Abstract
Cell migration is a complex molecular event that requires translocation of a large, stiff nucleus, oftentimes through interstitial pores of submicron size in tissues. Remarkable progress in the past decade has uncovered an ever-increasing array of diverse nuclear dynamics and underlying cytoskeletal control in various cell models. In many cases, the microtubule motors dynein and kinesin directly interact with the nucleus via the LINC complex and steer directional nuclear movement, while actomyosin contractility and its global flow exert forces to deform and move the nucleus. In this review, I focus on the synergistic interplay of the cytoskeletal motors and spatiotemporal sites of force transmission in various nuclear migration models, with a special focus on neuronal migration in the vertebrate brain.
Keywords: actomyosin, actin retrograde flow, microtubule motors, nesprins, nuclear envelope, force
Introduction
Cell migration is critical for various physiological and pathological events throughout life, including leukocyte extravasation and immune surveillance, tissue repair and renewal by epithelial cell migration, and vascular invasion of metastatic cancer cells. In addition, during normal development and morphogenesis, cells migrate from their birth-place to their final destination where they reassemble with other cells for integration into functional tissues. In developing mammals, neuronal migration is a fundamental step in the formation of efficient neural networks in the multi-layered cortices and nuclei of the brain. Neurons are generated in the germinal zones and migrate long distances — sometimes over a thousand cell-body lengths — in three-dimensional (3D) neural tissues. Failure or delay in neuronal migration thus results in brain malformation disorders such as type 1 lissencephaly (“smooth brain”), which is associated with severe mental retardation and epilepsy.1,2)
Delivery of the largest cargo in the cell, the nucleus, under the physical constraints of the surrounding cells and extracellular matrix (ECM) is a goal of paramount importance. Migrating neurons typically form a long leading process at the front and translocate the nucleus into the leading process. This movement of the nucleus, also known as nucleokinesis, is a fundamental step in cell migration. Molecular motors including the dynein, kinesin, and myosin families are critical for the transport of organelles in neuronal processes as previously reviewed in greater depth.3,4) The discovery of the evolutionarily conserved gene encoding the cytoplasmic dynein complex protein LIS1, an ortholog of a fungal gene that regulates nuclear positioning, as a gene responsible for type 1 lissencephaly led to a great breakthrough in our understanding of the mechanism of nuclear translocation.5,6) Subsequent studies have identified that many of the causal genes mutated in human brain malformation are involved in cytoskeleton dynamics during neuronal migration, such as Doublecortin (DCX), tubulin (TUBA1A, TUBA8, TUBB2B, and TUBB3), and Filamin A.1,2) A growing consensus is that the nucleus in a migrating neuron is associated with microtubules emanating from the leading process, and that it moves forward along the microtubules using dynein motor activity.7–10) Other studies have also implicated the contractile force of actomyosin in nuclear movement.11–15) However, competing hypotheses have been proposed using different neuron models to explain the sites and extent of cytoskeletal forces generated during nuclear migration. In this review, I will focus on recent studies examining the role of molecular motors during nuclear migration in various postmitotic cell models in vertebrates and invertebrates, with a special focus on neurons in vertebrate brains.
Passive nuclear translocation in mesenchymal cell migration
Adhesion-based migration of slow-moving mesenchymal cells.
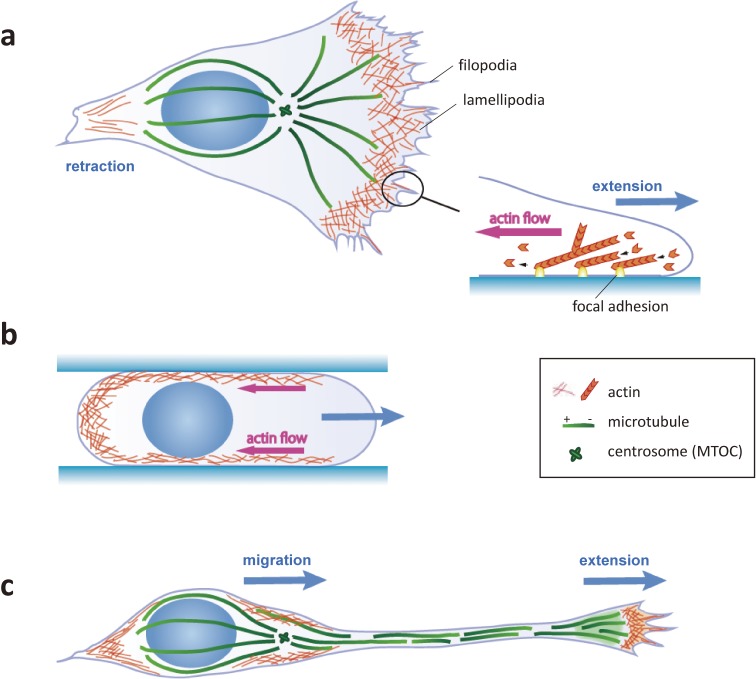
Cell migration steps are distinct in different cell types and environments. Migration of mesenchymal cells on flat surfaces is initiated by the formation of lamellipodia and/or filopodia, which bind to substratum-associated ECM proteins via integrin receptors on their plasma membrane (Fig. 1(a)). Activated integrins recruit cytoplasmic adaptors and signaling proteins to form a focal adhesion (FA) that ‘clutches’ dynamic filamentous actin (F-actin). F-actin filaments in migrating mesenchymal cells are polarized with their plus (barbed)-ends toward the cell periphery. These polarized F-actin filaments are continuously pushed toward the cell center by polymerization at their plus-ends against the plasma membrane, and by myosin II-dependent sliding. In turn, FAs can anchor F-actin and slow the retrograde flow, converting the plus-end elongation into a pushing force against the leading edge of the plasma membrane. Concurrently, weaker adhesions at the cell rear are disassembled and retracted toward the cell-center by the force of actin retrograde flow. As a consequence, the cell recovers its original shape and volume in a slightly advanced position. By repeating these steps, the plasma membrane moves forward and drags the nucleus and cytoplasm. Although the details of the individual steps can diverge among different cell types, the consensus appears to suggest that slow-moving mesenchymal cells on flat adhesive surfaces adopt an adhesion-based cell migration associated with passive nuclear translocation (for reviews, refs 16 and 17).
