Abstract
Glucosinolates have anti-carcinogenic properties. In the recent decades, the genetics of glucosinolate biosynthesis has been widely studied, however, the expression of specific genes involved in glucosinolate biosynthesis under exogenous phytohormone treatment has not been explored at the subspecies level in Brassica oleracea. Such data are vital for strategies aimed at selective exploitation of glucosinolate profiles. This study quantified the expression of 38 glucosinolate biosynthesis-related genes in three B. oleracea subspecies, namely cabbage, broccoli and kale, and catalogued associations between gene expression and increased contents of individual glucosinolates under methyl jasmonate (MeJA) and salicylic acid (SA) treatments. Glucosinolate accumulation and gene expression in response to phytohormone elicitation was subspecies specific. For instance, cabbage leaves showed enhanced accumulation of the aliphatic glucoiberin, progoitrin, sinigrin and indolic neoglucobrassicin under both MeJA and SA treatment. MeJA treatment induced strikingly higher accumulation of glucobrassicin (GBS) in cabbage and kale and of neoglucobrassicin (NGBS) in broccoli compared to controls. Notably higher expression of ST5a (Bol026200), CYP81F1 (Bol028913, Bol028914) and CYP81F4 genes was associated with significantly higher GBS accumulation under MeJA treatment compared to controls in all three subspecies. CYP81F4 genes, trans-activated by MYB34 genes, were expressed at remarkably high levels in all three subspecies under MeJA treatment, which also induced in higher indolic NGBS accumulation in all three subspecies. Remarkably higher expression of MYB28 (Bol036286), ST5b, ST5c, AOP2, FMOGS-OX5 (Bol031350) and GSL-OH (Bol033373) was associated with much higher contents of aliphatic glucosinolates in kale leaves compared to the other two subspecies. The genes expressed highly could be utilized in strategies to selectively increase glucosinolate compounds in B. oleracea subspecies. These results promote efforts to develop genotypes of B. oleracea and other species with enhanced levels of desired glucosinolates.
Keywords: glucosinolates, biosynthetic genes, expression analysis, Brassica oleracea, subspecies, methyl jasmonate, salicylic acid
1. Introduction
Glucosinolates are sulfur-rich secondary metabolites derived from amino acids and sugars that are biosynthesized in plant tissues. These molecules are widely produced in all oilseed and vegetable species of the order Brassicales, including Brassica oleracea [1]. In fact, the hydrolysis of glucosinolates imparts characteristic flavors to Brassica vegetables [2,3]. Myrosinase enzymes play the key role in hydrolysis of glucosinolates into bioactive and anti-carcinogenic products such as thiocyanates, isothiocyanates, nitrile and erucin [4,5,6]. Glucosinolate compounds help prevent cancer cell production in animal tissues by controlling the cell cycle and accelerating apoptosis [7]. Sulforaphane (an isothiocyanate) [8,9] and indole-3-carbinol (a product of isothiocyanate) [10] are strongly anti-carcinogenic, whereas phenethyl isothiocyanate plays an inhibitory role in the conversion of potential carcinogens from their native forms into carcinogenic forms [11,12]. The products of glucosinolate hydrolysis can also induce important detoxifying enzymes, for example, glutathione S-transferase and quinone reductase (QR) [8,13,14]. QR catalyzes the conversion of toxic quinones into stable and non-toxic hydroquinones, reducing oxidative cycling [15], and the activation of QR has often been used as a biomarker for cancer prevention. The products of glucosinolate hydrolysis also up-regulate other health-promoting bioactivities including anti-inflammatory activity in B. oleracea [16,17]. However, not all glucosinolate compounds play equivalent roles in human health and plant defense. For example, indolic glucobrassicin has the greatest antioxidative effect compared to other glucosinolates [18,19]. Moreover, an anti-nutritional effect, e.g., goitrogenic effect (anti-thyroid activity) of progoitrin in ruminant animals is also reported [20].
Elucidating the responses governing glucosinolate biosynthesis and accumulation with regard to exogenous factors is important in designing a strategy to produce Brassica vegetable varieties enriched in glucosinolates beneficial for human health and plant protection. A number of biotic and abiotic stresses increase the biosynthesis and accumulation of different types of glucosinolates in Brassica species. Exogenous application of jasmonic acid (JA) or salicylic acid (SA) is often used to mimic biotic stress. In notable number of studies, JA and SA applications have been shown to increase accumulation of beneficial biomolecules, including glucosinolates [21,22,23,24,25,26,27,28,29,30,31,32,33]. Methyl jasmonate (MeJA) can be utilized in fields for Brassica vegetable production to enhance human health-promoting glucosinolates and the market value of the products [25]. Experimental evidence suggests that MeJA-induced glucosinolates enhance QR activity and, thus, play anti-carcinogenic roles [27]. Exogenous MeJA has been reported to enhance particular indolic glucosinolates; for example, neoglucobrassicin significantly accumulated in the leaves of Brassica crops such as pak choi [34], cabbage [35], oilseed rape [36], broccoli [37], Chinese kale [38], oilseed mustard [39] and turnip [40]. By contrast, exogenous SA treatment has been reported to stimulate the biosynthesis and accumulation of all three types of glucosinolates: aromatic, indole, and aliphatic glucosinolates in Brassica crops [21,24,38,40]. Pre-treatment with MeJA four days before harvest significantly improves the contents of desirable glucosinolates in kale, broccoli and cauliflower without decreasing postharvest quality [25,26,27,28]. Accordingly, in our present study, we decided to apply MeJA and SA treatment four days before sampling to measure levels of aliphatic and indolic glucosinolates as well as the expression of genes involved in accumulation of glucosinolates.
In the Brassicaceae family, glucosinolate biosynthesis is accomplished through a specially featured three steps process, namely: (i) elongation of the amino acid side chain; (ii) core structure formation; and (iii) secondary modifications of side chains. MYB-transcription factor related genes that trans-activate the functions of several genes are vital for side chain elongation and core-structure formation [41]. A notable number of different gene loci are involved in secondary modifications of desulfo-glucosinolates to produce characteristically different glucosinolates, for example: ST5, GS-OX, GS-AOP, GS-OH are involved in aliphatic glucosinolate biosynthesis and CYP81, IGM are involved in indolic glucosinolate biosynthesis. It is therefore important to investigate which particular genes are involved in enhancing glucosinolates under exogenous phytohormone elicitation.
B. oleracea is an important Brassica vegetable species that includes a number of commercially valuable subspecies, such as cabbage, cauliflower, broccoli, kale, kohlrabi, and Brussels sprouts, among others. In B. oleracea, about 105 glucosinolate metabolism-related genes have been identified, including 22 catabolism-related genes [42]. Reverse transcription-PCR (RT-PCR)-based expression profiling of 84 genes associated with glucosinolate transcription, biosynthesis and breakdown recently revealed that not all of the genes are expressed in the edible organs of various B. oleracea subspecies [43]. However, expression patterns of glucosinolate biosynthetic genes after exposure to phytohormone elicitors has not been extensively explored in B. oleracea, although this information is needed for selective enhancement of healthful glucosinolate compounds. In this study, we have selected three economically important B. oleracea subspecies in Korea: cabbage, broccoli and kale.
Here, we aimed to relate the expression of glucosinolate biosynthesis genes in three selected B. oleracea subspecies to their glucosinolate contents with or without MeJA and SA treatment. Identifying the genes that underlie the higher glucosinolate biosynthesis mediated by exogenous MeJA and SA at the subspecies level will open a window to generate novel commercial cultivars of B. oleracea with enhanced contents of desired glucosinolates.
