Abstract
Coumarins are important plant-derived natural products with wide-ranging bioactivities and extensive applications. In this study, we evaluated for the first time the antibacterial activity and mechanisms of action of coumarins against the phytopathogen Ralstonia solanacearum, and investigated the effect of functional group substitution. We first tested the antibacterial activity of 18 plant-derived coumarins with different substitution patterns, and found that daphnetin, esculetin, xanthotol, and umbelliferone significantly inhibited the growth of R. solanacearum. Daphnetin showed the strongest antibacterial activity, followed by esculetin and umbelliferone, with MICs of 64, 192, and 256 mg/L, respectively, better than the archetypal coumarin with 384 mg/L. We further demonstrated that the hydroxylation of coumarins at the C-6, C-7 or C-8 position significantly enhanced the antibacterial activity against R. solanacearum. Transmission electron microscope (TEM) and fluorescence microscopy images showed that hydroxycoumarins may interact with the pathogen by mechanically destroying the cell membrane and inhibiting biofilm formation. The antibiofilm effect of hydroxycoumarins may relate to the repression of flagellar genes fliA and flhC. These physiological changes in R. solanacearum caused by hydroxycoumarins can provide information for integral pathogen control. The present findings demonstrated that hydroxycoumarins have superior antibacterial activity against the phytopathogen R. solanacearum, and thus have the potential to be applied for controlling plant bacterial wilt.
Keywords: Ralstonia solanacearum, bacterial wilt, hydroxycoumarins, antibacterial activity, biofilm inhibition, daphnetin, esculetin
1. Introduction
Bacterial wilt, caused by Ralstonia solanacearum, causes destructive economic losses to important crops such as tomato, potato, tobacco, and eggplant in tropical and subtropical regions [1,2]. It is estimated that bacterial wilt generates at least $1 billion in losses each year, with an especially devastating effect on staple crops in developing countries [3]. Currently, the main methods for controlling bacterial wilt are based on integrated pest management and chemical control. However the effectiveness of these methods diminishes with long-term field application [4]. For example, the traditional chemical control induces successive occurrence of pesticide-resistant strains and raises environmental safety concerns [5,6]. Due to the limitations of current control methods, the increasing host range of R. solanacearum and its complex pathogenicity and range of infectious conditions, it is difficult to develop effective measures to protect plants against R. solanacearum [7,8,9]. This situation demands alternative agents or measures to effectively control bacterial wilt.
More recently, biological control has been considered a promising management strategy for bacterial wilt, especially using plant-derived compounds or plant extracts [10,11,12]. These compounds exhibit the ability of suppressing soil-borne pathogens and promoting plant growth [13,14]. As previously reported, bacterial wilt was suppressed by lansiumamide B [10], flavonoids [11], palmarosa (Cymbopogon martini), lemongrass (C. citratus), and eucalyptus (Eucalyptus globulus) oils [14], and methyl gallate [15]. However, few studies have focused on the mechanism of the antibacterial action of these compounds against plant pathogenic bacteria, especially R. solanacearum. Moreover, coumarins, a group of promising biocontrol compounds, have not yet been tested against R. solanacearum.
Coumarins are natural compounds produced by a wide range of plant sources. Their structures comprise fused benzene and α-pyrone rings. Recently, coumarins have attracted extensive research interests due to their biological activities. Which include antibacterial, antifungal, anticoagulant, antioxidant, anticancer, and anti-inflammatory properties [16,17]. Studies have reported that coumarins exhibited strong antibacterial activity on both Gram-positive and Gram-negative bacteria, especially against Gram-negative bacteria, by damaging the cell membrane [17,18,19]. The advantages of coumarins as promising antibacterial compounds include: (1) a board spectrum of antibacterial activity; (2) they are secreted by plants as phytoalexins to defend against attacks from pathogens; (3) they are environmentally friendly and not susceptible to develop bacteria resistance. Our previous studies also indicated that plant-derived compounds like coumarin, protocatechuic aldehyde, resveratrol, and carvacrol had antibacterial activity against R. solanacearum. Furthermore, coumarins can serve as scaffolds for advanced design and synthesis of more active derivatives [20]. Taken together, these facts highlight the value of investigating the antibacterial activity of coumarins against R. solanacearum. Consequently, in this study, we tested the antibacterial activity of 18 coumarins against R. solanacearum, and found that hydroxycoumarins such as daphnetin and esculetin showed the strongest bioactivity. We further focused on the hydroxycoumarins to investigate the antibacterial mechanism of action, including biofilm inhibition and regulation of some virulence-associated genes of R. solanacearum. Our findings show that hydroxycoumarins have great potential as effective botanical bactericides for controlling bacterial wilt.