Figure 1.
Comparison of migration dynamics in mesenchymal cells and neurons. (a) Mesenchymal cells on 2D substrates migrate by precise coordination between extension at the front and retraction at the rear. The branched actin meshwork at the front of the cell is anchored to focal adhesions and exerts a pushing force against the plasma membrane. (b) Mesenchymal cells in confined 3D substrates migrate by pressure generated from the retrograde flow of cortical actin. (c) Neuronal migration consists of extension of the growth cone and translocation of the soma. Nuclear migration during somal translocation is driven by forces generated by the perinuclear cytoskeletons independent of growth cone extension.
Adhesion-independent migration driven by cortical actin retrograde flow.
Migrating cells in 3D tissues occasionally traverse through constricted spaces. It is known that some types of mesenchymal cells switch their mode of migration depending on the extracellular environment. During migration through narrow channels made from microfabricated PDMS substrates, these cells adopt round, amoeboid shapes and move faster than when on a flat surface and extending lamellipodia.18–21) Extensive studies have revealed that amoeboid migration does not require FA-dependent force transmission, but instead relies on the global retrograde flow of cortical actomyosin and counteracting friction between the cell membrane and non-specific substrates.22–24) When friction is strong enough to maintain the cell position, global actin flow concentrates myosin contractility at the cell rear, resulting in front expansion and net cell displacement due to an increase of intracellular pressure (Fig. 1(b)). The friction-based amoeboid migration propels the cell forward by a much smaller force (∼1 Pa) than adhesion-based motility, which generates forces of approximately 100 Pa that are transmitted to the substrate.22)
Thus, while adhesion-dependent and -independent mechanisms differ in their use of actin retrograde flow and rear contraction, both mechanisms involve passive nuclear transfer to the front of the cell with the bulk of the cytoplasm.
Microtubule-driven nuclear migration in developing neurons
Actin-based growth cone migration independent of nuclear movement.
Migratory neurons in the developing brain typically form one or a few long leading processes tipped by a large growth cone with broad lamellipodia and thin filopodia (Fig. 1(c)). The leading margin of the growth cone, the peripheral (P) domain, forms adhesions known as point contacts that are dependent on integrins and other cell adhesion molecules (for reviews, refs 25 and 26). Point contacts resemble FAs because of their ability to physically link to F-actin and transmit the force of actin polymerization toward the membrane protrusion. However, unlike mesenchymal cells, the neuronal growth cone advances independently of the cell body and elongates the leading process, which later differentiates into an axon or dendrite.27,28) Some neurons take on a bipolar shape by extending a trailing process on the opposite side of the cell body. Cortical pyramidal neurons extend a leading process and migrate along the radial glial fibers in the emerging neocortex. The standard mode of neuronal migration with one or a few leading processes is commonly known as locomotion.29) When the leading process reaches its destination, the nucleus starts to migrate without additional extension of the leading process. This mode of migration, known as somal translocation, is adopted by either early born pyramidal neurons or in the final step of locomoting neurons in the neocortex.29,30) Thus, neuronal nucleokinesis is not necessarily coupled to the leading edge extension or rear retraction. In contrast to mesenchymal cells, in which the nucleus is basically pushed forward by retraction of the cell rear by actomyosin contractility, nucleokinesis in neurons is propelled by a mechanism involving an intricate interplay among microtubules, actin, and their associated motors, which exert both pulling and pushing forces on the nucleus.
Dynein- and LINC complex-dependent nuclear migration in postmitotic neurons.
Migrating cells are highly polarized along the anterior-posterior axis, with the microtubule-organizing center (MTOC or centrosome) and Golgi apparatus typically positioned in front of the nucleus (Fig. 1(a), (c)). Thus, a large majority of microtubules orient their plus-ends toward the nucleus and minus-ends to the leading process. In neurons, cytoplasmic dynein links to the nuclear envelope and pulls the nucleus forward along microtubules by its minus-end-directed motor activity. Inhibition of cytoplasmic dynein or its regulator LIS1 (platelet-activating factor acetylhydrolase isoform 1b regulatory subunit 1 [PAFAH1B1]) attenuates nuclear migration in several types of postmitotic neurons.7,8,10) The link between cytoplasmic dynein and the nuclear envelope is mediated by the KASH (Klarsicht/ANC-1/Syne Homology) family proteins nesprin-1 and -2.31) KASH proteins span the outer nuclear membrane and associate with the cytoskeleton via their N-terminal cytoplasmic stretch. In nesprins-1/2, this cytoplasmic stretch binds to the microtubule motors dynein and kinesin as well as to actin. The C-terminal KASH domain on the other hand associates with SUN proteins that traverse the inner nuclear membrane and interact with lamins and chromatin in the nucleoplasm (Fig. 2(a)). As a result, KASH proteins and SUN proteins form the linker of nucleoskeleton and cytoskeleton (LINC) complex and transmit the cytoskeletal force to the nucleus.32–35)
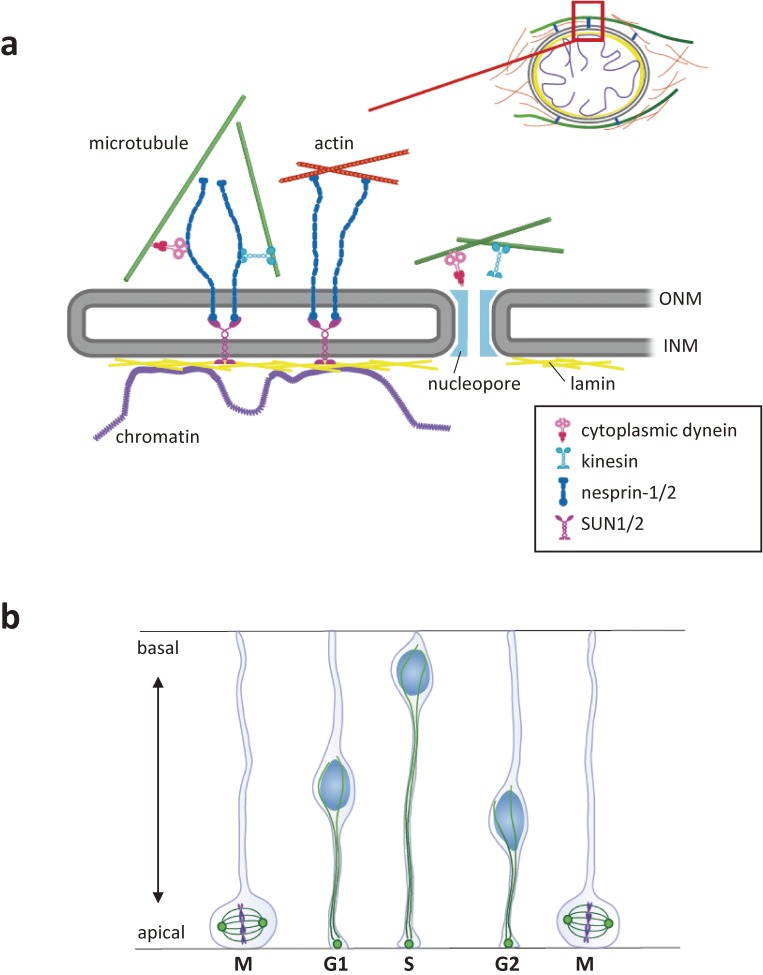
Figure 2.