2. Results
2.1. Subspecies-Specific Effects of Exogenous MeJA or SA on Glucosinolate Biosynthesis Gene Expression
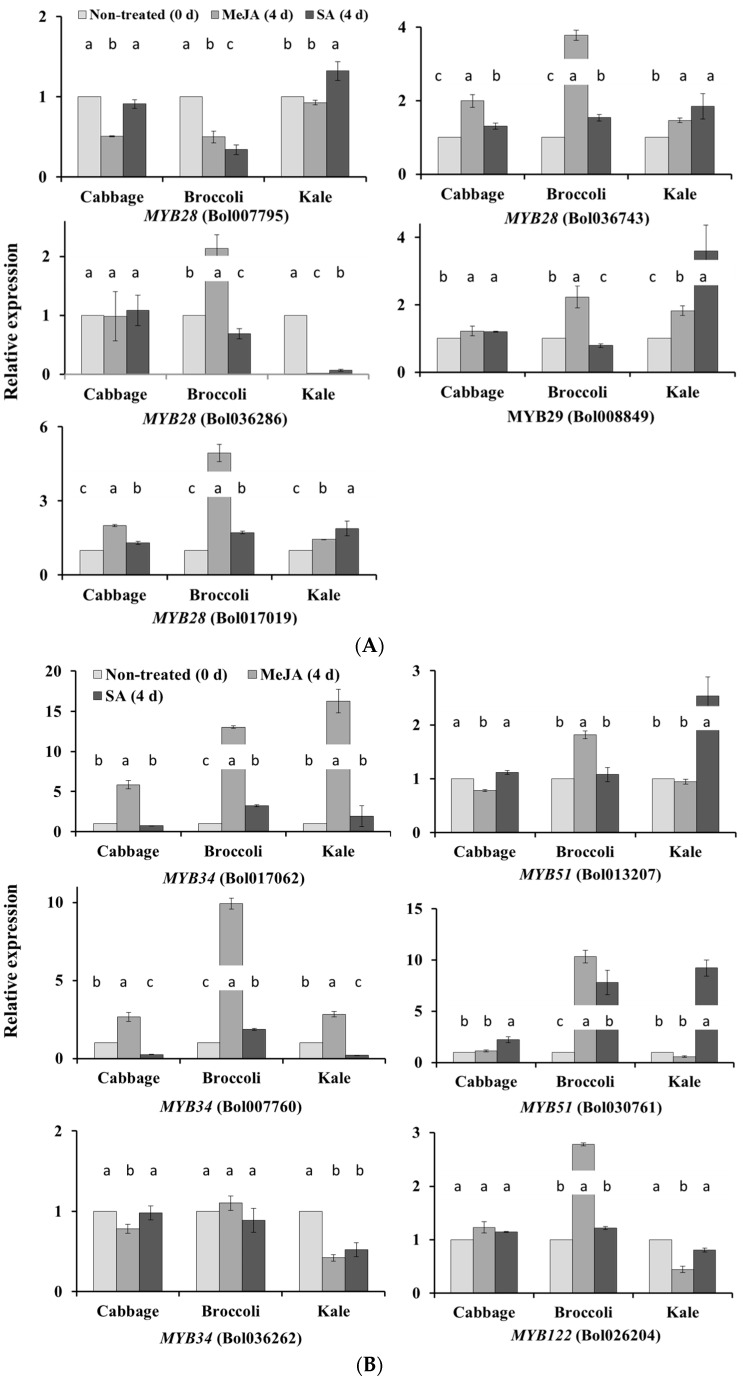
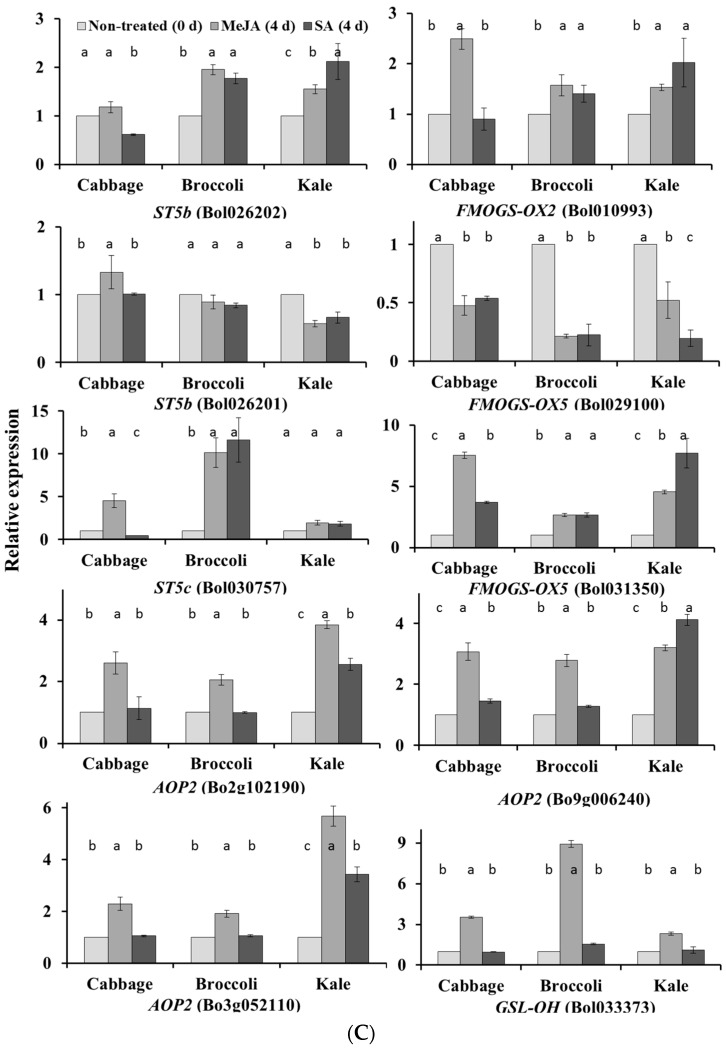
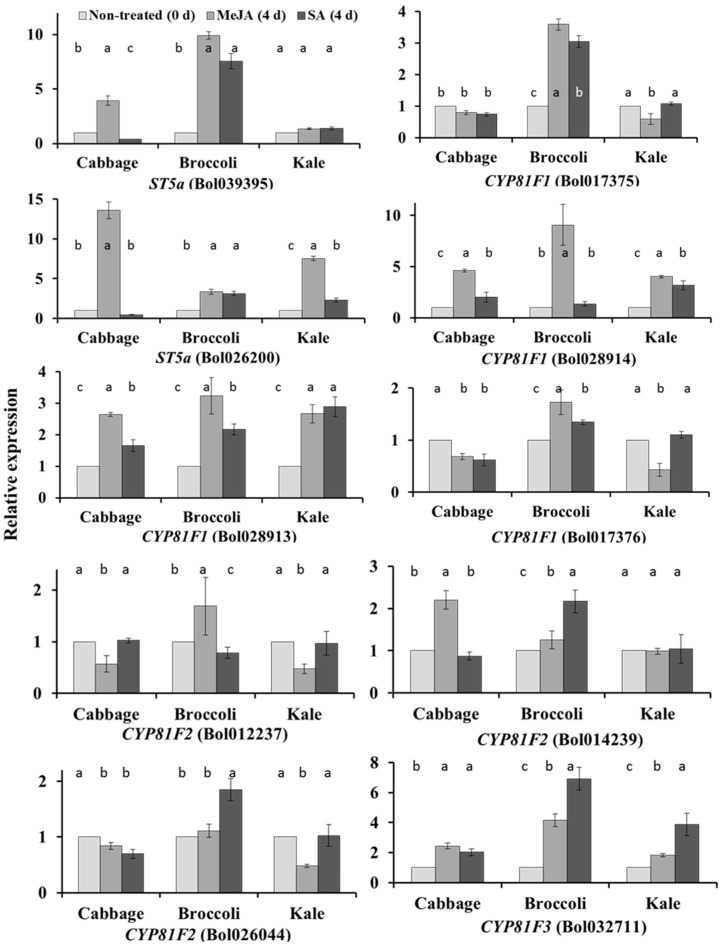
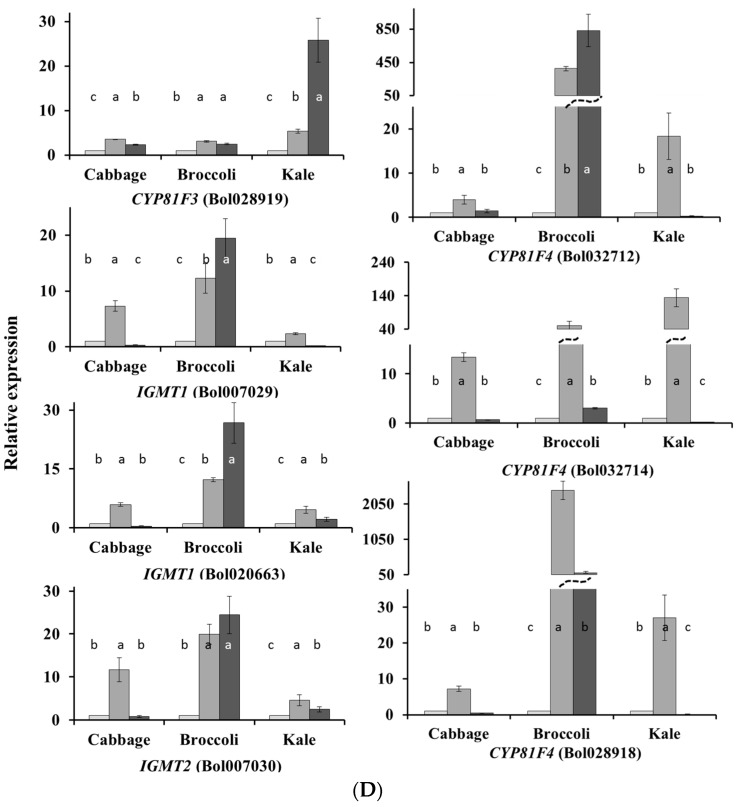
We carried out qPCR analysis of 38 glucosinolate biosynthesis-related genes from both aliphatic and indolic glucosinolate pathways (Figure 1). The results revealed that exogenous application of phytohormones affect the biosynthetic pathways in a variety-dependent manner. For example, the MYB28 gene Bol036743 was upregulated in broccoli leaves under MeJA treatment (p < 0.01, Figure 2A and Figure S1), whereas MYB28 genes Bol007795 in broccoli and Bol036286 in kale leaves were downregulated under both MeJA and SA treatment (p < 0.01 for both genes, Figure 2A and Figure S1). MYB51 genes Bol013207 and Bol030761 were upregulated in kale under SA treatment and MYB122 gene Bol026204 was upregulated in broccoli leaves under MeJA treatment (Figure 2B and Figure S1). Among the aliphatic glucosinolate biosynthesis-related genes, FMOGS-OX2 (Bol010993) and FMOGS-OX5 (Bol031350) were upregulated in cabbage leaves under MeJA treatment (Figure 2C and Figure S1). CYP81F1 gene Bol017375 was upregulated only in broccoli under both MeJA and SA treatment (Figure 2C and Figure S1). CYP81F4 genes Bol032712, Bol032714 and Bol028918 were downregulated under SA treatment in kale but upregulated in broccoli (Figure 2D and Figure S1). The more than 20-fold increase in expression of CYP81F3 gene Bol028919 in kale leaves under SA treatment was also remarkable (Figure S1). Broccoli was the most responsive to exogenous treatments in terms of the relative expression of both aliphatic and indolic transcription factor-related genes compared to cabbage and kale (Figure 2B and Figure S1).
