Abstract
Currently, a large number of anti-tumor drug delivery systems have been widely used in cancer therapy. However, due to the molecular complexity and multidrug resistance of tumors, monotherapies remain suboptimal. Thus, this study aimed to develop a multifunctional theranostic nanoplatform for effective cancer therapy.
Methods: Folic acid-modified silver sulfide@mesoporous silica core-shell nanoparticle was first modified with desthiobiotin (db) on the surface, then doxorubicin (DOX) was loaded into pore. Avidin was employed as "gatekeeper" to prevent leakage of DOX via desthiobiotin-avidin interaction. Db-modified survivin antisense oligonucleotide (db-DNA) which could inhibit survivin expression was then grafted on avidin at the outer layer of nanoparticle. DOX release and db-DNA dissociation were simultaneously triggered by overexpressing biotin in cancer cells, then combining PTT from Ag2S QD to inhibit tumor growth.
Results: This nanoprobe had satisfactory stability and photothermal conversion efficiency up to 33.86% which was suitable for PTT. Due to the good targeting ability and fluorescent anti-bleaching, its signal still existed at the tumor site after tail vein injection of probe into HeLa tumor-bearing nude mice for 48 h. In vitro and in vivo antitumor experiments both demonstrated that drug, gene and photothermal synergistic therapy significantly enhanced antitumor efficacy with minimal systemic toxicity.
Conclusion: Our findings demonstrate that this novel nanoplatform for targeted image-guided treatment of tumor and tactfully integrated chemotherapy, photothermal therapy (PTT) and gene therapy might provide an insight for cancer theranostics.
Keywords: mesoporous silica, fluorescence imaging, photothermal therapy, gene therapy, drug delivery system
Introduction
Malignant tumor affects human health worldwide 1. Although traditional chemotherapy still occupies a dominant position in clinical treatment, but due to the side effects caused by nonspecific distribution of chemotherapeutic drugs, and a large number of tumors appear multidrug resistance, the drawbacks of a single treatment mode are becoming increasingly apparent 2. Therefore, multi-mode combination therapy is hopeful to conquer cancer in the near future.
In recent years, the development of nanobiotechnology has brought new ideas for cancer therapy. Fluorescence imaging, as one molecular imaging technology, has attracted much attention owing to its high sensitivity, high resolution and short acquisition time. Since it can detect the dynamic change of tumor-related molecule in real time, it has great application prospect in early diagnosis 3. Ag2S QD with emission in NIR-II region solves the interference of auto-fluorescence from tissue and blood during in vivo fluorescent imaging 4-6. In addition, Ag2S QD with 1.1 eV band gap also possesses ideal light absorption efficiency and high photothermal conversion efficiency, therefore, it could be used to achieve simultaneous fluorescence imaging and photothermal therapy (PTT) 7-10. PTT as a rapid and efficient method for tumor therapy has attracted more attention, the photothermal agent has been used to convert the luminous energy into heat to make tumor regional hyperthermia (>41 ºC), thereby killing tumor cell without damaging normal tissue through the difference of temperature tolerance between normal tissue and tumor tissue 11-13.However, because of the uneven heat distribution of PTT, deep tumor treatment is insufficient and tumor is easy to relapse when used alone. Previous studies have shown that PTT can also increase cell membrane permeability and enhance the toxicity of chemotherapy drug, or trigger the immune response to inhibit tumor metastasis and recurrence 14-17. Therefore, PTT combined with other therapy has become a promising method for efficient tumor ablation and minimally invasive treatment 18.
Many drug delivery systems have been established in the past years including liposome 19, polymer 20, inorganic nanoparticle 21 and protein nanocarrier 22. Among these nanocarriers, mesoporous silica nanoparticle (MSN) has emerged as robust vehicle for drug delivery due to its high surface area, adjustable pore size, stable framework and excellent biocompatibility 23,24. Zink and co-workers synthesized polymer-modified MSN for DOX delivery, achieved high-efficiency drug release at the tumor site in vivo 25. In addition, the core-shell composite particles were prepared by coating mesoporous silica onto Fe3O4 26, gold 27 or upconversion nanoparticle 28 cores, thus giving MSN more novel applications. Stimulus-responsive drug controlled release system is an intelligent transportation system which can respond to various external or internal stimuli, such as pH 29, redox agent 30, enzyme 31, biomolecule 32, light 33 and temperature 27. Easily modified MSN as an ideal stimulating response drug controlled-release platform, can be simultaneously assembled with stimulus-responsive moiety and targeting moiety. Biotin, also known as vitamin H, owing to the rapid propagation, vigorous metabolism and abundant mitochondria of tumor cell, the endogenous biotin content in tumor cell is significantly higher than that in normal tissue cell 34. Remarkably, biotin can bind avidin with high specificity, and biotin-avidin system has been widely applied in biomedical fields. As a derivative of biotin, desthiobiotin also can bind avidin specifically, while its binding force is lower than that of biotin (dissociation constant Kd=10-11 and 10-15 mol/L, respectively) 35.
Gene interference based on nucleic acid such as antisense oligonucleotide and small interfering RNA, has a broad application prospect in tumor therapy 21,36. Survivin, an inhibitor of apoptosis protein is highly expressed in tumor cell and associated with chemotherapy resistance. Studies have shown that survivin acts on the junctions of various apoptosis pathways, directly inhibits the terminal effector protein Caspase-3 and Caspase-7, and prevents the apoptosis of tumor cell 37. Anti-survivin therapy is an attractive cancer therapy strategy for its notable targeting, specificity and safety.
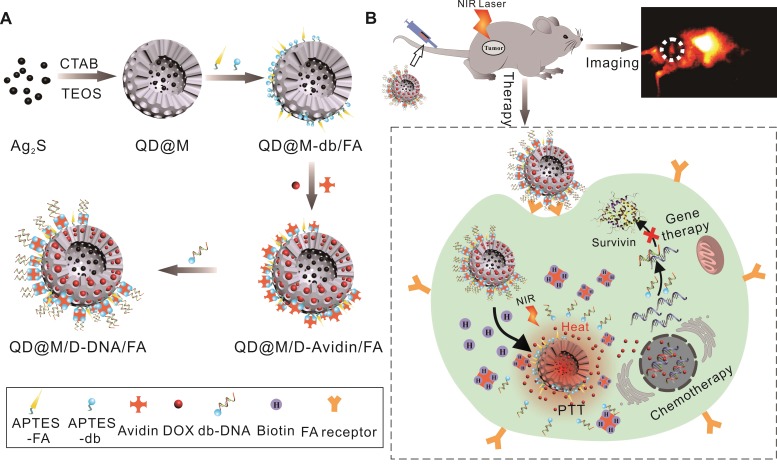
Hence, a novel multifunctional nanoplatform integrating fluorescence imaging/chemotherapy/ thermotherapy/gene therapy (QD@M/D-Avidin/ FA) was designed in this manuscript (Figure 1). Firstly, Ag2S QD was coated with mesoporous silica to prepare core-shell composite particle (QD@M), alternatively, folic acid (FA) and desthiobiotin (db) were both modified on its surface to obtain targeting nanoparticle QD@M-db/FA. Followed, classic anticancer drug, doxorubicin (DOX), was adsorbed into the mesoporous silica pore, and “gatekeeper” avidin with a size slightly larger than the pore diameter 38 was combined with desthiobiotin to block drug leakage. Since one avidin molecule has four binding sites, desthiobiotin-modified survivin antisense oligonucleotide (db-DNA) could be linked on the probe surface via the desthiobiotin-avidin interaction to get QD@M/D-DNA/FA. After the targeting probe was preferentially taken up by tumor cell through receptor-mediated endocytosis, DOX release and db-DNA dissociation were simultaneously triggered to induce apoptosis and inhibit tumor growth. Here, Ag2S QD was not only used to trace the transportation of nanoparticle in vivo, but also participated in PTT. Results indicated that this multifunctional nanoplatform had excellent biocompatibility and tumor targeting, and could effectively fight against cancer under the synergistic treatment of chemo-, photothermal and gene therapy. This novel multifunctional theranostic nanoplatform provides a new idea for high efficiency treatment of malignant tumor.
