Abstract
Context
Obesity has been shown to be unfavorable to skeletal microarchitecture when assessed by trabecular bone score (TBS). The influence of adiposity on skeletal microstructure in primary hyperparathyroidism (PHPT) has not yet been evaluated.
Objective
To investigate the effect of obesity on TBS and bone mineral density (BMD) in subjects with PHPT at baseline and through 2 years after parathyroidectomy.
Design
Prospective observational study.
Setting
Referral center.
Patients or Other Participants
Thirty men and women with PHPT undergoing parathyroid surgery.
Main Outcome Measures
TBS and BMD by dual-energy X-ray absorptiometry (DXA).
Results
There were notable improvements in lumbar spine and femoral neck BMD in the obese (lumbar spine: 4.3 ± 4.7%, femoral neck: 3.8 ± 6.6%; P < 0.05 for both) and nonobese subjects (lumbar spine: 3.8 ± 5.6%, femoral neck 3.1 ± 5.0%; P < 0.05 for both) but no marked change in TBS in either group at 24 months postparathyroidectomy. Obese subjects had fully degraded TBS values compared with the nonobese subjects, whose TBS values were minimally below normal throughout the study (baseline: 1.199 ± 0.086 vs 1.327 ± 0.099, respectively; P = 0.003; 24 months: 1.181 ± 0.061 vs 1.352 ± 0.114, respectively; P = 0.001), despite improvements in BMD.
Conclusions
The detrimental effect of obesity on TBS, an index of bone quality, was demonstrated in subjects with PHPT. Obesity was associated with fully degraded skeletal microarchitecture as measured by TBS in PHPT, despite similar values in bone density by DXA compared with nonobese subjects. TBS values did not improve postparathyroidectomy in either obese or nonobese subjects.
Spine and hip BMD improved in obese and nonobese subjects with PHPT through 2 years postparathyroidectomy, but there were no changes in their TBS scores with low values in the obese, only, persisting.
Trabecular bone score (TBS) is an indirect measure of bone microarchitecture that is derived from a textural analysis of pixel gray level variations in dual-energy X-ray absorptiometry (DXA) images of the lumbar vertebrae (1). TBS quantification is readily determined from software applied to the DXA image. It has become a convenient clinical tool to estimate skeletal microarchitecture (2). Better bone quality in vertebrae with dense trabeculae and good connectivity is reflected as a more homogenous pixel variation and a higher TBS, whereas poor bone quality with more disconnected trabeculae is reflected as a more heterogeneous pixel variation and a lower score (1, 3). TBS values have been shown to correlate with fracture risk, independent of bone mineral density (BMD) (4, 5).
Both vertebral and nonvertebral fracture risk are increased in primary hyperparathyroidism (PHPT) (6–8). Prior studies have shown that TBS values in PHPT are significantly lower than matched controls. Moreover, TBS assessment was shown to discriminate between patients with and without vertebral fractures (9, 10). The influence of adiposity on skeletal microstructure in PHPT is of interest because parathyroid hormone (PTH) influences osteoblast and adipocyte lineages through a common mesenchymal stem cell progenitor (11). Although TBS is inversely related to body mass index (BMI) in subjects without PHPT, whether a relationship exists in PHPT and/or differs from what has been described among normal subjects is not known. Moreover, if there are differences in TBS among normal and obese subjects with PHPT, it is not known if such differences are affected by curative parathyroidectomy. In this study, we examined TBS in obese and nonobese subjects with PHPT at baseline and prospectively 2 years after parathyroid surgery.
Materials and Methods
Patients
Thirty subjects with well-characterized PHPT undergoing parathyroidectomy were recruited from the Metabolic Bone Diseases Unit at Columbia University Medical Center (CUMC). Subjects were eligible if they had biochemical evidence of PHPT (hypercalcemia with elevated or inappropriately normal PTH) with at least 6 months of follow-up after surgery. Exclusion criteria included a clinical diagnosis of any chronic disorder affecting mineral metabolism such as Paget disease of bone, untreated thyroid disease, Cushing syndrome, diabetes mellitus, malabsorption syndrome, liver disease, renal disease (creatinine clearance <30 mL/min), current or recent pregnancy, or lactation. Current users of bisphosphonates or denosumab were also excluded as were subjects with a BMI >37 kg/m2 or <15 kg/m2. The cohort was grouped according to World Health Organization’s definition of obesity into two subgroups: BMI ≥30 kg/m2 (obese) and BMI <30 kg/m2 (nonobese). The study was approved by the Institutional Review Board of CUMC. Of the 30 subjects with baseline data, 25 participants (10 obese and 15 nonobese) had bone density and TBS measurements at 6 months, 28 participants (9 obese and 19 nonobese) at 12 months, 20 participants (7 obese and 13 nonobese) at 18 months, and 20 participants (9 obese and 11 nonobese) at 24 months.
Biochemical evaluation
Blood samples were drawn in a fasting state. Serum total calcium and albumin were analyzed using standard methods (Quest Diagnostics, Madison, NJ). Calcium values were corrected for low albumin (albumin <4.0 g/dL) using the standard equation: corrected calcium = [0.8 × (4.0 − patient's albumin)] + serum calcium level. Intact PTH was measured by immunoradiometric assay (Scantibodies, Santee, CA) in the Bone Marker Laboratory of the Metabolic Bone Diseases Unit at CUMC. The normal range was 14 to 66 pg/mL, and the interassay and intraassay coefficients were <7% and 5%, respectively. Propeptide of type I collagen (P1NP) was measured by immunoradiometric assay (Immunodiagnostic Systems, Scottsdale, AZ). The normal premenopausal range is 19 to 83 μg/L, and the interassay and intraassay coefficients of variation were 8.3% and 6.5%, respectively. Collagen type 1 cross-linked C-telopeptide (CTX) was measured by enzyme-linked immunosorbent assay (Immunodiagnostic Systems). The normal premenopausal range is 0.112 to 0.738 ng/mL, and interassay and intraassay coefficients of variation were 10.9% and 3%, respectively.
