Abstract
Background:
Folate receptors (FRs) facilitate embryonic uptake of folates and are important for proper early embryonic development. There is accumulating evidence that blocking FR autoantibodies contribute to developmental diseases. However, genetic factors associated with the expression of FR autoantibodies remain unknown.
Objective:
We investigated the effects of genetic polymorphisms in folate pathway genes on FR autoantibody titers in women.
Methods:
We recruited 302 pregnant women in China. The FR antigen-down immunoassay was used to measure levels of FR autoantibodies including human immunoglobulin G (IgG) and immunoglobulin M (IgM) in maternal plasma. Genotypes were identified by matrix-assisted laser desorption/ionization time of flight mass spectrometry and polymerase chain reaction methods. General linear model was used to analyze the effects of genetic variants on FR autoantibody levels.
Results:
Significant associations were observed between genotypic variations and levels of FR autoantibodies. Plasma levels of FR autoantibodies in women with the TT genotype at MTHFR rs1801133 were significantly higher than those of women with the CC genotype (IgG: β = 0.62, 95% CI 0.21–1.04; IgM: β = 0.42, 95% CI 0.12–0.72). For DNMT3A rs7560488, the level of FR autoantibody IgG significantly increased in the TT genotype compared with CC genotype (β = 0.90, 95% CI 0.20–1.59). For MTHFD2 rs828903, genotype GG was associated with elevated levels of FR autoantibody IgM compared to the AA genotype (β = 0.60, 95% CI 0.10–1.10). No association was detected between genetic variants of the DHFR gene with FR autoantibodies levels.
Conclusion:
Genetic variations in MTHFR, DNMT3A, and MTHFD2 genes were associated with elevated plasma levels of FR autoantibodies.
Keywords: autoantibody, folate receptor, genetic polymorphisms, IgG, IgM
1 |. INTRODUCTION
Folate is also referred to as vitamin B9 and is known to play important roles during embryonic development (De-Regil, Pena-Rosas, & Fernandez-Gaxiola, 2015). Supplementation with folic acid (FA) is recommended for women of child-bearing age to prevent selected birth defects, including the birth of babies with neural tube defects (NTDs) (Czeizel & Dudas, 1992). FA is an essential vitamin, meaning that it cannot be synthesized by the body, and deficiencies are common in the absence of folate supplementation or fortification programs. The folate receptor alpha (FR–α) has a high affinity for folate, and functions in the cellular folate uptake (Frye et al., 2016; Rijnboutt et al., 1996). Therefore, normal binding of folates to the FR–α is necessary for maternal uptake of folate and transport to the embryo during early development (Henderson, Perez, & Schenker, 1995; Piedra-hita et al., 1999; Rosenquist & Finnell, 2001; Weitman, Lark, & Coney, 1992).
There have been reports of maternal expression of an autoantibody (called the FA autoantibody) that can specifically bind to the FR and adversely affect cellular folate uptake in rats and humans (Coulam, 2000). Antibodies to FR administered to pregnant rats were shown to induce embryonic damage, and the distribution of the antibody to the FR in the embryonic and extra embryonic tissues was similar to that of the FR, suggesting that the FR antibody can specifically bind to this receptor (da Costa, Sequeira, Rothenberg, & Weedon, 2003). Scientists working in the area of ovarian and breast cancer research determined that the T-cell activation against FR can produce specific autoantibodies to FR in humans (Knutson et al., 2006). FR autoantibodies have been related to multiple different diseases, such as increasing the risk for complex birth defects including NTDs (Cabrera et al., 2008)and cleft lip and cleft palate (Bliek, Rothenberg, & Steegers-Theunissen, 2006), as well as autism (Ramaekers, Sequeira, Blau, & Quadros, 2008), and low fertility (Berrocal-Zaragoza et al., 2009). Rothenberg and colleagues first reported autoantibodies in the plasma of women who previously had a pregnancy complicated by NTDs that was bound to the FR and blocked the cellular uptake of folate in vitro (Rothenberg et al., 2004). Subsequently, several studies have now shown that FR autoantibodies are associated with an elevated risk of NTDs (Boyles et al., 2011; Cabrera et al., 2008; Yang et al., 2016).
Pathogenic autoimmune responses arise when functional proteins become modified or damaged and no longer recognized as self. Autoantibodies targeting these altered proteins may cross-react with the unmodified proteins, causing a loss of function. Two such post-translational modifications, N-and S-homocysteinylation, are directly tied to folate metabolism. Insufficient folate in the diet leads to low circulating levels in the blood, which causes homocysteine (Hcy) levels to rise. It has been reported that high levels of Hcy are associated with the induction of autoantibodies to N-homocysteinylated self-antigens (Undas et al., 2004, 2006). In healthy individuals, reduction in Hcy levels leads to reduction of autoantibodies targeting N-homocysteinylated proteins (Undas et al., 2006). The pathogenic implications of homocysteinylated FR are particularly intriguing, considering the potentially synergistic interaction between reduced folate levels, increased FR homocysteinylation, increased FR autoantibodies, and impaired folate transport. Therefore, we hypothesized that genetic variations in folate and Hcy metabolic pathways are associated with higher titers of FR auto-antibodies. Based on this hypothesis, we targeted genetic polymorphisms in selected genes involved in one carbon metabolism and methylation reactions including: 5,10-methylenetetrahydrofolate reductase (MTHFR), DNA (cytosine-5)-methyltransferase-3A (DNMT3A), bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (MTHFD2), and dihydrofolate reductase (DHFR), in order to investigate their effects on the levels of FR autoantibodies.
