Abstract
Objectives
The primary objective of this study was to examine the effect of vitamin supplementation on health-related quality of life and the risk of elevated depressive symptoms comparable to major depressive disorder (MDD) in HIV-positive pregnant women in Dar es Salaam, Tanzania.
Methods
From April 1995 to July 1997, 1078 HIV-positive pregnant women were enrolled in a randomized controlled trial. We examined the effects of vitamin supplementation on quality of life and the risk of elevated depressive symptoms, assessed longitudinally every 6–12 months.
Results
A substantial prevalence of elevated depressive symptoms (42%) was observed in HIV-positive pregnant women. Multivitamin supplementation (B-complex, C and E) demonstrated a protective effect on depression [relative risk (RR) = 0.78; P = 0.005] and quality of life [RR = 0.72 for social functioning (P = 0.001) and vitality (P = 0.0001); RR = 0.70 for role-physical (P = 0.002)]; however, vitamin A showed no effect on these outcomes.
Conclusions
Multivitamin supplementation (B-complex, C and E) resulted in a reduction in risk of elevated depressive symptoms comparable to MDD and improvement in quality of life in HIV-positive pregnant women in Tanzania.
Keywords: depression, HIV, quality of life, Tanzania, vitamin supplementation
Introduction
Of the 38.6 million individuals with HIV infection worldwide, approximately 64% (24.5 million) are from sub-Saharan Africa [1]. Despite the fact that sub-Saharan Africa bears the largest burden of HIV infection, access to antiretroviral (ARV) therapy for HIV remains limited, with only 310 000 patients receiving treatment by the end of 2004 [2]. This has resulted in dramatic decreases in life expectancy in some sub-Saharan African countries [3], largely as a result of premature mortality from untreated HIV infection. Limited access to ARV therapy has also resulted in excess morbidity from opportunistic infections as well as transmission of HIV, including mother-to-child transmission [2].
Given the excess morbidity and mortality related to HIV infection in resource-poor settings, there is a great need to identify strategies to improve the quality of life and related psychosocial outcomes of HIV-infected patients. Major depression has been shown to be highly prevalent among HIV-positive individuals [4–6] and has been associated with significant reductions in quality of life for these patients [7–13]. In addition, levels of depression or depressive symptoms have been shown to be elevated in HIV-positive as well as HIV-negative pregnant women accessing prenatal, prevention of mother-to-child transmission (PMTCT), or other healthcare services [14–17]. Some micronutrients, in particular the B-complex vitamins, have demonstrated a protective effect on depression; however, the studies in which these findings were obtained were largely cross-sectional and performed in HIV-negative populations [18–22].
Multivitamin supplementation (vitamins B-complex, C and E) has shown a protective effect on disease progression and HIV-related mortality in HIV-positive pregnant women in Dar es Salaam, Tanzania [23]. Provision of the multivitamins at the doses described in that trial may delay the need for antiretrovirals (ARVs), prolonging the ‘healthy’ phase of HIV disease. Promoting pre-ARV therapy, such as chemoprophylaxis, multivitamins and early treatment of opportunistic infections, can also increase access to services for HIV-infected individuals, increasing the likelihood that those individuals may later benefit from the mobilization of resources aimed at expanding access to ARVs in resource-poor settings.
If vitamin supplementation can reduce the burden of depression and improve quality of life in HIV-positive patients, it may also serve as an adjunctive therapy when ARVs are indicated. Given the protective effect of vitamin supplementation on HIV disease progression and mortality [23], the goal of the present analysis was to examine in the same study population the effect of these supplements on depression and health-related quality of life outcomes in HIV-positive pregnant women in Tanzania.
Methods
Study population and design
The study design was a double-blind, placebo-controlled trial to examine the effects of daily supplements of vitamin A (preformed vitamin A and beta carotene) and multivitamins (vitamins B-complex, C and E) on HIV-related outcomes, including perinatal transmission, disease progression, and other health outcomes. Pregnant women were counselled and tested for HIV and informed of their test results at selected health centres in Dar es Salaam. Women who were HIV-positive, resided in Dar es Salaam, were at 12–27 weeks’ gestation, and intended to stay in the city until at least 1 year after delivery were invited to enrol in the randomized controlled trial. From April 1995 to July 1997, 1078 HIV-positive pregnant women were enrolled in the trial. Women were randomly assigned to one of four arms based on a 2 × 2 factorial design: vitamin A alone (30 mg of beta carotene plus 5000 IU of preformed vitamin A); multivitamins excluding vitamin A (20 mg of B1, 20 mg of B2, 25 mg of B6, 100 mg of niacin, 50 mg of B12, 500 mg of C, 30 mg of vitamin E and 0.8 mg of folic acid); multivitamins plus vitamin A in the same doses above; or placebo. Women in the two groups that received vitamin A were given an additional oral dose of 200 000 IU of vitamin A at delivery; a placebo was given to the women at delivery for the other two groups. Study participants were followed monthly, and later quarterly, until August 2003 for an average of 38.7 months (median 44.4 months; standard deviation 24.7 months). T-cell subsets (including CD4) were measured at enrolment and every 6 months using the FACScount and FACSCAN systems (Becton Dickinson, San Jose, CA, USA). HIV disease stage was assessed on a monthly basis according to World Health Organization (WHO) criteria [24]. More detailed description of the study design and recruitment process has been provided elsewhere by Fawzi et al. [25,26].
