ABSTRACT
Skeletal muscle wasting has gained interest as a primary consequence of chronic kidney disease (CKD) due to the relationship between skeletal muscle mass, mortality and major adverse cardiovascular events in this population. The combination of reductions in physical function, skeletal muscle performance and skeletal muscle mass places individuals with CKD at greater risk of sarcopenia. Therefore the monitoring of skeletal muscle composition and function may provide clinical insight into disease progression. Dual-energy X-ray absorptiometry and bioelectrical impedance analysis are frequently used to estimate body composition in people with CKD within clinical research environments, however, their translation into clinical practice has been limited. Proxy measures of skeletal muscle quality can be obtained using diagnostic ultrasound, providing a cost-effective and accessible imaging modality to aid further clinical research regarding changes in muscle composition. Clinicians and practitioners should evaluate the strengths and limitations of the available technology to determine which devices are most appropriate given their respective circumstances. Progressive resistance exercise has been shown to improve skeletal muscle hypertrophy of the lower extremities, muscular strength and health-related quality of life in end-stage renal disease, with limited evidence available in CKD predialysis. Fundamental principles (i.e. specificity, overload, variation, reversibility, individuality) can be used in the development of more advanced programs focused on improving specific neuromuscular and functional outcomes. Future research is needed to determine the applicability of skeletal muscle monitoring in clinical settings and the feasibility and efficacy of more advanced resistance exercise approaches in those with CKD predialysis.
Keywords: CKD, exercise, pre-dialysis, resistance training, ultrasonography
INTRODUCTION
Chronic kidney disease (CKD) is a major health concern highlighted by the reported increased risks of mortality and comorbidities [1, 2]. Worldwide it is estimated that 10–15% of the general population is affected by CKD [1]. Mounting evidence shows loss of skeletal muscle mass and decrements in skeletal muscle performance as primary consequences of CKD [3–6]. The implications of skeletal muscle atrophy associated with CKD include increased risk for adverse cardiovascular events [7], dysregulation of glucose homeostasis [8], decreased force-generating capacity [9] and reductions in physical function and balance [10]. Moreover, reductions in skeletal muscle mass are strongly associated with the progression of CKD [6]. Given this relationship, assessment of skeletal muscle morphology and performance may provide valuable clinical insights into the pathophysiology of CKD and the potential risk for CKD progression.
Resistance exercise has garnered interest as a potential countermeasure for skeletal muscle impairments in CKD [9, 11–13]. Positive effects on skeletal muscle strength have been observed following resistance exercise in CKD, with equivocal effects on skeletal muscle hypertrophy [12, 13]. Despite a greater proportion of individuals with CKD being predialysis, the majority of evidence comes from studies in patients with end-stage renal disease (ESRD). This has left a paucity of evidence regarding the effects of resistance exercise in those with CKD predialysis [14]. Due to the multiplicity of variables to consider, fundamental principles (i.e. specificity, progressive overload and variation) should be used to increase the likelihood that specified outcomes are achieved when prescribing resistance exercise for CKD [15]. The objectives of this brief review are to (i) describe skeletal muscle consequences associated with CKD, (ii) discuss the importance of monitoring changes in skeletal muscle health and (iii) briefly present principles of exercise physiology and programming strategies to inform the design of resistance exercise to address neuromuscular impairments in CKD predialysis.
SIGNIFICANCE OF CKD
CKD is defined most commonly by decreased kidney function for a duration of at least 3 months as measured by an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 [2]. The chances of developing CKD are increased in those ≥50 years of age and are most common in those ≥70 years of age [16]. The most common risk factors for CKD include diabetes and high blood pressure followed by other factors such as cardiovascular disease, obesity, high cholesterol, lupus and family history [16]. The pathophysiology of CKD manifests in glomerulosclerosis, tubular atrophy and interstitial fibrosis, leading to reduced filtration capabilities of the kidneys [2]. This results in the accumulation and retention of uremic solutes thought to contribute to inflammation, immune dysfunction, vascular disease, platelet dysfunction and increased bleeding risk, reduced bone mineral density, altered drug metabolism, metabolic acidosis and skeletal muscle wasting [2, 4, 17].
The economic costs associated with CKD are significant, especially in patients with ESRD [18]. It is estimated that the socioeconomic burden is likely to continue to increase as a result of the aging population, prolonged survival among people with chronic diseases and the rising prevalence of frailty among other factors [19]. However, high economic costs have been reported as a result of CKD alone. Baumeister et al. [20] found that the high economic costs related to CKD were mainly due to excess inpatient care and drug costs and were independent of important comorbidities. Dialysis treatment is a major factor contributing to the high financial costs of CKD treatment. The average annual direct costs of dialysis treatment in Stage 5 CKD per patient has been reported to range between $30 000 and $60 000, with significantly higher costs associated with early dialysis initiation (10–14 mL/min/1.73 m2 versus 5–7 mL/min/1.73 m2) [21]. Thus, in addition to potential health and quality of life benefits, treatments capable of maintaining kidney function or delaying the onset of dialysis treatment would provide substantial socioeconomic benefit.
SKELETAL MUSCLE WASTING AND DYSFUNCTION IN CKD
Skeletal muscle wasting has gained interest as a primary consequence of CKD due to the relationship between skeletal muscle mass, mortality and major adverse cardiovascular events [3–5, 7, 22–26]. Carrero et al. [24] reported a 30% incident rate of muscle atrophy in patients starting dialysis with a hazard ratio of death of 2.62. Significant associations between loss of lean mass and kidney disease severity and physical function have also been reported [6, 27]. Maintenance of lean mass is dependent on the relationship between protein synthesis and protein degradation. In CKD, this relationship is altered favoring protein degradation and thus accelerating the rate at which skeletal muscle mass is lost [3, 4, 22]. The upregulation of the ubiquitin–proteasome system (UPS) is suggested to contribute to this process given its role in regulating protein degradation [3, 4, 22]. Other potential mechanisms include metabolic acidosis, insulin/insulin-like growth factor 1 (IGF-1), inflammation, appetite regulation and microRNA expression [4]. For example, decrements in microRNA 29a (miR-29a) and miR-29b levels, which are suggested to contribute to decreased muscle myogenesis and CKD-induced muscle atrophy through an upregulation of Yin Yang 1 (YY1), were observed in a CKD rodent model [28].