2 |. MATERIALS AND METHODS
2.1 |. Study population
The subjects were recruited from a population-based birth defects surveillance system in five rural counties of Shanxi Province (Taigu, Pingding, Xiyang, Shouyang, and Zezhou) in northern China between 2011 and 2013. The present study included 99 women with NTD-affected pregnancies and 203 control women whose pregnancies ended in term healthy newborns, or in terminated fetuses without congenital malformations. In-person interviews were performed by trained local health workers at the delivery hospitals within the first week of delivery or pregnancy termination. Information on maternal social demographic characteristics, reproductive history and periconceptional folic acid supplementation was collected. Samples of maternal venous blood were collected at delivery or at the time of the termination of the pregnancy. Blood cells and plasma were separated by centrifugation for DNA extraction and genotyping, and for measurement of FR autoantibodies, respectively. Aliquoted cells and plasma samples were stored at –80°C until they were used for these analyses. The study was approved by the institutional review board of Peking University and appropriate signed informed consent was obtained for all study subjects.
2.2 |. Assay for FA autoantibodies
FR autoantibodies were measured by immobilizing FR from human placenta to immunoassay plates and detecting diluted human serum immunoglobulin G (IgG) and immunoglobulin M (IgM) with the respective secondary antibodies, as previously described(Yang et al., 2016).
2.3 |. Identification of genotypes
We selected variants in the folate pathway related genes including: MTHFR, DNMT3A, MTHFD2, and DHFR with minimum allele frequency (MAF)>0.1 in the Chinese Han Beijing population (Table 1). Genomic DNA was prepared from peripheral leukocytes using Relax Gene blood DNA System (Relax Gene; TIANGEN, Beijing, China). The genotypes at MTHFR rs1801133 and rs1476413, DNMT3A rs7560488, MTHFD2 rs828903 and rs7340453 were determined by using the Sequenom MassARRAY MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry) system (Sequenom Inc., San Diego, CA, USA).
TABLE 1.
Selected variants in folate pathway genes
| SNP | Location | Chromosome | Gene | Nucleotide change | Type/comment | pa | MAF |
|---|---|---|---|---|---|---|---|
| rs1801133 | 11856378 | 1 | MTHFR | C→T | Exon, nonsynonymous | .271 | 0.418 |
| rs1476413 | 11852300 | 1 | MTHFR | A→G | Intron | .826 | 0.147 |
| rs7560488 | 25568821 | 2 | DNMT3A | C→T | Validated | .890 | 0.189 |
| rs828903 | 74209594 | 2 | MTHFD2 | A→G | Intron | .598 | 0.361 |
| rs7340453 | 74200888 | 2 | MTHFD2 | C→T | Intron | .987 | 0.390 |
| rs70991108 | 80654344 | 5 | DHFR | Deletion→insertion | Intron | .813 | 0.083 |
Note. SNP, single nucleotide polymorphisms; MAF, minimum allele frequency; MTHFR, 5,10-methylenetetrahydrofolate reductase; DNMT3A, DNA (cytosine-5)-methyltransferase-3A; MTHFD2, bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial; DHFR, dihydrofolate reductase.
p value of Hardy-Weinberg.
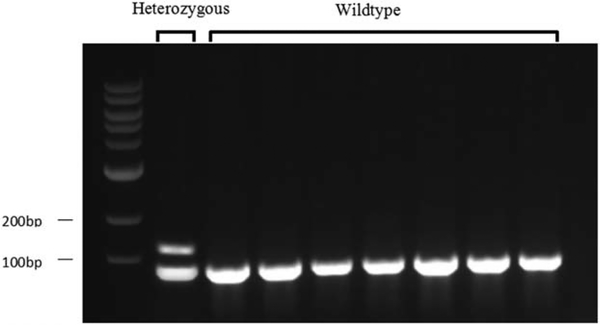
The DHFR 19bp-deletion/insertion (rs70991108) was genotyped as follows: Briefly, PCR used the forward primer 5′-CCACGGTCGGGGTACCTGGG-3′ and reverse primer 5′-AAAAGGGGAATCCAGTCGG-3′ for the DHFR 19bp-insertion and the forward primer 5′-ACGGTCGGGGTGGC CGACTC-3′ and reverse primer 5′-AAAAGGGGAATCCA GTCGG-3′ for the DHFR 19bp-deletion. The mixture was denatured at 95°C for 10 min, and the PCR reaction was performed for 35 cycles under the following conditions: denaturation at 95°C for 2 min, annealing at 58°C for 30 s, and extension at 72°C for 1 min and a final extension cycle of 72°C was for 5 min. There were two PCR reactions. PCR products were analyzed on an agarose gel (3%). A single fragment of 112 base pairs (bp) was identified as homozygous; two fragments of 112 and 93 bp were identified as heterozygous (Figure 1).