In addition to the HIV-related outcomes described above, a psychosocial assessment was performed throughout the follow-up period, starting with 2 months after enrolment on average (range 0.8–4 months), 2 months after delivery, every 6 months until 2001, and every 12 months thereafter. The mean number of psychosocial assessments was 6 (median 7; standard deviation 3.5). The psychosocial questionnaire assessed depression/anxiety symptoms; health-related quality of life; disability; HIV-related life events; perceived social support; and disclosure of HIV test result. Outcomes analysed for the current study included depression and health-related quality of life as described in more detail below.
Measures
Depressive symptoms
A validated subset of items from the Hopkins Symptom Checklist-25 (HSCL-25) was used to assess depressive symptoms comparable to major depressive disorder (MDD). The original scale included a 10-item anxiety scale and a 15-item depression scale [27]. The HSCL was validated in Tanzania to identify MDD in the study population. Kaaya et al. [28] developed a subscale that included eight of the original 25 items. This subscale demonstrated a sensitivity of 88% and a specificity of 89% for detecting MDD according to diagnostic and statistical manual of mental disorders, 4th edition (DSM-IV) criteria and included the following symptoms: feeling sad; feeling trapped or caught; difficulty falling asleep, staying asleep; worrying too much about things; heart pounding or racing; feeling hopeless about the future; faintness, dizziness or weakness; and crying easily. Each symptom was scored by severity, ranging from ‘1 = not at all’ to ‘4 = extremely’, and the overall scale score was based on the total score divided by the number of items. Women who had an average symptom score that was greater than 1.06 on this subscale were classified as having a symptom level comparable to MDD based on results from the validation study by Kaaya et al. [28].
Health-related quality of life
The Medical Outcomes Study Short Form-36 (SF-36) was used to assess health-related quality of life. The SF-36 has been used in a wide variety of settings in individuals suffering from a broad range of conditions, such as heart disease, diabetes and depression, as well as other illnesses [29]. The SF-36 has also been validated in a representative urban population in Tanzania [30]. Among adults in Dar es Salaam, Tanzania, individuals who were ‘healthy’ demonstrated significantly higher health-related quality of life scores compared with those who were ill or disabled [30]. This finding was observed for all eight dimensions of health-related quality of life that were included in the current analysis: physical functioning; role-physical; bodily pain; general health; vitality; social functioning; role-emotional; and mental health. We used the 25th percentile of these eight continuous subscales as the cut-off to define ‘poor’ health-related quality of life.
Statistical analysis
We compared characteristics at the time of enrolment for women on different regimens (i.e. multivitamins vs no multivitamins and vitamin A vs no vitamin A), including women’s age, education, HIV disease stage and CD4 count, using the GLM procedure of Statistical Analysis Software (SAS; SAS Institute, Cary, NC, USA) for continuous variables and the χ2 test for categorical variables (n = 1013). The percentages or mean scores of the depressive symptoms and health-related quality of life variables at first psychosocial assessment were calculated for 912 study participants who had this first assessment performed 2 months after enrolment on average. We also calculated the percentages and means for these psychosocial variables, stratifying by regimen status; P-values were generated for continuous and categorical variables based on the same procedures described above.
We assessed the effects of vitamin regimens on a level of depressive symptoms comparable to MDD and health-related quality of life by comparing the effect of multivitamins with no multivitamins as well as vitamin A with no vitamin A, given the factorial design of the trial; an ‘intent to treat’ analysis was performed. Generalized estimating equations (GEEs) [31] were employed with the genmod procedure of SAS statistical software. Exchangeable working covariance structure and a working binomial distribution were used. These associations were examined longitudinally, allowing for the examination of the incidence of elevated depressive symptom levels as well as ‘poor’ health-related quality of life during the follow-up period. We checked for interactions between multivitamins and vitamin A, and the modifiers of the regimens by baseline characteristics, including CD4 count, CD8 count, HIV disease stage, mid-upper arm circumference, total lymphocyte count, plasma vitamin A and E concentrations, haemoglobin concentration, viral load, social support, and participation in counselling (group and/or individual).
Results
Of 1078 women in the study, a total of 1013 had at least one psychosocial assessment and 912 had their first assessment 2 months after enrolment on average. The mean age of the HIV-positive pregnant women with at least one psychosocial assessment (n = 1013) was 25 years at baseline, with minimal variability by the multivitamins or vitamin A regimens (Table 1). In terms of other factors assessed at baseline, there were no statistically significant differences between the multivitamins and no multivitamins groups or between the vitamin A and no vitamin A groups; these factors included education, WHO disease stage, and CD4 count. The majority of HIV-positive women at baseline were at stage 1 of HIV disease (ranging from 80.1 to 82.2%) and had CD4 counts of 200 cells/µL or greater (ranging from 86.5 to 87.9%) (Table 1).
Table 1.
