Abstract
A new abeo-abietane diterpenoid, 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13-abietatetraen-3,7-dione (8), was isolated from the hydroalcoholic extract of the herb of Clerodendrum kiangsiense along with seven known diterpenoids (1–7). Their structures were identified on the basis of spectroscopic analyses including two-dimensional NMR and comparison with literature data. All of these compounds were evaluated for their cytotoxic activities against the growth of human cancer cells lines HL-60, SMMC-7721, A-549 and MCF-7 by the MTT assay. The results showed that cryptojaponol (4), fortunin E (6) and 8 exhibited significant cytotoxicity against four human cancer cell lines.
Keywords: Clerodendrum kiangsiense, abeo-abietane diterpenoid, cytotoxicity
1. Introduction
Abietane diterpenoids are a class of tricyclic diterpenoids, and have been isolated from plant species from the taxonomic families Verbenaceae, Lamiaceae, Taxaceae [1,2,3,4]. Clerodendrum is a genus of about 400 species in the family Verbenaceae, which mainly grows in tropical and warm temperate zones including Africa and Southern Asia. A few species are found in South America, Northern Australia and Eastern Asia [5]. Preparations of the leaves, branches and stems of Clerodendrum have been used in folk medicine to treat different diseases such as cancer, catarrhal affections of the lungs, fever, inflammation, skin diseases and asthma [6,7].
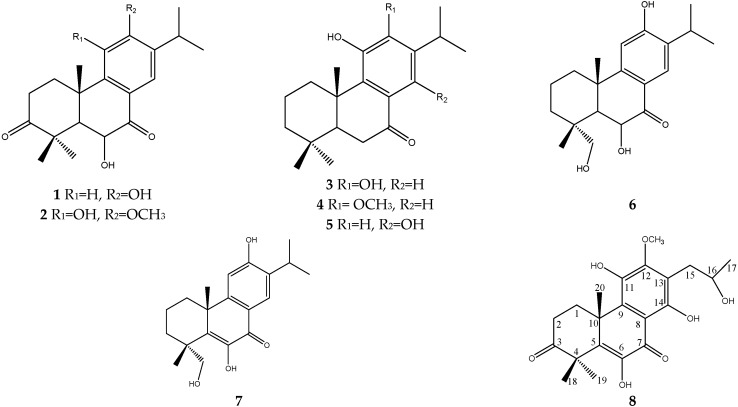
Phytochemical investigations on Clerodendrum species revealed various diterpenoids in the plants, which showed antibacterial and cytotoxic activities [8,9]. However, the phytochemical composition of the stems and roots of C. kiangsiense have not been full characterized. As a part of our ongoing effort to discover potential anticancer compounds from Chinese medicinal plants, the stems of C. kiangsiense, collected in Jiangxi Province, were investigated systematically. This has led to the isolation and structure elucidation of a new abeo-abietane diterpenoid (8) and seven known substances (1–7) (Figure 1). Compounds 1–8 were evaluated for their cytotoxicity against four cancer cell lines.
Figure 1.
Compounds isolated from Clerodendrum kiangsiense.
2. Results and Discussion
Compound 8 was isolated as colorless crystals from the EtOAc fraction of the ethanol extract of C. kiangsiense, it was assigned the molecular formula C21H26O7 (9 degrees of unsaturation) by HRESIMS (m/z 389.1597 [M − H]−, calcd. for C21H25O7−, 389.1599). The absorption bands in the UV spectrum (238, 285, 336, 381 nm) exhibited the presence of a benzene and a ketone. In the IR spectrum, two carbonyl signals were observed at 1660 and 1715 cm−1 in addition to the absorption peak at 3405 cm−1 for the hydroxyl moiety.