The migrating nucleus is anchored to the cytoskeletons via the LINC complex. (a) The LINC complex couples the nucleus with the cytoskeleton in the cytoplasm. KASH domain proteins (nesprins-1 to 4 and KASH5) traverse the outer nuclear membrane (ONM). Nesprins-1/2 bind to microtubule motors (cytoplasmic dynein and kinesin) and actin via their cytoplasmic stretch. The C-terminal KASH domain associates with the SUN domain of SUN1/2, which traverses the inner nuclear membrane (INM) and associates with the nuclear lamina (lamins and chromatin) in the nucleoplasm. Microtubule motors may also bind to the nucleopore complex. (b) Interkinetic nuclear migration is a cell cycle-dependent nuclear oscillation between the apical and basal ends of the ventricular zone in the developing mammalian neocortex. Microtubules emanate from the MTOC in the apical endfoot and are uniformly oriented with their plus-ends toward the basal side. Nesprins-1/2, SUN1/2, and bidirectional microtubule motors are implicated in the oscillatory movement of the nucleus.
The polarized arrangement of the MTOC and perinuclear microtubules excludes a direct role of the plus-end-directed motor activity of kinesin in unidirectional nuclear migration of postmitotic neurons. However, studies using dorsal root ganglion neurons have indicated that kinesin-1 motor KIF5B and the light chain KLC1 can indirectly regulate nuclear migration by transporting the cytoplasmic dynein complex to the microtubule plus-ends.36) Other studies using the developing cerebral cortex have demonstrated that kinesin-3 motor KIF1A facilitates the transport and secretion of neurotrophins that regulate the differentiation of surrounding newborn cortical neurons into bipolar migratory neurons.37)
In contrast, in migrating cerebellar granule cells, a substantial fraction of the perinuclear microtubules are dissociated from the MTOC and are arranged with mixed polarity around the nucleus.38,39) Indeed, a direct link between kinesin-1 (KIF5B and associated KLCs) and nesprins contributes to nuclear migration in cerebellar granule cells.39) Given the emerging diversity of cytoskeletal organization among neuronal types, further evaluation of the contribution of kinesin function to nuclear migration is necessary in various neuronal types and stages.
To-and-fro nuclear migration in neural progenitor cells regulated by opposing microtubule motors.
Neural progenitor cells in the developing cerebral cortex exhibit cell cycle-dependent, bidirectional nuclear migration between the apical and basal surface of the ventricular zone (Fig. 2(b)). This to-and-fro nuclear migration in non-migrating neural progenitor cells is known as interkinetic nuclear migration. The MTOC remains at the apical endfoot throughout the cell-cycle so that microtubules emanating from the MTOC are uniformly oriented with their plus-ends toward the basal side. Bidirectional movement of the nucleus may thus involve two opposing microtubule motors: basal-to-apical migration toward the MTOC powered by dynein, and apical-to-basal migration away from the MTOC by kinesin-3 motor KIF1A.40) It has been proposed that a combination of SUN1/2 and nesprins-1/2 recruits the dynein/LIS1 complex to the nuclear envelope during basal-to-apical migration, whereas SUN1/2 and nesprin-2 recruit kinesin during apical-to-basal migration.31) In addition to the LINC complex, interaction between dynein and kinesin with the nuclear envelope is also mediated by the nucleoporin-based nuclear pore complex proteins.41,42) Dynein-based, basal-to-apical migration has been consistently shown to involve a parallel pathway mediated by the nuclear pore components instead of the LINC complex (Fig. 2(a)).43)
On the other hand, many groups have indicated that apical-to-basal migration may occur passively as a consequence of active basal-to-apical migration of the surrounding progenitor cells crowding the apical ventricular zone.44–46) These conflicting interpretations highlight the need to carefully consider the contributions of both intrinsic and environmental variables on nucleokinesis in future studies.
Microtubule-driven nuclear motion in non-neuronal cells
Nuclear migration for proper positioning in polarized cells.
