-HT3 receptor antagonists. J. Mol. Model. 2007;13:121–131. doi: 10.1007/s00894-006-0131-1. [DOI] [PubMed] [Google Scholar]
- 13.Clark R.F., Zhang T., Wang X., Wang R., Zhang X., Camp H.S., Beutel B.A., Sham H.L., Gu Y.J. Phenoxythiazole derivatives as potent and selective acetyl Co-A carboxylase-2 inhibitors: Modulation of isozyme selectivity by incorporation of phenyl ring substituent. Bioorg. Med. Chem. Lett. 2007;17:1961–1965. doi: 10.1016/j.bmcl.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Andreani A., Rambaldi M., Mascellani G., Rugarli P. Synthesis and diuretic activity of imidazo [2,1-b]thiazole acetohydrazones. Eur. J. Med. Chem. 1987;22:19–22. doi: 10.1016/0223-5234(87)90169-3. [DOI] [Google Scholar]
- 15.Ergenc N., Capan G., Gunay N.S., Ozkirimli S., Gungor M., Ozbey S., Kendi E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. Pharm. Med. Chem. 1999;332:343–347. doi: 10.1002/(SICI)1521-4184(199910)332:10<343::AID-ARDP343>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Gomha S.M., Khalil K.D. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules. 2012;17:9335–9347. doi: 10.3390/molecules17089335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomha S.M., Abdel-aziz H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles. 2015;91:583–592. doi: 10.3987/COM-14-13146. [DOI] [Google Scholar]
- 18.Gomha S.M., Ahmed S.A., Abdelhamid A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one incorporating triazole moiety. Molecules. 2015;20:1357–1376. doi: 10.3390/molecules20011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbas I.M., Gomha S.M., Elneairy M.A.A., Elaasser M.M., Mabrouk B.K.A. Fused triazolo[4,3-a]pyrimidinones: Synthesis and biological evaluation as antimicrobial and anti-cancer agents. Turk. J. Chem. 2015;39:510–531. doi: 10.3906/kim-1501-144. [DOI] [Google Scholar]
- 20.Gomha S.M., Khalil K.D., El-Zanate A.M., Riyadh S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles. 2013;87:1109–1120. [Google Scholar]
- 21.Gomha S.M., Riyadh S.M., Mahmmoud E.A., Elaasser M.M. Synthesis, molecular mechanics calculations, and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles. 2015;91:1227–1243. [Google Scholar]
- 22.Gomha S.M., Abbas I.M., Elneairy M.A.A., Elaasser M.M., Mabrouk B.K.A. Antimicrobial and anticancer evaluation of novel synthetic tetracyclic system through Dimroth rearrangement. J. Serb. Chem. Soc. 2015;80:1251–1264. doi: 10.2298/JSC141222022G. [DOI] [Google Scholar]
- 23.Wang S., Meades C., Wood G., Osnowski A., Anderson S., Yuill R., Thomas M., Mezna M., Jackson W., Midgley C., et al. 2-Anilino-4-(thiazol-5-yl)pyrimidine CDK Inhibitors: Synthesis, SAR analysis, X-Ray Crystallography, and Biological Activity. J. Med. Chem. 2004;47:1662–1675. doi: 10.1021/jm0309957. [DOI] [PubMed] [Google Scholar]
- 24.Abdelhamid A.O., Zohdi H.F., Rateb N.M. Reactions with hydrazonoyl halides XXI: Reinvestigation of the reactions of hydrazonoyl bromides with 1,1-dicyanothioacetanilide. J. Chem. Res. 1999:184–185. doi: 10.1039/a807566b. [DOI] [Google Scholar]
- 25.Gomha S.M., Badrey M.G. A Convenient synthesis of some new thiazole and pyrimidine derivatives incorporating naphthalene moiety. J. Chem. Res. 2013;2:86–90. doi: 10.3184/174751912X13567137687630. [DOI] [Google Scholar]
- 26.Imrich J., Tomaščiková J., Danihel I., Kristian P., Böhm S., Klika K.D. Selective formation of 5- or 6-membered rings, 1,3-thiazolidin-4-one vs. 1,3-thiazin-4-one, from acridine thiosemicarbazides by the use of ethyne acid esters. Heterocycles. 2010;80:489–503. [Google Scholar]
- 27.Darehkordi A., Saidi K., Islami M.R. Preparation of heterocyclic compounds by reaction of dimethyl and diethyl acetylene dicarboxylate (DMAD, DEAD) with thiosemicarbazone derivatives. Arkivoc. 2007;1:180–188. [Google Scholar]
- 28.Nami N., Hosseinzadeh M., Rahimi E. Synthesis of some 3-substituted 1,2-dihydroindoles. Phosphorous Sulfur Silicon. 2008;183:2438–2442. doi: 10.1080/10426500801963855. [DOI] [Google Scholar]
- 29.Augoff K., Hryniewicz-Jankowska A., Tabola R. Lactate dehydrogenase-5: An old friend and a new hope in the war on cancer. Cancer Lett. 2015;358:1–7. doi: 10.1016/j.canlet.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Van Eerd J.P.F.M., Kreutzer E.K.J. Mouse anti-malaria PAN pLDH monoclonal antibody. Klinisch. Chem. Analisten Deel. 1996;2:138–139. [Google Scholar]
- 31.Fujiwara Y., Takenaka K., Kaliyama K. The characteristics of hepatocellular carcinoma with high levels of serum lactic dehydrogenase. Hepatogastroenterology. 1997;44:820–823. [PubMed] [Google Scholar]
- 32.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Jagt D.L.V., Semenza G.L., Dan C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Nat. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eweiss N.F., Osman A. Part II new routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980;17:1713–1717. doi: 10.1002/jhet.5570170814. [DOI] [Google Scholar]
- 34.Shawali A.S., Abdelhamid A.O. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976;49:321–327. doi: 10.1246/bcsj.49.321. [DOI] [Google Scholar]
- 35.Zund G., Ye Q., Hoerstrup S.P., Schoeberlein A., Schmid A.C., Grunenfelder J., Vogt P., Turina M. Tissue engineering in cardiovascular surgery: MTT, a rapid and reliable quantitative method to assess the optimal human cell seeding on polymeric meshes. Eur. J. Cardio Thorac. Surg. 1999;15:519–524. doi: 10.1016/S1010-7940(99)00068-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.