Abstract
OBJECTIVE:
Autism spectrum disorder (ASD) is diagnosed more often in boys than in girls; however, little is known about the nature of this sex/gender discrepancy or how it relates to diagnostic assessment practices. The present study examines the performance of the Social Communication Questionnaire (SCQ) in screening for ASD among boys and girls.
METHODS:
Data were drawn from the SUCCESS study, a population-based study of ASD prevalence among children 8-10 years of age. Analyses were conducted using SCQ data from 3520 children, with direct assessment data from 272 with elevated SCQ scores.
RESULTS:
A bifactor model based on DSM-5’s two ASD symptom domains fit the data well and performed slightly better for girls. In the general population sample, girls exhibited fewer social communication/interaction and restricted-repetitive behavior symptoms than boys. In the direct assessment sample, however, girls with ASD showed greater impairment in social communication/interaction than boys with ASD. Items pertaining to social communication/interaction problems at ages 4-5 were among the most diagnostically efficient overall and particularly for girls. Similarly, ROC analyses suggested that the SCQ performs adequately among boys and well among girls.
CONCLUSIONS:
Results support the use of the SCQ in screening for ASD but do not indicate sex/gender-specific cutoffs. Girls with ASD may exhibit pronounced intra-individual deficits in social communication/interaction compared to male peers with ASD and female peers without ASD. Although more research is needed, careful attention to social communication/interaction deficits around 4-5 years of age may be especially useful for assessing ASD in girls.
Keywords: Autism spectrum disorder, sex/gender differences, evidence-based assessment, screening and diagnosis, social development
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder defined by pervasive deficits in social communication and interaction and patterns of restricted, repetitive, stereotyped behaviors and interests (American Psychiatric Association [APA], 2013). Beyond these core criteria, however, there is considerable heterogeneity in the symptom presentations exhibited by children with ASD, including a variety of qualitative and quantitative differences (e.g., severity, language, cognitive ability, co-occurring problems). One critical factor in understanding ASD symptom variability is the role of sex/gender1 (e.g., Goldman, 2013; Lai et al., 2015).
Sex/gender differences in ASD are both over- and under-acknowledged. On the one hand, it has been known for decades that ASD is more common in boys than girls. Estimates suggest that the true sex/gender ratio is about 3.3 to 1 (based on higher-quality and population-screening studies; (Loomes, Hull, & Mandy, 2017) while the ratio among those clinically diagnosed is about 4.5 to 1 (based on administrative records; Christensen et al., 2016). On the other hand, little is known about the nature of sex/gender differences in symptom presentations among children with ASD. To the extent that ASD presents differently in boys versus girls, it is possible that girls with ASD are under-identified. Indeed, Loomes et al.’s (2017) finding that the magnitude of the sex/gender discrepancy is inversely related to study quality suggests that there is gender-related ascertainment bias in clinical diagnosis. Complicating matters further, the evidence base pertaining to ASD—and, by extension, the diagnostic criteria themselves—historically comes from research among overwhelmingly male samples (e.g., Edwards et al., 2012; Watkins et al., 2014). Thus, while there is clearly a need for more research to better understand sex/gender differences in ASD, it is also important to keep in mind that existing assessment tools and diagnostic criteria may contain sex/gender bias.
The present study seeks to advance the literature on both of these fronts: (a) to elucidate the extent and nature of sex/gender differences in ASD symptom presentations using comprehensive assessment methods and a population-based sample; and (b) to help understand the extent to which sex/gender bias may be operating in screening for ASD using the Social Communication Questionnaire (SCQ; Rutter & Bailey, 2003). To investigate these questions, we analyzed data from a large epidemiological sample of school-age children who were screened and assessed for ASD. Thus, the present analysis offers a unique lens for investigating ASD screening and symptom presentation among boys and girls with and without the diagnosis.
Sex/Gender Differences in ASD
Evidence has been mixed with respect to sex/gender differences in core ASD symptoms. Some studies indicate that boys have greater social and communicative problems compared to girls (e.g., Beggiato et al., 2017; Head et al., 2014; Hiller et al., 2016), while others show the opposite pattern (e.g., Carter et al., 2007; Frazier et al., 2014,a; Hartley & Sikora, 2009), and still others show no particular differences in this domain (e.g., Bölte et al., 2011; Holtmann et al., 2007; Mandy et al., 2012; May et al., 2016; Reinhardt et al., 2015; Szatmari et al., 2012). Similarly, some studies have found boys with ASD to exhibit higher levels of repetitive and stereotyped behaviors than girls (Beggiato et al., 2017; Bölte et al., 2011; Hartley, & Sikora, 2009; May et al., 2016; Szatmari et al., 2012) while others have found no differences in this domain (Carter et al. 2007; Holtmann et al. 2007; Reinhardt et al., 2015). In their meta-analysis, Van Wijngaarden-Cremers et al. (2014) found that among individuals with ASD beyond age 6, males show higher levels of restricted/repetitive behaviors and interests than females; no significant gender differences were found for social interaction or communication (Van Wijngaarden-Cremers et al., 2014). Narrative reviews (e.g., Kirkovski et al., 2013; Lai et al., 2015) have yielded similar conclusions.
In addition, there are clinically important sex/gender differences in ASD that are not related to the core diagnostic symptoms. Compared to boys, girls with ASD more often go undiagnosed or are diagnosed at a later age, particularly girls with less severe ASD symptoms and more intact language and cognitive skills (Beeger et al., 2013; Giarelli et al., 2010; Rutherford et al., 2016). Girls with ASD may also be better able to compensate for symptoms despite having persistent core deficits associated with ASD (Livingston & Happé, 2017), which might contribute to greater social “camouflage” (Hull et al. 2017). For example, some evidence suggests that girls with ASD perform better on measures of non-verbal communication, which may mask their symptoms (Rynkiewicz et al., 2016). Despite this compensation, research examining peer relationships found that boys and girls with ASD exhibit more similarities with each other than with their same-gender, typically developing peers; however girls with ASD appear to face more social, friendship, and language demands than boys with ASD (Dean et al., 2014). More broadly, girls can exhibit patterns of restricted interests and repetitive behaviors and social and communicative problems which might seem more socially acceptable than the patterns seen in boys with ASD (Lai et al., 2015). This could help explain why girls with ASD often have more severe behavioral, emotional, and cognitive problems compared to boys with ASD (e.g., Frazier et al., 2014a; Holtmann et al., 2007; Horiuchi et al., 2014; Stacy et al., 2014), and even compared to girls at risk for ASD who are not ultimately diagnosed (Dworzynski et al., 2012). That is, perhaps girls must exhibit more severe symptoms, impairment, or co-occurring problems to receive a diagnosis of ASD.
One possible explanation for these sex-gender differences is the “extreme male brain theory” of ASD (Baron-Cohen, 2002). After reviewing the evidence for behavioral sex/gender differences, Baron-Cohen concluded that on average, males exhibited weaknesses at empathizing and strengths at systematizing compared to females. Thus, ASD could be a disorder of the extreme male brain, characterized by low levels of empathizing traits (e.g., social-emotional understanding, pragmatic language, friendship development and maintenance) and high levels of systematizing traits (e.g., attention to detail, preference for rule-based systems and facts, preoccupation with cause-and-effect systems, and islets of ability; Baron-Cohen, 2002; Baron-Cohen et al., 2005). This theory has garnered some support by way of between-group behavioral differences (e.g., Stauder et al., 2011; Tan et al., 2015) and evidence linking masculinization and ASD traits to fetal testosterone exposure (e.g., Auyeung et al., 2009; Baron-Cohen et al, 2011). However, this account has also been criticized for being too biologically reductive and neglecting gender socialization processes (e.g., Buchen, 2011; Krahn & Fenton., 2012). Some evidence suggests that ASD is a gender-defiant disorder rather than disorder of masculinization (Bejerot et al., 2012), and other research suggests that normative sex differences in typically developing populations are absent in children with ASD (Park et al., 2012). Further research is needed to clarify these mixed findings.
Relevance to Screening
Much of the research summarized above has focused on children who have already received the diagnosis, sometimes with a non-ASD comparison group. Although such studies provide insight into clinical populations, they do relatively little to improve the assessment of boys and girls whose ASD diagnostic status is unknown. This is a major gap in the literature. Without addressing the nosological and diagnostic challenges pertaining to sex-gender considerations, any research on ASD based on existing assessment practices is subject to the underlying problem of not knowing how ASD should be defined and diagnosed in males compared to females (Lai et al., 2015). Thus, there is a need for rigorous population-based assessment research with attention to sex/gender. It is possible that systematic sex/gender differences could arise at any step in the assessment pipeline—from eliciting concerns about ASD to the results of diagnostic evaluations. Screening measures are particularly key for understanding sex/gender differences in symptom presentation and for addressing any systematic problems related to which children get referred for ASD evaluations. Improved interpretation of screening measures may lead to earlier identification for children in need of services. The SCQ (Rutter & Bailey, 2003) is one of the most widely researched and recommended parent-report screening measures for ASD in youth (Norris & Lecavalier, 2010; Ozonoff et al., 2005). While previous research investigated the general diagnostic utility of SCQ and similar measures for screening for ASD (e.g., ROC and sensitivity/specificity; Barnard-Brak et al., 2015; Chandler et al., 2007; Duvekot et al., 2015; Eaves et al., 2006; Ung et al., 2016), there has been little attention to sex/gender differences. The notable exception is that some authors have found evidence for little to no measurement invariance in the SCQ (Wei et al., 2015) or similar screening measures (Frazier et al., 2014b; Frazier & Hardan, 2017).
