Abstract
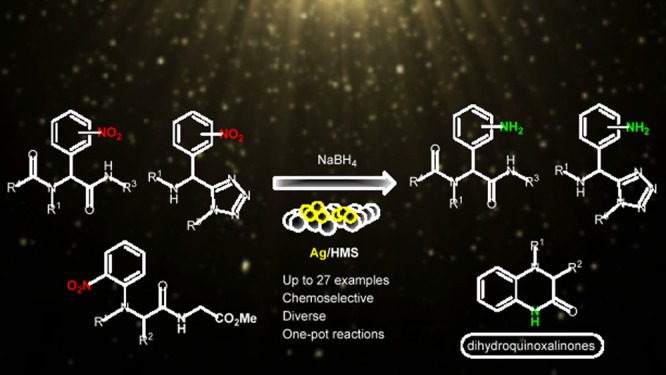
The catalytic efficacy of silver nanoparticles was investigated toward the chemoselective reduction of nitro-tetrazole or amino acid-substituted derivatives into the corresponding amines in high isolated yields. This highly efficient protocol was thereafter applied toward the multicomponent reaction synthesis of heterocyclic dihydroquinoxalin-2-ones with high isolated yields. The reaction proceeds with low catalyst loading (0.8–1.4 mol %) and under mild catalytic conditions, a very good functional-group tolerance, and high yields and can be easily scaled up to more than 1 mmol of product. Thus, the present catalytic methodology highlights a useful synthetic application. Different molecules are designed and accordingly synthesized with the current protocol that could play the role of inhibitors of the soluble epoxide hydrolase, an important target for therapies against hypertension or inflammation.
Introduction
The discovery of combinatorial methodologies to yield organic scaffolds in more efficient ways, such as waste reduction, cost, and less energy demanding, is a key challenge in synthetic chemistry. More specifically, the reduction of the nitro group may yield amines and this fundamental transformation is frequently used in the synthesis of pharmaceuticals, agrochemicals, dye intermediates, and pigments, as well as for a variety of fine chemicals.1 The most promising route for the conversion of −NO2 to −NH2 is the transition metal-catalyzed hydrogenation process,2 but the observed chemoselectivity is rather low, especially when other reducible groups are present. In this direction, noble metal nanoparticles such as gold (AuNPs)3,4 and silver (AgNPs),5,6 as well as cobalt, iron, and manganese-based compounds or oxides7−9 have been employed as catalysts in the reduction of nitro aromatic compounds. Both hydrogenation and transfer hydrogenation processes have been successfully used for the selective nitro group reduction to the corresponding amine but have significant drawback. The former requires high temperatures and H2 pressures and is applicable in monosubstituted aromatic nitro compounds; however, transfer hydrogenation is a chemoselective process employed at ambient conditions using several reducing agents such as borohydrides, hydrosilanes, CO/H2O, and HCOONH4.4 Therefore, the development of new protocols and synthetic strategies that will provide complex as well as functionalized molecules, not only efficient but also chemoselectively, environmentally benign as well as tolerant, including a diverse array of functional moieties and protecting groups, is of high interest and value.10,11
Multicomponent reactions (MCRs) are very often tagged as atom-economic, step-efficient with high exploratory power with regard to chemical space, processes.12,13 Using the MCR principle, rapid and easy access to organic scaffolds with high diversity (more than 500 different scaffolds) can be achieved. Consequently, the industrial and academic synthetic community uses this method to design and discover biologically active compounds applicable in medicinal chemistry and drug discovery.14 In addition, MCRs are the perfect example of a synthetic hub;13 they are highly compatible with a range of unprotected orthogonal functional groups, thus permitting subsequent transformations on a second level.12,15−17 The degree of diversity and complexity that can be achieved following this protocol is highly increased, and therefore the discovery of new methodologies that will postmodify the original MCR “core” is of great importance and value.
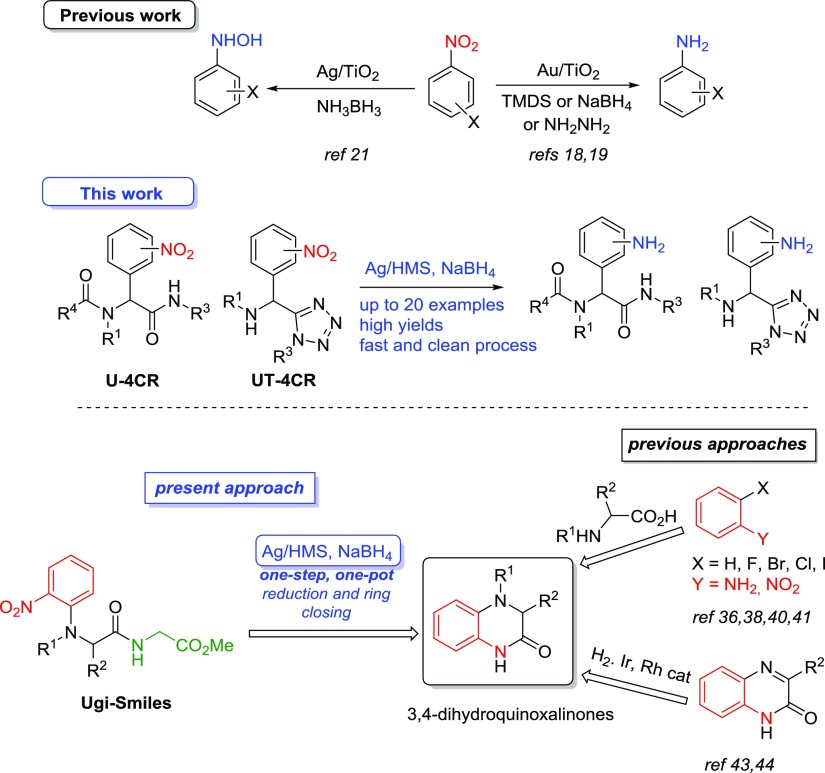
So far, we have demonstrated the catalytic efficacy of supported AuNPs and AgNPs on mesoporous titania and silica (Au/TiO2, Ag/TiO2, and Ag/HMS), toward the chemoselective reduction (via transfer hydrogenation) of a series of aromatic nitroarenes to the corresponding anilines and/or N-aryl hydroxylamines under mild conditions and applying fast and clean reaction processes.18−22 In all cases, simple borohydrides, boranes, hydrosilanes, and hydrazine were used as reducing agents (Scheme 1). Building on these findings, we attempt herein to combine—for the first time—the application of these catalytic systems in the highly diverse and complex environment of the MCR chemical space. More specifically, the Ag/HMS–NaBH4 system was employed to reduce selectively the nitro groups in the presence of other easily reducible moieties such as carbonyl, amide, ester, and halogen, without the need of a protecting group (Scheme 1). In addition, the one-pot synthesis of several N-substituted dihydroquinoxalin-2-ones was studied under the present catalytic conditions, providing an exceptional and mild synthetic approach based on the reported chemoselective transfer hydrogenation process of the multifunctional nitro precursors (Scheme 1).
Scheme 1. Silver-Catalyzed Transfer Hydrogenation Processes toward the Synthesis of Amine-Substituted MCR Scaffolds and Dihydroquinoxalinone Derivatives.
The present catalytic protocol is timely and of high interest as it has a selective and sustainable synthetic character permitting further diversification of the synthesized MCR libraries and giving access to multifunctional amines and dihydroquinoxalinones.
