Abstract
We report the draft genome sequence of Actinokineospora bangkokensis 44EHWT, the producer of the antifungal polyene compounds, thailandins A and B. The sequence contains 7.45 Mb, 74.1% GC content and 35 putative gene clusters for the biosynthesis of secondary metabolites. There are three gene clusters encoding large polyketide synthases of type I. Annotation of the ORF functions and targeted gene disruption enabled us to identify the cluster for thailandin biosynthesis. We propose a plausible biosynthetic pathway for thailandin, where the unusual butylmalonyl-CoA extender unit is incorporated and results in an untypical side chain.
Keywords: Actinokineospora bangkokensis, thailandin, polyene, biosynthetic gene cluster, butylmalonyl-CoA, draft genome sequence, genome mining
1. Introduction
Next generation sequencing and genome mining are powerful and rapid technologies to identify the genetic potential of a strain to synthesize secondary metabolites with various biological activities. One order known to produce many secondary metabolites with different bioactivities is the Actinomycetales. Under laboratory conditions only a few compounds are produced by a strain while their genomes comprise often more than 20 biosynthetic gene clusters. Cryptic clusters have been activated by heterologous expression [1], changing growth conditions [2] or by the manipulation of regulatory genes [3,4]. The knowledge of the genome sequence and the biosynthetic cluster composition of a secondary metabolite gives insights into the biosynthetic pathway. Therefore, it is a valuable tool for metabolic engineering to increase the production of a specific compound or to generate novel metabolites by combinatorial biosynthesis.
The genus Actinokineospora is a member of the order Actinomycetales and was introduced in 1988 as a separate genus [5]. The characteristics of this genus include having meso-diaminopimelic acid as component of their cell wall and the occurrence of menaquinone MK-9 (H4), phospholipids type II and iso-C16:0 fatty acids in their cell membrane. Until now only 16 strains of this genus have been identified. Thus, Actinokineospora belongs to the rare actinomycetes. Draft genome sequences are only available for A. spheciospongiae (GCA_000564855.1) [6], A. enzanensis (GCA_000374445.1) and A. inagensis (GCA_000482865.1).
The strain Actinokineospora bangkokensis 44EHWT was isolated from the rhizosphere soil of an elephant ear plant (Colocasia esculenta) in Bangkok (Thailand) [7]. It produces thailandins A and B, antifungal polyenes with 28 membered macrocyclic lactone ring with two methyl groups, seven free hydroxyl groups and five conjugated double bonds. In addition, thailandin A is O-rhamnosylated at position C15, where thailandin B has only a hydroxyl group. Both compounds show significant inhibition of anthracnose fungi and pathogenic yeast strains [8].
In this study, we performed whole genome sequencing of A. bangkokensis 44EHWT and successfully identified and verified the thailandin biosynthetic gene cluster. Herein, we report the putative biosynthetic pathway where the unusual butylmalonyl-CoA extender unit is incorporated into the polyketide chain.
2. Results
2.1. Draft Genome Sequence of Actinokineospora bangkokensis 44EHWT
A draft genome of A. bangkokensis 44EHWT was sequenced and aligned to 32 scaffolds and 79 contigs. The largest scaffold has 931,456 nucleotides. The genome sequence consists of 7,453,713 nucleotides with an overall GC content of 74.1%, ranging from 44.2%–87.4% (calculated for 500 bp fragments). The sequence contains 6287 coding sequences (CDS), 50 tRNAs, and four clustered regularly interspaced short palindromic repeats (CRISPRs) predicted by the NCBI prokaryotic genome annotation pipeline [9]. The genome coding density is 87.9% and the average gene length is 1030 bp. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession MKQR00000000. The version described in this paper is version MKQR01000000. An antiSMASH 3.0 [10] analysis revealed the presence of 35 gene clusters encoding secondary metabolite biosynthetic pathways (Table 1, Figure 1 and Appendix A, Table A1).
Table 1.
Genome features of A. bangkokensis 44EHWT.
| Feature | Property |
|---|---|
| total length | 7,453,713 bp |
| GC content | 74.1% |
| number of scaffold | 32 |
| number of contigs | 79 |
| CDS (total) | 6287 |
| genes (coding) | 6191 |
| tRNA | 50 |
| secondary metabolite biosynthetic gene clusters | 35 |
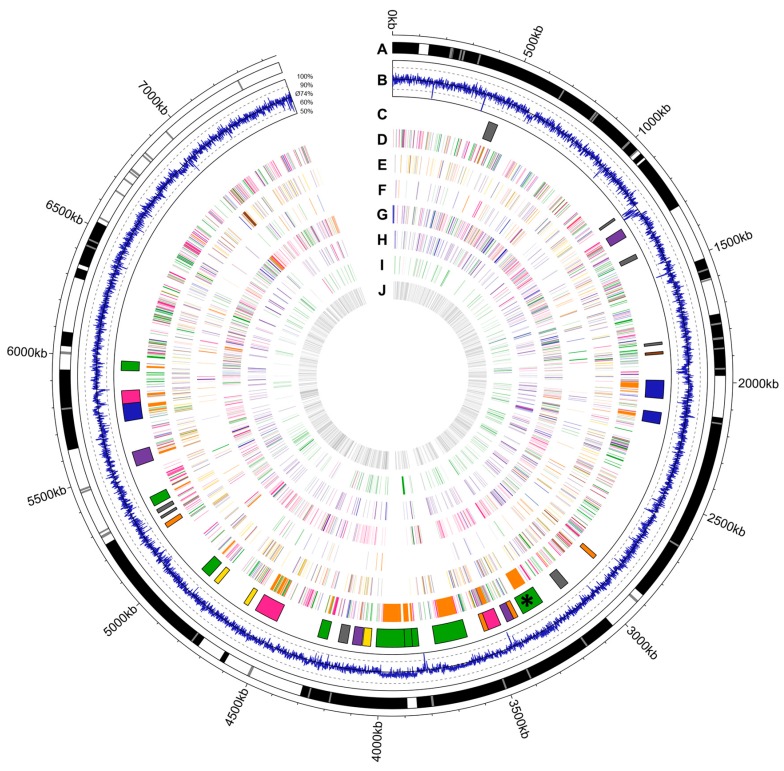
Figure 1.
Illustration of A. bangkokensis 44EHWT draft genome sequence. The genome has a size of 7.45 Mb. Circle A: Illustration of scaffold 1–32, shown in black and white, gaps are indicated in grey. Circle B: GC% content of 500 bp range, in 250 bp steps, between 50%–100%, line indicates average GC content of 74%. Circle C: Localization of putative secondary metabolite gene cluster, illustrated in PKS I (green), other PKS (purple), NRPS/PKS I (blue), other NRPS (pink), terpene (orange), siderophore (brown), lantipeptide (yellow) and other kind of cluster (grey); * Thailandin biosynthetic gene cluster is highlighted. Circle D: Localization of ORFs of general metabolism, subdivided into metabolism of amino acids (green), aromatic compounds (purple), fatty acids (blue), carbohydrates (pink), secondary metabolites (orange), and cofactors, vitamins and pigments (brown). Circle E: Localization of ORFs with putative modifying functions as carboxylases (green), dehydrogenases (purple), esterases (blue), hydratases (pink), hydrolases (orange), involved in redox reactions (brown), reductases (yellow) and transferases (grey). Circle F: Localization of ORFs putatively involved in ion metabolism, subdivided into metabolism of iron (green), phosphate (purple), sulfur (blue), nitrogen (pink) and other ions (orange). Circle G: Localization of ORFs putatively involved in replication/transcription/translation, subdivided into ORFs from nucleotide metabolism (green), protein-turnover and chaperons (purple), replication and repair (blue), transcription (pink), translation (orange) and tRNA metabolism (brown). Circle H: Localization of ORFs encoding putatively membrane proteins (green), transporters (purple), proteins involved in cell separation (blue) and from cell wall or membrane biosynthesis (pink). Circle I: Localization of ORFs putatively involved in defense and (stress-)response (green), from (pro-)phages (purple), for sporulation (blue) and communication (pink). Circle J: Localization of ORFs with unknown functions (grey).