Figure 1.
Schematic illustration of the synthesis route of QD@M/D-DNA/FA (A) and the tumor targeted fluorescence imaging and combined chemotherapy, photothermal and gene therapy (B).
Methods
Synthesis of hydrophobic Ag2S QD
The synthesis of Ag2S QD was modified according to the previous literature 13. A given amount of 76.8 mg Ag (DDTC), 30 g ODE and 6 g DT were successively added into a four-necked flask (100 mL) under Ar protection. Then, water in the system was removed with vigorous stirring and heating to 90 ºC for 10 min. The reaction was heated to 150 ºC and quenched by n-hexane after maintaining the temperature for 10 min. The solution was cooled to room temperature, centrifuged and washed twice with acetone. The hydrophobic Ag2S QD was re-dispersed in chloroform.
Synthesis of mesoporous silica encapsulated Ag2S (QD@M)
0.2 g CTAB and 6 mg Ag2S were dissolved in 10 mL water and homogenized with ultrasound. The mixture was heated to 70 ºC and stirred rapidly to completely evaporate chloroform to obtain clear and transparent brown Ag2S/CTAB dispersion system. Then 90 mL water and 3 mL ammonia were added, gently equilibrated at 40 ºC for 2 h. Vigorous agitation was carried out followed by rapidly adding 400 µL TEOS and 5 mL ethyl acetate to maintain 1 min, reduced the stirring speed and gently stirred for 6 h. The resulting QD@M was purified by repeated centrifugation (10000 rpm, 10 min) to remove the unreacted reagent by water and ethanol. The QD@M was re-dispersed in 50 mL ethanol containing ammonium nitrate (10 mg/mL) and heated to 75 ºC for 6 h to remove CTAB template, the same purification methods were used as mentioned above.
Synthesis of QD@M-db/FA
In order to couple FA and db to the surface of QD@M, FA silane precursor and db silane precursor were prepared 28. 15 mg FA or 7.28 mg db, 9.9 mg EDC and 9.9 mg NHS were dissolved in 10 mL DMSO followed by adding 20 µL APTES. The system was stirred for 8 h at room temperature to produce amino-functionalized FA or db silane precursor. Both precursors were mixtures containing FA or db coupled APTES and unreacted APTES, respectively. Then 100 mg QD@M was dissolved in 10 mL DMSO and further stirred for 24 h after mixing with above two systems. The final FA and db co-modified nanoparticle (QD@M-db/FA) was centrifuged (12000 rpm, 10 min) and washed twice with DMSO followed by washing 3 times with water. For control experiment, only db-functionalized silane precursor was added to obtain QD@M-db nanoparticle.
DOX loading and db-DNA coupling
20 mg QD@M-db/FA and 2 mg DOX was added into 7 mL water. After stirring for 24 h, 1 mL avidin (1 mg/mL) was added and stirred for 2 h to cap the pore. The mixture was repeated centrifugation (12000 rpm, 10 min) with PBS (0.1 M, pH 7.4) to clean free DOX to obtain DOX-loaded nanoparticle (QD@M/ D-Avidin/FA). FITC-loaded nanoparticle, QD@M/ FITC-Avidin/FA was prepared by same protocol for flow cytometry, and DOX-loaded QD@M/D-Avidin without modifying FA was also prepared as control. 1 mg QD@M/D-Avidin/FA was added in 50 µL hydrofluoric acid to dissolve mesoporous silica, and pH was adjusted to neutrality to determine DOX amount by analyzing fluorescence intensity at 590 nm. Loading content (%)=(DOX weight in probe)/(probe weight)×100% and encapsulation efficiency (%)= (DOX weight in probe)/(DOX initial weight)×100%. Then db-DNA was re-dispersed in QD@M/D-Avidin/FA at a final concentration of 1 µM. The mixture was stirred for 2 h to obtain DNA-modified probe (QD@M/D-DNA/FA). Ag+ concentration was measured by graphite furnace atomic absorption method.
Photothermal effect and photothermal conversion efficiency
200 µL QD@M at different concentrations (0-12 mg/mL and 0-200 μg/mL) was irradiated with 808 nm laser (1.5 W/cm2 and 2 W/cm2) for 5 min at a distance of 5 cm, and same concentration (3 mg/mL) QD@M was irradiated at different laser intensities (0.5-2.5 W/cm2). The temperature changes were recorded by thermal imager. Each experiment was repeated three times. To evaluate photothermal stability of probe, 300 µL QD@M was irradiated with laser (2 W/cm2) for 10 min and then allowed to cool for 10 min. In photothermal stability test, the system was naturally cooled after laser was irradiated for 10 min to stabilize temperature, the temperature was recorded and same sample was repeated six times. The photothermal conversion efficiency (η) was determined by following equation 39:
| η= [hS(Tmax-Tsurr)-Qdis]/I(1-10 A808), |
I was irradiation power to be 800 mW, A808 was absorption of probe at 808 nm (to be 0.89), Tmax-Tmin was 41 ºC, τs and hS were calculated to be 193.4 s and 6.52; same method measured water (300 μL) as a control and Qdis was calculated to be 30.84. On the basis of data, η could be obtained.
Biotin-promoted DOX Release
The release kinetics study was carried on by dialysis method, 1 mL QD@M/D-Avidin/FA solution (5 mg/mL) was dispersed in dialysis bag (MW 14000), and incubated in 20 mL PBS (0.01 M, pH 7.4, pH6.5, pH5.5) at 37 ºC. At the desired time point, the outside solution was taken out to measure the amount of released DOX via fluorescence spectrophotometer. In the experimental group, 0.15 mg biotin was added to the dialysis bag after 3 h of measurement, and 3 mg biotin was also added to the dialysate to maintain the consistency of inside and outside; the control group continued to dialyse without any treatment (n=3).
To evaluate the drug release profile in the cell, HeLa cells were seeded in 6-well plate (5×105 cells per well) and incubated for 24 h at 37 ºC under 5% CO2. Cells were treated with fresh culture medium in the presence of QD@M/FITC-Avidin/FA (CFITC=1 µg/ mL) for 2 h and then washed with PBS to remove unbound probe. The cells were continued to culture for desired time and analyzed by FC500 Flow cytometer (Beckman Coulter, USA). Each time point was repeated for 3 times.
In vitro fluorescence imaging
HeLa and A549 cells were seeded in 6-well plate and grown for 24 h. Replaced serum-free medium, QD@M-db/FA and QD@M-db (100 µg/mL) were cultured with cells for another 2 h. After terminating the culture, cells were washed with PBS for three times to remove free probe. Then cells were immobilized with 4% paraformaldehyde for 30 min and collected for fluorescence imaging.
For confocal imaging of cellular uptake of probe, HeLa and A549 cells were seeded in glass-bottom Petri dishes and incubated for 24 h. The cells were treated with QD@M/D-Avidin/FA and QD@M/ D-Avidin (CDOX=1 µg/mL) for another 2 h. Afterwards, cells were washed with PBS and added 20 µL DAPI to stain cell nucleus. The intracellular distribution of DOX was observed and imaged by FluoView FV1000 confocal microscopy (Olympus, Japan).
