Significance
The plant trans-Golgi network (TGN) is spatially separated from the Golgi and functions as an independent cargo sorting station. The molecular mechanism and significance of its generation and separation from the Golgi are largely unclear. This study identified a Golgi-localized component that is essential for TGN biogenesis and sheds light on the regulation of post-Golgi trafficking.
Keywords: TGN biogenesis, Golgi, vesicle trafficking, pollen tube growth
Abstract
The trans-Golgi network (TGN) is an essential tubular-vesicular organelle derived from the Golgi and functions as an independent sorting and trafficking hub within the cell. However, the molecular regulation of TGN biogenesis remains enigmatic. Here we identified an Arabidopsis mutant loss of TGN (lot) that is defective in TGN formation and sterile due to impaired pollen tube growth in the style. The mutation leads to overstacking of the Golgi cisternae and significant reduction in the number of TGNs and vesicles surrounding the Golgi in pollen, which is corroborated by the dispersed cytosolic distribution of TGN-localized proteins. Consistently, deposition of extracellular pectin and plasma membrane localization of kinases and phosphoinositide species are also impaired. Subcellular localization analysis suggests that LOT is localized on the periphery of the Golgi cisternae, but the mutation does not affect the localization of Golgi-resident proteins. Furthermore, the yeast complementation result suggests that LOT could functionally act as a component of the guanine nucleotide exchange factor (GEF) complex of small Rab GTPase Ypt6. Taken together, these findings suggest that LOT is a critical player for TGN biogenesis in the plant lineage.
The Golgi–trans-Golgi network (TGN) system serves as an essential crossroad of the biosynthetic and endocytic recycling pathways. The Golgi plays pivotal roles in sorting proteins and is also a major biosynthetic factory for cell wall polysaccharides and protein glycosylation in plants (1–3). It consists of stacks of cisternae that receive cargos at the cis-side from the endoplasmic reticulum (ER) and processes them before sorting at the TGNs into packaged vesicles destined for secretion or vacuoles (4, 5). Distinct from animal cells in which the TGNs locate on the trans-side of the Golgi, the plant TGN is a separate compartment from the Golgi and functions as an early endosome (EE) as well, thus called TGN/EE (6–8).
In plants, the TGN is verified to be an independent organelle that matures from the trans-most Golgi cisterna, as demonstrated by superresolution live imaging, cell fractionation, and electron microscopy-based studies (9, 10). Based on tomography study, the biogenesis of the TGN can be divided into two continuous stages in a coat-independent manner: cisternae peeling from the trans-side Golgi cisterna, at which stage the TGN is termed the Golgi-associated TGN (GA-TGN), and free TGN, which gives rise to proliferated smaller secretory vesicles (10, 11). Detachment of GA-TGNs from the Golgi is proposed to give rise to the free TGNs (10). Genetic studies and drug treatment have also evidenced the dynamic and physical association of the TGN with the Golgi (8, 12, 13). Several TGN-localized proteins have been implicated in regulating TGN detachment or function (14). However, it is still unclear how TGN biogenesis is regulated, and no Golgi-localized proteins have been discovered to take a role in this process.
Vesicular transport in yeasts, mammalian cells, and plants is known to be mainly determined by a large family of Ras-related GTPases known as Ypt in yeast and Rab proteins in the other two kingdoms. These small GTPases are activated by the guanine nucleotide exchange factor (GEF) that catalyzes the GDP-to-GTP switch at the donor or acceptor membrane (15). The GTP-bound Rabs are required for vesicle generation from the donor membrane and delivery to or fusion with the acceptor membrane. A considerable number of Rabs have been identified in flowering plants, with 57 members in Arabidopsis distributed in different compartments (16). GEFs for all Ypts have been identified in yeast, while compared with the largely extended Rab families, only a few GEFs have been identified in plants.
In flowering plants, pollen tubes are deployed to deliver the sperm cells to the embryo sac for double fertilization (17, 18). Membrane trafficking is critical for driving the tip growth of the pollen tube (19, 20). Distinct from other cell types, pollen tube growth requires faster and sustained deposition of the cell wall, proteins, and membrane materials for a shortened fertilization interval in angiosperms (21). The cell wall provides tensile and plastic mechanics as well as turgor pressure and membrane proteins that play roles in cell signaling (22). Most of these signaling and mechanical materials are packaged and sorted in the form of secretory vesicles from the Golgi apparatus to the TGN and then the plasma membrane (PM) and apoplast. Mutations affecting this trafficking pathway may impair pollen tube growth (23–25). Thus, understanding the molecular mechanism of vesicle secretion en route from the Golgi is important to plant reproduction and basic cell biology.
In this study, we identified a Golgi-localized RabGEF component loss of TGN (LOT) that regulates the TGN biogenesis from the Golgi and demonstrated its effect on the secretory and endocytic pathways in the pollen tube.
Results
LOT Regulates Pollen Tube Growth.
In a genetic screen of Ds transposon insertion mutants of Arabidopsis, we identified a male gametophyte defective mutant, kd329, with a distorted Ds segregation ratio. The Ds element contains an NPTII expressing cassette, which confers the Ds-mutated plants’ kanamycin resistance (KanR). Therefore, the gametophytic transmission can be tracked by the distorted segregation ratio of KanR to Kan sensitivity (KanR/KanS) of the seedlings on Murashige and Skoog media supplemented with kanamycin (26). In the self-pollination experiment of the heterozygous kd329+/−, the progeny displays a KanR/KanS ratio of 0.9:1 (1,244:1,392, n = 2,636), suggesting a gametophytic defect. In reciprocal test crosses with the wild-type (WT) plants, KanR/KanS is 0.9:1 (789:896, n = 1,685) when kd329+/− was used as the pollen recipient plant. However, the KanR/KanS ratio is 0.005:1 (10:2,031, n = 2041) when kd329+/− was used as the pollen donor plant (SI Appendix, Table S1). These results indicate that the kd329 mutation severely impairs the male gametophytic function.
