Abstract
Parkinson’s disease (PD) is the most common cause of movement disorders and is characterized by the progressive loss of dopaminergic neurons in the substantia nigra. It is increasingly recognized as a complex group of disorders presenting widely heterogeneous symptoms and pathology. With the exception of the rare monogenic forms, the majority of PD cases result from an interaction between multiple genetic and environmental risk factors. The search for these risk factors and the development of preclinical animal models are in progress, aiming to provide mechanistic insights into the pathogenesis of PD. This review summarizes the studies that capitalize on modeling sporadic (i.e., nonfamilial) PD using Drosophila melanogaster and discusses their methodologies, new findings, and future perspectives.
Keywords: sporadic Parkinson’s disease, Drosophila, animal model
1. Introduction
Parkinson’s disease (PD) is the second most frequent neurodegenerative disorder and affects 7–10 million individuals worldwide. It presents with motor symptoms, such as bradykinesia, rigidity, postural instability and resting tremor; however, it also manifests a broad spectrum of nonmotor symptoms, including sleep disturbances, mood disorders, and cognitive impairments [1,2]. A prominent pathology of PD is the loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc), which projects to the striatum. The resulting striatum DA deficiency leads to the characteristic motor dysfunction. Another histopathological hallmark of PD is the presence of abnormal aggregates (inclusions) that contain the α-synuclein (α-syn) protein, termed Lewy bodies and Lewy neurites [3], although many PD cases without Lewy body pathology have been reported [4,5]. No treatment strategy to cure or slow down PD progression is currently available. With a prevalence of approximately 1% at 60 years of age [6], a better understanding of PD pathogenesis for developing an improved treatment strategy is an increasing need in aging societies.
The role of genetics in the etiology of PD had long been debated as only approximately 15% of PD patients have a family history and no more than 10% of cases have Mendelian inheritance [1,7]. However, the identification of monogenic mutations that cause the Mendelian forms of PD has unequivocally placed genetic factors in the center of PD etiology. A positive family history can be explained by the exposure to common environmental factors, monogenic or polygenic inheritance, and a combination thereof [8]. Linkage studies of families with non-Mendelian inheritance of PD have provided evidence of gene-gene interaction as a PD risk factor in these families [9,10,11], which supports a genetic contribution to the non-monogenic familial PD.
PD cases that occur in individuals with no positive family history are defined as “sporadic PD”. However, this categorization does not exclude genetic factors as causative agents. It most likely has a multifactorial origin, which results from complex interactions of genetic and environmental factors. Sporadic PD can be monogenic because a dominant mutation may have occurred de novo or the mutation is recessive. With the exception of the monogenic forms of familial PD, which are frequently early-onset [12,13], familial and sporadic PDs are clinically indistinguishable [14,15]. Then, how do we go about modeling sporadic PD? Are the models for familial and sporadic PDs fundamentally different or based on similar strategies? To what extent do familial PD models provide relevant knowledge for sporadic cases and vice versa? This review aims to address these questions by introducing the literature, in which sporadic PD models were established or used for mechanistic or descriptive studies in Drosophila.
2. How to Model a Disease That Occurs Sporadically?
Sporadic PD, also referred to as idiopathic PD, is by definition a PD case whose cause is unknown. As such, it is intrinsically difficult to generate models through an approach that targets the “causal” genes or agents. Nevertheless, the recent progress in identifying candidate genetic and environmental risk factors for PD has made it possible to engineer animal models that carry genetic variations thought to increase the risk of PD, in the same way as most genetic models of familial PD were established. The analogous approach is the exposure to chemical agents known to be associated with an increased risk of PD. In addition, there may be a third category of sporadic PD models, which are developed by serendipity, such as the discovery of a mutant animal that exhibits a phenotypic similarity to PD. Furthermore, as sporadic PD is likely to be multifactorial and combinations among these strategies may result in a valid model.
On evaluating the value of animal models, it is worthwhile to perform a mental exercise imagining two extreme theoretical scenarios:
-
(A)
A genetically engineered animal model perfectly recapitulates a known risk genetic variant for PD, but shows no characteristics similar to those of PD.
-
(B)
A mutant animal was discovered to exhibit phenotypes similar to the cardinal characteristics of PD, but the mutant allele/gene is not known to be associated with PD risk.
Which model can be considered a model for sporadic PD?
In theory, an ideal model should possess similarities with the human disease in the (1) genetic basis; (2) pathological responses; (3) phenotypic endpoints; (4) underlying mechanism(s); and (5) response to known drugs with clinical efficacy. It should also be (6) predictive of human response to drugs [16]. However, there is a consensus in many disease research fields that no single animal model is expected to possess all these features. The two previously described scenarios exemplify this limitation. The lack of phenotypic similarity in (A) and known genetic association with PD in (B) may be viewed as weaknesses; however, given the heterogeneity and the multifactorial nature of sporadic PD, one can interpret the lack of phenotype in (A) as a missing interacting genetic or environmental factors for developing PD and speculate the existence of rare genetic variants similar to those in (B) in the human population. Thus, the apparent weakness can provide new opportunities for discovery that lead to a better understanding of the pathophysiology and underlying mechanisms of the disease. Therefore, I take a liberal position and propose to consider the animal models that possess any of the criteria (1) to (6) legitimate. On the basis of this premise, the current review recognizes animal models that possess one of the following features as sporadic PD models:
-
-
Based on chemical or genetic agents known to be associated with PD risk
-
-
Show functional impairments or loss of DA neurons, age-dependently
-
-
Exhibit behavioral abnormalities similar to the movement disabilities in PD
-
-
Responsive to symptomatic anti-PD medications, such as l-dopa
-
-
Show Lewy body pathology
These models may be roughly classified into three categories by the methodology: (1) chemically induced models; (2) targeted genetic models; and (3) serendipitous genetic models. Approaches that combine multiple methods within and among these categories to create more realistic models are also emerging with success.
3. Why Use Drosophila for Parkinson’s Disease Research?
Various animal and cell culture models of PD have been created and have substantially contributed to understanding the disease. Both in vivo and in vitro models are broadly categorized into toxin-induced or genetic models. As previously discussed, although none of the existing models fully recapitulates all pathological features of PD, each model provides opportunities to investigate certain aspects of the disease. For example, cellular models are convenient to investigate protein aggregates associated with neuronal death and test compounds for therapeutic applications. However, pathogenic processes that involve interactions among different cell types and tissues can only be studied in animal models [17]. Primates and rodents are widely used to create in vivo mammalian models, which provide excellent tools to elucidate the biochemical and neuronal mechanisms of key pathological features of PD, such as protein aggregation, DA neuron demise, and behavioral abnormality. Recent progress in mouse genetic tools that enable cell type-specific and temporal control of gene expression has further advanced our understanding of the molecular genetic basis of PD pathogenesis. However, although theoretically possible, it is practically difficult to apply in vivo mammalian models for genetic and drug screens because of the cost, ethical concerns, and the animal’s lifespan, which is generally longer than 2 years [18,19].
Drosophila melanogaster is a model organism that provides numerous advantages for biomedical research. The fly genome is smaller with substantially less gene redundancy than that of mammals, while it contains homologs of approximately 70% of human disease-related genes [20]. Advanced genetic tools enable mutagenesis, silencing, and the overexpression of the gene-of-interest in a cell-type specific and temporally controlled manner [21]. Thus, research in flies has proven potential to discover conserved genetic pathways relevant for human disease [22]. Flies are amenable for large-scale genetic and chemical screening, thus providing opportunities for not only understanding the genetic and molecular basis of diseases but also for drug discovery [23]. Moreover, the relatively short life cycle and lifespan of flies (approximately 10 days and 2 to 3 months, respectively) accelerate the study of age-related disorders, including PD.
The fly’s nervous system is simpler but shares many features in structure, organization, and function with mammalian counterparts. Flies exhibit various DA-dependent behaviors controlled by different subclasses of DA neurons (Figure 1), whose individual roles and connectivity are unfolding [24,25]. The startle-induced climbing assay and its variations (such as startle-induced negative geotaxis; SING) are the most commonly used methods to quantify a fly’s locomotor behavior in comparing the organismal phenotypes of flies and human movement disorders [26,27,28,29]. These assays are distinct from recording the natural tendency to climb, referred to as negative geotaxis; however, they monitor the rate of the climbing response of flies when knocked down to the bottom of a cylinder. The climbing response is normally rapid, and a failure to recover from the “shock” of being knocked-down suggests defects in the functioning of the nervous system in controlling initiation. Noteworthy, recent studies have demonstrated that at least a subset of DA neurons in the protocerebral anterior medial (PAM) cluster has important roles in the climbing response, similar to DA neurons in the mammalian SNpc, which makes flies an ever-relevant model system for the investigation of PD [26,30,31].
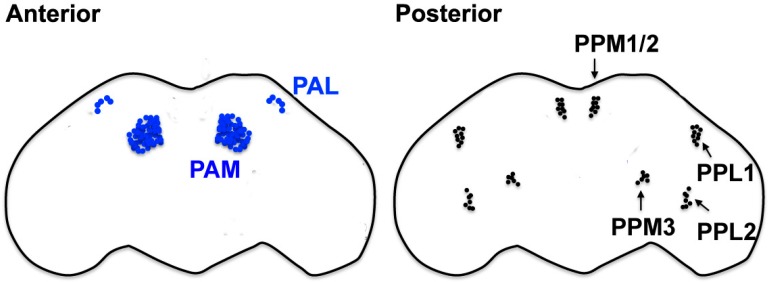
Figure 1.
The dopaminergic (DA) neurons in the adult fly brain. Schematics of the seven major clusters of DA neurons located in the anterior and posterior brain [24,25,36]. The protocerebral anterior lateral (PAL) and protocerebral anterior medial (PAM) clusters reside in the anterior brain. Major posterior clusters include the protocerebral posterior medial 1, 2, and 3 (PPM1, PPM2, and PPM3, respectively) and the protocerebral posterior lateral clusters 1 and 2 (PPL1 and PPL2, respectively).
The nonmotor symptoms of PD include olfactory dysfunction, mood disorders, cognitive impairments, sleep disorders, autonomic dysfunction, pain, and fatigue. These symptoms are common in early PD and are thought to be present prior to the onset of the motor symptoms [1]. Fly PD models have also been effectively used to investigate these non-motor symptoms, including sleep and circadian dysfunctions [32,33], visual deficits [34], and learning and memory abnormalities [33].
Despite these advantages, there are several limitations of Drosophila as a model organism to investigate PD. Flies are not the best organism for biochemical studies that require a large amount of homogeneous tissue. Flies do not have an adaptive immune system, which has important implications in the pathophysiology of PD [35]. Another notable limitation is the absence of a fly homolog of SNCA, the gene that encodes α-syn. Consequently, Lewy body pathology is not endogenously observed in flies. However, as subsequently discussed, the formation of Lewy body-like inclusions may be induced by the ectopic expression of human α-syn in flies [28], which indicates that the cellular machineries necessary for Lewy body formation and the pathophysiological impact of inclusions may be investigated in flies. In summary, Drosophila provides opportunities to rapidly discover the molecular-genetic mechanisms of PD and test therapeutics. Following up the discoveries made in flies with complementary studies using other animal and cellular models would be a promising path toward a comprehensive understanding of the disease and the development of new intervention strategies.
4. Chemically Induced Models of Sporadic PD
4.1. The Environmental Toxicology of PD
Epidemiological studies have suggested that several environmental factors, including pesticide exposure, rural living, well-water drinking, and agricultural occupation, increase the risk of PD [37]. Exposure to pesticides is the most consistently identified environmental risk factor for PD. A meta-analysis study reported a 62% increase in PD risk in a comparison of ever versus never pesticide exposure [38]. The pesticide exposure dose-dependently correlates with the risk of PD, which partly explains the increased risk in agricultural workers [39,40,41,42]. Although some inconsistency and heterogeneity among association studies have been reported [43,44], the idea to translate chronic pesticide use into animal models comes naturally. Indeed, the advent of the classic strategy to model sporadic PD using compounds found in pesticides dates before the expansion of the genetic models of PD.
Pesticides include insecticides, herbicides, and fungicides and encompass over 900 compounds that belong to many different chemical classes [45]. It is therefore tremendously difficult to determine which compounds pesticide users were exposed to. Consequently, only a small handful of chemicals have been used to develop PD models in animals. Rotenone and paraquat are among the most widely used.