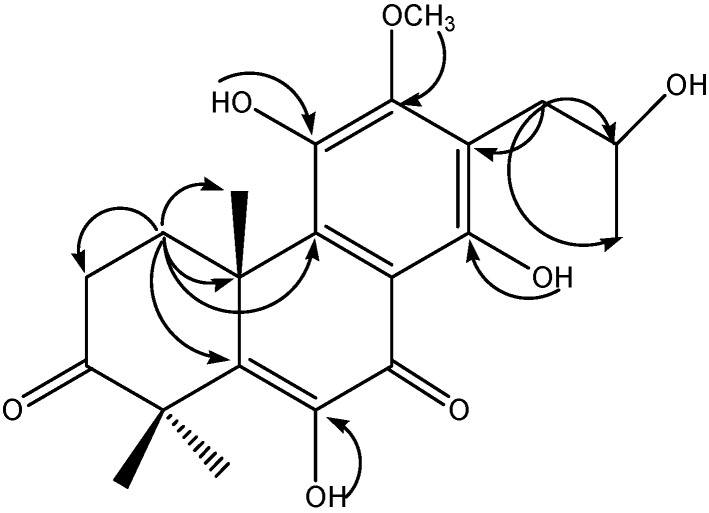
The 1H- and 13C-NMR spectroscopic data (Table 1) indicated the presence of four methyls [δH 1.28 (3H, d, J = 5.5 Hz, H-17), δH 1.53 (3H, s, H-18), δH 1.58 (3H, s, H-19) and δH 1.44 (3H, s, H-20)], a pair of doublet doublets at δH 1.80, 2.71, 2.73 and 3.32 (m, 1H each) corresponding to two methylene groups, one methine group at δH 4.18 (1H, m, H-16) together with one methoxyl at δH group 3.87 (3H, s, OMe-12), two ketone groups at δC 214.0 (C-3) and δC 183.6 (C-7), and six aromatic C-atom signals at δC 109.5, 118.7, 132.9, 139.3, 140.3 and 142.4. These data, together with other spectroscopic characteristics, suggested that 8 was a diterpenoid [10]. 1H-1H COSY correlations were observed from: H-1 (δH 1.80 and δH 3.32) to H-2 (δH 2.71 and δH 2.73); and H-17 (δH 1.28) through H-16 (δH 4.18) to H-15 (δH 2.83 and δH 2.91), in combination with HMBC correlations (Figure 2) between: H-20 (δH 1.44) to C-1 (δC 26.9), C-5 (δC 140.3), C-9 (δC 139.3), and C-10 (δC 40.6); H-15 (δH 2.83 and δH 2.91) to C-12 (δC 152.6), C-13 (δC 118.7), C-14 (δC 155.2), C-16 (δC 67.8) and C-17 (δC 23.8), which suggested that the oxygenated substituent was placed at the C-16 position (–CH2CH(OH)CH3), and the side chain of 8 is not an isopropyl but rather a 2-hudroxy-n-propyl group(CH3-17 shifted to C-16 from C-15); OH-6 (δH 6.86) to C-6 (δC 142.4) and C-7 (δC 183.6); OH-11 (δH 5.97) to C-11 (δC 132.9); OH-14 (δH 12.56) to C-8 (δC 109.5), C-13 (δC 118.7) and C-14 (δC 155.2); The 1H,13C long-range correlations between H-21 (δH 3.87) to C-12 (δC 152.6), therefore, compound 8 possesses an abeo-abietane diterpenoid framework with an OCH3 group on C-12. Thus, the structure of 8 was elucidated as 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13- abietatetraen-3,7-dione. (The NMR spectra of compound 8 are listed in the Supplementary Materials).
Table 1.
