Abstract
BACKGROUND & AIMS:
There have been few studies on the role of de novo lipogenesis in the development of nonalcoholic fatty liver disease (NAFLD). We used isotope analyses to compare de novo lipogenesis and fatty acid flux between individuals with NAFLD and those without, matched for metabolic factors (controls).
METHODS:
We studied subjects with metabolic syndrome and/or levels of alanine aminotransferase and aspartate aminotransferase >30 mU/L, using magnetic resonance spectroscopy to identify those with high levels (HighLF, n=13) or low levels of intrahepatic triacylglycerol (LowLF, n=11). Clinical and demographic information was collected from all participants, and insulin sensitivity was measured using the insulin-modified intravenous glucose tolerance test. Stable isotopes were administered and gas chromatography with mass spectrometry was used to analyze free (non-esterified) fatty acid (FFA) and triacylglycerol flux and lipogenesis.
RESULTS:
Individuals with HighLF (18.4%±3.6%) had higher plasma levels of FFA during the nighttime and concentrations of insulin than subjects with LowLF (3.1%±2.7%; P=.04 and P<.001, respectively). No differences were observed between groups in adipose flux of FFA (414±195 μmol/min for HighLF vs 358±105 μmol/min for LowLF; P=.41) or production of very low-density lipoprotein triacylglycerols from FFA (4.06±2.57 μmol/min vs 4.34±1.82 μmol/min; P=.77). By contrast, subjects with HighLF had more than 3-fold higher rates of de novo fatty acid synthesis than subjects with LowLF (2.57±1.53 μmol/min vs 0.78±0.42 μmol/min; P=.001). As a percentage of triacylglycerol palmitate, de novo lipogenesis was 2-fold higher in subjects with HighLF (23.2%±7.9% vs 10.1 %±6.7%; P<.001); this level was independently associated with the level of intrahepatic triacylglycerol (r=0.53; P=.007).
CONCLUSIONS:
By administering isotopes to individuals with NAFLD and control subjects, we confirmed that those with NAFLD increase synthesis of fatty acids. Subjects with NAFLD also had higher nocturnal plasma levels of FFA and did not suppress the contribution from de novo lipogenesis upon fasting. These findings indicate that lipogenesis might be a therapeutic target for NAFLD.
Keywords: lipid metabolism, fatty acid kinetics, obesity, diabetes
INTRODUCTION
The prevalence of NAFLD is increasing1 and over the next 10 years, this condition will become the most common liver disease in the United States2, 3. A comprehensive understanding of metabolic mechanisms leading to accumulation of intrahepatic triglycerides (TG) is critically needed to support the development of treatments for NAFLD. With regard to the flux of fatty acids (FA) through the blood, the liver exists as somewhat of an ‘innocent bystander’ - taking up a constant proportion (~25%) of the FA that flow to the organ4 and numerous studies have identified adipose insulin resistance as a key contributor to excess intrahepatic-TG5–8. Of note, most previous research focusing on FA flux in NAFLD has been conducted in the fasting state and thus, although postprandial insulin has been shown to be increased9, 10, little is known in NAFLD about how FA flux transitions between the fasted and fed states. For instance, if dietary FA are not cleared efficiently to the periphery, they can contribute to intrahepatic-TG11, 12 and postprandial hyperlipidemia has been demonstrated in this condition13. Further, dietary carbohydrates could provide a potentially important source of excess liver FA14 through the process of de novo lipogenesis.
Theoretically, de novo lipogenesis has been considered a minor contributor to TG synthesis in humans for a number of reasons. The first is that early published data were derived from fasting subjects15, 16 and the de novo lipogenesis pathway is suppressed by fasting17. Second, results from lean subjects, in which lipogenesis rates were found to be very low (e.g., <5% of liver-derived VLDL-TG palmitate) may not reflect values in obesity and insulin resistance18. The third reason for underestimation of the role of de novo lipogenesis in liver physiology is a technical one, in that the accurate assessment is dependent on a minimum length of isotope administration. Our lab14, 19–21 and others22, 23 have shown that a longer duration of labeling (>2d) is necessary to assess lipogenesis, possibly due to a delay in the movement of these FA through intrahepatocyte storage pools. Indeed, higher lipogenesis levels have been observed in lean, fasting subjects, if IV or oral administration of isotopes occurs concurrently with meal consumption, before the fasting measurement is made19–21. Such methodology, when used in insulin-resistant subjects, has shown that fasting lipogenesis is significantly greater than previously appreciated (>20%) and up to 5-fold higher in insulin-resistant, compared to insulin-sensitive individuals24. One last aspect of lipogenesis that contributes to its relevance in the pathology of intrahepatic-TG is that when the pathway is stimulated, the presence of a key intermediate of FA synthesis (malonyl-CoA) is thought to reduce the oxidation of fatty acids from any source25. This effect has been demonstrated in humans with fructose feeding, which increases lipogenesis and is associated with higher postprandial TG concentrations20, 26 and greater reesterification of dietary FA27, 28
Despite the comorbidity of insulin resistance in NAFLD, lipogenesis has been measured in only two small cohorts of patients by our group (n=9)14 and one other (n=5)29. Further, of the numerous investigations showing elevated adipose FFA flux as a prominent feature in NAFLD, a role for lipogenesis has been implicated indirectly30. In an elegant study by Fabbrini et al., it was noted that after labeling the plasma FFA pool into VLDL-TG, a high percentage of TG-FA remaining unlabeled7 (60%), which contributed significantly to increased VLDL-TG secretion. The sources of these nonsystemic FA were hypothesized to include those derived from visceral fat or intrahepatic depots, or from hepatic lipogenesis. However, direct confirmation has been challenging due to the complexity of measuring the different FA sources as they contribute to TG production rates. Accordingly, we have systematically quantified the sources of FA used for TG synthesis in subjects with either high levels of intrahepatic-TG or low liver fat, who were matched for similar adiposity and blood lipids. We used methodology optimized to accurately quantitate lipogenesis when liver lipid content was high and the subjects underwent multiple clinical assessments to determine whether particular characteristics would predict elevated intrahepatic-TG in NAFLD. The de novo lipogenesis pathway was found to be 3-fold higher in those with NAFLD and lipogenesis was the key feature associated with fatty liver. These findings provide strong support for targeting lipogenesis in the future development of therapies for this condition.