BMD
Areal BMD was measured at the lumbar spine (L1 to L4), total hip, femoral neck, and nondominant forearm by DXA (Hologic 4500A; Hologic Inc., Bedford, MA). Short-term, in vivo, precision error was 1.1% for L1 to L4, 2.4% for total hip and femoral neck, and 1.8% for the forearm. As the cohort consisted of premenopausal women and men <50 years of age, both absolute values as well as Z-scores for BMD (using sex- and age-matched reference data provided by the manufacturer) and T scores for BMD are presented.
TBS
TBS values were classified into three categories as follows (4, 12, 13): ≤1.2 (fully degraded microarchitecture); 1.21 to 1.34 (partially degraded microarchitecture); and ≥1.35 (normal). TBS was calculated from the L1 to L4 DXA image (Hologic Inc.) using TBS iNsight®, version 2.1 software (Med-Impas, Pessac, France). This newer software has an updated algorithm that adjusts for abdominal soft tissue based on BMI and is optimized for BMI within 15 to 37 kg/m2 (14).
Statistical analysis
Histograms were used to assess the normality of the distribution for continuous variables. The χ2 test or Fisher exact test was used for between-group comparisons for categorical variables and independent t tests for between-group comparisons for continuous variables. A linear mixed-effect model for repeated-measures approach was used for within-group comparisons of skeletal variables longitudinally across all time points (baseline and 6, 12, 18, and 24 months following surgery) for the entire cohort and the obese and nonobese subgroups. The correlation of TBS with BMI was assessed by the Pearson correlation test. All statistical tests were two-tailed, and a P <0.05 was considered significant. The statistical software R version 3.2 (http://www.r-project.org/) and SPSS 23.0 for Windows (IBM Corp., Armonk, NY) were used for the analyses. The data are presented as mean ± standard deviation (SD) for continuous variables unless otherwise stated. Data for categorical variables are presented as frequency and percentage.
Results
Demographic and biochemical indices
The baseline characteristics of the study population are shown in Table 1. The cohort (n = 30) was predominantly female (70.0%), with a mean age of 62.9 ± 12 years, consistent with the demographics of the disease. The majority were non-Hispanic white women (86.7%). On average, subjects had mild disease with mean corrected serum calcium 10.4 ± 0.6 mg/dL, mean serum PTH 90.6 ± 47 pg/mL, and mean serum 25-hydroxyvitamin D 45.7 ± 15 ng/mL. The majority (86.7%) of the study population met one or more surgical guidelines for parathyroidectomy (15). The obese group (n = 10) had a BMI of 33.4 ± 3 kg/m2 and was significantly heavier than the nonobese group (n = 20; 24.3 ± 4 kg/m2; P = 0.001). There were no noteworthy differences in age, sex, ethnicity, menopausal status, duration of disease, serum PTH, calcium, phosphate, vitamin D status, renal function, or bone turnover markers between groups at baseline. In addition, there were no differences in the history of fractures, prior history of bisphosphonate use, or proportion of subjects meeting criteria for parathyroidectomy between the two groups. All subjects had persistently normal serum calcium values postparathyroidectomy, demonstrating biochemical cure.
Table 1.
Baseline Characteristics of Study Population
| Reference Ranges | Total (n = 30) | Obese (n = 10) | Nonobese (n = 20) | P Value | |
|---|---|---|---|---|---|
| Age, y | 62.9 ± 12.1 | 65.3 ± 9.3 | 61.7 ± 13.4 | 0.454 | |
| Female, % | 21 (70.0) | 6 (60.0) | 15 (75.0) | 0.398 | |
| Postmenopausal, % | 18 (60.0) | 6 (60.0) | 12 (60.0) | 0.497 | |
| Years since menopause | 14.4 ± 7.4 | 12.5 ± 10.0 | 15.3 ± 6.0 | 0.458 | |
| Ethnicity, % | |||||
| White | 26 (86.7) | 10 (100.0) | 16 (80.0) | 0.435 | |
| Black | 1 (3.3) | 0 (0.0) | 1 (5.0) | ||
| Hispanic | 1 (3.3) | 0 (0.0) | 1 (5.0) | ||
| Asian | 2 (6.7) | 0 (0.0) | 2 (10.0) | ||
| Duration of PHPT, y | 6.46 ± 8.55 | 6.03 ± 7.30 | 6.67 ± 9.29 | 0.851 | |
| Meets surgical guidelines, % | 26 (86.7) | 10 (100.0) | 16 (80.0) | 0.129 | |
| History of fractures, % | 14 (46.7) | 4 (40.0) | 10 (50.0) | 0.605 | |
| Past use of bisphosphonates, % | 10 (33.3) | 2 (20.0) | 8 (40.0) | 0.273 | |
| Duration of use of bisphosphonates, y | 6.82 ± 3.74 | 5.50 ± 3.54 | 7.15 ± 3.95 | 0.608 | |
| BMI, kg/m2 | 27.3 ± 5.4 | 33.4 ± 2.5 | 24.3 ± 3.5 | 0.001 | |
| Weight, kg | 76.4 ± 17.0 | 92.0 ± 15.4 | 68.6 ± 11.7 | 0.001 | |
