Abstract
Background
VEGF plays a key role in tumor angiogenesis and immunosuppression. VEGF‐blocking has proven beneficial for EGFR mutant and wild‐type nonsquamous non‐small cell lung cancer (nonsq‐NSCLC); however, the number of cycles and treatment line yielding the optimal benefit are unknown.
Methods
We retrospectively analyzed the data of 115 patients with advanced/metastatic nonsq‐NSCLC administered at least one cycle of bevacizumab. The number of bevacizumab cycles was treated as a time‐dependent covariate. Predictors of overall survival (OS) were investigated.
Results
Bevacizumab was used as first‐line treatment in 47 (40.9%) patients, with a median of five cycles (range: 1–31). Eastern Cooperative Oncology Group performance status ≥ 2 (hazard ratio [HR] 4.78, 95% confidence interval [CI] 2.68–8.51; P < 0.001), wild‐type EGFR (HR 2.61, 95% CI 1.45–4.70; P = 0.001), and bleeding during bevacizumab treatment (HR 3.63, 95% CI 1.77–7.45; P < 0.001) were predictive of poor OS; the number of bevacizumab cycles and first‐line administration were not. In the wild‐type EGFR subgroup, the number of bevacizumab cycles (≥ 5 vs. 1–4) was associated with a significant OS benefit (HR 0.28, 95% CI 0.08–0.98; P = 0.044); first‐line administration also showed an OS benefit (HR 0.48, 95% CI 0.20–1.17; P = 0.105). A significant association between the number of cycles and EGFR status was identified (P = 0.046).
Conclusion
OS benefit is negatively affected by bleeding events in bevacizumab‐treated patients. Prolonged and early introduction of bevacizumab may provide an OS benefit for patients with wild‐type EGFR nonsq‐NSCLC.
Keywords: Bevacizumab, EGFR, NSCLC, VEGF
Introduction
The manifold composition of stromal cells, immune cells, and molecular factors in the microenvironment of non‐small cell lung cancer (NSCLC) plays a significant role in supporting initiation, progression, and metastasis.1, 2, 3 VEGF, one of the crucial molecular factors stimulating angiogenesis and extracellular matrix remodeling, has been shown to take a central part in the development of a wide array of solid cancer types.1, 4, 5 Recently, with increasing knowledge of the effects of inducing inhibitory checkpoint proteins and of promoting the accumulation of myeloid‐derived suppressor and T‐regulatory cells in tumor milieu, the immunosuppressive property of VEGF has also been recognized.6, 7, 8
Given the involvement of VEGF in these common features of cancer, the VEFG‐blocking agent bevacizumab has shown clinical efficacy for most non‐squamous (nonsq)‐NSCLC patients, regardless of the presence or absence of an EGFR driving mutation.9, 10, 11 However, no unique biomarkers or specific clinical profiles of fair consensus have been identified to distinguish the subgroup of patients that can particularly benefit from the VEFG‐blocking agent. In the JO25567 trial comparing erlotinib plus bevacizumab to erlotinib alone, each clinical study subgroup showed a relative risk reduction; therefore treatment to subgroup interaction was not evaluable, although patients with malignant pericardial or pleural effusion appeared to attain some additional efficacy with the add‐on of bevacizumab.11
While using a combination of the VEFG‐blocking agent with either chemotherapy or an EGFR‐tyrosine kinase inhibitor (TKI) for the treatment of nonsq‐NSCLC is clinically evident, the treatment length and timing of administering such an agent are less well understood. In view of the angiogenic and immunosuppressive role that VEGF plays in the tumor microenvironment, a continuous VEGF‐blocking strategy may provide clinical benefit to patients. Previous studies have addressed this issue focusing on maintenance treatment12, 13, 14 or treatment beyond progression15 rather than dealing with the actual number of treatment cycles used. Nadler et al. retrospectively analyzed 403 patients receiving first‐line chemotherapy plus bevacizumab and reported that those who subsequently underwent bevacizumab maintenance until progression had significantly better overall survival (OS) and progression‐free survival (PFS) compared to those who did not.13 On the other hand, a randomized phase IIb trial conducted by Takeda et al. showed that administering bevacizumab treatment beyond progression yielded a PFS benefit and also a trend of OS benefit, suggesting the possible prognostic advantage of keeping VEGF inhibited.15 Despite differences in the populations between the two studies, a similar benefit of continuous VEGF‐blocking was noted, which thereby raises the question of the number of bevacizumab treatment cycles that would provide most benefit.