EXPERIMENTAL PROCEDURES
The methods are briefly described here and have been published previously14, 31,32; the reader is referred to the Appendix for a detailed description of the study design, laboratory procedures, and calculations. Research subjects were recruited from local community health fairs and physician referrals to determine the role of metabolic syndrome in the development of NAFLD1. The initial screening criteria included characteristics of metabolic syndrome 33 and liver enzymes were also measured to increase the likelihood of finding subjects with NAFLD as evidenced by elevated levels. Based on the results of this initial screen, eligible subjects (with metabolic syndrome and/or ALT >30, AST >30) were invited to attend a more comprehensive screening visit to rule out diabetes and liver disease from other known causes, to obtain a medical and weight history, and measure intrahepatic-TG by 3.0 Tesla 1H-MRS1. Subjects were excluded if they smoked, had known metabolic abnormalities including elevated thyroid hormone levels, or elevated alcohol consumption (>140 g/wk for men and >70 g/wk for women). Eligible and interested subjects were recruited sequentially over a 4-yr period; 3 wks were needed for each subject’s in- and out-patient sample collection, followed by 5 wks of analytical analysis per subject studied. During the 3 wks of testing, each subject participated in two in-patient assessments; the first (admission #1) was designed to measure insulin sensitivity using a frequently-sampled, insulin-modified intravenous glucose tolerance test (IVGTT)34 and the second (admission #2) designed to measure FA metabolism (Appendix fig. 1). Subjects maintained habitual physical activity levels during the testing period and all foods and beverages were provided to subjects before and during both admissions based on their habitual dietary patterns (see Appendix). The goal sample size was 22 metabolic syndrome subjects with (n=11) and without (n=11) high liver fat and this number was powered to test an absolute difference in fasting de novo lipogenesis of 5% (SD of that difference of 3.6%), while allowing for a 10% drop-out rate during the 3 wks of research data acquisition. This study was approved by the UT Southwestern Medical Center IRB (#062007–025) and subjects provided written informed consent.
Study Design and Laboratory Procedures
For admission #1, subjects reported to the Clinical and Translational Research Center to measure glucose and insulin responses during an IVGTT34, body composition by DEXA, and intrahepatic-TG by MRS. Between admissions #1 and #2, subjects consumed deuterated water to measure hepatic lipogenesis31,32 For admission #2, each subject consumed a standardized evening meal containing 13C16-palmitate to trace meal fat incorporation into lipoprotein-TG and to trace spillover of dietary FA into the plasma FFA pool19. Subjects then fasted overnight and through the next morning (18h after the last meal) to allow for complete turnover of the plasma VLDL-TG pool. At midnight an IV infusion of 13C4-palmitate was initiated to measure the contribution of plasma FFA to hepatic-TG synthesis19 and the rate of adipose- FA and dietary spillover- FA flux (see below). The TG-FA composition and sources contributing to plasma VLDL- and liver-TG have been shown to be identical14, 35, and therefore the characteristics of VLDL-TG can be used to assess liver-TG fluxes. Energy expenditure and substrate oxidation were measured using indirect calorimetry. Immediately after blood collection, plasma was separated for measurement of FFA, TG, glucose, and insulin concentrations. Total TG-rich lipoproteins (TRL) were isolated from plasma by fixed-angle ultracentrifugation19. At midnight, TRL contain a mixture of chylomicron (from the previous evening meal) and hepatically-derived VLDL particles36. With progressive fasting, chylomicrons are cleared from the plasma such that TRL isolated 18h after the last meal contains very small concentrations of chylomicrons. In the present study, 18h fasting chylomicron apoB48 was 1/60th of post-meal TRL values (data not shown). Accordingly, this fraction isolated at and after midnight is referred to herein as TRL (containing both chylomicrons and VLDL), whereas after an extended fast (18h after the last meal), this fraction is referred to as VLDL, as is commonly done7, 16 Lipoprotein-TG and FFA were isolated from plasma and prepared for GC/MS and GC analysis12, 19 Fractional lipoprotein-TG turnover rates were calculated by modeling the rise to plateau enrichment of 13C4-palmitate into VLDL-TG19 and the assumptions for this model37 (e.g., the label used exclusively for hepatic-TG production) are presented in the Appendix.
Calculations and statistical analysis
TG- fatty acid sources – Using an established multiple stable-isotope procedure12, 14, 16, 19, the potential FA sources that contribute to lipoprotein-TG palmitate production (dietary fat, adipose FA, and hepatic lipogenesis) are each labeled with a different palmitate isotope, and expressed as both absolute concentration and a proportion to reflect intracellular-TG synthesis14. Note that even with an extended period of labeling, a proportion of VLDL-TG will remain unlabeled14 due to the use of visceral or intrahepatic stores. Plasma FFA sources include 1) adipose tissue, which supplies the majority of FFA in the fasting state, and 2) dietary FA, liberated from chylomicrons undergoing lipolysis in the plasma via lipoprotein lipase – the so-called spillover pathway19, 38. The rate of appearance of adipose-FA (Adipose RaFFA) was calculated using standard dilution equations and corrected for the presence of dietary spillover19. The quantity of FFA arising from meal spillover was determined by the presence of the dietary label (13C16-palmitate) in the FFA pool19. In the present study, we have extended this method to calculate the rate of appearance of spillover-FA (RaSpillover) using the known rate of infusion of 13C4-palmitate and appearance of 13C16-palmitate in the plasma FFA pool. Calculations and modeling (Appendix) were performed using Microsoft Excel (2000, Seattle, WA), GraphPad Prism (V6.01; La Jolla, CA), and SigmaPlot (V12.0; Systat Software Inc., San Jose, CA). The high liver-fat and low liver-fat groups were compared by unpaired t-test (data reported as mean ± SD); relationships between variables determined by Pearson correlation, and gender proportions compared with Fisher’s exact test. Interactions between subject characteristics and fatty liver status were tested by two-way ANOVA. Data varying by time were analyzed by repeated-measures ANOVA (and reported as mean ± SEM), with post hoc tests by Sidak’s multiple comparisons test. Significance was set at P<.05, and P<.10 reported as a trend.
