Abstract
A novel series of bis(1,3,4-thiadiazole) derivatives were synthesized in one step methodology with good yields by condensation reaction between bis-hydrazonoyl chloride 1 and various reagents. The structures of the prepared compounds were confirmed by spectral data (IR, NMR, and MS), and elemental analysis. The anticancer activity against human breast carcinoma (MCF-7) cancer cell lines was evaluated in MTT assay. The results revealed that the bis-thiadiazole derivatives 5c,d, 7b,c and 9c had higher antitumor activity than the standard drug Imatinib.
Keywords: bis-hydrazonoyl chlorides; bis(1,3,4-thiadiazole); anticancer activity; methyl arylidene dithiocarbamate; dipolar cycloaddition reaction
1. Introduction
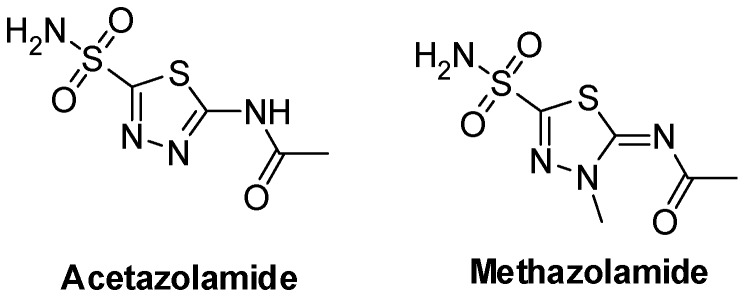
Many compounds having a 1,3,4-thiadiazole ring in their skeleton are capable of versatile pharmacological activities [1]. Also, thiadiazoles have been introduced as anticonvulsant [2], anti-parkinsonism [3], anti-histaminic [4] and anti-asthmatic [5], antitumor [6,7], analgesic [8], antimicrobial [9,10], and antitubercular [11,12,13] agents. Additionally, many drugs containing a 1,3,4-thiadiazole ring are available in the market, such as the carbonic anhydrase inhibitors Acetazolamide and Methazolamide (Figure 1).
Figure 1.
Examples of medications containing 1,3,4-thiadiazole ring.
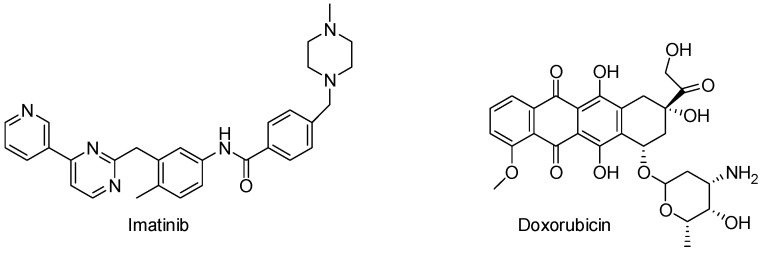
Imatinib (Figure 2) is a tyrosine-kinase inhibitor used to treat chronic myelogenous leukemia, gastrointestinal stromal tumors and a number of other malignancies. Doxorubicin (Figure 2) is used to treat some leukemias and Hodgkin’s lymphoma, cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, and multiple myeloma. In view of the medicinal importance of thiadiazole ring and as a continuation of our interest in the synthesis of a variety of thiadiazole derivatives for biological evaluation [14,15,16,17,18,19,20,21,22,23], we reported in the present study, the synthesis of a series of nine bis(1,3,4-thiadiazole) derivatives and their evaluation against breast cancer cell line (MCF-7) using Imatinib and Doxorubicin as a reference drugs.
Figure 2.
Structure of Imatinib and Doxorubicin.