2. Results and Discussion
2.1. Antibacterial Activity of Screened Coumarins against R. solanacearum
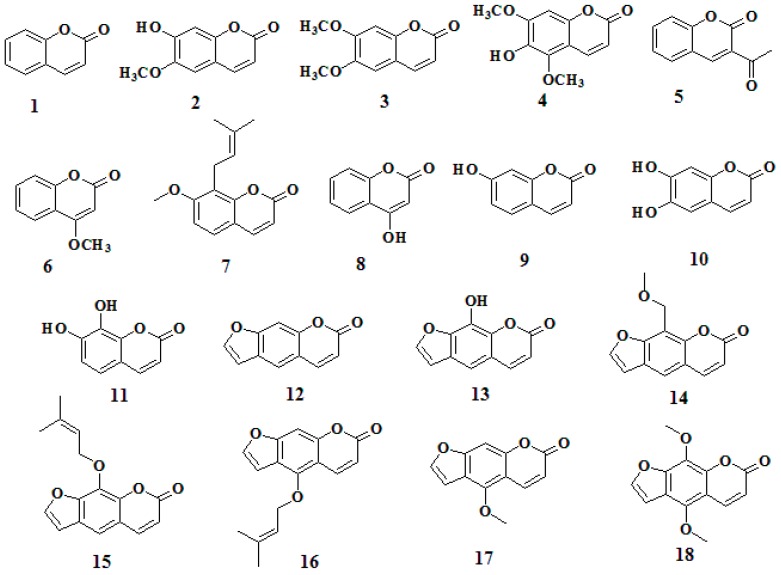
Initially, we evaluated the antibacterial activity of plant-derived compounds against R. solanacearum. Compared with thiodiazole copper treatment, umbelliferone, coumarin, and tea polyphenols exhibited strong antibacterial activity (Table S1). Further, in order to determine whether coumarin derivatives had antibacterial activity on R. solanacearum, we evaluated the antibacterial activity on R. solanacearum exposed to 18 coumarins at two dosage levels. The results showed that seven coumarins had strong antibacterial effects against R. solanacearum with an antibacterial rate over 50% after 24 h treatment at a dosage of 100 mg/L. Compared with thiodiazole copper treatment, among the screened coumarins, daphnetin (7,8-dihydroxycoumarin, 11, Figure 1) showed the highest antibacterial activity, followed by xanthotol (13), and esculetin (6,7-dihydroxycoumarin, 10) with antibacterial activity rates of 97.43%, 80.12%, and 71.44%, respectively (Table 1), significantly higher than the thiodiazole copper treatment with an antibacterial rate of 63.6%. Interestingly, hydroxyl substitution, especially on the C-6, C-7, or C-8 sites, seemed to enhance the antibacterial activity of coumarins against R. solanacearum. Thus we chose hydroxycoumarins, i.e., umbelliferone, esculetin and daphnetin, for further investigation of the detailed antibacterial mechanism.
Figure 1.
The chemical structures of the studied coumarins.
Table 1.
The antibacterial activity of coumarins against R. solanacearum.
| Number | Compound | Antibacterial Rate (%) (Mean ± SD) a | |
|---|---|---|---|
| 10 mg/L | 100 mg/L | ||
| 1 | Coumarin | 3.9 ± 1.1 * | 50.3 ± 3.3 * |
| 2 | Scopoletin | 1.5 ± 7.6 | 32.6 ± 4.9 * |
| 3 | Scoparone | 1.0 ± 7.0 | 17.3 ± 2.9 * |
| 4 | Isofraxidin | 2.5 ± 3.2 * | 0.7 ± 1.4 * |
| 5 | 3-Acetyl-2H-chromen-2-one | 6.3 ± 4.7 | 10.7 ± 2.7 * |
| 6 | 4-Methoxycoumarin | 7.3 ± 1.9 * | 54.1 ± 4.2 * |
| 7 | Osthole | 0.2 ± 1.9 * | 3.1 ± 3.3 * |
| 8 | 4-Hydroxycoumarin | 2.1 ± 5.7 * | 4.9 ± 6.6 * |
| 9 | Umbelliferone | 7.3 ± 3.0 | 59.7 ± 3.8 |
| 10 | Esculetin | 9.2 ± 2.8 | 71.4 ± 2.1 * |
| 11 | Daphnetin | 13.3 ± 3.0 | 97.4 ± 0.7 * |
| 12 | Psoralen | 13.1 ± 0.8 | 57.1 ± 8.8 |
| 13 | Xanthotol | 8.5 ± 5.4 | 80.1 ± 2.5 * |
| 14 | Xanthotoxin | 6.3 ± 5.0 | 36.3 ± 5.4 * |
| 15 | Imperatorin | 6.5 ± 2.9 * | 19.1 ± 2.8 * |
| 16 | Isoimperatorin | 4.1 ± 2.0 * | 23.9 ± 6.3 * |
| 17 | Bergapten | 4.7 ± 2.4 * | 21.9 ± 1.6 * |
| 18 | Isopimpinellin | 0.3 ± 1.6 * | 22.5 ± 5.1 * |
| Positive Control | Thiodiazole Copper | 12.2 ± 1.7 | 63.6 ± 2.9 |
a: The experiment was repeated in triplicates. Asterisks indicate statistically significant differences in antibacterial activity against R. solanacearum compared with thiodiazole copper treatment. (* indicates p < 0.05, Student’s t test).
2.2. MIC and MBC of Hydroxycoumarins against R. solanacearum
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of hydroxycoumarins against R. solanacearum were measured using the typical microdilution method. As shown in Table 2, daphnetin was the most effective compound against R. solanacearum, followed by esculetin, umbelliferone, and coumarin. The MIC of daphnetin was 64 mg/L, much lower than the value of the parent compound coumarin (384 mg/L), which indicated the inhibitory efficiency of daphnetin is 6-fold that of coumarin. The MICs of umbelliferone and esculetin were 256 and 192 mg/L, respectively. The minimum bactericidal concentrations (MBCs) of coumarins on R. solanacearum were defined as the lowest concentration of coumarins that prevent the growth of bacteria after sub-culture on agar media. As shown in Table 2, the MBCs of coumarin, umbelliferone, esculetin and daphnetin against R. solanacearum were 512, 384, 192, 64 mg/L, respectively.
Table 2.
The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of hydroxycoumarins against R. solanacearum in the 96-well polystyrene microtiter plates.
| Coumarins | MIC (mg/L) | MBC (mg/L) |
|---|---|---|
| Coumarin | 384 | 512 |
| Umbelliferone | 256 | 384 |
| Esculetin | 192 | 192 |
| Daphnetin | 64 | 64 |
Each experiment was repeated in three times.