Through thermal asymmetrical interlaced PCR and sequencing, the flanking sequence of Ds was obtained. Sequence analysis shows that the Ds element is inserted in the first exon of At1G50120, 4 bp downstream of the start codon (Fig. 1A and SI Appendix, Fig. S1A). We designated At1G50120 as LOT (see below) and mutant kd329 as lot. Additionally, a homozygous transfer DNA (T-DNA) insertion allele, Salk_130228 from Arabidopsis Biological Resource Center (ABRC) with T-DNA inserted in the sixth intron, still transcribes a substantial amount of the corresponding mRNA (SI Appendix, Fig. S1B). This suggests that Salk_130228 is a knockdown mutant, which may explain the lack of visible phenotype of the plants. Therefore, only the Ds mutation lot was further studied. In a screening for homozygotes, a homozygous plant for Ds that is dwarf and almost totally male sterile, bearing only scarce seeds (1 ± 0.5 seed per pod), was obtained among 300 plants (Fig. 1B).
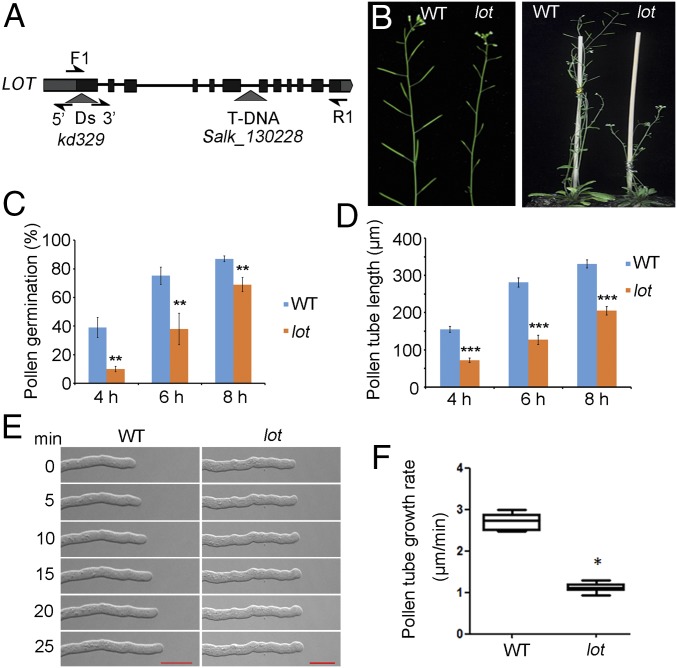
Fig. 1.
Characterization of lot and its phenotype. (A) Schematic diagram of Ds and T-DNA insertion. Black box, exon; black line, intron; gray box, untranslated region; arrow, primers used. (B) The lot plant is sterile and dwarf. (C and D) In vitro pollen germination and tube growth of lot at 4, 6, and 8 h after plating. Data are shown as means ± SD of three repeats (n > 1,000). Student’s t test, **P < 0.01; ***P < 0.001. (E) Time series of pollen tube growth. (Scale bar, 20 μm). (F) In vitro pollen tube growth rate of lot and WT. Data are shown as means ± SD of three repeats (n = 10 pollen tubes). Student’s t test, *P < 0.05.
To confirm that LOT is the causal gene for the phenotype, the fusion construct of the coding sequence of LOT and GFP driven by its native promoter (1,701 bp upstream of the start codon) was made and transformed into lot+/−. In T2 transgenic lot+/− plants expressing GFP-LOT, the KanR/KanS segregation ratio of five independent transgenic lines recovers from 0.9 to 1.7–2.8 (SI Appendix, Table S2). In the T3 plants, fertile homozygous lot plants carrying the transgene were isolated, which further confirms that LOT is the causal gene of the male defect of lot.
To investigate the biological basis of the decreased male gametophyte transmission efficiency, the pollen morphology and viability of lot homozygotes were examined by DAPI and Alexander staining, respectively. As the results have shown, lot pollen grains are morphologically normal with two generative nuclei and one vegetative nucleus at maturity (n = 1,000), indicating that pollen development of lot is normal (SI Appendix, Fig. S2). Further examination of pollen germination and tube growth in vitro showed that the germination ratio of lot is lower than that of the WT (Fig. 1C) and lot pollen tube length is shortened (Fig. 1D). The live imaging result suggests that even though the morphology of lot pollen tubes is normal, the growth rate is 1.2 μm/s, much slower than that of the WT (Fig. 1 E and F). However, this defect in pollen tube growth in vitro is not proportionally consistent with the severely impaired male gametophytic transmission (0.5%). Thus, we speculate that this may result from the low competence of lot pollen for fertilization in vivo.
To test this possibility, the pollen tube growth in the pistil was examined. Pollen grains were pollinated manually onto the WT pistil, and then at 12 h after pollination the pistils were stained with aniline blue, which labels the callose on the pollen tube. As shown, the WT pollen tubes grew to the base of the pistils, and every ovule in the pistil was targeted (Fig. 2A), while most of the pollen tubes from lot homozygotes were blocked in the style (Fig. 2 B and D). Only a few mutant pollen tubes extended to the transmitting tissue, but they failed to target the ovules (Fig. 2C). When the stigma was dissected, most of the blocked pollen tubes were observed to exhibit swollen or branching tips in the style (Fig. 2 E and F). In semi-in vitro conditions, lot pollen tubes never grew out of the cut style even after 8-h culturing on the medium (Fig. 2 G and H).