Baseline characteristics of HIV-positive pregnant Tanzanian women (n = 1013)
| Characteristics | Multivitamins |
Vitamin A |
||||
|---|---|---|---|---|---|---|
| Yes (n = 508) | No (n = 505) | P-value | Yes (n = 506) | No (n = 507) | P-value* | |
| Age (years) | 24.7 (4.7) | 24.7 (4.9) | 0.89 | 24.8 (4.8) | 24.7 (4.7) | 0.74 |
| Education (%) | 0.35 | 0.34 | ||||
| None or adult | 8.9 | 7.5 | 7.3 | 9.1 | ||
| Primary 1–4 years | 5.3 | 5.2 | 4.3 | 6.1 | ||
| Primary 5–8 years | 74.0 | 78.4 | 77.1 | 75.3 | ||
| >8 years | 11.8 | 8.9 | 11.3 | 9.5 | ||
| WHO disease stage (%) | 0.21 | 0.20 | ||||
| 1 | 81.9 | 80.4 | 80.1 | 82.2 | ||
| 2 | 17.5 | 17.9 | 18.1 | 17.2 | ||
| 3 | 0.6 | 1.7 | 1.8 | 0.6 | ||
| CD4 count (cells/µL) (%) | 0.52 | 0.09 | ||||
| 0–199 | 12.1 | 13.5 | 12.4 | 13.2 | ||
| 200–349 | 24.0 | 26.2 | 28.2 | 21.9 | ||
| ≥ 350 | 63.9 | 60.3 | 59.4 | 64.9 | ||
| CD4 count (cells/µL) | 424 (201) | 413 (199) | 0.40 | 414 (202) | 423 (197) | 0.51 |
Values are given as the percentage or mean (with standard deviation).
P-values were generated using the glm procedure of SAS for continuous variables and the χ2 test for categorical variables.
Given that the 1013 women with at least one psychosocial assessment were a subset of the 1078 women, we compared sociodemographic variables and other characteristics at baseline for the 1013 women with those for the remaining 65 who did not have at least one psychosocial assessment. The groups were comparable with respect to level of education. The difference for age was small and marginally significant (24.7 years vs 23.5 years; P = 0.05), with the 65 women younger on average. Also, the 65 women had a higher mean CD4 count (P = 0.007), and a greater proportion of these women were at HIV WHO stage 2 or higher (P = 0.03).
Table 2 shows data for the first psychosocial assessment performed 2 months after enrolment on average (n = 912). There was a fairly high burden of depressive symptoms comparable to MDD in this population (42.4%). However, mean scores for the eight dimensions of health-related quality of life were fairly good, with the physical functioning subscale demonstrating the highest score (94.2), followed by social functioning (93.1), mental health (90.6), and role-emotional (89.3). General health demonstrated the lowest overall score (80.8) in this population of HIV-positive pregnant women. For depressive symptoms, there were no significant differences at first assessment between the multivitamins and the no multi-vitamins groups or between the vitamin A and no vitamin A groups. Health-related quality of life scores were higher for the multivitamins group compared with the no multivitamins group; however, no differences in health-related quality of life were observed for the vitamin A vs no vitamin A groups.
Table 2.
Psychosocial characteristics of women at first psychosocial assessment (2 months after randomization on average; n = 912)
| Outcome | Overall | Multivitamins |
Vitamin A |
||||
|---|---|---|---|---|---|---|---|
| Yes (n = 457) | No (n = 455) | P-value | Yes (n = 461) | No (n = 451) | P-value* | ||
| Elevated level of depressive symptoms (%)† | 42.4 | 40.0 | 44.8 | 0.14 | 45.6 | 39.3 | 0.05 |
| Physical functioning | 94.2 (11.0) | 95.0 (10.3) | 93.4 (11.6) | 0.02 | 94.4 (10.9) | 94.0 (11.1) | 0.54 |
| Role-physical | 85.8 (31.3) | 90.0 (27.0) | 81.7 (34.6) | 0.0001 | 85.7 (31.5) | 85.9 (31.1) | 0.93 |
| Bodily pain | 85.2 (20.8) | 88.3 (18.2) | 82.1 (22.8) | 0.0001 | 85.0 (21.6) | 85.5 (20.1) | 0.69 |
| General health | 80.8 (18.9) | 83.2 (17.4) | 78.5 (20.0) | 0.0002 | 80.4 (19.1) | 81.3 (18.6) | 0.49 |
| Vitality | 88.1 (18.4) | 90.8 (15.9) | 85.3 (20.3) | 0.0001 | 88.0 (18.7) | 88.2 (18.2) | 0.87 |
| Social functioning | 93.1 (15.5) | 95.1 (12.3) | 91.0 (18.0) | 0.0001 | 92.6 (15.9) | 93.5 (15.1) | 0.39 |
| Role-emotional | 89.3 (27.8) | 92.1 (24.1) | 86.5 (30.8) | 0.002 | 88.0 (29.4) | 90.5 (26.0) | 0.17 |
| Mental health | 90.6 (16.5) | 92.2 (15.2) | 89.0 (17.5) | 0.004 | 89.8 (17.1) | 91.5 (15.8) | 0.12 |
Values are given as the percentage or mean (with standard deviation).
P-values were generated using the glm procedure of SAS for continuous variables and the χ2 test for categorical variables.
An elevated level of depressive symptoms is defined as having a score greater than 1.06 on the validated eight-item Hopkins Symptom Checklist.
