Little is known as to how white matter hyperintensities affect ischaemic stroke outcome and whether the effect differs by stroke subtype. Ryu et al. report a prominent association between white matter hyperintensities and stroke outcome in large-artery atherosclerosis patients, accompanied by early neurological deterioration and late recovery.
Keywords: white matter hyperintensities, magnetic resonance image, outcome, ischaemic stroke
Abstract
Leukoaraiosis or white matter hyperintensities are frequently observed on magnetic resonance imaging of stroke patients. We investigated how white matter hyperintensity volumes affect stroke outcomes, generally and by subtype. In total, 5035 acute ischaemic stroke patients were enrolled. Strokes were classified as large artery atherosclerosis, small vessel occlusion, or cardioembolism. White matter hyperintensity volumes were stratified into quintiles. Mean age (± standard deviation) was 66.3 ± 12.8, 59.6% male. Median (interquartile range) modified Rankin Scale score was 2 (1–3) at discharge and 1 (0–3) at 3 months; 16.5% experienced early neurological deterioration, and 3.3% recurrent stroke. The Cochran-Mantel-Haenszel test with adjustment for age, stroke severity, sex, and thrombolysis status showed that the distributions of 3-month modified Rankin Scale scores differed across white matter hyperintensity quintiles (P < 0.001). Multiple ordinal logistic regression analysis showed that higher white matter hyperintensity quintiles were independently associated with worse 3-month modified Rankin Scale scores; adjusted odds ratios (95% confidence interval) for the second to fifth quintiles versus the first quintile were 1.29 (1.10–1.52), 1.40 (1.18–1.66), 1.69 (1.42–2.02) and 2.03 (1.69–2.43), respectively. For large artery atherosclerosis (39.0%), outcomes varied by white matter hyperintensity volume (P = 0.01, Cochran-Mantel-Haenszel test), and the upper three white matter hyperintensity quintiles (versus the first quintile) had worse 3-month modified Rankin Scale scores; adjusted odds ratios were 1.45 (1.10–1.90), 1.86 (1.41–2.47), and 1.89 (1.41–2.54), respectively. Patients with large artery atherosclerosis were vulnerable to early neurological deterioration (19.4%), and the top two white matter hyperintensity quintiles were more vulnerable still: 23.5% and 22.3%. Moreover, higher white matter hyperintensities were associated with poor modified Rankin Scale improvement: adjusted odds ratios for the upper two quintiles versus the first quintile were 0.66 (0.47–0.94) and 0.62 (0.43–0.89), respectively. For small vessel occlusion (17.8%), outcomes tended to vary by white matter hyperintensitiy volume (P = 0.10, Cochran-Mantel-Haenszel test), and the highest quintile was associated with worse 3-month modified Rankin Scale scores: adjusted odds ratio for the fifth quintile versus first quintile, 1.98 (1.23–3.18). In this subtype, worse white matter hyperintensities were associated with worse National Institute of Health Stroke Scale scores at presentation. For cardioembolism (20.6%), outcomes did not vary significantly by white matter hyperintensity volume (P = 0.19, Cochran-Mantel-Haenszel test); however, the adjusted odds ratio for the highest versus lowest quintiles was 1.62 (1.09–2.40). Regardless of stroke subtype, white matter hyperintensities were not associated with stroke recurrence within 3 months of follow-up. In conclusion, white matter hyperintensity volume independently correlates with stroke outcomes in acute ischaemic stroke. There are some suggestions that stroke outcomes may be affected by leukoaraiosis differentially depending on stroke subtypes, to be confirmed in future investigations.
Introduction
Stroke is a leading cause of death and disability worldwide (Hong et al., 2013a). It is estimated that 25–74% of the 50 million stroke survivors require a certain degree of assistance or are fully dependent on caregivers after ictus (Miller et al., 2010). Being able to predict stroke outcomes is highly valuable in clinical management of stroke victims, and can guide the allocation of resources to improve outcomes.
In this study, we assess the influence of white matter hyperintensities (WMHs) on stroke outcomes. WMHs are the non-specific areas of increased signal seen on T2-weighted MRI studies of most older patients, and are thought to represent chronic small vessel ischaemic change in a majority of patients (Wardlaw et al., 2013). There is sparse literature on this topic, and only a few studies with relatively small sample sizes have indicated that increased WMHs are an independent risk factor for unfavourable outcome after acute ischaemic stroke (Arsava et al., 2009; Kissela et al., 2009; Liou et al., 2010; Henninger et al., 2012; Leonards et al., 2012). However, other studies suggested that the association between WMH severity and post-stroke outcome might be weak due to complex interactions between WMHs and outcome-related factors such as age, hypertension, and diabetes (Schiemanck et al., 2006; McAlpine et al., 2014).
The difficulties in determining the role of WMH on stroke outcome is partially the result of the way WMH is measured and described (usually using the non-volumetric Fazekas grading system) (Fazekas et al., 1987), and partly due to the heterogeneity of stroke as a disorder with multiple distinct subtypes and causes, all of which is important in terms of predicting stroke outcome.
Our study addresses this gap in the state of knowledge by investigating the impact of quantified WMH volumes on stroke outcomes, while stratifying by stroke subtype in a large number of patients, and measuring a variety of early and late outcome measures.
Materials and methods
We consecutively enrolled 5035 first-ever ischaemic stroke patients. Volumetric quantitative WMH measurements were obtained on each patient, and were correlated to modified Rankin Scale score at 3 months, stratified by stroke subtypes. To understand the mechanisms underlying stroke subtype-related differences in the influence of WMH on the 3-month functional outcome, we analysed inter-subtype differences in the initial neurological severity at admission, early neurological deterioration during the first 3 weeks after admission, and functional recovery until 3 months after stroke onset, along with stroke recurrence during the 3-month period.
Participants
This is a prospective multi-centre study involving 11 academic and regional stroke centres in Korea participating in the Korean Nationwide Image-based Stroke Database Project (Kim et al., 2011; Ryu et al., 2014). From May 2011 to December 2012, 8005 patients with ischaemic stroke who were admitted to the participating centres within 7 days after symptom onset were screened for participation to find patients with their first episode of confirmed, acute ischaemic stroke. To maintain a homogenous patient population, we excluded the following patients: previous stroke (defined as self-reported history of doctor-diagnosed stroke, n = 1436), pre-stroke modified Rankin Scale score of 2 or higher (n = 417), contraindication to MRI (n = 234), poor quality or unavailability of fluid attenuated inversion recovery (FLAIR) MRI (n = 636), MRI registration error (n = 25), and lost to follow-up (n = 222), leaving 5035 patients for analysis (Supplementary Fig. 1).
All patients underwent standard evaluation, treatment, and rehabilitation adhered to prespecified guidelines for ischaemic stroke (Kim et al., 2015). The institutional review boards of all participating centres approved this study. All patients or their legally authorized representatives provided a written informed consent for study participation.
Clinical data collection
Admission National Institutes of Health Stroke Scale (NIHSS) score, pre-stroke modified Rankin Scale score, and modified Rankin Scale score at 3 months after stroke were collected prospectively. Under a standardized protocol, we collected demographic data, prior medication history, laboratory data, and the presence of vascular risk factors including hypertension, diabetes mellitus, hyperlipidaemia, coronary artery disease, atrial fibrillation, and smoking history (Ryu et al., 2014). Stroke subtypes were determined by the consensus of experienced neurologists in each participating centre, using a validated MRI-based algorithm (Ko et al., 2014) based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria: large artery atherosclerosis, small vessel occlusion, cardioembolism, other determined, or undetermined stroke (Adams et al., 1993). Note was made of early neurological deterioration during the first 3 weeks after admission.