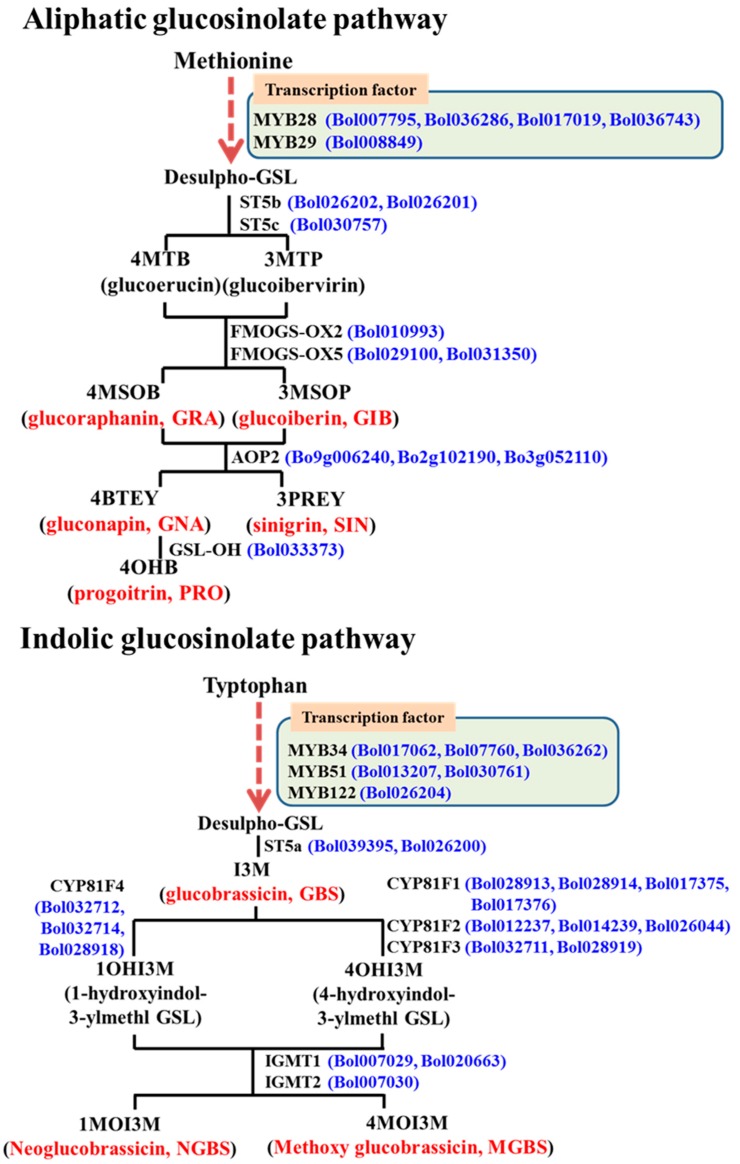
Figure 1.
The 38 genes (blue letters) analyzed in this work, with their postions in aliphatic and indolic glucosinolate (GSL, red letters) biosynthesis pathways. A total of 15 and 23 genes were selected from aliphatic and indolic glucosinolate biosynthesis pathways, respectively. Chemical structures of the glucosinolate compounds and intermediates are presented in Figure S2.
Figure 2.
Quantitative PCR analyses of the expression of glucosinolate biosynthesis genes under the exogenous application of MeJA and SA. Expression was normalized to that of actin and the values in control plants were set to 1. Each data point is the average for each of the three biological replicates with three technical replicates against each biological replicate. Vertical bars indicate standard deviation of the means. Different letters indicate statistically significant difference (p < 0.01). (A) Relative expression analyses of five aliphatic transcription factor-related genes; (B) Relative expression analyses of six indolic transcription factor-related genes; (C) Relative expression analyses of 10 aliphatic glucosinolate biosynthesis-related genes; (D) Relative expression analyses of 17 indolic glucosinolate biosynthesis-related genes.
2.2. Subspecies-Independent Effects of Exogenous MeJA or SA on Glucosinolate Biosynthesis Gene Expression
In addition to subspecies-specific gene expression, some genes were upregulated in all three subspecies under MeJA or SA treatment. For example, MYB34 genes Bol017062 and Bol007760 were notable, as they alone out of six indolic transcription factor-related genes, were upregulated in all three subspecies under MeJA treatment (p < 0.01 for both genes, Figure 2B and Figure S1). The level of increase ranged between 2- and 16-fold (Figure 2B). The FMOGS-OX5 gene Bol029100 was downregulated in all three subspecies. The majority of the indolic glucosinolate biosynthesis genes were induced by either MeJA or SA treatment (Figure 2D and Figure S1). The level of expression of CYP81F4 genes Bol032712, Bol032714 and Bol028918 were remarkably high (376-, 50- and 2434-fold upregulated in broccoli, respectively) in all three subspecies under MeJA treatment (Figure 2D and Figure S1).
2.3. Subspecies-Specific Glucosinolate Accumulation under MeJA or SA Treatment
HPLC analysis of leaves of the three subspecies detected eight different glucosinolates: five aliphatic glucosinolates, namely glucoiberin (GIB), progoitrin (PRO), glucoraphanin (GRA), sinigrin (SIN), and gluconapin (GNA), as well as three indolic glucosinolates: glucobrassicin (GBS), methoxyglucobrassicin (MGBS), and neoglucobrassicin (NGBS) (Table 1). Both MeJA and SA treatment increased the content of aliphatic GIB, PRO, SIN and indolic NGBS significantly in cabbage leaves (Table 1). SA treatment significantly increased the content of GNA in cabbage and that of GIB, GRA and MGBS in broccoli (Table 1). MeJA treatment significantly increased GBS in all three subspecies, SIN and NGBS in broccoli and NGBS in kale (Table 1). Notably, the content of all aliphatic glucosinolates in kale remained unaffected by exposure to exogenous MeJA or SA (Table 1). The content of GBS was 11-, 5- and 18-fold increased in cabbage, broccoli and kale leaves, respectively, under exogenous MeJA treatment compared to control plants (Table 1). The same treatment increased the content of NGBS by 4-, 158- and 19-fold in the cabbage, broccoli and kale leaves, respectively (Table 1). The SA treatment also increased the NGBS content by 4-fold in kale leaves (Table 1).
Table 1.
Contents of aliphatic and indolic glucosinolates (µmol·g−1·DW) produced under the exogenous application of MeJA and SA in three B. oleracea subspecies.
| Subspecies | Treatment | GIB | PRO | GRA | SIN | GNA | GBS | MGBS | NGBS |
|---|---|---|---|---|---|---|---|---|---|
| Cabbage | Control | 0.38 ± 0.17 b | 0.58 ± 0.66 b | 0.60 ± 0.29 a | 0.57 ± 0.25 b | 0.06 ± 0.07 b | 0.66 ± 0.20 b | bdl | 0.01 ± 0.004 b |
| MeJA | 1.63 ± 0.54 a | 2.33 ± 0.44 a | 1.27 ± 0.41 a | 1.58 ± 0.22 a | 0.19 ± 0.06 ab | 7.24 ± 3.16 a | bdl | 0.04 ± 0.006 a | |
| p (cabbage) | SA | 1.40 ± 0.41 a | 2.21 ± 0.18 a | 1.75 ± 0.77 a | 1.30 ± 0.13 a | 0.25 ± 0.02 a | 1.23 ± 1.23 b | bdl | 0.04 ± 0.007 a |
| Treatment | 0.02 | 0.006 | 0.09 | 0.003 | 0.016 | 0.008 | 0.002 | ||
| Broccoli | Control | 0.20 ± 0.02 b | 0.10 ± 0.027 a | 0.09 ± 0.009 b | 0.25 ± 0.062 ab | bdl | 0.29 ± 0.29 b | 0.01 ± 0.003 b | 0.04 ± 0.023 b |
| p (broccoli) | MeJA | 0.23 ± 0.015 ab | 0.10 ± 0.006 a | 0.08 ± 0.015 b | 0.40 ± 0.083 a | bdl | 1.46 ± 0.09 a | 0.11 ± 0.016 ab | 6.31 ± 2.07 a |
| SA | 0.28 ± 0.025 a | 0.13 ± 0.036 a | 0.14 ± 0.02 a | 0.14 ± 0.065 b | bdl | 0.20 ± 0.16 b | 0.19 ± 0.087 a | 0.11 ± 0.024 b | |
| Treatment | 0.012 | 0.37 | 0.007 | 0.011 | <0.001 | 0.016 | 0.001 | ||
| Kale | Control | 0.11 ± 0.025 a | 7.54 ± 5.02 a | 0.20 ± 0.087 a | 4.11 ± 2.25 a | 3.02 ± 1.63 a | 0.25 ± 0.102 b | 0.02 ± 0.002 a | 0.02 ± 0.01 b |
| MeJA | 0.41 ± 0.438 a | 6.20 ± 5.54 a | 1.67 ± 2.35 a | 6.37 ± 3.93 a | 2.23 ± 1.12 a | 4.52 ± 0.817 a | 0.08 ± 0.027 a | 0.38 ± 0.24 a | |
| SA | 0.10 ± 0.058 a | 5.48 ± 3.11 a | 0.63 ± 0.735 a | 2.89 ± 0.493 a | 2.54 ± 1.43 a | 0.45 ± 0.299 b | 0.08 ± 0.055 a | 0.07 ± 0.035 ab | |
| p (kale) | Treatment | 0.301 | 0.864 | 0.473 | 0.326 | 0.795 | <0.001 | 0.14 | 0.041 |
| p value | Subspecies | <0.001 | <0.001 | NS | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 |
Each data point is the average of three biological replicates ± standard deviation; p, probability values for statistical significance of treatment, subspecies and treatment × subspecies against each glucosinolate compound; NS, not significant; bdl, below detection limit. Different lower case letters indicate statistically significant differences (see Table S4 for the HPLC data).
2.4. Associations between Glucosinolate Contents and Gene Expression
2.4.1. MYB34, ST5a and CYP81 Gene Expression Is Related to GBS and NGBS Accumulation
Principal component analysis (PCA) indicated a probable association between indolic biosynthesis gene expression and glucosinolate contents (Table 2). PC2 coefficients explained 15% of the variation in the data, suggesting an association between higher GBS and NGBS contents and upregulation of ST5a (Bol026200), CYP81F1 (Bol028913), CYP81F1 (Bol028914), CYP81F4 (Bol032714), CYP81F4 (Bol028918) and downregulation of CYP81F2 (Bol026044) (Table 2). This PC accounted for significant treatment differences, where MeJA-treated samples had the largest and positive PC scores and both the control and SA-treated samples had negative PC scores (Table 2; see also Figure 2D). PC2 in the PCA between indolic glucosinolate content and expression level of indolic biosynthesis transcription factor genes also demonstrated an association between GBS content and MYB34 (Bol017062) expression with a significant treatment difference (Table S1). PC2 scores for that PC indicated that this variation is largely because of MeJA treatment (Table S1). PC1 and PC3, respectively explained 41.5% and 12.7% of the data variation between broccoli and the other two subspecies (Table 2). Exogenous MeJA treatment increased GBS most remarkably in cabbage and kale and NGBS in broccoli and that variation is largely explained by a negative relationship between GBS and NGBS content in PC3 (Table 1 and Table 2).