Apart from continuous VEGF blockade, the timing of VEGF inhibition, in terms of commencing bevacizumab at first‐line or beyond, remains an issue. Herbst et al. performed two second‐line studies. One showed that in patients who received the VEGF‐blocking strategy later still gained a trend of PFS and OS benefit compared to patients not treated with VEGR blockade,16 whereas the other study showed a PFS but not an OS benefit.17 Recently, better efficacy has also been reported in third‐line or further settings.18 However, whether the introduction of a VEGF‐blocking strategy at first‐line or later affects the survival outcome is not yet known.
We analyzed a cohort of advanced EGFR‐mutant and EGFR‐wild type nonsq‐NSCLC patients who received bevacizumab in combination with chemotherapy or EGFR‐TKIs at different lines and varying treatment cycles. The number of treatment cycles and the timing of starting bevacizumab were analyzed to determine an influence on survival outcome. This influence was also analyzed by clinical subgroups.
Methods
Patients
From January 2011 to November 2017, the data of 132 patients with advanced or metastatic NSCLC who received at least one cycle of bevacizumab‐based treatment (7.5 mg/kg, every 3 weeks) at Chang Gung Memorial Hospital were retrospectively reviewed. Twelve patients were excluded because of a short follow‐up duration (< 4 weeks) after bevacizumab‐based treatment and five were excluded because of a diagnosis of squamous cell carcinoma. The remaining 115 patients were eligible for the analysis (Fig 1a). Bevacizumab was initially combined with either chemotherapy or EGFR‐TKIs, and the physician in charge decided the treatment strategy, namely maintenance, treatment beyond progression, and line of treatment. The clinical profiles and the toxicities noted during bevacizumab‐based treatment were systemically reviewed. OS was defined as the interval between the date of commencing non‐curable intent treatment and the date of either death or the last follow‐up. The treatment response, defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.19 Toxicity was graded according to National Cancer Institute Common Toxicity Criteria, version 3.0.20 The ethics committee of Chang Gung Memorial Hospital, Linkou, Taiwan, approved the study.
Figure 1.
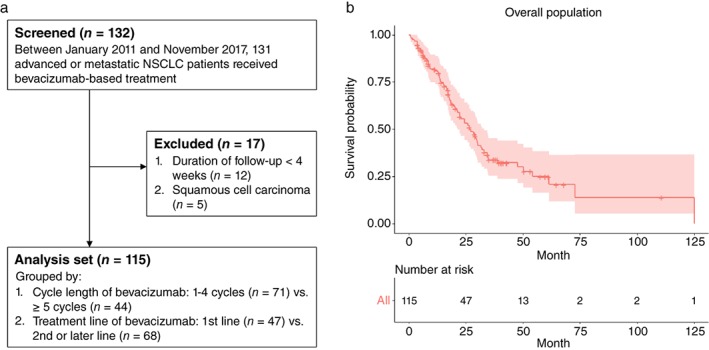
(a) Flow chart of the study population. (b) Kaplan–Meier curve with 95% confidence interval (red shade) of the overall survival of the study population. NSCLC, non‐small cell lung cancer.
Statistical analysis
A Mann–Whitney U test was used to determine the statistical significance between two groups of continuous variables and Fisher's exact tests were used for categorical variables. The median follow‐up duration was reported using the reverse Kaplan–Meier method. The number of cycles of bevacizumab treatment, which carried an intrinsic guarantee‐time bias,21 was treated as time‐dependent covariate, where R package survival (R Foundation for Statistical Computing, Vienna, Austria) was used to transform the covariate coded by the time of change in a timeframe fashion. R package survival was also used for the extended Kaplan–Meier method to estimate the survival curves22 and the hazard ratio (HR) was analyzed using the Cox regression model, where the proportional hazard assumption was confirmed for each covariate beforehand. All reported P values were two sided, with P < 0.05 considered statistically significant. All data were analyzed using SPSS version 10.1 (SPSS Inc., Chicago, IL, USA).
