Abstract
STUDY QUESTION
What are the general public’s reasons for being in favour of or against the use of genome modification for five potential applications?
SUMMARY ANSWER
Overall, 43 reasons for being in favour, 45 reasons for being against as well as 26 conditional reasons for the use of genome modification were identified.
WHAT IS KNOWN ALREADY
Various applications of somatic genome modification are progressing towards clinical introduction and several recent studies have reported on germline genome modification. This has incited a debate on ethical and legal implications and acceptability. There is a growing plea to involve the general public earlier on in the developmental process of science and (bio)technology including genome modification.
STUDY DESIGN, SIZE, DURATION
In April 2016, a cross-sectional survey was launched online among the Dutch general public. A documentary on genome modification on public television and calls in social media invited viewers and non-viewers, respectively, to participate.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The questionnaire introduced five potential future applications of genome modification: modified wheat for individuals with gluten intolerance; somatic modification for individuals with neuromuscular diseases; germline modification to prevent passing on a neuromuscular disease; germline modification to introduce resistance to HIV; and germline modification to increase intelligence. Participants were asked to indicate whether and why they would make use of genome modification in these scenarios. The reasons mentioned were analysed through content analysis by two researchers independently. The proportion of respondents that was willing to modify was described per scenario and associations with respondent characteristics were analysed.
MAIN RESULTS AND THE ROLE OF CHANCE
The survey was completed by 1013 participants. Forty-three reasons for being in favour, 45 reasons for being against as well as 26 conditional reasons for the use of genome modification were identified. These could be categorized into 14 domains: safety of the individuals concerned; effectiveness; quality of life of the individuals concerned; existence of a clinical need or an alternative; biodiversity and ecosystems; animal homo sapiens (i.e. relating to effects on humans as a species); human life and dignity; trust in regulation; justice; costs; slippery slope; argument of nature; parental rights and duties; and (reproductive) autonomy. Participants’ willingness to use genome modification was dependent on the application: most participants would eat modified wheat if gluten intolerant (74%), would use genome modification to cure his/her own neuromuscular disease (85%) and would apply germline modification to prevent passing on this neuromuscular disease (66%). A minority would apply germline modification to introduce resistance to HIV (30%) or increase intelligence (16%). Being young (odds ratio (OR) = 0.98 per year increase), being male (OR = 2.38), and having watched the documentary (OR = 1.82) were associated with being willing to apply genome modification in more scenarios.
LIMITATIONS, REASONS FOR CAUTION
Inquiring for reasons through open questions in a survey allowed for a larger sample size and intuitive responses but resulted in less depth than traditional face-to-face interviews. As the survey was disseminated through social media, the sample is not representative of the overall Dutch population, and hence the quantitative results should not be interpreted as such.
WIDER IMPLICATIONS OF THE FINDINGS
Further public consultation and a more in-depth ethical and societal debate on principles and conditions for responsible use of (germline) genome modification is required prior to future clinical introduction.
STUDY FUNDING/COMPETING INTEREST(S)
Funded by the University of Amsterdam and University Medical Centre Utrecht. No conflict of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: genetic engineering; CRISPR-Cas systems; mutation; germ cells; genome, human; humans; healthcare quality, access and evaluation; reproductive techniques; ethics; surveys and questionnaires
Introduction
The prospect of purposeful modification of DNA has been a source of both excitement and unease for decades. Although tools such as zinc finger nucleases and transcription activator-like effector nucleases for genome modification have been available for some time, the discovery of CRISPR-Cas9, given its specificity, efficiency, low-costs and ease in use, has represented a major step forward from previously available methods (Cong et al., 2013; Jinek et al., 2012).
WHAT DOES THIS MEAN FOR PATIENTS?
This article looks at the responses of the general public in Holland to the idea of altering human genes for a number of possible uses. The research team used a survey to ask people what they thought, and this accompanied a television documentary on the subject and a social media campaign.
It was explained that although science was still some way from the possibility of using gene editing for humans, there had been many developments in recent years and scientists were keen to understand the views of the general public on the issue. The survey covered a number of different scenarios and asked whether and why the participants would consider using gene modification.
A majority of people said they would be willing to use the technology to avoid passing on a neuromuscular disease, but they were least likely to want it to increase the intelligence of their future children. People had many different reasons for or against using gene modification. For example, some argued it would improve their well-being whereas others worried about safety or thought that alternatives to altering genes were more acceptable. Those who were most willing to consider gene editing were those who were young, male and those who had watched the accompanying documentary before completing the survey.
The researchers concluded that future studies could analyse more closely the reasons for the public’s thoughts on how and when altering human genes might be acceptable and that it would be useful to evaluate how best to involve the views of the public in emerging science in the future.
Genome modification has various types of possible applications. It can be used in agriculture to, for example, boost yields, protect against pests or enhance nutrient content (Gil-Humanes and Voytas, 2014; Wang et al., 2014; Jones, 2015), and some clustered regularly interspaced short palindromic repeats-CRISPR-associated protein-9 nuclease (CRISPR-Cas9) generated crops have already been cleared for commercial use by the US Department of Agriculture (Waltz, 2016a,b). A large number of human clinical trials have been performed or are underway in order to treat diseases in somatic cells (i.e. all cells that will not be passed on to future progeny) (Ginn et al., 2013). In contrast, germline genome modification, involving modification of nuclear DNA in germ cells or embryos such that all cells in the resulting child and its future progeny carry the modification, has thus far never been applied clinically. Five papers have recently described successful human germline genome modification of (non-viable) human embryos using CRISPR (Liang et al., 2015; Kang et al., 2016; Fogarty et al., 2017; Ma et al., 2017; Tang et al., 2017). Although the initial experiments revealed the techniques unsafe and ineffective, the latest experiments have shown remarkable progress and scientists expect to overcome the technical hurdles in the foreseeable future (Ishii, 2015; Lunshof, 2016; Olson, 2016; Smith et al., 2012).
As, with the discovery of CRISPR-Cas9, clinical applications of germline genome modification became more feasible, the debate on the ethical and legal implications of germline genome modification and its acceptability intensified (IBC, 2015; The Academy of Medical Sciences et al., 2015; National Academies of Sciences, 2017). So far, this debate has taken place mostly at international conferences as well as in the (academic) literature (Baltimore et al., 2015; Bosley et al., 2015; Lanphier et al., 2015). There is broad consensus among experts on the need to include more stakeholders in this debate, including the general public (Holdren et al., 2011; Baltimore, 2015; Bosley et al., 2015; IBC, 2015; The Academy of Medical Sciences et al., 2015; National Academies of Sciences, 2017). The general public itself also calls for public consultation before clinical applications (Scheufele et al., 2017). Including the general public is considered appropriate as this may improve the quality of decisions, the potential consequences theoretically affect everyone, stakeholders involved so far do not necessarily represent society, public involvement may help prevent abuse of the technology due to special interests, and public involvement may safeguard public trust in science. Despite not necessarily having complete or accurate information, the general public has been shown capable of holding complex social and ethical views and conducting sophisticated discussions on genetics (Kerr et al., 1998). Perceived risks and benefits have been repeatedly shown to affect public acceptability (Siegrist, 2000; Weisberg et al., 2017). As these differ, or are weighted differently, depending on the application, the acceptability of genome modification depends on the specific application (Frewer et al., 1997; Trust, 2005).