FIGURE 1.
Agarose gel electrophoresis for detecting genotypes at DHFR rs70991108. A single fragment of 112 bp was identified as homozygous; two fragments of 112 and 93 bp were identified as heterozygous
2.4 |. Statistical analysis
The Hardy-Weinberg equilibrium constant was assessed using the chi-squared (χ2) test. Pairwise linkage disequilibrium of genetic polymorphisms was estimated using the Haploview software program (version 4.0). Given that the distribution of the IgG and IgM was right-skewed, values of the IgG and IgM were transformed using the natural logarithm. Analysis of variance (ANOVA) was performed to detect differences in FR autoantibody levels among different study subjects. A general linear model was used to assess any possible association between genetic polymorphisms and FR autoantibody levels. Additionally, because the participants included women with NTD-affected pregnancies as well as women with normal pregnancy outcomes, a stratified analysis by cases and controls was also performed. A p value of <.05 was considered statistically significant. Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL), version 22.0 for Windows.
3 |. RESULTS
Maternal FR autoantibodies levels with respect to maternal population demographics are summarized in Table 2. There was no significant difference in FR autoantibodies levels among women of different maternal age, educational back-ground, occupation, prepregnancy BMI or between women with and without periconceptional folate supplementation (p>.05). Multipara women had significantly higher levels of FR autoantibodies than did primipara women (p<.05).
TABLE 2.
Characteristics of participants according to means of plasma FR autoantibodies in Shanxi Province, China, 2011–2013 (N = 302)
| IgGa |
IgMa |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | N | Mean | SD | pb | Mean | SD | pb |
| Maternalage (years) | .163 | .280 | |||||
| <25 | 154 | −0.20 | 1.12 | 0.09 | 0.82 | ||
| 25–29 | 83 | −0.01 | 1.11 | 0.29 | 0.78 | ||
| 30–34 | 46 | 0.08 | 1.11 | 0.05 | 0.87 | ||
| >35 | 17 | 0.33 | 1.19 | 0.07 | 0.73 | ||
| Education | .338 | .766 | |||||
| Primary or lower | 21 | 0.21 | 1.25 | 0.12 | 0.80 | ||
| Junior high | 190 | −0.04 | 1.12 | 0.17 | 0.84 | ||
| High school or above | 91 | −0.23 | 1.10 | 0.08 | 0.76 | ||
| Occupation | .344 | .267 | |||||
| Farmer | 238 | −0.05 | 1.11 | 0.15 | 0.82 | ||
| Officer | 38 | 0.01 | 1.12 | 0.24 | 0.71 | ||
| Others | 26 | −0.38 | 1.09 | 0.23 | 0.93 | ||
| Prepregnancy BMI (kg/m2) | .220 | .918 | |||||
| <18.5 | 37 | −0.45 | 1.04 | 0.03 | 0.74 | ||
| 18.5–24.9 | 192 | −0.01 | 1.12 | 0.17 | 0.74 | ||
| >25 | 73 | −0.10 | 1.14 | 0.12 | 0.98 | ||
| Parity | .012 | .042 | |||||
| 1 | 147 | −0.22 | 1.16 | 0.06 | 0.81 | ||
| >2 | 138 | 0.13 | 1.09 | 0.25 | 0.81 | ||
| Periconceptional folate supplementation | .092 | .281 | |||||
| Yes | 158 | −0.06 | 1.06 | 0.14 | 0.77 | ||
| No | 141 | −0.08 | 1.19 | 0.15 | 0.87 | ||
IgG and IgM index were transformed using the natural logarithm. bANOVA to examine differences in means.
The MTHFR rs1801133 and rs1476413, DNMT3A rs7560488, MTHFD2 rs828903 and rs7340453, DHFR rs70991108 genotypes were in H-W equilibrium (p>.05) among the study population. There was no linkage disequilibrium among these genetic polymorphisms (r2<.8). As shown in Table 3, the single nucleotide polymorphisms (SNPs) MTHFR rs1801133, DNMT3A rs7560488, and MTHFD2 rs828903 were highly correlated to FR autoantibodies levels. Plasma FR autoantibody in women with the TT genotype at MTHFR rs1801133were significantly higher (IgG: β = 0.62, 95% CI 0.21–1.04; IgM: β = 0.42, 95% CI 0.12–0.72) than those of women with the CC genotype. However, no differences in FR autoantibodies levels were found between the CT and CC genotypes at MTHFR rs1801133. For DNMT3A rs7560488, the level of FR autoantibody IgG significantly increased in TT genotype (β = 0.90, 95% CI 0.20–1.59) compared with CC genotype, whereas no significant difference was found between the CT and CC genotypes in terms of the levels of FR autoantibody IgG, or between TT/CT and CC in levels of FR autoantibody IgM. At the MTHFD2 rs828903 locus, genotype GG was associated with elevated plasma levels of FR autoantibody IgM (β = 0.60, 95% CI 0.10–1.10) compared to the AA genotype, whereas no significant difference was found between the AG and AA genotypes’ levels of FR autoantibody IgM, or between GG/AG and AA in levels of FR autoantibody IgG. The SNPs MTHFR rs1476413, MTHFD2 rs7340453 and DHFR rs70991108 polymorphisms had no association with FR autoantibodies levels.