In examining the effect of vitamin supplementation on a level of symptoms comparable to MDD and health-related quality of life, it was found that the multivitamin supplement demonstrated a reduced incidence of elevated depressive symptoms [relative risk (RR) 0.78; 95% confidence interval (CI) 0.66, 0.92]. A similar pattern was observed for health-related quality of life, in which the multivitamins had a protective effect with respect to reduced incidence of poor health-related quality of life (Table 3). The strongest protective effects were observed for the role-physical (RR 0.70; 95% CI 0.57, 0.88), vitality (RR 0.72; 95% CI 0.61, 0.84), and social functioning (RR 0.72; 95% CI 0.59, 0.88) dimensions of health-related quality of life. No associations between the vitamin A supplement and depressive symptoms or health-related quality of life were observed and no interactions between the multivitamins and vitamin A were demonstrated. In addition, for the effect of multivitamin supplements, we did not observe any effect modification by baseline characteristics, including CD4 count, HIV disease stage and haemoglobin concentration.
Table 3.
Effects of vitamin supplementation on psychosocial outcomes in HIV-positive pregnant women (n = 1013)
| Multivitamins compared with no multivitamins |
Vitamin A compared with no vitamin A |
|||
|---|---|---|---|---|
| Outcome | RR* (95% CI) | P-value | RR* (95% CI) | P-value |
| Elevated level of depressive symptoms† | 0.78 (0.66, 0.92) | 0.005 | 1.15 (0.97, 1.38) | 0.10 |
| Physical functioning | 0.76 (0.63, 0.90) | 0.002 | 1.00 (0.84, 1.20) | 0.98 |
| Role-physical | 0.70 (0.57, 0.88) | 0.002 | 1.01 (0.82, 1.26) | 0.91 |
| Bodily pain | 0.81 (0.69, 0.95) | 0.008 | 1.03 (0.88, 1.20) | 0.73 |
| General health | 0.77 (0.66, 0.90) | 0.0007 | 1.05 (0.90, 1.22) | 0.54 |
| Vitality | 0.72 (0.61, 0.84) | 0.0001 | 1.03 (0.88, 1.20) | 0.74 |
| Social functioning | 0.72 (0.59, 0.88) | 0.001 | 1.09 (0.90, 1.32) | 0.38 |
| Role-emotional | 0.80 (0.64, 1.01) | 0.06 | 1.13 (0.90, 1.42) | 0.31 |
| Mental health | 0.82 (0.70, 0.96) | 0.01 | 0.99 (0.84, 1.16) | 0.87 |
Relative risk (RR) of depression and ‘poor’ health-related quality of life (< 25th percentile for each subscale) during follow-up, estimated from generalized estimating equations (GEEs), controlled for the other regimen.
An elevated level of depressive symptoms is defined as having a score greater than 1.06 on the validated eight-item Hopkins Symptom Checklist.
CI, confidence interval.
Discussion
The prevalence of a level of depressive symptoms comparable to MDD in our cohort of HIV-positive pregnant women was 42.4% at first psychosocial assessment. This finding is consistent with those of other studies of HIV-positive women, which have demonstrated rates of major depression from 32 to 62% [4–6]. The level of depressive symptomatology in the present study was also comparable to that found in studies of HIV-positive and HIV-negative women accessing PMTCT services in South Africa (41%) [15] as well as HIV-positive women who had recently given birth in Thailand (43%) [32]. The results from the health-related quality of life subscale scores were either comparable to or higher than scores obtained from a random sample of adults from a large household survey conducted in Dar es Salaam, Tanzania (ranging from 62.9 to 93.8 vs 80.8 to 94.2 for the current study). It is important to note that the women in our study sample were primarily in the earlier stages of HIV disease and were younger on average than the study population included in the Dar es Salaam survey [30]. Asymptomatic HIV-infected patients in Venezuela demonstrated higher health-related quality of life scores compared with those with symptomatic HIV infection or AIDS [33]. The subscale scores of this asymptomatic HIV-positive study population from Venezuela were comparable to or lower than the mean scores presented by the current study population (ranging from 66.7 to 88.6) [33].
A number of studies have demonstrated an association between low levels of B vitamins and depressive symptoms or depression [18,19,21]. Clinical studies have shown systematically low folate levels in depressed patients [34– 36]. In middle-aged men in Finland there was nearly a 50% increase in the risk of depression for those who were in the lowest tertile of folate intake compared with those in the highest tertile [18]. Similarly, in a study of the general US population (ages 15–39 years), folate levels were on average significantly lower in those who had major depression compared with those who were not depressed [37]. There is also some indication of an association between vitamin B12 deficiency and depression [19,20]. Tiemeier et al. [19] reported that those with vitamin B12 deficiency were nearly 70% more likely to have a depressive disorder compared with those without B12 deficiency. Among HIV-positive and HIV-negative homosexual men, lower vitamin B12 level was associated with depressive symptoms; this association was also observed for major depressive disorder in a multivariate logistic regression model [22]. For other B-complex vitamins (vitamins B1, B2 and B6), Bell et al. [21] observed a trend towards a greater reduction in depressive symptoms and a larger increase in serum nortriptyline level for those patients who received the vitamin B complex [21].