Decrements in physical function are also commonly observed in individuals with CKD concomitant to losses in skeletal muscle mass [10, 29–32]. For example, patients with CKD predialysis were shown to experience reductions in strength, balance and gait speed, suggesting compromised physical function early in the disease process [31]. Further, physical function of patients with CKD not treated with dialysis is shown to be a stronger predictor of 3-year mortality than kidney function or commonly measured serum biomarkers [33]. Upon the initiation of dialysis, rapid and sustained declines in physical function are known to occur [29, 34]. For example, younger individuals on dialysis experienced poorer physical function compared with older individuals not on dialysis [29]. The reductions in physical function in the younger individuals with CKD on dialysis occurred independent of skeletal muscle mass loss [29]. Determining which factors contribute to declines in physical function will inform future treatment options to be introduced prior to or following dialysis initiation.
Individuals with CKD (both predialysis and ESRD) may be at greater risk of sarcopenia given the combined reductions in physical function, skeletal muscle performance and skeletal muscle mass [5, 6, 35–39]. While several definitions of sarcopenia exist, sarcopenia has been most recently defined as a syndrome classified by reductions in skeletal muscle mass plus a loss of physical function and/or skeletal muscle strength [40, 41]. Foley et al. [42] reported that the prevalence of sarcopenia, defined as the proportion of muscle mass to total body mass, increased with decreasing kidney function. Using the European Working Group on Sarcopenia in Old People (EWGSOP) criteria, elderly patients with ESRD demonstrated a high prevalence of sarcopenia (37% in men and 29.3% in women) [42, 43]. Similarly, Souza et al. [37] reported the prevalence of sarcopenia ranging between 11.9% and 28.7% using the EWGSOP and the Foundation for the National Institutes of Health Sarcopenia Project criteria in patients with CKD predialysis. The increased risk for sarcopenia further highlights the need for valid and reliable screening methods across the CKD spectrum. Additionally, the effects of exercise on sarcopenia in CKD are currently unknown.
CLINICALLY VIABLE APPROACHES TO ASSESSING CHANGES IN SKELETAL MUSCLE IN CKD
Monitoring skeletal muscle composition and function may provide clinical value given the concerns of skeletal muscle wasting and neuromuscular dysfunction in CKD and its relationship with mortality [23]. Dual-energy X-ray absorptiometry (DXA), computed tomography (CT), magnetic resonance imaging (MRI) and bioelectrical impedance analysis (BIA) are frequently used to estimate skeletal muscle morphology and body composition in people with CKD within clinical research environments. However, the translation of these imaging modalities into clinical practice for the purpose of body or tissue composition analysis has been limited [44, 45].
CT and MRI both allow for the assessment of skeletal muscle volume and cross-sectional area (CSA) [44, 45]. CT imaging allows for the differentiation of tissues in vivo based on attenuation characteristics, whereas MRI allows for tissue segmentation via water and fat proton resonance frequencies and relaxation times [44–46]. Advantages to these methods include their high-resolution, three-dimensional construction, regional and CSA assessments and the ability to provide measures of muscle quality [44, 45]. Previous studies using both CT and MRI have documented changes in skeletal muscle in patients with CKD [47–49]. For example, in a natural history study examining changes in skeletal muscle and fat CT imaging showed a greater loss of skeletal muscle CSA in predialysis CKD as compared with those receiving hemodialysis or peritoneal dialysis [47]. Similarly, MRI of the lower leg has been used to detect significant skeletal muscle atrophy in patients on hemodialysis [48]. Despite the valuable information obtained from CT and MRI, the high equipment cost, subject size restrictions and radiation exposure (i.e. CT) associated with these devices pose a challenge to their clinical application [44]. Moreover, the inherent difficulty of obtaining whole body estimates of skeletal muscle mass with CT and MRI confers distinct clinical advantages to DXA and BIA for body composition analysis.
DXA has been identified by sarcopenia consensus groups as the reference standard for whole body and regional estimates of fat mass and fat-free mass (FFM) [50, 51]. Renal disease, age, sex and nutritional status may alter states of hydration, imposing differing degrees of influence on DXA body composition estimate values of FFM [52]. Hydration status may exert a nominal effect on DXA FFM estimates in overweight and obese people [53] but result in significant differences in repeated measures involving lean individuals [54]. The analysis of body composition in community clinical settings often relies on BIA due its affordability, relatively safe usage and general portability. Electrical impedance generated via BIA provides the means to estimate body cell mass through the reactance (Xc) and total body water through the resistance (R) [55, 56]. Importantly, the calculation of FFM using BIA requires the use of validated equations that account for age, health status and racial/ethnic background [52, 55, 57]. Given that BIA is dependent on tissue-specific conductivity, altered states of hydration and chronic fluid imbalances adversely affect the accuracy and reliability of the body composition estimates [55, 58]. Therefore limitations to using BIA to assess post-exercise adaptations in people with CKD may be associated with peripheral edema, changes in diuretic use and the timing of hemodialysis procedures in those with ESRD. Promising alternate approaches to standard BIA include using the derived Xc and R for vector analysis or phase angle (φ) measurement. BIA phase angle has some prognostic utility for people with CKD and may reflect diminished muscle composition or lower body cell mass as a function of age or pathology [45, 59].