2.3. Hydroxycoumarins Inhibit the Growth of R. solanacearum
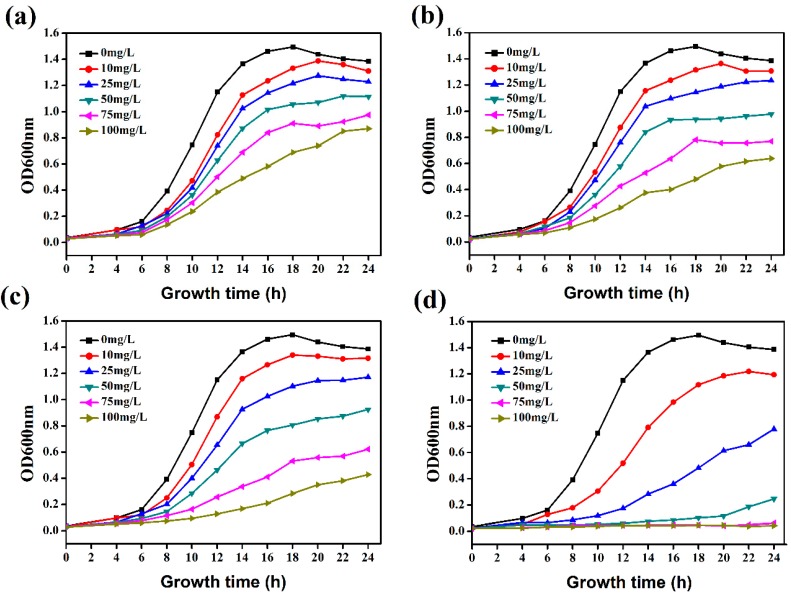
To further investigate the inhibitory effect of hydroxycoumarins and evaluate whether hydroxyl substitution enhances the antibacterial activity, we examined the growth curve of R. solanacearum exposed to the screened hydroxycoumarins like umbelliferone, esculetin, and daphnetin at concentrations ranging from 10 to 100 mg/L, and used the archetypal coumarin as the positive control. As shown in Figure 2, hydroxycoumarins, especially daphnetin and esculetin, siginificantly inhibited the growth of R. solanacearum through all the concentration range of 10 to 100 mg/L. Daphnetin almost completely stopped the bacterial growth at the concentration of 50–100 mg/L after 24 h incubation (Figure 2d). The growth of R. solanacearum was also inhibited by umbelliferone, esculetin (Figure 2a–c, and the antibacterial activity of hydroxycoumarins against R. solanaccearum increased with dosage. Further, to evaluate the quantitative correlation between the concentration and the antibacterial activity of coumarins against R. solanacearum, we simulated the linear relationship between the logarithmic value of coumarin concentration and the probability value of corrected antibacterial rate based on a turbidimeter test. After 12 and 24 h incubation, the growth of R. solanacearum entered the logarithmic phase and stable phase, respectively. As shown in Table 3, after 24 h incubation, the IC50 values of daphnetin and esculetin were 23.98 and 67.85 mg/L, respectively.
Figure 2.
The effect of coumarins at concentrations ranging from 10 to 100 mg/L on the growth curves of R. solanacearum: (a) coumarin; (b) umbelliferone; (c) esculetin; (d) daphnetin.
Table 3.
The IC50s of coumarins against R. solanacearum.
| Coumarins | 12 h | 24 h | ||||
|---|---|---|---|---|---|---|
| Toxicity Regression Equations | IC50 (mg/L) | R Value | Toxicity Regression Equations | IC50 (mg/L) | R Value | |
| Coumarin | Y = 1.0293X + 3.1841 | 57.11 | 0.9330 | Y = 1.2294X + 2.1747 | 198.64 | 0.9783 |
| Umbelliferone | Y = 1.501X + 2.6435 | 37.15 | 0.9820 | Y = 1.8022X + 1.4204 | 96.88 | 0.9683 |
| Esculetin | Y = 2.0185X + 2.1871 | 24.75 | 0.9712 | Y = 2.1808X + 1.0057 | 67.85 | 0.9807 |
| Daphnetin | Y = 2.0489X + 3.0719 | 8.73 | 0.9863 | Y = 3.2992X + 0.7286 | 23.98 | 0.9809 |
In summary, we can conclude that coumarins with hydroxylation in the position C-7 (umbelliferone) have strong antibacterial activity. An additional hydroxylation in position C-6 (esculetin) enhances the antibacterial activity, while an even greater improvement results from dihydroxylation in positions C-7 and C-8 (daphnetin). Coumarins have been proven to have multiple substitution sites, and different substitutions in these sites significantly affect the biological activity of coumarins [16,21,22]. Previous research found that coumarins with OH groups at C-7 or C-8 were better antibacterial compounds than the model molecules [23,24]. Daphnetin (7,8-dihydroxycoumarin) was the most active compound against the tested bacteria among 20 coumarins extract from Mexican tarragon or purchased [17]. In this case, it is probable that the two adjacent hydroxyl groups substituted on the C-7 and C-8 of the coumarin skeleton electronically activate the aromatic ring and increase the hydrophobic and lipophilicity, which consequently promote the combination of the hydroxycoumarins with the membrane of bacteria and enhance the activity against bacterial strains [17,25]. Other substituents were also proved to change the structure of compounds and affect their antibacterial activity. Four example, 2-aryl-4,5-dihydrothiazole analogues exhibited strong bioactivity on R. solanacearum unless a 2′-hydroxyl group was introduced on the 2-aryl substituent of the compound [26].
