Abstract
Antimicrobial peptides (AMPs) represent a vast array of molecules produced by virtually all living organisms as natural barriers against infection. Among AMP sources, an interesting class regards the food-derived bioactive agents. The whey protein lactoferrin (Lf) is an iron-binding glycoprotein that plays a significant role in the innate immune system, and is considered as an important host defense molecule. In search for novel antimicrobial agents, Lf offers a new source with potential pharmaceutical applications. The Lf-derived peptides Lf(1–11), lactoferricin (Lfcin) and lactoferrampin exhibit interesting and more potent antimicrobial actions than intact protein. Particularly, Lfcin has demonstrated strong antibacterial, anti-fungal and antiparasitic activity with promising applications both in human and veterinary diseases (from ocular infections to osteo-articular, gastrointestinal and dermatological diseases).
Keywords: antimicrobial peptides, lactoferricin, milk proteins, food safety
1. Introduction
Antimicrobial peptides (AMPs) represent a vast array of molecules produced by virtually all living organisms as a natural barrier against infection. They are a primitive defence mechanism found in a wide range of eukaryotic organisms, throughout the taxonomic scale, including mammals, invertebrates and plants [1,2,3,4]. AMPs exhibit a broad range of activities against Gram-negative and Gram-positive bacteria, fungi, viruses, and parasites. To date, 2645 AMPs from various sources have been listed in “The Antimicrobial Peptide Database” [5], a database dedicated to natural AMPs.
This rich source of antimicrobial agents has aroused growing interest, especially in the light of the decreasing effectiveness of antibiotics not only against severe infections, but also in treating common infectious diseases. Resistance to antibiotics has become a threat to global public health and is driving novel research into the development of new antimicrobial agents [6]. Innovation is thus needed not only for the development of new antibiotics (where, for example, the worldwide pipeline for new antibiotic classes active against highly resistant Gram-negative bacteria is almost dry) but also for combination therapies. By targeting different mechanisms of resistance simultaneously, combination therapy might help slow the emergence of resistance [7]. Moreover it has been strongly suggested that any synergy between these drugs and the immune response should be exploited in the treatment of bacterial infections [8].
Among AMP sources, an interesting class are the food-derived bioactive agents [9]. These AMP peptides together with other bioactive peptides are hidden inside food proteins (mainly milk proteins) and can be decrypted by proteolytic activity [10]. Dairy proteins, protein hydrolysates, and fermented dairy products have been shown to possess a wide spectrum of pharmacological activities: opioid, immunomodulatory, antimicrobial and antiviral, antithrombotic, growth-stimulating, and antihypertensive properties [11,12]. The class of AMP is particularly attracting and the “generally recognized as safe” (GRAS) status of food satisfies both consumers and industry. Research in the last twenty years has discovered several proteins and related peptides with interesting antimicrobial properties: milk proteins, such as β-lactoglobulin or α-lactalbumin and particularly lactoferrin (Lf) contain AMPs which have potential applications as pharmaceutical products [13]. The wealth of this research area is testified by specific conferences (e.g., XII International Conference on Lactoferrin) and a database named MilkAMP that contains all the physicochemical and microbiological characteristics of identified antimicrobial dairy peptides [14].
Recently, reports have demonstrated that the increased spectrum of activity of Lf and related peptides is larger than expected. Antiviral [12,15,16,17] and antiprotozoal activities [18] as well as tumor inhibition [19,20] and potent anti-inflammatory, anti-catabolic, and anti-oxidative effects [21] have been described. This article reviews the development of research on Lf-derived AMPs of bovine and human origin, and their applications, mainly as antimicrobial agents, for use by several administration routes and at different sites. Future prospects of lactoferrin-derived AMP will be also discussed.
2. Lactoferrin: Distribution in Body Fluids and Clinical Efficacy
The whey protein lactoferrin (Lf) is an 80 KDa iron-binding glycoprotein that plays a significant role in the innate immune system, and is considered to be an important host defense molecule. A wide range of physiological functions such as antiviral, [22] antimicrobial, [23] antifungal, anti-parasitic [24], immunomodulatory [25] and antioxidant activities have been identified [26,27]. In humans, Lf is one of the major proteins in all exocrine secretions, such as colostrum (where Lf is found at the highest concentration, i.e., 8 mg/mL), milk, tears, saliva, seminal and gastrointestinal fluids, nasal and bronchial mucosa, and plasma (0.2 µg/mL). Breast milk is the main source of Lf found in the gut of infants, and accounts for the initiation, development, and/or composition of the neonatal gut microbiota. Lf is also stored in the secondary granules of polymorphonuclear leukocytes and it has been reported that Lf regulates multiple signalling pathways to impart cytotoxic effects on cancer cell [19].
With regard to its antimicrobial effects, human Lf exhibits a very effective response against a wide range of bacteria, including species of Streptococcus, Salmonella, Shigella, Staphylococcus, and Enterobacter [28]. There is a vast literature describing the in vitro efficacy and animal-model benefits of Lf. The principal clinical results are reported below.
One of the first applications of Lf in humans was in infant formula, and this has been the object of systematic and critical reviews [29,30]. The majority of studies have shown Lf to be safe in preterm/term new-born, and in older infants; in these children, protection against enteric and neonatal infections are the most likely biologically relevant activities of Lf. Several clinical trials are currently ongoing (data from www.clinicaltrials.gov).
Lf was also administered in a trial concerning Giardia lamblia infection; this is among the most common protozoan infection of the human intestine causing severe diarrhoea. A cohort of children with Giardia infection fed Lf showed a lower prevalence of colonization and better growth as compared to unfed children [31].
Lactoferrin, naturally present in the saliva, is associated with host defense against oral pathogens. The clinical usefulness of bovine Lf (bLf) combined with proteolytic enzymes to treat oral candidiasis or dry mouth has been examined [32,33] and a mucoadhesive tablet containing Lf has been developed [34]. Wakabayashi et al. evaluated the in vitro effects of Lf-related agents on the growth and biofilm formation of two periodontopathic bacteria, Porphyromonas gingivalis and Prevotella intermedia, which reside as biofilms in the subgingival plaque [35]. bLf used in combination with four antibiotics reduced the amount of a preformed biofilm of P. gingivalis compared with the reduction achieved with antibiotics alone. Improved liposomal formulations of Lf and lactoperoxidase significantly reduced the caries incidence in rat models [36]. These results demonstrate the potential usefulness of Lf for the prevention and treatment of periodontal diseases and as adjunct therapy in such diseases.
Recently, Lf analysis was used as a sensitive assay for detecting the degree of periodontal inflammation and oxidative stress, and for monitoring the effects of periodontal therapy as well [37]. The Lf level was higher in the periodontitis group of patients compared to the healthy group, and the measured level decreased in the former group after periodontal therapy. Higher Lf levels were associated with higher values of clinical parameters (such as gingival index, plaque index, probing pocket depth, clinical attachment level) both before and after therapy. This overall research indicates that Lf plays an important role during periodontal disease, and crevicular Lf quantification could be a marker for detecting periodontal inflammation, oxidative stress, and monitoring periodontal therapy.
The role of fermented milk and whey proteins in controlled clinical trials regarding Helicobacter pylori infections was recently reviewed by Sachdeva [38]. Although the available evidence suggests that bLf is beneficial for H. pylori eradication (Recommendation Grade-A), unlike AMP derived from Lf [39], the extent of the documented benefit is small, and deserves further exploration. Other clinical trials on Lf are related to: (i) the prevention of hospital-acquired infections; (ii) the treatment of cystic fibrosis [40] in combination with hypothiocyanite; (iii) clinical efficacy against the common cold in combination with the IgG-rich fraction of whey proteins [41].
Regarding potential Lf side effects, advanced clinical trials, including the treatment for H. pylori infection [42], iron deficiency anemia in pregnant women [43] and new-born sepsis [44] did not report any significant adverse effects or intolerance.
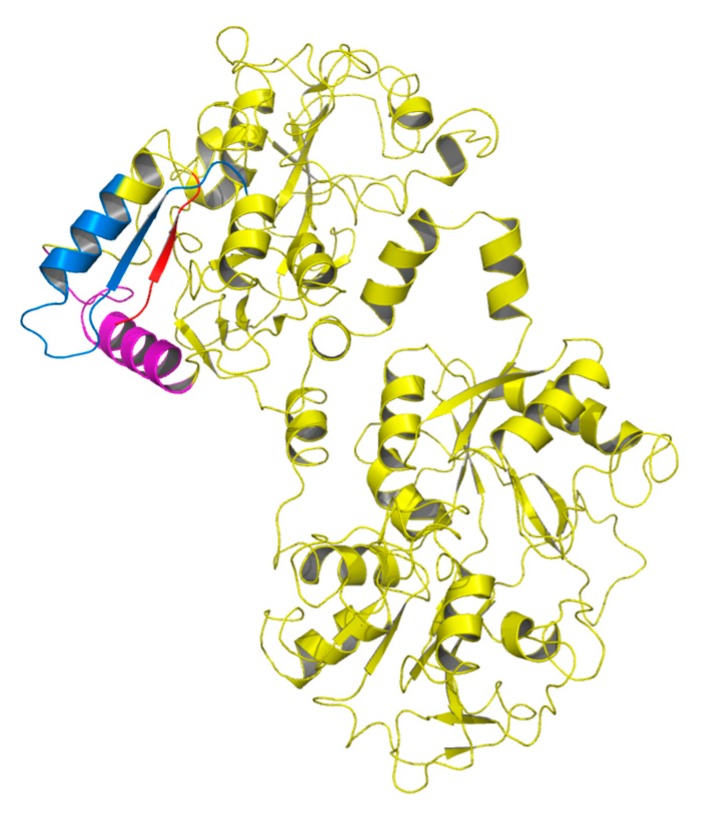
A number of functional peptides are produced from Lf by the action of proteolytic enzymes, and many Lf-derived antimicrobial peptides have been isolated and characterized (Figure 1); three have been studied in some detail [45]. They originate from the N-lobe of Lf, and their antimicrobial activity is chiefly linked to hydrophobicity, cationic charge, and helical conformation, which render these peptides amphiphilic molecules. Most of them cause membrane depolarization (like the antibiotics colistin and polimixin B) [46]. However, complex mechanisms, such as inhibition of the synthesis of macromolecules [47], and synergic action with host innate immunity compounds were also described [48]. The three peptides are Lf(1–11), lactoferricin (Lfcin) and lactoferrampin (LFampin); the alphabetic letter indicates the species of origin, e.g., (b) for bovine and (h) for human [49].
Figure 1.
Overall structure of lactoferrin showing positions of the functional peptides Lf(1–11) (red), lactoferrampin (pink), and lactoferricin (blue) in the N-terminal lobe.
3. Lf Derived Peptides: Lf(1–11)
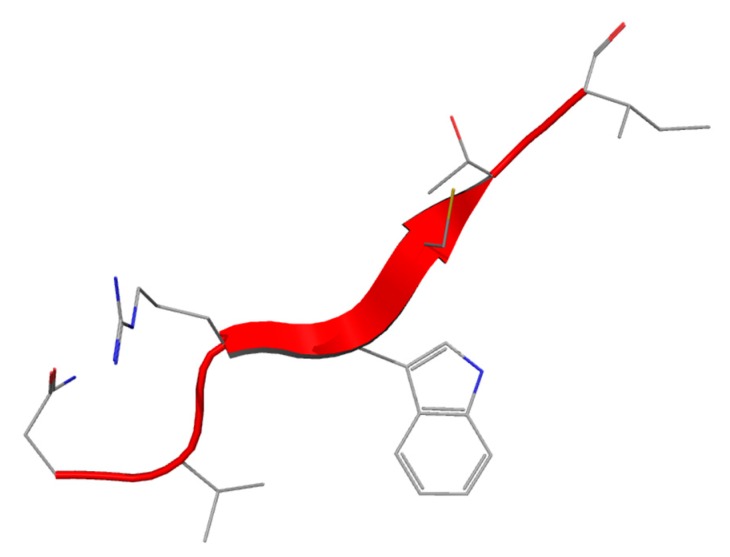
Lf(1–11) is the oligopeptide including the first eleven aminoacid residues of the Lf molecule (Figure 2). The sequence comparison of Lf(1–11) from six mammalian species (Table 1) shows that some important features such as the highly cationic nature of the peptide is maintained (pI > 11, ranging from 11.70 (bovine) to 12.5 (human)) and the hydrophobic residues valine and tryptophan V6 and W8 are conserved in all species. Investigation of the structural and dynamic properties in water and membrane-mimicking solvents show that hLf(1–11) effectively interacts with the membrane, whereas the control peptide (a scrambled analogue) did not show such conformation [40].
