Abstract
Background
Secretion from human basophils and mast cells requires the activity of SYK but expression of SYK is highly variable in the general population and this variability predicts the magnitude of IgE-mediated secretion. One known mechanism of modulating SYK expression in human basophils is aggregation of FceRI.
Objective
This study examines the possibility that functional auto-antibodies are present in a wide variety of subjects and in particular, subjects whose basophils poorly express SYK. It also tests whether any found antibodies could modulate SYK expression in maturing basophils and whether interaction with FcgRIIb/CD32b modulates the effect.
Methods
An experimental algorithm for classifying the nature of histamine release induced by serum from 3 classes of subjects was developed.
Results
The frequency of functional auto-antibodies that produce characteristics concordant with FceRI-mediated secretion was zero in 34 subjects without chronic spontaneous urticaria (CSU). In subjects with CSU, the frequency was lower than expected, approximately 7%. For the 5/68 unique CSU sera tested that contained anti-FceRI or anti-IgE Abs, these antibodies were found to induce down-regulation of SYK in both peripheral blood basophils and basophils developed from CD34+ progenitors. Blocking interaction of these antibodies with CD32b did not alter their ability to down-regulate SYK expression.
Conclusions
This study establishes that functional auto-antibodies to IgE/FceRI do not provide a good explanation for the variability in SYK expression in basophils in the general population. They do show that if antibodies with these characteristics are present, they are capable of modulating SYK expression in developing basophils.
Keyword list: Human, Basophil, Allergy, Fc Receptors
Introduction
IgE-mediated secretion from basophils and mast cells is a hallmark of the immediate hypersensitivity reaction. This receptor’s activation cascade is initiated by the aggregation of cell surface FceRI and the aggregation-induced assembly and activation of non-receptor kinases such as SYK [1]. We have been interested in the observation that expression of SYK in basophils is markedly reduced compared to other leukocytes and is highly variable in the general population [2]. This observation has functional consequences. First, it provides a reasonable explanation for the high variability in maximum IgE-mediated histamine release from basophils (in vitro) [2–4]. Second, it appears that the starting levels of SYK expression in basophils are predictive of the efficacy of omalizumab treatment in patients with asthma or peanut allergy [5, 6]. The implication of the latter observation is that basophils play a significant role in the expression of food allergy or asthma. For basophils, it is not yet clear whether the variability in SYK expression or the average low levels of SYK levels results from transcriptional or post-translational mechanisms. But one of the few known mechanisms for regulating SYK expression involves aggregation of FceRI [2, 7]. It is also known that the aggregation reaction may determine down-regulation of SYK even if the activation cascade is suppressed by co-engagement of CD32b on the basophil surface [8]. This possibility suggests that post-translational SYK regulation may occur in vivo without necessarily invoking mediator secretion.
The production of auto-antibodies as a driver of pathological states is a common finding in the field of immunological conditions. This is true for allergic diseases as well. In fact, for 20 years one dominant explanation for the existence of chronic spontaneous urticaria (or chronic idiopathic urticaria) has been the inappropriate production of antibodies to the high affinity IgE receptor, FceRI, or IgE antibody [9–12]. These antibodies could induce secretion from basophils or mast cells by inducing an aggregation reaction involving either IgE (bound to FceRI) or FceRI directly. But there is evidence that these auto-antibodies are also found at a high frequency in subjects without CIU/CSU [13].
Implicit to the thinking about auto-antibodies in CSU is that they are functionally active, i.e., can induce aggregation of FceRI and induce activation events that may lead to secretion. Postulating that the variability of SYK expression results from auto-antibodies would also require that they be able to induce aggregation and processing of SYK. Thus, in this study, functional antibodies will be the focus, in particular, auto-antibodies to either IgE or FceRIalpha. Taken together, the observations that 1) there may be auto-antibodies to IgE or FceRI, 2) that SYK is down-regulated by aggregation and 3) that co-engagement of CD32b can allow an aggregation reaction without secretion, suggest a hypothesis for the presence of variable SYK expression in basophils: naturally occurring IgE- or FceRI-specific antibodies interact with basophils to induce down-regulation of SYK and that secretion is ablated by simultaneously interacting with surface CD32b. A further qualification for the action of these antibodies is that the reaction occurs during maturation of the basophils in the bone marrow. In other words, the cells might emerge from the marrow having experienced prior aggregation and down104 regulation of SYK. Whether these antibodies are capable of this interaction requires direct testing of their actions in the presence and absence of CD32b blockade. One prediction from this hypothesis would be that non-CSU subjects would also express auto-antibodies since suppressed SYK expression is found in basophils of both non-atopic and atopic individuals without CSU. Therefore, one goal for this study was to determine the frequency of anti-IgE or anti-FceRI antibodies in healthy individuals and in particular, individuals whose basophils poorly express SYK.