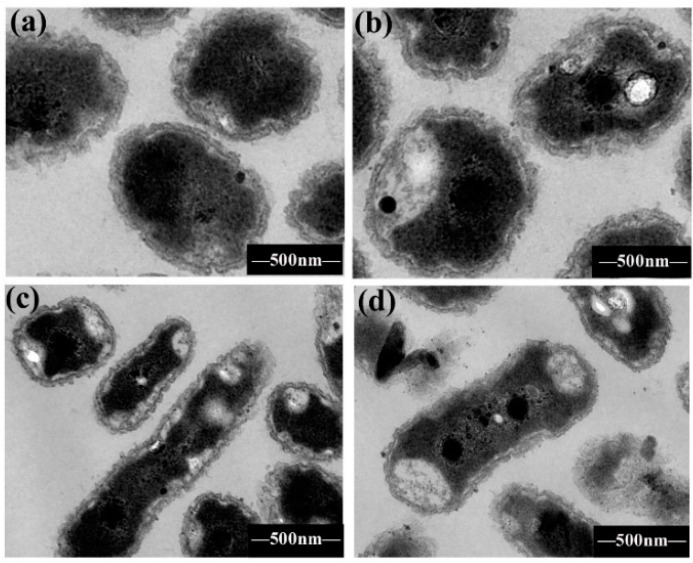
2.4. Bacterial Morphological Change by TEM
In order to further investigate the mechanism of antibacterial activity of coumarins, the cell morphology of R. solanacearum was monitored using TEM after treatment with hydroxycoumarins. The TEM images of R. solanacearum were taken after the addition of 50 mg/L hydroxycoumarins (umbelliferone, esculetin) and 25 mg/L daphnetin. According to Figure 3, daphnetin and esculetin induced irreversible damage to the cell membrane of R. solanacearum, producing irregular hollows in the cells. These results were in agreement with the observed antibacterial activity of hydroxycoumarins against R. solanacearum. However, the insignificant change caused by umbelliferone indicated other mechanisms leading to the bacterial death. Daphnetin and esculetin are widely distributed hydroxycoumarins throughout the plant kingdom with several promising biological activities, such as antibacterial and antioxidant, and phenolic activities [17,27]. Previous studies have identified several plant phenolic derivatives as promising antibacterial inhibitors of Escherichia coli, Hafnia alvei, and Xylella fastidiosa [28,29]. This is consistent with our finding that hydroxycoumarins have strong antibacterial activity against R. solanacearum. The antibacterial mechanism of phenolic compounds like carvacrol and thymol was attributed to membrane potential changes [30]. In agreement with these observations, our results also showed that daphnetin and esculetin induced the irreversible damage to the cell membrane of R. solanacearum. Literature reports indicate that activation of phenolic metabolites and phytoalexins could be expressed against pathogens which is considered to strongly limit the spread of invading pathogens [31]. Our findings suggest that hydroxycoumarins have superior antibacterial activity against the phytopathogen R. solanacearum, as the antibacterial activity of daphnetin and esculetin were significantly higher than that caused by thiodiazole copper treatment. Hydroxycoumarins had no effect on the germination of tobacco seed (Figure S2), and have low cytotoxicity on human cells [32], so the combination of antibacterial activity and low the cytotoxicity of hydroxycoumarins make them potentially useful agents for controlling plant bacterial wilt.
Figure 3.
TEM images of R. solanacearum cells treated with (a) DMSO; (b) umbelliferone; (c) esculetin; and (d) daphnetin, respectively. Overnight cultured bacterial suspension was diluted into B medium supplemented with coumarins (50 mg/L umbelliferone, 50 mg/L esculetin and 25 mg/L daphnetin) and incubated at 30 °C for 12 h.
2.5. Hydroxycoumarins Reduce Biofilm Formation of R. solanacearum
Like many plant pathogenic bacteria, R. solanacearum forms biofilm-like aggregations on host plant roots, contributing to the bacterial invasion and infection [33]. Previous studies have proved that coumarins like scopoletin, umbelliferone, esculetin, and daphnetin were phytoalexins secreted by plants to protect themselves from the attack of pathogenic bacteria [34,35]. Meanwhile, coumarins play an important role in the chemical defense strategy of plants [36]. We thus speculated that hydroxycoumarins were plant-microorganism interaction factors between plant and R. solanacearum, and hydroxycoumarins may have effects on biofilm formation.
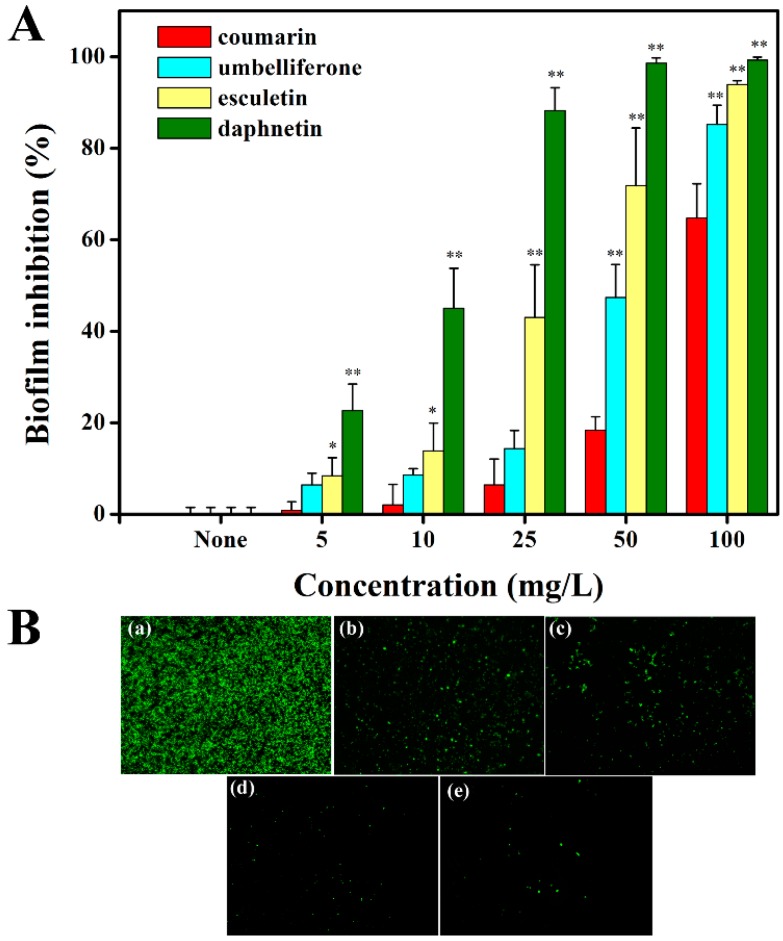
As shown in Figure 4a, the hydroxycoumarins significantly reduced the biofilm formation of R. solanacearum, especially esculetin and daphnetin. Specifically, daphnetin reduced biofilm formation by 99.22% at a concentration of 100 mg/L compared with 64.66% by coumarin treatment. Esculetin and umbelliferone also significantly reduced the biofilm formation at the concentration of 100 mg/L with inhibitory rates of 93.90% and 85.20%, respectively. The results showed that daphnetin exhibited the highest inhibitory ability on R. solanacearum biofilm formation, followed by esculetin, umbelliferone, and coumarin. The inhibitory activity of the coumarins was concentration-dependent. The motility of R. solanacearum plays an important role in biofilm formation, therefore the swimming motility under coumarin treatment was also investigated. The results showed that hydroxycouamrins like esculetin and daphnetin significantly repressed the swimming motility of R. solanacearum at a concentration of 50 mg/L (Figure S1).