NMR spectroscopic data for compound 8 in CDCl3.
| NO | δH (J in Hz) | δC | HMBC |
|---|---|---|---|
| 1 | 1.80, m, α 3.32, m, β |
26.9, CH2 | C-2, C-3, C-10, C-20 |
| 2 | 2.71, m, α 2.73, m, β |
33.2, CH2 | C-1, C-3, C-4, C-10 |
| 3 | 214.0, qC | ||
| 4 | 48.8, qC | ||
| 5 | 140.3, qC | ||
| 6 | 142.4, qC | ||
| 7 | 183.6, qC | ||
| 8 | 109.5, qC | ||
| 9 | 139.3, qC | ||
| 10 | 40.6, qC | ||
| 11 | 132.9, qC | ||
| 12 | 152.6, qC | ||
| 13 | 118.7, qC | ||
| 14 | 155.2, qC | ||
| 15 | 2.91, dd (13.8, 4.0) a 2.83, dd (13.7, 8.3) a |
32.9, CH2 | C-12, C-13, C-14, C-16, C-17 |
| 16 | 4.18, m | 67.8, CH | C-15, C-17 |
| 17 | 1.28, d (6.2) | 23.8, CH3 | C-15, C-16 |
| 18 | 1.58, s | 21.0, CH3 | C-3, C-4, C-5, C-19 |
| 19 | 1.53, s | 24.3, CH3 | C-3, C-4, C-5, C-18 |
| 20 | 1.44, s | 20.0, CH3 | C-1, C-5, C-9, C-10 |
| 21-OCH3 | 3.87, s | 61.7,OCH3 | C-12 |
a Assignments may be reversed.
Figure 2.
Key HMBC correlations for compound 8.
The seven known compounds were identified as mandarone A (1) [11], taxusabietane A (2) [12], 12-O-demethylcrypto-japonol (3) [13], cryptojaponol (4) [14], 11,14-dihydroxy-8,11,13-abietatrien-7-one (5) [15], fortunin E (6) [16], fortunin F (7) [16], by comparison of their spectra with those reported in the literature.
To investigate their cytotoxic activities, these compounds were evaluated using an MTT cytotoxicity assay against human myeloid leukemia (HL-60), hepatocellular carcinoma (SMMC-7721), lung cancer (A-549) and breast cancer (MCF-7) cell lines. The IC50 values of all eight compounds against the indicated cancer cells are summarized in Table 2. Compound 6 and 8 exhibited the strongest cytotoxicity to all cells, as its range of IC50 values was 1.8–5.0 μM. Additionally, A-549 was the most sensitive cell line to these types of compounds among all tested cancer cells because the IC50 values of all compounds against A-549 cells were close to 10 μM. Furthermore, the cytotoxicities of all the isolated compounds were comparable to the chemotherapeutic drug cisplatin [17], which suggests that these compounds might have promising potential to be anticancer agents.
Table 2.
Cytotoxic activities of compounds 1–8 isolated from C. kiangsiense (IC50 in μM).
| Compounds | HL-60 | SMMC-7721 | A-549 | MCF-7 |
|---|---|---|---|---|
| 1 | 12.5 ± 1.2 | 13.6 ± 0.8 | 7.4 ± 1.1 | 27.7 ± 2.2 |
| 2 | 18.6 ± 1.8 | 15.9 ±1.6 | 10.2 ± 1.2 | 15.4 ± 2.7 |
| 3 | 23.5 ± 2.0 | 33.0 ± 2.4 | 10.4 ± 1.3 | 19.2 ± 1.8 |
| 4 | 9.9 ± 0.9 | 6.7 ± 1.0 | 8.7 ± 0.8 | 10.0 ± 0.8 |
| 5 | 15.5 ± 1.9 | 15.7 ± 1.6 | 11.8 ± 2.4 | 22.4 ± 2.9 |
| 6 | 4.8 ± 0.5 | 3.8 ± 0.9 | 2.7 ± 0.7 | 5.0 ± 1.0 |
| 7 | 15.7 ± 1.8 | 5.8 ± 0.8 | 7.9 ± 0.7 | 19.2 ± 1.4 |
| 8 | 1.8 ± 0.3 | 4.9 ± 0.7 | 2.5 ± 0.7 | 3.1 ± 0.5 |
| Cisplatin | 4.2 ± 0.5 | 5.9 ± 0.9 | 9.8 ± 1.1 | 11.3 ± 1.0 |
Results are expressed as means of IC50 values (the concentration that reduced cell growth by 50%) in μM, and data were obtained from triplicate experiments.