Table 2.
Principal component analysis for indolic glucosinolate contents and relative expression of genes in three subspecies of B. oleracea in control, MeJA-treated and SA-treated plants. PC, principal component; p, statistical significance.
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 |
| GBS | −0.025 | 0.326 | −0.416 | 0.081 | −0.250 |
| MGBS | 0.253 | 0.031 | 0.030 | −0.017 | 0.345 |
| NGBS | 0.150 | 0.344 | 0.344 | −0.107 | −0.044 |
| ST5a (Bol026200) | 0.078 | 0.310 | 0.446 | 0.164 | −0.174 |
| ST5a (Bol039395) | 0.317 | 0.098 | 0.050 | 0.012 | −0.250 |
| CYP81F1 (Bol028913) | 0.199 | 0.259 | −0.101 | 0.387 | 0.013 |
| CYP81F1 (Bol028914) | 0.053 | 0.483 | 0.163 | −0.125 | 0.171 |
| CYP81F1 (Bol017375) | 0.305 | −0.007 | 0.197 | 0.096 | −0.165 |
| CYP81F1 (Bol017376) | 0.245 | −0.071 | 0.260 | 0.272 | −0.219 |
| CYP81F2 (Bol012237) | 0.155 | −0.065 | 0.250 | 0.423 | −0.147 |
| CYP81F2 (Bol014239) | 0.210 | −0.113 | −0.321 | −0.052 | 0.041 |
| CYP81F2 (Bol026044) | 0.244 | −0.285 | −0.036 | −0.125 | 0.230 |
| CYP81F3 (Bol032711) | 0.284 | −0.090 | −0.103 | −0.045 | 0.369 |
| CYP81F3 (Bol028919) | 0.016 | 0.084 | 0.019 | 0.554 | 0.541 |
| CYP81F4 (Bol032712) | 0.296 | −0.082 | −0.018 | −0.058 | −0.153 |
| CYP81F4 (Bol032714) | 0.004 | 0.326 | −0.182 | −0.122 | 0.237 |
| CYP81F4 (Bol028918) | 0.111 | 0.349 | 0.314 | −0.344 | 0.066 |
| IGMT1 (Bol007029) | 0.318 | −0.070 | −0.138 | −0.143 | −0.115 |
| IGMT1 (Bol020663) | 0.304 | −0.100 | −0.152 | −0.200 | 0.116 |
| IGMT2 (Bol007030) | 0.331 | 0.003 | −0.105 | −0.057 | −0.059 |
| % variation explained | 41.5 | 15.0 | 12.7 | 7.6 | 6.3 |
| p (subspecies) | <0.01 | 0.49 | <0.01 | 0.05 | <0.01 |
| p (treatment) | <0.01 | <0.01 | <0.01 | 0.46 | <0.01 |
| p (subspecies × treatment) | <0.01 | 0.019 | <0.01 | 0.03 | 0.16 |
| Source of Variation | Mean PC Scores (±Sd) | ||||
| Subspecies | |||||
| Cabbage | −1.26 ± 0.41 | −0.06 ± 0.22 | −0.57 ± 0.17 | −0.01 ± 0.26 | −0.58 ± 0.2 |
| Broccoli | 2.35 ± 0.41 | −0.15 ± 0.22 | 0.77 ± 0.17 | −0.47 ± 0.26 | −0.01 ± 0.2 |
| Kale | −1.09 ± 0.41 | 0.21 ± 0.22 | −0.19 ± 0.17 | 0.48 ± 0.26 | 0.59 ± 0.2 |
| Treatment | |||||
| Control | −1.57 ± 0.41 | −1.11 ± 0.22 | 0.63 ± 0.17 | −0.22 ± 0.26 | −0.45 ± 0.2 |
| MeJA | 0.76 ± 0.41 | 1.96 ± 0.22 | −0.61 ± 0.17 | −0.02 ± 0.26 | −0.30 ± 0.2 |
| SA | 0.81 ± 0.41 | −0.84 ± 0.22 | −0.01 ± 0.17 | 0.24 ± 0.26 | 0.75 ± 0.2 |
| Subspecies × treatment | |||||
| Cabbage × Control | −1.61 ± 0.71 | −1.09 ± 0.38 | 0.58 ± 0.30 | −0.21 ± 0.45 | −0.52 ± 0.35 |
| Cabbage × MeJA | −0.31 ± 0.71 | 1.47 ± 0.38 | −2.69 ± 0.30 | 0.24 ± 0.45 | −1.03 ± 0.35 |
| Cabbage × SA | −1.85 ± 0.71 | −0.56 ± 0.38 | 0.38 ± 0.30 | −0.05 ± 0.45 | −0.20 ± 0.35 |
| Broccoli × Control | −1.56 ± 0.71 | −1.13 ± 0.38 | 0.65 ± 0.30 | −0.23 ± 0.45 | −0.43 ± 0.35 |
| Broccoli × MeJA | 3.81 ± 0.71 | 2.52 ± 0.38 | 2.47 ± 0.30 | −0.25 ± 0.45 | −0.36 ± 0.35 |
| Broccoli × SA | 4.82 ± 0.71 | −1.85 ± 0.38 | −0.81 ± 0.30 | −0.93 ± 0.45 | 0.76 ± 0.35 |
| Kale × Control | −1.54 ± 0.71 | −1.12 ± 0.38 | 0.64 ± 0.30 | −0.23 ± 0.45 | −0.40 ± 0.35 |
| Kale × MeJA | −1.21 ± 0.71 | 1.87 ± 0.38 | −1.63 ± 0.30 | −0.05 ± 0.45 | 0.48 ± 0.35 |
| Kale × SA | −0.52 ± 0.71 | −0.11 ± 0.38 | 0.39 ± 0.30 | 1.71 ± 0.45 | 1.69 ± 0.35 |
2.4.2. CYP81F3 Gene Expression Is Related to MGBS Accumulation under SA Treatment
Similar to NGBS, accumulation of MGBS was subspecies specific (Table 1). The cabbage leaves contained no detectable MGBS (Table 1). Exogenous SA increased the MGBS content in broccoli leaves compared to control plants (Table 1). PC5 indicated a positive relationship between MGBS content and upregulation of CYP81F3 genes in broccoli leaves under SA treatments (Table 2, Figure 2D).
2.4.3. Expression of ST5c and FMOGS-OX5 Genes in Cabbage Is Related to Accumulation of Aliphatic Glucosinolates
We observed enhanced biosynthesis of GIB and a few other aliphatic glucosinolates in cabbage under both MeJA and SA treatment (Table 1). The GIB and GRA contents had similar relationships to the expression of transcription factor-related and aliphatic biosynthesis genes (Tables S2 and S3). The higher content of GIB and other aliphatic glucosinolates was associated with variation in expression levels of ST5c (Bol030757) and FMOGS-OX5 genes in treated cabbage plants compared to controls (Figure 2C).
2.5. Natural Variation in Glucosinolate Contents and Gene Expression
2.5.1. Glucosinolate Accumulation and Gene Expression in Kale Leaves
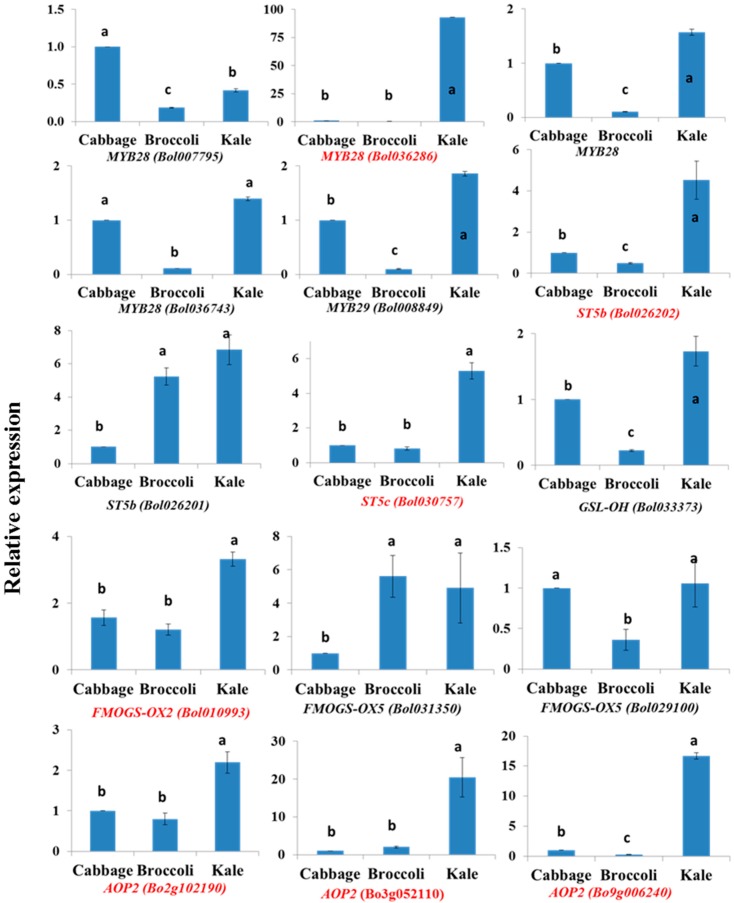
In control plants, kale leaves had remarkably higher contents of PRO, SIN and GNA compared to cabbage and broccoli, revealing significant subspecies variation (Figure S3 and Table 3). Kale leaves also contained significantly more MGBS whereas cabbage leaves had higher contents of GIB and GRA (Figure S3). The PRO content in kale leaves was 13- and 72-fold higher compared to cabbage and broccoli leaves, respectively (Table 3). GNA content was 53-fold higher in kale leaves compared to cabbage (Table 3), whereas broccoli leaves contained no detectable GNA (Table 1). Among the aliphatic transcription factor-related genes, MYB28 (Bol036286) showed 93- and 253-fold higher expression in kale leaves compared to cabbage and broccoli (Table 3, Figure 3). The expression of ST5b, ST5c and AOP2 genes was 2.2- to 20.5-fold and 1.3- to 66-fold greater in kale than in cabbage and broccoli, respectively (Table 3). In addition, FMOGS-OX5 (Bol031350) and GSL-OH (Bol033373) were expressed 4.9- and 7.8-fold more highly in kale leaves compared to cabbage and broccoli, respectively (Table 3, Figure 3).