While the SCQ demonstrates excellent psychometrics among school-age children (Chesnut et al., 2016; Norris & Lecavalier, 2010), its clinical and research utility is limited by its lack of subscales, yielding only a single total score. In developing the SCQ (Berument et al., 1999; Rutter & Bailey, 2003), the authors pulled items from the three ADI-R domains, offering one possible subscale structure; then they estimated a three-factor exploratory principal components analysis from their clinical sample of 200, offering a different possible structure; neither of these models has been validated for clinical or research purposes. Others (Wei et al., 2015) have subsequently adopted the SCQ’s exploratory model or developed their own (e.g., Gau et al., 2011). However, the most compelling and copious evidence from a variety of ASD measures (e.g., Frazier et al., 2008 2012; Frazier & Hardan, 2017; Mandy et al., 2012) supports the two-domain framework that was codified in DSM-5 (APA, 2013). For this reason, and to optimize the usefulness of our results, we examine a two-domain bifactor model of the SCQ.
The Present Study
In sum, the literature documents a large sex/gender discrepancy in ASD diagnoses and symptoms, with mixed evidence and explanations as to why. The present study investigates the extent and nature of sex/gender differences in ASD symptoms among a large epidemiological sample of school-age children and how these differences affect the SCQ in screening for ASD. Specifically, we examine (a) the prevalence of ASD markers in school-age children, overall and by sex/gender; (b) differences in SCQ results related to sex/gender and ASD diagnostic status, and their interaction; (c) the diagnostic efficiency of the SCQ in screening for ASD in boys and girls; and (d) whether different clinical cutoffs should be considered for boys and girls. Based on previous research, it was hypothesized that, among those with and without ASD diagnoses, boys would show higher ASD symptoms overall and particularly in restricted/ repetitive interests and behaviors. It was expected that this would lead to sex/gender-driven measurement problems, potentially detrimentally affecting the identification of girls’ ASD symptoms. Because diagnostic status was used as our criterion, this study could not examine sex/gender bias in the diagnostic construct, but rather focused on the performance of the SCQ. Results may help advance assessment practices and knowledge related to sex/gender differences in ASD or in the performance of the SCQ.
Methods
Participants
Data were drawn from the South Carolina Children’s Educational Surveillance Study (SUCCESS), a population-based study of ASD prevalence among school-age children. The study design and methodology has been detailed elsewhere (see Carpenter et al., 2016). The present analyses and descriptive statistics are based on all available data for children whose parent provided consent and fully completed the English version of the SCQ (n = 3520). The target population consisted of all children born in 2004 living in a three-county catchment area in coastal South Carolina. Participants were 8 to 10 years of age at the time of the initial screening. Those who were invited for a direct assessment were slightly older by the time their evaluation occurred (M = 10.3 years; SD = 0.5; range: 8.8 to 11.4).
Procedures (detailed below) were designed to obtain as large and representative a sample as possible, and preliminary results suggest a reasonable degree of representativeness was achieved. In the population-screening sample, racial/ethnic backgrounds were as follows (roughly similar to census estimates): 61% Non-Hispanic White, 27% Non-Hispanic Black, 6% Hispanic, 3% other, and 3% multiracial. In the direct assessment sample, racial/ethnic background proportions were as follows: 44% Non-Hispanic White, 37% Non-Hispanic Black, 13% Hispanic, 1% other, and 4% multiracial. Compared to girls, greater proportions of boys fell in the clinical range (SCQ ≥ 15) and in the at-risk range (8 ≥ SCQ < 15; see Table 1) during the screening, rendering them more likely to be eligible for a direct assessment (35% of boys vs. 24% of girls). Of these, boys (29%) were more likely to be invited to and ultimately complete an assessment compared to girls (22%). Thus, the gender ratio shifted from census-estimated 51% male in the population, to 49% male in the screening sample, to 65% male in the direct assessment sample. Sociodemographic variables were not used to adjust the population screening procedures, direct assessment sampling, clinical assessment, or analyses.
Table 1.
Descriptive statistics for SCQ by sex/gender, SCQ risk level, and diagnostic group
| Full Sample | Sex/Gender
Comparisons |
||
|---|---|---|---|
| Boys | Girls | ||
| Population Sample | n = 3520 | n = 1731 | n = 1789 |
| SCQ Total Score | |||
| M | 5.97 | 6.77 | 5.19 |
| M SE | 0.09 | 0.14 | 0.11 |
| SD | 5.41 | 5.91 | 4.76 |
| Median | 4.5 | 5 | 4 |
| Mode | 2 | 3 | 1 |
| Range | 0-36 | 0-36 | 0-34 |
| Skewness | 1.71 | 1.62 | 1.68 |
| Kurtosis | 3.88 | 3.23 | 4.03 |
| SCQ Risk Groups (%) | |||
| At risk (SCQ ≥ 15) | 7.1 | 9.4 | 4.9 |
| Subthreshold (8 ≤ SCQ ≤ 14) | 22.4 | 25.7 | 19.2 |
| Low risk (SCQ ≤ 7) | 70.5 | 64.9 | 75.9 |
| Direct Assessment Sample | n = 272 | n = 177 | n = 95 |
| SCQ Total Scores by ASD Groups | |||
| ASD+, M (SD) | 20.98 (6.86) | 20.45 (7.03) | 24.29 (4.75) |
| ASD−, M (SD) | 13.09 (4.66) | 13.38 (4.92) | 12.65 (4.24) |
| Distribution of ASD Groups (%) | |||
| ASD+ | 18.8 | 24.9 | 7.4 |
| ASD− | 81.3 | 75.1 | 92.6 |
Procedures
All procedures were approved by the researchers’ institutional review board. As described by Carpenter et al. (2016), a multi-phase sampling design was used. Procedures were designed to maximize participation rates from the entire population, including special education students. Extensive efforts were taken to ensure that the sample was as representative as possible, including steps to boost participation among students from ethnic minority and lower socioeconomic backgrounds. Recruitment and sampling procedures were developed based on the literature and in partnership with schools and organizations in the three-county catchment area. Ultimately, 123 of 127 public and private schools agreed to participate. Within a two-month period, families of eligible children received via their school an introductory letter; packet with cover letter, waiver, and SCQ; and up to two reminders. Parents were allowed to complete an online or paper version of the SCQ, or to decline. Incentives for responding were provided for students, parents, and teachers.
After completing the SCQ, a subset of participants was identified and invited for an in-person ASD assessment based on their SCQ scores. Given questions regarding the optimal cutoff value for the SCQ (Eaves et al., 2006; Norris & Lecavalier, 2010), all those in the “at-risk” range (SCQ ≥ 15; 100% invited; 44% completed; n = 112) and a randomly selected portion of those in the “subthreshold” range (8 ≤ SCQ ≤ 14; 69% invited; 20% completed; n = 160) were invited for a direct assessment. This included a separate informed consent and a comprehensive ASD diagnostic assessment (measures described below). These direct assessments were completed by doctoral-level psychologists with appropriate training and expertise in ASD evaluation. Participants’ ASD diagnostic status was determined based upon the integration of all assessment data. Examiners were not blinded to SCQ scores, but neither these nor sex/gender status were considerations for diagnostic decision-making. All cases were reviewed by the team on a weekly basis, with diagnostic ambiguity resolved by consensus. Examiners’ inter-rater reliability was 100% for case status.
From the census-estimated population of 8780 children, a total of 4185 survey responses were recorded, of which 3698 (42%) were usable data.2 The present analyses are based on data with complete responses on the English SCQ (excluding Spanish and partial SCQs), resulting in a final analytic sample of 3520 (40% of population), including 272 who ultimately completed a direct assessment.
Measures
Screening.
The lifetime SCQ was used to screen for ASD in the full sample. The SCQ is a brief, standardized checklist of 40 items pertaining to symptoms of ASD, including problems with communication and reciprocal social interaction, and restricted, repetitive, and stereotyped behaviors (Rutter & Bailey, 2003). All items are in a yes/no format; some ask if the child has ever exhibited the behavior, while others focus specifically on the time period of 4-5 years of age when symptoms of ASD may become more apparent. Items assess both atypical and typical behaviors, the latter being reverse-coded. Possible scores range from 0 to 39, with higher scores indicating greater likelihood of ASD. To minimize the possibility of parents recognizing questions as pertaining to ASD symptoms, the project was promoted as a study of child social development and the SCQ was licensed by the publisher to be presented as a “SUCCESS Questionnaire;” no changes were made to the SCQ instructions or items. The English Lifetime SCQ has demonstrated ample evidence of validity and reliability, including good specificity and sensitivity (Chandler et al., 2007; Chesnut et al., 2016). The SCQ had good internal consistency in the present study (Cronbach’s α = .82). As noted above, the SCQ does not have validated subscales. For the present analyses, three of the co-authors (two doctoral-level psychologists and one pre-doctoral psychology intern) with expertise in ASD evaluation divided the SCQ items into two subdomains mapping onto DSM-5 ASD criteria: (a) social communication and interaction (SCI) deficits (25 items; e.g., spontaneously used gestures, smiles back, talks to be friendly) and (b) restricted and repetitive behavior (RRB; 12 items, e.g., unusual special interests, odd mannerisms, repetitive language). Two items pertaining to self-injurious behavior (SCQ #17) and solitary make-believe play (SCQ #35), which are included in the total score, were not included in subdomain scores because there was no direct correspondence with DSM-5. This bifactor model was tested and supported via confirmatory factor analysis (see results).