Rotenone is a naturally occurring chemical found in the roots of tropical plants, commonly used as an insecticide. Being plant-derived and organic, it was originally thought to be harmless to humans when properly used. However, epidemiological studies have shown the increased risk for PD in rotenone users [46], and its use has been prohibited or restricted in many countries. It is a lipophilic compound that can freely cross the blood-brain barrier and cell membrane. The toxicity of rotenone is largely attributed to its effect on the inhibition of mitochondrial complex I activity [47], although other mechanisms, including microtubule depolymerization, may contribute to its toxicity [48].
Although not a common environmental toxin, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin) was the first chemical that demonstrated the role of neurotoxins in the etiology of “Parkinsonism” (a set of symptoms typically associated with PD). MPTP is a byproduct of the synthesis of a meperidine analog MPPP (1-methyl-4-phenyl-4-propionoxypiperidine), a synthetic opioid drug and was found to be the causative agent of symptoms similar to advanced PD in intravenous drug users [49]. MPTP crosses the blood-brain barrier and is converted to the toxic 1-methyl-4-phenylpyridinium ion (MPP+) by monoamine oxidase B (MAO-B) in glia. Due to its high affinity to the dopamine transporter (DAT), MPP+ is selectively taken up by DA neurons. Thus, MPTP exposure causes rapid, nonprogressive DA neuron loss and associated motor disabilities in humans, as well as in various animal models [50].
Clinically, MPTP exposure is one of the exclusion criteria for PD because of the known consequence of this drug’s action, which is acute and nonprogressive Parkinsonism, unlike in chronic PD [1]. However, the discovery of MPTP boosted a search for its analogs, including paraquat, one of the most widely used herbicides. Paraquat is structurally similar to MPP+, can cross the blood-brain barrier (BBB) and is likely to be taken up to DA neurons as a form of paraquat+ [51]. It is an oxidative stressor that, by acting as a redox cycler, produces superoxide radicals and other reactive oxygen species (ROS). Paraquat also increases superoxide radical production in mitochondria [52,53]. Several case-controlled epidemiological studies have shown an increased risk of PD with paraquat exposure [46,54].
4.2. The Use of Rotenone and Paraquat to Model Sporadic PD and Its Variations
Chronic rotenone exposure is one of the first toxin-based fly models of sporadic PD. Flies fed with a sublethal dose of rotenone (<750 μM) for 7 days show impaired locomotor ability in startle-induced climbing assays and neuronal loss selectively in several subclasses of DA neurons. The administration of Levodopa (l-dopa), the most effective symptomatic medication for PD, partially rescues rotenone-induced climbing defects, but not a neuronal loss. l-dopa can cross the BBB, be taken up by DA neurons and converted to DA by dopa decarboxylase. These results indicate that chronic rotenone exposure induces the selective loss of DA neurons, which leads to reduced DA signaling and locomotor deficits and thus recapitulates the key characteristics of PD [27]. Using a slightly modified protocol, another group also showed that a 10-day rotenone (250 μM) exposure induces the loss of DA neurons in the PPL1 and PAL clusters, which progresses at least up to 6 weeks after the treatment [55].
Similar to rotenone, paraquat has been frequently used in different model animals to test its causal relationship with the pathological characteristics of PD. Several studies in flies have consistently demonstrated that chronic paraquat exposure causes a DA neuron loss or functional impairments and locomotor deficits within hours to days, accompanied by a high lethality. Feeding flies with 20 mM paraquat induces locomotor defects within 12 h and a neuronal loss selectively in several classes of DA neurons as early as 6 h after the treatment. Despite these rapid and drastic effects, it does not induce obvious brain-wide tissue damage, judging from the overall morphology of the brain and cholinergic neurons [56]. Similarly, DA neuron loss in the PPL1 cluster was observed 24 h after the exposure to 10 mM paraquat [55]. It was also shown that 20 mM paraquat ingestion induces pathological morphological changes within 24 h in several subclasses (PPL2, PPL2, PPM1/2, PPM3, PAL, and PP2) of DA neurons, such as the shrinkage of cell bodies, which is indicative of functional impairments [57,58].
These chemically induced models have successfully shown the relevance of environmental agents, in particular, the pesticides rotenone and paraquat, in DA neuron demise and motor disabilities, but not without criticism. In general, toxins fail to recapitulate the progressive nature of neurodegeneration in PD, although rotenone-induced progressive DA neuron loss is reported [55]. An inevitable drawback is lethal (they are pesticides, after all); depending on the concentration and application route, rotenone and paraquat kill flies within hours to days. The dose of rotenone or paraquat that is effective to induce DA neuron loss falls within the range where the median survival time is 7 days or less during chronic exposure [55]. Furthermore, there are several discrepancies among the results of different studies, which may originate from the subtle differences in the toxin administration protocols in the laboratory environment. The absence of DA neuron loss after the chronic exposure of 500 μM rotenone or 100 μM paraquat for 7 to 10 days has been reported [59]. A study found a morphological sign of declined viability in DA neurons in flies exposed to 500 μM rotenone; however, there were no aberrant changes in DA neurons in flies treated with 20 mM paraquat for 72 h [60].
Nevertheless, these apparent shortcomings can be considered evidence that supports the multifactorial origin of sporadic PD. The inconsistency in the results is indicative of the necessity of synergism with other chemicals or genetic variants for DA neuron demise and locomotor deficits. It is extremely difficult to measure the dose of environmental toxin exposure in humans; however, it is unlikely to be comparable to the lethal or sublethal dose in the toxin ingestion experiments in flies. Thus, more realistic models of sporadic PD may be created by combining multiple chemicals of noneffective drug doses or exposing single or multiple chemicals to the flies of different genetic backgrounds. The synergism of chemical agents and genetic factors can be tested on known candidate genetic variants or by screening for genetic modifiers that enhance or suppress the effect of the chemicals.
In line with this view, numerous studies have examined the synergism of multiple compounds or compounds and genetic factors on PD risk. Maneb (manganese ethylenebisdithiocarbamate) and ziram (zinc dimethylbisdithiocarbamate) are commonly used fungicides that belong to the class of dithiocarbamates. Epidemiological studies that evaluated the effect of ambient exposure to pesticides found an increased risk of PD associated with maneb or ziram alone and a greater risk with the combined use of paraquat, maneb and ziram [61,62]. In flies, chronic exposure to 4 mM paraquat, 500 μM maneb, or 1 mM ziram (the dose that does not decrease survival) does not individually impair DA neuron viability or climbing ability. In contrast, the combined exposure of paraquat and maneb, but not paraquat and ziram, leads to DA neuron loss in the PPL1 cluster at 6 weeks [55,63].
The approach to combine toxin exposure and genetic manipulations in flies is increasingly common and has uncovered important molecular insights into the gene-environmental interactions that increase or decrease PD risk. For example, heat-shock protein-70 (Hsp-70) and a mutation in the methuselah gene have been shown to ameliorate paraquat-induced PD-like phenotypes in flies [58,64]. One study showed that RNAi-mediated knockdown of the gene that encodes tyrosine hydroxylase (TH), the rate-limiting enzyme for DA synthesis, in several subclasses of DA neurons extends the lifespan and partially suppresses DA neuron loss in rotenone-treated flies (500 µM chronic exposure). These results suggest that DA production enhances the deleterious effects of rotenone on the viability of DA neurons and the lifespan [65]. Similarly, another study found that the overexpression of Drosophila vesicular monoamine transporter (dVMAT), which is required for packaging DA into synaptic vesicles, protects DA neurons from rotenone-induced loss, presumably by reducing the cytosolic DA pool [55]. These findings partly explain why DA neurons are selectively sensitive to rotenone and are concordant with the hypothesis that DA metabolism is a contributing factor in the selective vulnerability of SNpc DA neurons in PD [66].
In contrast, several studies indicate the opposite relationship of DA production and toxicity in paraquat models of PD; that is, an increase in DA production confers protection against paraquat-induced neurotoxicity. Loss-of-function mutations in the Catecholamines-up (Catsup) gene, which encodes a negative regulator of DA production, unexpectedly promote the survival and protect DA neurons from degeneration in paraquat-treated flies [56]. Because the overexpression of dVMAT, which leads to the reduction of the cytosolic DA pool, does not enhance or exacerbate paraquat-induced DA neuron loss [55], the increase in extracellular DA rather than cytosolic DA likely reduces paraquat-induced neurotoxicity. Parallel with these observations was the finding that overexpression of the TH gene in DA neurons or serotonergic neurons equally promotes resistance against lethality in paraquat-treated flies [57]. This study also investigated how the increase in extracellular DA counteracts the paraquat-induced neurotoxicity by correlating the expression of the D1-like dopamine receptor, DAMB, and toxicity. DA signaling via DAMB typically increases the cytosolic Ca2+ levels, which, in excess, enhance the oxidative stress and excitotoxicity. However, they found that TH overexpression causes a compensatory downregulation of DAMB in postsynaptic glutamatergic neurons and thereby confers resistance against paraquat-induced neurotoxicity [57].
Thus, rotenone-induced neurotoxicity may be chiefly mediated by a cell-autonomous mechanism sensitive to the cytosolic DA levels. In contrast, the systemic neurotoxicity of paraquat, including the selective loss of DA neurons, is dependent on the extracellular DA and mediated by non-cell-autonomous mechanisms. The reason for this intriguing mechanistic difference is unknown; however, it may be partly attributable to the toxin’s biochemical mechanisms of action. Both rotenone and paraquat cause oxidative damage, but through distinct molecular events. The toxicity of rotenone is mainly caused by the inhibition of mitochondrial complex I [67], which results in a decline of energy production by the respiratory chain and increased ROS production [68]. In contrast, paraquat has little or no effect on mitochondrial complex I [69], and its toxicity is thought to be essentially due to its redox cycling. This leads to the production of ROS in the form of hydrogen peroxide (H2O2) and hydroxyl radicals (HO) and the oxidation of NADPH, the major source of reducing equivalents in the cell [70].
A recent study by Stephano et al. [71] showed that chronic exposure to 500 µM rotenone does not cause a significant DA neuron loss for at least up to 10 days, whereas the climbing ability declines from day 6 onwards. They took advantage of the absence of neuronal loss and isolated DA neurons after 3 days of rotenone treatment, the timepoint considered to be the presymptomatic phase, and analyzed their transcriptome. A differential gene expression analysis that compared the DA-neurons of rotenone-treated and control flies showed the gene expression signature, which indicated the activation of the MAPK/EGFR- and TGF-β signaling pathways and the inhibition of the Wnt signaling pathway in rotenone-treated flies, including a reduced expression of armadillo/β-catenin, a central component of Wnt signaling. Concordant with this finding, the overexpression of armadillo in DA neurons ameliorates rotenone-induced locomotor decline, which suggests a role for Wnt signaling in the pathogenesis of PD [71]. Further implications of this work include the potential use of transcriptomic datasets to identify prodromal disease biomarkers.
Mitochondrial uncoupling protein 2 (UCP2) belongs to a class of the anion transporter family present in the inner mitochondrial membrane. UCP2 uncouples the mitochondrial electrical transport chain from the phosphorylation of ADP to ATP synthesis by dissipating the proton gradient across the mitochondrial inner membrane. Through this process, UCP2 attenuates ROS production and protects cells from oxidative damage [72]. The overexpression of human UCP2 (hUCP2) in several subclasses of DA neurons ameliorates the locomotor decline and partially prevents DA neuron loss induced by chronic rotenone exposure [73], likely by counteracting the mitochondrial damage induced by rotenone [74]. Although whether endogenous Drosophila UCPs are involved in rotenone-induced toxicity remains unaddressed, these findings suggest UCP2 as a potential therapeutic target for PD.
In summary, Drosophila provides opportunities to delineate the toxin-specific mechanism of action on DA neurodegeneration and can provide new insights into understanding the PD of diverse etiological origins. Furthermore, the powerful genetic toolbox of Drosophila enables the identification of genetic modifiers of toxin-induced neurodegeneration with relative ease. It is anticipated that the investigation of the synergism between toxins and genetic variants will be increasingly popular to validate the role of common genetic variants and their underlying mechanisms in PD susceptibility. The toxin-genetic combined approach might even discover new genes, whose cryptic variations normally have little effect on human health, but increase PD risk in certain environmental conditions.