Table 3.
Fold increase in relative expression of aliphatic glucosinolate transcription factor and biosynthesis genes and glucosinolate contents in kale leaves compared to cabbage and broccoli leaves for the control plants. Fold increase was calculated based on mean values obtained from three biological replicates.
| Genes | Kale: Cabbage | Kale: Broccoli |
|---|---|---|
| Aliphatic Transcription Factor-Related | ||
| MYB28 (Bol007795) | 0.4 | 2.3 |
| MYB28 (Bol036286) | 93 | 253 |
| MYB28 (Bol017019) | 1.6 | 15.6 |
| MYB28 (Bol036743) | 1.4 | 12.9 |
| MYB29 (Bol008849) | 1.9 | 19.1 |
| Aliphatic Biosynthesis-Related | ||
| FMOGS-OX2 (Bol010993) | 3.3 | 2.7 |
| FMOGS-OX5 (Bol029100) | 1.1 | 2.9 |
| FMOGS-OX5 (Bol031350) | 4.9 | 0.9 |
| GSL-OH (Bol033373) | 1.7 | 7.8 |
| ST5b (Bol026202) | 4.5 | 9.1 |
| ST5b (Bol026201) | 6.8 | 1.3 |
| ST5c (Bol030757) | 5.3 | 6.5 |
| AOP2 (Bo2g102190) | 2.2 | 2.7 |
| AOP2 (Bo3g052110) | 20.5 | 10 |
| AOP2 (Bo9g006240) | 16.7 | 66 |
| Glucosinolate Compounds | ||
| GIB | 0.3 | 0.6 |
| PRO | 13 | 72 |
| GRA | 0.3 | 2.2 |
| SIN | 7.2 | 17 |
| GNA | 53 | - |
Figure 3.
Subspecies variation in aliphatic glucosinolate biosynthesis-related gene expression in the control cabbage, broccoli and kale leaves. Vertical bars indicate standard deviation of means. Different letters indicate statistically significant differences between subspecies at 1% level of significance in one-way ANOVA. Genes with red letters were highly expressed only in kale.
2.5.2. Cabbage Leaves Have Higher GIB and GRA Contents
GIB and GRA were at the highest levels in cabbage leaves compared to broccoli and kale leaves (Table 3, Figure S3). In addition, MYB28 (Bol007795) had significantly higher expression in cabbage compared to broccoli and kale (Figure 3).
Among the indolic glucosinolates, GBS was present at the highest levels in cabbage leaves, at 2.24- and 2.6-fold higher than in broccoli and kale, but no MGBS was detected in cabbage (Figure S3 and Table 4). Kale leaves had 17-fold more MGBS compared to broccoli, whereas the broccoli leaves contained 4.3- and 2.5-fold more NGBS compared to cabbage and kale, respectively (Figure S3 and Table 4). MYB34 (Bol007760, Bol036262), MYB51 (Bol013207) and MYB122 (Bol026204) showed more than two-fold higher expression in cabbage compared to the other two subspecies (Table 4). CYP81F4 (Bol032712) and CYP81F4 (Bol032714) had 2.7- and 316-fold higher transcript accumulation in cabbage compared to broccoli (Table 4). By contrast, ST5a, CYP81F2, CYP81F3, IGMT1 and IGMT2 showed higher expression in broccoli leaves compared to cabbage and kale (Table 4). CYP81F1 showed much higher relative expression in kale leaves compared to broccoli and cabbage (Table 4).
Table 4.
Upregulation of indolic glucosinolate transcription factor and biosynthesis genes in one subspecies compared to other two subspecies for the control plants.
| Gene Name | Accession Number | GBS (Cabbage) | NGBS (Broccoli) | MGBS (Kale) | |||
|---|---|---|---|---|---|---|---|
| Broccoli | Kale | Cabbage | Kale | Cabbage | Broccoli | ||
| MYB34 | Bol007760 | 2.5 | 20.4 | ||||
| Bol017062 | 1.0 | 2.7 | |||||
| Bol036262 | 3.4 | 2.7 | |||||
| MYB51 | Bol013207 | 2.0 | 3.0 | ||||
| Bol030761 | 0.3 | 1.9 | |||||
| MYB122 | Bol026204 | 2.2 | 3.1 | ||||
| ST5a | Bol026200 | 3.5 | 6.5 | ||||
| Bol039395 | 1.9 | 1.3 | |||||
| CYP81F4 | Bol032712 | 2.7 | 1.0 | ||||
| Bol032714 | 316 | 0.6 | |||||
| Bol028918 | 1.44 | 1.4 | |||||
| CYP81F1 | Bol017375 | 4.3 | 24 | ||||
| Bol017376 | 2.8 | 35 | |||||
| Bol028913 | 2.2 | 1.4 | |||||
| Bol028914 | 3.2 | 3.8 | |||||
| CYP81F2 | Bol012237 | 1.2 | 1.95 | ||||
| Bol014239 | 2.5 | 2.1 | |||||
| Bol026044 | 1.5 | 1.9 | |||||
| CYP81F3 | Bol028919 | 2.6 | 1.4 | ||||
| Bol032711 | 1.5 | 1.9 | |||||
| IGMT1 | Bol007029 | 3.98 | 5.1 | ||||
| Bol020663 | 3.5 | 2.35 | |||||
| IGMT2 | Bol007030 | 1.8 | 3.97 | ||||
| Content increased (fold) | 2.24 | 2.6 | 4.3 | 2.54 | α (infinity) | 17 | |
3. Discussion
The present study investigated the relative expression of 38 genes related to glucosinolate biosynthesis and measured glucosinolate contents in cabbage, broccoli and kale leaves under the exogenous treatment of MeJA and SA. Both glucosinolate accumulation and expression level of biosynthetic genes revealed subspecies-specific variations in B. oleracea.
3.1. Subspecies-Specific Response to Exogenous MeJA and SA Application
The effects of exogenous MeJA and SA application have been widely studied in different species and subspecies of the Brassicales order. The previous studies indicated that exogenous MeJA application enhances the production of indolic and aromatic glucosinolates in leaves of Arabidopsis thaliana [43,44,45,46], B. napus [21], B. juncea [39] and B. rapa [22], as well as in different subspecies of B. oleracea, for example, in broccoli leaves [25,47,48], turnip [40], and in cauliflower curds [25]. The MeJA and SA phytohormones in B. oleracea subspecies each enhanced accumulation of particular glucosinolates in a variety-dependent manner. In this study, under the exogenous application of MeJA, the contents of leaf indolic GBS and NGBS were enhanced in all three subspecies (Figure 3). However, the fold-increase of a particular glucosinolate in the edible organs might vary between subspecies compared to leaf tissues. In previous studies, increase of GBS and NGBS was approximately 2-fold and 3-fold higher, respectively, in cauliflower curds [25] compared to kale leaf tissues [27]. A notably higher accumulation of the indolic MGBS in broccoli under SA application confirmed that MeJA or SA have subspecies-specific effects towards the accumulation of that particular glucosinolate (Table 1). Other than indolic GBS and NGBS, 250 μM MeJA treatment four days prior to harvest increased the content of aliphatic glucoraphanin (GRA) and aromatic gluconasturtiin (GST) in broccoli [28,49].
In contrast to some other previous studies, e.g., Ku et al. [28] and Liu et al. [49], this study recorded an enhanced accumulation of the aliphatic glucosinolates GIB, PRO, SIN in cabbage under both MeJA and SA treatment and in broccoli under SA treatment. Baenas et al. [50] reported significantly increased GIB content in broccoli sprouts under 250 μM MeJA treatments but no significant change under a similar dose of SA. In another subspecies of B. oleracea var. alboglabra Bailey (Chinese kale), MeJA treatment increased indolic GBS and NGBS but SA treatment increased aliphatic GNA and SIN [40], suggesting that the signaling response of a particular B. oleracea subspecies is also elicitor-specific.
3.2. Association between Glucosinolate Accumulation and Gene Expression under Exogenous MeJA and SA
In this study, 36 out of 38 examined genes, the exceptions being CYP81F1 (Bol017376) and CYP81F2 (Bol012237), showed significant treatment variation in terms of relative expression under the exogenous application of MeJA and SA (Figure S1). Generally, increased gene expression of only one or a few genes under a phytohormone treatment was associated with higher glucosinolate biosynthesis. Guo et al. [44] conducted expression profiling of selected transcription factor and glucosinolate biosynthesis genes, revealing that some MYB transcription factor and glucosinolate biosynthesis genes are highly induced under combined application of JA and glucose. Our current study suggested that both indolic biosynthesis transcription factor-related genes and indolic biosynthesis genes themselves are largely regulated by JA-mediated pathways, leading to higher indolic glucosinolate production under MeJA treatment. In Arabidopsis, MeJA was reported to induce some CYP genes that enhanced the indolic glucosinolate production by 3- to 4-fold [24]. However, in some recent studies the BjuCYP83A1 and CYP79F1 genes were found to regulate aliphatic glucosinolate biosynthesis in B. juncea [51,52].