2.2. Identification and Verification of the Thailandin Biosynthetic Gene Cluster
Thailandins A and B were assumed to be synthesized by a polyketide synthase type I (PKS I). On the basis of the carbon chain length of the polyketide backbone, the PKS I is expected to have 14 modules. In the draft genome sequence of A. bangkokensis, three large PKS I clusters could be identified. Among these three clusters, cluster #11 encodes four PKSs comprising in total 14 modules. Cluster #16 has 20 modules, cluster #19 has at least 24 modules, yet it seems that the cluster is interrupted by the end of the scaffold. All three clusters have a higher GC content than the average genome. Cluster #11 has a GC content of 75.4%, cluster #16 and cluster #19 have 75.2% and 76.9%, respectively (Figure 1, circles B/C).
To verify that cluster #11 is responsible for thailandin biosynthesis, we interrupted the first PKS gene by a single crossover using a 3 kb internal fragment. Conjugation, electroporation as well as protoplast transformation was conducted for strain manipulation. However, only supplemention of MS plates with 10 mM CaCl2 could generate transconjugants, which were hygromycin resistant.
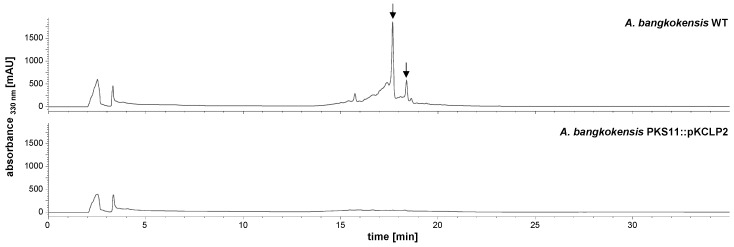
The recombinant strain A. bangkokensis PKS11::pKCLP2 was tested for the production of thailandins in comparison to the wildtype strain. HPLC/MS analysis of organic extracts demonstrated that thailandins were not produced in the mutant strain (Figure 2). The result confirms that cluster #11 is the thailandin biosynthetic gene cluster.
Figure 2.
HPLC chromatogram of A. bangkokensis WT (above) and recombinant strain with interrupted PKS cluster #11 (bellow). Arrows indicate peaks of thailandin A (retention time 17.7 min) and thailandin B (retention time 18.3 min). The recombinant strain A. bangkokensis PKS11::pKCLP2 does not produce thailandins anymore.
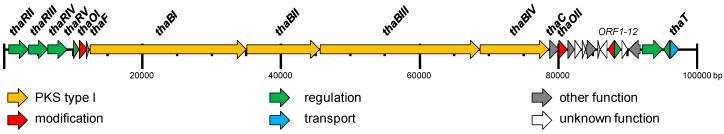
The thailandin biosynthetic gene cluster was further analyzed in detail (Figure 3, Table 2). It spans 96.1 kb with 25 open reading frames (ORFs), but the main cluster probably contains only 13 genes (thaRI-thaOII, thaT). The polyketide synthase is encoded by the four genes thaBI, thaBII, thaBIII and thaBIV. Beside them, the cluster encodes a crotonyl-CoA carboxylase/reductase (thaC), two monooxygenases (thaOI, thaOII), seven regulatory genes (thaRI-thaRIV; orf8/11/12), one MFS transporter (thaT), and further genes with various functions. By BLAST analysis the function of five ORFs could not be assumed.
Figure 3.
Genetic organization of the thailandin biosynthetic gene cluster in A. bangkokensis 44EHWT. The cluster spans a size of 96.1 kb with 25 open reading frames. Genes encoding the PKS I are yellow; genes encoding for modification are red; genes encoding for regulatory proteins are green; gene encoding a transporter is shown in blue; genes encoding other functions are grey; ORFs with unknown function are white.
Table 2.
Features of the thailandin biosynthetic gene cluster in A. bangkokensis 44EHWT.
| Gene | Protein-ID (PRJNA345323:) | Protein [aa] | Putative Product | Closest Similarity in the Databases (Identity %) |
|---|---|---|---|---|
| thaRI | BJP25_14755 | 953 | LuxR transcriptional regulator | Streptomyces himastatinicus (47%) |
| thaRII | BJP25_14760 | 923 | LuxR transcriptional regulator | Amycolatopsis azurea (38%) |
| thaRIII | BJP25_14765 | 921 | LuxR transcriptional regulator | Streptomyces sp. TAA204 (38%) |
| thaRIV | BJP25_14770 | 228 | LuxR transcriptional regulator | Allokutzneria albata (41%) |
| thaOI | BJP25_14775 | 402 | P450 monooxygenase | Streptomyces sp. LamerLS-31b (69%) |
| thaF | BJP25_14780 | 66 | ferredoxin | Streptomyces niger (73%) |
| thaBI | BJP25_14785 | 7524 | polyketide synthase type I | Streptomyces sp. MBT76 (60%) |
| thaBII | BJP25_14790 | 3501 | polyketide synthase type I | Streptomyces avermitilis (59%) |
| thaBIII | BJP25_14795 | 7685 | polyketide synthase type I | Streptomyces sp. NRRL B-24891 (54%) |
| thaBIV | BJP25_14800 | 3310 | polyketide synthase type I | Streptomyces avermitilis (58%) |
| thaC | BJP25_14805 | 418 | crotonyl-CoA carboxylase/reductase | Streptomyces durhamensis (71%) |
| thaOII | BJP25_14810 | 408 | P450 monooxygenase | Streptomyces avermitilis (65%) |
| orf1 | BJP25_14815 | 295 | phosphoesterase PA-phosphatase | Streptomyces regensis (70%) |
| orf2 | BJP25_14820 | 297 | hypothetical protein | Blastococcus sp. URHD0036 (70%) |
| orf3 | BJP25_14825 | 178 | hypothetical protein | Blastococcus sp. URHD0036 (60%) |
| orf4 | BJP25_14830 | 489 | chromosome segregation ATPase | Kibdelosporangium aridum (71%) |
| orf5 | BJP25_14835 | 172 | hypothetical protein | Lentzea sp. DHS C013 (65%) |
| orf6 | BJP25_14840 | 315 | hypothetical protein | Mycobacterium sp. Root135 (72%) |
| orf7 | BJP25_14845 | 297 | oxidoreductase | Nocardia niigatensis (63%) |
| orf8 | BJP25_14850 | 312 | LuxR transcriptional regulator | Saccharomonospora sp. CNQ490 (46%) |
| orf9 | BJP25_14855 | 300 | hypothetical protein | Actinokineospora spheciospongiae (69%) |
| orf10 | BJP25_14860 | 529 | serine protease | Actinokineospora spheciospongiae (73%) |
| orf11 | BJP25_14865 | 946 | LuxR transcriptional regulator | Actinokineospora inagensis (69%) |
| orf12 | BJP25_14870 | 294 | LysR transcriptional regulator | Alloactinosynnema sp. L-07 (70%) |
| thaT | BJP25_14875 | 405 | MFS transporter | Blastococcus saxobsidens (68%) |
2.3. Proposed Thailandin Biosynthetic Pathway
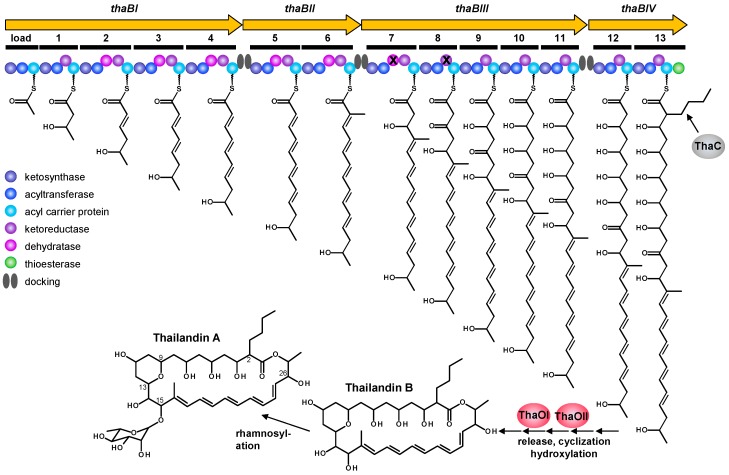
Further analysis of the thailandin biosynthetic gene cluster led to the putative biosynthetic pathway of the compounds (Figure 4). The polyketide synthase is composed of one loading module and thirteen extender modules encoded by thaBI-thaBIV. They encode for domains of acyl carrier proteins (ACP), acyltransferases (AT), ketosynthases (KS), dehydratases (DH), ketoreductases (KR), docking (Dock) and a thioesterase (TE). The assembly line order is ThaBI [(KS-AT-ACP)-(KS-AT-KR-ACP)-(KS-AT-DH-KR-ACP)-(KS-AT-DH-KR-ACP)-(KS-AT-DH-KR-ACP)-Dock], ThaBII [Dock-(KS-AT-DH-KR-ACP)-(KS-AT-DH-KR-ACP)-Dock], ThaBIII [Dock-(KS-AT-DH-KR-ACP)-(KS-AT-KR-ACP)-(KS-AT-KR-ACP)-(KS-AT-KR-ACP)-(KS-AT-KR-ACP)-Dock] and ThaBIV [Dock-(KS-AT-KR-ACP)-(KS-AT-KR-ACP)-TE]. Futhermore, docking domains were identified between the PKS enzymes.