Study of in vitro safety
For in vitro comparison of QD@M-db/FA and QD@M-db, MTT assay was used to analysis cell viability of HeLa and A549 cells. Cells were seeded in 96-well plate (1×104 cells per well) and incubated overnight. Subsequently, cells were treated with different amounts of QD@M-db/FA and QD@M-db (12.5, 25, 50, 100 and 200 µg/mL) for 24 h. After medium was removed, cells were incubated with 20 µL MTT (5 mg/mL) for another 4 h. The medium was discarded and 150 µL DMSO was added to each well to solubilize formazan crystal, ELX808IU microplate reader (Biotek, USA) was used to measure the absorption at 490 nm. Untreated cells were used as negative control.
Apoptosis assay
The extent of apoptosis in vitro was determined by Annexin V-FITC/propidium iodide (PI) double staining apoptosis assay. HeLa cells were seeded in 6-well plate and cultured overnight. The cells were transfected by QD@M-DNA/FA or Lipofectamine 2000 at the same concentration of db-DNA (200 nM) for 4 h. After cleaning, they were cultured with DMEM complete medium for another 24 h. At the end point, cells were washed with cooled PBS and suspended in 100 µL Annexin V binding buffer. 10 µL Annexin V-FITC and 5 µL PI were added and incubated at room temperature for 15 min in dark. Each tube was supplemented 400 µL binding buffer for analysis. Unstained cells and cells stained with Annexin V-FITC or PI were also prepared in parallel. The flow cytometry experiment was repeated for 3 times.
Western blot analysis of inhibition of survivin
To evaluate the inhibition of survivin by db-DNA, above apoptosis cells were subjected to western blot to verify protein reduction. In brief, cells were lysed in cell lysis buffer and collected by centrifugation. The proteins were then separated by 10% SDS-PAGE and electrotransferred onto PVDF membrane. The membrane was blocked with TBST containing 5% nonfat dry milk and then incubated with primary antibody overnight at 4 ºC. After washing with TBST, the membrane was hybridized with secondary antibody for 1 h at room temperature. Anti-GAPDH antibody was used as loading control.
Immunohistochemistry (IHC) analysis of inhibition of survivin
To evaluate the inhibition of survivin by db-DNA, HeLa tumor-bearing nude mice were intratumorally injected QD@M-DNA and free DNA, respectively. Tumors were obtained after 24 h, tissue sections were prepared and stained by Cy3 labeled anti-survivin antibody. In IHC, blue was nucleus of DAPI staining and red was survivin labeled by Cy3.
In vitro antitumor therapy
HeLa and A549 cells were seeded in 24-well plate (2×105 cells per well) and cultured overnight to analyse PTT in vitro. The culture medium was removed and cells were treated with QD@M-db/FA, QD@M-db and QD@M/D-DNA/FA (100 µg/mL) for 4 h. After washing with PBS to remove unbound probe, each well was added 200 µL PBS and irradiated with 808 nm laser (2 W/cm2) for 10 min. The cells were stained with calcein and observed under fluorescence microscope.
Combined antitumor therapy in vitro
The combined antitumor therapy was studied based on MTT assay. HeLa cells were seeded in 96-well plate and cultured overnight. Then cells were exposed to various probes with different concentrations (0.63, 1.25, 2.5 and 5 µg/mL DOX) for 4 h and irradiated with 808 nm laser (2 W/cm2) for 10 min or without any treatment. Then cells were grown for another 24 h and measured cell viability.
In vivo fluorescence imaging and thermal imaging
4-5 weeks Balb/C nude mice (male, SPF grade) were injected subcutaneously with 100 μL resuspended HeLa cells (1×106) or A549 cells (2×106) PBS to induce tumor formation. The tumor began to be treated when its volume reached ~100 mm3 (0.5×length×width2). For in vivo fluorescence imaging, QD@M-db/FA or QD@M-db (60 mg/kg) were administered by tail vein injection in tumor-bearing mice and acquired fluorescence signals at different time points (0, 2, 4, 8, 12, 24 and 48 h). For thermal imaging, HeLa tumor-bearing nude mice were intratumorally injected with PBS (50 µL) or QD@M-db/FA (50 µL, 20 mg/mL). 808 nm laser was used to irradiate the tumor site after 24 h of incubation and the temperature was recorded by thermal imager.
In vivo combined antitumor efficacy and safety evaluation
HeLa tumor-bearing nude mice with an average volume of ~200 mm3 were randomly divided into five groups (n=6) and intratumorally injected with 50 µL different probes: (I) PBS, (II) QD@M/D-Avidin/FA, (III) QD@M-DNA/FA, (IV) QD@M/D-DNA/FA and (V) QD@M/D-DNA/FA. 5 injections were done at 1, 5, 9, 13 and 17 d respectively, and group II, III and V were irradiated with 808 nm laser (2 W/cm2) for 10 min at 2 d after the first injection. One mouse from each group was randomly sacrificed after laser irradiation. The tumors were fixed with 4% paraformaldehyde for H&E and IHC staining. The probe concentration was 20 mg/mL, DOX was 600 µg/mL and db-DNA was 76.4 µg/mL. The tumor volume and body weight of mice were measured for 30 d. When the experiments were finished, mice were sacrificed and major organs (hearts, livers, spleens, lungs, kidneys and small intestines) were collected, weighed and fixed with 4% paraformaldehyde. Each sample was stained with hematoxylin/eosin (H&E), and then subjected to a microscope to investigate biocompatibility of probe in these tissues. Visceral index was calculated as follows, organ mass/mice mass×100%. All animal experiments were approved by Animal Experimental Ethics Committee of Huazhong University of Science and Technology.
Hemolysis assay
Blood compatibility was evaluated with hemolysis assay. Fresh mice blood was extracted from orbital vein and stabilized with heparin. After accumulating to 2 mL, whole blood was diluted with PBS to 4 mL and centrifuged at 10016 rpm for 5 min to isolate red blood cells (RBCs). The RBCs were further washed and finally diluted to 20 mL PBS. Different concentrations of QD@M-db/FA were incubated with RBCs at 37 ºC for 4 h, water as positive control and PBS as negative control. The absorbance of the supernatants from each group was measured using a microplate reader at 570 nm. The hemolysis percentage was calculated as follows:
| hemolysis percentage = (ODtest-ODnegative control) /(ODpositive control-ODnegative control)×100%. |
Statistics
Statistical analyses were performed by the Student's t-test or one-way analysis of variance (ANOVA) using SPSS 20.0, with significance levels as follows, *: p<0.05, **: p<0.01. Error bars represent standard deviations.
