Abstract
Successful alkylations of the nitrogen of ethyl indol-2-carboxylate were carried out using aq. KOH in acetone. The respective N-alkylated acids could be obtained without separating the N-alkylated esters by increasing the amount of KOH and water. The use of NaOMe in methanol led to transesterification instead of the alkylation, while the use of NaOEt led to low yields of the N-alkylated acids. Hydrazinolysis of the ester gave indol-2-carbohydrazide which then was allowed to react with different aromatic aldehydes and ketones in ethanol catalyzed by acetic acid. Indol-2-thiosemicarbazide was used in a heterocyclization reaction to form thiazoles. The new structures were confirmed using NMR, mass spectrometry and X-ray single crystal analysis.
Keywords: ethyl indol-2-carboxylate, alkylation, hydrazinolysis, single-crystal X-ray diffraction
1. Introduction
Indole derivatives have been a topic of substantial research interest and continue to be one of the most active areas of heterocyclic chemistry, particularly due to their natural occurrence and pharmacological activities [1]. Indole derivatives also occur widely in many natural products such as those obtained from plants [2], fungi [3], and marine organisms [4]. The isolation, biological evaluation, and chemical properties of natural products have attracted the attention of organic chemists, medicinal chemists, biologists and pharmacists as well as led to optimization of highly efficient and economical synthetic routes.
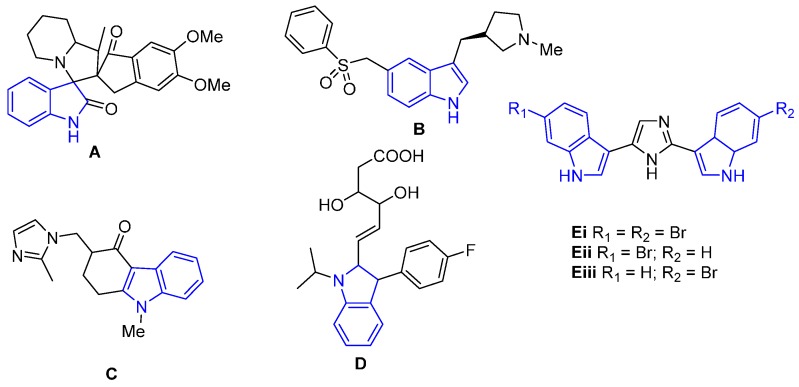
At present there are several thousand indole alkaloids described, including simple and more complex functionalized indole compounds [5]. The growing importance of substituted indoles (Figure 1) in the field of medicinal chemistry as potential chemotherapeutic agents and their implication for pro-drug design have been previously reported [6,7,8,9,10,11,12,13,14,15].
Figure 1.
Biologically active compounds incorporating an indole scaffold.
The indazoline A is an indole derivative inhibitor of acetylcholinesterase used to treat Alzheimer’s disease [16]. The indole derivative eletriptan (B) is an anti-migraine drug. A process route for the synthesis of eletriptan published by Pfizer starts from a preformed bromoindole [17]. Fluvastatin (C) is a member of the statin drug class, used to treat hypercholesterolemia and to prevent cardiovascular diseases. It has also been shown to exhibit antiviral activity against hepatitis C [18]. Ondansetron (D) is a indole derivative used mainly as an antiemetic [19]. It is indicated for the prevention of acute nausea and vomiting associated with cancer chemotherapy [20].
Bis-indole alkaloids are an important structural class due to their high degree of biological activity. For example, the nortopsentins Ei-iii exhibit in vitro cytotoxicity against P388 cells with IC50 (inhibitory concentration) values of 7.6, 7.8, and 1.7 µg/mL, respectively [21,22,23].
Given the significant pharmacological activities associated with these heterocycles, and in order to contribute to the development of the chemistry of indole [24,25,26,27,28,29,30,31,32], we were interested in the synthesis of new heterocyclic polyfunctional indole derivative systems using alkylation reactions.
2. Results
Alkylation of the nitrogen of the indole ring in indole-containing compounds requires strong bases to generate the indole anion [33]. Protecting the nitrogen of the indole ring in ethyl 1H-indol-2-carboxylate requires special care to avoid the ester hydrolysis before the alkylation. KOH in anhydrous DMSO was used for the alkylation of nitrogen of the indole esters [34]. Herein, we describe the alkylation of the indole nitrogen using aq. KOH in acetone. In this method we can control the reaction to give the alkylated esters or the alkylated acids in the same reaction process, with the additional benefit of the ease of solvent removal after reaction completion.
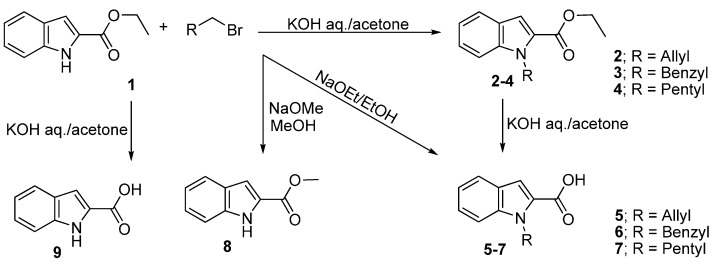
Reaction of ethyl indol-2-carboxylate (1) with allyl bromide and benzyl bromide in the presence of aq. KOH (3.0 mmol/0.1 mL H2O/10 mL acetone) and stirring for two hours at 20 °C afforded ethyl 1-allyl-1H-indole-2-carboxylate (2) and ethyl 1-benzyl-1H-indole-2-carboxylate (3) in excellent yields. The corresponding alkylated carboxylic acids 1-allyl-1H-indol-2-carboxylic acid (5) and 1-benzyl-1H-indol-2-carboxylic acid (6) were obtained in high yields directly without separating the alkylated esters by increasing the amount of aq. KOH and refluxing for one hour. Alkylation with amyl bromide seems to be slow, since it took about eight hours to give ethyl 1-pentyl-1H-indole-2-carboxylate (4) under the same conditions. Moreover, a considerable amount of 1H-indol-2-carboxylic acid (9) was detected. The alkylated acids 5–7 were also obtained in excellent yields from the hydrolysis of the respective esters 2–4 using aqueous KOH in acetone. The use of NaOMe in methanol led to transesterification to afford methyl indol-2-carboxylate (8) instead of NH alkylation, whereas using NaOEt in ethanol gave the acids 5 and 6 in low to moderate yields in the case of the of 1 with allyl and benzyl bromides whereas, in case of amyl bromide a high yield of 1H-indol-carboxylic acid 9 was obtained (Scheme 1, Table 1).
Scheme 1.
Alkylation of indole nitrogen, transesterification and ester hydrolysis.
Table 1.
