Abstract
STUDY QUESTION
What is the recommended management of women with recurrent pregnancy loss (RPL) based on the best available evidence in the literature?
SUMMARY ANSWER
The guideline development group formulated 77 recommendations answering 18 key questions on investigations and treatments for RPL, and on how care should be organized.
WHAT IS KNOWN ALREADY
A previous guideline for the investigation and medical treatment of recurrent miscarriage was published in 2006 and is in need of an update.
STUDY DESIGN, SIZE, DURATION
The guideline was developed according to the structured methodology for development of ESHRE guidelines. After formulation of key questions by a group of experts, literature searches and assessments were performed. Papers published up to 31 March 2017 and written in English were included. Cumulative live birth rate, live birth rate and pregnancy loss rate (or miscarriage rate) were considered the critical outcomes.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Based on the collected evidence, recommendations were formulated and discussed until consensus was reached within the guideline group. A stakeholder review was organized after finalization of the draft. The final version was approved by the guideline group and the ESHRE Executive Committee.
MAIN RESULTS AND THE ROLE OF CHANCE
The guideline provides 38 recommendations on risk factors, prevention and investigations in couples with RPL, and 39 recommendations on treatments. These include 60 evidence-based recommendations – of which 31 were formulated as strong recommendations and 29 as conditional – and 17 good practice points. The evidence supporting investigations and treatment of couples with RPL is limited and of moderate quality. Of the evidence-based recommendations, only 10 (16.3%) were supported by moderate quality evidence. The remaining recommendations were supported by low (35 recommendations: 57.4%), or very low quality evidence (16 recommendations: 26.2%). There were no recommendations based on high quality evidence. Owing to the lack of evidence-based investigations and treatments in RPL care, the guideline also clearly mentions investigations and treatments that should not be used for couples with RPL.
LIMITATIONS, REASONS FOR CAUTION
Several investigations and treatments are offered to couples with RPL, but most of them are not well studied. For most of these investigations and treatments, a recommendation against the intervention or treatment was formulated based on insufficient evidence. Future studies may require these recommendations to be revised.
WIDER IMPLICATIONS OF THE FINDINGS
The guideline provides clinicians with clear advice on best practice in RPL, based on the best evidence available. In addition, a list of research recommendations is provided to stimulate further studies in RPL. One of the most important consequences of the limited evidence is the absence of evidence for a definition of RPL.
STUDY FUNDING/COMPETING INTEREST(S)
The guideline was developed and funded by ESHRE, covering expenses associated with the guideline meetings, with the literature searches and with the dissemination of the guideline. The guideline group members did not receive payment. J.E. reports position funding from CARE Fertility. S.L. reports position funding from SpermComet Ltd. S.M. reports research grants, consulting and speaker’s fees from GSK, BMS/Pfizer, Sanquin, Aspen, Bayer and Daiichi Sankyo. S.Q. reports speaker’s fees from Ferring. The other authors report no conflicts of interest.
ESHRE Pages are not externally peer reviewed. This article has been approved by the Executive Committee of ESHRE.
Keywords: recurrent pregnancy loss, ESHRE, guideline, evidence based, recurrent miscarriage, treatment, diagnosis, GRADE
Introduction
Recurrent pregnancy loss (RPL) is defined as the loss of two or more pregnancies. The exact prevalence of RPL is difficult to estimate, but most studies report that RPL affects 1–2% of women.
An evidence-based guideline for the investigation and medical treatment of recurrent miscarriage was published in 2006 on behalf of the ESHRE Special Interest Group (SIG) Early Pregnancy and Implantation (Jauniaux et al., 2006). Since this guideline needed updating, the SIG Early Pregnancy initiated the development of the ESHRE guideline on the management of RPL.
This guideline offers best practice advice on the care of couples confronted with RPL. Furthermore, the guideline provides an overview of the treatments for RPL that are currently offered to couples, and which of those are recommended. Recommendations are also formulated on the investigations that could be helpful to identify the origin of the pregnancy losses and to select patients for possible therapeutic targets.
What does this mean for patients?
This European guideline looks at how best to care for people who have experienced recurrent pregnancy loss based on the evidence currently available.
Recurrent pregnancy loss is defined as the loss of two or more pregnancies, and it affects around 1–2% of couples. The guideline states that the emotional impact needs to be considered, and that there is a need for more research looking at the impact on men.
The guidance explains that providing people with information is essential, and that a specialist outpatient clinic should offer investigations, support and, if possible, treatment. Staff should be experienced and should have appropriate listening skills. The guidance stresses that it should be made clear from the start that there may not always be relevant treatments for recurrent pregnancy loss.
The guideline explains that age is a key factor in recurrent pregnancy loss, which is more common in women who are over 40 years old. It gives the lifestyle advice that should be provided to men and women, and explains that there is no evidence that stress is a direct cause of pregnancy loss. It details the investigations and interventions, which should – and should not – be carried out, and gives some recommendations for research, making it clear that in many areas there is limited evidence and an urgent need for further studies. A patient leaflet based on the Guideline is available on the ESHRE website https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Recurrent-pregnancy-loss.aspx
Materials and Methods
The guideline was developed according to a well-documented methodology that is universal to ESHRE guidelines (Vermeulen, 2014).
In short, 18 key questions were formulated by the Guideline Development Group (GDG), with input from patient organizations (Fertility Europe, Miscarriage Association UK), and structured in PICO format (Patient, Intervention, Comparison, Outcome). For each question, databases (PUBMED/MEDLINE and the Cochrane library) were searched from inception to 31 March 2017, with a limitation to studies written in English. From the literature searches, studies were selected based on the PICO questions, assessed for quality and summarized in evidence tables and summary of findings tables (for interventions with at least two studies per outcome). Cumulative live birth rate, live birth rate and pregnancy loss rate (or miscarriage rate) were considered the critical outcomes. GDG meetings were organized where the evidence and draft recommendations were presented by the assigned GDG member, and discussed until consensus was reached within the group.
Each recommendation was labelled as strong or conditional and a grade was assigned based on the strength of the supporting evidence (High ⊕⊕⊕⊕ – Moderate ⊕⊕⊕○ Low ⊕⊕○○ – Very low ⊕○○○). In the absence of evidence, the GDG formulated no recommendation or a good practice points (GPP) based on clinical expertise (Table I).
Table I.
Interpretation of strong versus conditional recommendations in the GRADE approach.*
| Implications for | Strong recommendation | Conditional recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians |
|
|
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making will require substantial debate and involvement of various stakeholders. |
The guideline draft and an invitation to participate in the stakeholder review was published on the ESHRE website. In addition, all relevant stakeholders received a personal invitation to review by e-mail. We received 307 comments from 23 reviewers, representing 15 countries, two national societies (Royal College of Obstetricians and Gynaecologists, and Italian Society of Gynecology and Obstetrics Sigo – L’Associazione degli Ostetrici e Ginecologi Ospedalieri Italiani – Associazione Ginecologi Universitari Italiani) and one international research group (ESHRE/European Society for Gynaecological Endoscopy[ESGE] CONgenital UTerine Anomalies Group). All comments were processed by the GDG, either by adapting the content of the guideline and/or by replying to the reviewer. The review process was summarized in the review report which is published on the ESHRE website (www.eshre.eu/guidelines).
This guideline will be considered for update 4 years after publication, with an intermediate assessment of the need for updating 2 years after publication.
Results
Key questions and recommendations
The current document summarizes all the key questions and the recommendations from the guideline ‘Management of Recurrent Pregnancy Loss’. Further background information and the supporting evidence for each recommendation can be found in the full version of the guideline available at http://www.eshre.eu/Guidelines-and-Legal/Guidelines.
Definition and terminology
A pregnancy loss is defined as the spontaneous demise of a pregnancy before the foetus reaches viability. The term therefore includes all pregnancy losses from the time of conception until 24 weeks of gestation.
There has been significant debate in the literature and in the GDG on the definition of RPL and, more specifically, the extent to which this definition needs to be extended or constricted based on the number of losses and whether these are consecutive or not.
The GDG concluded that a diagnosis of RPL could be considered after the loss of two or more pregnancies.
This definition includes pregnancy losses both after spontaneous conception and ART, but excludes ectopic and molar pregnancies (if identified as such) and implantation failure.
The GDG would like to stress the importance of the issue and the need for further scientific research (including epidemiological studies on the effect of various RPL definitions on diagnosis, prognosis and treatment).