3. Materials and Methods
3.1. General Experimental Procedures
Melting points were measured using a XT-4 micro melting point apparatus (Beijing, China). Optical rotations were determined at 25 °C on a JASCO (Tokyo, Japan) P2000 polarimeter. UV data were measured using a Shimadzu UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan). IR spectra were recorded on a Nicolet 380 FT-IR spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). NMR spectra were recorded on a Bruker Avance III 500 spectrometer (Bruker, Bremen, Germany), using TMS as internal standard, Chemical shifts are reported as δ values and the coupling constants (J) are in Hz. HRESIMS data were obtained on a Agilent 6210 TOF-MS mass spectrometer; HPLC (Amersham Biosciences, GE Healthcare Life Science, Santa Clara, CA, USA), Waters 1525 semi-preparative HPLC system (Waters Co. Ltd., Milford, MA, USA) coupled with a Waters 2996 photodiode array detector. A Kromasil C18 preparative HPLC column (250 × 10 mm, 5 μm) was used; Column chromatography was carried out on silica gel (Qing Dao Hai Yang Chemical Group Co., Qingdao, China; 200–300 mesh) and Sephadex LH-20 (Amersham Biosciences). TLC analyses were carried out on silica gel 60 F254 (Merck, Darmstadt, Germany) and RP-18 F254s (Merck) plates. Compounds were detected by UV and 30% H2SO4 spraying reagent followed by heating at 105 °C for 1–2 min.
3.2. Plant Material
The stems of C. kiangsiense were collected on the Wugong Mountain of Pingxiang City, Jiangxi Province, China, in September 2010, and identified by Chunhui Dai in Zhejiang Academy of Traditional Chinese Medicine. A voucher specimen (No. 201007) has been deposited in the Key Laboratory for Genetic Improvement and Quality Control of Medical Plants of Zhejiang Province.
3.3. Extraction and Isolation
The air-dried powder of the stems (7.6 kg) of C. kiangsiense was extracted by 90% ethanol (30 L × 3) at 65 °C. The solvents were combined and evaporated to dryness under vacuum at 50 °C to afford a gummy residue (110 g). This residue was suspended in H2O (1000 mL) and partitioned with petroleum ether (1000 mL × 3, 19 g), ethyl acetate (EtOAc) (1000 mL × 3, 32 g), and n-BuOH (1000 mL × 3, 9 g), successively. The EtOAc fraction (19 g) was subjected to silica-gel column chromatography (CC) with a step gradient elution of petroleum ether–EtOAc (20:1 to 1:4, v/v) to afford 8 fractions (Fr.1–Fr.8). Fr.2 was repeatedly separated by silica gel column chromatography eluted with a petroleum ether–EtOAc gradient (20:1 to 4:1, v/v) to give compound 1 (13 mg), compound 3 (7 mg) and a fraction that was a mixture of three compounds. Further chromatographic purification on Sephadex LH-20 CC with a methanol afforded compound 2 (9 mg), compound 8 (9 mg) and compound 4 (10 mg). The separation of Fr.3 on silica gel eluted with EtOAc:petroleum ether (10:1 to 2:1, v/v) afforded a mixture (40 mg) of compounds 5 (7 mg), 6 (7 mg) and 7 (16 mg), which was separated by prep-HPLC using an acetonitrile–water mixture (30:70 v/v).
Compound 8: colorless crystals; m.p. 284–285 °C; +15.7° (c 0.1, MeOH); UV λmax: 238, 285, 336, 381 nm; IR (KBr) νmax: 3405, 1715, 1660, 1605, 1581, 1500, 1457, 1382, 1331 cm−1; 1H- and 13C-NMR (CDCl3) spectroscopic data, see Table 1; HRESIMS (negative ion mode) m/z 389.1597 [M − H]− (calcd. for C21H25O7−, 389.1599).