A wide range of methods are used to estimate postexercise changes in skeletal muscle. The selected assessment method is governed by cost, accessibility, testing burden and measurement capabilities, as well as test analytics such as accuracy, reliability and responsiveness. Skeletal muscle adaptations in people with CKD have been characterized using DXA following low-intensity strengthening exercises featuring calisthenics and ankle weights [60], measuring total body potassium upon completion of a progressive resistance exercise (PRE) regimen during a period of restricted protein intake [61] and estimating muscle mass using CT scanning after a high-intensity PRE regimen [9]. Methods of body composition and muscle tissue analysis beyond DXA and BIA confer some advantages concerning the assessment of muscle morphology and morphemetry [62]. Changes in muscle morphology attributable to high levels of intramuscular adipose tissue have been associated with impaired lower extremity muscle performance and declines in functional performance [63, 64]. Estimates of intramuscular adipose tissue measured via CT scanning may be a responsive measure of skeletal muscle adaptations to strengthening exercise in people with CKD—even in the absence of muscle hypertrophy [9]. However, this observation is equivocal and requires additional study to better understand the usefulness of postintervention CT attenuation values regarding PRE program efficacy in those with kidney disorders [65].
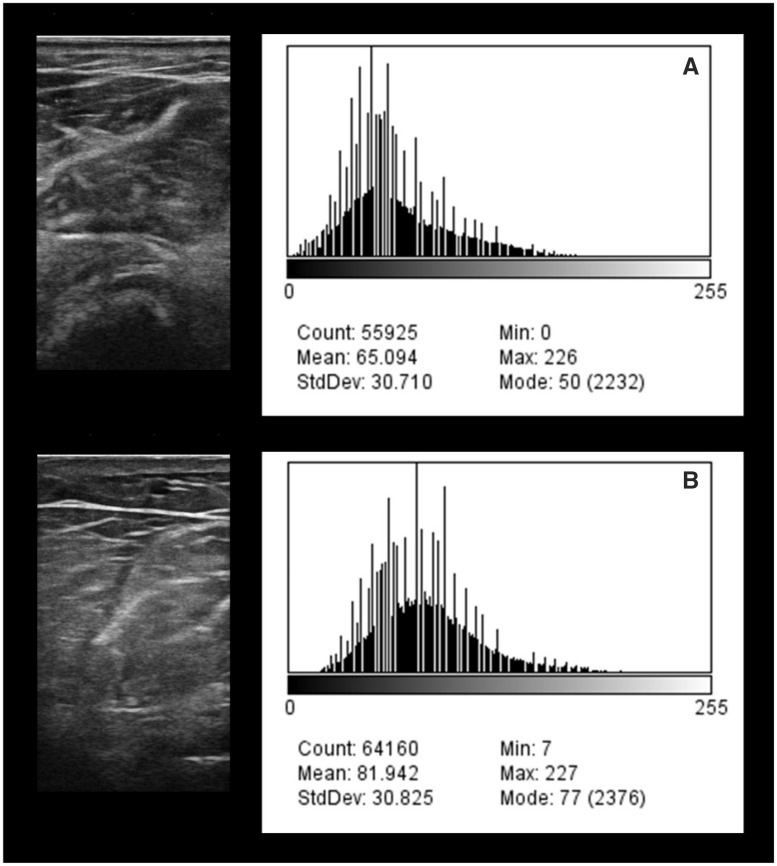
Diagnostic ultrasound has been utilized to obtain proxy measures of skeletal muscle quality providing a cost-effective and accessible imaging modality to aid further clinical research regarding postexercise changes in muscle composition [66–70]. Skeletal muscle quality via computer-aided gray-scale analysis of echogenicity is shown to be independently associated with muscle strength [69, 70]. In addition, echogenicity has demonstrated the potential for a greater magnitude of associations with scaled peak force when compared with age [69]. Figure 1 depicts diagnostic ultrasound images of the mobile wad compartment acquired over the surface of the brachioradialis in a patient with CKD Stage 3 predialysis (Figure 1A) and CKD Stage 4 predialysis (Figure 1B). Gray-scale histogram analysis of the axial view scans was calculated using ImageJ (version 1.48; National Institutes of Health, Bethesda, MD, USA). The histogram in Figure 1A is shifted to the left and the gray-scale values are lower compared with Figure 1B. These ultrasound features suggest that the muscle tissue of the brachioradialis in the patient with CKD Stage 3 may have a better composition profile compared with the muscle tissue of the patient with CKD Stage 4. These examples demonstrate the potential clinical application of diagnostic ultrasound for assessing muscle quality with CKD progression. Further investigations are needed to confirm the validity and reliability of such measures.
FIGURE 1:
Exemplar musculoskeletal ultrasound images and gray-scale histograms of the proximal forearm in men with CKD. (A) The ultrasound scan and gray-scale histogram of a 65-year-old man with Stage 3 CKD with a grip strength value of 0.53 (scaled to body weight) and a Short Physical Performance Battery score of 11. (B) The ultrasound measures of a 71-year-old man with Stage 4 CKD with a grip strength value of 0.15 and a Short Physical Performance Battery score of 7.
Postexercise changes in muscle morphology may differ based on exercise program elements such as mode of muscle action, movement velocity, relative workload, exercise volume and program duration [71]. Diagnostic ultrasound allows for the analysis of postexercise changes in muscle thickness and area, fascicle length and pennation angle [72]. Additionally, specialized diagnostic ultrasound modes such as Doppler may characterize focal muscle blood flow, and quantitative image acquisition and analysis techniques allow for muscle volume estimates [73–77]. Imaging modalities also allow for the assessment of nonuniform adaptations involving skeletal muscle architecture that vary based on the dominant mode of muscle action used during a PRE regimen [71, 72]. Clinicians and practitioners alike should evaluate the strengths and limitations of the available technology to determine which devices are most appropriate.