Direct assessment.
Consistent with recommendations (e.g., Ozonoff et al., 2005), multiple instruments and methods were used in the diagnostic evaluation. First, a structured ASD diagnostic interview was administered to a primary caregiver. This interview was developed for the SUCCESS study to assess current and lifetime symptoms of ASD using an integrated set of criteria that is compatible with DSM-IV and DSM-5. Second, the Autism Diagnostic Observation Schedule, 2nd ed. (ADOS-2; Lord, Luyster, Gotham, & Guthrie, 2012) was administered. The ADOS-2 is a semi-structured, standardized test, commonly considered a “gold standard” instrument in ASD assessment. The ADOS-2 facilitates direct observation of ASD-related behaviors across several developmentally appropriate tasks and items, yielding a total score representing the likelihood of ASD and the severity of symptoms. The ADOS-2 and its predecessors have substantial evidence for validity, reliability, and utility in assessing ASD (Gotham et al., 2008; Lord et al., 2000, 2012; Molloy et al., 2011). Standard ADOS-2 procedures were followed, with modules determined by the child’s expressive language abilities (96% were Module 3). Finally, a variety of additional measures were administered assessing broadband (e.g., CBCL/TRF) and narrowband (e.g., Social Responsiveness Scale, 2nd ed.) symptoms, adaptive (Vineland-2) and cognitive functioning (e.g., Kaufman Brief Intelligence Test, 2nd ed.), language (Children’s Communication Checklist, 2nd ed.), medical and educational history, and demographics (see Carpenter et al., 2016). DSM-5 ASD diagnoses were determined using clinical best-estimate procedures incorporating all available data, with primary consideration to the diagnostic interview and ADOS-2 results (Carpenter et al., 2016).
Analytic Plan
Descriptive statistics of SCQ scores and items were inspected overall and by sex/gender and diagnostic subgroups. Group differences in SCQ scores and item endorsements were estimated using t-tests (Cohen’s d effect sizes), ANOVAs (partial η2), and chi-square tests (Cramers’ V). Hypothesized SCQ factor structures were assessed via confirmatory factor analysis (CFA), with model fit evaluated through collective consideration of the Root Mean Square Error of Approximation (RMSEA), and Confirmatory Fit Index (CFI) and Tucker Lewis Fit Index (TLI). Following recent recommendations (Kline, 2016; Little, 2013), fit indices were interpreted collectively as continuous measures with approximate thresholds (rather than strict cutoffs) for adequate model fit as follows: CFI/TLI ≥ .90 and RMSEA ≤ .08. CFAs were estimated in Mplus Version 7 (Muthén & Muthén, 2012) using weighted least squares (WLSMV). All other analyses were conducted in SPSS Version 24 (IBM, 2016).
The diagnostic utility of SCQ scores and items were examined through receiver operating characteristic (ROC) analyses and diagnostic efficiency statistics as follows: sensitivity (proportion of those with ASD with positive test result3out of all those with positive result), specificity (proportion of those without ASD with negative test result out of all those with negative result), positive predictive value (PPV; likelihood of ASD diagnosis given a positive test result), negative predictive values (NPV; likelihood of no ASD diagnosis given negative test result), and diagnostic likelihood ratios (DLRs; calculated as [sensitivity] / [1-specificity]). Clinically, DLR values represent the most concise estimate of diagnostic probability. DLRs around 1 indicate no change in the probability of the diagnosis, whereas higher DLRs represent increases in the probability of the diagnosis (e.g., DLRs 2, 5, 10 correspond to 15%, 30%, and 45% increases, respectively), and DLRs below 1 represent decreasing probability. These estimates should be interpreted according to the pre-test and post-test probabilities of the population being considered (e.g., the probability of ASD diagnosis in a clinical setting vs. in the general population). Finally, ROC analyses were also utilized to consider diagnostic efficiency of the SCQ among boys and girls. Complex sampling weights were considered but were not used because they had little influence on other analyses, suggesting that the data are sufficiently representative for non-epidemiological analyses.
Results
Confirmatory Factor Analysis
The single-factor CFA model (i.e., original SCQ scoring, with all 39 items loading onto a single construct) fit the data poorly, χ2 (df = 702) = 17064.53, p < .001, RMSEA = 0.081 (90% CI: 0.080, 0.082), CFI = 0.711, TLI = .695. By contrast, a bifactor model (i.e., with the single-factor plus subdomain factors of SCI and RRB) showed acceptable fit, χ2 (df = 665) = 6056.07, p < .001, RMSEA = 0.048 (90% CI: 0.047, 0.049), CFI = 0.905, TLI = 0.894. The WLSMV-adjusted Δ χ2 test was significant, Δ χ2 (df = 37) = 4305.26, p < .001, confirming that the bifactor model fit the data better than the single-factor model, and supporting the use of the two subdomain scores and the total score in subsequent analyses. Factorial invariance by sex/gender could not be examined due to non-convergence of multiple-group models. Thus, the bifactor model was estimated separately by sex/gender, showing adequate fit for both boys (χ2 (df = 665) = 3442.52, p < .001, RMSEA = 0.049 (90% CI: 0.048, 0.051), CFI = 0.905, TLI = 0.894) and girls (χ2 (df = 665) = 2595.03, p < .001, RMSEA = 0.040 (90% CI: 0.039, 0.042), CFI = 0.924, TLI = 0.916). This model appears to show better fit for girls than for boys (e.g., non-overlapping RMSEA CIs).
SCQ Results: Population Screening Sample
Descriptive statistics for SCQ scores are presented in Table 1 and item endorsement frequencies presented in Figure 1. As shown, markers of ASD symptoms were commonly endorsed in the general population. Over 50% of boys and girls had 4 or more SCQ items endorsed, and about a quarter of the items were endorsed by at least 20% of the sample. Boys had higher SCQ scores than girls, t(3518) = 8.77, p < .001, Cohen’s d = 0.29, and greater variability in the distribution of scores. A similar pattern was observed for the SCQ risk subgroups; 35.1% of boys fell in the elevated ranges (“at-risk” or “subthreshold”), compared to only 24.1% of girls, χ2 (2, N = 3520) = 56.48, p < .001, Cramer’s V = 0.13. Boys also had higher scores than girls for both the RRB domain t(3518) = 24.75, p < .001 (boys M = 2.52, SD = 2.83; girls M = 2.00, SD = 2.58, Cohen’s d = 0.19) and for the SCI domain t(3518) = 57.23, p < .001 (boys M = 3.97, SD = 4.01; girls M = 3.04, SD = 3.20, Cohen’s d = 0.26).
Figure 1.
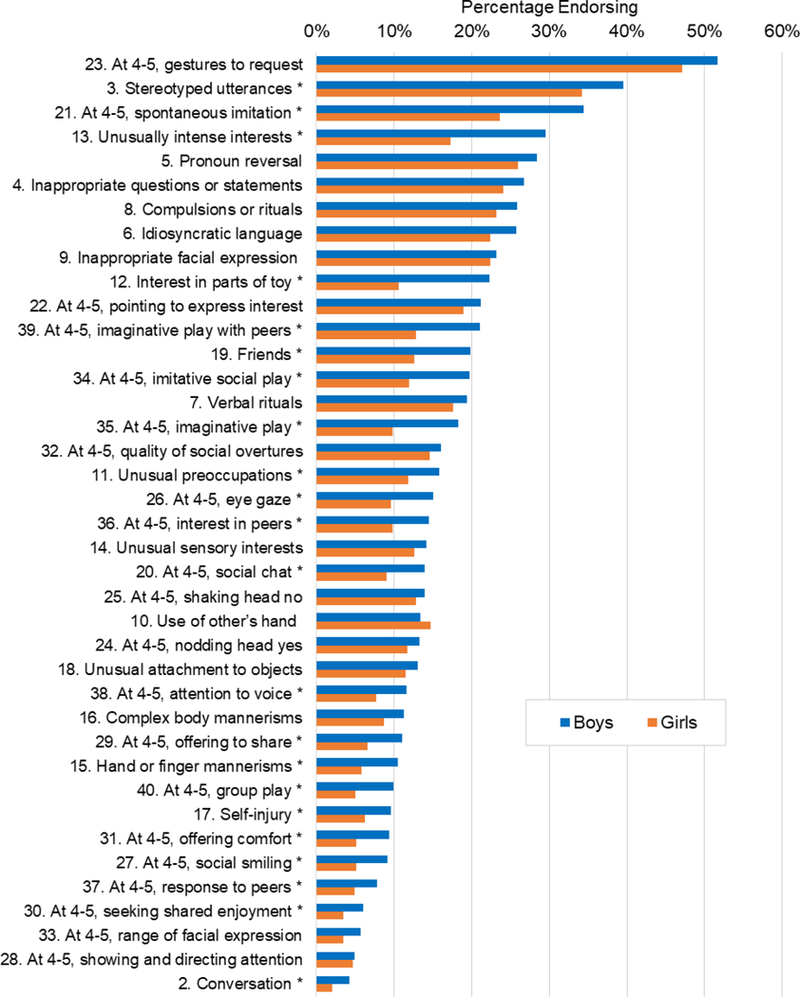
Prevalence of ASD markers in school-age children by sex/gender in the general population. *Significant difference between boys and girls after family-wise Bonferoni adjustment (ps < .0013).