This paper aims to gain insight into the reasons of the Dutch general public for being in favour or against using genome modification for five potential clinical applications.
Materials and Methods
The Dutch general public is an interesting population to study as familiarity with gene therapy is the highest in the European Union (EU) (73%, as compared to the EU average of 45%) (Gaskell et al., 2006) and a lack of public education has been flagged as a major limitation of previous studies (Blendon et al., 2016). Simultaneously, disapproval of gene therapy research is only slightly different from the EU average (25% as compared to 29%) (EC, 2010).
Cross-sectional surveys are in the Netherlands exempt from Institute Review Board approval.
Questionnaire
A study-specific questionnaire was developed. It was designed to cover applications which have previously been shown to differ in their acceptability: curing diseases versus enhancement (Robillard et al., 2014) and curing genetic diseases versus curing preventable diseases (Kalfoglou et al., 2005). Furthermore, previous studies showed differences between germline or somatic applications (Trust, 2005), and human versus plant applications (Crne-Hladnik et al., 2009). To cover these axes, the following five scenarios were included: modified wheat for individuals with gluten intolerance (Barro et al., 2016; Shewry and Tatham, 2016); somatic modification for individuals with neuromuscular diseases (Tabebordbar et al., 2016); germline modification to prevent passing on a neuromuscular disease (Long et al., 2014; Liang et al., 2015); germline modification to introduce resistance to HIV (Samson et al., 1996; Kang et al., 2016); or germline modification to increase intelligence (intelligence is in part genetically determined (Plomin and Spinath, 2004)). Participants were asked to consider that each of these scenarios would be applicable to them, and to indicate whether they would use genome modification (yes/no) and why they would or would not (open question) (Macer et al., 1995). Of note, surveys frequently operationalized genomic enhancement as improving intelligence, and mimicking that allowed for comparison to previous studies (Congress, 1987; Macer et al., 1995; Kalfoglou et al., 2005; Meisenberg, 2009; Muller and Shepherd, 2009; Criger and Fekken, 2013; Robillard et al., 2014; Funk et al., 2016; McCaughey et al., 2016). We did inform respondents that this application is scientifically still far-fetched.
Finally, participants were asked for their gender, age and whether they had watched the documentary on genome modification (see below). Information on the purpose of this study, as well as the state-of-the-art and feasibility of the presented scenarios, was provided (see Supplementary Data for the translated questionnaire, the announcement of the study, and the background information provided).
The questionnaire was reviewed by an expert panel composed for this study including a clinician (S.H.), a biologist (S.R.), two science journalists and a senior editor of a Dutch popular science media outlet. The expert panel focused on scientific accuracy, non-directive phrasing (Molewijk et al., 2003), understandable use of language for the lay public, and feasibility (e.g. sufficient background information provided, acceptable administration time). Amongst others, the panel decided to refer to genome modification as ‘genetic modification’, despite appreciating the differences in the terms, as this is common practice in Dutch language, which has not yet adopted the term ‘genome modification’ (e.g. not part of dictionaries, Wikipedia, lay vocabulary). To ensure proper understanding, the documentary explained CRISPR-Cas9 as a new, highly effective technology to cut-and-paste specific pieces of DNA.
Data collection
The survey was launched online on 30 March 2016 and was accessible for 4 weeks through the website of ‘de Kennis van Nu’, a Dutch popular science media outlet. It was released together with a documentary (which was available online as well as aired twice on Dutch television) providing background information on genome modification. The documentary covered recent advancements of CRISPR-Cas9 in genome modification and potential germline, somatic and agriculture applications. In the documentary, the interviewed scientists called for the public to express their views by filling out the survey. Additionally, calls on social media invited viewers and non-viewers to comment on genome modification through the survey.
Data analysis
The proportion of respondents being willing to use modification was described per scenario and an ordinal regression analysis assessed whether respondent characteristics affected the number of scenarios in which participants were willing to apply genome modification. Data were analysed with the Statistical Package for the Social Sciences (SPSS 22.0) (IBM, Chicago, IL, USA).
The responses to the open questions were analysed using phenomenology methodology involving multiple readings to understand the context; highlighting meaningful units of text that were relevant to the research question; clustering meaningful units of text into distinct reasons and overarching domains; contextualizing the identified domains (i.e. checking for consistency with the full response to maintain the context) (Hycner, 1985; Graneheim and Lundman, 2004). The identified reasons were organised into reasons for being in favour of using genome modification, reasons for being against using genome modification, and conditional reasons for using genome modification. Reasons representing flipsides of the same coin were highlighted, for example: ‘genome modification would not impose a high treatment burden’ and ‘genome modification would impose a high treatment burden’. For data presentation, examples of patient quotations were selected and translated.
Finally, the reasons for and against germline genome modification identified by the general public were compared to reasons identified by experts as reported in a recent systematic literature review, and novel considerations were flagged (van Dijke et al., 2017).
Results
Respondents
A total of 1013 participants filled out the questionnaire (Table I). About half (54%) of the respondents were male. The respondents’ age ranged from 11 to 90 years, with a mean of 44 years. Most respondents (69%) had watched the documentary on genome modification.
Table I.
Characteristics of respondents in the survey on views of the Dutch general public on the use of genome modification.
| Respondent characteristics | Proportion % (n) |
|---|---|
| Gender | |
| Male | 54% (n = 542/1013) |
| Female | 46% (n = 471/1013) |
| Age (mean ± SD in years) | 44 ± 19 |
| Watched television documentary on GM | |
| Yes | 69% (n = 704/1013) |
| No | 31% (n = 309/1013) |
GM: genome modification.
Willingness to use genome modification
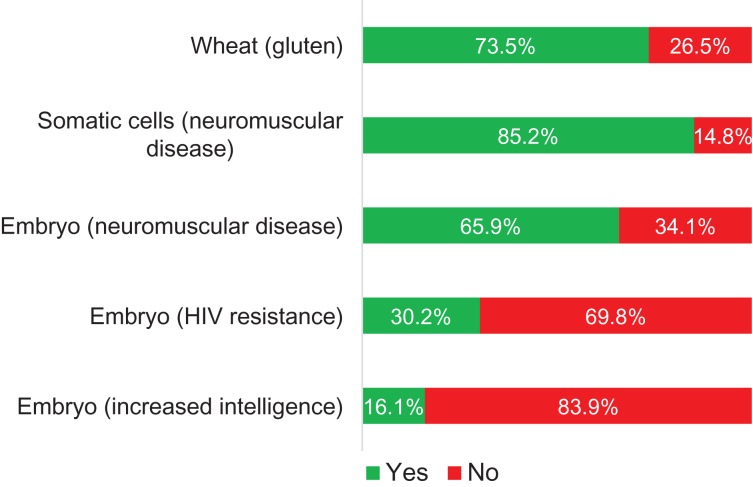
The differences in respondents’ willingness to alter the human germline for different purposes or use genome modification for non-germline purposes is reported in Fig. 1. A majority of participants (66%) was willing to use germline modification to prevent passing on a neuromuscular disease. Participants were least willing to use germline modification to increase intelligence of their embryo (16%). However, the scenarios that did not involve human germline modification were considered acceptable by more participants. About 1 in 10 participants considered modification either unacceptable in all scenarios (8%) or acceptable in all scenarios (11%).
Figure 1.