RESISTANCE EXERCISE IN CKD
Aerobic and resistance exercise, both alone and in combination, have shown beneficial outcomes on health and function in those with CKD [78]. Specifically, regular exercise is seen to improve physical fitness, walking capacity, cardiovascular outcomes, some nutritional parameters and health-related quality of life (HRQoL) [78]. According to the findings from a systematic review and meta-analysis, PRE significantly improved skeletal muscle hypertrophy of the lower extremities, muscular strength and HRQoL [12]. These findings were corroborated in a systematic review of patients with ESRD that reported skeletal muscle hypertrophy, increased lower-body strength and improved aspects of HRQoL in response to resistance exercise [13]. However, to date, limited evidence exists on the effects of resistance exercise in patients with CKD predialysis [11, 79]. The available evidence in this subpopulation of CKD posits the potential for PRE to increase skeletal muscle hypertrophy [80, 81] and strength [61, 81] with no indication of exacerbated inflammatory responses [82].
Despite promising findings of the effects of resistance exercise on neuromuscular health and function in CKD, large variations exist among the protocols investigated. Differences in exercise program design and exercise mode make it difficult to decipher which elements of the exercise regime are most essential. For example, the type of external load applied has included free-weight dumbbells [9], weight machines [80, 81], ankle cuffs/weights [9, 60, 83], elastic bands [9, 84] and pneumatic equipment [85]. The duration of interventions has ranged from 8 weeks to 6 months. Regarding workload assignment, exercise intensity has been defined and monitored according to ratings of perceived exertion and relative exercise intensity (i.e. percentage repetition maximum), while training volume has typically been prescribed at 3 sets of 8–15 repetitions [13]. The lack of standardization across studies complicates data interpretation, limiting the understanding of the potential benefit of resistance exercise for those with CKD.
PRACTICAL CONSIDERATIONS FOR THE DESIGN OF RESISTANCE EXERCISE
Recommendations for maintaining or enhancing skeletal muscle fitness
The American College of Sports Medicine (ACSM) recommends that resistance exercise be performed using 1–4 sets of 8–12 repetitions (2–3 days/week), at loads between 60% and 80% of an individual’s 1-repetition maximum (1-RM), for improving general or overall muscular fitness (Table 1) [86–88]. The current recommendations for resistance exercise specific to CKD are in accordance with the ACSM guidelines [89–92]. These guidelines propose the use of 8–10 multijoint exercises per session performed two times per week. Exercise intensity is encouraged to range between 60% and 70% of a person’s 1-RM or 5-RM with a minimum of 1 set of 10–15 repetitions completed. A gradual increase in volume (i.e. progressing to 2–4 sets per exercise) is also encouraged with 2–3 min of rest between sets and at least 48 h rest between exercise sessions (Table 2). While such recommendations seem to be sufficient for enhancing overall muscular fitness, the inclusion of advanced exercise program designs may be necessary when attempting to target specific neuromuscular characteristics. Such programs may also allow for individualization of exercise programs based on personal factors and CKD severity.
Table 1.
Resistance exercise recommendations for older adults
| Neuromuscular target |
|||||
|---|---|---|---|---|---|
| Hypertrophy | Strength | Power | |||
| Modality | Free weights; machines | Machine-based | Free weights; machines | Machine-based | Free weights; machines |
| Frequency | 2–3 days/week on nonconsecutive days | 3 days/week on nonconsecutive days | 2–3 days/week on nonconsecutive days | 2 days/week on nonconsecutive days | 2–3 days/week on nonconsecutive days |
| Intensity | 60–80% 1-RM | 51–69% 1-RM | 60–80% 1-RM | 70–79% 1-RM | 30–60% 1-RM |
| Training volume | 1–3 sets/exercise; 8–12 repetitions/set | 2–3 sets/exercise; 7–9 repetitions/set | 1–3 sets/exercise; 8–12 repetitions/set | 2–3 sets/exercise; 7–9 repetitions/set | 1–3 sets/exercise; 6–10 repetitions/set |
| Contraction velocity | Slow to moderate | N/A | Slow to moderate | N/A | High |
| Rest intervals | 1–3 min between sets | 120 ss between sets | 1–3 min between sets | 60 ss between sets | 1–3 min between sets |
| Duration | N/A | 50–53 weeks | N/A | 50–53 weeks | N/A |
| Additional comments | Multiple- and single-joint exercises | 6 s time under tension per repetition; 2.5 s rest between repetitions | Multiple- and single-joint exercises | 6 s time under tension per repetition; 4 s rest between repetitions | Should be conducted in combination with training to improve strength; multiple- and single-joint exercises |
Resistance exercise recommendations for enhancing muscular hypertrophy, strength, and power for older adults as proposed by the American College of Sports Medicine (ACSM) [90] and a systematic review and meta-analysis performed by Borde et al. [92].
1-RM, 1 repetition maximum; min, minute; N/A, data not available.
Table 2.
Resistance exercise recommendations for Chronic Kidney Disease
| Neuromuscular target |
|||
|---|---|---|---|
| Muscular fitness | |||
| Modality | Weight-bearing activity, thera-bands, machines and free weights | N/A | N/A |
| Frequency | 2 days/week on nonconsecutive days | ≥ 2 days/week on nonconsecutive days | 2 days/week |
| Intensity | 60–70% 1-RM | N/A | 60–70% 1-RM or 5-RM |
| Training volume | 1 set/exercise; 8–12 exercises; 10–15 repetitions/set | 8–10 exercises involving major muscle groups; 10–15 repetitions/exercise | Minimum of 1 set of 10–15 repetitions; gradually increase to 2–4 sets; choose 8–10 different exercises to work major muscle groups |
| Contraction velocity | N/A | N/A | N/A |
| Rest intervals | N/A | N/A | 2–3 minutes between sets; ≥ 48 hours between sessions |
| Duration | N/A | N/A | N/A |
| Additional comments | Flexibility exercise can be performed 5–7 days/week for a duration of 10 min/session | N/A | Multijoint exercises affecting more than one muscle group and targeting agonist and antagonist muscle |
Guiding principles for resistance exercise prescription
Fundamental principles can be used to guide resistance exercise prescription. These principles include specificity, progressive overload, variation, reversibility and individuality [15]. Specificity refers to the similarities between the training stimulus being applied and the physiological adaptation of interest. This includes (i) muscle actions involved, (ii) speed of the muscle contraction, (iii) range of motion, (iv) muscle groups emphasized, (v) bioenergetic requirements and (vi) training load (i.e. intensity and volume) [15]. Progressive overload describes gradual, planned increases in training stimuli to promote continued gains in health and performance. Variation describes the systematic manipulation of one or more training variables at specified times throughout the training process [15]. Variation differs from traditional progressive overload in that certain training variables may be reduced or removed within a given period of the training cycle. Reversibility describes the loss of exercise-induced adaptations following the secession of exercise and individuality refers to the unique individual responses to a given exercise stimulus. By using such principles, informed decisions can be made during the planning and design of exercise interventions to ensure the safety of the participant while maximizing health and functional benefits.