Regarding terminology, the GDG concludes to use the term Recurrent Pregnancy Loss and to reserve ‘recurrent miscarriage’ to describe cases where all pregnancy losses have been confirmed as intrauterine miscarriages. The terms spontaneous abortion, chemical pregnancy and blighted ovum are ambiguous and should be avoided (Kolte et al., 2015a).
Organization of care
Pregnancy loss is a significant negative life event and the repetitive nature of RPL may intensify the grief experienced. Studies have mostly focused on women, and there is a need for studies on the emotional impact of RPL on men. Clinicians and clinics should take the psychosocial needs of couples faced with RPL into account when offering and organizing care for these couples.
How should care for RPL patients be organized?
A dedicated RPL clinic is an outpatient clinic that offers specialist investigations, support and (if possible) treatment of couples with RPL. Information provision is one of the important aims of a RPL clinic. Investigations do not necessarily lead to treatment options and this should be clear from the beginning. The elements required in a RPL clinic are experienced staff members with appropriate listening skills and appropriate imaging facilities. The first visit at the clinic should allow time for the clinician to review the patient’s history, to answer questions and to propose a plan for investigations and, perhaps, treatment. The first visit is the opportunity to provide general information about RPL incidence, causes and investigations, and to link it to the patient’s history. Staff should be aware that many women with RPL will already have information from a variety of sources, and some explanation and re-education may be needed.
There should be individual evaluation of the investigations appropriate to each woman or couple, based on age, fertility/sub-fertility, pregnancy history, family history, previous investigations and/or treatments. In addition, care should be tailored to the psychological needs of the couples (Musters et al., 2013).
Risk factors and health behaviour modifications
What are the known risk factors of RPL?
| Women should be sensitively informed that the risk of pregnancy loss is lowest in women aged 20 to 35 years (Cauchi et al., 1991; Lund et al., 2012). | Strong ⊕⊕○○ |
| Women should be sensitively informed that the risk of pregnancy loss rapidly increases after the age of 40 years (Grande et al., 2012; Lund et al., 2012). | Strong ⊕⊕○○ |
| Stress is associated with RPL, but couples should be informed that there is no evidence that stress is a direct cause of pregnancy loss (Nelson et al., 2003; Nepomnaschy et al., 2006; Li et al., 2012; Kolte et al., 2015b; Plana-Ripoll et al., 2016). | Strong ⊕○○○ |
Are health behaviour modifications relevant for reducing the risk of pregnancy loss in women with a history of RPL?
| Couples with RPL should be informed that smoking could have a negative impact on their chances of a live birth, and therefore cessation of smoking is recommended. | GPP |
| Couples with RPL should be informed that maternal obesity or being significantly underweight is associated with obstetric complications and could have a negative impact on their chances of a live birth and on their general health (Lashen et al., 2004; Zhang et al., 2010; Boots and Stephenson, 2011; Lo et al., 2012; Boots et al., 2014). | Strong ⊕⊕○○ |
| Striving for a healthy normal range BMI is recommended. | GPP |
| Couples with RPL should be informed that excessive alcohol consumption is a possible risk factor for pregnancy loss and a proven risk factor for foetal problems (foetal alcohol syndrome) (Maconochie et al., 2007; Andersen et al., 2012; Avalos et al., 2014). | Strong ⊕⊕○○ |
| Couples with RPL should be advised to limit alcohol consumption. | GPP |
There was insufficient evidence for recommendations on other lifestyle factors, including exercise (Schlussel et al., 2008; Hegaard et al., 2016) and caffeine intake (Maconochie et al., 2007; Stefanidou et al., 2011).
Investigations in RPL
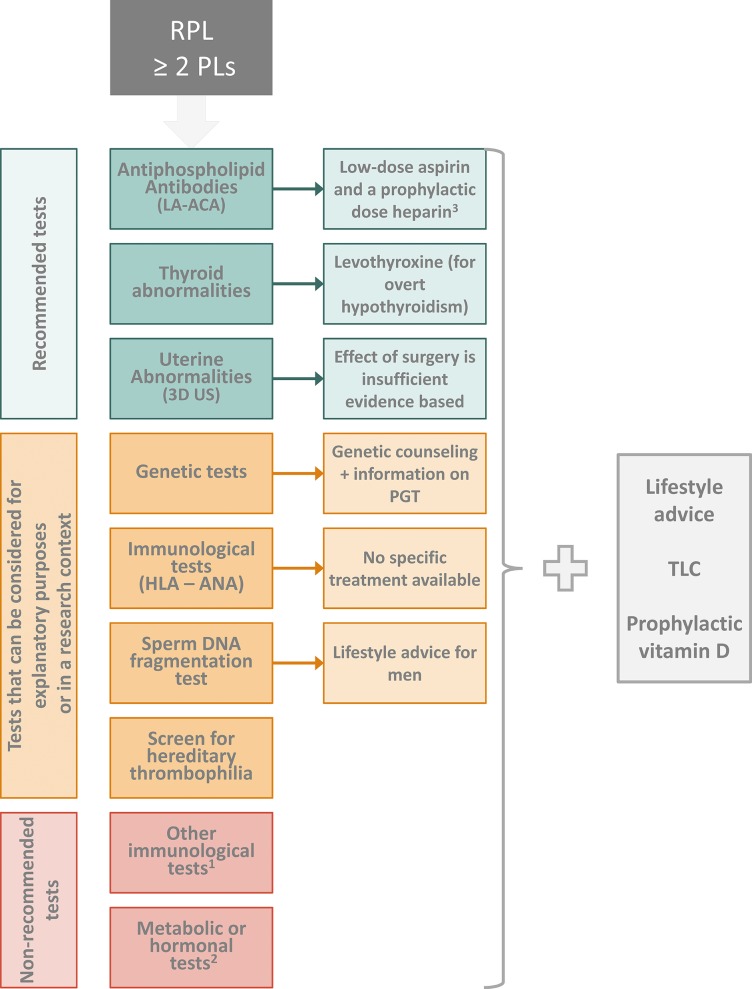
A summary of all recommended investigations and treatments is available in Fig. 1.
| Medical and family history could be used to tailor diagnostic investigations in RPL. | GPP |
| The guideline development group (GDG) recommends to base prognosis on the number of preceding pregnancy losses and female age (Brigham et al., 1999; Lund et al., 2012; Kaandorp et al., 2014; Egerup et al., 2016). | Strong ⊕⊕⊕○ |
Figure 1.
Pictorial summary of the recommendations for investigations and treatments of couples with recurrent pregnancy loss.
- 1: Including anti-HY antibodies, Natural Killer (NK) cell testing, anti-HLA antibodies.
- 2: Including cytokine testing/polymorphisms, assessment of polycystic ovary syndrome (PCOS), fasting insulin and fasting glucose, prolactin testing, ovarian reserve testing, luteal phase insufficiency testing, androgen testing, LHtesting, homocysteine plasma levels.
- 3: Low-dose aspirin and heparin are recommended after three or more pregnancy losses, or in the context of a clinical trial.
RPL: recurrent pregnancy loss. LA: lupus anticoagulant. ACA: anticardiolipin antibodies. 3D US: 3D ultrasound. PGT: preimplantation genetic testing. ANA: antinuclear antibody. TLC: tender loving care.
What is the value of screening for genetic factors in the diagnosis of RPL?
| Genetic analysis of pregnancy tissue is not routinely recommended but it could be performed for explanatory purposes (Hogge et al., 2003; Bernardi et al., 2012; Foyouzi et al., 2012; van den Berg et al., 2012). | Conditional ⊕⊕○○ |
| For genetic analysis of the pregnancy tissue, array-based comparative genomic hybridization (array-CGH) is recommended based on a reduced maternal contamination effect (Robberecht et al., 2009). | Strong ⊕⊕○○ |
| Parental karyotyping is not routinely recommended in couples with RPL. It could be carried out after individual assessment of risk (Franssen et al., 2006; Barber et al., 2010) (Franssen et al., 2005; Sugiura-Ogasawara et al., 2008; Flynn et al., 2014). | Conditional ⊕⊕○○ |
What is the value of thrombophilia screening in women with RPL?
| For women with RPL, we suggest not to screen for hereditary thrombophilia unless in the context of research, or in women with additional risk factors for thrombophilia (Bradley et al., 2012). | Conditional ⊕⊕⊕○ |
| For women with RPL, we recommend screening for antiphospholipid antibodies (lupus anticoagulant [LA], and anticardiolipin antibodies [ACA IgG and IgM]), after two pregnancy losses (Miyakis et al., 2006; Opatrny et al., 2006). | Strong ⊕⊕○○ |
| For women with RPL, screening for β2 glycoprotein I antibodies (aβ2GPI) can be considered after two pregnancy losses. | GPP |
What is the value of immunological screening in the diagnosis of RPL?
