Abstract
Ferulic acid in ester form has shown a stronger ability in ameliorating certain pathological conditions and inhibiting lipid oxidation. In present study, a solvent-free and reduced pressure evaporation system was developed for lipase-catalyzed synthesis of 2-ethylhexyl ferulate (2-EF) from ferulic acid and 2-ethylhexanol. A Box-Behnken design with response surface methodology (RSM) and artificial neural network (ANN) was selected to model and optimize the process. Based on the yields of 2-EF, reaction temperature was shown to be the most important process factor on the molar conversion among all variables. The residual values and the coefficient of determination (R2) calculated from the design data indicated that ANN was better than RSM in data fitting. Overall, the present lipase-catalyzed approach for 2-EF synthesis at low reaction temperature in a reduced pressure evaporation system shows high 2-EF production efficiency. Notably, this approach can reduce the enzyme denaturation and ferulic acid oxidation that usually occur during long-term biosynthetic operations at high temperature.
Keywords: 2-ethylhexyl ferulate, lipase, reduced pressure evaporation system, response surface methodology, artificial neural network
1. Introduction
Ferulic acid (FA) belongs to the family of phenolic acids and is very abundant in grains, fruits and vegetables. Studies have shown that FA displays excellent antioxidant [1], ultraviolet-absorbing activities [2,3] and potential health benefits against cardiovascular problems, inflammatory diseases and cancer [4]. However, the solubility of FA in hydrophobic solvents is low, thus limiting its utilization in the pharmaceutical, cosmetic and food industries. In order to increase utilization of FA, lipophilization is needed, where enzymatic esterification with alcohols has proved to be a promising approach [5,6,7,8]. In recent years, many studies have report that the enzymatic synthesis of hydrophobic derivatives of FA can increase its oil-solubility [9,10,11], and some of them have been applied for preparation of emulsion-based creams due to their anti-inflammatory activity [12]. FA esters, the hydrophobic derivatives of FA, have also demonstrated other bioactivities such as antioxidant [13], anticonvulsant [14], synergistic photobactericidal effects [15], and antithrombotic activities [16], as well as inhibition of the atherogenic effects of leptin [17]. Interestingly, FA esters possess better inhibitory effect on lipid oxidation than FA [18].
Enzymatic biosynthesis of these compounds has attracted much attention in recent years, and it may be a better approach in comparison to conventional chemical processes [19,20,21,22,23]. It has been reported that continuous synthesis of alkyl ferulate by immobilized lipase shows great conversion rates (approximately 90%) at 90 °C after two days [21]. It should be kept in mind that FA is susceptible to oxidation under high temperatures. Novozym®435 had been used in the lipase-catalyzed synthesis of ferulate esters, but the yield was only 17% after several days [22]. Generally, solvent-free reaction systems are applied in the lipase-catalyzed synthesis of ferulate esters to decrease the reaction time and increase the yield [20,23]. The solvent-free system is a simple mixture of substrates, offering advantages including maximization of substrate concentration, greater volumetric production, and cost savings in reactor design and product separation. In addition, the boiling point of the component liquids is lower in a reduced pressure evaporation system than that in the atmosphere at the same temperature, suggesting that the byproducts with relative low vapor pressure physical characteristics are more prone to be eliminated in the reduced pressure system. This may increase the efficiency of the lipase-catalyzed reaction. Therefore, a reduced pressure evaporation system combined with a solvent-free system may be an excellent strategy to overcome the problems of long reaction time and low yields of FA esters synthesis. So far, the literature regarding the lipase-catalyzed esterification of ferulic acid in a reduced pressure evaporation system is still limited. Notably, there have been no reports regarding lipase-catalyzed synthesis of 2-ethylhexylferulate (2-EF), a common FA ester with great inhibitory effect on lipid oxidation, which has been widely used as the antioxidant in the food and cosmetic industries.
Recently, response surface methodology (RSM) and an artificial neural network (ANN) approach have been applied for optimization and process modeling. The development of an optimum enzymatic synthesis procedure to improve the yield conversion to reduce production costs would be attractive for manufacturers and consumers. RSM and ANN have been successfully applied to model and optimize extraction of lignans [24], curdlan production [25], and biodiesel production [26]. In this study, a reduced pressure evaporation system was used to synthesize 2-ethylhexyl ferulate (2-EF) under solvent free conditions. RSM and ANN were employed to investigate the effects of different reaction variables (reaction time, reaction temperature, and enzyme amount) on the response (yield %), and to obtain the optimal conditions to solve the problems of long reaction time and low yield of FA esters.
2. Results and Discussion
The present study applied RSM and ANN for experimental design of enzymatic synthesis of 2-EF in a solvent-free system. The molar ratio of FA and 2-ethylhexanol (the reactants) was 1:129, and the independent parameters selected for the experimental design were: reaction time (X1), enzyme amount (X2), and reaction temperature (X3). Their ranges and levels are given in Table 1.