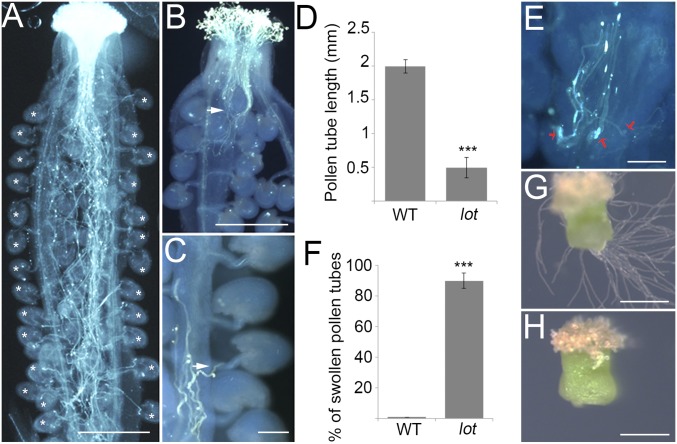
Fig. 2.
The lot pollen tubes grow slower in vivo. (A) WT pollen tubes in the WT pistil. Asterisks, targeted ovules. (B) The lot pollen tubes in the WT pistil. Arrow, where most pollen tubes reached. (C) The lot pollen tubes fail to target the ovule. Arrow, failed pollen tube. (D) Pollen tube length in vivo. Data are shown as means ± SD of three repeats (n = 20 pistils). Student’s t test, ***P < 0.001. (E) Swelled lot pollen tubes in the style. (F) Statistics of E. Data are shown as means ± SD of three repeats (n = 120 pollen tubes). Student’s t test, ***P < 0.001. (G) WT pollen tubes grow out of the WT cut style. (H) The lot pollen tubes fail to penetrate out of the WT style. (Scale bars in A, B, G, and H, 400 μm; C and E, 100 μm.)
Together, these results suggest an indispensable role of LOT in pollen tube growth in vivo, and different tip morphologies of the lot pollen tube in vivo and in vitro imply a pollen–stigma interaction defect.
LOT Is Widely Expressed and Encodes a Golgi-Localized Protein.
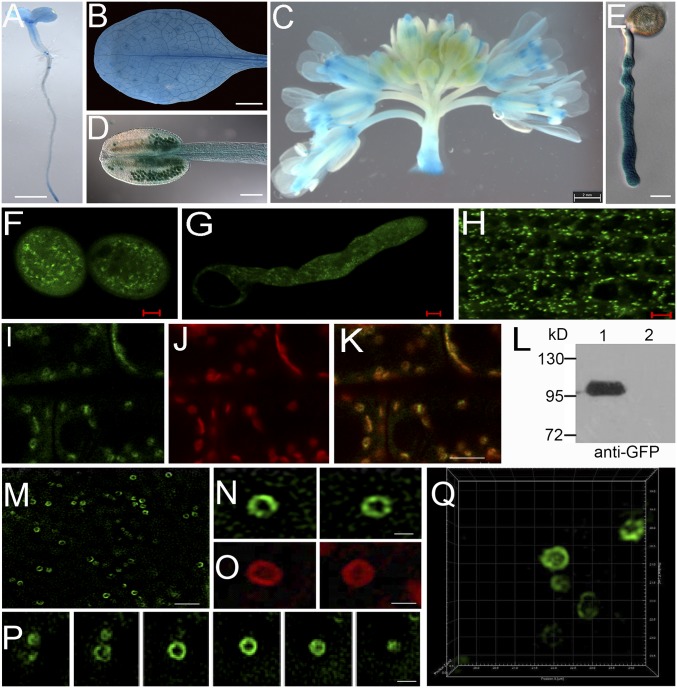
To determine the subcellular localization and expression pattern of LOT, a genomic fragment including the native promoter and coding region fused C-terminally with β-glucuronidase (GUS) or GFP was introduced to the lot heterozygotes. The KanR/KanS segregation ratio was highly elevated in the T2 transgenic progeny (SI Appendix, Table S3), suggesting that the C-terminal tagged LOT also functions properly in vivo. GUS staining showed that GUS activity is detected in the root tip, lateral root primordium, leaf, stamen filament, pollen, and pollen tube (Fig. 3 A–E). In the genetically rescued GFP-LOT and LOT-GFP plants, the fusion proteins display punctate structures in the pollen grain, pollen tube, and root cells (Fig. 3 F–H). To further dissect the subcellular localization, the plant expressing Golgi marker 35S:ST-mCherry (sialyltransferase-mCherry) was crossed with GFP-LOT transgenic plants. ST-mCherry and GFP-LOT totally colocalize in the same punctate structure in root cells, which indicates that LOT is localized on the Golgi apparatus (Fig. 3 I–K). Immunoblot assay with GFP-LOT seedlings showed that GFP-LOT is ∼100 kDa, as predicted (Fig. 3L). Furthermore, superresolution microscopy was deployed to obtain clearer observation of LOT on the Golgi (Fig. 3M). The series of optical sections (Fig. 3 N and P) and projection images (Fig. 3Q and Movie S1) showed that GFP-LOT is localized on the rim of the Golgi apparatus, similar to the pattern of ST-mCherry (Fig. 3O).
Fig. 3.
The expression pattern and subcellular localization of LOT. (A–E) GUS staining of pLOT: gLOT-GUS-3UTR plants. (A) Seedling. (B) Leaf. (C) Inflorescence. (Scale bar in A–C, 1 mm). (D) Anther. (Scale bar, 100 μm.) (E) Pollen tube. (Scale bar, 10 μm.) (F–H) Image of GFP from pLOT: gLOT-GFP-3UTR plants. (F) Pollen. (G) Pollen tube. (H) Root. (Scale bar, 5 μm.) (I–K) Colocalization of GFP-LOT and ST-mCherry in roots. (Scale bar, 1 μm.) (L) Immunoblot of GFP-LOT fusion protein. 1, GFP-LOT plants; 2, WT plants. kD, kilodalton. (M–Q) GFP-LOT and ST-mCherry in the root by superresolution microscopy. (M) GFP-LOT image with lower magnification. (Scale bar, 2 μm.) (N) Enlarged view of M. (Scale bar, 0.7 μm.) (O) ST-mCherry. (Scale bar, 0.7 μm.) (P) Stacked image series of GFP-LOT. (Scale bar, 0.5 μm.) (Q) Three-dimensional projection of stacked images of GFP-LOT.