Considerations for targeting specific neuromuscular outcomes
The prescription of workload assignment is dictated by the neuromuscular or functional outcomes of interest. For example, a resistance exercise regime may aim to improve skeletal muscle hypertrophy, strength or power, as well as functional outcomes such as gait speed or walking distance. Due to the lack of information on resistance exercise programming in CKD, evidence concerning older adults is often used to guide the exercise prescription for this patient population [58, 89–91]. For enhancing muscular strength and hypertrophy it is recommended that 1–3 sets per exercise be performed using a slow to moderate lifting velocity with loads corresponding to 60–80% 1-RM for 8–12 repetitions with rest periods of 1–3 min [86]. Further, dose–response relationships have been identified for training period, intensity, time under tension (i.e. the duration of each repetition) and rest between sets, highlighting the importance of these variables for promoting muscular strength and morphological (i.e. CSA, volume, thickness) adaptations in older adults [87, 93–95]. For enhancing lean body mass, programs consisting of higher volume were associated with the greatest increases, [93] while maximal strength favors training at higher intensities (i.e. 60–80% 1-RM) [87, 94, 95].
Power training (i.e. resistance exercise using low loads performed at high contraction velocities) has been appealing to rehabilitation and exercise professionals given the strong relationship between muscular power and functional outcomes in older adults [96]. To improve muscular power, it is recommended that training include 1–3 sets per exercise performed using high lifting velocities with loads corresponding to 30–60% 1-RM for 6–10 repetitions with rest periods of 1–3 min [86]. However, no consensus regarding optimal loading for the maximization of muscular power in older adults currently exists [95, 97]. In mobility-limited elderly adults, high-velocity resistance training using light loads (i.e. 40% 1-RM) on pneumatic devices resulted in similar improvements in power output and physical performance compared with power training using heavy resistance (i.e. 70% 1-RM) [97]. Therefore, training both the force and velocity components of the force–velocity curve seems beneficial for improving muscular power in older adults as proposed by the ACSM [86]. Contraction velocity and volume were found to be critical factors for increasing muscular power [98]. High-velocity contractions were superior to slow and moderate velocities while lower training volume was associated with greater improvements in muscular power [98]. Currently the available evidence on the effects of resistance exercise for improving muscular power in CKD is scarce. Therefore studies investigating power training interventions in those with CKD are warranted.
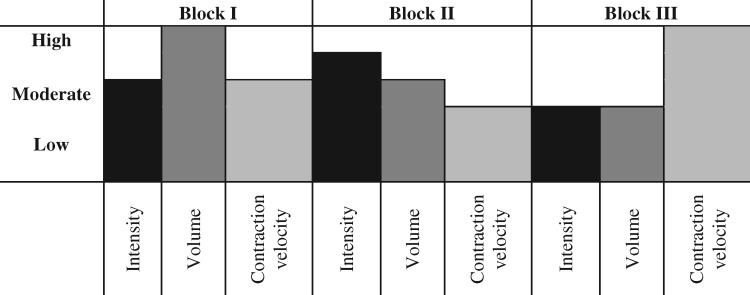
A major challenge working with clinical populations is the safety and tolerability of increasing workloads. As seen in Table 2 [89–91], exercise intensities between 50% and 80% of 1-RM are most effective for eliciting gains in muscle hypertrophy and strength [88]. Depending on disease severity, comorbidities, age, fitness level, genetics, and social and psychological factors, such intensities may not be feasible for those with CKD when initiating a resistance exercise program. Sequencing exercise intensity in a manner that focuses on the development of neuromuscular capacity may overcome this challenge. The application of periodized exercise has been used in clinical and rehabilitation settings as a way to manage exercise intensity [99, 100]. Periodization models provide a conceptual basis describing the systematic manipulations of exercise stimuli in an attempt to account for the accumulation of fatigue and stagnation in training adaptations [15, 101]. Resistance exercise intervention design informed by a block periodization model is presented in Table 3. Training parameters are prescribed in accordance with the neuromuscular outcome of interest and assumes translation of adaptations from one training block to the next (i.e. phase potentiation) [101]. The concept of block periodization aims to develop muscular work capacity, strength and power during different periods of training in a sequential manner (Figure 2). While this provides one example of the organization of workload based on specified outcomes, future research is required to determine the feasibility, efficacy and effectiveness of such approaches in this subpopulation of CKD.
Table 3.
Theoretical example of a block periodization model for resistance exercise adapted for individuals with CKD predialysis
| Block | I (Weeks 1–4) | II (Weeks 5–8) | III (Weeks 9–12) |
|---|---|---|---|
| Emphasis | Work capacity | Maximal strength | Muscular power |
| Intensity | 70% 1-RM | 75% 1-RM | 40% 1-RM |
| Volume | 3 × 12 | 3 × 10 | 3 × 6 |
| Contraction velocity | Slow to moderate | Slow to moderate | High |
FIGURE 2:
Block periodization model depicting intensity, volume and contraction velocity across the training period.