Results and Discussion
Synthesis and characterization of QD@M-DNA/FA
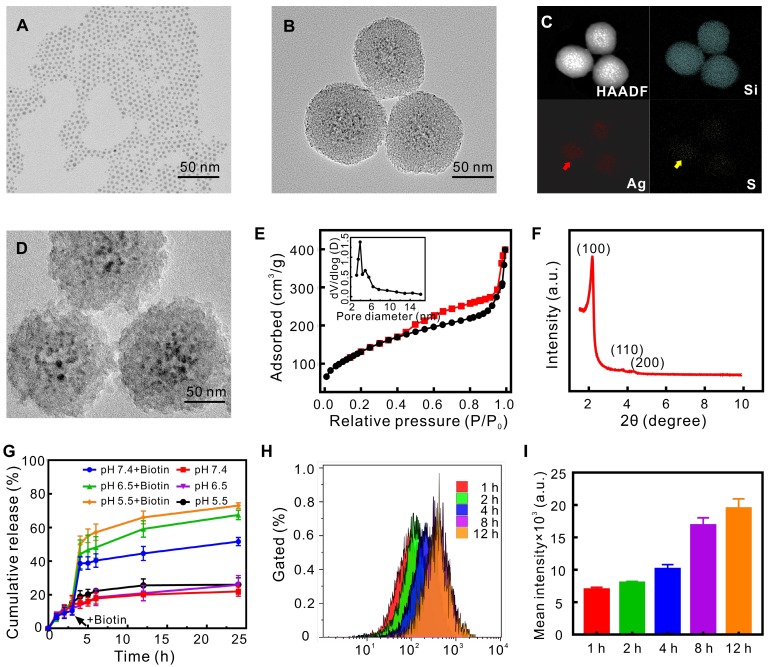
Through the sol-gel reaction, the uniform spherical QD@M nanoparticle (Figure 2B) employed Ag2S QDs (Figure 2A, 6.5 nm) as core covered by 30-40 nm thick mesoporous silicon as shell, EDX spectrum (Figure S1A) and elemental mapping images (Figure 2C) showed the proportion and distribution of elements. Typical IV type isotherm of N2 adsorption/desorption isotherm curve (Figure 2E) and small angle XRD pattern (Figure 2F, two-dimensional hexagonal symmetry with space group of p6mm) demonstrated an ordered mesoporous characteristic. BET surface area and total pore volume were 602.4 m2/g and 0.51 cm3/g respectively, and the size of whole particle was about 102 nm and pore size calculated by BJH method was 3.29 nm (Figure 2E, inset). We also found that concentrations of Ag2S and TEOS could affect the shape of QD@M and the thickness of silicon layer, indicating higher concentration of Ag2S QD would result in thinner silicon layer and adhesion of particles, higher TEOS concentration would result in larger particle and thicker silicon layer (Figure S2). Accordingly, 6 mg Ag2S QD and 400 µL TEOS were selected to prepare satisfactory dispersible nanoparticle (Figure S1B).
Figure 2.
Characterization of nanoparticles and evaluation of drug controlled release capacity. TEM images of Ag2S QD (A), QD@M (B) and QD@M-DNA/FA (D); high-angle annular dark field image (HAADF), Si, Ag and S elemental mapping images of QD@M (C); nitrogen adsorption-desorption isothermal curve (E) and pore size distribution (inset); small-angle X-ray diffraction of QD@M (F); DOX release curves of QD@M/D-Avidin/FA with and without biotin of different pH (G); intracellular fluorescence intensity (H) and mean intensity (I) were measured by flow cytometry at different time points after QD@M/FITC-Avidin/FA endocytosis in HeLa cells (n=3).
Fluorescence spectrum showed that emission of QD@M was about 1080 nm in NIR-II region under 808 nm excitation, the fluorescence quantum yield was about 3.4±0.75% (Figure S3A). Owing to the protection of silicon layer, photo-bleaching resistance of Ag2S QD after encapsulation was improved (Figure S3A, inset). UV-Vis absorbance spectra was used to demonstrate FA modification and DOX loading (Figure S3B), db-modified nitrocellulose membranes (NC membranes) was introduced to confirm that avidin was successfully coupled through desthiobiotin-avidin interaction (Figure S3C, p<0.01). Zeta potential (Figure S3D) and hydrate particle size (Figure S3E) of QD@M/D-DNA/FA were -21.3±0.3 mV and 190.1±1.17 nm, respectively, the content of Ag2S in QD@M/D-DNA-FA was calculated to be 7.8% (w/w). The achieved negative charge has been previously shown to be optimal for a long-lasting in vivo circulation time. Besides, Carboxyfluorescein (FAM) labeled db-DNA was used to indicate that db-DNA was mainly modified to probe surface by desthiobiotin-avidin interaction, and a small amount was also directly adsorbed by electrostatic adsorption (Figure S3F). Particle stability test illustrated that temperature (in the range of 4-37 ºC) and storage environment had little effect on QD@M-DNA/FA (Figure S4). The morphology of QD@M after incubation in DMEM (10% FBS, pH 7.4), PBS of pH 6.5 and pH 5.5 for 30 d was also explored by TEM (Figure S5), the result showed that silicon shell structure was degraded under acidic condition. As tumor was acidic, it provided good microenvironment for the later drug release and degradation of probe.
Ag2S QD had good photothermal conversion efficiency, and the photothermal ability of QD@M was explored by detecting temperature changes under 808 nm laser irradiation. The results showed that photothermal effect of QD@M was concentration dependent (Figure S6A, C) and laser power intensity dependent character (Figure S6B, D). More importantly, QD@M did not show significant decrease in photothermal conversion after continuous laser irradiation for six cycles (Figure S6E). The photothermal conversion efficiency (η) of QD@M was 33.86% (Figure S6F). TEM results also revealed that silicon shell skeleton did not scatter, and still maintained core-shell structure (Figure S7), showing an ideal photostability of QD@M. Therefore, QD@M had an outstanding photothermal effect and photostability for PTT.
Biotin-dependent drug release
In our targeting drug delivery system, avidin was used as a “gatekeeper” to control drug and db-DNA release. As the affinity of biotin toward avidin was higher than that of db, biotin would replace db and competitively bind avidin, making it leave from pore entrance and release the drug in pore, and dissociate db-DNA from avidin too. Here, DOX was adsorbed into pore, the loading rate and encapsulation efficiency reached up to 2.87±0.13% and 51.33±5.79%, respectively. To investigate the controlled release behavior of our designed system, drug release effect in the presence and absence of biotin in vitro was simulated under different pH conditions (Figure 2G). Under acidic conditions that mimicked the tumor microenvironment (pH 6.5 and pH 5.5), the release rate of each group was about 10% at 3 h before biotin was added; in the presence of biotin, the cumulative release of DOX from QD@M/D-Avidin/ FA was increased significantly to about 67.44±2.73% (pH 6.5) and 72.94±1.723% (pH 5.5), respectively, at 24 hours posttreatment, which was higher than that without biotin (approximately 25%). The release profiles of DOX from QD@M/D-Avidin/FA in neutral buffer (pH 7.4) were also examined, the release profiles similar to those observed under acidic conditions. Moreover, the cumulative release of DOX under acidic conditions showed an increased compared with that under neutral condition. Tumor tissue was under acidic pH and express maximal biotin to trigger the release of DOX. When drug-loaded probe entered into tumor cell, drug could be released rapidly under the both stimulations to achieve killing ability of drug. To verify this speculation, QD@M/FITC-Avidin/FA was prepared by loading fluorescein isothiocyanate (FITC) instead of DOX. Flow cytometry was used to detect fluorescence intensity of FITC in cell which was continuously cultured for different time slot (Figure 2H, I). It was found that intracellular fluorescence gradually increased with time and stabilized after 8 h. Because the fluorescence of FITC in pore was inhibited since Ag2S QD affected the fluorescence of nearby fluorescent dye, and the recovery of FITC fluorescence was the indication of its release from pore into cytoplasm. Taken together, our smart drug delivery system exhibited an effective control of drug release and presented great potential for tumor therapy.
FA receptor-mediated endocytosis of QD@M/D-Avidin/FA
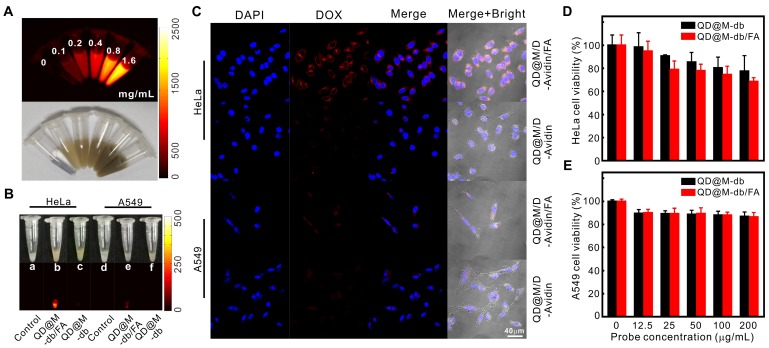
Firstly, the NIR-II fluorescence imaging property of QD@M was investigated. It was found that when the concentration increased, the fluorescence intensity enhanced gradually, indicating that the fluorescence of QD@M was concentration-dependent (Figure 3A).