Results and Discussion
In this work, three variants of one of the most well-known isocyanide-based MCRs12,13,23 were employed; such as the Ugi-Smiles,24−26 the Ugi-tetrazole (UT-4CR),27−33 and the classical Ugi reaction (U-4CR).32,34,35 We highly diversified the aforementioned reactions including a variety of functional groups (their synthetic schemes are presented in detail in Supporting Information). For the catalytic reductions, we employed commercially available supported AuNPs Au/TiO2, as well as the salts AgNO3 and AgOTf and the synthesized mesoporous catalysts Ag/TiO2 and Ag/HMS.21,22 The commercial catalyst Au/TiO2 features a ca. 1 wt % Au loading and exhibits an average AuNP size of about 2–3 nm. Mesoporous Ag/TiO2 composite with an AgNP loading amount of 4 wt % and an average size of about 4–7 nm was prepared by photochemical deposition of AgNPs on the surface of NP-based mesoporous titania.21 Also, Ag/HMS with AgNP loading amounts of 10, 30, and 50 wt % and the size of particles ranged between 15 and 30 nm (Table S1) were synthesized by the in situ deposition/reduction with a mixture of ethanolamine and ethylenediamine, described in details in our previous work on the selective reduction of azines to benzyl hydrazones.22 For selected transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images, see also Supporting Information (Figure S1).
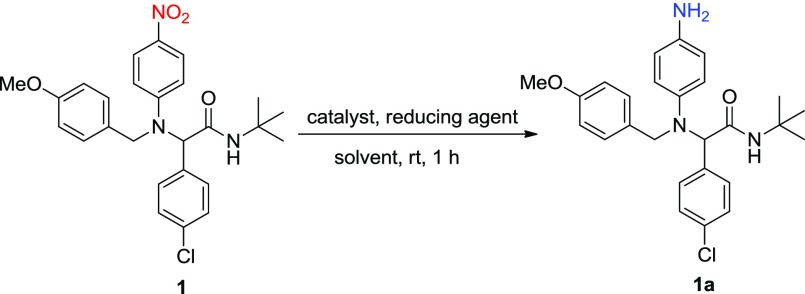
The reduction of nitro multifunctional derivative 1 toward the corresponding amine 1a was initially investigated in order to optimize the reaction conditions, using supported AgNPs as the catalyst, different hydrides, solvents, and catalysts (Table 1). Under the present conditions described below, the common reducing agents NaBH4, LiAlH4, and NaH were found to be inactive in the absence of catalyst (Table 1, entries 1–4); furthermore, degradation products (cleavage of the tert-amide of the anisole group) were observed by the 1H NMR of the crude mixtures in the two latter cases. In addition, the presence of only 1,1,3,3-tetramethyl disiloxane (TMDS) does not lead to any reduction process (Table 1, entry 5).
Table 1. Evaluation of Catalysts, Reducing Agents, and Solvents in the Catalytic Reduction of 1 into 1a.
| entry | catalysta | solventa | reducing agenta | time (h) | 1%/1a%b |
|---|---|---|---|---|---|
| 1 | MeOH | NaBH4 | 18 | 100/0 | |
| 2 | THF/MeOH | NaBH4 | 18 | 100/0 | |
| 3c | THF | LiAlH4 | 18 | 43/0 | |
| 4c | THF | NaH | 18 | 47/0 | |
| 5 | THF/MeOH | TMDS | 24 | 100/0 | |
| 6 | Au/TiO2 | THF/MeOH | NaBH4 | 1 | 0/>99 |
| 7 | Au/TiO2 | THF/MeOH | TMDS | 1 | 0/>99 |
| 8 | Au/TiO2 | MeOH | TMDS | 1 | 0/>99 |
| 9 | Ag/TiO2 | THF/MeOH | NaBH4 | 18 | 3/97 |
| 10 | Ag/TiO2 | MeOH | NaBH4 | 18 | 5/95 |
| 11 | Ag/HMS(10) | MeOH | NaBH4 | 18 | 0/>99 |
| 12 | Ag/HMS(10) | THF/MeOH | NaBH4 | 1 | 0/>99 |
| 13d | Ag/HMS(10) | THF/MeOH | NaBH4 | 1 | 60/40 |
| 14 | Ag/HMS(10) | THF/MeOH | TMDS | 1 | 100/0 |
| 15 | Ag/HMS(10) | THF/MeOH | Et3SiH | 18 | 100/0 |
| 16 | Ag/HMS(30) | THF/MeOH | NaBH4 | 1 | 0/>99 |
| 17 | Ag/HMS(50) | THF/MeOH | NaBH4 | 1 | 5/95 |
| 18 | HMS | THF/MeOH | NaBH4 | 1 | 100/0 |
| 19e | AgNO3 | THF/MeOH | NaBH4 | 1 | 2/98 |
| 20e | AgOTf | THF/MeOH | NaBH4 | 1 | 7/93 |
Conditions: 20 mg of the Au/TiO2 or 10 mg of the Ag/TiO2 (ca. 0.8 mol %) or 3 mg of the Ag/HMS (ca. 1.4, 4, and 7 mol %), 0.2 mmol of 1, 0.8 mmol of the hydrosilanes or 0.4 mmol of the sodium borohydride, 1 mL of solvent mixture, at rt.
Relative yields of 1 and 1a at appropriate time measured by 1H NMR of the crude reduction mixture.
Unidentified products missing either the tert-amide or the anisole group from its structure as determined by 1H NMR in some cases.
Equimolar amount of the reducing agent was used.
All the salts were used in 20 mmol %.
To our delight, NaBH4 and TMDS in the presence of Au/TiO2, using an equimolar mixture of solvents THF/MeOH or only MeOH, provided an efficient reduction, Table 1, entries 6–8). In addition, Ag NPs (Ag/TiO2) were found to catalyze the quantitative formation of the corresponding amine 1a using NaBH4 as the reducing agent (Table 1, entries 9 and 10). However, for better solubility of the starting material, we continue our further screening tests with the equimolar mixture of THF/MeOH. To compare the catalytic activity of the present synthesized Ag/HMS catalysts, we studied the reduction of 1, with Ag/HMS (x), (x = 10, 30, and 50%), catalysts in the presence of NaBH4 and the equimolar mixture of methanol/THF as solvent. All catalysts found to lead the transfer hydrogenation process to completion in a fast and clean manner, in high isolated yield >95%, without the requirement of any chromatographic purification of the product 1a (Table 1, entries 11–17). The Ag/HMS (10) and the Ag/HMS (30) catalysts, with an average of AgNP size below 20 nm, showed the best catalytic activity in terms of the conversion and selectivity of 1a in the presence of two equivalents of NaBH4 (Table 1, entries 12 and 16). This result supports a high relationship between the silver loading amount and the Ag particle size with the reaction selectivity toward 1. In comparison, yields dramatically decreased when an equimolar amount of NaBH4 was used (Table 1, entry 13); however in the absence of AgNPs (HMS) no conversion of 1 to 1a was observed (Table 1, entry 18). On the other hand, in the presence of hydrosilanes TMDS and Et3SiH, no reactions occurred (Table 1, entries 14 and 15). Finally, the presence of AgNO3 and AgOTf salts leads to the significant formation of 1a in 98 and 93% isolated yields, although 20 mol % was used in each case (Table 1, entries 19 and 20). These findings suggest that AgNPs with the size of <15 nm were found to catalyze the reduction process in the presence of NaBH4, within short reaction time and under mild conditions, results that are in good agreement with previous studies.21,22 On the basis of these findings, herein we attempt to combine—for the first time—the application of this simple catalytic system in the highly diverse and complex environment of the MCR chemical space, without the need of a protecting group, as well as into the facile one-pot synthesis of substituted 3,4-dihydroquinoxalinones, molecules with significant biological activity.