5. Genetic Models of Sporadic PD
The identification of several causative genes for rare monogenic forms of PD, such as SNCA (α-synuclein) [75], Leucine-rich repeat kinase 2 (LRRK2) [76], Parkin [77], PTEN-induced putative kinase 1 (PINK1) [78] and DJ-1 [79], overturned the traditional view that PD is not hereditary and unequivocally demonstrated the major role of genetics in PD etiology [80]. Furthermore, the past decade has seen considerable progress in identifying genetic risk variants associated with PD through linkage analysis or genome-wide association studies (GWAS). Recent GWAS meta-analyses have identified 41 loci, encompassing a total of 72 candidate genes, associated with sporadic PD risk [81,82]. Over 70% of the PD risk loci have protein altering or cis-QTL variants, which suggests that changes in the function or expression levels of candidate genes are associated with the increased PD risk (Table 1). Importantly, in agreement with previous studies, these studies found polymorphic variants of SNCA and LRRK2 in association with sporadic PD. These studies also confirmed MAPT and GBA as PD-susceptibility factors [83].
Table 1.
The candidate genetic risk factors for Parkinson’s disease (PD) identified in a recent genome-wide association study (GWAS) meta-analysis. The table is adapted from [81]. Genes in bold font are also linked to the monogenic forms of familial PD.
| Chromosome | Gene |
|---|---|
| 1 | GBA, NUCKS1, SLC41A1, ITPKB, SIPA1L2 |
| 2 | IL1R2, TMEM163, CCNT2, SCN3A, STK39 |
| 3 | CHMP2B, MCCC1, SATB1, NCKIPSD, CCDC71, ALAS1, TLR9, DNAH1, BAP1, PHF7, NISCH, STAB1, ITIH3, ITIH4 |
| 4 | TMEM175, DGKQ, FAM200B, CD38, FAM47E, SNCA, ANK2, CAMK2D |
| 5 | ELOVL7 |
| 6 | ZNF184, HLA−DRB6, HLA−DQA1 |
| 7 | KLHL7, NUPL2, GPNMB |
| 8 | MICU3, CTSB, SORBS3, PDLIM2, C8orf58, BIN3 |
| 9 | SH3GL2 |
| 10 | FAM171A1, BAG3 |
| 11 | DLG2, MIR4697 |
| 12 | LRRK2, OGFOD2 |
| 14 | GCH1, TMEM229B, GALC |
| 15 | VPS13C |
| 16 | ZNF646, KAT8, COQ7, TOX3 |
| 17 | ARHGAP27, CRHR1, SPPL2C, MAPT, STH, KANSL1, ATP6V0A1, PSMC3IP |
| 18 | SYT4 |
| 19 | LSM7 |
| 20 | DDRGK1 |
The discovery of genetic factors implicated in PD risk has motivated the generation of genetic models in both vertebrate and invertebrate animals [18,84]. These models have been instrumental in advancing our understanding of the pathophysiology and molecular mechanisms of PD. In flies, the most common approach to generate genetic models is to introduce causal or disease-associated genetic variations in their fly homologs or express human genes in wild-type or mutant forms. The latter is applicable only for dominant mutations. These methodologies are used to model both familial and sporadic PD. Moreover, the genetic models based on the familial PD-linked genes that are also implicated in sporadic PD (i.e., SNCA and LRRK2) are often not specifically designated as sporadic PD models. This is in line with the view that the boundaries between familial and sporadic PD are increasingly blurred. The following sections will first discuss the targeted genetic models based on familial PD-causal genes, whose common variants are associated with sporadic PD risk. Second, the models developed based on the genetic susceptibility factors associated with PD will be introduced. Finally, the genetic models of sporadic PD developed through serendipity will be discussed.
5.1. Familial PD-Linked Genes in Sporadic PD: SNCA and LRRK2
One of the most notable discoveries with regard to the genetics of sporadic PD is that some low-penetrance variants of the familial PD-linked genes, SNCA and LRRK2, are also present in sporadic cases [7,83,85]. SNCA, which encodes α-syn, is a causal gene of autosomal dominant familial PD. At least five pathogenic mutations (A30P, E46K, A53T, H50Q, and G51D), as well as gene duplications and triplications have been identified in approximately 1% of PD families [75,86,87,88,89,90]. SNCA dominant mutations are extremely rare; however, the discovery that α-syn is the major constituent of Lewy bodies [3], the pathological hallmark of most familial and sporadic PD, prompted the search for SNCA polymorphisms in sporadic cases. Duplications have been found in apparently sporadic cases [91,92], as well as in asymptomatic carriers [91,93], with an estimated penetrance of approximately 40% [91,94]. These findings suggest the presence of other genetic modifiers or environmental factors in the pathogenesis of PD due to SNCA duplication. An association of PD risk with polymorphisms in the promoter region and 3′ UTR of the SNCA gene is also reported [95,96,97]. The duplications and variants likely increase the expression levels of α-syn protein, thereby contributing to PD pathogenesis [98,99,100]. A recent work that evaluated the SNPs in the cis-regulatory elements of the SNCA gene on the gene expression levels and pathogenesis broadly confirms this conclusion [101].
α-Syn is abundantly expressed in the nervous system and enriched in the presynaptic terminals [102]. It is highly soluble and unfolded in aqueous solutions; however, it can self-assemble to form fibrils. Multiple lines of evidence suggest that the soluble prefibrillar form of α-syn oligomers, rather than larger aggregates, are the toxic species mediating the cellular dysfunction and degeneration [103]. α-Syn toxicity is rooted in its propensity for oligomerization, and disease-causing mutants are more prone to oligomerization than wild-type α-Syn [103]. Recent studies have also shown that α-syn oligomers can be released from neurons and spread in a prion-like manner by seeding with the soluble α-syn of the recipient cells [104,105,106,107].
Although Drosophila does not have SNCA homologs, because genetic and molecular evidence indicates that toxic gain-of-function mechanisms underlie α-syn-linked PD, the transgenic overexpression of human α-syn in flies provides a useful tool to investigate the pathogenesis of α-syn-linked PD. The overexpression of the human wild-type or mutant form of α-syn was the first PD model ever created in flies [28]. Similar strategies have been used in numerous studies to date and have shown that the overexpression of wild-type or mutant human α-syn impairs the function or survival of DA neurons and causes PD-like locomotor deficits [28,30,108,109,110,111], although some discrepancies exist regarding its effect on DA neuron loss [60,112,113].
Mutations in LRRK2 are the most frequent cause of PD, found in approximately 10% of autosomal dominant familial PD and 4% of sporadic cases worldwide [114]. The most common mutation, G2019S, is found in 4% of familial and 1% of apparently sporadic cases. The estimated penetrance of the G2019S mutation ranges from approximately 30 to 70%, depending on the age and ethnic background [115]. The G2019S mutation is particularly frequent in sporadic PD patients among Ashkenazi Jews (13%) [116] and North African Arabs (41%) [117]. No disease case caused by LRRK2 deletion or multiplication has been reported, and the disease phenotypes of homozygous LRRK2 mutant carriers are similar to those of heterozygous carriers [118]. Overall, these findings suggest that LRRK2 G2019S manifests PD by gain-of-function mechanisms that involve interactions with other genetic or environmental factors.
LRRK2 encodes a large 2527 amino-acid protein that contains multiple conserved domains, including a GTPase domain, a kinase domain, Armadillo, an Ankyrin repeat, a leucine-rich repeat, MAPKKK (MAP kinase kinase kinase), and WD-40 domains [119]. The G2019S mutation resides in the MAPKKK domain, and the mutation increases the kinase activity by at least two- to three-fold in vitro [120,121]. Drosophila LRRK (dLRRK) is the unique homolog of human LRRK2. Consistent with the notion that LRRK2 gain-of-function mutations cause PD, dLRRK null mutant flies show little or no defects in the viability of DA neurons [122], although some controversy exists [123]. Numerous transgenic fly lines expressing the wild-type or mutant form of dLRRK or human LRRK2 have been generated in an attempt to model dominantly inherited PD, with varying consequences in neuronal loss and locomotion. Pan-neuronal or DA neuron-targeted expression of human LRRK2 G2019S mutants, the genetic model relevant for sporadic PD, causes the selective age-dependent loss of DA neurons, locomotor deficits, and premature mortality [124,125,126,127,128].
Given that there are many excellent reviews summarizing the findings on α-syn or LRRK2 models in flies (such as [129,130,131,132]), instead of listing findings from individual papers, this review elects to briefly discuss the overarching insights from a large body of work based on these models. One of the emerging notions is the diversity of the mechanisms underlying the DA neuron demise. To date, there is no single unified theory explaining the pathogenic mechanism of α-syn overexpression or LRRK2 G2019S mutation; however, various intracellular machineries are implicated. Studies using Drosophila models have established a bewildering list of mechanisms involved in the α-syn pathogenicity. These mechanisms include alterations in the endoplasmic reticulum (ER)-Golgi trafficking and synaptic vesicle formation [133,134], glutathione abundance [111], glucose metabolism [135], actin cytoskeleton that leads to impaired mitochondrial dynamics [110], mitochondrial integrity and function [136], macroautophagy [137], and elevated oxidative stress levels [138,139,140].
An increasing number of potential LRRK2 phosphorylation targets involved in PD pathogenesis has been identified in the studies that used the fly model of LRRK2-linked PD, including the glycogen synthase kinase 3b (GSK 3b) [125], microtubule-binding protein 1B (MAP1B) homolog Futsch [141], endophilin A (EndoA) [142,143], eukaryotic initiation factor 4E-binding protein (4E-BP) [124], and ribosome protein s15 [144]. The phosphorylation of these targets by LRRK2 dysregulates cellular processes, such as dendritic and synaptic morphology, synaptic endocytosis, macroautophagy, and translational machinery. In addition, genetic and functional interaction assays have indicated several factors involved in the neurotoxicity of LRRK2, such as vacuolar protein sorting 35 (VPS35) [145,146], RAB7L1 [147], and ArfGAP1 [148,149], which are involved in vesicular trafficking, retromer, the lysosomal pathway, and miRNA-mediated translational inhibition.
The discovery of these numerous targets and pathways leads us to the next important questions: how are the multiple pathways related? Do they additively or synergistically act to disrupt cellular homeostasis, thereby leading to the eventual cell death? One connecting point may be the mitochondrial dysfunction, which has been proposed as a central pathogenic mechanism of PD in an increasing number of studies [150]. A healthy mitochondrial network is maintained by multiple quality control mechanisms, including the fission-fusion dynamics and clearance of damaged mitochondria via the autophagy-lysosome pathway, termed mitophagy [151]. Studies in flies have demonstrated that regulators of mitophagy, including the sterol regulatory element binding transcription factor 1 (SREBF1) gene known to be associated with the risk of sporadic PD, modulate the DA neuron toxicity caused by the mutations in familial PD-linked genes, PINK1, parkin, and LRRK2. These studies contribute evidence to the notion that sporadic and monogenic familial PD have common pathological mechanisms that converge on the abnormal mitochondrial homeostasis and mitophagy dysfunction [152,153,154].
Given the clinical, geographic, and ethnic heterogeneity of PD patients, it is also possible that different pathogenic mechanisms are triggered depending on the genetic background, environmental conditions, or in a state-dependent manner. To date, there is no clear view as to why the same mutation (e.g., LRRK2 G2019S) is causal to both familial and sporadic PD. Understanding whether and how different pathogenic mechanisms interact will help comprehend the mechanistic differences between familial and sporadic PD, for which Drosophila models can provide useful tools.
Both α-syn and LRRK2 G2019S models are based on overexpression via the use of binary systems, such as GAL4/UAS and LexA/LexAop [21]. Although the results obtained by the misexpression of foreign genes must be interpreted with a grain of salt, carefully controlled misexpression experiments can be useful to analyze the cell-type specificity of toxicity and neuroanatomical correlates with behavioral phenotypes, as well as to distinguish cell-autonomous from noncell-autonomous consequences of overexpressed genes. A prime example is the use of α-syn overexpression to map the neuronal subtypes relevant for climbing defects, which identified a subgroup of DA neurons within the PAM cluster, thus suggesting functional parallels between this group of neurons with DA neurons in the SNpc [30]. Another example is the overexpression of LRRK2 G2019S in DA neurons, which leads to noncell-autonomous degeneration in the visual system and the loss of visual function [155]. With the use of advanced genetic tools that enable gene expression in a spatially and temporarily tunable fashion, it is likely that the misexpression strategy will provide further insights into the mechanisms that underlie PD associated with gain-of-function mutations.