LOT Is Required for TGN Formation and Golgi Structure Maintenance.
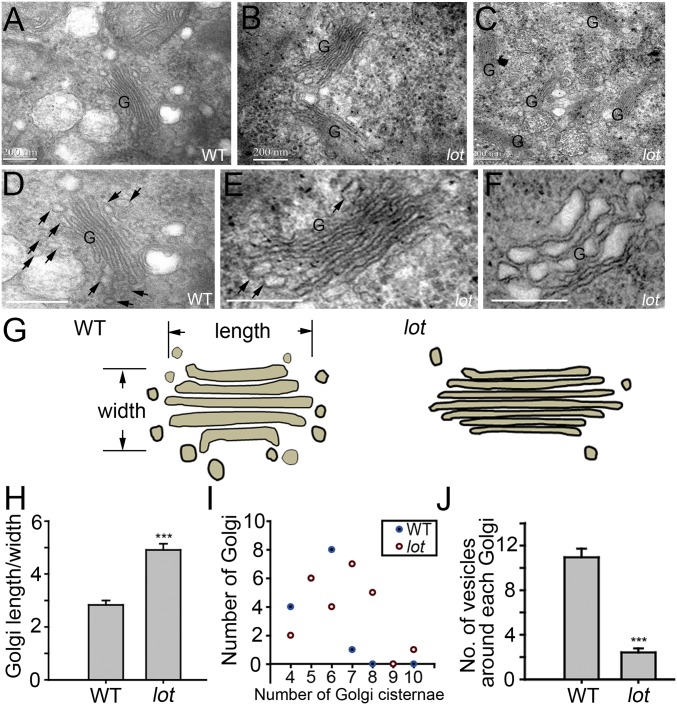
To further investigate the function of LOT on the Golgi, we performed high-pressure freezing and freeze-substitution-based transmission electron microscopy (TEM) analysis with pollen grains to examine the ultrastructure of the WT and lot. TEM analysis revealed that the WT pollen grains display a regular Golgi morphology, with vesicles of different sizes around the Golgi (Fig. 4 A and D and SI Appendix, Fig. S3). By contrast, whole-cell survey suggested that most of the Golgi in lot pollen exhibits overstacking and elongated Golgi cisternae with narrower space in between (Fig. 4 B, C, and E and SI Appendix, Fig. S4). In addition, a small proportion (<5%) of the cisternae, without vesicles around the Golgi, is dilated (Fig. 4F), which was not observed in the WT. Nevertheless, it could not be ruled out that the dilated structure is caused by the sectioning angle. A 3D reconstruction would be able to clarify this caveat. Statistical results suggest that the length of the Golgi cisternae is longer, and the numbers of cisternae in the Golgi stack are increased in lot (Fig. 4 G–I). Importantly, vesicles surrounding the Golgi are drastically reduced (Fig. 4J), implying defective vesicle budding from the Golgi. These results suggest the possibility of defective formation of TGN and vesicles.
Fig. 4.
The lot causes reduced TGNs, vesicles, and deformed Golgi. (A–F) TEM images of the Golgi in WT (A and D) and lot (B, C, E, and F) pollen. (D) Zoomed-in image of A. (E) Zoomed-in image of B. Arrows, vesicular structures around the Golgi (G). (Scale bar, 200 nm.) (G) Diagram of the Golgi structure and surrounding vesicles. (H) Ratio of Golgi length/width in WT (n = 21) and lot (n = 26) pollen. Data are shown as means ± SD. Student’s t test, ***P < 0.01. (I) Number of Golgi of different cisternae numbers in the WT (n = 19) and lot (n = 25). (J) Quantification of vesicles around the Golgi. Areas of (800 nm × 400 nm) square with a Golgi centered were scored for the vesicle numbers in WT and lot. (n = 21 Golgi for WT; n = 26 Golgi for lot). Data are shown as means ± SD. Student’s t test, ***P < 0.01.
To confirm this speculation, the TGN-specific maker YFP-RabA4b (27) and endosome marker mCherry-RabA5D (28) driven by the pollen-specific LAT52 promoter and UBQ10 promoter, respectively, were introduced to track the number and distribution of TGN/EE in the pollen grain and pollen tube. RabA4b labels budding vesicles that peel off from the GA-TGN, and the knockout mutant of RabA4b displays morphological defects of the GA-TGN and fewer budding vesicles from these GA-TGNs (10). Our results show that these two markers are dispersive in the cytosol of lot instead of on the punctate TGN/EE as in the WT (Fig. 5 A–C). As Rab proteins dynamically recycle between the membrane and cytosol by the switch of GTP to GDP binding, we further introduced transmembrane TGN marker protein VTI12, which is a component of Q-SNARE (28, 29). Similarly, VTI12 is also dispersed in the cytosol of lot pollen and the pollen tube (Fig. 5 D–G). These results corroborate the observation of TGN deficiency in lot pollen by TEM.
Fig. 5.