Table 1.
Box-Behnken design and observed experimental data for 3-level-3-factor response surface analysis.
| Treatment No. a | Experimental Variables | Observed Molar Conversion (%) | ||
|---|---|---|---|---|
| X1 (h) | X2 (PLU) | X3 (°C) | ||
| 1 | 16 | 500 | 80 | 85.7 ± 3.4 |
| 2 | 16 | 1000 | 70 | 92.4 ± 3.1 |
| 3 | 16 | 1000 | 70 | 92.0 ± 2.8 |
| 4 | 16 | 1000 | 70 | 91.7 ± 2.3 |
| 5 | 8 | 500 | 70 | 68.7 ± 4.1 |
| 6 | 24 | 500 | 70 | 82.3 ± 3.2 |
| 7 | 8 | 1500 | 70 | 80.4 ± 2.1 |
| 8 | 24 | 1500 | 70 | 98.8 ± 1.6 |
| 9 | 16 | 500 | 60 | 39.8 ± 2.7 |
| 10 | 8 | 1000 | 60 | 42.7 ± 3.3 |
| 11 | 16 | 1500 | 60 | 44.8 ± 4.2 |
| 12 | 16 | 1500 | 80 | 98.9 ± 2.5 |
| 13 | 24 | 1000 | 60 | 64.2 ± 2.1 |
| 14 | 8 | 1000 | 80 | 86.3 ± 2.8 |
| 15 | 24 | 1000 | 80 | 98.7 ± 1.9 |
a The treatments were run in a random order.
2.1. RSM Model
The RSM Box-Behnken procedure was employed to fit the second-order polynomial equation to the experimental data (Table 1). Among the various treatments, the highest molar conversion (98.9% ± 2.5%) was observed in treatment 12 (reaction temperature of 80 °C, enzyme amount of 1500 PLU and reaction time of 16 h), and the lowest molar conversion (42.7% ± 3.3%) was observed in treatment 10 (reaction temperature of 60 °C, enzyme amount of 1000 PLU and reaction time of 8 h). The second-order polynomial equation obtained was as follows:
| Y = −968.26667 + 3.60052X1 + 26.28208X2 + 0.039783X3 − 0.027812X1X2 + 0.0003X1X3 + |
| 0.0004X2X3 − 0.028971X12 − 0.17154X22 − 0.0000305167X32 |
The analysis of variance (ANOVA), represented in Table 2, indicates that this second-order polynomial model was highly significant and adequate to represent the actual relationship between the response (molar conversion) and the significant variables with a very small p value (<0.001) and a satisfactory coefficient of determination (R2 = 0.9852). The ANOVA results of responses reveal an insignificant “lack of fit” for p > 0.05. Therefore, these models were adequate for prediction within the range of variables employed. Furthermore, the ANOVA results indicate that the linear term of X1 and X3 and the square term of X22 and X32 had significant (p < 0.05) influence on molar conversion. The interaction terms had less influence (p > 0.05).
Table 2.
ANOVA for synthetic variables pertaining to response percent molar conversion.
| Source | Sum of Squares | Degree of Freedom | Prob > F a |
|---|---|---|---|
| Model | 22.50 | 9 | 0.0005 |
| Linear | |||
| X1 | 1.87 | 1 | 0.0033 |
| X2 | 0.85 | 1 | 0.0614 |
| X3 | 14.72 | 1 | <0.0001 |
| Interaction | |||
| X1X2 | 0.01 | 1 | 0.7297 |
| X1X3 | 0.17 | 1 | 0.1762 |
| X2X3 | 0.02 | 1 | 0.5973 |
| Square | |||
| X12 | 0.02 | 1 | 0.6545 |
| X22 | 0.79 | 1 | 0.0188 |
| X32 | 4.33 | 1 | 0.0005 |
| Lack of fit | 15.20 | 3 | 0.09 |
| Pure error | 0.99 | 2 | |
| Total error | 16.19 | 5 |
a Prob > F: level of significance.
