Abstract
Autosomal dominant myopathy, Paget disease of bone, and dementia constitute a unique disorder (MIM 605382). Here we describe the clinical, biochemical, radiological, and pathological characteristics of 49 affected (23 male, 26 female) individuals from four unrelated United States families. Among these affected individuals 90% have myopathy, 43% have Paget disease of bone, and 37% have premature frontotemporal dementia. EMG shows myopathic changes and muscle biopsy reveals nonspecific myopathic changes or blue-rimmed vacuoles. After candidate loci were excluded, a genome-wide screen in the large Illinois family showed linkage to chromo-some 9 (maximum LOD score 3.64 with marker D9S301). Linkage analysis with a high density of chromosome 9 markers generated a maximum two-point LOD score of 9.29 for D9S1791, with a maximum multipoint LOD score of 12.24 between D9S304 and D9S1788. Subsequent evaluation of three additional families demonstrating similar clinical characteristics confirmed this locus, refined the critical region, and further delineated clinical features of this unique disorder. Hence, autosomal dominant inclusion body myopathy (HIBM), Paget disease of bone (PDB), and frontotemporal dementia (FTD) localizes to a 1.08–6.46 cM critical interval on 9p13.3–12 in the region of autosomal recessive IBM2.
Keywords: autosomal dominant, hereditary inclusion body myopathy, Paget disease of bone, fronto-temporal dementia, amyotropic lateral sclerosis, limb-girdle-muscular dystrophy, rimmed vacuoles, IBM2, chromosome 9p13.3–12
Autosomal dominant hereditary inclusion body myopathies (HIBMs) compose a clinically and genetically heterogeneous group of disorders characterized by late-onset weakness of the shoulder and pelvic girdle and normal to slightly elevated serum creatine kinase (CK) levels (1,2). EMG typically reveals myopathic abnormalities often associated with irritative changes (2). Myopathic changes include blue-rimmed vacuoles in the cytoplasm and occasionally in the nucleus.
Clinical and molecular studies have identified at least four loci for HIBM: autosomal dominant forms IBM1 (MIM 147420), IBM3 (MIM 605637), and a recently identified HIBM with distal weakness and early respiratory failure (3), and the autosomal recessive form, IBM2 (MIM 600737). IBM1 encompasses several reports of autosomal dominant inclusion body myopathy for which the chromosomal locus has not been identified (1,2). Dominant IBM3, with joint contractures and ophthalmoplegia, has been mapped in a large Swedish family to chromo-some 17p13.1, a region containing the myosin heavy-chain gene cluster (4). Subsequent studies of affected individuals in this family identified a mis-sense mutation in the motor domain of MYHC2A, the major myosin isoform found in the abnormal muscle fibers (5). Recessive IBM2 has been finely mapped to a region on chromosome 9p13–p12 (6,7). This region overlaps with the locus for Nonaka myopathy described in a Japanese family presenting with a recessive form of distal myopathy with rimmed vacuoles (DMRV [MIM 605820]), and it has been suggested that IBM2 and Nonaka myopathy are allelic variants (8).
Paget disease of bone (PDB) is a common disorder, seldom seen before the age of 50 years, characterized by abnormal, overactive osteoclasts that are unusually large, excessively multinucleated and often contain paramyxovirus-like inclusions (9). Despite frequent familial occurrences of PDB, genetic linkage to chromosome 18q has only recently been established for one extremely rare autosomal dominant variant of PDB (PDB2 [MIM 602080]) called familial expansile osteolysis (FEO) (10). Mutation in the TNFRSF11A gene, which encodes RANK (Receptor Activator of Nuclear Factor kappa-B), causes FEO(11). RANK is a transmembrane receptor essential for osteoclastogenesis, and is activated by its ligand, Osteoclast Differentiation Factor (ODF). More recently, in a linkage study of 24 large French Canadian families, Laurin et al. mapped two novel loci for autosomal dominant PDB at 5q31 and 5q35–qter(12).
Frontotemporal dementia (FTD) accounts for a substantial proportion of cases of primary degenerative dementia occurring before age 65 years (13). Disproportionately impaired executive or other frontal lobe functions, associated with changes in behavior and conduct, early in the course of the illness with relative sparing of memory and visuospatial abilities provide strong support for the diagnosis of FTD (14, 15). There is localized atrophy of the frontal and anterior temporal lobes. Perhaps 38–45% of all FTD cases have a strong hereditary component and 80% of these have an autosomal dominant inheritance (16). FTD is genetically heterogeneous, with three loci previously identified. A family in which affected members developed disinhibition-dementiaparkinsonism-amyotrophy complex was linked in 1994 to a locus on chromosome 17 (17). Subsequently, the disease in 8/25 families with similar features was found to map to chromosome 17q21–22 (MIM 600274) (18, 19). Sequencing of the tau gene in the critical region identified missense mutations and mutations in the 5′ splice site of exon 10 in families demonstrating considerable clinical variability (20–22). Brown et al. mapped a familial nonspecific dementia to a region of chromosome 3 in a large Danish family (23). Linkage to chromosome 9q21–q22 has also been established in combined autosomal dominant FTD and amyotrophic lateral sclerosis (ALS) (24) (MIM 105550), the gene for which is unknown.
We previously reported a large family (Fig. 1) presenting with the unique combination of myopathy and PDB (25) (MIM 605382). A series of muscle biopsies in one individual was indicative of HIBM. Nine other patients had nonspecific myopathic changes. Most affected individuals had associated PDB, and dementia of the frontotemporal type was present in two affected individuals. Loci for limb girdle muscular dystrophy (LGMD), PDB, and amyotropic lateral sclerosis were excluded (25). Additional members of this family as well as three additional families (Figs. 2, 3, and 4) with combinations of HIBM, PDB, and FTD were subsequently identified. Clinical delineation of this unique disorder in these four families, as well as molecular data supporting localization of the gene to chromosome 9p13.3–p12, a novel locus for PDB and FTD, is described in this report.