Nuclear migration is also observed in stationary cells for proper intracellular positioning, particularly in mitotic cells and large cells with bulk cytoplasm such as early embryonic cells. In many cases, microtubules and their associated motors are involved in this nuclear positioning. For instance, during a series of cell rearrangements of the hypodermal layers in C. elegans larvae, long hypodermal precursor cells move their nuclei from one end of the cell to the other through the cytoplasm that is wedged between the body wall muscle and cuticle.47,48) The nuclei are actively deformed and pass through constrictions by a process dependent on dynein and kinesin-1 (KIF5 homologue UNC-116 associated with KLC2) bound to the KASH protein UNC-83 and SUN protein UNC-84.49) Because microtubules in hypodermal precursor cells are uniformly oriented with their minus-ends pointing ventrally, dynein plays a major role in the ventral migration of the nucleus during the larval stages. By contrast, nuclei move dorsally in embryonic hypodermal precursor cells, using kinesin-1 as the predominant motor, whereas dynein drives short, back-stepping movement.50) How the oppositely directed motors contribute to nuclear transport against the direction of the uniformly oriented microtubules remains to be elucidated. It is possible that dynamic, short, bi-directional movements by opposing motors might adjust the precise nuclear position and help it pass through the narrow interstitial pores, a process that generates high mechanical stress.51)
Multinucleated myocytes provide another example of nuclear positioning guided by microtubule motors. Their nuclei are evenly spaced along the long-axis of a large muscle cell to ensure sufficient transcriptional capacity and intracellular molecular transport throughout the entire cell volume.52) Studies using C2C12 myoblasts have indicated that the nuclei in newly fused myotube cells migrate and rotate in 3D while they rearrange themselves at regular intervals. In these cells microtubules are of mixed polarity, along which the nuclei are translocated by the synergistic actions of dynein and kinesin-1 (the KIF5B and KLC1/2 complex) and their associated nesprins-1/2. Inhibition of either of the microtubule motors thus leads to disruption of regular nuclear positioning.53,54)
One notable exception to microtubule-dependent nuclear positioning is seen in Drosophila oocytes. The oocyte nucleus migrates from the posterior to the anterior of the cell for asymmetric localization of the mRNAs that encode body axis determinants.55,56) Instead of microtubule motors, polymerizing microtubules emanating from the MTOC behind the nucleus push directly against the nucleus and move it into position.57) It should be also noted that a more recent study has suggested that the nucleus can migrate within the oocyte via multiple routes, some of which may utilize microtubule motors.58)
Rotational motion of the nucleus driven by microtubule motors.
During force transmission, microtubules are likely anchored to multiple points on the nuclear envelope primarily via the LINC complex. Whilst nuclear displacement is induced when the net force acts on the center of mass, unbalanced forces result in torque and drive nuclear rotation. Indeed, nuclei frequently rotate during rearrangement in the abovementioned multinucleated muscle cells. Nuclear rotation is powered by the same driving force used for nuclear translocation, which is generated by dynein and kinesin-1 (KIF5B and KLC1/2) associated with nesprins-1/2.53,54) Nuclear rotation is also seen in migrating fibroblasts in culture, where it might contribute to the maintenance of nuclear centrality.59) In contrast to the 3D rotation of round nuclei in muscle cells, nuclei are flattened in cultured fibroblasts and rotate in 2D parallel to the dish surface. Rotation of fibroblast nuclei is driven by dynein motors; however, the involvement of kinesin has not yet been evaluated.
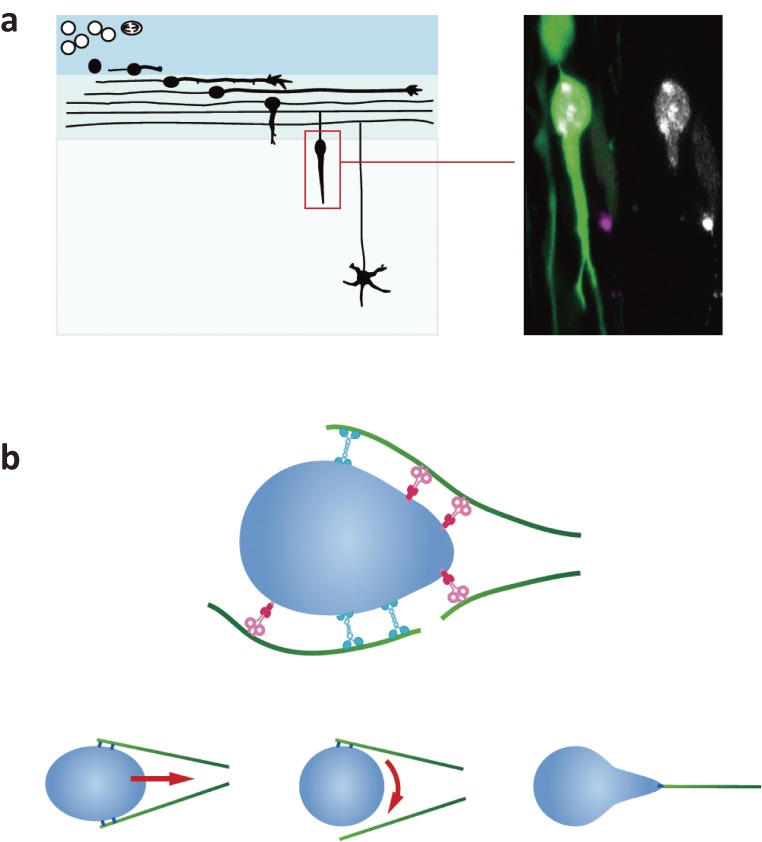
Live imaging studies using cerebellar granule cells have shown remarkable deformation and rotation of the nucleus during migration through narrow intercellular spaces in neural tissues (Fig. 3(a)).39) The axis of the rotation is dependent on the direction of nuclear migration and microtubule arrangement. Nuclear rotation in neurons is much faster (∼50°/min) than what is observed during nuclear positioning in myotubes (<6°/min) and fibroblasts (<10°/min). Evidence suggests that microtubules dynamically bind to small points on the nuclear envelope via kinesin-1 motor KIF5B and cytoplasmic dynein, by which they can induce sharpening, rotation, and translocation of the nucleus depending on the positions of the force points (Fig. 3(b)). The physiological significance of rotation in neuronal migration is still unclear, but it might help optimize nuclear and cytoskeletal positioning for smooth translocation in the confined spaces of neural tissue.
Figure 3.
Nuclear dynamics during migration of cerebellar granule cells. (a) left: Granule cell migration in the developing cerebellar cortex. Granule cells are born in the external granule layer (EGL) and first migrate along the surface of the brain primordium. They then turn and migrate into the emerging cortex and migrate to the internal granule layer (IGL). right: Migrating granule cells transfected with EGFP (green) and heterochromatin protein 1β (HP1β) conjugated with mCherry (white). The single-channel view of the HP1β signal on the right shows drastic deformation of the nucleus. (b) Hypothetical interaction of the nucleus and microtubules in migrating granule cells. Microtubules of mixed polarity interact with small points on the nuclear envelope via dynein and kinesin-1. Nuclear translocation, rotation, and deformation are evoked depending on the force positions.