3.4. Anti-Proliferative Activity
The percentage of growth inhibition was determined using a MTT colorimetric technique to measure four viable cells (HL-60 human myeloid leukemia, SMMC-7721 hepatocellular carcinoma, A-549 lung cancer and MCF-7 breast cancer) with minor modification [18,19]. A total of 5000–10,000 exponential phase cells per well were seeded onto a 96-well plate for 24 h, Each tumor cell line was exposed to a test compound at concentrations of 0.0624, 0.32, 1.6, 8, and 40 μM in DMSO in triplicate for 72 h, with cisplatin as the positive control. Briefly, 100 μL of a MTT working solution (1 mg/mL) was added into each well and incubated at 37 °C for 4 h and then the medium was removed. The converted dye formazan was solubilized with 150 μL acidic isopropanol (0.04 M HCl in absolute isopropanol) and each concentration was tested in triplicate. The absorbance was then measured at a wavelength of 570 nm using a microplate reader (Multiskan Spectrum, Thermo Electron Corporation, Vantaa, Finland). The dose resulting in 50% inhibition of cell growth (IC50) was calculated by the Reed and Muench method.
4. Conclusions
In the present study, a new abeo-abietane diterpenoid, 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13-abietatetraen-3,7-dione (8) was isolated from C. kiangsiense by chromatographic separation of a 90% EtOH extract of its stems. In addition, 7 known compounds (1–7) were also obtained. The structures of compounds 1–8 were determined by spectroscopical data interpretation. The isolated compounds were subsequently evaluated for cytotoxic activities against HL-60 human myeloid leukemia, SMMC-7721 hepatocellular carcinoma, A-549 lung cancer and MCF-7 breast cancer cells, respectively. Compounds 4, 6 and 8 exhibited the strongest cytotoxicity to all cells. Additionally, A-549 was the most sensitive cell line to these types of compounds among all tested cancer cells. Furthermore, the cytotoxicities of the isolated compounds were comparable to the chemotherapeutic drug cisplatin, which suggests that C. kiangsiense and its constituents could be useful sources of candidates for the development of anticancer medicines.
Acknowledgments
Financial support from the Project of Zhejiang Provincial Natural Science Foundation (LQ14C010003, LQ13C200007), the Research Foundation of Education Bureau of Zhejiang Province (Y201327898), the Hangzhou Science and Technology Development Plan (20140432B06) are gratefully acknowledged.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/1/86/s1.
Author Contributions
Mingfeng Xu and Lu’e Shi planned the work; Shengjia Wang, Ouya Jia and Qin Zhu performed the experiments; Shengjia Wang and Ouya Jia analyzed the data; Mingfeng Xu and Qin Zhu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Gazim Z.C., Rodrigues F., Amorin A.C., de Rezende C.M., Sokovic M., Tesevic V., Vuckovic I., Krstic G., Cortez L.E., Colauto N.B., et al. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules. 2013;19:514–524. doi: 10.3390/molecules19010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yue J.R., Feng D.Q., Xu Y.K. A new triterpenoid bearing octacosanoate from the stems and roots of Clerodendrum philippinum var. simplex (Verbenaceae) Nat. Prod. Res. 2015;29:1228–1234. doi: 10.1080/14786419.2015.1023725. [DOI] [PubMed] [Google Scholar]
- 3.Bharitkar Y.P., Hazra A., Shah S., Saha S., Matoori A.K., Mondal N.B. New flavonoid glycosides and other chemical constituents from Clerodendrum phlomidis leaves: Isolation and characterisation. Nat. Prod. Res. 2015;29:1850–1856. doi: 10.1080/14786419.2015.1009457. [DOI] [PubMed] [Google Scholar]