Results
Baseline patient characteristics
Among the 115 patients, 53 (46.1%) were male, 29 (25.2%) were smokers or ex‐smokers, and 67 (58.3%) had EGFR‐mutated nonsq‐NSCLC (Table 1). Eighty‐four (73.0%) patients were at Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1 when they underwent bevacizumab‐based treatment and the median number of bevacizumab cycles was five (range: 1–31). Eighty‐six (74.8%) patients initially received bevacizumab in combination with chemotherapy and 29 (25.2%) in combination with EGFR‐TKIs. Bevacizumab was used as first‐line treatment in 47 (40.9%) patients and as second‐line or later settings in 68 (59.1%). The adverse grade 3 events noted during bevacizumab‐based treatment included: bleeding in 11 (9.6%), proteinuria in 4 (3.5%), venous thrombosis in 3 (2.6%), and hypertension in 23 (20.0%) patients. Outcomes of the bevacizumab‐based combination were: PR in 42 (36.5%), SD in 20 (17.3%), and PD in 53 (46.0%) patients. The median OS was 26.6 months (Fig 1b) and the median follow‐up duration was 40.3 months.
Table 1.
Clinical characteristics of study subjects
| Variable, N (%) | Overall population (n = 115) |
|---|---|
| Age, median (range), year | 58 (26–82) |
| Gender (male) | 53 (46.0) |
| Smoker/ex‐smoker | 29 (25.2) |
| ECOG PS | |
| 0, 1 | 84 (73.0) |
| ≥ 2 | 31 (27.0) |
| Staging | |
| III | 11 (9.6) |
| IV | 104 (90.4) |
| EGFR status | |
| Mutation | 67 (58.3) |
| Wild type | 36 (31.3) |
| Unknown | 12 (10.4) |
| Regimen combination | |
| Chemotherapy | 86 (74.8) |
| EGFR‐TKI | 29 (25.2) |
| Comorbidity | |
| Chronic liver disease | 9 (7.8) |
| Chronic kidney disease | 4 (3.5) |
| Adverse effects† | |
| Proteinuria | 4 (3.4) |
| Hypertension | 23 (20.0) |
| Bleeding | 11 (9.6) |
| Venous thrombosis | 3 (2.6) |
| Treatment response | |
| Partial response | 42 (36.5) |
| Stable disease | 20 (17.3) |
| Progression disease | 53 (46.0) |
Grade 3.
ECOG PS, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor.
Number of treatment cycles and line of bevacizumab
To determine whether the number of cycles and the timing of bevacizumab administration had an influence on survival outcomes, patients were grouped by treatment strategies: the number of cycles (≥ 5 vs. 1–4) and the line (first‐line vs. second or later) (Table 2). Patients administered ≥ 5 cycles of bevacizumab were observed to have significantly better ECOG PS (PS 0–1, 93.2% vs. 60.6%; P < 0.001), were younger (55 [46–61] vs. 59 [52–69]; P = 0.043), and had a higher response rate (59.1% vs. 22.5%; P < 0.001) compared to patients administered 1–4 cycles. Patients who received bevacizumab in the first‐line setting also had a significantly better ECOG PS (PS 0–1, 87.2% vs. 63.2%; P = 0.002), were more often male (59.6% vs. 36.8%; P = 0.026), with wild‐type EGFR (48.9% vs. 19.1%; P = 0.001), and had a higher response rate (48.9% vs. 27.9%; P = 0.030) compared to patients administered bevacizumab in the second or later line setting.
Table 2.