Figure 4.
Putative thailandin biosynthetic pathway. The PKS encoding genes thaBI-thaBIV are shown in yellow; they encode for 14 modules (one loading module and 13 extender modules); the order of catalytic domains is shown in circles and color code (left), proposed X inactive domains; the single extender units are emphasized in bold. The crotonyl-CoA carboxylase/reductase ThaC is involved in butylmalonyl-CoA biosynthesis. The mature polyketide chain is released by the thioesterase domain of ThaBIV and cyclized. Furthermore, the polyene compound is hydroxylated by ThaOI or ThaOII and finally rhamnosylated, resulting in thailandin A.
All acyltransferase domains have the typical GHSxG-Motif [11]. Except for the ATs of module 6 and 13, they all show specificity to the extender unit malonyl-CoA (x = LVIFAM). In the loading module, the AT domain has also malonyl-CoA-specificity. There, malonyl-CoA is probably decarboxylated by the KS of the loading module to provide an acetyl starter unit for transfer onto the first extension module. Like in other PKS systems with a KS domain in the loading module, the common cysteine of “condensing” KS domains is occupied by a glutamine in the active site [12].
The sequence of the AT domain of module 6 shows specificity to methylmalonyl-CoA (x = Q), which is consistent with the thailandin structure. In the last module, module 13, it is predicted that the extender unit ethylmalonyl-CoA is incorporated. In comparison with the polyene structure, we would suppose butylmalonyl-CoA as the unusual extender unit. Downstream of thaBIV, the gene thaC is located. The encoded protein is assumed to have a crotonyl-CoA carboxylase/reductase activity. It was shown, that crotonyl-CoA carboxylases/reductases are essential for the biosynthesis of various substituted malonyl-CoA extender units. They catalyze the NADPH-dependent carboxylation of α,β-unsaturated acyl-thioesters [13,14,15,16,17]. In the thailandin gene cluster, thaC encodes for such an enzyme, likely involved in the biosynthesis of butylmalonyl-CoA.
In module 7, a KS and a DH domain are located. Because of the hydroxyl group at C15 in the thailandin molecule, the DH domain is apparently inactive. This hydroxyl group is later used for the attachment of rhamnose moiety. The DH7 domain contains the conserved HxxxGxxxxP motif found in active DH domains, but possesses alterations of the GYxYGPxF, LPFxW, and Dxxx(Q/H) motifs [18].
After biosynthesis, the mature polyketide chain is released from the PKS and cyclized via the action of a thioesterase domain located at the C-terminal end of ThaBIV. The other cyclization takes place between C9 and C13. In other polyene biosynthetic pathways, this cyclization is formed by a keto and a hydroxyl group building a hemiketal ring [19,20]. Therefore, we assume that the KR domain of module 8 must be inactive, yet it contains all conserved amino acids of type A ketoreductases [21,22,23]. Following, the hydroxyl group could be transferred from C13 to C14 by an epoxide intermediate, which could be catalyzed by one of the two cytochrome P450 monooxygenases ThaOI or ThaOII. Alternatively, the tetrahydropyran ring could be generated by oxa-Michael addition on an α,β-unsaturated thioester intermediate. Therefore, special dehydratases and pyran-forming cyclases are required [24,25,26], which are not present within the cluster.
The other monooxygenase is likely responsible for hydroxylation of C26. For their activity, they require electrons from NADH, often mediated by ferredoxin. In the thailandin cluster, downstream of thaOI, the gene thaF is located, encoding for ferredoxin. Finally, thailandin B is rhamnosylated and results in thailandin A. Within the cluster no gene encodes for an enzyme with glycosyltransferase activity. A putative major faciliate transporter encoded near the structural genes, thaT, is suggested to be responsible for the transport of thailandin out of the producing organism. The other ORFs near the biosynthetic genes are unlikely to have an important role in the thailandin biosynthesis.
3. Discussion
Actinokineospora bangkokensis 44EHWT produces the polyene compounds thailandin A and thailandin B [8]. Thailandin B is probably the precursor of the A-form, because thailandin A is further rhamnosylated. Both compounds show activity against pathogenic fungal strains with minimum inhibitory concentrations ranging between 16–32 μg/mL [8].
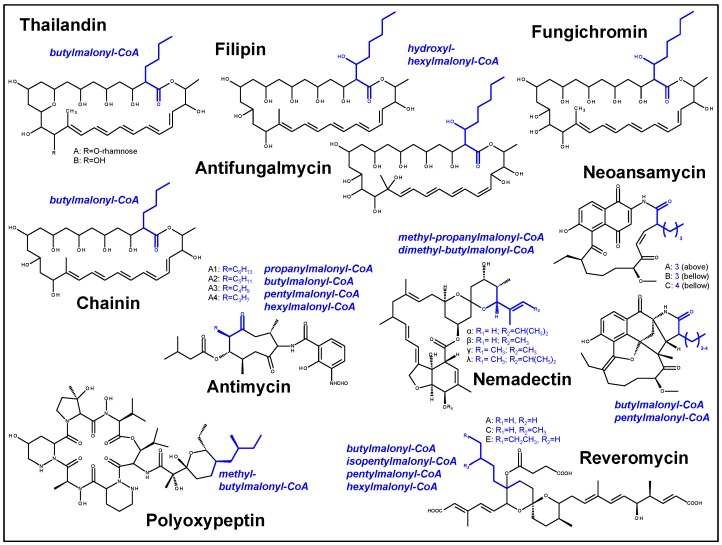
Polyene compounds are efficient antibiotics because they directly target the fungal plasma membrane by interacting with the main sterol, ergosterol, which often results in membrane permeabilization [27]. In addition, further acitivities of various polyene compounds have been demonstrated. The clinical application of these antifungal compounds is complicated by their low water-solubility and dose-dependent side effects, notably nephrotoxicity [28]. Many studies have been done to modify existing molecules in order to improve them. Thereby important structural elements were identified. Accordingly, the polyol [29] and polyene regions [30], the sugar modification, mainly by the aminoglycoside d-mycosamine [31,32], the exocyclic carboxyl group [30,33], and an additional aromatic heptaen side chain, which leads to haemolytic activity [34], seem to be particularly important for selective toxicity and activity. The modification or the loss of the D-mycosamine sugar moiety led to significant reduced antifungal activity [31,32]. Thailandin A has a rhamnose instead of d-mycosamine modification, but has also higher MIC compared to amphotericin B. Surprisingly, although thailandin B is not modified by a sugar moiety, it has even better antifungal activity than thailandin A [8]. In addition, both compounds do not have the exocyclic carboxyl group. In contrast to other polyenes, thailandins have an additional short side chain at C2 similar to chainin [35], filipin [36], fungichromin [37] and antifungalmycin [38] (Figure 5).