The willingness of participants in survey to use genome modification in different scenarios. Reporting the results of a sample of 1013 participants that is not representative of the overall Dutch general public.
Being young (odds ratio (OR) = 0.98 per year increase), being male (OR = 2.38), and having watched the documentary on this topic (OR = 1.82) were associated with being willing to use genome modification in more scenarios (Table II).
Table II.
Respondents’ characteristics determining the number of applications in which participants are willing to use GM.
| Respondent characteristic | Adjusted odds ratio | 95% CI | P-value |
|---|---|---|---|
| Being male | 2.38 | 1.90–2.98 | <0.001 |
| Age (per year increase) | 0.98 | 0.98–0.99 | <0.001 |
| Having watched documentary | 1.82 | 1.42–2.31 | <0.001 |
Reasons for being in favour or against using genome modification
A total of 6333 meaningful units (i.e. text describing a motivation in favour or against genome modification) were identified. Between zero (n = 83; only filled out the quantitative questions) and eight meaningful units were identified per person.
The meaningful units could be categorized into 43 reasons for being in favour of using genome modification, 45 reasons for being against using genome modification, and 26 conditional reasons for using genome modification (114 in total; Supplementary Table SI). Of these, 39 reasons have only been raised by participants in the scenario’s in which the germline is altered (Supplementary Table S1).
Table III reports on the five most frequently reported reasons for and against using genome modification for each application. Improving the quality of life of the person directly involved was the most frequently reported advantage of all applications. Experiencing the ‘yuck-factor’ (i.e. a feeling of horror, revulsion or disgust) or the explicit lack thereof, or even the ‘wow factor’, was in the top five most frequently mentioned reasons both for and against using all applications. The possibility of negative long-term consequences for society and the unnaturalness of genome modification were in the top five of four applications. Unacceptable health risks was among the top three most frequently mentioned reasons against using four applications. The availability of alternatives was the most frequently mentioned reason against using three applications.
Table III.
The five most frequently mentioned reasons per application.
| Application | Order | Reasons provided (n = x)* | ||
|---|---|---|---|---|
| In favour | Conditions | Against | ||
| Wheat (gluten) | 1 | GM would improve my/my child’s quality of life (n = 344) | I would only use GM if it would not pose unacceptable health risks (n = 78) | GM would not be acceptable as there are alternatives available to obtain the same result (n = 82) |
| 2 | GM incites the ‘wow factor’ and/or does not incite the ‘yuck-factor’ (n = 102) | I would only use GM if it would not do ecological harm (n = 15) | GM would pose unacceptable health risks (n = 53) | |
| 3 | GM would merely raise the speed and efficiency of a naturally occurring process (n = 77) | I would only use GM if there are no alternatives available to obtain the same result (n = 8)/I would only use GM if the extent of the manipulation is limited (n = 8) | GM would have negative long-term consequences for society (n = 43)/GM would be too unnatural (n = 43) | |
| 4 | GM would not pose health risks (n = 63) | I would only use GM if it would not affect biodiversity (n = 7) | GM would cause harm to ecosystems (n = 18) | |
| 5 | GM would not be inherently different than currently accepted vaccines and medications (n = 29) | I would only find genetic modification acceptable if it would be applied for disease rather than enhancement (n = 6) | GM incites the ‘yuck-factor’ (i.e. a feeling of horror, revulsion or disgust)/GM would not cure the cause of the problem but merely its expression (n = 17) | |
| Somatic (neuromuscular disease) | 1 | GM would improve my/my child’s quality of life (n = 578) | I would only use GM if it would not pose unacceptable health risks (n = 82) | GM incites the ‘yuck-factor’ (i.e. a feeling of horror, revulsion or disgust) (n = 34) |
| 2 | GM would pose limited health risks but those are acceptable considering the benefits (n = 59) | I would only use GM if it would improve my/my child’s quality of life (n = 41) | GM would pose unacceptable health risks (n = 32) | |
| 3 | GM incites the ‘wow factor’ and/or does not incite the ‘yuck-factor’ (n = 47) | I would only use GM if potential consequences are limited to myself (n = 22) | GM would be unacceptable because disease has a purpose in life (n = 19) | |
| 4 | GM would not be inherently different than currently accepted vaccines and medications (n = 41) | I would only find GM acceptable if it would be applied for disease rather than enhancement (n = 16) | GM would be too unnatural/GM would have negative long-term consequences for society (n = 14) | |
| 5 | GM would have consequences that are limited to myself (n = 31) | I would only use GM if it would be an effective treatment (n = 11) | GM would not (sufficiently) improve my/my child’s quality of life (n = 13) | |
| Germline (neuromuscular disease) | 1 | GM would improve my/my child’s quality of life (n = 366) | I would only use GM if it would not pose unacceptable health risks (n = 77) | GM would not be acceptable as there are alternatives available to obtain the same result (n = 188) |
| 2 | GM is a moral obligation as I do not have the right to withhold my child from the possibilities created by germline modification (n = 54) | I would only use GM if it would improve my/my child’s quality of life (n = 27) | GM would pose unacceptable health risks (n = 58) | |
| 3 | GM is better than, or an acceptable alternative to, current options (n = 37) | I would only find GM acceptable if it would be applied for disease rather than enhancement (n = 24) | GM incites the ‘yuck-factor’ (i.e. a feeling of horror, revulsion, or disgust) (n = 57) | |
| 4 | GM incites the ‘wow factor’ and/or does not incite the ‘yuck-factor’ (n = 29) | I would only use GM if there are no alternatives available to obtain the same result (n = 14) | GM is unacceptable as I do not have the right to decide on modifying genes on behalf of my unborn child (n = 31) | |
| 5 | GM would improve the quality of life of the family and friends of the cured individual (n = 25) | I would only use GM if the risk of acquiring the disease is high (n = 10) | GM prevents the disease, and this is not preferable over curing the disease if it manifests/GM would have negative long-term consequences for society/GM would be too unnatural (n = 18) | |
| Germline (HIV resistance) | 1 | GM would improve my/my child’s quality of life (n = 145) | I would only use GM if it would not pose unacceptable health risks (n = 26) | GM would not be acceptable as there are alternatives available to obtain the same result (n = 292) |
| 2 | GM would reduce the frequency of or eradicate diseases (n = 34) | I would only find GM acceptable if it would be applied for disease rather than enhancement (n = 17) | GM would not be necessary as the risk of acquiring the disease is low (n = 100) | |
| 3 | GM would not be inherently different than currently accepted vaccines and medications (n = 22) | I would only use GM if the risk of acquiring the disease is high (n = 16) | GM would pose unacceptable health risks (n = 64) | |
| 4 | GM incites the ‘wow factor’ and/or does not incite the ‘yuck-factor’ (n = 18) | I would only use GM if it would improve my/my child’s quality of life (n = 5) | GM incites the ‘yuck-factor’ (i.e. a feeling of horror, revulsion, or disgust) (n = 62) | |
| 5 | GM would be an effective treatment (n = 16) | I would only use GM if there are no alternatives available to obtain the same result/I would only use GM if costs of GM are not high for the individual (n = 3) | GM would start a slippery slope towards morally unacceptable applications (n = 54) | |
| Germline (increased intelligence) | 1 | GM would improve my/my child’s quality of life (n = 72) | I would only find GM acceptable if it would be applied for disease rather than enhancement (n = 107) | GM would not (sufficiently) improve my/my child’s quality of life (n = 188) |
| 2 | GM would allow its users to contribute more to society (n = 39) | I would only use GM if it would improve my/my child’s quality of life (n = 38) | GM would have negative long-term consequences for society (n = 103) | |
| 3 | GM would not have much effect as it would merely shift the normal curve to a higher average (n = 15) | I would only use GM if not using it would make me fall behind (n = 20) | GM would reduce human diversity which is important for the functioning of society (n = 96) | |
| 4 | GM incites the ‘wow factor’ and/or does not incite the ‘yuck-factor’ (n = 10) | I would only use GM if it would not pose unacceptable health risks (n = 12) | GM incites the ‘yuck-factor’ (i.e. a feeling of horror, revulsion, or disgust) (n = 92) | |
| 5 | GM would merely raise the speed and efficiency of a naturally occurring process (n = 7) | I would only use GM if it would be accessible for everyone (n = 6) | GM would be too unnatural (n = 53) | |
*See Supplementary Table SI for all reasons provided.