2. Results and Discussion
2.1. Chemistry
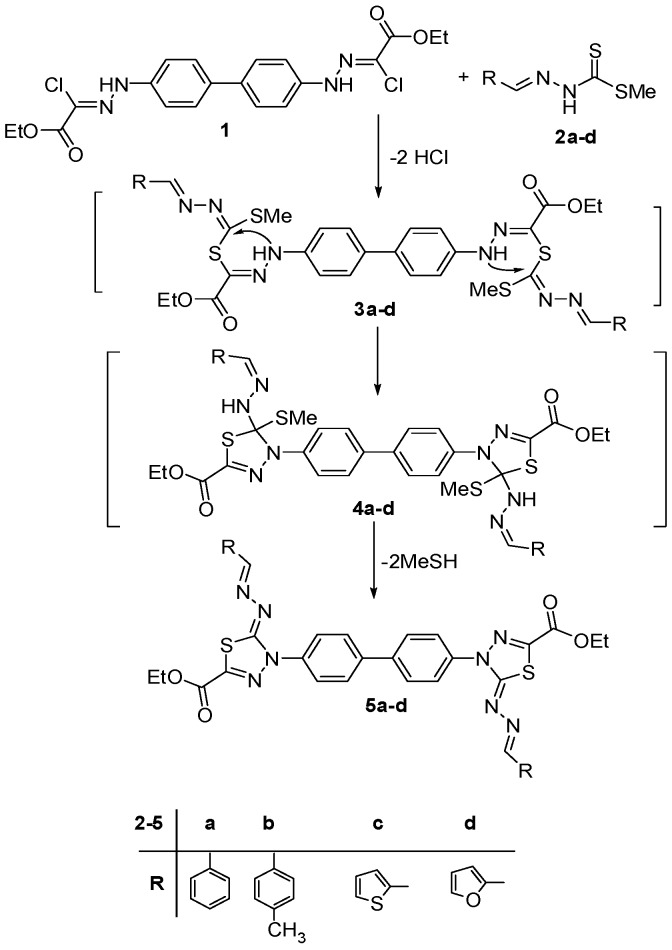
A mixture of bis-hydrazonoyl chloride 1 [24] and the appropriate methyl arylidene dithiocarbamate 2 [25,26,27,28] was stirred at room temperature for 30 min to afford the corresponding bis(1,3,4-thiadiazole) derivative 5 (Scheme 1). These reactions were assumed to start in each case, via S-alkylation with the elimination of two hydrochloric molecules, to afford the non-isolable bis-alkylated intermediate 3, which underwent intramolecular Michael-type addition to give intermediate 4. Elimination of two methanethiol molecules from 4 gave the final product 5. The structures of the synthesized products 5a–d were elucidated based on their spectral (IR, MS, 1H- and 13C-NMR) and elemental analyses Their IR spectra showed, in each case, an absorption band in the region 1708–1733 cm−1 due to the carbonyl of the ester group. Also, their 1H-NMR spectrum exhibited a triplet signals in the region δ 1.29–1.32 and a quartet signals in the region δ 4.34–4.35 due to CH2 and CH2 protons of the ethoxy groups, in addition to the aromatic signals. The mass spectra of the compounds 5a–d showed, in each case, a peak due to their molecular ions.
Scheme 1.
Synthesis of bis-thiadiazole derivatives 5a–d.
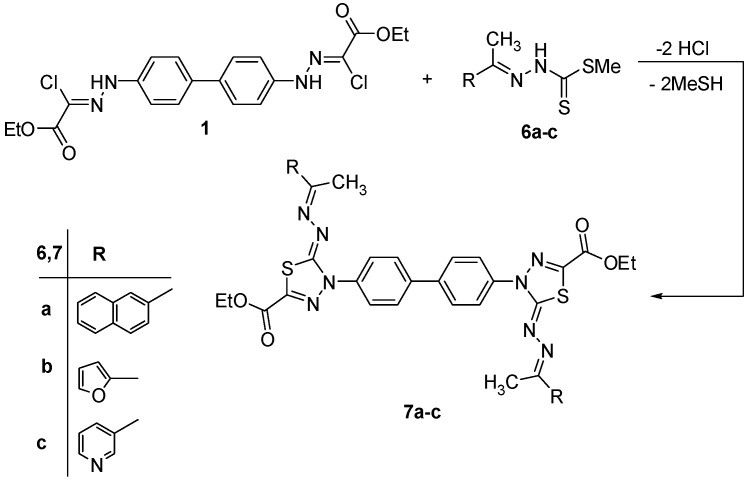
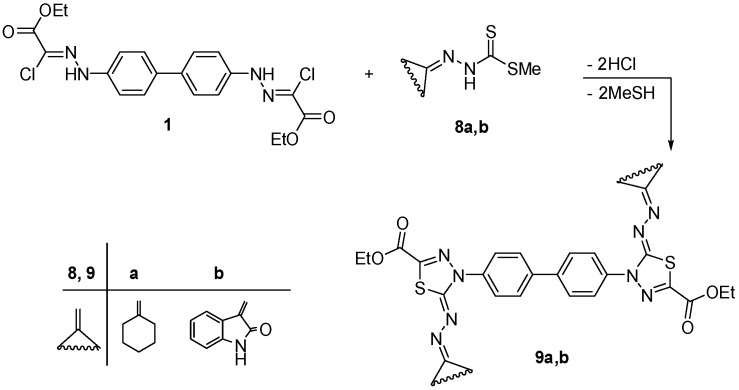
Also, the synthesis of combinatorial libraries of bis(1,3,4-thiadiazole) derivatives permits the testing of the biological activities of a vast array of these compounds. So, we intended to repeat the latter experiment again using bis-hydrazonoyl chloride 1 with the appropriate methyl arylidene dithiocarbamate 6a–c and 8a,b, under the same experimental conditions, which led to the corresponding bis-thiadiazoles 7a–c and 9a,b, respectively (Scheme 2 and Scheme 3). The structures of these products 7a–c and 9a,b were verified by their spectral and elemental analysis.
