Abstract
People living in the tropical and subtropical regions of the world face an enormous health burden due to mosquito-borne diseases such as malaria, dengue fever, and filariasis. Historically and today, targeting mosquito vectors with, primarily, insecticide-based control strategies have been a key control strategy against major mosquito-borne diseases. However, the success to date of such approaches is under threat from multiple insecticide resistance mechanisms while vector control (VC) options are still limited. The situation therefore requires the development of innovative control measures against major mosquito-borne diseases. Transinfecting mosquitos with symbiotic bacteria that can compete with targeted pathogens or manipulate host biology to reduce their vectorial capacity are a promising and innovative biological control approach. In this review, we discuss the current state of knowledge about the association between mosquitoes and Wolbachia, emphasizing the limitations of different mosquito control strategies and the use of mosquitoes' commensal microbiota as innovative approaches to control mosquito-borne diseases.
1. Introduction
Mosquitoes of the Anopheles, Aedes, and Culex genera include a number of main vector species of protozoan, virus, and nematode pathogens [1]. Therefore, since their first association with the transmission of such pathogens to humans and other vertebrates in the late nineteenth century [1], targeting mosquito vectors to interrupt the transmission of diseases has been a key control strategy against major mosquito-borne diseases such as malaria, yellow fever, dengue, chikungunya fever, and Zika virus infection. During the first quarter of the twentieth century, mosquito control activities were primarily based on source reduction, through larviciding using petroleum oils and larvivorous fish, together with environmental-based management [2]. With the advent of Dichlorodiphenyltrichloroethane (DDT) and the discovery of its insecticidal properties in the early 1940s, began the chemical era of vector control (VC) with mainly DDT-based interventions, both as larvicide and adulticide [3]. The publication of “Silent Spring” by Rachel Carson in 1962 raised public concerns about the use of DDT, characterized by high mammalian toxicity, poisoning risks to nontarget organisms, persistence in the biosphere surface, and an accumulation in food-chains [2]. Increasing public concerns about Persistent Organic Pollutants (POPs) led to DDT being banned. Fortunately, in the 1980s, a few years before the prohibition of DDT, synthetic pyrethroids compounds were added to the arsenal of public health insecticides [4]. Between 2000 and 2015, pyrethroid-treated bed-nets (ITNs), indoor residual spraying (IRS) with residual insecticides, and other insecticide-based strategies were widely used as front-line tools against the vectors of malaria and other mosquito-borne diseases [5] in an Integrated Vector Management (IVM) framework [3]. However, despite controversial, growing multiple insecticide resistance mechanisms threaten to reverse the progresses made so far to eliminate or control main mosquito-borne diseases [1, 6]. In this context, attention has turned toward research onto biological control, transgenic and paratransgenic approaches as potential alternatives, or complements to current chemical strategies [7].
Mosquito transgenesis is based on genetic modifications to introduce novel elements into the mosquito genomes. According to Abraham et al. [8], the two major transgenic approaches are (i) the genetic suppression or limitation of the vectors' ability to serve as competent hosts for parasite development, thus decreasing or eliminating their ability to transmit pathogens (vector competence), and (ii) the genetic suppression of insect populations by reducing the lifespans of known vectors. These approaches can, potentially, be used to control mosquito populations by reducing their ability to transmit human or animal pathogens [9]. For instance, Anopheles gambiae and Anopheles stephensi, the respective main malarial vectors in Africa and Asia, have been successfully engineered to interfere with malaria parasites, to stop or at least reduce transmission of the disease [10, 11]. Nowadays, there is a huge potential for transgenic vector control strategies. However, genetic manipulation tends to reduce the fitness of the modified mosquitoes thus reducing the chance of successfully spreading of genes of interest among natural populations of the targeted vector species [12]. Moreover, failure of spreading transgenes (Weill M. personal communication), mutation, and recombination rates could seriously undermine the feasibility or durability of such an approach as anticipated for the engineered geminivirus aiming to protect crops in the agricultural sector [5].
The recent discovery of a number of symbiotic bacteria inhabiting the gut and/or reproductive tissues of arthropods has opened the way for innovative control strategies against some of the major vector-borne diseases [13, 14]. Indeed, bacterial symbionts associated with mosquitoes can directly exert a pathogenic effect on their host [15], interfere with its reproduction [16, 17], and reduce vector competence [18]. Furthermore, the use of genetically modified bacteria to deliver antiparasite molecules has several advantages over the use of genetically modified vectors [19]. Strategies to exploit symbiotic microorganisms to control vector-borne diseases are known as paratransgenesis, i.e., the generation of engineered symbionts expressing antiparasite molecules [20]. Moreover, organisms that are able to manipulate their host biology and even shorten their lifespan may be of the highest interest for use as biological control agents.
Over the last decade, the focus has been put upon symbiotic microorganisms to identify potential candidates which could be used in new vector control approaches. Among the most promising candidates, several strains of the genus Wolbachia, a dominant endosymbiotic bacterium of numerous insects including major vectors of zoonotic pathogens, are of highest interest for the scientific community. Indeed, Wolbachia is a maternally inherited that can infect mosquitoes' reproductive organs to self-sustain itself in host populations, but also somatic tissues where pathogens development occurs and compete with them. It is, therefore, an interesting biological control agent which can be used to stop or prevent the transmission of several vertebrate pathogens to humans and domestic animals [21].
In this review, we discuss the current state of knowledge about the association between mosquitoes and Wolbachia, emphasizing the limitation of different mosquito control strategies and the use of mosquitoes' commensal microbiota as innovative approaches to control mosquito-borne diseases.
2. Methodology
2.1. Search Methods
Peer-reviewed literature search was conducted using online databases including PubMed, Bibliovie, INSERM databases, Web of Knowledge, and Google Scholar for articles. Gray literature searches were conducted using World Health Organization (WHO) webpage. The key search terms used was different combinations of “mosquito”, “wolbachia”, “biological control”, “control”, and “mosquito-borne diseases” using the Boolean operator “OR”, and combinations between concepts used the logical “AND”.
2.2. Data Screening
All documents were quickly checked to assess their relevance to the project using information in the title and the abstract. A subset of all relevant documents was selected, sorted by section, further reviewed and compiled in the manuscript.
3. Main Text
3.1. The Genus of Wolbachia (Alphaproteobacteria)
3.1.1. Description, Classification and Phylogeny
Bacteria of the genus Wolbachia are obligate intracellular Gram-negative bacteria belonging to the Alphaproteobacteria class (Table 1) found into the cytoplasmic vacuoles inside the cells of their insects, isopods, mites, arachnids and nematodes hosts [22]. The genus was first discovered in 1924 by Marshall Hertig and S. Burt Wolbach in the reproductive organs of Culex pipiens [23], then subsequently described by Hertig in 1936, who named the genus after his collaborator [24].
Table 1.
Taxonomic classification of Wolbachia.
| Taxa | Names |
|---|---|
| Domain | Bacteria |
| Phylum | Proteobacteria |
| Class | Alphaproteobacteria |
| Subclass | Rickettsidae |
| Order | Rickettsiales |
| Family | Rickettsiaceae |
| Genus | Wolbachia |
| Type species | Wolbachia pipientis, Hertig 1936 |
Wolbachia pipientis is the unique valid species of the genus. Noteworthily, the two other species that have been previously described as belonging to the genus Wolbachia [25]: Wolbachia melophagi and Wolbachia persica were removed latter on [26]. W. melophagi is now considered as nomen nudum, because no strain of this species has been found to date. While W. persica, which was isolated from the soft tick Argas persicus, was erroneously attributed to the genus as revealed by phylogenetic analysis of the 16S rRNA gene showing its close relatedness to the genus Francisella [27].