RESULTS
Subjects were separated phenotypically as having low liver-fat (LowLF) or high liver-fat (HighLF)1 (table 1). LowLF and HighLF groups were matched for anthropometrics and plasma biochemistries, except for fasting insulin concentrations, which were higher in the HighLF group, as expected39. This higher insulin resulted in a greater calculated AdipoIR (an index of adipose insulin resistance6) in the HighLF subjects (table 1). Given differences in body composition, anthropometric variables for men and women were compared between groups, but were not significantly different (P>.05). When the dietary intakes of the groups were measured they were also found to be similar (table 1). Physical activity analysis revealed no subjects engaged in vigorous activity at any time during 4d of wearing the accelerometer. Indeed, for 70–80% of each day, subjects were sedentary. Neither fasting energy expenditures nor substrate oxidation rates were different between the groups (table 2) and none of the indices derived from the IVGTT (SI, AIRg, disposition index) were different (table 2).
Table 1.
Anthropometric characteristics, and biochemical and dietary intake data of subjects categorized by normal (LowLF) or elevated (HighLF) liver fat
| Characteristics | LowLF (n=11) | HighLF (n=13) | P-value |
|---|---|---|---|
| Liver Fat (%) | 3.1 ± 2.7 | 18.4 ± 3.6 | < .001 |
| Males : Females | 4:7 | 6:7 | .70 |
| Anthropometrics | |||
| BMI (kg/m2) | 35.3 ± 7.7 | 34.9 ± 5.2 | .89 |
| Weight (kg) | 103 ± 22 | 92 ± 18 | .19 |
| Body fat (%) | 39.7 ± 10.5 | 39.2 ± 6.8 | .89 |
| Lean body mass (%) | 57.6 ± 10.1 | 58.2 ± 6.6 | .86 |
| Trunk body fat (%) | 20.1 ± 5.3 | 21.4 ± 4.5 | .50 |
| Waist:hip ratio | 0.92 ± 0.07 | 0.96 ± 0.09 | .20 |
| Fasting Biochemical Data a | |||
| ALT (U/L) | 45 ± 35 | 66 ± 44 | .20 |
| AST (U/L) | 36 ± 27 | 51 ± 39 | .29 |
| HbA1c (%) | 5.8 ± 0.2 | 5.9 ± 0.3 | .12 |
| Glucose (mmol/L) | 5.1 ± 0.5 | 5.5 ± 0.8 | .20 |
| Insulin (mU/L) | 7 ± 4 | 11 ± 5 | .05 |
| AdipoIR b | 4.1 ± 2.0 | 7.2 ± 3.4 | .01 |
| Plasma FFA (mmol/L) | 0.57 ± 0.15 | 0.66 ± 0.12 | .11 |
| â-hydroxybutyrate (μmol/L) | 114 ± 90 | 89 ± 61 | .42 |
| Plasma TG (mmol/L) | 1.24 ± 0.57 | 1.51 ± 0.61 | .28 |
| Total cholesterol (mmol/L) | 5.11 ± 0.35 | 5.16 ± 0.86 | .87 |
| LDL-cholesterol (mmol/L) | 3.13 ± 0.29 | 3.27 ± 0.78 | .59 |
| HDL-cholesterol (mmol/L) | 1.26 ± 0.40 | 1.21 ± 0.17 | .70 |
| Dietary Intake | |||
| Total energy intake (kcal/kg BW/day) | 25.7 ± 6.6 | 26.2 ± 6.2 | .84 |
| Fat (%E) | 35 ± 8 | 36 ± 7 | .80 |
| Carbohydrate (%E) | 48 ± 10 | 47 ± 10 | .88 |
| Protein (%E) | 17 ± 4 | 17 ± 5 | .96 |
| Total sugars (%E) | 24 ± 13 | 22 ± 8 | .59 |
| Physical Activity | |||
| Steps/day c | 6451 ± 2968 | 5254 ± 3452 | .43 |
| Daily activity c | |||
| % of day Sedentary | 79.8 ± 8.8 | 79.0 ± 10.5 | .85 |
| % of day in Light Activity | 15.0 ± 6.0 | 16.8 ± 7.8 | .56 |
| % of day in Moderate Activity | 5.2 ± 3.5 | 4.2 ± 3.1 | .50 |
Data are mean ± SD. Abbreviations: %E, percent of total energy intake; BW, body weight.
Biochemistry data represent the average concentrations of these analytes at 0600 and 0800.
AdipoIR was calculated by multiplying the fasting plasma FFA concentration by the fasting insulin concentration 6.
METs were calculated based on counts per minute of activity. Sedentary activity corresponded to 0-1 MET, light activity 1-3 MET, and moderate activity 3-6 MET58. No detection of vigorous activity was found in any subjects’ activity analyses.
Table 2.