SAFETY CONSIDERATIONS FOR RESISTANCE EXERCISE
It is recommended that exercise prescription for clinical populations should involve a multidisciplinary approach to ensure the safety of the participant while maximizing potential benefits [58]. This may include a team of medical experts, rehabilitation professionals and exercise specialists. Due to the high risk of cardiovascular disease associated with CKD, patients should consult with their physician prior to engaging in exercise. For a complete list of absolute and relative contraindications to resistance exercise and testing see Smart et al. [90] and the ACSM’s Guidelines for Exercise Testing and Prescription [92]. All resistance exercise programs should be individually tailored for each person by a multidisciplinary team. Lower training intensities and volumes and longer exposure to given workloads may be required at the initiation of training. Furthermore, training should be progressed cautiously and informed by individualized responses [92]. The principles outlined above provide additional guidance to ensure patient safety through appropriate exercise progression while still promoting specific adaptations to meet the desired goals.
CONCLUSIONS
CKD is a complex condition that poses a severe threat to skeletal muscle health and function. Monitoring changes in skeletal muscle health might provide critical information regarding the overall health status of patients with CKD regardless of stage. Several options for monitoring skeletal muscle in clinical settings are currently available. Most notably, BIA and diagnostic ultrasound offer promising approaches due to the mobility and minimal space requirements for such technology. Inclusion of skeletal muscle assessments during routine appointments may aid in the understanding of disease progression following the diagnosis of CKD.
Currently, limited information is available regarding the application of resistance exercise in CKD predialysis. While resistance exercise has been shown to be beneficial for combating decrements in skeletal muscle health and function, questions remain concerning the responsiveness of skeletal muscle to various loading stimuli and the feasibility of such schemes in this patient population. Periodization models may offer a useful framework for the planning exercise regimes based on CKD stage and functional abilities to ensure patient safety through appropriate progression while still working towards achieving patient goals. Future research should seek to better understand the impact of resistance exercise to improve muscular power and the relationship between long-term resistance exercise and disease progression.
FUNDING
This publication was supported by the Veterans Affairs (VA) Center for Innovation (AM-251 12-11-2015), Georgetown–Howard Universities Center for Clinical and Translational Science Consortium (NIH/NCATS; UL1TR000101), VA Office of Academic Affiliations (38 U.S.C 7406) and the Rehabilitation Research & Development Service at the VA Office of Research and Development (IK2RX001854-01).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Levin A, Tonelli M, Bonventre J. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917 [DOI] [PubMed] [Google Scholar]
- 2. Webster AC, Nagler EV, Morton RL. et al. Chronic kidney disease. Lancet 2017; 389: 1238–1252 [DOI] [PubMed] [Google Scholar]
- 3. Workeneh BT, Mitch WE.. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010; 91: 1128S–1132S [DOI] [PubMed] [Google Scholar]
- 4. Wang XH, Mitch WE.. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014; 10: 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avin KG, Moorthi RN.. Bone is not alone: the effects of skeletal muscle dysfunction in chronic kidney disease. Curr Osteoporos Rep 2015; 13: 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Y, Hellberg M, Svensson P. et al. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant 2018; 33: 342–348 [DOI] [PubMed] [Google Scholar]
- 7. Harada K, Suzuki S, Ishii H. et al. Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol 2017; 119: 1275–1280 [DOI] [PubMed] [Google Scholar]
- 8. Janssen I, Ross R.. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging 2005; 9: 408–419 [PubMed] [Google Scholar]
- 9. Cheema B, Abas H, Smith B. et al. Progressive Exercise for Anabolism in Kidney Disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 2007; 18: 1594–601 [DOI] [PubMed] [Google Scholar]
- 10. Delgado C, Shieh S, Grimes B. et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol 2015; 42: 134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gould DW, Graham-Brown MP, Watson EL. et al. Physiological benefits of exercise in pre-dialysis chronic kidney disease: exercise in chronic kidney disease. Nephrology 2014; 19: 519–527 [DOI] [PubMed] [Google Scholar]
- 12. Cheema BS, Chan D, Fahey P. et al. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med 2014; 44: 1125–1138 [DOI] [PubMed] [Google Scholar]
- 13. Chan D, Cheema BS.. Progressive resistance training in end-stage renal disease: systematic review. Am J Nephrol 2016; 44: 32–45 [DOI] [PubMed] [Google Scholar]
- 14. MacKinnon HJ, Feehally J, Smith AC.. A review of the role of exercise and factors affecting its uptake for people with chronic kidney disease (CKD) not requiring renal replacement therapy. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015; 36: 37–46 [PubMed] [Google Scholar]
- 15. Kraemer WJ, Ratamess NA.. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc 2004; 36: 674–688 [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2017 [Google Scholar]
- 17. Massy Z, Drueke T.. Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J Nephrol 2017; 30: 629–34 [DOI] [PubMed] [Google Scholar]
- 18. Klarenbach SW, Tonelli M, Chui B. et al. Economic evaluation of dialysis therapies. Nat Rev Nephrol 2014; 10: 644–652 [DOI] [PubMed] [Google Scholar]
- 19. Vanholder R, Annemans L, Brown E. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 20. Baumeister SE, Böger CA, Krämer BK. et al. Effect of chronic kidney disease and comorbid conditions on health care costs: a 10-year observational study in a general population. Am J Nephrol 2010; 31: 222–229 [DOI] [PubMed] [Google Scholar]
- 21. Harris A, Cooper BA, Li JJ. et al. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis 2011; 57: 707–715 [DOI] [PubMed] [Google Scholar]
- 22. Thomas SS, Mitch WE.. Mechanisms stimulating muscle wasting in chronic kidney disease: the roles of the ubiquitin-proteasome system and myostatin. Clin Exp Nephrol 2013; 17: 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vega A, Abad S, Macías N. et al. Low lean tissue mass is an independent risk factor for mortality in patients with stages 4 and 5 non-dialysis chronic kidney disease. Clin Kidney J 2017; 10: 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carrero JJ, Chmielewski M, Axelsson J. et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 2008; 27: 557–564 [DOI] [PubMed] [Google Scholar]