| Intact PTH, ng/mL | 14–66 | 90.6 ± 47.2 | 103.2 ± 62.0 | 84.6 ± 38.9 | 0.341 |
| Corrected calcium, mg/dL | 8.6–10.2 | 10.4 ± 0.6 | 10.4 ± 0.6 | 10.3 ± 0.6 | 0.695 |
| Albumin, g/dL | 3.5–5.5 | 4.6 ± 0.3 | 4.5 ± 0.2 | 4.6 ± 0.4 | 0.253 |
| 25OHD, ng/mL | 30–100 | 45.7 ± 14.7 | 48.6 ± 20.6 | 43.4 ± 8.6 | 0.552 |
| 1,25OHD, pg/mL | 16–65 | 65.7 ± 21.5 | 68.5 ± 15.3 | 64.2 ± 24.4 | 0.567 |
| Creatinine, μmol/L | 53–106 | 76.1 ± 19.2 | 74.3 ± 14.2 | 77.0 ± 21.6 | 0.720 |
| Estimated GFR, mL/min | >60 | 81.3 ± 17.5 | 81.8 ± 17.2 | 81.1 ± 18.1 | 0.921 |
| P1NP, μg/L | 19–83 | 66.2 ± 36.3 | 66.0 ± 30.2 | 66.3 ± 40.5 | 0.983 |
| C-telopeptide, ng/mL | 0.112–0.738 | 0.66 ± 0.35 | 0.75 ± 0.41 | 0.61 ± 0.32 | 0.348 |
| Phosphate, mg/dL | 2.5–4.5 | 2.83 ± 0.44 | 2.78 ± 0.46 | 2.86 ± 0.44 | 0.638 |
| Lumbar spine BMD, g/cm2 | 0.938 ± 0.16 | 0.936 ± 0.19 | 0.939 ± 0.15 | 0.963 | |
| Lumbar spine T-score | −1.12 ± 1.5 | −1.24 ± 1.8 | −1.07 ± 1.4 | 0.782 | |
| Lumbar spine Z-score | 0.25 ± 1.6 | 0.13 ± 1.9 | 0.31 ± 1.5 | 0.778 | |
| Femoral neck BMD, g/cm2 | 0.686 ± 0.11 | 0.702 ± 0.15 | 0.678 ± 0.09 | 0.655 | |
| Femoral neck T-score | −1.59 ± 0.9 | −1.47 ± 1.2 | −1.64 ± 0.7 | 0.646 | |
| Femoral neck Z-score | −0.27 ± 0.9 | −0.11 ± 1.2 | −0.35 ± 0.8 | 0.574 | |
| Total hip BMD, g/cm2 | 0.828 ± 0.13 | 0.847 ± 0.15 | 0.819 ± 0.12 | 0.579 | |
| Total hip T-score | −1.10 ± 0.9 | −0.98 ± 1.1 | −1.15 ± 0.8 | 0.633 | |
| Total hip Z-score | −0.11 ± 0.9 | −0.00 ± 1.1 | −0.17 ± 0.8 | 0.644 | |
| Distal radius BMD, g/cm2 | 0.636 ± 0.10 | 0.633 ± 0.11 | 0.638 ± 0.09 | 0.895 | |
| Distal radius T-score | −1.58 ± 1.5 | −1.89 ± 1.6 | −1.42 ± 0.5 | 0.447 | |
| Distal radius Z-score | −0.08 ± 1.4 | −0.39 ± 1.7 | 0.08 ± 1.3 | 0.414 |
Abbreviations: GFR, glomerular filtration rate; 1,25OHD, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D.
BMD
At baseline, lumbar spine BMD (g/cm2) and Z-scores were normal in both the obese (BMD 0.936 ± 0.19 g/cm2; Z-score 0.13 ± 1.9; T-score −1.24 ± 1.8) and nonobese subjects (BMD 0.939 ± 0.15 g/cm2; Z-score 0.31 ± 1.5; T-score −1.07 ± 1.4) and were not significantly different between the two groups (Table 1). After parathyroidectomy, there was a marked improvement in lumbar spine BMD in the nonobese group noted at 6 months and obese group at 12 months that persisted through 24 months [Fig. 1(b)]. The improvement in lumbar spine BMD was similar between the obese and nonobese groups. At 24 months, there was a 4.3 ± 5% increase in the obese group and a 3.8 ± 6% increase in the nonobese group (P = 0.813) (Table 2).
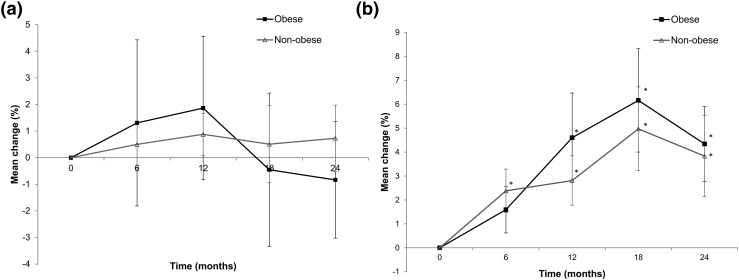
Figure 1.
(a) Percentage change from baseline in TBS and (b) percentage change in lumbar spine BMD through 24 months in obese (squares) and nonobese (triangles) subjects with PHPT after parathyroidectomy. Data are mean ± SE. *P < 0.05 compared with baseline.
Table 2.
Areal BMD (g/cm2) of the Lumbar Spine, Femoral Neck, Total Hip, and Distal Radius at Baseline and Percentage Change Through 24 Months After Parathyroid Surgery for the Entire Cohort and Obese and Nonobese Subgroups
| BMD
(g/cm2) |
Percentage Change
From Baseline (%) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
6 Mo |
12 Months |
18 Months |
24 Months |
|||||||||||
| Total (n = 30) | Obese (n = 10) | Nonobese (n = 20) | Total (n = 25) | Obese (n = 10) | Nonobese (n = 15) | Total (n = 27) | Obese (n = 9) | Nonobese (n = 18) | Total (n = 20) | Obese (n = 7) | Nonobese (n = 13) | Total (n = 20) | Obese (n = 9) | Nonobese (n = 11) | |
| Lumbar spine | 0.938 ± 0.16 | 0.936 ± 0.19 | 0.939 ± 0.15 | 2.03 ± 3.3a | 1.59 ± 3.1 | 2.38 ± 3.5a | 3.39 ± 4.9a | 4.60 ± 5.6a | 2.81 ± 4.5a | 5.38 ± 6.0a | 6.16 ± 5.7a | 4.97 ± 6.3a | 4.02 ± 5.1a | 4.34 ± 4.7a | 3.83 ± 5.6a |
| Femoral neck | 0.686 ± 0.11 | 0.702 ± 0.15 | 0.678 ± 0.09 | 1.39 ± 5.0 | 1.88 ± 6.7 | 1.07 ± 3.7 | 2.05 ± 5.1a | 2.01 ± 7.0 | 2.07 ± 4.2a | 4.31 ± 4.3a | 4.39 ± 4.5a | 4.24 ± 4.4a | 3.35 ± 5.6a | 3.79 ± 6.6a | 3.05 ± 5.0a |
| Total hip | 0.828 ± 0.13 | 0.847 ± 0.15 | 0.819 ± 0.12 | 1.77 ± 3.0a | 1.98 ± 1.9a | 1.64 ± 3.6a | 2.17 ± 3.1a | 2.25 ± 2.1a | 2.11 ± 3.6a | 2.68 ± 4.0a | 3.28 ± 3.6a | 2.34 ± 4.3a | 3.44 ± 4.4a | 4.30 ± 4.0a | 2.77 ± 4.8a |
| Distal radius | 0.636 ± 0.10 | 0.633 ± 0.11 | 0.638 ± 0.09 | −0.41 ± 3.1 | −0.81 ± 3.5 | −0.19 ± 2.9 | −0.19 ± 3.1 | −0.65 ± 4.2 | 0.06 ± 2.4 | −0.46 ± 3.96 | −1.22 ± 4.0 | −0.01 ± 3.8 | 0.22 ± 3.6 | −0.14 ± 4.3 | 0.45 ± 3.0 |
Data are mean ± SD.