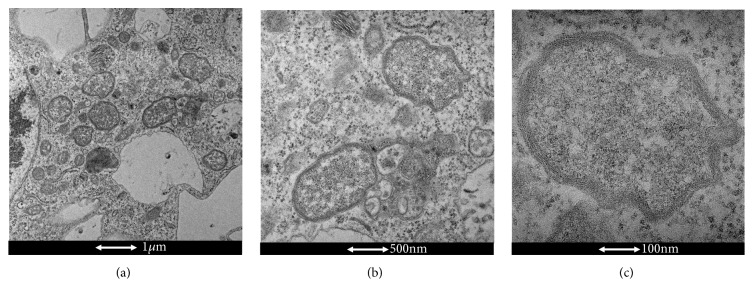
Morphologically, Wolbachia is a pleomorphic bacterium (Figure 1) that appears as small rods [0.5–1.3 μl in length] and coccoid forms [0.25–1 μl]; large forms [1–1.8 μl in diameter] growing inside vacuoles in the cytoplasm of host cells [28]. Despite its Gram-negative cell wall structure, Wolbachia is poorly stained by Gram staining, but can be well visualized using the Diff-Quik and May-Grunwald-Giemsa staining methods. Using the Gimenez stain, they can also be visualized as dark-blue structures within a blue-green cytoplasm [29]. Since they do not form morulae and exclusively infect arthropods and filarial nematodes, Wolbachia are easily differentiated from other closely related genera [28].
Figure 1.
Electron microscopy of Wolbachia. (a) Wolbachia cocci (Scale bar: 1 μm). (b) Zoom of two Wolbachia cells (Scale bar: 500 nm). (c) Zoom of a single Wolbachia cell (Scale bar: 100 nm). (by El Hadji Amadou Niang).
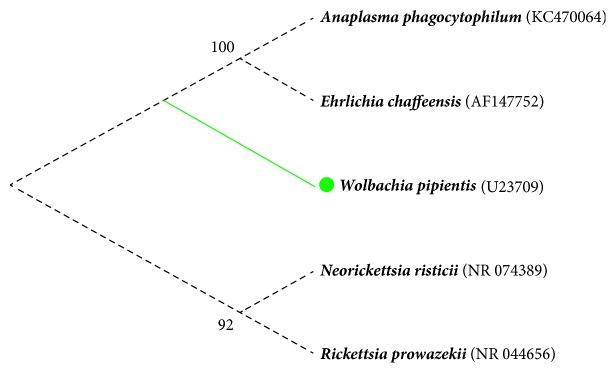
Phylogenetic analysis of the 16S rRNA gene (Figure 2), showed that W. pipientis, the nomen of the genus, forms a monophyletic clade within the Alphaproteobacteria class, closely related to the Anaplasma, Ehrlichia and Neorickettsia genera of the Anaplasmataceae family [28].
Figure 2.
Molecular Phylogenetic analysis of Anaplasmataceae by Maximum Likelihood method. The evolutionary history was inferred using the Maximum Likelihood method based on the Tamura-Nei model [30]. The tree with the highest log likelihood (-4338.5700) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with the higher log likelihood value. The analysis involved five nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 1,411 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 [31].
Further analysis based on the 16S rRNA and the Wolbachia Surface Protein (wsp) genes, were used to cluster the species into finer taxonomic scales. A system based on the level of similarity in the wsp gene sequences has been proposed for strain grouping. So far, 16 main evolutionary lineages from different host taxa known as “supergroups” have been identified and designated by the letters A to Q, with the exception of G [22]. The usefulness of such an assemblage remains controversial and the suggestion of splitting W. pipientis into multiple species has its pros and cons [32, 33].
Typically, the supergroups A and B are widely spread across many arthropod taxa [34]. Their common ancestor has probably diverged approximately 58–67 million years ago, at a time when all modern Arthropoda orders already existed [22]. The supergroups C and D are obligate and beneficial endosymbionts in some filarial nematodes [34–37]. While the F supergroup is peculiar and includes both nematode and arthropod Wolbachia strains [37–40]. More specific to certain host lineages, strains in supergroup E have been reported from Collembola [41, 42], in H from termites [40], and in M and N from aphids [43]. Further distinct supergroups have been identified either in nematodes or arthropods [34].
3.1.2. Obligate Intracellular Lifestyle
A range of microbial pathogens interact with their host in numerous and complex ways. Many are extracellular, while others invade organs and multiply within specific vector cells [44]. Wolbachia belongs to the latter group and has an original lifestyle as an obligate intracellular symbiont (endosymbiont) in close relationship with infected eukaryotic cells [45]. In arthropods, Wolbachia grow inside vacuoles often within the cytoplasm in the host's reproductive cells. However, they can also be found in somatic tissues, including nervous tissue and hemocytes [28]. Growing research has provided exciting insights into various aspects of the Wolbachia's biology [46]. One of the most obvious consequences of their presence inside reproductive cells is the facilitation of their transovarian transmission to their host's offspring. Analysis of the sequenced genomes of several members of the a-Proteobacteria group, to which belong the Wolbachia genus, has also provided greater understanding of their reductive genome evolution and antigenic variation as well as how they manipulate host cells [44]. However, the intracellular lifestyle has led to the loss of several genes as a consequence of the reduced genome size, varying from 1.1 Mb to 1.5 Mb, including less than 1000 protein-coding genes [45]. Furthermore, it has been reported that intracellular symbionts, such as Wolbachia, transfer genes into the host nucleus and vice versa [47, 48]. Leclercq et al. [49] showed high affinity between coding sequences of the f-element of the common pillbug (Armadillidium vulgare) with a large piece of the genome of the feminizing wVulC Wolbachia strain. Symbionts may also acquire genes from other symbionts [47]. The high level of genetic exchange in Wolbachia mentioned above suggests that its core genome is completed by an extensive auxiliary genome. As explained by Ishmael et al. [50], the core genome contains all the housekeeping genes shared by all (or almost all) sequenced strains for a given taxon, while all other genes constitute the auxiliary genome, encompassing the genetic variation within the species.
3.1.3. Host Reproductive Manipulation
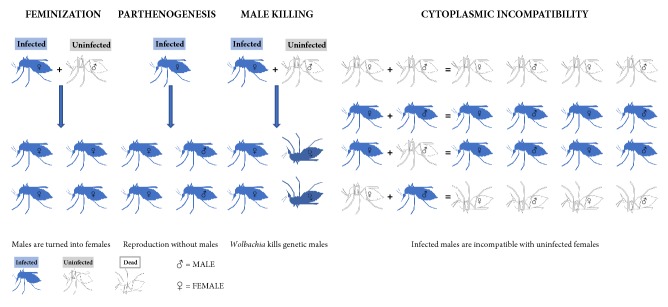
Wolbachia are typically transmitted vertically through host eggs and alter host biology in diverse ways. They induce reproductive manipulations (Figure 3), such as (i) the feminization of infected males (i.e., turning genetic males into females); (ii) induced parthenogenesis (i.e., reproduction without males); (iii) killing of infected males; and (iv) cytoplasmic incompatibility (i.e., modification of sperm from infected males resulting in embryonic defects and death) [34, 51].
Figure 3.
Different phenotypes of Wolbachia's host reproductive manipulation.
(1) Feminization. Infected males are “dead ends” for Wolbachia inheritance, because they do not transmit Wolbachia infection to their offspring. Thus, converting infected male offspring into females increases the potential for Wolbachia to be transmitted to the next generation. The phenomenon was first described in isopods such as Armadillidium vulgare and more recently in insects [52], where it involves different mechanisms, operating at the embryonic stage [51, 52]. In several species of terrestrial isopod within the order Oniscidae, Wolbachia invade the androgenic gland. The hypertrophied gland is then inhibited, causing genetic males to develop as females [53]. Among insects, feminizing strains have been reported in Ostrinia furnacalis (Lepidoptera) and in Eurema hecabe (Lepidoptera) and Zyginidia pullula (Hemiptera), in which the involved mechanisms remain unclear [51].
(2) Parthenogenesis. Another beneficial strategy to increase the maternal inheritance of Wolbachia is to induce the production of female offspring without fertilization by sperm, a process known as parthenogenesis (thelytoky). Wolbachia-induced female parthenogenesis is less common and has only been documented in haplodiploid species such as thrips (Thysanoptera), mites (Acari) and wasps (Hymenoptera) [51]. In these organisms, males normally develop from unfertilized haploid eggs (arrhenotokous parthenogenesis), whereas females develop from fertilized diploid eggs. Wolbachia disrupt the cells' early embryonic development, doubling the number of chromosomes in the unfertilized haploid eggs and rendering them diploid. This leads to development as an asexually produced female, so that infected females produce twice as many daughters as uninfected ones, allowing their cytoplasm to be transmitted to twice as many granddaughters as possible [54].