Kinetic measures of metabolism
| LowLF (n=11) | HighLF (n=13) | P-value | |
|---|---|---|---|
| Energy Expenditure and Substrate Oxidation a | |||
| Energy expenditure (kcal/kg BW/day) | 14.4 ± 2.9 | 15.5 ± 2.4 | .32 |
| Glucose oxidation (mg/kg BW/min) | 1.07 ± 0.35 | 0.96 ± 0.36 | .48 |
| Fat oxidation (mg/kg BW/min) | 0.55 ± 0.18 | 0.68 ± 0.19 | .10 |
| Insulin Sensitivity Measures b | |||
| SI (10−4 * min−1 per μU/mL) | 3.1 ± 1.4 | 2.2 ± 1.4 | .14 |
| AIRg (μU/mL * min) | 568 ± 407 | 489 ± 304 | .60 |
| Disposition index (unitless) | 1431 ± 668 | 962 ± 637 | .09 |
| Plasma FFA Kinetics c | |||
| Total RaFFA μmol/kg FM/min) | 9.98 ± 2.80 | 12.73 ± 6.30 | .20 |
| Adipose RaFFA μmol/kg FM/min) | 9.57 ± 2.65 | 12.28 ± 6.17 | .19 |
| RaSpillover μmol/kg FM/min) | 0.37 ± 0.29 | 0.43 ± 0.23 | .53 |
| Mean FFA residence time (min) d | 6.54 ± 1.66 | 6.75 ± 2.97 | .84 |
| T½ life (min) | 4.5 ± 1.2 | 4.7 ± 2.1 | .84 |
| VLDL-TG Concentrations and Kinetics e | |||
| VLDL-TG concentration (mmol/L) | 0.39 ± 0.22 | 0.60 ± 0.29 | .07 |
| Total Production Rate (ìmol/min) | 8.6 ± 3.0 | 11.0 ± 4.5 | .16 |
| TG Production from Adipose (ìmol/min) | 4.34 ± 1.82 | 4.06 ± 2.57 | .77 |
| TG Production from Evening Meal (ìmol/min) | 0.46 ± 0.24 | 0.56 ± 0.25 | .39 |
| TG Production from Lipogenesis (ìmol/min) | 0.78 ± 0.42 | 2.57 ± 1.53 | .001 |
| Fractional Catabolic Rate (% / hr) | 28.0 ± 11.1 | 24.8 ± 7.6 | .44 |
| T½ life (h) | 2.9 ± 1.2 | 3.1 ± 1.3 | .65 |
| Clearance Rate (mL/min) | 27.0 ± 12.2 | 20.1 ± 6.3 | .11 |
Data are mean ± SD. Abbreviations: SI, insulin sensitivity index; AIRg, acute insulin response to glucose, RaFFA, rate of appearance of FFA.
Fasting energy expenditure and substrate oxidation were determined at 0800.
Data are calculated from the frequently-sampled insulin-modified IVGTT using MINMOD software.
Plasma FFA kinetics were determined by the average values taken at the end of the study sampling period (1030, 1145).
Mean residence time is the average time a FFA molecule spends in the volume of distribution.
n=11/group; two subjects in the high liver fat (LF) group were excluded from this analysis because they had VLDL-TG concentrations that were more than 2 SD greater than the mean of all other subjects.
Plasma insulin, glucose and FFA concentrations, and sources of plasma FFA
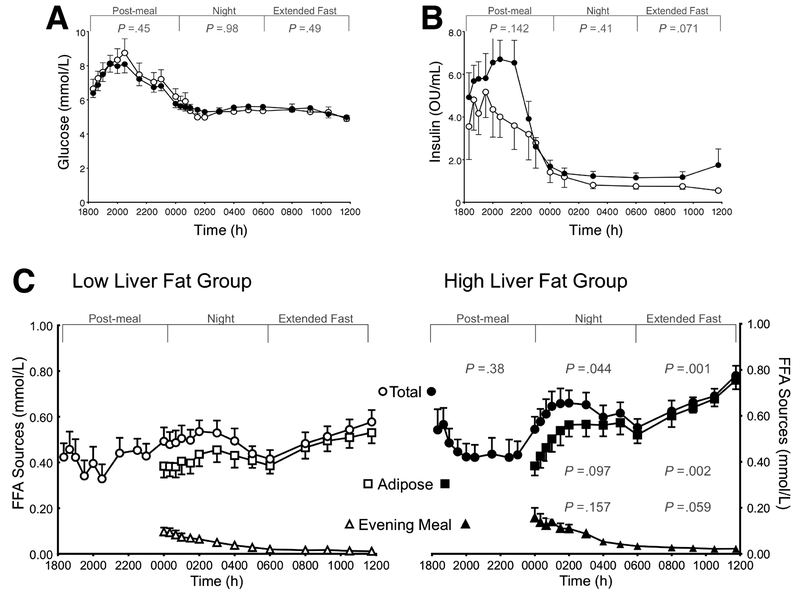
During the 18h of testing, plasma glucose concentrations showed similar dynamics between the groups (fig. 1A). Insulin concentrations in both groups were highly variable among the subjects in the post-evening meal phase; the groups were similar during the night, but the HighLF group tended to exhibit a rise in insulin during the morning fast (P=.07, fig. 1B). Figure 1C shows the FFA concentrations and sources of plasma FFA from midnight until 12 noon. Immediately after the evening meal, total FFA concentrations were not different between the groups (P=.38), but during the night, total concentrations were, on average 30% higher in the HighLF group (P=.044) due primarily to greater adipose-derived FFA (P=.097).
Figure 1. Dynamics of plasma glucose, insulin, and fatty acids.
Data are presented as mean ± SEM. The 18h study period was divided into three segments for analysis: 1800 to midnight (post-evening meal), midnight to 0600 (at night), and 0600 to 1200 (during the extended fast). Panels A displays glucose and (B) insulin concentrations, which were not different between the groups during any of the segments. Panel C: postprandial changes in total plasma free fatty acid (FFA) concentrations (total; circles), those derived from adipose lipolysis (adipose; squares) or from evening-meal spillover (evening meal; triangles) in subjects with High liver fat (HighLF, filled symbols) and Low liver fat (LowLF, open symbols). P-values represent significant differences between the HighLF and LowLF groups for the FFA sources within the designated time period (determined by repeated measures ANOVA).
During the morning fast, total FFA concentrations were significantly higher in the HighLF group (27%, P=. 001) due to a greater adipose-derived FFA (P=.002), and a tendency for greater circulating residual meal-derived FFA at this time (0600-1200, P=.059). Similarly, the calculated rates of appearance of FFA during the night revealed that the peak RaSpillover between midnight and 0300 was two-fold greater in the HighLF group compared to the LowLF group (3.27±2.17 vs. 1.82±1.06 μmol/kg FM/min, respectively, P=.056, data not shown). At the end of the extended fast, neither total RaFFA, adipose RaFFA , nor RaSpillover rates were different between the groups (table 2).