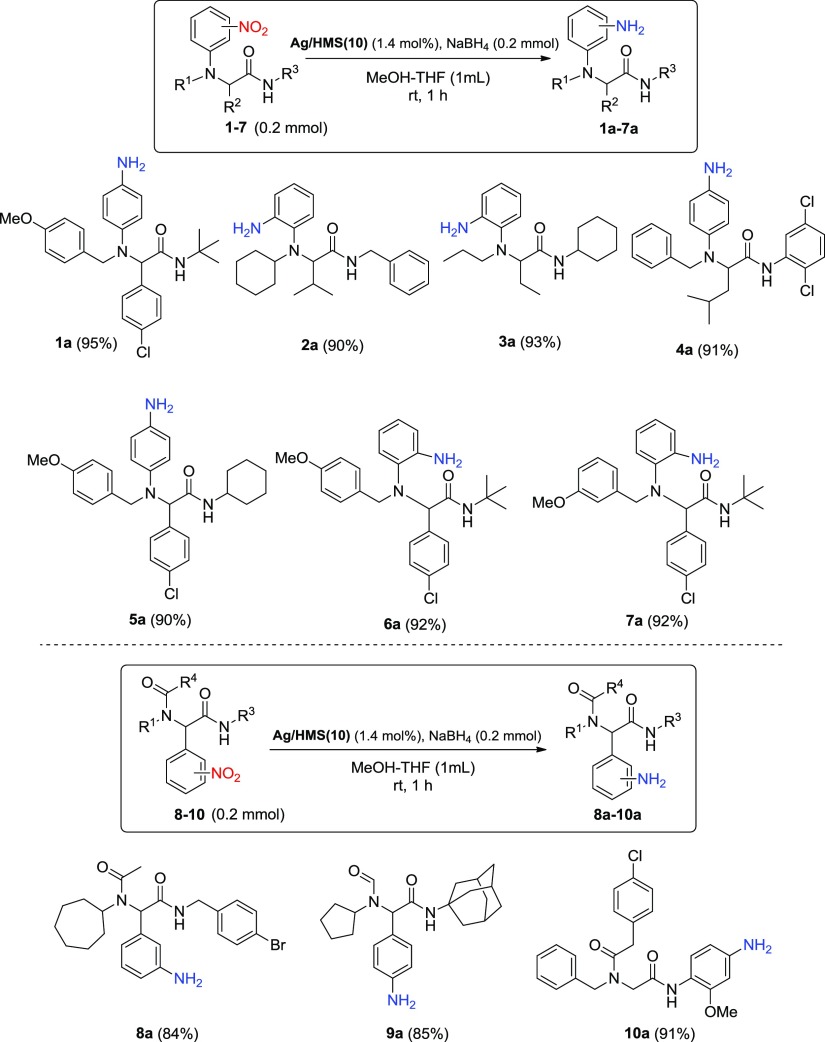
In order to determine if the present examined catalytic reduction processes can also be applicable to a variety of multifunctional nitro compounds, different starting materials were synthesized 1–7 and 8–10 and tested under the above two catalytic system, the Ag/HMS(10)–NaBH4 (Scheme 2). To our surprise, the corresponding amine derivatives 1a–10a were formed as the only product, in high conversions (92–98%, based on nitro consumption, results not shown) as measured by 1H NMR from the crude reduction mixture and isolated yields in 84–95%, as shown in Scheme 2 (values in parentheses corresponds to the isolated yields under the Ag/HMS(10)–NaBH4 catalytic system). Interestingly, not only amide bonds but also other easily reducible moieties as chloro, aldehyde, or methyl ketone under the present conditions remained intact. These results support unambiguously the high catalytic activity of the used catalytic system Ag/HMS(10)–NaBH4 toward the chemoselective reduction of such multifunctional nitro derivatives to the corresponding amines.
Scheme 2. Ag/HMS(10)–NaBH4 Catalyzed Chemoselective Reduction of the Multifunctional Nitro Compounds 1–10 Synthesized by the Ugi-Smiles and Ugi-4C Reactions.
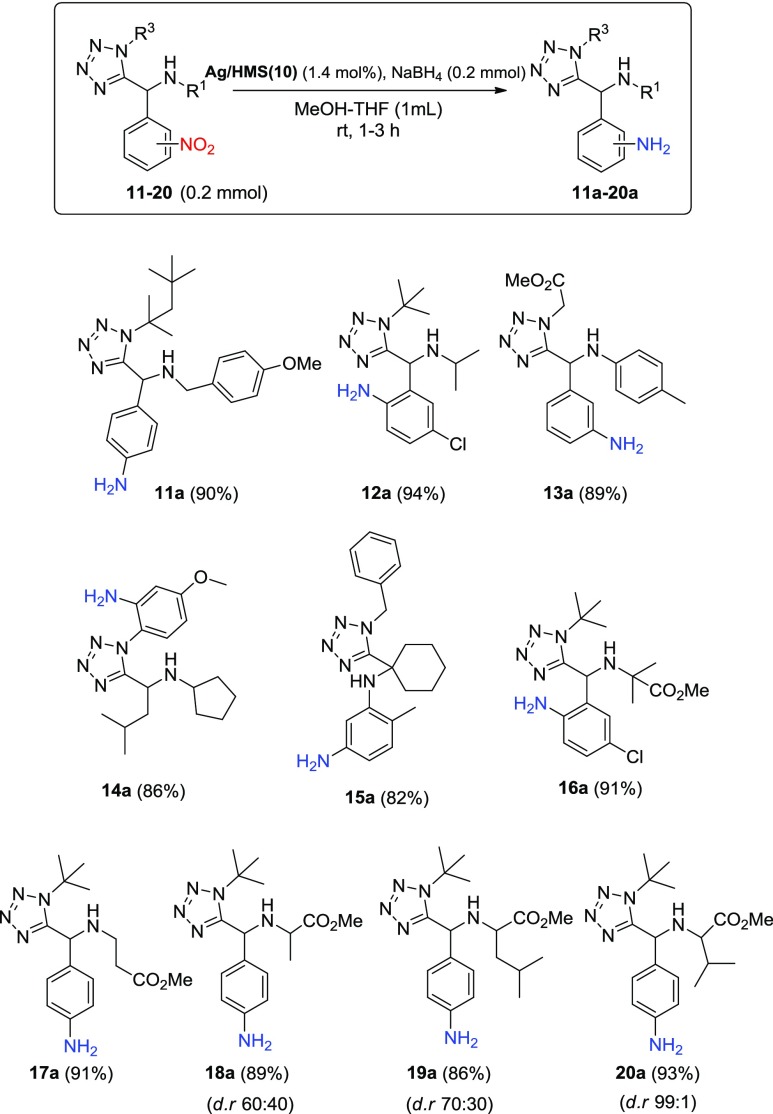
On the basis of this outcome, the present research was extended to more complicated multifunctional nitro derivatives, such as substituted tetrazoles (11–20), bearing also the corresponding methylesters of the amino acids β-alanine, alanine, leucine, and valine for example compounds 16–20 (Scheme 3). Remarkably, the reduction processes using the Ag/HMS(10)–NaBH4 do not affect either the chemical functionality of the tetrazole ring or the chloro and methyl ester groups, giving the corresponding multifunctional amines (11a–20a) as the only products in high isolated yields (86–94%) and selectivity (>99%), after short reaction time (1–3 h). The measured diastereoselectivity (dr) of the amines 18a–20a corresponds to the initial ratio of the corresponding starting nitro compounds 18–20, as observed by 1H NMR (see Supporting Information). These results support further the high activity and chemoselectivity of the present catalytic system toward the nitro group reduction of a series of multifunctional biological active molecules, a facile and useful procedure plausibly applicable for a multi-gram-scale synthesis.