| HLA determination in women with RPL is not recommended in clinical practice. Only HLA class II determination (HLA-DRB1*15:01 and HLA-DQB1*05:01/05:2) could be considered in Scandinavian women with secondary RPL after the birth of a boy, for prognostic purposes (Nielsen et al., 2009). | Conditional ⊕⊕○○ |
| Measurement of anti-HY antibodies in women with RPL is not recommended in clinical practice (Nielsen et al., 2010). | Conditional ⊕⊕○○ |
| Cytokine testing should not be used in women with RPL in clinical practice (Mueller-Eckhardt et al., 1994; Calleja-Agius et al., 2012; Lee et al., 2013). | Strong ⊕⊕○○ |
| Cytokine polymorphisms should not be tested in women with RPL (Choi and Kwak-Kim, 2008; Medica et al., 2009). | Strong ⊕⊕⊕○ |
| Antinuclear antibodies (ANA) testing could be considered for explanatory purposes (Christiansen, 1996; Ogasawara et al., 1996; Stern et al., 1998; Kaider et al., 1999; Matsubayashi et al., 2001; Bustos et al., 2006; Giasuddin et al., 2010; Ticconi et al., 2010; Cavalcante et al., 2014; Molazadeh et al., 2014; Hefler-Frischmuth et al., 2017). | Conditional ⊕⊕○○ |
| There is insufficient evidence to recommend natural killer (NK) cell testing of either peripheral blood or endometrial tissue in women with RPL (Chao et al., 1995, Souza et al., 2002, Shakhar et al., 2006; Hadinedoushan et al., 2007, Karami et al., 2012, Lee et al., 2013). | Strong ⊕○○○ |
| Testing anti-HLA antibodies in women with RPL is not recommended (Lashley et al., 2013). | Strong ⊕⊕⊕○ |
What is the value of screening for metabolic/endocrinological abnormalities in the diagnosis of RPL?
| Thyroid screening (thyroid-stimulating hormone [TSH] and thyroid peroxidase [TPO]-antibodies) is recommended in women with RPL (Rao et al., 2008; van den Boogaard et al., 2011). | Strong ⊕⊕⊕○ |
| Abnormal thyroid-stimulating hormone (TSH) and thyroid peroxidase [TPO]-antibody levels should be followed up by thyroxine (T4) testing in women with RPL (van den Boogaard et al., 2011; Lazarus et al., 2014). | Strong ⊕⊕⊕○ |
| Assessment of polycystic ovary syndrome (PCOS), fasting insulin and fasting glucose is not recommended in women with RPL to improve next pregnancy prognosis (Rai et al., 2000; Craig et al., 2002; Wang et al., 2011; Maryam et al., 2012; Chakraborty et al., 2013; Ispasoiu et al., 2013; Kazerooni et al., 2013). | Strong ⊕⊕○○ |
| Prolactin testing is not recommended in women with RPL in the absence of clinical symptoms of hyperprolactinemia (oligo/amenorrhoea) (Bussen et al., 1999) (Triggianese et al., 2015) (Li et al., 2013). | Conditional ⊕⊕○○ |
| Ovarian reserve testing is not routinely recommended in women with RPL (Bussen et al., 1999; Hofmann et al., 2000; Prakash et al., 2006; Atasever et al., 2016). | Strong ⊕⊕○○ |
| Luteal phase insufficiency testing is not recommended in women with RPL (Balasch et al., 1986; Jordan et al., 1994; Stephenson, 1996; Ogasawara et al., 1997; Badawy and Westpfal, 2000; Li et al., 2000). | Strong ⊕⊕○○ |
| Androgen testing is not recommended in women with RPL (Watson et al., 1993; Okon et al., 1998; Rai et al., 2000; Nardo et al., 2002; Cocksedge et al., 2008; Kazerooni et al., 2013). | Strong ⊕⊕○○ |
| LH testing is not routinely recommended in women with RPL (Sagle et al., 1988; Regan et al., 1990; Carp et al., 1995; Rai et al., 2000; Prakash et al., 2006; Kazerooni et al., 2013). | Strong ⊕○○○ |
| Measurement of homocysteine plasma levels is not routinely recommended in women with RPL (Nelen et al., 2000; Alonso et al., 2002; Zammiti et al., 2008; Creus et al., 2013; Puri et al., 2013; Lee et al., 2016). | Strong ⊕○○○ |
Even though one study showed a significant prevalence of vitamin D deficiency in women with RPL, there are no indications that vitamin D status is a contributing factor for RPL (Ota et al., 2014). Moreover, there is no report of an association between vitamin D status and miscarriage, and hence testing of vitamin D status is not recommended for women with RPL. Irrespective of RPL, vitamin D supplementation is nowadays frequently prescribed in pregnant women.
What is the value of anatomical investigations in the diagnosis of RPL?
| All women with RPL should have an assessment of the uterine anatomy (Saravelos et al., 2008; Chan et al., 2011a, b; Venetis et al., 2014; Grimbizis et al., 2016). | Strong ⊕⊕○○ |
| The preferred technique to evaluate the uterus is transvaginal 3D ultrasound (3D US), which has a high sensitivity and specificity, and can distinguish between septate uterus and bicorporeal uterus with normal cervix (former American Fertility Society classification (AFS) bicornuate uterus) (Saravelos et al., 2008; Ghi et al., 2009; Caliskan et al., 2010). | Conditional ⊕⊕○○ |
| Sonohysterography (SHG) is more accurate than hysterosalpingography (HSG) in diagnosing uterine malformations. It can be used to evaluate uterine morphology when 3D ultrasound (3D US) is not available, or when tubal patency has to be investigated (Saravelos et al., 2008). | Conditional ⊕⊕○○ |
| If a Müllerian uterine malformation is diagnosed, further investigation (including investigation of the kidneys and urinary tract) should be considered (Oppelt et al., 2007; Ramanathan et al., 2016). | Conditional ⊕⊕○○ |
| MRI is not recommended as first line option for the assessment of uterine malformations in women with RPL, but can be used where 3D ultrasound (3D US) is not available (Oppelt et al., 2007; Saravelos et al., 2008; Chan et al., 2011b). | Conditional ⊕⊕○○ |
Does the quality of the male gametes contribute to RPL?
| In the male partner, it is suggested to assess life style factors (smoking, alcohol consumption, exercise pattern, and body weight). | GPP |
| Assessing sperm DNA fragmentation in couples with RPL can be considered for explanatory purposes, based on indirect evidence (Robinson et al., 2012). | Conditional ⊕⊕○○ |
Prognosis and treatment
What is the value of information on medical and family history in establishing the prognosis of RPL?
| The guideline development group (GDG) recommends to base prognosis on the number of preceding pregnancy losses and female age (Brigham et al., 1999; Lund et al., 2012; Kaandorp et al., 2014; Egerup et al., 2016). | Strong ⊕⊕⊕○ |
| Prognostic tools (Lund et al., 2012) (Brigham et al., 1999) can be used to provide an estimate of subsequent chance of live birth in couples with unexplained RPL. | GPP |
Which therapeutic interventions should be offered to couples with RPL due to genetic/chromosomal causes to increase live birth rate?
| All couples with results of an abnormal foetal or parental karyotype should receive genetic counselling. | GPP |
| All couples with results of an abnormal foetal or parental karyotype may be informed about the possible treatment options available including their advantages and disadvantages. | GPP |
The limited evidence for preimplantation genetic testing in couples with RPL shows no clear benefit of treatment (Franssen et al., 2011; Musters et al., 2011; Ikuma et al., 2015).
Which therapeutic interventions should be offered to couples with RPL and thrombophilia to increase the chance of a live birth?
| For women with hereditary thrombophilia and a history of RPL, we suggest not to use antithrombotic prophylaxis unless in the context of research, or if indicated for venous thromboembolism (VTE) prevention (Skeith et al., 2016). | Conditional ⊕⊕○○ |
| For women who fulfil the laboratory criteria of antiphospholipid syndrome (APS) and have a history of three or more pregnancy losses, we suggest administration with low dose aspirin (75–100 mg/day), starting before conception, and a prophylactic dose heparin (unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) starting at date of a positive pregnancy test, over no treatment (Empson et al., 2005; Mak et al., 2010; Ziakas et al., 2010). | Conditional ⊕○○○ |
| The guideline development group (GDG) suggests offering anticoagulant treatment for women with two pregnancy losses and antiphospholipid syndrome (APS), only in the context of clinical research. | GPP |
Which therapeutic interventions should be offered to couples with RPL with suspicion of immunological background to increase live birth rate?