Definition of early neurological deterioration and late recurrence of stroke
The neurological status of the patients was assessed by trained neurologists on a daily basis. Early neurological deterioration was defined as any new neurological symptoms/signs or any neurological worsening occurring during the admission and/or within 3 weeks after stroke onset (Jeong et al., 2015; Kim et al., 2015). We collected early neurological deterioration cases using the following criteria: (i) an increment in the total NIHSS score of ≥2 points; (ii) an increment in the consciousness score (1a–1c) of NIHSS ≥1; (iii) an increment in the motor score (5a–6b) of NIHSS ≥1; or (iv) any new neurological deficit (even if unmeasurable by NIHSS scores). Early neurological deterioration was categorized into stroke progression, early stroke recurrence, transient ischaemic attack, symptomatic haemorrhagic transformation (with 4 or more point increase in NIHSS score) (Brott et al., 1992), unknown and others. Detailed information is provided in Supplementary Table 1. Early (within 3 weeks) or late (from 3 weeks to 3 months after index stroke) stroke recurrence was defined as rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 h or longer or leading to death, with no apparent cause other than of vascular origin (Kim et al., 2015).
MRI registration and analysis
Brain MRI was performed on 1.5 T (n = 4327) or 3.0 T (n = 708) MRI systems. FLAIR image protocols were: echo time 76–160 ms, repetition time 6000–11 000 ms, voxel size 1 × 1 × 3 ∼ 1 × 1 × 7 mm3, interslice gap 0–2.25 mm, field of view 250 mm, and matrix size 256 × 256. Diffusion-weighted MRI protocols were b-values of 0 and 1000 s/mm2, repetition time 2400–9000 ms, echo time 50–99 ms, voxel size 1 × 1 × 3 ∼ 1 × 1 × 5 mm3, interslice gap 0–2 mm. All scans were transferred to the Korean Brain MRI Data Centre for central data storage and quantitative analysis. As previously reported (Kim et al., 2011; Ryu et al., 2014), FLAIR and diffusion-weighted MRI were converted into a patient-independent quantitative visual format. In brief, brain template images (1 × 1 × 1 mm3 voxels) were chosen from the Montreal Neurological Institute template within the range of −63.5 to 74.5 mm in the z-axis of Talairach space. After normalization of images, each patient’s high signal intensity lesions on FLAIR and diffusion-weighted MRI were semi-automatically segmented and registered onto the brain templates under close supervision by vascular neurologists.
In the segmentation and registration of FLAIR WMHs, only chronic lesions were registered by excluding high signal lesions due to acute infarction, as previously published (Ryu et al., 2014). When chronic lesions on FLAIR and acute lesions on diffusion-weighted MRI overlapped, the extent and distribution of FLAIR WMH contralateral to the location of acute infarct served as a reference to determine what volumes to include and exclude, by assuming a symmetric distribution of WMHs across the midline.
During the process of quantification for WMH volume and acute infarct volume, the inter-rater variability was minimal. Intra-observer correlation coefficients were high, ranging from 0.987 to 0.995 for WMH on FLAIR and 0.836 to 0.977 for infarct volume on diffusion-weighted MRI. WMH volume on FLAIR and acute infarct volume on diffusion-weighted MRI were calculated as a percentage of brain volume by dividing the number of voxels in the lesions (FLAIR or diffusion-weighted MRI) over the total number of brain voxels, with corrections applied to account for the differences in scan slice thicknesses by adjusting the denominators as previously described (Ryu et al., 2014).
Data analysis and statistics
First, we investigated the impact of WMH on 3-month modified Rankin Scale score in the entire patient population and in each of the groups defined by stroke subtype. Then, we analysed inter-subtype differences by: (i) initial NIHSS score at admission; (ii) early neurological deterioration during the first 3 weeks after stroke onset; and (iii) late functional recovery that was defined as either (a) modified Rankin Scale improvement after discharge until 3 months after stroke onset; or (b) early neurological deterioration-adjusted 3-month modified Rankin Scale score. We also studied early and late stroke recurrences during the 3-month period following the initial infarct.
Data are presented as mean [standard deviation (SD)], median [interquartile range (IQR)], and number (percentage), as appropriate. Quantified WMH volumes were sorted a priori into quintiles. The quintile categorization for the entire study population was used for all relevant statistical analyses, regardless of stratification by stroke subtypes. Parametric data were assessed using either Student’s t-test or ANOVA for continuous variables. For non-parametric data, either the Wilcoxon Rank-Sum test or the Kruskal-Wallis test was used. Either the χ2 test or Fisher’s exact test was used to examine the association between categorical variables. Some data were missing for body mass index (3.5%), haemoglobin (0.2%), fasting glucose (5.7%), total cholesterol (2.2%), and infarct volume on diffusion-weighted MRI (5.3%). Multiple imputation with a multivariate normal regression method (Markov chain Monte Carlo procedure) was used to impute the missing data (Lavori et al., 1995). Covariates used in each of the multiple imputation models included age, sex, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, previous use of antiplatelet, prior use of statin, stroke subtype, and WMH volume.
To test for a relationship between the quintiles of WMHs and distributions of 3-month modified Rankin Scale scores, we used Cochran-Mantel-Haenszel test with adjustment of age, sex, admission NIHSS, and thrombolysis (Shuaib et al., 2007; Mishra et al., 2010). The Cochran-Mantel-Haenszel test is a non-parametric method, allowing for the analysis of the modified Rankin Scale as an ordinal outcome rather than a binary one (Stokes et al., 2000). To measure the strength of the association between modified Rankin Scale scores and WMH quintiles, we also performed multiple ordinal logistic regression analysis (Bath et al., 2007) with modified Rankin Scale scores as an ordinal outcome variable. In this multivariable analysis, a common odds ratio (OR) and its 95% confidence interval (CI) for each quintile of WMHs (versus the lowest quintile) was obtained. Covariates with P < 0.2 in bivariable analyses for all patients were entered into the multivariable model. For the large artery atherosclerosis group, additional multivariable analyses were performed to further adjust for the severity of symptomatic intracranial or extracranial artery stenosis: absence versus presence of significant (>50%) stenosis or occlusion in the large artery relevant to acute infarcts on diffusion-weighted MRI, which had been assessed by using magnetic resonance or CT angiograms, and registered to our multi-centre image-based stroke database. Furthermore, to account for within-hospital clustering, we used generalized estimating equations with an exchangeable working correlation matrix. For the generalized estimating equations, modified Rankin Scale scores at 3 months were dichotomized into two groups of favourable (score 0–2) versus unfavourable (score 3–6).
The relationship between log-transformed WMH volume and admission NIHSS score in each of the stroke subtype groups was analysed by multiple linear regression model after accounting for covariates with P < 0.2 in simple linear regression analysis for the entire study population. The association between WMH quintiles and early neurological deterioration was tested by binary logistic regression analysis with adjustment for the same covariates that were used in the aforementioned ordinal logistic regression analysis. To evaluate the association between WMH quintiles and late recovery after discharge, the patients with modified Rankin Scale score of 0 at discharge were excluded, and then categorized to two groups: improved (lower 3-month modified Rankin Scale score compared with discharge modified Rankin Scale score) versus stationary or aggravated (the same or higher 3-month modified Rankin Scale score compared with discharge modified Rankin Scale score). To examine whether this functional recovery was related to WMH quintiles, binary logistic regression analysis was performed after accounting for the occurrence of early neurological deterioration and the covariates that were used in the ordinal logistic regression analysis. There could be variability in the time-to-discharge, which may partly depend on stroke subtypes, thereby affecting the results of the above analysis on the late recovery. Thus, further ordinal logistic regression analysis was performed to investigate the association between WMH quintiles and 3-month modified Rankin Scale scores in each group of stroke subtypes after additionally adjusting for early neurological deterioration. Data were analysed using SAS 9.3 software (SAS Institute, Cary, North Carolina, USA) and STATA software 13.0 (STATA Corp., College Station, Texas, USA), and P < 0.05 were considered statistically significant.