Scheme 3. Ag/HMS(10)–NaBH4-Catalyzed Chemoselective Reduction of the Nitro Multifunctional Tetrazoles 11–15 and Amino Acid-Functionalized Derivatives 16–20.
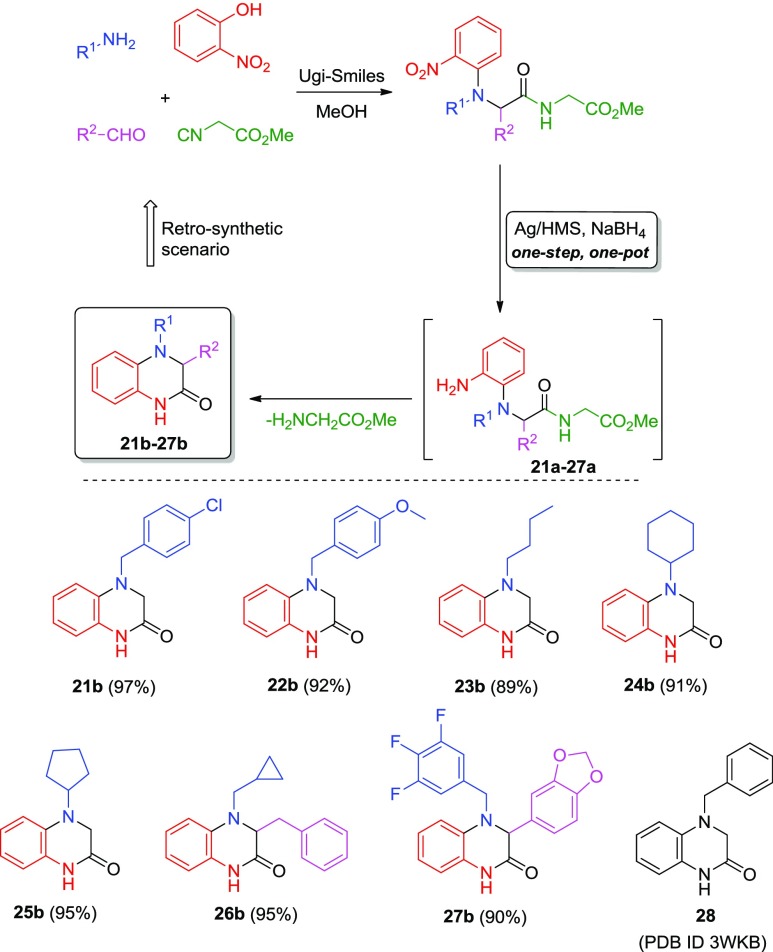
The synthetic value of the present reductive catalytic systems was verified by performing a one-pot C–N cyclization reaction of the in situ formed ortho-amino derivatives 21a–27a, forming a series of novel dihydroquinoxalin-2-ones (21b–27b, Scheme 4). Dihydroquinoxalino-2-ones are present in many bioactive compounds with anticancer36−38 and anti-inflammatory39 properties, as well as against neurological diseases.40 There are mainly three different synthetic approaches; the majority of the dihydroquinaxolinone derivatives is accessed through an o-halogen substituted nitro or aniline phenyl group and its subsequent reaction with amino acids37,39,41,42 or the reduction of quinoxaline derivatives through a transition metal-catalyzed asymmetric hydrogenation43−45 (Scheme 1). The aforementioned processes involve, on average, more than three sequential steps, protecting groups, Lewis acids and in many cases expensive starting materials. In addition to these two methods, few examples have been described in the literature with different approaches for example reactions of o-phenylenodiamine (oPDM) toward dihydroquinoxaline derivatives.35,36 Herein, a mild and efficient retrosynthetic scheme is proposed based on the above heterogeneous catalytic systems including the one-pot ring closing pathway of the in situ formed amine derivative (Scheme 4).
Scheme 4. One-Pot Synthesis of Dihydroquinoxalin-2-ones (21b–27b) Using the Ag/HMS Heterogeneous Transfer Hydrogenation Catalytic Processes.
Thus, the Ag/HMS(10)–NaBH4-catalyzed transfer hydrogenation process of the ortho-nitro substituted multifunctional compounds performed toward to the corresponding amines (21a–27a) in 0.2 mmol reaction scale. In all cases, a direct formation of the corresponding cyclized products dihydroquinoxalino-2-one (21b–27b), through an intramolecular transamidation pathway (intramolecular C–N cyclization process), was observed by thin layer chromatography (TLC). It is worth noting that during the reaction pathway, a mixture mainly containing the cyclic and the starting amine was determined by 1H NMR of the crude reaction mixture (see Figure S2). It is also worth noting that this pathway was observed when the methyl carbamate group existed in the starting material structure (21–27), which is eliminated as glycine methyl ester (a good living group) under the intramolecular nucleophilic pathway. Finally, high isolated yields were measured in all cases (89–96%) within a reaction time ranged between 5 and 12 h, results that support the clean and mild efficacy of the later heterogeneous catalytic process.
The observed retrosynthetic scenario is based on the facile MCR synthesis of the starting nitro compound by an Ugi-Smiles reaction of the readily available o-nitrophenols, amines, aldehydes, and glycine methyl ester isocyanide in high yields (Scheme 4). The subsequently catalytic results shown in Scheme 4 indicated that the corresponding cyclized products (21b–27b) were formed in good to high isolated yields. This spontaneous in situ reduction-cyclization pathway occurred within short reaction time and under ambient conditions supports further the synthetic value of the present transfer hydrogenation reactions using Ag/HMS–NaBH4 as the heterogeneous catalytic systems. To the best of our knowledge, there is a study in the literature reported on the Ugi-Smiles adduct transformation to the quinoxaline derivatives under hydrogenation conditions with Pd/C (10 mol %) as the catalyst and acidic conditions using p-toluenesulfonic acid.46 In all cases, prolonged reaction times and multistep workup protocols were required until the final product isolation; compared to the present heterogeneous, fast, mild, and lab-scale transfer hydrogenation process is catalyzed by AgNPs.
On the basis of these results, the Ag/HMS–NaBH4 catalytic system was further tested for possible lab-scale production of dihydroquinoxalino-2-ones from the corresponding multicomponent amines. For this reason, 1 mmol of the nitro derivative 22 was reduced in the presence of Ag/HMS 10% wt (20 mg, 1.8 mol %) with 2 mmol of NaBH4 in 6 mL MeOH/THF = 1:1. After completion of the reduction (ca. 120 min based on TLC analysis), 50 mg of silica gel was added into the reaction mixture and left under stirring for appropriate time (monitored by TLC) to afford the corresponding cyclized product 22b. After that, the mixture was filtered and the filtrate was evaporated under vacuum to afford, after chromatographic purification (see Experimental Section), the corresponding dihydroquinoxalin-2(1H)-one derivative 22b in pure forms with 78% isolated yield based on the corresponding nitro amount. This result corresponds to a high turnover number of 43, as measured from the ratio of product 22b (mmol)/supported Ag/HMS (mmol).