Figure 5.
Examples for polyketides with unusual extender units. Unusual extender units are written in italic, shown blue in the chemical structure.
The genus Actinokineospora belong to the group of rare Actinomycetales with great potential to produce novel secondary metabolites. Only 16 strains of this genus are known to date. Many studies have been carried out to categorize these strains, yet studies into their secondary metabolite production is limited (Table A2). Recently, new antitrypanosomal and antioxidant compounds actinosporins were isolated from A. spheciospongia EG49T [39,40]. Co-cultivation of this strain with Nocardiopsis sp. RV163 led to induction of further secondary metabolite biosynthesis [41]. Furthermore, only three other Actinokineospora genomes have been sequenced, A. enzanensis DSM 44649T (ID 1120934) (8119858 bp, GC 70.8%, 37 predicted gene cluster), A. inagensis DSM 44258T (ID 1120935) (7278759 bp, GC 70.2%, 34 predicted gene cluster) and A. spheciospongia EG49T (ID 909613) (7529476 bp, GC 72.8%, 36 predicted gene cluster). In this study, we sequenced the genome of A. bangkokensis 44EHWT. The draft genome has 7.5 Mb with 74.1% GC content, which is significantly higher than the other Actinokineospora genomes. It is also remarkable, that there are many regions within the genome with a GC content <50%, as well as the three large PKS I gene clusters have an overall higher GC content of 75.4% (cluster #11, thailandin cluster), 75.2% (cluster #16) and 76.9% (cluster #19). This indicates a high frequeny of horizontal DNA transfer during evolution.
The antiSMASH analysis of the genome revealed 35 putative secondary metabolite gene clusters. The detailed evaluation of the PKS encoding clusters led to the assumption that cluster #11 should be responsible for thailandin biosynthesis. This hypothesis was proved by targeted inactivation of the first PKS gene thaBI. For genetic manipulation, different protocols were conducted without success. Finally, the supplementation of 10 mM CaCl2 resulted in the mutant strain via conjugation. The addition of CaCl2 could also increase conjugation frequency of several Streptomyces strains [42]. Noteworthy, A. bangkokensis is resistant against the commonly used antibiotics apramycin and spectinomycin.
The thailandin biosynthetic gene cluster encodes next to the PKS enzymes for proteins with regulatory function, post-polyketide modification, one transporter and few other proteins. Remarkably, there is no gene within the cluster which encodes a glycosyltransferase. In other polyene biosynthetic gene clusters, the genes for biosynthesis of the sugar moiety and the glycosyltransferase are present [43]. In the genome of A. bangkokensis 45 glycosyltransferase genes could be identified, of which two genes are in the PKS cluster #19 and nine in cluster #16, respectively. One of them should catalyze the rhamnosylation of the thailandin aglycon.
Beside modules 6 and 13, the bioinformatic analysis identified malonyl-CoA as extender unit, which corresponds to the chemical structure of thailandin. In module 6, methylmalonyl-CoA should be incorporated. In the last module we postulated the incorporation of butylmalonyl-CoA. The high diversity among polyketides is caused by the number of modules in the PKS assembly line, the presence of reducing domains, and other modifying enzymes, whereas malonyl-CoA, methylmalonyl-CoA or ethylmalonyl-CoA are usually incorporated. Therefore, butylmalonyl-CoA is an unusual extender unit. From structural analysis, we would also suppose the incorporation of butylmalonyl-CoA in chainin biosynthesis, but so far there is no cluster information available. The usage of unusual extender units is also postulated in few other biosynthetic pathways, but often the acyltransferases seem to be less specific, incorporating not only one defined unit. The AT domain of RevA of the reveromycin biosynthesis putatively uses butylmalonyl-CoA, isopentylmalonyl-CoA, pentylmalonyl-CoA or hexylmalonyl-CoA [44]. In the biosynthesis of neoansamycin A-C, pentylmalonyl-CoA or butylmalonyl-CoA is incorporated [45]. Different acylmalonyl-CoA extender units are proposed on the basis of various derivatives in both antimycin [46] and nemadectin [47]. In polyoxypeptin, methylbutylmalonyl-CoA is putatively used as an extender unit [48]. In the polyene compounds fungichromin, filipin and antifungalmycin, an unusual extender like hydroxyl- hexylmalonyl-CoA should be incorporated. These secondary products with their unusual extender units are shown in Figure 5. The incorporation of the lengthened extender unit in thailandin, chainin, filipin, fungichromin and antifungalmycin leads to the particular C2 side chain of the molecules, which is not common in other polyene compounds. Further structural studies would shed new light on polyene–fungus interaction.
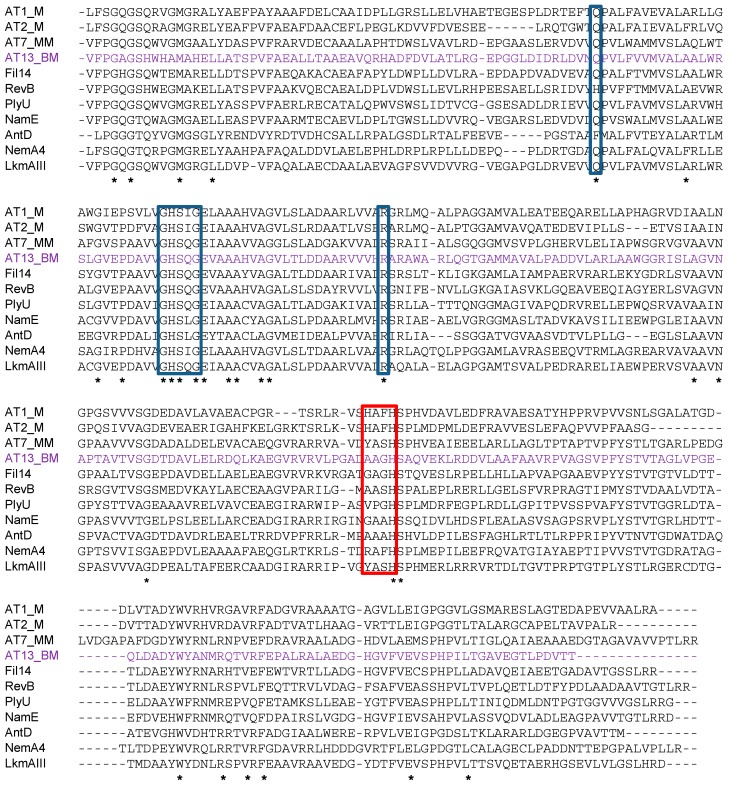
The sequences of the described acyltransferases were aligned (Figure A1). The alignment indicates that a later motif may encode for this specificity. Whereas acyltransferases with malonyl-CoA specificity have a HAFH-motif, the sequences differ in the these acyltransferases. The AT13 of the thailandin biosynthesis pathway has a GHSQH- and a AAGH-motif.