5.2. Models Based on Known Genetic Risk Factors: GBA, MAPT, and GAK
5.2.1. GBA
Homozygous or compound heterozygous mutations in the GBA gene that encodes β-glucocerebrosidase (GCase) cause Gaucher disease. Gaucher disease is a rare lipid storage disorder, and these patients are known to have an increased risk for PD. Moreover, single heterozygous mutations are associated with an increased risk of PD [7,81,83]. Approximately 300 mutations in GBA have been found to cause Gaucher diseases. Including the two common mutations associated with PD, N370S, and L444P, GBA mutations are considered to be loss-of-function mutations that lead to a deficiency in GCase. GCase is a lysosomal enzyme synthesized in the ER that undergoes N-linked glycosylation and folding and subsequently traffics to lysosomes through the Golgi. GBA mutations result in the accumulation of the lipid substrates of GCase [156]. Furthermore, the accumulation of misfolded mutant GCase causes ER stress and triggers the unfolded protein response (UPR). The resulting accumulation of misfolded protein, including that of α-syn, is thought to increase the risk of PD [157,158].
Drosophila has two homologs of the GBA gene, dGBA1a, and dGBA1b, both of which have approximately 50% similarity to GCase. Given that disease-causing GBA variants are loss-of-function alleles, several dGBA1a/b gene knockout or knockdown models that inactivate either or both of the dGBA1 genes have been generated [159,160,161,162]. In addition, the finding that many mutations, including PD-associated N370S and L444P, are likely to be dominant-negative has led to the creation of transgenic flies that express wild-type or mutant human GBA [158,163,164]. The phenotypes of these loss-of-function and misexpression models are roughly in agreement in that they show a reduced lifespan, locomotor impairments, protein aggregation, and neurodegeneration. The dGBA1a gene expression is restricted in the midgut, whereas dGBA1b is ubiquitously expressed [165]. This finding explains the observation that dGBA1a deletion is not detrimental, whereas the dGBA1b loss-of-function leads to the deleterious phenotypes [159,166].
In contrast, the results are less consistent with regard to DA neuron loss in the dGBA1 models. Some studies have indicated the loss of adult DA neurons [161,163,164], whereas other studies did not show DA neuron loss, although brain vacuolization was observed in dGBA1 loss-of-function models [159]. One study reported that in flies expressing α-syn, the dGBA1b mutation increases the misfolding of α-syn and mildly enhances α-syn-dependent DA neuron loss; however, other phenotypes of the dGBA1b mutation are unaffected by the presence of α-syn. Therefore, DA neuron degeneration is primarily the consequence of α-syn overexpression, and α-Syn and dGBA1 do not synergistically impair neuronal integrity [159], although a recent study contradicts this conclusion [167]. The mechanism regarding how dGBA1 deficiency promotes abnormal protein aggregation is also under debate. One study proposed that dGBA1b deficiency causes autophagy defects that lead to the accumulation of protein aggregates [166], whereas another report found no evidence of autophagy defects, but an increase in extracellular vesicles, which is thought to spread the protein aggregates in the dGBA1b null mutants [168].
Although these differences warrant further studies to clarify the contribution of dGBA1 in DA neuron degeneration and its underlying mechanisms, fly models have been effectively used to demonstrate that small molecule pharmacological chaperones that cross the BBB, ambroxol, and isofagomine, suppress GCase misfolding, reduce ER stress, and ameliorate the GBA mutant phenotype in vivo. These results provide supporting evidence that ER stress is a pathogenic process in GBA mutants and suggest a therapeutic potential of small molecule chaperons in GBA-associated PD [161,163]. Collectively, the dGBA1 mutation and hGBA misexpression models provide useful tools to better understand the molecular mechanisms that underlie the pathogenic process caused by GBA mutations and develop disease-modifying therapeutics.
5.2.2. MAPT
The MAPT gene encodes the microtubule-associated protein tau essential for tubulin polymerization and microtubule stability [169]. Accumulation of the misfolded tau protein is a pathological characteristic of a spectrum of neurological disorders referred to as tauopathies, including Alzheimer’s disease, progressive supranuclear palsy, corticobasal degeneration, and frontotemporal dementia. Although the majority of tauopathies occur sporadically, more than 50 pathogenic MAPT mutations were identified in approximately 150 families with tauopathies [170]. Tauopathies associated with MAPT mutations include familial and sporadic frontotemporal dementia (FTD) [171,172] (also referred to as “frontotemporal lobar degeneration with MAPT mutation (FTLD-tau)”) [173]. Intriguingly, Parkinsonism is a common comorbidity in patients of familial and sporadic tauopathies, and more than 50% of patients of Alzheimer’s disease manifest Lewy body pathology [174]. Furthermore, numerous studies, including GWAS meta-analyses, have confirmed the association of the MAPT locus and an increased PD risk in Caucasian populations [81,175]. These findings support the emerging view that tauopathies and synucleinopathies (associated with α-syn inclusions), the two disease classes characterized by the accumulation of pathological protein aggregates, belong to a continuum of disorders with significantly overlapping clinical features and pathogenic mechanisms [176].
The MAPT locus lies within a genomic region that has two conserved haplotypes, H1 and H2, defined by numerous single nucleotide polymorphisms [177]. H1 and H2 haplotypes cover the entire MAPT gene, differ in orientation and do not recombine [178] as they originate from a 900 kb inversion [179]. The H1 haplotype of the MAPT gene is associated with a risk of PD [180,181], as well as in sporadic tauopathies [182]. It has been shown that the H1 haplotype has a greater expression of the tau protein than H2 [183,184], and the H2 haplotype has a protective effect in neurodegeneration [185].
Drosophila tau (dtau) is a unique homolog of the MAPT gene. Given the central role of tau in tauopathies, it is not surprising that much of the related work on the fly is based on the overexpression of Drosophila or the human tau protein in wild-type or mutant forms. These models have been effectively used to investigate the toxicity of tau inclusions and their underlying mechanisms at the cellular and organismal levels (reviewed in References [186,187]). Deletion of the tau gene is not detrimental by itself and does not increase the toxicity of human amyloid-β on the fly’s lifespan [188], although tau deletion is reported to decrease the axonal microtubule number and density in larval and adult nervous systems [189]. One study demonstrated that, by ectopically expressing human tau and α-syn in the retina, DA neurons, and larval neuromuscular junction, tau overexpression enhances α-syn-mediated DA neuron loss. Conversely, α-syn overexpression enhances tau-induced motor dysfunction, microtubule disorganization, and axonal transport defects, among other neuronal dysfunctions [190]. These results are in agreement with the existence of the interaction between tauopathy and synucleinopathy and provide insights into the mechanism that underlies the role of tau in PD pathogenesis. Because the H1 haplotype is very polymorphic and includes subhaplotypes, defining specific genetic variants that associate with the increased risk of PD remains a major challenge [191,192]. Thus, fly models based on the polymorphisms in the MAPT gene associated with PD risk, rather than overexpression, are yet to be generated.
5.2.3. GAK
A chromosomal locus that contains the GAK gene has been repeatedly found in GWAS [175,193]. Like many other genetic risk factors identified in GWAS, the association of GAK with PD risk may depend on the ethnic background and is replicated mainly in Caucasian populations [81,194]. GAK encodes cyclin-G-associated kinase, the association partner of cyclin-G and CDK5 and is involved in diverse cellular mechanisms, including clathrin-mediated endocytosis and mitotic progression [195]. The PD-associated GAK gene polymorphism reduces the GAK protein levels and correlates with the increase in α-syn expression [196]. A recent work demonstrated that RNAi-mediated silencing of auxilin (aux), the fly homolog of GAK, in DA neurons leads to age-dependent locomotor impairments, a reduced lifespan and progressive neuronal loss in several subclasses of DA neurons. Moreover, aux RNAi accelerates the onset and progression of DA neuron loss caused by α-syn overexpression [197]. These results support that GAK is a PD risk factor and aux gene silencing in flies provides a valid model to investigate the function of GAK in PD pathogenesis, including the potential synergism with α-syn toxicity.
5.3. Serendipitous Fly Models of Sporadic PD: Fer2 and Dfoxo Mutants
To date, only a few fly models that provide tools to investigate sporadic PD have been generated by serendipity. This may seem like an unusual path to model a human disease, compared to the targeted approach of using knowledge regarding the genetic and chemical factors relevant to the disease. However, historically, many animal models have been developed by serendipity, typically through the careful phenotypic analysis of mutant animals carrying spontaneous mutations or induced by forward genetics, and have contributed to the study of diseases [198]. This approach has the potential to fill the knowledge gap in the genetic factors involved in the disease pathogenesis. Using a statistical model termed genome-wide complex trait analysis (GCTA), which quantifies the “missing heritability” in PD, it was estimated that genetic factors could explain up to 27% of PD cases. In contrast, only 3–5% of cases have been attributed to the single nucleotide polymorphisms (SNPs) identified in GWAS [199]. This finding suggests that many more genetic variants that influence PD risk and could represent novel therapeutic targets may have yet to be discovered. The bottom-up approach transitioning from mutant phenotypic characterization to gene discovery has the potential to open a new avenue in PD research.
The bHLH-transcription factor Fer2 (p48-related 2) in Drosophila shares a high homology with the mammalian transcription factors p48/ptf1a and Ferd3l (Nato3) within the bHLH domain. Both p48/ptf1a and Ferd3l are involved in the control of neuronal development [200,201,202]. In the fly brain, Fer2 is expressed in a limited number of neurons, including a subgroup of circadian pacemaker neurons [203,204,205] and two subclasses of DA neurons, PAM and PAL [26,206]. Intriguingly, hypomorphic mutants of Fer2 exhibit PD-like phenotypes typical for fly models of PD. These phenotypes include locomotor deficits that can be improved by l-dopa administration, a reduced lifespan and a progressive DA neuron loss in the PAM cluster. Because fewer numbers of PAM neurons are born in both strong and milder hypomorphic mutants of Fer2, the role of Fer2 in PAM neuron development is indisputable. However, the knockdown of Fer2 restricted in adulthood demonstrated that Fer2 plays an essential role in the survival of adult DA neurons independent of its role in the development. Furthermore, it was also shown that oxidative stress elicited by the oxidants H2O2 or paraquat induces DA neuron loss specifically in PAM neurons in Fer2 mutant flies. At the cellular level, the Fer2 mutation causes abnormalities in the mitochondrial morphology and autophagosome formation, the well-known pathogenic molecular events implicated in PD, which suggests that Fer2 controls the expression of genes involved in multiple mechanisms that are normally protective of DA neurons [26,207]. Collectively, these results suggest that Fer2 mutant flies are useful for investigating the mechanisms of DA neuron demise, particularly the gene-environmental interaction with the presence of oxidative stress, thereby providing an opportunity to investigate pathogenic processes in PD.
The forkhead box family of transcription factors (FOXOs) are regulators of diverse biological pathways, including the stress response, metabolism and cellular homeostasis and are implicated in numerous diseases [208]. Drosophila has a unique homolog of FOXO, encoded by the dfoxo gene. Although no direct association of polymorphisms in FOXO factors with PD risk has been identified in the human population, changes in the gene expression levels in PD patients [209] and studies in rodents and flies suggest the role of FOXO factors in the survival of DA neurons in aged animals and PD models [210,211,212,213,214,215,216]. In addition, it was recently found that dfoxo null mutants and Fer2 mutants are phenotypically similar, showing the selective loss of PAM cluster DA neurons, locomotor deficits, and a reduced lifespan. Both Fer2 and dfoxo mutants exhibit mitochondrial damage and an autophagy defect specifically in PAM neurons. A genetic interaction analysis showed that the loss of function of Fer2 and dfoxo additively, but not synergistically, aggravates DA neuron loss. Consistent with the finding that mitochondria and autophagy are commonly affected in both mutants, the overexpression of one gene can rescue the DA neuron loss caused by the mutation of the other gene. Interestingly, however, in contrast to Fer2 mutants, oxidative stress does not enhance the DA neuron loss in dfoxo mutants. These results suggest that the activities of Fer2 and dfoxo are regulated by different cellular stressors; however, their downstream pathways converge onto the process that leads to mitochondrial biology and autophagy, thereby promoting the survival of DA neurons in the PAM cluster [207].