Moreover, our synthesis gives an easy access to inhibitors of the soluble epoxide hydrolase (sEH), an important target for therapies against hypertension or inflammation; demonstrating this potential, derivatives 21b and 22b were designed and accordingly synthesized with our protocol based on the cocrystalized compound 28 (PDB ID 3WKB), which was identified as a potent fragment (IC50 = 61 μM, LE = 0.32) of the aforementioned enzyme inhibition (Figure 1).38 To our delight, this is the first example in the literature that describes the synthesis in the lab scale of such heterocyclic molecules based on the present gold- or silver-based heterogeneous catalytic conditions.
Figure 1.
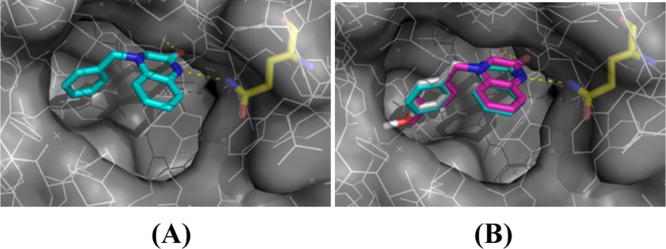
(A) Crystal structure of compound 28 (PDB ID 3WKB) bound to sEH which forms hydrogen bonds with Gln384 and a water molecule; (B) alignment of compound 28 with the newly synthesized compounds 21b and 22b.
Conclusions
In conclusion, we have demonstrated that silica-supported AgNPs can be applied to more complicated organic compounds, containing the highly diverse and complex environment of the MCR chemical space, and catalyze the selective reduction of a series of such multifunctional nitro-compounds into the corresponding amines employing NaBH4 as reducing agents. Among the tested catalytic systems, the Ag/HMS(10) with an average of AgNP size below 15 nm showed the best catalytic activity in terms of the conversion and selectivity of the amine formation. The catalyst was found to be highly chemoselective even for more complex molecules such as tetrazole and amino acid-substituted derivatives. The present Ag/HMS–NaBH4 catalytic system represents an expeditious approach toward the production of the heterocyclic dihydroquinoxalin-2-ones with good-to-high isolated yields, even to larger-scale, via the one-pot ring closing pathway based on the chemoselective reduction of the nitro group to the corresponding amine. The present heterogeneous catalytic procedure gives an easy access to synthesize inhibitors of the sEH for therapies against hypertension or inflammation.
Experimental Section
General
All the solvents and reagents were purchased from Sigma-Aldrich, Fluorochem, Acros and were used without further purification. Thin layer chromatography was performed on silica gel plates (0.20 mm thick, particle size 25 μm). Nuclear magnetic resonance spectra were recorded on Agilent 500 {1H NMR (500 MHz), 13C NMR (126 MHz)}. Chemical shifts for 1H NMR were reported as δ values and coupling constants were in hertz (Hz). The following abbreviations were used for spin multiplicity: s = singlet, br s = broad singlet, d = doublet, t = triplet, q = quartet, quin = quintet, dd = double of doublets, ddd = double doublet of doublets, m = multiplet. Mass spectra (HRMS) were determined on an electrospray ionization mass spectrometry (ESI-MS), by using a Thermo Fisher Scientific (Bremen, Germany) model LTQ Orbitrap Discovery MS, at a flow rate of 10 μL/min using a syringe pump. The infusion experiments were run using a standard ESI source operating in a positive ionization mode. Source operating conditions were a 3.7 kV spray voltage and a 300 °C heated capillary temperature.
Preparation of Catalysts
The HMS mesoporous silica was synthesized based on previously reported methods.47,48 Dodecylamine (DDA), which was used as the mesostructure directing agent (template), was dissolved in ethanol followed by addition of water in a final ratio of water/ethanol = 80:20, v/v. Tetraethyl orthosilicate (TEOS), used as the silica source, was then added slowly, and the mixture was stirred for 30 min at room temperature followed by hydrothermal aging in sealed PP bottles, in a shaker bath, at 65 °C for 24 h. The white product was filtered, washed with water, and dried at room temperature and at 90 °C for 3 h. The reaction mole stoichiometry was 1TEOS/0.23DDA/9.0EtOH/95H2O. The organic template was removed from the dried as-synthesized samples by calcination at 600 °C for 4 h in air.
The metallic silver (Ag0)-supported catalysts were prepared by the method using amines as reductants. The precursor silver compound was as-synthesized silver oxalate (from AgNO3 and oxalic acid), which was solubilized in aqueous solution of ethylenediamine (65 vol %). The amount of silver oxalate was adjusted so that the final silver loading of the catalysts to be 10, 30, or 50 wt % Ag/HMS. Aqueous solution of ethanolamine was then added to the ethylene diamine solution, followed by addition of HMS silica under continuous stirring at room temperature. The molar ratio of ethylenediamine and ethanolamine to Ag was equal to 3 and 0.5, respectively. The suspension formed was further left under stirring at room temperature for 1 h, after which the temperature was gradually raised up to 120 °C under vacuum in order to induce the reduction of silver (occurred at about 70–80 °C) and evaporate water and most of the amines. The collected paste was dried at 100 °C in air and was then calcined at 350 °C for 3 h in order to remove the residual organic compounds.