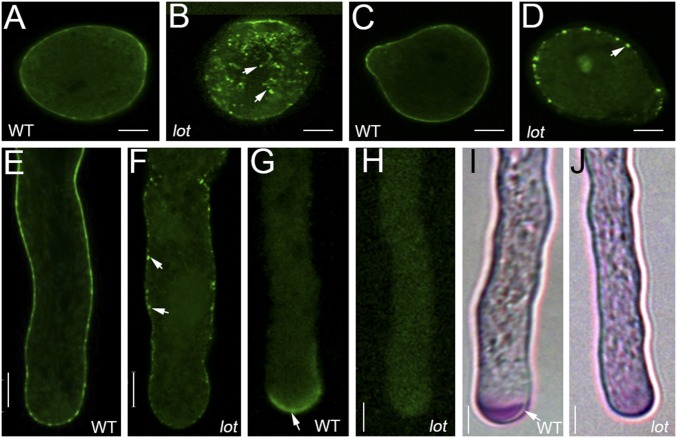
The TGN formation in lot pollen tubes is disrupted. (A and B) Confocal images of YFP-RabA4b in WT (A) and lot (B) pollen tubes. (C) Pollen from mCherry-RabA5D; lot+/−. (D–G) mCherry-VTI12 in WT (D) and lot (E) pollen and WT (F) and lot (G) pollen tubes. (Scale bar, 5 μm.) (H–M) Confocal images of FM4-64 staining (H and I) and the YFP signal (J–M) in pollen tubes. YFP-ARA6 in WT (J) and lot (K). YFP-ARA7 in WT (L) and lot (M). mCITRINE-1×TUBBY in WT (N) and lot (O). mCITRINE-2×PH(FAPP1) in WT (P) and lot (Q). N–Q, insets, pollen grain. (Scale bar, 5 μm.)
The lack of TGNs may also disrupt the endocytic process due to the reduced pool of TGN/EE compartments. To test this hypothesis, we tracked the endocytosis of lot pollen tubes with FM4-64, a lipophilic probe that labels the outer leaflet of the PM. Under in vitro germination conditions, the WT pollen tubes internalized the dye within 5 min (Fig. 5H), while endocytosis was not obvious even after 30 min of staining in lot pollen tubes (Fig. 5I). This result indicates that the endocytic pathway is also impaired in lot pollen tubes. To further analyze the endocytic pathway, we introduced the late endosome/multivesicular body (MVB) markers ARA6 and ARA7, which label the homotypically fused endosomes (30–32). In WT pollen tubes, YFP-ARA6 and YFP-ARA7 label the late endosomes (Fig. 5 J and L), while in lot pollen tubes, they display a dispersed pattern with no visible late endosomal compartments (Fig. 5 K and M). This result is consistent with the lack of TGN/EE in lot pollen tubes. As MVBs progress and integrate with the vacuole, one intriguing question is whether the vacuole formation in lot is also disrupted. To investigate this question, we introduced the vacuole marker mCherry-VAMP711 (28). The results suggest that no obvious abnormal vacuole pattern was observed in lot pollen tubes (SI Appendix, Fig. S5). One possible explanation is that the ER, not the Golgi and TGN, is the major membrane source of vacuole biogenesis (33). Together, these results suggest that the TGN biogenesis is impaired in lot.
Membrane-associated Rab GTPases regulate membrane trafficking events in Rab-GTP bound active state by specifically recruiting cytosolic effectors. TGN-localized RabA4b recruits PI4Kβ, which catalyzes the formation of PI(4)P, the precursor of PI(4,5)P2 (34). PI(4,5)P2 is required for PM targeting of diverse proteins, which in turn affects exocytosis and endocytosis and, subsequently, pollen tube growth (35). Thus, we raised the possibility that failed generation or targeting of phosphoinositide species might be the cause of defective endocytosis. To test this hypothesis, we examined their distribution in lot pollen tubes using phosphoinositide-specific biosensors mCITRINE-1×TUBBY and mCITRINE-2×PH (FAPP1), which label PI(4,5)P2 and PI(4)P, respectively. In the WT pollen tube, both phosphoinositides are targeted to the PM of pollen grains and tubes (Fig. 5 N and P), while in lot, they are distributed evenly in the cytosol (Fig. 5 O and Q). This result supports the speculation that the failed endocytosis may be caused by, or at least correlated with, the impaired PI(4,5)P2 targeting on the PM due to the lack of TGNs.
To investigate whether the localization of Golgi-resident proteins is affected in lot pollen, three Golgi-resident proteins were examined: COG3, which plays key roles in Golgi structure (23) in pollen; Got1 (28); and EMP12 (36). In the pollen populations from WT and lot+/− plants, consistent localization patterns for all three markers were observed, which is similar to what is found in the literature (23, 36) (SI Appendix, Fig. S6). These results suggest that lot mutation most likely affects the biogenesis of the TGN from the Golgi, although the localization of the Golgi resident proteins is normal.
Secretion Is Defective in lot Pollen Tubes.
The Golgi is the main synthetic station of cell wall polysaccharides and glycolipids for the PM as well as glycoprocessing of proteins. Deposition of new cell wall materials and the PM targeting of receptor-like kinases derived from the Golgi are indispensable for pollen tube growth (22, 37). To elucidate the direct cause of defective growth of lot pollen tubes, we analyzed the secretion of a cell surface receptor kinase and pectin deposition. First, we examined the expression and localization of PRK2, a member of the receptor-like kinases required for pollen tube growth (38). PRK2-GFP is localized continuously on the PM in the WT pollen tube (Fig. 6 A, C, and E); however, in the lot pollen tube, it appears to be localized on punctuate structures at and/or beneath the PM (Fig. 6 B, D, and F).
Fig. 6.
Biosynthetic secretion in lot pollen tubes is defective. (A–F) Confocal images of PRK2-GFP in WT (A, C, and E) and lot (B, D, and F) pollen or pollen tubes. Arrows, punctate structures. (Scale bar, 5 μm.) (G and H) Pollen tubes of WT (G) and lot (H) stained with the JIM7 antibody. Arrows, pollen tube tip. (Scale bar, 5 μm.) (I and J) Pollen tubes of WT (I) and lot (J) stained with ruthenium red. Arrows, pollen tube tip. (Scale bar, 5 μm.)