Comparing the reasons for and against germline genome modification identified by the general public to those previously identified by experts (van Dijke et al., 2017), revealed that the public identified 20 additional arguments (Supplementary Table SI). For instance, the general public flagged the concern that after germline genome modification, parents would no longer be full genetic parents (as the inserted ‘normal’ copy replacing the defective gene would not be their own).
The reasons could be categorized into 14 domains, which are listed and exemplified with specific reasons and direct citations in Table IV.
Table IV.
Domains of the reasons provided in favour and against using GM.
| Domain* | Examples of reasons provided | |||||
|---|---|---|---|---|---|---|
| In favour | Condition | Against | ||||
| Reason | Citation | Reason | Citation | Reason | Citation | |
| Safety of the individuals concerned | GM would pose limited health risks but those are acceptable considering the benefits | ‘I would certainly take the risk; the alternative is way too dangerous’ (#295, E_NMD) | I would only use GM if it would not pose unacceptable health risks | ‘If it would be a 100% safe, I would consider it’ (#559, E_INT) | GM would pose unacceptable health risks | ‘You know what you have, but not what you’re going to get [from the GM], and that could be worse.’ (#31, S_NMD) |
| Effectiveness | GM would cure the cause of the problem | ‘This way you tackle the root of the problem and are not just relieving the symptoms like we’ve been doing so far’ (#402, S_NMD) | I would only use GM if it would be an effective treatment | ‘A condition would be it having a reasonable chance of success’ (#158, S_NMD) | GM would not allow for me to become a full genetic parent since modified genes are passed on | ‘The child would be partially mine, partially my wife’s and partially of science and I wouldn’t want that’ (#885, E_INT) |
| Quality of life of the individuals concerned | GM would prevent disease’s suffering in upcoming generations | ‘To improve the health of upcoming generations’ (#987, E_NMD) | I would only use GM if it would improve my/my child’s quality of life | ‘Only for serious diseases that cause lots of limitations’ (#169, E_NMD) | GM would eliminate some of the joys of parenthood | ‘It’s precious not to know what your child will be like’ (#1011, E_INT) |
| Existence of a clinical need or alternative | GM would not be inherently different than currently accepted vaccines and medications | ‘We also have vaccination programs. This is a logical next step’ (#804, E_HIV) | I would only use GM if the risk of acquiring the disease is high | ‘If I would live in a country where HIV is more prevalent’ (#678, E_HIV) | GM would not be acceptable as there are alternatives available to obtain the same result | ‘My kid should just learn to use a condom’ (#520, E_HIV) |
| Biodiversity and ecosystems | GM would not affect biodiversity | ‘It won’t overrun species of wheat with gluten’ (#914, W_GLU) | I would only use GM if it would not do ecological harm | ‘If it doesn’t harm nature’ (#839, W_GLU) | GM would reduce human diversity which is important for the functioning of society | ‘If we’ll end up with only scientists, we’ll miss the rest very badly and mankind will still go extinct’ (#113, E_INT) |
| Animal homo sapiens (i.e. relating to effects on humans as a species) | GM would improve mankind’s chance of survival | ‘Building better people and a stronger future for our species’ (#990, E_HIV) | I would only use GM if there was no alternative to eradicate the disease | ‘As long as there are no other cures for HIV, this is the only way to get to a disease-free world’ (#908, E_HIV) | GM would have negative long-term consequences for society | ‘Wasn’t there a movement that was pursuing this last century, which only brought death and destruction (1940–45)?’ (#365, E_INT) |
| Human life and dignity | GM [of the germline] would not affect a human because the embryo does not yet have that status | ‘An embryo is not a fully grown human. Before it is actually born, it may still be modified.’ (#627, E_NMD) | I would only use GM if ethical issues were addressed | ‘Of course, you’ll have to ask the ethical questions…’ (#367, W_GLU) | GM incites the ‘yuck-factor’ (i.e. a feeling of horror, revulsion, or disgust) | ‘Modifying human embryo’s gives me the chills’ (#509, E_INT) |
| Trust in regulation | GM would not be offered unless it was established as being a good treatment | ‘If it is allowed, it’s safe’ (#698, W_GLU) | I would only use GM if it would be well-regulated | ‘If it’s well monitored by the government’ (#905, W_GLU) | GM would be subject to special interests (commercial, regimes, terrorist), which may cause exploitation of vulnerable individuals | ‘It won’t be long until governments will interfere and start making rules. The chance that totalitarian regimes will misuse it is huge’ (#683, E_INT) |
| Justice | GM would improve equality as everyone would have access to beneficial genetic traits | ‘Because the sick want healthy children just like anyone else (#414, E_NMD) | I would only use GM if not using it would make me fall behind | ‘If the rest of the world is doing ‘it’, you have no other choice’ (#587, E_INT) | GM would increase segregation | ‘Being strong and smart are important conditions for a good and successful life. I wonder what happens if you can buy those things. I imagine it will cause segregation. A gab between those who can afford it, and those who can’t’ (#608, E_INT) |
| Costs | GM would lower healthcare costs | ‘Such a disease entails substantial societal costs. If this leads to a more productive life and less (medical) expenses, this is a win-win situation’ (#241, S_NMD) | I would only use GM if costs of GM are not high for the individual | ‘Depending on how much it would cost…’ (#934, E_HIV) | GM would increase healthcare costs | It would make healthcare, which is already too expensive, entirely unaffordable (#842, S_NMD) |
| Slippery slope | GM would strictly be applied to those purposes considered acceptable | ‘A list of which diseases are and are not allowed will have to be created. No matter how bad some cases may be, you don’t want to just do everything’ (#429, E_NMD) | I would only use GM if it would not change societal norms and induce a slippery slope effect | ‘As long as modification doesn’t become the norm’ (#438, E_NMD) | GM would start a slippery slope towards morally unacceptable applications | ‘If you allow this, where will it end?’ (#587, E_INT) |
| Argument of nature | GM would merely raise the speed and efficiency of a naturally occurring process | ‘Mankind will always be evolving whether we speed this up with GM or not, we’ll become smarter anyways’ (#883, E_INT) | I would only use GM if the extent of the manipulation is limited | ‘I also want them to have their own characteristics so I wouldn’t do too much with it [GM]’ (#776, E_INT) | GM would be too unnatural | ‘It’s important to, however hard it is, respect nature’s boundaries and not to bend them for your own benefit’ (#975, S_NMD) |
| Parental rights and duties | GM is a moral obligation as I do not have the right to withhold my child from the possibilities created by germline modification | ‘Allowing your child to be born with an abnormality if you could have done something should be a crime. They should put these people in prison and make them pay for the health expenses for life’ (#631, E_NMD) | N/A | N/A | GM is unacceptable as I do not have the right to decide on modifying genes on behalf of my unborn child | ‘You shouldn’t be allowed to decide that for your unborn child’ (#359, E_HIV) |
| (Reproductive) autonomy | GM would fall under my right for autonomy and thus should not be prohibited | ‘My body, my life, my decision’ (#755, S_NMD) | I would only use GM if it remains a free choice to do so | ‘I should never be required to do it. It will become problematic once e.g. health insurance starts demanding it and there will be social pressure’ (#755, E_NMD) | GM would create choices and thereby responsibilities that people are not able to carry out | ‘Once you will have these choices, you may experience guilt complexes for things you attributed to if they go wrong. That’s why you shouldn’t be asked in the first place’ (#245, E_NMD) |
*See Supplementary Table SI for all reasons provided.