Clinical characteristics of the patients stratified by the number of treatment cycles and the line of bevacizumab
| Variables, N (%) | Treatment cycle | Treatment line | ||||
|---|---|---|---|---|---|---|
| ≥ 5 cycles (n = 44) | 1–4 cycles (n = 71) | P | First‐line (n = 47) | Second or later line (n = 68) | P | |
| Age, median (range), year | 55 (46–61) | 59 (52–69) | 0.043 | 57 (49–64) | 59 (51–65) | 0.914 |
| Gender (male) | 19 (43.1) | 34 (47.9) | 0.765 | 28 (59.6) | 25 (36.8) | 0.026 |
| Smoker/ex‐smoker | 11 (25.0) | 18 (25.4) | 0.858 | 16 (34.0) | 13 (19.1) | 0.111 |
| ECOG PS | ||||||
| 0, 1 | 41 (93.2) | 43 (60.6) | < 0.001 | 41 (87.2) | 43 (63.2) | 0.008 |
| ≥ 2 | 3 (6.8) | 28 (39.4) | 6 (12.8) | 25 (36.8) | ||
| Staging | ||||||
| III | 4 (8.7) | 7 (9.9) | 0.909 | 6 (12.8) | 5 (7.4) | 0.517 |
| IV | 42 (91.3) | 64 (90.1) | 41 (87.2) | 63 (92.3) | ||
| EFGR status | ||||||
| Mutation | 21 (47.7) | 46 (64.7) | 0.096 | 21 (44.7) | 46 (67.6) | 0.003 |
| Wild type | 19 (43.2) | 17 (23.9) | 23 (48.9) | 13 (19.1) | ||
| Unknown | 4 (9.0) | 8 (11.2) | 3 (6.4) | 9 (13.2) | ||
| Regimen of combination | ||||||
| Chemotherapy | 35 (79.5) | 51 (71.8) | 0.481 | 35 (74.5) | 51 (75.0) | 0.878 |
| EGFR‐TKI | 9 (20.5) | 20 (21.2) | 12 (25.5) | 17 (25.0) | ||
| Comorbidity | ||||||
| Chronic liver disease | 3 (6.8) | 6 (8.5) | 1.000 | 2 (4.3) | 7 (10.3) | 0.405 |
| Chronic kidney disease | 2 (4.5) | 2 (2.8) | 1.000 | 1 (2.1) | 3 (4.4) | 0.889 |
| Adverse effects† | ||||||
| Proteinuria | 1 (2.2) | 3 (4.2) | 0.133 | 2 (4.3) | 2 (2.9) | 0.153 |
| Hypertension | 9 (20.5) | 14 (19.7) | 9 (19.1) | 14 (20.6) | ||
| Bleeding (Grade 3) | 2 (4.5) | 9 (12.7) | 2 (4.3) | 9 (13.2) | ||
| Venous thrombosis | 3 (6.8) | 0 | 3 (6.3) | 0 | ||
| Treatment response | ||||||
| Partial response | 26 (59.1) | 16 (22.5) | < 0.001 | 23 (48.9) | 19 (27.9) | 0.004 |
| Stable disease | 12 (27.3) | 8 (11.3) | 11 (23.4) | 9 (13.2) | ||
| Progressive disease | 6 (13.6) | 47 (66.2) | 13 (27.7) | 40 (58.8) | ||
Grade 3.
ECOG PS, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor.
Analysis of predictors of survival outcome
A Cox regression model was subsequently analyzed to determine the predictors of OS (Table 3). In univariate analysis, neither age (HR 1.02, 95% confidence interval [CI] 0.99–1.03; P = 0.502) nor male gender (HR 1.20, 95% CI 0.75–1.93; P = 0.453) had an impact on OS, whereas ECOG PS (PS ≥ 2, HR 4.09, 95% CI 2.49–6.71; P < 0.001) and bleeding events during bevacizumab treatment (HR 2.38, 95% CI 1.43–3.98; P = 0.001) were significant predictive factors of poor OS. Wild‐type EGFR (HR 1.64, 95% CI 0.95–2.83; P = 0.075) and brain metastasis in patients administered bevacizumab (HR 1.62, 95% CI 0.98–2.67; P = 0.058) also presented a trend as negative predictors of OS. Neither ≥ 5 cycles of bevacizumab treatment (HR 0.96, 95% CI 0.53–1.72; P = 0.884) nor the first‐line use of bevacizumab (HR 1.16, 95% CI 0.70–1.92; P = 0.573) was predictive of OS. In multivariate analysis, ECOG PS (PS ≥ 2, HR 4.78, 95% CI 2.68–8.51; P < 0.001), wild‐type EGFR (HR 2.61, 95% CI 1.45–4.70; P = 0.001), and bleeding events during bevacizumab treatment (HR 3.63, 95% CI 1.77–7.45; P < 0.001) remained predictive of poor OS.
Table 3.