Figure 3.
Targeting and in vitro biocompatibility of probes. Fluorescence and white light images of QD@M at different concentrations (A); NIR fluorescence images of HeLa and A549 cells incubated with QD@M-db/FA and QD@M-db (B); confocal fluorescence images of HeLa and A549 cells incubated with QD@M/D-Avidin/FA and QD@M/D-Avidin (C); survival of HeLa (D) and A549 (E) cells incubated with QD@M-db/FA and QD@M-db at different concentrations.
Efficient cellular uptake was an essential requirement for treatment efficacy of multifunctional nanoparticle. Modifying the targeting ligand on surface of nanoparticle was an effective way to increase probe uptake by receptor-mediated endocytosis. To test targeting specificity of probe, HeLa and A549 cells with high and low expression of FA receptor were used as positive and negative cells, respectively. QD@M-db/FA and QD@M/D-Avidin/ FA were used as positive probes, while no FA-coupled QD@M-db and QD@M/D-Avidin were used as negative probes. After these probes were incubated with both cells for 2 h, NIR-II fluorescence images revealed that HeLa cells incubated with QD@M-db/ FA exhibited the strongest fluorescence signal (Figure 3B), significantly higher than other groups. Although A549 cells incubated with QD@M-db/FA also had weak fluorescence, which should be caused by non-specific adsorbed probe. Confocal images showed that QD@M/D-Avidin/FA transported the most DOX into HeLa cells (Figure 3C), and the red fluorescence signal of DOX was mainly distributed in cytoplasm, a few into nucleus because of the short incubation time. While there was a certain amount of red fluorescence in A549 cells incubated with QD@M/D-Avidin/FA, and the other two groups were very few, confirming that FA-modified probe was subject to FA receptor-mediated endocytosis. In consequence, FA-modified multifunctional probe had excellent targeting properties. Besides, the above-mentioned experimental cells continued to be cultured for 6h and 12h after treatment and washing. With the release of DOX triggered by biotin in cells, DOX began to enter nucleus after 6h and most drugs entered the nucleus after 12 h of treatment (Figure S8). The intracellular release and treatment of the drug were studied.
As a potential drug delivery system, biocompatibility of probe was the key to assessment. HeLa and A549 cells were incubated with different concentrations of QD@M-db/FA or QD@M-db for 24 h, respectively. MTT results showed that survival rate of HeLa cells remained 68.5±3.4% when QD@M-db/FA concentration reached 200 μg/mL, and that of A549 cells was 86.3±4.1%, probably due to the uptake of more targeting probe by HeLa cells (Figure 3D, E). It was also found that the targeting property of probe had an effect on cell survival. After incubation of HeLa cells with 200 μg/mL QD@M-db/FA and QD@M-db, cell survival rate of probe with FA ligand was lower than that of probe without FA ligand (77.6±13.5%), whereas there was no significant difference in cell survival rate for A549 cells incubation with different probes.
Investigation on in vitro antitumor effect
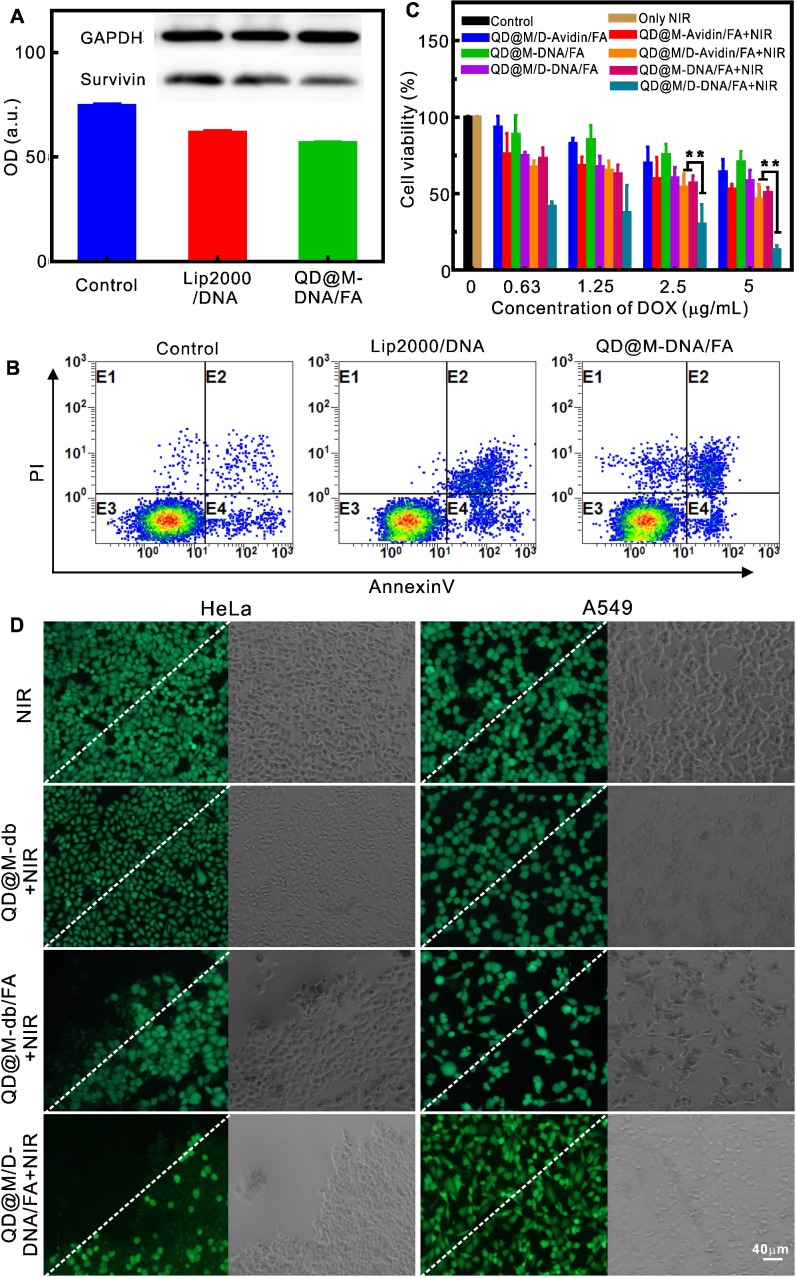
The treatment of tumor by multifunctional nanoplatform QD@M/D-DNA/FA included three parts: chemotherapy, photothermal therapy and apoptosis of survivin antisense oligonucleotide. To verify the ability of probe to deliver nucleic acid, QD@M-DNA/FA and commercialized Lip 2000 were compared to transfect survivin antisense oligonucleotide. The expression of survivin protein in HeLa cells were detected by western blot (Figure 4A), and both of them had almost the same gene silencing effect on survivin. Flow cytometry was also used to examine apoptosis by using Annexin V-FITC and PI double staining (Figure 4B). DNA-loaded QD@M-DNA/FA treated group yielded approximately 14.7% apoptosis induction rate, and similar with Lip2000 treatment (16.9%), demonstrating that this DNA deliver system could knockdown gene expression and induce apoptosis as an adjuvant therapy. In addition, immunohistochemistry (IHC) was used to analyze the survivin inhibitions in QD@M-DNA/FA and free DNA treated tumors, and it was found that the inhibition of survivin was better when DNA was delivered into tissue cells by QD@M-DNA/FA probe (Figure S9).
Figure 4.