Scheme 2.
Synthesis of bis-thiadiazole derivatives 7a–c.
Scheme 3.
Synthesis of bis-thiadiazole derivatives 9a,b.
2.2. Pharmacology
Biological Screening of the Synthesized Bis-thiadiazoles for Their Cytotoxic Activity
The in vitro growth inhibitory rates (%) and inhibitory growth activity (as measured by IC50) of the newly synthesized bis-thiadiazoles were determined against human breast carcinoma cell line (MCF-7) in comparison with the well-known anticancer standard drugs doxorubicin and Imatinib (Gleevec®), using MTT viability assay. Data generated were used to plot a dose-response curve with which the concentration (μM) of test compounds required to kill 50% of the cell population (IC50) was determined. Cytotoxic activity was expressed as the mean IC50 of three independent experiments. The difference between inhibitory activities of all bis-thiadiazoles with different concentrations was statistically significant p < 0.001. Table 1 shows the antitumor activities of the tested bis-thiadiazoles compared with reference standard drugs evaluated using MTT assay on breast cancer cell line (MCF-7).
Table 1.
The antitumor activities of the tested bis-thiadiazoles 5a–d, 7a–c, 9a,b.
| Compd. No. | R | IC50 (µg/mL) |
|---|---|---|
| 5a | 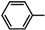 |
45.2 ± 1.4 |
| 5b | 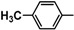 |
37.5 ± 1.7 |
| 5c | 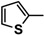 |
18.4 ± 1.2 |
| 5d | 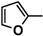 |
20.2 ± 0.6 |
| 7a | 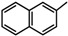 |
145.1 ± 3.4 |
| 7b | 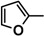 |
21.7 ± 1.5 |
| 7c | 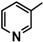 |
16.9 ± 1.7 |
| 9a | 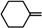 |
80.0 ± 2.0 |
| 9b | 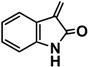 |
20.7 ± 2.4 |
| Doxorubicin | ---- | 0.8 ± 0.1 |
| Imatinib | ---- | 24.5 ± 0.3 |
The previous results lead to the following conclusions.
The bis-thiadiazole derivatives 5c,d, 7b,c and 9b had higher antitumor activity than the standard drug Imatinib.
The bis-thiadiazole derivatives 5a,b and 9a have moderate activity.
The bis-thiadiazole derivative 7a has poor antitumor activity against human breast carcinoma cell line (MCF-7).
The heterocyclic rings such as pyridine in 7c, thiophene in 5c, furan in 5d, 7b and indole in 9b are necessary to have the higher antitumor activity.
