Abstract
Background
Alice in Wonderland syndrome (AIWS) is a rare sensory perception disorder, most often caused by migraine in adults. We aimed to characterize the clinical characteristics of AIWS in a cohort of vestibular migraine (VM) patients.
Methods
Retrospective chart review of patients diagnosed with VM seen between August 2014 and January 2018.
Results
Seventeen patients were identified (10 women) with a median age at onset of 45 years (range 15–61 years), and median age at presentation of 49 years (range 17–63 years). Eighty-two percent reported 1 AIWS symptom, 12% reported 3 symptoms, and 6% described 2 symptoms. The most common symptom was visual distortions (47%), followed by extrapersonal misperceptions (41%) and somesthetic distortions (29%). Most AIWS occurred during VM episodes (77%). Eleven patients were seen in follow-up; 10 described complete or partial resolution of both AIWS and VM with migraine preventive therapy, while 1 experienced complete resolution of VM but continued to have AIWS. Neuro-otologic abnormalities improved in 2 patients.
Conclusions
This study characterizes the clinical features of AIWS in patients with VM. We observed several rare and highly unusual AIWS misperceptions (frosted-glass vision, underwater vision, dolly zoom effect, sensation of the brain coming out of the head, closed-eye visual hallucinations, and headlight glare–induced marco/microsomatognosia), and resolution or improvement in AIWS and VM with migraine preventive treatment.
Alice in Wonderland syndrome (AIWS) is a rare disorder causing distorted perceptions of time and space, vision, hearing, and somesthetic sensations similar to the experiences of the protagonist of Lewis Carroll's classic novel Alice's Adventures in Wonderland.1–5 Hermann Oppenheim was the first to describe body image distortions in a migraineurs,6 but a comparison to Alice's experiences was first drawn by Caro Lippman,7 who also pointed out that Lewis Carroll had migraine. Subsequently, John Todd8 coined the term “the syndrome of Alice in Wonderland” to aptly describe these misperceptions, and to also draw attention to Lewis Carroll's history of migraine.
AIWS is most frequently due to Epstein-Barr virus infections in children, but in adults, the most common cause is migraine (occurring in approximately 15% of migraineurs).1,2 The most typical distortions are visual (about 75%), which include micropsia (objects appearing smaller than they are), macropsia (appearing larger), teleopsia (appearing farther), and pelopsia (appearing closer). Somesthetic distortions are the second most common manifestation (10%), and include macrosomatognosia (the body feeling bigger than it actually is) and microsomatognosia (feeling smaller than one is). Infrequently, some describe altered time perceptions (time moving too slowly or quickly), auditory distortions (involving pitch, tone, and volume; or hearing voices, or music), and extrapersonal misperceptions (derealization, depersonalization, out-of-body experiences).1–5
Vestibular migraine (VM) is a disorder characterized by episodic vestibular symptoms associated with migrainous features, with a lifetime prevalence of 1%, and a 1-year prevalence of 0.9% in the general population.9 While the relationship of AIWS to migraine headache is well-recognized in the majority of publications on the subject,1,2 we underscore the association of AIWS with VM.
Methods
We conducted a retrospective chart review of patients seen in the Vestibular & Neuro-Visual Disorders Clinic of the University of Texas Southwestern Neurology Department by a single physician (SCB) between August 2014 and January 2018. From 121 patients who met the Bárány Society/International Headache Society criteria for VM,10 a total of 17 patients with symptoms consistent with AIWS were identified (10 women, 7 men).
The charts were reviewed for the following information: sex, age at onset of VM and AIWS, age at presentation, relevant medical history, characteristics of VM, description of AIWS distortions, relevant family history, brain MRI, EEG, and neurologic and bedside neuro-otologic examination findings. The neuro-otologic examination consisted of assessing dynamic visual acuity (utilizing a Snellen chart), ocular alignment (for skew deviation), ocular ductions, ocular versions, the presence of nystagmus in primary gaze, gaze-holding (in eccentric left, right, up and downgaze), smooth pursuit, saccade speed and accuracy, head-impulse tests, and vestibulo-ocular reflex suppression; this was followed by examining the patient under binocular infrared video goggles (RealEyes, MicroMedical Technologies, Chatham, IL) for nystagmus with removal of fixation, tragal compression, Valsalva maneuver, head-shaking, mastoid vibration, hyperventilation, and positional testing (right/left Dix-Hallpike, head-hanging, supine, and supine head right/left).
Brain MRI was considered unremarkable if normal or if only incidental abnormalities were discovered (e.g., cysts; scattered, nonenhancing subcortical white matter lesions).