The results of Fer2 and dfoxo mutants highlight the complex interaction between multiple genetic and environmental factors that affect DA neuron viability and its impact on the pathogenesis of PD. It will be of substantial interest to determine whether their homologs, downstream or interacting genes in humans have a causal role in PD pathogenesis.
6. Conclusions
An increasing body of work to model sporadic PD in flies has verified candidate environmental and genetic risk factors and provided insights into their roles in the pathogenesis of PD. The current list of genetic risk variants is by no means exhaustive to explain all sporadic PD cases, and as the expansion and technological improvement of GWAS continues, many more susceptibility loci are expected to be identified and prompt the generation of genetic animal models of sporadic PD in the coming years. Furthermore, more unbiased approaches going from phenotype to genotype, including forwarding genetic screens, remain valuable to identify novel genetic factors that modify the risk or severity of PD. Fly models continue to provide effective tools to better understand whether and how different molecular pathways involved in the degenerative process interplay, which is the knowledge necessary to develop an efficient and potentially personalized treatment strategy for this complex disorder.
Abbreviations
| PD | Parkinson’s disease |
| DA | dopamine |
| SNpc | substantia nigra pars compacta |
| α-syn | α-synuclein |
| SING | startle-induced negative geotaxis |
| PAM | protocerebral anterior medial |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin |
| MPPP | 1-methyl-4-phenyl-4-propionoxypiperidine |
| MPP+ | 1-methyl-4-phenylpyridinium ion |
| MAO-B | monoamine oxidase B |
| DAT | dopamine transporter |
| BBB | blood brain barrier |
| ROS | reactive oxygen species |
| Maneb | manganese ethylenebisdithiocarbamate |
| ziram | zinc dimethylbisdithiocarbamate |
| Hsp-70 | heat-shock protein-70 |
| PAL | protocerebral anterior lateral |
| PPM | protocerebral posterior medial |
| PPL | protocerebral posterior lateral |
| dVMAT | Drosophila vesicular monoamine transporter |
| Catsup | Catecholamines-up |
| DAMB | D1-like dopamine receptor |
| TH | tyrosine hydroxylase |
| H2O2 | hydrogen peroxide |
| HO | hydroxyl radicals |
| UCP2 | uncoupling protein 2 |
| LRRK2 | leucine-rich repeat kinase 2 |
| PINK1 | PTEN-induced putative kinase 1 |
| GWAS | genome-wide association study |
| MAPKKK | MAP kinase kinase kinase |
| GSK 3b | glycogen synthase kinase 3b |
| MAP1B | microtubule-binding protein 1B |
| EndoA | endophilin A |
| 4E-BP | eukaryotic initiation factor 4E-binding protein |
| VPS35 | vacuolar protein sorting 35 |
| SREBF1 | sterol regulatory element binding transcription factor 1 |
| GCase | β-glucocerebrosidase |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| FTD | frontotemporal dementia |
| GCTA | genome-wide complex trait analysis |
| SNP | single nucleotide polymorphism |
| Fer2 | p48-related 2 |
Funding
This research was funded by the European Research Council, grant number ERC-2012-StG_20111109, Project number 311194 and the Swiss National Science Foundation, grant number 31003A_169548.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15(Suppl. 1):14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 4.Kalia L.V., Lang A.E., Hazrati L.N., Fujioka S., Wszolek Z.K., Dickson D.W., Ross O.A., Van Deerlin V.M., Trojanowski J.Q., Hurtig H.I., et al. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015;72:100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulopoulos M., Levy O.A., Alcalay R.N. The neuropathology of genetic Parkinson’s disease. Mov. Disord. 2012;27:831–842. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte C., Gasser T. Genetic basis of Parkinson’s disease: Inheritance, penetrance, and expression. Appl. Clin. Genet. 2011;4:67–80. doi: 10.2147/TACG.S11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foltynie T., Sawcer S., Brayne C., Barker R.A. The genetic basis of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2002;73:363–370. doi: 10.1136/jnnp.73.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeStefano A.L., Golbe L.I., Mark M.H., Lazzarini A.M., Maher N.E., Saint-Hilaire M., Feldman R.G., Guttman M., Watts R.L., Suchowersky O., et al. Genome-wide scan for Parkinson’s disease: The Gene PD Study. Neurology. 2001;57:1124–1126. doi: 10.1212/WNL.57.6.1124. [DOI] [PubMed] [Google Scholar]
- 10.Pankratz N., Nichols W.C., Uniacke S.K., Halter C., Murrell J., Rudolph A., Shults C.W., Conneally P.M., Foroud T., Parkinson Study G. Genome-wide linkage analysis and evidence of gene-by-gene interactions in a sample of 362 multiplex Parkinson disease families. Hum. Mol. Genet. 2003;12:2599–2608. doi: 10.1093/hmg/ddg270. [DOI] [PubMed] [Google Scholar]
- 11.Scott W.K., Nance M.A., Watts R.L., Hubble J.P., Koller W.C., Lyons K., Pahwa R., Stern M.B., Colcher A., Hiner B.C., et al. Complete genomic screen in Parkinson disease: Evidence for multiple genes. JAMA. 2001;286:2239–2244. doi: 10.1001/jama.286.18.2239. [DOI] [PubMed] [Google Scholar]
- 12.Gasser T. Mendelian forms of Parkinson’s disease. Biochim. Biophys. Acta. 2009;1792:587–596. doi: 10.1016/j.bbadis.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez D.G., Reed X., Singleton A.B. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J. Neurochem. 2016;139(Suppl. 1):59–74. doi: 10.1111/jnc.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba Y., Markopoulou K., Putzke J.D., Whaley N.R., Farrer M.J., Wszolek Z.K., Uitti R.J. Phenotypic commonalities in familial and sporadic Parkinson disease. Arch. Neurol. 2006;63:579–583. doi: 10.1001/archneur.63.4.579. [DOI] [PubMed] [Google Scholar]
- 15.Papapetropoulos S., Adi N., Ellul J., Argyriou A.A., Chroni E. A prospective study of familial versus sporadic Parkinson’s disease. Neurodegener. Dis. 2007;4:424–427. doi: 10.1159/000107702. [DOI] [PubMed] [Google Scholar]
- 16.McGonigle P., Ruggeri B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014;87:162–171. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Falkenburger B.H., Saridaki T., Dinter E. Cellular models for Parkinson’s disease. J. Neurochem. 2016;139(Suppl. 1):121–130. doi: 10.1111/jnc.13618. [DOI] [PubMed] [Google Scholar]
- 18.Blesa J., Przedborski S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagmag S.A., Tripathi N., Shukla S.D., Maiti S., Khurana S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2015;9:503. doi: 10.3389/fnins.2015.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey U.B., Nichols C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venken K.J., Simpson J.H., Bellen H.J. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wangler M.F., Yamamoto S., Bellen H.J. Fruit flies in biomedical research. Genetics. 2015;199:639–653. doi: 10.1534/genetics.114.171785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Hernandez I., Scheenaard E., Pollarolo G., Gonzalez C. The translational relevance of Drosophila in drug discovery. EMBO Rep. 2016;17:471–472. doi: 10.15252/embr.201642080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao Z., Davis R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto S., Seto E.S. Dopamine dynamics and signaling in Drosophila: An overview of genes, drugs and behavioral paradigms. Exp. Anim. 2014;63:107–119. doi: 10.1538/expanim.63.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bou Dib P., Gnagi B., Daly F., Sabado V., Tas D., Glauser D.A., Meister P., Nagoshi E. A conserved role for p48 homologs in protecting dopaminergic neurons from oxidative stress. PLoS Genet. 2014;10:e1004718. doi: 10.1371/journal.pgen.1004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coulom H., Birman S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004;24:10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feany M.B., Bender W.W. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 29.Nichols C.D., Becnel J., Pandey U.B. Methods to assay Drosophila behavior. J. Vis. Exp. 2012 doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemensperger T., Issa A.R., Pech U., Coulom H., Nguyen M.V., Cassar M., Jacquet M., Fiala A., Birman S. A single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of Parkinson disease. Cell Rep. 2013;5:952–960. doi: 10.1016/j.celrep.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Sun J., Xu A.Q., Giraud J., Poppinga H., Riemensperger T., Fiala A., Birman S. Neural Control of Startle-Induced Locomotion by the Mushroom Bodies and Associated Neurons in Drosophila. Front. Syst. Neurosci. 2018;12:6. doi: 10.3389/fnsys.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajula Balija M.B., Griesinger C., Herzig A., Zweckstetter M., Jackle H. Pre-fibrillar alpha-synuclein mutants cause Parkinson’s disease-like non-motor symptoms in Drosophila. PLoS ONE. 2011;6:e24701. doi: 10.1371/journal.pone.0024701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julienne H., Buhl E., Leslie D.S., Hodge J.J.L. Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor Parkinson’s disease phenotypes. Neurobiol. Dis. 2017;104:15–23. doi: 10.1016/j.nbd.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afsari F., Christensen K.V., Smith G.P., Hentzer M., Nippe O.M., Elliott C.J., Wade A.R. Abnormal visual gain control in a Parkinson’s disease model. Hum. Mol. Genet. 2014;23:4465–4478. doi: 10.1093/hmg/ddu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannarkat G.T., Boss J.M., Tansey M.G. The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinsons Dis. 2013;3:493–514. doi: 10.3233/JPD-130250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartenstein V., Cruz L., Lovick J.K., Guo M. Developmental analysis of the dopamine-containing neurons of the Drosophila brain. J. Comp. Neurol. 2017;525:363–379. doi: 10.1002/cne.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noyce A.J., Bestwick J.P., Silveira-Moriyama L., Hawkes C.H., Giovannoni G., Lees A.J., Schrag A. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Mark M., Brouwer M., Kromhout H., Nijssen P., Huss A., Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ. Health Perspect. 2012;120:340–347. doi: 10.1289/ehp.1103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ascherio A., Chen H., Weisskopf M.G., O’Reilly E., McCullough M.L., Calle E.E., Schwarzschild M.A., Thun M.J. Pesticide exposure and risk for Parkinson’s disease. Ann. Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 40.Dick F.D., De Palma G., Ahmadi A., Scott N.W., Prescott G.J., Bennett J., Semple S., Dick S., Counsell C., Mozzoni P., et al. Environmental risk factors for Parkinson’s disease and parkinsonism: The Geoparkinson study. Occup. Environ. Med. 2007;64:666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elbaz A., Clavel J., Rathouz P.J., Moisan F., Galanaud J.P., Delemotte B., Alperovitch A., Tzourio C. Professional exposure to pesticides and Parkinson disease. Ann. Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- 42.Van Maele-Fabry G., Hoet P., Vilain F., Lison D. Occupational exposure to pesticides and Parkinson’s disease: A systematic review and meta-analysis of cohort studies. Environ. Int. 2012;46:30–43. doi: 10.1016/j.envint.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Bellou V., Belbasis L., Tzoulaki I., Evangelou E., Ioannidis J.P. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat. Disord. 2016;23:1–9. doi: 10.1016/j.parkreldis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Breckenridge C.B., Berry C., Chang E.T., Sielken R.L., Jr., Mandel J.S. Association between Parkinson’s Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0151841. doi: 10.1371/journal.pone.0151841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casida J.E. The greening of pesticide-environment interactions: Some personal observations. Environ. Health Perspect. 2012;120:487–493. doi: 10.1289/ehp.1104405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner C.M., Kamel F., Ross G.W., Hoppin J.A., Goldman S.M., Korell M., Marras C., Bhudhikanok G.S., Kasten M., Chade A.R., et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherer T.B., Richardson J.R., Testa C.M., Seo B.B., Panov A.V., Yagi T., Matsuno-Yagi A., Miller G.W., Greenamyre J.T. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi W.S., Kruse S.E., Palmiter R.D., Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc. Natl. Acad. Sci. USA. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langston J.W., Ballard P., Tetrud J.W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 50.Meredith G.E., Rademacher D.J. MPTP mouse models of Parkinson’s disease: An update. J. Parkinsons Dis. 2011;1:19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rappold P.M., Cui M., Chesser A.S., Tibbett J., Grima J.C., Duan L., Sen N., Javitch J.A., Tieu K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Natl. Acad. Sci. USA. 2011;108:20766–20771. doi: 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cocheme H.M., Murphy M.P. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 53.Hosamani R., Muralidhara Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction. Arch. Insect Biochem. Physiol. 2013;83:25–40. doi: 10.1002/arch.21094. [DOI] [PubMed] [Google Scholar]