Cox regression analysis of overall survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.02 (0.99–1.03) | 0.502 | — | — |
| Gender (Male) | 1.20 (0.75–1.93) | 0.453 | — | — |
| Smoker | 0.94 (0.53–1.68) | 0.833 | — | — |
| Brain metastasis | 1.62 (0.98–2.67) | 0.058 | 1.58 (0.89–2.81) | 0.117 |
| ECOG PS ≥ 2 | 4.09 (2.49–6.71) | < 0.001 | 3.95 (2.22–7.01) | < 0.001 |
| Wild‐type EGFR | 1.64 (0.95–2.83) | 0.075 | 2.96 (1.61–5.44) | 0.001 |
| First‐line use of bevacizumab | 1.16 (0.70–1.92) | 0.575 | — | — |
| Cycles of bevacizumab ≥ 5 | 0.96 (0.53–1.71) | 0.883 | — | — |
| Bleeding event | 2.38 (1.43–3.98) | 0.001 | 2.27 (1.25–4.11) | 0.007 |
CI, confidence interval; HR, hazard ratio.
Subgroup analysis of the treatment cycle and line of bevacizumab
As the number of treatment cycles and line of bevacizumab did not influence the survival outcome in the overall population, we analyzed whether there was any influence in clinical subgroups. A forest plot showed that the administration of ≥ 5 cycles of bevacizumab was associated with a significant survival benefit in the wild‐type EGFR subgroup compared to 1–4 cycles (HR 0.28, 95% CI 0.08–0.98; P = 0.044) (Fig 2a), although there was no significant difference in the clinical profiles between these groups (Table S1, Supporting Information). No beneficial effect of ≥ 5 treatment cycles was observed in the EGFR mutation subgroup (HR 1.12, 95% CI 0.52–2.42; P = 0.777); thus a significant association between the number of cycles and EGFR status was noted (P = 0.046). Regarding the treatment line, a forest plot showed that first‐line use of bevacizumab was not associated with a significant survival benefit compared to second or later line use, regardless of EGFR mutation status (Fig 2b); however, a trend of better survival was observed in the wild‐type EGFR subgroup (HR 0.48, 95% CI 0.20–1.17; P = 0.105). In addition, while significantly poorer survival was associated with first‐line bevacizumab use in the PS 0–1 subgroup (Fig 2b), a biased wild‐type EGFR disposition toward first‐line bevacizumab was noted in this subgroup, which invalidated the finding (Table S2, Supporting Information).
Figure 2.
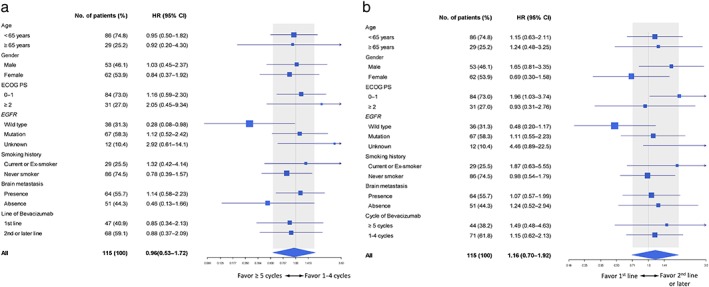
Forest plot of treatment to subgroup analysis. (a) Number of bevacizumab treatment cycles: ≥ 5 versus 1–4 cycles. (b) First‐line versus second or later lines of bevacizumab. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NSCLC, non‐small cell lung cancer.
Survival estimate of treatment by EGFR mutation status
As subgroup analysis revealed an association between bevacizumab treatment strategy and EGFR status, the Kaplan–Meier estimator was subsequently analyzed. In wild‐type EGFR patients, the median OS was significantly longer in patients treated with ≥ 5 cycles compared to 1–4 cycles (22.8 vs. 13.5 months; P = 0.048) (Fig 3a), whereas the median OS of the first‐line treatment group was numerically but not statistically longer than the second or later line group (22.8 vs. 12.9 months; P = 0.097) (Fig 3c). In the patients with EGFR mutations, the median OS was similar regardless of the number of cycles (28.4 vs. 29.4 months; P = 0.777) (Fig 3b) or the treatment line (30.0 vs. 29.2 months; P = 0.771) (Fig 3d).
Figure 3.