All patients were prescribed a migraine preventive of their choice (after discussing the benefits and potential side effects of available medications with the treating physician). The clinical course of VM and AIWS was documented in those patients who returned for follow-up care.
Standard protocol approvals, registrations, and patient consents
The University of Texas Southwestern Medical Center Institutional Review Board approved this retrospective study, with waiver of informed consent.
Data availability
De-identified data not published within this article will be made available by request from any qualified investigator.
Results
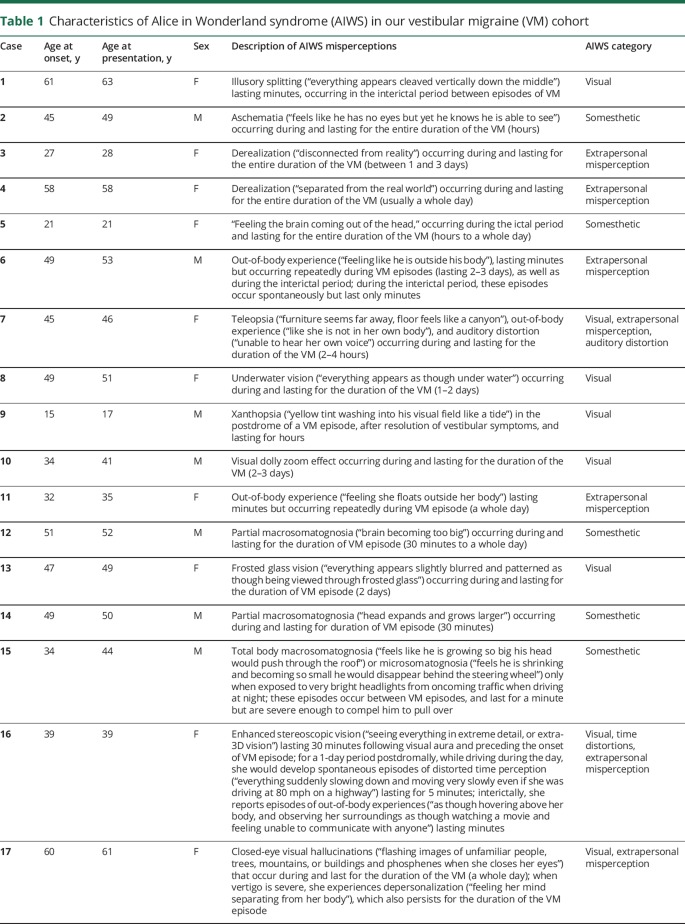
The descriptions of AIWS symptoms in our cohort are summarized in table 1. In all 17 patients, AIWS and VM began simultaneously. Median age at onset was 45 years (range 15–61 years) (mean 42.1 years) and median age at presentation was 49 years (range 17–63 years) (mean 44.5 years).
Table 1.
Characteristics of Alice in Wonderland syndrome (AIWS) in our vestibular migraine (VM) cohort
Most reported only 1 AIWS sensory misperception (14 patients); patients 7 and 16 reported 3 distinct distortions, while patient 17 described 2 misperceptions. The most common distortion was visual (8 patients, 47%), followed by extrapersonal misperceptions (7 patients, 41%), and somesthetic distortions (5 patients, 29%). Only 1 patient experienced auditory distortion (patient 7) and 1 experienced altered time perception (patient 16). Most AIWS distortions occurred during VM episodes (13 patients, 77%) and lasted for the duration of the VM attack. AIWS occurred following the visual aura in 1 patient and lasted 30 minutes (patient 16). It was a manifestation of the postdrome in 2 (patients 9 and 16). Interictal AIWS lasting minutes (between VM episodes) were reported by 4 patients (patients 1, 6, 15, and 16).
Four patients (24%) reported migraine without aura prior to the onset of VM and AIWS. None reported prior psychotic disorders, epilepsy, encephalitis, head trauma/concussion, or illicit drug use. All adult patients denied any symptoms suggestive of AIWS during childhood.