3. Materials and Methods
3.1. Chemistry
3.1.1. General
Measurements of the melting points were carried out on Electrothermal IA 9000 series digital melting point apparatus (Bibby Sci. Lim. Stone, Staffordshire, UK). The IR spectra were recorded in potassium bromide discs on a Pye Unicam SP 3300 and Shimadzu FT-IR 8101 PC infrared spectrophotometer (Shimadzu, Tokyo, Japan). 1H-NMR and 13C-NMR spectra were measured in deuterated dimethyl sulfoxide (DMSO-d6) using a Varian Gemini 300 NMR spectrometer (Varian, Inc., Karlsruhe, Germany). Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer (Tokyo, Japan) at 70 eV. Measurements of the elemental analysis were carried out at the Microanalytical Centre of Cairo University, Giza, Egypt. All reactions were followed by TLC (Silica gel, Merck, Kenilworth, NJ, USA). The biological evaluation of the products was carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt. Bis-hydrazonoyl chloride 1 [24] and the methyl arylidene dithiocarbamate 2, 6, 8 [25,26,27,28] were prepared as described in the literature.
3.1.2. Synthesis of 1,3,4-Thiadiazoline Derivatives 5a–d, 7a–c and 9a,b
Triethylamine (0.14 mL, 2 mmol) was added dropwise with stirring to a mixture of bis-hydrazonoyl chloride 1 (0.451 g, 1 mmol) and the appropriate methyl arylidene dithiocarbamate 2a–d, 6a–c and 8a,b (2 mmol) in ethanol (20 mL) for 30 min. The resulting solid product was collected and recrystallized from DMF to give the corresponding products 5a–d, 7a–c and 9a,b, respectively.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-(benzylidenehydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (5a). Yellow solid, (73% yield), m.p. 194–196 °C; IR (KBr) νmax 3032, 2964 (C-H), 1730 (C=O), 1609 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.32 (t, 6H, J = 7.2 Hz, 2CH2CH3), 4.35 (q, 4H, J = 7.2 Hz, 2CH2CH3), 7.47–7.88 (m, 14H, Ar-H), 8.04 (d, 4H, J = 7.2 Hz, Ar-H); 8.47 (s, 2H, 2CH=N); 13C-NMR (DMSO-d6) δ 13.5(CH3), 61.5 (CH2), 117.1, 123.4, 126.8, 127.8, 128.1, 129.9, 134.5, 138.2, 142.3, 145.1, 153.6 (Ar-C), 162.5 (C=O). Anal. Calcd. for C36H30N8O4S2 (702.80): C, 61.52; H, 4.30; N, 15.94. Found: C, 61.38; H, 4.19; N, 15.77%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-((4-methylbenzylidene)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (5b). Yellow solid, (75% yield), m.p. 186–188 °C; IR (KBr) νmax 3049, 2974 (C-H), 1725 (C=O), 1610 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.29 (t, 6H, J = 6.9 Hz, 2CH2CH3), 2.35 (s, 6H, 2CH3), 4.34 (q, 4H, J = 6.9 Hz, 2CH2CH3), 7.21 (d, 4H, J = 7.5 Hz, Ar-H), 7.66 (d, 4H, J = 7.5 Hz, Ar-H), 7.84 (d, 4H, J = 8.7 Hz, Ar-H), 8.05 (d, 4H, J = 8.7 Hz, Ar-H), 8.43 (s, 2H, 2CH=N); 13C-NMR (DMSO-d6) δ 14.4, 22.3 (2CH3), 61.8 (CH2), 115.5, 122.7, 126.9, 127.3, 128.9, 131.2, 139.0, 139.7, 146.5, 147.8, 153.4 (Ar-C), 163.1 (C=O); MS m/z (%) 730 (M+, 4), 627 (9), 457 (26), 226 (41), 119 (100), 87 (73), 59 (69). Anal. Calcd. for C38H34N8O4S2 (730.86): C, 62.45; H, 4.69; N, 15.33. Found: C, 62.36; H, 4.60; N, 15.24%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-((thiophen-2-ylmethylene)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (5c). Yellow solid, (74% yield), m.p. 251–253 °C; IR (KBr) νmax 3041, 2980 (C-H), 1733 (C=O), 1598 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.31 (t, 6H, J = 6.9 Hz, 2CH2CH3), 4.34 (q, 