Figure 1 presents the frequency of item endorsement by sex/gender. The most frequently endorsed items overall included not using gestures at 4-5, using odd or repetitive speech, not spontaneously copying others at 4-5, getting pronouns mixed up, and making socially inappropriate questions or statements. The least frequently endorsed items included not responding positively to other children at 4-5, not showing things to parents at 4-5, not wanting parents to join in her/his enjoyment 4-5, showing limited range of facial expressions at 4-5, and not able to have a to and fro conversation. After adjusting for multiple comparisons (family-wise Bonferroni approach; all ps < .0013), about half of the items showed significantly pronounced gender differences; in all cases, boys were rated as having higher symptom counts than girls. As shown in Figure 1, items with the greatest sex/gender differences include interest in parts of toys, unusually intense interests, not having a best friend, odd mannerisms such as hand flapping, odd/repetitive language, and a variety of social deficits at 4-5 years of age (e.g., make believe/imaginative games, spontaneously copying/joining others).
SCQ Results: Direct Assessment Sample
Among those who completed an in-person diagnostic evaluation, there were significant differences in the frequencies with which boys and girls were diagnosed with ASD, χ2 (1, N = 272) =12.41, p < .001, Cramer’s V = 0.21, with one-quarter (24.9%) of boys assessed receiving the diagnosis compared to only 7.4% of girls assessed. A 2×2 ANOVA revealed a significant difference in SCQ scores between those with and without a diagnosis of ASD, F(1, 268) = 72.46, p < .001, partial η2 = .213. Although there was no main effect for sex/gender (p = .160) after ASD diagnosis was included in the model, there was a significant interaction between sex/gender and diagnostic status, F(1, 268) = 4.32, p = .039, partial η2 = .016. On average, girls diagnosed with ASD had SCQ scores approximately four points higher than boys diagnosed with ASD, whereas boys and girls without the diagnosis did not differ in their SCQ scores. (See Table 1 for all descriptive statistics concerning total SCQ scores.)
Next, ANOVAs were re-estimated for the RRB and SCI symptom domains. In the RRB model, there was only a main effect for ASD diagnosis in the expected direction, F(1, 268) = 6.22, p = .013, partial η2 = .023, with diagnosed children showing higher levels of RRB (M = 7.18, SD = 3.17) compared to non-diagnosed children (M = 5.17, SD = 3.11), with no main effect or interaction for sex/gender (ps > .4). In the SCI model, however, there was a significant interaction between diagnostic status and gender, F(1, 268) = 7.67, p = .006, partial η2 = .028, such that girls with ASD had SCI scores that were over four points higher (M = 16.71, SD = 3.90) than boys with ASD (M = 12.23, SD = 5.41), whereas the pattern ran in the opposite direction for non-diagnosed girls (M = 6.93, SD = 4.17) and boys (M = 7.74, SD = 4.31). There was also a significant main effect for diagnostic status, F(1, 268) = 55.57, p < .001, partial η2 = .172, with those with ASD showing greater SCI scores than those without. There was a marginal main effect for gender, F(1, 268) = 3.69, p = .056, partial η2 = .014, with boys showing slightly higher levels of SCI overall (M = 8.86, SD = 4.99) compared to girls (M = 7.65, SD = 4.86).
Diagnostic Efficiency and Clinical Cutoffs
The sensitivity, specificity, PPVs, NPVs, and DLRs are present overall and by sex/gender at the item level in Table 2, and for the total SCQ scores (various cutoffs) in Table 3. At the item level, DLRs were relatively higher (>2) for items related to spontaneous showing, sharing, initiation of joint attention, shared enjoyment, cooperative, imaginative, and spontaneous play, positive social response, not smiling back, interest in same-age peers, comforting parents, limited range of facial expressions, and odd mannerisms such as hand flapping. Regarding sex/gender differences, DLRs were higher for girls compared to boys on items relating to seeking shared enjoyment, pointing, nodding and shaking yes and no, sharing and showing, make believe games, talking to be friendly, having a to and fro conversation, cooperative games with children not looking when parent spoke, little interest in same-age peers, and not spontaneously copying others. Only a few items showed greater DLRs for boys compared to girls, including odd mannerisms such as hand-flapping, and whole-body movements (e.g., spinning, bouncing). Notably, for both boys and girls the most diagnostically efficient items were those pertaining to social-communication/interaction behaviors at 4-5 years of age.
Table 2.
Diagnostic efficiency of SCQ items for identifying ASD in boys and girls sorted by full sample DLR (highest to lowest)
| Item | Symptom Domain |
Full Sample |
Boys |
Girls |
DLR Diff |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | DLR | Sens | Spec | PPV | NPV | DLR | Sens | Spec | PPV | NPV | DLR | ||||
| 33 | At 4-5, range of facial expression | SCI | .45 | .85 | .40 | .87 | 2.93 | .45 | .86 | .51 | .83 | 3.18 | .43 | .83 | .17 | .95 | 2.51 | 0.67 |
| 40 | At 4-5, group play | SCI | .76 | .73 | .39 | .93 | 2.82 | .73 | .72 | .46 | .89 | 2.61 | 1.00 | .74 | .23 | 1.00 | 3.83 | −1.22 |
| 37 | At 4-5, response to peers | SCI | .59 | .79 | .39 | .89 | 2.77 | .59 | .80 | .50 | .86 | 3.02 | .57 | .76 | .16 | .96 | 2.39 | 0.63 |
| 30 | At 4-5, seeking shared enjoyment | SCI | .41 | .85 | .39 | .86 | 2.76 | .36 | .83 | .41 | .80 | 2.10 | .71 | .89 | .33 | .98 | 6.29 | −4.19 |
| 31 | At 4-5, offering comfort | SCI | .57 | .78 | .37 | .89 | 2.56 | .55 | .78 | .45 | .84 | 2.50 | .71 | .77 | .20 | .97 | 3.14 | −0.64 |
| 27 | At 4-5, social smiling | SCI | .53 | .78 | .36 | .88 | 2.39 | .52 | .76 | .42 | .83 | 2.17 | .57 | .81 | .19 | .96 | 2.96 | −0.79 |
| 15 | Hand or finger mannerisms | RRB | .55 | .76 | .35 | .88 | 2.29 | .57 | .77 | .45 | .84 | 2.44 | .43 | .75 | .12 | .94 | 1.71 | 0.73 |
| 29 | At 4-5, offering to share | SCI | .61 | .73 | .34 | .89 | 2.24 | .57 | .71 | .39 | .83 | 1.94 | .86 | .76 | .22 | .99 | 3.59 | −1.65 |
| 36 | At 4-5, interest in peers | SCI | .69 | .67 | .33 | .90 | 2.11 | .66 | .65 | .38 | .85 | 1.87 | .86 | .72 | .19 | .98 | 3.02 | −1.15 |