Conditions of indole nitrogen alkylation and ester hydrolysis.
| Entry | Reactant | R-Br | Base | T (°C) | Time (h) | Product | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | Allyl-Br | KOH (3.0 mmol/0.1 mL·H2O) | 20 | 2.0 | 2 | 85 |
| 2 | 1 | Benzyl-Br | KOH (3.0 mmol/0.1 mL·H2O) | 20 | 2.0 | 3 | 94 |
| 3 | 1 | Amyl-Br | KOH (3.0 mmol/0.1 mL·H2O) | 20 | 8.0 | 4/9 | 60/30 |
| 4 | 2 | - | KOH (6.0 mmol/1.0 mL·H2O) | 60 | 1.0 | 5 | 95 |
| 5 | 3 | - | KOH (6.0 mmol/1.0 mL·H2O) | 60 | 1.0 | 6 | 97 |
| 6 | 4 | - | KOH (6.0 mmol/1.0 mL·H2O) | 60 | 1.0 | 7 | 90 |
| 7 | 1 | Allyl-Br | NaOEt (6.0 mmol)/EtOH | 60 | 2 | 5 | 35 |
| 8 | 1 | Benzyl-Br | NaOEt (6.0 mmol)/EtOH | 60 | 2 | 6 | 40 |
| 9 | 1 | Amyl-Br | NaOEt (6.0 mmol)/EtOH | 60 | 2 | 9 | 90 |
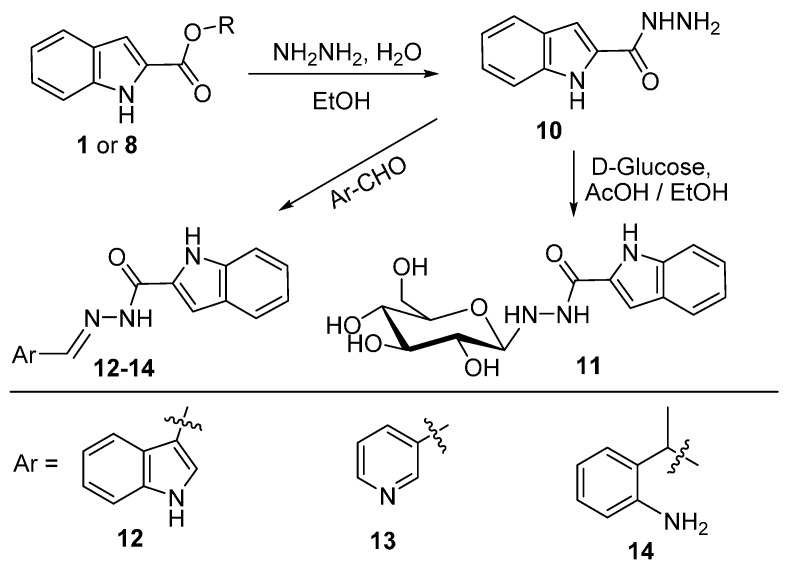
Hydrazinolysis of either ethyl or methyl esters 1 or 8 afforded indol-2-carbohydrazide (10). The hydrazide 10 was reacted with D-glucose, indol-3-carboxyaldehyde, pyridine-3-carboxyaldehyde and 2′-aminoacetophenone in ethanol and drops of acetic acid to yield N′-β-d-glucopyranosyl-1H-indole-2-carbohydrazide (11), N′-((1H-indol-3-yl)methylene)-1H-indole-2-carbohydrazide (12), N′-(pyridin-3-ylmethylene)-1H-indole-2-carbohydrazide (13) and N′-(1-(2-aminophenyl)ethylidene)-1H-indole-2-carbohydrazide (14), respectively (Scheme 2).
Scheme 2.
Reaction of indol-2-carbohydrazide 10 with aldehydes and ketones.
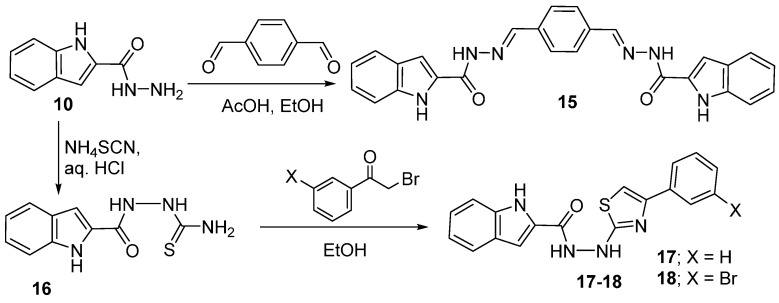
Moreover, hydrazide 10 was reacted with terephthalaldehyde under the same conditions to give the bis-indolyl product 15. A thiosemicarbazide 16 was obtained from the hydrazide 10 and served as adduct for cyclization with two phenacyl bromides to afford indolylcarbonylhydrazino-thiazoles 17 and 18 (Scheme 3).
Scheme 3.
Synthesis of thiazoles 17 and 18.
2.1. Structural Analysis
All NMR spectra showed the indole CH protons between δ 7.00 and 7.70 ppm and all indole carbons from δ 103.0 to 138.0 ppm.
2.2. Alkylated Ester Analysis
The formation of ethyl N-alkylated indol-2-carboxylates 2–4 was confirmed by the disappearance of the indole NH proton signal from the 1H-NMR of these compounds, the presence of ethoxy group signals (-OCH2CH3) at δ 1.30 and 4.30 ppm and the respective carbons in 13C-NMR at δ 15.0 and 46.7 ppm in addition to the ester carbonyl group around δ 162.0 ppm. Moreover, new signals appeared in the NMR spectra which are characteristic for the new groups and can be summarized as follows: in compound 2 the signals of the allyl group appeared as two doublets at δ 4.81 and 5.06 ppm for the olefinic CH2 with coupling constants δ 16.8 and 10.2 Hz, respectively, the NCH2 appeared at 5.23 ppm and the corresponding carbon (NCH2) appeared at δ 46.7 ppm, whereas, the remaining olefinic CH appeared as multiplet at δ 5.97–6.07 ppm. The benzylated ester 3 showed the NCH2 protons as a singlet at δ 5.87 ppm and the respective carbon at δ 47.6 ppm in addition to the phenyl protons and carbons. The pentylated ester 4 showed characteristic signals for the pentyl group protons at δ 0.83, 1.23–1.29, 1.68–1.70 and 4.55 ppm and the corresponding carbons appeared at δ 14.3, 22.3, 28.8, 30.4 and 44.4 ppm.
2.3. Hydrolyzed Ester Data Analysis
Hydrolysis of the esters with time was deduced from the disappearance of the signals of the ethoxy group in the NMR of 5–7 and instead a new broad signal appeared around δ 12.90 ppm for COOH, in addition to characteristic allyl, benzyl and pentyl signals.
Transesterification and formation of the methyl ester 8 was confirmed by 1H-NMR by observing a singlet signal at δ 3.88 ppm for the ester methyl group and the indole NH at δ 11.91 ppm. The respective methyl carbon appeared in 13C-NMR at δ 52.2 ppm and the ester carbonyl appeared at δ 162.3 ppm. The acid 9 lacked any alkyl signals and showed the C=O at δ 163.0 ppm, in addition to the remaining expected protons and carbons.
2.4. Hydrazinolysis of the Esters and the Related Products Analysis
Hydrazinolysis of either indol-2-carboxylate 1 or 2 led to the formation of indol-2-carboxylic acid hydrazide (10). The structure of 10 was confirmed by NMR which showed only two signals at δ 4.52 and 9.78 ppm for the -NH-NH2 group, whereas the carbonyl group appeared at δ 161.7 ppm. The 1H-NMR (DMSO-d6 + D2O) of 11 showed the anomeric proton as a doublet at 3.87 ppm with a coupling constant value of 8.7 Hz which confirms the β-configuration. The corresponding anomeric carbon appeared in 13C-NMR at 90.9 ppm and the carbonyl carbon signal appeared at 161.3 ppm.