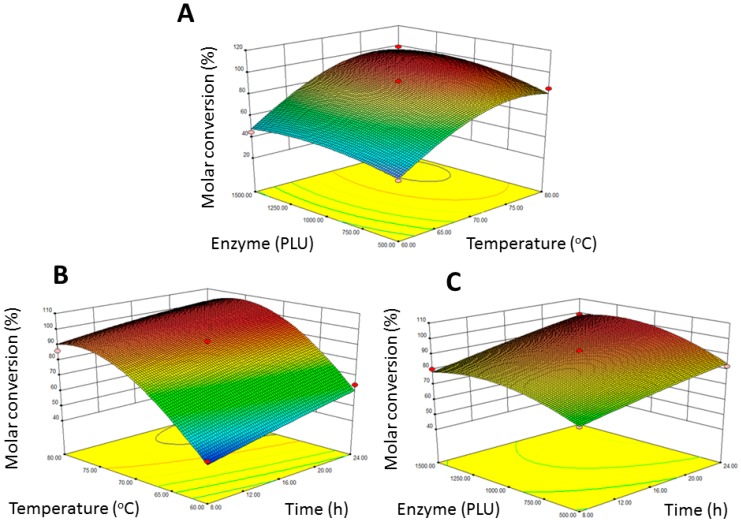
The effect of varying reaction temperature and enzyme amount on transesterification efficiency at a constant reaction time of 16 h was shown in Figure 1A. With the lowest reaction temperature (60 °C) and enzyme amount (500 PLU), a 2-EF molar conversion of 39.8% was obtained. Low molar conversions are also found in a previous report on lipase-catalyzed synthesis of ferulate esters at the temperature of 60 °C, enzyme amount of 100 mg, and time of 240 h [22]. The low molar conversion of ferulate esters can be improved by using a reduced pressure evaporation system. In the reduced pressure evaporation system, when the enzyme amount was increased to 1500 PLU and the reaction temperature was increased to 80 °C, the molar conversion of 2-EF was increased to 98.9 (Figure 1A). The great increase in molar conversion can result from the impact of solvent-free and reduced pressure evaporation system which increases the reaction rate of lipase-catalyzed biosynthesis by elevating the substrate concentration and eliminating byproducts with low vapor pressure physical characteristics. Figure 1B shows the effects of reaction time and reaction temperature on 2-EF biosynthesis at a constant enzyme amount (1000 PLU). At the lowest reaction time (8 h) and reaction temperature (60 °C), the molar conversion was 42.7% ± 3.3%. Figure 1C shows the effects of reaction time and enzyme amount on 2-EF biosynthesis at a constant reaction temperature. In this regard, enzyme amount had an insignificant effect on molar conversion, while reaction time had only a partial effect on molar conversion. Taken together, the molar conversion significantly increased when the reaction temperature and reaction time increased. As the ANOVA results shown in Table 2 indicate, reaction temperature and reaction time are important factors in Novozym®435-catalyzed reactions, which is in accordance with our previous works [27]. However, it has been reported that higher reaction temperature might decrease the lipase activity [21]. Thus, a reduced pressure evaporation system can be a good solution for lipase-catalyzed synthesis of FA esters in order to obtain a higher conversion at a lower reaction temperature.
Figure 1.
Response surface plot for the relationships between molar conversion and experimental variables. Response surface plot showing the relationships between molar conversion of 2-ethylhexyl ferulate and reaction parameters: (A) reaction temperature and enzyme amount; (B) reaction temperature and reaction time and (C) reaction time and enzyme amount.
2.2. ANN Model
ANN is a promising modeling methodology for non-linear multivariate process/reaction [28]. In this study, the data generated from the Box-Behnken design (Table 1) were employed to generate an ANN model for the enzymatic 2-EF synthesis. The proposed ANN consisted of three layers in the present work: an input layer with four neurons (reaction time, reaction temperature, and enzyme amount), a hidden layer with several neurons and an output layer containing one output neuron (molar conversion). The feed-forward neural network was trained by using incremental back propagation (IBP), batch backpropagation (BBP) and Levenberg-Marquardt algorithm (LM) or genetic algorithm (GA), respectively. All neurons from the hidden layer and output layer were calculated by a sigmoid transfer function. Different numbers of neurons, from three to six, were examined. The various architectures for ANN models were developed using NeuralPower software (Version 2.5, CPC-X Software, USA; available from Internet: http://www.brothersoft.com/neuralpower-download-21356.html), and the best one was selected based on the maximization of the R2 value and the minimization of the RMSE value.
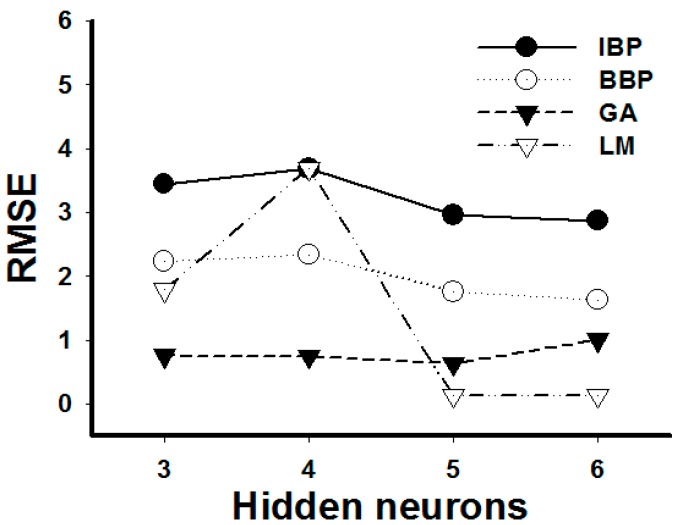
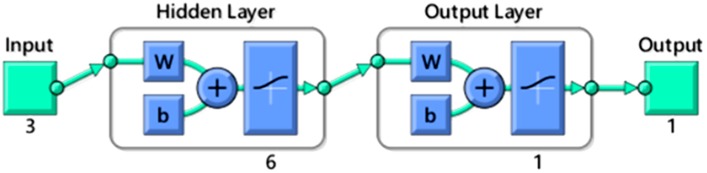
All results from the test were evaluated statistically based on the RMSE values. According to Figure 2, the best performance network was trained using a LM algorithm with a sigmoid transfer function, which exhibited a lower and stable RMSE. It is noted that the curve of the RMSE levels off at five hidden neurons. Therefore, the best ANN model in this study is determined to be a multilayer feed forward connection, trained by a LM algorithm using a sigmoid transfer function that consists of a 3–6–1 topology, as shown in Figure 3. This learning was acquired with RMSE = 0.79, R2 = 0.99.