Next, we observed the cell wall deposition using the carbohydrate antibody JIM7 and ruthenium red to visualize pectin with a high level of methylesterification (39). In the WT, the JIM7 antibody and ruthenium red predominantly label the apex of pollen tubes with a dome shape (Fig. 6 G and I), while in lot, no positive staining was observed in the pollen tube tip (Fig. 6 H and J). Receptor-like kinases, like PRK2, are synthesized in the ER and processed in the Golgi and then transported to the PM through TGNs. Pectin is synthesized and processed in the Golgi and then secreted to the extracellular matrix by the bulk secretion pathway, instead of the Golgi-TGN-PM/cell wall pathway (40, 41). Other reports also suggest that a defect in the TGN also impairs pectin secretion (42). Collectively, these results imply that lot disrupts the Golgi and post-Golgi secretion pathways.
LOT Functions as a Component of GTP Exchange Factor of Rab GTPase.
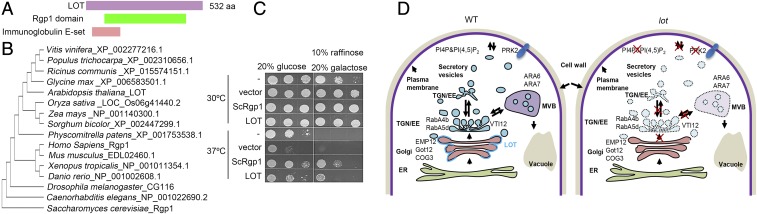
LOT contains 532 amino acids and encodes an unidentified protein with two overlapping domains that could be identified from the known domain repertoires: a predicted Rgp1 domain (InterPro: IPR014848) that is found only among its orthologs in other organisms and an Ig E-set (InterPro: IPR014756) domain (Fig. 7A). The Rgp1 protein was suggested to function as a functional component of GEF of Ypt6 and Rab6 in yeast and mammalian cells, respectively (43, 44). Single-copy orthologs of Rgp1 are found in all eukaryotes (Fig. 7B), although Rgp1 in Saccharomyces cerevisiae and LOT share only 14.6% identity and 27% similarity in the amino acid sequence. In contrast to the biological function of LOT in TGN biogenesis, Rgp1 in yeast and mammalian cells functions in endosome-to-Golgi retrograde trafficking (43–45). To elucidate whether LOT is a functional component of RabGEF, we performed a yeast complementation assay with the yeast rgp1 mutant which shows a temperature-sensitive growth defect at 37 °C (44). The yeast rgp1 mutant cells transformed with LOT indeed can survive at 37 °C (Fig. 7C). This indicates that LOT can functionally replace the yeast Rgp1 as a component of RabGEF.
Fig. 7.
LOT shares conserved molecular function with yeast Rgp1. (A) The protein structure of LOT. (B) Phylogenetic tree of LOT orthologs. (C) LOT rescues the growth defect of the rpg1 yeast mutant. (D) A proposed model of LOT in TGN and vesicle formation. In WT pollen tubes, LOT is required for the activation of Rab GTPases on the Golgi periphery to promote TGN formation. In lot pollen tubes, Rab GTPases and possibly other proteins fail to be activated or recruited to the Golgi, thus causing failed vesicle formation or budding and lack of TGN. The lack of TGN and vesicles causes failed secretion and endocytosis, which leads to the pollen tube growth defect.
Discussion
In summary, we demonstrated that LOT regulates Golgi-derived TGN formation and is essential for the entire post-Golgi trafficking during pollen tube growth (Fig. 7D). GFP-LOT is localized on the rim of Golgi cisternae and might regulate vesicle formation therein, which is indispensable for TGN formation. The overstacking of Golgi cisternae and the proper Golgi localization of EMP12 exported from the ER network in lot suggest that the cargo input from the ER to cis-cisternae may be unimpaired. Furthermore, the dispersed distribution of the TGN markers implies that the earlier stage of TGN formation before TGN detachment is likely defective in lot. An early report suggested the role of PI4Kβ1/2 in TGN detachment since loss of PI4Kβ1 and PI4Kβ2, which catalyze PI(4)P generation, causes TGN aggregation at the trans-side of the Golgi and fewer TGN budding profiles (34). Such Golgi-associated TGN aggregation was not observed in lot, which suggests a mechanism distinct from PI4Kβ1/2. Thus, LOT provides a novel clue to study how the cisternae rim contributes to the TGN formation, which may also be valuable to the understanding of intra-Golgi trafficking.
Secretion and endocytosis underline the cellular basis for pollen tube growth. In lot plants, pollen tube growth is blocked in the style due to failed TGN formation from the Golgi that causes the depletion of secretory vesicles which are the carriers of the post-Golgi traffic, as well as the endocytic pathway. Thus, the entire post-Golgi trafficking of cargo repertoires, such as phospholipids, cell wall components, and membrane-bound proteins, is abolished. Of note, the lot pollen tubes swell in the style but not in vitro, implying that LOT regulates the pollen tube–style interaction. The failed secretion of PRK2 suggests that more receptor-like kinases are possibly defective in PM targeting in lot, which could contribute to the failed male–female interaction. In Arabidopsis, three clades of Rab subfamilies have been confirmed to be present on the Golgi, and each clade contains members highly expressed in pollen. Among the Golgi-localized Rabs, only the RabD2 subfamily has been determined to function in pollen development, germination, and pollen tube growth (46). However, none of their GEF activators have been identified. The Arabidopsis RabH1b was able to complement the temperature-sensitive growth defect of yeast ypt6 (47), and activation of Ypt6/Rab6 requires Rgp1 (44), which can be functionally replaced by LOT in the yeast complementation assay, as shown above. In Arabidopsis, the ortholog of Ypt6 is the RabH1 subfamily, among which four members have been indicated to be expressed in pollen and none have been functionally studied (48). RABH1b and RABH1c localize on the Golgi and physically interact with Golgi-tethering proteins (49–51). Rgp1 and Ric1 form a functional GEF complex in mammalian cells and yeast (44, 45), while the two orthologs of Ric1 in Arabidopsis are large proteins which are difficult to express and purify in our hands. Further endeavor is needed to clarify the potential relationship between LOT and Rab GTPases on the Golgi, as well as their downstream effectors.