TG-rich lipoprotein TG concentrations and fatty acid sources used for TG synthesis
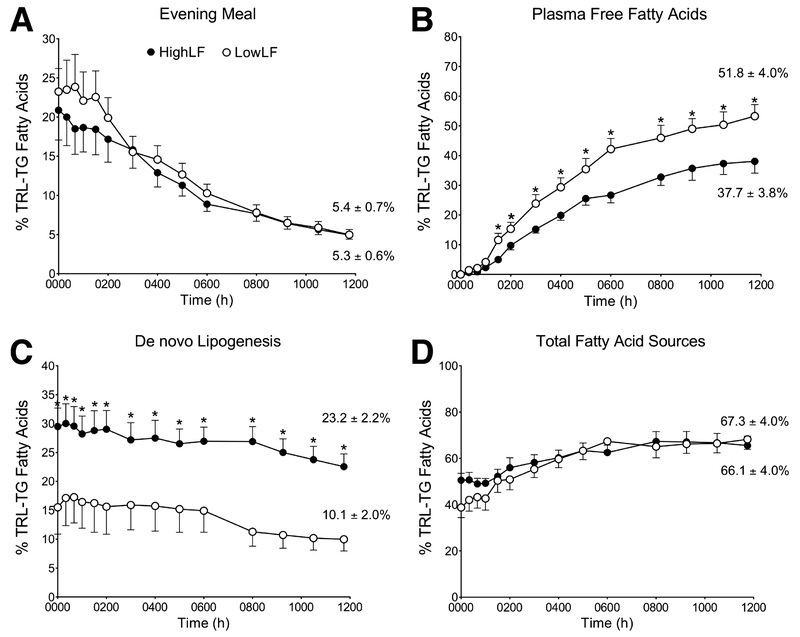
Fasting VLDL-TG concentrations were approximately 50% higher in the HighLF group (table 2). Findings on the contribution of each identified source of FA present in lipoprotein-TG are presented as the relative input into the TG pool over time in figure 2. As shown in figure 2A, previous evening-meal fat represented ca. 22% of FA in TG at midnight for both groups. This label would be carried in chylomicron remnants made following the evening meal and/or in VLDL made from the recycling of chylomicron-TG through the liver11, 12, 19 The proportion of meal-derived FA decayed throughout the night (fig. 2A) and at the end of the experiment the percentages measured by GC/MS were similar between the HighLF and LowLF groups (5.3±0.6% and 5.4±0.7%, respectively, P=.88). The concentration of TRL apoB48 was measured during fasting and some residual apoB48 was found after the morning fast, without appreciable differences between the groups (2.3±1.6 vs. 1.9±1.2 nmol/L, P=.51). Based on these apoB48 concentrations, we estimated the fasting contribution of chylomicron-TG to the TRL fraction, which was calculated to be 5.9±3.9% and 4.0±4.0% for the HighLF and LowLF respectively (P=0.32). These values agreed with the proportion measured by GC/MS described above (e.g., 5.3% and 5.4%).
Figure 2. Proportion of fatty acid sources becoming labeled in TRL-TG from midnight to noon in subjects with low or high liver fat.
Data represent the proportions of fatty acids arising from the (A) evening meal, (B) plasma FFA pool, (C) de novo lipogenesis, and (D) the proportion of total fatty acid sources accounted for in TRL particles at the end of the experiment. Values reported on the right edge of panels A-D represent the average of the last two measurements taken in the fasting state (e.g., 1030 and 1145). Data are presented as mean ± SEM; asterisks denote significant differences between groups at individual time points (P<.05); open circles = Low liver fat (LowLF) group; filled circles = High liver fat (HighLF) group.
The proportion of lipoprotein-TG arising from the plasma FFA pool was significantly lower in the HighLF compared to the LowLF group from 0130 onward (fig. 2B, P<.01). With regard to lipogenesis (fig. 2C), at midnight the fractional contribution was 72% higher in subjects with HighLF (29.5±3.2% and 17.1 ±4.2%, respectively, P=.037) and these group differences were significant at every time point thereafter (P<.05). Fractional contribution from lipogenesis decayed throughout the night for both groups, in agreement with other studies22, 23. However, within the HighLF group, every value from midnight until 1030 was higher than fasting values at 1030 and 1145 (P<.05). In other words, it took at least 16.5h without food for the HighLF subjects to significantly suppress lipogenesis down to basal levels. By contrast, in the LowLF group the fasting level of lipogenesis was reached by at least 0800. The final fasting lipogenesis values at 12 noon were more than doubled in the HighLF group compared to the LowLF group (fig. 2C, P<.001). Finally, for the sum of all the isotopically-identified FA sources found at 1200 (fig. 2D), an identical total proportion of VLDL-TG was identified in the HighLF (66±4%) and LowLF (67±4%) subjects (P=.84). Fatty acid sources remaining unlabeled are anticipated7, 14, 16 and could be derived from visceral stores or previously stored dietary and plasma-derived FA used to make intrahepatic-TG droplets prior to the hours of labeling 7.