However, as we began the search for natural antibodies to test, it became clear that the ability of sera to directly activate basophils was not always related to aggregation of IgE or FceRI and that the frequency of sera with properties consistent with activation of FceRI was much lower than published accounts suggest. This study established a different set of criteria for assessing the presence of functional auto-antibodies in sera that can induce secretion from human basophils and then explored whether they can act to modify SYK expression in maturing human basophils.
Materials & Methods
General methods
The online repository includes descriptions of methods in common use or methods previously published.
Sera
There were three categories of subjects providing serum for this study. The serum from non-CSU subjects was generally obtained from subjects whose basophils were well-characterized for their releasing properties, including IgE-mediated histamine release. These characteristics will be noted. The serum from CSU subjects was divided into two groups and all sera were provided by the laboratory of Dr. Sarbjit Saini. These subjects were clinically well-characterized with respect to the expression of chronic spontaneous urticaria. The sera were not always obtained during active disease states. The study began with a general selection of CSU subjects without regard to the presence of basopenia at the time of blood draw (i.e., basopenia may or may not have been present). Subsequent to obtaining this group of sera, a second request for sera was made for subjects for whom basopenia was known to be present. Retrospectively, 3 of the sera transmitted to us for the second group were from subjects in the first CSU group.
Definition of Positivity
For a serum to have induced histamine release, a threshold was chosen that was based on the general variability of results from a large number of screens. The algorithm used to set a threshold of 6% (above spontaneous release) is discussed in the online repository. Due to technical issues related to the need to precipitate protein before analyzing samples on the auto-analyzer, the maximum concentration of serum tested was 25%. To get a sense of the serum titer in these screening tests, a concentration of 5% was included.
Drug sensitivity and similarity index calculation
Partially enriched basophils were stimulated in the presence of 25% or 5% serum following a 5 minute incubation with the four agents tested, PP1 (PP2 was also tested in pilot experiments but suffered the same problems with serum found for PP1), NVP-QAB205, PCI-32765 and LY294002 (respectively, a src-family kinase inhibitors, a SYK inhibitor, BTK inhibitor and a PI3K inhibitor, all IgE-mediated release-specific inhibitors). For the final testing the concentrations of the latter 3 drugs were 0.75 μM, 75 nM, and 7.5 μM respectively. Positive controls included 1 μg/ml of anti-IgE Ab, 6061P, in PAGCM or in 25% negative serum (extensive testing of several negative sera established a single serum that was not found to induce release under a variety of circumstances). To calculate a similarity index when determining if a particular serum behaved towards the drugs tested in a manner similar to 6061P, the ratio of the response of (6061P+drug)/(6061P-drug) was calculated, define as ADR. Calculate the ratio of (serum+drug)/(serum-drug), define as SDR. The similarity index is (1-SDR)/(1-ADR). This value is 1.0 when drug inhibits serum as effectively as it inhibits 6061P. To account for the noise in the assay, indices greater than 0.9 were considered “similar” to the behavior with 6061P. A similar metric was calculated for the cross-desensitization experiments.
Results
Auto-antibodies to FceRI or IgE
The frequency that one finds sera that induce release from basophils ex vivo appears variable among studies [10–13]. On the nature of positivity, our approach was cautious. For the assay itself, the goal was to choose donors whose basophils were sensitive to stimulation through IgE or FceRI. The online repository also discusses results on whether ABO incompatibility was a technical issue. While it was concluded that AB/Rh compatibility was probably not a factor in the ability of serum to induce secretion, for the current purposes ‘recipient’ basophils were all O type.
The criterion for declaring a serum as causing release was developed from analysis of noise in the assay (see methods). In addition, a positive serum was required to repeat with a different basophil donor. There were 29 single positives from 102 distinct sera, for a frequency of 28% (41% in the CSU groups), only 13 that repeated positive with distinct basophil donors, a frequency of 13% (or approximately one-half of the single positives). Because we then engaged in additional testing with each positive serum, replication for true positives was a result usually based on multiple experiments. Single positives received no further testing.
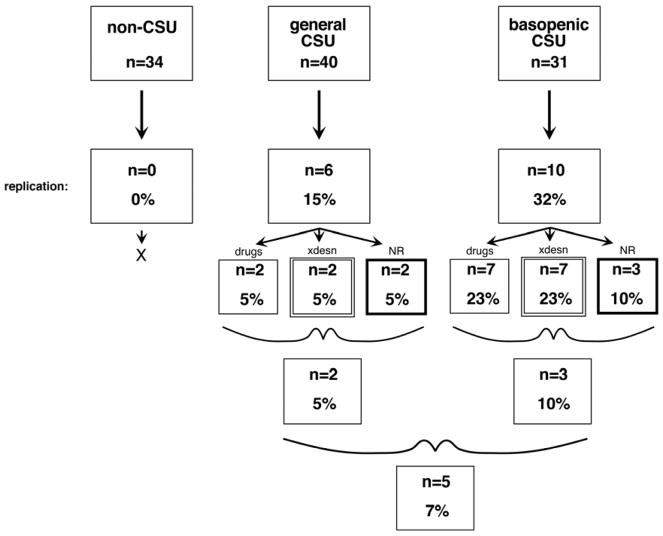
Figures 1 and online figure E1 summarize the results and extends the summary to three categories of serum donor, those without CSU, those with CSU but chosen without regard to the presence of basopenia and those with CSU selected a priori for coincident basopenia. There were no consistent positives in the non-CSU group but consistent positives were observed in those with CSU.