Nuclear migration driven by actomyosin motility
Actomyosin forces during nuclear migration in neurons.
Although it is well established that microtubules and associated motors power nuclear translocation via the LINC complex, actomyosin also plays important roles in nuclear migration in neurons (Fig. 4(a)).
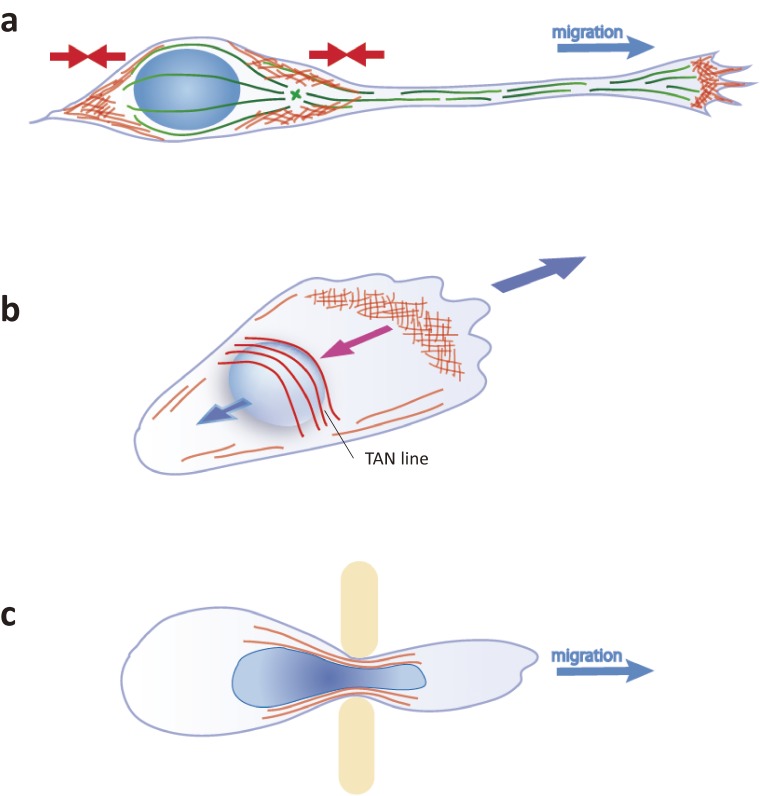
Figure 4.
Actin-dependent forces during cell migration. (a) In migrating neurons with a long leading process, the nucleus is either pushed or pulled by actomyosin-driven forces, depending on myosin II localization. (b) Mesenchymal cells move the nucleus rearward by the retrograde flow of TAN lines at the onset or reorientation of migration. (c) Some immune cells and cancer cells pass through submicron pores between vascular endothelial cells. The nucleus is covered by actin meshwork and is protected from damage caused by strong shear stress at the constriction.
Inhibitory interneurons in the neocortex and olfactory bulb are born in the ventral and anterior telencephalon and migrate horizontally along the brain surface (tangential axis) to their final destinations in the dorsal and rostral telencephalon.60,61) During the tangential migration of these interneurons in organotypic slice or 3D Matrigel cultures, myosin II is heavily enriched at the cell rear and pushes the cell body forward during nucleokinesis (Fig. 4(a)).13–15) Myosin II activity at the rear has also been implicated in interkinetic nuclear migration of neural progenitor cells.62)
On the other hand, in migrating cerebellar granule cells, the leading process is a major site for actomyosin function. Pharmacological studies as well as traction force microscopy have defined the leading process as a major actomyosin contraction center that can generate a traction force to pull the nucleus.63,64) Live imaging studies have shown myosin II-driven anterograde actin flow in the leading process,11,12) which may indirectly promote nuclear migration by mediating transport of plasma membrane receptors.65) Actin in the proximal leading process may also complex with and pull the microtubules that are anchored to the nuclear surface.66,67) Actomyosin regulation in the leading process is also involved in the radial migration of cortical neurons.68) Thus, it remains of great interest to clarify whether actomyosin drives nucleokinesis via direct interaction with the nuclear envelope in neurons.
Actin-driven nuclear migration via direct linkage to the nucleus.
Unlike neurons, a direct link between actin and the LINC complex mediates nuclear migration in mesenchymal cells. In the scratch-wound assay in cultured fibroblasts, the cells at the wound-front first polarize to form lamellipodia, and reorient the MTOC toward the wound gap by rearward translocation of the nucleus.69,70) This rearward nuclear migration in the polarizing cells is driven by a retrograde flow of actin that directly associates with the SUN2/nesprin-2 complex at the nuclear envelope, forming structures called transmembrane actin-associated nuclear (TAN) lines (Fig. 4(b)). As expected, depletion or inhibition of SUN2 or nesprin-2 resulted in slower migration in these cells.
When primary human fibroblasts are cultivated in an anisotropic, linearly elastic 3D matrix, cells slenderize and form a blunt, cylindrical protrusion of about nuclear diameter instead of a flat lamellipodium. Nuclear migration is powered by actomyosin at the cell front, which binds to nesprin-3 through vimentin. Because molecular diffusion from the cell rear is physically hampered by the nucleus, the forward motion of the nucleus functions as a piston, pressurizing the cytoplasm and pushing the leading edge forward.71,72)
Nuclear deformation in confined tissue.