(3) Male Killing. In Coleoptera, Lepidoptera, Diptera (Insecta) and Pseudoscorpiones (Arachnida), Wolbachia induce male killing of infected females' male progeny. This phenotype, occurring mainly during embryogenesis, provides fitness benefits to the female progeny in terms of the competition for resources. -induced male killing occurs through lethal feminization. Indeed, when Insight into the mechanism has shown that Wolbachia infected mothers were treated with tetracycline to remove Wolbachia, genetic males survive, whereas in the presence of Wolbachia, genetic males become feminized and die during larval development [51, 54].
(4) Cytoplasmic Incompatibility (CI). Wolbachia-induced cytoplasmic incompatibility (CI) is the most commonly described reproductive manipulation phenotype. Reproductive incompatibility between populations of the Culex pipiens mosquito was first reported in the 1950s, but Wolbachia was only identified as the causative agent in the 1970s [55]. This phenotype comprises two distinct components: Wolbachia-induced modification of sperm during spermatogenesis and rescue of this modification in embryos infected with the same strain [51]. The incompatible cross, due to the asynchrony of the male and female pronuclei phases at the initial stage of mitosis, occurs when Wolbachia-infected males mate with uninfected females (unidirectional CI). Bidirectional CI occurs when both partners are infected by different but incompatible Wolbachia strains, causing cross lethality in both directions. CI has been widely described in numerous arthropod host species infected by Wolbachia strains belonging to both the A and B supergroups [56].
3.1.4. Wolbachia spp. and Insects
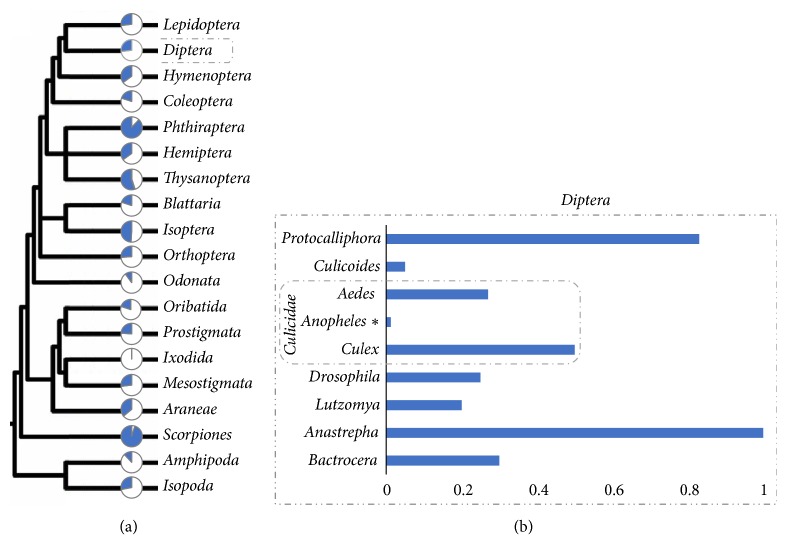
The “pandemic” nature of Wolbachia [46] resulting in their widespread distribution in various invertebrate hosts (Figure 4) is explained by their ability to manipulate host reproduction, but also by their ability to move horizontally across species boundaries [51, 54]. It has been estimated that different strains of Wolbachia may infect more than 65% of insect species [34]. Among these, several mosquito species belonging to different genera have been found carrying different strains.
Figure 4.
Wolbachia in arthropods. (Modified from Russell & Steiner 2012, Myrmecological News Journal [57]). (a) Graph illustrating Wolbachia-infected (Blue shaded portion) and Wolbachia-uninfected (white portion) proportions by host taxon. (b) Histogram highlighting the frequencies Wolbachia infection of some dipteral families. The asterisk (∗) indicates the recent discovery of native Wolbachia within the Anopheles genus.
3.2. Mosquitoes (Diptera, Culicidae)
3.2.1. Taxonomy, Classification and Phylogeny
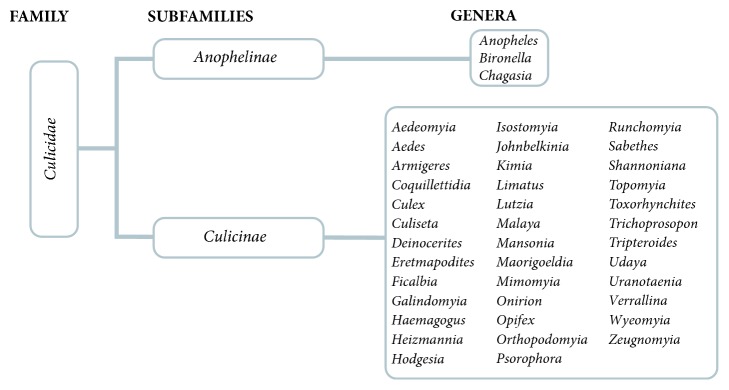
Mosquitoes are a monophyletic group that belongs to the order of Diptera (Table 2 and Figure 5) [58]. The origin and phylogenetic history of the family of Culicidae dates back to the Mesozoic Era. It is estimated that the main lineages of current mosquitoes date from the early Cretaceous period (145-100 million years) [58, 59].
Table 2.
Taxonomic classification of mosquitoes (Diptera: Culicidae).
| Taxa | Names |
|---|---|
| Kingdom | Animalia |
| Phylum | Arthropoda |
| Class | Insecta |
| Order | Diptera |
| Suborder | Nematocera |
| Infraorder | Culicomorpha |
| Superfamily | Culicoidea |
| Family | Culicidae Meigen, 1818 |
| Sub-families | Anophelinae, Culicinae |
| Genera (112) | Culex, Aedes, Anopheles, etc. |
Figure 5.
Classification of mosquitoes (DIPTERA: CULICIDAE) (by El Hadji Amadou Niang).
The family of Culicidae is a large and abundant group which is distributed from tropical latitudes to temperate regions, well beyond the Arctic Circle. It includes approximately 4,000 species, classified into two subfamilies and 112 genera. The subfamily Anophelinae has three genera and Culicinae has 109 genera, segregated into 11 tribes [58].
Mosquitoes are of prime medical and veterinary importance. In nearly all Culicidae species, only females feed on vertebrates, because of their need for blood to produce their offspring. During blood-sucking, a complex salivary secretion facilitates feeding but also enables several pathogens (viruses, protozoa, and nematode worms) to be directly injected into the capillaries of their vertebrate hosts [60].
3.2.2. Mosquitoes of Medical and Veterinary Importance
Mosquito-borne diseases such as malaria, filariases, dengue, chikungunya, Zika, and West Nile fevers represent significant medical and veterinary problems around the world and lead to major economic problems [61]. Table 3 summarises some of the most devastating mosquito-borne diseases [59].
Table 3.
Main diseases transmitted by mosquito.
| Diseases | Pathogens | Genera of main vectors | Vertebrate hosts | Reservoir hosts |
|---|---|---|---|---|
| Yellow fever | Yellow Fever virus (Flavivirus) |
Aedes Haemagogus Sabethes
|
Humans | Monkeys |
| Dengue fever | Dengue (D1, D2, D3, D4) viruses (Flavivirus) |
Aedes | Humans | Monkeys |
| Chikungunya | Chikungunya virus (Alphavirus) | Aedes | Humans | Monkeys |
| Zika fever | Zika virus (ZIKV) (Flavivirus) |
Aedes | Humans | Monkeys |
| Rift Valley fever | Rift Valley fever virus (Phlebovirus) |
Aedes, Culex | Sheep, Goats, Humans | Bats |
| West Nile Fever | West Nile virus (Flavivirus) |
Culex | Horses, Humans | Birds |
| Equine Encephalitis | Equine Encephalitis virus (Alphavirus, Flavivirus) |
Culex | Horses | Birds |
| Japanese Encephalitis | Japanese Encephalitis virus (Flavivirus) |
Culex | Horses, Humans | Pigs and wild birds |
| Saint Louis Encephalitis | Saint Louis Encephalitis virus (Flavivirus) |
Culex | Humans, Animals | Birds |
| Malaria | P. falciparum, P. vivax, P. malariae, P. ovale, P. knowlesi | Anopheles | Humans | Monkeys for P. knowlesi |
| Lymphatic filariasis | Wuchereria bancrofti, Brugia malaya | Anopheles, Aedes, Culex | Humans | Wild mammals for B. malaya |
3.2.3. Wolbachia and Mosquitoes
Among Culicidae, two types of Wolbachia infections can be distinguished: natural Wolbachia infections and transinfected mosquito lines.