W_GLU: Response to the scenario ‘I would eat modified wheat if I would be gluten intolerant’.
S_NMD: Response to the scenario ‘I would cure own neuromuscular disease to prevent ending up in a wheelchair’.
E_NMD: Response to the scenario ‘I would modify my embryo to prevent passing on a severe neuromuscular disease’.
E_HIV: Response to the scenario ‘I would introduce resistance to HIV in my embryo’.
E_INT: Response to the scenario ‘I would increase the intelligence of my embryo’.
Discussion
Studying the general publics’ perspectives on germline genome modification has been frequently called for (Baltimore, 2015; Bosley et al., 2015; IBC, 2015; The Academy of Medical Sciences et al., 2015; National Academies of Sciences, 2017). Such a study is timely considering that CRISPR/Cas9 brings clinical applications of germline genome modification closer, which means both international and national governance should be installed now (Baltimore, 2015; Bosley et al., 2015; IBC, 2015; National Academies of Sciences, 2017; The Academy of Medical Sciences et al., 2015).
The study has several limitations. First, research comparing open questionnaire questions to individual/group interviews is limited and conflicting (Bowling et al., 1993; Bowling, 1996; Dolan et al., 1999; Hanratty and Lawlor, 1999; Shiell et al., 2000; Contandriopoulos, 2004; Delli-Carpini et al., 2004). For example, questionnaires allow for a larger sample size and intuitive responses, but allow for less in-depth understanding of participants’ perspectives than interviews. Second, we could not confirm understanding of the provided information and scenarios. Although it is still unclear whether and how knowledge of genome modification effects its acceptability (Trust, 2005; Muller and Shepherd, 2009; Scheufele et al., 2017), proper understanding is important for the validity of responses (Bradburn et al., 1982). Therefore, we attempted to facilitate understanding through providing balanced information and a documentary. Third, we questioned specific applications (e.g. HIV resistance) instead of broad categories (e.g. preventable disease), to ensure respondents considered comparable scenarios. However, the specific diseases may not represent the entire category as, for example, the severity of the disease determines acceptability (Evans et al., 2005; Trust, 2005; Xiang et al., 2015). Fourth, as the survey was disseminated through social media, our respondents were not representative and likely interested in the topic (Eysenbach and Wyatt, 2002). Fifth, despite using two independent reviewers, content analysis of the responses involves interpretation and the potential for misinterpretation. Sixth, for accurate representation, we report all reasons provided by the participants even though some may be fallacious (e.g. genome modification would not cure the cause of the problem but merely its expression). Furthermore, we report on their frequency of being spontaneously mentioned, which portrays attention, rather than importance. For example, a respondent mentioning safety concerns in only the first three scenarios does not necessarily imply that safety was no longer important in the final two scenarios.
Our identification and structured analysis of the reasons mentioned by members of the general public added to the existing literature. Many previous studies quantified the importance of only a preselected, limited number of attitudes/reasons to the general public (Singer et al., 1998; Siegrist, 2000; Trust, 2005; Meisenberg, 2009; Muller and Shepherd, 2009; Criger and Fekken, 2013; Robillard et al., 2014; Xiang et al., 2015; Funk et al., 2016; Weisberg et al., 2017). A number of previous studies from different countries did qualitatively inquire for reasons (Congress, 1987; Macer, 1992; Macer et al., 1995; Frewer et al., 1997; Lewis et al., 1997; Iredale et al., 2003, 2006; Kalfoglou et al., 2005; Massarani and de Castro Moreira, 2005; Crne-Hladnik et al., 2009; Funk et al., 2016; McCaughey et al., 2016). However, most of them lack the precision that scientists might prefer (Blendon et al., 2016) and/or had substantial methodological limitations, such as not reporting on a structured qualitative analysis (Frewer et al., 1997; Iredale et al., 2003; Massarani and de Castro Moreira, 2005; McCaughey et al., 2016); only reporting on a selection of the obtained domains (Funk et al., 2016); only inquiring for reasons against genome modification (Congress, 1987); not differentiating between germline modification and other reproductive genetic technologies (Kalfoglou et al., 2005); and only including high school students (average age 17 years) (Lewis et al., 1997; Iredale et al., 2006; Crne-Hladnik et al., 2009). The fact that the general public identified 20 arguments that were not mentioned by experts as reported in a systematic overview of the literature (van Dijke et al., 2017) supports the added value of public consultations.
Referring to the content of the reasons that were identified in this survey, the advantage of improving the quality of life of those directly affected, and the disadvantage of potential health risks were frequently and consistently mentioned for the different scenarios. This is similar to the reasons brought forward by professionals (van Dijke et al., 2017). Interestingly, the ‘yuck-factor’, or the explicit lack thereof, was in the top five of reasons for and against all applications. It would be interesting to qualitatively explore the roots of these gut feelings, which by themselves may not form a moral argument but with time may be articulated, weighed, and either endorsed or dismissed (Midgley, 2000).
Although specific arguments were used for specific scenarios and various arguments have only been mentioned in the scenario’s that reflect altering the germline, there were no structural differences in the top five listed arguments of germline and non-germline applications. The following section discusses differences in the top five most frequently reported reasons of the five applications. Interestingly, participants often argued for modifying wheats by stating this would not pose health risks and that modified wheats would not be commercially available if they would not be safe. This may be related to the explanation in the documentary about regulatory approval of modified foods, and/or knowledge otherwise obtained regarding regulations (safety assessment by the European Food Safety Authority) and the reassuring safety data of currently available genetically engineered crops (NASEM, 2016). Specific disadvantages that were frequently addressed in relation to modified wheats included the potential harm to ecosystems and not curing the cause of the problem (i.e. individuals would still be intolerant to gluten). Indeed, these reasons seem less applicable to the other modifications, which are in human. Of note, there is no conclusive evidence on cause-and-effect relationships relating to genetically engineered crops and environmental problems (NASEM, 2016).
Reasonably, the consequences being limited to the individual was used to advocate for somatic modification. Without necessarily being specific to somatic modification, the benefits outweighing the risks and diseases having a purpose in life were only among the top five reasons this application. This may partially be an artefact of structuring in groups of top five responses (e.g. diseases having a purpose in life was more frequently raised against introducing HIV resistance but did not make it in that top five). Alternatively, respondents may feel more comfortable making these types of analyses for themselves than for others.