TABLE 3.
Association between selected gene variants and FR autoantibodies levels (n = 302)
| IgGa |
IgMa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | N | Mean | SD | β | 95%CI | p | Mean | SD | μ | 95%CI | p |
| rs1801133 (MTHFR) | |||||||||||
| Wildtype (CC) | 105 | −0.23 | 1.17 | Ref | −0.01 | 0.80 | Ref | ||||
| Heterozygous (CT) | 140 | −0.10 | 1.07 | 0.20 | −0.10, 0.49 | .198 | 0.14 | 0.83 | 0.14 | −0.07, 0.36 | .197 |
| Homozygous (TT) | 57 | 0.24 | 1.11 | 0.62 | 0.21, 1.04 | .003 | 0.40 | 0.70 | 0.42 | 0.12, 0.72 | .006 |
| rs1476413 (MTHFR) | |||||||||||
| Wildtype (AA) | 218 | −0.09 | 1.11 | Ref | 0.10 | 0.83 | Ref | ||||
| Heterozygous (AG) | 84 | −0.09 | 1.10 | −0.20 | −0.53, 0.12 | .224 | 0.22 | 0.76 | 0.19 | −0.22, 0.26 | .873 |
| rs7560488 (DNMT3A) | |||||||||||
| Wildtype (CC) | 199 | −0.16 | 1.11 | Ref | 0.15 | 0.82 | Ref | ||||
| Heterozygous (CT) | 93 | 0.05 | 1.11 | 0.25 | −0.03, 0.52 | .076 | 0.10 | 0.81 | 0.01 | −0.19, 0.21 | .945 |
| Homozygous (TT) | 10 | 0.66 | 1.01 | 0.90 | 0.20, 1.59 | .012 | 0.52 | 0.48 | 0.45 | −0.06, 0.96 | .081 |
| rs828903 (MTHFD2) | |||||||||||
| Wildtype (AA) | 121 | −0.23 | 1.11 | Ref | 0.10 | 0.81 | Ref | ||||
| Heterozygous (AG) | 145 | −0.02 | 1.15 | 0.01 | −0.37, 0.39 | .974 | 0.10 | 0.86 | −0.10 | −0.38, 0.18 | .478 |
| Homozygous (GG) | 36 | 0.29 | 0.92 | 0.45 | −0.24, 1.14 | .200 | 0.48 | 0.58 | 0.60 | 0.10, 1.10 | 0.018 |
| rs7340453 (MTHFD2) | |||||||||||
| Wildtype (CC) | 115 | −0.26 | 1.12 | Ref | 0.08 | 0.85 | Ref | ||||
| Heterozygous (CT) | 142 | −0.19 | 1.16 | 0.21 | −0.17, 0.59 | .273 | 0.14 | 0.80 | 0.10 | −0.17, 0.38 | .462 |
| Homozygous (TT) | 45 | 0.16 | 0.96 | 0.13 | −0.50, 0.76 | .689 | 0.28 | 0.73 | −0.22 | −0.68,0.24 | .343 |
| rs70991108 (DHFR) | |||||||||||
| Wildtype (del/del) | 252 | −0.08 | 1.08 | Ref | 0.11 | 0.78 | Ref | ||||
| Heterozygous (del/ins) | 50 | −0.09 | 1.13 | 0.005 | −0.33, 0.34 | .979 | 0.14 | 0.82 | −0.08 | −0.32, 0.17 | .528 |
With adjustment for parity of women; IgG and IgM index were transformed using the natural logarithm.
Additionally, a stratified analysis was performed on samples from women with NTD-affected pregnancies and women with normal pregnancies. In NTDs, DNMT3A rs7560488 genotypes were significantly correlated to levels of FR autoantibody IgG (Table 4). FR autoantibody IgG levels were significantly higher in the TT genotype compared with CC of DNMT3A rs7560488, whereas no significant difference was found between the CC and CT genotypes in terms of levels of FR autoantibody IgG. No association was found in other SNPs or with the DHFR rs70991108 polymorphism with respect to FR autoantibodies levels. In control samples, the SNPs MTHFR rs1801133 and MTHFD2 rs828903 were significantly correlated to FR autoantibodies concentrations (Table 4). Among the three possible genotypes at rs1801133, the FR autoantibody level of women with the TT and CT genotypes were significantly higher than that of women with the CC genotype, respectively. For MTHFD2 rs828903, FR autoantibody IgM levels were significantly higher in the GG genotype compared with the AA genotype samples. No association was found in other SNPs or in DHFR rs70991108 polymorphisms related to FR autoantibodies levels.
TABLE 4.