Absolute contributions of fatty acid sources to TRL-TG
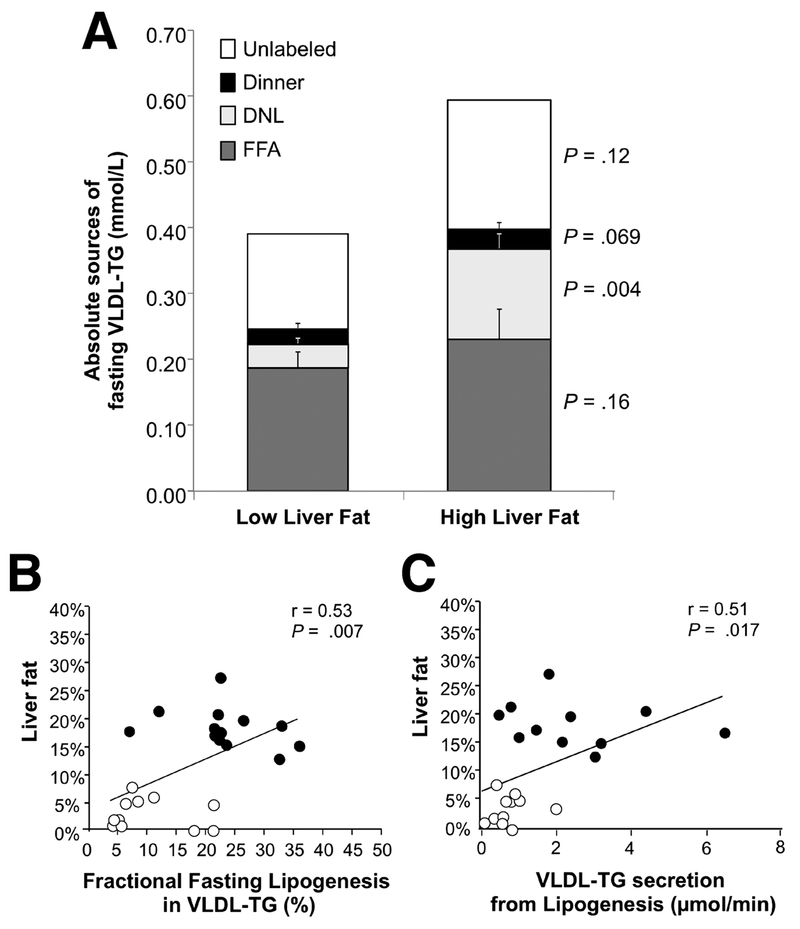
While the proportional contribution of FA sources to VLDL-TG reflects intracellular lipid synthesis14, 35, the absolute quantity of VLDL-TG derived from each source (mmol/L) reflects a balance of the processes of TG secretion, lipoprotein turnover, and clearance from the plasma. As shown in figure 3A, at the end of fasting, the absolute quantities of VLDL-TG made from adipose FFA flux were not different between the HighLF and LowLF groups (0.23±0.05 vs. 0.19±0.03 mmol/L, respectively; P=.44), in agreement with their similar RaFFA rates. This was also true for the residual TG derived from the evening meal (0.03±0.01 and 0.02±0.01 mmol/L, P=.38). The significant difference between the groups was found in the absolute quantity of FA made via de novo lipogenesis, which was 3.5-fold higher in the HighLF group (0.14±0.03 mmol/L) compared to the LowLF group (0.04±0.01 mmol/L, P=.001). The quantity of unidentified VLDL-FA was not different between the groups (P=.22). When the kinetic measurements of VLDL-TG production were applied to these FA sources, the contribution of the de novo lipogenesis pathway was again evident as a 3-fold increase in TG production from this source (table 2). No significant differences were found between the groups in production rate of total-TG or from adipose and meal FA, while the production of TG from lipogenesis was more than three-fold higher in the HighLF group (P=.004). Fractional TG catabolic, TG half-life, and clearance rates were similar between the groups (table 2).
Figure 3. Absolute contributions of fatty acid sources to fasting VLDL-TG in subjects with low or high liver fat and the relationships between liver fat with de novo lipogenesis.
A) The absolute concentrations of fatty acids arising from the evening meal, plasma FFA pool, de novo lipogenesis, and the amount remaining unlabeled in fasting VLDL-TG particles. Values represent the mean group data from each subject in which the last two measurements were taken in the fasting state (e.g., 1030 and 1145) and data from these two time points were averaged for that subject. Relationships between liver fat content and de novo lipogenesis represented as B) newly-synthesized fatty acid in VLDL-TG in units of mmol/L and C) as a fraction of VLDL-TG from lipogenesis (%). Open circles, Low liver fat (LF) group; filled circles, High liver fat (LF) group.
Relationships between fatty acid flux, lipoprotein-TG sources and intrahepatic-TG
Among the factors tested for prediction of liver-TG accrual in these subjects, no associations were found between intrahepatic-TG and BMI, trunk fat, fasting glucose and insulin (data not shown). By contrast, intrahepatic-TG was positively associated with de novo lipogenesis, when lipogenesis was expressed as an absolute quantity of lipogenic FA within VLDL-TG (r=0.60, P=.003) and as a proportion of FA VLDL-TG (fig. 3B). Similarly, intrahepatic-TG was positively correlated with fasting TG secretion arising from lipogenic FA (μmol/min) (fig. 3C).
DISCUSSION
The present study represents the first direct confirmation of the significant role of de novo lipogenesis in the etiology of fatty liver disease. Compared to subjects with LowLF, those with HighLF demonstrated a doubling of the fractional contribution from de novo lipogenesis to VLDL-FA (10.1 ±6.7% vs 23.2±7.9%, respectively, P<.001), a 3-fold higher rate of production of VLDL-TG from lipogenesis, accounted for through extended labeling of the lipogenic pathway and, among all subjects, a positive association between fasting lipogenesis and intrahepatic-TG.
It should be noted that the importance of the de novo lipogenesis pathway in human physiology has been the subject of debate40. Indeed, in lean individuals in energy balance, the contribution of lipogenesis to liver-TG synthesis in the fasting state is low, <5% of VLDL-TG16,17. However, over the past 10 years, data from our lab14, 19 and others17, 18, 23, 41 have demonstrated a significant stimulation of this pathway in the fed state (>25% of VLDL-TG), particularly when dietary carbohydrates are more simple in structure42, consumed in liquid form42, and enter the body at a higher rate12. Furthermore, numerous studies have now documented significant elevations in both fasting and fed lipogenesis in obesity18, insulin resistance41,43, 44, and diabetes23, 24 Previously, indirect evidence of elevated lipogenesis in NAFLD has come from the findings of Fabbrini et al7 and Sevastianova et al30 and muscle insulin resistance has been hypothesized to contribute to NAFLD by shifting dietary carbohydrate away from muscle glycogen synthesis and into hepatic lipogenesis 43 Direct evidence of the role of lipogenesis in NAFLD has come from our lab14 and Diraison et al29, however these observations were made in a small group of subjects (n=5–9) and lacked an obese, non-NAFLD comparison group. By matching control and NAFLD subjects closely for body composition and blood chemistries, the present study significantly strengthens our previous observations in NAFLD14 and underscores elevated lipogenesis as a prominent characteristic of this condition.