Results
Baseline characteristics and univariate analyses
In this study on 5035 patients with first-ever ischaemic stroke, mean age was 66.3 (SD 12.8) and 59.6% were male. Compared with these patients, the excluded patients who had first-ever ischaemic stroke but whose MRIs or 3-month modified Rankin Scale score were unavailable (n = 895 and 222, respectively; Supplementary Fig. 1) were likely to be older and female, and have diabetes and more severe stroke symptoms (Supplementary Table 2). Between the 1.5 T and 3.0 T groups, WMH volumes were not statistically different (median 0.66 versus 0.70; P = 0.32). Baseline characteristics stratified by WMH quintiles were summarized in Table 1. Patients with higher WMH quintiles were likely to be older and female, and to have hypertension, atrial fibrillation and coronary artery disease compared to those with lower WMH quintiles. Patients with higher WMH quintiles had higher NIHSS score at admission and were less likely to receive thrombolysis compared to those with lower WMH quintiles. In regard to stroke subtypes, 1965 (39.0%) had large artery atherosclerosis, 895 (17.8%) small vessel occlusion and 1035 (20.6%) cardioembolism.
Table 1.
Baseline characteristics according to WMH quintiles in 5035 patients with first-ever acute ischaemic stroke
| WMH quintiles | P-valuea | Adjusted P-valuec | |||||
|---|---|---|---|---|---|---|---|
| First (n = 1007) | Second (n = 1007) | Third (n = 1007) | Fourth (n = 1007) | Fifth (n = 1007) | |||
| Range, % of brain | <0.31 | 0.32–0.52 | 0.52–0.86 | 0.87–1.56 | >1.56 | ||
| Median WMH, % of brain | 0.21 (0.15–0.26) | 0.41 (0.36–0.46) | 0.66 (0.79–0.75) | 1.15 (0.99–1.34) | 2.37 (1.88–3.23) | <0.001b | |
| Age (years) | 55.6 (13.0) | 62.8 (12.0) | 67.9 (10.6) | 71.3 (9.7) | 74.0 (9.4) | <0.001 | NA |
| Sex (male) | 667 (66.2%) | 659 (65.4%) | 606 (60.2%) | 587 (58.3%) | 484 (48.1%) | <0.001 | NA |
| Hypertension | 494 (49.1%) | 589 (58.5%) | 665 (66.0%) | 726 (72.1%) | 776 (77.1%) | <0.001 | <0.001 |
| Diabetes | 209 (20.8%) | 272 (27.0%) | 310 (30.8%) | 292 (29.0%) | 280 (27.8%) | <0.001 | 0.03 |
| Hyperlipidemia | 316 (31.4%) | 326 (32.4%) | 333 (33.1%) | 309 (30.7%) | 303 (30.1%) | 0.61 | 0.25 |
| Smoking | 468 (46.5%) | 432 (42.9%) | 392 (38.9%) | 373 (37.0%) | 286 (28.4%) | <0.001 | 0.46 |
| Coronary artery disease | 61 (6.1%) | 74 (7.4%) | 88 (8.7%) | 110 (10.9%) | 92 (9.1%) | 0.001 | 0.46 |
| Atrial fibrillation | 158 (15.7%) | 175 (17.4%) | 218 (21.7%) | 211 (20.1%) | 227 (22.5%) | <0.001 | <0.001 |
| Stroke subtype | |||||||
| Large artery atherosclerosis | 378 (37.5%) | 397 (39.4%) | 390 (38.7%) | 405 (40.2%) | 395 (39.2%) | <0.001 | 0.01 |
| Small vessel occlusion | 165 (16.4%) | 180 (17.9%) | 198 (19.7%) | 197 (19.6%) | 155 (15.4%) | ||
| Cardioembolism | 201 (20.0%) | 190 (18.9%) | 215 (21.4%) | 211 (21.0%) | 218 (21.7%) | ||
| Undetermined | 200 (19.9%) | 223 (22.1%) | 190 (18.9%) | 181 (18.0%) | 227 (22.5%) | ||
| Other determined | 63 (6.3%) | 17 (1.7%) | 14 (1.4%) | 13 (1.3%) | 12 (1.2%) | ||
| Prior use of statin | 91 (9.0%) | 105 (10.4%) | 118 (11.7%) | 126 (12.5%) | 118 (11.7%) | 0.11 | 0.98 |
| Previous use of antiplatelet | 165 (16.4%) | 178 (17.7%) | 222 (22.1%) | 225 (22.3%) | 259 (25.7%) | <0.001 | 0.30 |
| Thrombolysis | 200 (19.9%) | 191 (19.0%) | 175 (17.4%) | 177 (17.6%) | 167 (16.6%) | 0.32 | 0.006 |
| Admission NIHSS score | 3 (1–6) | 3 (1–7) | 3 (2–8) | 4 (2–7) | 4 (2–8) | <0.001b | NA |
| Infarct volume, % of brain | 0.26 (0.05–1.14) | 0.16 (0.04–0.96) | 0.14 (0.04–0.88) | 0.16 (0.04–0.94) | 0.16 (0.04–0.93) | 0.23b | NA |
| Body mass index, kg/m2 | 23.9 (3.0) | 23.9 (3.3) | 23.8 (3.3) | 23.4 (3.2) | 23.3 (3.5) | <0.001 | 0.006 |
| Haemoglobin, g/dl | 14.1 (2.0) | 13.9 (1.9) | 13.5 (2.0) | 13.3 (2.0) | 13.2 (1.8) | 0.03 | 0.15 |
| Fasting glucose, mmol/l | 6.4 (2.9) | 6.7 (2.7) | 6.9 (3.0) | 6.8 (2.8) | 6.7 (3.1) | 0.001 | 0.02 |
| Total cholesterol, mmol/l | 4.7 (1.1) | 4.8 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.0) | 0.23 | 0.38 |
NA = not available. Data are mean (SD), number (percentage), or median (IQR). Some data were missing for body mass index (3.5%), haemoglobin (0.2%), fasting glucose (5.7%), total cholesterol (2.2%), and infarct volume on diffusion-weighted MRI (5.3%).
aP-values by ANOVA or χ2 test, unless otherwise indicated.
bKruskal–Wallis test.
cAdjusted for age and sex.
Association between WMH quintiles and 3-month modified Rankin Scale for the entire study population
The Cochran-Mantel-Haenszel test with adjustment for age, admission NIHSS score, sex, and thrombolysis showed a significant difference between the distribution of 3-month modified Rankin Scale scores in each of the second to fifth WMH quintiles and that in the first WMH quintile (all P < 0.05, Table 2). In addition, the distribution of 3-month modified Rankin Scale scores was significantly different across the quintiles of WMH (Cochran-Mantel-Haenszel P < 0.001 for the overall effect of WMH quintiles on 3-month modified Rankin Scale scores, Table 2).
Table 2.
Bivariable and multivariable analyses between quintiles of WHM volume and 3-month modified Rankin Scale score after first-ever acute ischaemic stroke
| Modified Rankin Scale score at 3 months | Cochran-Mantel- Haenszela | Ordinal logistic regression modelb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | P-value | Crude OR (95% CI) | Adjustedc OR (95% CI) | |
| First quintile | 37% | 29% | 13% | 8% | 6% | 2% | 5% | Reference | Reference | Reference |
| Second quintile | 29% | 28% | 16% | 10% | 7% | 4% | 6% | 0.04 | 1.42 (1.21–1.66) | 1.29 (1.10–1.52) |
| Third quintile | 24% | 28% | 15% | 12% | 10% | 5% | 6% | 0.001 | 1.81 (1.55–2.12) | 1.40 (1.18–1.66) |
| Fourth quintile | 21% | 23% | 18% | 15% | 9% | 7% | 7% | <0.001 | 2.26 (1.93–2.64) | 1.69 (1.42–2.02) |
| Fifth quintile | 18% | 21% | 17% | 16% | 12% | 7% | 9% | <0.001 | 2.96 (2.53–3.46) | 2.03 (1.69–2.43) |
aCochran-Mantel-Haenszel test with adjustment for age, sex, admission NIHSS score, and thrombolysis. P-value for the overall effect of WMH quintiles on the distributions of modified Rankin Scale scores was < 0.001.
bOrdinal logistic regression analysis using 3-month modified Rankin Scale scores for the imputed dataset (n = 5035).
cAdjusted for age, admission NIHSS score, sex, body mass index, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, prior use of statin, thrombolysis, haemoglobin, total cholesterol, fasting glucose, and log-transformed infarct volume (on diffusion-weighted MRI).