Germline modification to prevent a neuromuscular disease raised two arguments that were not in the other top fives: parents having the duty not to withhold their child from this possibility and it improving the quality of life of the family of the affected individual. Arguably, these arguments are indeed more relevant here, due to the combination of the severity and unavoidability of the disease, and the notion that parenthood entails special obligations towards one’s offspring. Of note, although we can speculate about the strong moral claim that genome modification is a moral duty (which participants only state in relation to germline modification), it would be interesting to study why this duty is perceived and what the extents of this duty are. Additionally, genome modification being a better or acceptable alternative to current options is especially relevant here as alternatives (PGD or terminating the pregnancy) are also ethically contested (Porteus and Dann, 2015). The view that preventing a disease is not preferable over curing a manifested disease should theoretically also apply for the HIV scenario, where it was indeed mentioned more frequently although not as frequent as to come out in the top five. Finally, parents not having the right to decide on behalf of their child is not necessity linked to this specific germline application, and it only being in this top five may be an artefact of there being less arguments against curing severe genetic diseases (National Academies of Sciences, 2017).
Unsurprisingly, specifically germline genome modification to introduce HIV resistance, our operationalization of curing preventable diseases, was considered less necessary because of the low risk of acquiring the disease. Interestingly, introducing HIV resistance, and not the germline genome modification scenario that was questioned first (neuromuscular disease), most incited the slippery slope argument. In contrast, professionals use this argument against any germline application (Lanphier et al., 2015). It is unclear why introducing HIV resistance specifically was applauded for reducing the prevalence of diseases and for its effectiveness, as genome modification could do the same for genetic diseases. Perhaps respondents associate infectious diseases with eradication more easily.
Using germline genome modification for enhancement, operationalised as increasing intelligence, incited more considerations about our species and the society than the other applications. More specifically, the top five included concerns about losing valuable human diversity, concerns about falling behind if others use it, the consideration that shifting the normal curve would not have much effect, and the advantage that it could allow users to contribute more to society. Similar types of considerations are voiced by the general public about pharmacological cognitive enhancement (Schelle et al., 2014).
Previous scholars have suggested that the arguments brought forward in the debate on human germline modification are not new, but are in fact similar to arguments used in relation to other novel technologies, such as PGD (Harris, 2016; Tonkens, 2011). However, even when the arguments are not new, this does not diminish the need to reflect on human germline modification, as sometimes a difference in degree can amount to a difference in kind.
In line with previous studies, a significant proportion of responders (40–90%) indicated being willing to use genome modification (Congress, 1987; Macer et al., 1995; Singer et al., 1998; Xiang et al., 2015; Funk et al., 2016). Our finding that willingness to use genome modification depends on the scenario in which it is used corresponds to previous findings. More specifically, curing diseases is considered more acceptable than enhancement (Congress, 1987; Macer et al., 1995; Lewis et al., 1997; Singer et al., 1998; Iredale et al., 2003; Evans et al., 2005; Kalfoglou et al., 2005; Massarani and de Castro Moreira, 2005; Meisenberg, 2009; Muller and Shepherd, 2009; Criger and Fekken, 2013; Robillard et al., 2014; Xiang et al., 2015; Blendon et al., 2016; Funk et al., 2016; McCaughey et al., 2016; Scheufele et al., 2017). Curing genetic diseases is considered more acceptable than curing diseases that may be prevented (Singer et al., 1998; Kalfoglou et al., 2005). Somatic modification is considered more acceptable than germline modification (Lewis et al., 1997; Commission, 2000; Iredale et al., 2003; Trust, 2005; Crne-Hladnik et al., 2009; Blendon et al., 2016; Funk et al., 2016) although some studies highlight the difference as small (Macer et al., 1995; Meisenberg, 2009) and two studies found no distinction at all (Congress, 1987; Scheufele et al., 2017). Even though modifying plants was seen as more acceptable than somatic human modification in some older studies (Congress, 1987; Frewer et al., 1997), this was not the case in our, and other more recent studies, which may be related to the lower clinical need (alternative to a gluten-free wheat versus a neuromuscular disease leading to needing a wheelchair) (Crne-Hladnik et al., 2009; Gaskell et al., 2006).
That men are more likely to support the technology is consistent with previous studies in various other countries (Evans et al., 2005; Meisenberg, 2009; Criger and Fekken, 2013; Xiang et al., 2015; McCaughey et al., 2016). This may be related to women having less trust in the institutions involved (Siegrist, 2000) or women perceiving the applications for genome modification as less valuable (Siegrist, 2000; Crne-Hladnik et al., 2009) or the risks as higher (Xiang et al., 2015). In addition, two previous studies also report that being young (in various countries) is associated with more favourable attitudes towards certain applications of human genome modification (McCaughey et al., 2016; Weisberg et al., 2017). Age was not significant in another US study, however, in this study the age-range may have been limited as all participants were students (Meisenberg, 2009).
In our study, there was a positive association between watching the documentary and acceptability of genome modification. Several explanations may apply. First, it is possible that watching the documentary increased knowledge on genome modification and more knowledge increases acceptability. However, a previous UK study found that increased knowledge from watching a documentary had little effect on attitudes towards gene therapy (Trust, 2005). At this point, the evidence for a positive association between knowledge and acceptability is insufficient (Trust, 2005; Muller and Shepherd, 2009; Scheufele et al., 2017). Second, although the documentary aimed to avoid this, it could have framed genome modification positively. However, a former study showed that framing did not affect participants’ attitudes towards genome modification (Weisberg et al., 2017). Third, people with a priori more positive attitudes towards genome modification may have been more likely to electively watch the documentary.
Future research
This study revealed a large number of reasons for the general public to use, or refrain from using, genome modification. Qualitative interviews with individuals or focus groups may provide more in-depth understanding into these reasons. Future quantitative studies may provide insight into which reasons carry the largest weight in the deliberations of representative samples of the general public in various countries. Furthermore, respondents phrasing acceptability as conditional to certain aspects incites interest in determining more exact levels of acceptability (e.g. what risk of off-target effects that would be considered acceptable for which indication?). In addition, studying the impact of more respondent characteristics, genetic literacy, preparatory study information and of recent media attention on perspectives may be relevant.
Moreover, it seems invaluable to incorporate empirical data in an ethical analysis when one wants to make well-informed and pro-active normative claims about a certain practice (Dunn et al., 2012). Therefore, the data provided here and elsewhere may provide a valuable input for further ethical analysis.
Finally, we should evaluate the efficacy of various approaches of public engagement and how these can and should be incorporated into policy-making (National Academies of Sciences, 2017).
Implications for future practice
Further public consultation and a more in-depth ethical and societal debate on principles and conditions for responsible use of germline genome modification is required to develop balanced oversight prior to future clinical introduction. Our study, and another non-representative international survey (McCaughey et al., 2016), provide some preliminary evidence that moral intuitions of the general public support professional statements, which state that the acceptability of germline genome editing should be considered per application rather than banned altogether (Chan et al., 2015; National Academies of Sciences, 2017). Experts in the field and policy-makers should consider the reasons provided by the general public for or against germline genome modification in their deliberations on acceptability and appropriate and balanced oversight (Holdren et al., 2011).