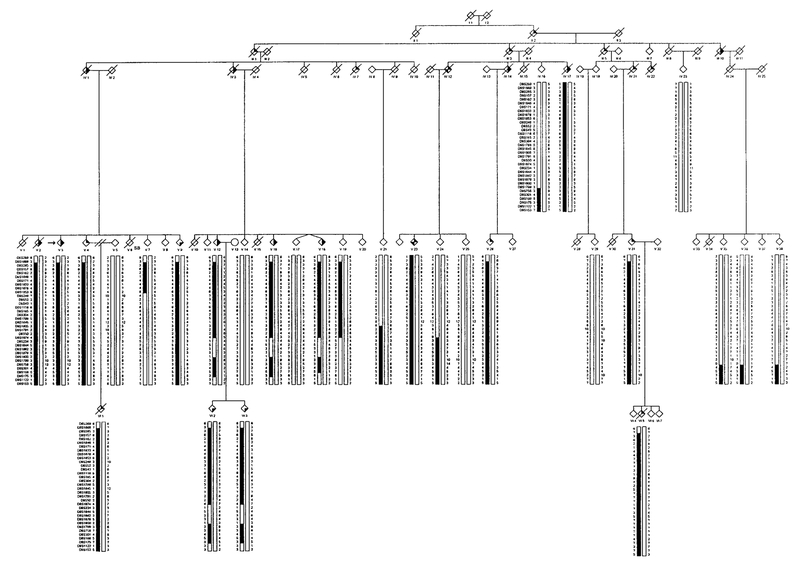
FIG. 1.
Partial pedigree of Family 1 affected with autosomal dominant HIBM and PDB showing genotype and haplotype analysis for markers on chromosome 9.  , IBM;
, IBM;  , PDB;
, PDB;  , dementia. The proband is indicated with an arrow on the left.
, dementia. The proband is indicated with an arrow on the left.
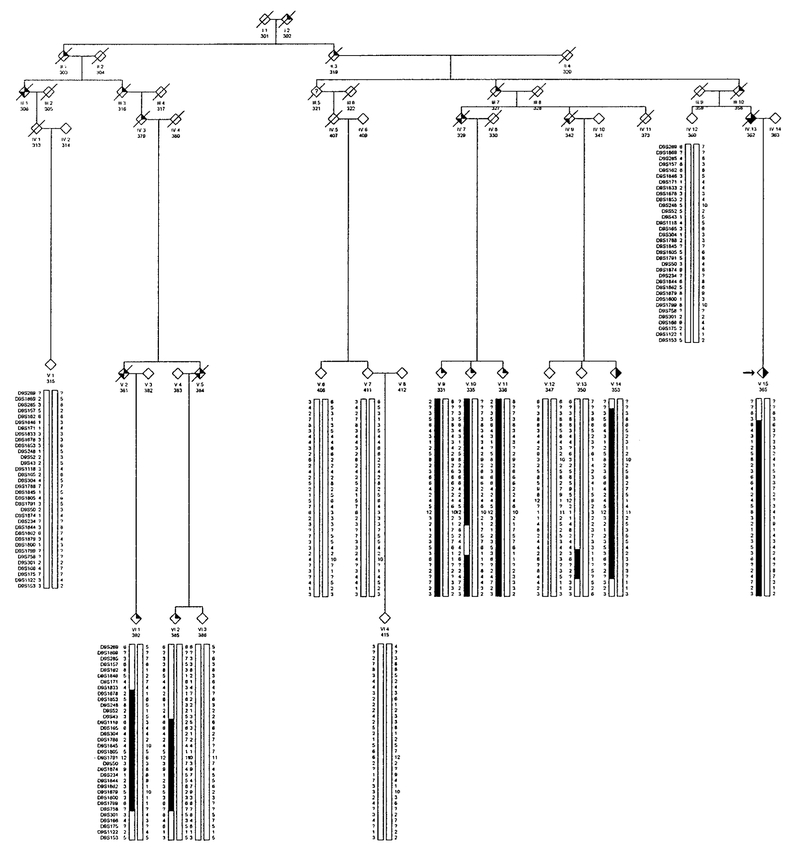
FIG. 2.
Partial pedigree of Family 2 affected with autosomal dominant HIBM and PDB showing genotype and haplotype analysis for markers on chromosome 9.  , IBM;
, IBM;  , PDB;
, PDB;  , dementia. The proband is indicated with an arrow on the left.
, dementia. The proband is indicated with an arrow on the left.
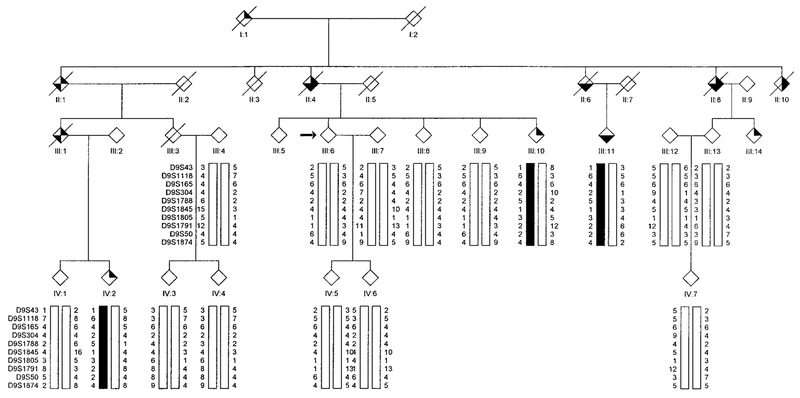
FIG. 3.
Partial pedigree of Family 3 affected with autosomal dominant HIBM and PDB showing genotype and haplotype analysis for markers on chromosome 9.  , IBM;
, IBM;  , PDB;
, PDB;  , dementia. The proband is indicated with an arrow on the left.
, dementia. The proband is indicated with an arrow on the left.
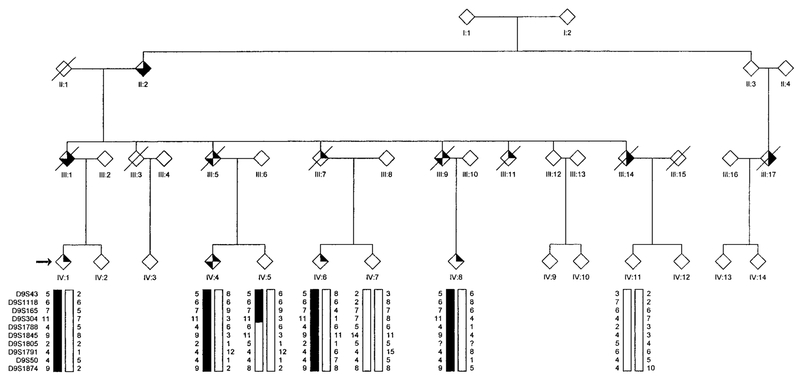
FIG. 4.