- 25. Honda H, Qureshi AR, Axelsson J. et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr 2007; 86: 633–638 [DOI] [PubMed] [Google Scholar]
- 26. Desmeules S, Levesque R, Jaussent I. et al. Creatinine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant 2004; 19: 1182–1189 [DOI] [PubMed] [Google Scholar]
- 27. McIntyre CW, Selby NM, Sigrist M. et al. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant 2006; 21: 2210–2216 [DOI] [PubMed] [Google Scholar]
- 28. Wang XH, Hu Z, Klein JD. et al. Decreased miR-29 suppresses myogenesis in CKD. J Am Soc Nephrol 2011; 22: 2068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcus RL, LaStayo PC, Ikizler TA. et al. Low physical function in maintenance hemodialysis patients is independent of muscle mass and comorbidity. J Ren Nutr 2015; 25: 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansen KL, Doyle J, Sakkas GK. et al. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am J Physiol Regul Integr Comp Physiol 2005; 289: R805–R813 [DOI] [PubMed] [Google Scholar]
- 31. Hiraki K, Yasuda T, Hotta C. et al. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol 2013; 17: 225–231 [DOI] [PubMed] [Google Scholar]
- 32. Lewis MI, Fournier M, Wang H. et al. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol 2012; 112: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roshanravan B, Robinson-Cohen C, Patel KV. et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurella Tamura M, Covinsky KE, Chertow GM. et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361: 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moorthi RN, Avin KG.. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017; 26: 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Androga L, Sharma D, Amodu A. et al. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int Rep 2017; 2: 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Souza VAd, Oliveira D, Barbosa SR. et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One 2017; 12: e0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pereira RA, Cordeiro AC, Avesani CM. et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015; 30: 1718–1725 [DOI] [PubMed] [Google Scholar]
- 39. Isoyama N, Qureshi AR, Avesani CM. et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9: 1720–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McLean RR, Kiel DP.. Developing consensus criteria for sarcopenia: an update. J Bone Miner Res 2015; 30: 588–592 [DOI] [PubMed] [Google Scholar]
- 41. Cruz-Jentoft AJ, Baeyens JP, Bauer JM. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foley RN, Wang C, Ishani A. et al. Kidney function and sarcopenia in the united states general population: NHANES III. Am J Nephrol 2007; 27: 279–286 [DOI] [PubMed] [Google Scholar]
- 43. Kim J-K, Choi SR, Choi MJ. et al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr 2014; 33: 64–68 [DOI] [PubMed] [Google Scholar]
- 44. Carrero JJ, Johansen KL, Lindholm B. et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 2016; 90: 53–66 [DOI] [PubMed] [Google Scholar]
- 45. Heymsfield SB, Gonzalez MC, Lu J. et al. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc 2015; 74: 355–366 [DOI] [PubMed] [Google Scholar]
- 46. Goodpaster BH, Thaete FL, Kelley DE.. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 2000; 904: 18–24 [DOI] [PubMed] [Google Scholar]
- 47. John SG, Sigrist MK, Taal MW. et al. Natural history of skeletal muscle mass changes in chronic kidney disease stage 4 and 5 patients: an observational study. PLoS One 2013; 8: e65372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA.. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int 2003; 63: 291–297 [DOI] [PubMed] [Google Scholar]
- 49. Segura-Ortí E, Gordon PL, Doyle JW. et al. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res 2018; 27: 579–596 [DOI] [PubMed] [Google Scholar]
- 50. Cooper C, Fielding R, Visser M. et al. Tools in the assessment of sarcopenia. Calcif Tissue Int 2013; 93: 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buckinx F, Landi F, Cesari M. et al. Pitfalls in the measurement of muscle mass: a need for a reference standard: measurement of muscle mass. J Cachexia Sarcopenia Muscle 2018; 9: 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fosbøl MØ, Zerahn B.. Contemporary methods of body composition measurement. Clin Physiol Funct Imaging 2015; 35: 81–97 [DOI] [PubMed] [Google Scholar]
- 53. Bredella MA, Ghomi RH, Thomas BJ. et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 2010; 18: 2227–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Toomey CM, McCormack WG, Jakeman P.. The effect of hydration status on the measurement of lean tissue mass by dual-energy X-ray absorptiometry. Eur J Appl Physiol 2017; 117: 567–574 [DOI] [PubMed] [Google Scholar]
- 55. Kyle UG, Piccoli A, Pichard C.. Body composition measurements: interpretation finally made easy for clinical use. Curr Opin Clin Nutr Metab Care 2003; 6: 387–393 [DOI] [PubMed] [Google Scholar]
- 56. Walter-Kroker A, Kroker A, Mattiucci-Guehlke M. et al. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr J 2011; 10: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harris-Love MO, Adams B, Hernandez HJ. et al. Disparities in the consequences of sarcopenia: implications for African Americans veterans. Front Physiol 2014; 5: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hernandez H, Obamwonyi G, Harris-Love M.. Physical therapy considerations for chronic kidney disease and secondary sarcopenia. J Funct Morphol Kinesiol 2018; 3: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soares V, Avelar IS de, Andrade SR de S. et al. Body composition of chronic renal patients: anthropometry and bioimpedance vector analysis. Rev Lat Am Enfermagem 2013; 21: 1240–1247 [DOI] [PubMed] [Google Scholar]
- 60. Chen JLT, Godfrey S, Ng TT. et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant 2010; 25: 1936–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Castaneda C, Gordon PL, Uhlin KL. et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized controlled trial. Ann Intern Med 2001; 135: 965–976 [DOI] [PubMed] [Google Scholar]
- 62. Harris-Love MO, Monfaredi R, Ismail C. et al. Quantitative ultrasound: measurement considerations for the assessment of muscular dystrophy and sarcopenia. Front Aging Neurosci 2014; 6: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Landi F, Liperoti R, Russo A. et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012; 31: 652–658 [DOI] [PubMed] [Google Scholar]