| 34 | At 4-5, imitative social play | SCI | .75 | .64 | .32 | .92 | 2.06 | .75 | .58 | .37 | .88 | 1.78 | .71 | .73 | .17 | .97 | 2.62 | −0.84 |
| 28 | At 4-5, showing and directing attention | SCI | .35 | .82 | .32 | .85 | 2.00 | .30 | .84 | .38 | .78 | 1.87 | .71 | .80 | .22 | .97 | 3.49 | −1.62 |
| 20 | At 4-5, social chat | SCI | .57 | .71 | .32 | .88 | 1.99 | .50 | .72 | .37 | .81 | 1.80 | 1.00 | .70 | .21 | 1.00 | 3.38 | −1.58 |
| 26 | At 4-5, eye gaze | SCI | .75 | .62 | .31 | .91 | 1.98 | .73 | .62 | .39 | .87 | 1.90 | .86 | .64 | .16 | .98 | 2.36 | −0.46 |
| 35 | At 4-5, imaginative play | -- | .63 | .68 | .31 | .89 | 1.98 | .59 | .65 | .36 | .83 | 1.67 | .86 | .74 | .21 | .98 | 3.28 | −1.61 |
| 38 | At 4-5, attention to voice | SCI | .57 | .70 | .31 | .88 | 1.90 | .52 | .70 | .37 | .82 | 1.74 | .86 | .70 | .19 | .98 | 2.90 | −1.16 |
| 17 | Self-injury | -- | .33 | .82 | .30 | .84 | 1.84 | .34 | .81 | .38 | .79 | 1.81 | .29 | .83 | .12 | .94 | 1.68 | 0.13 |
| 16 | Complex body mannerisms | RRB | .49 | .72 | .29 | .86 | 1.78 | .52 | .74 | .40 | .83 | 2.04 | .29 | .69 | .07 | .92 | .93 | 1.11 |
| 14 | Unusual sensory interests | RRB | .61 | .66 | .29 | .88 | 1.77 | .59 | .67 | .37 | .83 | 1.79 | .71 | .64 | .14 | .97 | 1.96 | −0.17 |
| 39 | At 4-5, imaginative play with peers | SCI | .73 | .59 | .29 | .90 | 1.76 | .70 | .55 | .34 | .85 | 1.56 | .86 | .65 | .16 | .98 | 2.43 | −0.87 |
| 18 | Unusual attachment to objects | RRB | .45 | .74 | .28 | .85 | 1.72 | .41 | .77 | .38 | .80 | 1.81 | .71 | .68 | .15 | .97 | 2.24 | −0.43 |
| 24 | At 4-5, nodding head yes | SCI | .41 | .75 | .28 | .85 | 1.65 | .36 | .74 | .31 | .78 | 1.38 | .71 | .77 | .20 | .97 | 3.14 | −1.76 |
| 11 | Unusual preoccupations | RRB | .69 | .58 | .27 | .89 | 1.63 | .68 | .56 | .34 | .84 | 1.54 | .71 | .61 | .13 | .96 | 1.85 | −0.31 |
| 13 | Unusually intense interests | RRB | .82 | .48 | .27 | .92 | 1.57 | .86 | .44 | .34 | .91 | 1.55 | .57 | .52 | .09 | .94 | 1.20 | 0.35 |
| 21 | At 4-5, spontaneous imitation | SCI | .59 | .62 | .26 | .87 | 1.53 | .57 | .56 | .30 | .80 | 1.30 | .71 | .69 | .16 | .97 | 2.33 | −1.03 |
| 19 | Friends | SCI | .43 | .71 | .26 | .84 | 1.49 | .41 | .69 | .31 | .78 | 1.33 | .57 | .74 | .15 | .96 | 2.19 | −0.86 |
| 10 | Use of other’s hand | SCI | .35 | .76 | .25 | .84 | 1.47 | .32 | .77 | .32 | .77 | 1.41 | .57 | .74 | .15 | .96 | 2.19 | −0.78 |
| 8 | Compulsions or rituals | RRB | .76 | .47 | .25 | .90 | 1.44 | .80 | .46 | .33 | .87 | 1.47 | .57 | .49 | .08 | .93 | 1.12 | 0.35 |
| 22 | At 4-5, pointing to express interest | SCI | .51 | .65 | .25 | .85 | 1.44 | .43 | .62 | .27 | .77 | 1.13 | 1.00 | .69 | .21 | 1.00 | 3.26 | −2.13 |
| 2 | Conversation | SCI | .16 | .89 | .23 | .84 | 1.43 | .15 | .87 | .26 | .77 | 1.18 | .20 | .92 | .13 | .95 | 2.43 | −1.25 |
| 7 | Verbal rituals | RRB | .68 | .52 | .22 | .89 | 1.41 | .67 | .56 | .31 | .85 | 1.52 | .80 | .45 | .08 | .97 | 1.45 | 0.07 |
| 25 | At 4-5, shaking head no | SCI | .39 | .71 | .24 | .84 | 1.38 | .34 | .68 | .26 | .76 | 1.08 | .71 | .76 | .19 | .97 | 2.99 | −1.91 |
| 12 | Interest in parts of toy | RRB | .57 | .58 | .24 | .85 | 1.37 | .61 | .56 | .31 | .81 | 1.38 | .29 | .63 | .06 | .92 | .76 | 0.62 |
| 6 | Idiosyncratic language | RRB | .59 | .53 | .20 | .86 | 1.25 | .56 | .50 | .25 | .79 | 1.13 | .80 | .56 | .10 | .98 | 1.84 | −0.71 |
| 9 | Inappropriate facial expression | SCI | .35 | .71 | .22 | .83 | 1.22 | .36 | .73 | .31 | .78 | 1.34 | .29 | .68 | .07 | .92 | .90 | 0.44 |
| 3 | Stereotyped utterances | RRB | .77 | .33 | .19 | .88 | 1.15 | .77 | .35 | .26 | .83 | 1.18 | .80 | .29 | .06 | .96 | 1.13 | 0.05 |
| 23 | At 4-5, gestures to request | SCI | .63 | .41 | .20 | .83 | 1.06 | .64 | .36 | .25 | .75 | 1.00 | .57 | .48 | .08 | .93 | 1.09 | −0.09 |
| 4 | Inappropriate questions or statements | SCI | .59 | .40 | .17 | .83 | .99 | .59 | .43 | .24 | .78 | 1.04 | .60 | .36 | .05 | .94 | .94 | 0.10 |
| 5 | Pronoun reversal | RRB | .55 | .41 | .16 | .82 | .93 | .54 | .48 | .24 | .78 | 1.03 | .60 | .32 | .05 | .93 | .88 | 0.15 |
| 32 | At 4-5, quality of social overtures | SCI | .22 | .69 | .14 | .79 | .69 | .20 | .67 | .17 | .72 | .62 | .29 | .72 | .07 | .93 | 1.01 | −0.39 |
Note. Sens = sensitivity, spec = specificity, PPV = positive predictive value, NPV = negative predictive value, DLR = diagnostic likelihood ratio.
Table 3.
Diagnostic efficiency estimates at varying SCQ total score cutoff values
| SCQ Cutoff Value |
Full
Sample |
Sex/Gender
Comparisons |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys |
Girls |
|||||||||||||||
| Sens | Spec | PPV | NPV | DLR | Sens | Spec | PPV | NPV | DLR | Sens | Spec | PPV | NPV | DLR | ||
| 9 | .98 | .14 | .21 | .97 | 1.13 | .98 | .09 | .26 | .92 | 1.07 | 1.00 | .21 | .09 | 1.00 | 1.26 | |
| 10 | .96 | .26 | .23 | .97 | 1.30 | .96 | .24 | .29 | .94 | 1.26 | 1.00 | .30 | .10 | 1.00 | 1.42 | |
| 11 | .92 | .38 | .26 | .95 | 1.49 | .91 | .37 | .32 | .92 | 1.44 | 1.00 | .40 | .12 | 1.00 | 1.66 | |
| 12 | .84 | .48 | .27 | .93 | 1.62 | .82 | .46 | .33 | .88 | 1.51 | 1.00 | .51 | .14 | 1.00 | 2.04 | |
| 13 | .80 | .54 | .29 | .92 | 1.74 | .77 | .52 | .35 | .87 | 1.61 | 1.00 | .57 | .16 | 1.00 | 2.31 | |
| 14 | .80 | .61 | .32 | .93 | 2.07 | .77 | .61 | .40 | .89 | 1.98 | 1.00 | .61 | .17 | 1.00 | 2.59 | |
| 15 | .80 | .68 | .37 | .94 | 2.50 | .77 | .66 | .43 | .90 | 2.29 | 1.00 | .71 | .21 | 1.00 | 3.39 | |
| 16 | .76 | .73 | .39 | .93 | 2.82 | .73 | .72 | .46 | .89 | 2.62 | 1.00 | .74 | .23 | 1.00 | 3.83 | |
| 17 | .75 | .78 | .44 | .93 | 3.43 | .71 | .77 | .51 | .89 | 3.12 | 1.00 | .80 | .28 | 1.00 | 4.88 | |
| 18 | .73 | .81 | .46 | .93 | 3.73 | .68 | .80 | .53 | .88 | 3.36 | 1.00 | .82 | .30 | 1.00 | 5.49 | |
| 19 | .67 | .87 | .55 | .92 | 5.26 | .61 | .86 | .59 | .87 | 4.29 | 1.00 | .90 | .44 | 1.00 | 9.80 | |
| 20 | .61 | .91 | .61 | .91 | 6.72 | .57 | .90 | .66 | .86 | 5.81 | .86 | .92 | .46 | .99 | 1.78 | |
| 21 | .57 | .94 | .67 | .90 | 8.98 | .52 | .92 | .70 | .85 | 6.95 | .86 | .95 | .60 | .99 | 18.86 | |
Note. Estimates for the standard SCQ cutoff value are in bold. Sens = sensitivity, spec = specificity, PPV = positive predictive value, NPV = negative predictive value, DLR = diagnostic likelihood ratio.