Figure 2.
Structure of Lf(1–11) peptide.
Table 1.
Comparison of amino acid sequences of Lf(1–11) in Lf of different mammalian species.
| Species | Sequence |
|---|---|
| Human | |
| Cow | |
| Buffalo | |
| Horse | |
| Goat | |
| Camel |
For the different amino acids, the colours indicate: blue = positively charged (R, K, H); black = negatively charged (D, E); red = hydrophobic (I, L, V, A, P, M F, Y, W); green = hydrophilic (S, T, E, Q, N, C and G).
3.1. In Vitro Antibacterial and Anti-Fungal Activity of Lf(1–11)
In a recent study, hLf(1–11) (milkAMP database id = LFH0004) was investigated for its ability to treat infections caused by multi-drug-resistant bacteria. The results showed that hLf(1–11) was active against various Gram positive bacteria such as Staphylococcus spp. (including MRSA), and Streptococcus mitis (MIC values ranged from 1.6 to 6.3 µg/mL) as well as Gram-negative bacteria: Acinetobacter baumannii, Pseudomonas spp., Klebsiella spp. and E. coli (MIC values 6.3 to 12.5 µg/mL). As far as Yeasts are concerned in Candida spp. (MIC > 12.5 µg/mL) were found [49].
This peptide’s anti-fungal action mechanism appears to involve a particular sequence of events. The peptide interacts with structural elements of the plasma membrane of the blastoconidia, and is taken up in an energy-dependent manner. Inside the cell it triggers the energized mitochondria to synthesize and secrete ATP, and the peptide is released extracellularly, where it interacts with surface ATP binding sites, resulting in pore formation. These events induce progression towards cell death, possibly involving mitochondria [50]. Another study demonstrated the involvement of fungal endogenous thiols and reactive oxygen species (ROS) in the candidacidal activity exerted by hLf(1–11). In this study, hLf(1–11) caused a decrease in the internal thiol levels of Candida albicans by 20% and an increase in the level of its production of ROS in a dose-dependent manner; a correlation between ROS production and candidacidal activity was also found [51].
Little has been reported concerning the synergistic effects of hLf(1–11) with antibiotics. It has been observed that Lf or hLf(1–11), added at sub-inhibitory concentrations to antifungal agents such as clotrimazole, ketoconazole, fluconazole, or itraconazole, reduces the minimum inhibitory concentrations of these agents against Candida species [52]. The peptide was highly active against fluconazole-resistant Candida albicans at non-candidacidal concentrations, acted synergistically with fluconazole against this yeast and a fluconazole-sensitive C. albicans strain, as well as against C. glabrata, C. krusei, C. parapsilosis and C. tropicalis. When these yeasts were exposed to hLf(1–11) for five minutes and then incubated with fluconazole, they were effectively killed, while no candidacidal activity was observed when they were incubated first with fluconazole and then exposed to the peptide: this shows that the candidacidal activity is initiated by the peptide, whereas fluconazole is only required during the effector phase [53].
3.2. Main in Vivo Results in Human and Animal Models
hLf(1–11) showed activity with 2.5 log reduction against an antibiotic-resistant S. aureus strain (at 12 μM) [54]; it retained its activity when bound to bone cements used clinically [55]. In rabbit model trials using cement containing hLf(1–11), two studies [56,57] revealed a significant reduction of bone-injected S. aureus ATCC 10832 bacteria or S. aureus (W234) compared with the control group. The results clearly showed that hLf(1–11) has the ability to reduce osteomyelitis, with microbiological results similar to those of gentamicin [57]. In an earlier study, the intravenous injection of hLf(1–11) at 0.1 or 1 nmol successfully reduced murine muscle infections (1 × 107 CFU) caused by the methicillin-resistant S. aureus strain 2141 (MRSA), Klebsiella pneumoniae ATCC 43816 [54], S. aureus 25923 [58], and various multidrug-resistant Acinetobacter baumannii [59].
In an interesting report using labelled hLf(1–11), the administration routes of the peptide were compared (oral, i.p., i.v. and s.c.) on mice infected with multidrug resistant S. aureus [60]. A dose-dependent effect was observed with increasing i.v. doses, from 0.04 to 40 mg/kg of body weight, with maximal reduction of viable bacteria in infected thigh muscle of 4 log CFU/g of tissue compared with control. hLf(1–11) was rapidly removed from the circulation after i.v. administration (blood half-life t = 5–10 min) through the kidneys. This administration route produced the highest concentration in muscle tissue (0.9% ID/g). Removal was slower for orally-administered peptide, and 38% ID/g of the radioactivity still remained in the stomach and 40% in the intestine 2 h after administration [60].
Following the promising in vitro antifungal results, in vivo activity of hLf(1–11) against fluconazole-resistant C. albicans was investigated using neutrocytopenic mice infected with C. albicans Y01-19. A reduction of clinical signs and symptoms of the infection was observed at a dose 0.4 μg/kg of body weight, much lower than that found in in vitro experiments [49]. The most likely explanation for the levelling off of the antifungal effects of hLf(1–11) is that the peptide induces multiple processes that contribute differently to its antifungal activity. Furthermore, administering hLf(1–11) at up to 10,000 times the therapeutic dose produced no significant adverse or toxic effects, and consequently the therapeutic window of the peptide is very wide.
The safety of Lf(1–11) was investigated during the first three trials conducted in humans using ascending doses of the peptide. The investigation was done in healthy volunteers and to patients receiving autologous haematopoietic stem-cell transplantation following conditioning with high-dose melphalan for multiple myeloma or lymphoplasmocytic lymphoma. Intravenous doses of up to 5 mg daily for 5 days showed a very favourable side-effect profile. The only undesirable effect was an elevation of transaminases, which may be due to hLf(1–11), although current data does not allow any cause-effect relationship to be postulated [61].
The safety and tolerability of hLF(1–11) is under investigation in some clinical studies, sponsored by AM-Pharma, but currently no other recruiting studies are on-going (from www.clinicaltrials.org database) thus the future of pharmaceutical applications appears unclear.
4. Lf-Derived Peptides: Lactoferricin
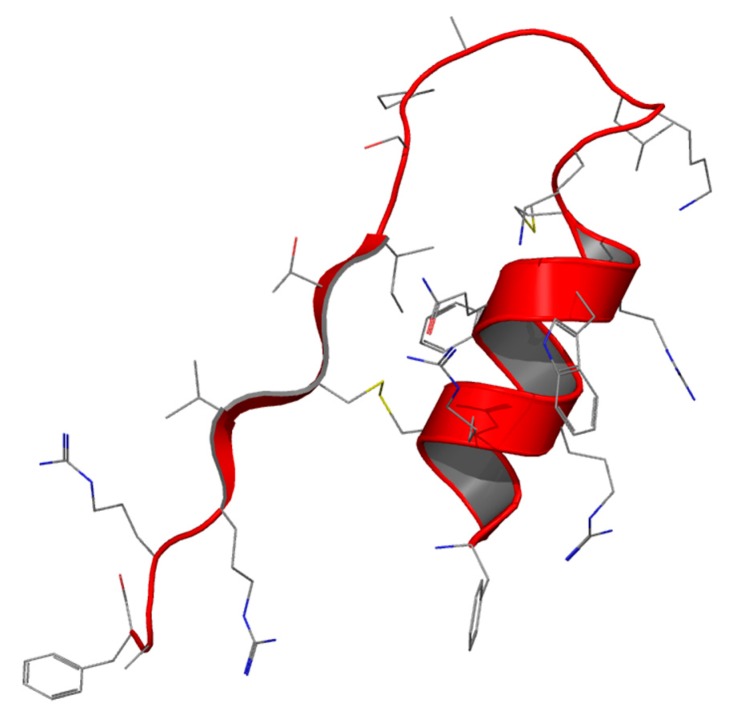
After Lf proteolysis by pepsin under acidic conditions, a 25-residue peptide (Lf amino acid residues 17–41) lactoferricin (Lfcin) was identified [62] The peptide has an abundance of basic amino acids including lysine and arginine, as well as hydrophobic residues like tryptophan and phenylalanine. In all Lfcins (derived from different mammalian species) a loop region with an intramolecular disulfide bridge is present (milkAMP database id = LFB0084) (Figure 3) [62]. Table 2 shows the structure of Lfcin of different origins [63]. The current hLfcin sequence contains this loop, but in this case a second disulfide bond extends the overall structure, which is about twice as long as bLfcin. hLfcin is composed of two fragments corresponding to 1–11 and 12–47 connected by a disulfide bridge [62,64] (milkAMP database id = LFH0009).
Figure 3.
Structure of Lfcin peptide.
Table 2.
Comparison of aminoacidic sequences of Lfcin from Lf of different mammalian species.
| Species | Sequence |
|---|---|
| Human | TCQQNGSCDS |
| Cow | CQGSTC |
| Buffalo | CHQGSTC |
| Horse | CQNGSSCTS |
| Goat | SCYQQGSTCTS |
| Camel | CQQGSTCTS |
| Mouse | ECQNEMVGGSCSS |
For the different amino acids, the colours indicate: blue = positively charged (R, K, H); black = negatively charged (D, E); red = hydrophobic (I, L, V, A, P, M F, Y, W); green = hydrophilic (S, T, E, Q, N, C and G).
Both bLfcin and hLfcin exist as an amphipathic α-helix in Lf but, after pepsin digestion, the former is transformed into amphipathic β-sheet hairpin in an aqueous environment, whereas the latter (bulkier model) also possess a parallel β-sheet that is lost after digestion, preserving the α-helix [64]. The retention of the α-helical region in hLfcin may be directly related to the additional length of the peptide. The ability of Lfcin to form amphipathic structures with net hydrophobic and positively-charged faces (Table 2) is a trait shared with other peptides having antimicrobial activity [65].
4.1. In Vitro Antibacterial and Anti-Fungal Activity
The first study, in 1992, on Lfcin as an AMP reported it to be a more potent antibacterial and anti-fungal agent than intact Lf; it was shown to cause a rapid loss of colony-forming capacity in most of its targets [66]. Comparing the activity of Lfcin from different species (cow, mouse and goat) showed that bLfcin was the most potent [63]. For example, the MIC of bLfcin against certain E. coli strains was found to be around 30 μg/mL whereas a MIC of with 100 μg/mL was detected for hLfcin. bLfcin showed bactericidal activity against a range of Gram-positive and Gram-negative bacteria [67], whereas hLfcin only exerted bacteriostatic activity [62]. The wide range of activities are shown in Table 3; the MIC as antibacterial, antifungal and anti-protozoarian activities are taken from the milkAMP database [14].
Table 3.