4H, J = 7.2 Hz, 2CH2CH3), 7.14–7.73 (m, 6H, Ar-H), 7.84 (d, 4H, J = 8.4 Hz, Ar-H), 8.07 (d, 4H, J = 8.4 Hz, Ar-H), 8.61 (s, 2H, 2CH=N); 13C-NMR (DMSO-d6) δ 14.4 (CH3), 62.7 (CH2), 115.5, 123.4, 127.0, 128.0, 130.4, 132.4, 133.3, 139.1, 143.1, 158.3, 159.8 (Ar-C), 165.0 (C=O); MS m/z (%) 714 (M+, 7), 542 (16), 364 (45), 171 (83), 109 (100), 62 (68). Anal. Calcd. for C32H26N8O4S4 (714.86): C, 53.76; H, 3.67; N, 15.67. Found: C, 53.69; H, 3.50; N, 15.43%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-((furan-2-ylmethylene)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (5d). Yellow solid, (70% yield), m.p. 283–285 °C; IR (KBr) νmax 3050, 2973 (C-H), 1732 (C=O), 1616 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.32 (t, 6H, J = 7.2 Hz, 2CH2CH3), 4.35 (q, 4H, J = 7.2 Hz, 2CH2CH3), 7.08–7.71 (m, 6H, Ar-H), 7.87 (d, 4H, J = 8.4 Hz, Ar-H), 8.05 (d, 4H, J = 8.4 Hz, Ar-H), 8.62 (s, 2H, 2CH=N); MS m/z (%) 682 (M+, 3), 368 (16), 224 (29), 152 (35), 80 (100), 52 (84). Anal. Calcd. for C32H26N8O6S2 (682.73): C, 56.30; H, 3.84; N, 16.41. Found: C, 56.17; H, 3.81; N, 16.28%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-((1-(naphthalen-2-yl)ethylidene)hydrazono)-4,5-dihydro- 1,3,4-thiadiazole-2-carboxylate) (7a). Yellow solid, (72% yield), m.p. 243–245 °C; IR (KBr) νmax 3046, 2924 (C-H), 1718 (C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.32 (t, 6H, J = 7.2 Hz, 2CH2CH3), 2.49 (s, 6H, 2CH3), 4.35 (q, 4H, J = 7.2 Hz, 2CH2CH3), 7.46–8.19 (m, 20H, Ar-H), 8.34 (s, 2H, Naphthalene-H1); MS m/z (%) 830 (M+, 17), 378 (88), 263 (29), 177 (48), 127 (100), 63 (71). Anal. Calcd. for C46H38N8O4S2 (830.98): C, 66.49; H, 4.61; N, 13.48. Found: C, 66.36; H, 4.49; N, 13.41%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-((1-(furan-2-yl)ethylidene)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (7b). Yellow solid, (73% yield), m.p. 259–261 °C; IR (KBr) νmax 3055, 2978, 2928 (C-H), 1715 (C=O), 1601 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.34 (t, 6H, J = 7.2 Hz, 2CH2CH3), 2.32 (s, 6H, 2CH3), 4.35 (q, 4H, J = 7.2 Hz, 2CH2CH3), 6.63 (d, 2H, J = 2.7 Hz), 7.02 (d, 2H, J = 2.7 Hz), 7.39 (m, 2H), 7.87 (d, 4H, J = 9.0 Hz, Ar-H), 8.05 (d, 4H, J = 9.0 Hz, Ar-H); 13C-NMR (DMSO-d6) δ 14.4, 15.1(2CH3), 62.3 (CH2), 116.5, 122.6, 127.9, 128.3, 128.9, 133.2, 138.0, 142.7, 146.7, 153.0, 157.8 (Ar-C), 163.4(C=O); MS m/z (%) 710 (M+, 20), 447 (22), 226 (29), 109 (52), 59 (100). Anal. Calcd. for C34H30N8O6S2 (710.78): C, 57.45; H, 4.25; N, 15.76. Found: C, 57.32; H, 4.21; N, 15.53%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-((1-(pyridin-3-yl)ethylidene)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (7c). Yellow solid, (70% yield), m.p. 263–265 °C; IR (KBr) νmax 3059, 2922 (C-H), 1736 (C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.31 (t, 6H, J = 7.2 Hz, 2CH2CH3), 2.49 (s, 6H, 2CH3), 4.32 (q, 4H, J = 7.2 Hz, 2CH2CH3), 7.48–7.75 (m, 2H, Pyridine-H), 7.84 (d, 4H, J = 8.4 Hz, Ar-H), 8.07 (d, 4H, J = 8.4 Hz, Ar-H), 8.29–9.05 (m, 6H, Pyridine-H); 13C-NMR (DMSO-d6) δ 14.6, 15.3 (2CH3), 62.6 (CH2), 114.2, 115.5, 120.5, 127.3, 128.0, 130.8, 132.2, 134.2, 142.1, 143.5, 152.6, 159.8 (Ar-C), 167.1 (C=O); MS m/z (%): 732 (M+, 3), 415 (24), 276 (37), 205 (64), 152 (60), 84 (100). Anal. Calcd. for C36H32N10O4S2 (732.83): C, 59.00; H, 4.40; N, 19.11 Found: C, 58.86; H, 4.31; N, 19.02%.