As shown in Table 3, at the existing clinical cutoff of 15, the SCQ demonstrated good sensitivity and NPV, moderate specificity and DLR, and poor PPV. These lower values were driven by low PPV for both boys and girls (reflecting the high proportion of non-ASD cases above the cutoff) and low specificity particularly for boys (reflecting the high proportion of ASD cases below the cutoff). Similar patterns can be seen when alternative cutoffs are considered, with higher thresholds leading to better specificity, NPVs, and DLRs and poorer sensitivity and PPVs, and vice versa for lower thresholds. Figure 2 presents the ROC curves and the distribution of SCQ scores by sex/gender and diagnostic status. The SCQ showed better sensitivity and specificity for the identification of girls with ASD (AUC = .977). Still, results showed a good AUC for boys (.791) and for the overall sample (.824). While it is clear that the SCQ performed differently in boys and girls in this sample, it is difficult to discern specific cutoffs based on the observed data due to the truncated range and qualitative symptom differences as noted above. Strictly speaking, the optimal tradeoff between sensitivity and specificity (i.e., maximizing the AUC) falls between 15 (boys) and 19 (girls). However, a visual inspection of Figure 2 indicates that using cutoffs this high would have resulted in ten boys (but zero girls) with ASD going unidentified in the directly assessed sample. To the extent that screening measures should prioritize sensitivity, these results do not provide compelling evidence for developing gender-specific cutoffs or changing the overall clinical cutoff
Figure 2.
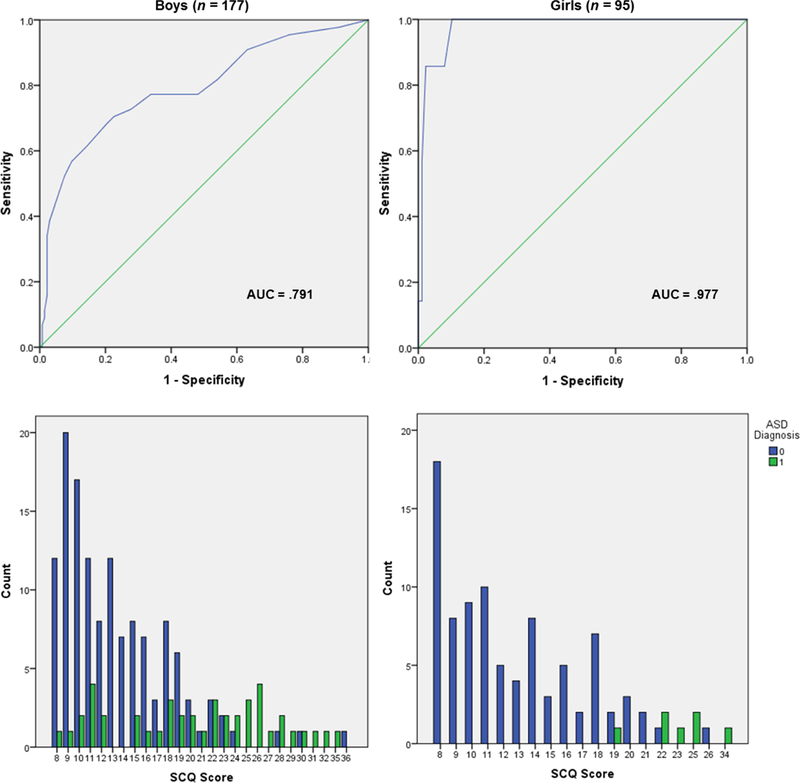
ROC curves and frequency distributions of SCQ scores by sex/gender and diagnosis.
Discussion
This study investigated sex/gender differences in ASD symptoms in a large sample of school-age children assessed for ASD. By applying rigorous assessment methods to a population-based sample, this design helps advance the literature beyond descriptions of sex/gender differences among diagnosed populations, toward useful clinical and research recommendations for diagnostic assessment. Our results coalesce around one interesting conclusion: unlike their typically developing peers, girls with ASD have higher SCQ scores overall, specifically greater social communication problems, compared to boys with ASD. This pattern is only partially consistent with our hypotheses and may help explain other aspects of our results, as discussed below.
In the population sample, boys received higher SCQ scores than girls both overall and in the SCI and RRB domains, a finding which is roughly consistent with prior research (e.g., Van Wijngaarden-Cremers et al., 2014). Among those with ASD, however, girls showed higher SCI scores than boys. This is consistent with the notion that girls may need to exhibit more severe difficulties to receive an ASD diagnosis—a pattern which has been found on a variety of variables in previous studies (e.g., Dworzynski et al., 2012; Frazier et al., 2014a; Holtmann et al., 2007; Horiuchi et al., 2014; Russell et al, 2011; Stacy et al., 2014). Our results suggest that, among those likely to be referred for assessment, there might be little or no sex/gender differences in levels of RRB, whether overall or in terms of an interaction with diagnostic status. Thus, despite the robust sex/gender differences in RRB in the population, these particular behaviors do not appear to be associated with sex/gender differences or differentially contribute to an ASD diagnosis for boys more than girls or vice versa. Overall, these results are in line with Park et al.’s (2012) finding that normative sex/gender differences may be absent in children with ASD.
Item-level analyses offer further insight into these findings, with some of the least frequently endorsed items tending to be most useful for screening. Specifically, items pertaining to children’s spontaneous interaction and socially oriented behaviors at ages 4-5 appear to be among the most diagnostically efficient (showing good sensitivity and specificity) for informing a diagnosis of ASD in all children, and particularly for girls. Persistent SCI deficits appear to be a relatively sensitive and specific marker for differentiating girls with ASD from their typically developing female peers. Interestingly, hallmark RRB features of ASD were generally not among the most diagnostically efficient items; only odd mannerisms such as hand flapping showed good diagnostic efficiency for boys and girls.
The bifactor SCQ model fit the data better than the single factor model, and slightly better for girls than for boys. Although research examining measurement invariance is limited, studies have found similar item functioning and measurement invariance in the SCQ and other screening measures (Frazier & Hardan, 2017; Wei et al., 2015). Direct comparisons of the SCQ and SRS-2 might be particularly useful. Previous research suggests that age is a key factor affecting the performance of ASD symptom scales and screening measures; different patterns of results have been found and other measures might perform better among children roughly 6 years of age and younger (Barnard-Brak et al., 2015; Van Wijngaarden-Cremers et al., 2014). However, in the present sample of 8- to 10-year-old children, results supported the SCQ’s bifactor structure and two-domain conceptualization of ASD put forth in DSM-5 (Frazier et al., 2012; Mandy et al., 2012; APA, 2013). This suggests that there may be utility in further validation and clinical/research use of these two subscales derived from the SCQ. While the SCQ items have previously been subdivided according to a variety of different exploratory (Berument et al., 1999; Gau et al., 2011) and conceptual/confirmatory (Rutter & Bailey, 2003; Wei et al., 2015) approaches, the present results accord with the largest body of evidence, these results add to a body of evidence suggesting that ASD symptoms can be differentiated into a general domain with two subdomains, with implications for research and clinical assessment. For example, the SCQ could be refined not only as a screening tool, but also as secondary instrument to help support or rule out ASD symptom domains for diagnosis. Due to the different number of items, however, (25 for SCI vs. 12 for RRB), results should be interpreted with caution and future work may be needed to render these scales more comparable.
ROC results show that the SCQ performs adequately as a diagnostic instrument for boys and girls, especially for girls. These findings are consistent with the factor analysis, subdomain, and item-level sex/gender differences described above. These results do not provide a compelling reason to alter the existing cutoff for boys or girls. However, caution in both directions is warranted: for boys and girls alike, scores falling in the “at-risk” range (≥15) are more likely to be false positives than true positives (probability of true positive = 43% and 21%, respectively); and among boys, scores falling below this cutoff (in the “subthreshold” range) still had a 10% probability of diagnosis. Thus, the SCQ should be interpreted cautiously and probabilistically. Clinically, an SCQ score of 15 or higher is associated with a small but clinically significant increase in the probability of ASD. As scores increase beyond 15, the probability of ASD increases proportionately, particularly for girls. Scores between 11 and 15 may increase the probability enough to warrant careful clinical judgment (e.g., Corsello et al., 2007; Eaves et al., 2006). Overall, at the recommended threshold of 15, the sensitivity and NPV were good, the specificity and DLR were moderate, and the PPV poor. Thus, for both boys and girls, a positive value (above the cutoff) does not necessarily predict a diagnosis of ASD; indeed, a majority of cases above this cutoff were false-positives. And for boys in particular, ASD cases were common among those in the subthreshold range. Again, if the full range of possible SCQ scores were represented in the data, these values might differ. As a screening instrument within the broader assessment context, false negatives might be more clinically detrimental than false positives given that the latter only indicates the need for further assessment.
We interpret our sex/gender-discrepant findings as evidence for qualitative rather than quantitative differences in ASD symptom presentations between boys and girls. The pattern of sex/gender symptom differences observed among those with ASD (SCI, boys < girls; RRB, boys = girls) is qualitatively distinct from the pattern observed in the general population (SCI, boys > girls; RRB, boys > girls). These results differ from the conclusions of a recent meta-analysis by Hull et all (2016) which found no difference in RRB, and equivocal evidence for social impairment (Hull et al., 2016). In general, our findings are broadly consistent with the well-established sex/gender differences in ASD prevalence (e.g., Christensen et al., 2016; Loomes et al., 2017), but do not align neatly with existing theories in the literature which attempt to explain this discrepancy. For example, if the extreme male brain theory (Baron-Cohen, 2002) were supported, we might expect similar patterns of male-female levels of SCI (related to empathizing) and RRB (related to systematizing) in those with ASD as in those in the full population sample; this was not the case. Similarly, we did not find evidence for a female camouflage effect (e.g., Hull et al., 2017; Livingston & Happé, 2017; Rynkiewicz et al., 2016) insofar as parents did not rate girls diagnosed with ASD as possessing compensatory social skills (i.e., lower SCI scores) which might obfuscate their symptoms. It is possible that camouflage may still exist in settings that parents typically do not actively observe, such as educational settings. Notably, the pattern of results shown in Figure 2 suggests it may be more difficult to differentiate ASD vs. non-ASD status in boys than in girls.