Partial pedigree of Family 4 affected with autosomal dominant HIBM and PDB showing genotype and haplotype analysis for markers on chromosome 9.  , IBM;
, IBM;  , PDB;
, PDB;  , dementia. The proband is indicated with an arrow on the left.
, dementia. The proband is indicated with an arrow on the left.
MATERIALS AND METHODS
Consent was obtained from each subject prior to participation. Clinical studies were approved by the Springfield Committee for Research Involving Human Subjects. Individuals included in the analysis were over age 18 years. Members of these families were examined for muscular weakness, PDB, and dementia through clinical, biochemical, and radiological studies. A diagnosis of myopathy was based on the presence of weakness on physical examination and, in some patients, EMG changes or biopsy findings suggestive of myopathy. Skeletal surveys including skull, spine, hips, long bones, hands and feet for PDB, measurements of alkaline phosphatase (ALP), and CK were performed at St. John’s Hospital, Springfield, Illinois. Quantification of bone-specific alkaline phosphatase (B-ALP) and osteocalcin levels in serum and pyridinoline/deoxypyridinoline collagen crosslinking metabolites in urine was performed by Quest Diagnostics (San Juan Capistrano, CA) and Emory University, (Atlanta, GA), respectively.
Several of the affected individuals underwent comprehensive neuropsychological assessments comprised of tests assessing language, visuospatial processing, episodic memory, working memory, executive control, and dementia severity (26). The diagnosis of dementia in deceased individuals was confirmed from their medical records.
DNA Marker Analysis
Peripheral venous blood was obtained by standard venipuncture. Genomic DNA was extracted from leukocytes by means of the PUREGENE DNA Isolation Kit (Gentra Systems; Minneapolis, MN). In cases where blood samples could not be obtained, buccal scrapings were collected and processed for DNA using standard methods. For individuals VI:1 and VI:5 of pedigree 1, archival histology specimens or autopsy tissue provided the source of DNA used in genetic analysis. Specifically, genomic DNA was extracted from 4-mm histology slides of the patient’s liver. A modified extraction protocol was developed, using a combination of published tissue extraction protocols (27–29).
Linkage Analysis
DNA from 39 individuals in Family 1 (9 affected, 24 unaffected, 6 spouses) was submitted for a genome-wide search to the National Heart, Lung, and Blood Institute Mammalian Genotyping Service (Marshfield, WI). A total of 402 microsatellite markers spanning the entire human genome were analyzed in this genome-wide screen. The power to detect linkage with the available pedigree was evaluated by simulating a marker locus with five alleles of equal frequency (heterozygosity = 0.8) completely linked to the disease locus (theta = 0.0) utilizing the SLINK program (30,31). All individuals included in the analysis were above 18 years of age. The disease was modeled as an autosomal dominant trait with age-dependent reduced penetrance. It was assumed that there were no phenocopies. The four independent risk classes were identified and the estimated penetrance values were as follows: I, 0–19 years old, 0.01; II, 20–29 years old, 0.10; III, 30–34 years old, 0.80; and IV, >35 years, 0.98. Because the trait is rare, a disease allele frequency of 0.00001 was used in the analysis. Two-point linkage analysis was carried out using the MLINK program of the FASTLINK computer package (32). Multipoint linkage analysis was carried out with SIMWALK2 (30,31). The marker-allele frequencies were estimated from the data by means of both observed and reconstructed genotypes of founders within the pedigrees. Linkage was considered established to a locus if a positive LOD score of 3 was obtained and was considered excluded if a LOD score of −2 was obtained.
RESULTS
Clinical Studies
Forty-nine affected individuals (23 males, 26 females), ranging in age from 10 years to 69 years, have been identified in the four autosomal dominant families (Tables 1 and 2). Of the affected individuals, 44/49 have myopathy, 21/49 have PDB, and 18/49 have dementia.
TABLE 1.
Clinical Data of Affected Individuals
| Pedigree No. |
Age (years) |
Deceased | Sex | PDB | IBM | FTD | Age DX PDB (years) |
Age DX IMB (years) |
Age DX FTD (years) |
|---|---|---|---|---|---|---|---|---|---|
| Family 1 (N = 18) | |||||||||
| IV:1 | 59 | + | M | + | + | − | − | 51 | − |
| IV:3 | 61 | + | M | + | + | − | 61 | 52 | − |
| IV:12 | 69 | + | F | − | + | + | − | 50 | 60 |
| IV: 17 | 64 | + | M | + | + | − | 37 | 40 | − |
| V:2 | 50 | + | M | + | + | − | 38 | 38 | − |
| V:3 | 48 | − | F | + | + | − | 34 | 44 | − |
| V:4 | 47 | − | M | − | + | − | − | 45 | − |
| V:9 | 40 | − | F | + | − | − | 39 | − | 39 |
| V:12 | 57 | − | M | + | + | − | 42 | 54 | − |
| V:16 | 49 | − | F | + | + | − | 44 | 48 | − |
| V:18 | 47 | − | F | + | + | − | 45 | 45 | − |
| V:23 | 45 | − | M | − | + | + | − | 44 | 42 |
| V:26 | 46 | − | M | − | + | − | − | 40 | − |
| V:31 | 56 | − | F | − | + | − | − | 49 | − |
| VI: 1 | 10 | + | M | − | + | − | − | 3 | − |
| VI:3 | 32 | − | F | + | − | − | 31 | − | − |
| VI:5 | 38 | + | M | − | + | − | − | 21 | − |
| VI:2 | 33 | − | M | + | − | − | 31 | − | − |