P < 0.05 as compared with baseline.
Femoral neck BMD and Z-scores in the obese subjects (BMD 0.702 ± 0.15 g/cm2; Z-score −0.11 ± 1.2; T-score −1.47 ± 1.2) were not significantly different from the nonobese subjects (BMD 0.678 ± 0.09 g/cm2; Z-score −0.35 ± 0.8; T-score −1.64 ± 0.7) at baseline (Table 1). After parathyroidectomy, there was an improvement in femoral neck BMD at 12 months in the nonobese group and an improvement at 18 months in the obese group. There were no significant between-group differences. At 24 months, there was a 3.8 ± 7% increase in femoral neck BMD in the obese group and a 3.1 ± 5% increase in the nonobese group (P = 0.960) (Table 2). Of note, there was a 6-month delay in BMD improvement among the obese as compared with the nonobese at the lumbar spine and femoral neck.
Total hip BMD and Z-scores in the obese subjects (BMD 0.847 ± 0.15 g/cm2; Z-score −0.00 ± 1.1; T-score −0.98 ± 1.1) were not significantly different from the nonobese subjects (BMD 0.819 ± 0.12 g/cm2; Z-score −0.17 ± 0.8; T-score −1.15 ± 0.8) at baseline (Table 1). After parathyroidectomy, there was an improvement in total hip BMD at 6 months in both the obese and nonobese groups. There were no noteworthy between-group differences. At 24 months, there was a 4.3 ± 4% increase in total hip BMD in the obese group and a 2.8 ± 5% increase in the nonobese group (P = 0.629) (Table 2).
Baseline distal radius BMD and Z-scores in the obese group (BMD 0.633 ± 0.11 g/cm2; Z-score −0.39 ± 1.7; T-score −1.89 ± 1.6) were also not different from the nonobese group (BMD 0.638 ± 0.09 g/cm2; Z-score +0.08 ± 1.3; T-score −1.42 ± 0.5) (Table 1). Following parathyroidectomy, there were no notable differences in BMD in either the obese or nonobese groups.
TBS
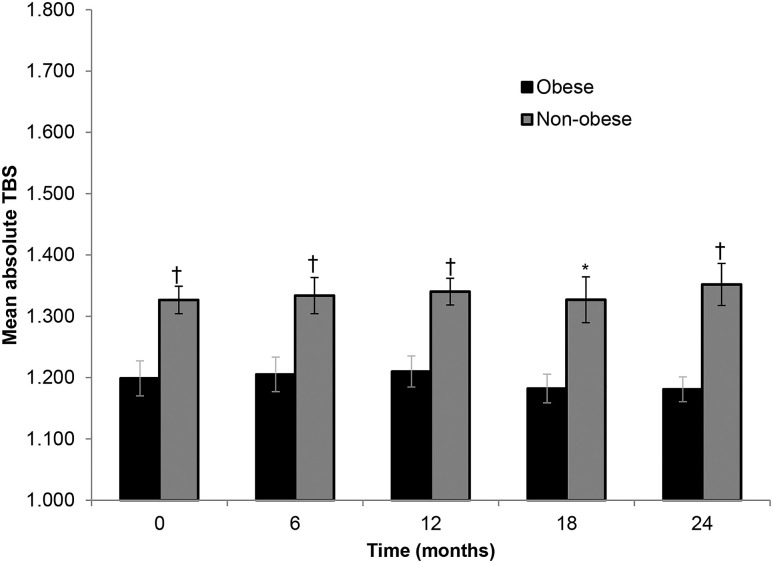
At baseline, the mean TBS in the obese group reflected a fully degraded microarchitectural classification (1.199 ± 0.09) and was significantly lower than the value in the nonobese group, which showed a minimally degraded microarchitecture classification (1.327 ± 0.10; P = 0.003) (Fig. 2). After parathyroidectomy, there were no notable differences from baseline for the study population as a whole or for the obese and nonobese subgroups [Fig. 1(a)]. However, the mean TBS value remained significantly lower in the obese group than the nonobese group throughout the 24 months. There was an inverse relationship between TBS and BMI using baseline values (r = −0.476; P = 0.009).
Figure 2.
Absolute values in TBS at baseline and through 24 months after parathyroid surgery in obese and nonobese subjects with PHPT. Data are mean ± SE. *P < 0.05 between obese and nonobese; †P < 0.01 between obese and nonobese.
Effect of age on BMD and TBS
Using a linear mixed-effects model, age was not significantly interactive with BMD at the lumbar spine (P = 0.23), femoral neck (P = 0.59), total hip (P = 0.93), and distal radius (P = 0.90). However, there was a significant interaction with age and TBS in the entire cohort (P = 0.01). When stratified by age 50, the values for TBS at baseline and 6, 12, 18, and 24 months were significantly higher in the younger group than the older group. In addition, older nonobese had significantly higher TBS values than the older obese subgroup across all time points.