Evaluation of the therapy effect in vitro. Western blot analysis of intracellular survivin protein after Lip 2000 and QD@M-DNA/FA transfected antisense oligonucleotide (A); flow cytometry of the apoptosis induced by Lip 2000 and QD@M-DNA/FA transfection of antisense oligonucleotide (B) (n=3) ; MTT assay of survival rate of HeLa cells treated with different probes (C); fluorescence imaging of calcein staining HeLa and A549 cells irradiated by laser (2 W/cm2) for 10 min after incubation with QD@M-db, QD@M-db/FA and QD@M/D-DNA/FA for 2 h (D), the white dashed line was the irradiation boundary. **: p<0.01.
Previous studies confirmed that tumor cell would necrosis due to heat-induced protein denaturation when temperature exceeded 50 ºC, and our probe with low concentration showed the potential of cell photothermal therapy under 808 nm laser irradiation (Figure S10), so the photothermal effect of our probe in cells was also assessed. The results showed that HeLa cells incubated with QD@M-db/ FA exhibited obvious cell death after staining with calcein (Figure 4D), while the same treatment by negative QD@M-db without FA ligand showed no apparent cell death. In addition, no cell death occurred in A549 cells, and laser irradiation alone could not kill tumor cell. Hence, the targeting probe designed by us had excellent targeting property and good PTT effect on FA receptor high expression tumor cell. Furthermore, the HeLa cell treated with QD@M/D-DNA/FA showed cell death in laser irradiated area and partial apoptosis in non-irradiated area due to the action of DOX and antisense oligonucleotide.
The combined therapeutic effect of QD@M/ D-DNA/FA on HeLa cells was evaluated by MTT assay (Figure 4C). It should be noted that since unit probe carried a certain amount of drug, DOX concentration here indicated probe concentration. In accordance with photothermal fluorescence imaging (Figure 4D), laser irradiation alone had no effect on cell survival. The results revealed that in the absence of laser irradiation, when DOX, oligonucleotide or both were delivered to cells, survival rate decreased gradually with the increase of probe concentration; as its concentration reached 5 μg/mL, cell survival rates decreased to 64.03±8.11, 70.42±7.39 and 58.11±7.18%, respectively. Comparison of these results showed that antisense oligonucleotide had the weakest killing effect on tumor cell, which was due to its low apoptosis efficiency, and synergistic effect of the two modes of action was relatively better. Under laser irradiation, 5 μg/mL QD@M-Avidin/FA without DOX and antisense oligonucleotide could reduce cell survival to 52.63±3.45% owing to its good PTT effect. Meanwhile, once PTT was combined with DOX or antisense oligonucleotide, the lower concentration of probe (0.63 μg/mL) exhibited high cytotoxicity (67.1±4.1 and 72.98±7.03%). This trend was more obvious at high concentration. When probe concentration reached up to 5 μg/mL, survival rates of cells combined laser irradiation with DOX or antisense oligonucleotide were 46.2±9.8 and 50.6±3.3%, while the combination of the three was only 13.04±2.77%, which was significantly different compared with the former two (p<0.01) (Figure 4C). Together, the above results proved that the synergistic effect of QD@M/ D-DNA/FA carrying DOX and survivin antisense oligonucleotide under laser irradiation was superior to other treatment groups in tumor cell therapy.
In vivo targeted NIR fluorescence imaging and antitumor therapy
Due to high NIR fluorescence quantum yield of Ag2S QD, fluorescence imaging performance of probe targeting tumor in vivo was further investigated. QD@M-db/FA was injected into HeLa tumor-bearing nude mice through tail vein (Figure 5B). Fluorescence signal began to enhance at the tumor site after 2 h, and continued to increase over time. The signal was brightest during 8-12 h and could still be observed after 48 h. To assess the targeting ability of probe in vivo, HeLa and A549 tumor-bearing nude mice were intravenously injected with same concentration of QD@M-db/FA and QD@M-db, respectively. As shown in Figure S11, compared with other experimental groups, FA ligand-modified QD@M-db/FA exhibited the strongest signal and more persistent enrichment for HeLa tumor (high FA receptor expression), indicating that our targeting probe was an excellent fluorescence tracer probe owing to its strong fluorescence signal and long residence time at tumor site.
Figure 5.
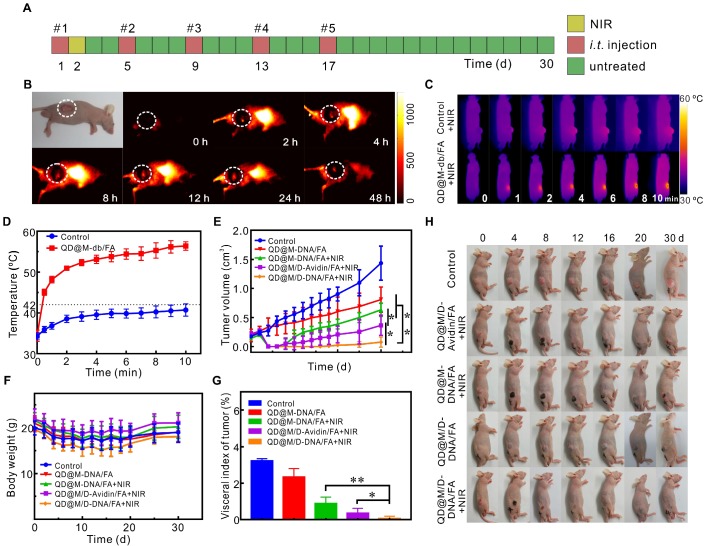
The fluorescence imaging and tumor treatment in vivo. Schematic illustration of treatment route (A); fluorescence imaging at different time points after tail vein injection of QD@M-db/FA into HeLa tumor-bearing nude mice (B); infrared thermal imaging results (C) and temperature variation curves (D) of tumor site irradiated by laser after intratumoral injection of PBS and QD@M-db/FA for 24 h; tumor growth after different treatments (E); body weights (F) and images (H) of mice with various treatments during 30 d; ratio of tumor mass to body weight in different groups at end of treatment (G). n=5, *: p<0.05, **: p<0.01.
Encouraged by the excellent synergistic therapeutic effect in cell experiments of probe, the combined tumor therapy of probe was further carried out in vivo. To verify the potential of probe to induce hyperthermia effect in vivo, tumor was injected with PBS or QD@M-db/FA, and the temperature of both increased under laser irradiation (Figure 5C). After 10 min exposure, the temperature reached to 41.2 and 54.4 ºC, respectively (Figure 5D), the latter temperature could be sufficient to kill tumor cells while the former was harmless to tumor. For purpose of improving therapeutic effect, HeLa tumor-bearing nude mice were intratumorally injected with different probes for 5 times every 4 d 40,41, and in order to take maximum advantage of DOX and antisense oligonucleotide, laser irradiation was administered at 24 h after the first injection (Figure 5A). Analyzing the change in tumor volume (Figure 5E), it was found the group of QD@M/D-DNA/FA without laser irradiation showed moderate inhibition of tumor growth compared with control group due to synergistic effect of chemotherapy and gene therapy. In the experimental group irradiated by laser, the tumor tissues all suffered scab formation and exfoliation after heating up (Figure 5H). Unfortunately, group III mice treated with photothermal and gene therapy began to recur at 8 d and the similar phenomenon occurred in group II at 10 d which dealt with chemo-photothermal therapy (Figure 5E). Moreover, all the mice in these two groups relapsed and tumor volume began to increase continuously, illustrating that tumor therapeutic effect of combining photothermal with gene therapy or chemotherapy was not satisfactory. It could also be found that tumor volume in group III grew faster than that in group II (p<0.05), which was consistent with the experimental result at cellular level that gene therapy was less effective than chemotherapy. This phenomenon might be caused by the partial degradation of antisense oligonucleotide, which affected gene silencing efficiency. Moreover, photothermal heating could increase the permeability of cell membrane, promoting endocytosis of probe and inducing drug release, thus improved chemotherapy effect. Excitingly, the mice injected with co-delivery system QD@M/D-DNA/FA coupled with laser irradiation (group V) obtained the most efficient antitumor effect. Only one mouse regrew tumor after 20 d in the whole experimental period, and there was significant difference between group V and group III (p<0.01) in inhibiting tumor growth, also for group II (p<0.05). The excellent antitumor effect achieved here might be due to the synergistic treatment of chemo-, gene-, and photothermal therapeutic effect. The same results were also found in H&E and IHC staining sections of tumors from each treatment group (Figure S12). Compared with the non-irradiated group IV, the laser-irradiated groups had a larger area of cell necrosis; compared with the control group, the experimental groups had inhibitory effect on survivin expression, while the group II without gene therapy had less inhibitory effect than other treatment groups. Mice were sacrificed after 30 d treatment and tumor masses were measured to calculate tumor index (Figure 5G, tumor index=tumor weight/body weight×100%). Similar to the previous results, the combined effect of chemo-, gene and photothermal therapy was significantly better than those of other groups (p<0.05 or p<0.01). In addition, no noticeable body weight loss was observed in all groups during treatment process (Figure 5F), indicating outstanding biocompatibility of probe and the treatment had no obvious toxicity to mice. All in all, above results demonstrated that QD@M/D-DNA/FA combined with laser irradiation achieved significant therapeutic effect and prolonged the prognosis.