Strengths, Limitations, and Implications
One strength of the present study is that comprehensive, multi-method assessment practices were used, which minimizes the possibility of results being influenced by bias or error. In other words, if the present gender-discrepant results reflect assessment error, then it is likely an underlying problem in ASD diagnostic criteria and sassessment tools in general rather than the particulars of the present study. Herein lies the dilemma articulated by Lai et al. (2015): because our existing conceptualizations and instruments (including SCQ, ADOS-2, and DSM-5 criteria) are derived from predominately male ASD samples, there remains a challenging problem of “the chicken and the egg.” That is, to the extent that ASD symptoms truly manifest differently in boys compared to girls, studies such as this are not able to ascertain this difference. Broader research is needed to understand the qualitative nature of SCI and RRB among typically and atypically developing girls.
This does not, however, rule out the possibility of informant bias affecting SCQ scores, and this should be considered in interpreting these results. Parents’ perceptions of SCI deficits in boys and girls are likely influenced by sex/gender expectations relative to typically developing same-sex peers. This is consistent with previous research suggesting that the social relationships between boys and girls with ASD are more similar than relationships with their same-gendered peers (Dean et al., 2014). Similarly, bias may be operating in items pertaining to 4-5 years of age, as these rely on parents’ recall of behaviors occurring several years ago. The possibility of response bias might be illustrated in the rates at which different items were endorsed. Approximately half of children were rated as not using gestures at 4-5 years of age, suggesting that parents may be misinterpreting this item. This may be a limitation of the yes/no format of the SCQ, which some parents might struggle with. For example, Frazier et al. (2010) found that 5.1% of unaffected siblings were rated by their caregivers with SCQ score of 15 or higher. Thus, results of single items should be interpreted cautiously.
Additional limitations should be noted. Using SCQ scores as the basis for direct assessment sampling results in an artificially truncated range and distribution of SCQ scores, which would not be seen if all participants received all measures. Thus, there may be children with ASD with scores in the low risk range (SCQ < 8) who were missed, while those in the at-risk range (SCQ ≥ 15) were more likely to be invited and assessed than those in the subthreshold range (8 ≤ SCQ ≤ 14). Second, despite our large overall sample size, our direct assessment sample was relatively small in terms of gender-by-diagnosis subgroups, with 177 boys (only 25% of whom had ASD) and 95 girls (only 7% of whom had ASD). A related consequence is that the completion of in-person assessments may have been higher due to greater levels of parental concern; indeed, response rate was higher among the at-risk group (44%) compared to the subthreshold group (29%). Although these data are considered to be a representative sample from a weighted epidemiological study, the present results should not be generalized to the entire population in an epidemiological manner (e.g., complex survey weights were not used in analyses). Rather, these findings can be interpreted simply as results of screening and assessment analyses conducted among the observed data with its limitations as noted above. However, these limitations are also reflective of a larger strength of this study—representative sampling of a population of over 8,000 children, with nearly 50% participation and inclusion of subthreshold children so as to not miss more mildly affected cases.
Third, this study did not use a well-validated, published diagnostic interview. Rather, the evaluations used an unpublished structured diagnostic interview designed to map onto both DSM-IV and DSM-5 criteria, assessing lifetime and present symptoms within a reasonable administration time. Lastly, a larger and more pernicious problem is the possibility of sex/gender bias in the diagnosis of ASD itself. The present study (and much of ASD research) relies upon diagnostic criteria which have developed over the years from research largely among boys with ASD. The present study utilized a rigorous assessment protocol to ascertain the diagnosis; however, to the extent that the ASD construct is gender-biased, these results cannot shed light on the nature of that bias and only highlight the need for broader research.
These findings have several implications for clinical and research assessment practices. First, elevated scores on the SCQ should be taken seriously regardless of sex/gender. It may be the case that clinically some girls with ASD are overlooked due to their perceived strengths in certain domains. These are important questions for a diagnostic evaluation. During the screening phase, however, a high score should not overridden based on other perceived strengths. Additionally, responses to specific items should be interpreted according to their developmental and social context. Careful attention might be given to items addressing social-communication and interaction behaviors at 4-5 years of age. In particular, girls with ASD may exhibit pronounced intra-individual SCI deficits compared to both their male peers with ASD and their female peers without ASD. Finally, positive results on screening measures should not be interpreted as indicating a diagnosis, but only a need for a more comprehensive evaluation.
Footnotes
Following Lai et al. (2015), we use both terms (sex and gender) together when referring to differences between boys and girls throughout most of this article to acknowledge it is not clear whether biological sex at birth or social gender constructs are the key variable; and both likely play a role.
The other 487 screeners were excluded due to duplicate submissions, not living in the surveillance area in 2012, declined participation, or insufficient number of SCQ responses for scoring.
Positive test result = SCQ score falling on or above the cutoff value. In standard usage, the SCQ cutoff is 15. We adopt this cutoff value as a default, but also consider alternative cutoff values where specified.
Contributor Information
Spencer C. Evans, Email: scevans@fas.harvard.edu.
Andrea D. Boan, Email: boan@musc.edu.
Catherine Bradley, Email: bradlecc@musc.edu.
Laura A. Carpenter, Email: carpentl@musc.edu.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.) Arlington, VA: Author. [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, & Hackett G (2009). Fetal testosterone and autistic traits. British Journal of Psychology, 100, 1–22. [DOI] [PubMed] [Google Scholar]
- Barnard-Brak L, Brewer A, Chesnut S, Richman D, & Schaeffer AM (2016). The sensitivity and specificity of the social communication questionnaire for autism spectrum with respect to age. Autism Research, 9(8), 838–845. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S (2002). The extreme male brain theory of autism. Trends in Cognitive Sciences, 6, 248–254. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, & Belmonte MK (2005). Sex differences in the brain: implications for explaining autism. Science, 310, 819–823. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, & Knickmeyer R (2011). Why are autism spectrum conditions more prevalent in males? PLoS Biology, 9, e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, & Koot HM (2013). Sex differences in the timing of identification among children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Beggiato A, Peyre H, Maruani A, Scheid I, Rastam M, Amsellem F, ... & Delorme R (2017). Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Research, 10, 680–689 [DOI] [PubMed] [Google Scholar]
- Bejerot S, Eriksson JM, Bonde S, Carlström K, Humble MB, & Eriksson E (2012). The extreme male brain revisited: Gender coherence in adults with autism spectrum disorder. The British Journal of Psychiatry, 201, 116–123. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A (1999). Autism screening questionnaire: diagnostic validity. The British Journal of Psychiatry, 175, 444–451. [DOI] [PubMed] [Google Scholar]
- Bölte S, Duketis E, Poustka F, & Holtmann M (2011). Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism, 15, 497–511. [DOI] [PubMed] [Google Scholar]
- Buchen L (2011). Scientists and Autism: When geeks meet. Nature, 479, 25–27. [DOI] [PubMed] [Google Scholar]
- Carpenter LA, Boan AD, Wahlquist AE, Cohen A, Charles J, Jenner W, & Bradley CC (2016). Screening and direct assessment methodology to determine the prevalence of Autism Spectrum Disorders. Annals of Epidemiology, 26, 395–400. [DOI] [PubMed] [Google Scholar]
- Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, & Tager-Flusberg H (2007). Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37, 86–97. [DOI] [PubMed] [Google Scholar]
- Chandler S, Charman T, Baird G, Simonoff E, Loucas T, Meldrum D, ... & Pickles A. (2007). Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 46, 1324–1332. [DOI] [PubMed] [Google Scholar]
- Chesnut SR, Wei T, Barnard-Brak L, & Richman DM (2016). A meta-analysis of the social communication questionnaire: Screening for autism spectrum disorder Autism Advance online publication. doi:1362361316660065. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KV, Bilder D, Charles J, Constantino JN, ... & Lee LC (2016). Prevalence and characteristics of Autism Spectrum Disorder among children aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and Mortality Weekly Report. Surveillance Summaries, 65, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal BL, & Lord C (2007). Between a ROC and a hard place: decision making and making decisions about using the SCQ. Journal of Child Psychology and Psychiatry, 48, 932–940. [DOI] [PubMed] [Google Scholar]
- Dean M, Kasari C, Shih W, Frankel F, Whitney R, Landa R, ... & Harwood R (2014). The peer relationships of girls with ASD at school: comparison to boys and girls with and without ASD. Journal of Child Psychology and Psychiatry, 55, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvekot J, van der Ende J, Verhulst FC, & Greaves-Lord K (2015). The screening accuracy of the parent and teacher-reported social responsiveness scale (SRS): comparison with the 3Di and ADOS. Journal of Autism and Developmental Disorders, 45, 1658–1672. [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happé F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Child & Adolescent Psychiatry, 51, 788–797. [DOI] [PubMed] [Google Scholar]