One of the remarkable observations in this study is the striking increase, up to 2435-fold, of CYP81F4 genes under MeJA application in all three subspecies (Figure 2D; [24]). The corresponding increase in GBS in under the same treatment indicated that the upregulation of CYP81F4 might have an association with enhanced GBS biosynthesis under exogenous MeJA application. In B. juncea, Augustine and Bisht [39] reported a 9-fold increase in GBS at 48 h of MeJA treatment. When the Arabidopsis indolic glucosinolate biosynthesis pathway was engineered into Nicotiana benthamiana, CYP81F4 was found to be the key gene responsible for the production of 1-methoxy-3-indolylmethyl glucosinolate from the initial 3-indolylmethyl glucosinolate (Figure 2D; [53]). CYP81F4 has been suggested to have a significant role in the conversion of GBS to NGBS (Figure 2D, Table 2; [24,26]). In our study, the increase in GBS and NGBS in all three subspecies (Table 1) indicated that while CYP81F4 likely functions in conversion of GBS to NGBS, some other enzymes involved might be induced by the transcription factors that induce biosynthesis of GBS. PC2 in Table S1, for the two highest coefficients of GBS content and MYB34 expression indicated that higher GBS biosynthesis under MeJA treatment is possibly induced by MYB34 (Figure 2B). Analysis of knockdown mutants in the presence of JA and SA along with other signaling compounds indicated that MYB34 is a key regulator in JA signaling and MYB51 is the central regulator in SA signaling, whereas MYB122 plays a minor role in JA-induced glucosinolate biosynthesis [54]. However, in this study, high upregulation of MYB51 genes Bol013207 and Bol030761 and CYP81F3 (Bol028919) in kale leaves under SA treatment with a corresponding increase in NGBS content only in the kale indicated that the effect of SA is subspecies specific (Figure 2B,D, Table 1). CYP81F genes are involved in methoxylation that converts GBS to 4-methoxy-GBS, which has antifungal properties [55,56]. In addition, experimental evidence suggests that CYP81F3, similar to CYP81F2, catalyzes the conversion of I3M to 4OHI3M [53,57]. Such conversion might enhance NGBS biosynthesis in kale under SA treatment.
The observed increase in contents of GIB and some other aliphatic glucosinolate under MeJA treatment in cabbage was consistent with the approximately 67% increase in B. juncea seedlings after 24 h of MeJA treatment reported by Augustine and Bisht [39]. Under both MeJA and SA treatment, MYB29 trans-activates the biosynthesis gene ST5b and also induces expression of transcription factors that upregulate the gene encoding the second enzyme in the glucosinolate biosynthesis pathway [54]. MYB28 (Bol017019, Bol036743) and FMOGS-OX5 (Bol031350) showed the highest level of relative expression in cabbage compared to control (Figure 2A,C). A significantly higher level of upregulation of CYP81F4 gene in broccoli and kale compared to cabbage (Figure 2D) might be associated with higher accumulation of NGBS in those two subspecies.
3.3. Natural Variation in Glucosinolate Biosynthesis and Gene Expression at the Sub-Species Level
In a recent study with 25 kale varieties collected from three diverse geographical locations indicated that kale leaves are rich in aliphatic glucosinolates compared to reference broccoli but the wild landraces contained comparatively less glucosinolates compared to its commercial varieties [58]. Similarly, we observed a higher content of aliphatic glucosinolates PRO, SIN and GNA in the leaves of the control kale plants, indicating a natural subspecies-related variation in glucosinolate contents (Table 1). However, the corresponding greater relative expression of MYB28 (Bol036286), ST5b, ST5c and AOP2 genes indicated that the regulation of the aliphatic glucosinolate biosynthesis pathway varies between B. oleracea subspecies (Figure 3). In a recent study, expression of the kale BoMYB29 protein in Arabidopsis enhanced expression of aliphatic glucosinolate biosynthesis genes and simultaneously enhanced content of GRA, indicating that kale BoMYB29 gene is a key regulator of methylsulphinyl glucosinolate biosynthesis [59]. Yi et al. [42] reported variation in glucosinolate content in the edible organs of four different subspecies of B. oleracea. Our results show that similar conclusions can be drawn for indolic glucosinolate biosynthesis based on contents and simultaneous expression of related genes (Table 2 and Table 4). Thus, a comparatively higher accumulation of GBS in cabbage might be associated with higher level of expression of MYB34, MYB51 (Bol013207) and MYB122 genes; a comparatively higher accumulation of MGBS in kale might be associated with relatively higher upregulation of ST5a (Bol026202), IGMT1 and IGMT2 genes; and a comparatively higher accumulation of NGBS in broccoli might be associated with CYP81F1 genes (Figure 2, Table 2).
Other notable observations in this study were: (i) the broccoli leaves unusually measured higher amount of PRO and SIN (Table 1) which were often absent or trace in amount in previous studies [60,61] that could explain both genotypic and environmental variation; (ii) absence of GNA in broccoli (Table 1) might be associated with deletion of the enzyme sitting on the pathway branching point; (iii) in cabbage, missing MGBS might be a consequence of deletion of one of IGMT1 or IGMT2 enzymes. Three AOP2 genes in this study were relatively less expressed in broccoli compared to cabbage and kale but among them Bo9g006240 was the least expressed gene (Figure 3) that might have been regulated biosynthesis of GNA in broccoli. The evolutionary or ecological reasons for the sub-species specific variation in glucosinolate content of B. oleracea species and species-specific responses to hormone treatments is a subject of further investigation whereas such evolutionary variation to date has been discussed at the species level in Brassicaceae [42]. The genes identified herein to be associated with glucosinolate accumulation are strong candidates to be exploited for selective accumulation of desired glucosinolates in B. oleracea subspecies.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
One variety from each of the three different subspecies of B. oleracea L. was selected for this study (Table S5). Seeds were obtained from Asia Seed Co., Ltd. (Seoul, Korea). The seeds were sown and seedlings were raised in garden soil mixture in a plant culture room at 25 ± 1 °C, 60% relative humidity and 80–120 μmol·m−2·s−1 light intensity. Seedlings were transferred at four weeks of age to a glasshouse. The plants were allowed to grow for another three months before imposing MeJA or SA treatments.
4.2. MeJA and SA Treatment
Two different treatments were applied on four-month-old, glasshouse-grown plants to study variation in the expression level of glucosinolate biosynthesis genes under exogenous treatment of MeJA and SA compared to “control” plants. The two treatments were 250 μM MeJA [25,28] and 800 μM SA [47], both of which were applied as solutions prepared in 0.1% Triton X-100. Control plants were sprayed with only 0.1% Triton X-100. Three plants of each variety were sprayed with 500 mL solution for each treatment. The plants were allowed to grow for four days before sample collection. Leaf samples were chosen for subspecies comparison as the level of glucosinolate-related gene expression was reported to be organ-specific [62,63]. The relative expression of treated samples for each subspecies was estimated by comparison to control plants. The content of glucosinolates was determined for both treated and control samples. Three biological replicates were destructively harvested each plant. Samples were frozen in liquid nitrogen and preserved at −80 °C until use for further analysis.
4.3. Primer Design for Glucosinolate Biosynthesis Genes
A total of 38 genes related to glucosinolate biosynthesis were selected for relative expression analysis under three treatments (Table 1). Among them, five and six genes respectively were aliphatic and indolic transcription factor-related, 10 and 17 were aliphatic and indolic glucosinolate biosynthesis-related genes. Primers were previously designed and their efficiency was tested by Robin et al. [64] (Table S6).
4.4. cDNA Synthesis and Real-Time Quantitative PCR (qPCR) Analysis
An RNeasy mini kit (Catalogue No. 74106, Qiagen, Valencia, CA, USA) was used to extract the total RNA of the leaf samples. cDNA was synthesized from the total RNA of each sample using a PrimeScript-based kit (Takara Bio, Inc., Shiga, Japan). iTaq™ SYBR® Green Super-mix was used with ROX (Bio-Rad, Hercules, CA, USA) for real-time PCR. The reaction volume was 20 µL where 1 µL cDNA of 60 ng·µL−1 of each sample was used. The targeted DNA segment was amplified by denaturation at 95 °C for 10 min, followed by 40 cycles of amplification with denaturation at 94 °C for 20 s, annealing at 58 °C for 20 s and a final incubation and signal acquisition at 72 °C for 25 s (see Figure S4 for melting curves). The fluorescence was recorded for each sample at the end of each of the 40 cycles. LightCycler96 software was used for quantification (Cq) analysis (Roche, Mannheim, Germany). The relative expression level was calculated by the comparative 2−ΔΔCt method [65]. The actin gene, GenBank accession No. JQ435879 that is expressed in all subspecies (Forward sequence: GTCGCTATTCAAGCTGTTCTCT; Reverse sequence: GAGAGCTTCTCCTTGATGTCTC) was the housekeeping gene [66]. A heat map was generated using Gene Cluster 3.0 [67] and Java TreeView [68] software using the log-transformed data of the relative expression level of glucosinolate biosynthesis-related genes under MeJA and SA treatment.
4.5. Desulfo-Glucosinolate Extraction for HPLC Analysis
The modified HPLC protocol, previously used by Yi et al. [42] and Robin et al. [64], was used to extract desulfo-glucosinolates from the treated and control leaf samples. Methanol treated frozen leaf tissue of about 10 g was powdered. The processed samples were initially incubated for 10 min at 70 °C. The samples were then kept at room temperature for 1 h. To eliminate structural components of the tissues and proteins the samples were then centrifuged at 10,000× g for 8 min at 4 °C. An anion-exchange chromatography was conducted with the collected supernatant. The process of centrifugation and anion-exchange chromatography was repeated twice and the supernatants from three steps were composited in a 5-mL tube. The pooled supernatants were the crude glucosinolates. To conduct a desulfation process 0.5 mL 50 mM barium acetate and 0.5 mL 50 mM lead acetate was mixed with the crude glucosinolates. In this step, the solution was centrifuged at 2000× g for 10 min. The samples were then loaded into a 0.5 M sodium acetate pre-equilibrated DEAE-Sephadex column. Prior to desulfation, the crude glucosinolate samples were rinsed with distilled water. Then, 250 μL aryl sulfatase was added to the column to commence desulfation process. The process was continued for 16 h before starting elution of desulfated glucosinolates with 1 mL distilled water. The eluted desulfo-glucosinolates was further purified by configuring at a high speed of 20,000× g for 4 min at 4 °C and filtering through a PTFE filter (13 mm, 0.2 μm, Advantec, Pleasanton, CA, USA). The desulfo-glucosinolate samples were then analyzed in a Waters 2695 HPLC system (Waters, Milford, MA, USA) equipped with a C18 column (Zorbax Eclipse XBD C18, 4.6 mm × 150 mm, Agilent Technologies, Palo Alto, CA, USA). Water and acetonitrile were used as mobile phase solvents during HPLC analysis of desulfated and purified glucosinolates. The purified desulfo-glucosinolates were detected using PDA 996 UV-visible detector (Waters) at a wavelength of 229 nm. A standard curve prepared for commercial sinigrin was used to quantify the detected glucosinolates. Mass spectrometry analysis (HPLC/MS, Agilent 1200 series, Agilent Technologies) facilitated the identification of individual glucosinolate molecules (HPLC/MS, Agilent 1200 series, Agilent Technologies) (Table S7).