No immunological biomarker, except for high-titre antiphospholipid antibodies, can be used for selecting couples with RPL for specific immunological treatments.
Which therapeutic interventions should be offered to couples with RPL AND metabolic or hormonal abnormalities to increase live birth rate?
| Overt hypothyroidism arising before conception or during early gestation should be treated with levothyroxine in women with RPL (Stagnaro-Green et al., 2011; Khan et al., 2017). | Strong ⊕⊕○○ |
| There is conflicting evidence regarding treatment effect of levothyroxine for women with subclinical hypothyroidism and RPL. Treatment of women with subclinical hypothyroidism (SCH) may reduce the risk of miscarriage, but the potential benefit of treatment should be balanced against the risks (Negro et al., 2010; Bernardi et al., 2013). | Conditional ⊕⊕○○ |
| If women with subclinical hypothyroidism and RPL are pregnant again, thyroid-stimulating hormone (TSH) level should be checked in early gestation (7–9 weeks AD), and hypothyroidism should be treated with levothyroxine. | GPP |
| If women with thyroid autoimmunity and RPL are pregnant again, thyroid-stimulating hormone (TSH) level should be checked in early gestation (7–9 weeks gestational age), and hypothyroidism should be treated with levothyroxine. | GPP |
| There is insufficient evidence to support treatment with levothyroxine in euthyroid women with thyroid antibodies and RPL outside a clinical trial (Vissenberg et al., 2012). | Conditional ⊕⊕○○ |
| There is insufficient evidence to recommend the use of progesterone to improve live birth rate in women with RPL and luteal phase insufficiency (Coomarasamy et al., 2015). | Conditional ⊕⊕⊕○ |
| There is insufficient evidence to recommend the use of hCG to improve live birth rate in women with RPL and luteal phase insufficiency (Morley et al., 2013). | Conditional ⊕⊕○○ |
| There is insufficient evidence to recommend metformin supplementation in pregnancy to prevent pregnancy loss in women with RPL and glucose metabolism defects (Zolghadri et al., 2008). | Conditional ⊕○○○ |
| Bromocriptine treatment can be considered in women with RPL and hyperprolactinemia to increase live birth rate (Hirahara et al., 1998). | Conditional ⊕○○○ |
| Preconception counselling in women with RPL could include the general advice to consider prophylactic vitamin D supplementation | GPP |
Controlled ovarian stimulation by human menopausal gonadotrophins could be beneficial for decreasing the chance of a next pregnancy loss in women with RPL diagnosed with luteal phase insufficiency (Li et al., 2001), but the GDG decided that the evidence was too limited to support recommending controlled ovarian stimulation in women with RPL but without polycystic ovary syndrome (PCOS).
Which therapeutic interventions should be offered to women with RPL and uterine abnormalities to increase live birth rates?
| Whether hysteroscopic septum resection has beneficial effects (improving live birth rates, and decreasing miscarriage rates, without doing harm), should be evaluated in the context of surgical trials in women with RPL and septate uterus (Rikken et al., 2017). | Conditional ⊕○○○ |
| Metroplasty is not recommended for bicorporeal uterus with normal cervix (former American Fertility Society classification (AFS) bicornuate uterus) and RPL (Bailey et al., 2015; Sugiura-Ogasawara et al., 2015). | Strong ⊕○○○ |
| Uterine reconstruction is not recommended for hemi-uterus (former American Fertility Society classification (AFS) unicornuate uterus) and RPL (Jaslow, 2014). | Strong ⊕○○○ |
| There is insufficient evidence in favour of metroplasty in women with bicorporeal uterus and double cervix (former American Fertility Society classification (AFS) didelphic uterus) and RPL (Bailey et al., 2015). | Conditional ⊕○○○ |
| There is insufficient evidence supporting hysteroscopic removal of submucosal fibroids or endometrial polyps in women with RPL (Pritts et al., 2009; Lieng et al., 2010; Salim et al., 2011; Jaslow, 2014). | Conditional ⊕○○○ |
| Surgical removal of intramural fibroids is not recommended in women with RPL. There is insufficient evidence to recommend removing fibroids that distort the uterine cavity (Pritts et al., 2009; Jaslow, 2014). | Conditional ⊕○○○ |
| There is insufficient evidence of benefit for surgical removal of intrauterine adhesions for pregnancy outcome. After hysteroscopic removal of intrauterine adhesions in women with RPL, precautions have to be taken to prevent recurrence of adhesions (Kodaman and Arici, 2007; Jaslow, 2014). | Conditional ⊕○○○ |
| Women with a history of second-trimester pregnancy losses and suspected cervical weakness should be offered serial cervical sonographic surveillance. | Strong ⊕⊕○○ |
| In women with a singleton pregnancy and a history of recurrent second-trimester pregnancy loss attributable to cervical weakness, a cerclage could be considered. There is no evidence that this treatment increases perinatal survival. | Conditional ⊕⊕○○ |
Which therapeutic interventions should be offered to couples with RPL due to male factor to increase live birth rate?
| Couples with RPL should be informed that smoking, alcohol consumption, obesity and excessive exercise could have a negative impact on their chances of a live birth, and therefore cessation of smoking, a normal body weight, limited alcohol consumption and a normal exercise pattern is recommended. | GPP |
| Sperm selection is not recommended as a treatment in couples with RPL. | GPP |
| Antioxidants for men have not been shown to improve the chance of a live birth (Showell et al., 2014). | Conditional ⊕○○○ |
Which therapeutic interventions should be offered to couples with unexplained RPL to increase live birth rate?
| Lymphocyte immunization therapy should not be used as treatment for unexplained RPL as it has no significant effect and there may be serious adverse effects (Wong et al., 2014). | Strong ⊕⊕○○ |
| Intravenous immunoglobulin (IvIg) is not recommended as a treatment of RPL (Egerup et al., 2015). | Strong ⊕⊕○○ |
| Glucocorticoids are not recommended as a treatment of unexplained RPL or RPL with selected immunological biomarkers (Tang et al., 2013; Gomaa et al., 2014). | Strong ⊕⊕○○ |
| Heparin or low dose aspirin are not recommended, as there is evidence that they do not improve live birth rate in women with unexplained RPL (de Jong et al., 2014). | Strong ⊕⊕⊕○ |
| Low dose folic acid is routinely started preconceptionally to prevent neural tube defects, but it has not been shown to prevent pregnancy loss in women with unexplained RPL. | Strong ⊕⊕○○ |
| Vaginal progesterone does not improve live birth rates in women with unexplained RPL (Coomarasamy et al., 2015) (Saccone et al., 2017). | Conditional ⊕⊕⊕○ |
| There is insufficient evidence to recommend intralipid therapy for improving live birth rate in women with unexplained RPL. | Strong ⊕○○○ |
| There is insufficient evidence to recommended granulocyte-colony stimulating factor (G-CSF) in women with unexplained RPL (Scarpellini and Sbracia, 2009). | Conditional ⊕⊕○○ |
| There is no evidence to recommended endometrial scratching in women with unexplained RPL. | GPP |
Which therapeutic interventions could be offered to all couples with RPL, irrespective of a cause, to increase live birth rates?
| If women with RPL ask about using multivitamin supplements, they should be advised on multivitamin supplements that are safe in pregnancy. | GPP |
Discussion
This ESHRE guideline on the management of RPL aims to supply healthcare providers with the best available evidence for the investigation and treatment of women with RPL.
All recommendations in the guideline were formulated after an assessment of the best available evidence in the literature and discussion within the GDG, taking into account the balance of benefits versus harms, patient preferences, clinicians’ expertise and resource use. The guideline includes 77 recommendations, including 60 evidence-based recommendations – of which 31 were formulated as strong recommendations and 29 as conditional – and 17 good practice points. Evidence supporting investigations and treatment of couples with RPL is limited and of moderate quality. Of the evidence-based recommendations, only 10 (16.3%) were supported by moderate quality evidence. The remaining recommendations were supported by low (35 recommendations (57.4%)), or very low quality evidence (16 recommendations (26.2%)). There were no recommendations based on high quality evidence.