- Eaves LC, Wingert HD, Ho HH, & Mickelson EC. (2006). Screening for autism spectrum disorders with the Social Communication Questionnaire. Journal of Developmental and Behavioral Pediatrics, 27, S95–S103. [DOI] [PubMed] [Google Scholar]
- Edwards TL, Watkins EE, Lotfizadeh AD, & Poling A (2012). Intervention research to benefit people with autism: How old are the participants?. Research in Autism Spectrum Disorders, 6, 996–999. [Google Scholar]
- Frazier TW, & Hardan AY (2017). Equivalence of symptom dimensions in females and males with autism. Autism, 21, 749–759. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, & Hardan AY (2014). Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. Journal of the American Academy of Child & Adolescent Psychiatry, 53, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, & Constantino JN (2014). Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism, 18, 31–44. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Kubu CS, Sinclair L, & Rezai A (2008). Exploratory and confirmatory factor analysis of the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders, 38, 474–480. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Sinclair L, Kubu CS, Law P, Rezai A, ... & Eng C (2010). Autism spectrum disorders as a qualitatively distinct category from typical behavior in a large, clinically ascertained sample. Assessment, 17, 308–320. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Speer L, Embacher R, Law P, Constantino J, ... & Eng C. (2012). Validation of proposed DSM-5 criteria for autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SSF, Lee CM, Lai MC, Chiu YN, Huang YF, Kao JD, & Wu YY (2011). Psychometric properties of the Chinese version of the Social Communication Questionnaire. Research in Autism Spectrum Disorders, 5, 809–818. [Google Scholar]
- Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, & Mandell D (2010). Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disability and Health Journal, 3, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S (2013). Opinion: Sex, gender and the diagnosis of autism—A biosocial view of the male preponderance. Research in Autism Spectrum Disorders, 7, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, ... & Sigman M. (2008). A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. Journal of the American Academy of Child & Adolescent Psychiatry, 47, 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, & Sikora DM (2009). Sex differences in autism spectrum disorder: An examination of developmental functioning, autistic symptoms, and coexisting behavior problems in toddlers. Journal of Autism and Developmental Disorders, 39, 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, & Stokes MA (2014). Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism, 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2016). Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism, 20, 75–84. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Bölte S, & Poustka F (2007). Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Developmental Medicine & Child Neurology, 49, 361–366. [DOI] [PubMed] [Google Scholar]
- Horiuchi F, Oka Y, Uno H, Kawabe K, Okada F, Saito I, ... & Ueno SI (2014). Age-and sex-related emotional and behavioral problems in children with autism spectrum disorders: Comparison with control children. Psychiatry and Clinical Neurosciences, 68, 542–550. [DOI] [PubMed] [Google Scholar]
- Hull L, Mandy W, & Petrides KV (2016). Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism, 21, 706–727. [DOI] [PubMed] [Google Scholar]
- Hull L, Petrides KV, Allison C, Smith P, Baron-Cohen S, Lai MC, & Mandy W (2017). “Putting on my best normal”: Social camouflaging in adults with autism spectrum conditions. Journal of Autism and Developmental Disorders, 47, 2519–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM (2016). SPSS Statistics for Windows Version 24. Armonk, NY: IBM. [Google Scholar]
- Kirkovski M, Enticott PG, & Fitzgerald PB (2013). A review of the role of female gender in autism spectrum disorders. Journal of Autism and Developmental Disorders, 43, 2584–2603. [DOI] [PubMed] [Google Scholar]
- Kline RB (2016). Principles and practice of structural equation modeling. Guilford. [Google Scholar]
- Krahn TM, & Fenton A (2012). The extreme male brain theory of autism and the potential adverse effects for boys and girls with autism. Journal of Bioethical Inquiry, 9, 93–103. [DOI] [PubMed] [Google Scholar]
- Lai DC, Tseng YC, Hou YM, & Guo HR (2012). Gender and geographic differences in the prevalence of autism spectrum disorders in children: Analysis of data from the national disability registry of Taiwan. Research in Developmental Disabilities, 33, 909–915. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo M, Auyeung B, Chakrabarti B, & Baron-Cohen S, (2015) Sex/gender differences and autism: Setting the scene for future research. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD (2013). Longitudinal structural equation modeling. Guilford Press. [Google Scholar]
- Livingston LA, & Happé F (2017). Conceptualising compensation in neurodevelopmental disorders: Reflections from autism spectrum disorder. Neuroscience & Biobehavioral Reviews, 80, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 466–474 [DOI] [PubMed] [Google Scholar]
- Lord C, Luyster R, Gotham K, & Guthrie W (2012). Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2) Manual. Torrence, CA: Western Psychological Services. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, ... & Rutter M (2000). The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Mandy WP, Charman T, & Skuse DH (2012). Testing the construct validity of proposed criteria for DSM-5 autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 41–50. [DOI] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, & Skuse D (2012). Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders, 42, 1304–1313. [DOI] [PubMed] [Google Scholar]
- May T, Cornish K, & Rinehart NJ (2016). Gender profiles of behavioral attention in children with autism spectrum disorder. Journal of Attention Disorders, 20, 627–635. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Murray DS, Akers R, Mitchell T, & Manning-Courtney P (2011). Use of the Autism Diagnostic Observation Schedule (ADOS) in a clinical setting. Autism, 15, 143–162. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2012). Mplus User’s Guide Sixth Edition Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Norris M, & Lecavalier L Screening accuracy of level 2 autism spectrum disorder rating scales. (2010). A review of selected instruments. Autism, 14, 263–284. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Goodlin-Jones BL, & Solomon M (2005). Evidence-based assessment of autism spectrum disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34, 523–540. [DOI] [PubMed] [Google Scholar]
- Park S, Cho SC, Cho IH, Kim BN, Kim JW, Shin MS, ... & Yoo HJ. (2012). Sex differences in children with autism spectrum disorders compared with their unaffected siblings and typically developing children. Research in Autism Spectrum Disorders, 6, 861–870. [Google Scholar]
- Reinhardt VP, Wetherby AM, Schatschneider C, & Lord C (2015). Examination of sex differences in a large sample of young children with Autism Disorder and typical development. Journal of Autism and Developmental Disorders, 45, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G, Steer C, & Golding J (2011). Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Social Psychiatry and Psychiatric Epidemiology, 46, 1283–1293. [DOI] [PubMed] [Google Scholar]
- Rutherford M, McKenzie K, Johnson T, Catchpole C, O’Hare A, McClure I, ... & Murray A (2016). Gender ratio in a clinical population sample, age of diagnosis and duration of assessment in children and adults with autism spectrum disorder. Autism, 20, 628–634. [DOI] [PubMed] [Google Scholar]
- Rutter M, & Bailey A (2003). Social Communication Questionnaire. Torrance, CA: WPS. [Google Scholar]
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, & Baron-Cohen S (2016). An investigation of the ‘female camouflage effect’in autism using a computerized ADOS-2 and a test of sex/gender differences. Molecular Autism, 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy ME, Zablotsky B, Yarger HA, Zimmerman A, Makia B, & Lee L-C (2014). Sex differences in co-occurring conditions of children with autism spectrum disorders Autism, 18, 965–974. [DOI] [PubMed] [Google Scholar]
- Stauder JEA, Cornet LJM, & Ponds RWHM (2011). The Extreme Male Brain theory and gender role behaviour in persons with an autism spectrum condition. Research in Autism Spectrum Disorders, 5, 1209–1214. [Google Scholar]
- Szatmari P, Liu XQ, Goldberg J, Zwaigenbaum L, Paterson AD, Woodbury-Smith M, ... & Thompson A (2012). Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159, 5–12. [DOI] [PubMed] [Google Scholar]
- Tan DW, Russell-Smith SN, Simons JM, Maybery MT, Leung D, Ng HL, & Whitehouse AJ (2015). Perceived Gender Ratings for High and Low Scorers on the Autism-Spectrum Quotient Consistent with the Extreme Male Brain Account of Autism. PloS One, 10, e0131780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung D, Johnco C, McBride NM, Howie F, Scalli L, & Storch EA (2016). Optimizing the screening of autism spectrum disorders in outpatient clinics: An examination of the Social Communication Questionnaire-Lifetime. Research in Autism Spectrum Disorders, 27, 21–28. [Google Scholar]
- Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, & Van der Gaag RJ (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 44, 627–635. [DOI] [PubMed] [Google Scholar]
- Watkins EE, Zimmermann ZJ, & Poling A (2014). The gender of participants in published research involving people with autism spectrum disorders. Research in Autism Spectrum Disorders, 8, 143–146. [Google Scholar]
- Wei T, Chesnut SR, Barnard-Brak L, & Richman D (2015). Psychometric analysis of the Social Communication Questionnaire using an item-response theory framework: implications for the use of the lifetime and current forms. Journal of Psychopathology and Behavioral Assessment, 37, 469–480. [Google Scholar]