(1) Natural Wolbachia Infections. The interest in the Wolbachia genus has renewed when the biological connection between cytoplasmic incompatibility and Wolbachia infection was established and documented by Yen & Barr [55] in the early 1970s. Subsequently, Yen [62] reported the presence of Wolbachia within the ovaries and eggs of mosquito members of the Aedes scutellaris group (Aedes cooki, Aedes polynesiensis, Aedes albopictus, and Aedes riversi). In 2002, while screening several mosquitoes species, Ricci et al. [63] found arthropod strains of Wolbachia in Culex modestus, Culex pipiens, and Coquillettidia richiardii, while three other mosquitoes (Aedes cinereus, Aedes detritus, and Ae. geniculatus) were infected with filarial strains previously described from Dirofilaria immitis, and two mosquitoes (Aedes punctor and Culex torrentium) were positive for both arthropod and filarial strains. Later, the development of PCR and sequencing techniques has led to the discovery of many other Wolbachia strains from several other mosquito species.
A recent meta-analysis of the distribution of Wolbachia in mosquitoes indicated that, of 185 mosquito screened, 31.4% was Wolbachia-infected but also demonstrated the nonrandom distribution of Wolbachia among different mosquito taxa [64]. Indeed, Wolbachia was found in 39.5% of the 147 mosquito species screened, but never in Ae. aegypti, the primary vector of Dengue, Chikungunya, and Zika viruses [9, 64]. Moreover, prior to 2014 no Wolbachia infection has been documented in 38 anopheline species, including several important malarial vector species (An. gambiae, An. arabiensis, An. funestus, An. stephensi, An. culicifacies, An. dirus, An. Albimanus, and An. darlingi), which has led to the previous believe that Wolbachia were unable to infect Anopheles species until their very recent discovery in natural populations of Anopheles gambiae and Anopheles coluzzii in Burkina Faso [21, 65] and in Mali (Gomes et al. 2017). More recently Ayala et al. [66] and Jeffries et al. [67] have revealed that native Wolbachia infections was wider than expected among natural anopheline populations with at least 16 species naturally infected (Table 4). Furthermore, Ayala et al. [66] revealed a large diversity of Wolbachia strains in wild anopheline populations, which offers an unexpected opportunity to discover suitable phenotypes to suppress Plasmodium transmission and/or to manipulate Anopheles reproduction and reduces the malaria burden in Africa [66].
Table 4.
Native Wolbachia infections in natural mosquito populations.
| Host Taxa | Supergroups | Strains | GeneBank # | References | ||
|---|---|---|---|---|---|---|
| Subfamily | Genera | Species | wsp | |||
|
| ||||||
| Culicinae | Culex | Cx p. pipiens | B | wPip | AF020060 | Hertig and Wolbach 1924, Zhou et al., 1998 |
| Cx p. quinquefasciatus | B | wPip | AF020061 | Zhou et al., 1998 | ||
| Cx. brevipalpis | A | wBre | AF317477 | Ruang-Areerate et al., 2003 | ||
| Cx. (Eumelanomyia) spp. | A | wEum | AF317480 | |||
| Cx. fuscocephala | B | wFus | AF317481 | |||
| Cx. Gelidus | B | wGel | AF317482 | |||
| Cx (Lophoceraomyia) spp. | A | WLop | AF317490 | |||
| Cx. sitiens | B | wSit | AF317491 | |||
| Cx. modestus | B | wPip | - | Ricci et al., 2002 | ||
| Cq. richiardii | B | wCon | - | |||
| Cx. torrentium | B, C | wPip, wDi | - | |||
| Aedes | Ae. albopictus | A, B | wAlbA, wAlbB | AF020059, AF020059 | Wright and Wang 1980; Zhou et al., 1998 | |
| Ae. albotaeniatus | wAlbo | AF317475 | Ruang-Areerate et al., 2003 | |||
| Ae. craggi | WCrag | AF317478 | ||||
| Ae. novoniveus | wNov | AF317484 | ||||
| Ae. niveus | wNiv | AF317485 | ||||
| Ae. pseudalbopictus, | wPseu | AF317487 | ||||
| Ae. perplexus | wPerp | AF317486 | ||||
| Ae. cooki | - | Yen 1975; Dean & Dobson (2004); Takken & Koenraadt 2013 |
||||
| Ae. polynesiensis | - | |||||
| Ae. riversi | - | |||||
| Ae. cinereus | C | wDi | - | Ricci et al., 2002 | ||
| Ae. detritus | C | wDi | - | |||
| Ae. geniculatus | C | wDi | - | |||
| Ae. punctor | B, C | wPip, wDi | - | |||
| Ae. fluviatilis | B | wFlu | GQ981315 | Moreira et al., 2009 | ||
| Armigeres | Arm. subalbatus | A | wSub | AF317488 | Ruang-Areerate et al., 2003 | |
| Mansonia | Mn. uniformis | B | wUnif | AF317493 | ||
| Mn. indiana | B | wInd | AF317492 | |||
| Coquillettidia | Cq. crassipes | B | wCra | AF317479 | ||
| Anophelinae | Anopheles | An. gambiae | A, B | wAnga-BF, wAnga-Mali | KJ728739-MF944223 | Baldini et al., 2014; Shaw et al., 2016; Gomes et al., 2017 |
| An. coluzzii | A, B | wAnga-BF, wAnga-Mali | KJ728755-MF944223 | |||
| An. arabiensis | A, B | wAnga | KJ728739- KJ728755 | Shaw et al., 2016 | ||
| An. funestus | A, B | wAnfu | - | Niang et al., 2018 [68], Ayala et al., 2018 | ||
| An. carnevalei | A,B | - | - | Ayala et al., 2018 | ||
| An. coustani | B | - | - | |||
| An. hancocki | B | - | - | |||
| An. implexus | B | - | - | |||
| An. jebudensis | B | - | - | |||
| An. marshallii | B | - | - | |||
| An. moucheti | B | - | - | |||
| An. nigeriensis | B | - | - | |||
| An. nili | B | - | - | |||
| An. paludis | B | - | - | |||
| An. vinckei | A,B | - | - | |||
(2) Mosquito Transinfection. The absence of natural infection in some dominant vector species has been a limiting factor for the potential operational use of Wolbachia to control vectors and the diseases they transmit. The ability of the bacterium to adapt to new intracellular environments has been exploited to transinfect vector species of medical and veterinary importance [9]. Transinfection via embryonic microinjection was used to transfer several Wolbachia strains into Ae. albopictus [64] and Ae. aegypti. For instance, the life-shortening strain of Wolbachia (wMelPop-CLA) from Drosophila melanogaster was successfully and stably introduced into Ae. aegypti to reduce its life-span. Given the proof that wMelPop strain being protective against RNA viruses in Drosophila, its derivate has been used latter to block Dengue, Chikungunya transmitted by Ae. aegypti, while the wMel Wolbachia strain (wMel_Br) has been used successfully against Zika infections in Brazil [69, 70]. Contrary to the complex Wolbachia-Arbovirus vectors, current views about the impact of Wolbachia on Plasmodium infections are almost entirely based on artificially transfected mosquito models [71]. In the Anophelinae sub-family, Wolbachia transinfection has been successful in Anopheles gambiae [72, 73] and in Anopheles stephensi [74], respectively major vectors of human malaria in Africa and the Middle East, and South Asia [75, 76].