Multiple ordinal logistic regression analysis showed that the quintiles of WMHs were independently associated with 3-month modified Rankin Scale score after adjusting for age, admission NIHSS score, sex, body mass index, hypertension, diabetes, hyperlipidaemia, smoking, atrial fibrillation, coronary artery disease, prior use of statin, thrombolysis, serum haemoglobin, serum cholesterol, fasting glucose, and log-transformed diffusion-weighted MRI infarct volume, all of which were P < 0.2 in each of the bivariable analyses (Table 2 and Supplementary Tables 3). In the adjusted model, compared with the first quintile of WMH volume as a reference, ORs (95% CI) of higher modified Rankin Scale score for the second to fifth quintiles of WMH were 1.29 (1.10–1.52), 1.40 (1.18–1.66), 1.69 (1.42–2.02) and 2.03 (1.69–2.43), respectively. The use of a generalized estimating equation produced similar results in terms of the prediction of modified Rankin Scale score 0–2 versus 3–6 (favourable versus unfavourable outcome) by WMH quintiles (Supplementary Table 4).
Association between WMH quintiles and 3-month modified Rankin Scale for each stroke subtype
In large artery atherosclerosis stroke, the Cochran-Mantel-Haenszel test showed significant differences in the distributions of 3-month modified Rankin Scale scores for the third to fifth WMH quintiles (versus the first quintile, all P < 0.05; Table 3). The overall effect of WMH quintiles on the distributions of 3-month modified Rankin Scale scores was also significant (Cochran-Mantel-Haenszel test P = 0.01 for the overall effect of WMH quintiles; Table 3). The adjusted OR (95% CI; P-value) for the second to fifth quintiles versus the first quintile by multiple ordinal logistic regression analysis were 1.26 (0.97–1.64; 0.08), 1.45 (1.10–1.90; 0.008), 1.86 (1.41–2.47;< 0.001), and 1.89 (1.41–2.54; <0.001), respectively (P-value for trend <0.001), suggesting a dose-response relationship between the WMH quintiles and 3-month modified Rankin Scale scores (Table 3). This dose-response relationship remained significant after further adjustment for the severity of symptomatic intracranial or extracranial artery stenosis (P-value for trend <0.001, Supplementary Table 5).
Table 3.
Multivariable analysis between quintiles of WMH volume and 3-month modified Rankin Scale score after stratification by stroke subtype
| Large artery atherosclerosis | Small vessel occlusion | Cardioembolism | ||||
|---|---|---|---|---|---|---|
| (n = 1965) | (n = 895) | (n = 1035) | ||||
| OR (95% CI)a | CMH P-valueb | OR (95% CI)a | CMH P-valueb | OR (95% CI)a | CMH P-valueb | |
| First quintile | Reference | Reference | Reference | |||
| Second quintile | 1.26 (0.97–1.64) | 0.95 | 1.17 (0.77–1.76) | 1.00 | 1.21 (0.83–1.75) | 0.62 |
| Third quintile | 1.45 (1.10–1.90) | 0.04 | 1.46 (0.98–2.19) | 0.95 | 1.19 (0.81–1.73) | 0.71 |
| Fourth quintile | 1.86 (1.41–2.47) | 0.01 | 1.37 (0.89–2.13) | 0.03 | 1.23 (0.84–1.82) | 0.44 |
| Fifth quintile | 1.89 (1.41–2.54) | 0.004 | 1.98 (1.23–3.18) | 0.74 | 1.62 (1.09–2.40) | 0.92 |
| P-value | <0.001c | 0.01d | 0.17c | 0.10d | 0.47c | 0.19d |
Data for patients with undetermined (n = 1021) or other determined (n = 119) strokes are not shown.
aOrdinal logistic regression analysis using 3-month modified Rankin Scale scores for the imputed dataset, after adjustment for age, admission NIHSS score, sex, body mass index, hypertension, diabetes, hyperlipidaemia, smoking, coronary artery disease, atrial fibrillation, prior use of statin, thrombolysis, haemoglobin, total cholesterol, fasting glucose, and log-transformed infarct volume on diffusion-weighted MRI.
bCochran-Mantel-Haenszel (CMH) test with adjustment for age, sex, admission NIHSS score, and thrombolysis.
cχ2 trend test across quintiles.
dP-value from the Cochran-Mantel-Haenszel test for the overall effect of WMH quintiles on the distributions of modified Rankin Scale scores.
In small vessel occlusion stroke, the Cochran-Mantel-Haenszel test showed a significant difference in the distributions of 3-month modified Rankin Scale scores only for the fourth WMH quintile (versus the first quintile, P = 0.03; Table 3), and the overall effect of WMH quintiles on the distributions of 3-month modified Rankin Scale scores did not reach a statistical significance (P = 0.10; Table 3). Multiple ordinal logistic regression analysis showed that, when compared with large artery atherosclerosis stroke, a similar, but weaker, dose-response relationship tended to be observed in small vessel occlusion stroke also (Table 3). There also seemed to be a threshold effect, because the association between the highest WMH quintile (relative to the first quintile) and higher 3-month modified Rankin Scale scores was solely significant and relatively strong (adjusted OR 1.98, 95% CI 1.23–3.18, P = 0.005), while the third and fourth quintiles showed only a trend toward a relatively weak association with the worse functional outcome (1.46, 0.98–2.19, P = 0.07 for the third quintile; 1.37, 0.89–2.13, P = 0.16 for the fourth quintile).
In cardioembolism stroke, the Cochran-Mantel-Haenszel test did not show a significant difference in the distributions of 3-month modified Rankin Scale scores for any of the second to fifth WMH quintiles (versus the first quintile; Table 3). The overall effect of WMH quintiles on the distributions of 3-month modified Rankin Scale scores was not significant either (Table 3). Ordinal logistic regression analysis showed that a threshold effect but no dose-response relationship was observed; however, the association between the highest quintile and higher modified Rankin Scale scores was relatively weak (adjusted OR 1.62, 95% CI 1.09–2.40, P = 0.016; Table 3).
Association between WMH volume and the initial neurological severity at admission
In the entire study population, WMH volume was not associated with the admission NIHSS score by multiple linear regression analysis (Table 4 and Supplementary Table 6). In small vessel occlusion stroke, however, increased WMH volume was independently associated with the admission NIHSS score (coefficient 0.251, 95% CI 0.060–0.441, P = 0.01) after being adjusted for covariates (Table 4), but this was not true for other stroke subtypes. In addition, age was independently related to the admission NIHSS score in all subtypes other than small vessel occlusion.
Table 4.
Multiple linear regression analysis between admission NIHSS score and WMH volume, with/without stratification by stroke subtype
| All patients | Large artery atherosclerosis | Small vessel occlusion | Cardioembolism | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 5035) | (n = 1965) | (n = 895) | (n = 1035) | |||||
| Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||
| Log-transformed WMH | 0.050 | 0.54 | −0.019 | 0.88 | 0.251 | 0.01 | −0.208 | 0.35 |
| (-0.109–0.210) | (−0.258–0.220) | (0.060–0.441) | (−0.644–0.229) | |||||
| Age | 0.048 | <0.001 | 0.056 | <0.001 | 0.013 | 0.07 | 0.081 | <0.001 |
| (0.037–0.060) | (0.037–0.075) | (−0.001–0.027) | (0.046–0.117) | |||||
| Log-transformed infarct volume | 1.759 | <0.001 | 1.519 | <0.001 | 0.738 | <0.001 | 2.079 | <0.001 |
| (1.673–1.846) | (1.382–1.657) | (0.457–1.012) | (1.867–2.292) | |||||
Coefficient (95% CI) were derived from imputed dataset (n = 5035). Results are from multiple linear regression analysis using the NIHSS score as a dependent variable. Data for patients with undetermined (n = 1021) or other determined (n = 119) strokes are not shown. WMH volume and infarct volume (on diffusion-weighted MRI) were transformed into a logarithmic scale. Covariates with P < 0.2 in the simple linear regression analysis for the entire study population (age, sex, hypertension, smoking, coronary artery disease, atrial fibrillation, previous use of antiplatelet, thrombolysis, log-transformed WMH volume, log-transformed infarct volume, body mass index, haemoglobin, fasting glucose, and total cholesterol) were entered into the multivariable model (Supplementary Table 6).