- 54.Kamel F., Tanner C., Umbach D., Hoppin J., Alavanja M., Blair A., Comyns K., Goldman S., Korell M., Langston J., et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am. J. Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 55.Lawal H.O., Chang H.Y., Terrell A.N., Brooks E.S., Pulido D., Simon A.F., Krantz D.E. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudhuri A., Bowling K., Funderburk C., Lawal H., Inamdar A., Wang Z., O’Donnell J.M. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cassar M., Issa A.R., Riemensperger T., Petitgas C., Rival T., Coulom H., Iche-Torres M., Han K.A., Birman S. A dopamine receptor contributes to paraquat-induced neurotoxicity in Drosophila. Hum. Mol. Genet. 2015;24:197–212. doi: 10.1093/hmg/ddu430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shukla A.K., Pragya P., Chaouhan H.S., Tiwari A.K., Patel D.K., Abdin M.Z., Chowdhuri D.K. Heat shock protein-70 (Hsp-70) suppresses paraquat-induced neurodegeneration by inhibiting JNK and caspase-3 activation in Drosophila model of Parkinson’s disease. PLoS ONE. 2014;9:e98886. doi: 10.1371/journal.pone.0098886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meulener M., Whitworth A.J., Armstrong-Gold C.E., Rizzu P., Heutink P., Wes P.D., Pallanck L.J., Bonini N.M. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr. Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 60.Navarro J.A., Hessner S., Yenisetti S.C., Bayersdorfer F., Zhang L., Voigt A., Schneuwly S., Botella J.A. Analysis of dopaminergic neuronal dysfunction in genetic and toxin-induced models of Parkinson’s disease in Drosophila. J. Neurochem. 2014;131:369–382. doi: 10.1111/jnc.12818. [DOI] [PubMed] [Google Scholar]
- 61.Costello S., Cockburn M., Bronstein J., Zhang X., Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am. J. Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang A., Costello S., Cockburn M., Zhang X., Bronstein J., Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur. J. Epidemiol. 2011;26:547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin C.A., Barajas A., Lawless G., Lawal H.O., Assani K., Lumintang Y.P., Nunez V., Krantz D.E. Synergistic effects on dopamine cell death in a Drosophila model of chronic toxin exposure. Neurotoxicology. 2014;44:344–351. doi: 10.1016/j.neuro.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla A.K., Pragya P., Chaouhan H.S., Patel D.K., Abdin M.Z., Kar Chowdhuri D. Mutation in Drosophila methuselah resists paraquat induced Parkinson-like phenotypes. Neurobiol. Aging. 2014;35:2419. doi: 10.1016/j.neurobiolaging.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Bayersdorfer F., Voigt A., Schneuwly S., Botella J.A. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol. Dis. 2010;40:113–119. doi: 10.1016/j.nbd.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Mosharov E.V., Larsen K.E., Kanter E., Phillips K.A., Wilson K., Schmitz Y., Krantz D.E., Kobayashi K., Edwards R.H., Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherer T.B., Betarbet R., Testa C.M., Seo B.B., Richardson J.R., Kim J.H., Miller G.W., Yagi T., Matsuno-Yagi A., Greenamyre J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J.A., Robinson J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 69.Richardson J.R., Quan Y., Sherer T.B., Greenamyre J.T., Miller G.W. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 70.Bus J.S., Gibson J.E. Paraquat: Model for oxidant-initiated toxicity. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stephano F., Nolte S., Hoffmann J., El-Kholy S., von Frieling J., Bruchhaus I., Fink C., Roeder T. Impaired Wnt signaling in dopamine containing neurons is associated with pathogenesis in a rotenone triggered Drosophila Parkinson’s disease model. Sci. Rep. 2018;8:2372. doi: 10.1038/s41598-018-20836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brand M.D., Esteves T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Islam R., Yang L., Sah M., Kannan K., Anamani D., Vijayan C., Kwok J., Cantino M.E., Beal M.F., Fridell Y.W. A neuroprotective role of the human uncoupling protein 2 (hUCP2) in a Drosophila Parkinson’s disease model. Neurobiol. Dis. 2012;46:137–146. doi: 10.1016/j.nbd.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 74.Hwang R.D., Wiemerslage L., LaBreck C.J., Khan M., Kannan K., Wang X., Zhu X., Lee D., Fridell Y.W. The neuroprotective effect of human uncoupling protein 2 (hUCP2) requires cAMP-dependent protein kinase in a toxin model of Parkinson’s disease. Neurobiol. Dis. 2014;69:180–191. doi: 10.1016/j.nbd.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 75.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 76.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 78.Valente E.M., Abou-Sleiman P.M., Caputo V., Muqit M.M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A.R., Healy D.G., et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 79.Bonifati V., Rizzu P., van Baren M.J., Schaap O., Breedveld G.J., Krieger E., Dekker M.C., Squitieri F., Ibanez P., Joosse M., et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 80.Farrer M.J. Genetics of Parkinson disease: Paradigm shifts and future prospects. Nat. Rev. Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 81.Chang D., Nalls M.A., Hallgrimsdottir I.B., Hunkapiller J., van der Brug M., Cai F., International Parkinson’s Disease Genomics Consortium. 23andMe Research Team. Kerchner G.A., Ayalon G., et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nalls M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., DeStefano A.L., Kara E., Bras J., Sharma M., et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lesage S., Brice A. Role of mendelian genes in “sporadic” Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18(Suppl. 1):S66–S70. doi: 10.1016/S1353-8020(11)70022-0. [DOI] [PubMed] [Google Scholar]
- 84.Pienaar I.S., Gotz J., Feany M.B. Parkinson’s disease: Insights from non-traditional model organisms. Prog. Neurobiol. 2010;92:558–571. doi: 10.1016/j.pneurobio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Lesage S., Brice A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 86.Appel-Cresswell S., Vilarino-Guell C., Encarnacion M., Sherman H., Yu I., Shah B., Weir D., Thompson C., Szu-Tu C., Trinh J., et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov. Disord. 2013;28:811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 87.Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., Przuntek H., Epplen J.T., Schols L., Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 88.Lesage S., Anheim M., Letournel F., Bousset L., Honore A., Rozas N., Pieri L., Madiona K., Durr A., Melki R., et al. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 2013;73:459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 89.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 90.Zarranz J.J., Alegre J., Gomez-Esteban J.C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atares B., et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 91.Ahn T.B., Kim S.Y., Kim J.Y., Park S.S., Lee D.S., Min H.J., Kim Y.K., Kim S.E., Kim J.M., Kim H.J., et al. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 92.Troiano A.R., Cazeneuve C., Le Ber I., Bonnet A.M., Lesage S., Brice A. Re: Alpha-synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;71:1295. doi: 10.1212/01.wnl.0000338435.78120.0f. [DOI] [PubMed] [Google Scholar]
- 93.Nishioka K., Ross O.A., Ishii K., Kachergus J.M., Ishiwata K., Kitagawa M., Kono S., Obi T., Mizoguchi K., Inoue Y., et al. Expanding the clinical phenotype of SNCA duplication carriers. Mov. Disord. 2009;24:1811–1819. doi: 10.1002/mds.22682. [DOI] [PubMed] [Google Scholar]
- 94.Nishioka K., Hayashi S., Farrer M.J., Singleton A.B., Yoshino H., Imai H., Kitami T., Sato K., Kuroda R., Tomiyama H., et al. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann. Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 95.Kruger R., Vieira-Saecker A.M., Kuhn W., Berg D., Muller T., Kuhnl N., Fuchs G.A., Storch A., Hungs M., Woitalla D., et al. Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann. Neurol. 1999;45:611–617. doi: 10.1002/1531-8249(199905)45:5<611::AID-ANA9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 96.Mueller J.C., Fuchs J., Hofer A., Zimprich A., Lichtner P., Illig T., Berg D., Wullner U., Meitinger T., Gasser T. Multiple regions of alpha-synuclein are associated with Parkinson’s disease. Ann. Neurol. 2005;57:535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- 97.Tan E.K., Tan C., Shen H., Chai A., Lum S.Y., Teoh M.L., Yih Y., Wong M.C., Zhao Y. Alpha synuclein promoter and risk of Parkinson’s disease: Microsatellite and allelic size variability. Neurosci. Lett. 2003;336:70–72. doi: 10.1016/S0304-3940(02)01178-3. [DOI] [PubMed] [Google Scholar]
- 98.Chiba-Falek O., Nussbaum R.L. Effect of allelic variation at the NACP-Rep1 repeat upstream of the alpha-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum. Mol. Genet. 2001;10:3101–3109. doi: 10.1093/hmg/10.26.3101. [DOI] [PubMed] [Google Scholar]
- 99.Eriksen J.L., Przedborski S., Petrucelli L. Gene dosage and pathogenesis of Parkinson’s disease. Trends Mol. Med. 2005;11:91–96. doi: 10.1016/j.molmed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 100.McCarthy J.J., Linnertz C., Saucier L., Burke J.R., Hulette C.M., Welsh-Bohmer K.A., Chiba-Falek O. The effect of SNCA 3’ region on the levels of SNCA-112 splicing variant. Neurogenetics. 2011;12:59–64. doi: 10.1007/s10048-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soldner F., Stelzer Y., Shivalila C.S., Abraham B.J., Latourelle J.C., Barrasa M.I., Goldmann J., Myers R.H., Young R.A., Jaenisch R. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature. 2016;533:95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.George J.M. The synucleins. Genome Biol. 2002;3:REVIEWS3002. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stefanis L. Alpha-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2:a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brundin P., Melki R. Prying into the Prion Hypothesis for Parkinson’s Disease. J. Neurosci. 2017;37:9808–9818. doi: 10.1523/JNEUROSCI.1788-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luk K.C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peelaerts W., Bousset L., Van der Perren A., Moskalyuk A., Pulizzi R., Giugliano M., Van den Haute C., Melki R., Baekelandt V. Alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 108.Auluck P.K., Chan H.Y., Trojanowski J.Q., Lee V.M., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 109.Chen L., Feany M.B. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 110.Ordonez D.G., Lee M.K., Feany M.B. Alpha-synuclein Induces Mitochondrial Dysfunction through Spectrin and the Actin Cytoskeleton. Neuron. 2018;97:108–124. doi: 10.1016/j.neuron.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trinh K., Moore K., Wes P.D., Muchowski P.J., Dey J., Andrews L., Pallanck L.J. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson’s disease. J. Neurosci. 2008;28:465–472. doi: 10.1523/JNEUROSCI.4778-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pesah Y., Burgess H., Middlebrooks B., Ronningen K., Prosser J., Tirunagaru V., Zysk J., Mardon G. Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of alpha-Synuclein in Drosophila. Genesis. 2005;41:154–159. doi: 10.1002/gene.20106. [DOI] [PubMed] [Google Scholar]
- 113.Whitworth A.J., Wes P.D., Pallanck L.J. Drosophila models pioneer a new approach to drug discovery for Parkinson’s disease. Drug Discov. Today. 2006;11:119–126. doi: 10.1016/S1359-6446(05)03693-7. [DOI] [PubMed] [Google Scholar]