Activities of bLfcin against different bacterial, fungal and parasite species. References from [14].
| Gram Positive | MIC (µg/mL) | Gram Negative | MIC (µg/mL) |
| Bacillus subtilis ATCC 6633 | 0.6–2 | Escherichia coli IID-861 | 6–50 |
| B. subtilis 2116 | 7.8 | E. coli ATCC 25922 | 3.3–30 |
| B. subtilis IFO-3009 | 2 | E. coli O111 | 6–12 |
| B. cereus MMI-272 | 9 | E. coli O157:H7-A | 8 |
| B. cereus 6349 | 15.6 | E. coli O157:H7-B | 8 |
| B. circulans JCM-2504T | 0.6 | E. coli O157:H7-C | 10 |
| B. sphaericus 7585 | 1.9 | E. coli O157:H7-D | 8 |
| Staphylococcus aureus JCM-2151 | 6-25 | E. coli O157 wild type | 7.8 |
| S. aureus JCM-2179 | 6 | E. coli CL99 1–2 | 4 |
| S. aureus JCM-2413 | 18 | E. coli K12 UB1005 | 1.6 |
| S. aureus ATCC 25923 | 30 | E. coli K12 UB1005 DC-2 | 1.6 |
| S. aureus ATCC 29213 | 6.6 | E. coli 7275 | 7.8 |
| S. aureus 8530 | 15.6 | E. coli 10418 | 3.9 |
| S. aureus 8532 | 15.6 | E. coli wild type | 15.6–31.2 |
| S. aureus R1 (antib-res) | 12 | Pseudomonas aeruginosa MMI-603 | 12–24 |
| S. epidermidis JCM-2414T | 3–6 | P. aeruginosa IFO-3445 | 12–18 |
| S. epidermidis 4276 | 7.8 | P. aeruginosa IFO-3446 | 24 |
| S. haemolyticus JCM-2416T | 1 | P. aeruginosa IFO-3448 | 45 |
| S. hominus JCM-2419T | 3 | P. aeruginosa IFO-3452 | 30 |
| S. sp. wild type | 7.8–15.6 | P. aeruginosa ATCC-2783 | 3.3 |
| Listeria monocytogenes IDF-Ib | 0.6 | P. aeruginosa PAO | 3.3 |
| L. monocytogenes JCM-7673 | 1 | P. aeruginosa 10662 | 31.2 |
| L. monocytogenes JCM-7674 | 3 | P. aeruginosa wild type | 15.6–31.2 |
| L. monocytogenes EGD | 1.6 | P. putida wild type | 15.6 |
| L. monocytogenes 4b | 6.6 | P. cepacia wild type | 250–500 |
| L. monocytogenes 5105 | 1.9 | P. fluorescens wild type | 15.6 |
| Streptococcus bovis JCM-5672 | 3–6 | Pseudomonas fluorescens IFO-14160 | >60 |
| S. mutans JCM-5705T | 6 | Salmonella enteritidis IID-604 | 12–18 |
| S. mutans JCM-5175 | 6 | S. typhimurium SH7641 | 1.6 |
| S. mutans JCM-5176 | 3 | S. typhimurium SL696 | 5 |
| S. thermophilus ATCC-19258 | 3 | S. typhimurium 6749 | 1.6 |
| S. lactis ATCC-19435 | 3 | S.montevideo SL5222 | 3 |
| S. cremoris ATCC-9265 | 3 | S. newport 5751 | 7.8 |
| Lactobacillus. casei MMI-114 | 12 | S. typhi wild type | 7.8–15.6 |
| Corynebacterium ammoniagenes JCM-1306 | 0.3 | S. enteritidis wild type | 7.8 |
| C. renale JCM-1322 | 1 | Yersinia enterocolitica IID-981 | 6–24 |
| C. diphtheriae JCM-1310 | 18 | Y. enterocolitica wild type | 62.5 |
| Clostridium perfringens ATCC-6013 | 24 | Proteus vulgaris JCM 1668T | 12–45 |
| C. paraputrificum MMI-25 | 3 | P. vulgaris wild type | 500 |
| Micrococcus sp. wild type | 7.8–31.2 | P. vulgaris 4635 | 500 |
| Bifidobacterium adolescentis ATCC-15703 | no | P. mirabilis wild type | 250–500 |
| B. breve ATCC-15700 | no | P. mirabilis NCTC-60 | >200 |
| B. longum ATCG15707 | no | P. mirabilis ATCC 35659 | >1000 |
| B. infantis ATCC-15697 | no | P. rettgeri wild type | 250 |
| B. bifidum ATCC-15696 | >60 × 103 | P. sp. wild type | 500 |
| Klebsiella pneumoniae 418 | 500 | Klebsiella pneumoniae JCM 1662T | 9 |
| Enterococcus faecalis ATCCE 19433 | >60 | K. pneumoniae JCM-16623 | 12 |
| Enterococcus sp. wild type | 62.5–125 | K. pneumoniae 5055 | 15.6 |
| K. pneumoniae wild type | 15.6–62.5 | ||
| K. pneumoniae 418 | 500 | ||
| Shigella flexneri 5452 | 3.9 | ||
| Shigella flexneri wild type | 3.9 | ||
| Shigella sonnei wild type | 7.8 | ||
| Enterobacter intermedius wild type | 15.6 | ||
| Enterobacter aerogenes wild type | 125 | ||
| Enterobacter cloacae wild type | 70.3 | ||
| Enterobacter sp. wild type | 7.8 | ||
| Serratia sp. wild type | 500 | ||
| S. marcescens wild type | 500 | ||
| S. liquefaciens wild type | 500 | ||
| Citrobacter freundii wild type | 7.8–62.5 | ||
| C. diversus wild type | 62.5 | ||
| Bacteroides distasonis MMI-M602 | no | ||
| B. vulgatus MMI-S601 | no | ||
| Yeast | MIC (µg/mL) | Filamentous Fungi | MIC (µg/mL) |
| Candida. albicans TIMM 0154 | 25 | Trichophyton mentagrophytes TIMM 1189 | 12 |
| Candida albicans TIMM 1768 | 12.5–400 | T. mentagrophytes TIMM 2789 | 6.3 |
| C. albicans TIMM 3164 | 400 | T. mentagrophytes IFO 5466 | 30 |
| C. albicans TIMM 3315 | 50 | T. mentagrophytes IFO 5812 | 30 |
| C. albicans TIMM 3317 | 200 | T. mentagrophytes IFO 5974 | 45 |
| C. albicans JCM1542T | 24 | T. mentagrophytes | 5 |
| C. albicans JCM2072 | 30 | T. rubrum IFO 6203 | 24 |
| C. albicans JCM2075 | 45 | T. rubrum IFO 32409 | 13 |
| C. albicans JCM2076 | 24 | T. tonsurans | 5–40 |
| C. albicans JCM2374 | 24 | T. violaceum | 40 |
| C. albicans JCM2900 | 24 | T. rubrum | >80 |
| C. albicans JCM2901 | 45 | T. shoenleinii | >80 |
| C. albicans JCM2902 | 60 | Nannizzia incurvata JCM 1906 | 18 |
| C. albicans JCM2904 | 45 | N. otae JCM 1909 | 60 |
| C. albicans 6372 | 0.8 | N. gypsea JCM 1903 | >60 |
| C. albicans 6434 | 0.8 | Penicillium pinophilum JCM 5593 | 45 |
| C. albicans ATCC 90028 | 400 | P. vermiculatum JCM 5595 | 45 |
| C. albicans wild type | 7.8–21.67 | P. notatum | >80 |
| C. parapsilosis wild type | 7.8–80 | P. expansum | >80 |
| C. tropicalis | 0.31–1.25 | Aspergillus versicolor | 10 |
| C. glabrata | 120 | A. fumigatus JCM 1917 | >60 |
| C. guilliermondii | A. niger JCM 5546 | >60 | |
| C. kefyr | 2.5–10 | A. fumigatus | >80 |
| C. krusei | 10–20 | A. niger | >80 |
| Cryptococcus uniguttulatus JCM 3685 | 6 | A. flavus | >80 |
| C. curvatus JCM 1532T | 9 | A. clavatus | >80 |
| C. albidus JCM 8252 | 24 | Fusarium moniliforme | 2.5–5 |
| C. neoformans | 0.63 | Absidia corymbifera | 40 |
| Trichosporon cutaneum JCM 2466 | 18 | Microsporum canis | 40 |
| T. cutaneum | 1.25–2.5 | M. gypseum | 20–40 |
| Saccharomyces cerevisiae | 0.63 | Epidermophyton floccosum | 0.31-2.5 |
| Fonsecaea pedrosoi | 5 | ||
| Exophiala dermatitidis | 2 | ||
| Phialophora verrucosa | 5–10 | ||
| Cladosporium trichoides | 0.63–1.2 | ||
| Paracoccidioides brasiliensis | 5 | ||
| Sporothrix schenckii | 2–10 | ||
| Rhizopus oryzae JCM 5557 | >60 | ||
| Mucor circinelloides | >80 | ||
| M. racemosus | >80 | ||
| Parasite | LD50 (µM) | ||
| Giardia lamblia | 2.8 | ||
| Entamoeba histolytica | 647 | ||
| Toxoplasma gondii | no |
Very recently, data have been reported on Lfcin and other Lf peptides derived from Lf active against Mycobacterium avium [68]. Both human and bovine Lfcins, as well as Lf(1–11), were active against M. avium strains of different virulence, the bovine peptide being more active than its human counterpart. However, some strains, such as Pseudomonas fluorescens, Enterococcus faecalis, and Bifidobacterium bifidum, were found to be resistant to Lfcin [69].
The antibacterial activity of Lfcin is thought to involve the disordering and alteration of the permeability of the bacterial membrane, resulting in inhibition of macromolecular biosynthesis and ultimately cell death [70]. In terms of the structure-activity relationship, the mechanism of action of Lfcin has been attributed to the 11-amino-acid amphipathic α-helical region [71], and the importance of the initial electrostatic interaction is highlighted by the high overall positive charge of these peptides; a net charge of at least +4 is necessary for optimal antibacterial activity [72]. This is confirmed by the fact that murine Lfcin (mLfcin), which contains two glutamine residues, lacks antibacterial activity (Table 2) [63] and also by the increased activity of C-terminally amidated undecapeptides derived from various Lfcins [72]. Since arginine can interact both electrostatically and through multiple hydrogen bonds with the negatively-charged surface of bacteria, it is thought that this amino acid is the most effective for targeting the peptide to the bacterial membrane. In addition, the guanidinium group adds bulk to the side chain, thereby potentially contributing to membrane disruption [71]. Once the positively charged residues bring Lfcin into contact with the bacterial cell, the hydrophobic residues (in particular tryptophan) interact with the lipophilic portion of the membrane, becoming embedded in its surface and destabilizing the packing of the phospholipids. At least two tryptophan residues (the best value being with three residues) are required to ensure a maximal thinning of the membrane in a certain radius around the peptide [73].
On Gram-negative bacteria, antimicrobial peptides act on lipopolysaccharides, whereas on Gram-positive bacteria Lfcin acts on lipoteichoic and teichoic acids. Studies have indicated that bLfcin leads to depolarization of the cell membrane without causing the lysis of the cells [70], that it exerts its bactericidal effect initially by acting on the bacterial cell surface, and subsequently on the cytoplasmic contents [74].
The role of disulfide is not yet fully understood, and Liu et al. [74] recently failed to find a significant difference in MICs between the disulfide-bridged peptide and its linear counterpart, while another study showed three-times-higher activity for the disulfide-bridged peptide [63]. Intracellular targets, such as phosphoenolpyruvate carboxylase, were detected using E. coli proteome chips, indicating that one of Lfcin’s mechanisms of action may be associated with pyruvate metabolism [75]. Furthermore, bLfcin inhibits phosphorylation of the response regulators and of the two-component system’s (TCSs) cognate sensor kinases. The role of the TCS is to protect the integrity of bacterial cell membranes against antimicrobial peptides [76]. However, the homologous examination of response regulators and sensor kinases in probiotics, including Bifidobacterium and Lactobacillus, showed that this motif was not present, suggesting that bLfcin may have only marginal effects on the microbioma probiotics in the intestine [76].
In addition to antimicrobial properties, Lfcin of human and bovine origin has also been found to be effective in inhibiting the classical complement pathway. Both Lf and Lfcin increase interleukin-8 release from polymorphonucleate leukocytes, suggesting their immunomodulatory function [77]. This implies a role of these peptides in suppressing the inflammatory effects caused by bacteria [78]. Lfcin was found to be produced in the human stomach, indicating that this peptide is generated in vivo for host defense [79].
In regard to its toxicity on eukaryotic cells, measured as haemolytic activity, bLfcin displayed slight hemolytic activity at a concentration of 64 µg/mL, exhibiting a marked antimicrobial activity against most of the test bacteria [74]. However, a study of the relationship between structure and activity found that the undecapeptide structure is essentially non-haemolytic, but that undecapeptides containing more than three tryptophan residues produced 50% haemolysis of human red blood cells at concentrations above 400 μg/mL (>230 μM) [72]. Toxicity seems also related to increased hydrophobicity. In a strategy to improve antimicrobial activity of Lfcin peptides were linked by N-acylation to hydrophobic chains. The derivatives resulted in higher antibacterial activity but also in higher toxicity towards eukaryotic cells [80,81].
Apart from having a broad antibacterial spectrum, bLfcin was also found to be efficacious against yeasts, such as Candida albicans, Cryptococcus uniguttulatus, C. curvatus, C. albidus and Trichosporon cutaneum [67,82] (see Table 3). The anti-fungal mechanism has not yet been fully clarified. bLfcin interacts with and disrupts the integrity of the Candida spp. membrane within the range 18 to 60 µg/mL (MIC). Cells of nine different strains exposed to bLfcin exhibited severe ultra-structural damage, reflecting the peptide’s induction of an autolytic response [83].