4.6. Statistical Analysis
The relative expression level of each gene and contents of each glucosinolate in each subspecies under three treatments was analyzed via the generalized linear model (GLM) using MINITAB 15 statistical software (Minitab Inc., State College, PA, USA). Subspecies-to-subspecies variation was analyzed similarly. A principal component analysis was conducted taking either aliphatic or indolic glucosinolate content and the corresponding relative expression level of biosynthesis genes as a set of variables. PC scores obtained under three treatment and three subspecies combinations were also analyzed using a two-way ANOVA following the GLM procedure using MINITAB 15 statistical software. For separating means of three treatments under each subspecies a Tukey’s pairwise comparison test was conducted.
5. Conclusions
This study investigated the expression analysis of 38 glucosinolate biosynthesis-related genes and also estimated contents of glucosinolates under the exogenous treatment of MeJA and SA in B. oleracea subspecies. A subspecies-specific response in glucosinolate accumulation and gene expression was observed under MeJA or SA treatment. The increased accumulation of a particular glucosinolate was generally associated with upregulation of specific genes under MeJA or SA treatment. The deduced association between particular glucosinolate biosynthesis and gene expression improves our understanding of underlying genetics of glucosinolate accumulation under the exposure of biotic elicitors. The non-treated kale leaves measured strikingly higher content of PRO, SIN and GNA that was associated with higher expression level of aliphatic biosynthetic genes including AOP2, indicating natural variation in their glucosinolate biosynthesis.
Acknowledgments
We thank the Asia Seed Co., Ltd., Republic of Korea for providing B. oleracea seeds. This study was supported by the Golden Seed Project (Center for Horticultural Seed Development, No. 213003-04-4-SB110) of the Ministry of Agriculture, Food and Rural affairs in the Republic of Korea (MAFRA).
Abbreviations
- GIB
glucoiberin
- PRO
progoitrin
- GRA
glucoraphanin
- SIN
sinigrin
- GNA
gluconapin
- GBS
glucobrassicin
- MGBS
methoxyglucobrassicin
- NGBS
neoglucobrassicin
- 3MTP
3-methylthiopropyl glucosinolate (glucoiberverin)
- 4MTB
4-methylthiobutyl glucosinolate (glucoerucin)
- 3MSOP
3-(methylsulfinyl)propyl glucosinolate (glucoiberin)
- 4MSOB
4-(methylsulfinyl)butyl GSL (glucoraphanin)
- Sinigrin
allyl glucosinolate
- 3BTEY
3-butenyl glucosinolate (Gluconapin)
- 4OHB
4-hydroxybutyl glucosinolate (progoitrin)
- I3M
3-indolylmethyl glucosinolate (glucobrassicin)
- 4OHI3M
4-hydroxy-3-indolylmethyl glucosinolate (4-hydroxyglucobrassicin)
- 1MOI3M
1-methoxy-3-indolylmethyl glucosinolate (neoglucobrassicin)
- 4MOI3M
4-methoxy-3-indolylmethyl glucosinolate (4-methoxyglucobrassicin)
- IGM
indole GSL modifier
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/10/1417/s1.
Author Contributions
I.-S.N., J.-I.P. and B.H.H. conceived and designed the study. K.Y. and G.-E.Y. managed the experimental plants, collected samples, prepared cDNA, qPCR analysis and conducted HPLC analysis. A.H.K.R. provided technical advice to G.-E.Y. and K.Y. conducted in silico analysis and wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Seeds of the genotypes from three subspecies are available from the authors.
References
- 1.Rodman J.E., Karol K.G., Price R.A., Sytsma K.J. Molecules, morphology, and Dahlgren’s expanded order Capparales. Syst. Bot. 1993;21:289–307. doi: 10.2307/2419660. [DOI] [Google Scholar]
- 2.Schonhof I., Krumbein A., Brückner B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Mol. Nutr. Food Res. 2004;48:25–33. doi: 10.1002/food.200300329. [DOI] [PubMed] [Google Scholar]
- 3.Padilla G., Cartea M.E., Velasco P., de Haro A., Ordás A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry. 2007;68:536–545. doi: 10.1016/j.phytochem.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Rask L., Andréasson E., Ekbom B., Eriksson S., Pontoppidan B., Meijer J. Plant Molecular Evolution. Springer; Dordrecht, The Netherlands: 2000. Myrosinase: Gene Family Evolution and Herbivore Defense in Brassicaceae; pp. 93–113. [PubMed] [Google Scholar]
- 5.Wittstock U., Halkier B.A. Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of l-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J. Biol. Chem. 2000;275:14659–14666. doi: 10.1074/jbc.275.19.14659. [DOI] [PubMed] [Google Scholar]
- 6.Bones A.M., Rossiter J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006;67:1053–1067. doi: 10.1016/j.phytochem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Hayes J.D., Kelleher M.O., Eggleston I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008;47:73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Kensler T.W., Cho C.G., Posner G.H., Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keck A.S., Finley J.W. Cruciferous vegetables: Cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr. Cancer Ther. 2004;3:5–12. doi: 10.1177/1534735403261831. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.H., Park S., Lim Y.P., Kim S.J., Park J.T., An G. Metabolite profiles of glucosinolates in cabbage varieties (Brassica oleracea var. capitata) by season, color, and tissue position. Hortic. Environ. Biotechnol. 2014;55:237–247. [Google Scholar]
- 11.Hecht S.S. Inhibition of carcinogenesis by isothiocyanates 1*. Drug Metab. Rev. 2000;32:395–411. doi: 10.1081/DMR-100102342. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima M., Yoshida R., Shimada N., Yamazaki H., Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab. Dispos. 2001;29:1110–1113. [PubMed] [Google Scholar]
- 13.Kang Y.-H., Pezzuto J.M. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Meth. Enzymol. 2004;382:380–414. doi: 10.1016/S0076-6879(04)82021-4. [DOI] [PubMed] [Google Scholar]
- 14.Rose P., Faulkner K., Williamson G., Mithen R. 7-Methylsulfinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis. 2000;21:1983–1988. doi: 10.1093/carcin/21.11.1983. [DOI] [PubMed] [Google Scholar]
- 15.Matusheski N.V., Swarup R., Juvik J.A., Mithen R., Bennett M., Jeffery E.H. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006;54:2069–2076. doi: 10.1021/jf0525277. [DOI] [PubMed] [Google Scholar]
- 16.Lin W., Wu R.T., Wu T., Khor T.-O., Wang H., Kong A.-N. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008;76:967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnesen C., Eggleston I.M., Hayes J.D. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 18.Mithen R. Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica. 1992;63:71–83. doi: 10.1007/BF00023913. [DOI] [Google Scholar]
- 19.Cabello-Hurtado F., Gicquel M., Esnault M.A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. 2012;132:1003–1009. doi: 10.1016/j.foodchem.2011.11.086. [DOI] [Google Scholar]
- 20.Mawson R., Heaney R.K., Zdunczyk Z., Kozlowska H. Rapeseed meal-glucosinolates and their antinutritional effects Part 4. Goitrogenicity and internal organs abnormalities in animals. Mol. Nutr. Food Res. 1994;38:178–191. doi: 10.1002/food.19940380210. [DOI] [PubMed] [Google Scholar]
- 21.Kiddle G.A., Doughty K.J., Wallsgrove R.M. Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J. Exp. Bot. 1994;45:1343–1346. doi: 10.1093/jxb/45.9.1343. [DOI] [Google Scholar]
- 22.Doughty K.J., Kiddle G.A., Pye B.J., Wallsgrove R.M., Pickett J.A. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry. 1995;38:347–350. doi: 10.1016/0031-9422(94)00653-B. [DOI] [Google Scholar]
- 23.Bartlet E., Kiddle G., Williams I., Wallsgrove R. Proceedings of the 10th International Symposium on Insect-Plant Relationships. Springer; Dordrecht, The Netherlands: 1999. Wound-Induced Increases in the Glucosinolate Content of Oilseed Rape and Their Effect on Subsequent Herbivory by a Crucifer Specialist; pp. 163–167. [Google Scholar]
- 24.Mikkelsen M.D., Petersen B.L., Glawischnig E., Jensen A.B., Andreasson E., Halkier B.A. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol. 2003;131:298–308. doi: 10.1104/pp.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku K.M., Choi J.-H., Kushad M.M., Jeffery E.H., Juvik J.A. Pre-harvest methyl jasmonate treatment enhances cauliflower chemoprotective attributes without a loss in postharvest quality. Plant Foods Hum. Nutr. 2013;68:113–117. doi: 10.1007/s11130-013-0356-y. [DOI] [PubMed] [Google Scholar]
- 26.Ku K.M., Choi J.H., Kim H.S., Kushad M.M., Jeffery E.H. Methyl Jasmonate and 1-Methylcyclopropene Treatment Effects on Quinone Reductase Inducing Activity and Post-Harvest Quality of Broccoli. PLoS ONE. 2013;8:e77127. doi: 10.1371/journal.pone.0077127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku K.M., Jeffery E.H., Juvik J.A. Exogenous methyl jasmonate treatment increases glucosinolate biosynthesis and quinone reductase activity in kale leaf tissue. PLoS ONE. 2014;9:e103407. doi: 10.1371/journal.pone.0103407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ku K.M., Jeffery E.H., Juvik J.A. Optimization of methyl jasmonate application to broccoli florets to enhance health-promoting phytochemical content. J. Sci. Food Agric. 2014;94:2090–2096. doi: 10.1002/jsfa.6529. [DOI] [PubMed] [Google Scholar]