Changes in bone turnover markers and the relationship with BMD and TBS
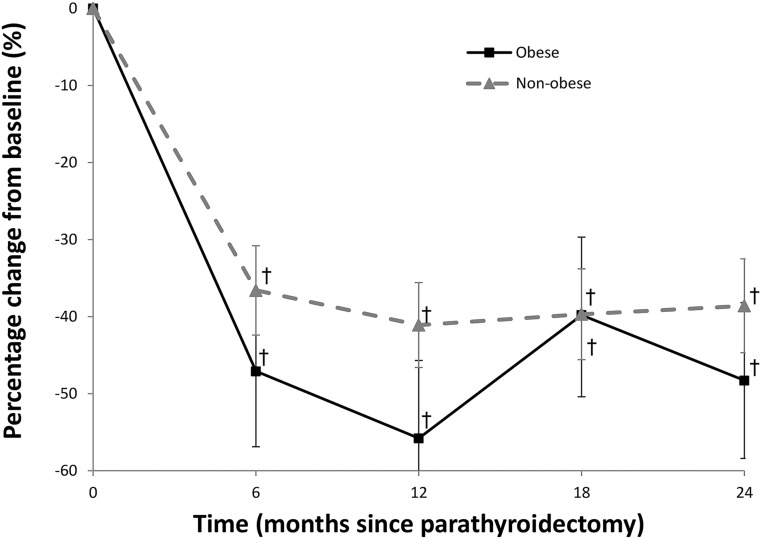
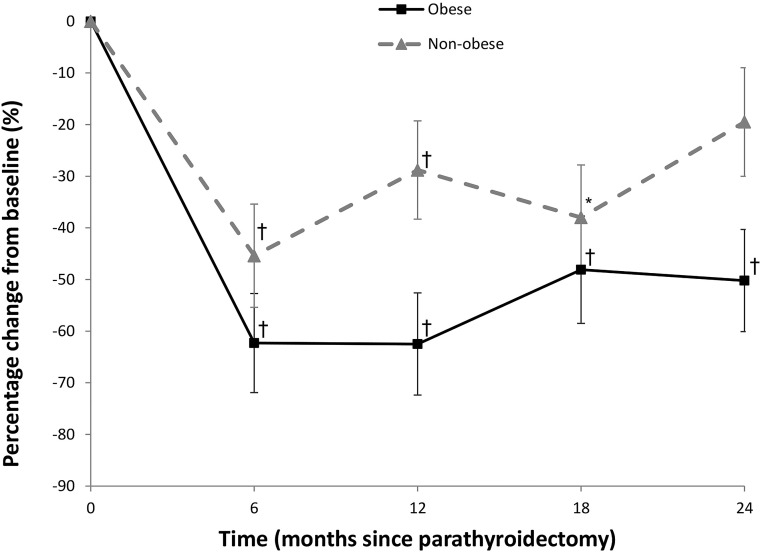
Both markers of bone resorption (CTX) and bone formation (P1NP) are significantly reduced postparathyroidectomy. There is no difference in the bone formation marker P1NP between the obese and nonobese group across the 2 years of observation (Fig. 3). Moreover, there was no difference in the bone resorption marker CTX at the 6- and 18-month time points between the obese and nonobese group. However, at month 12, the obese subjects had a transiently larger decrease in CTX (absolute difference −33.7 ± 14.7%; P = 0.02) and at month 24 (absolute difference −30.8 ± 15.3%; P = 0.048) compared with the nonobese group (Fig. 4).
Figure 3.
Percentage change in markers of bone formation (P1NP) in subjects with PHPT through 24 months postparathyroidectomy. †P < 0.001 compared with baseline.
Figure 4.
Percentage change in markers of bone resorption (C-telopeptide, CTX) in subjects with PHPT through 24 months postparathyroidectomy. *P < 0.05 compared with baseline; †P < 0.001 compared with baseline.
BMD: lumbar spine
With the exception of the 6-month postparathyroidectomy time point, changes in CTX were negatively correlated with changes in lumbar spine BMD (12 months: r = −0.413, P = 0.05; 18 months, r = −0.511, P = 0.03; and 24 months: r = −0.548, P = 0.02). The correlation between lumbar spine BMD and the bone formation marker P1NP was not as consistent, with significant changes only being seen at 18 months (r = −0.570; P = 0.01) and 24 months (r = −0.541; P = 0.02).
BMD: total hip
In the relationship between total hip BMD and CTX, there was a 6-month displacement in time with the change in total hip BMD occurring 6 months after the change in CTX: 6-month CTX vs 12-month total hip BMD (r = −0.439; P = 0.047); 12-month CTX vs 18-month total hip BMD (r = −0.556; P = 0.02); and 18-month CTX vs 24-month total hip BMD (r = −0.630; P = 0.007). For P1NP, the only time point that was correlated with a change in total hip BMD was at 18 months (r = −0.461; P = 0.047).
BMD: femoral neck
Similar to the relationship between CTX and total hip BMD, the relationship between the change in CTX and femoral neck BMD correlated significantly at each time point but was displaced in time by 6 months: 6-month CTX vs 12-month femoral neck BMD: r = −0.594, P = 0.005; 12-month CTX vs 18-month femoral neck BMD: r = −0.534, P = 0.023; and 18-month CTX vs 24-month femoral neck BMD at 24 months: r = −0.502, P = 0.04. For P1NP, the only time point that was correlated with a change in femoral neck BMD was 6 months: r = −0.473; P = 0.03.
BMD distal radius
There was no noteworthy correlation between changes in bone turnover markers and changes at the distal one-third radius BMD site at any time points at baseline or postparathyroidectomy.
TBS
There was no notable correlation between bone turnover markers and TBS at any time points at baseline or postparathyroidectomy.
Discussion
Our results demonstrate the detrimental effect of obesity on TBS, a clinically useful index of bone quality, in subjects with PHPT. By TBS, obese subjects showed evidence of fully degraded trabecular microarchitecture, despite similar values in BMD by DXA compared with nonobese subjects. Following successful parathyroid surgery, obese subjects had persistently low TBS at 24 months despite improvement in BMD at the lumbar spine and femoral neck. In the nonobese subjects, TBS was only minimally reduced at baseline and persisted at this level throughout the 24-month post–parathyroid surgery period. Similarly, nonobese subjects had an improvement in BMD at the lumbar spine and femoral neck even though TBS values remained unchanged. Additionally, although the magnitude of increase was similar, there was a notable 6-month delay in BMD improvement among the obese as compared with the nonobese at the lumbar spine and femoral neck but not at the total hip and distal radius.