Association between WMH quintiles and the incidence of early neurological deterioration
About 17% of the entire study population (831/5035) had early neurological deterioration (mostly progression of ischaemia); 83% or 74% of early neurological deterioration occurred within 3 days after admission or symptom onset, respectively. The upper three WMH quintiles (relative to the lowest quintile) were more frequently involved (Table 5). In large artery atherosclerosis stroke, early neurological deterioration was more frequent in the top two WMH quintiles compared with the lowest quintile; the adjusted ORs were 1.99 (95% CI 1.31–3.01) and 1.81 (1.17–2.79) for the fourth and fifth quintiles (P for trend = 0.02) by multiple binary logistic regression analyses, respectively. In other subtypes, WMH volume quintiles were not associated with early neurological deterioration. If we restrict the definition of early neurological deterioration to what occurred within 72 h after admission, early neurological deterioration occurred in 13.7% (690/5035) of patients, and the results are materially unchanged (Supplementary Table 7).
Table 5.
Multivariable analysis between quintiles of WMH volume and early (onset to 3 weeks) neurological deterioration with/without stratification by stroke subtype
| All patients | Large artery atherosclerosis | Small vessel occlusion | Cardioembolism | |
|---|---|---|---|---|
| (n = 5035) | (n = 1965) | (n = 895) | (n = 1035) | |
| Early neurological deterioration, no | 4204 (83.5%) | 1584 (80.6%) | 800 (89.4%) | 843 (81.5%) |
| Early neurological deterioration, yes | 831 (16.5%) | 381 (19.4%) | 95 (10.6%) | 192 (18.6%) |
| Progression | 606 (12.0%) | 289 (14.7%) | 74 (8.3%) | 128 (12.4%) |
| Recurrence | 69 (1.4%) | 33 (1.7%) | 1 (0.1%) | 21 (2.0%) |
| Haemorrhagic transformation | 50 (1.0%) | 11 (0.6%) | 1 (0.1%) | 26 (2.5%) |
| Unknown | 85 (1.7%) | 41 (2.1%) | 19 (2.1%) | 10 (1.0%) |
| Others | 21 (0.4%) | 7 (0.4%) | 0 | 7 (0.7%) |
| WMH quintile | Adjusted OR of early neurological deterioration (95% CI) | |||
| First quintile | Reference | Reference | Reference | Reference |
| Second quintile | 1.12 (0.86–1.46) | 1.16 (0.77–1.73) | 1.10 (0.47–2.58) | 0.80 (0.45–1.43) |
| Third quintile | 1.32 (1.01–1.73) | 1.45 (0.96–2.19) | 1.68 (0.75–3.72) | 0.99 (0.56–1.74) |
| Fourth quintile | 1.49 (1.13–1.96) | 1.99 (1.31–3.01) | 1.79 (0.78–4.09) | 0.87 (0.49–1.57) |
| Fifth quintile | 1.47 (1.11–1.95) | 1.81 (1.17–2.79) | 2.05 (0.86–4.89) | 0.91 (0.51–1.62) |
| P* for trend | 0.04 | 0.02 | 0.60 | 0.97 |
ORs (95% CI) were derived from imputed dataset (n = 5035). Data for patients with undetermined (n = 1021) or other determined (n = 119) strokes are not shown. Binary logistic regression analysis was used with early neurological deterioration (categorical yes/no) as a dependent variable. ORs were adjusted for age, admission NIHSS score, sex, body mass index, hypertension, diabetes, hyperlipidemia, smoking, coronary artery disease, atrial fibrillation, prior use of statin, haemoglobin, total cholesterol, fasting glucose, and log-transformed infarct volume (on diffusion-weighted MRI). *χ2 trend test across quintiles.
A total of 910 (18.1%) patients received recanalization therapy. Among them, 566 received intravenous tissue plasminogen activator therapy only, 136 underwent intra-arterial interventions only, and 208 received combined therapy. Symptomatic haemorrhagic transformation occurred in 37 (4.8%) patients among those who received tissue plasminogen activator therapy (n = 774). As shown in the Supplementary Table 8, WMHs were not significantly associated with symptomatic haemorrhagic transformation after intravenous tissue plasminogen activator therapy with or without intra-arterial intervention. However, it is notable that haemorrhagic transformation in the tissue plasminogen activator-only group tended to be less frequent in the lowest quintile (0.9%) compared with the upper four quintiles (3.4∼8.6%; P = 0.08).
Association between WMH quintiles and early or late stroke recurrence
During the 3-month observation period after a first-ever ischaemic stroke, recurrent stroke was observed in 3.3% of the entire study population (167/5035). The subtype of recurrent stroke was the same as the subtype of the index stroke in about half the cases (Supplementary Table 9). WMH quintiles were not associated with stroke recurrence (P = 0.56, Supplementary Table 9). In patients with large artery atherosclerosis, small vessel occlusion, and cardioembolism strokes, the recurrence rate was 4.1%, 0.9%, and 3.8%, respectively. In all the subtypes, WMH quintiles were not significantly associated with early or late stroke recurrence.
WMH quintiles and functional recovery from discharge to 3-months
About 43% of the study subjects with a modified Rankin Scale score of 1 or more at discharge (1874/4337), when examined again at 3 months, were found to have a further improvement of modified Rankin Scale score compared with the level at discharge [median (IQR), 1 (0–2) versus 2 (2–3)]. The other 57% (2463/4337) had the same or higher 3-month modified Rankin Scale score [3 (1–4)], compared with the level at discharge [2 (1–4)]. As WMH quintile increased, the number of patients with modified Rankin Scale improvements decreased in a dose-dependent manner (53.3%, 46.6%, 42.2%, 40.1%, and 34.6%, respectively; P for trend < 0.001). Using binary logistic regression analysis, compared with the first WMH quintile as a reference, adjusted OR of the modified Rankin Scale improvement was 0.81 (95% CI, 0.66–1.00), 0.81 (0.65–1.00) and 0.67 (0.54–0.84) for the third, fourth and fifth quintiles, respectively (Table 6). This relationship was pronounced in large artery atherosclerosis stroke, where compared with the lowest WMH quintile, the fourth and fifth quintiles showed a significant negative association with modified Rankin Scale improvement (adjusted ORs 0.66, 95% CI 0.47–0.94 and 0.62, 0.43–0.89, respectively, P for trend = 0.03). In small vessel occlusion and cardioembolism strokes, WMH quintiles were not significantly associated with the modified Rankin Scale improvement.
Table 6.
The association between WMH volume quintiles and modified Rankin Scale improvements from discharge to 3 months after stroke after excluding patients with discharge modified Rankin Scale of 0
| All | Large artery atherosclerosis | Small vessel occlusion | Cardioembolism | |
|---|---|---|---|---|
| (n = 4337) | (n = 1711) | (n = 741) | (n = 906) | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| No. of patients with improvement | 1874 (43.2%) | 794 (46.4%) | 371 (50.1%) | 320 (35.3%) |
| First quintile | Reference | Reference | Reference | Reference |
| Second quintile | 0.86 (0.70–1.05) | 0.75 (0.54–1.03) | 0.74 (0.45–1.24) | 1.08 (0.67–1.72) |
| Third quintile | 0.81 (0.66–1.00) | 0.76 (0.54–1.07) | 0.91 (0.55–1.50) | 0.68 (0.41–1.11) |
| Fourth quintile | 0.81 (0.65–1.00) | 0.66 (0.47–0.94) | 1.27 (0.75–2.15) | 0.91 (0.55–1.48) |
| Fifth quintile | 0.67 (0.54–0.84) | 0.62 (0.43–0.89) | 1.13 (0.64–2.00) | 0.73 (0.44–1.21) |
| P* for trend | 0.03 | 0.03 | 0.13 | 0.13 |
Data are presented as number (percentage) or adjusted OR (95% CI). Results are from binary logistic regression analysis using the improvement of modified Rankin Scale score from discharge to 3 months (categorical yes/no) as a dependent variable. Patient’s modified Rankin Scale score was defined as improved if his/her 3-month modified Rankin Scale score was lower than discharge modified Rankin Scale score. At discharge, 698 patients (large artery atherosclerosis 254, small vessel occlusion 154, cardioembolism 129, undetermined 134, and other determined 27) had modified Rankin Scale score of 0, and these patients were excluded from the analysis. ORs were adjusted for age, sex, body mass index, admission NIHSS score, coronary artery disease, hypertension, diabetes, hyperlipidaemia, smoking, atrial fibrillation, prior use of statin, haemoglobin, total cholesterol, fasting glucose, log-transformed infarct volume on diffusion-weighted MRI, and early neurological deterioration. *χ2 trend test across quintiles.