Studies on rare actinomycetes such as Actinokineospora are very promising in order to identify novel secondary metabolites. The genome of A. bangkokensis 44EHWT revealed 34 other secondary metabolite biosynthetic gene clusters indicating that this strain can produce more compounds. In addition, the examination of PKS systems with unusual extender units is important. The knowledge of the specificity of acyltransferases and the underlying sequence motifs gives basics to modify compounds by combinatorial biosynthesis.
4. Materials and Methods
4.1. Bacterial Growth Condition
A. bangkokensis 44EHWT was incubated at 28 °C in TSB medium (CASO Bouillon 30 g/L; Carl Roth GmbH, Karlsruhe, Germany) for 3–5 days, 180 rpm. For production analysis, the strain was pre-cultivated in TSB medium for 2 days at 28 °C and then further cultivated in 100 mL HA medium (0.4% yeast extract, 1% malt extract, and 0.4% glucose; pH 7.4) for 8 days.
4.2. Extraction of Secondary Metabolites
After cultivation in HA medium the culture broth was centrifuged (3500× g, 10 min, 4 °C). The pH of the supernatant was adjusted to pH 4 by addition of HCl (1 M) and extracted by shaking vigorously with an equal volume of ethyl acetate for 30 min. The organic phase was evaporated to dryness using rotary evaporation at 240 bar. The extract was dissolved in MeOH and analyzed by HPLC/MS.
4.3. Analysis of Secondary Metabolite Production by HPLC/MS
The extract was analyzed by a HPLC system equipped with a photodiode array detector (200–600 nm) a mass spectrometer (1100 Series, Agilent Technologies, Waldbronn, Germany) The separation was done by usage of a XBridge™ C18 column (4.6 × 100 mm) with precolumn (4.6 × 20 mm) on a non-linear 0.5% AcOH-CH3CN:H2O gradient ranged from 20% to 95% at a flow rate of 0.5 mL/min. Thailandin A has UV/vis maxima at 325, 340, and 358 nm and a mass of 754 g/mol, thailandin B has 608 g/mol.
4.4. Isolation of Genomic DNA
The strain A. bangkokensis 44EHWT was incubated in TSB-Media for 4 days, at 28 °C and 180 rpm. Accordingly, 15 mL of culture was centrifuged (3500× g, 10 min, 4 °C) and pellet was washed in 15 mL H2O and finally resuspended in 15 mL SET buffer (75 mM NaCl, 25 mM EDTA, 20 mM Tris/HCl pH 8) with lysozyme (100 µg/mL). After incubation for 30 min at 37 °C, RNase (100 µg/mL) was added and further incubated for additional 2 h at 37 °C. Afterwards proteinase K and SDS were added to obtain a final concentration of 100 µg/mL and 0.5%, respectively, and incubated at 50 °C overnight. The DNA was extracted by the addition of equal volume of phenol/chloroform/isoamyl alcohol (25:24:1), inverting carefully for 10 min. After centrifugation (2000× g, 20 min, 4 °C), the aqueous phase was transferred into a new tube and the last step was repeated two times. Two volumes of isopropanol (100%) were added to the aqueous phase and genomic DNA was spooled by a glass Pasteur pipette. The DNA was washed in EtOH (70%), dried and finally dissolved in 1 mL H2O.
4.5. Genome Sequencing of A. bangkokensis 44EHWT
The genome of A. bangkokensis 44EHWT was sequenced twice. Eurofins Genomics GmbH (Ebersberg, Germany) sequenced the genome using 454 technologies and assembled the reads by Newbler to 247 contigs and 56 scaffolds. In addition, the genome was sequenced at the Center for Biotechnology at the University of Bielefeld, Bielefeld, Germany, using Illumina-HiSeq 1000 technology. Initially, all reads were assembled to a draft genome of 206 contigs and 105 scaffolds using GS de novo assembler version 3.0 (Roche, Branford, CT, USA). The genome was further assembled to 7,453,713 bp genome sequence.
4.6. Genome Annotation and Identification of the Thailandin Biosynthetic Gene Cluster
The automatic functional annotation results were obtained using the NCBI prokaryotic genome annotation pipeline [9]. In addition, the ORFs were categorized into different subsystems manually. Secondary metabolite gene clusters were identified by antiSMASH 3.0 [10]. The genes were further analyzed by BLAST [49].
4.7. Visualization of the Genome of A. bangkokensis 44EHWT
The genome was visualized by Circos, generated with the R package circlize [50,51]. Therefore, the scaffolds with gaps were visualized, the GC content in 500 bp ranges (each 250 bp), the predicted secondary metabolite gene clusters and annotated ORFs grouped by function.
4.8. Cloning of Single Crossover Vector pKCLP2_PKS11
For the inactivation of thaBI of the PKS cluster #11, a 3087 bp internal fragment of the PKS I gene was amplified using the primers TTATACTGCAGACCGAGGACGAGGTCATC and ATCGGGAGAACTAGACGAACAG by PCR. The PCR product was firstly cloned into pUC19 (Stratagene, La Jolla, CA, USA). Subsequently, it was cut by PstI/EcoRV and cloned into the final vector pKCLP2 [52]. For cloning experiments E. coli XL1 Blue (Stratagene) was used.
4.9. Genetic Manipulation of A. bangkokensis 44EHWT
The single crossover vector pKCLP2-PKS11 was transferred into E. coli ET12567 (dam-, dcm-, hsdM-, hsdR-) [53], which carries the conjugative plasmid pUZ8002. For intergeneric conjugation, A. bangkokensis was grown at 28 °C for 2 days in TSB media. The mating mixture was spread on MS agar plates (2% mannitol, 2% soy flour, 2% agarose) containing 10 mM CaCl2. The plates were incubated at 28 °C for 16–20 h and overlaid with 1 mL H2O containing (0.2 mg phosphomycin and 0.05 mg hygromycin). The plates were further incubated at 28 °C for 6 days. The exconjugants were analyzed for thailandin production.
4.10. Alignment of Sequences
The sequences of thailandin acyltransferases and other acyltransferase from biosynthetic pathways with proposed unusual extender units were aligned by Clustal Omega [54] and compared in detail.
Acknowledgments
This work was supported by a research grant from National Research Council of Thailand and partially supported by Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (grant no. Ph.D./0314/2551) to W.P. and B.I.
Appendix
Table A1.
Putative secondary metabolite biosynthetic gene clusters in A. bangkokensis 44EHWT.