4.2. Synergy with Antibacterial and Anti-Fungal Drugs
Synergistic effects, deriving from the combination of active agents with different modes of action, provide an attractive therapeutic option (see Table 4). The loss of inner membrane integrity may promote the uptake of other agents, for example antibiotics or other antibacterial peptides, leading to synergy with conventional antibiotics. In this respect, Lfcins also appear to affect the cytoplasm physiology of fungal cells. In addition, some azole-resistant C. albicans strains are inhibited by fluconazole or itraconazole to a greater extent in the presence of relatively low concentrations of Lf or bLfcin. Conversely, no cooperative effect with non-azole types of antifungal agents, such as amphotericin B or 5-flucytosine, was observed. These findings indicate that LF or bLFcin may play a valuable role in inhibiting the mycelial form of azole-resistant C. albicans [52,84].
Table 4.
Synergistic effects of Lf AMPs [13].
| Lf Peptide | Drug | Bacterial, Fungal, Parasite Species | Refs |
|---|---|---|---|
| Lf(1–11) | Fluconazole | Candida sp. | [53] |
| bLfcin | Clotrimazole, ketoconazole, itraconazole, fluconazole | C. albicans | [52] |
| Cecropin A, aureomycin | E. coli | [74] | |
| Aureomycin | S. aureus | [74] | |
| Fluconazole, itraconazole | C. albicans | [84] | |
| Erythromycin | E. coli | [85] | |
| Ciprofloxacin, ceftazidime, gentamicin | S. aureus, E. coli | [86] | |
| Ciprofloxacin, norfloxacin | E. coli | [87] | |
| Minocycline, acid cholic, cysteine, various acylglycerols, β-cyclodextrin | S. aureus | [88] | |
| Metronidazole | Entamoeba histolytica | [89] | |
| Nisin, Lf | E. coli, S. epidermidis | [90] | |
| Lfampin | Ampicillin | S. aureus | [91] |
Based on Lfcin’s high antibacterial and antifungal activity, in order to identify possible synergic therapeutic strategies, Lf peptides have been tested in association with drugs. Following initial evaluations, Vorland et al. examined whether bLfcin interfered with the action of various antibiotics against E. coli and S. aureus [85]. For what regards E. coli, marked synergism was observed with erythromycin, partial synergism with penicillin G, vancomycin, and gentamicin, whereas there was no effect with cycloserine or colistin. As far as S. aureus is concerned, only penicillin G acted in partial synergism with bLfcin, whereas bLfcin antagonized vancomycin and gentamicin also at low concentrations. The difference in activity is related to the different mechanisms of action of antibiotics on both the membranes and the intracellular targets. In some cases, however, bLfcin may facilitate the uptake of antibiotics across the cell envelope.
Longhi et al. analysed the susceptibility to fluoroquinolones of uropathogenic E. coli strains, and the influence of bLfcin on the activity of norfloxacin and ciprofloxacin against these strains [87]. The research revealed synergistic, partial synergistic, or indifferent effects depending on the tested E. coli strains. Furthermore these results demonstrated that the association of fluoroquinolones with bLfcin could allow the use of these therapeutic agents at lower concentrations for a reasonable number of E. coli strains.
Liu et al. found a synergistic effect between bLfcin and aureomycin and cecropin A, on E. coli. No synergy, but rather independent action, was found in all bacteria tested when combining bLfcin and neomycin. No antagonism was observed between the antibacterial agents used and bLfcin against E. coli, S. aureus and P. aeruginosa. The MICs for the antibiotics markedly decreased when the peptide was added, as expected, indicating that bLfcin might act in combination with these antimicrobial agents against E. coli, S. aureus and P. aeruginosa [74].
Wakabayashi investigated the effects of bLfcin combined with antibiotics or various other compounds against S. aureus [88]. Among conventional antibiotics, minocycline increased the bactericidal activity of bLfcin against S. aureus, but methicillin, ceftizoxime, and sulfamethoxazole-trimethoprim did not have such an effect. The combination of minocycline and bLfcin had synergistic effects against three antibiotic-resistant strains of S. aureus. Further, 33 compounds were screened, including acids and salts, alcohols, sugars, lipids, amino acids, proteins and peptides, were tested in combination with bLfcin and among these medium-chain (8, 12, 14 carbons) monoacylglycerols increased the bactericidal activity of bLfcin against three S. aureus strains.
A cooperative action of milk proteins was investigated by Lopez-Esposito [90]. In this study Lf, bLfcin, and alphaS2-casein were assayed against E. coli, S. epidermidis, Listeria monocytogenes and Salmonella cholerae-suis, in combination with nisin. This bacteriocin produced by Lactocococcus lactis spp. lactis is primarily active against Gram-positive bacteria, and has found practical application as a food preservative. The synergy was observed for the combination Lf and bLfcin against E. coli and S. epidermidis. When bLfcin was combined with nisin, an antagonistic effect was found against E. coli, whereas the synergy index achieved against S. epidermidis revealed an additive interaction. Murata et al. [92] subsequently identified a milk RNAse protein (15 kDa) able to double or quadruple the antimicrobial activity of bovine Lf and LFcin against G-negative and positive bacteria.
4.3. Anti-Parasitic Activities of Lfcin
A synergistic effect against Entamoeba histolytica, was found by Leon-Sicairos et al. between metronidazole and Lf or bLfcin: in the presence of 31 µM of Lf or 323 µM of bLfcin, approximately one-third to one-fifth of the full metronidazole concentration effectively killed the majority of amoebas. This result opens promising perspectives for reducing the dose of antiamoebic drug in patients [89].
The ascertained giardicidal activity of bLf has the limit to be hampered by metal ions. It has been demonstrated that the most efficient activity against Giardia, is due to bLfcin. The latter also has the advantage, as compared to bLf to be insensitive to the inhibiting action of metals, except for Fe3+ [93]. The study authors opined that, as the intestinal lumen has little free iron, it is likely that Lf-derived peptides remain active within the human small intestine.
Treatment of Toxoplasma gondii with bLfcin leads to inactivation of the parasite’s ability to penetrate the host cell [94]. This protective effect in mice was confirmed by Isamida et al. [95] using oral or i.p. administration of the peptide (5 mg and 0.1 mg respectively). Subsequently, Omata et al. observed that sporozoites of T. gondii and Eimeria stiedai pre-incubated with Lfcin showed decreased penetration activity of mouse embryonal cells; mice inoculated with 105 sporozoites preincubated with Lfcin showed a higher survival rate than those inoculated with the same number of untreated sporozoites [18].
4.4. Preclinical and Clinical Trials of Lfcin
On the basis of in vitro data confirming bLfcin as the best performing peptide, with broad-range activities and low MIC values, several preclinical trials were run; the principal results are reported below, classified by disease treated or by administration route.
4.4.1. Ocular Infections
Recently, Oo et al. [86] reported that two antibiotics (ciprofloxacin and ceftazidime) act synergistically with bLfcin against various strains of P. aeruginosa isolated from corneal infections in vitro. In mouse corneas, the addition of bLfcin as adjuvant enhanced the activity of ciprofloxacin, with 1.5 log reduction of bacterial growth compared with negative control, although the peptide alone had no significant effect. Moreover, bLfcin with or without ciprofloxacin appeared able to reduce myeloperoxidase activity and consequently host inflammatory response in vivo.
More recently, an ex vivo study on the efficacy of the combination of bLfcin with other antifungal agents was performed. The aim was to assess the performance of such a combination in the inhibition of evaluated of biofilm formation of three fungal strains, Aspergillus fumigatus, Fusarium solani, and Candida albicans, isolated from patients with keratitis [96]. Biofilm eradication is now a major challenge, to overcome the incidence of drug resistance in keratitis treatment. This disease constitutes one of the most important causes of ocular morbidity and sight loss in developing nations, as well as in contact lens users, as reported in recent epidemics of Fusarium spp. keratitis. bLfcin, in combination with antifungal agents, increased the susceptibility to fluconazole, voriconazole, and amphotericin B and, when added to a lens-care solution, drastically reduced the mature biofilm on contact lenses. This finding suggested that bLfcin might be a promising candidate for clinical use in improving the susceptibility of biofilm to antifungals, and could also be used as an antibiofilm-antifungal additive in lens-care solutions.
4.4.2. Osteo-Articular Diseases
In addition to antibacterial and antifungal uses, bLfcin’s anti-catabolic and anti-inflammatory effects were recently assessed in ex vivo experiments on human articular cartilage and synovium [97]. bLfcin demonstrated chondroprotective properties, damping the inflammatory response in synovial fibroblasts, and may thus be seen as a promising molecule in the prevention and/or treatment of degenerative joint diseases. Additionally, considering its antimicrobial activities, bLfcin may also bring therapeutic benefits to septic arthritis.
4.4.3. Gastro-Intestinal Diseases
The first reports of the bacteriostatic effects of Lf and Lf pepsine-hydrolysate (LFH) after per os administration, concerned Enterobacteriaceae in the gut environment [98], The same author demonstrated the efficacy of bLf and LFH to control and limit the growth of different strains of Clostridium spp. in a mouse model [99].
4.4.4. Dermatological Diseases
Lf and bLfcin have been tested as possible therapeutic agents for the treatment of dermatophytosis, one of the most common infectious diseases of the stratum corneum. The in vitro MIC of Lf and Lfcin against Trichophyton spp. demonstrated a great variability, depending on the strain tested and medium used, in contrast of what observed with the antifungal drug griseofulvin which gave constant and reproducible results. The efficacy of oral Lf administration was assessed on guinea pigs experimentally infected with Tinea corporis and T. pedis. The study authors assumed that Lf did not have any direct antifungal activity, but that it enhanced the inflammatory response involving cell-mediated immunity required for the cure of dermatophytosis [100].
4.5. Veterinary Applications
Mastitis is one of the most significant and costly diseases in dairy cattle. It is an intramammary bacterial infections caused by several bacteria, with S. aureus, Streptococcus uberis, and Streptococcus dysgalactiae being the most frequently involved pathogens. Intramammary treatment with Lf was not satisfactory for overcoming beta-lactam resistant S. aureus infection. However, Lf co-administered with penicillin G increased the cure rate (from 12.5% to 33%), reducing beta-lactamase activity in resistant S. aureus strains [101]. The strong activity against mastitis pathogens of AMP and bLfcin, in particular, has spurred interest in their potential application to the control of udder infections. In an in vivo trial, Kawai et al. tested an infusion of LFH in cows affected by subclinical mastitis, caused by various bacteria, including E. coli and staphylococci [102]. The results showed a significant reduction of bacteria in the mammary tissue already on day one after infusion, and eradication of the disease after 14 days. Recently, bLfcin exhibited in vitro biocidal activity against both the algae Prototheca zopfii, the causative agent of protothecal mastitis, and several yeasts causing fungal mastitis isolated from clinical cases of bovine mastitis However these promising in vitro findings must be confirmed in vivo [103]. In a different approach, bLfcin was expressed in goat mammary gland, using a plasmid vector as preventive therapy [104]. The results confirmed the persistence of the peptide in goat’s milk for 6 days after injection of the recombinant plasmid, but with variations in the concentration after 3 days. Milk produced during this period was active in vitro against S. aureus ATCC 25923 and E. coli D12K31.
hLf and Lfcin have also been studied as components of dietary supplementation in livestock, and particularly in pigs. Tang et al. analysed the role of Lfcin to replace colistin sulphate in a study on piglets weaned at 21 days of age and challenged with enterotoxigenic E. coli, that examined gut microflora, circulating cytokines, and intestinal mucosal morphology [105]. When cipB-Lfcin, a fusion protein that releases Lfcin in the animal stomach, was given as a dietary supplement at the dose of 100 mg/kg, the development of villus-crypt architecture of the intestinal mucosa was observed. Because Lfcin can decrease the concentration of E. coli and keep the gut tissue healthy, pigs fed with cipB-Lfcin had lower serum levels of circulating cytokines than pigs fed a standard or control diet [95]. Further studies of the use of these peptides as an alternative to antimicrobial growth promoters in pig production comprised construct cipB-Lfcin-Lframpin [106]. Piglets fed with construct (100 mg/kg) or control diets were challenged with enterotoxigenic E. coli. The AMP diet enhanced growth performance to a similar extent as did colistin sulphate. Analogous results were obtained using fusion protein containing bLfcin or Lfampin produced by a recombinant of the yeast Pichia pastoris [107,108].