When cells migrate in 3D tissues through restrictive extracellular matrices and small interstitial spaces, the nucleus is unable to advance passively by simple withdrawal of the cell rear but instead must be strongly squeezed through narrow pores. Leukocytes and tumor cells pass through submicron pores of blood or lymph vascular endothelia that are much smaller than their diameters.32,73,74) Squeezing the large nucleus through such a narrow confinement requires extensive nuclear deformation. Recent studies have demonstrated that the formation of perinuclear actin structures controls nuclear shape and limits nuclear envelope damage from the physical environmental pressure (Fig. 4(c)). Deformation of dendritic cell nuclei is aided by a perinuclear branched actin network nucleated by Arp2/3.75) In the hypodermal cells of C. elegans, Arp2/3 may interact with the nuclear surface via the actin regulator TOCA-1 and help squeeze nuclei through constrictions.49,76–78)
More recently, accumulating evidence indicates that the nucleus is not just passively transported by cytoskeletal forces but actively pushes its way through narrow spaces in crowded tissues by dynamically regulating its own physical properties. During the extreme nuclear deformation of extra- or intravasating leukocytes and cancer cells, the nuclear envelope experiences transient rupture, mixing nuclear and cytoplasmic contents. This nuclear envelope rupture is rapidly repaired by ESCRT III (endosomal sorting complexes required for transport) and nuclear lamins to minimize DNA damage.79,80) Regulation of the mechanical properties of the migratory nucleus has been extensively reviewed elsewhere.34,81,82)
Conclusion
As described here, there is considerable diversity in cytoskeletal architecture and motor control for many models of nuclear migration. While the apparent diversity may be mostly attributed to cell-type differences, contradictory models of functional requirements of cytoskeletal components have been proposed for the same migration models. This appears to be partly due to differences in experimental design and the technical limits of conventional approaches for resolving the spatio-temporal interplay of cytoskeletal components.
Generally speaking, microtubule motors exert forces to small points on the nuclear envelope via assembly with the LINC complex, and thereby precisely steer the nucleus into the correct direction and position. It is notable that dynein and kinesin are not simply segregated into exclusive “traffic lanes” of microtubules, but can synergistically drive one-way nuclear movement along uniformly oriented microtubules. A comprehensive review by Hancock has summarized the possible ‘co-dependence’ mechanisms of how opposing motors bound to the same cargo mutually regulate the motor activity and/or tether counterpart motors to microtubules.83) Such coordinated activities of opposing motors are particularly important for nuclear migration in narrow spaces. In contrast to the steering forces of microtubule motors, the strong contractile force and global flow of actomyosin instead act on a broad area of the cell and/or nucleus, resulting in massive nuclear deformation and translocation. The force transmission of actomyosin does not necessarily require direct interaction with the nuclear envelope, rather it can influence intracellular transport and cell shape, or regulate the positioning of microtubules anchored to the nucleus.
The dynamic interactions and force generation of molecular motors are physicochemical reactions occurring in the nanometer and subsecond scales. New integrated approaches are thus needed to clarify the precise loci, timing, and extent of force generation by the molecular motors during nuclear migration. Emerging techniques for site-directed manipulation of molecular expression and function, combined with high resolution live-imaging beyond the diffraction limit, would help us to construct an integrated model for cytoskeletal control of nuclear migration.
Acknowledgements
I thank Dr. Kelly Kawabata Galbraith for critical reading of the manuscript.
Profile
Mineko Kengaku is currently a Professor at the Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University. She was born in Kanagawa, Japan, in 1966 and graduated from The University of Tokyo (Department of Zoology, Faculty of Science) in 1989. She obtained her Ph.D. in Basic Medicine from The University of Tokyo in 1995, working on the molecular mechanism underlying axis formation in the developing Xenopus brain, under the supervision of Profs. Kunitaro Takahashi and Harumasa Okamoto. She performed post-doctoral research in the laboratory of Prof. Cliff Tabin at Harvard Medical School between 1995 and 1997. Her post-doctoral research focused on understanding the molecular signals regulating axis formation and morphogenesis in the chick limb bud. She then joined the laboratory of Dr. Tomoo Hirano at Kyoto University as a junior faculty member, where she started her current studies on cell motility control of developing neurons in mouse brain. She moved to the RIKEN Brain Science Institute in 2004 to establish her first independent research group. She joined the current institute as an Associate Professor in 2008 and was promoted to full Professor in 2012. Her major research focus is on the polarity formation and motility control of differentiating neurons during cortex formation in the mammalian brain.
References
- 1).Moon H.M., Wynshaw-Boris A. (2013) Cytoskeleton in action: lissencephaly, a neuronal migration disorder. Wiley Interdiscip. Rev. Dev. Biol. 2, 229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Manzini M.C., Walsh C.A. (2011) What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr. Opin. Genet. Dev. 21, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Maday S., Twelvetrees A.E., Moughamian A.J., Holzbaur E.L.F. (2014) Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron 84, 292–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Hirokawa N., Niwa S., Tanaka Y. (2010) Molecular Motors in Neurons: Transport Mechanisms and Roles in Brain Function, Development, and Disease. Neuron 68, 610–638. [DOI] [PubMed] [Google Scholar]
- 5).Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W.B., et al. (1993) Isolation of a Miller-Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717–721. [DOI] [PubMed] [Google Scholar]
- 6).Hirotsune S., Fleck M.W., Gambello M.J., Bix G.J., Chen A., Clark G.D., et al. (1998) Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat. Genet. 19, 333–339. [DOI] [PubMed] [Google Scholar]
- 7).Tsai J.-W., Bremner K.H., Vallee R.B. (2007) Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970–979. [DOI] [PubMed] [Google Scholar]
- 8).Umeshima H., Hirano T., Kengaku M. (2007) Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc. Natl. Acad. Sci. U.S.A. 104, 16182–16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Tanaka T., Serneo F.F., Higgins C., Gambello M.J., Wynshaw-Boris A., Gleeson J.G. (2004) Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 165, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Shu T., Ayala R., Nguyen M.-D., Xie Z., Gleeson J.G., Tsai L.-H. (2004) Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44, 263–277. [DOI] [PubMed] [Google Scholar]