| Cluster # | Type Secondary Metabolite | Scaffold | from [bp] | to [bp] |
|---|---|---|---|---|
| 1 | other | 5 | 222,242 | 266,147 |
| 2 | butyrolactone | 9 | 112,144 | 123,172 |
| 3 | PKS II/ectoine | 9 | 173,085 | 217,111 |
| 4 | nucleosid | 10 | 89,791 | 111,566 |
| 5 | bacteriocin | 13 | 79,417 | 91,126 |
| 6 | siderophore | 13 | 123,673 | 135,433 |
| 7 | trans AT-PKS/NRPS/other | 14 | 21,256 | 106,054 |
| 8 | NRPS/PKS I | 15 | 12,027 | 65,701 |
| 9 | terpene | 16 | 2354 | 23,460 |
| 10 | other | 17 | 25,281 | 68,715 |
| 11 * | PKS I THAILANDIN CLUSTER | 17 | 179,992 | 276,120 |
| 12 | terpene | 17 | 304,613 | 326,544 |
| 13 | PKS III | 17 | 319,674 | 361,110 |
| 14 | NRPS/ladderane/arylpolyene | 17 | 392,681 | 462,423 |
| 15 | terpene | 17 | 454,050 | 475,513 |
| 16 * | oligosaccharide/PKS I | 17 | 557,011 | 710,221 |
| 17 | PKS I | 17 | 785,384 | 815,172 |
| 18 | PKS I | 18 | 1 | 35,881 |
| 19 * | PKS I/oligosaccharide | 19 | 1 | 133,613 |
| 20 | lantipeptide | 19 | 158,947 | 196,476 |
| 21 | PKS II | 19 | 200,846 | 243,937 |
| 22 | other | 19 | 266,838 | 310,212 |
| 23 | PKS I | 19 | 361,716 | 408,222 |
| 24 | NRPS/lantipeptide | 20 | 198,722 | 311,653 |
| 25 | lassopeptide | 22 | 18,493 | 40,971 |
| 26 | lantipeptide | 23 | 93,884 | 117,519 |
| 27 | other PKS/PKS I | 23 | 147,485 | 196,457 |
| 28 | terpene | 23 | 444,400 | 466,643 |
| 29 | butyrolactone | 23 | 494,036 | 505,109 |
| 30 | indole | 24 | 1 | 18,253 |
| 31 | PKS I | 24 | 37,982 | 83,864 |
| 32 | other PKS | 24 | 239,959 | 308,466 |
| 33 | NRPS/PKS I | 25 | 71,292 | 162,064 |
| 34 | ladderane/NRPS | 25 | 153,235 | 214,305 |
| 35 | PKS I | 26 | 1 | 44,677 |
* cluster encoding for large PKS I enzymes.
Table A2.
Identified strains of the genus Actinokineospora.
| Strain | Product | Genome Sequence * | Literature |
|---|---|---|---|
| A. auranticolor IFO 16518 | ? | - | [55] |
| A. baliensis ID03-0561T | ? | - | [56] |
| A. bangkokensis 44EHWT | thailandins A and B | this study MKQR01000000 |
[7,8] |
| A. cianjurensis ID03-0810T | ? | - | [56] |
| A. cibodasensis ID03-0784T | ? | - | [56] |
| A. diospyrosa NRRL B-24047 | ? | - | [57] |
| A. enzanensis IFO 16517 | ? | GCA_000374445.1 | [55] |
| A. fastidiosa NRRL B-16697 | macrobicyclic peptide antibiotic | - | [58,59,60] |
| A. globicatena NRRL B-24048 | ? | - | [57] |
| A. guangxiensis GK-6T | ? | - | [61] |
| A. inagensis NRRL B-24050 | ? | GCA_000482865.1 | [57] |
| A. mzabensis PAL84 | ? | - | [62] |
| A. riparia IFO 14541 | compound with antimycoplasmic activity | - | [63] |
| A. soli YIM 75948T | ? | - | [64] |
| A. spheciospongiae EG49 | actinosporins A–D, products from co-cultivation |
GCA_000564855.1 | [6,39,40,41,65,66] |
| A. terrae IFO 15668 | ? | - | [57] |
* GenBank assembly accession number.
Figure A1.
Alignment of acyltransferases with different AT-specificities. AT1-M, AT of thailandin biosynthesis of module 1 with malonyl-CoA specificity; AT2-M, AT of thailandin biosynthesis of module 2 with malonyl-CoA specificity; AT6-MM, AT of thailandin biosynthesis of module 6 with methylmalonyl-CoA specificity; AT13-M, AT of thailandin biosynthesis of module 13 with proposed butylmalonyl-CoA specificity (violet); Fil14, AT of filipin biosynthesis with proposed specificity to hexylmalonyl-CoA; AntD, AT of antimycin biosynthesis with specificity to propanylmalonyl-CoA, butylmalonyl-CoA, pentylmalonyl-CoA and hexylmalonyl-CoA; NemA4, AT of nemadectin biosynthesis with specificity to methyl-propanylmalonyl-CoA and dimethyl-butylmalonyl-CoA; RevB, AT of reveromycin biosynthesis with specificity to butylmalonyl-CoA, isopentylmalonyl-CoA, pentylmalonyl-CoA and hexylmalonyl-CoA; NamE, AT of neoansamycin biosynthesis with specificity to butylmalonyl-CoA and pentylmalonyl-CoA; PlyU, AT of polyoxypeptin biosynthesis with specificity to methyl-butylmalonyl-CoA; motifs highlighted by boxes in blue and red; * indicates highly conserved aa.
Author Contributions
A.G. conceived and designed the experiments and wrote the paper; S.F. analyzed genome sequence; M.E.G.R. conducted cloning; B.I. did cultural work; W.P. and A.B. reviewed experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Strain Actinokineospora bangkokensis 44EHWT is available from the author W.P.
References
- 1.Gomez-Escribano J.P., Bibb M.J. Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: From genome mining to manipulation of biosynthetic pathways. J. Ind. Microbiol. Biotechnol. 2014;41:425–531. doi: 10.1007/s10295-013-1348-5. [DOI] [PubMed] [Google Scholar]
- 2.Bode H.B., Bethe B., Höfs R., Zeeck A. Big effects from small changes: possible ways to explore nature’s chemical diversity. Chembiochem. 2002;3:619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Gao C., Hindra, Mulder D., Yin C., Elliot M.A. Crp is a global regulator of antibiotic production in Streptomyces. MBio. 2012;3 doi: 10.1128/mBio.00407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessner A., Heitzler T., Zhang S., Klaus C., Murillo R., Zhao H., Vanner S., Zechel D.L., Bechthold A. Changing biosynthetic profiles by expressing bldA in Streptomyces strains. ChemBioChem. 2015;16:2244–2252. doi: 10.1002/cbic.201500297. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa T. Actinokineospora: A new genus of the Actinomycetales. Actinomycetologica. 1988;2:31–45. doi: 10.3209/saj.2_31. [DOI] [Google Scholar]
- 6.Harjes J., Ryu T., Abdelmohsen U.R., Moitinho-Silva L., Horn H., Ravasi T., Hentschel U. Draft genome sequence of the antitrypanosomally active sponge-associated bacterium Actinokineospora sp. strain EG49. Genome Announc. 2014;2 doi: 10.1128/genomeA.00160-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Intra B., Matsumoto A., Inahashi Y., Omura S., Takahashi Y., Panbangred W. Actinokineospora bangkokensis sp. nov., isolated from rhizospheric soil. Int. J. Syst. Evol. Microbiol. 2013;63:2655–2660. doi: 10.1099/ijs.0.047928-0. [DOI] [PubMed] [Google Scholar]
- 8.Intra B., Greule A., Bechthold A., Euanorasetr J., Paululat T., Panbangred W. Thailandins A and B, new polyene macrolactone compounds isolated from Actinokineospora bangkokensis strain 44EHWT, possessing antifungal activity against anthracnose fungi and pathogenic yeasts. J. Agric. Food Chem. 2016;64:5171–5179. doi: 10.1021/acs.jafc.6b01119. [DOI] [PubMed] [Google Scholar]
- 9.Tatusova T., DiCuccio M., Badretdin A., Chetvernin V., Nawrocki E.P., Zaslavsky L., Lomsadze A., Pruitt K.D., Borodovsky M., Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber T., Blin K., Duddela S., Krug D., Kim H.U., Bruccoleri R., Lee S.Y., Fischbach M.A., Müller R., Wohlleben W., et al. antiSMASH 3.0—A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–W243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav G., Gokhale R.S., Mohanty D. Computational approach for prediction of domain organization and substrate specificity of modular polyketide synthases. J. Mol. Biol. 2003;328:335–363. doi: 10.1016/S0022-2836(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 12.Bisang C., Long P.F., Corte´s J., Westcott J., Crosby J., Matharu A.-L., Cox R.J., Simpson T.J., Staunton J., Leadlay P.F. A chain initiation factor common to both modular and aromatic polyketide synthases. Nature. 1999;401:502–505. doi: 10.1038/46829. [DOI] [PubMed] [Google Scholar]