As for communication with the general public, it seems unfeasible to discuss all potential reasons for or against germline genome modification (as there are over a hundred (van Dijke et al., 2017)). Unfortunately, this implies the need to make a selection when providing information, which introduces the potential for biasing the public’s views that experts need to be cognizant of. The results of our study can provide some assistance, as one may wish to address frequently considered and/or fallacious reasons, as well as reasons considered important by professionals but uncommonly reflected on by the public. Although finding a neutral balance between information overload and information deficit may be challenging, maintaining a public dialogue is crucial.
Supplementary Material
Acknowledgements
We thank the participants as well as Saar Slegers, Marcia van Woensel, Valentijn van der Lende, and their colleagues for working with us on dissemination of the questionnaire.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Authors’ roles
S.H. contributed to all aspects of the study. N.A.A.G. contributed to the data analysis and critical discussion. A.L.B. contributed to the critical discussion. S.R. contributed to the study design and critical discussion.
Funding
University of Amsterdam and University Medical Centre Utrecht.
Conflict of interest
None.
Disclaimer
The views expressed are the authors’ own and do not necessarily reflect those of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
References
- Baltimore D. The Purpose of the Summit International Summit on Human Gene Editing: Commissioned Papers. Washington: U.S. National Academy of Sciences, U.S. National Academy of Medicine, Royal Society, Chinese Academy of Sciences, 2015, 3–5. [Google Scholar]
- Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, Corn JE, Daley GQ, Doudna JA, Fenner M et al. . Biotechnology. A prudent path forward for genomic engineering and germline gene modification. Science 2015;348:36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barro F, Iehisa JC, Gimenez MJ, Garcia-Molina MD, Ozuna CV, Comino I, Sousa C, Gil-Humanes J. Targeting of prolamins by RNAi in bread wheat: effectiveness of seven silencing-fragment combinations for obtaining lines devoid of coeliac disease epitopes from highly immunogenic gliadins. Plant Biotechnol J 2016;14:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendon RJ, Gorski MT, Benson JM. The public and the gene-editing revolution. N Engl J Med 2016;374:1406–1411. [DOI] [PubMed] [Google Scholar]
- Bosley KS, Botchan M, Bredenoord AL, Carroll D, Charo RA, Charpentier E, Cohen R, Corn J, Doudna J, Feng G et al. . CRISPR germline engineering—the community speaks. Nat Biotechnol 2015;33:478–486. [DOI] [PubMed] [Google Scholar]
- Bowling A. Health care rationing: the public’s debate. Br Med J 1996;312:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A, Jacobson B, Southgate L. Explorations in consultation of the public and health professionals on priority setting in an inner London health district. Soc Sci Med 1993;37:851–857. [DOI] [PubMed] [Google Scholar]
- Bradburn NM, Sudman S, Wansink B. Asking Questions: A Practical Guide to Questionnaire Design. A Wiley Imprint, San Francisco: Jossey-Bass, 1982. [Google Scholar]
- Chan S, Donovan PJ, Douglas T, Gyngell C, Harris J, Lovell-Badge R, Mathews DJ, Regenberg A, Hinxton G. Genome editing technologies and human germline genetic modification: The Hinxton Group Consensus Statement. Am J Bioeth 2015;15:42–47. [DOI] [PubMed] [Google Scholar]
- Commission HG Public Attitudes to Human Genetic Information—People’s Panel Quantitative Study conducted for the Human Genetics Commission. 2000.
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA et al. . Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congress US New Developments in Biotechnology: Public Perceptions of Biotechnology. Washington, DC: United States Congress. Office of Technology Assessment, 1987. [Google Scholar]
- Contandriopoulos D. A sociological perspective on public participation in health care. Soc Sci Med 2004;58:321–330. [DOI] [PubMed] [Google Scholar]
- Criger B, Fekken CG. Human Germline Engineering: a study of attitudes among Canadian University Students and the American Public. Int J Humanit Soc Sci 2013;3:148–159. [Google Scholar]
- Crne-Hladnik H, Peklaj C, Košmelj K, Hladnik A, Javornik B. Assessment of Slovene secondary school students’ attitudes to biotechnology in terms of usefulness, moral acceptability and risk perception. Public Underst Sci 2009;18:747–758. [Google Scholar]
- Delli-Carpini MX, Cook FL, Jacobs JR. Public deliberation, discursive participation, and citizen engagement: a review of the empirical literature. Annual Review of Political Science 2004;7:315–344. [Google Scholar]
- Dolan P, Cookson R, Ferguson B. Effect of discussion and deliberation on the public’s views of priority setting in health care: focus group study. Br Med J 1999;318:916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M, Sheehan M, Hope T, Parker M. Toward methodological innovation in empirical ethics research. Camb Q Healthc Ethics 2012;21:466–480. [DOI] [PubMed] [Google Scholar]
- EC Biotechnology report 2010. European Commission, Bruxelles.
- Evans MDR, Kelley J, Zanjani ED. The ethics of gene therapy and abortion: public opinion. Fetal DiagnTher 2005;20:223–234. [DOI] [PubMed] [Google Scholar]
- Eysenbach G, Wyatt J. Using the Internet for surveys and health research. J Med Internet Res 2002;4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty NME, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, Lea R, Elder K, Wamaitha SE, Kim D et al. . Genome editing reveals a role for OCT4 in human embryogenesis. Nature 2017;550:67–73. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewer LJ, Howard C, Shepherd R. Public concerns in the United Kingdom about general and specific applications of genetic engineering: risk, benefit, and ethics. Sci Technol Hum Values 1997;22:98–124. [DOI] [PubMed] [Google Scholar]
- Funk C, Kennedy B, Sciupac EP 2. U.S. public opinion on the future use of gene editing. US Public Wary of Biomedical Technologies to ‘Enhance’ Human Abilities 2016. Pew Research Center.
- Gaskell G, Stares S, Allansdottir A, Allum N, Corchero C, Fischler C, Hampel J, Jackson J, Kronberger N, Mejlgaard N et al. . Europeans and Biotechnology in 2005: Patterns and Trends. Luxembourg: Publications Office of the European Union, 2006. [Google Scholar]
- Gil-Humanes J, Voytas DF. Wheat rescued from fungal disease. Nat Biotechnol 2014;32:886–887. [DOI] [PubMed] [Google Scholar]
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 2013;15:65–77. [DOI] [PubMed] [Google Scholar]
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004;24:105–112. [DOI] [PubMed] [Google Scholar]
- Hanratty B, Lawlor D. Effect of discussion and deliberation on public’s views of priority setting. More data are needed for readers to make judgment about study. Br Med J 1999;319:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. Germline Modification and the burden of human existence. Camb Q Healthc Ethics 2016;25:6–18. [DOI] [PubMed] [Google Scholar]
- Holdren JP, Sunstein CR, Siddiqui IA Memorandum: principles for regulation and oversight of emerging technologies. 2011.
- Hycner RH. Some guidelines for the phenomenological analysis of interview data. Hum Stud 1985;8:279–303. [Google Scholar]
- IBC Report of the IBC on updating its reflection on the human genome and human rights In United Nations Educational SaCO and Committee IB (eds). 2015, Paris.