The NMR spectra of 12, 13 showed the -CH=N- proton around δ 8.60 ppm whereas, the NMR of 14 showed the methyl protons at 2.43 ppm and the respective methyl carbon at 15.5 ppm in addition to the remaining aromatic signals. The NMR spectra of 15 showed a singlet signal at δ 8.51 ppm for the two –CH=N- protons and two signals at δ 11.86 and 12.02 ppm for indole and hydrazide NHs, besides all aromatic protons and carbons signals.
2.5. Thiazole Structural Analysis
The 1H-NMR spectra of thiazoles 17 and 18 showed all aromatic CH protons of thiazole, indole and phenyl in the range of δ 7.07–7.83 ppm, while the three NH protons appeared as broad signals at δ 9.69, 10.87 and 11.76 ppm. In addition, the 13C-NMR of 17 and 18 showed the carbonyl carbons at δ 161.42 and 161.35 ppm, respectively.
2.6. X-ray Diffraction Analysis
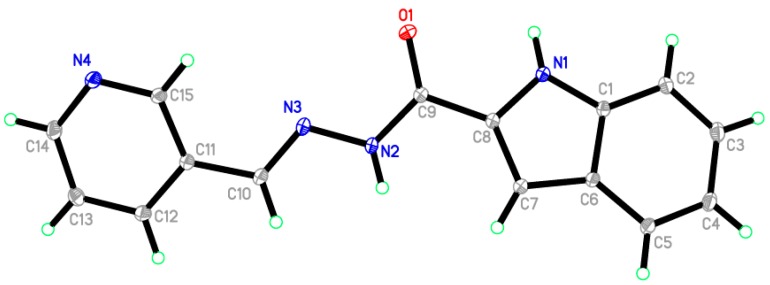
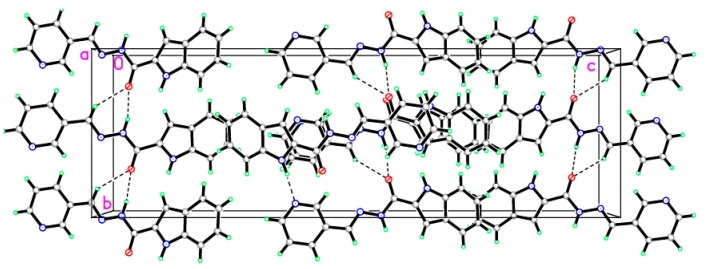
The structure of 13 was confirmed by X-ray crystal structural analysis. The crystallographic data, conditions retained for the intensity data collection and some features of the structure refinements are listed in Table 2. Selected interatomic distances and bond angles are given in Table 3. The unit cell of the titled compound contains one molecule. All of the bond lengths and bond angles in the phenyl rings are in the normal range. The indole ring (C1—C8/N1) forms a dihedral angle of 28.05° with the pyridine-3-yl ring (C11-C14/N4/C15). The title compound exists in trans configuration with respect to the C10=N3 bond [1.2805 (15) Å] as shown in Figure 2. In the crystal structure (Figure 3), molecules are linked via three intermolecular N1—H1N1···N4, N2—H1N2···O1 and C10—H10A···O1 hydrogen bonds in b axis (Table 4).
Table 2.
The crystal structure and refinement data of compound 13.
| Crystal Data | |
| Chemical formula | C15H12N4O |
| Mr | 264.29 |
| Crystal system, space group | Orthorhombic, Pbca |
| Temperature (K) | 100 |
| a, b, c (Å) | 7.8503 (2), 10.1780 (3), 31.9226 (9) |
| V (Å3) | 2550.63 (12) |
| Z | 8 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.09 |
| Crystal size (mm) | 0.47 × 0.35 × 0.23 |
| Data Collection | |
| Diffractometer | Bruker APEX-II D8 venture diffractometer |
| Absorption correction | Multi-scan, SADABS Bruker 2014 |
| Tmin, Tmax | 0.844, 0.881 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 17978, 2927, 2645 |
| Rint | 0.032 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.037, 0.104, 1.04 |
| No. of reflections | 2927 |
| No. of parameters | 190 |
| No. of restraints | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e·Å−3) | 0.36, −0.22 |
Table 3.
Selected bond lengths and bond angles in compound 13.
| Atoms | Å, ° | Atoms | Å, ° |
|---|---|---|---|
| O1—C9 | 1.2301 (14) | N2—C9 | 1.3594 (15) |
| N1—C1 | 1.3700 (14) | N3—C10 | 1.2805 (15) |
| N1—C8 | 1.3773 (15) | N4—C14 | 1.3430 (16) |
| N2—N3 | 1.3771 (14) | N4—C15 | 1.3376 (14) |
| C1—N1—C8 | 108.34 (9) | N1—C8—C9 | 117.81 (10) |
| N3—N2—C9 | 119.20 (9) | O1—C9—C8 | 121.67 (10) |
| N2—N3—C10 | 115.30 (10) | N2—C9—C8 | 114.68 (10) |
| C14—N4—C15 | 117.59 (10) | O1—C9—N2 | 123.62 (10) |
| N1—C1—C6 | 108.28 (9) | N3—C10—C11 | 119.51 (10) |
| N1—C1—C2 | 129.58 (11) | N4—C14—C13 | 122.99 (12) |
| N1—C8—C7 | 110.20 (9) | N4—C15—C11 | 123.74 (10) |
Figure 2.
ORTEP diagram of the titled compound 11 drawn at 50% ellipsoids for non-hydrogen atoms.
Figure 3.
A view of the crystal packing down the b axis for the title compound. Dotted lines indicates the intermolecular interaction.
Table 4.
Hydrogen-bond geometry (Å, °) in compound 13.
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| N1—H1N1···N4i | 0.915(17) | 1.975(17) | 2.8856(14) | 173.4(14) |
| N2—H1N2···O1ii | 0.915(17) | 2.135(17) | 2.9909(13) | 155.4(14) |
| C10—H10A···O1ii | 0.9300 | 2.4300 | 3.2266(14) | 144.00 |
| Symmetry codes: (i) x − 1/2, −y + 3/2, −z + 1; (ii) –x + 3/2, y + 1/2, z. | ||||
3. Experimental Section
3.1. General Details
Melting points were measured with a Stuart melting-point apparatus (SMP10, Bibby Scientific Ltd., Staffordshire, UK) in open capillaries and are uncorrected. Flash chromatography was done on silica gel 60 (230–400 mesh ASTM). TLC was performed on silica gel 60 F254 (Merck Millipore, Darmstadt, Germany) and spots were detected by absorption of UV light. 1H-NMR spectra were recorded on Advanced NMR spectrometers (Bruker Biospin, Fallanden, Switzerland) at 300–600 MHz whereas 13C-NMR spectra were recorded on the same instruments at 75–150 MHz, with TMS as internal standard. Mass spectra were obtained using MAT312 (ThermoFinnigan GmbH, Tokyo, Japan) and a JMS.600H (Jeol, Tokyo, Japan) instruments for EIMS; HRMS spectra were recorded on a Thermo Finnigan MAT 95XP and Jeol JMS HX110 and ESI on an Ion Trap 6320 mass detector (Agilent Technologies, Wilmington, DE, USA). IR spectra were recorded using KBr discs on a Bruker FT-IR IFS 48 spectrophotometer (Bruker Optics, Ettlingen, Germany).