Figure 2.
Learning curves of ANN model at different hidden neurons. The training set uses sigmoid transfer function in the hidden layer and output layer based on increment back propagation (IBP), batch back propagation (BBP), Levenberg-Marquardt algorithm (LM) or genetic algorithm (GA).
Figure 3.
The optimal architecture of multilayer feed forward neural network. The network contains three inputs (reaction temperature, reaction time and enzyme amount), one hidden layer with six nodes and one output (molar conversion of 2-ethylhexyl ferulate). W means weight; b means basic input/output system (BIOS).
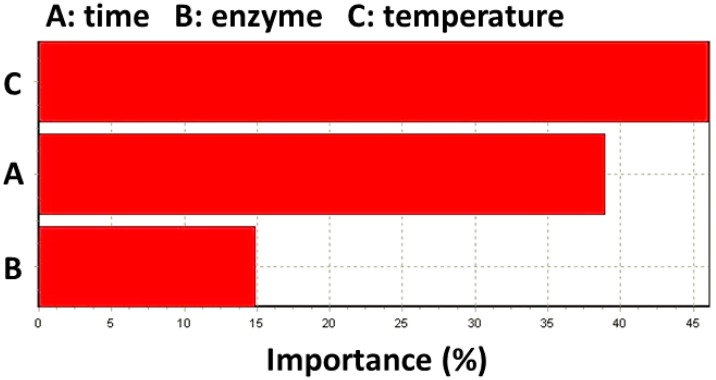
According to Figure 4, the reaction time and reaction temperature displayed great impact on the conversion with a relative importance of approximate 39% and 46%, respectively. These results are in accordance with the ones obtained with RSM.
Figure 4.
Importance of experimental variables on lipase-catalyzed synthesis of 2-ethylhexyl ferulate.
2.3. Comparison between RSM and ANN and Optimization for Experimental Condition
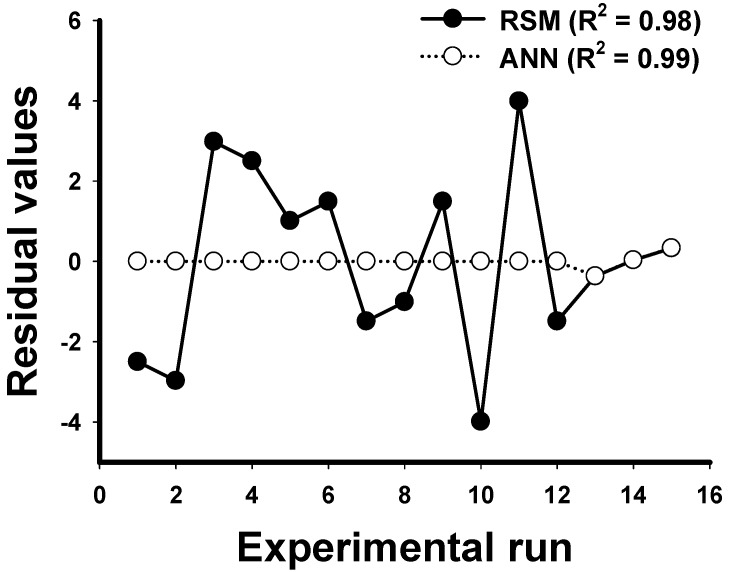
The distribution of the residual values obtained from the experimental data of Box-Behnken design experiments minus the related computed RSM and ANN data are shown in Figure 5. It was observed that the deviations of residual values showed a relatively small variation in the ANN model when compared with the RSM model. This suggested that ANN was better than RSM in data fitting.
Figure 5.
The distribution of residual values for each experimental run of Box-Behnken design experiments employing the RSM and ANN models. Coefficient of determination (R2) calculated from the design data for RSM and ANN models were 0.98 and 0.99, respectively. Residual values: predicted values minus actual values.