- 114.Berg D., Schweitzer K.J., Leitner P., Zimprich A., Lichtner P., Belcredi P., Brussel T., Schulte C., Maass S., Nagele T., et al. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson’s disease. Pt 12Brain. 2005;128:3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- 115.Healy D.G., Falchi M., O’Sullivan S.S., Bonifati V., Durr A., Bressman S., Brice A., Aasly J., Zabetian C.P., Goldwurm S., et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozelius L.J., Senthil G., Saunders-Pullman R., Ohmann E., Deligtisch A., Tagliati M., Hunt A.L., Klein C., Henick B., Hailpern S.M., et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 117.Lesage S., Durr A., Tazir M., Lohmann E., Leutenegger A.L., Janin S., Pollak P., Brice A., French Parkinson’s Disease Genetics Study G. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N. Engl. J. Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 118.Ishihara L., Warren L., Gibson R., Amouri R., Lesage S., Durr A., Tazir M., Wszolek Z.K., Uitti R.J., Nichols W.C., et al. Clinical features of Parkinson disease patients with homozygous leucine-rich repeat kinase 2 G2019S mutations. Arch. Neurol. 2006;63:1250–1254. doi: 10.1001/archneur.63.9.1250. [DOI] [PubMed] [Google Scholar]
- 119.Cookson M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu M., Kang S., Ray S., Jackson J., Zaitsev A.D., Gerber S.A., Cuny G.D., Glicksman M.A. Kinetic, mechanistic, and structural modeling studies of truncated wild-type leucine-rich repeat kinase 2 and the G2019S mutant. Biochemistry. 2011;50:9399–9408. doi: 10.1021/bi201173d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nichols R.J., Dzamko N., Morrice N.A., Campbell D.G., Deak M., Ordureau A., Macartney T., Tong Y., Shen J., Prescott A.R., et al. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 2010;430:393–404. doi: 10.1042/BJ20100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang D., Tang B., Zhao G., Pan Q., Xia K., Bodmer R., Zhang Z. Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol. Neurodegener. 2008;3:3. doi: 10.1186/1750-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee S.B., Kim W., Lee S., Chung J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem. Biophys. Res. Commun. 2007;358:534–539. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- 124.Imai Y., Gehrke S., Wang H.Q., Takahashi R., Hasegawa K., Oota E., Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin C.H., Tsai P.I., Wu R.M., Chien C.T. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ss. J. Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Z., Wang X., Yu Y., Li X., Wang T., Jiang H., Ren Q., Jiao Y., Sawa A., Moran T., et al. A Drosophila model for LRRK2-linked parkinsonism. Proc. Natl. Acad. Sci. USA. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ng C.H., Mok S.Z., Koh C., Ouyang X., Fivaz M.L., Tan E.K., Dawson V.L., Dawson T.M., Yu F., Lim K.L. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J. Neurosci. 2009;29:11257–11262. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Venderova K., Kabbach G., Abdel-Messih E., Zhang Y., Parks R.J., Imai Y., Gehrke S., Ngsee J., Lavoie M.J., Slack R.S., et al. Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum. Mol. Genet. 2009;18:4390–4404. doi: 10.1093/hmg/ddp394. [DOI] [PubMed] [Google Scholar]
- 129.Li T., Yang D., Sushchky S., Liu Z., Smith W.W. Models for LRRK2-Linked Parkinsonism. Parkinsons Dis. 2011;2011:942412. doi: 10.4061/2011/942412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mizuno H., Fujikake N., Wada K., Nagai Y. alpha-Synuclein Transgenic Drosophila as a Model of Parkinson’s Disease and Related Synucleinopathies. Parkinsons Dis. 2010;2011:212706. doi: 10.4061/2011/212706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whitworth A.J. Drosophila models of Parkinson’s disease. Adv. Genet. 2011;73:1–50. doi: 10.1016/B978-0-12-380860-8.00001-X. [DOI] [PubMed] [Google Scholar]
- 132.Xiong Y., Yu J. Modeling Parkinson’s Disease in Drosophila: What Have We Learned for Dominant Traits? Front Neurol. 2018;9:228. doi: 10.3389/fneur.2018.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Breda C., Nugent M.L., Estranero J.G., Kyriacou C.P., Outeiro T.F., Steinert J.R., Giorgini F. Rab11 modulates alpha-synuclein-mediated defects in synaptic transmission and behaviour. Hum. Mol. Genet. 2015;24:1077–1091. doi: 10.1093/hmg/ddu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F., et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Knight A.L., Yan X., Hamamichi S., Ajjuri R.R., Mazzulli J.R., Zhang M.W., Daigle J.G., Zhang S., Borom A.R., Roberts L.R., et al. The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell Metab. 2014;20:145–157. doi: 10.1016/j.cmet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Butler E.K., Voigt A., Lutz A.K., Toegel J.P., Gerhardt E., Karsten P., Falkenburger B., Reinartz A., Winklhofer K.F., Schulz J.B. The mitochondrial chaperone protein TRAP1 mitigates alpha-Synuclein toxicity. PLoS Genet. 2012;8:e1002488. doi: 10.1371/journal.pgen.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Issa A.R., Sun J., Petitgas C., Mesquita A., Dulac A., Robin M., Mollereau B., Jenny A., Cherif-Zahar B., Birman S. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain. Autophagy. 2018;14:1898–1910. doi: 10.1080/15548627.2018.1491489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barone M.C., Sykiotis G.P., Bohmann D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis. Model Mech. 2011;4:701–707. doi: 10.1242/dmm.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Botella J.A., Bayersdorfer F., Schneuwly S. Superoxide dismutase overexpression protects dopaminergic neurons in a Drosophila model of Parkinson’s disease. Neurobiol. Dis. 2008;30:65–73. doi: 10.1016/j.nbd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 140.Wang B., Liu Q., Shan H., Xia C., Liu Z. Nrf2 inducer and cncC overexpression attenuates neurodegeneration due to alpha-synuclein in Drosophila. Biochem. Cell Biol. 2015;93:351–358. doi: 10.1139/bcb-2015-0015. [DOI] [PubMed] [Google Scholar]
- 141.Lee S., Liu H.P., Lin W.Y., Guo H., Lu B. LRRK2 kinase regulates synaptic morphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J. Neurosci. 2010;30:16959–16969. doi: 10.1523/JNEUROSCI.1807-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matta S., Van Kolen K., da Cunha R., van den Bogaart G., Mandemakers W., Miskiewicz K., De Bock P.J., Morais V.A., Vilain S., Haddad D., et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75:1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 143.Soukup S.F., Kuenen S., Vanhauwaert R., Manetsberger J., Hernandez-Diaz S., Swerts J., Schoovaerts N., Vilain S., Gounko N.V., Vints K., et al. A LRRK2-Dependent EndophilinA Phosphoswitch Is Critical for Macroautophagy at Presynaptic Terminals. Neuron. 2016;92:829–844. doi: 10.1016/j.neuron.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 144.Martin I., Kim J.W., Lee B.D., Kang H.C., Xu J.C., Jia H., Stankowski J., Kim M.S., Zhong J., Kumar M., et al. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell. 2014;157:472–485. doi: 10.1016/j.cell.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Inoshita T., Arano T., Hosaka Y., Meng H., Umezaki Y., Kosugi S., Morimoto T., Koike M., Chang H.Y., Imai Y., et al. Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum. Mol. Genet. 2017;26:2933–2948. doi: 10.1093/hmg/ddx179. [DOI] [PubMed] [Google Scholar]
- 146.Linhart R., Wong S.A., Cao J., Tran M., Huynh A., Ardrey C., Park J.M., Hsu C., Taha S., Peterson R., et al. Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson’s disease mutant of Leucine-Rich Repeat Kinase 2 (LRRK2) Mol. Neurodegener. 2014;9:23. doi: 10.1186/1750-1326-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.MacLeod D.A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B.D., Marder K.S., Honig L.S., Clark L.N., Small S.A., et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gehrke S., Imai Y., Sokol N., Lu B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 2010;466:637–641. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xiong Y., Yuan C., Chen R., Dawson T.M., Dawson V.L. ArfGAP1 is a GTPase activating protein for LRRK2: Reciprocal regulation of ArfGAP1 by LRRK2. J. Neurosci. 2012;32:3877–3886. doi: 10.1523/JNEUROSCI.4566-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bose A., Beal M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016;139(Suppl. 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 151.Ni H.M., Williams J.A., Ding W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hsieh C.H., Shaltouki A., Gonzalez A.E., Bettencourt da Cruz A., Burbulla L.F., St. Lawrence E., Schule B., Krainc D., Palmer T.D., Wang X. Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ivatt R.M., Sanchez-Martinez A., Godena V.K., Brown S., Ziviani E., Whitworth A.J. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc. Natl. Acad. Sci. USA. 2014;111:8494–8499. doi: 10.1073/pnas.1321207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ryan B.J., Hoek S., Fon E.A., Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: From familial to sporadic disease. Trends Biochem. Sci. 2015;40:200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 155.Hindle S., Afsari F., Stark M., Middleton C.A., Evans G.J., Sweeney S.T., Elliott C.J. Dopaminergic expression of the Parkinsonian gene LRRK2-G2019S leads to non-autonomous visual neurodegeneration, accelerated by increased neural demands for energy. Hum. Mol. Genet. 2013;22:2129–2140. doi: 10.1093/hmg/ddt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grabowski G.A. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 157.Fishbein I., Kuo Y.M., Giasson B.I., Nussbaum R.L. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Pt 12Brain. 2014;137:3235–3247. doi: 10.1093/brain/awu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maor G., Rencus-Lazar S., Filocamo M., Steller H., Segal D., Horowitz M. Unfolded protein response in Gaucher disease: From human to Drosophila. Orphanet J. Rare Dis. 2013;8:140. doi: 10.1186/1750-1172-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davis M.Y., Trinh K., Thomas R.E., Yu S., Germanos A.A., Whitley B.N., Sardi S.P., Montine T.J., Pallanck L.J. Glucocerebrosidase Deficiency in Drosophila Results in alpha-Synuclein-Independent Protein Aggregation and Neurodegeneration. PLoS Genet. 2016;12:e1005944. doi: 10.1371/journal.pgen.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawasaki H., Suzuki T., Ito K., Takahara T., Goto-Inoue N., Setou M., Sakata K., Ishida N. Minos-insertion mutant of the Drosophila GBA gene homologue showed abnormal phenotypes of climbing ability, sleep and life span with accumulation of hydroxy-glucocerebroside. Gene. 2017;614:49–55. doi: 10.1016/j.gene.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 161.Maor G., Cabasso O., Krivoruk O., Rodriguez J., Steller H., Segal D., Horowitz M. The contribution of mutant GBA to the development of Parkinson disease in Drosophila. Hum. Mol. Genet. 2016;25:2712–2727. doi: 10.1093/hmg/ddw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Suzuki M., Fujikake N., Takeuchi T., Kohyama-Koganeya A., Nakajima K., Hirabayashi Y., Wada K., Nagai Y. Glucocerebrosidase deficiency accelerates the accumulation of proteinase K-resistant alpha-synuclein and aggravates neurodegeneration in a Drosophila model of Parkinson’s disease. Hum. Mol. Genet. 2015;24:6675–6686. doi: 10.1093/hmg/ddv372. [DOI] [PubMed] [Google Scholar]