Anxiety disorder was diagnosed in 43%, and depression in 31%, by the referring physicians or primary care providers. Psychotropic medications were used by 4 (patients 7, 9, 15, and 16). Patient 7 was prescribed escitalopram but stopped it due to side effects after 6 days. Patient 9 was prescribed quetiapine 50 mg at bedtime by his pediatrician a year prior to the onset of AIWS. It was discontinued 6 months before he was seen in our clinic without any effect on AIWS and VM. Patient 15 took citalopram 10 mg daily 8 years after the onset of AIWS and VM, without any effect on the frequency or severity of his symptoms. Patient 16 started escitalopram 10 mg daily 4 years prior to the onset of AIWS and VM and remained on it; AIWS and VM episodes were only controlled with migraine preventive therapy (see later). Fourteen (82%) expressed apprehension that AIWS symptoms indicated they had, or would lead to the diagnosis of, psychiatric illness. Typical statements used by patients before describing their symptoms include “You will think I am crazy but…,” “I hope I am not going crazy but…,” and “I need you to tell me if I am going crazy.” Patients 1, 9, and 13 (who experienced visual distortions) did not share a similar fear, and found their experiences more fascinating than worrisome. Patient 1 had researched her illusory splitting on the Internet and correctly attributed it to migraine.
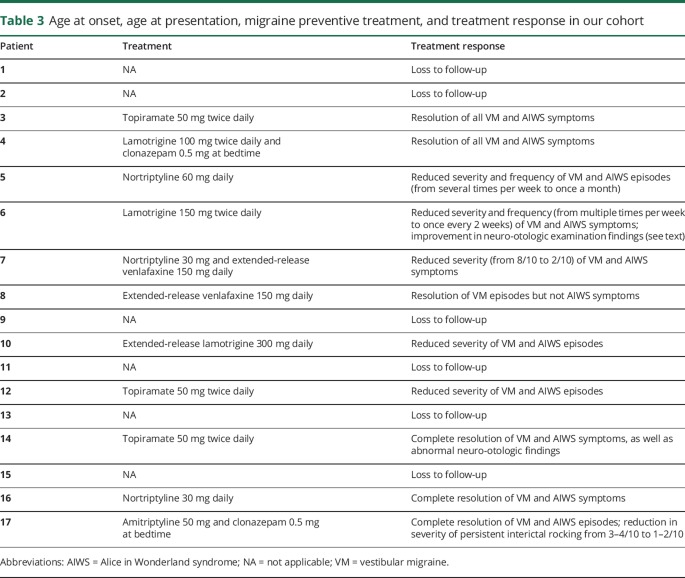
A family history of migraine was reported by 53%; 3 reported a family history of symptoms suggestive of VM (vestibular symptoms associated with migrainous features). Interestingly, patient 10 reported that his sister had similar VM and AIWS symptoms as his; no other patient described any family history of AIWS symptoms. No family history of psychotic illness or epilepsy was reported. Table 2 summarizes the abovementioned details.
Table 2.
Demographic information, pertinent medical history, family history, test results, and examination findings in our cohort with vestibular migraine (VM) and Alice in Wonderland syndrome (AIWS)
Neurologic examination was normal in all patients. Neuro-otologic examination was abnormal in 3 patients (18%). Patient 6 had left-beating nystagmus with removal of visual fixation, which increased with mastoid vibration, right-beating nystagmus with hyperventilation, and upbeat nystagmus in the right Dix-Hallpike position. Patient 14 revealed downbeat nystagmus with mastoid vibration, hyperventilation, and horizontal head shaking, as well as in the head-hanging and Dix-Hallpike positions. Patient 17 had right-beating nystagmus with mastoid vibration, and downbeat nystagmus with hyperventilation.
Brain MRI was unremarkable in all cases. Incidental findings were recorded in patient 3 (cerebellar tonsillar ectopia), patient 6 (a small cerebellar vermal subarachnoid cyst), patient 7 (a few scattered subcortical fluid-attenuated inversion recovery hyperintensities), and patient 12 (asymptomatic Chiari I malformation). The tonsillar ectopia in patient 3 and Chiari I malformation in patient 12 were considered unremarkable since both patients had normal neurologic and neuro-otologic examination, and denied any symptoms provoked by pressure maneuvers. EEG was unremarkable in all patients.