- 11).Solecki D.J., Trivedi N., Govek E.-E., Kerekes R.A., Gleason S.S., Hatten M.E. (2009) Myosin II Motors and F-Actin Dynamics Drive the Coordinated Movement of the Centrosome and Soma during CNS Glial-Guided Neuronal Migration. Neuron 63, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).He M., Zhang Z.H., Guan C.B., Xia D., Yuan X.B. (2010) Leading Tip Drives Soma Translocation via Forward F-Actin Flow during Neuronal Migration. J. Neurosci. 30, 10885–10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Schaar B.T., McConnell S.K. (2005) Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. U.S.A. 102, 13652–13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Bellion A., Baudoin J.-P., Alvarez C., Bornens M., Métin C. (2005) Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Martini F.J., Valdeolmillos M. (2010) Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J. Neurosci. 30, 8660–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Case L.B., Waterman C.M. (2015) Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 14, 3–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., et al. (2003) Cell Migration: Integrating Signals from Front to Back. Science 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- 18).Lämmermann T., Bader B.L., Monkley S.J., Worbs T., Wedlich-Söldner R., Hirsch K., et al. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55. [DOI] [PubMed] [Google Scholar]
- 19).Panková K., Rösel D., Novotný M., Brábek J. (2010) The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell. Mol. Life Sci. 67, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Petrie R.J., Gavara N., Chadwick R.S., Yamada K.M. (2012) Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 197, 439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Paluch E.K., Aspalter I.M., Sixt M. (2016) Focal Adhesion-Independent Cell Migration. Annu. Rev. Cell Dev. Biol. 32, 469–490. [DOI] [PubMed] [Google Scholar]
- 22).Bergert M., Erzberger A., Desai R.A., Aspalter I.M., Oates A.C., Charras G., et al. (2015) Force transmission during adhesion-independent migration. Nat. Cell Biol. 17, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Liu Y.-J., Le Berre M., Lautenschlaeger F., Maiuri P., Callan-Jones A., Heuzé M., et al. (2015) Confinement and Low Adhesion Induce Fast Amoeboid Migration of Slow Mesenchymal Cells. Cell 160, 659–672. [DOI] [PubMed] [Google Scholar]
- 24).Ruprecht V., Wieser S., Callan-Jones A., Smutny M., Morita H., Sako K., et al. (2015) Cortical Contractility Triggers a Stochastic Switch to Fast Amoeboid Cell Motility. Cell 160, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Kerstein P.C., Nichol R.H., IV, Gomez T.M. (2015) Mechanochemical regulation of growth cone motility. Front. Cell. Neurosci. 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Tojima T., Hines J.H., Henley J.R., Kamiguchi H. (2011) Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 12, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Hatanaka Y., Zhu Y., Torigoe M., Kita Y., Murakami F. (2016) From migration to settlement: the pathways, migration modes and dynamics of neurons in the developing brain. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 92, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Kawaji K., Umeshima H., Eiraku M., Hirano T., Kengaku M. (2004) Dual phases of migration of cerebellar granule cells guided by axonal and dendritic leading processes. Mol. Cell. Neurosci. 25, 228–240. [DOI] [PubMed] [Google Scholar]
- 29).Nadarajah B., Parnavelas J.G. (2002) Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 3, 423–432. [DOI] [PubMed] [Google Scholar]
- 30).Marín O., Valiente M., Ge X., Tsai L.-H. (2010) Guiding Neuronal Cell Migrations. Cold Spring Harb. Perspect. Biol. 2, a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Zhang X., Lei K., Yuan X., Wu X., Zhuang Y., Xu T., et al. (2009) SUN1/2 and Syne/Nesprin-1/2 Complexes Connect Centrosome to the Nucleus during Neurogenesis and Neuronal Migration in Mice. Neuron 64, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Friedl P., Wolf K., Lammerding J. (2011) Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 23, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Rajgor D., Shanahan C.M. (2013) Nesprins: from the nuclear envelope and beyond. Expert Rev. Mol. Med. 15, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Gerlitz G., Bustin M. (2011) The role of chromatin structure in cell migration. Trends Cell Biol. 21, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Starr D.A., Fridolfsson H.N. (2010) Interactions Between Nuclei and the Cytoskeleton Are Mediated by SUN-KASH Nuclear-Envelope Bridges. Annu. Rev. Cell Dev. Biol. 26, 421–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Yamada M., Toba S., Takitoh T., Yoshida Y., Mori D., Nakamura T., et al. (2010) mNUDC is required for plus-end-directed transport of cytoplasmic dynein and dynactins by kinesin-1. EMBO J. 29, 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Carabalona A., Hu D.J.-K., Vallee R.B. (2016) KIF1A inhibition immortalizes brain stem cells but blocks BDNF-mediated neuronal migration. Nat. Neurosci. 19, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Rao A.N., Falnikar A., O’Toole E.T., Morphew M.K., Hoenger A., Davidson M.W., et al. (2016) Sliding of centrosome-unattached microtubules defines key features of neuronal phenotype. J. Cell Biol. 213, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Wu Y.K., Umeshima H., Kurisu J., Kengaku M. (2018) Nesprins and opposing microtubule motors generate a point force that drives directional nuclear motion in migrating neurons. Development 145, dev158782. [DOI] [PubMed] [Google Scholar]
- 40).Tsai J.-W., Lian W.-N., Kemal S., Kriegstein A.R., Vallee R.B. (2010) Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat. Neurosci. 13, 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Splinter D., Tanenbaum M.E., Lindqvist A., Jaarsma D., Flotho A., Yu K.L., et al. (2010) Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol. 8, e1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Bolhy S., Bouhlel I., Dultz E., Nayak T., Zuccolo M., Gatti X., et al. (2011) A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J. Cell Biol. 192, 855–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Hu D.J.-K., Baffet A.D., Nayak T., Akhmanova A., Doye V., Vallee R.B. (2013) Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell 154, 1300–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Okamoto M., Namba T., Shinoda T., Kondo T., Watanabe T., Inoue Y., et al. (2013) TAG-1-assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat. Neurosci. 16, 1556–1566. [DOI] [PubMed] [Google Scholar]
- 45).Norden C., Young S., Link B.A., Harris W.A. (2009) Actomyosin Is the Main Driver of Interkinetic Nuclear Migration in the Retina. Cell 138, 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Kosodo Y., Suetsugu T., Suda M., Mimori-Kiyosue Y., Toida K., Baba S.A., et al. (2011) Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 30, 1690–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Francis R., Waterston R.H. (1991) Muscle cell attachment in Caenorhabditis elegans. J. Cell Biol. 114, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Cox E.A., Hardin J. (2004) Sticky worms: adhesion complexes in C. elegans. J. Cell Sci. 117, 1885–1897. [DOI] [PubMed] [Google Scholar]