Physicochemical Characterization
The Ag content of the catalysts was determined by inductive coupled plasma–atomic emission spectroscopy using a Plasma 400 (PerkinElmer) spectrometer, equipped with a Cetac6000AT+ ultrasonic nebulizer. Nitrogen adsorption/desorption experiments at −196 °C were performed using an automatic volumetric sorption analyzer (Autosorb-1MP, Quantachrome). Powder X-ray diffraction (XRD) experiments were conducted on a Shimadzu XRD 7000 diffractometer using a Cu Kα X-ray radiation operating at 45 kV and 100 mA. TEM experiments were carried out in a JEOL 2011 high-resolution transmission electron microscope operating at 200 kV, with a point resolution of 0.23 nm and Cs = 1.0 mm. SEM experiments were performed on a JEOL 6300 microscope.22
Synthetic Procedure of the Nitro-Substituted MCR Compounds via the Ugi-Smiles Reaction
A solution of the corresponding aldehyde (5.0 mmol), nitro-substituted phenol (5.0 mmol), and amine (5.0 mmol) in methanol (5 mL) was stirred at room temperature for 15 min. Subsequently, isocyanide (5.0 mmol) was added and the reaction was stirred at room temperature for approximately 20 h. After reaction completion, monitored by TLC, the reaction mixture was concentrated in vacuo and the residue was purified by column chromatography using silica and eluent solvent, a mixture of hexane/ethyl acetate in a ratio from 5:1 to 1:3, affording the desired compounds 1–7 and 21–27 in good-to-high yields.46
Synthetic Procedure of the Nitro-Substituted MCR Compounds via the Ugi-Tetrazole (UT-4CR) Reaction
A solution of the corresponding aldehyde or ketone (5.0 mmol) and amine or amino acid methylester (5.0 mmol) in methanol (5 mL) was stirred at room temperature for 15 min. Subsequently, isocyanide (5.0 mmol) and TMSN3 (5.0 mmol, 665 μL) were added and the reaction mixture was stirred at room temperature for approximately 20 h. After reaction completion, monitored by TLC, the reaction mixture was concentrated in vacuo, and the residue was purified by column chromatography using silica and eluent solvent, a mixture of hexane/ethyl acetate in a ratio from 5:1 to 1:5, affording the desired compounds 11–20 in high yields.49
Synthetic Procedure of the Nitro-Substituted MCR Compounds via the Ugi-Four Component (U-4CR) Reaction
A solution of the corresponding aldehyde (5.0 mmol), carboxylic acid (5.0 mmol), and amine (5.0 mmol) in methanol (5 mL) was stirred at room temperature for 15 min. Subsequently, isocyanide (5.0 mmol) was added and the reaction was stirred at room temperature for approximately 20 h. After reaction completion, monitored by TLC, the reaction mixture was concentrated in vacuo and the residue was purified by column chromatography using silica and eluent solvent, a mixture of hexane/ethyl acetate in a ratio from 5:1 to 1:2, affording the desired compounds 8–10 in good-to-high yields.46
General Catalytic Reduction of the Nitro-Substituted Derivatives to the Corresponding Amines 1a–27a
To a sealed tube containing the corresponding nitro-substituted compounds (0.2 mmol) in 0.5 mL of methanol and 0.5 mL of THF, NaBH4 (0.4 mmol) and Ag/HMS, 10 % wt (1.4 mol %, 3 mg), were added. The reaction mixture was stirred at room temperature for the appropriate time. After reaction completion (monitored by TLC), the slurry was filtered through a short pad of Celite and silica gel to withhold the catalyst using MeOH (∼10 mL) as an eluent. The solvent was then evaporated under vacuum, and the residue was separated by column chromatography using silica gel and the mixture solvent hexane/EtOAc from 10:1 to 1:1 as the eluent to give the corresponding amines 1a–27a in pure form. It is worth noting that the amines in most cases were unstable during the chromatographic purification procedure; for this reason, neutralized silica (with the addition of a few drops of triethylamine in the eluent solvent mixture) was used for the column chromatography, as well as pretreated CDCl3 with K2CO3 was used to accomplished the NMR spectra.
General Catalytic Procedure for Synthesis of the Hydroquinoxalin-2(1H)-one Derivatives 21b–27b
To a sealed tube containing the corresponding nitro compounds (0.2 mmol) in 0.5 mL of methanol and 0.5 mL of THF, NaBH4 (0.4 mmol) and Ag/HMS, 10% wt (1.4 mol %, 3 mg), were added. The reaction mixture was stirred at room temperature for the appropriate time, and it was monitored by TLC. After that, the slurry was filtered to remove the catalyst and the residue was washed with methanol (∼5 mL). The filtrate was evaporated under vacuum to afford, after chromatographic purification as described above with hexane/ethyl acetate from 30:1 to 2:1 as the eluent, the corresponding dihydroquinoxalin-2(1H)-one derivatives 21b–27b in pure forms.
Acknowledgments
The Empeirikeion Foundation for financial support is acknowledged (I.N.L.). The program of State Scholarships Foundation (I.K.Y.) for graduate studies is acknowledged (I.D.). The work was financially supported (A.D.) by the NIH (1R01GM097082-01) and by Innovative Medicines Initiative (grant agreement no. 115489). This project has also received funding from the European Union’s Horizon 2020 research and innovation program under MSC ITN “Accelerated Early stage drug dIScovery” (AEGIS), grant agreement no. 675555. We thank Prof. G. E. Kostakis (Sussex University, UK) for valuable scientific comments. We thank Dr. C. Gabriel (AUTh, Department of Chemical Engineering) for performed the HRMS experiments.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02749.
Physicochemical characteristics of HMS mesoporous silica and 10, 30, and 50% w/w Ag/HMS; TEM and SEM images; and copies of 1H, 13C, and NOESY-1D NMR spectra of the products (PDF)
Author Contributions
D.I. and T.Z.-T. contributed equally. All authors have given approval to the final version of the manuscript; I.N.L. conceived and designed the experiments.
The authors declare no competing financial interest.
Supplementary Material
References
- Orlandi M.; Brenna D.; Harms R.; Jost S.; Benaglia M. Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org. Process Res. Dev. 2016, 22, 430–445. 10.1021/acs.oprd.6b00205. [DOI] [Google Scholar]
- Blaser H.-U.; Steiner H.; Studer M. Selective catalytic hydrogenation of functionalized nitroarenes: An update. ChemCatChem 2009, 1, 210–221. 10.1002/cctc.200900129. [DOI] [Google Scholar]
- a Takale B. S.; Bao M.; Yamamoto Y. Gold nanoparticle (AuNPs) and gold nanopore (AuNPore) catalysts in organic synthesis. Org. Biomol. Chem. 2014, 12, 2005–2027. 10.1039/c3ob42207k. [DOI] [PubMed] [Google Scholar]; b Liu X.; He L.; Liu Y.-M.; Cao Y. Supported gold catalysis: From small molecule activation to green chemical synthesis. Acc. Chem. Res. 2014, 47, 793–804. 10.1021/ar400165j. [DOI] [PubMed] [Google Scholar]; c Mikami Y.; Dhakshinamoorthy A.; Alvaro M.; García H. Catalytic activity of unsupported gold nanoparticles. Catal. Sci. Technol. 2013, 3, 58–69. 10.1039/c2cy20068f. [DOI] [Google Scholar]; d Corma A.; Garcia H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. 10.1039/b707314n. [DOI] [PubMed] [Google Scholar]