One of the most important consequences of the limited evidence, is the absence of evidence for a definition of RPL. An evidence-based definition was not feasible. Furthermore, for most investigations and treatments, there are no data on when investigations and/or treatment should be started, whether it can be postponed until after a next pregnancy loss, and whether the care of couples with primary versus secondary, or consecutive versus non-consecutive losses should be approached differently. For most investigations and treatments, the decision on when to start investigations or treatment will have to be decided by the doctor and the couple, as the result of shared decision-making, and be compliant with available resources.
A second consequence of the limited evidence is the number of recommendations specifying investigations and treatments to be applied in a research context rather than routine clinical practice. The current guideline contains three recommendations on interventions to be applied in a research context only. In the 2006 guideline, five treatments were listed as requiring more RCTs. Four of these treatments (progesterone, IvIg, folic acid and donor leucocyte immunization) are currently believed not to improve the chance of a live birth in couples with RPL. The fifth, aspirin/heparin, is recommended as treatment for women with APS and three pregnancy losses, but more research is now needed in women with APS and two losses, or women with RPL and hereditary thrombophilia.
Third, the lack of evidence-based investigations and treatments has resulted in a significant research wastage in RPL care. Therefore, the guideline also clearly mentions investigations and treatments that should not be used for couples with RPL (Fig. 1). Some of these treatments are not recommended because they have been shown to be ineffective for increasing the chance of a live born baby in couples with RPL, while others have not been studied in couples with RPL, or were shown to have significant adverse events. Similarly, several investigations are currently being applied to couples with RPL while they have no benefit to the couples.
It is clear that evidence-based practice in RPL is not yet feasible as studies are lacking. The current guideline clearly exposes areas where more research is necessary and a research agenda has been developed, with the aim of stimulating research on RPL and more specifically on the questions in urgent need of an answer (Supplementary Fig. S1). While awaiting evidence and evidence-based recommendations, GPPs are provided to support clinicians in routine practice.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Supplementary Material
Acknowledgements
The Guideline Development Group would like to thank invited experts Peter Bisschop for providing helpful comments on thyroid abnormalities, and Grigoris Grimbizis for checking the chapters on uterine malformations. The guideline development group also acknowledges the help of many clinicians and patient organizations who refereed the content of the Guideline and submitted helpful comments to the draft version.
Authors’ roles
M.G. chaired the guideline development group and hence fulfilled a leading role in collecting the evidence, writing the manuscript and dealing with reviewer comments. N.V., as methodological expert, performed all literature searches for the guideline, provided methodological support and coordinated the guideline development. R.B.A. represented the patient perspective in the guideline group. All other authors, listed in alphabetical order, as guideline group members, contributed equally to the manuscript, by drafting key questions, synthesizing evidence, writing the different parts of the guideline and discussing recommendations until consensus within the group was reached.
Funding
The study has no external funding; all costs for meetings were covered by ESHRE.
Conflict of interest
J.E. reports position funding from CARE Fertility. S.L. reports position funding from SpermComet Ltd. S.M. reports research grants, consulting and speaker’s fees from GSK, BMS/Pfizer, Sanquin, Aspen, Bayer and Daiichi Sankyo. S.Q. reports speaker’s fees from Ferring. The other authors reported no conflicts of interest.
References
- Alonso A, Soto I, Urgelles MF, Corte JR, Rodriguez MJ, Pinto CR. Acquired and inherited thrombophilia in women with unexplained fetal losses. Am J Obstet Gynecol 2002;187:1337–1342. [DOI] [PubMed] [Google Scholar]
- Andersen AM, Andersen PK, Olsen J, Gronbaek M, Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol 2012;41:405–413. [DOI] [PubMed] [Google Scholar]
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R et al. . GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–725. [DOI] [PubMed] [Google Scholar]
- Atasever M, Soyman Z, Demirel E, Gencdal S, Kelekci S. Diminished ovarian reserve: is it a neglected cause in the assessment of recurrent miscarriage? A cohort study. Fertil Steril 2016;105:1236–1240. [DOI] [PubMed] [Google Scholar]
- Avalos LA, Roberts SC, Kaskutas LA, Block G, Li DK. Volume and type of alcohol during early pregnancy and the risk of miscarriage. Subst Use Misuse 2014;49:1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy SZ, Westpfal EM. Frequency of etiological factors and cost effectiveness of the work up for patients with history of recurrent pregnancy loss. Early Pregnancy 2000;4:253–260. [PubMed] [Google Scholar]
- Bailey AP, Jaslow CR, Kutteh WH. Minimally invasive surgical options for congenital and acquired uterine factors associated with recurrent pregnancy loss. Womens Health (Lond Engl) 2015;11:161–167. [DOI] [PubMed] [Google Scholar]
- Balasch J, Creus M, Marquez M, Burzaco I, Vanrell JA. The significance of luteal phase deficiency on fertility: a diagnostic and therapeutic approach. Hum Reprod 1986;1:145–147. [DOI] [PubMed] [Google Scholar]
- Barber JC, Cockwell AE, Grant E, Williams S, Dunn R, Ogilvie CM. Is karyotyping couples experiencing recurrent miscarriage worth the cost? Bjog 2010;117:885–888. [DOI] [PubMed] [Google Scholar]
- Bernardi LA, Cohen RN, Stephenson MD. Impact of subclinical hypothyroidism in women with recurrent early pregnancy loss. Fertil Steril 2013;100:1326–1331. [DOI] [PubMed] [Google Scholar]
- Bernardi LA, Plunkett BA, Stephenson MD. Is chromosome testing of the second miscarriage cost saving? A decision analysis of selective versus universal recurrent pregnancy loss evaluation. Fertil Steril 2012;98:156–161. [DOI] [PubMed] [Google Scholar]
- Boots CE, Bernardi LA, Stephenson MD. Frequency of euploid miscarriage is increased in obese women with recurrent early pregnancy loss. Fertil Steril 2014;102:455–459. [DOI] [PubMed] [Google Scholar]
- Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med 2011;29:507–513. [DOI] [PubMed] [Google Scholar]
- Bradley LA, Palomaki GE, Bienstock J, Varga E, Scott JA. Can Factor V Leiden and prothrombin G20210A testing in women with recurrent pregnancy loss result in improved pregnancy outcomes? Results from a targeted evidence-based review. Genet Med 2012;14:39–50. [DOI] [PubMed] [Google Scholar]
- Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod 1999;14:2868–2871. [DOI] [PubMed] [Google Scholar]
- Bussen S, Sutterlin M, Steck T. Endocrine abnormalities during the follicular phase in women with recurrent spontaneous abortion. Hum Reprod 1999;14:18–20. [DOI] [PubMed] [Google Scholar]
- Bustos D, Moret A, Tambutti M, Gogorza S, Testa R, Ascione A, Prigoshin N. Autoantibodies in Argentine women with recurrent pregnancy loss. Am J Reprod Immunol 2006;55:201–207. [DOI] [PubMed] [Google Scholar]
- Caliskan E, Ozkan S, Cakiroglu Y, Sarisoy HT, Corakci A, Ozeren S. Diagnostic accuracy of real-time 3D sonography in the diagnosis of congenital Mullerian anomalies in high-risk patients with respect to the phase of the menstrual cycle. J Clin Ultrasound 2010;38:123–127. [DOI] [PubMed] [Google Scholar]
- Calleja-Agius J, Jauniaux E, Muttukrishna S. Inflammatory cytokines in maternal circulation and placenta of chromosomally abnormal first trimester miscarriages. Clin Dev Immunol 2012;2012:175041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp HJ, Hass Y, Dolicky M, Goldenberg M, Mashiach S, Rabinovici J. The effect of serum follicular phase luteinizing hormone concentrations in habitual abortion: correlation with results of paternal leukocyte immunization. Hum Reprod 1995;10:1702–1705. [DOI] [PubMed] [Google Scholar]