| Mean | 47.2 | 7 | 7F/11M | 11 | 15 | 2 | 40.2 | 40.8 | 49.5 |
| Family 2 (N = 11) | |||||||||
| IV:7 | 64 | + | F | − | + | + | − | 35 | 61 |
| IV:9 | 58 | + | F | − | + | + | − | 42 | 53 |
| IV:13 | 60 | + | M | + | + | + | 45 | 45 | 55 |
| V:5 | 59 | + | F | − | + | + | − | 45 | 51 |
| V:9 | 54 | − | M | − | + | − | − | 36 | − |
| V:10 | 51 | − | F | − | + | − | − | 36 | − |
| V:11 | 47 | − | M | − | + | − | − | 45 | − |
| V:14 | 47 | − | F | + | + | − | 46 | 44 | − |
| V.15 | 41 | − | M | + | + | − | 39 | 40 | − |
| VI:1 | 55 | − | F | − | + | − | − | 43 | − |
| VI:2 | 43 | − | F | − | + | − | − | 41 | − |
| Mean | 53.7 | 4 | 7F/4M | 3 | 11 | 4 | 43.3 | 41.1 | 55 |
| Family 3 (N = 10) | |||||||||
| II:1 | 64 | + | F | + | + | + | 55 | 40 | 58 |
| II:4 | 69 | + | M | + | + | + | 29 | 51 | 55 |
| II:6 | 57 | + | F | + | − | + | 41 | − | 50 |
| II:8 | 59 | + | M | + | + | + | 45 | 42 | 54 |
| II:10 | 63 | + | M | + | + | − | 49 | 57 | − |
| III:1 | 66 | + | F | − | + | + | − | 57 | 55 |
| II:10 | 49 | − | F | − | + | − | − | 49 | − |
| II:11 | 56 | − | F | + | − | + | 34 | 52 | |
| II:14 | 56 | − | F | − | + | + | − | 46 | 46 |
| IV:2 | 48 | − | F | − | + | − | − | 39 | − |
| Mean | 58.7 | 6 | 3M/7F | 6 | 8 | 7 | 42.2 | 47.6 | 52.9 |
| Family 4 (N = 10) | |||||||||
| III:1 | 63 | + | M | + | + | + | 50 | 40 | 55 |
| III:5 | 64 | + | M | − | + | + | − | 45 | 50 |
| III:7 | 56 | + | F | − | + | − | − | 52 | − |
| III:9 | 66 | + | M | − | + | + | − | 52 | 58 |
| III:11 | 59 | + | M | − | + | − | − | 45 | − |
| III:14 | 64 | + | F | − | + | + | 52 | 40 | 50 |
| IV: 1 | 56 | − | F | − | + | − | − | 40 | − |
| IV:4 | 69 | − | F | − | + | + | − | 66 | 62 |
| IV:6 | 60 | − | F | − | + | − | − | 55 | − |
| IV:8 | 55 | − | M | − | + | − | − | 41 | − |
| Mean | 61.2 | 6 | 5M/5F | 1 | 10 | 5 | 51 | 47.6 | 53.3 |
| All families | |||||||||
| Mean | 53.7 | 23 | 23M/26E | 21 | 44 | 18 | 42.2 | 44.0 | 53.7 |
TABLE 2.
Laboratory Data of Affected Individuals
| Case | AP | CK | PYR | DPYR | EMG | Radiography | Muscle biopsy |
|---|---|---|---|---|---|---|---|
| Family 1 | |||||||
| IV:1 | 431 | 84 | NA | NA | NA | NA | Myopathy, end stage |
| IV:3 | 1535 | NA | NA | NA | NA | Spine, pelvis | NA |
| IV:17 | 1508 | 220 | 15.6 | 3.2 | Myopathic; irritative | Widespread | Myopathy, vacuoles |
| V:2 | 240 | 82 | NA | NA | NA | NA | NA |
| V:3 | 1137 | 60 | 231.4 | 55.8 | NA | Spine, pelvis | NA |
| V:4 | 93 | 220 | 79.9 | 22.4 | Myopathic | — | Myopathy |
| V:9 | 203 | 102 | 95.5 | 30 | — | Spine | — |
| V:12 | 786 | 268 | 116.1 | 37.5 | Myopathic | Spine, pelvis, skull | Myopathy |
| V:16 | 354 | 98 | 79.4 | 15 | Myopathic | Spine, pelvis | Atrophic fibers |
| V:18 | 329 | NA | 25.2 | 5.6 | NA | Spine | NA |
| V:23 | 153 | 96 | 25.8 | 4.2 | Myopathic | — | NA |
| V:26 | 88 | 1145 | 57.1 | 11.2 | Myopathic | Early in pelvis | Myopathy, end stage |
| V:31 | 138 | 111 | 19.5 | 3.9 | Normal | NA | Myopathy |
| VI:1 | NA | NA | NA | NA | Normal | — | Myopathy |
| VI:2 | 1724 | 90 | 115.3 | 42.9 | Myopathic | Spine, pelvis, skull | NT |
| VI: 3 | 370 | 150 | 32.4 | 4.4 | — | Spine | — |
| VI: 5 | 308 | 115 | NA | NA | Myopathic; irritative | NA | Atrophic muscle fibers, angulated |
| Mean | 587.3 | 202.9 | 74.4 | 19.7 | |||
| Family 2 | |||||||
| IV:7 | NA | NA | NA | NA | Myotonic-type discharges | — | Myopathy |
| V:5 | 76 | 61 | NA | NA | Myopathic | — | Atrophic muscle fibers, angulated |
| V:9 | 99 | 40 | NA | NA | NA | — | NA |
| V:10 | 69 | 188 | 131.2 | 24.9 | Myo(.onic-type discharges | — | NA |
| V:11 | 85 | 255 | 80.6 | 13.4 | NA | — | NA |
| V:14 | 184 | 109 | 46.8 | 9.9 | NA | Skull | — |
| V:15 | 663 | 181 | 54.4 | 6.7 | Myopathic; irritative | Spine, pelvis, humerus, tibia | Myopathy; rimmed vacuoles |
| VI:1 | 98 | 315 | 53.6 | 16.7 | Myopathic | — | NA |
| VI: 2 | 117 | 126 | 20.9 | 4.9 | NA | — | NA |
| Mean | 182.3 | 164.1 | 73.3 | 14.2 | |||
| Family 3 | |||||||
| II:1 | NA | NA | NA | NA | NA | Widespread Paget | NA |
| II:6 | NA | NA | NA | NA | NA | Spine | NA |
| II:8 | NA | NA | NA | NA | NA | Skull, pelvis | NA |
| III:1 | NA | NA | NA | NA | D enervation | — | Myopathy; vacuoles |
| III:10 | 108 | 131 | NA | NA | Myopathy | — | NA |
| III:11 | 836 | 35 | NA | NA | NA | Paget | NA |
| III:14 | 98 | 136 | NA | NA | Myopathic | — | Myopathy; rimmed vacuoles |
| IV:2 | 63 | 160 | 40.9 | 6.5 | Myopathic; irrigative | — | NA |
| Mean | 276.3 | 115.5 | 40.9 | 6.5 | |||
| Family 4 | |||||||
| IV:1 | 167 | 40 | Myopathic; irritative | — | Myopathy; rimmed vacuoles | ||
| IV:4 | 90 | 101 | 139.9 | 26.7 | Myopathic; irritative | — | Myopathy; rimmed vacuoles |
| IV:6 | 85 | 89 | 35.4 | 5.5 | NT | — | NA |
| IV:8 | 225 | 99 | 66.2 | 14.0 | Myopathic; irritative | NA | NA |
| Mean | 141.8 | 82.3 | 80.5 | 15.4 | |||
| All families | |||||||
| Mean | 389.4 | 163.6 | 88.2 | 16.6 | |||
Note. AP, alkaline phosphatase, normal range, 30–130 IU/L; CK, creatine kinase, normal range, 20.0–222.0 U/L; PYR, pyridinoline, normal range, 23.2–43.2 μmol/mol; DYPR, normal range, 6.4–11 mmol/mol; EMG, electromyography. Myopathic: small amplitude, brief, polyphasic action potentials present. Irritative: spontaneous fibrillations or positive sharp waves present. NA, not available; NT, not tested.