Association between selected gene variants and FR autoantibodies in NTDs and Controls
| IgGa |
IgMa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | N | Mean | SD | β | 95%CI | p | Mean | SD | β | 95%CI | p |
| NTD Cases(99) | |||||||||||
| rs1801133 (MTHFR) | |||||||||||
| Wildtype (CC) | 31 | 0.63 | 0.94 | Ref | 0.55 | 0.68 | Ref | ||||
| Heterozygous (CT) | 45 | 0.42 | 1.07 | −0.13 | −0.62, 0.49 | .592 | 0.45 | 0.92 | −.20 | −0.57, 0.18 | .312 |
| Homozygous (TT) | 23 | 0.74 | 0.85 | 0.16 | −0.43, 0.75 | .601 | 0.64 | 0.55 | −.04 | −0.50, 0.42 | .867 |
| rs1476413 (MTHFR) | |||||||||||
| Wildtype (AA) | 69 | 0.50 | 1.10 | Ref | 0.49 | 0.83 | Ref | ||||
| Heterozygous (AG) | 30 | 0.70 | 0.92 | 0.29 | −0.18, 0.77 | .228 | 0.61 | 0.98 | .26 | −0.11, 0.63 | .170 |
| rs7560488 (DNMT3A) | |||||||||||
| Wildtype (CC) | 62 | 0.43 | 1.01 | Ref | 0.57 | 0.74 | Ref | ||||
| Heterozygous (CT) | 32 | 0.71 | 0.94 | 0.35 | −0.10, 0.80 | .127 | 0.40 | 0.86 | −.13 | −0.48, 0.22 | .451 |
| Homozygous (TT) | 5 | 1.21 | 0.58 | 0.88 | 0.02, 1.75 | .044 | 0.76 | 0.54 | .21 | −0.46, 0.88 | .545 |
| rs828903 (MTHFD2) | |||||||||||
| Wildtype (AA) | 37 | 0.55 | 1.06 | Ref | 0.67 | 0.74 | Ref | ||||
| Heterozygous (AG) | 46 | 0.54 | 1.00 | −0.42 | −1.11, 0.28 | .238 | 0.34 | 0.86 | −.12 | −0.26, 0.19 | .384 |
| Homozygous (GG) | 16 | 0.66 | 0.77 | −0.68 | −2.03, 0.67 | .322 | 0.71 | 0.41 | −.47 | −0.51, 0.58 | .082 |
| rs7340453 (MTHFD2) | |||||||||||
| Wildtype (CC) | 39 | 0.46 | 1.11 | Ref | 0.54 | 0.95 | Ref | ||||
| Heterozygous (CT) | 41 | 0.58 | 0.97 | 0.38 | −0.31, 1.07 | .279 | 0.45 | 0.70 | .45 | −0.08,0.99 | .097 |
| Homozygous (TT) | 19 | 0.71 | 0.77 | 0.94 | −0.33, 2.21 | .146 | 0.66 | 0.44 | .51 | −0.47, 1.50 | .307 |
| rs70991108 (DHFR) | |||||||||||
| Wildtype (del/del) | 84 | 0.54 | 1.02 | Ref | 0.48 | 0.93 | Ref | ||||
| Heterozygous (del/ins) | 15 | 0.56 | 0.98 | −0.01 | −0.56, 0.53 | .959 | 0.53 | 0.74 | −.11 | −0.54, 0.31 | .590 |
| Controls (203) | |||||||||||
| rs1801133 (MTHFR) | |||||||||||
| Wildtype (CC) | 74 | −0.59 | 1.07 | Ref | −0.24 | 0.74 | Ref | ||||
| Heterozygous (CT) | 95 | −0.33 | 0.99 | 0.34 | 0.01, 0.67 | .044 | −0.003 | 0.76 | .27 | 0.03, 0.51 | .027 |
| Homozygous (TT) | 34 | −0.10 | 1.15 | 0.76 | 0.25, 1.28 | .004 | 0.23 | 0.75 | .54 | 0.17, 0.92 | .004 |
| rs1476413 (MTHFR) | |||||||||||
| Wildtype (AA) | 149 | −0.36 | 1.06 | Ref | −0.08 | 0.77 | Ref | ||||
| Heterozygous (AG) | 54 | −0.53 | 1.06 | −0.25 | −0.55, 0.86 | .267 | 0.01 | 0.75 | −.11 | −0.39, 0.18 | .469 |
| rs7560488 (DNMT3A) | |||||||||||
| Wildtype (CC) | 137 | −0.42 | 1.05 | Ref | −0.04 | 0.79 | Ref | ||||
| Heterozygous (CT) | 61 | −0.30 | 1.04 | 0.10 | −0.20, 0.41 | .505 | −0.07 | 0.73 | −.03 | −0.26, 0.19 | .765 |
| Homozygous (TT) | 5 | 0.11 | 1.11 | 0.48 | −0.44, 1.40 | .304 | 0.27 | 0.34 | .30 | −0.37, 0.97 | .376 |
| rs828903 (MTHFD2) | |||||||||||
| Wildtype (AA) | 84 | −0.58 | 0.95 | Ref | −0.16 | 0.67 | Ref | ||||
| Heterozygous (AG) | 99 | −0.27 | 1.13 | 0.09 | −0.32, 0.50 | .974 | −0.01 | 0.84 | .26 | −0.23, 0.37 | .649 |
| Homozygous (GG) | 20 | −0.01 | 0.93 | 0.51 | −0.21, 1.23 | .200 | 0.30 | 0.64 | .17 | 0.21, 1.26 | .006 |
| rs7340453 (MTHFD2) | |||||||||||
| Wildtype (CC) | 76 | −0.62 | 0.93 | Ref | −0.15 | 0.69 | Ref | ||||
| Heterozygous (CT) | 101 | −0.26 | 1.15 | 0.26 | −0.15, 0.68 | .209 | 0.02 | 0.81 | .08 | −0.22, 0.38 | .602 |
| Homozygous (TT) | 26 | −0.24 | 0.89 | 0.10 | −0.56, 0.77 | .761 | 0.004 | 0.77 | −.29 | −0.78, 0.19 | .235 |
| rs70991108 (DHFR) | |||||||||||
| Wildtype (del/del) | 168 | −0.33 | 1.01 | Ref | −0.05 | 0.66 | Ref | ||||
| Heterozygous (del/ins) | 35 | −0.41 | 1.06 | −0.01 | −0.39, 0.37 | .957 | −0.05 | 0.79 | −.07 | −0.35, 0.20 | .597 |
With adjustment for parity of women; IgG and IgM index were transformed using the natural logarithm
4 |. DISCUSSION
In this study, we identified genomic variations in MTHFR, DNMT3A, MTHFD2 and DHFR genes, and proposed that variations in the genes of the folate pathway may be important contributors to the expression of FR autoantibodies levels in women. We found that variations at MTHFR rs1801133, DNMT3A rs7560488, and MTHFD2 rs828903 were associated with titers of FR autoantibodies.