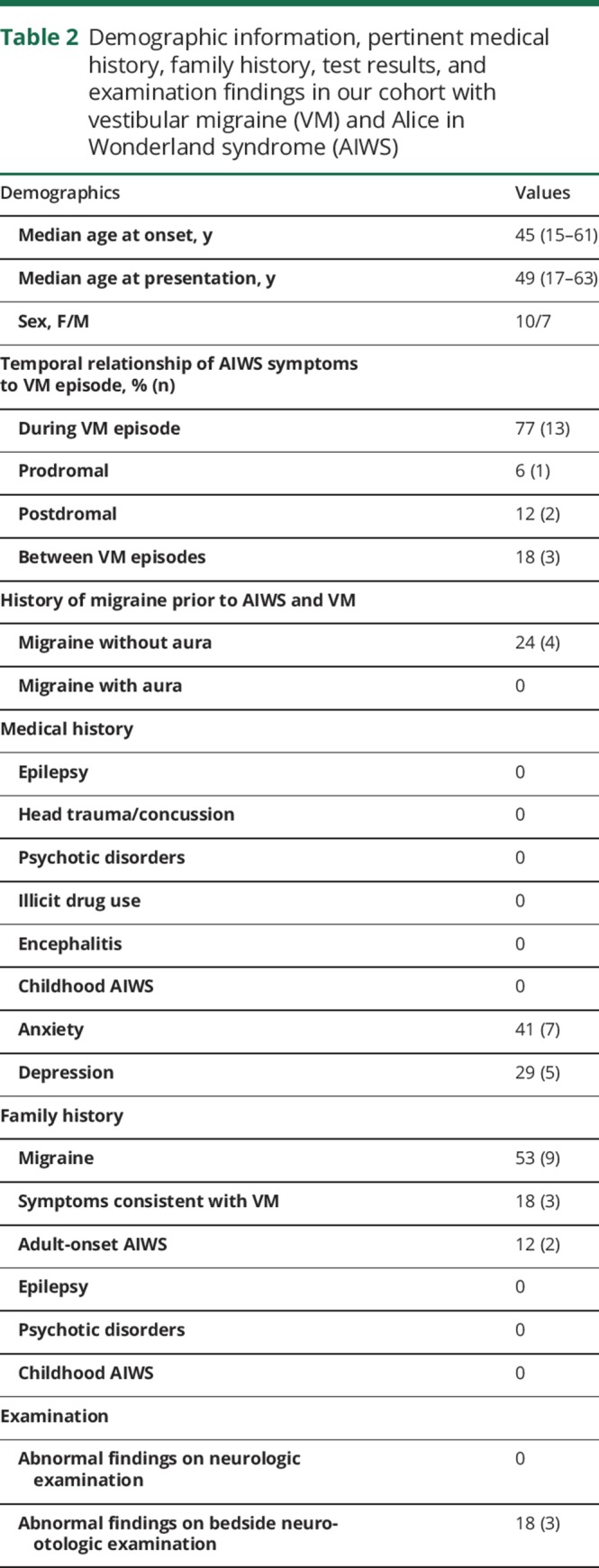
Table 3 summarizes the treatment and treatment responses of our cohort. The response to migraine prophylactic therapy could not be evaluated in 6 patients (patients 1, 2, 9, 11, 13, and 15) who did not return for follow-up visits. Of the remaining 11, migraine preventives resulted in complete resolution of both VM and AIWS symptoms in 55% (patients 3, 4, 5, 14, 16, and 17); reduced severity or frequency of both VM and AIWS episodes in 36% (patients 6, 7, 10, 12); and resolution of VM but not AIWS symptoms in 1 patient (patient 8). Eight patients were well-controlled on monotherapy (3 on topiramate, 2 on lamotrigine, 2 on nortriptyline, and 1 on venlafaxine), and 3 required 2 medications for good control (patient 4—lamotrigine and clonazepam; patient 7—nortriptyline and venlafaxine; patient 17—amitriptyline and clonazepam). In the 2 patients with abnormal neuro-otologic findings, migraine prevention therapy resulted in improvement of the findings in patient 6, and complete resolution of all abnormalities in patient 14. Patient 6 had resolution of spontaneous left-beating nystagmus with removal of visual fixation, but continued to have right-beating nystagmus with hyperventilation, left-beating nystagmus with mastoid vibration, and left-beating nystagmus in the left Dix-Hallpike position. The findings in patient 17 did not change with migraine prevention therapy.
Table 3.