Golgi-localized LOT regulates the Golgi-derived TGN biogenesis, a role that has not been identified in its animal and yeast homologs. The yeast Rgp1-activated Ypt6 has been recognized to mediate the binding of endosome-derived vesicles to the late Golgi through the SNARE protein, that is, the endosome-to-late Golgi retrograde trafficking (43). In human cells, Rgp1 also functions as a component of GEF in the TGN-localized small GTPase Rab6A, which regulates tethering of endosome-derived vesicles to the trans-Golgi cisternae for retrograde transport of the internalized proteins by endocytosis (43, 45). In animal cells, TGN is associated with the Golgi at the trans-face, and the cargo sorting at the TGN to endosome is accompanied by endosome-to-TGN retrograde trafficking of a catalog of factors, such as cargo receptors and SNAREs. However, in plants, the TGN is an independent compartment separated from the Golgi and functions as a genuine early endosome, which might explain the lack of an endosome-to-TGN retrograde pathway. In this cellular context, LOT likely maintains the GEF molecular function but is adapted to function in the TGN biogenesis from the Golgi. However, whether the plant lineage indeed lacks TGN/EE-to-Golgi retrograde trafficking and the role of LOT orthologs in yeast and animals in TGN biogenesis still require further investigation.
In summary, this work identified LOT as a potential RabGEF on the Golgi and provided a foundation for dissecting the molecular mechanisms that underpin TGN formation and the sorting regulation therein. Second, this work evidenced the cellular significance of Golgi in cargo trafficking and endocytosis and provided valuable material for the study of Golgi-dependent and -independent membrane trafficking pathways.
Materials and Methods
Plant Materials and Growth Conditions.
Salk_130228 was obtained from ABRC; kd329 was selected from a Ds insertion mutant pool. See SI Appendix, Materials and Methods for details.
Phenotype Analysis.
For in vivo pollen tube growth, pollen grains were manually pollinated on the WT pistil and fixed 12 h after pollination with ethanol and acetic acid (3:1) for 2 h. See SI Appendix, Materials and Methods for details.
Plasmid Construction.
See SI Appendix, Materials and Methods for details.
Western Blot, Immunofluorescence, and Microscopy.
Total proteins were resolved with SDS/PAGE and immunodetected with anti-GFP. See SI Appendix, Materials and Methods for details.
LOT Protein Sequence and Phylogenetic Analysis.
For bioinformatics analysis, see SI Appendix, Materials and Methods for details.
Yeast Complementation Assay.
For constructs and the following analysis, see SI Appendix, Materials and Methods for details.
Supplementary Material
Acknowledgments
We thank Prof. Yan Zhang (Shandong Agriculture University) for kindly providing the PRK2-GFP and YFP-ARA6 and -ARA7 seeds, Prof. Yi-Hua Zhou [Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences] for kindly providing the JIM7 antibody, Prof. Pelham (Medical Research Council Laboratory of Molecular Biology, Cambridge) for kindly providing the rgp1 yeast mutant, Prof. Yiqun Bao (Nanjing Agriculture University) for kindly providing the Golgi markers, Prof. Shilai Bao (IGDB) for the critical reading of the manuscript, and the staff of the live-cell imaging facilities of IGDB for assistance with TEM, superresolution, and confocal microscopy. This work is supported by the National Natural Science Foundation of China (Grant 31330053 to W.-C.Y., and Grants 31622010 and 31270351 to H.-J.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Z.Y. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809206115/-/DCSupplemental.
References
- 1.Faso C, Boulaflous A, Brandizzi F. The plant Golgi apparatus: Last 10 years of answered and open questions. FEBS Lett. 2009;583:3752–3757. doi: 10.1016/j.febslet.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ, Brandizzi F. The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology. 2016;26:940–949. doi: 10.1093/glycob/cww044. [DOI] [PubMed] [Google Scholar]
- 3.Nakano A, Luini A. Passage through the Golgi. Curr Opin Cell Biol. 2010;22:471–478. doi: 10.1016/j.ceb.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol. 2013;14:382–392. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang I. Sorting and anterograde trafficking at the Golgi apparatus. Plant Physiol. 2008;148:673–683. doi: 10.1104/pp.108.124925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann U, Brandizzi F, Hawes C. Protein transport in plant cells: In and out of the Golgi. Ann Bot. 2003;92:167–180. doi: 10.1093/aob/mcg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viotti C, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura T, Suda Y, Ueda T, Nakano A. Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol. 2014;55:694–703. doi: 10.1093/pcp/pcu010. [DOI] [PubMed] [Google Scholar]
- 10.Kang BH, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA. Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic. 2011;12:313–329. doi: 10.1111/j.1600-0854.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 11.Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gendre D, et al. Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc Natl Acad Sci USA. 2011;108:8048–8053. doi: 10.1073/pnas.1018371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirakawa M, et al. Continuous vascular ring (COV1) is a trans-Golgi network-localized membrane protein required for Golgi morphology and vacuolar protein sorting. Plant Cell Physiol. 2014;55:764–772. doi: 10.1093/pcp/pct195. [DOI] [PubMed] [Google Scholar]