- 49).Bone C.R., Chang Y.-T., Cain N.E., Murphy S.P., Starr D.A. (2016) Nuclei migrate through constricted spaces using microtubule motors and actin networks in C. elegans hypodermal cells. Development 143, 4193–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Fridolfsson H.N., Ly N., Meyerzon M., Starr D.A. (2010) UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev. Biol. 338, 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Wu Y.K., Kengaku M. (2018) Dynamic interaction between microtubules and the nucleus regulates nuclear movement during neuronal migration. J. Exp. Neurosci. 12, doi: 10.1177/1179069518789151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Bruusgaard J.C., Liestøl K., Ekmark M., Kollstad K., Gundersen K. (2004) Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J. Physiol. 551, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Wilson M.H., Holzbaur E.L.F. (2012) Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J. Cell Sci. 125, 4158–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Wilson M.H., Holzbaur E.L.F. (2015) Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 142, 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).González-Reyes A., Elliott H., Johnston D.S. (1995) Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375, 654–658. [DOI] [PubMed] [Google Scholar]
- 56).Roth S., Shira Neuman-Silberberg F., Barcelo G., Schüpbach T. (1995) cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 81, 967–978. [DOI] [PubMed] [Google Scholar]
- 57).Zhao T., Graham O.S., Raposo A., Johnston D.S. (2012) Growing microtubules push the oocyte nucleus to polarize the Drosophila dorsal-ventral axis. Science 336, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Tissot N., Lepesant J.-A., Bernard F., Legent K., Bosveld F., Martin C., et al. (2017) Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte. Nat. Commun. 8, 15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Levy J.R., Holzbaur E.L.F. (2008) Dynein drives nuclear rotation during forward progression of motile fibroblasts. J. Cell Sci. 121, 3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Marín O., Müller U. (2014) Lineage origins of GABAergic versus glutamatergic neurons in the neocortex. Curr. Opin. Neurobiol. 26, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Tanaka D.H., Nakajima K. (2012) Migratory pathways of GABAergic interneurons when they enter the neocortex. Eur. J. Neurosci. 35, 1655–1660. [DOI] [PubMed] [Google Scholar]
- 62).Leung L., Klopper A.V., Grill S.W., Harris W.A., Norden C. (2011) Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development 138, 5003–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Jiang J., Zhang Z.-H., Yuan X.-B., Poo M.-M. (2015) Spatiotemporal dynamics of traction forces show three contraction centers in migratory neurons. J. Cell Biol. 209, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Umeshima H., Nomura K.-I., Yoshikawa S., Hörning M., Tanaka M., Sakuma S., et al. (2018) Local traction force in the proximal leading process triggers nuclear translocation during neuronal migration. Neurosci. Res. doi: 10.1016/j.neures.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 65).Wang D., She L., Sui Y.-N., Yuan X.-B., Wen Y., Poo M.-M. (2012) Forward transport of proteins in the plasma membrane of migrating cerebellar granule cells. Proc. Natl. Acad. Sci. U.S.A. 109, E3558–E3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Hutchins B.I., Wray S. (2014) Capture of microtubule plus-ends at the actin cortex promotes axophilic neuronal migration by enhancing microtubule tension in the leading process. Front. Cell. Neurosci. 8, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Trivedi N., Stabley D.R., Cain B., Howell D., Laumonnerie C., Ramahi J.S., et al. (2017) Drebrin-mediated microtubule–actomyosin coupling steers cerebellar granule neuron nucleokinesis and migration pathway selection. Nat. Commun. 8, 14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Yang T., Sun Y., Zhang F., Zhu Y., Shi L., Li H., et al. (2012) POSH localizes activated Rac1 to control the formation of cytoplasmic dilation of the leading process and neuronal migration. Cell Rep. 2, 640–651. [DOI] [PubMed] [Google Scholar]
- 69).Gomes E.R., Jani S., Gundersen G.G. (2005) Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463. [DOI] [PubMed] [Google Scholar]
- 70).Luxton G.W.G., Gomes E.R., Folker E.S., Vintinner E., Gundersen G.G. (2010) Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 329, 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Petrie R.J., Koo H., Yamada K.M. (2014) Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Stroka K.M., Jiang H., Chen S.-H., Tong Z., Wirtz D., Sun S.X., et al. (2014) Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Heuzé M.L., Vargas P., Chabaud M., Berre M., Liu Y.-J., Collin O., et al. (2013) Migration of dendritic cells: physical principles, molecular mechanisms, and functional implications. Immunol. Rev. 256, 240–254. [DOI] [PubMed] [Google Scholar]
- 74).Paul C.D., Mistriotis P., Konstantopoulos K. (2016) Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Thiam H.R., Vargas P., Carpi N., Crespo C.L., Raab M., Terriac E., et al. (2016) Perinuclear Arp2/3-driven actin polymerization enables nuclear deformation to facilitate cell migration through complex environments. Nat. Commun. 7, 10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Chang Y.-T., Dranow D., Kuhn J., Meyerzon M., Ngo M., Ratner D., et al. (2013) toca-1 is in a novel pathway that functions in parallel with a SUN-KASH nuclear envelope bridge to move nuclei in Caenorhabditis elegans. Genetics 193, 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Xiong H., Mohler W.A., Soto M.C. (2011) The branched actin nucleator Arp2/3 promotes nuclear migrations and cell polarity in the C. elegans zygote. Dev. Biol. 357, 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Skau C.T., Waterman C.M. (2015) Specification of architecture and function of actin structures by actin nucleation factors. Annu. Rev. Biophys. 44, 285–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Denais C.M., Gilbert R.M., Isermann P., McGregor A.L., te Lindert M., Weigelin B., et al. (2016) Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Raab M., Gentili M., de Belly H., Thiam H.R., Vargas P., Jimenez A.J., et al. (2016) ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362. [DOI] [PubMed] [Google Scholar]
- 81).McGregor A.L., Hsia C.-R., Lammerding J. (2016) Squish and squeeze — the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 40, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Swift J., Discher D.E. (2014) The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 127, 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Hancock W.O. (2014) Bidirectional cargo transport: moving beyond tug of war. Nat. Rev. Mol. Cell. Biol. 15, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]