Physical examination reveals proximal muscle weakness of the limb muscles with distal weakness or winging of the scapulae (Fig. 5) in some individuals. Affected individuals walk with an exaggerated lumbar lordosis and have difficulty lifting their arms above their head and going up stairs. Tendon reflexes are often diminished. Onset of weakness is usually in adulthood, at a mean age of 42.7 years (range 3–66 years), but varies widely, even within families. The pattern of weakness is often patchy and mildly asymmetric and may occur in proximal, distal, or facial muscles. Proximal weakness was present in all patients with myopathy. Mild distal weakness, affecting either foot dorsiflexion or small hand muscles, was found in several patients. Weakness was noted in some, but not other family members in muscles of the face, tongue, posterior neck, scapular fixators, and intrinsic muscles of the hands. The most common change on EMG was myopathy with brief small polyphasic action potentials found in all affected patients who were studied. Irritative features, fibrillations, and positive sharp waves were also seen in most patients. Nerve conduction studies were normal. Muscle biopsies reveal myopathic changes and/or rimmed vacuoles. CK levels were not very helpful in screening for myopathy, as they were abnormal in only 4 individuals [mean 163.6 U/L, range 35–1145 U/L (normal 20–222 U/L)]. Nerve conduction studies were normal. The muscle disease is progressive, causing death at a mean of 58 years due to respiratory and cardiac failure.
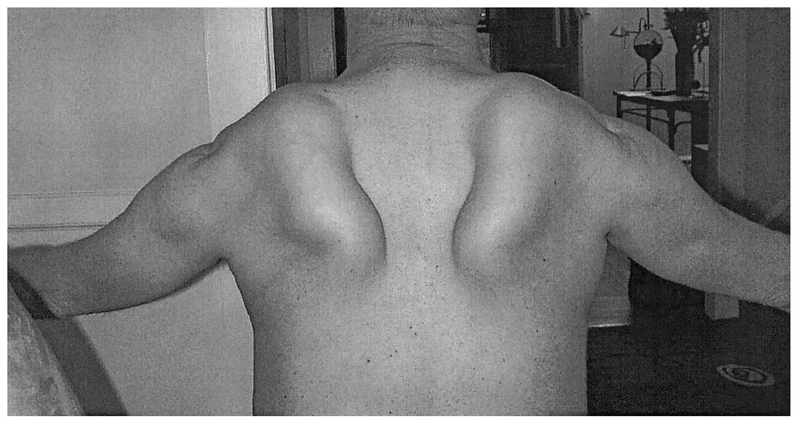
FIG. 5.
Figure of a 55-year-old male with proximal myopathy showing atrophy of the deltoid and striking winging of the scapulae while attempting to raise his arms.
Paget disease of bone also presents early (mean42.2 years, range 29 – 61 years) with typical distribution in the spine, pelvis and skull. Laboratory testing reveals elevated alkaline phosphatase in individuals with PDB [mean 389.3 U/L, range 63–1724 U/L (normal 30–130 IU/L)]. Urine pyridino-line and deoxypyridinoline levels were also elevated in individuals with PDB. The osteocalcin level was not found to be helpful in evaluating PDB, as it was elevated in only one affected individual (mean 30.8 ng/mL, range 10–54.6 ng/mL, normal 8–52 ng/mL).
Onset of dementia in 18 affected individuals occurred on average at 54 years (range 39–62 years). A detailed case history of individual V:23 in Family 1 specifies clinical progression and neuropsychological testing for FTD. Case histories of 3 living individuals in two additional families illustrate the variability in the clinical features of the frontotemporal dementia. History and medical records obtained from deceased relatives indicate that the dementia is characterized by increased agitation, anomia, apathy, and personality change with relative sparing of the memory. Many patients reported having visual or auditory hallucinations. Speech typically becomes more economical, eventually leading to mutism in some individuals.
Family 1.
Clinical features of this family of German ancestry were previously reported (25). Eighteen affected individuals have been identified, seven of whom have died prematurely. The majority have both myopathy and PDB. The muscle disorder was first diagnosed as Limb-girdle muscular dystrophy (LGMD) due to evidence of proximal weakness and myopathic changes on muscle biopsy. Subsequently, one individual (IV:17) had a muscle biopsy showing chronic, patchy myopathic features and rimmed vacuoles, but no inflammation, a pattern more characteristic of HIBM than of inclusion body myositis. The muscle disease is painless; however, several individuals have complained of cramps in their legs and back. The PDB usually manifests at a relatively early age, before myopathy, and is distributed primarily in the spine and pelvis. Dementia has been a prominent component in past generations. A detailed case report of affected individual (V:23), age 45 years, who inherited dementia and myopathy from his mother is presented below.
Case history.