- 29.Zang Y.X., Ge J.L., Huang L.H., Gao F., Lv X.S., Zheng W.W., Hong S.B., Zhu Z.J. Leaf and root glucosinolates profiles of Chinese cabbage (Brassica rapa ssp. pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. J. Zhejiang Univ. Sci. B. 2015;16:696–708. doi: 10.1631/jzus.B1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zang Y., Zhang H., Huang L., Wang F., Gao F., Lv X., Zhu Z. Glucosinolate enhancement in leaves and roots of pak choi (Brassica rapa ssp. chinensis) by methyl jasmonate. Hortic. Environ. Biotechnol. 2015;56:830–840. doi: 10.1007/s13580-015-0079-0. [DOI] [Google Scholar]
- 31.Bruinsma M., van Dam N.M., Van Loon J.J., Dicke M. Jasmonic acid-induced changes in Brassica oleracea affect oviposition preference of two specialist herbivores. J. Chem. Ecol. 2007;33:655–668. doi: 10.1007/s10886-006-9245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demole E., Lederer E., Mercier D.E. Isolement et determination de la structure du jasmonate de methyle, constituant odorant characteristique de l’essence de jasmin. Helv. Chim. Acta. 1962;45:675–685. doi: 10.1002/hlca.19620450233. [DOI] [Google Scholar]
- 33.Scognamiglio J., Jones L., Letizia C.S., Api A.M. Fragrance material review on methyl jasmonate. Food Chem. Toxicol. 2012;50:S572–S576. doi: 10.1016/j.fct.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 34.Wiesner M., Hanschen F.S., Schreiner M., Glatt H., Zrenner R. Induced production of 1-methoxy-indol-3-ylmethyl glucosinolate by jasmonic acid and methyl jasmonate in sprouts and leaves of Pak Choi (Brassica rapa ssp. chinensis) Int. J. Mol. Sci. 2013;14:14996–15016. doi: 10.3390/ijms140714996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz V.A., Justen V.L., Bode A.M., Schuster T., Wang M. Glucosinolate enhancement in cabbage induced by jasmonic acid application. HortScience. 2010;45:1188–1191. [Google Scholar]
- 36.Loivamäki M., Holopainen J.K., Nerg A.M. Chemical changes induced by methyl jasmonate in oilseed rape grown in the laboratory and in the field. J. Agric. Food Chem. 2004;52:7607–7613. doi: 10.1021/jf049027i. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Balibrea S., Moreno D.A., García-Viguera C. Genotypic effects on the phytochemical quality of seeds and sprouts from commercial broccoli cultivars. Food Chem. 2011;125:348–354. doi: 10.1016/j.foodchem.2010.09.004. [DOI] [Google Scholar]
- 38.Sun B., Yan H., Liu N., Wei J., Wang Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012;131:519–526. doi: 10.1016/j.foodchem.2011.09.016. [DOI] [Google Scholar]
- 39.Augustine R., Bisht N.C. Biotic elicitors and mechanical damage modulate glucosinolate accumulation by co-ordinated interplay of glucosinolate biosynthesis regulators in polyploid Brassica juncea. Phytochemistry. 2015;117:43–50. doi: 10.1016/j.phytochem.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Smetanska I., Krumbein A., Schreiner M., Knorr M. Influence of salicylic acid and methyl jasmonate on glucosinolate levels in turnip. J. Hortic. Sci. Biotechnol. 2007;82:690–694. doi: 10.1080/14620316.2007.11512292. [DOI] [Google Scholar]
- 41.Celenza J.L., Quiel J.A., Smolen G.A., Merrikh H., Silvestro A.R., Normanly J., Bender J. The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 2005;137:253–262. doi: 10.1104/pp.104.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S., Liu Y., Yang X., Tong C., Edwards D., Parkin I.A. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014;5 doi: 10.1038/ncomms4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi G.E., Robin A.H.K., Yang K., Park J.I., Kang J.G., Yang T.J. Identification and expression analysis of glucosinolate biosynthetic genes and estimation of glucosinolate contents in edible organs of Brassica oleracea subspecies. Molecules. 2015;20:13089–13111. doi: 10.3390/molecules200713089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo R., Shen W., Qian H., Zhang M., Liu L., Wang Q. Jasmonic acid and glucose synergistically modulate the accumulation of glucosinolates in Arabidopsis thaliana. J. Exp. Bot. 2013;64:5707–5719. doi: 10.1093/jxb/ert348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jost R., Altschmied L., Bloem E., Bogs J., Gershenzon J., Hähnel U. Expression profiling of metabolic genes in response to methyl jasmonate reveals regulation of genes of primary and secondary sulfur-related pathways in Arabidopsis thaliana. Photosynth. Res. 2005;86:491–508. doi: 10.1007/s11120-005-7386-8. [DOI] [PubMed] [Google Scholar]
- 46.Mewis I., Appel H.M., Hom A., Raina R., Schultz J.C. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005;138:1149–1162. doi: 10.1104/pp.104.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H.S., Juvik J.A. Effect of selenium fertilization and methyl jasmonate treatment on glucosinolate accumulation in broccoli florets. J. Am. Soc. Hortic. Sci. 2011;136:239–246. [Google Scholar]
- 48.Schreiner M., Krumbein A., Knorr D., Smetanska I. Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 2011;59:1400–1405. doi: 10.1021/jf103585s. [DOI] [PubMed] [Google Scholar]
- 49.Liu A.G., Juvik J.A., Jeffery E.H., Berman-Booty L.D., Clinton S.K., Erdman J.W.J. Enhancement of broccoli indole glucosinolates by methyl jasmonate treatment and effects on prostate carcinogenesis. J. Med. Food. 2014;17:1177–1182. doi: 10.1089/jmf.2013.0145. [DOI] [PubMed] [Google Scholar]
- 50.Baenas N., Villaño D., García-Viguera C., Moreno D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016;204:314–319. doi: 10.1016/j.foodchem.2016.02.144. [DOI] [PubMed] [Google Scholar]
- 51.Meenu Augustine R., Majee M., Pradhan A.K., Bisht N.C. Genomic origin, expression differentiation and regulation of multiple genes encoding CYP83A1, a key enzyme for core glucosinolate biosynthesis, from the allotetraploid Brassica juncea. Planta. 2015;241:651–665. doi: 10.1007/s00425-014-2205-0. [DOI] [PubMed] [Google Scholar]
- 52.Sharma M., Mukhopadhyay A., Gupta V., Pental D., Pradhan A.K. BjuBCYP79F1 Regulates Synthesis of Propyl Fraction of Aliphatic Glucosinolates in Oilseed Mustard Brassica juncea: Functional Validation through Genetic and Transgenic Approaches. PLoS ONE. 2016;11:e0150060. doi: 10.1371/journal.pone.0150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfalz M., Mikkelsen M.D., Bednarek P., Olsen C.E., Halkier B.A., Kroymann J. Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell. 2011;23:716–729. doi: 10.1105/tpc.110.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frerigmann H., Gigolashvili T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant. 2014;7:814–828. doi: 10.1093/mp/ssu004. [DOI] [PubMed] [Google Scholar]
- 55.Bednarek P., Pislewska-Bednarek M., Svatos A., Schneider B., Doubsky J. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- 56.Kim J.H., Lee B.W., Schroeder F.C., Jander G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid) Plant J. 2008;54:1015–1026. doi: 10.1111/j.1365-313X.2008.03476.x. [DOI] [PubMed] [Google Scholar]
- 57.Pfalz M., Vogel H., Kroymann J. The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell. 2009;21:985–999. doi: 10.1105/tpc.108.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hahn C., Müller A., Kuhnert N., Albach D. Diversity of Kale (Brassica oleracea var. sabellica): Glucosinolate Content and Phylogenetic Relationships. J. Agric. Food Chem. 2016;64:3215–3225. doi: 10.1021/acs.jafc.6b01000. [DOI] [PubMed] [Google Scholar]
- 59.Araki R., Hasumi A., Nishizawa O.I., Sasaki K., Kuwahara A., Sawada Y., Saito K. Novel bioresources for studies of Brassica oleracea: Identification of a kale MYB transcription factor responsible for glucosinolate production. Plant Biotechnol. J. 2013;11:1017–1027. doi: 10.1111/pbi.12095. [DOI] [PubMed] [Google Scholar]
- 60.Meyer M., Adam S.T. Comparison of glucosinolate levels in commercial broccoli and red cabbage from conventional and ecological farming. Eur. Food Res. Technol. 2008;226:1429–1437. doi: 10.1007/s00217-007-0674-0. [DOI] [Google Scholar]
- 61.Tian Q., Rosselot R.A., Schwartz S.J. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography—Electrospray ionization—Tandem mass spectrometry. Anal. Biochem. 2005;343:93–99. doi: 10.1016/j.ab.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 62.Gigolashvili T., Yatusevich R., Rollwitz I., Humphry M., Gershenzon J., Flügge U.I. The plastidic bile acid transporter 5 is required for the biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana. Plant Cell. 2009;21:1813–1829. doi: 10.1105/tpc.109.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y.B., Li X., Kim S.J., Kim H.H., Lee J., Kim H. MYB transcription factors regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp. pekinensis) Molecules. 2013;18:8682–8695. doi: 10.3390/molecules18078682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robin A.H.K., Yi G.E., Laila R., Yang K., Park J.I., Kim H.R., Nou I.S. Expression Profiling of Glucosinolate Biosynthetic Genes in Brassica oleracea L. var. capitata Inbred Lines Reveals Their Association with Glucosinolate Content. Molecules. 2016;21:787. doi: 10.3390/molecules21060787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 66.Nawaz I., Iqbal M., Hakvoort H.W., Bliek M., de Boer B., Schat H. Expression levels and promoter activities of candidate salt tolerance genes in halophytic and glycophytic Brassicaceae. Environ. Exp. Bot. 2014;99:59–66. doi: 10.1016/j.envexpbot.2013.10.006. [DOI] [Google Scholar]
- 67.De Hoon M., Imoto S., Miyano S. Cluster 3.0. University of Tokyo, Human Genome Center; Tokyo, Japan: 2002. [Google Scholar]
- 68.Saldanha A.J. Java Treeview—Extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.