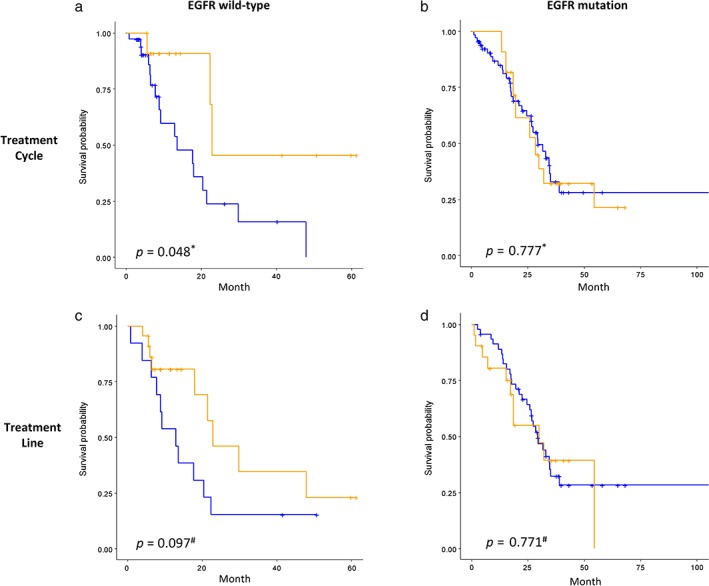
Kaplan–Meier curve analysis of EGFR status (column side) to bevacizumab treatment (row side). *P value of Cox‐regression model; # P value of log‐rank test. (a,b) Number of treatment cycles: ( ) 1–4, (
) 1–4, ( ) ≥ 5. (c,d) Treatment line: (
) ≥ 5. (c,d) Treatment line: ( ) second or later line, (
) second or later line, ( ) first‐line.
) first‐line.
Discussion
This study evaluated the predictive factors of OS in a cohort of bevacizumab‐treated nonsq‐NSCLC patients with a disposition of wild type and mutant EGFR at an approximate 1:2 ratio. In addition to the commonly recognized negative OS predictors (ECOG PS ≥ 2 and wild‐type EGFR), bleeding events during bevacizumab treatment were also identified as negatively associated with OS. Although treatment with ≥ 5 bevacizumab cycles was not predictive of OS in the overall study population, it was associated with positive OS in EGFR wild‐type patients. This result indicates that treatment with ≥ 5 cycles of bevacizumab yields a higher OS benefit/risk reduction in wild‐type EGFR patients, thus a differential OS impact between the number of bevacizumab cycles and EGFR mutation status was identified.
Although previous studies have shown the efficacy of VEGF blockade using bevacizumab in both EGFR‐mutant and EGFR‐wild type nonsq‐NSCLC patients, our results suggest that the extent of the beneficial effect from prolonged and early VEGF blockade may be different between these two molecular phenotypes. One of the possible reasons underlying this distinction can be linked to differences in the tumor microenvironment. Recent studies of immune checkpoint inhibitors showed that tumors with EGFR sensitizing mutations had significantly lower response rates compared to wild‐type EGFR tumors,23 as a result of less abundant immune cell infiltration and weaker immunogenicity in the EGFR‐mutant tumor microenvironment.24 The lower infiltration of immune cells, including CD8 T and CD4 T regulatory and myeloid‐derived suppressor cells, indicates a reduction in the number of target cells that VEGF can engage to augment immunosuppression through the VEGF/VEFG‐R2 signaling pathway,6, 7, 25 thereby diminishing the role VEGF plays in this microenvironment context and leading to moderation of the beneficial effect of bevacizumab. In line with this, treatments using VEGF and VEGFR blocking agents in combination with checkpoint protein inhibitors, mainly for non‐driving mutation NSCLCs, are being actively investigated with encouraging results.26, 27, 28
The relatively modest benefit of bevacizumab treatment in EGFR‐mutant tumors was also shown in the JO25567 trial,29 where the addition of bevacizumab to erlotinib did not decrease the risk of death as opposed to erlotinib alone (HR 0.81, 95% CI 0.53–1.23; P = 0.327) and yielded similar median OS (47.0 vs. 47.4 months) in the two groups, although the combination of bevacizumab plus erlotinib had previously shown significantly better efficacy.11 This result also explains why the prolonged and early VEGF‐blocking strategy did not yield a positive effect on OS in our study; as EGFR‐mutant nonsq‐NSCLC patients accounted for approximately 60% of our study population, the positive effect on OS of the wild‐type EGFR subjects was thus diluted.