Several possible mechanisms may explain the association between vitamin B12, folate levels and depression. Both vitamin B12 and folate are involved in methylation processes that can impact upon neurotransmitter metabolism [38]. The availability of a folate metabolite (5,6,7,8-tetrahydrobiopterin) affects the metabolism of serotonin (5-HT); low central nervous system concentrations of 5-HT have been linked to depressive illness [38]. Cerebral spinal fluid (CSF) 5-hydroxyindole acetic acid (5-HIAA), a metabolite of 5-HT, was lower in a group of depressed patients who were folate deficient compared with control patients with neurological disorders; interestingly, among depressed patients in the same study population, red cell folate was positively correlated with CSF 5-HIAA levels [35].
The association between low vitamin B12 and folate levels and depression may also indicate the role of oxidative stress. Widner et al. [39] suggest that the relationship between reduced folate availability and disturbed monoamine metabolism that is found in depression may manifest as elevated homocysteine levels, which can be linked through enhanced oxidative stress. Bottiglieri et al. [40] observed elevated levels of homocysteine in 52% of depressed inpatients studied; depressed patients also demonstrated a higher mean value of total plasma homocysteine compared with normal and neurological control groups. Among those with major depression, oxidative stress in red blood cell membranes and decreased antioxidant defences have also been observed [41,42].
In contrast to the B vitamins, there is limited empirical evidence to support the association between vitamins C and E and depression or depressive symptoms, despite the fact that they are also antioxidants. In a small placebo-controlled crossover trial (n = 23) in patients with mood disorders (major depression and bipolar illness), large doses of ascorbic acid (3g) were associated with a reduction in severity of depression compared with placebo [43]. Similarly, in case reports of four patients, provision of intravenous vitamin C resulted in a reduction of depressive symptoms [44]. Maes et al. [41] found that vitamin E level was significantly lower in depressed patients compared with healthy controls; however, among those who were depressed there was no association between vitamin E and severity of depressive symptoms [41]. In elderly people in Japan, a reduction of depressive symptoms was associated with an increase in alpha-tocopherol over a 4-year period, but this was observed only in men [45]. No association between vitamin E level and depressive symptoms was observed in a large cohort (n = 3884) of adults aged 60 years and older who participated in the population-based Rotterdam Study in the Netherlands [46].
In terms of quality of life, there are relatively few studies that have examined the association between vitamin supplementation and health-related quality of life. A randomized placebo-controlled study of the effects of vitamin B12 was performed in individuals with elevated plasma methylmalonic acid in Denmark [47]. These authors found that only one of eight dimensions of health-related quality of life had improved with administration of vitamin B12 (general health) in this population [47]. Similarly, in a trial of pravastatin and vitamin E, no significant changes on health-related quality of life scores were observed for vitamin E after 12 months of therapy [48]. Among patients with congestive heart failure who received vitamin E as part of a clinical trial, no changes in quality of life were observed after 12 weeks of treatment [49].
In the present study, multivitamin supplementation (vitamins B-complex, C and E) demonstrated a protective effect on the incidence of a level of depressive symptoms comparable to MDD and poor health-related quality of life in HIV-positive pregnant women in Dar es Salaam, Tanzania. There are a number of pathways in which this observation can be explained. First, based on the existing literature, it is possible that the folate and vitamin B12 elements of the multivitamin had a protective effect on depressive symptoms in this population. For folate in particular, this association may have occurred through two possible mechanisms: increases in folate levels may have resulted in an increase in 5-HT/5-HIAA in this population; alternatively, the multivitamins may have resulted in a reduction in oxidative stress (Fig. 1). As indicated in the literature cited above [34,38,39,41,42], both of these mechanisms might account for the reduction in risk of depressive symptoms. Although the multivitamin supplementation may have directly impacted health-related quality of life, there is minimum prior evidence for this type of association. Therefore, it is more likely that the reduction in depressive symptoms resulted in improvements in quality of life; the association between depression and quality of life has also been reported in a number of studies in HIV-positive individuals [7–13].
Fig. 1.
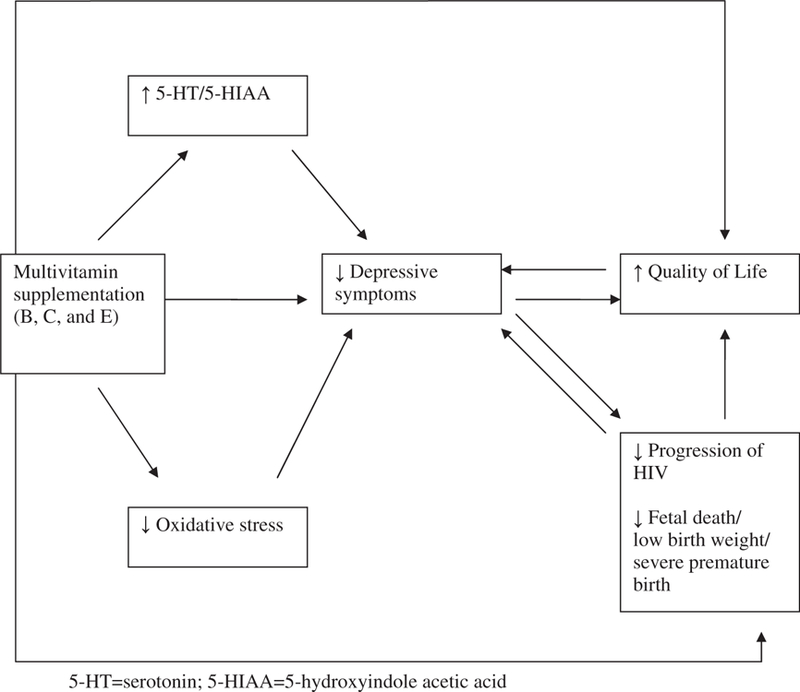
Potential mechanisms for the effect of multivitamin supplementation on depressive symptoms and health-related quality of life.