3.2. General Procedure for the Alkylation of Ethyl Indol-2-carboxylate (1)
A solution of ethyl indol-2-carboxylate (1, 1.0 mmol) and aq. KOH (3.0 mmol) in acetone (10 mL) was stirred at 20 °C for half hour, then the appropriate alkylating agent (1.1 mmol) was added and stirring was continued for 2 h to give 2 and 3 and for eight hours to give 4. The solvent was removed, water was added and organic layer was extracted using ethyl acetate. The products were purified using column chromatography (ethyl acetate/hexane 1:9).
3.3. Hydolysis of the Ester and Formation of Acids 5–7
Method a: the above procedure was followed until the alkylation was complete, then KOH (6.0 mmol in 1.0 mL·H2O) was added and the reaction mixture refluxed one hour, the solvent removed, cold water added, and acidified. The ppt was collected and purified by crystallization from ethanol in the case of 5 and 6 and from hexane in the case of 7.
Method b: a solution of the appropriate ester 2–4 and KOH (6.0 mmol in 1.0 mL·H2O) and acetone (10 mL) was refluxed for one hour, then the above purification process was followed.
Method c: A mixture of ethyl indol-2-carboxylate (1, 1.0 mmol) and NaOEt (3.0 mmol) in ethanol (10 mL) was stirred for half an hour then, alkylating agent was added and the mixture heated under reflux for two hours and the above purification process followed.
Ethyl 1-allyl-1H-indole-2-carboxylate (2): Colorless oil, Rf 0.65 (ethyl acetate/n-hexane 1:9); 1H-NMR (DMSO-d6, 600 MHz) δ 1.33 (t, 3H, J 6.3 Hz, CH3), 4.32 (q, 2H, OCH2CH3), 4.81 (d, 1H, Jtrans 16.8 Hz, -NCH2-CH=CHH), 5.06 (d, 1H, Jcis 10.2 Hz, -NCH2-CH=CHH), 5.23 (s, 2H, -NCH2-CH=CH2), 5.97–6.07 (m, 1H, -NCH2-CH=CHH), 7.15 (dd, 1H, J4,5 7.8, J5,6 7.2 Hz, H-5Indol), 7.32–7.34 (m, 2H, H-3Indol, H-6Indol), 7.56 (d, 1H, J6,7 8.4 Hz, H-7Indol), 7.71 (d, 1H, J4,5 7.8 Hz, H-4Indol); 13C-NMR (DMSO-d6, 150 MHz) δ 14.7 (CH3), 46.7 (-NCH2-CH=CH2), 60.9 (OCH2CH3), 110.7 (C-3Indol), 111.6 (C-7Indol), 116.2 (-NCH2-CH=CH2), 121.1 (C-5Indol), 122.9 (C-4Indol), 125.5 (C-6Indol), 125.9 (C-2Indol), 127.6 (C-3aIndol), 135.0 (-NCH2-CH=CH2), 139.2 (C-7aIndol), 161.6 (C=O); LRMS-ESI+ m/z (int. %): 58.7 (6), 79.3 (55), 101.1 (11), 142.1 (100), 182.0 (10), 216.9 (31), 230 (18 for M + H).
Ethyl 1-benzyl-1H-indole-2-carboxylate (3): White ppt, m.p. 52–53 °C (lit. [26] 55–56 °C), Rf 0.61 (ethyl acetate/n-hexane 1:9); 1H-NMR (DMSO-d6, 600 MHz) δ 1.28 (t, 3H, J 7.2 Hz, CH3), 4.27 (q, 2H, OCH2CH3), 5.87 (s, 2H, -NCH2-Ph), 7.05 (d, 2H, J 7.8 Hz, 2 HPh), 7.14–7.33 (m, 5H, H-5Indol, H-6Indol, 3 HPh), 7.40 (s, 1H, H-3Indol), 7.57 (d, 1H, J6,7 8.4 Hz, H-7Indol), 7.74 (d, 1H, J4,5 7.8 Hz, H-4Indol); 13C-NMR (DMSO-d6, 150 MHz) δ 14.5 (OCH2CH3), 47.6 (NCH2-Ph), 60.9 (OCH2CH3), 111.1, 111.8 (C-3Indol, C-7Indol), 121.3 (C-5Indol), 123.0 (C-4Indol), 125.7 (C-6Indol), 126.1 (C-2Indol), 126.7 (2 CHPh), 127.5 (CHPh) 127.7 (C-3aIndol), 128.9 (2 CHPh), 138.9, 139.6 (CPh, C-7aIndol), 161.7 (C=O).
Ethyl 1-pentyl-1H-indole-2-carboxylate (4): Colorless oil, Rf 0.75 (ethyl acetate/n-hexane 1:9); 1H-NMR (DMSO-d6, 600 MHz) δ 0.83 (t, 3H, J 6.3 Hz, CH3), 1.23–1.29 (m, 4H, 2 CH2), 1.34 (t, 3H, J 6.6 Hz, OCH2CH3), 1.68–1.70 (m, 2H, CH2), 4.33 (q, 2H, OCH2CH3), 4.55 (t, 2H, J 6.6 Hz, -NCH2-), 7.13 (dd, 1H, J4,5 7.8, J5,6 7.2 Hz, H-5Indol), 7.28 (s, 1H, H-3Indol), 7.34 (dd, 1H, J5,6 7.2, J6,7 8.4 Hz, H-6Indol), 7.59 (d, 1H, J6,7 8.4 Hz, H-7Indol), 7.68 (d, 1H, J4,5 7.8 Hz, H-4Indol); 13C-NMR (DMSO-d6, 150 MHz) δ 14.3, 14.6 (2 CH3), 22.3, 28.8, 30.4 (3 CH2), 44.4 (NCH2-), 60.8 (OCH2CH3), 110.1, 111.4 (C-3Indol, C-7Indol), 120.9 (C-5Indol), 122.8 (C-4Indol), 125.4 (C-6Indol), 125.8 (C-2Indol), 127.5 (C-3aIndol), 139.2 (C-7aIndol), 161.7 (C=O).
1-Allyl-1H-indole-2-carboxylic acid (5): White solid, m.p. 178–179 °C, Rf 0.51 (ethyl acetate/n-hexane 3:7); 1H-NMR (DMSO-d6, 600 MHz) δ 4.81 (d, 1H, Jtrans 17.4 Hz, -NCH2-CH=CHH), 5.05 (d, 1H, Jcis 10.2 Hz, -NCH2-CH=CHH), 5.24 (d, 2H, J 1.2 Hz, -NCH2-CH=CH2), 5.96–6.00 (m, 1H, -NCH2-CH=CHH), 7.13 (dd, 1H, J4,5 7.8, J5,6 7.2 Hz, H-5Indol), 7.27 (s, 1H, H-3Indol), 7.32 (dd, 1H, J5,6 7.2, J6,7 8.4 Hz, H-6Indol), 7.53 (d, 1H, J6,7 8.4 Hz, H-7Indol), 7.69 (d, 1H, J4,5 7.8 Hz, H-4Indol), 12.92 (br.s, 1H, -COOH); 13C-NMR (DMSO-d6, 150 MHz) δ 46.5 (-NCH2-CH=CH2), 110.5, 111.5 (C-3Indol, C-7Indol), 116.1 (-NCH2-CH=CH2), 120.9 (C-5Indol), 122.9 (C-4Indol), 125.2 (C-6Indol), 126.0 (C-2Indol), 128.4 (C-3aIndol), 135.2 (-NCH2-CH=CH2), 139.2 (C-7aIndol), 163.2 (C=O); LRMS-ESI− m/z (int. %): 116.0 (20), 155.9 (50), 199.9 (100 for M − H).