- Cauchi MN, Pepperell R, Kloss M, Lim D. Predictors of pregnancy success in repeated miscarriage. Am J Reprod Immunol 1991;26:72–75. [DOI] [PubMed] [Google Scholar]
- Cavalcante MB, Costa FD, Araujo Junior E, Barini R. Risk factors associated with a new pregnancy loss and perinatal outcomes in cases of recurrent miscarriage treated with lymphocyte immunotherapy. J Matern Fetal Neonatal Med 2014;28:1082–6. [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Goswami SK, Rajani S, Sharma S, Kabir SN, Chakravarty B, Jana K. Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS One 2013;8:e64446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound Obstet Gynecol 2011. a;38:371–382. [DOI] [PubMed] [Google Scholar]
- Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, Coomarasamy A. The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update 2011. b;17:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao KH, Yang YS, Ho HN, Chen SU, Chen HF, Dai HJ, Huang SC, Gill TJ 3rd. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am J Reprod Immunol 1995;34:274–280. [DOI] [PubMed] [Google Scholar]
- Choi YK, Kwak-Kim J. Cytokine gene polymorphisms in recurrent spontaneous abortions: a comprehensive review. Am J Reprod Immunol 2008;60:91–110. [DOI] [PubMed] [Google Scholar]
- Christiansen OB. A fresh look at the causes and treatments of recurrent miscarriage, especially its immunological aspects. Hum Reprod Update 1996;2:271–293. [DOI] [PubMed] [Google Scholar]
- Cocksedge KA, Saravelos SH, Wang Q, Tuckerman E, Laird SM, Li TC. Does free androgen index predict subsequent pregnancy outcome in women with recurrent miscarriage? Hum Reprod 2008;23:797–802. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, Gupta P, Dawood F, Koot YE, Bender Atik R et al. . A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med 2015;373:2141–2148. [DOI] [PubMed] [Google Scholar]
- Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril 2002;78:487–490. [DOI] [PubMed] [Google Scholar]
- Creus M, Deulofeu R, Penarrubia J, Carmona F, Balasch J. Plasma homocysteine and vitamin B12 serum levels, red blood cell folate concentrations, C677T methylenetetrahydrofolate reductase gene mutation and risk of recurrent miscarriage: a case-control study in Spain. Clin Chem Lab Med 2013;51:693–699. [DOI] [PubMed] [Google Scholar]
- de Jong PG, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev 2014:Cd004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non-consecutive losses? Hum Reprod 2016;31:2428–2434. [DOI] [PubMed] [Google Scholar]
- Egerup P, Lindschou J, Gluud C, Christiansen OB, ImmuReM IPD Study Group . The effects of intravenous immunoglobulins in women with recurrent miscarriages: a systematic review of randomised trials with meta-analyses and trial sequential analyses including individual patient data. PLoS One 2015;10:e0141588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev 2005:Cd002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn H, Yan J, Saravelos SH, Li TC. Comparison of reproductive outcome, including the pattern of loss, between couples with chromosomal abnormalities and those with unexplained repeated miscarriages. J Obstet Gynaecol Res 2014;40:109–116. [DOI] [PubMed] [Google Scholar]
- Foyouzi N, Cedars MI, Huddleston HG. Cost-effectiveness of cytogenetic evaluation of products of conception in the patient with a second pregnancy loss. Fertil Steril 2012;98:151–155. [DOI] [PubMed] [Google Scholar]
- Franssen MT, Korevaar JC, Leschot NJ, Bossuyt PM, Knegt AC, Gerssen-Schoorl KB, Wouters CH, Hansson KB, Hochstenbach R, Madan K et al. . Selective chromosome analysis in couples with two or more miscarriages: case-control study. BMJ 2005;331:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen MT, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ 2006;332:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen MT, Musters AM, van der Veen F, Repping S, Leschot NJ, Bossuyt PM, Goddijn M, Korevaar JC. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: a systematic review. Hum Reprod Update 2011;17:467–475. [DOI] [PubMed] [Google Scholar]
- Ghi T, Casadio P, Kuleva M, Perrone AM, Savelli L, Giunchi S, Meriggiola MC, Gubbini G, Pilu G, Pelusi C et al. . Accuracy of three-dimensional ultrasound in diagnosis and classification of congenital uterine anomalies. Fertil Steril 2009;92:808–813. [DOI] [PubMed] [Google Scholar]
- Giasuddin AS, Mazhar I, Haq AM. Prevalence of anticardiolipin antibody in Bangladeshi patients with recurrent pregnancy loss. Bangladesh Med Res Counc Bull 2010;36:10–13. [DOI] [PubMed] [Google Scholar]
- Gomaa MF, Elkholy AG, El-Said MM, Abdel-Salam NE. Combined oral prednisolone and heparin versus heparin: the effect on peripheral NK cells and clinical outcome in patients with unexplained recurrent miscarriage. A double-blind placebo randomized controlled trial. Arch Gynecol Obstet 2014;290:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande M, Borrell A, Garcia-Posada R, Borobio V, Munoz M, Creus M, Soler A, Sanchez A, Balasch J. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod 2012;27:3109–3117. [DOI] [PubMed] [Google Scholar]
- Grimbizis GF, Di Spiezio Sardo A, Saravelos SH, Gordts S, Exacoustos C, Van Schoubroeck D, Bermejo C, Amso NN, Nargund G, Timmerman D et al. . The Thessaloniki ESHRE/ESGE consensus on diagnosis of female genital anomalies. Hum Reprod 2016;31:2–7. . and Gynecol Surg 2016;2013: 20112016. [DOI] [PubMed] [Google Scholar]
- Hadinedoushan H, Mirahmadian M, Aflatounian A. Increased natural killer cell cytotoxicity and IL-2 production in recurrent spontaneous abortion. Am J Reprod Immunol 2007;58:409–414. [DOI] [PubMed] [Google Scholar]
- Hefler-Frischmuth K, Walch K, Hefler L, Tempfer C, Grimm C. Serologic markers of autoimmunity in women with recurrent pregnancy loss. Am J Reprod Immunol 2017;77 10.1111/aji.12635. [DOI] [PubMed] [Google Scholar]
- Hegaard HK, Ersboll AS, Damm P. Exercise in pregnancy: first trimester risks. Clin Obstet Gynecol 2016;59:559–567. [DOI] [PubMed] [Google Scholar]
- Hirahara F, Andoh N, Sawai K, Hirabuki T, Uemura T, Minaguchi H. Hyperprolactinemic recurrent miscarriage and results of randomized bromocriptine treatment trials. Fertil Steril 1998;70:246–252. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Khoury J, Thie J. Recurrent pregnancy loss and diminished ovarian reserve. Fertil Steril 2000;74:1192–1195. [DOI] [PubMed] [Google Scholar]
- Hogge WA, Byrnes AL, Lanasa MC, Surti U. The clinical use of karyotyping spontaneous abortions. Am J Obstet Gynecol 2003;189:397–400. ; discussion 400-392. [DOI] [PubMed] [Google Scholar]
- Ikuma S, Sato T, Sugiura-Ogasawara M, Nagayoshi M, Tanaka A, Takeda S. Preimplantation genetic diagnosis and natural conception: a comparison of live birth rates in patients with recurrent pregnancy loss associated with translocation. PLoS One 2015;10:e0129958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispasoiu CA, Chicea R, Stamatian FV, Ispasoiu F. High fasting insulin levels and insulin resistance may be linked to idiopathic recurrent pregnancy loss: a case-control study. Int J Endocrinol 2013;2013:576926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslow CR. Uterine factors. Obstet Gynecol Clin North Am 2014;41:57–86. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Farquharson RG, Christiansen OB, Exalto N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod 2006;21:2216–2222. [DOI] [PubMed] [Google Scholar]
- Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril 1994;62:54–62. [DOI] [PubMed] [Google Scholar]
- Kaandorp SP, van Mens TE, Middeldorp S, Hutten BA, Hof MH, van der Post JA, van der Veen F, Goddijn M. Time to conception and time to live birth in women with unexplained recurrent miscarriage. Hum Reprod 2014;29:1146–1152. [DOI] [PubMed] [Google Scholar]
- Kaider AS, Kaider BD, Janowicz PB, Roussev RG. Immunodiagnostic evaluation in women with reproductive failure. Am J Reprod Immunol 1999;42:335–346. [DOI] [PubMed] [Google Scholar]
- Karami N, Boroujerdnia MG, Nikbakht R, Khodadadi A. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol 2012;95:87–92. [DOI] [PubMed] [Google Scholar]