Figure 4.
Effects of coumarins on biofilm formation of R. solanacearum. Biofilm inhibition (%) was quantified after treatment with different concentrations of coumarins at 30 °C for 24 h in the 96-well plates (A); (* indicated p < 0.05, ** indicated p < 0.01). Fluorescence microscope imaging of biofilm formation of R. solanacearum using FITC-ConA tagged with extracellular polysaccharide in 24-well polystyrene microtiter plate (B); Fluorescence microscope imaging of R. solanacearum with (a) 50 mg/L DMSO (control treatment); (b) coumarin (50 mg/L); (c) umbelliferone (50 mg/L); (d) esculetin (50 mg/L); (e) daphnetin (50 mg/L).
To further validate the effect of hydroxycoumarins on R. solanacearum biofilm formation, the extracellular polysaccharide (EPS) involved in biofilm formation was measured in 24-well polystyrene microtiter plates with or without coumarins, using FITC-ConA as a tag which can be excited at wavelength of 488 nm (Ar laser) to generate a bright green fluorescence [37]. Daphnetin and esculetin dramatically reduced the biofilm formation compared with control treatment (Figure 4b). Daphnetin almost completely inhibited the biofilm formation (Figure 4e). The results agreed with the biofilm formation assay.
2.6. Hydroxycoumarins Repress Virulence-Associated Genes of R. solanacearum
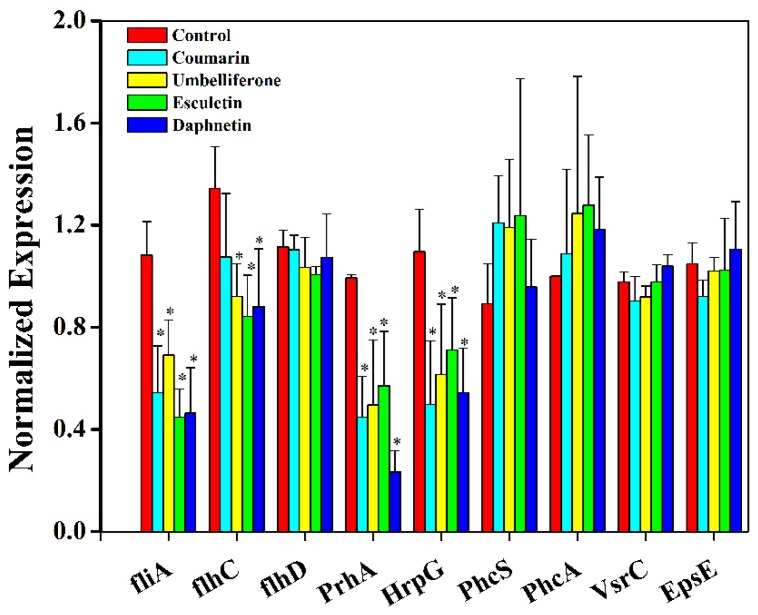
Thanks to our increasing knowledge about R. solanacearum, the virulence-associated genes were comprehensively and thoroughly studied in previous studies [38,39]. For example, the regulating and structural flagellar genes, fliA, flhC and flhD, participate in the regulation of swimming motility of R. solanacearum [40]. In a preliminary study, we found that hydroxycoumarins, especially daphnetin, repressed the swimming motility and reduced the biofilm formation. In order to investigate the molecular mechanism responsible for swimming motility and biofilm inhibition, we measured the expression of the main virulence-associated genes in R. solanacearum cells treated or not with coumarins using qRT-PCR. The results indicated that the expression of fliA and flhC was significantly repressed by umbelliferone, esculetin, and daphnetin (Figure 5), but the hydroxycoumarins did not have effect on flhD and VsrC which also contribute to the swimming motility of R. solanacearum. This result implied that coumarins (umbelliferone, esculetin, and daphnetin) reduce biofilm formation through repressing the swimming motility by repressing some flagellar genes.
Figure 5.
Expression of some virulence-associated genes of R. solanacearum were quantified by qRT-PCR treated with or without coumarins. R. solanacearum was cultured in B medium treated with DMSO or the coumarins using the IC50 concentration (coumarin 58.09 mg/L, umbelliferone 37.15 mg/L, esculetin 24.15 mg/L and daphnetin 8.73 mg/L). SerC was used as the reference gene to normalize the gene expression using the ∆∆Cq method. The results reflect three biological replicates and error bars indicate the standard deviation. (* indicated p < 0.05)
In addition, coumarins had no effect on the expression of EpsE, PhcA, VsrC, and PhcS. Interestingly, coumarins significantly repressed the expression of PrhA and HrpG, which are the main regulators of the type III secretion system (T3SS) of R. solanacearum. The result indicated that hydroxycoumarins like daphnetin, esculetin, and umbelliferone have the potential to be the T3SS inhibitors. The regulation pathway of hydroxycoumarins on T3SS of R. solanacearum needs further investigation.
3. Experimental Section
3.1. Bacterial Strains and Coumarins
The R. solanacearum (phylotype I, race1, biovar 3) was used throughout the study [41]. The experiments were conducted at 30 °C in rich B medium [42]. All coumarins (Table 1) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO). The final concentration of coumarins in DMSO was 10 mg/mL.
3.2. Antibacterial Biological Assay to Screen Active Compounds
The antibacterial activity of coumarins against R. solanacearum was evaluated by the turbidimeter test method with minor modifications [43]. Briefly, 50 µL overnight-cultured bacterial suspension adjusted to OD600 = 1.0 was inoculated in 10 mL rich B medium supplemented with coumarins to generate concentrations of 10 mg/L or 100 mg/L. DMSO (100 µL) was used as control treatment. The flasks were kept rotating on a shaker at 180 rpm and 30 °C for 24 h. The antibacterial rate of R. solanacearum supplemented with coumarins were calculated according to the following equation:
| (1) |
where A0: Corrected OD600 values of the control treatment; A1: Corrected OD600 values of the coumarin treatment. All assays were carried out at least three times in biological repeats.