1-Benzyl-1H-indole-2-carboxylic acid (6): White solid, m.p. 190–191 °C (lit. [26] 194–196 °C), Rf 0.50 (ethyl acetate/n-hexane 3:7); 1H-NMR (DMSO-d6, 600 MHz) δ 5.89 (s, 2H, -NCH2-Ph), 7.04 (d, 2H, J 6.6 Hz, 2 HPh), 7.12–7.29 (m, 5H, H-5Indol, H-6Indol, 3 HPh), 7.34 (s, 1H, H-3Indol), 7.54 (d, 1H, J6,7 8.4 Hz, H-7Indol), 7.72 (d, 1H, J4,5 7.8 Hz, H-4Indol), 12.99 (br.s, 1H, -COOH); 13C-NMR (DMSO-d6, 150 MHz) δ 47.4 (NCH2-Ph), 110.9, 111.7 (C-3Indol, C-7Indol), 121.1 (C-5Indol), 122.8 (C-4ndol), 125.4 (C-6Indol), 126.1 (C-2Indol), 126.7 (2 CHPh), 127.4 (CHPh) 128.7 (C-3aIndol), 128.9 (2 CHPh), 139.1, 139.4 (CPh, C-7aIndol), 163.4 (C=O); LRMS-ESI+ m/z (int. %): 142.1 (10), 156.9 (30), 169.9 (11), 178.8 (13), 252.0 (100 for M + H).
1-Pentyl-1H-indole-2-carboxylic acid (7): White solid, m.p. 91–92 °C, Rf 0.43 (ethyl acetate/n-hexane 3:7); 1H-NMR (CDCl3, 400 MHz) δ 0.93 (t, 3H, J 6.9 Hz, CH3), 1.28–1.42 (m, 4H, 2 CH2), 1.82–1.89 (m, 2H, CH2), 4.61 (t, 2H, J 7.5 Hz, NCH2), 7.19 (dd, 1H, J5,6 7.4, J4,5 8.0 Hz, H-5Indol), 7.40 (dd, 1H, J5,6 7.4, J6,7 8.1 Hz, H-6Indol), 7.45 (d, 1H, J6,7 8.1 Hz, H-7Indol), 7.51 (s, 1H, H-3Indol), 7.74 (d, 1H, J4,5 8.0 Hz, H-4Indol); IR (KBr): νmax/cm−1 2500–3500 (OH acid), 1684.1 (C=O acid).
3.4. Transesterification Procedures
A mixture of ethyl indol-2-carboxylate 1 (1.0 mmol) with or without the alkylating agents and NaOMe (4.0 mmol) in methanol (10 mL) was stirred for one hour. The mixture was acidified and the precipitate was collected, dried and crystallized from ethanol or purified by sublimation.
Methyl 1H-indole-2-carboxylate (8): Yield: 89% as colorless needle crystals, m.p. 149–150 °C, Rf 0.28 (ethyl acetate/n-hexane 1:9); 1H-NMR (DMSO-d6, 600 MHz) δ 3.88 (s, 3H, CH3), 7.09 (dd, 1H, J4,5 7.8, J5,6 7.2 Hz, H-5Indol), 7.18 (s, 1H, H-3Indol), 7.27 (dd, 1H, J5,6 7.2, J6,7 8.4 Hz, H-6Indol), 7.49 (d, 1 H, J6,7 8.4 Hz, H-7Indol), 7.66 (d, 1H, J4,5 7.8 Hz, H-4Indol), 11.91 (s, 1H, NHIndol); 13C-NMR (DMSO-d6, 150 MHz) δ 52.2 (CH3), 108.3 (C-3Indol), 113.1 (C-7Indol), 120.7 (C-5Indol), 122.5 (C-4Indol), 125.1 (C-6Indol), 127.2, 127.5 (C-2Indol, C-3aIndol), 137.9 (C-7aIndol), 162.3 (C=O); LRMS-ESI− m/z (int. %): 89.0 (6), 113.1 (7), 145.9 (5), 158.9 (10), 173.9 (100 for M − H).
3.5. Synthesis of 9 Following Methods b: Starting with 1 or 8
1H-Indole-2-carboxylic acid (9): Yield: 55% as yellow solid, m.p. 203–204 °C, Rf 0.30 (ethyl acetate/n-hexane 3:7); 1H-NMR (DMSO-d6, 600 MHz) δ 7.06 (dd, 1H, J4,5 7.8, J5,6 7.2 Hz, H-5Indol), 7.11 (s, 1H, H-3Indol), 7.24 (dd, 1H, J5,6 7.2, J6,7 8.4 Hz, H-6Indol), 7.46 (d, 1H, J6,7 8.4 Hz, H-7Indol), 7.65 (d, 1H, J4,5 7.8 Hz, H-4Indol), 11.74 (s, 1H, NHIndol); 13C-NMR (DMSO-d6, 150 MHz) δ 107.79, 112.96 (C-3Indol, C-7Indol), 120.4 (C-5Indol), 122.4 (C-4ndol), 124.7 (C-6Indol), 127.3, 128.9 (C-2Indol, C-3aIndol), 137.7 (C-7aIndol), 163.3 (C=O); LRMS-ESI− m/z (int. %): 115.9 (25), 159.8 (100 for M + H).
3.6. Hydrazide Formation
Either ethyl indol-2-carboxylate 1 or methyl indol-2-carboxylate 8 was refluxed with hydrazine hydrate in ethanol (4 h), the formed ppt was collected and crystalized from 95% ethanol.
1H-Indole-2-carbohydrazide (10): Yield: 90% as colorless crystals, m.p. 247–248 °C (lit. [17,18]), Rf 0.43 (9:1 CH2Cl2/MeOH); 1H-NMR (DMSO-d6, 600 MHz): δ 4.52 (s, 2H, NH2), 7.03 (dd, 1H, J5,6 7.2, J4,5 7.8 Hz, H-5Indol), 7.10 (s, 1H, H-3Indol), 7.18 (dd, 1H, J5,6 7.2, J6,7 7.8 Hz, H-6Indol), 7.45 (d, 1H, J6,7 7.8 Hz, H-7Indol), 7.60 (d, 1H, J4,5 7.8 Hz, H-4Indol), 9.78 (s, 1H, NH), 11.60 (s, 1H, NHIndol); 13C-NMR (DMSO-d6, 150 MHz): δ 102.3 (C-3Indol), 112.7 (C-7Indol), 120.2 (C-5Indol), 121.9 (C-4Indol), 123.6 (C-6Indol), 127.6, 131.0, 136.8 (C-2Indol, C-3aIndol, C-7aIndol), 161.7 (C=O). LRMS-ESI− m/z (Int. %): 115.9 (6), 173.9 (100 for M − H).