- a Mitsudome T.; Kaneda K. Gold nanoparticle catalysts for selective hydrogenations. Green Chem. 2013, 15, 2636. 10.1039/c3gc41360h. [DOI] [Google Scholar]; b Stratakis M.; Garcia H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. 10.1021/cr3000785. [DOI] [PubMed] [Google Scholar]; c Zhang Y.; Cui X.; Shi F.; Deng Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2012, 112, 2467–2505. 10.1021/cr200260m. [DOI] [PubMed] [Google Scholar]
- Abbiati G.; Rossi E. Silver and gold-catalyzed multicomponent reactions. Beilstein J. Org. Chem. 2014, 10, 481–513. 10.3762/bjoc.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dong X.-Y.; Gao Z.-W.; Yang K.-F.; Zhang W.-Q.; Xu L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. 10.1039/c5cy00285k. [DOI] [Google Scholar]; b Bhosale M. A.; Bhanage B. M. Silver Nanoparticles: Synthesis, Characterization and their Application as a Sustainable Catalyst for Organic Transformations. Curr. Org. Chem. 2015, 19, 708–727. 10.2174/1385272819666150207001154. [DOI] [Google Scholar]; c El-Nour K. M. M. A.; Eftaiha A.; Al-Warthan A.; Ammar R. A. A. Synthesis and applications of silver nanoparticles. Arabian J. Chem. 2010, 3, 135–140. [Google Scholar]; d Díez-González S.; Nolan S. P. Copper, Silver, and Gold Complexes in Hydrosilylation Reactions. Acc. Chem. Res. 2008, 41, 349–358. 10.1021/ar7001655. [DOI] [PubMed] [Google Scholar]
- a Jagadeesh R. V.; Wienhöfer G.; Westerhaus F. A.; Surkus A.-E.; Pohl M.-M.; Junge H.; Junge K.; Beller M. Efficient and highly selective iron-catalyzed reduction of Nitroarenes. Chem. Commun. 2011, 47, 10972–10974. 10.1039/c1cc13728j. [DOI] [PubMed] [Google Scholar]; b Jagadeesh R. V.; Surkus A.-E.; Junge H.; Pohl M.-M.; Radnik J.; Rabeah J.; Huan H.; Schunemann V.; Bruckner A.; Beller M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076. 10.1126/science.1242005. [DOI] [PubMed] [Google Scholar]; c Jagadeesh R. V.; Stemmler T.; Surkus A.-E.; Junge H.; Junge K.; Beller M. Hydrogenation using iron oxide-based nanocatalysts for the synthesis of amines. Nat. Protoc. 2015, 10, 548–557. 10.1038/nprot.2015.025. [DOI] [PubMed] [Google Scholar]; d Junge K.; Wendt B.; Shaikh N.; Beller M. Iron-catalyzed selective reduction of nitroarenes to anilines using organosilanes. Chem. Commun. 2009, 1769–1771. 10.1039/b924228g. [DOI] [PubMed] [Google Scholar]; e Wienhöfer G.; Baseda-Krüger M.; Ziebart C.; Westerhaus F. A.; Baumann W.; Jackstell R.; Junge K.; Beller M. Hydrogenation of nitroarenes using defined iron-phosphine catalysts. Chem. Commun. 2013, 49, 9089–9091. 10.1039/c3cc42983k. [DOI] [PubMed] [Google Scholar]
- a Jagadeesh R. V.; Junge H.; Beller M. Green synthesis of nitriles using non-noble metal oxides-based nanocatalysts. Nat. Commun. 2014, 5, 4123. 10.1038/ncomms5123. [DOI] [PubMed] [Google Scholar]; b Jagadeesh R. V.; Stemmler T.; Surkus A.-E.; Bauer M.; Pohl M.-M.; Radnik J.; Junge K.; Junge H.; Brückner A.; Beller M. Cobalt-based nanocatalysts for green oxidation and hydrogenation processes. Nat. Protoc. 2015, 10, 916–926. 10.1038/nprot.2015.049. [DOI] [PubMed] [Google Scholar]; c Westerhaus F. A.; Jagadeesh R. V.; Wienhöfer G.; Pohl M.-M.; Radnik J.; Surkus A.-E.; Rabeah J.; Junge K.; Junge H.; Nielsen M.; Brückner A.; Beller M. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. 10.1038/nchem.1645. [DOI] [PubMed] [Google Scholar]
- Recent selected reviews:; a Renaud J.-L.; Gaillard S. Recent advances in iron- and cobalt-complex-catalyzed tandem/consecutive processes involving hydrogenation. Synthesis 2016, 48, 3659–3683. 10.1055/s-0035-1562791. [DOI] [Google Scholar]; b Pellissier H.; Clavier H. Enantioselective cobalt-catalyzed transformations. Chem. Rev. 2014, 114, 2775–2823. 10.1021/cr4004055. [DOI] [PubMed] [Google Scholar]; c Filonenko G. A.; van Putten R.; Hensen E. J. M.; Pidko E. A. Catalytic (de)hydrogenation promoted by non-precious metals - Co, Fe and Mn: recent advances in an emerging field. Chem. Soc. Rev. 2018, 47, 1459–1483. 10.1039/c7cs00334j. [DOI] [PubMed] [Google Scholar]
- Trost B. The atom economy--a search for synthetic efficiency. Science 1991, 254, 1471–1477. 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- Kolesnikov P. N.; Yagafarov N. Z.; Usanov D. L.; Maleev V. I.; Chusov D. Ruthenium-catalyzed reductive amination without an external hydrogen source. Org. Lett. 2015, 17, 173–175. 10.1021/ol503595m. [DOI] [PubMed] [Google Scholar]
- Dömling A.; Wang W.; Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- Slobbe P.; Ruijter E.; Orru R. V. A. Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm 2012, 3, 1189. 10.1039/c2md20089a. [DOI] [Google Scholar]
- Zarganes-Tzitzikas T.; Chandgude A. L.; Dömling A. Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 2015, 15, 981–996. 10.1002/tcr.201500201. [DOI] [PubMed] [Google Scholar]
- Cioc R. C.; Ruijter E.; Orru R. V. A. Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. 10.1039/c4gc00013g. [DOI] [Google Scholar]
- Touré B. B.; Hall D. G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486. 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]
- Gkizis P. L.; Stratakis M.; Lykakis I. N. Catalytic activation of hydrazine hydrate by gold nanoparticles: Chemoselective reduction of nitro compounds into amines. Catal. Commun. 2013, 36, 48–51. 10.1016/j.catcom.2013.02.024. [DOI] [Google Scholar]
- Fountoulaki S.; Daikopoulou V.; Gkizis P. L.; Tamiolakis I.; Armatas G. S.; Lykakis I. N. Mechanistic Studies of the Reduction of Nitroarenes by NaBH4 or Hydrosilanes Catalyzed by Supported Gold Nanoparticles. ACS Catal. 2014, 4, 3504–3511. 10.1021/cs500379u. [DOI] [Google Scholar]
- Fountoulaki S.; Gkizis P. L.; Symeonidis T. S.; Kaminioti E.; Karina A.; Tamiolakis I.; Armatas G. S.; Lykakis I. N. Titania-supported gold nanoparticles catalyze the selective oxidation of amines into nitroso compounds in the presence of hydrogen peroxide. Adv. Synth. Catal. 2016, 358, 1500–1508. 10.1002/adsc.201500957. [DOI] [Google Scholar]
- Andreou D.; Iordanidou D.; Tamiolakis I.; Armatas G.; Lykakis I. Reduction of Nitroarenes into Aryl Amines and N-Aryl hydroxylamines via Activation of NaBH4 and Ammonia-Borane Complexes by Ag/TiO2 Catalyst. Nanomaterials 2016, 6, 54. 10.3390/nano6030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charistoudi E.; Kallitsakis M. G.; Charisteidis I.; Triantafyllidis K. S.; Lykakis I. N. Selective Reduction of Azines to Benzyl Hydrazones with Sodium Borohydride Catalyzed by Mesoporous Silica-Supported Silver Nanoparticles: A Catalytic Route towards Pyrazole Synthesis. Adv. Synth. Catal. 2017, 359, 2949–2960. 10.1002/adsc.201700442. [DOI] [Google Scholar]
- Dömling A.; Ugi I. Multicomponent reactions with isocyanides. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. . [DOI] [PubMed] [Google Scholar]