- 163.Sanchez-Martinez A., Beavan M., Gegg M.E., Chau K.Y., Whitworth A.J., Schapira A.H. Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci. Rep. 2016;6:31380. doi: 10.1038/srep31380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schondorf D.C., Ivanyuk D., Baden P., Sanchez-Martinez A., De Cicco S., Yu C., Giunta I., Schwarz L.K., Di Napoli G., Panagiotakopoulou V., et al. The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson’s Disease. Cell Rep. 2018;23:2976–2988. doi: 10.1016/j.celrep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 165.Robinson S.W., Herzyk P., Dow J.A., Leader D.P. FlyAtlas: Database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 2013;41:D744–D750. doi: 10.1093/nar/gks1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kinghorn K.J., Gronke S., Castillo-Quan J.I., Woodling N.S., Li L., Sirka E., Gegg M., Mills K., Hardy J., Bjedov I., et al. A Drosophila Model of Neuronopathic Gaucher Disease Demonstrates Lysosomal-Autophagic Defects and Altered mTOR Signalling and Is Functionally Rescued by Rapamycin. J. Neurosci. 2016;36:11654–11670. doi: 10.1523/JNEUROSCI.4527-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Abul Khair S.B., Dhanushkodi N.R., Ardah M.T., Chen W., Yang Y., Haque M.E. Silencing of Glucocerebrosidase Gene in Drosophila Enhances the Aggregation of Parkinson’s Disease Associated alpha-Synuclein Mutant A53T and Affects Locomotor Activity. Front. Neurosci. 2018;12:81. doi: 10.3389/fnins.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thomas R.E., Vincow E.S., Merrihew G.E., MacCoss M.J., Davis M.Y., Pallanck L.J. Glucocerebrosidase deficiency promotes protein aggregation through dysregulation of extracellular vesicles. PLoS Genet. 2018;14:e1007694. doi: 10.1371/journal.pgen.1007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weingarten M.D., Lockwood A.H., Hwo S.Y., Kirschner M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Orr M.E., Sullivan A.C., Frost B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol. Sci. 2017;38:637–648. doi: 10.1016/j.tips.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Poorkaj P., Bird T.D., Wijsman E., Nemens E., Garruto R.M., Anderson L., Andreadis A., Wiederholt W.C., Raskind M., Schellenberg G.D. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 172.Van Swieten J., Spillantini M.G. Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol. 2007;17:63–73. doi: 10.1111/j.1750-3639.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mackenzie I.R., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J., Kovacs G.G., Ghetti B., Halliday G., Holm I.E., et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Galpern W.R., Lang A.E. Interface between tauopathies and synucleinopathies: A tale of two proteins. Ann. Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- 175.Rhodes S.L., Sinsheimer J.S., Bordelon Y., Bronstein J.M., Ritz B. Replication of GWAS associations for GAK and MAPT in Parkinson’s disease. Ann. Hum. Genet. 2011;75:195–200. doi: 10.1111/j.1469-1809.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moussaud S., Jones D.R., Moussaud-Lamodiere E.L., Delenclos M., Ross O.A., McLean P.J. Alpha-synuclein and tau: Teammates in neurodegeneration? Mol. Neurodegener. 2014;9:43. doi: 10.1186/1750-1326-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Baker M., Litvan I., Houlden H., Adamson J., Dickson D., Perez-Tur J., Hardy J., Lynch T., Bigio E., Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 178.Pastor P., Ezquerra M., Tolosa E., Munoz E., Marti M.J., Valldeoriola F., Molinuevo J.L., Calopa M., Oliva R. Further extension of the H1 haplotype associated with progressive supranuclear palsy. Mov. Disord. 2002;17:550–556. doi: 10.1002/mds.10076. [DOI] [PubMed] [Google Scholar]
- 179.Stefansson H., Helgason A., Thorleifsson G., Steinthorsdottir V., Masson G., Barnard J., Baker A., Jonasdottir A., Ingason A., Gudnadottir V.G., et al. A common inversion under selection in Europeans. Nat. Genet. 2005;37:129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- 180.Martin E.R., Scott W.K., Nance M.A., Watts R.L., Hubble J.P., Koller W.C., Lyons K., Pahwa R., Stern M.B., Colcher A., et al. Association of single-nucleotide polymorphisms of the tau gene with late-onset Parkinson disease. JAMA. 2001;286:2245–2250. doi: 10.1001/jama.286.18.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pastor P., Ezquerra M., Munoz E., Marti M.J., Blesa R., Tolosa E., Oliva R. Significant association between the tau gene A0/A0 genotype and Parkinson’s disease. Ann. Neurol. 2000;47:242–245. doi: 10.1002/1531-8249(200002)47:2<242::AID-ANA16>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 182.Pittman A.M., Fung H.C., de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum. Mol. Genet. 2006;15:R188–R195. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- 183.Kwok J.B., Teber E.T., Loy C., Hallupp M., Nicholson G., Mellick G.D., Buchanan D.D., Silburn P.A., Schofield P.R. Tau haplotypes regulate transcription and are associated with Parkinson’s disease. Ann. Neurol. 2004;55:329–334. doi: 10.1002/ana.10826. [DOI] [PubMed] [Google Scholar]
- 184.Myers A.J., Pittman A.M., Zhao A.S., Rohrer K., Kaleem M., Marlowe L., Lees A., Leung D., McKeith I.G., Perry R.H., et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 2007;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 185.Trabzuni D., Wray S., Vandrovcova J., Ramasamy A., Walker R., Smith C., Luk C., Gibbs J.R., Dillman A., Hernandez D.G., et al. MAPT expression and splicing is differentially regulated by brain region: Relation to genotype and implication for tauopathies. Hum. Mol. Genet. 2012;21:4094–4103. doi: 10.1093/hmg/dds238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gistelinck M., Lambert J.C., Callaerts P., Dermaut B., Dourlen P. Drosophila models of tauopathies: What have we learned? Int. J. Alzheimers Dis. 2012;2012:970980. doi: 10.1155/2012/970980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Papanikolopoulou K., Skoulakis E.M. The power and richness of modelling tauopathies in Drosophila. Mol. Neurobiol. 2011;44:122–133. doi: 10.1007/s12035-011-8193-1. [DOI] [PubMed] [Google Scholar]
- 188.Burnouf S., Gronke S., Augustin H., Dols J., Gorsky M.K., Werner J., Kerr F., Alic N., Martinez P., Partridge L. Deletion of endogenous Tau proteins is not detrimental in Drosophila. Sci. Rep. 2016;6:23102. doi: 10.1038/srep23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Talmat-Amar Y., Arribat Y., Parmentier M.L. Vesicular Axonal Transport is Modified In Vivo by Tau Deletion or Overexpression in Drosophila. Int. J. Mol. Sci. 2018;19:744. doi: 10.3390/ijms19030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roy B., Jackson G.R. Interactions between Tau and alpha-synuclein augment neurotoxicity in a Drosophila model of Parkinson’s disease. Hum. Mol. Genet. 2014;23:3008–3023. doi: 10.1093/hmg/ddu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Caffrey T.M., Wade-Martins R. Functional MAPT haplotypes: Bridging the gap between genotype and neuropathology. Neurobiol. Dis. 2007;27:1–10. doi: 10.1016/j.nbd.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zabetian C.P., Hutter C.M., Factor S.A., Nutt J.G., Higgins D.S., Griffith A., Roberts J.W., Leis B.C., Kay D.M., Yearout D., et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson’s disease. Ann. Neurol. 2007;62:137–144. doi: 10.1002/ana.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pankratz N., Beecham G.W., DeStefano A.L., Dawson T.M., Doheny K.F., Factor S.A., Hamza T.H., Hung A.Y., Hyman B.T., Ivinson A.J., et al. Meta-analysis of Parkinson’s disease: Identification of a novel locus, RIT2. Ann. Neurol. 2012;71:370–384. doi: 10.1002/ana.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tseng W.E., Chen C.M., Chen Y.C., Yi Z., Tan E.K., Wu Y.R. Genetic variations of GAK in two Chinese Parkinson’s disease populations: A case-control study. PLoS ONE. 2013;8:e67506. doi: 10.1371/journal.pone.0067506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shimizu H., Nagamori I., Yabuta N., Nojima H. GAK, a regulator of clathrin-mediated membrane traffic, also controls centrosome integrity and chromosome congression. Pt 17J. Cell Sci. 2009;122:3145–3152. doi: 10.1242/jcs.052795. [DOI] [PubMed] [Google Scholar]
- 196.Dumitriu A., Pacheco C.D., Wilk J.B., Strathearn K.E., Latourelle J.C., Goldwurm S., Pezzoli G., Rochet J.C., Lindquist S., Myers R.H. Cyclin-G-associated kinase modifies alpha-synuclein expression levels and toxicity in Parkinson’s disease: Results from the GenePD Study. Hum. Mol. Genet. 2011;20:1478–1487. doi: 10.1093/hmg/ddr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Song L., He Y., Ou J., Zhao Y., Li R., Cheng J., Lin C.H., Ho M.S. Auxilin Underlies Progressive Locomotor Deficits and Dopaminergic Neuron Loss in a Drosophila Model of Parkinson’s Disease. Cell Rep. 2017;18:1132–1143. doi: 10.1016/j.celrep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 198.Davisson M.T. Discovery genetics: Serendipity in basic research. ILAR J. 2005;46:338–345. doi: 10.1093/ilar.46.4.338. [DOI] [PubMed] [Google Scholar]
- 199.Keller M.F., Saad M., Bras J., Bettella F., Nicolaou N., Simon-Sanchez J., Mittag F., Buchel F., Sharma M., Gibbs J.R., et al. Using genome-wide complex trait analysis to quantify ‘missing heritability’ in Parkinson’s disease. Hum. Mol. Genet. 2012;21:4996–5009. doi: 10.1093/hmg/dds335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hoshino M., Nakamura S., Mori K., Kawauchi T., Terao M., Nishimura Y.V., Fukuda A., Fuse T., Matsuo N., Sone M., et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 201.Ono Y., Nakatani T., Minaki Y., Kumai M. The basic helix-loop-helix transcription factor Nato3 controls neurogenic activity in mesencephalic floor plate cells. Development. 2010;137:1897–1906. doi: 10.1242/dev.042572. [DOI] [PubMed] [Google Scholar]
- 202.Pascual M., Abasolo I., Mingorance-Le Meur A., Martinez A., Del Rio J.A., Wright C.V., Real F.X., Soriano E. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc. Natl. Acad. Sci. USA. 2007;104:5193–5198. doi: 10.1073/pnas.0605699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Abruzzi K.C., Zadina A., Luo W., Wiyanto E., Rahman R., Guo F., Shafer O., Rosbash M. RNA-seq analysis of Drosophila clock and non-clock neurons reveals neuron-specific cycling and novel candidate neuropeptides. PLoS Genet. 2017;13:e1006613. doi: 10.1371/journal.pgen.1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kula-Eversole E., Nagoshi E., Shang Y., Rodriguez J., Allada R., Rosbash M. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. USA. 2010;107:13497–13502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nagoshi E., Sugino K., Kula E., Okazaki E., Tachibana T., Nelson S., Rosbash M. Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat. Neurosci. 2010;13:60–68. doi: 10.1038/nn.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Croset V., Treiber C.D., Waddell S. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife. 2018;7:e34550. doi: 10.7554/eLife.34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tas D., Stickley L., Miozzo F., Koch R., Loncle N., Sabado V., Gnagi B., Nagoshi E. Parallel roles of transcription factors dFOXO and FER2 in the development and maintenance of dopaminergic neurons. PLoS Genet. 2018;14:e1007271. doi: 10.1371/journal.pgen.1007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Eijkelenboom A., Burgering B.M. FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 209.Dumitriu A., Latourelle J.C., Hadzi T.C., Pankratz N., Garza D., Miller J.P., Vance J.M., Foroud T., Beach T.G., Myers R.H. Gene expression profiles in Parkinson disease prefrontal cortex implicate FOXO1 and genes under its transcriptional regulation. PLoS Genet. 2012;8:e1002794. doi: 10.1371/journal.pgen.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Domanskyi A., Alter H., Vogt M.A., Gass P., Vinnikov I.A. Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance. Front. Cell. Neurosci. 2014;8:275. doi: 10.3389/fncel.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hwang S., Song S., Hong Y.K., Choi G., Suh Y.S., Han S.Y., Lee M., Park S.H., Lee J.H., Lee S., et al. Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO. PLoS Genet. 2013;9:e1003412. doi: 10.1371/journal.pgen.1003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kanao T., Venderova K., Park D.S., Unterman T., Lu B., Imai Y. Activation of FoxO by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Hum. Mol. Genet. 2010;19:3747–3758. doi: 10.1093/hmg/ddq289. [DOI] [PubMed] [Google Scholar]
- 213.Kittappa R., Chang W.W., Awatramani R.B., McKay R.D. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007;5:e325. doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Koh H., Kim H., Kim M.J., Park J., Lee H.J., Chung J. Silent information regulator 2 (Sir2) and Forkhead box O (FOXO) complement mitochondrial dysfunction and dopaminergic neuron loss in Drosophila PTEN-induced kinase 1 (PINK1) null mutant. J. Biol. Chem. 2012;287:12750–12758. doi: 10.1074/jbc.M111.337907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pino E., Amamoto R., Zheng L., Cacquevel M., Sarria J.C., Knott G.W., Schneider B.L. FOXO3 determines the accumulation of alpha-synuclein and controls the fate of dopaminergic neurons in the substantia nigra. Hum. Mol. Genet. 2014;23:1435–1452. doi: 10.1093/hmg/ddt530. [DOI] [PubMed] [Google Scholar]
- 216.Pristera A., Lin W., Kaufmann A.K., Brimblecombe K.R., Threlfell S., Dodson P.D., Magill P.J., Fernandes C., Cragg S.J., Ang S.L. Transcription factors FOXA1 and FOXA2 maintain dopaminergic neuronal properties and control feeding behavior in adult mice. Proc. Natl. Acad. Sci. USA. 2015;112:E4929–E4938. doi: 10.1073/pnas.1503911112. [DOI] [PMC free article] [PubMed] [Google Scholar]