In addition, there is evidence that the multivitamin supplements reduced progression of HIV disease [23] and the risk of fetal loss, low birth weight, and severe preterm birth [50] among women in the present study population, which may also have resulted in an improvement in quality of life and a reduction in the risk of depressive symptoms in these HIV-positive pregnant women. Given the potential impact of depression on the immune system [51–53], some researchers have examined the association between depression and the progression of HIV disease. Whereas some studies have demonstrated a link between depression and HIV disease progression [5,54,55], others have not [56–58]. The association between depression and HIV disease progression is currently being analysed in the present study population. If such a relationship exists in the current study population, then a reduction in depression may have had an impact on the rate of progression of HIV disease.
There are a number of limitations in the present study. Firstly, given that the multivitamin supplement included vitamins B-complex, C and E, it is not possible to tease apart the effect of specific vitamins on the level of depressive symptoms and quality of life in this population. Although prior literature provides more support for the association between B-complex vitamins and depression, one cannot infer that association in a definitive manner from this study. Secondly, while we can present different potential mechanisms for the impact of the multivitamins on depression and quality of life, we are not able to provide a definitive interpretation. This is reflected in Fig. 1, where various explanations for this association are delineated. While it is difficult to tease apart depression from HIV-related symptoms, the confluence of the two is minimized in this study as only one of the eight items from the HSCL-revised scale can also serve as a somatic symptom of HIV disease (faintness, dizziness or weakness). Although diagnosis of MDD by a psychiatrist was not feasible for this large study, the validation substudy [28] provided data that allowed us to identify women who demonstrated a level of depressive symptoms comparable to major depression. While elevated levels of depressive symptoms have been documented in HIV-positive as well as HIV-negative pregnant women [14–17], as all of the women in the study were pregnant at the time of enrolment this should not pose a threat to internal validity. However, there are limitations in generalizability of these results to nonpregnant populations, although we anticipate that the results would be relevant for pregnant women in other resource-poor settings. Strengths of the study are the randomized controlled trial design to minimize the effects of confounding and bias and the longitudinal follow up which allowed examination of the direction of the relationship between vitamin supplementation and depression as well as health-related quality of life outcomes.
In conclusion, multivitamin supplementation (vitamins B-complex, C and E) but not supplementation with vitamin A resulted in a reduction in a level of depressive symptoms comparable to MDD and improvement in quality of life in HIV-positive pregnant women in Tanzania. This finding should be explored further, particularly in terms of the potential impact of multivitamin supplementation on depression in HIV-negative populations in Tanzania and other resource-limited settings. For HIV-positive populations, we recommend providing multivitamin supplementation (vitamins B-complex, C and E) at the doses described in this study, given the observed reduction in risk of depressive symptoms and improvement in quality of life. For patients who require ARVs, multivitamin supplementation may serve as an adjunctive therapy to potentially enhance quality of life. In addition, reducing the risk of depression and improving quality of life among HIV-positive patients may positively impact utilization of ARVs. Cook et al. [59] observed that women with high levels of depressive symptoms and poor mental health quality of life were less likely to utilize highly active antiretroviral therapy (HAART) and those who received mental health services had increased probability of accessing HAART. This intervention has the capacity to prevent disease progression and mortality [23] and it has been demon-strated to reduce the burden of depression and improve quality of life in patients with HIV infection.
Acknowledgements
We are indebted to the mothers and children, the field teams, including physicians, nurses, midwives and supervisors, the laboratory staff and the administrative staff who made the study possible. This work was supported by the National Institute of Child Health and Human Development (NICHD R01 32257), the National Institute of Mental Health (NIMH R03 MH55451) and the Fogarty International Center at the National Institutes of Health. Hoffmann-La Roche donated the raw material that was used for preparing the vitamin and placebo tablets.
References
- 1.UNAIDS. 2006 Report on the Global AIDS Epidemic http://www.UNAIDS.org 2006.
- 2.Joint United Nations Programme on HIV/AIDS/World Health Organization (UNAIDS/WHO). “3 by 5” Progress Report, December 2004 http://www.who.int/3by5/en/ProgressReportfinal.pdf 2004.