3.3. Determination of MIC and MBC
The bacteriostatic and bactericidal activities of coumarins against R. solanacearum were examined by the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) methods. The experiments were performed in 96-well polystyrene microtiter plates with minor modifications [44,45]. Briefly, 1 µL of overnight-cultured bacterial suspension adjusted to OD600 = 1.0 was inoculated in 198 µL rich B medium, and supplemented with 1 µL triphenyl tetrazolium chloride (TTC) as the indicator of R. solanacearum growth [46]. The concentrations of coumarins were adjusted to 512, 398, 256, 192, 128, 64, 32, 16, 8, 4, 2, and 1 mg/L. The polystyrene microtiter plate was incubated without shaking for 12 h at 30 °C. TTC was. After addition of TTC and incubation, the MIC was defined as the lowest concentration of compounds at which no pink color appeared. The MBC was defined as the lowest concentration of coumarins that prevented the growth of bacteria after sub-culturing on agar media. All assays were carried out at least three times in biological repeats.
3.4. Bacterial Growth Curve
The growth curve of R. solanacearum was determined as in a previous study with minor modifications [41]. Briefly, the overnight-cultured bacterial suspension (OD600 ≈ 1.0) was inoculated in 25 mL rich B medium supplemented with coumarins (coumarin, umbelliferone, esculetin, daphnetin) to generate a final concentration of 10, 25, 50, 75, or 100 mg/L. The control treatment contained 100 µL DMSO. Cell density was detected by measuring the optical density (OD) at 600 nm every two hours during the 24-h cultivation. All samples were performed in triplicates and calculated to obtain an averaged value. The antibacterial rate of R. solanacearum supplemented with coumarins after incubated for 12 and 24 h were calculated using the equation mentioned above.
3.5. Biofilm Assay
The biofilm formation assay of R. solanacearum was performed in 96-well polystyrene microtiter plates as previously reported [40]. Briefly, 1 µL overnight-cultured bacterial suspension adjusted to OD600 = 1.0 was inoculated in 199 µL B medium supplemented with coumarins (coumarin, umbelliferone, esculetin, and daphnetin) to generate a final concentration of 5, 10, 25, 50, or 100 mg/L. The polystyrene microtiter plate was incubated without shaking for 24 h at 30 °C. Biofilms were stained with crystal violet and dissolved in 95% ethanol and quantified by absorbance at 530 nm (OD530). The experiment was performed in at least six replicate wells and average values were calculated. All assays were carried out at least three times in biological repeats.
3.6. Swimming Motility Assay
The swimming motility of R. solanacearum was measured on semi-solid motility media as previously reported [47]. Medium plates contain 1% tryptone and 0.325% ager. Coumarins (50 mg/L) were added into motility medium and DMSO was used as control treatment. An overnight R. solanacearum culture was adjusted to OD600 = 0.1 and 3 µL bacterial suspension was dropped on the plate. Motility was visualized as a white halo and measured after 1–2 days of incubation at 30 °C. All assays were carried out at least three times in biological repeats.
3.7. Fluorescence Microscopy Imaging
The biofilm formation of R. solanacearum was further investigated by fluorescence microscopy using FITC-ConA tagged with extracellular polysaccharide (EPS) [37]. Briefly, the experiment was performed in 24-well polystyrene microtiter plate with or without coumarins (50 mg/L), and incubated without shaking for 12 h at 30 °C. The biofilm formation was stained with 20 µL FITC-ConA for 30 min in the dark. Static biofilm formation was visualized by inverted fluorescence microscope (Axio Observer D1, Zeiss, Jena, Germany) using an excitation wavelength of 488 nm (Ar laser) and a 200 × objective. The biofilm formation was evaluated by the number of green points in the fluorescence microscopy images.
3.8. Cell Morphology Observation with TEM
To measure the morphology of R. solanacearum after hydroxycoumarins treatments, a transmission electron microscope (TEM) was used as previously reported with some modifications [48]. Briefly, an overnight R. solanacearum culture was diluted to an OD600 of 0.05 in B medium supplemented with hydroxycoumarins (50 mg/L umbelliferone, 50 mg/L esculetin and 25 mg/L daphnetin) and incubated at 30 °C for 12 h. The treated bacterial cells were collected after centrifugation at 6000 rpm, fixed with 2.5% glutaraldehyde, post-fixed with 1% aqueous OsO4 (Fluka, Los Angeles, CA, USA) and washed with 0.1 M, PH 7.0 phosphate buffers. Thin sections containing the cells were placed on copper grids and observed under TEM microscope (FEI, Brno, Czech Republic).
3.9. RNA Isolation and Quantitative Real-Time RT-PCR
The effect of coumarins on the expression of virulence-associated genes of R. solanacearum was evaluated as previously reported [41]. Briefly, overnight-cultured R. solanacearum suspension adjusted to OD600 = 1.0 was inoculated in fresh B medium with coumarins or DMSO (the IC50 of coumarins against R. solanacearum after incubated for 12 h was listed in Table 3), then incubated on a shaker at 180 rpm and 30 °C for 6 h. The treated bacterial cells were collected after centrifugation. Total RNA was isolated using TRNzol reagent (Tiangen Biotech Co. Ltd., Beijing, China). qRT-PCR was used to evaluate the normalized expression of flagellar genes (fliA, flhC and flhD), and virulence-associated genes (PrhA, HrpG, PhcS, PhcA, VsrC, and EpsE) [40,41]. The primers of the tested genes used in this study were list in Table S2 and the housekeeping gene SerC was used as the control [49]. qRT-PCR was performed on the CFX96 Manager (Bio-Rad) using an Sso Fast™ EvaGreen® Supermix (Bio-Rad, Hercules, CA, USA). All assays were carried out at least three times in biological repeats.