Early neurological deterioration-adjusted 3-month modified Rankin Scale score
Time-to-discharge differed depending on the stroke subtypes as expected. Compared to small vessel occlusion stroke [median (IQR) 5.6 (4.3–8.0) days], large artery atherosclerosis [7.3 (5.3–10.7) days] and cardioembolism [8.6 (6.0–13.9) days] strokes had longer hospital stay (Supplementary Table 10). Because we captured early neurological deterioration in 3 weeks after the admission, we analysed further using ordinal logistic regression analysis to reassess the association between WMH quintiles and 3-month modified Rankin Scale scores in each group of stroke subtypes after additionally adjusting for early neurological deterioration (Supplementary Table 11). In large artery atherosclerosis stroke, compared with the lowest quintile, the top two WMH quintiles were again significantly associated with higher 3-month modified Rankin Scale scores (adjusted OR 1.66, 95% CI 1.26–2.21, P < 0.001 for the fourth quintile; adjusted OR 1.75, 1.30–2.34, P < 0.001 for the fifth quintile). In small vessel occlusion stroke, the additional adjustment for early neurological deterioration revealed that the highest WMH quintile relative to the lowest quintile had a significant and strong relationship with 3-month modified Rankin Scale scores (adjusted OR 1.84, 1.14–2.96; P = 0.012). In cardioembolism stroke, the fifth quintile of WMHs remained to be related to 3-month modified Rankin Scale scores (adjusted OR 1.68, 1.13–2.50; P = 0.011).
Discussion
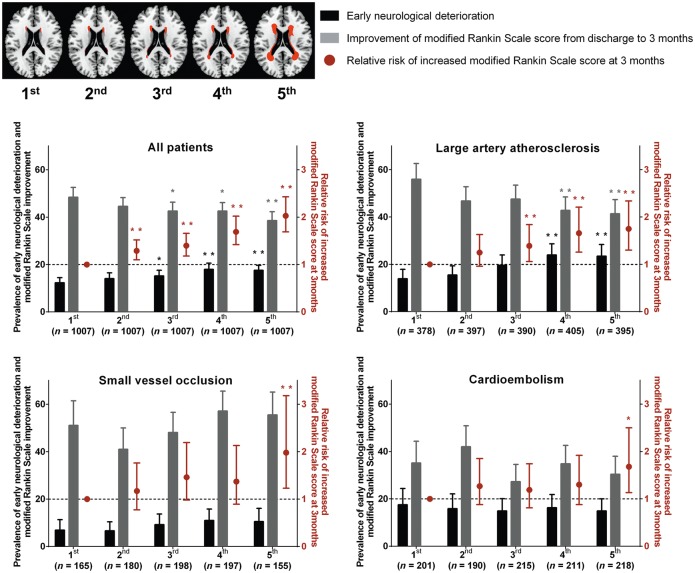
Our study reveals that high WMH burden substantially impacts stroke outcomes, and that the impact may be different depending on stroke subtype. We show convincing evidence that higher WMH quintiles are associated in a dose-dependent manner with worse outcomes and higher modified Rankin Scale scores at 3 months, using data from a prospective multi-centre quantitative MRI study on 5035 first-ever ischaemic stroke patients, even after adjusting for covariates in multivariable analyses. These results held true for the entire study population but the stroke subtype that was affected the most by increased WMH seemed to be large artery atherosclerosis stroke, where higher WMH volumes (the upper three quintiles) were associated with higher 3-month modified Rankin Scale scores, probably due to more frequent early neurological deterioration and worse late recovery. In small vessel occlusion stroke, higher WMH volumes (the highest quintile) were associated with higher 3-month modified Rankin Scale scores, probably due to more severe initial neurological severity and worse late recovery. In cardioembolism stroke, higher WMH volumes (the highest quintile) were weakly associated with higher 3-month modified Rankin Scale scores, to which worse late recovery seemed to have contributed. Regardless of stroke subtype, WMH quintiles were not associated with stroke recurrence during the 3 months after the onset of index stroke. These results are graphically summarized in Fig. 1.
Figure 1.
Graphical summary of the major results of this study. Predicted prevalence of early neurological deterioration/post-discharge improvement of modified Rankin Scale score and relative risk of increased modified Rankin Scale score at 3 months: the impact of WMH volume, generally and by stroke subtype. Top left: Topographical frequency-volume maps that were generated by using the quantitative magnetic resonance data of the 5035 patients of this study, as previously reported in our study (n = 2699 patients with first-ever acute ischaemic stroke) using the Kim statistical WMH scoring system: a graphical reference system allowing the quantitative estimation of the severity of WMHs as a percentile rank score (Ryu et al., 2014). First to fifth (WMH volume quintile) images correspond to 10, 30, 50, 70 and 90 percentile maps, respectively. Predicted prevalence of early neurological deterioration and improvement of modified Rankin Scale score (from discharge to 3 months) were derived from multiple logistic regression analysis with adjustment for age, NIHSS score, sex, body mass index, hypertension, diabetes, hyperlipidemia, smoking, coronary artery disease, atrial fibrillation, prior use of statin, haemoglobin, total cholesterol, fasting glucose, and log-transformed infarct volume on diffusion-weighted image. Black or grey bars represent predicted prevalence with 95% CI (left y-axis). Red dots and lines indicate adjusted ORs with 95% CI for the relative risk of increased modified Rankin Scale score at 3 months by ordinal logistic regression analysis. *P < 0.05 and **P < 0.01 compared with the first quintile of WMHs.
About 17% of the study population had early neurological deterioration, and a high WMH burden (fourth and fifth WMH quintiles) in large artery atherosclerosis stroke was associated with about a 90% higher risk of having early neurological deterioration compared with the first quintile. Given that the majority of the early neurological deterioration was due to stroke progression, this finding is in line with a previous study reporting that WMH was a predictor of infarct growth (Ay et al., 2008), which could be facilitated by the reduction of vascular density and reduced cerebral blood flow that is known to be associated with advanced WMH (Debette and Markus, 2010).
Microcirculatory dysfunction contributes to the aggravation of WMH (Wardlaw et al., 2013). An animal study also demonstrated that microcirculatory failure contributed to white matter damage in a transgenic mouse model for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (Joutel et al., 2010), where WMH is the earliest and consistent MRI change preceding the onset of ischaemic and cognitive symptoms (Lambert et al., 2016). In our study, the association between WMH burden and poor outcome may be partly attributable to WMH-related microcirculatory alterations potentially causing: pericyte-mediated capillary no-reflow and blood–brain barrier damage (Hall et al., 2014), impairment in the maintenance or enhancement of cerebral blood flow/collateral flow (Bisschops et al., 2003), or decline in cerebrovascular angiogenesis (Jickling et al., 2009).
Previous studies have shown the association between WMH and haemorrhagic transformation after tissue plasminogen activator-mediated thrombolysis (Neumann-Haefelin et al., 2006; Willer et al., 2015), albeit one study did not support the presence of the association (Aries et al., 2010). A similar association was not clearly observed in our study, where unlike in the other studies WMH volumes were quantified and stratified into quintiles. Currently, it is not recommended to use WMHs in the risk assessment of thrombolysis (Karaszewski et al., 2015). We suggest that further prospective studies are required to clarify this issue, particularly when considering the relatively infrequent haemorrhagic transformation in patients with the lowest WMH quintile versus upper four quintiles after intravenous tissue plasminogen activator therapy without intra-arterial intervention.