With regard to using a VEGF‐blocking strategy for wild‐type EGFR tumors, our findings suggest that a prolonged rather than an early VEGF blockade was more beneficial. A recent study of the combination of bevacizumab to salvage treatment at third‐line or beyond showed improved efficacy compared to the salvage treatment alone, confirming that the clinical benefit of a VEGF‐blocking strategy is preserved, even when introduced at later lines.18 On the other hand, Takeda et al. revealed the significance of prolonged VEGF blockade, reporting that failure of prior treatment containing bevacizumab does not prevent continuous use in further lines of treatment as VEGF may still play a role.15
The present study further identified that bleeding during bevacizumab treatment was a negative factor for OS. No fatal or central nervous system bleeding was observed, although the frequency of brain metastasis (55.7%) tended to be higher in our study population. The 11 (9.6%) patients who experienced bleeding events (9 [81.8%] from the gastrointestinal tract, 2 [18.2%] from hemoptysis) discontinued bevacizumab. The overall rate of bleeding events tended to be higher than in previous reports,30, 31 but whether this is associated with the underlying comorbidity of chronic liver and kidney disease in the study population (11.3%) requires further evaluation.
A limitation of the present study was the inherent bias as a result of the retrospective and heterogeneous nature of the study population, such as the molecular profile of EGFR. It was difficult to determine the differential responses to treatment associated with different tumor molecular phenotypes from previous studies because they included large numbers of EGFR mutant or wild‐type patients. However, at the expense of a homogeneous EGFR mutation profile, the present study revealed a differential OS impact between the number of bevacizumab cycles and EGFR mutation status.
In conclusion, prolonged and early VEGF blockade is an effective therapeutic approach for nonsq‐NSCLC, and caution must be taken in the event of bleeding. Patients with wild‐type EGFR tumors may particularly benefit from this treatment strategy.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Clinical characteristics of wild‐type EGFR subjects stratified by the number of treatment cycles of bevacizumab.
Table S2. Clinical characteristics of Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1 of subjects stratified by the bevacizumab treatment line.
References
- 1. Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol 2018; 15: 366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gkretsi V, Stylianopoulos T. Cell adhesion and matrix stiffness: Coordinating cancer cell invasion and metastasis. Front Oncol 2018; 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–22. [DOI] [PubMed] [Google Scholar]
- 4. Karnezis T, Shayan R, Caesar C et al VEGF‐D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012; 21: 181–95. [DOI] [PubMed] [Google Scholar]
- 5. Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med 2011; 17: 347–62. [DOI] [PubMed] [Google Scholar]
- 6. Huang Y, Chen X, Dikov MM et al Distinct roles of VEGFR‐1 and VEGFR‐2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 2007; 110: 624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voron T, Colussi O, Marcheteau E et al VEGF‐A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015; 212: 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng PH, Chen KY, Huang YC et al Bevacizumab reduces S100A9‐positive MDSCs linked to intracranial control in patients with EGFR‐mutant lung adenocarcinoma. J Thorac Oncol 2018; 13: 958–67. [DOI] [PubMed] [Google Scholar]
- 9. Sandler A, Gray R, Perry MC et al Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006; 355: 2542–50. [DOI] [PubMed] [Google Scholar]
- 10. Reck M, von Pawel J, Zatloukal P et al Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAil. J Clin Oncol 2009; 27: 1227–34. [DOI] [PubMed] [Google Scholar]
- 11. Seto T, Kato T, Nishio M et al Erlotinib alone or with bevacizumab as first‐line therapy in patients with advanced non‐squamous non‐small‐cell lung cancer harbouring EGFR mutations (JO25567): An open‐label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15: 1236–44. [DOI] [PubMed] [Google Scholar]
- 12. Niho S, Kunitoh H, Nokihara H et al Randomized phase II study of first‐line carboplatin‐paclitaxel with or without bevacizumab in Japanese patients with advanced non‐squamous non‐small‐cell lung cancer. Lung Cancer 2012; 76: 362–7. [DOI] [PubMed] [Google Scholar]