- Kazerooni T, Ghaffarpasand F, Asadi N, Dehkhoda Z, Dehghankhalili M, Kazerooni Y. Correlation between thrombophilia and recurrent pregnancy loss in patients with polycystic ovary syndrome: a comparative study. J Chin Med Assoc 2013;76:282–288. [DOI] [PubMed] [Google Scholar]
- Khan I, Okosieme OE, Lazarus JH. Current challenges in the pharmacological management of thyroid dysfunction in pregnancy. Expert Rev Clin Pharmacol 2017;10:97–109. [DOI] [PubMed] [Google Scholar]
- Kodaman PH, Arici A. Intra-uterine adhesions and fertility outcome: how to optimize success? Curr Opin Obstet Gynecol 2007;19:207–214. [DOI] [PubMed] [Google Scholar]
- Kolte AM, Bernardi LA, Christiansen OB, Quenby S, Farquharson RG, Goddijn M, Stephenson MD, Eshre Special Interest Group EP . Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod 2015. a;30:495–498. [DOI] [PubMed] [Google Scholar]
- Kolte AM, Olsen LR, Mikkelsen EM, Christiansen OB, Nielsen HS. Depression and emotional stress is highly prevalent among women with recurrent pregnancy loss. Hum Reprod 2015. b;30:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod 2004;19:1644–1646. [DOI] [PubMed] [Google Scholar]
- Lashley EE, Meuleman T, Claas FH. Beneficial or harmful effect of antipaternal human leukocyte antibodies on pregnancy outcome? A systematic review and meta-analysis. Am J Reprod Immunol 2013;70:87–103. [DOI] [PubMed] [Google Scholar]
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014;3:76–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Na BJ, Kim JY, Hur SE, Lee M, Gilman-Sachs A, Kwak-Kim J. Determination of clinical cellular immune markers in women with recurrent pregnancy loss. Am J Reprod Immunol 2013;70:398–411. [DOI] [PubMed] [Google Scholar]
- Lee GS, Park JC, Rhee JH, Kim JI. Etiologic characteristics and index pregnancy outcomes of recurrent pregnancy losses in Korean women. Obstet Gynecol Sci 2016;59:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TC, Ding SH, Anstie B, Tuckerman E, Wood K, Laird S. Use of human menopausal gonadotropins in the treatment of endometrial defects associated with recurrent miscarriage: preliminary report. Fertil Steril 2001;75:434–437. [DOI] [PubMed] [Google Scholar]
- Li W, Ma N, Laird SM, Ledger WL, Li TC. The relationship between serum prolactin concentration and pregnancy outcome in women with unexplained recurrent miscarriage. J Obstet Gynaecol 2013;33:285–288. [DOI] [PubMed] [Google Scholar]
- Li W, Newell-Price J, Jones GL, Ledger WL, Li TC. Relationship between psychological stress and recurrent miscarriage. Reprod Biomed Online 2012;25:180–189. [DOI] [PubMed] [Google Scholar]
- Li TC, Spuijbroek MD, Tuckerman E, Anstie B, Loxley M, Laird S. Endocrinological and endometrial factors in recurrent miscarriage. Bjog 2000;107:1471–1479. [DOI] [PubMed] [Google Scholar]
- Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand 2010;89:992–1002. [DOI] [PubMed] [Google Scholar]
- Lo W, Rai R, Hameed A, Brailsford SR, Al-Ghamdi AA, Regan L. The effect of body mass index on the outcome of pregnancy in women with recurrent miscarriage. J Family Community Med 2012;19:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund M, Kamper-Jorgensen M, Nielsen HS, Lidegaard O, Andersen AM, Christiansen OB. Prognosis for live birth in women with recurrent miscarriage: what is the best measure of success? Obstet Gynecol 2012;119:37–43. [DOI] [PubMed] [Google Scholar]
- Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage--results from a UK-population-based case-control study. Bjog 2007;114:170–186. [DOI] [PubMed] [Google Scholar]
- Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxford) 2010;49:281–288. [DOI] [PubMed] [Google Scholar]
- Maryam K, Bouzari Z, Basirat Z, Kashifard M, Zadeh MZ. The comparison of insulin resistance frequency in patients with recurrent early pregnancy loss to normal individuals. BMC Res Notes 2012;5:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H, Sugi T, Arai T, Kondo A, Suzuki T, Izumi S, McIntyre JA, Makino T. Different antiphospholipid antibody specificities are found in association with early repeated pregnancy loss versus recurrent IVF-failure patients. Am J Reprod Immunol 2001;46:323–329. [DOI] [PubMed] [Google Scholar]
- Medica I, Ostojic S, Pereza N, Kastrin A, Peterlin B. Association between genetic polymorphisms in cytokine genes and recurrent miscarriage – a meta-analysis. Reprod Biomed Online 2009;19:406–414. [DOI] [PubMed] [Google Scholar]
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL et al. . International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- Molazadeh M, Karimzadeh H, Azizi MR. Prevalence and clinical significance of antinuclear antibodies in Iranian women with unexplained recurrent miscarriage. Iran J Reprod Med 2014;12:221–226. [PMC free article] [PubMed] [Google Scholar]
- Morley LC, Simpson N, Tang T. Human chorionic gonadotrophin (hCG) for preventing miscarriage. Cochrane Database Syst Rev 2013:Cd008611. [DOI] [PubMed] [Google Scholar]
- Mueller-Eckhardt G, Mallmann P, Neppert J, Lattermann A, Melk A, Heine O, Pfeiffer R, Zingsem J, Domke N, Mohr-Pennert A. Immunogenetic and serological investigations in nonpregnant and in pregnant women with a history of recurrent spontaneous abortions. German RSA/IVIG Study Group. J Reprod Immunol 1994;27:95–109. [DOI] [PubMed] [Google Scholar]
- Musters AM, Koot YE, van den Boogaard NM, Kaaijk E, Macklon NS, van der Veen F, Nieuwkerk PT, Goddijn M. Supportive care for women with recurrent miscarriage: a survey to quantify women’s preferences. Hum Reprod 2013;28:398–405. [DOI] [PubMed] [Google Scholar]
- Musters AM, Repping S, Korevaar JC, Mastenbroek S, Limpens J, van der Veen F, Goddijn M. Pregnancy outcome after preimplantation genetic screening or natural conception in couples with unexplained recurrent miscarriage: a systematic review of the best available evidence. Fertil Steril 2011;95:2153–2157. , 2157.e2151-2153. [DOI] [PubMed] [Google Scholar]
- Nardo LG, Rai R, Backos M, El-Gaddal S, Regan L. High serum luteinizing hormone and testosterone concentrations do not predict pregnancy outcome in women with recurrent miscarriage. Fertil Steril 2002;77:348–352. [DOI] [PubMed] [Google Scholar]
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab 2010;95:1699–1707. [DOI] [PubMed] [Google Scholar]
- Nelen WL, Blom HJ, Steegers EA, den Heijer M, Eskes TK. Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril 2000;74:1196–1199. [DOI] [PubMed] [Google Scholar]
- Nelson DB, Grisso JA, Joffe MM, Brensinger C, Shaw L, Datner E. Does stress influence early pregnancy loss? Ann Epidemiol 2003;13:223–229. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci USA 2006;103:3938–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HS, Steffensen R, Varming K, Van Halteren AG, Spierings E, Ryder LP, Goulmy E, Christiansen OB. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet 2009;18:1684–1691. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Wu F, Aghai Z, Steffensen R, van Halteren AG, Spierings E, Christiansen OB, Miklos D, Goulmy E. H-Y antibody titers are increased in unexplained secondary recurrent miscarriage patients and associated with low male: female ratio in subsequent live births. Hum Reprod 2010;25:2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara M, Aoki K, Kajiura S, Yagami Y. Are antinuclear antibodies predictive of recurrent miscarriage? Lancet 1996;347:1183–1184. [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Kajiura S, Katano K, Aoyama T, Aoki K. Are serum progesterone levels predictive of recurrent miscarriage in future pregnancies? Fertil Steril 1997;68:806–809. [DOI] [PubMed] [Google Scholar]
- Okon MA, Laird SM, Tuckerman EM, Li TC. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril 1998;69:682–690. [DOI] [PubMed] [Google Scholar]
- Opatrny L, David M, Kahn SR, Shrier I, Rey E. Association between antiphospholipid antibodies and recurrent fetal loss in women without autoimmune disease: a metaanalysis. J Rheumatol 2006;33:2214–2221. [PubMed] [Google Scholar]
- Oppelt P, von Have M, Paulsen M, Strissel PL, Strick R, Brucker S, Wallwiener D, Beckmann MW. Female genital malformations and their associated abnormalities. Fertil Steril 2007;87:335–342. [DOI] [PubMed] [Google Scholar]