A recent independent single-centre study showed that patients with severe WMHs are at higher risk of early stroke recurrence (<90 days) in large artery atherosclerosis but not in the cardioembolism and small vessel occlusion subtypes (Kim et al., 2014b). In addition, severe WMH was shown to increase the risk of symptomatic or asymptomatic acute infarction in patients undergoing carotid stenting as well as endarterectomy (Rostamzadeh et al., 2014). However, in the present multi-centre study, the severity of WMH was not related to stroke recurrence (<90 days) in patients with large artery atherosclerosis stroke as well as in those with other subtype strokes.
Our data suggest that high WMH burdens may affect late functional recovery after the acute large artery atherosclerosis stroke had been stabilized. Compared with the first WMH quintile, the fourth and fifth quintiles were associated with ∼35% lower chances of further modified Rankin Scale improvement after discharge until 3 months after stroke. When adjusted for early neurological deterioration, the top two quintiles were associated with about 1.7- and 1.8-fold higher chances of having a 1-point higher modified Rankin Scale score at 3 months compared with the first quintile. Peri-infarct areas that suffer white matter disease may have a relatively low potential for post-stroke functional recovery (Helenius and Henninger, 2015), possibly due to reduced spare capacity to compensate for ischaemic damage.
WMH could affect post-stroke outcome by disrupting motor/cognitive networks that are important for learning and neurorehabilitation (Valdes Hernandez Mdel et al., 2013). In a meta-analysis of 22 longitudinal studies, WMHs were clearly associated with progressive cognitive impairment, and a 2-fold increase in the risk of dementia (Wardlaw et al., 2015). WMH-related cognitive/executive dysfunction may impair not only motor learning but also active participation in rehabilitation and adherence to treatment guidelines, thus leading to poor functional recovery.
In small vessel occlusion stroke only, higher WMH volume was associated with a higher initial NIHSS score independently of age and infarct volume, indicating a relatively strong influence of WMH on the initial manifestation of this stroke subtype. These results are partly in conflict with a recent study comprised of 312 patients that demonstrated that advanced WMH was associated with higher NIHSS scores in non-single small subcortical infarcts (mainly large artery atherosclerosis and cardioembolism strokes) but not in single small subcortical infarcts (mainly small vessel occlusion stroke) (Helenius and Henninger, 2015). The discrepancy may be explained by: (i) the smaller study population; (ii) the qualitative versus quantitative measurement of WMH; and/or (iii) biological/ethnic differences in the patient populations. Compared with larger large artery atherosclerosis or cardioembolism infarcts, which frequently involve cortex as well as subcortex, a small lacunar infarct in the distal distribution of penetrating vessels that supply subcortical or periventricular areas will have a higher chance of the ‘entire’ lesion being located within or adjacent to the extensive subcortical or periventricular WMH. Recently, incident lacunes were shown to preferentially localize to the edge of WMH (Duering et al., 2013). The closer spatial relationship with WMH may contribute to turning lacunar infarcts that would otherwise remain asymptomatic or mildly symptomatic, rather than sizeable large artery atherosclerosis or cardioembolism infarcts, into symptomatic or more severely symptomatic stroke events, respectively. The capacity to withstand acute ischaemic injury may be largely dependent on the integrity of white matter tracts connecting different parts of the brain (Arsava et al., 2011). However, further investigation is required to confirm if higher WMH volume is related to the location of lacunar infarcts presenting with more severe clinical features at stroke onset.
In small vessel occlusion stroke, only the highest WMH quintile (relative to the lowest quintile) was independently associated with about 2-fold higher chance of having a 1-point higher modified Rankin Scale score at 3 months. A threshold effect is suspected, but further studies in larger numbers of patients will be required to assess this. Alternately, the distinctive feature associated with the highest quintile, i.e. the strongest and solely significant aggravation of 3-month modified Rankin Scale score by the fifth quintile relative to the first quintile, might suggest the existence of not only volumetric but also histopathological differences between ‘advanced’ WMH and ‘mild to moderate’ WMH. We hypothesize that advanced WMH will more likely contain not only a cavitated (distinct) type of silent lacunes but also non-cavitated (stealth) type of silent infarcts, which do not stand out from other WMH lesions on conventional brain MRI (Smith et al., 2012; van Veluw et al., 2013). A first-ever ischaemic stroke in patients with prior silent infarcts would predispose the patient to have relatively severe neurological deficits both initially and/or finally.
In cardioembolism stroke, the highest WMH quintile relative to the lowest quintile was associated with ∼1.6-fold higher chance of having a 1-point higher modified Rankin Scale score at 3 months. Cardioembolism stroke frequently manifests with relatively dense and severe neurological deficits (Hong et al., 2013b), not allowing WMHs to have any significant impact on functional outcomes. In addition, less frequent involvement of subcortical white matter in cardioembolism stroke than in large artery atherosclerosis stroke (Cho et al., 2010) could be another explanation for the differential impact. A wider area of dysfunctional peri-infarct brain tissue recruited during the process of post-stroke recovery may cause a poor functional outcome, thereby increasing the impact of WMH on 3-month modified Rankin Scale score in large artery atherosclerosis stroke than in cardioembolism stroke.
This study has several strengths and limitations. A large sample size allowed us to investigate the impact of quantitatively measured WMH volume on post-stroke outcomes with sufficient statistical power to allow stratification by stroke subtype. All data including outcomes were prospectively captured in every participating centre and audited weekly; early neurological deterioration and modified Rankin Scale score at 3 months were thus collected with a minimal loss of information using a well-structured protocol. The present nationwide study consecutively enrolled Korean stroke patients who underwent brain MRI. About 90% of all ischaemic stroke patients underwent brain MRI (Kim et al., 2014a), and there is a bias for critically ill patients who were unable to tolerate MRI to have been excluded from the study. Thus, the results of this study may not be directly generalizable to all stroke patients or other ethnic groups.
The sample size of the large artery atherosclerosis group is ∼2-fold higher than that of the small vessel occlusion and cardioembolism groups. Sample size can affect a confidence interval, and consequently the statistical significance, favouring the large artery atherosclerosis group in our study. However, considering the widths of the confidence intervals of odds ratios in Table 3 (i.e. the variability of the size of the effects of WMH volume quintiles on favourable versus unfavourable outcomes in each stroke subtype), it is estimated that the different sample sizes do not appear to have affected the study results by critically altering the statistical significance.
Common risk factors of WMH and large artery atherosclerosis may have confounded our results. In the present study, multivariable analyses were used to adjust post-stroke outcome results for a variety of vascular risk factors, many of which could be shared by WMH and large artery atherosclerosis. Previous studies showed that the severity of intracranial or extracranial artery stenosis was not associated with the severity of WMH (Potter et al., 2012; Schulz et al., 2013). Furthermore, we demonstrated that the association between WMH and early neurological deterioration or 3-month modified Rankin Scale score in large artery atherosclerosis stroke patients was independent of the presence versus absence of symptomatic arterial steno-occlusion.
In multivariable analysis, an additional adjustment for infarct location as well as for the infarct size and cardiovascular risk factors may need to be considered for a better estimation of WMH-related effects on post-stroke outcomes. Also, 3 months may not be sufficient to detect stroke recurrence and further studies are needed to explore the stroke subtype-dependent impact of WMH on longer-term outcomes.
Lastly, although the use of volumetric WMH data is scientifically preferable to simple rating scales, it may be less applicable to clinical practice. It would be useful to know whether the use of a simple white matter grading scale gives the same results as the volumetric data. Alternatively, one could use a graphical WMH grading (Kim statistical WMH scoring) system that we recently developed (Ryu et al., 2014), which allows for estimating volumetric WMH quintiles in individual patients, followed by predicting their post-stroke outcomes based on the results of the present study (Fig. 1).