In order to validate and test the extrapolative capability of both the ANN and RSM models, a completely new set of five experiments was conducted on an experimental range which does not belong to the design data sets. The experimental and predicted values of the response for both the ANN and RSM models are given in Table 3. The performance of the newly constructed ANN and RSM models were statistically measured, in terms of AAD and RMSE values. The RMSEs for RSM and ANN were found as 7.322 and 5.152. These results indicate that the RSM prediction has a greater deviation than the ANN prediction. The AAD for RSM and ANN was found to be 10.046 and 9.430. From the results, it was observed that predictive power of ANN was found to be more powerful than RSM.
Table 3.
Validation and comparison of RSM- and ANN-modeling biocatalysis.
| Run | Independent Variable | Molar Conversion (%) | ||||
|---|---|---|---|---|---|---|
| X1 (h) | X2 (PLU) | X3 (°C) | Experimental | RSM Predicted | ANN Predicted | |
| 1 | 14 | 1250 | 75 | 93.2 | 98.8 | 95.0 |
| 2 | 15 | 750 | 65 | 76.8 | 71.2 | 73.5 |
| 3 | 10 | 850 | 75 | 74.6 | 90 | 85.3 |
| 4 | 9 | 1400 | 65 | 65.4 | 65.1 | 62.2 |
| 5 | 20 | 950 | 60 | 51.7 | 56.9 | 56.3 |
| AAD | 10.046 | 9.430 | ||||
| RMSE | 7.322 | 5.152 | ||||
Ridge max analysis can be used to determine optimum operating conditions by computing the estimated ridge of the maximum response for increasing the radius from the center of the original design. As shown in Table 4, the optimum condition for lipase-catalyzed synthesis of 2-EF determined by the ridge max analysis of RSM was reaction time of 23 h, reaction temperature of 71 °C and enzyme amount of 1422 PLU. A verification experiment performed at this condition obtained a molar conversion of 99.43%. Notably, higher molar conversion (99.74) was observed when the experiment was performed using the ANN-modeled optimal conditions (Table 4).
Table 4.
The optimal trial obtained from RSM and ANN models.
| Model | Optimal Condition | Relative Conversion (%) | |||
|---|---|---|---|---|---|
| X1 (h) | X2 (PLU) | X3 (°C) | Experimental Value | Predicted Value | |
| RSM | 23 | 1422 | 71 | 99.43 | 102.74 |
| ANN | 24 | 1485 | 78 | 99.74 | 99.82 |
2.4. Evaluation of Enzyme Reusability
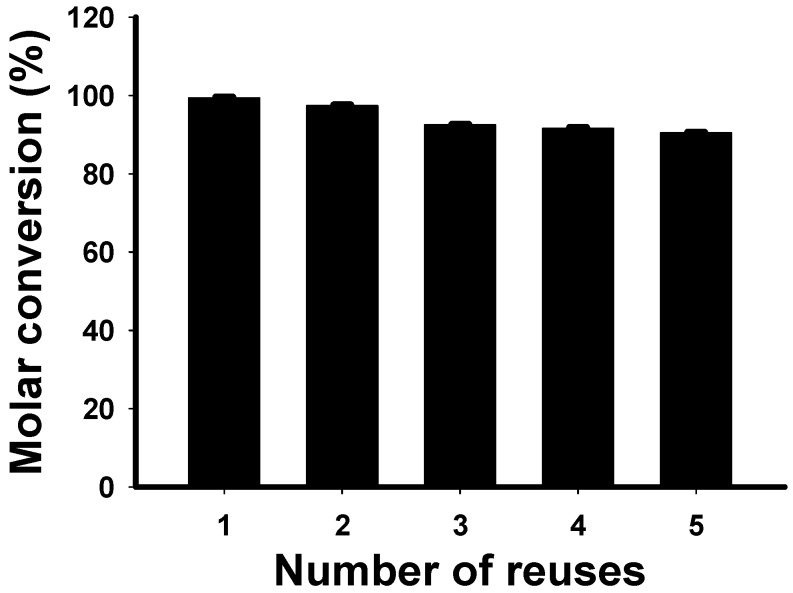
To examine the enzyme reusability, the ability of reused immobilized lipase for 2-EF synthesis was investigated under optimum conditions (Figure 6). After reaction, the mixture was filtered through a 0.45 μm membrane. The immobilized lipase was then recovered from the membrane, washed three times with fresh reaction media and dried before reuse in the next batch. After the immobilized lipase was reused five times, the molar conversion of 2-EF still remained at about 90.4%. This result showed that the immobilized lipase remained stable through long-term 2-EF exposure. Thus, the result demonstrated that the lipase could be effectively applied for 2-EF synthesis in the reduced pressure evaporation system and that the stability was high enough to permit reuse.
Figure 6.
Reusability of Novozym®435 in the synthesis of 2-ethylhexyl ferulate performed under optimum conditions.