- 14.Rosquete MR, Davis DJ, Drakakaki G. The plant trans-Golgi network: Not just a matter of distinction. Plant Physiol. 2018;176:187–198. doi: 10.1104/pp.17.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segev N. Ypt and Rab GTPases: Insight into functions through novel interactions. Curr Opin Cell Biol. 2001;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 16.Vernoud V, Horton AC, Yang Z, Nielsen E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 2003;131:1191–1208. doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant. 2013;6:1018–1036. doi: 10.1093/mp/sst061. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Yang WC. RLKs orchestrate the signaling in plant male-female interaction. Sci China Life Sci. 2016;59:867–877. doi: 10.1007/s11427-016-0118-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Yang Z. Tip growth: Signaling in the apical dome. Curr Opin Plant Biol. 2008;11:662–671. doi: 10.1016/j.pbi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung AY, Wu HM. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- 21.Williams JH. Novelties of the flowering plant pollen tube underlie diversification of a key life history stage. Proc Natl Acad Sci USA. 2008;105:11259–11263. doi: 10.1073/pnas.0800036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hepler PK, Rounds CM, Winship LJ. Control of cell wall extensibility during pollen tube growth. Mol Plant. 2013;6:998–1017. doi: 10.1093/mp/sst103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan X, et al. Arabidopsis COG complex subunits COG3 and COG8 modulate Golgi morphology, vesicle trafficking homeostasis and are essential for pollen tube growth. PLoS Genet. 2016;12:e1006140. doi: 10.1371/journal.pgen.1006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LY, et al. The Arabidopsis alkaline ceramidase TOD1 is a key turgor pressure regulator in plant cells. Nat Commun. 2015;6:6030. doi: 10.1038/ncomms7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, et al. Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol Plant. 2013;6:1131–1148. doi: 10.1093/mp/sst084. [DOI] [PubMed] [Google Scholar]
- 26.Sundaresan V, et al. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 1995;9:1797–1810. doi: 10.1101/gad.9.14.1797. [DOI] [PubMed] [Google Scholar]
- 27.Thole JM, Vermeer JE, Zhang Y, Gadella TW, Jr, Nielsen E. Root hair defective4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana. Plant Cell. 2008;20:381–395. doi: 10.1105/tpc.107.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geldner N, et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 2009;59:169–178. doi: 10.1111/j.1365-313X.2009.03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV. Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell. 2001;12:3733–3743. doi: 10.1091/mbc.12.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda T, Yamaguchi M, Uchimiya H, Nakano A. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 2001;20:4730–4741. doi: 10.1093/emboj/20.17.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, He J, Lee D, McCormick S. Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiol. 2010;152:2200–2210. doi: 10.1104/pp.109.142349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee GJ, Sohn EJ, Lee MH, Hwang I. The Arabidopsis rab5 homologs rha1 and ara7 localize to the prevacuolar compartment. Plant Cell Physiol. 2004;45:1211–1220. doi: 10.1093/pcp/pch142. [DOI] [PubMed] [Google Scholar]
- 33.Viotti C, et al. The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant Cell. 2013;25:3434–3449. doi: 10.1105/tpc.113.114827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preuss ML, et al. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, et al. Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell. 2010;22:4031–4044. doi: 10.1105/tpc.110.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao C, et al. The Golgi-localized Arabidopsis endomembrane protein12 contains both endoplasmic reticulum export and Golgi retention signals at its C terminus. Plant Cell. 2012;24:2086–2104. doi: 10.1105/tpc.112.096057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li HJ, Meng JG, Yang WC. Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod. 2018;31:31–41. doi: 10.1007/s00497-018-0324-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim HU, et al. New pollen-specific receptor kinases identified in tomato, maize and Arabidopsis: The tomato kinases show overlapping but distinct localization patterns on pollen tubes. Plant Mol Biol. 2002;50:1–16. doi: 10.1023/a:1016077014583. [DOI] [PubMed] [Google Scholar]
- 39.Szumlanski AL, Nielsen E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell. 2009;21:526–544. doi: 10.1105/tpc.108.060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micheli F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, et al. A distinct pathway for polar exocytosis in plant cell wall formation. Plant Physiol. 2016;172:1003–1018. doi: 10.1104/pp.16.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gendre D, et al. Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell. 2013;25:2633–2646. doi: 10.1105/tpc.113.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 44.Siniossoglou S, Peak-Chew SY, Pelham HRB. Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J. 2000;19:4885–4894. doi: 10.1093/emboj/19.18.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pusapati GV, Luchetti G, Pfeffer SR. Ric1-Rgp1 complex is a guanine nucleotide exchange factor for the late Golgi Rab6A GTPase and an effector of the medial Golgi Rab33B GTPase. J Biol Chem. 2012a;287:42129–42137. doi: 10.1074/jbc.M112.414565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng J, Ilarslan H, Wurtele ES, Bassham DC. AtRabD2b and AtRabD2c have overlapping functions in pollen development and pollen tube growth. BMC Plant Biol. 2011;11:25. doi: 10.1186/1471-2229-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bednarek SY, et al. A small GTP-binding protein from Arabidopsis thaliana functionally complements the yeast YPT6 null mutant. Plant Physiol. 1994;104:591–596. doi: 10.1104/pp.104.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, et al. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansen JN, Chow CM, Moore I, Hawes C. AtRAB-H1b and AtRAB-H1c GTPases, homologues of the yeast Ypt6, target reporter proteins to the Golgi when expressed in Nicotiana tabacum and Arabidopsis thaliana. J Exp Bot. 2009;60:3179–3193. doi: 10.1093/jxb/erp153. [DOI] [PubMed] [Google Scholar]
- 50.Latijnhouwers M, et al. Localization and domain characterization of Arabidopsis golgin candidates. J Exp Bot. 2007;58:4373–4386. doi: 10.1093/jxb/erm304. [DOI] [PubMed] [Google Scholar]
- 51.Osterrieder A, et al. Fluorescence lifetime imaging of interactions between Golgi tethering factors and small GTPases in plants. Traffic. 2009;10:1034–1046. doi: 10.1111/j.1600-0854.2009.00930.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.