Figure 1.
Criteria for classification of sera as containing functional auto-antibodies to FceRI or IgE. ‘drugs’ = 3 IgE-pathway-specific drugs, ‘xdesn’ = cross-desensitization with known aggregating anti-IgE Ab, ‘NR’ inability to stimulate basophils of the non-releasing phenotype.
Notably, there was no evidence for histamine-releasing activity in the healthy non-CSU group (n=34); this group included both allergic (25%) and non-allergic subjects (75%). For these serum donors, there was information about the release induced by an optimal concentration of anti-IgE Ab/6061P from their basophils as well; it was as variable as previous studies have found (average = 40± 28%, C.V. = 0.70) and 6 (17%) of the donors fit the non-releaser category (2.0± 0.4% release vs. 48± 5% for releasers). Thus, the donors who had non-releasing basophils did not also have sera that induced release (see below as well). (The online repository presents results using IL-3 treated recipient basophils. These experiments show that sera from non-CSU subjects can induce release that is not IgE-mediated.)
To determine if the induced release operated through an FceRI-like mechanism the chosen positive sera were examined with 4 different assays. Three of these assays were used on each of the multiply positive sera and the final assay used for the sera that passed the first 3. The online repository presents these results (figures E2–E5). The 4 tests included 1) the ability of 3 known IgE-signaling pathway drugs (SYK, BTK and PI3K inhibitors) to inhibit serum-induced release, 2) the ability to cross-desensitize serum-induced release with a known anti-IgE Ab, 3) the inability of sera to induce release in basophils with the non-releasing phenotype and 4) the ability of IgE- or FceRIalpha-sepharose (and not HSA-sepharose) to adsorb the auto-antibody activity. Only 5 sera passed these tests.
Auto-antibody sensitivity to CD32b engagement
One goal for these experiments was to determine if human anti-FceRI or anti-IgE antibodies interacted significantly with CD32b. However, this necessitated first understanding how nonspecific IgG (nsIgG) could interfere with the extrinsic specific antibodies interacting with FcgRIIb/CD32b (or FcgRIIa/CD32a) since serum nsIgG is only avoidable if it can be diluted sufficiently to stop its interference with CD32b binding [14]. The online repository includes experiments addressing this issue (figure E7). It was determined that if sera were used as stimuli, that testing would require sera concentrations be less than 2%. Of the 5 sera with the expected anti-IgE or anti-FceRIalpha characteristics, only 2 were of sufficient titer to allow study with serum concentrations ≤2%. This was important because it reduces the nonspecific IgG concentrations to less than 150 μg/ml. As noted in the preliminary studies in the online repository, this concentration may not significantly interfere with FcgRIIb/CD32b binding, while inclusion of Ab10523 (a CD32b selective high affinity Ab [8]), would interfere.
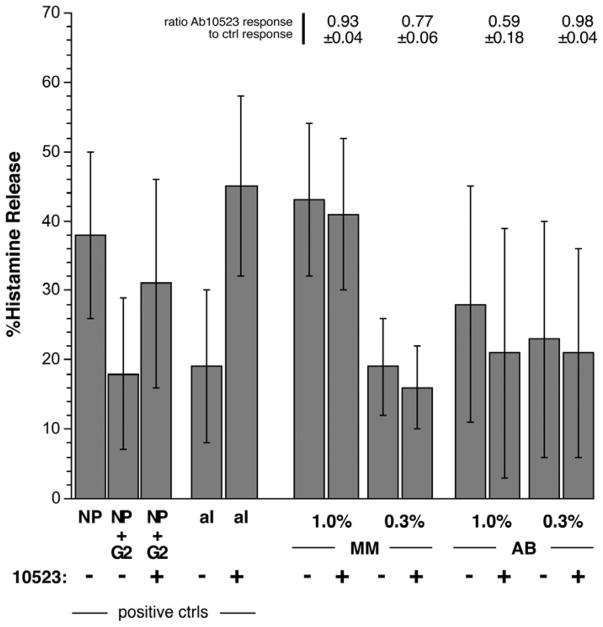
For these experiments, there were two positive controls that were used to insure that a FcgRIIb/CD32b-mediated inhibition of histamine release was observable under the conditions of the experiment. The cells were treated to dissociate a portion of the endogenous IgE and resensitized with a nitrophenyl (NP)-specific IgE. As we have shown previously [14], the mixing of NP-specific IgG2 at an appropriate concentration with NP-BSA prior to stimulation inhibits histamine release caused by NP-BSA and that this inhibition is partially or fully reversible by blocking FcgRIIb/CD32b with Ab10523 (anti-FcgRIIb/CD32b). A second positive control was that noted in the online repository, testing for release with a supraoptimal concentration of goat polyclonal anti-IgE Ab ± Ab10523 [8]. As above, the inclusion of Ab10523 (anti-FcgRIIb/CD32b) should enhance the response to goat anti-IgE Ab. As shown in figure 2, both of these controls demonstrated the expected behavior for Ab10523 (anti-FcgRIIb/CD32b), reversing NP-specific IgG2 inhibition (with paired analysis, reversal was 62± 14%, p = 0.0016) and markedly enhancing the goat polyclonal anti-IgE Ab response (p=0.0009). It should be noted that these positive control conditions were performed in the presence of a 1% negative serum (to replicate the conditions for the two test sera).