Conclusion
In conclusion, this is a multi-centre quantitative brain MRI study to demonstrate that advanced WMH affects post-stroke outcomes both in the general stroke population, and with possibly differential impact in different stroke subtypes. We suggest that caregivers use the knowledge of WMH to provide better and more personalised care, by identifying those patients at higher risk for early neurological deterioration and by more clearly assessing the potential of functional recovery.
Funding
This study was supported by grants from ‘National Centre for Standard Reference Data, Ministry of Trade, Industry & Energy’, ‘Ministry of Health & Welfare (HI12C1847; Korea Healthcare Technology R&D Project)’, and ‘Global Research Lab (GRL) program (NRF-2015K1A1A2028228) of the National Research Foundation’, funded by the Korean government, Republic of Korea.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- NIHSS
National Institutes of Health Stroke Scale
- FLAIR
fluid-attenuated inversion recovery
- WMH
white matter hyperintensity
References
- Adams HP Jr Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- Aries MJ, Uyttenboogaart M, Vroomen PC, De Keyser J, Luijckx GJ. tPA treatment for acute ischaemic stroke in patients with leukoaraiosis. Eur J Neurol 2010; 17: 866–70. [DOI] [PubMed] [Google Scholar]
- Arsava EM, Bayrlee A, Vangel M, Rost NS, Rosand J, Furie KL, et al. Severity of leukoaraiosis determines clinical phenotype after brain infarction. Neurology 2011; 77: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009; 72: 1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008; 39: 1409–13. [DOI] [PubMed] [Google Scholar]
- Bath PM, Gray LJ, Collier T, Pocock S, Carpenter J. Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke 2007; 38: 1911–15. [DOI] [PubMed] [Google Scholar]
- Bisschops RH, Klijn CJ, Kappelle LJ, van Huffelen AC, van der Grond J. Collateral flow and ischemic brain lesions in patients with unilateral carotid artery occlusion. Neurology 2003; 60: 1435–41. [DOI] [PubMed] [Google Scholar]
- Brott TG, Haley EC Jr Levy DE, Barsan W, Broderick J, Sheppard GL, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 1992; 23: 632–40. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Yang JH, Jung YH, Kim YD, Choi HY, Nam HS, et al. Cortex-sparing infarctions in patients with occlusion of the middle cerebral artery. J Neurol Neurosurg Psychiatry 2010; 81: 859–63. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duering M, Csanadi E, Gesierich B, Jouvent E, Herve D, Seiler S, et al. Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease. Brain 2013; 136: 2717–26. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–6. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius J, Henninger N. Leukoaraiosis burden significantly modulates the association between infarct volume and national institutes of health stroke scale in ischemic stroke. Stroke 2015; 46: 1857–63. [DOI] [PubMed] [Google Scholar]
- Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis 2012; 33: 525–31. [DOI] [PubMed] [Google Scholar]
- Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the Korean stroke society and clinical research center for stroke. J Stroke 2013a; 15: 2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KS, Lee J, Bae HJ, Lee JS, Kang DW, Yu KH, et al. Greater stroke severity predominates over all other factors for the worse outcome of cardioembolic stroke. J Stroke Cerebrovasc Dis 2013b; 22: e373–80. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke 2015; 46: 687–91. [DOI] [PubMed] [Google Scholar]
- Jickling G, Salam A, Mohammad A, Hussain MS, Scozzafava J, Nasser AM, et al. Circulating endothelial progenitor cells and age-related white matter changes. Stroke 2009; 40: 3191–6. [DOI] [PubMed] [Google Scholar]
- Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 2010; 120: 433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaszewski B, Houlden H, Smith EE, Markus HS, Charidimou A, Levi C, et al. What causes intracerebral bleeding after thrombolysis for acute ischaemic stroke? Recent insights into mechanisms and potential biomarkers. J Neurol Neurosurg Psychiatry 2015; 86: 1127–36. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke 2014a; 9: 514–18. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Park JM, Kang K, Lee SJ, Ko Y, Kim JG, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke 2015; 17: 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DE, Park KJ, Schellingerhout D, Jeong SW, Ji MG, Choi WJ, et al. A new image-based stroke registry containing quantitative magnetic resonance imaging data. Cerebrovasc Dis 2011; 32: 567–76. [DOI] [PubMed] [Google Scholar]
- Kim GM, Park KY, Avery R, Helenius J, Rost N, Rosand J, et al. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke 2014b; 45: 479–85. [DOI] [PubMed] [Google Scholar]
- Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke 2009; 40: 530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y, Lee S, Chung JW, Han MK, Park JM, Kang K, et al. MRI-based algorithm for acute ischemic stroke subtype classification. J Stroke 2014; 16: 161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Benjamin P, Zeestraten E, Lawrence AJ, Barrick TR, Markus HS. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain 2016; 139 (Pt 4): 1136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavori PW, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Stat Med 1995; 14: 1913–25. [DOI] [PubMed] [Google Scholar]
- Leonards CO, Ipsen N, Malzahn U, Fiebach JB, Endres M, Ebinger M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke 2012; 43: 3046–51. [DOI] [PubMed] [Google Scholar]
- Liou LM, Chen CF, Guo YC, Cheng HL, Lee HL, Hsu JS, et al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis 2010; 29: 22–7. [DOI] [PubMed] [Google Scholar]
- McAlpine H, Churilov L, Mitchell P, Dowling R, Teo S, Yan B. Leukoaraiosis and early neurological recovery after intravenous thrombolysis. J Stroke Cerebrovasc Dis 2014; 23: 2431–6. [DOI] [PubMed] [Google Scholar]
- Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke 2010; 41: 2402–48. [DOI] [PubMed] [Google Scholar]
- Mishra NK, Lyden P, Grotta JC, Lees KR. Thrombolysis is associated with consistent functional improvement across baseline stroke severity: a comparison of outcomes in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 2010; 41: 2612–17. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke 2006; 37: 2463–6. [DOI] [PubMed] [Google Scholar]
- Potter GM, Doubal FN, Jackson CA, Sudlow CL, Dennis MS, Wardlaw JM. Lack of association of white matter lesions with ipsilateral carotid artery stenosis. Cerebrovasc Dis 2012; 33: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamzadeh A, Zumbrunn T, Jongen LM, Nederkoorn PJ, Macdonald S, Lyrer PA, et al. Predictors of acute and persisting ischemic brain lesions in patients randomized to carotid stenting or endarterectomy. Stroke 2014; 45: 591–4. [DOI] [PubMed] [Google Scholar]
- Ryu WS, Woo SH, Schellingerhout D, Chung MK, Kim CK, Jang MU, et al. Grading and interpretation of white matter hyperintensities using statistical maps. Stroke 2014; 45: 3567–75. [DOI] [PubMed] [Google Scholar]
- Schiemanck SK, Kwakkel G, Post MW, Kappelle LJ, Prevo AJ. Predicting long-term independency in activities of daily living after middle cerebral artery stroke: does information from MRI have added predictive value compared with clinical information? Stroke 2006; 37: 1050–4. [DOI] [PubMed] [Google Scholar]
- Schulz UG, Gruter BE, Briley D, Rothwell PM. Leukoaraiosis and increased cerebral susceptibility to ischemia: lack of confounding by carotid disease. J Am Heart Assoc 2013; 2: e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med 2007; 357: 562–71. [DOI] [PubMed] [Google Scholar]
- Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012; 11: 272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. 2nd edn. Cary, NC: SAS Institute; 2000. [Google Scholar]
- Valdes Hernandez Mdel C, Booth T, Murray C, Gow AJ, Penke L, Morris Z, et al. Brain white matter damage in aging and cognitive ability in youth and older age. Neurobiol Aging 2013; 34: 2740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, Spliet WG, Hendrikse J, Luijten PR, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab 2013; 33: 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015; 4: 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer L, Havsteen I, Ovesen C, Christensen AF, Christensen H. Computed tomography–verified leukoaraiosis is a risk factor for post-thrombolytic hemorrhage. J Stroke Cerebrovasc Dis 2015; 24: 1126–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.