- 3.World Bank. Confronting AIDS: Public Priorities in a Global Epidemic, Revised Edition. New York, NY: Oxford University Press, 1999. [Google Scholar]
- 4.Cook JA, Grey D, Burke J et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health 2004; 94: 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ickovics JR, Hamburger ME, Vlahov D et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. J Am Med Assoc 2001; 285: 1466–1474. [DOI] [PubMed] [Google Scholar]
- 6.Moore J, Schuman P, Schoenbaum E, Boland B, Solomon L, Smith D. Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. AIDS 1999; 13: 2459–2468. [DOI] [PubMed] [Google Scholar]
- 7.Tostes MA, Chalub M, Botega NJ. The quality of life of HIV-infected women is associated with psychiatric morbidity. AIDS Care 2004; 16: 177–186. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M, Nishimura K, Inoue T, Kimura S, Oka S. QoL Research Group of the AIDS Clinical Centre and Eight Regional AIDS Treatment Hospitals in Japan. A discriminative study of health-related quality of life assessment in HIV-1-infected persons living in Japan using the Multidimensional Quality of Life Questionnaire for persons with HIV/AIDS. Int J STD AIDS 2004; 15: 107–115. [DOI] [PubMed] [Google Scholar]
- 9.Kemmler G, Schmied B, Shetty-Lee A et al. Quality of life of HIV-infected patients: psychometric properties and validation of the German version of the MQOL-HIV. Qual Life Res 2003; 12: 1037–1050. [DOI] [PubMed] [Google Scholar]
- 10.Riley ED, Bangsberg DR, Perry S, Clark RA, Moss AR, Wu AW. Reliability and validity of the SF-36 in HIV-infected homeless and marginally housed individuals. Qual Life Res 2003; 12: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 11.Tate D, Paul RH, Flanigan TP et al. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care STDs 2003; 17: 115–120. [DOI] [PubMed] [Google Scholar]
- 12.Molassiotis A, Callaghan P, Twinn SF, Lam SW. Correlates of quality of life in symptomatic HIV patients living in Hong Kong. AIDS Care 2001; 13: 319–334. [DOI] [PubMed] [Google Scholar]
- 13.Sherbourne CD, Hays RD, Fleishman JA et al. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry 2000; 157: 248–254. [DOI] [PubMed] [Google Scholar]
- 14.Blaney NT, Fernandez MI, Ethier KA, Wilson TE, Walter E, Koenig LJ. Psychosocial and behavioral correlates of depression among HIV-infected pregnant women. AIDS Patient Care STDs 2004; 18: 405–415. [DOI] [PubMed] [Google Scholar]
- 15.Rochat TJ, Richter LM, Doll HA, Buthelezi NP, Tomkins A, Stein A. Depression among pregnant rural South African women undergoing HIV testing. J Am Med Assoc 2006; 295: 1376–1378. [DOI] [PubMed] [Google Scholar]
- 16.Ethier KA, Ickovics JR, Fernandez MI, Wilson TE, Royce RA, Koenig LJ. The Perinatal Guidelines Evaluation Project HIV and Pregnancy Study: overview and cohort description. Public Health Rep 2002; 117: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwalombota M The effect of pregnancy in HIV-infected women. AIDS Care 2002; 14: 431–433. [DOI] [PubMed] [Google Scholar]
- 18.Tolmunen T, Voutilainen S, Hintikka J et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr 2003; 133: 3233–3236. [DOI] [PubMed] [Google Scholar]
- 19.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry 2002; 159: 2099–2101. [DOI] [PubMed] [Google Scholar]
- 20.Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B(12) deficiency and depression in physically disabled older women. epidemiologic evidence from the Women’s Health and Aging Study. Am J Psychiatry 2000; 157: 715–721. [DOI] [PubMed] [Google Scholar]
- 21.Bell IR, Edman JS, Morrow FD et al. Brief communication. Vitamin B1, B2, and B6 augmentation of tricyclic antidepressant treatment in geriatric depression with cognitive dysfunction. J Am Coll Nutr 1992; 11: 159–163. [PubMed] [Google Scholar]
- 22.Baldewicz TT, Goodkin K, Blaney NT et al. Cobalamin level is related to self-reported and clinically rated mood and to syndromal depression in bereaved HIV-1( 1 ) and HIV-1( ) homosexual men. J Psychosom Res 2000; 48: 177–185. [DOI] [PubMed] [Google Scholar]
- 23.Fawzi WW, Msamanga GI, Spiegelman D et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med 2004; 351: 23–32. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). Interim proposal for a WHO staging system for HIV infection and disease. Wkly Epidemiol Rec 1990; 65: 221–244. [PubMed] [Google Scholar]
- 25.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, Hunter DJ. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials 1999; 20: 75–90. [DOI] [PubMed] [Google Scholar]
- 26.Fawzi WW, Msamanga GI, Wei R et al. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child’s morbidity and CD4 1cell counts. Clin Infect Dis 2003; 36: 1053–1062. [DOI] [PubMed] [Google Scholar]
- 27.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry 1974; 7: 79–110. [DOI] [PubMed] [Google Scholar]
- 28.Kaaya SF, Smith Fawzi MC, Mbwambo JK, Lee B, Msamanga GI, Fawzi W. Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatr Scand 2002; 106: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware J, Snow K, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide Boston, MA: The Health Institute, 1993. [Google Scholar]