Figure 2.
Effect of Ab10523 (blocking Ab for FcgRIIb/CD32b) on sera-induced secretion. Two methods were u sed to demonstrate within the same cell preparations that blocking FcgRIIb/CD32b with Ab10523 (anti-FcgRIIb/CD32b) under known conditions would alter the response to stimuli. These positive controls (positive ctrls, left side of plot) are described in the text. Using similar conditions, the cells were stimulated with sera MM and AB at the concentrations shown in the presence or absence of Ab10523 (anti-FcgRIIb/CD32b). The bars plot the average histamine release for the 4 experiments and the average ratios (response in the presence of Ab10523/response without Ab) for the 4 experiments are shown above the bars.
MM (an anti-IgE-like serum) and AB (an anti-FceRIalpha-like serum) were tested at 1% and 0.3% concentrations. There was no effect, or possibly some inhibition of release, by the inclusion of Ab10523 (anti-FcgRIIb/CD32b) (figure 2).
Influence of IgE/Fc-like serum on SYK levels in peripheral blood basophils
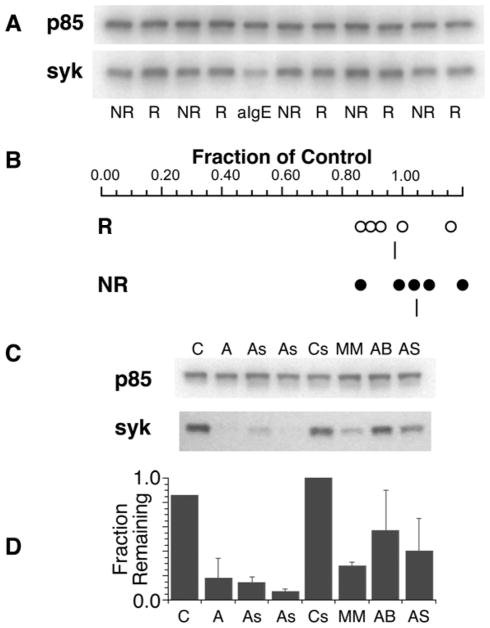
Stimulation of human basophils through the IgE-mediated pathways induces the loss of SYK expression [7]. The process is relatively slow but even low levels of stimulation induce SYK loss when they don’t induce histamine release. Therefore, this can prove a useful test for low levels of stimulation. The assay was performed with impure cells using flow cytometry to detect SYK expression or with purified cells using Western blotting to detect SYK expression. For context, the cells were stimulated with anti-IgE Ab/6061P diluted into a putatively negative serum so that the final serum concentration (2%) was similar to the negative control and the other tested sera. Five sera from releasers and five sera from non-releasers were tested. By either flow cytometry or Western blotting, there was no significant loss of SYK expression (relative to a control containing no serum) for all 10 sera while there was a 6061P-induced a loss of 61± 5% of the SYK. Notably, there was no difference between releaser and non-releaser sera (figure 3A&B).
Figure 3.
SYK expression in purified human basophils cultured overnight with serum (see methods) from subjects whose basophils were characterized as non-releasers or releasers. Panel A: Example western blot for SYK expression, NR = serum from non-releaser phenotype, R = serum from releaser phenotype, aIgE = known aggregator 6061P/anti-IgE Ab. The protein p85 (a subunit of PI3K) is used to normalize results (see methods). Panel B: summary analysis of 2 experiments. The serum effects were calculated relative to media control band intensity. Five releaser and 5 non-releaser sera were tested in overnight cultures and the ratios plotted. Panel C: Example western blot analysis for SYK expression, C = media control, A = anti-IgE Ab, As = suboptimal anti-IgE Ab, Cs = control serum (known not to induce secretion), MM, AB, AS = sera known to induce release by the criteria established for FceRI/IgE-mediated release. Overnight cultures as described for panel A. Panel D: average results of the conditions aligned in panel C (n=3, 2 experiments using Western analysis and 1 using flow cytometric analysis).