Age at onset, age at presentation, migraine preventive treatment, and treatment response in our cohort
Discussion
AIWS was described by 14% of the patients diagnosed with VM in our clinic, close to the estimated 15% prevalence rate of AIWS in migraine patients.1 AIWS disturbances began with the onset of VM in all our patients. The mean age at onset was 42.1 years, which is close to the mean age of 40 years for adult-onset AIWS,1 but higher than the mean age at onset of VM reported by others (35–38 years).11,12
Similar to other studies,1,5,13 most of our patients experienced visual distortions. Of the 42 visual distortions previously described in AIWS,1 those experienced by our cohort include illusory splitting (patient 1), xanthopsia (patient 9), and enhanced stereoscopic vision (patient 16). Four rare and very unusual visual distortions were described. Patient 10 described the “dolly zoom” effect, a distortion of visual perspective produced by zooming the camera lens onto an object while simultaneously moving the camera farther from that object in such a way as to maintain the object's size in the frame throughout, causing the background to change in size relative to the object of interest. The dolly zoom effect was first conceived of and used in Alfred Hitchcock's film Vertigo.14 Patient 8 described “underwater vision” and patient 13 reported “frosted-glass vision.” Another curious symptom was patient 17's closed-eye visual hallucinations, hallucinations that only occur when a patient's eyes are closed and immediately disappear with eye opening. Closed-eye visual hallucinations have previously been reported following general anesthesia, local anesthesia with lidocaine, and atropine poisoning.15–19 While hallucinations are not strictly a sensory misperception, these closed-eye visual hallucinations may be considered an AIWS symptom in view of its link to the patient’s VM attacks, and because hallucinations (e.g., zoopsia) have been considered manifestations of AIWS in previous publications.3,8
An unusual finding in our cohort is the high proportion (41%) with extrapersonal misperceptions (4 with out-of-body experiences, 2 with derealization, 1 with depersonalization) compared to other migraine-related AIWS studies.1–4 In fact, while the most common type of AIWS misperceptions in our cohort was visual in nature, the most frequent AIWS symptom described was an out-of-body experience (OBE). It is likely that the higher proportion of extrapersonal misperceptions in our cohort may be related to the vestibular symptoms accompanying the migraines (and not just headaches), in view of the fascinating relationship between vestibular symptoms and such phenomena. Derealization/depersonalization symptoms are not infrequent in peripheral vestibular disorders.20,21 Patients experiencing OBE frequently report associated vestibular symptoms, regardless of the etiology of the OBE22; in fact, OBE have been observed in both central and peripheral vestibular disorders.23 Since peripheral vestibular disorders can provoke extrapersonal misperceptions, we cannot completely discount their presence in our cohort as the consequence of vertigo, rather than AIWS. However, it is important to note that the vast majority of OBE arising from peripheral vestibular disorders only occur with the first episode of dizziness/vertigo.23 In contradistinction, the patients in our cohort experienced recurrent extrapersonal misperceptions with every VM episode, as well as between VM episodes (i.e., independent of any vertigo), suggesting a migrainous etiology instead. The temporo-parietal junction has been identified as the neurologic source of OBE22,24–26; this location is not only part of the vestibular cortex (explaining the relationship between vestibular symptoms and OBE), but has also been implicated in AIWS (see later).
A greater proportion of our cohort also reported somasthetic distortions (29%) compared to other studies (approximately 10%).5,13,27 Partial macrosomatognosia was reported by 2 (patients 12 and 14). Patient 2 experienced an unusual form of aschematia where he felt “he had no eyes.” Aschematia was also described by Todd8; 1 patient reported that her hands “drop off and disappear” and another described “one or other of his arms was missing.” The distortions experienced by patients 5 and 15 in our cohort are highly unusual. Patient 5 described the unusual sensation of her “brain coming out of her head” during VM attacks. Patient 15 experienced whole-body macrosomatognosia and microsomatognosia between VM episodes that were only triggered by very bright headlights from oncoming traffic when driving at night; all other reported cases of AIWS are spontaneous, and to our knowledge, not triggered by bright lights. Photophobia is an integral part of migraine, and bright lights are a well-known trigger for migraine headaches.28–31 One hypothesized pathway involves a population of intrinsically photosensitive retinal ganglion cells (ipRGCs) that directly communicate with the posterior, lateral posterior, and intergeniculate thalamic nuclei (a pathway responsible for exacerbation of migraine headache by light)32; these nuclei, in turn, are connected with the primary and secondary somatosensory cortices, which also form part of the central vestibular network.32,33 It is possible that the somesthetic misperceptions triggered by headlight glare in patient 15 arise from photoactivation of ipRGCs that subsequently induces abnormal activity within the vestibular cortices (analogous to how light exacerbates migraine headaches), causing the distorted body spatial representation that characterized his AIWS.
In pediatric studies, AIWS symptoms are typically self-limiting and cease within weeks or months,13,27 but in some, continue well into adulthood.4 All our patients reported that AIWS began at the same time as VM symptoms, and continued to experience the symptoms until the time of presentation; no adults reported childhood AIWS. We followed the treatment response of 11 after starting migraine preventive therapy. All of them reported either complete resolution or partial improvement in both VM and AIWS, except for patient 8, who experienced complete resolution of VM but not AIWS. This suggests that migraine preventive therapy can ameliorate AIWS symptoms, and could potentially be used to help those who find their AIWS symptoms distressing. Interestingly, in the 2 patients with abnormal neuro-otologic examination findings, the improvement in VM and AIWS was also accompanied by improvement in these abnormalities.