In vivo biocompatibility and safety
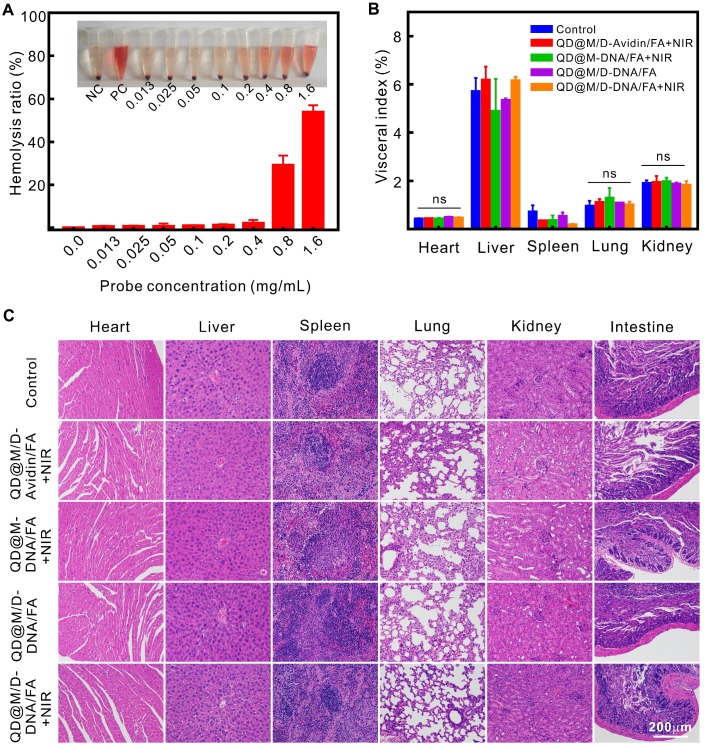
The excellent multifunctional nanoplatform not only needed stable imaging and high-efficiency therapeutic effect, but also required satisfactory biosecurity in vivo. Since probe needed to participate in blood circulation after injection, hemolysis by nanoparticle was a crucial consideration. The result showed that slight hemolysis began to occur when the concentration of QD@M-db/FA as high as 0.8 mg/mL (Figure 6A), suggesting that tail vein injection of probe could be nontoxic toward erythrocyte in vivo.
Figure 6.
Evaluation of blood compatibility and biological safety. Hemolysis of QD@M-FA (A); visceral index of major organs at the end of different treatment groups (B); H&E staining of major organs at the end of different treatment groups (C). ns: no statistical significance.
The toxicity of tumor therapy on mice was evaluated by comparing organ index and H&E staining of each group after treatment. Visceral index was the ratio of viscera weight and body weight, it was relatively constant under normal condition, and it would change while organ was damaged, the increase of visceral index indicated congestion, edema or hyperplasia, and decrease illustrated organ atrophy or other degenerative diseases. The data revealed that there was no apparent difference in heart, lung and kidney index between control group and experimental group (Figure 6B), there might be a little damage in liver and spleen since both visceral index slightly increased or decreased. As liver and spleen were the main metabolic or immune organ, it was speculated that liver and spleen captured and enriched probe which leading to the slight abnormality. The enrichment phenomena could be certified in fluorescence imaging (Figure 5B). Compared to the control group, neither noticeable damage nor inflammation was observed from H&E staining images of major organs (Figure 6C). The heart did not present reduction of fibrosis or vacuolation, pulmonary alveoli was significant without fibrosis or congestion, and clear and complete glomerular structure could be seen in kidneys. In conclusion, the drug delivery system for combination therapy could greatly suppress tumor growth without any apparent side effect.
Conclusions
In summary, a multifunctional nanoplatform (QD@M/D-DNA/FA) with the ability of tumor targeting fluorescence imaging and combining chemotherapy, PTT and gene therapy was successfully designed and constructed. The probe employed Ag2S QD with NIR-II fluorescence and photothermal properties as core covered by mesoporous silica as shell. The pore loaded with drug was capped by desthiobiotin-avidin complex, survivin antisense oligonucleotide and FA were grafted onto the probe surface for gene therapy and targeting tumor. In vitro and in vivo experiments proved that the probe had satisfactory biocompatibility and targeting fluorescence imaging ability, and could effectively inhibit tumor growth under the synergistic effect of biotin-responsive drug controlled release, PTT and gene-induced apoptosis. This novel theranostic platform combined synergistic therapy and real-time imaging might hold promise for efficient tumor therapy.
Supplementary Material
Supplementary figures.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFA0700501), the National Natural Science Foundation of China (Grant No. 81471697, 81771878), and the Fundamental Research Funds for the Central Universities (Hust: 2016YXMS253, 2017KFXKJC002, 2018KFYXKJC048). We also thank the Analytical and Testing Center (HUST) and the Center for Nanoscale Characterization & Devices (CNCD) at WNLO of HUST for the help of measurement.
Abbreviations
- Ag(DDTC)
Diethyldithiocarbamic acid silver salt
- APTES
3-aminopropyltriethoxysilane
- CTAB
hexadecyltrimethylammonium bromide
- db
desthiobiotin
- DOX
doxorubicin
- FA
folic acid
- FAM
carboxyfluorescein
- H&E
hematoxylin/eosin
- IHC
immunohistochemistry
- MSN
mesoporous silica nanoparticle
- NC
nitrocellulose membranes
- QDs
quantum dots
- PTT
photothermal therapy
- PDI
polydispersity index
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TEOS
tetraethylorthosilicate.