3.2.4. Vector Control Approaches for the Control of Mosquito-Borne Diseases
In the past century, significant advances have been made in controlling main vector-borne diseases. Malaria disappeared from the northern hemisphere, diseases such as typhus, Bartonella, and yellow fever prevalence were drastically reduced in many countries with effective vector control methods [9]. Despite these successes, there are currently no effective vaccines against dengue, filariasis, or malaria, and specific treatments are only available for malaria and some filariases. Historically and today, targeting mosquito vectors has been a key control strategy against major mosquito-borne diseases. Vector control is an essential component of mosquito-borne disease prevention and control. Its aim is to interrupt or eliminate local transmission or reduce vulnerability to disease and prevent secondary infections from introduced diseases and prevent outbreaks. Before the Second World War, vector control was predominantly based on the environmental control of the proliferation of mosquitoes [3]. The so-called “chemical period” then began with the advent of DDT and other organochlorine pesticides in the late 1940s. During this period, widescale spraying of the indoor surfaces of houses and shelters drastically reduced the number of malarial mosquitoes and other insects and led to the successful eradication of malaria in the United States, European countries, the Soviet Union, South East Asia, India, and South America [4, 77]. But the Malaria Eradication Programme failed in several malarial pilot areas in the African continent, due to the extremely high malaria heterogeneity and vector behavioral plasticity [78]. However, during the past decade there has been a global renewed focus on vector control with the widespread use of impregnated (LLINs) and sprayed materials (IRS), particularly against malaria vectors. Large community-based distribution and/or IRS campaigns have led to significant ITN and IRS coverage in several African countries, resulting in a substantial drop in the prevalence of malaria in that region [3]. However, to make vector control more effective, cost effective, ecologically sound, and sustainable the WHO adopted in 2004 the Global Strategic Framework on Integrated Vector Management (IVM) as the first step towards the search and the implementation of new approaches to control vectors and the diseases they transmit [3]. Defined as “a rational decision-making process for the optimal use of resources for vector control,” IVM is not a new concept since its basic principles have been used over the past century in the USA through the vast network of Mosquito Abatement Districts implemented to protect people from nuisance-biting and vector species of mosquitoes [79]. Lately, the WHO called for the strengthening of IVM as one of the strategic areas for action in the global plan framework to combat neglected tropical diseases for 2008–2015.
Although insecticides have been successful in controlling vectors, current ecological and environmental protection standards make insecticide-based strategies unsustainable, due to the adverse effects of many insecticides on nontarget species, their environmental impact, the contamination of soil and water and the development of selective processes, and subsequent mosquito resistance to insecticides [1]. Moreover, a number of malaria prevention and control tools currently available are quite expensive, while arbovirus vector management also has to face significant challenges, due to the peculiar traits of Aedes vectors, which have huge physiological and ecological plasticity making them difficult to control [80]. A broad spectrum of resistance to insecticides has evolved in the Culex genus, involving both “Metabolic” (enhanced esterase, glutathione-S-transferase, or p450 monooxygenase activities) and “Target Site” (modification of the acetylcholinesterase; the GABA receptors; or the sodium channels) mechanisms [81]. There is, therefore, an urgent need for effective alternative vector control strategies that can be used on a large scale and which are environmentally friendly. This is critical to sustaining control efforts and to achieving the goal of malaria elimination. Potential alternatives or complementary strategies to current core interventions include genetic control approaches, using refractory mosquitoes to replace vector populations or the release of mosquitoes carrying a lethal gene to suppress the targeted populations [1]. In addition to transgenic mosquitoes, paratransgenic and biological control approaches provide concrete possibilities for innovative vector control strategies [7].
3.2.5. Biological Control of Mosquito-Borne Diseases
Beyond the paratransgenic VC approaches taking advantage of the naturally/transinfected mosquitoes microbiota and defined as the use of symbiotic organisms naturally inhabit or successfully introduced into mosquitoes to deliver an effector molecule to inhibit, compete or kill the pathogen in insects [1, 9], their utilisation to directly interfere with or modulate vector immunity against pathogens constitutes a Biological approach to control MBD. The feasibility of the paratransgenic approach was demonstrated by Durvasula et al. [82], when they successfully transformed a commensal symbiont in the hindgut lumen of Rhodnius prolixus, Rhodococcus rhodnii, to express the cecropin A protein to kill the causative agent of Chagas disease, and Trypanosoma cruzi inside their host. Similarly, the recent use of the life-shortening Wolbachia wMelPop-CLA strain is a prelude for an innovative Biological approach to control MBD. Indeed, intracellular bacteria such as Wolbachia that can manipulate their host biology, including their immune system, are unduly regarded as promising innovative biocontrol approach to control insect-transmitted diseases. Therefore, several studies have attempted to show the potential for Wolbachia to be used in such a strategy to control mosquito-transmitted diseases [74]. Wolbachia has several characteristics, including the capacity to perturb insect ecology, behaviour, and physiology, making it one of the best candidates for blocking, or at least significantly reducing, the transmission of pathogens of medical and veterinary importance [21]. However, before the operational implementation of any Wolbachia-based approach, an important prerequisite is to better characterize all potential the strain of the genus and their host manipulation phenotypes which could make them good biocontrol agents candidates, to develop predictive models, and to perform a comprehensive risk assessment for their use to control mosquitoes and disease they transmit. As stated before, Wolbachia-transinfection technology has already shown promise in controlling the transmission of arboviruses by Ae. aegypti using different Wolbachia strains which can shorten vector lifespans, limit susceptibility to infection, and induce cytoplasmic incompatibility to reduce vector density. Furthermore, in An. gambiae and An. stephensi, the presence of Wolbachia appears to negatively impact the Plasmodium developmental cycle and egg laying [21, 74, 83]. Although potentially eligible as an inovative weapon, our knowledge of Wolbachia-mediated antiparasite mechanisms is fragmented, if not completely lacking. A significant delay in the virus-induced mortality of the pathogenic Drosophila C, Cricket paralysis and Flock House virus have been related to the presence of Wolbachia in the host. Johnson hypothesized that by reducing the viral load Wolbachia endosymbionts enhance host survival [84]. However, since different Wolbachia strains affect a wide variety of insect viruses this likely suggests that the underlying mechanisms are not pathogen specific/Wolbachia interactions but involve putatively broad processes targeting a wide-range of viral types, including competition for resources and the upregulation of hosts' immune responses.
(1) Wolbachia-Based Approach to Control Arboviruses Diseases. A new era for controlling arboviruses started with the successful introduction of the life-shortening wMelPop-CLA Wolbachia strain into Ae. aegypti to reduce its natural populations life span [85, 86]. Primary data gathered from field trials in Australia has made it possible to validate theoretical models for Wolbachia population dynamics and has demonstrated the feasibility and sustainability of such a strategy to control mosquito populations and the diseases they transmit [87]. However, barriers to dispersal responsible for a slower than anticipated spread of transinfected Aedes aegypti mosquito in Cairns (Australia) [88] should be taken into account in future releases. Moreover, how Wolbachia strains of interest interfere with pathogens is a critical aspect that needs to be better understood when dealing with Wolbachia-based approaches. Several authors have attempted to unravel the basis of Wolbachia pathogen blocking. To that aim, Terradas and McGraw discussed the possible mechanistic basis of Wolbachia-mediated pathogen blocking and have evaluated the existence of evidences from field mosquitoes and related insects [89]. They showed that the amount of Wolbachia inside host cells and tissues appears to correlate with the strength of Wolbachia-mediated blocking. They revealed that the highly replicative Wolbachia strain (wMelPop) by exhibiting great cellular loads causes tissue damage thus inducing near perfect blocking in Ae. aegypti [89]. Another possible mode of action through which Wolbachia interferes with pathogen infection is by priming the host immune system, with the preactivation of the immune response which could then theoretically protect the insect from a range of pathogens. Gene regulation is another way by which Wolbachia modulates the host immune system as demonstrated by recent studies about the potential role of the Vago protein on the innate immune pathways of Culex quinquefasciatus and Ae. aegypti to restrict West Nile and dengue virus replication [90]. For instance, Asad et al. have shown that in Wolbachia-infected cells, knocking-down the Vago1 gene led to significant increases in DENV replication with no effect on Wolbachia density, and concluded that in Ae. aegypti the induction of the AeVago1 protein, mediated by Wolbachia in infected cells, may function as a host factor to suppress DENV replication [90].