A high proportion of our cohort carried a diagnosis of anxiety disorder (43%) and depression (31%), which is unsurprising in view of the relationship of mood disorders with migraine and VM.34–36 The vast majority of our patients (82%) expressed apprehension that the AIWS were symptomatic of, or would lead physicians to diagnose them with, a psychiatric illness, similar to Corbett's37 observations. This underscores the need for neurologists to be aware of AIWS, and allay patient fears that the distortions of AIWS indicate mental illness.
A history of migraine without aura was reported by 24% of our cohort, consistent with other studies in VM that observe a history of migraine headaches preceding the onset of VM.38 Similar to our study, a family history of migraine was present in the studies by Liu et al.13 and Weidenfeld and Borusiak27; one patient in each of these studies also reported a family member with childhood AIWS. None of our patients reported family members with childhood AIWS in childhood but intriguingly, one described similar AIWS and VM symptoms in an adult family member (his sister).
While many publications on AIWS recognize its relationship with migraine headaches, we specifically highlight the oft-overlooked occurrence of AIWS in VM. Upon reviewing Todd's8 seminal case series, it is highly likely that 5 of the 6 patients he described experienced vestibular symptoms in conjunction with migraine attacks, and thus, most likely had VM. Four had vertigo; one had “giddiness with nausea,” tilt illusion (“feeling that she was either going up or down hill even though walking on a flat surface”), and imbalance (“a tendency to lurch into articles of furniture”).8 Two patients in Lippman's7 cohort also most likely had VM; one would “teeter and reel as though drunk” before, during, or after a migraine headache, while another had migraine headaches associated with “staggering, imbalance on sitting or standing.” Looking back even further, Oppenheim's 1913 patient with body image distortion experienced “spontaneous dizziness” accompanying migraine headaches.6 As such, it is important to emphasize how an association between AIWS and VM was observed long before VM was conceived as a distinct clinical entity.
The precise localization of AIWS remains unclear; case reports and series in AIWS have implicated widespread and different parts of the brain including the frontal, temporal, parietal, and occipital lobes.1–3 This disparity may be due to the different sensory modalities affected by these distortions; for example, it is unlikely that visual distortions share an exact neurologic localization as extrapersonal misperceptions. The relationship between VM and AIWS may provide clues as to the location of the neural substrate responsible for generating this unusual disorder. The associative cortical regions responsible for generating 3D bodily self-consciousness (i.e., self-location and first-person perspective) need to continuously integrate real-time signals from exteroception (visual, auditory), somatosensation (tactile, proprioceptive), interoception (e.g., thermal, nociception), and vestibular information,39 and are the most likely source of AIWS misperceptions. The neural structures involved in this multisensory integration include the posterior parietal cortex, medial superior temporal region, parietoinsular vestibular cortex, and temporo-parietal junction, which also form core components in the cortical vestibular network.33,39,40 In fact, there is evidence that the vestibular system plays a central role in whole-body spatial representation, body part representation, self-motion perception, mental spatial representations, as well as the relationship between the external world and a global full-body representation.39 While the precise pathophysiology of VM is unclear, one hypothesis is cortical spreading depression (i.e., short-lasting neuronal depolarization spreading over adjacent cortical areas, followed by a longer-lasting inhibition of neuronal activity) that involves the aforementioned multisensory integration cortical regions38; it is not inconceivable that the dysfunctional neuronal activity affecting these same structures, which are critical to body schema, would cause the highly conspicuous distortions that characterize AIWS.
Limitations
Six patients (35%) were lost to follow-up. Since migraine prophylactic choice and dosage were based on patient preference, comorbid conditions, and possible side effects, different medications and dosages were used; however, the improvement observed with different classes of migraine preventives (tricyclic antidepressants, antiepileptics, and selective norepinephrine reuptake inhibitors) indicate that these benefits were related to migraine control. Description of AIWS and VM in family members was based on patient accounts; while a direct interview of the affected family members would have been optimal, they were unavailable for this.
Conclusion
We describe a unique cohort of 17 patients with VM and AIWS; the majority experienced visual distortions but a large percentage described extrapersonal and somesthetic misperceptions. Several highly unusual AIWS distortions were reported by our cohort (frosted-glass vision, underwater vision, dolly zoom effect, sensation of the brain coming out of the head, closed-eye visual hallucinations, and headlight glare–induced marco/microsomatognosia). Migraine preventive therapy resulted in either complete or partial control of both VM and AIWS symptoms.