A further study investigated the effects of bLfcin on performance, faecal score, and dry matter of weaned piglets orally challenged with enterotoxigenic E. coli F4, confirming bLfcin’s activity combined with its absence of toxicity [109].
Recently, an in vivo study comprised treating dogs affected by external otitis with bacterial and yeast overgrowth, using an emulsion containing bLfcin, verbascoside and glycerophosphoinositol lysine [110]. After 7 days’ treatment, there was a significant decrease in microbial overgrowth, together with a clinical improvement in the otitis, suggesting the therapeutic combination possessed synergistic antibacterial and antifungal activity. An in vitro study in the present authors’ laboratory confirmed the anti-fungal activity of bLfcin against Malassezia spp. isolated from dogs or cats affected by fungal otitis (unpublished data).
4.6. Lfcin for Food Applications
On the basis of its wide spectrum of activity, bLfcin is also a promising candidate as preservative in various foods and beverages. The main applications of AMP are well summarized in a recent review [13]. Chiefly considering bLfcin and LFH, the principal studies examined were as follows:
4.6.1. Dairy products
Quintieri and co-workers [111] evaluated the effect of bLfcin to control spoilage by mesophilic bacteria in Mozzarella cheese and, more recently, that of LFH in controlling P. fluorescens, responsible for cheese pigmentation in high-moisture Mozzarella cheese. LFH (rich in bLfcin) efficiently counteracted the chromatic spoilage of cheese throughout the storage time [112]. The inclusion of LFH in the holding liquid did not significantly affect the viable cell counts of useful microorganisms; however, resistance of AMPs to proteases becomes a major concern, because they rapidly degrade in food matrices, especially those containing lactic acid bacteria whose proteolytic attitude is very strong. This aspect strongly limits their action time. For example, bLfcin was reported to have a short half-life in contact with food microbial communities [111,112]. For these reasons, bLfcin resistance to microbial proteolysis was also assessed using different strains of yogurt starter (Streptococcus thermophilus and Lactobacillus delbrueckii sp. bulgaricus strains). However in acidic conditions (pH 4.5) the bLfcin resistance to hydrolysis was confirmed [113].
4.6.2. Oenological applications
Recently, the use of both bLf and bLfcin was examined, in a study that assessed their control over food spoilage bacteria and yeasts. Enrique et al. [114] evaluated both in vitro, and subsequently in micro-vinification experiments, the antimicrobial activity exerted by LFH and one synthetic peptide derived from bLf (bLfcin17e31) against wine-related Gram-positive spoilage bacteria. The study authors demonstrated the additional killing effect of bLfcin17e31 on bacterial cells belonging to Pediococcus damnosus and Oenococcus oeni, already stressed by changes in chemical composition of the must caused by Saccharomyces cerevisiae fermentation.
4.6.3. Fruits and vegetables
Recently, both bLf and bLfcin were investigated for their potential in controlling spoilage pathogens in fruits and vegetables. Tests carried out on mandarins with bLfcin and Lf (17–31) showed a significant protection against Penicillium digitatum at a concentration close to the in vitro MIC value [115]. More recently Baruzzi et al. demonstrated the interesting activity of bLfcin in controlling microbial spoilage in ready-to-eat leafy vegetables during cold storage [116]. These studies introduce alternative approaches to fungicides in fruit and vegetable production.
4.6.4. Meat products
bLfcin is currently also being assessed to control food-borne pathogens in meat matrices. Initial studies on the addition of bLfcin to ground meat inoculated with enterohemorrhagic E. coli O157:H7 did not significantly reduce the bacterial population [117], although LFH exerted antimicrobial action on this strain in controlled experiments [118]. Similar results were obtained on beef plates and adipose tissue after treatment by immersion in a solution of 10 μg/mL of bLfcin [119]. The possibility of combining LfcinB and high pressure to reduce colonization of chicken fillets preinoculated with Pseudomonas fluorescens ATCC948, Listeria monocytogenes CECT5725 and E. coli O157:H7 strain CECT 4972 was evaluated, although if failed to produce a relevant reduction [120,121]. In another approach, LFH was applied to ground beef and meat fractions [122]. In conclusion of this chapter it is worth highlighting that the strategy of employing milk-derived AMPs to control spoilage bacteria and food-borne infections not only satisfies the need to reduce the use of antibiotics and food preservatives but also allows to reduce the use of salt and sugars with benefits for consumer’s health.
5. Lf Derived Peptides: Lactoferrampin
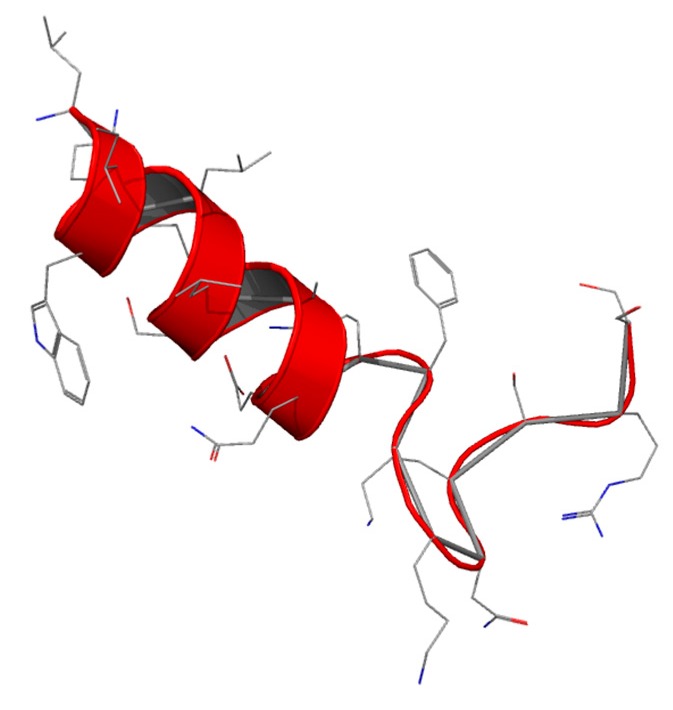
Lactoferrampin (Lfampin) has recently been identified as being of potential interest by sequence scanning, based on the common feature of antimicrobial peptides: a highly positive charge, a hydrophobic domain and hence an amphipathic character (Figure 4).
Figure 4.
Structure of Lfampin.
Lfampin comprises residues 268–284 in the N1 domain of Lf, and was found to be located in close proximity to Lfcin. However, the bactericidal activity of LFampin differs from that of bLfcin, although both peptides share amphipathic and cationic features; they have a markedly different amino acid composition and chain length, and therefore their structures differ greatly. The sequence comparison of Lfampin from six different species shows a uniform preponderance of cationic amino acid residues among hydrophobic residues (Table 5). As an antimicrobial peptide Lfampin plays a key role in membrane-mediated activities of Lf [123]. Following the initial discovery of LFampin 268–284 (produced by solid-phase chemistry), a slightly longer sequence (produced by action of a single endopeptidase on bLf) was published that included an additional N-terminal helix cap region, Asp-Leu-Ile. It was found that the longer peptide LFampin 265–284 had higher activity [123]. For both peptides, the helical conformation was found to be critical for effectiveness against Gram-positive bacteria [124].
Table 5.
Comparison of amino acid sequences of Lfampin from Lf from six species.
| Species | Sequence |
|---|---|
| Human | WLLAFP |
| Cow | WLLAF |
| Buffalo | WLLAF |
| Horse | WLLAF |
| Goat | WLLAF |
| Camel | WLLVAFP |
For the different amino acids, the colours indicate: blue = positively charged (R, K, H); black = negatively charged (D, E); red = hydrophobic (I, L, V, A, P, M F, Y, W); green = hydrophilic (S, T, E, Q, N, C and G).
The sequence comparison of Lfampin from six different species shows a uniform preponderance of positively-charged residues among the hydrophobic domain containing tryptophan, a residue that is involved in membrane insertion.
Lfampin exhibits broad antimicrobial action against several Gram-positive and Gram-negative bacteria, yeast and parasites: data taken from the MilkAMP database are reported in Table 6 [14,123].
Table 6.
Activities of Lfampin 265–284 and 268–285 [14].
| Gram-Positive | LC50 (µM) | Gram-Negative | LC50 (µM) | Yeast | LC50 (µM) |
|---|---|---|---|---|---|
| Streptococcus sanguis SK4 | 4.8 | E. coli K12 | 5.8 | Candida albicans 315 ATCC 10231 | 0.7–2.1 |
| Bacillus subtilis ATCC 9372 | 5.5–20 | E. coli O157:H7 | 25 | ||
| S. aureus MRSA | 20 | E. coli MREPEC | 20 | Parasite | |
| Streptococcus mutans | 5.5 | E. coli EPEC E2348/69 | 20 | Leishmania donovani | 25.3 |
| Actinomyces naeslundii ATCC 12104 | 4.3 | P. aeruginosa Pak | 7 | ||
| P. aeruginosa PAO | 5.8–15 |
Although no advanced preclinical trials or applications have yet been reported, the study by Flores et al. shows that the combination of ampicillin with LFcin 17–30 or LFampin 265–284 enhanced the inhibitory effect on growth of S. aureus (99.9%) for both peptides, suggesting that a synergistic effect occurs. These data strongly suggest that LFcin 17–30 and LFampin 265–284 act synergistically with antibiotics against multi drug resistant S. aureus strains in vitro [91].
6. Conclusions
With the steady increase in the number of multidrug-resistant pathogens, many patients are looking to alternative medicine instead of classical antibiotics and antimicrobial agents. It has thus become necessary to explore natural resources for new, alternative and/or complementary medicines. In this search for novel antimicrobial agents for the future, Lf, a multifunctional protein that participates in a range of important physiological processes, offers a new source with potential pharmaceutical applications. Moreover, Lf fragments or derivatives are still being explored, through both chemical synthesis and tryptic digestion, as has recently been described [125].
The best demonstrated effects of Lf and Lf AMP in the treatment of various infectious diseases caused by bacteria, fungi, and protozoa in humans and animals have been described; however, considerable research remains to be done to achieve a better understanding of Lf and Lf-derived AMPs activity. Actually, the functional peptides till now produced from Lf and studied, Lf(1–11), Lfcin and LFampin, are still underexplored but promising results begin to be obtained suggesting new interesting antimicrobial actions that can be exploited.
Lf(1–11) is active in vitro and in vivo against various bacteria and yeast although showing few synergistic effects with antibiotics. Lfcin displays a higher efficacy potent as an antibacterial, anti-fungal and antiparasitic agent than intact Lf, particularly in ocular infections, osteo-articular, gastrointestinal and dermatological diseases. bLfcin is the most potent Lfcin, and is also used in veterinary medicine. Bovine mastitis, intestinal infections in piglets, and canine dermatitis are the most important in vivo applications.
Both bLf and bLfcin have recently been tested for food applications due to their significant antibacterial and antifungal activities, combined with a wide safety profile. They find interesting applications in food preservation from both spoilage and pathogenic bacteria and fungi. This strategy may allow to reduce the use of chemical preservatives, reducing food loss due to spoilage, and lead to the development of “novel” food products (with lower salt content, less acidic, etc.) able to satisfy the demands of consumers and industry.
Further, the ascertained protective roles of bLfcin, which have been demonstrated against pathogens, and inflammation, but also cancer, make the molecule a centerpiece for the development and application of drug candidates and functional foods. Natural origin, safety, marked activity, and possible use on the industrial scale, are the main features that make Lfcin interesting and promising as a therapeutic agent.
LFampin exhibits broad antimicrobial action against several gram-positive and gram-negative bacteria, yeasts, and parasites, but no advanced preclinical trials or applications have been reported to date.
On the other hand, the overall research in this field reveals some bottlenecks such as the synergy between Lfcin-derived peptides and antibiotics, which is relevant, in vitro but still far from optimal for in vivo therapeutic use [126,127]. Combinations of milk AMPs with a greater number of antimicrobials have to be tested, as they provide new directions to control pathogens growth. Systematic evaluations on in vivo models, selecting different pathogens, pathologies and administration routes should be welcomed. Another important issue is the cell-penetrating potential of AMPs as vectors for intracellular targets [128].
Because Lfcin and Lfampin are spatially close in location, a generation of chimera peptides containing the same sequences has been produced. Recent studies have shown that chimera peptides exert stronger antimicrobial activity, as well as antiparasitic activity [91,129,130,131]. The construction of chimera peptides, based on the active regions of Lf, may be a promising approach possibly leading to interesting and improved activity. Nevertheless, some shortcoming of such chimera might stem from the difficulty of producing bulk quantities, not fully disclosed toxicity, and stability issues.