In contrast, 3 of the CSU-derived sera that were shown to contain anti-FceRI or anti-IgE-like properties induced significant loss of SYK in these cultures. Combining the results where flow or Western blotting was used, these 3 sera induced a 56± 9% loss of SYK (6061 induced a 85± 5% loss). Two of the CSU sera had a high enough titer that a 1.2% dilution could be used (therefore, nonspecific IgG should be approximately 180 μg/ml, low enough that it shouldn’t interfere with binding of an anti-IgE or anti-FceRI-like antibody, see above). For these two sera, the overnight incubation was done ± Ab10523 (anti-FcgRIIb/CD32b) to block interaction with FcgRIIb/CD32b. Based on previous studies [8] showing that SYK loss was not affected by signaling changes downstream of SYK, loss of SYK was not expected to be affected in this experimental design and for the CSU-sera (average reduction to 0.29± 0.02 of control) or anti-IgE Ab/6061P or goat anti-IgE diluted into negative serum, average reduction to 0.30± 0.08 of control), Ab10523 (anti-FcgRIIb/CD32b) did not change the loss of SYK (for CSU-sera, average reduction to 0.30± 0.05 of control and for anti-IgE Abs, reduction to 0.30± 0.02 of control).
Influence of IgE/Fc-like serum on SYK levels in maturing basophils
Although there was no evidence that non-CSU sera could induce secretion or alter SYK expression in overnight cultures of PBB, the original purpose of this study was to determine whether a serum with demonstrable auto-Ab to FceRI could modify SYK expression in maturing basophils. Previous studies on the maturation of basophils from CD34+ progenitors showed that chronic aggregation could induce a loss of SYK expression without changes in cell surface FceRI density or granulation (as measured by alcian blue positivity or histamine content) [15]. A similar experimental design was used to examine one of the better serums from the above studies and specifically a serum that appeared to be an anti-FceRIalpha Ab. In addition, Ab10523 (anti-FcgRIIb/CD32b) was added in one set of conditions to test whether blockade of FcgRIIb/CD32b interaction would alter the results. To do these experiments, the serum needed to have a high titer so that the nonspecific IgG levels would be low. One of the 4 sera studied above, AS, was shown above to induce SYK loss in the PBB at low enough concentrations to test the effect of Ab10523. This serum was also shown to bind well to unoccupied FceRI. The positive control in these studies was a concentration of Ab22E7 (anti-FceRI Ab) that was chosen to be suboptimal for induction of histamine release (to better test the low end of stimulation for an effect on SYK expression). For these experiments, conditions included media alone, Ab22E7 (anti-FceRI), a ‘negative’ serum± Ab10523 (anti-FcgRIIb/CD32b) and AS serum± Ab10523 (anti-FcgRIIb/CD32b). Both negative serum and AS serum were tested at 1% and 2% concentrations. Not shown in the figure, inclusion of serum (negative or AS) increased the number of alcian blue-positive cells (1.65± 0.065, p=0.009) and total histamine content of the culture cell pellets, with a slight increase in viability in the cultures.
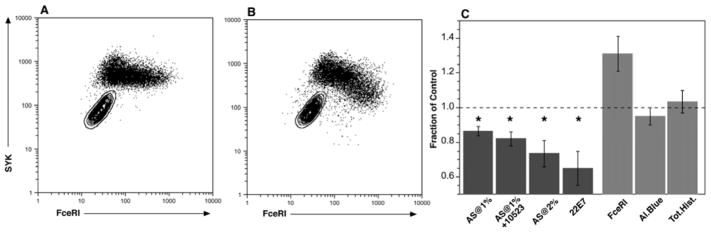
Figure 4 shows the 2D plots of FceRI vs. SYK expression for negative control serum (panel A) and 1% AS serum (panel B) for one of 3 experiments and panel C summarizes the results for the 3 experiments. Relative to negative serum, AS serum induced a loss of SYK and the pattern at 1% was similar to the pattern observed in previous studies (and different from the pattern with negative control serum); greater loss at higher densities of FceRI (thus the negative slope in the dot plot) [15]. Greater loss occurred at 2% serum and Ab10523 did not alter the results (with statistical significance). For context, the Ab22E7 (anti-FceRI) results showed a greater decrease. Also as observed previously, there was no effect on alcian blue counts (between negative control serum and AS), total histamine content, or on cell surface FceRI expression (the apparent increase with AS was not statistically significant, p = 0.095).
Figure 4.
SYK expression in 15 day culture-derived basophils treated with AS serum ± Ab10523 (anti-FcgRIIb/Cd32b). The various stimuli were added at day 3 of culture and analysis of SYK done by flow cytometry. Panel A; flow cytometry of cells treated with control serum at 1% (serum previously determined to not induce release). Panel B: flow cytometry of cells treated with AS serum at 1% (known to induce release). Panel C: summary results from 2–3 experiments (n=3 for 1% and n=2 for 2% serum), expressed relative to control serum, 22E7 (anti-FceRI Ab) at a concentration previously determined to induce 5% of maximum release. For the AS treated cells, combining results for both 1% and 2% concentrations, FceRI = surface FceRI expression (n=3), Al.Blue = counts of alcian blue positive cells (n=3), Tot.Hist. = total histamine content (n=2).