No previous studies have shown that genetic polymorphisms in the folate and Hcy metabolic pathway are associated with levels of maternal FR autoantibodies. In our study, we found that the genotypes of MTHFR polymorphisms were related to the levels of FR autoantibodies produced. The thermolabile protein MTHFR is of great importance for the regulation of available 5-MTHF, which serves as the main circulating folate required for Hcy remethylation (Finnell, Shaw, Lammer, & Volcik, 2002). Mutations at MTHFR rs1801133 can result in 50%–60% reduced enzyme activity (van der Put et al., 1998), which can have significant developmental consequences. Previous studies have shown that Hcy levels were significantly higher in the TT genotype at MTHFR rs1801133 compared to that in the CT and CC (Cai, Yin, Yang, Zhang, & Cheng, 2014). The FR protein contains several putative surface lysine residues which may be susceptible to the posttranslational modification known as homocysteinylation (Cabrera et al., 2008). Studies have shown the potential for the formation of a neo-antigen as the FR is modified, inducing the maternal system to create autoantibodies against this altered FR protein (Jakubowski, 2005). Our results demonstrate that mutations at MTHFR rs1801133 were associated with elevated FR autoantibodies. Plasma FR autoantibody titers in women with the TT genotype at MTHFR rs1801133 were significantly higher than that of women with the CC genotype. Mutations at MTHFR rs1801133 are related to elevated Hcy concentrations which may promote homocysteinylation of the FR such that the modified FAs may act as neo-antigens capable of inducing the production of FR autoantibodies.
DNA (cytosine-5)-methyltransferase-3A (DNMT3A) belongs to a family of genes that encode enzymes involved in the de novo methylation of S-adenosyl methionine during development (Ding et al., 2012). The TT genotype at DNMT3A rs7560488 has been previously found to be associated with an increased risk of myelomeningocele (Pangilinan et al., 2012). Our study was the first to link the TT genotype at rs7560488 with increased levels of maternal FR autoantibodies.
MTHFD2 plays an important role in folate metabolism by oxidizing one carbon units and recycling the folate cofactor required by the glycine-synthesizing enzyme serine hydroxylmethyltransferase (SHMT2) (Hol et al., 1998). It has been reported that genetic variations in MTHFD2 were associated with an increased risk of NTDs (Shaw et al., 2009). Our study found that there was a significant difference in the levels of FR autoantibodies among the three rs828903 genotypes.
DHFR encodes enzymes which are essential for the conversion of folic acid to active folate needed for one-carbon metabolism (Nazki, Sameer, & Ganaie, 2014). Studies have investigated a 19bp deletion/insertion with mixed results. One study found that the DHFR intronic 19-bp deleted allele may be a protective NTD genetic factor (Parle-McDermott et al., 2007). Another group showed that the deleted allele was modestly associated with an increased maternal risk of NTDs (Johnson et al., 2004). Unfortunately, neither study explained just how this variant might functionally influence NTD susceptibility. The estimated MAF was 0.085 with an absence of cases or controls with homozygote insertions in the population of our study. There was no association between DHFR 19bp deletion/insertion (rs70991108) and FR autoantibodies in this study. No assumptions can be made specifically for the impact of DHFR homozygous insertions for FR autoantibody levels, due to the general rarity of these individuals in this population.
The identification of high titers of FR autoantibodies in clinical samples with gene interactions associated with NTD risk provides strengthening support for the biological significance of autoantibodies beyond mere association. Managing FR autoimmunity can potentially influence the management of human fertility and pregnancy (Shapira, Sequeira, & Quadros, 2015). These data also support additional testing of the proposed mechanisms involving post-translational modification of FR and opens the possibility of developing intervention strategies that reduce FR autoantibodies before and during critical stages of development. Reducing the risk of NTDs via FA supplementation or reducing FR autoantibodies titers and understanding why some pregnancies escape this prevention strategy has broad implications for the estimated 300,000 infants born with NTDs annually worldwide.