Diethyl 4,4'-(biphenyl-4,4'-diyl)bis(5-(cyclohexylidenehydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (9a). Yellow solid, (72% yield), m.p. 233–235 °C; IR (KBr) νmax 3032, 2929 (C-H), 1716 (C=O), 1616 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.34 (t, 6H, J = 7.2 Hz, 2CH2CH3), 1.61 (m, 12H, 6CH2), 2.34 (m, 4H, 2CH2), 2.63 (m, 4H, 2CH2), 4.38 (q, 4H, J = 7.2 Hz, 2CH2CH3), 7.88 (d, 4H, J = 8.4 Hz, Ar-H), 8.04 (d, 4H, J = 8.4 Hz, Ar-H); 13C-NMR (DMSO-d6) δ 14.4 (CH3), 25.0, 27.5, 35.2, 63.4 (CH2), 116.1, 122.6, 126.2, 133.6, 137.2, 146.5, 160.0 (Ar-C), 163.1(C=O); MS m/z (%): 686 (M+, 6), 415 (16), 226 (24), 194 (52), 104 (79), 90 (100). Anal. Calcd. for C34H38N8O4S2 (686.85): C, 59.45; H, 5.58; N, 16.31. Found: C, 59.25; H, 5.39; N, 16.22%.
Ethyl 4-(4'-((Z)-5-(ethoxycarbonyl)-2-((2-oxoindolin-3-ylidene)hydrazono)-1,3,4-thiadiazol-3(2H)-yl)biphenyl-4-yl)-5-((Z)-(2-oxoindolin-3-ylidene)hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (9b). Yellow solid, (67% yield), m.p. 266–268 °C; IR (KBr) νmax 3344 (NH), 3033, 2978 (C-H), 1724, 1681 (2C=O), 1607 (C=N) cm−1; 1H-NMR (DMSO-d6) δ 1.34 (t, 6H, J = 6.9 Hz, 2CH2CH3), 4.42 (q, 4H, J = 6.9 Hz, 2CH2CH3), 6.85–8.01 (m, 16H, Ar-H), 10.65 (s, br, 2H, 2NH, D2O-exchangeable); 13C-NMR (DMSO-d6) δ 14.3 (CH3), 62.6 (CH2), 114.2, 115.5, 117.5, 120.5, 123.5, 124.1, 126.9, 127.3, 130.8, 131.2, 134.2, 142.1,159.8 (Ar-C), 163.4, 170.6 (2C=O); MS m/z (%): 784 (M+, 4), 599 (18), 392 (26), 257 (38), 166 (68), 70 (100), 58 (42). Anal. Calcd. for C38H28N10O6S2 (784.82): C, 58.15; H, 3.60; N, 17.85. Found: C, 58.08; H, 3.47; N, 17.69%.
3.2. Pharmacology
Anticancer Activity
The cytotoxic evaluation of the synthesized compounds was carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt according to the reported method [29].
4. Conclusions
Various bis(1,3,4-thiadiazole) derivatives were synthesized in efficient and easy protocol. Biological studies revealed that bis-thiadiazole derivatives 5c,d, 7b,c and 9b had higher antitumor activity than the standard drug Imitanib.
Acknowledgments
The authors extend their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding this Prolific Research group (PRG-1437-29).
Author Contributions
A.O.A., S.M.G. and N.A.K. designed research; A.O.A., S.M.G., N.A.K. and Y.N.M. performed research, analyzed the data, wrote the paper and approved the final manuscript.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Footnotes
Sample Availability: Samples of the compounds 5a–d, 7a–c and 9a,b are available from the authors.