Individual V:23 was evaluated at the Memory Diagnostic Center (MDC) at Washington University Medical Center at age 44 years for a 6-year history of the gradual onset and progression of unexplained language difficulties. There was no history of trauma. Despite worsening language, he continued to be employed as a delivery person for a distributing company. Previous evaluations yielded unremarkable serum chemistries, CK, ALP, blood counts, vitamin B12 level, and thyroid status. An electroencephalogram was unrevealing. A head magnetic resonance image (MRI) study 3 years prior to evaluation showed mild diffuse cerebral atrophy. Dysnomia, comprehension deficits, and paraphasic errors were evident on examination; speech generally was fluent but was devoid of content words. There was no right-left disorientation, finger agnosia, or visuoperceptual deficits. He was oriented for time and place. Errors were present on simple calculations; for example, this high school graduate stated that there were “six” nickels in 35¢ and “24” quarters in $6.75. Psychometric testing was confounded by the language deficits. Adjusting for aphasia, episodic memory was minimally impaired (e.g., he recalled 3 of 5 items of a name and address). Scores on standard and verbally mediated brief cognitive tests included 10 for Mini-Mental State Examination (33) (range from best to worst: 0–30) and 8 for the Short Blessed Test (34) (range from best to worst: 0–28). Performance on other measures is shown in Table 3. The neurologic examination was remarkable only for proximal bilateral lower extremity weakness with a positive Gower’s sign and inability to tandem walk and for the “frontal release” signs of positive jaw jerk and snout reflex. There were no extrapyramidal signs. The clinical diagnosis was semantic dementia (subtype of frontotemporal dementia).
TABLE 3.
Psychometric Performance
| Evaluation | Normsa | Patient |
|---|---|---|
| Verbal fluency (No. animals named in 60s) | 18 | 3 |
| Boston naming test (confrontation naming) | 15 | 4 |
| Word list recall | 7 | 4 |
| Logical memory (episodic memory) | 9 | 4 |
| Trailma king A (speeded psychomotor performance) | 42 s | 110 s |
| Digit symbol (speeded psychomotor performance) | 45 | 22 |
Based on the performance of 83 healthy control participants age 64–83 years (mean age ± standard deviation = 72 ± 5 years) in Washington University’s Alzheimer’s Disease Research Center(59). Values are expressed as means ± standard deviations. For all but Trailmaking A, where an increase indicates worsening performance, a lower score indicates poorer performance.
Shortly after the initial evaluation, repeat head MRI again showed only mild generalized atrophy. He was terminated from his job because of comprehension difficulties. Self-care deteriorated; he inadvertently missed spots when shaving. He did not benefit from a trial of an oral cholinesterase inhibitor. He was, however, able to operate home appliances and continued to drive a motor vehicle and shop for a limited number of items. Reexamination 8 months after the initial evaluation confirmed progressive aphasia with inability to repeat, logorrhea with paraphasias, auditory comprehension deficits for even one-step commands, alexia, and agraphia, and cognitive test performance worsened on both brief measures; the Mini Mental State score now was 6, and the Short Blessed Test score was 19. The neurologic examination was unchanged.
Family 2.
Eleven affected individuals in this family also of German decent have been identified. All affected individuals have weakness. Variability in the manifestation of the disease is a striking feature within this family, as different components of the disorder were present among relatives. The myopathic component in affected individuals had been diagnosed as LGMD, Kugelberg-Welander syndrome, facioscapulohumeral muscular dystrophy (FSH), ALS, and most recently as IBM. Muscle in five individuals revealed features of IBM with rimmed vacuoles. EMGs showed both myopathic and irritative changes, and individuals IV:7 and V:5 also had myotonic discharges. PDB has been identified in three family members and has a typical distribution in the spine, pelvis, and skull. Dementia had been diagnosed in four now deceased individuals: IV:13, IV:7, V:5, and IV:9 with onset at a mean age of 55 years (range 51–61 years) and early demise at age 60.2 years (58–64 years).
Family 3.
Family 3 is a six-generation family of English extraction with 10 affected individuals, including 6 deceased individuals, and 6 with associated FTD. Many individuals in the family were diagnosed with “muscular dystrophy.” Rimmed vacuoles were detected in the muscle of individuals III:1 and III:14. EMGs show myopathic and irritative changes. The majority of the symptoms of the muscle disorder begin at a mean age of 47.6 years (range 39–57 years) and death occurs at a mean age of 63 years (range 59–69 years). Distribution of the PDB is found in the skull, spine, and pelvis.
Onset of dementia typically follows that of HIBM or PDB and is characterized by a marked change in personality, anomia, and eventual mutism in most individuals. Two living individuals in this family have advanced dementia associated with their disease. Individual III:11 had early features of dementia at an age of 52 years and characterized by a dramatic personality change which included apathy, increased agitation, fixation, and confusion. Symptoms of dementia rapidly progressed within 2 years to apraxia, aphasia, and abulia. Brain MRI and CT revealed cerebral atrophy with prominence of the cerebral sulci and third and lateral ventricles. Presenilin-1 gene mutation testing was negative. This individual also has mild muscle weakness and has asymptomatic PDB of the spine since age 32 years. Individual III:14 had difficulty seeing due to visual field constriction at age 46 years and then began showing signs of dementia characterized by memory loss, apraxia, and depression. An MRI at 50 years revealed generalized cortical atrophy and a left occipitoparietal infarction. Testing for Huntington disease and myotonic dystrophy was negative. Gait disturbance and mild muscle weakness are also present with an EMG showing myopathic changes and a specimen of muscle revealing myopathy with rimmed vacuoles.
Family 4.
This Jewish family of Romanian extraction has 10 affected individuals, 6 of whom are deceased. Some members of this family experienced early weakness in proximal muscles whereas others had early involvement of distal legs or facial muscles. Weakness is mildly asymmetric in several individuals and posterior neck muscles are severely affected in advanced stages of the disorder. Two patients have scapular winging typical of FSH dystrophy whereas other patients with similar degrees of weakness have little or no scapular winging. Muscle histology reveals myopathic changes with variation in muscle fiber size, increased endomysial connective tissue, and rimmed vacuoles, but no inflammation. EMGs show an irritative myopathy with small motor unit potentials, fibrillations, and positive sharp waves. One individual suffered rapid progression of the muscle disease and was erroneously diagnosed with ALS (III:7). Dementia has also been a prominent component of the disorder in this family. Individual IV:4 is currently wheelchair bound from proximal limb-girdle weakness and has marked winging of her scapulae. She developed language, behavior, and cognitive changes in her 60s. When evaluated neuropsychologically at University of Pennsylvania Medical School recently at age 68 she presented with prominent language disturbance (primary progressive aphasia) and impaired executive control skills, but relatively preserved visuospatial abilities and episodic memory, this pattern being suggestive of FTD.