In summary, we found that genetic variations in the MTHFR, DNMT3A, and MTHFD2 genes were associated with high levels of FR autoantibodies in maternal plasma. Our study provides the first evidence that genetic variations in the folate pathway may play an important role in the extent of FR autoantibody production. Further studies are needed to elucidate the mechanism by which genetic variations in the folate pathway affect the levels of FR autoantibodies, examine genenutrient-immune interactions and determine why certain women are prone to produce blocking antibodies.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (Grant Number: 81472987 and 81773441); Beijing Natural Science Foundation (Grant Number: 7162094); and the National Key Research and Development Program, Ministry of Science and Technology, P.R. China (Grant Number: 2016YFC1000501). Dr. Finnell was supported by Changjiang Scholar Professorship to Fudan University and by NIH grant HD067244, HD081216, and HD083809.
Funding information
National Natural Science Foundation of China, Grant/Award Number: 81472987 and 81773441; Beijing Natural Science Foundation, Grant/Award Number: 7162094; National Key Research and Development Program, Ministry of Science and Technology, P.R. China, Grant/Award Number: 2016YFC1000501; Changjiang Scholar, Professorship to Fudan University (to Dr. Finnell); NIH, Grant/Award Number: HD067244, HD081216, and HD083809 (to Dr. Finnell)
Footnotes
CONFLICT OF INTEREST
No conflict of interest.
REFERENCES
- Berrocal-Zaragoza MI, Fernandez-Ballart JD, Murphy MM, Cavalle-Busquets P, Sequeira JM, & Quadros EV (2009). Association between blocking folate receptor autoantibodies and subfertility. Fertility and Sterility, 91(4), 1518–1521. https://doi.org/10.1016/j.fertnstert.2008.08.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliek JB, Rothenberg SP, & Steegers-Theunissen RP (2006). Maternal folate receptor autoantibodies and cleft lip and/or palate. International Journal of Gynecology & Obstetrics, 93(2), 142–143. https://doi.org/10.1016/j.ijgo.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Boyles AL, Ballard JL, Gorman EB, McConnaughey DR, Cabrera RM, Wilcox AJ, … Finnell RH (2011). Association between inhibited binding of folic acid to folate receptor alpha in maternal serum and folate-related birth defects in Norway. Human Reproduction, 26(8), 2232–2238. https://doi.org/10.1093/humrep/der144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera RM, Shaw GM, Ballard JL, Carmichael SL, Yang W, Lammer EJ, & Finnell RH (2008). Autoantibodies to folate receptor during pregnancy and neural tube defect risk. Journal of Reproductive Immunology, 79(1), 85–92. https://doi.org/10.1016/j.jri.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Yin L, Yang F, Zhang L, & Cheng J (2014). Association between Hcy levels and the CBS844ins68 and MTHFR C677T polymorphisms with essential hypertension. Biomedical Reports, 2(6), 861–868. https://doi.org/10.3892/br.2014.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulam CB (2000). Understanding the immunobiology of pregnancy and applying it to treatment of recurrent pregnancy loss. Early Pregnancy (Online), 4(1), 19–29. [PubMed] [Google Scholar]
- Czeizel AE, & Dudas I (1992). Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. New England Journal of Medicine, 327(26), 1832–1835. https://doi.org/10.1056/nejm199212243272602 [DOI] [PubMed] [Google Scholar]
- da Costa M, Sequeira JM, Rothenberg SP, & Weedon J (2003). Antibodies to folate receptors impair embryogenesis and fetal development in the rat. Birth Defects Research Part A: Clinical and Molecular Teratology, 67(10), 837–847. https://doi.org/10.1002/bdra.10088 [DOI] [PubMed] [Google Scholar]
- De-Regil LM, Pena-Rosas JP, & Fernandez-Gaxiola AC (2015). Effects and safety of periconceptional oral folate supplementation for preventing birth defects. The Cochrane Database of Systematic Reviews, (12), Cd007950 https://doi.org/10.1002/14651858.CD007950.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YB, He JL, Liu XQ, Chen XM, Long CL, & Wang YX (2012). Expression of DNA methyltransferases in the mouse uterus during early pregnancy and susceptibility to dietary folate deficiency. Reproduction, 144(1), 91–100. https://doi.org/10.1530/rep-12-0006 [DOI] [PubMed] [Google Scholar]
- Finnell RH, Shaw GM, Lammer EJ, & Volcik KA (2002). Does prenatal screening for 5,10-methylenetetrahydrofolate reductase (MTHFR) mutations in high-risk neural tube defect pregnancies make sense? Genetic Testing, 6(1), 47–52. https://doi.org/10.1089/109065702760093915 [DOI] [PubMed] [Google Scholar]
- Frye RE, Delhey L, Slattery J, Tippett M, Wynne R, Rose S, … Quadros E (2016). Blocking and binding folate receptor alpha autoantibodies identify novel autism spectrum disorder subgroups. Frontiers in Neuroscience, 10, 80 https://doi.org/10.3389/fnins.2016.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GI, Perez T, & Schenker S (1995). Maternal-to-fetal transfer of 5-methyltetrahydrofolate by the perfused human placental cotyledon: Evidence for a concentrative role by placental folate receptors in fetal folate delivery. Journal of Laboratory and Clinical Medicine, 126(2), 184–203. [PubMed] [Google Scholar]