4. Conclusions
In conclusion, this study evaluated the antibacterial activity of 18 plant-derived coumarins against R. solanacearum. Moreover, this is the first study to investigate the antibacterial activity and mechanism of action of hydroxycoumarins (umbelliferone, esculetin, and daphnetin) against R. solanacearum. Among the tested compounds, daphnetin exhibited the best antibacterial activity, followed by esculetin and umbelliferone, which showed that dihydroxylation in positions C-7 and C-8 of the coumarin structure enhanced the bactericidal activity against R. solanacearum. The mechanism of antibacterial action of hydroxycoumarins could be at least partially attributed to the destruction of the cell membrane. Hydroxycoumarins also significantly reduced the biofilm formation and repressed the swimming motility of R. solanacearum, resulting in weaker pathogenicity. The molecular mechanism of biofilm inhibition was shown to be related to the down-regulation of the regulating and structural flagellar genes (fliA and flhC). In addition, coumarins repressed the expression of the type III secretion system genes such as HrpG and PrhA. Future studies are expected to assess the antibacterial activity of hydroxycoumarins against other important agricultural pathogenic bacteria, and to design and synthesize coumarins which have better biological activity based on the hydroxycoumarins model. These investigations on the antibacterial and antibiofilm activities of hydroxycoumarins have shown that daphnetin and esculetin have the potential to be effective bacterial wilt control agents and applied as a new strategy to control plant bacterial diseases.
Acknowledgments
This work was supported by the key Projects of the China National Tobacco Corporation (110201202002, 110201502019) and the National Natural Science Foundation of China (31572041).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/4/468/s1.
Author Contributions
W.D. conceived and designed the experiments; L.Y., S.L., B.G., W.D. performed the experiments and analyzed the date; L.Y., W.D. wrote the paper; D.W., Y.X., J.C. revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–18 are available from the authors.
References
- 1.Genin S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytologist. 2010;187:920–928. doi: 10.1111/j.1469-8137.2010.03397.x. [DOI] [PubMed] [Google Scholar]
- 2.De Oliveira Monteiro F.M. Ph.D. Thesis. Universitat de Barcelona; Barcelona, Spain: 2013. Environmental Cues Controlling the Pathogenicity of “Ralstonia Solanacearum” on Plants: Señales Ambientales Que Determinan la Patogenicidad de “Ralstonia Solanacearum” en Plantas. [Google Scholar]
- 3.Elphinstone J.G., Allen C., Prior P., Hayward A.C. Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex. American Phytopathological Society; St Paul, MN, USA: 2005. The current bacterial wilt situation: A global overview; pp. 9–28. [Google Scholar]
- 4.Yuliar Y.A.N., Toyota K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015;30:1. doi: 10.1264/jsme2.ME14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny T. Plant-Associated Bacteria. Springer; Dordrecht, The Netherlands: 2006. Plant pathogenic Ralstonia species; pp. 573–644. [Google Scholar]
- 6.Lin Y., He Z., Rosskopf E.N., Conn K.L., Powell C.A, Lazarovits G. A nylon membrane bag assay for determination of the effect of chemicals on soilborne plant pathogens in soil. Plant Dis. 2010;94:201–206. doi: 10.1094/PDIS-94-2-0201. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Liu X., Han H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B Biointerfaces. 2013;103:136–142. doi: 10.1016/j.colsurfb.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 8.Álvarez B., Biosca E.G., López M.M. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Volume 1 Formatex Research Center; Badajoz, Spain: 2010. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. [Google Scholar]
- 9.Hayward A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991;29:65–87. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- 10.Li L., Feng X., Tang M., Hao W., Han Y., Zhang G., Wan S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum) Microbiol. Res. 2014;169:522–526. doi: 10.1016/j.micres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X., Mei W., Gong M., Zuo W., Bai H., Dai H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules. 2011;16:9775–9782. doi: 10.3390/molecules16129775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan M.A.E., Bereika M.F.F., Abo-Elnaga H.I.G., Sallam M.A.A. Direct antimicrobial activity and induction of systemic resistance in potato plants against bacterial wilt disease by plant extracts. Plant Pathol. J. 2009;25:352–360. doi: 10.5423/PPJ.2009.25.4.352. [DOI] [Google Scholar]
- 13.Abo-Elyousr K.A., Seleim M.A., Abd-El-Moneem K.M., Saead F.A. Integrated effect of Glomus mosseae and selected plant oils on the control of bacterial wilt disease of tomato. Crop Prot. 2014;66:67–71. doi: 10.1016/j.cropro.2014.07.022. [DOI] [Google Scholar]
- 14.Paret M.L., Cabos R., Kratky B.A., Alvarez A.M. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010;94:521–527. doi: 10.1094/PDIS-94-5-0521. [DOI] [PubMed] [Google Scholar]
- 15.Fan W.W., Yuan G.Q., Li Q.Q., Lin W. Antibacterial mechanisms of methyl gallate against Ralstonia solanacearum. Australas. Plant Pathol. 2014;43:1–7. doi: 10.1007/s13313-013-0234-y. [DOI] [Google Scholar]
- 16.Barot K.P., Jain S.V., Kremer L., Singh S., Ghate M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015;24:2771–2798. doi: 10.1007/s00044-015-1350-8. [DOI] [Google Scholar]
- 17.Céspedes C.L., Avila J.G., Martínez A., Serrato B., Calderón-Mugica J.C., Salgado-Garciglia R. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida) J. Agric. Food Chem. 2006;54:3521–3527. doi: 10.1021/jf053071w. [DOI] [PubMed] [Google Scholar]
- 18.Saleem M., Nazir M., Ali M.S., Hussain H., Lee Y.S., Riaz N., Jabbar A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010;27:238–254. doi: 10.1039/B916096E. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y.M. Master’s Thesis. Southwest University; Chongqing, China: 2015. The Antibacterial Effects and Mechanism of Several Botanical Compounds against Ralstonia solanacearum. [Google Scholar]