- El Kaïm L.; Grimaud L.; Oble J. Phenol Ugi-Smiles Systems: Strategies for the Multicomponent N-Arylation of Primary Amines with Isocyanides, Aldehydes, and Phenols. Angew. Chem., Int. Ed. 2005, 44, 7961–7964. 10.1002/anie.200502636. [DOI] [PubMed] [Google Scholar]
- El Kaim L.; Grimaud L. Beyond the Ugi reaction: less conventional interactions between isocyanides and iminium species. Tetrahedron 2009, 65, 2153–2171. 10.1016/j.tet.2008.12.002. [DOI] [Google Scholar]
- Neochoritis C. G.; Dömling A. Towards a facile and convenient synthesis of highly functionalized indole derivatives based on Multi-Component Reactions. Org. Biomol. Chem. 2014, 12, 1649–1651. 10.1039/c4ob00166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugi I. Neuere Methoden der präparativen organischen Chemie IV Mit Sekundär-Reaktionen gekoppelte α-Additionen von Immonium-Ionen und Anionen an Isonitrile. Angew. Chem. 1962, 74, 9–22. 10.1002/ange.19620740103. [DOI] [Google Scholar]
- Gunawan S.; Hulme C. Bifunctional building blocks in the Ugi-azide condensation reaction: a general strategy toward exploration of new molecular diversity. Org. Biomol. Chem. 2013, 11, 6036–6046. 10.1039/c3ob40900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas-Galindo L.; Islas-Jácome A.; Alvarez-Rodríguez N.; El Kaim L.; Gámez-Montaño R. Synthesis of 2-Tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a one-pot Ugi-Azide/Pictet–Spengler process. Synthesis 2013, 46, 49–56. 10.1055/s-0033-1340051. [DOI] [Google Scholar]
- Zarganes-Tzitzikas T.; Patil P.; Khoury K.; Herdtweck E.; Dömling A. Concise Synthesis of Tetrazole-Ketopiperazines by Two Consecutive Ugi Reactions. Eur. J. Org. Chem. 2015, 51–55. 10.1002/ejoc.201403401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; Khoury K.; Herdtweck E.; Dömling A. MCR synthesis of a tetracyclic tetrazole scaffold. Bioorg. Med. Chem. 2015, 23, 2699–2715. 10.1016/j.bmc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Patil P.; Kurpiewska K.; Kalinowska-Tluscik J.; Dömling A. Two Cycles with One Catch: Hydrazine in Ugi 4-CR and Its Postcyclizations. ACS Comb. Sci. 2017, 19, 193–198. 10.1021/acscombsci.7b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; Zhang J.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Hydrazine in the Ugi tetrazole reaction. Synthesis 2016, 48, 1122–1130. 10.1055/s-0035-1561353. [DOI] [Google Scholar]
- Huang Y.; Khoury K.; Chanas T.; Dömling A. Multicomponent synthesis of diverse 1,4-benzodiazepine scaffolds. Org. Lett. 2012, 14, 5916–5919. 10.1021/ol302837h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C.; Peng J.; Morton G.; Salvino J. M.; Herpin T.; Labaudiniere R. Novel safety-catch linker and its application with a Ugi/De-BOC/Cyclization (UDC) strategy to access carboxylic acids, 1,4-benzodiazepines, diketopiperazines, ketopiperazines and dihydroquinoxalinones. Tetrahedron Lett. 1998, 39, 7227–7230. 10.1016/s0040-4039(98)01593-7. [DOI] [Google Scholar]
- Galal S. A.; Abdelsamie A. S.; Soliman S. M.; Mortier J.; Wolber G.; Ali M. M.; Tokuda H.; Suzuki N.; Lida A.; Ramadan R. A.; El Diwani H. I. Design, synthesis and structure-activity relationship of novel quinoxaline derivatives as cancer chemopreventive agent by inhibition of tyrosine kinase receptor. Eur. J. Med. Chem. 2013, 69, 115–124. 10.1016/j.ejmech.2013.07.049. [DOI] [PubMed] [Google Scholar]
- Tanimori S.; Nishimura T.; Kirihata M. Synthesis of novel quinoxaline derivatives and its cytotoxic activities. Bioorg. Med. Chem. Lett. 2009, 19, 4119–4121. 10.1016/j.bmcl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Amano Y.; Yamaguchi T.; Tanabe E. Structural insights into binding of inhibitors to soluble epoxide hydrolase gained by fragment screening and X-ray crystallography. Bioorg. Med. Chem. 2014, 22, 2427–2434. 10.1016/j.bmc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhao L.; Xu B.; Yang L.; Zhang J.; Zhang H.; Zhou J. Design, synthesis and biological evaluation of dihydroquinoxalinone derivatives as BRD4 inhibitors. Bioorg. Chem. 2016, 68, 236–244. 10.1016/j.bioorg.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Gitto R.; Barreca M. L.; De Luca L.; Chimirri A. New Trends in the development of AMPA receptor antagonists. Expert Opin. Ther. Pat. 2004, 14, 1199–1213. 10.1517/13543776.14.8.1199. [DOI] [Google Scholar]
- Kanyiva K. S.; Horiuchi M.; Shibata T. Metal-Free N-H/C-H Coupling for Efficient Asymmetric Synthesis of Chiral Dihydroquinoxalinones from Readily Available α-Amino Acids. Eur. J. Org. Chem. 2018, 1067–1070. 10.1002/ejoc.201800012. [DOI] [Google Scholar]
- Neagoie C.; Krchňák V. Piperazine amide linker for cyclative cleavage from solid support: Traceless synthesis of dihydroquinoxalin-2-ones. ACS Comb. Sci. 2012, 14, 399–402. 10.1021/co300023b. [DOI] [PubMed] [Google Scholar]
- Xue Z.-Y.; Jiang Y.; Peng X.-Z.; Yuan W.-C.; Zhang X.-M. The First General, Highly Enantioselective Lewis Base Organo- catalyzed Hydrosilylation of Benzoxazinones and Quinoxalinones. Adv. Synth. Catal. 2010, 352, 2132–2136. 10.1002/adsc.201000274. [DOI] [Google Scholar]
- Núñez-Rico J. L.; Vidal-Ferran A. [Ir(P–OP)]-Catalyzed Asymmetric Hydrogenation of Diversely Substituted C=N-Containing Heterocycles. Org. Lett. 2013, 15, 2066–2069. 10.1021/ol400854a. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Qiu R.; Xue X.; Pan Y.; Xu C.; Li H.; Xu L. Versatile (Pentamethylcyclopentadienyl)rhodium-2,2′-Bipyridine (Cp*Rh-bpy) Catalyst for Transfer Hydrogenation of N-Heterocycles in Water. Adv. Synth. Catal. 2015, 357, 3529–3537. 10.1002/adsc.201500491. [DOI] [Google Scholar]
- El Kaïm L.; Oble J.; Gizzi M.; Grimaud L. Ugi-Smiles access to quinoxaline derivatives. Heterocycles 2007, 73, 503–517. 10.3987/com-07-s(u)26. [DOI] [Google Scholar]
- a Karakoulia S. A.; Triantafyllidis K. S.; Lemonidou A. A. Preparation and characterization of vanadia catalysts supported on non-porous, microporous and mesoporous silicates for oxidative dehydrogenation of propane (ODP). Microporous Mesoporous Mater. 2008, 110, 157–166. 10.1016/j.micromeso.2007.10.027. [DOI] [Google Scholar]; b Pauly T. R.; Liu Y.; Pinnavaia T. J.; Billinge S. J. L.; Rieker T. P. Textural mesoporosity and the catalytic activity of mesoporous molecular sieves with wormhole framework structures. J. Am. Chem. Soc. 1999, 121, 8835–8842. 10.1021/ja991400t. [DOI] [Google Scholar]; c Pauly T. R.; Pinnavaia T. J. Pore size modification of mesoporous HMS molecular sieve silicas with wormhole framework structures. Chem. Mater. 2001, 13, 987–993. 10.1021/cm000762t. [DOI] [Google Scholar]
- Rouquerol F.; Rouquerol J.; Sing K.. Copyright, in Adsorption by Powders and Porous Solids; Elsevier: San Diego, 1999; p iv. [Google Scholar]
- Kroon E.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Cleavable β-Cyanoethyl Isocyanide in the Ugi Tetrazole Reaction. Org. Lett. 2016, 18, 4762–4765. 10.1021/acs.orglett.6b01826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.