The role of insulin resistance appears central to NAFLD development, particularly when it occurs in multiple tissues6, 8. The present data join an established literature supporting adipose insulin resistance as the key contributor to the development of NAFLD5, 7 In the present study, a measure of adipose insulin resistance, the AdipoIR6 provided evidence of excess FA release in the subjects with HighLF, yet the greater availability of plasma FFA did not contribute to significantly greater liver-TG secretion in the form of VLDL. This disconnect between plasma FA flux and VLDL-TG secretion rates has been recently highlighted by Koutsari et al. 45. These findings raise a key unanswered question, “What is the fate of excess plasma FA in the liver?” We have recently shown in a subset of these same subjects46, that those with HighLF exhibited greater rates of liver TCA cycle activity, which is a major pathway of FA utilization. Increased TCA cycle activity would supply the energy needed to support the elevated gluconeogenesis and excess hepatic glucose production47, which we46 and others48, 49 have demonstrated in NAFLD. In the context of these observations, the higher lipogenesis observed here in the HighLF subjects, fits into an overall picture of hepatic insulin resistance in which excess glucose carbon flux provides a common metabolic mechanism for both elevated glucose production and de novo lipogenesis. Indeed, in rodent studies, hepatic insulin resistance is characterized by a bifurcation of insulin signaling in which glucose production is resistant to insulin’s inhibitory effects whereas lipogenesis is not, and is, in fact, stimulated50.
Mounting data have supported the concept that stimulation of lipogenesis can lead to an increased hepatic lipid burden by multiple distinct mechanisms. These include the fact that 1) elevated lipogenesis is the end result of a high flux of carbons down glycolysis (as occurs in fructose feeding, for example20, 26) supplying excess triose phosphates, which serve as the glycerol backbone for TG synthesis51; 2) stimulation of lipogenesis produces FA directly through the fatty acid synthase enzyme complex52, while at the same time 3) elevating intracellular malonyl-CoA concentrations which should inhibit FA oxidation25. If FA oxidation is actually increased in NAFLD 46 this would suggest that the production of malonyl-CoA is insufficient to have a suppressive effect. Lastly, the primary product of lipogenesis is palmitate, a saturated FA24, which may promote inflammation and endoplasmic reticulum stress53, 54.
The strengths of the present study included the comprehensive assessments of characteristics that impact intrahepatic-TG, and the similarity between groups for adiposity, food intake, and physical activity. The primary study limitation relates to the nature of tracer studies, as these intensive protocols typically limit the sample size. Furthermore, the fact that the lipogenesis pathway was labeled longer than the dietary or plasma NEFA pools leaves some question as to the true contribution of the previously unlabeled diet and adipose flux to the unlabeled TG pools remaining at the end of the study. However, within a given source (e.g., diet or lipogenesis), both groups of subjects underwent the same timing of label administration and yet only the fractional contribution from lipogenesis was different between the LowLF and HighLF groups. In addition to these findings, more research is needed to understand the relative lack of circadian control of lipogenesis in NAFLD and whether elevated nocturnal insulin concentrations play a role in the continued stimulation of this pathway during fasting.
As the understanding of NAFLD has evolved, scientists have questioned whether it is excess lipolysis or lipogenesis that causes fatty liver disease55. The present data join the large literature currently available demonstrating that elevated lipogenesis, in combination with excess adipose fatty acid release, is a significant contributor to this condition. Excess lipolysis is found in other conditions such as hypertriglyceridemia and obesity, but the distinctive elevation of lipogenesis in NAFLD suggests a causative role. As a result, these data provide strong support for the recent emergence of carbohydrate restriction as a goal of dietary therapy56, 57. Whether lipogenesis, as a target for pharmaceutical development, will benefit fatty liver has yet to be determined.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to express their appreciation to the research subjects for their participation, to the staff of the Clinical Translational Research Center, and to Dora Bradford, RN-C, WHNP for recruitment and screening of the subjects and excellent patient care. The authors would also like to thank Maressa Valdez, RD, for subject recruitment, clinical coordination, and data collection, and to Joseph Lee, BS, Yelena Hovhannisyan, MS, and Kimberly Borke, MD, for data generation, analysis, and management. We thank Drs. Jay Horton and Scott Grundy for support, Dr. Elizabeth Murphy for advice in planning the D2O labeling procedures, and Dr. Robert Phair for insightful discussions of the data.
Financial Support:
National Institutes of Health grant RL1DK081187 (Elizabeth Parks, PI), the Task Force for Obesity Research at Southwestern (TORS) NIH UL1DE019584 (Jay Horton, PI), and the Clinical Translational Science Award, NIH/NCATS UL1-RR024982 (Robert Toto, PI).
Abbreviations
- apoB48
Apolipoprotein B48
- FA
fatty acids
- FFA
plasma free (non-esterified) fatty acids
- GC/MS
gas chromatography/mass spectrometry
- IHTG
intrahepatic triacylglycerols
- MIDA
mass isotopomer distribution analysis
- 1H-MRS
magnetic resonance spectroscopy
- NAFLD
non-alcoholic fatty liver disease
- RaFFA
rate of appearance of plasma free fatty acids
- RaSpillover
rate of appearance of free fatty acids from lipoprotein-TG lipolysis
- SI
Insulin sensitivity as calculated using the MINMOD program
- TG
triacylglycerols
- TRL
TG-rich lipoproteins
- VLDL
very low-density lipoproteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author names in bold designate shared co-first authors.
None of the authors had any conflicts of interest.