- Hol FA, Put NMJ, Geurds MPA, Heil SG, Trijbels FJM, Hamel BCJ, … Blom HJ (1998). Molecular genetic analysis of the gene encoding the trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase) in patients with neural tube defects. Clinical Genetics, 53(2), 119–125. [DOI] [PubMed] [Google Scholar]
- Jakubowski H (2005). Anti-N-homocysteinylated protein autoantibodies and cardiovascular disease. Clinical Chemistry and Laboratory Medicine, 43(10), 1011–1014. https://doi.org/10.1515/cclm.2005.177 [DOI] [PubMed] [Google Scholar]
- Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, & Buyske S (2004). New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): A risk factor for spina bifida acting in mothers during pregnancy? American Journal of Medical Genetics, 124a(4), 339–345. https://doi.org/10.1002/ajmg.a.20505 [DOI] [PubMed] [Google Scholar]
- Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, … Kalli KR (2006). T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. Journal of Clinical Oncology, 24(26), 4254–4261. https://doi.org/10.1200/jco.2006.05.9311 [DOI] [PubMed] [Google Scholar]
- Nazki FH, Sameer AS, & Ganaie BA (2014). Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene, 533(1), 11–20. https://doi.org/10.1016/j.gene.2013.09.063 [DOI] [PubMed] [Google Scholar]
- Pangilinan F, Molloy AM, Mills JL, Troendle JF, ParleMcDermott A, Signore C, … Brody LC (2012). Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Medical Genetics, 13(1), 62 doi.org/10.1186/1471-2350-13-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle-McDermott A, Pangilinan F, Mills JL, Kirke PN, Gibney ER, Troendle J, … Brody LC (2007). The 19-bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR) may decrease rather than increase risk for spina bifida in the Irish population. American Journal of Medical Genetics Part A, 143a (11), 1174–1180. https://doi.org/10.1002/ajmg.a.31725 [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, … Finnell RH (1999). Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nature Genetics, 23(2), 228–232. https://doi.org/10.1038/13861 [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Sequeira JM, Blau N, & Quadros EV (2008). A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Developmental Medicine & Child Neurology, 50(5), 346–352. https://doi.org/10.1111/j.1469-8749.2008.02053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, & Strous GJ (1996). Endocytosis of GPI-linked membrane folate receptor-alpha. The Journal of Cell Biology, 132(1–2), 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist TH, & Finnell RH (2001). Genes, folate and homocysteine in embryonic development. The Proceedings of the Nutrition Society, 60(1), 53–61. [PubMed] [Google Scholar]
- Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, Weedon J, & Quadros EV (2004). Autoantibodies against folate receptors in women with a pregnancy complicated by a neuraltube defect. New England Journal of Medicine, 350(2), 134–142. https://doi.org/10.1056/NEJMoa031145 [DOI] [PubMed] [Google Scholar]
- Shapira I, Sequeira JM, & Quadros EV (2015). Folate receptor autoantibodies in pregnancy related complications. Birth Defects Research Part A: Clinical and Molecular Teratology, 103(12), 1028–1030. https://doi.org/10.1002/bdra.23436 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Lu W, Zhu H, Yang W, Briggs FBS, Carmichael SL, … Finnell RH (2009). 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Medical Genetics, 10(1), 49 https://doi.org/10.1186/14712350-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undas A, Perla J, Lacinski M, Trzeciak W, Kazmierski R, & Jakubowski H (2004). Autoantibodies against N-homocysteinylated proteins in humans: implications for atherosclerosis. Stroke, 35(6), 1299–1304. https://doi.org/10.1161/01.STR.0000128412.59768.6e [DOI] [PubMed] [Google Scholar]
- Undas A, Stïpien E, Glowacki R, Tisonczyk J, Tracz W, & Jakubowski H (2006). Folic acid administration and antibodies against homocysteinylated proteins in subjects with hyperhomocysteinemia. Thrombosis and Haemostasis, 96(09), 342–347. https://doi.org/10.1160/th06-04-0228 [DOI] [PubMed] [Google Scholar]
- van der Put NMJ, Gabre€els F, Stevens EMB, Smeitink JAM, Trijbels FJM, Eskes TKAB, … Blom HJ (1998). A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? American Journal of Human Genetics, 62(5), 1044–1051. https://doi.org/10.1086/301825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman SD, Lark RH, & Coney LR (1992). Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Research, 52(12), 3396–3401. [PubMed] [Google Scholar]
- Yang N, Wang L, Finnell RH, Li Z, Jin L, Zhang L, … Ren, A. (2016). Levels of folate receptor autoantibodies in maternal and cord blood and risk of neural tube defects in a Chinese population. Birth Defects Research Part A: Clinical and Molecular Teratology, 106(8), 685–695. https://doi.org/10.1002/bdra.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]