Furthermore, besides the molecules object of the present review report, research on different proteolytic fractions of whey proteins (from different mammalian species) lead to continuous discovery of new peptides possessing antimicrobial activities. Hence, the research on such molecules, the evaluation of their spectrum of activities and their in vitro and in vivo synergy with conventional therapeutics, represent the main goals for the future production of novel peptides.
In conclusion, in recent years, peptides have attracted increasing interest as a new therapeutic approach. They play a key-role due to their wide availability and the broad spectrum of activities, which control many biochemical processes. More than sixty peptide drugs have reached the market, and several hundred new therapeutic peptides are now in preclinical and clinical development. However, the possible ready enzymatic degradation and the low cellular uptake greatly restrict the use of these compounds as pharmaceutical agents.
A key-factor for enhancing stability and cellular permeability is the application of rational design to partially modify chemical and physical properties of peptides. The challenge is therefore to improve biological and pharmacological activity by making appropriate structural modifications on functional groups present in the amino acids of natural peptides. We believe that in the future, once these transformations will be achieved, the new molecules would benefit of significant stability and cell permeability, which combined with the high efficiency, specificity and security of the natural molecules will ensure success of peptide drugs.
Acknowledgments
The work was partially funded by MIUR-University of Torino “Fondi Ricerca Locale (ex-60%)”. We express gratitude to Frances Cooper for revising the manuscript and to Sonia Zorniotti for Pymol structural representation of Lf and derivatives.
Abbreviations
The following abbreviations are used in this manuscript:
| AMPs | antimicrobial peptides |
| Lf | lactoferrin |
| Lfcin | lactoferricin |
| Lfampin | lactoferrampin |
| MIC | minimal inhibition concentration |
| LFH | lactoferrin hydrolysate by pepsin |
Conflicts of Interest
Candioli Farmaceutici is the funding sponsor in writing the manuscript.
References
- 1.Reddy K.V.R., Yedery R.D., Aranha C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Tam J.P., Wang S., Wong K.H., Tan W.L. Antimicrobial peptides from plants. Pharmaceuticals. 2015;8:711–757. doi: 10.3390/ph8040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock R.E.W., Sahl H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 4.Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G., Li X., Wang Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37(Suppl. 1):D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., Vlieghe E., Hara G.L., Gould I.M., Goossens H., et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 7.Gill E.E., Franco O.L., Hancock R.E.W. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 2015;85:56–78. doi: 10.1111/cbdd.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anuforom O., Wallace G.R., Piddock L.V. The immune response and antibacterial therapy. Med. Microbiol. Immunol. 2015;204:151–159. doi: 10.1007/s00430-014-0355-0. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen H., Pihlanto A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003;9:1297–1308. doi: 10.2174/1381612033454892. [DOI] [PubMed] [Google Scholar]
- 10.Atanasova J., Moncheva P., Ivanova I. Proteolytic and antimicrobial activity of lactic acid bacteria grown in goat milk. Biotechnol. Biotechnol. Equip. 2014;28:1073–1078. doi: 10.1080/13102818.2014.971487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nongonierma A.B., FitzGerald R.J. Bioactive properties of milk proteins in humans: A review. Peptides. 2015;73:20–34. doi: 10.1016/j.peptides.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Ng T.B., Cheung R.C.F., Wong J.H., Wang Y., Ip D.T.M., Wan D.C.C., Xia J. Antiviral activities of whey proteins. Appl. Microbiol. Biotechnol. 2015;99:6997–7008. doi: 10.1007/s00253-015-6818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Théolier J., Fliss I., Jean J., Hammami R. Antimicrobial peptides of dairy proteins: From fundamental to applications. Food Rev. Int. 2014;30:134–154. doi: 10.1080/87559129.2014.896017. [DOI] [Google Scholar]
- 14.Théolier J., Fliss I., Jean J., Hammami R. MilkAMP: A comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. Technol. 2014;94:181–193. doi: 10.1007/s13594-013-0153-2. [DOI] [Google Scholar]
- 15.Andersen J.H., Jenssen H., Gutteberg T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antivir. Res. 2003;58:209–215. doi: 10.1016/S0166-3542(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 16.Shestakov A., Jenssen H., Nordström I., Eriksson K. Lactoferricin but not lactoferrin inhibit herpes simplex virus type 2 infection in mice. Antivir. Res. 2012;93:340–345. doi: 10.1016/j.antiviral.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Mistry N., Drobni P., Näslund J., Sunkari V.G., Jenssen H., Evander M. The anti-papillomavirus activity of human and bovine lactoferricin. Antivir. Res. 2007;75:258–265. doi: 10.1016/j.antiviral.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Omata Y., Satake M., Maeda R., Saito A., Shimazaki K., Yamauchi K., Uzuka Y., Tanabe S., Sarashina T., Mikami T. Reduction of the Infectivity of Toxoplasma gondii and Eimeria stiedai Sporozoites by Treatment with Bovine Lactoferricin. J. Vet. Med. Sci. 2001;63:187–190. doi: 10.1292/jvms.63.187. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Lima C.F., Rodrigues L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014;72:763–773. doi: 10.1111/nure.12155. [DOI] [PubMed] [Google Scholar]
- 20.Riedl S., Rinner B., Schaider H., Lohner K., Zweytick D. Killing of melanoma cells and their metastases by human lactoferricin derivatives requires interaction with the cancer marker phosphatidylserine. BioMetals. 2014;27:981–997. doi: 10.1007/s10534-014-9749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.-S., Ellman M.B., Yan D., An H.S., Kc R., Li X., Chen D., Xiao G., Cs-Szabo G., Hoskin D.W., et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J. Cell. Physiol. 2013;228:1884–1896. doi: 10.1002/jcp.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlutti F., Pantanella F., Natalizi T., Frioni A., Paesano R., Polimeni A., Valenti P. Antiviral properties of lactoferrin—A natural immunity molecule. Molecules. 2011;16:6992–7012. doi: 10.3390/molecules16086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Embleton N.D., Berrington J.E., McGuire W., Stewart C.J., Cummings S.P. Lactoferrin: Antimicrobial activity and therapeutic potential. Semin. Fetal Neonat. Med. 2013;18:143–149. doi: 10.1016/j.siny.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Leboffe L., Giansanti F., Antonini G. Antifungal and antiparasitic activities of lactoferrin. Anti-Infect. Agents Med. Chem. 2009;8:114–127. doi: 10.2174/187152109787846105. [DOI] [Google Scholar]
- 25.Siqueiros-Cendón T., Arévalo-Gallegos S., Iglesias-Figueroa B.F., García-Montoya I.A., Salazar-Martínez J., Rascón-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014;35:557–566. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanwar J.R., Roy K., Patel Y., Zhou S.F., Singh M.R., Singh D., Nasir M., Sehgal R., Sehgal A., Singh R.S., et al. Multifunctional iron bound lactoferrin and nanomedicinal approaches to enhance its bioactive functions. Molecules. 2015;20:9703–9731. doi: 10.3390/molecules20069703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brock J.H. Lactoferrin-50 years on. Biochem. Cell Biol. 2012;90:245–251. doi: 10.1139/o2012-018. [DOI] [PubMed] [Google Scholar]
- 28.Arnold R.R., Brewer M., Gauthier J.J. Bactericidal activity of human lactoferrin: Sensitivity of a variety of microorganisms. Infect. Immun. 1980;28:893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochoa T.J., Pezo A., Cruz K., Chea-Woo E., Cleary T.G. Clinical studies of lactoferrin in children. Biochem. Cell Biol. 2012;90:457–467. doi: 10.1139/o11-087. [DOI] [PubMed] [Google Scholar]
- 30.Sharma D., Shastri S. Lactoferrin and neonatology-role in neonatal sepsis and necrotizing enterocolitis: Present, past and future. J. Mater. Fetal Neonat. Med. 2016;29:763–770. doi: 10.3109/14767058.2015.1017463. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa T.J., Chea-Woo E., Campos M., Pecho I., Prada A., McMahon R.J., Cleary T.G. Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children. Clin. Infect. Dis. 2008;46:1881–1883. doi: 10.1086/588476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masci J.R. Complete response of severe, refractory oral candidiasis to mouthwash containing lactoferrin and lysozyme. AIDS. 2000;14:2403–2404. doi: 10.1097/00002030-200010200-00023. [DOI] [PubMed] [Google Scholar]
- 33.Gil-Montoya J.A., Guardia-López I., González-Moles M.A. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth—A pilot study. Gerodontology. 2008;25:3–9. doi: 10.1111/j.1741-2358.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 34.Kuipers M.E., Heegsma J., Bakker H.I., Meijer D.K.F., Swart P.J., Frijlink E.W., Eissens A.C., de Vries-Hospers H.G., van Den Berg J.J.M. Design and fungicidal activity of mucoadhesive lactoferrin tablets for the treatment of oropharyngeal candidosis. Drug Deliv. 2002;9:31–38. doi: 10.1080/107175402753413154. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi H., Yamauchi K., Kobayashi T., Yaeshima T., Iwatsuki K., Yoshie H. Inhibitory effects of lactoferrin on growth and biofilm formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob. Agents Chemother. 2009;53:3308–3316. doi: 10.1128/AAC.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez-Gomis J., Fernández-Solanas A., Viñas M., González P., Planas M.E., Sánchez S. Effects of topical application of free and liposome-encapsulated lactoferrin and lactoperoxidase on oral microbiota and dental caries in rats. Arch. Oral Biol. 1999;44:901–906. doi: 10.1016/S0003-9969(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 37.Yadav N., Lamba A.K., Thakur A., Faraz F., Tandon S., Pahwa P. Effect of periodontal therapy on lactoferrin levels in gingival crevicular fluid. Aust. Dent. J. 2014;59:314–320. doi: 10.1111/adj.12203. [DOI] [PubMed] [Google Scholar]
- 38.Sachdeva A., Rawat S., Nagpal J. Efficacy of fermented milk and whey proteins in helicobacter pylori eradication: A review. World J. Gastroenterol. 2014;20:724–737. doi: 10.3748/wjg.v20.i3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dial E.J., Hall L.R., Serna H., Romero J.J., Fox J.G., Lichtenberger L.M. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. Dig. Dis. Sci. 1998;43:2750–2756. doi: 10.1023/A:1026675916421. [DOI] [PubMed] [Google Scholar]
- 40.Alaxia, Sas. [(accessed on 7 June 2016)]. Available online: http://www.alaxia-pharma.eu/alx-009/
- 41.Vitetta L., Coulson S., Beck S.L., Gramotnev H., Du S., Lewis S. The clinical efficacy of a bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: A double blind randomized study. Complement. Ther. Med. 2013;21:164–171. doi: 10.1016/j.ctim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 42.De Bortoli N., Leonardi G., Ciancia E., Merlo A., Bellini M., Costa F., Mumolo M.G., Ricchiuti A., Cristiani F., Santi S., et al. Helicobacter pylori eradication: A randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am. J. Gastroenterol. 2007;102:951–956. doi: 10.1111/j.1572-0241.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 43.Nappi C., Tommaselli G.A., Morra I., Massaro M., Formisano C., di Carlo C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: A prospective controlled randomized study. Acta Obstet. Gynecol. Scand. 2009;88:1031–1035. doi: 10.1080/00016340903117994. [DOI] [PubMed] [Google Scholar]