As a follow-up to the FcgRIIb/CD32b portion of the experiment, the expression of FcgRIIb/CD32b in these maturing basophil cultures was examined. Its expression was near zero in the original CD34+ cells and increased with considerable variability in rate in different preparations but was nevertheless present by day 9 of culture (data not shown).
Discussion
These studies were undertaken to determine if the sera of subjects whose basophils poorly expressed SYK would contain auto-antibodies to FceRI or IgE that could explain the suppressed levels of SYK expression. In the same context, the question could be expanded to ask whether heterogeneity of SYK expression in the general population could be explained by the presence of functional auto-antibodies. This hypothesis was developed because it has been demonstrated that aggregation of FceRI/IgE is one mechanism for reducing SYK in both mature peripheral blood basophils and in maturing culture-derived basophils [2, 7]. There is also evidence that suggests that the sera of healthy subjects may contain auto-antibodies to FceRI or IgE [9–13]. The hypothesis was predicated on finding auto-antibodies that were functionally active for inducing either secretion or, at a minimum, inducing loss of SYK. To provide a context for this search, subjects with clinically demonstrable CSU were used to act as a positive control because frequencies for finding these auto-antibodies in subjects with this condition are reported to be as high as 50%. After initial screening it became clear that a better definition of antibody activity that could induce secretion was needed. It also became clear that the bioassay – basophil activation by a foreign serum – was sensitive to factors not related to FceRI or IgE crosslinking. By the end of the study, the criteria for a serum containing a functionally active anti-IgE or anti-FceRI antibody had expanded to 6 conditions. Including the 4 tests noted in the results, two additional tests were: 5) sera able to be depleted of their activity by specific adsorption with either IgE- or FceRIalpha-bearing solid matrix, and 6) inducing the loss of SYK expression in overnight cultures of basophils.
With these considerations, no serum from non-CSU subjects was found to contain functional auto-antibodies. Notably, this included sera from subjects whose basophils showed near zero levels of IgE-mediated histamine release (and therefore little SYK expression). There were also no differences between sera from non-allergic and allergic subjects. This result suggests that the variability in SYK expression in the non-CSU population (i.e., 99% of the population) is not readily explained by the variable presence of functional auto-antibodies to FceRI or IgE.
Previous studies on the presence of anti-FceRI or anti-IgE Abs in sera from non-CSU subjects used various binding assays (e.g., an IEMA-formatted assay, Western blotting, etc) to detect these antibodies [9–13, 16]. Because the current hypothesis required crosslinking-capable antibodies, the distinction between the current results and previous results may reflect the difference in ability to crosslink IgE vs. simple binding. One early study suggested that antibodies with this characteristic exist and are not functionally active [17]. This study found that it was possible to induce histamine release from many individuals by simply stimulating the cells with an anti-IgG antibody. Since there are no known activating IgG receptors on basophils [14, 18, 19], it could be concluded that IgG is bound by other means, possibly bound to structures like IgE or FceRI, especially since the characteristics of the release induced by anti-IgG Ab were similar to anti-IgE Ab-induced release [17]. It would be surprising to find no examples of auto-antibodies in the serum from atopic subjects if this interpretation were correct unless these antibodies can bind well but not induce aggregation without the assistance of a secondary crosslinking reagent like anti-IgG Ab. For this previously published study, other than a strong association of these antibodies with subjects with allergic diseases, there was no identified consequence to possessing the antibodies. A more recent study [16] identified anti-IgE antibodies in sera using an ELISA-based assay and determined that some of these antibodies were functionally active although a consequence of this activity in the subjects from which they were derived was not discussed. In contrast, if auto-antibodies are functionally active in patients with CIU/CSU, there would seem to be a pathological consequence to their presence (although concordance between their presence and disease expression is poor [10–12]). These studies were limited to a single clinic population and although the patients were well characterized, a broader study, potentially at other sites, to establish whether this algorithm for identifying positive sera would yield a similar frequency of auto-antibody occurrence at other geographical regions. It is also possible that the threshold for positivity used in this study (to make possible the SYK studies) excluded some weakly positive sera.
The current study also suggests that the frequency of functionally active auto-antibodies in the CSU population was lower than anticipated. Instead of a frequency of ≈50%, this study suggests a frequency of 7%. Although this is a far lower frequency than expected, the few sera found pass a stringent set of requirements and clearly possess functional auto-antibodies to either IgE or FceRI. With these sera in hand, it was possible to demonstrate that they can act to induce a reduction in SYK expression in both mature and developing basophils in CD34+ cultures. As found previously, an interesting aspect of the reduction in the CD34 cultures was the loss of SYK expression while retaining FceRI expression and the granularity (alcian blue positivity/histamine content) of the maturing cell [15]. In addition, in neither peripheral blood basophils or CD34-derived basophil cultures did blockade of CD32b modify the ability of the antibodies to induce the loss of SYK. This was the behavior expected from previous studies of the signaling requirements for CD32b-mediated inhibition [8] but these results served to strengthen the hypothesis that an auto-antibody could induce a change in SYK expression without interference from Fc-region binding to CD32b. However, the prediction might be different for SYK down-regulation vs. histamine release [8] and the observations that CD32b blockade effected neither outcome suggests that interaction with CD32b is minimal regardless of the conditions or outcome metrics of the experiments.