N′-β-d-Glucopyranosyl-1H-indole-2-carbohydrazide (11): Yield: 60% as white solid, m.p. 208–210 °C, Rf 0.10 (MeOH/DCM 1.5:8.5); 1H-NMR (DMSO-d6 + D2O, 300 MHz) δ 3.02–3.24 (m, 4H, H-5Glc, H-6Glc, H-3Glc, H-4Glc), 3.44 (dd, 1H, J5,6 6, J6,6′ 17.7 Hz, H-6′Glc), 3.66 (dd, 1H, J 9.9 Hz, H-2Glc), 3.87 (d, 1H, J 8.7 Hz, H-1Glc), 7.02 (dd, 1H, J4,5 8.1, J5,6 7.2 Hz, H-5Indol), 7.16–7.23 (m, 2H, H-3Indol, H-6Indol), 7.42 (d, 1H, J6,7 8.1 Hz, H-7Indol), 7.59 (d, 1H, J4,5 8.1 Hz, H-4Indol); 13C-NMR (DMSO-d6 + D2O, 150 MHz) δ 61.3, 70.3, 71.2, 76.6, 78.1, 90.9 (6 CGlc), δ 103.3 (C-3Indol), 112.25 (C-7Indol), 119.8 (C-2Indol), 121.5 (C-5Indol), 123.4 (C-4ndol), 126.9, 129.7, 136.5 (C-6Indol, C-3aIndol, C-7aIndol), 161.3 (C=O); HRMS (+ESI) calcd for C15H20N3O6 [M+]: 338.1352. Found: 338.1348.
3.7. Condensation of Hydrazide with Aromatic Aldehydes and Ketones
A mixture of hydrazide 10 (1.0 mmol) and the appropriate aldehyde or ketone (1.1 mmol) in ethanol (5.0 mL) containing acetic acid (0.5 mL) was refluxed until the ppt appeared. The ppt was filtered and crystalized from DMF or DMF/EtOH mixture.
N'-((1H-Indol-3-yl)methylene)-1H-indole-2-carbohydrazide (12): Yield: 82% as yellowish white needle-like crystals, m.p. 277–278 °C, Rf 0.49 (ethyl acetate/n-hexane 6:4); 1H-NMR (DMSO-d6, 600 MHz): δ 7.08–7.23 (m, 5H), 7.30–7.49 (m, 2H), 7.68 (d, 1H, J 6.6 Hz), 7.86 (s, 1H), 8.32 (d, 1H, J 6.0 Hz), 8.65 (s, 1H), 11.56, 11.61, 11.70 (3s, 1H, 3 NH); 13C-NMR (DMSO-d6, 150 MHz): δ 103.3, 112.2, 112.3, 112.8, 120.3, 120.9, 122.06, 122.4, 123.1, 123.9, 124.8, 127.6, 130.7, 131.2, 137.2, 137.5, 145.0, 157.6 (C=O); LRMS-ESI+ m/z (Int. %): 79.4 (6), 303.1 (100 for M + H).
N′-(Pyridin-3-ylmethylene)-1H-indole-2-carbohydrazide (13): Yield: 71% as colorless needle crystals, m.p. 250–251 °C, Rf 0.35 (ethyl acetate/n-hexane 6:4); 1H-NMR (DMSO-d6, 600 MHz): δ 7.07–7.70 (m, 6H), 8.18 (d, 1H, J 4.8 Hz), 8.52 (s, 1H), 8.63 (s, 1H), 8.90 (s, 1H), 11.83, 12.07 (2 s, 2H, NH, NHIndol); 13C-NMR (DMSO-d6, 150 MHz): δ 104.3 (C-3Indol), 112.9 (C-7Indol), 120.5 (C-5Indol), 122.3 (C-4Indol), 124.5 (C-6Indol, CHPyridin), 127.4, 130.5, 130.8, 133.9, 137.4, 144.8, 151.1, 158.2 (C-2Indol, C-3aIndol, C-7aIndol, 3 CHPyridin, 2 CPyridin), 160.8 (C=O). LRMS-ESI− m/z (Int. %): 79.3 (8), 265.0 (100 for M + H).
N'-(1-(2-Aminophenyl)ethylidene)-1H-indole-2-carbohydrazide (14): Yield: 60% as colorless scale crystals, m.p. 207–209 °C, Rf 0.82 (ethyl acetate/n-hexane 6:4); 1H-NMR (DMSO-d6, 600 MHz): δ 2.43 (s, 3H, CH3), 6.56 (t, 1H, J 7.2 Hz), 6.77 (d, 1H, J 7.8 Hz), 7.07–7.09 (m, 2H), 7.23–7.26 (m, 2H), 7.41 (s, 1H), 7.48 (d, 1H, J 7.8 Hz), 7.50 (d, 1H, J 8.4 Hz), 7.69 (d, 1H, J 7.8 Hz), 10.84 (s, H, NH), 11.79 (s, H, NHIndol); 13C-NMR (DMSO-d6, 150 MHz): δ 15.5 (CH3), 104.9, 112.8, 115.0, 116.6, 118.1, 120.4, 122.2, 124.3, 127.5, 129.6, 130.1, 130.6, 148.5, 157.3, 158.7 (2 C=O).
N′,N′′-(1,4-Phenylenebis(methan-1-yl-1-ylidene))bis(1H-indole-2-carbohydrazide) (15): Yield: 90% as yellowish white solid, m.p. 332–333 °C, Rf 0.77 (ethyl acetate); 1H-NMR (DMSO-d6, 600 MHz): δ 7.09 (dd, 2H, J5,6 7.2, J4,5 7.8 Hz, 2 H-5Indol), 7.25 (dd, 2H, J5,6 7.2, J6,7 6.6 Hz, 2 H-6Indol), 7.25 (s, 2H, 2 H-3Indol), 7.50 (d, 2H, J6,7 7.8 Hz, 2 H-7Indol), 7.70 (br, 2H, 2 H-4Indol), 7.87 (s, 4H, 4 HPh), 8.51 (s, 2H, -N=CH), 11.86 (s, 2H, 2 NHIndol), 12.02 (s, 2H, 2 NH); 13C-NMR (DMSO-d6, 150 MHz): δ 104.30 (2 C-3Indol), 112.9 (2 C-7Indol), 120.5 (2 C-5Indol), 122.3 (2 C-4Indol), 124.4 (2 C-6Indol), 127.5, 128.0, 130.5 (2 C-2Indol, 2 C-3aIndol, 4 CHPh), 136.2, 137.4 (2 CPh, 2 C-7aIndol), 146.9, 158.2 (2 CH=N, 2 C=O). LRMS-ESI+ m/z (Int. %): 79.3 (100), 101.0 (20), 142.1 (62), 237.0 (66), 280.0 (30), 341.9 (12), 448.8 (10 for M + H).
3.8. Synthesis of N′-(4-Aryl-1,3-thiazol-2-yl)-1H-indole-2-carbohydrazides 17, 18
A mixture of 1-[(1H-Indol-2-yl)-carbonyl]-thiosemicarbazide (16 [29], 1.0 mmol) and the respective phenacyl bromide (1.1 mmol) in ethanol (10 mL) was stirred at room temperature for 25 min. A precipitate was formed, filtered, and then recrystallized from ethanol.