- Ota K, Dambaeva S, Han AR, Beaman K, Gilman-Sachs A, Kwak-Kim J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum Reprod 2014;29:208–219. [DOI] [PubMed] [Google Scholar]
- Plana-Ripoll O, Parner E, Olsen J, Li J. Severe stress following bereavement during pregnancy and risk of pregnancy loss: results from a population-based cohort study. J Epidemiol Community Health 2016;70:424–429. [DOI] [PubMed] [Google Scholar]
- Prakash A, Li TC, Laird S, Nargund G, Ledger WL. Absence of follicular phase defect in women with recurrent miscarriage. Fertil Steril 2006;85:1784–1790. [DOI] [PubMed] [Google Scholar]
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril 2009;91:1215–1223. [DOI] [PubMed] [Google Scholar]
- Puri M, Kaur L, Walia GK, Mukhopadhhyay R, Sachdeva MP, Trivedi SS, Ghosh PK, Saraswathy KN. MTHFR C677T polymorphism, folate, vitamin B12 and homocysteine in recurrent pregnancy losses: a case control study among North Indian women. J Perinat Med 2013;41:549–554. [DOI] [PubMed] [Google Scholar]
- Rai R, Backos M, Rushworth F, Regan L. Polycystic ovaries and recurrent miscarriage--a reappraisal. Hum Reprod 2000;15:612–615. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Kumar D, Khanna M, Al Heidous M, Sheikh A, Virmani V, Palaniappan Y. Multi-modality imaging review of congenital abnormalities of kidney and upper urinary tract. World J Radiol 2016;8:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Lakshmi A, Sadhnani MD. Prevalence of hypothyroidism in recurrent pregnancy loss in first trimester. Indian J Med Sci 2008;62:357–361. [PubMed] [Google Scholar]
- Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet 1990;336:1141–1144. [DOI] [PubMed] [Google Scholar]
- Rikken JF, Kowalik CR, Emanuel MH, Mol BW, Van der Veen F, van Wely M, Goddijn M. Septum resection for women of reproductive age with a septate uterus. Cochrane Database Syst Rev 2017:CD008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht C, Schuddinck V, Fryns JP, Vermeesch JR. Diagnosis of miscarriages by molecular karyotyping: benefits and pitfalls. Genet Med 2009;11:646–654. [DOI] [PubMed] [Google Scholar]
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, Kirkman-Brown J, Coomarasamy A. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908–2917. [DOI] [PubMed] [Google Scholar]
- Saccone G, Schoen C, Franasiak JM, Scott RT Jr., Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta-analysis of randomized, controlled trials. Fertil Steril 2017;107:430–438 e433. [DOI] [PubMed] [Google Scholar]
- Sagle M, Bishop K, Ridley N, Alexander FM, Michel M, Bonney RC, Beard RW, Franks S. Recurrent early miscarriage and polycystic ovaries. BMJ 1988;297:1027–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Won H, Nesbitt-Hawes E, Campbell N, Abbott J. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol 2011;18:569–581. [DOI] [PubMed] [Google Scholar]
- Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum Reprod Update 2008;14:415–429. [DOI] [PubMed] [Google Scholar]
- Scarpellini F, Sbracia M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Hum Reprod 2009;24:2703–2708. [DOI] [PubMed] [Google Scholar]
- Schlussel MM, Souza EB, Reichenheim ME, Kac G. Physical activity during pregnancy and maternal-child health outcomes: a systematic literature review. Cad Saude Publica 2008;24:s531–s544. [DOI] [PubMed] [Google Scholar]
- Shakhar K, Rosenne E, Loewenthal R, Shakhar G, Carp H, Ben-Eliyahu S. High NK cell activity in recurrent miscarriage: what are we really measuring? Hum Reprod 2006;21:2421–2425. [DOI] [PubMed] [Google Scholar]
- Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev 2014:Cd007411. [DOI] [PubMed] [Google Scholar]
- Skeith L, Carrier M, Kaaja R, Martinelli I, Petroff D, Schleussner E, Laskin CA, Rodger MA. A meta-analysis of low-molecular-weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood 2016;127:1650–1655. [DOI] [PubMed] [Google Scholar]
- Souza SS, Ferriani RA, Santos CM, Voltarelli JC. Immunological evaluation of patients with recurrent abortion. J Reprod Immunol 2002;56:111–121. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011;21:1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidou EM, Caramellino L, Patriarca A, Menato G. Maternal caffeine consumption and sine causa recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol 2011;158:220–224. [DOI] [PubMed] [Google Scholar]
- Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 1996;66:24–29. [PubMed] [Google Scholar]
- Stern C, Chamley L, Hale L, Kloss M, Speirs A, Baker HW. Antibodies to beta2 glycoprotein I are associated with in vitro fertilization implantation failure as well as recurrent miscarriage: results of a prevalence study. Fertil Steril 1998;70:938–944. [DOI] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M, Aoki K, Fujii T, Fujita T, Kawaguchi R, Maruyama T, Ozawa N, Sugi T, Takeshita T, Saito S. Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J Hum Genet 2008;53:622–628. [DOI] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M, Lin BL, Aoki K, Maruyama T, Nakatsuka M, Ozawa N, Sugi T, Takeshita T, Nishida M. Does surgery improve live birth rates in patients with recurrent miscarriage caused by uterine anomalies? J Obstet Gynaecol 2015;35:155–158. [DOI] [PubMed] [Google Scholar]
- Tang AW, Alfirevic Z, Turner MA, Drury JA, Small R, Quenby S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum Reprod 2013;28:1743–1752. [DOI] [PubMed] [Google Scholar]
- Ticconi C, Rotondi F, Veglia M, Pietropolli A, Bernardini S, Ria F, Caruso A, Di Simone N. Antinuclear autoantibodies in women with recurrent pregnancy loss. Am J Reprod Immunol 2010;64:384–392. [DOI] [PubMed] [Google Scholar]
- Triggianese P, Perricone C, Perricone R, De Carolis C. Prolactin and natural killer cells: evaluating the neuroendocrine-immune axis in women with primary infertility and recurrent spontaneous abortion. Am J Reprod Immunol 2015;73:56–65. [DOI] [PubMed] [Google Scholar]
- van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta 2012;1822:1951–1959. [DOI] [PubMed] [Google Scholar]
- van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011;17:605–619. [DOI] [PubMed] [Google Scholar]
- Venetis CA, Papadopoulos SP, Campo R, Gordts S, Tarlatzis BC, Grimbizis GF. Clinical implications of congenital uterine anomalies: a meta-analysis of comparative studies. Reprod Biomed Online 2014;29:665–683. [DOI] [PubMed] [Google Scholar]
- Vermeulen N, de Sutter P, Nelen WLDM Manual for ESHRE Guideline Development. 2014, www.eshre.eu/guidelines.
- Vissenberg R, van den Boogaard E, van Wely M, van der Post JA, Fliers E, Bisschop PH, Goddijn M. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Hum Reprod Update 2012;18:360–373. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao H, Li Y, Zhang J, Tan J, Liu Y. Relationship between recurrent miscarriage and insulin resistance. Gynecol Obstet Invest 2011;72:245–251. [DOI] [PubMed] [Google Scholar]
- Watson H, Kiddy DS, Hamilton-Fairley D, Scanlon MJ, Barnard C, Collins WP, Bonney RC, Franks S. Hypersecretion of luteinizing hormone and ovarian steroids in women with recurrent early miscarriage. Hum Reprod 1993;8:829–833. [DOI] [PubMed] [Google Scholar]
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev 2014:Cd000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammiti W, Mtiraoui N, Mahjoub T. Lack of consistent association between endothelial nitric oxide synthase gene polymorphisms, homocysteine levels and recurrent pregnancy loss in tunisian women. Am J Reprod Immunol 2008;59:139–145. [DOI] [PubMed] [Google Scholar]
- Zhang BY, Wei YS, Niu JM, Li Y, Miao ZL, Wang ZN. Risk factors for unexplained recurrent spontaneous abortion in a population from southern China. Int J Gynaecol Obstet 2010;108:135–138. [DOI] [PubMed] [Google Scholar]
- Ziakas PD, Pavlou M, Voulgarelis M. Heparin treatment in antiphospholipid syndrome with recurrent pregnancy loss: a systematic review and meta-analysis. Obstet Gynecol 2010;115:1256–1262. [DOI] [PubMed] [Google Scholar]
- Zolghadri J, Tavana Z, Kazerooni T, Soveid M, Taghieh M. Relationship between abnormal glucose tolerance test and history of previous recurrent miscarriages, and beneficial effect of metformin in these patients: a prospective clinical study. Fertil Steril 2008;90:727–730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.