- 44.Manzoni P., Rinaldi M., Cattani S., Pugni L., Romeo M.G., Messner H., Stolfi I., Decembrino L., Laforgia N., Vagnarelli F., et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA J. Am. Med. Assoc. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 45.Sinha M., Kaushik S., Kaur P., Sharma S., Singh T.P. Antimicrobial Lactoferrin Peptides: The Hidden Players in the Protective Function of a Multifunctional Protein. Int. J. Pept. 2013;2013 doi: 10.1155/2013/390230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohner K., Blondelle S.E. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 2005;8:241–256. doi: 10.2174/1386207053764576. [DOI] [PubMed] [Google Scholar]
- 47.Ulvatne H., Samuelsen Ø., Haukland H.H., Krämer M., Vorland L.H. Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS Microbiol. Lett. 2004;237:377–384. doi: 10.1111/j.1574-6968.2004.tb09720.x. [DOI] [PubMed] [Google Scholar]
- 48.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 49.Brouwer C.P.J.M., Rahman M., Welling M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides. 2011;32:1953–1963. doi: 10.1016/j.peptides.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Lupetti A., Paulusma-Annema A., Welling M.M., Senesi S., van Dissel J.T., Nibbering P.H. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob. Agents Chemother. 2000;44:3257–3263. doi: 10.1128/AAC.44.12.3257-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lupetti A., Paulusma-Annema A., Senesi S., Campa M., van Dissel J.T., Nibbering P.H. Internal thiols and reactive oxygen species in candidacidal activity exerted by an N-terminal peptide of human lactoferrin. Antimicrob. Agents Chemother. 2002;46:1634–1639. doi: 10.1128/AAC.46.6.1634-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wakabayashi H., Abe S., Okutomi T., Tansho S., Kawase K., Yamaguchi H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996;40:821–825. doi: 10.1111/j.1348-0421.1996.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 53.Lupetti A., Paulusma-Annema A., Welling M.M., Dogterom-Ballering H., Brouwer C.P.J.M., Senesi S., van Dissel J.T., Nibbering P.H. Synergistic activity of the N-terminal peptide of human lactoferrin and fluconazole against Candida species. Antimicrob. Agents Chemother. 2003;47:262–267. doi: 10.1128/AAC.47.1.262-267.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nibbering P.H., Ravensbergen E., Welling M.M., van Berkel L.A., van Berkel P.H.C., Pauwels E.K.J., Nuijens J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001;69:1469–1476. doi: 10.1128/IAI.69.3.1469-1476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stallmann H.P., Faber C., Slotema E.T., Lyaruu D.M., Bronckers A.L.J.J., Nieuw Amerongen A.V., Wuisman P.I.J.M. Continuous-release or burst-release of the antimicrobial peptide human lactoferrin 1–11 (hLF1–11) from calcium phosphate bone substitutes. J. Antimicrob. Chemother. 2003;52:853–855. doi: 10.1093/jac/dkg443. [DOI] [PubMed] [Google Scholar]
- 56.Stallmann H.P., Faber C., Bronckers A.L.J.J., Nieuw Amerongen A.V., Wuismann P.I.J.M. Osteomyelitis prevention in rabbits using antimicrobial peptide hLF1–11-or gentamicin-containing calcium phosphate cement. J. Antimicrob. Chemother. 2004;54:472–476. doi: 10.1093/jac/dkh346. [DOI] [PubMed] [Google Scholar]
- 57.Faber C., Stallmann H.P., Lyaruu D.M., Joosten U., von Eiff C., van Nieuw Amerongen A., Wuisman P.I.J.M. Comparable efficacies of the antimicrobial peptide human lactoferrin 1–11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob. Agents Chemother. 2005;49:2438–2444. doi: 10.1128/AAC.49.6.2438-2444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nibbering P.H., Welling M.M., Paulusma-Annema A., Brouwer C.P.J.M., Lupetti A., Pauwels E.K.J. 99mTc-labeled UBI 29–41 peptide for monitoring the efficacy of antibacterial agents in mice infected with Staphylococcus aureus. J. Nucl. Med. 2004;45:321–326. [PubMed] [Google Scholar]
- 59.Dijkshoorn L., Brouwer C.P.J.M., Bogaards S.J.P., Nemec A., van den Broek P.J., Nibbering P.H. The synthetic N-terminal peptide of human lactoferrin, hLF(1–11), is highly effective against experimental infection caused by multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2004;48:4919–4921. doi: 10.1128/AAC.48.12.4919-4921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brouwer C.P.J.M., Welling M.M. Various routes of administration of 99mTc-labeled synthetic lactoferrin antimicrobial peptide hLF 1–11 enables monitoring and effective killing of multidrug-resistant Staphylococcus aureus infections in mice. Peptides. 2008;29:1109–1117. doi: 10.1016/j.peptides.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Van der Velden W.J., van Iersel T.M., Blijlevens N.M., Donnelly J.P. Safety and tolerability of the antimicrobial peptide human lactoferrin 1–11 (hLF1–11) BMC Med. 2009;7 doi: 10.1186/1741-7015-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- 63.Vorland L.H., Ulvatne H., Andersen J., Haukland H.H., Rekdal Ø., Svendsen J.S., Gutteberg T.J. Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand. J. Infect. Dis. 1998;30:513–517. doi: 10.1080/00365549850161557. [DOI] [PubMed] [Google Scholar]
- 64.Hunter H.N., Ross Demcoe A., Jenssen H., Gutteberg T.J., Vogel H.J. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrob. Agents Chemother. 2005;49:3387–3395. doi: 10.1128/AAC.49.8.3387-3395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Epand R.M., Vogel H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta Biomembr. 1999;1462:11–28. doi: 10.1016/S0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 66.Yamauchi K., Tomita M., Giehl T.J., Ellison Iii R.T. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 1993;61:719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gifford J.L., Hunter H.N., Vogel H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva T., Magalhaes B., Maia S., Gomes P., Nazmi K., Bolscher J.G.M., Rodrigues P.N., Bastos M., Gomes M.S. Killing of Mycobacterium avium by Lactoferricin peptides: Improved activity of arginine- and d-amino-acid-containing molecules. Antimicrob. Agents Chemother. 2014;58:3461–3467. doi: 10.1128/AAC.02728-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellamy W., Takase M., Wakabayashi H., Kawase K., Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992;73:472–479. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- 70.Ulvatne H., Haukland H.H., Olsvik O., Vorland L.H. Lactoferricin B causes depolarization of the cytoplasmic membrane of Escherichia coli ATCC 25922 and fusion of negatively charged liposomes. FEBS Lett. 2001;492:62–65. doi: 10.1016/S0014-5793(01)02233-5. [DOI] [PubMed] [Google Scholar]
- 71.Kang J.H., Lee M.K., Kim K.L., Hahm K.S. Structure-biological activity relationships of 11-residue highly basic peptide segment of bovine lactoferrin. Int. J. Pept. Protein Res. 1996;48:357–363. doi: 10.1111/j.1399-3011.1996.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 72.Strøm M.B., Rekdal Ø., Svendsen J.S. The effects of charge and lipophilicity on the antibacterial activity of undecapeptides derived from bovine lactoferricin. J. Pept. Sci. 2002;8:36–43. doi: 10.1002/psc.365. [DOI] [PubMed] [Google Scholar]
- 73.Strøm M.B., Erik Haug B., Rekdal Ø., Skar M.L., Stensen W., Svendsen J.S. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell Biol. 2002;80:65–74. doi: 10.1139/o01-236. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Han F., Xie Y., Wang Y. Comparative antimicrobial activity and mechanism of action of bovine lactoferricin-derived synthetic peptides. BioMetals. 2011;24:1069–1078. doi: 10.1007/s10534-011-9465-y. [DOI] [PubMed] [Google Scholar]
- 75.Tu Y.H., Ho Y.H., Chuang Y.C., Chen P.C., Chen C.S. Identification of lactoferricin B intracellular targets using an Escherichia coli proteome chip. PLoS ONE. 2011;6:e28197. doi: 10.1371/journal.pone.0028197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho Y.H., Sung T.C., Chen C.S. Lactoferricin B inhibits the phosphorylation of the two-component system response regulators BasR and CreB. Mol. Cell. Proteom. 2012;11 doi: 10.1074/mcp.M111.014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinoda I., Takase M., Fukuwatari Y., Shimamura S., Köller M., König W. Effects of Lactoferrin and Lactoferricin® on the Release of Interleukin 8 from Human Polymorphonuclear Leukocytes. Biosci. Biotechnol. Biochem. 1996;60:521–523. doi: 10.1271/bbb.60.521. [DOI] [PubMed] [Google Scholar]
- 78.Samuelsen Ø., Haukland H.H., Ulvatne H., Vorland L.H. Anti-complement effects of lactoferrin-derived peptides. FEMS Immunol. Med. Microbiol. 2004;41:141–148. doi: 10.1016/j.femsim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Kuwata H., Yip T.T., Tomita M., Hutchens T.W. Direct evidence of the generation in human stomach of an antimicrobial peptide domain (lactoferricin) from ingested lactoferrin. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998;1429:129–141. doi: 10.1016/S0167-4838(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 80.Wakabayashi H., Matsumoto H., Hashimoto K., Teraguchi S., Takase M., Hayasawa H. N-acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob. Agents Chemother. 1999;43:1267–1269. doi: 10.1128/aac.43.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zweytick D., Pabst G., Abuja P.M., Jilek A., Blondelle S.E., Andrä J., Jerala R., Monreal D., Martinez de Tejada G., Lohner K. Influence of N-acylation of a peptide derived from human lactoferricin on membrane selectivity. Biochim. Biophys. Acta (BBA) Biomembr. 2006;1758:1426–1435. doi: 10.1016/j.bbamem.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Bellamy W., Wakabayashi H., Takase M., Kawase K., Shimamura S., Tomita M. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 1993;182:97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- 83.van der Kraan M.I.A., van Marle J., Nazmi K., Groenink J., van’t Hof W., Veerman E.C.I., Bolscher J.G. M., Amerongen A.V.N. Ultrastructural effects of antimicrobial peptides from bovine lactoferrin on the membranes of Candida albicans and Escherichia coli. Peptides. 2005;26:1537–1542. doi: 10.1016/j.peptides.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 84.Wakabayashi H., Abe S., Teraguchi S., Hayasawa H., Yamaguchi H. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob. Agents Chemother. 1998;42:1587–1591. doi: 10.1128/aac.42.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vorland L.H., Osbakk S.A., Perstølen T., Ulvatne H., Rekdal Ø., Svendsen J.S., Gutteberg T.J. Interference of the antimicrobial peptide lactoferricin B with the action of various antibiotics against Escherichia coli and Staphylococcus aureus. Scand. J. Infect. Dis. 1999;31:173–177. doi: 10.1080/003655499750006236. [DOI] [PubMed] [Google Scholar]
- 86.Oo T.Z., Cole N., Garthwaite L., Willcox M.D.P., Zhu H. Evaluation of synergistic activity of bovine lactoferricin with antibiotics in corneal infection. J. Antimicrob. Chemother. 2010;65:1243–1251. doi: 10.1093/jac/dkq106. [DOI] [PubMed] [Google Scholar]
- 87.Longhi C., Marazzato M., Conte M.P., Iebba V., Schippa S., Seganti L., Comanducci A. Effect of lactoferricin on fluoroquinolone susceptibility of uropathogenic Escherichia coli. J. Antibiot. 2009;62:109–111. doi: 10.1038/ja.2008.22. [DOI] [PubMed] [Google Scholar]
- 88.Wakabayashi H., Teraguchi S., Tamura Y. Increased Staphylococcus-killing activity of an antimicrobial peptide, lactoferricin B, with minocycline and monoacylglycerol. Biosci. Biotechnol. Biochem. 2002;66:2161–2167. doi: 10.1271/bbb.66.2161. [DOI] [PubMed] [Google Scholar]
- 89.León-Sicairos N., Reyes-López M., Ordaz-Pichardo C., de la Garza M. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem. Cell Biol. 2006;84:327–336. doi: 10.1139/o06-060. [DOI] [PubMed] [Google Scholar]