- 13. Nadler E, Yu E, Ravelo A, Sing A, Forsyth M, Gruschkus S. Bevacizumab treatment to progression after chemotherapy: Outcomes from a U.S. community practice network. Oncologist 2011; 16: 486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou C, Wu YL, Chen G et al BEYOND: A randomized, double‐blind, placebo‐controlled, multicenter, phase III study of first‐line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2015; 33: 2197–204. [DOI] [PubMed] [Google Scholar]
- 15. Takeda M, Yamanaka T, Seto T et al Bevacizumab beyond disease progression after first‐line treatment with bevacizumab plus chemotherapy in advanced nonsquamous non‐small cell lung cancer (West Japan Oncology Group 5910L): An open‐label, randomized, phase 2 trial. Cancer 2016; 122: 1050–9. [DOI] [PubMed] [Google Scholar]
- 16. Herbst RS, O'Neill VJ, Fehrenbacher L et al Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small‐cell lung cancer. J Clin Oncol 2007; 25: 4743–50. [DOI] [PubMed] [Google Scholar]
- 17. Herbst RS, Ansari R, Bustin F et al Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non‐small‐cell lung cancer after failure of standard first‐line chemotherapy (BeTa): A double‐blind, placebo‐controlled, phase 3 trial. Lancet 2011; 377: 1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu B, Zhou X, Liu Y et al Comparison of chemotherapy plus bevacizumab vs. chemotherapy alone as third‐line treatment or beyond for advanced non‐small cell lung cancer: A propensity score‐matched analysis. Oncol Lett 2018; 15: 5671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 20. Trotti A, Colevas AD, Setser A et al CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment Semin Radiat Oncol 2003; 13: 176–81. [DOI] [PubMed] [Google Scholar]
- 21. Giobbie‐Hurder A, Gelber RD, Regan MM. Challenges of guarantee‐time bias. J Clin Oncol 2013; 31: 2963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time‐varying covariate with an extended Kaplan‐Meier estimator. Am Stat 2005; 59: 301–7. [Google Scholar]
- 23. Lee CK, Man J, Lord S et al Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung cancer‐a meta‐analysis. J Thorac Oncol 2017; 12: 403–7. [DOI] [PubMed] [Google Scholar]
- 24. Dong ZY, Zhang JT, Liu SY et al EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD‐1 blockade in non‐small cell lung cancer. Oncoimmunology 2017; 6 (11): e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terme M, Pernot S, Marcheteau E et al VEGFA‐VEGFR pathway blockade inhibits tumor‐induced regulatory T‐cell proliferation in colorectal cancer. Cancer Res 2013; 73: 539–49. [DOI] [PubMed] [Google Scholar]
- 26. Chau I, Bendell JC, Calvo E et al Ramucirumab (R) plus pembrolizumab (P) in treatment naive and previously treated advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: A multi‐disease phase I study. J Clin Oncol 2017. (Suppl); 35: Abstract 4046. [Google Scholar]
- 27. Reck M, Socinski MA, Cappuzzo F et al Primary PFS and safety analyses of a randomized phase III study of carboplatin + paclitaxel +/− bevacizumab, with or without atezolizumab in 1L non‐squamous metastatic NSCLC (IMPOWER150). Ann Oncol 2017; 28 (Suppl 11): mdx760.002. [Google Scholar]
- 28. Herbst RS, Martin‐Liberal J, Calvo E et al Previously treated advanced NSCLC cohort from a multi‐disease phase 1 study of ramucirumab (R) plus pembrolizumab(P): Efficacy and safety data. Ann Oncol 2017; 28 (Suppl 2: mdx091.010): ii28–51. [Google Scholar]
- 29. Yamamoto N, Seto T, Nishio M et al Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first‐line treatment for advanced EGFR mutation‐positive non‐squamous non‐small‐cell lung cancer (NSCLC): Survival follow‐up results of JO25567. J Clin Oncol 2018; 36 (Suppl: Abstract 9007). [DOI] [PubMed] [Google Scholar]
- 30. Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: A meta‐analysis. Oncology 2010; 79 (1–2): 27–38. [DOI] [PubMed] [Google Scholar]
- 31. Hang XF, Xu WS, Wang JX et al Risk of high‐grade bleeding in patients with cancer treated with bevacizumab: A meta‐analysis of randomized controlled trials. Eur J Clin Pharmacol 2011; 67: 613–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics of wild‐type EGFR subjects stratified by the number of treatment cycles of bevacizumab.
Table S2. Clinical characteristics of Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1 of subjects stratified by the bevacizumab treatment line.