Muscle Pathology
Common histopathologic features disclosed by muscle biopsies in these four families included myopathic changes and rimmed vacuoles. Typical myopathic features were variation in muscle fiber size and mildly increased endomysial connective tissue in patchy regions of the specimen. Focal large regions of “myopathic grouping” (Fig. 6) are a distinctive histopathological feature that is common in HIBM, but unusual in sporadic inclusion body myositis. Rimmed vacuolar inclusion bodies were present in some patients in all families. Immuno-staining for components of the dystrophin-sacroglycan complex, emerin and desmin, was normal in all biopsy specimens studied. Ultrastructural examination of muscle in Family 2 has been previously reported showing atrophic and vacuolated muscle fibers containing abundant cytoplasmic paired helical filaments with epitopes of phosphorylated tau, congophilia, abnormal accumulation of β-amyloid precursor protein (βAPP) epitopes, and accumulation of apolipoprotein E (ApoE) (35).
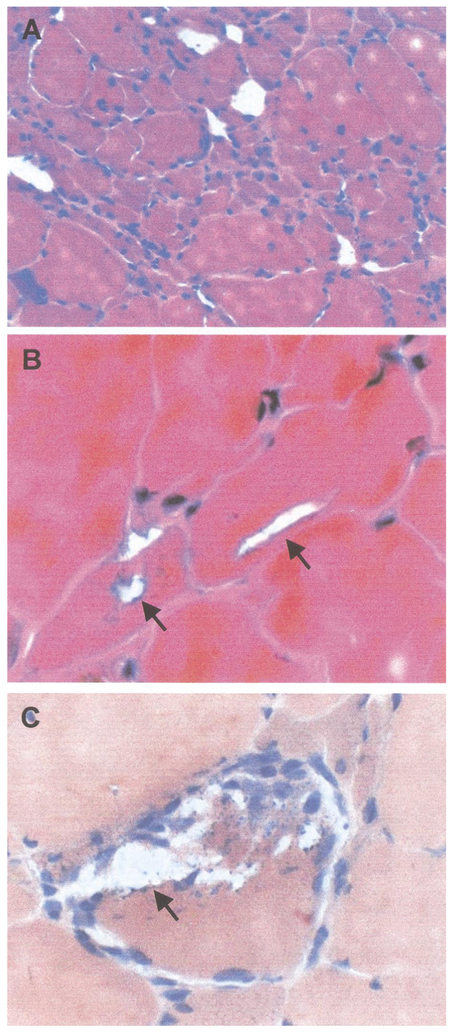
FIG. 6.
Muscle biopsy analysis. Figure 5A is an H&E stain which illustrates the myopathic features with variation in fiber size and grouped regions of muscle fiber atrophy. Figure 5B is also an H&E stain which shows blue-rimmed vacuoles in small and large muscle fibers. Figure 5C is stained with Congo red and illustrates blue rimmed vacuoles with punctate, blue staining debris.
Brain Pathology
Autopsy of two affected individuals in Family 3 revealed severe brain atrophy and degeneration. Individual III:1 was a 66-year-old woman with proximal weakness and progressive dementia and individual II:8 was a 59-year-old man with myopathy, marked winging of the scapulae, extensive PDB and dementia. Decrease in cortical thickness, dilatation of the ventricular system, and loss of white matter with small cyst-like spaces were noted. Microscopically, acute changes consisting predominately of several small micro-abscesses with a small amount of cerebral edema, focal and minimal thickening of the meninges, and increased numbers of glial cells involving both white and gray matter were also reported. Thinning of the cerebral cortex was present. The number of neurons was reduced and remaining neurons appeared shrunken.
Molecular Studies
Linkage analysis.
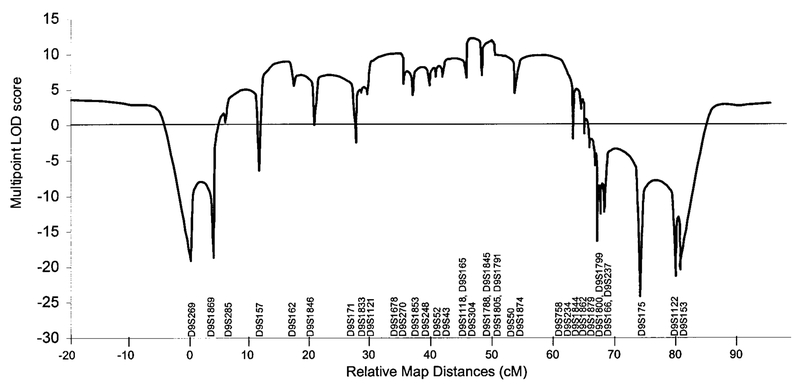
Linkage analysis excluded loci for LGMD, Edstrom myopathy, PDB, and cardiomyopathy (25). The ALS locus on 21q was also excluded in view of its previous association with PDB (36). In fact, a genome-wide search showed linkage to chromosome 9 with marker D9S301 (maximum LOD = 3.64) in Family 1. No other region was identified with a LOD score greater than 3. Table 4 presents the results from two-point linkage analyses of genotype data from all families obtained with a series of markers spanning D9S301. The maximum LOD score generated from the combined genotype data was 9.29 for marker D9S1791. Multipoint linkage results from the combined analysis of families 1 and 2 are presented in Fig. 7. A maximum multipoint LOD score of 12.24 was obtained for the interval defined by markers D9S304 and D9S1788.
TABLE 4.