Author contributions
S.C. Beh contributed to the study conceptualization, data gathering, data interpretation, drafting of the manuscript, and revision of the manuscript. S. Masrour contributed to data gathering, data interpretation, and revision of the manuscript. S.V. Smith contributed to data gathering, data interpretation, and revision of the manuscript. D.I. Friedman contributed to data interpretation and revision of the manuscript.
Study funding
No targeted funding reported.
Disclosure
S.C. Beh and S. Masrour report no disclosures. S.V. Smith serves as an on-track neurologic consultant for American Medical Response, NASCAR Safety Team, and receives research support from Fight for Sight, North American Neuro-Ophthalmology Society, American Headache Society, and International Headache Academy. D.I. Friedman serves on scientific advisory boards for Supernus, Teva Pharmaceuticals, Alder Biopharmaceuticals, Amgen, Biohaven Pharmaceuticals, Allergan, Eli Lilly, Avanir, electroCore, Zosano, and Autonomic Technologies, Inc.; has received funding for travel and/or speaker honoraria from electroCore and Autonomic Technologies, Inc.; serves on the editorial board of Neurology Reviews, as Associate Editor for Headache, and contributing author and senior advisor, Migraine Center of Excellence, Medscape; serves on speakers' bureaus for Allergan, Avanir, electroCore, Amgen, Teva, and Supernus; receives research support to his institution from Merck, electroCore, Autonomic Technologies, Inc., Eli Lilly, and Zosano; has participated in medico-legal cases; and is on the Board of Directors of the American Headache Society and on the Medical Advisory Board of the Spinal CSF Leak Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Alice in Wonderland syndrome (AIWS) is a rare disorder causing distorted perceptions of time and space, vision, hearing, and somesthetic sensations.
→ The most common cause of AIWS in children is Epstein-Barr infection, while migraine is the most common cause in adults.
→ The manifestations of AIWS are often bizarre and cause patients to worry that they have, or will be labeled by their physician as having, a psychiatric disorder.
→ AIWS most likely localizes to the cortical regions involved in generating 3D bodily awareness through multisensory integration (visual, proprioceptive, vestibular).
→ An appropriate migraine preventive may help migraine patients who are experiencing frequent or very distressing AIWS misperceptions.
References
- 1.Blom JD. Alice in Wonderland syndrome. Neurol Clin Pract 2016;6:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Toole P, Modestino EJ. Alice in Wonderland syndrome: a real life version of Lewis Carroll’s novel. Brain Dev 2017;39:470–474. [DOI] [PubMed] [Google Scholar]
- 3.Farooq O, Fine EJ. Alice in Wonderland syndrome: a historical and medical review. Pediatr Neurol 2017;77:5–11. [DOI] [PubMed] [Google Scholar]
- 4.Dooley JM, Augustine HF, Gordon KE, Brna PM, Westby E. Alice in Wonderland and other migraine associated phenomena: evolution over 30 years after headache diagnosis. Ped Neurol 2014;51:321–323. [DOI] [PubMed] [Google Scholar]
- 5.Lanska JR, Lanska DJ. Alice in Wonderland syndrome: somesthetic vs visual perceptual disturbance. Neurology 2013;80:1262–1264. [DOI] [PubMed] [Google Scholar]
- 6.Fine EJ. The Alice in Wonderland syndrome. Prog Brain Res 2013;206:143–156. [DOI] [PubMed] [Google Scholar]
- 7.Lippman CW. Certain hallucinations peculiar to migraine. J Nerv Ment 1952;116:346–351. [DOI] [PubMed] [Google Scholar]
- 8.Todd J. The syndrome of Alice in wonderland. Can Med Assoc J 1955;73:701–704. [PMC free article] [PubMed] [Google Scholar]
- 9.Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–1033. [DOI] [PubMed] [Google Scholar]
- 10.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167–172. [DOI] [PubMed] [Google Scholar]
- 11.Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–441. [DOI] [PubMed] [Google Scholar]
- 12.Teggi R, Colombo B, Albera R, et al. Clinical features, familial history, and migraine precursors in patients with definite vestibular migraine: the VM-Phenotypes Projects. Headache 2018;58:534–544. [DOI] [PubMed] [Google Scholar]
- 13.Liu AM, Liu JG, Liu GW, Liu GT. “Alice in Wonderland” syndrome: presenting and follow-up characteristics. Pediatr Neurol 2014;51:317–320. [DOI] [PubMed] [Google Scholar]
- 14.Lyttelton O. 5 Things You Might Not Know About Alfred Hitchcock's Masterpiece “Vertigo”. IndieWire; Available at: indiewire.com/2012/05/5-things-you-might-not-know-about-alfred-hitchcocks-masterpiece-vertigo-110734/. Accessed February 2, 2018. [Google Scholar]