- 20.Lin M.H., Chou Y.S., Tsai Y.J., Chou D.S. Antioxidant properties of 5, 7-dihydroxycoumarin derivatives in in vitro cell-free and cell-containing systems. J. Exp. Clin. Med. 2011;3:126–131. doi: 10.1016/j.jecm.2011.04.006. [DOI] [Google Scholar]
- 21.Jurd L., Corse J., King A.D., Bayne H., Mihara K. Antimicrobial properties of 6,7-dihydroxy-, 7,8-dihydroxy-, 6-hydroxy-and 8-hydroxycoumarins. Phytochemistry. 1971;10:2971–2974. doi: 10.1016/S0031-9422(00)97334-5. [DOI] [Google Scholar]
- 22.Nazari Z.E., Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 23.Konc J.T., Hejchman E., Kruszewska H., Wolska I., Maciejewska D. Synthesis and pharmacological activity of O-aminoalkyl derivatives of 7-hydroxycoumarin. Eur. J. Med. Chem. 2011;46:2252–2263. doi: 10.1016/j.ejmech.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Khan R., Bhat K.A., Raja A.F., Shawl A.S., Alam M.S. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall. ex G. Don, Ericaceae. Rev. Bras. Farmacogn. 2010;20:886–890. [Google Scholar]
- 25.Kabeya L.M., da Silva C.H., Kanashiro A., Campos J.M., Azzolini A.E.C., Polizello A.C.M., Pupo M.T., Lucisano-Valim Y.M. Inhibition of immune complex-mediated neutrophil oxidative metabolism: A pharmacophore model for 3-phenylcoumarin derivatives using GRIND-based 3D-QSAR and 2D-QSAR procedures. Eur. J. Med. Chem. 2008;43:996–1007. doi: 10.1016/j.ejmech.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Tan F., Shi B., Li J., Wu W., Zhang J. Design and synthesis of new 2-aryl-4, 5-dihydro-thiazole analogues: In vitro antibacterial activities and preliminary mechanism of action. Molecules. 2015;20:20118–20130. doi: 10.3390/molecules201119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witaicenis A., Seito L.N., da Silveira Chagas A., de Almeida L.D., Luchini A.C., Rodrigues-Orsi P., Cestari S.H., Di Stasi L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. 2014;21:240–246. doi: 10.1016/j.phymed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Turkmen N., Velioglu Y.S., Sari F., Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–496. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddox C.E., Laur L.M., Tian L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010;60:53–58. doi: 10.1007/s00284-009-9501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Zhou F., Ji B.P., Pei R.S., Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008;47:174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- 31.Hassan S., Mathesius U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012;63:3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- 32.Kawase M., Sakagami H., Hashimoto K., Tani S., Hauer H., Chatterjee S.S. Structure-cytotoxic activity relationships of simple hydroxylated coumarins. Anticancer Res. 2002;23:3243–3246. [PubMed] [Google Scholar]
- 33.Yao J., Allen C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 2007;189:6415–6424. doi: 10.1128/JB.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kai K., Shimizu B.I., Mizutani M., Watanabe K., Sakata K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry. 2006;67:379–386. doi: 10.1016/j.phytochem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Gnonlonfin G.B., Sanni A., Brimer L. Review scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012;31:47–56. doi: 10.1080/07352689.2011.616039. [DOI] [Google Scholar]
- 36.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strathmann M., Wingender J., Flemming H.C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods. 2002;50:237–248. doi: 10.1016/S0167-7012(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 38.Genin S., Denny T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012;50:67–89. doi: 10.1146/annurev-phyto-081211-173000. [DOI] [PubMed] [Google Scholar]
- 39.Peeters N., Guidot A., Vailleau F., Valls M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013;14:651–662. doi: 10.1111/mpp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L., Xu J., Xu J., Zhang H., He L., Feng J. TssB is essential for virulence and required for type VI secretion system in Ralstonia solanacearum. Microb. Pathog. 2014;74:1–7. doi: 10.1016/j.micpath.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Wu D., Ding W., Zhang Y., Liu X., Yang L. Oleanolic acid induces the type III secretion system of Ralstonia solanacearum. Front. Microbiol. 2015;6:1466. doi: 10.3389/fmicb.2015.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimochi T., Zhang Y., Kiba A., Hikichi Y., Ohnishi K. Expression of hrpG and activation of response regulator HrpG are controlled by distinct signal cascades in Ralstonia solanacearum. J. Gen. Plant Pathol. 2009;75:196–204. doi: 10.1007/s10327-009-0157-1. [DOI] [Google Scholar]
- 43.Dalgaard P., Ross T., Kamperman L., Neumeyer K., McMeekin T.A. Estimation of bacterial growth rates from turbidimetric and viable count data. Int. J. Food Microbiol. 1994;23:391–404. doi: 10.1016/0168-1605(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 44.Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48(Suppl. 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 45.Smyth T., Ramachandran V.N., Smyth W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents. 2009;33:421–426. doi: 10.1016/j.ijantimicag.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Galal A.A., Kehil Y.E.I., El-Daoudi Y.H., Shihata Z.A., Ouf M.F. A Comparative Study on the Identification of Races and Biovars of some Egyptian Isolates of Ralsionia solanaceamm. Egypt J. Phytopathol. 2003;31:103–117. [Google Scholar]
- 47.Tans-Kersten J., Brown D., Allen C. Swimming motility, a virulence trait of Ralstonia solanacearum, is regulated by FlhDC and the plant host environment. Mol. Plant Microbe Interact. 2004;17:686–695. doi: 10.1094/MPMI.2004.17.6.686. [DOI] [PubMed] [Google Scholar]
- 48.Chen J., Peng H., Wang X., Shao F., Yuan Z., Han H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale. 2014;6:1879–1889. doi: 10.1039/C3NR04941H. [DOI] [PubMed] [Google Scholar]
- 49.Monteiro F., Genin S., van Dijk I., Valls M. A luminescent reporter evidences active expression of Ralstonia solanacearum type III secretion system genes throughout plant infection. Microbiology. 2012;158:2107–2116. doi: 10.1099/mic.0.058610-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.