(2) Wolbachia-Based Approach to Control Malaria. As reported in the last World Malaria Report 2017, despite significant progress made since 2000, the rate of decline of malaria has stalled and even reversed in some region since 2014 [91]. Reasons for this are the spread of resistance of parasite to antimalarial drugs and vectors to insecticides [4]. Beside the implementation of a strategic insecticide resistance monitoring for malaria endemic countries, the WHO's Global plan for insecticide resistance management in malaria vectors (GPIRM) highlighted also the need for the development of innovative approaches for sustainable vector control at global scale [92]. As a response to that, attention has been drawn to mosquitoes' microbiota and their potential impact on host fitness and parasite evolution [93]. Wolbachia-mediated parasite interference in other insect systems has raised the exciting possibility of using them to control or limit the spread of malaria. However, the development of Wolbachia-based antimalarial strategies has been impeded by the lack of stable Wolbachia infections in natural anopheline populations, as well as the failure to establish stable inherited transinfections in anopheline mosquitoes. Both issues have recently been overcome with the successful establishment of a stable Wolbachia strain wAlbB infection in Anopheles stephensi, an important malarial vector in Asia [74], and the recent report of stable Wolbachia infections in natural populations of two important malarial vectors, Anopheles gambiae and Anopheles coluzzii, in Burkina Faso [65]. Furthermore, Shaw et al. showed that the wAnga strain stably infects reproductive tissues (ovaries), and certainly somatic tissues where the Plasmodium development occurs, and where it may effectively compete for resources or upregulate the immune response to effectively kill the malaria parasite [21]. Similar results were reported recently in Mali with a new anopheline Wolbachia strain (wAnga-Mali) [83]. Interestingly, experimental infection showed that wAnga-Mali has strong impact on late sporozoites stages and reduces malaria transmission [83]. Both studies showed the potential for the release of Wolbachia-infected mosquitoes as a promising strategy to reduce malaria transmission, but also raised the great limitation due to the apparent lack of clear Cytoplasm Incompatibility [21] to ensure released population self-sustenance in the nature. The recent discovery of native Wolbachia infections in 16 out of 25 wild African Anopheles species, including both vectors and non-vectors of malaria confirm that natural Wolbachia infection in anopheline mosquitoes is more common than expected [66, 67]. This offers an unprecedented opportunity to further studies the diversity of anopheline Wolbachia strains to identify suitable phenotypes naturally impeding the development of Plasmodium parasites in mosquitoes, especially among Wolbachia strains associated with non-malaria vectors.
4. Conclusions and Future Directions
This review discussed the current state of knowledge about the association between mosquitoes and Wolbachia, emphasizing the limitation of different mosquito control strategies and the use of mosquitoes' commensal/introduced microbiota as innovative VC intervention against mosquito-borne diseases.
In summary,
Several human, animal and zoonotic diseases are transmitted by mosquitoes of the Anopheles, Aedes, and Culex genera. Insecticide-based vector control tools/strategies are keys components in the fight against major mosquito-borne diseases.
The increasing emergence/ resurgence of mosquito-borne diseases such as malaria, yellow fever, dengue, chikungunya, and Zika fevers, and the spread of drug resistant parasites and insecticide resistant mosquito strains threatens the sustainability of current control methods and stresses the urgent need for the development of additional control methods for mosquito-borne diseases.
Wolbachia is one of the most promising mosquito symbionts for innovative vector control approaches. Wolbachia has several characteristics which can be used in such a strategy to reduce host fitness and competes or kills the pathogens.
Wolbachia was first discovered in 1924 and described in 1936 by Marshall Hertig and S. Burt Wolbach in the reproductive organs of Culex pipiens. The “pandemic” nature of Wolbachia results from their ability to manipulate host reproduction and to move horizontally across species' boundaries.
About 31.4% of mosquito species naturally harbour one or several Wolbachia strains. Moreover, it is now possible to stably transinfect mosquito vector species of medical and veterinary importance with nonnative Wolbachia strains which can shorten vector lifespan, limit susceptibility to infection, or induce cytoplasmic incompatibility to reduce vector density.
Wolbachia-based approach is certainly a promising innovative strategy for mosquito vector control. However, our knowledge of Wolbachia-mediated antiparasite mechanisms is fragmented if not entirely lacking.
Additional studies, including laboratory experiments, semifield, and field trial on several mosquito vector species in different geographical population urgently need to be reinforced to better understand Wolbachia-mediated antiparasite mechanisms and interaction between hosts and parasites but also to provide empirical data to test theoretical models for Wolbachia population dynamics, and demonstrate the feasibility and sustainability of Wolbachia-based approach approaches to control mosquito and diseases they transmit.
Acknowledgments
The authors thank Dr Florian M. Steiner, Editor in Chief of Myrmecological News Journal, and Dr Jacob A. Russell of Drexel University for allowing them to reproduce their Fig 1 of Myrmecology News 16: 7-23.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Wilke A. B. B., Marrelli M. T. Paratransgenesis: A promising new strategy for mosquito vector control. Parasites & Vectors. 2015;8(1) doi: 10.1186/s13071-015-0959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulla M. S. Mosquito control then, now, and in the future. Journal of the American Mosquito Control Association. 1994;10(4):574–575. [PubMed] [Google Scholar]
- 3.WHO. Handbook for Integrated Vector Management. Geneva, Switzerland: WHO Press; 2013. [Google Scholar]
- 4.Sougoufara S., Doucouré S., Sembéne P. M. B., Harry M., Sokhna C. Challenges for malaria vector control in sub-Saharan Africa: Resistance and behavioral adaptations in Anopheles populations. Journal of Vector Borne Diseases. 2017;54(1):4–15. [PubMed] [Google Scholar]
- 5.Karunamoorthi K., Sabesan S. Insecticide resistance in insect vectors of disease with special reference to mosquitoes: a potential threat to global public health. Health Scope. 2013;2(1):4–18. doi: 10.17795/jhealthscope-9840. [DOI] [Google Scholar]
- 6.Alout H., Labbé P., Chandre F., Cohuet A. Malaria Vector Control Still Matters despite Insecticide Resistance. Trends in Parasitology. 2017;33(8):610–618. doi: 10.1016/j.pt.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Mancini M. V., Spaccapelo R., Damiani C., et al. Paratransgenesis to control malaria vectors: A semi-field pilot study. Parasites & Vectors. 2016;9(1) doi: 10.1186/s13071-016-1427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham E. G., Cha S.-J., Jacobs-Lorena M. Towards the genetic control of insect vectors: An overview. Entomological Research. 2007;37(4):213–220. doi: 10.1111/j.1748-5967.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takken W., Koenraadt C. J. Ecology of Parasite-Vector Interactions. Wageningen, the Netherlands: Wageningen Academic Publishers; 2013. [Google Scholar]
- 10.Grossman G. L., Rafferty C. S., Clayton J. R., Stevens T. K., Mukabayire O., Benedict M. Q. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Molecular Biology. 2001;10(6):597–604. doi: 10.1046/j.0962-1075.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 11.Catteruccia F., Nolan T., Loukeris T. G., et al. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405(6789):959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- 12.Catteruccia F., Godfray J. C. H., Crisanti A. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299(5610):1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- 13.Favia G., Ricci I., Damiani C., et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proceedings of the National Acadamy of Sciences of the United States of America. 2007;104(21):9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favia G., Ricci I., Marzorati M., et al. Bacteria of the genus asaia: A potential paratransgenic weapon against malaria. Advances in Experimental Medicine and Biology. 2008;627:49–59. doi: 10.1007/978-0-387-78225-6_4. [DOI] [PubMed] [Google Scholar]