Mast cell SYK is also regulated by IgE-mediated stimulation but past experience with natural tissue mast cells has shown them to be either as sensitive or less sensitive (cell surface density of IgE required for a response) than basophils (ref). Because it wasn’t examined, it is possible that some sera that were classified as negative for inducing an IgE-mediated response on basophils may behave differently with mast cells but there is no expectation for this result.
Anecdotally, for the 5 sera that showed demonstrable functional auto-antibodies to IgE or FceRIalpha, the basophil responses of these same donors were a mixture of releasers and non-releasers. Four of the five of the subjects with serum-induced histamine release classified as having anti-IgE/FceRI antibodies had non-releasing basophils. The remaining 8 (8/13) whose sera failed some of the tests had basophils that were evenly split between releasers and non-releasers. Previous studies [13, 20] have suggested the frequency of non-releasers in the CSU population is 30–40%, so the 80% frequency in the auto-antibody group (n=5) appears high (chi-square against the Baker study [21] yields p=0.054, and against the Rauber study [20], p = 0.04) but compatible with having auto-antibodies to IgE/FceRI. A prospective study would be needed to determine if the titer of auto-antibodies validated with this study’s algorithm would predict the releasing phenotype of the subject’s basophils.
It was clear that there are technical issues when serum from one subject is used to stimulate leukocytes from another subject. Although there was some concern regarding ABO antibodies, the preliminary studies suggested this was not a problem. However, it was also clear that there are un-identified incompatibilities between sera and non-self basophils (see the ABO compatibility section of the methods in the online repository). This problem was least apparent when using sera from non-CSU subjects unless the basophils were first cultured overnight with IL-3. Under these conditions basophils became responsive to all sera and histamine release induced by the sera was insensitive to inhibition with a BTK inhibitor. This phenomenon was not explored further but suggests that shifting basophil phenotypes (e.g, generated by IL-3 exposure) can complicate studies using sera. The problem was more apparent when using sera from CSU subjects. The frequency of any response with sera from the two CSU groups was approximately 41%. This is very similar to findings from other studies. However, after testing for repeatability and IgE/FceRI-mediated characteristics, the frequency dropped significantly to 7%. There were a variety of failure points, from non-repeating results to not being inhibited by signal transduction inhibitors that should alter IgE/FceRI signaling. The most puzzling result were 4 sera that passed two of the tests for IgE/FceRI-mediated characteristics but failed the non-releaser test. Because there was no further study of these unusual sera, there is no conclusion about the mechanisms that underlay the result. But on a practical level, it appears that there are multiple ways that sera can activate basophils that overlap with IgE/FceRI-mediated release but not necessarily be activation through IgE/FceRI.
Some of the sera that showed demonstrable auto-antibodies were re-examined using different blood draw dates from the same patients. This was only briefly explored; in 3 instances where additional serum samples were obtained 1 year apart or more from the tested serum, the positive results did not repeat. In one instance, a recent patient, serum samples spaced by only 2 months were very similar in characteristics. The sampling is too limited to relate these results to clinical status but the variability has practical implications as well as highlighting the ephemeral nature of these auto-antibodies.
In summary, one important conclusion of these studies is that there is no evidence for functional auto-antibodies with specificity for IgE or FceRI in non-CSU subjects that could modify SYK expression levels in their circulating basophils. Since SYK expression is highly variable in all subjects, it appears that functional auto-antibodies provide a poor explanation for this variability. A second conclusion is that functional auto-antibodies can be found in sera of subjects with CSU although if functionality on a FceRI-bearing cell type is the behavior of interest, the frequency appears to be below 10%. But these antibodies do have the capability of modifying SYK expression in a developing basophil and they don’t appear to interact with FcgRIIb/CD32b.
Supplementary Material
Key Messages.
Auto-antibodies to either IgE or FceRIalpha can induce down-regulation of SYK in either mature or maturing human basophils.
The frequency of functional auto-antibodies in subjects without chronic spontaneous urticaria is near zero and in subjects with chronic spontaneous urticaria is only 7%. Therefore, auto-antibodies to IgE or FceRIalpha do not explain the wide variation of SYK expression in the general population.
Acknowledgments
Funding: NIH grant AI100952 and AI116658
Portions of this study were made possible by generous access to serum from patients with chronic spontaneous urticaria collected by the laboratory of Dr. Sarbjit Saini and Ms. Kristin Chichester. I thank Valerie Alexander for her excellent technical assistance. This work was support by NIH grant AI100952 and AI116658.