3. Experimental Section
3.1. Materials
Immobilized lipase Novozym®435 (10,000 U/g; propyl laurate units (PLU)) from Candida antarctica B (EC3.1.1.3) supported on a macroporous acrylic resin was purchased from NovoNor disk Bioindustrials Inc. (Copenhagen, Denmark). Ferulic acid (FA), 2-ethylhexanol, 2-methyl-2-butanol, methanol and acetic acid were purchased from the Sigma Chemical Co. (St. Louis, MO, USA). A 4 Å molecular sieve was purchased from Davison Chemical (Baltimore, MD, USA). 2-ethylhexyl ferulate (2-EF) was purchased from Shanghai Richem International Co., Ltd. (Shanghai, China). All the other reagents and chemicals, unless otherwise noted, were of analytical grade.
3.2. Enzymatic 2-ethylhexyl Ferulate Synthesis
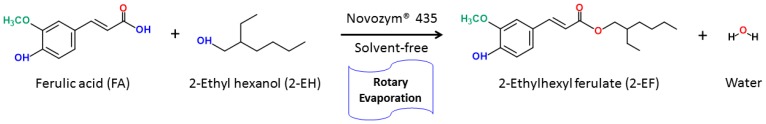
FA was dissolved in 2-ethylhexanol (1 mL) to the concentration of 0.05 M in the fingernails emissions glass flask that contained Novozym®435 of a rotary evaporator (EYELA N-1100, Tokyo Rikakikai Co., Ltd., Bohemia, NY, USA), which operated at 80 rpm and 560 torr under different experimental conditions. The esterification of FA with 2-EF catalyzed by Novozym®435 is represented in Scheme 1.
Scheme 1.
Enzymatic synthesis of 2-ethylhexyl ferulate.
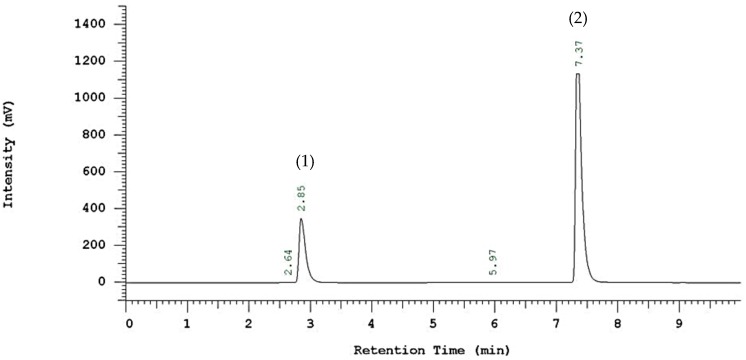
After incubation, liquid samples were withdrawn from the reaction mixture for analysis of 2-EF by high-performance liquid chromatography (HPLC). The sample was diluted with hexanol/2-methyl-2-butanol (1:150), and was injected (30 μL) into a HPLC (Hitachi L-7400; Tokyo, Japan) equipped with UV detector and Thermo C18 column (250 mm × 4.6 mm, Agilent, Santa Clara, CA, USA). Separations were carried out by a gradient elution with 0.1% acetic acid and methanol, the flow rate was set to 1.0 mL/min, and 2-EF was detected under UV light at 325 nm as shown in Figure 7. Calibration curves were prepared from the FA and 2-EF standards based on the peak areas. The molar conversion was defined as (mmol of 2-EF production per mmol of initial FA) × 100%.
Figure 7.
HPLC chromatogram of ferulic acid and its ester form. (1) ferulic acid; (2) 2-ethylhexyl ferulate.
3.3. Box-Behnken Design
A Box-Behnken design for 15 experimental runs was employed in present study. To avoid bias, the 15 runs were performed in a totally random order. The variables and their levels selected for the study of 2-EF biosynthesis were: reaction temperature (60 °C–80 °C), enzyme amount (500–1500 PLU) and reaction time (8–24 h), which were coded as shown in Table 5. Table 1 shows the independent factors (xi), levels and experimental design. Each experimental point was carried out in duplicate. Statistical parameters mentioned in present study including coefficient of determination (R2), root-mean-square error (RMSE), and absolute average deviation (AAD) were calculated by following Equations (1) to (3):
| (1) |
where Ypre is the predicted 2-EF yield (by either RSM or ANN), Yexp is the observed 2-EF yield and Ym is the average 2-EF yield.
| (2) |
where Ypre and Yexp are the predicted and experimental response, respectively, and n is the number of experiments.
| (3) |
where Ypre and Yexp are the predicted and experimental response, respectively, and n is the number of experiments.
Table 5.
Coding of experimental parameters and related levels.
| Parameters | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Reaction time (h) | X1 | 8 | 16 | 24 |
| Enzyme amount (PLU) a | X2 | 500 | 1000 | 1500 |
| Reaction temperature (°C) | X3 | 60 | 70 | 80 |
a PLU means propyl laurate units.