- 13.Erb T.J., Berg I.A., Brecht V., Müller M., Fuchs G., Alber B.E. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc. Natl. Acad. Sci. USA. 2007;104:10631–10636. doi: 10.1073/pnas.0702791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erb T.J., Brecht V., Fuchs G., Müller M., Alber B.E. Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase. Proc. Natl. Acad. Sci. USA. 2009;106:8871–8876. doi: 10.1073/pnas.0903939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quade N., Huo L., Rachid S., Heinz D.W., Müller R. Unusual carbon fixation gives rise to diverse polyketide extender units. Nat. Chem. Biol. 2012;8:117–124. doi: 10.1038/nchembio.734. [DOI] [PubMed] [Google Scholar]
- 16.Sandy M., Rui Z., Gallagher J., Zhang W. Enzymatic synthesis of dilactone scaffold of antimycins. ACS Chem. Biol. 2012;7:1956–1961. doi: 10.1021/cb300416w. [DOI] [PubMed] [Google Scholar]
- 17.Wilson M.C., Moore B.S. Beyond ethylmalonyl-CoA: The functional role of crotonyl-CoA-carboxylase/reductase homologs in expanding polyketide diversity. Nat. Prod. Rep. 2012;29:72–86. doi: 10.1039/C1NP00082A. [DOI] [PubMed] [Google Scholar]
- 18.Keatinge-Clay A. Crystal structure of the erythromycin polyketide synthase dehydratase. J. Mol. Biol. 2008;384:941–953. doi: 10.1016/j.jmb.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caffrey P., Lynch S., Flood E., Finnan S., Oliynyk M. Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes. Chem. Biol. 2001;8:713–723. doi: 10.1016/S1074-5521(01)00046-1. [DOI] [PubMed] [Google Scholar]
- 20.Fjærvik E., Zotchev S.B. Biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei. Appl. Microbiol. Biotechnol. 2005;67:436–443. doi: 10.1007/s00253-004-1802-4. [DOI] [PubMed] [Google Scholar]
- 21.Caffrey P. Conserved amino acid residues correlating with ketoreductase stereospecificity in modular polyketide synthases. ChemBioChem. 2003;4:654–657. doi: 10.1002/cbic.200300581. [DOI] [PubMed] [Google Scholar]
- 22.Keatinge-Clay A.T., Stroud R.M. The structure of a ketoreductase determines the organization of the β-carbon processing enzymes of modular polyketide synthases. Structure. 2006;14:737–748. doi: 10.1016/j.str.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Bonnett S.A., Whicher J.R., Papireddy K., Florova G., Smith J.L., Reynolds K.A. Structural and stereochemical analysis of a modular polyketide synthase ketoreductase domain required for the generation of a cis-alkene. Chem. Biol. 2013;20:772–783. doi: 10.1016/j.chembiol.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pöplau P., Frank S., Morinaka B.I., Piel J. An enzymatic domain for the formation of cyclic ethers in complex polyketides. Angew. Chem. Int. Ed. 2013;52:13215–13218. doi: 10.1002/anie.201307406. [DOI] [PubMed] [Google Scholar]
- 25.Berkhan G., Hahn F. A dehydratase domain in ambruticin biosynthesis displays additional activity as a pyran-forming cyclase. Angew. Chem. Int. Ed. 2014;53:14240–14244. doi: 10.1002/anie.201407979. [DOI] [PubMed] [Google Scholar]
- 26.Luhavaya H., Dias M.V.B., Williams S.R., Hong H., de Oliveira L.G., Leadlay P.F. Enzymology of pyran ring A formation in salinomycin biosynthesis. Angew. Chem. Int. Ed. 2015;54:13622–13625. doi: 10.1002/anie.201507090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiński D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014;43:453–467. doi: 10.1007/s00249-014-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanos V., Cataldi L. Amphotericin B-induced nephrotoxicity: A review. J. Chemother. 2000;12:463–470. doi: 10.1179/joc.2000.12.6.463. [DOI] [PubMed] [Google Scholar]
- 29.Tevyashova A.N., Olsufyeva E.N., Solovieva S.E., Printsevskaya S.S., Reznikova M.I., Trenin A.S., Galatenko O.A., Treshalin I.D., Pereverzeva E.R., Mirchink E.P., et al. Structure-antifungal activity relationships of polyene antibiotics of the amphotericin B group. Antimicrob. Agents Chemother. 2013;57:3815–3822. doi: 10.1128/AAC.00270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brautaset T., Sletta H., Nedal A., Borgos S.E.F., Degnes K.F., Bakke I., Volokhan O., Sekurova O.N., Treshalin I.D., Mirchink E.P., et al. Improved antifungal polyene macrolides via engineering of the nystatin biosynthetic genes in Streptomyces noursei. Chem. Biol. 2008;15:1198–1206. doi: 10.1016/j.chembiol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Huang X., Zhou X., Bai L., He J., Jeong K.J., Lee S.Y., Deng Z. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem. Biol. 2003;10:1065–1076. doi: 10.1016/j.chembiol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Palacios D.S., Dailey I., Siebert D.M., Wilcock B.C., Burke M.D. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl. Acad. Sci. USA. 2011;108:6733–6738. doi: 10.1073/pnas.1015023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gary-Bobo C.M. Polyen-sterol interaction and selective toxicity. Biochimie. 1989;71:37–47. doi: 10.1016/0300-9084(89)90129-6. [DOI] [PubMed] [Google Scholar]
- 34.Cybulska B., Bolard J., Seksek O., Czerwinski A., Borowski E. Identification of the structural elements of amphotericin B and other polyene macrolide antibiotics of the hepteane group influencing the ionic selectivity of the permeability pathways formed in the red cell membrane. Biochim. Biophys. Acta. 1995;1240:167–178. doi: 10.1016/0005-2736(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 35.Pandey R.C., Narasimhachari N., Rinehart K.L., Millington D.S. Polyene antibiotics. IV. Structure of chainin. J. Am. Chem. Soc. 1972;94:4306–4310. doi: 10.1021/ja00767a045. [DOI] [PubMed] [Google Scholar]
- 36.Ceder O., Ryhage R. The structure of filipin. Acta Chem. Scand. 1964;18:558–560. doi: 10.3891/acta.chem.scand.18-0558. [DOI] [Google Scholar]
- 37.Shih H.-D., Liu Y.-C., Hsu F.-L., Mulabagal V., Dodda R., Huang J.-W. Fungichromin: A substance from Streptomyces padanus with inhibitory effects on Rhizoctonia solani. J. Agric. Food Chem. 2003;51:95–99. doi: 10.1021/jf025879b. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y.-F., Wei S.-J., Zhang Z.-P., Zhan T.-H., Tu G.-Q. Antifungalmycin, an antifungal macrolide from Streptomyces padanus 702. Nat. Products Bioprospect. 2012;2:41–45. doi: 10.1007/s13659-011-0037-1. [DOI] [Google Scholar]