N′-(4-Phenyl-1,3-thiazol-2-yl)-1H-indole-2-carbohydrazide (17): Yield: 68% as pink shiny crystals, m.p. 245–247 °C, Rf 0.68 (ethyl acetate/n-hexane 6:4); 1H-NMR (DMSO-d6, 400 MHz) δ 7.07 (dd, 1H, J4,5 8.0, J5,6 7.6 Hz, H-5Indol), 7.19–7.29 (m, 4H, H-3Indol, H-6Indol, H-4Thiazol, CHPh), 7.38 (dd, 2H, J 7.2, J 8.0 Hz, 2HPh), 7.45 (d, 1H, J6,7 8.0 Hz, H-7Indol), 7.66 (d, 1H, J4,5 8.0 Hz, H-4Indol), 7.83 (d, 2H, J 7.6 Hz, 2HPh), 9.69 (br. s, 1H, NH), 10.87 (br. s, 1H, NH), 11.76 (br. s, 1H, NHIndol); 13C-NMR (DMSO-d6, 100 MHz) δ 103.16, 103.69 (C-3Indol, CHThiazol), 112.43 (C-7Indol), 120.06 (C-5Indol), 121.83 (C-4Indol), 123.93 (C-6Indol), 125.60 (2 CHPh), 126.98 (C-3aIndol), 127.53 (CHPh), 128.60 (2 CHPh), 129.19 (C-2Indol), 134.69 (CPh), 136.8 (C-7aIndol), 150.71 (C-4Thiazol), 161.42 (C=O), 172.71 (C-2Thiazol)); HRMS (EI) calcd for C18H14N4OS [M+.]: 334.0888. Found: 334.0890.
N′-(4-(3-Bromophenyl)-1,3-thiazol-2-yl)-1H-indole-2-carbohydrazide (18): Yield: 60% as pink shiny crystals, m.p. 250–251 °C, Rf 0.73 (ethyl acetate/n-hexane 6:4); 1H-NMR (DMSO-d6, 300 MHz) δ 7.07 (dd, 1H, J4,5 7.8, J5,6 7.5 Hz, H-5Indol), 7.19–7.27 (m, 2H, H-3Indol, H-6Indol), 7.35 (dd, 1H, J 7.8, J 8.1 Hz, CHPh), 7.42–7.48 (m, 3H, H-7Indol, H-4Thiazol, CHPh), 7.66 (d, 1H, J4,5 7.8 Hz, H-4Indol), 7.84 (d, 1H, J 7.5 Hz, 2HPh), 8.02 (s, 1H, CHPh), 9.75 (br. s, 1H, NH), 10.89 (br. s, 1H, NH), 11.77 (br. s, 1H, NHIndol); 13C-NMR (DMSO-d6, 100 MHz) δ 103.67, 104.71 (C-3Indol, CHThiazol), 112.37 (C-7Indol), 120.00 (C-5Indol), 121.78 (C-4Indol), 122.02 (CPh), 123.90 (C-6Indol), 124.42 (CHPh), 126.90 (C-3aIndol), 128.09 (CHPh), 129.05 (C-2Indol), 130.30, 130.76 (2 CHPh), 134.75, 136.84 (C-7aIndol, CPh), 148.87 (C-4Thiazol), 161.35 (C=O), 172.81 (C-2Thiazol); HRMS (EI) calcd for C18H13N4OSBr [M+]: 411.9993. Found: 411.9954.
3.9. X-ray Crystallography
Compound 13 was obtained as single crystals by slow evaporation of ethanol. Data were collected on a Bruker APEX-II D8 Venture area diffractometer (Bruker AXS GmbH, Karlsruhe, Germany), equipped with graphite monochromatic Mo Kα radiation at 100(2) K. Cell refinement and data reduction were carried out by Bruker SAINT. SHELXS-97 [33,34,35,36] was used to solve structure. The final refinement was carried out by full-matrix least-squares techniques with anisotropic thermal data for nonhydrogen atoms on F2. CCDC 1438576 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
4. Conclusions
In summary, using NaOMe did not catalyze the alkylation of the ethyl indole-2-carboxylate (NH), and instead led to transesterification. The alkylation succeeded by the use of aq. KOH in acetone and is time dependent. Hydrazinolysis of ethyl or methyl indol-2-carboxylate afforded indol-2-carbohydrazide, which reacted with some aromatic aldehydes and ketones to form hydrazones. Indol-2-thiosemicarbazide was used for the synthesis of thiazoles. Further biological evaluations of the synthesized compounds 2–18 are underway.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this Research group NO (RGP-1436-038).
Author Contributions
A.T.A.B and E-S.H.E.-A conceived and designed the experiments; A.T.A.B performed the experiments; A.T.A.B, and E-S.H.E.-A. Analyzed the data; A.B., and H.A.G. contributed reagents/materials/analysis tools; A.B., and H.A.G. carried out the X-ray single crystal; A.T.A.B and A.B. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2–18 are available from the authors.
References
- 1.Sundberg R.J. Indoles. Academic Press; New York, NY, USA: 1996. [Google Scholar]
- 2.Robert M.F., Wink M. Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum; London, UK: 1998. [Google Scholar]
- 3.Von Nussbaum F. Stephacidin B—A new stage of complexity within prenylated indole alkaloids from fungi. Angew. Chem. Int. Ed. 2003;42:3068–3071. doi: 10.1002/anie.200301646. [DOI] [PubMed] [Google Scholar]
- 4.Pindur U., Lemster T. Advances in marine natural products of the indole and annelated indole series: Chemical and biological aspects. Curr. Med. Chem. 2001;8:1681–1698. doi: 10.2174/0929867013371941. [DOI] [PubMed] [Google Scholar]
- 5.Hesse M. Alkaloids. Nature’s Curse or Blessing. Wiley—VCH; Weinheim, Germany: 2002. [Google Scholar]
- 6.Sharma V., Kumar P., Pathak D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010;47:491–502. doi: 10.1002/jhet.349. [DOI] [Google Scholar]
- 7.Premanathan M., Radhakrishnan S., Kulangiappar K., Singaravelu G., Thirumalaiarasu V., Sivakumar T., Kathiresan K. Antioxidant & anticancer activities of isatin (1H-indole-2,3-dione), isolated from the flowers of Couroupita guianensis Aubl. Indian J. Med. Res. 2012;136:822–826. [PMC free article] [PubMed] [Google Scholar]
- 8.Islam M.R., Mohsin M. Synthesis of isatin, 5-chloroisatin and their Δ2–1, 3, 4 oxadiazoline derivatives for comparative cytotoxicity study on brine shrimp. Bangladesh J. Pharmacol. 2007;2:7–12. [Google Scholar]
- 9.Matesic L., Locke J.M., Bremner J.B., Pyne S.G., Skropeta D., Ranson M., Vine K.L. N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg. Med. Chem. 2008;16:3118–3124. doi: 10.1016/j.bmc.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Gao N., Kramer L., Rahmani M., Dent P., Grant S. The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydroindol-2-one (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism. Mol. Pharmacol. 2006;70:645–655. doi: 10.1124/mol.106.024505. [DOI] [PubMed] [Google Scholar]
- 11.Wang F., Fang Y., Zhu T., Zhang M., Lin A., Gu Q., Zhu W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron. 2008;64:7986–7991. doi: 10.1016/j.tet.2008.06.013. [DOI] [Google Scholar]
- 12.Lane M.E., Yu B., Rice A., Lipson K.E., Liang C., Sun L., Wadler S. A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 2001;61:6170–6177. [PubMed] [Google Scholar]
- 13.Patyna S., Laird A.D., Mendel D.B., O'Farrell A.M., Liang C., Guan H., Grazzini M. SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol. Cancer Ther. 2006;5:1774–1782. doi: 10.1158/1535-7163.MCT-05-0333. [DOI] [PubMed] [Google Scholar]