- 64. Goodpaster BH, Carlson CL, Visser M. et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol 2001; 90: 2157–2165 [DOI] [PubMed] [Google Scholar]
- 65. Cheema B, Abas H, Smith B. et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis 2007; 50: 574–584 [DOI] [PubMed] [Google Scholar]
- 66. Pillen S, Tak RO, Zwarts MJ. et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 2009; 35: 443–446 [DOI] [PubMed] [Google Scholar]
- 67. Harris-Love MO, Seamon BA, Teixeira C. et al. Ultrasound estimates of muscle quality in older adults: reliability and comparison of photoshop and imagej for the grayscale analysis of muscle echogenicity. PeerJ 2016; 4: e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Akima H, Hioki M, Yoshiko A. et al. Intramuscular adipose tissue determined by T1-weighted MRI at 3T primarily reflects extramyocellular lipids. Magn Reson Imaging 2016; 34: 397–403 [DOI] [PubMed] [Google Scholar]
- 69. Ismail C, Zabal J, Hernandez HJ. et al. Diagnostic ultrasound estimates of muscle mass and muscle quality discriminate between women with and without sarcopenia. Front Physiol 2015; 6: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fukumoto Y, Ikezoe T, Yamada Y. et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol 2012; 112: 1519–1525 [DOI] [PubMed] [Google Scholar]
- 71. Franchi MV, Atherton PJ, Reeves ND. et al. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol 2014; 210: 642–654 [DOI] [PubMed] [Google Scholar]
- 72. Narici M, Franchi M, Maganaris C.. Muscle structural assembly and functional consequences. J Exp Biol 2016; 219: 276–284 [DOI] [PubMed] [Google Scholar]
- 73. English C, Fisher L, Thoirs K.. Reliability of real-time ultrasound for measuring skeletal muscle size in human limbs in vivo: a systematic review. Clin Rehabil 2012; 26: 934–944 [DOI] [PubMed] [Google Scholar]
- 74. Klauser AS, Peetrons P.. Developments in musculoskeletal ultrasound and clinical applications. Skeletal Radiol 2010; 39: 1061–1071 [DOI] [PubMed] [Google Scholar]
- 75. Abe T, Loenneke JP, Thiebaud RS.. Morphological and functional relationships with ultrasound measured muscle thickness of the lower extremity: a brief review. Ultrasound 2015; 23: 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Obst SJ, Boyd R, Read F. et al. Quantitative 3-D ultrasound of the medial gastrocnemius muscle in children with unilateral spastic cerebral palsy. Ultrasound Med Biol 2017; 43: 2814–2823 [DOI] [PubMed] [Google Scholar]
- 77. Walton JM, Roberts N, Whitehouse GH.. Measurement of the quadriceps femoris muscle using magnetic resonance and ultrasound imaging. Br J Sports Med 1997; 31: 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heiwe S, Jacobson SH.. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 10: CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Barcellos FC, Santos IS, Umpierre D. et al. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J 2015; 8: 753–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Castaneda C, Gordon PL, Parker RC. et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004; 43: 607–616 [DOI] [PubMed] [Google Scholar]
- 81. Watson EL, Greening NJ, Viana JL. et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis 2015; 66: 249–257 [DOI] [PubMed] [Google Scholar]
- 82. Watson EL, Viana JL, Wimbury D. et al. The effect of resistance exercise on inflammatory and myogenic markers in patients with chronic kidney disease. Front Physiol 2017; 8: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Johansen KL. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 2006; 17: 2307–2314 [DOI] [PubMed] [Google Scholar]
- 84. Song W-J, Sohng K-Y.. Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. J Korean Acad Nurs 2012; 42: 947. [DOI] [PubMed] [Google Scholar]
- 85. Dong J, Sundell MB, Pupim LB. et al. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr 2011; 21: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Garber CE, Blissmer B, Deschenes MR. et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359 [DOI] [PubMed] [Google Scholar]
- 87. Johansen KL, Painter P.. Exercise in individuals with CKD. Am J Kidney Dis 2012; 59: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smart NA, Williams AD, Levinger I. et al. Exercise & Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J Sci Med Sport 2013; 16: 406–411 [DOI] [PubMed] [Google Scholar]
- 89. Roshanravan B, Gamboa J, Wilund K.. Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis 2017; 69: 837–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, Tenth Edition. Philadelphia: Wolters Kluwer, 2018 [Google Scholar]
- 91. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009; 41: 687–708 [DOI] [PubMed] [Google Scholar]
- 92. Borde R, Hortobágyi T, Granacher U.. Dose–response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med 2015; 45: 1693–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Peterson MD, Sen A, Gordon PM.. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 2011; 43: 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Peterson MD, Rhea MR, Sen A. et al. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010; 9: 226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Steib S, Schoene D, Pfeifer K.. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc 2010; 42: 902–914 [DOI] [PubMed] [Google Scholar]
- 96. Reid KF, Fielding RA.. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 2012; 40: 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reid KF, Martin KI, Doros G. et al. Comparative effects of light or heavy resistance power training for improving lower extremity power and physical performance in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 2015; 70: 374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Straight CR, Lindheimer JB, Brady AO. et al. Effects of resistance training on lower-extremity muscle power in middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med 2016; 46: 353–364 [DOI] [PubMed] [Google Scholar]
- 99. Harris-Love MO, Seamon BA, Gonzales TI. et al. Eccentric exercise program design: a periodization model for rehabilitation applications. Front Physiol 2017; 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lorenz DS, Reiman MP, Walker JC.. Periodization: current review and suggested implementation for athletic rehabilitation. Sports Health Multidiscip Approach 2010; 2: 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cunanan AJ, DeWeese BH, Wagle JP. et al. The general adaptation syndrome: a foundation for the concept of periodization. Sports Med Auckl NZ 2018; 48: 787–797 [DOI] [PubMed] [Google Scholar]