Few studies have used TBS as a clinical index of bone quality in PHPT. In a cross-sectional study of 22 postmenopausal women from our group, we showed a discordance between TBS and BMD, demonstrating that TBS identified more patients with osteoporosis or osteopenia than did lumbar spine BMD (13). In this prospective study, we further show the negative influence of adiposity on TBS. Even with lumbar spine BMD relatively preserved in both the obese and nonobese subjects, TBS was discordant and considerably lower in the obese participants. Longitudinal studies with TBS in PHPT are limited. In a small cohort of 20 Italian subjects undergoing parathyroidectomy, TBS Z-scores improved significantly from −3.03 ± 1.17 to −1.63 ± 0.37 at 2 years (9). In contrast, we did not observe an improvement in TBS values after parathyroidectomy in our cohort as a whole as well as the obese and nonobese subgroups. Potential reasons for these differences among the two studies include differences in the characteristics of the study populations. The Italian cohort had more severe biochemical PHPT with lower 25-hydroxyvitamin D levels, higher PTH levels, and worse areal bone densities at the spine and hip. Of note, the BMD Z-scores at the lumbar spine and femoral neck in the Italian cohort were ∼1 SD below the values in our cohort. The baseline TBS Z-score in the Italian cohort (with reference to French normative data) was −3.03 ± 1.17, whereas the baseline absolute TBS in this study was 1.287 ± 0.111, which corresponds to at most 1 SD away from the same French reference cohort and ∼2 SDs higher than the Italian cohort (16).
The difference between the rapid improvements in BMD in both obese and nonobese subjects and the lack of any change by TBS after parathroidectomy is noteworthy in this cohort of subjects with relatively mild PHPT. This observation could well reflect the point that changes in skeletal microstructure, as detected by TBS, may take longer to be demonstrable than simple improvement in bone mineral content. The ability to see this difference in kinetics of change over time may be a particular feature of the mild PHPT state that allowed us to make this observation. It is reasonable to expect that longer follow-up will eventually show an improvement in TBS scores, but whether the time course of this change will be different between the obese and nonobese subjects remains to be seen.
Novel imaging techniques have dispelled the notion that trabecular sites are preserved in mild asymptomatic PHPT when evaluated by DXA. Studies employing high-resolution peripheral qualitative computed tomography show that both cortical as well as trabecular microarchitecture are detrimentally affected (17, 18). Our results as well as those from previous studies demonstrate that TBS is low in patients with mild asymptomatic disease (10, 13). Even when derived from the lumbar spine, a site that is conventionally regarded as a trabecular site, it must be noted that TBS does not only indirectly reflect the trabecular microstructure but rather indirectly reflects a composite of both cortical as well as trabecular microstructure. Silva et al. (13) have shown that TBS correlated with all cortical and trabecular high-resolution peripheral qualitative computed tomography indices except for trabecular thickness and trabecular stiffness at the radius and with similar findings at the weight-bearing tibia after adjusting for weight.
Although a direct association between BMI and BMD is well documented (19–21), and an association between low BMI and increased fracture risk is clearly established (22), the relationship between adiposity and fracture risk is less clear. The high prevalence of obesity in postmenopausal women with fragility fractures does not support the notion that adiposity is protective against fractures (23). One study demonstrated that after adjusting for the mechanical loading effect of body weight on the skeleton, there was an inverse relationship between fat mass and bone mass (24). In a meta-analysis of multiple prospective population-based cohorts, the relationship between BMI and fracture risk was nonlinear, with a marked increase in risk for the underweight and a modest decrease in the obese (25). However, studies have not consistently shown a lower overall fracture risk, with some studies showing an increase in fractures associated with obesity that may be site-specific (26–30).
The influence of adiposity on skeletal microarchitecture may be dependent on fat distribution. The type of fat distribution, whether gynoid or android, can influence the direction of the relationship between TBS and obesity. In men, there is an inverse relationship between TBS and BMI (r = −0.452; P < 0.0001) (31). This relationship is not as clear in women, however. Some studies reported the same inverse association in women (32–34), although conflicting results were noted in a study of postmenopausal women from Korea (35). In that study, TBS was instead positively related to BMI (r = 0.099; P < 0.001), and the investigators further showed that TBS was positively associated with gynoid fat mass (r = 0.086; P < 0.05) but negatively related to android fat mass (r = −0.106; P < 0.05). Likewise, other studies have confirmed that android fat distribution or visceral fat is detrimental to bone health (36, 37). As it has been previously shown that postmenopausal women with PHPT had a more android pattern of fat distribution than age-matched controls (38), the inverse relationship seen in our study may be consistent with existing evidence, although we did not classify subjects by fat distribution in this study.
As adipocytes and osteoblasts originate from the same pluripotent mesenchymal stem cells, PTH may have a complex role in energy homeostasis through its effect on adipocyte differentiation and by influencing the process of adipogenesis (39). PTH can direct the mesenchymal stem cell toward the osteoblast lineage and away from adiopogenesis (11). Given that PTH has dual anabolic and catabolic effects depending on its interaction with different states of the PTH/PTH-related protein receptor, it is plausible that the direction of the mesenchymal stem cell fate may differ with chronic exposure to excessive PTH as in PHPT (40). An increase in bone marrow adipocytes has been shown to reflect a worsened skeletal microarchitecture in aging and osteoporosis as well as in other diseases (41–43). This detrimental effect on skeletal microarchitecture may possibly be compounded by obesity (39, 44).
We also note the observation that the vitamin D levels were similar between the obese and nonobese subjects. This is likely because most of the subjects (76.7%) were on vitamin D supplementation. We did not note any difference in the proportion of vitamin D supplement users between the obese (80%) and nonobese groups (75%) (P = 0.760). Similarly, there was no difference in the median weekly dosage of vitamin D supplementation between the obese [7000 (5250 to 13,575 IU/week)] and nonobese [5000 (0 to 14,000 IU/week)] groups (P = 0.327). The interesting normalization of 25-hydroxyvitamin D levels in patients with modern-day PHPT has previously been reported by Walker et al. (45) In that cohort, a majority of subjects were taking vitamin D supplements. Our study, however, makes this observation among both obese and nonobese cohorts with PHPT.
We recently reported substantial reductions in bone turnover markers postparathyroidectomy (46). Although obesity has been associated with a global suppression of bone turnover in patients without PHPT (47, 48), we did not detect any difference in the bone formation marker P1NP between the obese and nonobese group across the 2 years of observation, except for bone resorption marker CTX, demonstrating a transiently larger decrease at 12 and 24 months in patients with PHPT. The decrease in CTX has been related to improvement in areal BMD, consistent with existing data (49), and has shown that the change in CTX was contemporaneous with the changes in BMD at the spine, whereas there was a 6-month displacement in time with changes in BMD at the hip.