4. Conclusions
The present study demonstrates for the first time that immobilized C. antarctica lipase (Novozym®435) is able to synthesize 2-ethylhexyl ferulate (2-EF). A Box-Behnken design with RSM and ANN was selected to model and optimize the biocatalysis. Both RSM and ANN models for the 2-EF synthesis were built, and ANN was better than RSM in data fitting and prediction. The optimum synthesis condition modeled by ANN was in a reaction time of 24 h, reaction temperature of 78 °C and enzyme amount of 1485 PLU. The conversion of 2-EF under this optimum condition was verified to be 99.74%. The reduced pressure evaporation system combined with solvent free reaction conditions can decrease the reaction time and reaction temperature for ferulic acid ester synthesis.
Acknowledgments
The authors are very grateful to the Ministry of Science and Technology of Taiwan, ROC for supporting this research (Grants No. MOST 104-2221-E-005-061-MY3 and MOST 104-2218-E-022-001-MY2).
Author Contributions
Yawo-Kuo Twu and Chwen-Jen Shieh conceived and designed the experiments; Kuo-Chuan Huang performed the experiments and analyzed the data; Kuo-Chuan Huang, Ying Li and Chwen-Jen Shieh wrote the paper. Jer-An Lin and Chia-Hung Kuo assisted paper revision.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of 2-ethylhexyl ferulate are available from the authors.
References
- 1.Liu J., Wen X.Y., Lu J.F., Kan J., Jin C.H. Free radical mediated grafting of chitosan with caffeic and ferulic acids: Structures and antioxidant activity. Int. J. Biol. Macromol. 2014;65:97–106. doi: 10.1016/j.ijbiomac.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Choo W.S., Birch E.J. Radical scavenging activity of lipophilized products from lipase-catalyzed transesterification of triolein with cinnamic and ferulic acids. Lipids. 2009;44:145–152. doi: 10.1007/s11745-008-3242-x. [DOI] [PubMed] [Google Scholar]
- 3.Itagaki S., Kurokawa T., Nakata C., Saito Y., Oikawa S., Kobayashi M., Hirano T., Iseki K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009;114:466–471. doi: 10.1016/j.foodchem.2008.09.073. [DOI] [Google Scholar]
- 4.Mancuso C., Santangelo R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014;65:185–195. doi: 10.1016/j.fct.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Guyot B., Bosquette B., Pina M., Graille J. Esterification of phenolic acids from green coffee with an immobilized lipase from Candida antarctica in solvent-free medium. Biotechnol. Lett. 1997;19:529–532. doi: 10.1023/A:1018381102466. [DOI] [Google Scholar]
- 6.Stamatis H., Sereti V., Kolisis F.N. Studies on the enzymatic synthesis of lipophilic derivatives of natural antioxidants. J. Am. Oil Chem. Soc. 1999;76:1505–1510. doi: 10.1007/s11746-999-0193-1. [DOI] [Google Scholar]
- 7.Vosmann K., Weitkamp P., Weber N. Solvent-free lipase-catalyzed preparation of long-chain alkyl phenylpropanoates and phenylpropyl alkanoates. J. Agric. Food Chem. 2006;54:2969–2976. doi: 10.1021/jf060052t. [DOI] [PubMed] [Google Scholar]
- 8.Stamatis H., Sereti V., Kolisis F.N. Enzymatic synthesis of hydrophilic and hydrophobic derivatives of natural phenolic acids in organic media. J. Mol. Catal. B Enzym. 2001;11:323–328. doi: 10.1016/S1381-1177(00)00016-3. [DOI] [Google Scholar]
- 9.Chigorimbo-Murefu N.T.L., Riva S., Burton S.G. Lipase-catalysed synthesis of esters of ferulic acid with natural compounds and evaluation of their antioxidant properties. J. Mol. Catal. B Enzym. 2009;56:277–282. doi: 10.1016/j.molcatb.2008.05.017. [DOI] [Google Scholar]
- 10.Laszlo J.A., Compton D.L. Enzymatic glycerolysis and transesterification of vegetable oil for enhanced production of feruloylated glycerols. J. Am. Oil Chem. Soc. 2006;83:765–770. doi: 10.1007/s11746-006-5012-3. [DOI] [Google Scholar]
- 11.Sun S., Shan L., Jin Q., Liu Y., Wang X. Solvent-free synthesis of glyceryl ferulate using a commercial microbial lipase. Biotechnol. Lett. 2007;29:945–949. doi: 10.1007/s10529-007-9338-1. [DOI] [PubMed] [Google Scholar]