- 39.Abdelmohsen U., Cheng C., Viegelmann C., Zhang T., Grkovic T., Ahmed S., Quinn R., Hentschel U., Edrada-Ebel R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs. 2014;12:1220–1244. doi: 10.3390/md12031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grkovic T., Abdelmohsen U.R., Othman E.M., Stopper H., Edrada-Ebel R., Hentschel U., Quinn R.J. Two new antioxidant actinosporin analogues from the calcium alginate beads culture of sponge-associated Actinokineospora sp. strain EG49. Bioorg. Med. Chem. Lett. 2014;24:5089–5092. doi: 10.1016/j.bmcl.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 41.Dashti Y., Grkovic T., Abdelmohsen U., Hentschel U., Quinn R. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs. 2014;12:3046–3059. doi: 10.3390/md12053046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X.-K., Jin J.-L. Crucial factor for increasing the conjugation frequency in Streptomyces netropsis SD-07 and other strains. FEMS Microbiol. Lett. 2014;357:99–103. doi: 10.1111/1574-6968.12507. [DOI] [PubMed] [Google Scholar]
- 43.Aparicio J.F., Caffrey P., Gil J.A., Zotchev S.B. Polyene antibiotic biosynthesis gene clusters. Appl. Microbiol. Biotechnol. 2003;61:179–188. doi: 10.1007/s00253-002-1183-5. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi S., Toyoda A., Sekiyama Y., Takagi H., Nogawa T., Uramoto M., Suzuki R., Koshino H., Kumano T., Panthee S., et al. Reveromycin A biosynthesis uses RevG and RevJ for stereospecific spiroacetal formation. Nat. Chem. Biol. 2011;7:461–468. doi: 10.1038/nchembio.583. [DOI] [PubMed] [Google Scholar]
- 45.Li S., Li Y., Lu C., Zhang J., Zhu J., Wang H., Shen Y. Activating a cryptic ansamycin biosynthetic gene cluster to produce three new naphthalenic octaketide ansamycins with n-pentyl and n-butyl side chains. Org. Lett. 2015;17:3706–3709. doi: 10.1021/acs.orglett.5b01686. [DOI] [PubMed] [Google Scholar]
- 46.Seipke R.F., Patrick E., Hutchings M.I. Regulation of antimycin biosynthesis by the orphan ECF RNA polymerase sigma factor σ AntA. PeerJ. 2014;2:e253. doi: 10.7717/peerj.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter G.T., Nietsche J.A., Hertz M.R., Williams D.R., Siegel M.M., Morton G.O., James J.C., Borders D.B. LL-F28249 antibiotic complex: A new family of antiparasitic macrocyclic lactones. Isolation, characterization and structures of LL-F28249 α, β, δ, λ. J. Antibiot. (Tokyo) 1988;41:519–529. doi: 10.7164/antibiotics.41.519. [DOI] [PubMed] [Google Scholar]
- 48.Du Y., Wang Y., Huang T., Tao M., Deng Z., Lin S. Identification and characterization of the biosynthetic gene cluster of polyoxypeptin A, a potent apoptosis inducer. BMC Microbiol. 2014;14:30. doi: 10.1186/1471-2180-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madden T. The NCBI Handbook. 2nd ed. National Center for Biotechnology Information; Bethesda, MD, USA: 2003. The BLAST Sequence Analysis Tool. Chapter 16. [Google Scholar]
- 50.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Z., Gu L., Eils R., Schlesner M., Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 52.Petzke L., Bechthold A. Ph.D. Thesis. Albert-Ludwigs-University of Freiburg; Freiburg im Breisgau, Germany: 2010. Transgenese in Streptomyceten: Transposons, Rekombinasen und Meganukleasen. [Google Scholar]
- 53.MacNeil D.J., Gewain K.M., Ruby C.L., Dezeny G., Gibbons P.H., MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-M. [DOI] [PubMed] [Google Scholar]
- 54.Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otoguro M., Hayakawa M., Yamazaki T., Tamura T., Hatano K., Iimura Y. Numerical phenetic and phylogenetic analyses of Actinokineospora isolates, with a description of Actinokineospora auranticolor sp. nov. and Actinokineospora enzanensis sp. nov. Actinomycetologica. 2001;15:30–39. doi: 10.3209/saj.15_30. [DOI] [Google Scholar]
- 56.Lisdiyanti P., Otoguro M., Ratnakomala S., Lestari Y., Hastuti R.D., Triana E., Katsuhiko A., Widyastuti Y. Actinokineospora baliensis sp. nov., Actinokineospora cibodasensis sp. nov. and Actinokineospora cianjurensis sp. nov., isolated from soil and plant litter. Int. J. Syst. Evol. Microbiol. 2010;60:2331–2335. doi: 10.1099/ijs.0.013276-0. [DOI] [PubMed] [Google Scholar]
- 57.Tamura T., Hayakawa M., Nonomura H., Yokota A., Hatano K. Four new species of the genus Actinokineospora: Actinokineospora inagensis sp. nov., Actinokineospora globicatena sp. nov., Actinokineospora terrae sp. nov., and Actinokineospora diospyrosa sp. nov. Int. J. Syst. Bacteriol. 1995;45:371–378. doi: 10.1099/00207713-45-2-371. [DOI] [Google Scholar]
- 58.Celmer W.D., Cullen W.P., Moppett C.E., Routien J.B., Shibakawa R., Tone J. Antibiotics Produced by Species of Pseudonocardia. 4031206 A. U.S. Patent. 1977 Jun 21;
- 59.Henssen A., Kothe H.W., Kroppenstedt R.M. Transfer of Pseudonocardia azurea and Pseudonocardia fastidiosa to the genus Amycolatopsis, with emended species description. Int. J. Syst. Bacteriol. 1987;37:292–295. doi: 10.1099/00207713-37-3-292. [DOI] [Google Scholar]
- 60.Labeda D.P., Price N.P., Tan G.Y.A., Goodfellow M., Klenk H.-P. Emended description of the genus Actinokineospora (Hasegawa 1988) and transfer of Amycolatopsis fastidiosa (Henssen et al. 1987) as Actinokineospora fastidiosa comb. nov. Int. J. Syst. Evol. Microbiol. 2010;60:1444–1449. doi: 10.1099/ijs.0.016568-0. [DOI] [PubMed] [Google Scholar]
- 61.Wu H., Liu B. Actinokineospora guangxiensis sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 2015;65:4650–4654. doi: 10.1099/ijsem.0.000627. [DOI] [PubMed] [Google Scholar]
- 62.Aouiche A., Bouras N., Mokrane S., Zitouni A., Schumann P., Spröer C., Sabaou N., Klenk H.-P. Actinokineospora mzabensis sp. nov., a novel actinomycete isolated from Saharan soil. Antonie Van Leeuwenhoek. 2015;107:291–296. doi: 10.1007/s10482-014-0328-8. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa T. Studies on motile arthrospore-bearing rare actinomycetes. Actinomycetologica. 1991;5:64–71. doi: 10.3209/saj.5_64. [DOI] [Google Scholar]
- 64.Tang X., Zhou Y., Zhang J., Ming H., Nie G.-X., Yang L.-L., Tang S.-K., Li W.-J. Actinokineospora soli sp. nov., a thermotolerant actinomycete isolated from soil, and emended description of the genus Actinokineospora. Int. J. Syst. Evol. Microbiol. 2012;62:1845–1849. doi: 10.1099/ijs.0.035832-0. [DOI] [PubMed] [Google Scholar]
- 65.Abdelmohsen U.R., Pimentel-Elardo S.M., Hanora A., Radwan M., Abou-El-Ela S.H., Ahmed S., Hentschel U. Isolation, phylogenetic analysis and anti-infective activity screening of marine sponge-associated actinomycetes. Mar. Drugs. 2010;8:399–412. doi: 10.3390/md8030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kämpfer P., Glaeser S.P., Busse H.-J., Abdelmohsen U.R., Ahmed S., Hentschel U. Actinokineospora spheciospongiae sp. nov., isolated from the marine sponge Spheciospongia vagabunda. Int. J. Syst. Evol. Microbiol. 2015;65:879–884. doi: 10.1099/ijs.0.000031. [DOI] [PubMed] [Google Scholar]