There are several important limitations to this study, most notably the small sample size and a smaller subset of subjects with complete data points (n = 15) over the 2-year observation. In the subanalysis of subjects for whom data were available at all time points, similar robust improvements in BMD contrasting a lack of change in TBS were noted, which substantiates the overall conclusion of the study involving all subjects. We were not able to classify subjects by fat distribution or evaluate the effect of merely being overweight but not obese. There are also concerns that increased soft tissue mass in obese individuals over the vertebrae may artifactually reduce TBS estimates (50). However, TBS values in our study were calculated using TBS iNsight®, version 2.1 software, with an updated algorithm that adjusts for abdominal soft tissue based on BMI. This system is optimized for BMI within 15 to 37 kg/m2, the BMI range of our subjects (51). There is also no age-, sex-, and weight-matched control population, and comparisons made in the analyses were with subjects’ baseline values and between obese and nonobese subjects. The conclusions drawn are thus limited to patients with PHPT who have undergone parathyroidectomy.
Acknowledgments
Financial Support: This study was supported by National Institutes of Health Grant DK32333 to J.P.B.
Disclosure Summary: N.E.C. receives research support from Shire. J.P.B. is a consultant for Amgen, Shire, Radius, and Ultragenyx. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BMD
bone mineral density
- BMI
body mass index
- CTX
collagen type 1 cross-linked C-telopeptide
- CUMC
Columbia University Medical Center
- DXA
dual-energy X-ray absorptiometry
- P1NP
propeptide of type I collagen
- PHPT
primary hyperparathyroidism
- PTH
parathyroid hormone
- SD
standard deviation
- TBS
trabecular bone score
References
- 1. Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29(3):518–530. [DOI] [PubMed] [Google Scholar]
- 2. Shevroja E, Lamy O, Kohlmeier L, Koromani F, Rivadeneira F, Hans D. Use of trabecular bone score (TBS) as a complementary approach to dual-energy X-ray absorptiometry (DXA) for fracture risk assessment in clinical practice. J Clin Densitom. 2017;20(3):334–345. [DOI] [PubMed] [Google Scholar]
- 3. Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42(4):775–787. [DOI] [PubMed] [Google Scholar]
- 4. Hans D, Goertzen AL, Krieg M-A, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–2769. [DOI] [PubMed] [Google Scholar]
- 5. Leslie WD, Johansson H, Kanis JA, Lamy O, Oden A, McCloskey EV, Hans D. Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int. 2014;25(9):2271–2277. [DOI] [PubMed] [Google Scholar]
- 6. Khosla S, Melton LJ III, Wermers RA, Crowson CS, O’Fallon Wm, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14(10):1700–1707. [DOI] [PubMed] [Google Scholar]
- 7. Vestergaard P, Mollerup CL, Frøkjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321(7261):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vignali E, Viccica G, Diacinti D, Cetani F, Cianferotti L, Ambrogini E, Banti C, Del Fiacco R, Bilezikian JP, Pinchera A, Marcocci C. Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94(7):2306–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eller-Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Verga U, Scillitani A, Beck-Peccoz P, Chiodini I. Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol. 2013;169(2):155–162. [DOI] [PubMed] [Google Scholar]
- 10. Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, Pepe J, Diacinti D, Piemonte S, Carnevale V, Minisola S. “Trabecular Bone Score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone. 2013;53(1):154–159. [DOI] [PubMed] [Google Scholar]
- 11. Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, Baron R, Bronson RT, Horowitz MC, Wu JY, Bilezikian JP, Dempster DW, Rosen CJ, Lanske B. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017;25(3):661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24(1):77–85. [DOI] [PubMed] [Google Scholar]
- 13. Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, Udesky J, Cremers S, Sarquis M, Guo XD, Hans D, Bilezikian JP. Trabecular bone score (TBS)--a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2013;98(5):1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schacter GI, Leslie WD, Majumdar SR, Morin SN, Lix LM, Hans D. Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: the Manitoba BMD cohort. Osteoporos Int. 2017;28(11):3199–3203. [DOI] [PubMed] [Google Scholar]
- 15. Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dufour R, Heraud A. Lumbar spine micro-architecture in Caucasian French women derived from DXA: TBS normative data. J Clin Densitom. 2009;12(3):377–378. [Google Scholar]
- 17. Hansen S, Beck Jensen JE, Rasmussen L, Hauge EM, Brixen K. Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: A case-control study using HR-pQCT. J Bone Miner Res. 2010;25(9):1941–1947. [DOI] [PubMed] [Google Scholar]
- 18. Stein EM, Silva BC, Boutroy S, Zhou B, Wang J, Udesky J, Zhang C, McMahon DJ, Romano M, Dworakowski E, Costa AG, Cusano N, Irani D, Cremers S, Shane E, Guo XE, Bilezikian JP. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res. 2013;28(5):1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14(9):1622–1627. [DOI] [PubMed] [Google Scholar]
- 20. Marcus R, Greendale G, Blunt BA, Bush TL, Sherman S, Sherwin R, Wahner H, Wells B. Correlates of bone mineral density in the postmenopausal estrogen/progestin interventions trial. J Bone Miner Res. 1994;9(9):1467–1476. [DOI] [PubMed] [Google Scholar]
- 21. Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–573. [DOI] [PubMed] [Google Scholar]
- 22. Mpalaris V, Anagnostis P, Goulis DG, Iakovou I. Complex association between body weight and fracture risk in postmenopausal women. Obes Rev. 2015;16(3):225–233. [DOI] [PubMed] [Google Scholar]
- 23. Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25(2):292–297. [DOI] [PubMed] [Google Scholar]