References
- 1.Hu Y., Li C.Y., Wang X.M., Yang Y.H., Zhu H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014;114:5572–5610. doi: 10.1021/cr400131u. [DOI] [PubMed] [Google Scholar]
- 2.Yar M.S., Akhter M.W. Synthesis and anticonvulsant activity of substituted oxadiazole and thiadiazole derivatives. Acta Pol. Pharm. 2009;66:393–397. [PubMed] [Google Scholar]
- 3.Azam F., Ibn-Rajab I.A., Alruiad A.A. Adenosine A2A receptor antagonists as novel anti-parkinsonian agents: a review of structure-activity relationships. Die Pharm. 2009;64:771–775. doi: 10.1002/chin.201011263. [DOI] [PubMed] [Google Scholar]
- 4.Lalezari I., Shafiee A., Badaly A., Salimi M.M., Khoyi M.A., Abtahi F., Zarrindast M.R. Synthesis and pharmacological activity of 5-substituted 2-(N,N-dialkylaminoethyl)amino- and 2-N-methyl piperazinyl-1,3,4-thiadiazoles. J. Pharm. Sci. 1975;64:1250–1252. doi: 10.1002/jps.2600640732. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya P., Leonard J.T., Roy K. Exploring QSAR of thiazole and thiadiazole derivatives as potent and selective human adenosine A3 receptor antagonists using FA and GFA techniques. Bioorg. Med. Chem. 2005;15:1159–1165. doi: 10.1016/j.bmc.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J., Xuan L., Zhao H., Cheng J., Fu X., Li S., Jing F., Liu Y., Chen B. Synthesis and antitumor activities of 1,3,4-thiadiazole derivatives possessing benzisoselenazolone scaffold. Chem. Res. Chin. Univ. 2014;30:764–769. doi: 10.1007/s40242-014-4080-4. [DOI] [Google Scholar]
- 7.Kumar D., Kumar N.M., Chang K.-H., Shah K. Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010;45:4664–4668. doi: 10.1016/j.ejmech.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Mathew V., Keshavayya J., Vaidya V.P., Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur. J. Med. Chem. 2007;42:823–840. doi: 10.1016/j.ejmech.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Noolvi M.N., Patel H.M., Kamboj S., Cameotra S.S. Synthesis and antimicrobial evaluation of novel 1,3,4-thiadiazole derivatives of 2-(4-formyl-2-methoxyphenoxy) acetic acid. Arab. J Chem. 2012 doi: 10.1016/j.arabjc.2012.02.003. [DOI] [Google Scholar]
- 10.Chawla A., Chawla P., Baghel U.S. Synthesis and antimicrobial evaluation of 5-aryl-1,3,4-thiadiazole-2-ylamine derivatives. World J. Pharm. Sci. 2016;4:110–114. [Google Scholar]
- 11.Karakus S., Rollas S. Synthesis and antituberculosis activity of new N-phenyl-N′-[4-(5-alkyl/arylamino-1,3,4-thiadiazole-2- yl)phenyl]thioureas. Farmaco. 2002;57:577–581. doi: 10.1016/S0014-827X(02)01252-1. [DOI] [PubMed] [Google Scholar]
- 12.Oruc E.E., Rollas S., Kandermirli F., Shvets N., Dimoglo A.S. 1,3,4-Thiadiazole derivatives: Synthesis, structure elucidation, and structure-antituberculosis activity relationship investigation. J. Med. Chem. 2004;47:6760–6767. doi: 10.1021/jm0495632. [DOI] [PubMed] [Google Scholar]
- 13.Foroumadi A., Kargar Z., Sakhteman A., Sharifzadeh Z., Feyzmohammadi R., Kazemi M., Shafiee A. Synthesis and antimycobacterial activity of some alkyl [5-(nitroaryl)-1,3,4-thiadiazol-2-ylthio]propionates. Bioorg. Med. Chem. Lett. 2006;16:1164–1167. doi: 10.1016/j.bmcl.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 14.Darwish E.S., Kheder N.A., Farag A.M. Synthesis and antimicrobial evaluation of some new pyridine based heterocycles. Heterocycles. 2010;81:2247–2256. [Google Scholar]