- 15.Fisher CM. Visual hallucinations and racing thoughts on eye closure after minor surgery. Arch Neurol 1991;48:1091–1092. [DOI] [PubMed] [Google Scholar]
- 16.Fisher CM. Visual hallucinations on eye closure with atropine toxicity: a neurological analysis and comparison with other visual hallucinations. Can J Neurol Sci 1991;18:18–27. [DOI] [PubMed] [Google Scholar]
- 17.Eissa A, Baker RA, Knight JL. Closed-eye visual hallucinations after coronary artery bypass grafting. J Cardiothorac Vasc Anesth 2005;19:217–219. [DOI] [PubMed] [Google Scholar]
- 18.Otomo S, Sugita M, Yano T. Visual hallucinations on eye closure after orthopedic surgery under general anesthesia. J Anesth 2008;22:439–442. [DOI] [PubMed] [Google Scholar]
- 19.Weinschenk K, Schwartz AC. A case report of closed-eye visual hallucinations. Psychosomatics 2011;52:86–87. [DOI] [PubMed] [Google Scholar]
- 20.Sang FY, Jauregui-Renaud K, Green DA, Bronstein AM, Gresty MA. Depersonalization/derealisation symptoms in vestibular disease. J Neurol Neurosurg Psychiatry 2006;77:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jauregui-Renaud K, Sang FY, Gresty MA, Green DA, Bronstein AM. Depersonalization/derealisation symptoms and updating orientation in patients with vestibular disease. J Neurol Neurosurg Psychiatry 2008;79:276–283. [DOI] [PubMed] [Google Scholar]
- 22.Blanke O, Landis T, Spinelli L, Seeck M. Out-of-body experience and autoscopy of neurological origin. Brain 2004;127:243–258. [DOI] [PubMed] [Google Scholar]
- 23.Lopez C, Elziere M. Out-of-body experience in vestibular disorders: a prospective study of 210 patients with dizziness. Cortex 2018;104:193–206. [DOI] [PubMed] [Google Scholar]
- 24.Blanke I, Mohr C, Michel CM, et al. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J Neurosci 2005;25:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith AM, Messier C. Voluntary out-of-body experience: an fMRI study. Front Hum Neurosci 2014;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bos EM, Spoor JK, Smits M, Schouten JW, Vincent AJ. Out-of-body experience during awake craniotomy. World Neurosurg 2016;92:e9–13. [DOI] [PubMed] [Google Scholar]
- 27.Weidenfeld A, Borusiak P. Alice-in-Wonderland syndrome: a case-based update and long-term outcome in nine children. Childs Nerv Syst 2011;27:893–896. [DOI] [PubMed] [Google Scholar]
- 28.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27:394–402. [DOI] [PubMed] [Google Scholar]
- 29.Hauge AW, Kirchmann M, Olesen J. Characterization of consistent triggers of migraine with aura. Cephalalgia 2011;31:416–438. [DOI] [PubMed] [Google Scholar]
- 30.Bekkelund SI, Hindberg K, Bashari H, Godliebsen F, Alstadhaug KB. Sun-induced migraine attacks in an Arctic population. Cephalalgia 2011;31:992–998. [DOI] [PubMed] [Google Scholar]
- 31.Charles A. The evolution of a migraine attack: a review of recent evidence. Headache 2012;53:413–419. [DOI] [PubMed] [Google Scholar]
- 32.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandt T, Strupp M, Dieterich M. Towards a concept of disorders of “higher vestibular function.” Front Intergr Neurosci 2014;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Best C, Eckhardt-Henn A, Tschan R, et al. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes: results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65. [DOI] [PubMed] [Google Scholar]
- 35.Best C, Tschan R, Eckhardt-Henn A, Dieterich M. Who is at risk for ongoing dizziness and psychological strain after a vestibular disorder? Neuroscience 2009;164:1579–1587. [DOI] [PubMed] [Google Scholar]
- 36.Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep 2012;16:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbett JJ. Symposium on neuro-ophthalmology: neuro-ophthalmic complications of migraine and cluster headaches. Neurol Clin 1983;1:973–995. [PubMed] [Google Scholar]
- 38.Von Brevern M, Lempert T. Vestibular migraine. Handb Clin Neurol 2016;137:301–316. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer C, Serino A, Blanke O. The vestibular system: a spatial reference for bodily self-consciousness. Front Integr Neurosci 2014;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitlock JR. Posterior parietal cortex. Curr Biol 2017;27:R691–R695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data not published within this article will be made available by request from any qualified investigator.