- 15.Schnepf E., Crickmore N., Van Rie J., et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Reviews. 1998;62(3):775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zchori-Fein E., Gottlieb Y., Kelly S. E., et al. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proceedings of the National Acadamy of Sciences of the United States of America. 2001;98(22):12555–12560. doi: 10.1073/pnas.221467498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabalou S., Riegler M., Theodorakopoulou M., Stauffer C., Savakis C., Bourtzis K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proceedings of the National Acadamy of Sciences of the United States of America. 2004;101(42):15042–15045. doi: 10.1073/pnas.0403853101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beard C. B., Dotson E. M., Pennington P. M., Eichler S., Cordon-Rosales C., Durvasula R. V. Bacterial symbiosis and paratransgenic control of vector-borne Chagas disease. International Journal for Parasitology. 2001;31(5-6):621–627. doi: 10.1016/S0020-7519(01)00165-5. [DOI] [PubMed] [Google Scholar]
- 19.Riehle M. A., Jacobs-Lorena M. Using bacteria to express and display anti-parasite molecules in mosquitoes: Current and future strategies. Insect Biochemistry and Molecular Biology. 2005;35(7):699–707. doi: 10.1016/j.ibmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Coutinho-Abreu I. V., Zhu K. Y., Ramalho-Ortigao M. Transgenesis and paratransgenesis to control insect-borne diseases: Current status and future challenges. Parasitology International. 2010;59(1):1–8. doi: 10.1016/j.parint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw W. R., Marcenac P., Childs L. M., et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nature Communications. 2016;7 doi: 10.1038/ncomms11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilinsky Y., Kosterin O. E. Molecular diversity of Wolbachia in Lepidoptera: Prevalent allelic content and high recombination of MLST genes. Molecular Phylogenetics and Evolution. 2017;109:164–179. doi: 10.1016/j.ympev.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Hertig M., Wolbach S. B. Studies on Rickettsia-Like Micro-Organisms in Insects. The Journal of Medical Research. 1924;44(3):329–374. [PMC free article] [PubMed] [Google Scholar]
- 24.Hertig M. The Rickettsia, Wolbachia pipientis (Gen. Et Sp. N.) and Associated Inclusions of the Mosquito, Culex pipiens. Parasitology. 1936;28(4):453–486. doi: 10.1017/S0031182000022666. [DOI] [Google Scholar]
- 25.Skerman V. B. D., McGowan V., Sneath P. H. A. Approved lists of bacterial names. International Journal of Systematic Bacteriology. 1980;30(1):225–420. doi: 10.1099/00207713-30-1-225. [DOI] [Google Scholar]
- 26.Lo N., Paraskevopoulos C., Bourtzis K., et al. Taxonomic status of the intracellular bacterium Wolbachia pipientis. International Journal of Systematic and Evolutionary Microbiology. 2007;57(3):654–657. doi: 10.1099/ijs.0.64515-0. [DOI] [PubMed] [Google Scholar]
- 27.Forsman M., Sandstrom G., Sjostedt A. Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. International Journal of Systematic Bacteriology. 1994;44(1):38–46. doi: 10.1099/00207713-44-1-38. [DOI] [PubMed] [Google Scholar]
- 28.La Scola B., Bandi C., Raoult D. Bergeys Manual of Systematics of Archaea and Bacteria. Chichester, UK: John Wiley & Sons, Ltd; 2015. Wolbachia; pp. 1–12. [Google Scholar]
- 29.Fenollar F., La Scola B., Inokuma H., Dumler J. S., Taylor M. J., Raoult D. Culture and Phenotypic Characterization of a Wolbachia pipientis Isolate. Journal of Clinical Microbiology. 2003;41(12):5434–5441. doi: 10.1128/JCM.41.12.5434-5441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993 doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S., Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochemistry and Molecular Biology. 2005;35(7):721–727. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Ramírez-Puebla S. T., Servín-Garcidueñas L. E., Ormeño-Orrillo E., et al. A response to Lindsey et al. "Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al.". Systematic and Applied Microbiology. 2016;39(3):223–225. doi: 10.1016/j.syapm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Lindsey A. R. I., Bordenstein S. R., Newton I. L. G., Rasgon J. L. Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al. Systematic and Applied Microbiology. 2016;39(3):220–222. doi: 10.1016/j.syapm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glowska E., Dragun-Damian A., Dabert M., Gerth M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae) Infection, Genetics and Evolution. 2015;30:140–146. doi: 10.1016/j.meegid.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Henkle-Dührsen K., Eckelt V. H., Wildenburg G., Blaxter M., Walter R. D. Gene structure, activity and localization of a catalase from intracellular bacteria in Onchocerca volvulus1Note: Nucleotide sequence data reported in this paper are available in the EMBL, GenBank and DDJB databases under the accession numbers X82176, AF069070 and AF069069.1. Molecular and Biochemical Parasitology. 1998;96(1-2):69–81. doi: 10.1016/S0166-6851(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 36.Sironi M., Bandi C., Sacchi L., Sacco B. D., Damiani G., Genchi C. Molecular evidence for a close relative of the arthropod endosymbiont Wolbachia in a filarial worm. Molecular and Biochemical Parasitology. 1995;74(2):223–227. doi: 10.1016/0166-6851(95)02494-8. [DOI] [PubMed] [Google Scholar]
- 37.Taylor M. J., Bilo K., Cross H. F., Archer J. P., Underwood A. P. 168 rDNA phylogeny and ultrastructural characterization of Wolbachia intracellular bacteria of the filarial nematodes Brugia malayi, B. pahangi, and Wuchereria bancrofti. Experimental Parasitology emphasizes. 1999;91(4):356–361. doi: 10.1006/expr.1998.4383. [DOI] [PubMed] [Google Scholar]
- 38.Lo N., Casiraghi M., Salati E., Bazzocchi C., Bandi C. How Many Wolbachia Supergroups Exist? Molecular Biology and Evolution. 2002;19(3):341–346. doi: 10.1093/oxfordjournals.molbev.a004087. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto J. M., Feinstein J., Rasgon J. L. Wolbachia infections in the Cimicidae: Museum specimens as an untapped resource for endosymbiont surveys. Applied and Environmental Microbiology. 2006;72(5):3161–3167. doi: 10.1128/AEM.72.5.3161-3167.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell B. C., Bragg T. S., Turner C. E. Phylogeny of symbiotic bacteria of four weevil species (coleoptera: curculionidae) based on analysis of 16S ribosomal DNA. Insect Biochemistry and Molecular Biology. 1992;22(5):415–421. doi: 10.1016/0965-1748(92)90136-3. [DOI] [Google Scholar]
- 41.Vandekerckhove T. T. M., Watteyne S., Willems A., Swings J. G., Mertens J., Gillis M. Phylogenetic analysis of the 16S rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for wolbachial taxonomy. FEMS Microbiology Letters. 1999;180(2):279–286. doi: 10.1016/S0378-1097(99)00499-1. doi: 10.1016/S0378-1097(99)00499-1. [DOI] [PubMed] [Google Scholar]
- 42.Czarnetzki A. B., Tebbe C. C. Detection and phylogenetic analysis of Wolbachia in Collembola. Environmental Microbiology. 2004;6(1):35–44. doi: 10.1046/j.1462-2920.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z., Su X.-M., Wen J., Jiang L.-Y., Qiao G.-X. Widespread infection and diverse infection patterns of Wolbachia in Chinese aphids. Insect Science. 2014;21(3):313–325. doi: 10.1111/1744-7917.12102. [DOI] [PubMed] [Google Scholar]
- 44.Darby A. C., Cho N.-H., Fuxelius H.-H., Westberg J., Andersson S. G. E. Intracellular pathogens go extreme: genome evolution in the Rickettsiales. Trends in Genetics. 2007;23(10):511–520. doi: 10.1016/j.tig.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Le P. T., Pontarotti P., Raoult D. Alphaproteobacteria species as a source and target of lateral sequence transfers. Trends in Microbiology. 2014;22(3):147–156. doi: 10.1016/j.tim.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Saridaki A., Bourtzis K. Wolbachia: more than just a bug in insects genitals. Current Opinion in Microbiology. 2010;13(1):67–72. doi: 10.1016/j.mib.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Duron O., Hurst G. D. D. Arthropods and inherited bacteria: From counting the symbionts to understanding how symbionts count. BMC Biology. 2013;11, article no. 45 doi: 10.1186/1741-7007-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moran N. A. Tracing the evolution of gene loss in obligate bacterial symbionts. Current Opinion in Microbiology. 2003;6(5):512–518. doi: 10.1016/j.mib.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Leclercq S., Thézé J., Chebbi M. A., et al. Birth of a W sex chromosome by horizontal transfer of Wolbachia bacterial symbiont genome. Proceedings of the National Acadamy of Sciences of the United States of America. 2016;113(52):15036–15041. doi: 10.1073/pnas.1608979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishmael N., Hotopp J. C. D., Loanidis P., et al. Extensive genomic diversity of closely related wolbachia strains. Microbiology. 2009;155(7):2211–2222. doi: 10.1099/mic.0.027581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werren J. H., Baldo L., Clark M. E. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6(10):741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 52.Kageyama D., Nishimura G., Hoshizaki S., Ishikawa Y. Feminizing Wolbachia in an insect, Ostrinia furnacalis (Lepidoptera: Crambidae) Heredity. 2002;88(6):444–449. doi: 10.1038/sj/hdy/6800077. doi: 10.1038/sj/hdy/6800077. [DOI] [PubMed] [Google Scholar]