References
- 1.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2009;31:100–10. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan W, Yung B, Huang P, Chen X. Nanotechnology for multimodal synergistic cancer therapy. Chem Rev. 2017;117:13566–638. doi: 10.1021/acs.chemrev.7b00258. [DOI] [PubMed] [Google Scholar]
- 3.Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng. 2017;1:0010. [Google Scholar]
- 4.Chen G, Tian F, Zhang Y, Zhang Y, Li C, Wang Q. Tracking of transplanted human mesenchymal stem cells in living mice using near-infrared Ag2S quantum dots. Adv Funct Mater. 2014;24:2481–8. [Google Scholar]
- 5.Li C, Li F, Zhang Y, Zhang W, Zhang X-E, Wang Q. Real-time monitoring surface chemistry-dependent in vivo behaviors of protein nanocages via encapsulating an NIR-II Ag2S quantum dot. ACS Nano. 2015;9:12255–63. doi: 10.1021/acsnano.5b05503. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Zhang Y, Wang M, Zhang Y, Chen G, Li L. et al. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials. 2014;35:393–400. doi: 10.1016/j.biomaterials.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Du Y, Xu B, Fu T, Cai M, Li F, Zhang Y. et al. Near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J Am Chem Soc. 2010;132:1470–1. doi: 10.1021/ja909490r. [DOI] [PubMed] [Google Scholar]
- 8.Sadovnikov S, Gusev A. Recent progress in nanostructured silver sulfide: from synthesis and nonstoichiometry to properties. J Mater Chem A Mater. 2017;5:17676–704. [Google Scholar]
- 9.Jiang P, Tian Z-Q, Zhu C-N, Zhang Z-L, Pang D-W. Emission-tunable near-infrared Ag2S quantum dots. Chem Mater. 2011;24:3–5. [Google Scholar]
- 10.Yang T, Tang Ya, Liu L, Lv X, Wang Q, Ke H. et al. Size-dependent Ag2S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy. ACS Nano. 2017;11:1848–57. doi: 10.1021/acsnano.6b07866. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Hong G, Zhang Y, Chen G, Li F, Dai H. et al. Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano. 2012;6:3695–702. doi: 10.1021/nn301218z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong G, Robinson JT, Zhang Y, Diao S, Antaris AL, Wang Q. et al. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew Chem Int Ed Engl. 2012;124:9956–9. doi: 10.1002/anie.201206059. [DOI] [PubMed] [Google Scholar]
- 13.Qin M-Y, Yang X-Q, Wang K, Zhang X-S, Song J-T, Yao M-H. et al. In vivo cancer targeting and fluorescence-CT dual-mode imaging with nanoprobes based on silver sulfide quantum dots and iodinated oil. Nanoscale. 2015;7:19484–92. doi: 10.1039/c5nr05620a. [DOI] [PubMed] [Google Scholar]
- 14.Lu N, Huang P, Fan W, Wang Z, Liu Y, Wang S. et al. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials. 2017;126:39–48. doi: 10.1016/j.biomaterials.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WC. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv Mater. 2008;20:3832–8. [Google Scholar]
- 16.Kim H, Kim WJ. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small. 2014;10:117–26. doi: 10.1002/smll.201202636. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater. 2014;26:8154–62. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Wang D, Yu H, Wang M, Liu J, Feng B. et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano. 2016;10:3496–508. doi: 10.1021/acsnano.5b07706. [DOI] [PubMed] [Google Scholar]
- 19.Wei T, Liu J, Ma H, Cheng Q, Huang Y, Zhao J. et al. Functionalized nanoscale micelles improve drug delivery for cancer therapy in vitro and in vivo. Nano Lett. 2013;13:2528–34. doi: 10.1021/nl400586t. [DOI] [PubMed] [Google Scholar]
- 20.Fiandra L, Mazzucchelli S, De Palma C, Colombo M, Allevi R, Sommaruga S. et al. Assessing the in vivo targeting efficiency of multifunctional nanoconstructs bearing antibody-derived ligands. ACS Nano. 2013;7:6092–102. doi: 10.1021/nn4018922. [DOI] [PubMed] [Google Scholar]
- 21.Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin S. et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata M, Narahara S, Kawano T, Hamano N, Piao JS, Kang J-H. et al. Design and function of engineered protein nanocages as a drug delivery system for targeting pancreatic cancer cells via neuropilin-1. Mol Pharm. 2015;12:1422–30. doi: 10.1021/mp5007129. [DOI] [PubMed] [Google Scholar]
- 23.Kecht J, Schlossbauer A, Bein T. Selective functionalization of the outer and inner surfaces in mesoporous silica nanoparticles. Chem Mater. 2008;20:7207–14. [Google Scholar]
- 24.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- 25.Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI. et al. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011;5:4131–44. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T. et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl. 2008;47:8438–41. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 27.An J, Yang XQ, Cheng K, Song XL, Zhang L, Li C. et al. In vivo computed tomography/photoacoustic imaging and NIR-triggered chemo-photothermal combined therapy based on a gold nanostar-, mesoporous silica-, and thermosensitive liposome-composited nanoprobe. ACS Appl Mater Interfaces. 2017;9:41748–59. doi: 10.1021/acsami.7b15296. [DOI] [PubMed] [Google Scholar]
- 28.Fan W, Shen B, Bu W, Chen F, Zhao K, Zhang S. et al. Rattle-structured multifunctional nanotheranostics for synergetic chemo-/radiotherapy and simultaneous magnetic/luminescent dual-mode imaging. J Am Chem Soc. 2013;135:6494–503. doi: 10.1021/ja312225b. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z, Meng H, Wang N, Donovan MJ, Fu T, You M. et al. A controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew Chem Int Ed Engl. 2013;52:7487–91. doi: 10.1002/anie.201302557. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Nguyen KT, Borah P, Ang CY, Zhao Y. Functional silica nanoparticles for redox-triggered drug/ssDNA co-delivery. Adv Healthc Mater. 2012;1:690–7. doi: 10.1002/adhm.201200123. [DOI] [PubMed] [Google Scholar]
- 31.Huo M, Wang L, Chen Y, Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8:357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Xu C, Wen L, Han MK, Xiao B, Zhou J. et al. A hyaluronidase-responsive nanoparticle-based drug delivery system for targeting colon cancer cells. Cancer Res. 2016;76:7208–18. doi: 10.1158/0008-5472.CAN-16-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Sreejith S, Zhao Y. Spacer intercalated disassembly and photodynamic activity of zinc phthalocyanine inside nanochannels of mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2013;5:12860–8. doi: 10.1021/am404578h. [DOI] [PubMed] [Google Scholar]
- 34.Russell-Jones G, McTavish K, McEwan J, Rice J, Nowotnik D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J Inorg Biochem. 2004;98:1625–33. doi: 10.1016/j.jinorgbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Chivers CE, Crozat E, Chu C, Moy VT, Sherratt DJ, Howarth M. A streptavidin variant with slower biotin dissociation and increased mechanostability. Nat Methods. 2010;7:391. doi: 10.1038/nmeth.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu M, Meng Q, Chen Y, Zhang L, Li M, Cai X. et al. Large pore-sized hollow mesoporous organosilica for redox-responsive gene delivery and synergistic cancer chemotherapy. Adv Mater. 2016;28:1963–9. doi: 10.1002/adma.201505524. [DOI] [PubMed] [Google Scholar]
- 37.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 38.Schlossbauer A, Kecht J, Bein T. Biotin-avidin as a protease-responsive cap system for controlled guest release from colloidal mesoporous silica. Angew Chem Int Ed Engl. 2009;121:3138–41. doi: 10.1002/anie.200805818. [DOI] [PubMed] [Google Scholar]
- 39.Roper DK, Ahn W, Hoepfner M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J Phys Chem C Nanomater Interfaces. 2007;111:3636–41. doi: 10.1021/jp064341w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan S, Yang Y, Zhang C, Zhao N, Xu FJ. NIR-responsive polycationic gatekeeper-cloaked hetero-nanoparticles for multimodal imaging-guided triple-combination therapy of cancer. Small. 2017;13:1603133. doi: 10.1002/smll.201603133. [DOI] [PubMed] [Google Scholar]
- 41.Meng Y, Wang S, Li C, Qian M, Yan X, Yao S. et al. Photothermal combined gene therapy achieved by polyethyleneimine-grafted oxidized mesoporous carbon nanospheres. Biomaterials. 2016;100:134–42. doi: 10.1016/j.biomaterials.2016.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.