- 24. Zhao L-J, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92(5):1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ III, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–1338. [DOI] [PubMed] [Google Scholar]
- 26. Compston JE, Flahive J, Hosmer DW, Watts NB, Siris ES, Silverman S, Saag KG, Roux C, Rossini M, Pfeilschifter J, Nieves JW, Netelenbos JC, March L, LaCroix AZ, Hooven FH, Greenspan SL, Gehlbach SH, Díez-Pérez A, Cooper C, Chapurlat RD, Boonen S, Anderson FA Jr, Adami S, Adachi JD; GLOW Investigators . Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J Bone Miner Res. 2014;29(2):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Díez-Pérez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi JD, Rossini M, Lacroix AZ, Roux C, Sambrook PN, Siris ES; Glow Investigators . Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nielson CM, Marshall LM, Adams AL, LeBlanc ES, Cawthon PM, Ensrud K, Stefanick ML, Barrett-Connor E, Orwoll ES; Osteoporotic Fractures in Men Study Research Group . BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS). J Bone Miner Res. 2011;26(3):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu Y-H, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83(1):146–154. [DOI] [PubMed] [Google Scholar]
- 30. Johansson H, Kanis JA, Odén A, McCloskey E, Chapurlat RD, Christiansen C, Cummings SR, Diez-Perez A, Eisman JA, Fujiwara S, Glüer CC, Goltzman D, Hans D, Khaw KT, Krieg MA, Kröger H, LaCroix AZ, Lau E, Leslie WD, Mellström D, Melton LJ III, O’Neill TW, Pasco JA, Prior JC, Reid DM, Rivadeneira F, van Staa T, Yoshimura N, Zillikens MC. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–233. [DOI] [PubMed] [Google Scholar]
- 31. Romagnoli E, Lubrano C, Carnevale V, Costantini D, Nieddu L, Morano S, Migliaccio S, Gnessi L, Lenzi A. Assessment of trabecular bone score (TBS) in overweight/obese men: effect of metabolic and anthropometric factors. Endocrine. 2016;54(2):342–347. [DOI] [PubMed] [Google Scholar]
- 32. Leslie WD, Krieg MA, Hans D; Manitoba Bone Density Program . Clinical factors associated with trabecular bone score. J Clin Densitom. 2013;16(3):374–379. [DOI] [PubMed] [Google Scholar]
- 33. Aloia JF, Mikhail M, Usera G, Dhaliwal R, Islam S. Trabecular bone score (TBS) in postmenopausal African American women. Osteoporos Int. 2015;26(3):1155–1161. [DOI] [PubMed] [Google Scholar]
- 34. Iki M, Tamaki J, Sato Y, Winzenrieth R, Kagamimori S, Kagawa Y, Yoneshima H. Age-related normative values of trabecular bone score (TBS) for Japanese women: the Japanese Population-based Osteoporosis (JPOS) study. Osteoporos Int. 2015;26(1):245–252. [DOI] [PubMed] [Google Scholar]
- 35. Kim JH, Choi HJ, Ku EJ, Hong AR, Kim KM, Kim SW, Cho NH, Shin CS. Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos Int. 2016;27(3):1161–1168. [DOI] [PubMed] [Google Scholar]
- 36. Lv S, Zhang A, Di W, Sheng Y, Cheng P, Qi H, Liu J, Yu J, Ding G, Cai J, Lai B. Assessment of fat distribution and bone quality with trabecular bone score (TBS) in healthy Chinese men. Sci Rep. 2016;6:24935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grey AB, Evans MC, Stapleton JP, Reid IR. Body weight and bone mineral density in postmenopausal women with primary hyperparathyroidism. Ann Intern Med. 1994;121(10):745–749. [DOI] [PubMed] [Google Scholar]
- 39. Roca-Rodríguez MM, El Bekay R, Garrido-Sanchez L, Gómez-Serrano M, Coin-Aragüez L, Oliva-Olivera W, Lhamyani S, Clemente-Postigo M, García-Santos E, de Luna Diaz R, Yubero-Serrano EM, Fernández Real JM, Peral B, Tinahones FJ. Parathyroid hormone-related protein, human adipose-derived stem cells adipogenic capacity and healthy obesity. J Clin Endocrinol Metab. 2015;100(6):E826–E835. [DOI] [PubMed] [Google Scholar]
- 40. Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, Votta B, Stroup GB, Kumar S, Nuttall ME. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39(6):1361–1372. [DOI] [PubMed] [Google Scholar]
- 41. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. [DOI] [PubMed] [Google Scholar]
- 42. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18(10):1319–1328. [DOI] [PubMed] [Google Scholar]
- 43. Grey A. Thiazolidinedione-induced skeletal fragility--mechanisms and implications. Diabetes Obes Metab. 2009;11(4):275–284. [DOI] [PubMed] [Google Scholar]
- 44. Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10(12):737–748. [DOI] [PubMed] [Google Scholar]
- 45. Walker MD, Cong E, Lee JA, Kepley A, Zhang C, McMahon DJ, Bilezikian JP, Silverberg SJ. Low vitamin D levels have become less common in primary hyperparathyroidism. Osteoporos Int. 2015;26(12):2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cusano NE, Rubin MR, Silva BC, Tay WD, Williams JM, Agarwal S, Omeragic B, Guo XE, Bilezikian JP. Skeletal microstructure and estimated bone strength improve following parathyroidectomy in primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(1):196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viljakainen H, Ivaska KK, Paldánius P, Lipsanen-Nyman M, Saukkonen T, Pietiläinen KH, Andersson S, Laitinen K, Mäkitie O. Suppressed bone turnover in obesity: a link to energy metabolism? A case-control study. J Clin Endocrinol Metab. 2014;99(6):2155–2163. [DOI] [PubMed] [Google Scholar]
- 48. Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Müller R, Zhao B, Guo X, Lang T, Saeed I, Liu XS, Guo XE, Cremers S, Rosen CJ, Stein EM, Nickolas TL, McMahon DJ, Young P, Shane E. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98(6):2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christiansen P, Steiniche T, Brixen K, Hessov I, Melsen F, Heickendorff L, Mosekilde L. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999;25(2):237–244. [DOI] [PubMed] [Google Scholar]
- 50. Amnuaywattakorn S, Sritara C, Utamakul C, Chamroonrat W, Kositwattanarerk A, Thamnirat K, Ongphiphadhanakul B. Simulated increased soft tissue thickness artefactually decreases trabecular bone score: a phantom study. BMC Musculoskelet Disord. 2016;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martineau P, Leslie WD. Trabecular bone score (TBS): Method and applications. Bone. 2017;104:66–72. [DOI] [PubMed] [Google Scholar]