- Iredale R, Dolan G, McDonald K, Kirk M. Public attitudes to human gene therapy: a pilot study in Wales. Community Genet 2003;6:139–146. [DOI] [PubMed] [Google Scholar]
- Iredale R, Longley M, Thomas C, Shaw A. What choices should we be able to make about designer babies? A Citizens’ Jury of young people in South Wales. Health Expect 2006;9:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T. Germ line genome editing in clinics: the approaches, objectives and global society. Brief Funct Genomic 2015;16:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012;337:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HD. Regulatory uncertainty over genome editing. Nat Plants 2015;1:14011. [DOI] [PubMed] [Google Scholar]
- Kalfoglou AL, Doksum T, Bernhardt B, Geller G, LeRoy L, Mathews DJ, Evans JH, Doukas DJ, Reame N, Scott J et al. . Opinions about new reproductive genetic technologies: hopes and fears for our genetic future. Fertil Steril 2005;83:1612–1621. [DOI] [PubMed] [Google Scholar]
- Kang X, He W, Huang Y, Yu Q, Chen Y, Gao X, Sun X, Fan Y. Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. Assist Reprod Genet 2016;33:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A, Cunningham-Burley S, Amos A. The new genetics and health: mobilizing lay expertise. Public Underst Sci 1998;7:41–60. [DOI] [PubMed] [Google Scholar]
- Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don’t edit the human germ line. Nature 2015;519:410–411. [DOI] [PubMed] [Google Scholar]
- Lewis J, Driver R, Leach J, Wood-Robinson C Working Paper 7—Opinions On And Attitudes Towards Genetic Engineering: Acceptable Limits A: The Discussion Task. 1997. University of Leeds, Centre for Studies in Science and Mathematics Education.
- Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y et al. . CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 2015;6:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 2014;345:1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunshof JE. Human germ line editing-roles and responsibilities. Protein Cell 2016;7:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Marti-Gutierrez N, Park S-W, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R et al. . Correction of a pathogenic gene mutation in human embryos. Nature 2017;548:413–419. advance online publication. [DOI] [PubMed] [Google Scholar]
- Macer DRJ. Public acceptance of human gene therapy and perceptions of human genetic manipulation. Hum Gene Ther 1992;3:511–518. [DOI] [PubMed] [Google Scholar]
- Macer DR, Akiyama S, Alora AT, Asada Y, Azariah J, Azariah H, Boost MV, Chatwachirawong P, Kato Y, Kaushik V et al. . International perceptions and approval of gene therapy. Hum Gene Ther 1995;6:791–803. [DOI] [PubMed] [Google Scholar]
- Massarani L, de Castro Moreira I. Attitudes towards genetics: a case study among Brazilian high school students. Public Underst Sci 2005;14:201–212. [DOI] [PubMed] [Google Scholar]
- McCaughey T, Sanfilippo PG, Gooden GE, Budden DM, Fan L, Fenwick E, Rees G, MacGregor C, Si L, Chen C et al. . A global social media survey of attitudes to human genome editing. Cell Stem Cell 2016;18:569–572. [DOI] [PubMed] [Google Scholar]
- Meisenberg G. Designer babies on tap? Medical students’ attitudes to pre-implantation genetic screening. Public Understand Sci 2009;18:149–166. [DOI] [PubMed] [Google Scholar]
- Midgley M. Biotechnology and monstrosity. Why we should pay attention to the ‘yuk factor’. Hastings Cent Rep 2000;30:7–15. [PubMed] [Google Scholar]
- Molewijk AC, Stiggelbout AM, Otten W, Dupuis HM, Kievit J. Implicit normativity in evidence-based medicine: a plea for integrated empirical ethics research. Health Care Anal 2003;11:69–92. [DOI] [PubMed] [Google Scholar]
- Muller C, Shepherd D. Attitudes towards reproductive technologies for humans. Kōtuitui: N. Z. J. Soc. Sci. Online 2009;4:225–238. [Google Scholar]
- NASEM Genetically Engineered Crops: Experiences and Prospects. Washington, DC: National Academies of Sciences, Engineering, Medicine, 2016, 606. [PubMed] [Google Scholar]
- National Academies of Sciences E, Medicine. Human Genome Editing: Science, Ethics, and Governance. 2017. [PubMed]
- Olson S. International Summit on Human Gene Editing: A Global Discussion. The National Academies Press. Washington, DC, 2015. 10.17226/21913. [DOI] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Intelligence: genetics, genes, and genomics. J Pers Soc Psychol 2004;86:112–129. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Dann CT. Genome editing of the germline: broadening the discussion. Mol Ther 2015;23:980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard JM, Roskams-Edris D, Kuzeljevic B, Illes J. Prevailing public perceptions of the ethics of gene therapy. Hum Gene Ther 2014;25:740–746. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C et al. . Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996;382:722–725. [DOI] [PubMed] [Google Scholar]
- Schelle KJ, Faulmüller N, Caviola L, Hewstone M. Attitudes toward pharmacological cognitive enhancement—a review. Front Syst Neurosci 2014;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, Hardy BW. U.S. attitudes on human genome editing. Science 2017;357:553–554. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Tatham AS. Improving wheat to remove coeliac epitopes but retain functionality. J Cereal Sci 2016;67:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiell A, Seymour J, Hawe P, Cameron S. Are preferences over health states complete? Health Econ 2000;9:47–55. [DOI] [PubMed] [Google Scholar]
- Siegrist M. The influence of trust and perceptions of risks and benefits on the acceptance of gene technology. Risk Anal 2000;20:195–203. [DOI] [PubMed] [Google Scholar]
- Singer E, Corning A, Lamias M. Trends: genetic testing, engineering, and therapy: awareness and attitudes. Public Opin Q 1998;62:633–664. [Google Scholar]
- Smith KR, Chan S, Harris J. Human germline genetic modification: scientific and bioethical perspectives. Arch Med Res 2012;43:491–513. [DOI] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA et al. . In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016;351:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Zeng Y, Du H, Gong M, Peng J, Zhang B, Lei M, Zhao F, Wang W, Li X et al. . CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Genet Genomics 2017;292:525–533. [DOI] [PubMed] [Google Scholar]
- The Academy of Medical Sciences , Association of Medical Research Charities, Cancer Research UK, BBSRC Bioscience for the Future, Medical Research Council, Progress Education, Wellcome Trust, Institute WTS. Genome editing in human cells—initial joint statement. 2015.
- Tonkens R. Parental wisdom, empirical blindness, and normative evaluation of prenatal genetic enhancement. J Med Philos 2011;36:274–295. [DOI] [PubMed] [Google Scholar]
- Trust W. What do People Think About Gene Therapy? London: Wellcome Trust, 2005. [Google Scholar]
- van Dijke I, Bosch L, Bredenoord AL, Cornel M, Repping S, Hendriks S The ethics of clinical applications of germline genome modification: a systematic review of reasons. Under review2017. [DOI] [PMC free article] [PubMed]
- Waltz E. CRISPR-edited crops free to enter market, skip regulation. Nat Biotechnol 2016. a;34:582. [DOI] [PubMed] [Google Scholar]
- Waltz E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016. b;532:293. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu J-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 2014;32:947–951. [DOI] [PubMed] [Google Scholar]
- Weisberg SM, Badgio D, Chatterjee A. A CRISPR New World: attitudes in the public toward innovations in human genetic Modifcation. Front Public Health 2017;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Xiao L, Gou Z, Li M, Zhang W, Wang H, Feng P. Survey of attitudes and ethical concerns related to gene therapy among medical students and postgraduates in China. Hum Gene Ther 2015;26:841–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.