- 15.Farag A.M., Kheder N.A., Mabkhot Y.N. Synthesis and antimicrobial evaluation of new pyrazole, thiophene, thiazole and 1,3,4-thiadiazole derivatives incorporating pyrimidine ring. Heterocycles. 2009;78:1787–1798. doi: 10.3987/COM-09-11682. [DOI] [Google Scholar]
- 16.Kheder N.A., Riyadh S.M., Asiry A.M. Azoles and bis-Azoles: Synthesis and biological evaluation as antimicrobial and anti-cancer agents. Chem. Pharm. Bull. 2013;61:504–510. doi: 10.1248//cpb.c12-00939. [DOI] [PubMed] [Google Scholar]
- 17.Gomha S.M., Badrey M.G., Edrees M.M. Heterocyclisation of 2,5-diacetyl-3,4-disubstituted- thieno[2,3-b]thiophene bis-thiosemicarbazones leading to bis-thiazoles and bis-1,3,4-thiadiazoles as anti-breast cancer agents. J. Chem. Res. 2016:120–125. doi: 10.3184/174751916X14537182696214. [DOI] [Google Scholar]
- 18.Dawood K.M., Gomha S.M. Synthesis and anti-cancer activity of 1,3,4-thiadiazole and 1,3-thiazole derivatives having 1,3,4-oxadiazole moiety. J. Heterocycl. Chem. 2015;52:1400–1405. doi: 10.1002/jhet.2250. [DOI] [Google Scholar]
- 19.Gomha S.M., Riyadh S.M., Mahmmoud E.A., Elaasser M.M. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinyl pyridine) as a novel copolymer basic catalyst. Heterocycles. 2015;91:1227–1243. [Google Scholar]
- 20.Gomha S.M., Abdelrazek F.M., Abdelrahman A.H., Metz P. Synthesis of some novel thiazole, thiadiazole and 1,4-phenylene-bis-thiazole derivatives. Heterocycles. 2016;92:954–967. doi: 10.3987/COM-16-13443. [DOI] [Google Scholar]
- 21.Gomha S.M., Abdel-aziz H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles. 2015;91:583–592. doi: 10.3987/COM-14-13146. [DOI] [Google Scholar]
- 22.Gomha S.M., Salah T.A., Abdelhamid A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015;146:149–158. doi: 10.1007/s00706-014-1303-9. [DOI] [Google Scholar]
- 23.Gomha S.M., Ahmed S.A., Abdelhamid A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin- 5(1H)-one incorporating triazole moiety. Molecules. 2015;20:1357–1376. doi: 10.3390/molecules20011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheder N.A., Darwish E.S. Diethyl 2,2′-[Biphenyl-4,4′-diyldihydrazin-2-yl-1-ylidene]bis (chloroacetate) Molbank. 2014;2014:M813. doi: 10.3390/M813. [DOI] [Google Scholar]
- 25.Mahapatra M.K., Kulandaivelu U., Saiko P., Graser G., Szekeres T., Andrei G., Snoeck R., Balzarini J., Jayaprakash V. Methyl-2-arylidene hydrazinecarbodithioates: Synthesis and biological activity. Chem. Pap. 2013;67:650–656. doi: 10.2478/s11696-013-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Audrieth L.F., Scott E.S., Kippur P.S. Hydrazine derivatives of the carbonic and thiocarbonic acids. I. the preparation and properties of thiocarbohydrazide. J. Org. Chem. 1954;19:733–741. doi: 10.1021/jo01370a006. [DOI] [Google Scholar]
- 27.Klayman D.L., Bartosevich J.F., Griffin T.S., Mason C.J., Scovill J.P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979;22:855–862. doi: 10.1021/jm00193a020. [DOI] [PubMed] [Google Scholar]
- 28.Scovill J.P., Klayman D.L., Franchino C.F. 2-Acetylpyridine thiosemicarbazones. 4. Complexes with transition metals as antimalarial and antileukemic agents. J. Med. Chem. 1982;25:1261–1264. doi: 10.1021/jm00352a036. [DOI] [PubMed] [Google Scholar]
- 29.Gomha S.M., Edrees M.M., El-Arab E.E. Synthesis and preliminary in vitro cytotoxic evaluation of some novel bis-heterocycles incorporating thienothiophene. J. Heterocycl. Chem. 2016 doi: 10.1002/jhet.2636. [DOI] [Google Scholar]