- 90.López-Expósito I., Pellegrini A., Amigo L., Recio I. Synergistic effect between different milk-derived peptides and proteins. J. Dairy Sci. 2008;91:2184–2189. doi: 10.3168/jds.2007-0037. [DOI] [PubMed] [Google Scholar]
- 91.Flores-Villasenor H., Canizalez-Roman A., Reyes-Lopez M., Nazmi K., de la Garza M., Zazueta-Beltran J., Leon-Sicairos N., Bolscher J.G.M. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals. 2010;23:569–578. doi: 10.1007/s10534-010-9306-4. [DOI] [PubMed] [Google Scholar]
- 92.Murata M., Wakabayashi H., Yamauchi K., Abe F. Identification of milk proteins enhancing the antimicrobial activity of lactoferrin and lactoferricin. J. Dairy Sci. 2013;96:4891–4898. doi: 10.3168/jds.2013-6612. [DOI] [PubMed] [Google Scholar]
- 93.Turchany J.M., Aley S.B., Gillin F.D. Giardicidal activity of lactoferrin and N-terminal peptides. Infect. Immun. 1995;63:4550–4552. doi: 10.1128/iai.63.11.4550-4552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanaka T., Omata Y., Saito A., Shimazaki K., Yamauchi K., Takase M., Kawase K., Igarashi I., Suzuki N. Toxoplasma gondii: Parasiticidal effects of bovine lactoferricin against parasites. Exp. Parasitol. 1995;81:614–617. doi: 10.1006/expr.1995.1157. [DOI] [PubMed] [Google Scholar]
- 95.Isamida T., Tanaka T., Omata Y., Yamauchi K., Shimazaki K.I., Saito A. Protective effect of lactoferricin against Toxoplasma gondii infection in mice. J. Vet. Med. Sci. 1998;60:241–244. doi: 10.1292/jvms.60.241. [DOI] [PubMed] [Google Scholar]
- 96.Sengupta J., Saha S., Khetan A., Sarkar S.K., Mandal S.M. Effects of lactoferricin B against keratitis-associated fungal biofilms. J. Infect. Chemother. 2012;18:698–703. doi: 10.1007/s10156-012-0398-3. [DOI] [PubMed] [Google Scholar]
- 97.Yan D., Chen D., Shen J., Xiao G., van Wijnen A.J., Im H.J. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. J. Cell. Physiol. 2013;228:447–456. doi: 10.1002/jcp.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teraguchi S., Ozawa K., Yasuda S., Shin K., Fukuwatari Y., Shimamura S. The bacteriostatic effects of orally administered bovine lactoferrin on intestinal Enterobacteriaceae of SPF mice fed bovine milk. Biosci. Biotechnol. Biochem. 1994;58:482–487. doi: 10.1271/bbb.58.482. [DOI] [PubMed] [Google Scholar]
- 99.Teraguchi S., Shin K., Ozawa K., Nakamura S., Fukuwatari Y., Tsuyuki S., Namihira H., Shimamura S. Bacteriostatic effect of orally administered bovine lactoferrin on proliferation of Clostridium species in the gut of mice fed bovine milk. Appl. Environ. Microbiol. 1995;61:501–506. doi: 10.1128/aem.61.2.501-506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wakabayashi H., Uchida K., Yamauchi K., Teraguchi S., Hayasawa H., Yamaguchi H. Lactoferrin given in food facilitates dermatophytosis cure in guinea pig models. J. Antimicrob. Chemother. 2000;46:595–601. doi: 10.1093/jac/46.4.595. [DOI] [PubMed] [Google Scholar]
- 101.Lacasse P., Lauzon K., Diarra M.S., Petitclerc D. Utilization of lactoferrin to fight antibiotic-resistant mammary gland pathogens. J. Anim. Sci. 2008;86(Suppl. 13):66–71. doi: 10.2527/jas.2007-0216. [DOI] [PubMed] [Google Scholar]
- 102.Kawai K., Nagahata H., Lee N.Y., Anri A., Shimazaki K. Effect of infusing lactoferrin hydrolysate into bovine mammary glands with subclinical mastitis. Vet. Res. Commun. 2003;27:539–548. doi: 10.1023/A:1026039522286. [DOI] [PubMed] [Google Scholar]
- 103.Kawai K., Shimazaki K., Higuchi H., Nagahata H. Antibacterial activity of bovine lactoferrin hydrolysate against mastitis pathogens and its effect on superoxide production of bovine neutrophils. Zoonoses Public Health. 2007;54:160–164. doi: 10.1111/j.1863-2378.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J.X., Zhang S.F., Wang T.D., Guo X.J., Hu R.L. Mammary gland expression of antibacterial peptide genes to inhibit bacterial pathogens causing mastitis. J. Dairy Sci. 2007;90:5218–5225. doi: 10.3168/jds.2007-0301. [DOI] [PubMed] [Google Scholar]
- 105.Tang Z.R., Sun Z.H., Tang X.S., Feng Z.M., Zhou D., Xiao D.F., Zhang B., Li L.L. Dietary bovine lactoferricin improved gut microflora, benefited intestinal mucosal morphology and inhibited circular cytokines delivery in 21-day weaned piglets. J. Appl. Anim. Res. 2011;39:153–157. doi: 10.1080/09712119.2011.565570. [DOI] [Google Scholar]
- 106.Tang Z., Yin Y., Zhang Y., Huang R., Sun Z., Li T., Chu W., Kong X., Li L., Geng M., Tu Q. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009;101:998–1005. doi: 10.1017/S0007114508055633. [DOI] [PubMed] [Google Scholar]
- 107.Tang X.S., Shao H., Li T.J., Tang Z.R., Huang R.L., Wang S.P., Kong X.F., Wu X., Yin Y.L. Dietary supplementation with bovine lactoferrampin-lactoferricin produced by Pichia pastoris fed-batch fermentation affects intestinal microflora in weaned piglets. Appl. Biochem. Biotechnol. 2012;168:887–898. doi: 10.1007/s12010-012-9827-0. [DOI] [PubMed] [Google Scholar]
- 108.Tang X., Fatufe A.A., Yin Y., Tang Z., Wang S., Liu Z., Xinwu, Li T.J. Dietary supplementation with recombinant lactoferrampin-lactoferricin improves growth performance and affects serum parameters in piglets. J. Anim. Vet. Adv. 2012;11:2548–2555. [Google Scholar]
- 109.Hou Z.P., Wang W.J., Liu J.X., Liu G., Liu Z.Q., Souffrant W.B., Urubschurov V., Fan J. Effects of lactoferricin B and cecropin P1 on growth performance, faecal score and dry matter in weaned piglets orally challenged with enterotoxigenic Escherichia coli F4. J. Food Agric. Environ. 2011;9:275–280. [Google Scholar]
- 110.Vercelli A., Fanton N., Bruni N., Vergnano D., Bigliati M., Cornegliani L. Use of lactoferricin, verbascoside and glycerophosphoinositol emulsion in otitis externa treatment: A pilot study. Veterinaria. 2015;29:49–57. [Google Scholar]
- 111.Quintieri L., Caputo L., Monaci L., Deserio D., Morea M., Baruzzi F. Antimicrobial efficacy of pepsin-digested bovine lactoferrin on spoilage bacteria contaminating traditional Mozzarella cheese. Food Microbiol. 2012;31:64–71. doi: 10.1016/j.fm.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 112.Caputo L., Quintieri L., Bianchi D.M., Decastelli L., Monaci L., Visconti A., Baruzzi F. Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiol. 2015;46:15–24. doi: 10.1016/j.fm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 113.Paul M., Somkuti G.A. Hydrolytic breakdown of lactoferricin by lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2010;37:173–178. doi: 10.1007/s10295-009-0660-6. [DOI] [PubMed] [Google Scholar]
- 114.Enrique M., Manzanares P., Yuste M., Martínez M., Vallés S., Marcos J.F. Selectivity and antimicrobial action of bovine lactoferrin derived peptides against wine lactic acid bacteria. Food Microbiol. 2009;26:340–346. doi: 10.1016/j.fm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 115.Muñoz A., Marcos J.F. Activity and mode of action against fungal phytopathogens of bovine lactoferricin-derived peptides. J. Appl. Microbiol. 2006;101:1199–1207. doi: 10.1111/j.1365-2672.2006.03089.x. [DOI] [PubMed] [Google Scholar]
- 116.Baruzzi F., Pinto L., Quintieri L., Carito A., Calabrese N., Caputo L. Efficacy of lactoferricin B in controlling ready-to-eat vegetable spoilage caused by Pseudomonas spp. Int. J. Food Microbiol. 2015;215:179–186. doi: 10.1016/j.ijfoodmicro.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 117.Venkitanarayanan K.S., Zhao T., Doyle M.P. Antibacterial effect of lactoferricin B on Escherichia coli O157:H7 in ground beef. J. Food Prot. 1999;62:747–750. doi: 10.4315/0362-028x-62.7.747. [DOI] [PubMed] [Google Scholar]
- 118.Murdock C., Chikindas M.L., Matthews K.R. The pepsin hydrolysate of bovine lactoferrin causes a collapse of the membrane potential in Escherichia coli O157:H7. Probiot. Antimicrob. Proteins. 2010;2:112–119. doi: 10.1007/s12602-010-9039-2. [DOI] [PubMed] [Google Scholar]
- 119.Ransom J.R., Belk K.E., Sofos J.N., Stopforth J.D., Scanga J.A., Smith G.C. Comparison of intervention technologies for reducing Escherichia coli O157:H7 on beef cuts and trimmings. Food Prot. Trends. 2003;23:24–34. [Google Scholar]
- 120.Del Olmo A., Calzada J., Nuñez Manuel M. Effect of lactoferrin and its derivatives against gram-positive bacteria in vitro and, combined with high pressure, in chicken breast fillets. Meat Sci. 2012;90:71–76. doi: 10.1016/j.meatsci.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Del Olmo A., Calzada J., Nuñez M. Effect of lactoferrin and its derivatives, high hydrostatic pressure, and their combinations, on Escherichia coli O157:H7 and Pseudomonas fluorescens in chicken filets. Innov. Food Sci. Emerg. Technol. 2012;13:51–56. doi: 10.1016/j.ifset.2011.07.016. [DOI] [Google Scholar]
- 122.Del Olmo A., Morales P., Nuñez M. Bactericidal activity of lactoferrin and its amidated and pepsin-digested derivatives against Pseudomonas fluorescens in ground beef and meat fractions. J. Food Prot. 2009;72:760–765. doi: 10.4315/0362-028x-72.4.760. [DOI] [PubMed] [Google Scholar]
- 123.Van Der Kraan M.I.A., Groenink J., Nazmi K., Veerman E.C.I., Bolscher J.G.M., Nieuw Amerongen A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides. 2004;25:177–183. doi: 10.1016/j.peptides.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 124.Haney E.F., Lau F., Vogel H.J. Solution structures and model membrane interactions of lactoferrampin, an antimicrobial peptide derived from bovine lactoferrin. Biochim. Biophys. Acta Biomembr. 2007;1768:2355–2364. doi: 10.1016/j.bbamem.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 125.Rastogi N., Nagpal N., Alam H., Pandey S., Gautam L., Sinha M., Shin K., Manzoor N., Virdi J.S., Kaur P., et al. Preparation and antimicrobial action of three tryptic digested functional molecules of bovine lactoferrin. PLoS ONE. 2014;9:e90011. doi: 10.1371/journal.pone.0090011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Théolier J., Hammami R., Labelle P., Fliss I., Jean J. Isolation and identification of antimicrobial peptides derived by peptic cleavage of whey protein isolate. J. Funct. Foods. 2013;5:706–714. doi: 10.1016/j.jff.2013.01.014. [DOI] [Google Scholar]
- 127.Sanchez-Gomez S., Japelj B., Jerala R., Moriyon I., Fernandez Alonso M., Leiva J., Blondelle S.E., Andra J., Brandenburg K., Lohner K., et al. Structural features governing the activity of lactoferricin-derived peptides that act in synergy with antibiotics against Pseudomonas aeruginosa in vitro and in vivo. Antimicrob. Agents Chemother. 2010;55:218–228. doi: 10.1128/AAC.00904-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Durzyńska J., Przysiecka Ł., Nawrot R., Barylski J., Nowicki G., Warowicka A., Musidlak O., Goździcka-Józefiak A. Viral and other cell-penetrating peptides as vectors of therapeutic agents in medicine. J. Pharmacol. Exp. Ther. 2015;354:32–42. doi: 10.1124/jpet.115.223305. [DOI] [PubMed] [Google Scholar]
- 129.López-Soto F., León-Sicairos N., Nazmi K., Bolscher J.G., de la Garza M. Microbicidal effect of the lactoferrin peptides Lactoferricin17–30, Lactoferrampin265–284, and Lactoferrin chimera on the parasite Entamoeba histolytica. BioMetals. 2010;23:563–568. doi: 10.1007/s10534-010-9295-3. [DOI] [PubMed] [Google Scholar]
- 130.León-Sicairos N., Angulo-Zamudio U.A., Vidal J.E., López-Torres C.A., Bolscher J.G.M., Nazmi K., Reyes-Cortes R., Reyes-López M., de la Garza M., Canizalez-Román A. Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin. BioMetals. 2014;27:969–980. doi: 10.1007/s10534-014-9775-y. [DOI] [PubMed] [Google Scholar]
- 131.Bolscher J., Nazmi K., van Marle J., van’T Hof W., Veerman E. Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem. Cell Biol. 2012;90:378–388. doi: 10.1139/o11-085. [DOI] [PubMed] [Google Scholar]