REFERENCES
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 2.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 3.Wattacheril J, Chalasani N. Nonalcoholic fatty liver disease (NAFLD): is it really a serious condition? Hepatology 2012;56:1580–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iozzo P, Turpeinen AK, Takala T, et al. Defective liver disposal of free fatty acids in patients with impaired glucose tolerance. J Clin Endocrinol Metab 2004;89:3496–502. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–1192. [DOI] [PubMed] [Google Scholar]
- 6.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012;55:1389–1397. [DOI] [PubMed] [Google Scholar]
- 7.Fabbrini E, Mohammed BS, Magkos F, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008;134:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korenblat KM, Fabbrini E, Mohammed BS, et al. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008;134:1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mager DR, Mazurak V, Rodriguez-Dimitrescu C, et al. A meal high in saturated fat evokes postprandial dyslipemia, hyperinsulinemia, and altered lipoprotein expression in obese children with and without nonalcoholic fatty liver disease. J Parenter Enteral Nutr. 2012;December 5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Manchanayake J, Chitturi S, Nolan CJ, et al. Postprandial hyperinsulinemia is universal in non-diabetic patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:510–516. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar B, Carey PE, Snaar JE, et al. Real-time assessment of postprandial fat storage in liver and skeletal muscle in health and type 2 diabetes. Am J Physiol Endocrinol Metab 2005;288:E789–97. [DOI] [PubMed] [Google Scholar]
- 12.Timlin MT, Barrows B, Parks EJ. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes 2005;54:2694–2701. [DOI] [PubMed] [Google Scholar]
- 13.Matikainen N, Mänttäri S, Westerbacka J, et al. Postprandial lipemia associates with liver fat content. J Clin Endocrinol Metab 2007;92:3052–3059. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr 1999;53:S53–S65. [DOI] [PubMed] [Google Scholar]
- 16.Parks EJ, Krauss RM, Christiansen MP, et al. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest 1999;104:1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz JM, Neese RA, Turner S, et al. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest 1995;96:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques-Lopes I, Ansorena D, Astiasaran I, et al. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr 2001;73:253–261. [DOI] [PubMed] [Google Scholar]
- 19.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab 2006;91:1446–1452. [DOI] [PubMed] [Google Scholar]
- 20.Parks EJ, Skokan LE, Timlin MT, et al. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 2008;138:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timlin MT, Parks EJ. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am J Clin Nutr 2005;81:35–42. [DOI] [PubMed] [Google Scholar]
- 22.Hudgins LC, Hellerstein MK, Seidman CE, et al. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res 2000;41:595–604. [PubMed] [Google Scholar]
- 23.Vedala A, Wang W, Neese RA, et al. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res 2006;47:2562–2574. [DOI] [PubMed] [Google Scholar]
- 24.Wilke MS, French MA, Goh YK, et al. Synthesis of specific fatty acids contributes to VLDL-triacylglycerol composition in humans with and without type 2 diabetes. Diabetologia 2009;52:1628–1637. [DOI] [PubMed] [Google Scholar]
- 25.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18. [DOI] [PubMed] [Google Scholar]
- 26.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts R, Bickerton AS, Fielding BA, et al. Reduced oxidation of dietary fat after a short term high-carbohydrate diet. Am J Clin Nutr 2008;87:824–831. [DOI] [PubMed] [Google Scholar]
- 28.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 2007;85:1511–1520. [DOI] [PubMed] [Google Scholar]
- 29.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 2003;29:478–485. [DOI] [PubMed] [Google Scholar]
- 30.Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012;96:727–34. [DOI] [PubMed] [Google Scholar]
- 31.Murphy EJ. Stable isotope methods for the in vivo measurement of lipogenesis and triglyceride metabolism. J Anim Sci 2006;84 Suppl: E94–E104. [DOI] [PubMed] [Google Scholar]
- 32.Turner SM, Murphy EJ, Neese RA, et al. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab 2003;285:E790–E803. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol 2006;21:1–6. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Roman MA, Lapidot SA, Phair RD, et al. Insulin activation of plasma nonesterified fatty acid uptake in metabolic syndrome. Arterioscler Thromb Vasc Biol 2012;32:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peter A, Cegan A, Wagner S, et al. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 2009;55:2113–2120. [DOI] [PubMed] [Google Scholar]
- 36.Schneeman BO, Kotite L, Todd KM, et al. Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc Natl Acad Sci U S A 1993;90:2069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson BW. Use of stable isotopically labeled tracers for studies of metabolic kinetics: an overview. Metabolism 1997;46:322–329. [DOI] [PubMed] [Google Scholar]
- 38.Muthusamy K, Nelson RH, Singh E, et al. Effect of insulin infusion on spillover of meal-derived Fatty acids. J Clin Endocrinol Metab 2012;97:4201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz-Lopez C, Lomonaco R, Orsak B, et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 2012;35:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrell GC. PNPLeAse get the fats right: does lipogenesis or lipolysis cause NASH? Hepatology 2010;52:818–21. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz JM, Linfoot P, Dare D, et al. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003;77:43–50. [DOI] [PubMed] [Google Scholar]
- 42.Hudgins LC, Siedman CE, Diakun J, et al. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am J Clin Nutr 1998;67:631–639. [DOI] [PubMed] [Google Scholar]
- 43.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 2007;104:12587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutsari C, Mundi MS, Ali AH, et al. Systemic free fatty acid disposal into very low-density lipoprotein triglycerides. Diabetes 2013; February 22 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunny NE, Parks EJ, Browning JD, et al. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 2011;14:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest 1999;103:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844–1850. [DOI] [PubMed] [Google Scholar]
- 49.Glass LC, Cusi K, Berria R, et al. Pioglitazone improvement of fasting and postprandial hyperglycaemia in Mexican-American patients with Type 2 diabetes: a double tracer OGTT study. Clin Endocrinol (Oxf) 2010;73:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A 2010;107:3441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siler SQ, Neese RA, Parks EJ, et al. VLDL-triglyceride production after alcohol ingestion, studied using [2–13C1] glycerol. J Lipid Res 1998;39:2319–2328. [PubMed] [Google Scholar]
- 52.Shimano H, Horton JD, Hammer RE, et al. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest 1996;98:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003;100:3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 2006;291:E275–81. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology 2006;130:1343–6. [DOI] [PubMed] [Google Scholar]
- 56.Kirk E, Reeds DN, Finck BN, et al. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009;136:1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haufe S, Engeli S, Kast P, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011;53:1504–1514. [DOI] [PubMed] [Google Scholar]
- 58.Crouter SE, Dellavalle DM, Horton M, et al. Validity of the Actical for estimating free-living physical activity. Eur J Appl Physiol 2011;111:1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.