- 14.Li H.H., Zhang X.H., Tan J.Z., Chen L.L., Liu H., Luo X.M., Jiang H.L. Design, synthesis, antitumor evaluations and molecular modeling studies of novel 3,5-substituted indolin-2-one derivatives. Acta Pharmacol. Sin. 2007;28:140–152. doi: 10.1111/j.1745-7254.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 15.Juranić Z., Anastasova F., Juranić I., Stanojković T., Radulović S., Vuletić N. Antiproliferative action of isatine-beta-thiocarbohydrazone and N-ethylisatine-beta-thiocarbohydrazone on human PBMC and on two neoplastic cell lines. Exp. Clin. Cancer Res. 1999;18:317–324. [PubMed] [Google Scholar]
- 16.Terry A.V., Buccafusco J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 17.Brandes J.L., Kudrow D., Cady R., Tiseo P.J., Sun W., Sikes C.R. Eletriptan in the early treatment of acute migraine: Influence of pain intensity and time of dosing. Cephalalgia. 2005;25:735–742. doi: 10.1111/j.1468-2982.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- 18.Bader T., Fazili J., Madhoun M., Aston C., Hughes D., Rizvi S., Seres K., Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am. J. Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 19.Tramer M.R., Reynolds D.J.M., Moore R.A., McQuay H.J. Efficacy, Dose-Response, and Safety of Ondansetron in Prevention of Postoperative Nausea and Vomiting A Quantitative Systematic Review of Randomized Placebo-controlled Trials. J. Am. Soc. Anesthesiol. 1997;87:1277–1289. doi: 10.1097/00000542-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mancini I., Guella G., Dbitus C., Waikedre J., Pietra F. From inactive nortopsentin D, a novel bis (indole) alkaloid isolated from the axinellid sponge Dragmacidon sp. from deep waters south of new caledonia, to a strongly cytotoxic derivative. Helv. Chim. Acta. 1996;79:2075–2082. doi: 10.1002/hlca.19960790804. [DOI] [Google Scholar]
- 21.Miyake F.Y., Yakushijin K., Horne D.A. A concise synthesis of topsentin A and nortopsentins B and D. Org. Lett. 2000;2:2121–2123. doi: 10.1021/ol000124g. [DOI] [PubMed] [Google Scholar]
- 22.Sakemi S., Sun H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis [indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991;56:4304–4307. doi: 10.1021/jo00013a044. [DOI] [Google Scholar]
- 23.Kawasaki I., Yamashita M., OHTA S. Total Synthesis of Nortopsentins AD, Marine Alkaloids. Chem. Pharm. Bull. 1996;44:1831–1839. doi: 10.1248/cpb.44.1831. [DOI] [Google Scholar]
- 24.El-Wareth A., Sarhan A.O. On the synthesis and reactions of indole-2-carboxylic acid hydrazide. Monatsh. Chem. 2001;132:753–763. doi: 10.1007/s007060170091. [DOI] [Google Scholar]
- 25.Boraei A.T.A. A new direct synthetic access to 4-amino-2-N-(glycosyl/propyl)-1,2,4-triazole-3-thiones via hydrazinolysis of 3-N-((acylated glycosyl)/allyl)-1,3,4-oxadiazole-2-thiones. Arkivoc. 2016;3:71–81. [Google Scholar]
- 26.El Ashry E.S.H., El Tamany E.S.H., El Fattah M.E.D.A., Aly M.R., Boraei A.T. Synthesis of New Functionalized 2-Alkylsulfanyl-5-(1H-indol-2-yl)-1,3,4-Oxadiazole and a Facile Thio-Aza-Claisen Rearrangement of the S-Allyl Analog. Lett. Org. Chem. 2009;6:462–469. doi: 10.2174/157017809789124902. [DOI] [Google Scholar]
- 27.El Ashry E.S.H., Fattah M.E.D.A., Aly M.R., Boraei A.T., Duerkop A. A new synthetic access to 2-N-(glycosyl) thiosemicarbazides from 3-N-(glycosyl) oxadiazolinethiones and the regioselectivity of the glycosylation of their oxadiazolinethione precursors. Beilstein J. Org. Chem. 2003;9:135–146. doi: 10.3762/bjoc.9.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Ashry E.S.H., El Tamany E.S.H., Abd El Fattah M.E.D., Aly M.R., Boraei A.T., Mesaik M.A., Soomro S. Immunomodulatory properties of S-and N-alkylated 5-(1H-indol-2-yl)-1,3,4-oxadiazole-2(3H)-thione. J. Enzyme Inhib. Med. Chem. 2013;28:105–112. doi: 10.3109/14756366.2011.636361. [DOI] [PubMed] [Google Scholar]
- 29.El Ashry E.S.H., El Fattah M.E.D.A., Boraei A.T., El-Nabi H.M.A. Regioselective synthesis, characterization and antimicrobial evaluation of S-glycosides and S,N-diglycosides of 1,2-dihydro-5-(1H-indol-2-yl)-1,2,4-triazole-3-thione. Eur. J. Med. Chem. 2013;66:106–113. doi: 10.1016/j.ejmech.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 30.Barakat A., Islam M.S., Al Majid A.M.A., Al-Othman Z.A. Highly enantioselective Friedel–Crafts alkylation of indoles with α, β-unsaturated ketones with simple Cu(II)–oxazoline–imidazoline catalysts. Tetrahedron. 2013;69:5185–5192. doi: 10.1016/j.tet.2013.04.063. [DOI] [Google Scholar]
- 31.Islam M.I., Al-Majid A.M., Al-Othman Z.A., Barakat A. Highly enantioselective Friedel-Crafts alkylation of indole with electron deficient trans-β-nitroalkenes under simple Zn(II)-oxazoline- imidazoline catalysts. Tetrahedron Asymmetry. 2014;25:245–251. doi: 10.1016/j.tetasy.2013.11.018. [DOI] [Google Scholar]
- 32.Al-Majid A.M., Islam M.I., Barakat A., Al-Agamy M.H.M., Naushad Mu. Facile and promising method for Michael addition of indole and pyrrole to electron deficient trans-β-nitroolefins catalyzed by Feist’s acid: Preliminary study of anti-microbial activity. Sci. World J. 2014 doi: 10.1155/2014/649197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottoni O., Cruz R., Alves R. Alves. Efficient and simple methods for the introduction of the sulfonyl, acyl and alky protecting groups on the nitrogen of the indole and its derivatives. Tetrahedron. 1998;54:13915–11398. doi: 10.1016/S0040-4020(98)00865-5. [DOI] [Google Scholar]
- 34.Sechi M., Derudas M., Dallocchio R., Dessì A., Bacchi A., Sannia L., Carta F., Palomba M., Ragab O., Chan C., et al. Design and synthesis of novel indole beta-diketo acid derivatives as HIV-1 integrase inhibitors. J. Med. Chem. 2004;47:5298–5310. doi: 10.1021/jm049944f. [DOI] [PubMed] [Google Scholar]
- 35.Sheldrick G.M. A short history of SHELX. Acta Cryst. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 36.Spek A.L. Structure validation in chemical crystallography. Acta Cryst. 2009;D65:148–155. doi: 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]