Footnotes
Disclosures: Dr MacGlashan discloses short-term consultancy for Sixal, Inc. and Boehringer-Ingelheim
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vilarino N, MacGlashan D., Jr Transient transfection of human peripheral blood basophils. J Immunol Methods. 2005;296:11–8. doi: 10.1016/j.jim.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.MacGlashan DW., Jr Relationship Between Syk and SHIP Expression and Secretion from Human Basophils in the General Population. J Allergy Clin Immunol. 2007;119:626–633. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder JT, Bieneman AP, Chichester KL, Keet CA, Hamilton RG, MacGlashan DW, Jr, Wood R, Frischmeyer-Guerrerio PA. Spontaneous basophil responses in food-allergic children are transferable by plasma and are IgE-dependent. J Allergy Clin Immunol. 2013;132:1428–31. doi: 10.1016/j.jaci.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puan KJ, Andiappan AK, Lee B, Kumar D, Lai TS, Yeo G, Bercin D, Starke M, Haase D, Lum J, Chew FT, Connolly J, Wong SC, Zolezzi F, Poidinger M, Wang Y, Rotzschke O. Systematic characterization of basophil anergy. Allergy. 2017;72:373–384. doi: 10.1111/all.12952. [DOI] [PubMed] [Google Scholar]
- 5.MacGlashan DW, Jr, Savage JH, Wood RA, Saini SS. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol. 2012;130:1130–1135 e5. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacGlashan DW, Jr, Saini SS. Syk expression and IgE-mediated histamine release in basophils as biomarkers for predicting the clinical efficacy of omalizumab. J Allergy Clin Immunol. 2017;139:1680–1682 e10. doi: 10.1016/j.jaci.2016.12.965. [DOI] [PubMed] [Google Scholar]
- 7.MacGlashan D, Miura K. Loss of syk kinase during IgE-mediated stimulation of human basophils. J Allergy Clin Immunol. 2004;114:1317–24. doi: 10.1016/j.jaci.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Macglashan D, Jr, Moore G, Muchhal U. Regulation of IgE-mediated signalling in human basophils by CD32b and its role in Syk down-regulation: basic mechanisms in allergic disease. Clin Exp Allergy. 2014;44:713–23. doi: 10.1111/cea.12155. [DOI] [PubMed] [Google Scholar]
- 9.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 10.Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G, Woisetschlager M, Kinet JP, Stingl G. Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2606–12. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi Y, Kaplan AP. Mechanisms of autoimmune activation of basophils in chronic urticaria. J Allergy Clin Immunol. 2001;107:1056–62. doi: 10.1067/mai.2001.115484. [DOI] [PubMed] [Google Scholar]
- 12.Soundararajan S, Kikuchi Y, Joseph K, Kaplan AP. Functional assessment of pathogenic IgG subclasses in chronic autoimmune urticaria. J Allergy Clin Immunol. 2005;115:815–21. doi: 10.1016/j.jaci.2004.12.1120. [DOI] [PubMed] [Google Scholar]
- 13.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil Phenotypes in Chronic Idiopathic Urticaria in Relation to Disease Activity and Autoantibodies. J Invest Dermatol. 2008;128:1956–1963. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 14.MacGlashan D, Jr, Hamilton RG. Parameters determining the efficacy of CD32 to inhibit activation of FcepsilonRI in human basophils. J Allergy Clin Immunol. 2016;137:1256–8. e1–11. doi: 10.1016/j.jaci.2015.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishmael SS, MacGlashan DW., Jr Syk expression in peripheral blood leukocytes, CD34+ progenitors, and CD34-derived basophils. J Leukoc Biol. 2010;87:291–300. doi: 10.1189/jlb.0509336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan YC, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, Fang C, Dodev TS, Wu SY, Ying S, Corrigan CJ, Gould HJ. “Auto-anti-IgE”: naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol. 2014;134:1394–1401 e4. doi: 10.1016/j.jaci.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenstein LM, Kagey-Sobotka A, White JM, Hamilton RG. Anti-human IgG causes basophil histamine release by acting on IgG-IgE complexes bound to IgE receptors. J Immunol. 1992;148:3929–3936. [PubMed] [Google Scholar]
- 18.Kepley CL, Cambier JC, Morel PA, Lujan D, Ortega E, Wilson BS, Oliver JM. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000;106:337–48. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 19.Cassard L, Jonsson F, Arnaud S, Daeron M. Fcgamma receptors inhibit mouse and human basophil activation. J Immunol. 2012;189:2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- 20.Rauber MM, Pickert J, Holiangu L, Mobs C, Pfutzner W. Functional and phenotypic analysis of basophils allows determining distinct subtypes in patients with chronic urticaria. Allergy. 2017;72:1904–1911. doi: 10.1111/all.13215. [DOI] [PubMed] [Google Scholar]
- 21.Baker R, Vasagar K, Ohameje N, Gober L, Chen SC, Sterba PM, Saini SS. Basophil histamine release activity and disease severity in chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2008;100:244–9. doi: 10.1016/S1081-1206(10)60449-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.