- 4.Lee W.S., Kim J.R., Han J.M., Jang K.C., Sok D.E., Jeong T.S. Antioxidant activities of abietane diterpenoids isolated from Torreya nucifera leaves. J. Agric. Food Chem. 2006;54:5369–5374. doi: 10.1021/jf060896c. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q., Hu H.J., Li P.F., Yang Y.B., Wu L.H., Chou G.X., Wang Z.T. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and alpha-glucosidase. Phytochemistry. 2014;103:196–202. doi: 10.1016/j.phytochem.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Panthong A., Kanjanapothi D., Taesotikul T., Wongcome T., Reutrakul V. Anti-inflammatory and antipyretic properties of Clerodendrum petasites S. Moore. J. Ethnopharmacol. 2003;85:151–156. doi: 10.1016/S0378-8741(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 7.Patel J.J., Acharya S.R., Acharya N.S. Clerodendrum serratum (L.) Moon—A review on traditional uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2014;154:268–285. doi: 10.1016/j.jep.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 8.Waliullah T.M., Yeasmin A.M., Alam A., Islam W., Hassan P. In vitro antimicrobial study for biological evaluation of Clerodendrum infortunatum Linn. Recent Pat. Anti-Infect. Drug Discov. 2015;10:98–104. doi: 10.2174/1574891X10666150512104405. [DOI] [PubMed] [Google Scholar]
- 9.Xu R.L., Wang R., Ding L., Shi Y.P. New cytotoxic steroids from the leaves of Clerodendrum trichotomum. Steroids. 2013;78:711–716. doi: 10.1016/j.steroids.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Xie W.D., Li X., Zhao J.H., Liu Y.H., Row K.H. Abietane diterpenoids from Isodon inflexus. Phytochemistry. 2012;81:153–158. doi: 10.1016/j.phytochem.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Inaba Y., Hasuda T., Hitotsuyanagi Y., Aoyagi Y., Fujikawa N., Onozaki A., Watanabe A., Kinoshita T., Takeya K. Abietane diterpenoids and a sesquiterpene pyridine alkaloid from Euonymus lutchuensis. J. Nat. Prod. 2013;76:1085–1090. doi: 10.1021/np400110g. [DOI] [PubMed] [Google Scholar]
- 12.Yang S.J., Fang J.M., Cheng Y.S. Diterpenes from Taxus mairei. Phytochemistry. 1998;49:2037–2043. doi: 10.1016/S0031-9422(98)00443-9. [DOI] [Google Scholar]
- 13.Rodriguez B. Structural and spectral assignment by two-dimensional NMR of two new derivatives of the abietane diterpenoid taxodione. Magn. Reson. Chem. 2005;43:97–99. doi: 10.1002/mrc.1503. [DOI] [PubMed] [Google Scholar]
- 14.Fronza M., Murillo R., Slusarczyk S., Adams M., Hamburger M., Heinzmann B., Laufer S., Merfort I. In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011;19:4876–4881. doi: 10.1016/j.bmc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 15.Min Z.D., Jiang H., Liang J.Y. Studies on the taxane diterpenes of the heartwood from Taxus mairei. Yao Xue Xue Bao. 1989;24:673–677. [PubMed] [Google Scholar]
- 16.Yao S., Tang C.P., Ke C.Q., Ye Y. Abietane diterpenoids from the bark of Cryptomeria fortunei. J. Nat. Prod. 2008;71:1242–1246. doi: 10.1021/np8002063. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X.D., Ni W., Yan H., Li G.T., Zhong H.M., Li Y., Liu H.Y. Daphnane-type diterpenoid glucosides and further constituents of Euphorbia pilosa. Chem. Biodivers. 2014;11:760–766. doi: 10.1002/cbdv.201300154. [DOI] [PubMed] [Google Scholar]
- 18.Liang Z., Zhang T., Zhang X., Zhang J., Zhao C. An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules. 2015;20:1424–1433. doi: 10.3390/molecules20011424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caamal-Fuentes E.E., Peraza-Sanchez S.R., Torres-Tapia L.W., Moo-Puc R.E. Isolation and identification of cytotoxic compounds from Aeschynomene fascicularis, a Mayan medicinal plant. Molecules. 2015;20:13563–13574. doi: 10.3390/molecules200813563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.