- 12.Nazare A.C., de Faria C.M., Chiari B.G., Petronio M.S., Regasini L.O., Silva D.H., Correa M.A., Isaac V.L., da Fonseca L.M., Ximenes V.F. Ethyl ferulate, a component with anti-inflammatory properties for emulsion-based creams. Molecules. 2014;19:8124–8139. doi: 10.3390/molecules19068124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha F.V., Gomes B.S., Neto B.S., Ferreira A.R., de Sousa D.P., E Martins M.D., Oliveira F.A. Ferulic acid ethyl ester diminished Complete Freund’s Adjuvant-induced incapacitation through antioxidant and anti-inflammatory activity. Naunyn Schmiedebergs Arch. Pharmacol. 2015;389:117–130. doi: 10.1007/s00210-015-1180-8. [DOI] [PubMed] [Google Scholar]
- 14.Machado K.C., Oliveira G.L., Machado K.C., Islam M.T., Junior A.L., de Sousa D.P., Freitas R.M. Anticonvulsant and behavioral effects observed in mice following treatment with an ester derivative of ferulic acid: Isopentyl ferulate. Chem. Biol. Interact. 2015;242:273–279. doi: 10.1016/j.cbi.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Shirai A., Kajiura M., Omasa T. Synergistic photobactericidal activity based on ultraviolet-A irradiation and ferulic acid derivatives. Photochem. Photobiol. 2015;91:1422–1428. doi: 10.1111/php.12507. [DOI] [PubMed] [Google Scholar]
- 16.Yang X.Z., Diao X.J., Yang W.H., Li F., He G.W., Gong G.Q., Xu Y.G. Design, synthesis and antithrombotic evaluation of novel dabigatran prodrugs containing methyl ferulate. Bioorg. Med. Chem. Lett. 2013;23:2089–2092. doi: 10.1016/j.bmcl.2013.01.126. [DOI] [PubMed] [Google Scholar]
- 17.Tsai Y.C., Lee Y.M., Hsu C.H., Leu S.Y., Chiang H.Y., Yen M.H., Cheng P.Y. The effect of ferulic acid ethyl ester on leptin-induced proliferation and migration of aortic smooth muscle cells. Exp. Mol. Med. 2015;47:e180. doi: 10.1038/emm.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuzaki H., Hisamoto M., Hirose K., Akiyama K., Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 19.Giuliani S., Piana C., Setti L., Hochkoeppler A., Pifferi P.G., Williamson G., Faulds C.B. Synthesis of pentylferulate by a feruloyl esterase from Aspergillus niger using water-in-oil microemulsions. Biotechnol. Lett. 2001;23:325–330. doi: 10.1023/A:1005629127480. [DOI] [Google Scholar]
- 20.Sun S., Song F., Bi Y., Yang G., Liu W. Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: Optimized by response surface methodology. J. Biotechnol. 2012;164:340–345. doi: 10.1016/j.jbiotec.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y., Kimura Y., Kadota M., Tsuno T., Adachi S. Continuous synthesis of alkyl ferulate by immobilized Candida antarctica lipase at high temperature. Biotechnol. Lett. 2006;28:1471–1474. doi: 10.1007/s10529-006-9113-8. [DOI] [PubMed] [Google Scholar]
- 22.Compton D.L., Laszlo J.A., Berhow M.A. Lipase-catalyzed synthesis of ferulate esters. J. Am. Oil Chem. Soc. 2000;77:513–519. doi: 10.1007/s11746-000-0082-9. [DOI] [Google Scholar]
- 23.Sun S., Zhu S., Bi Y. Solvent-free enzymatic synthesis of feruloylated structured lipids by the transesterification of ethyl ferulate with castor oil. Food Chem. 2014;158:292–295. doi: 10.1016/j.foodchem.2014.02.146. [DOI] [PubMed] [Google Scholar]
- 24.Guo T., Su D., Huang Y., Wang Y., Li Y.H. Ultrasound-assisted aqueous two-phase system for extraction and enrichment of Zanthoxylum armatum lignans. Molecules. 2015;20:15273–15286. doi: 10.3390/molecules200815273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafigh S.M., Yazdi A.V., Vossoughi M., Safekordi A.A., Ardjmand M. Optimization of culture medium and modeling of curdlan production from Paenibacillus polymyxa by RSM and ANN. Int. J. Biol. Macromol. 2014;70:463–473. doi: 10.1016/j.ijbiomac.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Maran J.P., Priya B. Comparison of response surface methodology and artificial neural network approach towards efficient ultrasound-assisted biodiesel production from muskmelon oil. Ultrason. Sonochem. 2015;23:192–200. doi: 10.1016/j.ultsonch.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Huang K.C., Li Y., Twu Y.K., Shieh C.J. High efficient synthesis of enzymatic 2-ethylhexyl ferulate at solvent-free and reduced pressure evaporation system. J. Mater. Sci. Chem. Eng. 2015;3:33–40. doi: 10.4236/msce.2015.36006. [DOI] [Google Scholar]
- 28.Tao Y., Wu D., Zhang Q.A., Sun D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014;21:706–715. doi: 10.1016/j.ultsonch.2013.09.005. [DOI] [PubMed] [Google Scholar]