- 30.Wyss K, Wagner AK, Whiting D et al. Validation of the Kiswahili version of the SF-36 Health Survey in a representative sample of an urban population in Tanzania. Qual Life Res 1999; 8: 111–120. [DOI] [PubMed] [Google Scholar]
- 31.Diggle PJ, Liang KY, Zeger SI. Analysis of Longitudinal Data London, UK: Oxford University Press, 1994. [Google Scholar]
- 32.Bennetts A, Shaffer N, Manopaiboon C et al. Determinants of depression and HIV-related worry among HIV-positive women who have recently given birth, Bangkok, Thailand. Social Sci Med 1999; 49: 737–749. [DOI] [PubMed] [Google Scholar]
- 33.Bastardo YM, Kimberlin CL. Relationship between quality of life, social support and disease-related factors in HIV-infected persons in Venezuela. AIDS Care 2000; 12: 673–684. [DOI] [PubMed] [Google Scholar]
- 34.Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 1997; 154: 426–428. [DOI] [PubMed] [Google Scholar]
- 35.Bottiglieri T, Hyland K, Laundy M et al. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med 1992; 22: 871–876. [DOI] [PubMed] [Google Scholar]
- 36.Abou-Saleh MT, Coppen A. Serum and red blood cell folate in depression. Acta Psychiatr Scand 1989; 80: 78–82. [DOI] [PubMed] [Google Scholar]
- 37.Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US population. Psychother Psychosom 2003; 72: 80–87. [DOI] [PubMed] [Google Scholar]
- 38.Folate Bottiglieri T., vitamin B12, and neuropsychiatric disorders. Nutr Rev 1996; 54: 382–390. [DOI] [PubMed] [Google Scholar]
- 39.Widner B, Fuchs D, Leblhuber F, Sperner-Unterweger B. Does disturbed homocysteine and folate metabolism in depression result from enhanced oxidative stress? J Neurol Neurosurg Psychiatry 2001; 70: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry 2000; 69: 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maes M, De Vos N, Pioli R et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord 2000; 58: 241–246. [DOI] [PubMed] [Google Scholar]
- 42.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry 1998; 43: 315–319. [DOI] [PubMed] [Google Scholar]
- 43.Anonymous. Vanadium vitamin C and depression. Nutr Rev 1982; 40: 293–295. [DOI] [PubMed] [Google Scholar]
- 44.Cocchi P, Silenzi M, Calabri G, Salvi G. Antidepressant effect of vitamin C. Pediatrics 1980; 65: 862–863. [PubMed] [Google Scholar]
- 45.Shibata H, Kumagai S, Watanabe S, Suzuki T. Relationship of serum cholesterols and vitamin E to depressive status in the elderly. J Epidemiol 1999; 9: 261–267. [DOI] [PubMed] [Google Scholar]
- 46.Tiemeier H, Hofman A, Kiliaan AJ, Meijer J, Breteler MM. Vitamin E and depressive symptoms are not related: The Rotterdam Study. J Affect Disord 2002; 72: 79–83. [DOI] [PubMed] [Google Scholar]
- 47.Hvas AM, Juul S, Nexo E, Ellegaard J. Vitamin B-12 treatment has limited effect on health-related quality of life among individuals with elevated plasma methylmalonic acid: a randomized placebo-controlled study. J Intern Med 2003; 253: 146–152. [DOI] [PubMed] [Google Scholar]
- 48.Carlsson CM, Papcke-Benson K, Carnes M, McBride PE, Stein JH. Health-related quality of life and long-term therapy with pravastatin and tocopherol (vitamin E) in older adults. Drugs Aging 2002; 19: 793–805. [DOI] [PubMed] [Google Scholar]
- 49.Keith ME, Jeejeebhoy KN, Langer A et al. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am J Clin Nutr 2001; 73: 219–224. [DOI] [PubMed] [Google Scholar]
- 50.Fawzi WW, Msamanga GI, Spiegelman D et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998; 351: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 51.Reichlin S Neuroendocrine–immune interactions. N Engl J Med 1993; 329: 1246–1253. [DOI] [PubMed] [Google Scholar]
- 52.Stein M, Miller AH, Trestman RL. Depression, the immune system, and health and illness. Findings in search of meaning. Arch Gen Psychiatry 1991; 48: 171–177. [DOI] [PubMed] [Google Scholar]
- 53.Evans DL, Leserman J, Pedersen CA et al. Immune correlates of stress and depression. Psychopharmacol Bull 1989; 25: 319–324. [PubMed] [Google Scholar]
- 54.Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men. A 2-year follow-up study. Arch Gen Psychiatry 1997; 54: 279–285. [DOI] [PubMed] [Google Scholar]
- 55.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. J Am Med Assoc 1993; 270: 2568–2573. [PubMed] [Google Scholar]
- 56.Lyketsos CG, Hoover DR, Guccione M et al. Depressive symptoms as predictors of medical outcomes in HIV infection. Multicenter AIDS Cohort Study. J Am Med Assoc 1993; 270: 2563–2567. [PubMed] [Google Scholar]
- 57.Perry S, Fishman B, Jacobsberg L, Frances A. Relationships over 1 year between lymphocyte subsets and psychosocial variables among adults with infection by human immunodeficiency virus. Arch Gen Psychiatry 1992; 49: 396–401. [DOI] [PubMed] [Google Scholar]
- 58.Rabkin JG, Williams JB, Remien RH, Goetz R, Kertzner R, Gorman JM. Depression, distress, lymphocyte subsets, and human immunodeficiency virus symptoms on two occasions in HIV-positive homosexual men. Arch Gen Psychiatry 1991; 48: 111–119. [DOI] [PubMed] [Google Scholar]
- 59.Cook JA, Cohen MH, Burke J et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr 2002; 30: 401–409. [DOI] [PubMed] [Google Scholar]