- 53.Vandekerckhove T. T. M., Watteyne S., Bonne W., et al. Evolutionary trends in feminization and intersexuality in woodlice (Crustacea, Isopoda) infected with Wolbachia pipientis (α-Proteobacteria) Belgian Journal of Zoology. 2003;133(1):61–69. [Google Scholar]
- 54.Charlat S., Hurst G. D. D., Merçot H. Evolutionary consequences of Wolbachia infections. Trends in Genetics. 2003;19(4):217–223. doi: 10.1016/S0168-9525(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 55.Yen J. H., Barr A. R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. [31] Nature. 1971;232(5313):657–658. doi: 10.1038/232657a0. [DOI] [PubMed] [Google Scholar]
- 56.Hurst G. D. D., Jiggins F. M., Pomiankowski A. Which way to manipulate host reproduction? Wolbachia that cause cytoplasmic incompatibility are easily invaded by sex ratio-distorting mutants. The American Naturalist. 2002;160(3):360–373. doi: 10.1086/341524. [DOI] [PubMed] [Google Scholar]
- 57.Russell J. A. The ants (Hymenoptera: Formicidae) are unique and enigmatic hosts of prevalent Wolbachia (Alphaproteobacteria) symbionts. Myrmecological News. 2012;16:7–23. [Google Scholar]
- 58.Harbach R. E. The Culicidae (Diptera): A review of taxonomy, classification and phylogeny. Zootaxa. 2007;(1668):591–638. [Google Scholar]
- 59.Duvallet G. D. D. (1958-. . . . ). Fontenille, and V. (1956-. . . . ). Robert. Entomologie médicale et vétérinaire. 2017 [Google Scholar]
- 60.Reiter P. Climate change and mosquito-borne disease. Environmental Health Perspectives. 2001;109(1):141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iturbe-Ormaetxe I., Walker T., O' Neill S. L. Wolbachia and the biological control of mosquito-borne disease. EMBO Reports. 2011;12(6):508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yen J. H. Transovarial transmission of rickettsia-like microorganisms in mosquitoes. Annals of the New York Academy of Sciences. 1975;266(1):152–161. doi: 10.1111/j.1749-6632.1975.tb35096.x. [DOI] [PubMed] [Google Scholar]
- 63.Ricci I., Cancrini G., Gabrielli S., D'Amelio S., Favia G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): Large polymerase chain reaction survey and new identifications. Journal of Medical Entomology. 2002;39(4):562–567. doi: 10.1603/0022-2585-39.4.562. [DOI] [PubMed] [Google Scholar]
- 64.Bourtzis K., Dobson S. L., Xi Z., et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Tropica. 2014;132(1):S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Baldini F., Segata N., Pompon J., et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nature Communications. 2014;5, article no. 3985 doi: 10.1038/ncomms4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ayala D., Akone-Ella O., Rahola N., et al. Natural Wolbachia infections are common in the major malaria vectors in Central Africa. bioRxiv. 2018 doi: 10.1101/343715.343715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeffries C. L., Lawrence G. G., Golovko G., et al. Novel Wolbachia strains in Anopheles malaria vectors from Sub-Saharan Africa. bioRxiv. 2018 doi: 10.1101/338434.338434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niang E. H., Bassene H., Makoundou P., Fenollar F., Weill M., Mediannikov O. First report of natural wolbachia infection in wild anopheles funestus population in senegal. Malaria Journal. 2018;17(1):p. 408. doi: 10.1186/s12936-018-2559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreira L. A., Iturbe-Ormaetxe I., Jeffery J. A., et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 70.Dutra H. L. C., Rocha M. N., Dias F. B. S., Mansur S. B., Caragata E. P., Moreira L. A. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host & Microbe. 2016;19(6):771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zélé F., Nicot A., Duron O., Rivero A. Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. Journal of Evolutionary Biology. 2012;25(7):1243–1252. doi: 10.1111/j.1420-9101.2012.02519.x. [DOI] [PubMed] [Google Scholar]
- 72.Kambris Z., Blagborough A. M., Pinto S. B., et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in anopheles gambiae. PLoS Pathogens. 2010;6(10) doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes G. L., Koga R., Xue P., Fukatsu T., Rasgon J. L., Schneider D. S. Wolbachia Infections Are Virulent and Inhibit the Human Malaria Parasite Plasmodium Falciparum in Anopheles Gambiae. PLoS Pathogens. 2011;7(5):p. e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bian G., Joshi D., Dong Y., et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340(6133):748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 75.Sinka M. E., Bangs M. J., Manguin S., et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasites & Vectors. 2010;3(1, article 117) doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinka M. E., Bangs M. J., Manguin S., et al. A global map of dominant malaria vectors. Parasites & Vectors. 2012;5(1) doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruce-Chwatt L. J. Malaria research and eradication in the USSR. A review of Soviet achievements in the field of malariology. Bulletin of the World Health Organization. 1959;21:737–772. [PMC free article] [PubMed] [Google Scholar]
- 78.Molineaux L., Gramiccia G. he Garki project: Research on The Epidemiology And Control of Malaria in The Sudan Savanna of West Africa. 1980. [Google Scholar]
- 79.Beier J. C., Keating J., Githure J. I., Macdonald M. B., Impoinvil D. E., Novak R. J. Integrated vector management for malaria control. Malaria Journal. 2008;7(Suppl 1):p. S4. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benelli G., Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitology Research. 2016;115(5):1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 81.Rivero A., Vézilier J., Weill M., Read A. F., Gandon S., Manchester M. Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem? PLoS Pathogens. 2010;6(8):p. e1001000. doi: 10.1371/journal.ppat.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durvasula R. V., Gumbs A., Panackal A., et al. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proceedings of the National Acadamy of Sciences of the United States of America. 1997;94(7):3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomes F. M., Hixson B. L., Tyner M. D. W., et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proceedings of the National Acadamy of Sciences of the United States of America. 2017;114(47):12566–12571. doi: 10.1073/pnas.1716181114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson K. N. The impact of Wolbachia on virus infection in mosquitoes. Viruses. 2015;7(11):5705–5717. doi: 10.3390/v7112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McMeniman C. J., Lane R. V., Cass B. N., et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323(5910):141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 86.Hoffmann A. A., Montgomery B. L., Popovici J., et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–459. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 87.Jiggins F. M. The spread of Wolbachia through mosquito populations. PLoS Biology. 2017;15(6):p. e2002780. doi: 10.1371/journal.pbio.2002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt T. L., Filipovic I., Hoffmann A. A., Raic G., Filipović I., Rašić G. Fine-scale landscape genomics of Aedes aegypti reveals loss of Wolbachia transinfection, dispersal barrier and potential for occasional long distance movement. BioRxiv. 2017 doi: 10.1101/103598. [DOI] [Google Scholar]
- 89.Terradas G., McGraw E. A. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Current Opinion in Insect Science. 2017;22:37–44. doi: 10.1016/j.cois.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Asad S., Parry R., Asgari S. Upregulation of Aedes aegypti Vago1 by Wolbachia and its effect on dengue virus replication. Insect Biochemistry and Molecular Biology. 2018;92:45–52. doi: 10.1016/j.ibmb.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 91.WHO. World Malaria Report 2017. Geneva, Switzerland; 2017. [Google Scholar]
- 92.WHO. Global Plan for Insecticide Resistance Management in Malaria Vectors. WHO; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zélé F., Nicot A., Berthomieu A., Weill M., Duron O., Rivero A. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proceedings of the Royal Society B Biological Science. 2014;281(1779) doi: 10.1098/rspb.2013.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]