Combined Two-Point Linkage Analysis in Four Families with HIBM and PDB
| Marker | LOD score at recombination fraction θ = | Marker | LOD score at recombination fraction θ = | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.10 | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.10 | ||
| D9S1853 | −0.68 | 2.75 | 3.02 | 3.14 | 3.20 | 3.23 | 3.09 | D9S1805 | 8.45 | 8.40 | 8.33 | 8.24 | 8.14 | 8.01 | 7.27 |
| 1 | 2.10 | 2.16 | 2.19 | 2.21 | 2.21 | 2.21 | 2.09 | 1 | 3.74 | 3.79 | 3.82 | 3.83 | 3.83 | 3.81 | 3.62 |
| 2 | −2.79 | 0.59 | 0.82 | 0.93 | 0.99 | 1.02 | 1.00 | 2 | 1.95 | 1.89 | 1.84 | 1.79 | 1.74 | 1.68 | 1.41 |
| D9S52 | 4.24 | 4.19 | 4.13 | 4.08 | 4.01 | 3.95 | 3.59 | 3 | 1.32 | 1.31 | 1.29 | 1.28 | 1.26 | 1.24 | 1.12 |
| 1 | 3.91 | 3.87 | 3.83 | 3.78 | 3.73 | 3.68 | 3.36 | 4 | 1.44 | 1.41 | 1.38 | 1.34 | 1.31 | 1.28 | 1.12 |
| 2 | 0.32 | 0.31 | 0.30 | 0.29 | 0.28 | 0.27 | 0.23 | D9S1791 | 9.29 | 9.11 | 8.92 | 8.72 | 8.54 | 8.33 | 7.30 |
| D9S43 | 3.42 | 6.70 | 6.85 | 6.87 | 6.84 | 6.78 | 6.22 | 1 | 4.66 | 4.60 | 4.54 | 4.47 | 4.41 | 4.33 | 3.94 |
| 1 | 6.81 | 6.70 | 6.59 | 6.47 | 6.36 | 6.24 | 5.60 | 2 | 2.96 | 2.88 | 2.81 | 2.73 | 2.65 | 2.58 | 2.19 |
| 2 | −3.39 | −0.00 | 0.26 | 0.40 | 0.48 | 0.54 | 0.63 | 3 | 1.86 | 1.81 | 1.75 | 1.70 | 1.65 | 1.59 | 1.33 |
| 3 | 2.00 | 1.97 | 1.93 | 1.90 | 1.86 | 1.82 | 1.62 | 4 | −0.19 | −0.18 | −0.18 | −0.18 | −0.17 | −0.17 | −0.15 |
| 4 | 0.43 | 0.43 | 0.43 | 0.43 | 0.42 | 0.42 | 0.38 | D9S50 | 6.86 | 6.71 | 6.57 | 6.43 | 6.27 | 6.13 | 5.34 |
| D9S165 | 7.67 | 7.57 | 7.46 | 7.34 | 7.21 | 7.08 | 6.35 | 1 | 3.66 | 3.60 | 3.54 | 3.48 | 3.41 | 3.34 | 2.98 |
| 1 | 6.11 | 6.02 | 5.94 | 5.85 | 5.75 | 5.66 | 5.13 | 2 | 1.18 | 1.15 | 1.12 | 1.09 | 1.06 | 1.03 | 0.87 |
| 2 | 0.44 | 0.45 | 0.45 | 0.45 | 0.45 | 0.44 | 0.38 | 3 | 2.17 | 2.11 | 2.06 | 2.00 | 1.94 | 1.89 | 1.60 |
| 3 | 1.46 | 1.43 | 1.39 | 1.35 | 1.32 | 1.28 | 1.10 | 4 | −0.15 | −0.15 | −0.15 | −0.14 | −0.14 | −0.13 | −0.11 |
| 4 | −0.34 | −0.33 | −0.32 | −0.31 | −0.31 | −0.30 | −0.26 | D9S1874 | 2.94 | 6.13 | 6.24 | 6.39 | 6.39 | 6.37 | 5.99 |
| D9S1118 | 8.61 | 8.43 | 8.25 | 8.06 | 7.87 | 7.68 | 6.69 | 1 | 3.93 | 3.91 | 3.88 | 3.84 | 3.80 | 3.76 | 3.50 |
| 1 | 4.40 | 4.34 | 4.28 | 4.21 | 4.14 | 4.07 | 3.67 | 2 | −2.76 | 0.46 | 0.71 | 0.83 | 0.90 | 0.94 | 0.97 |
| 2 | 1.64 | 1.58 | 1.52 | 1.47 | 1.42 | 1.36 | 1.10 | 3 | 0.92 | 0.93 | 0.93 | 0.94 | 0.94 | 0.94 | 0.91 |
| 3 | 2.00 | 1.94 | 1.89 | 1.83 | 1.77 | 1.72 | 1.44 | 4 | 0.85 | 0.83 | 0.80 | 0.78 | 0.75 | 0.73 | 0.61 |
| 4 | 0.57 | 0.57 | 0.56 | 0.55 | 0.54 | 0.53 | 0.48 | D9S1844 | 4.29 | 4.19 | 4.09 | 3.99 | 3.89 | 3.80 | 3.30 |
| D9S304 | 8.27 | 8.10 | 7.95 | 7.78 | 7.60 | 7.42 | 6.47 | 1 | 2.60 | 2.55 | 2.51 | 2.47 | 2.42 | 2.38 | 2.15 |
| 1 | 5.05 | 4.96 | 4.88 | 4.79 | 4.69 | 4.60 | 4.09 | 2 | 1.69 | 1.64 | 1.58 | 1.53 | 1.47 | 1.42 | 1.15 |
| 2 | 1.52 | 1.46 | 1.40 | 1.35 | 1.29 | 1.23 | 0.95 | D9S234 | −3.58 | 6.16 | 6.59 | 6.77 | 6.84 | 6.85 | 6.51 |
| 3 | 1.15 | 1.12 | 1.10 | 1.07 | 1.05 | 1.02 | 0.89 | 1 | −0.90 | 4.91 | 5.11 | 5.18 | 5.20 | 5.18 | 4.89 |
| 4 | 0.55 | 0.56 | 0.57 | 0.57 | 0.57 | 0.57 | 0.54 | 2 | −2.68 | 1.25 | 1.48 | 1.59 | 1.64 | 1.67 | 1.63 |
| D9S1788 | 3.33 | 6.63 | 6.76 | 8.21 | 6.72 | 6.64 | 6.01 | D9S1862 | 1.09 | 4.86 | 5.02 | 5.06 | 5.04 | 5.00 | 4.62 |
| 1 | −1.67 | 1.76 | 2.03 | 2.16 | 2.24 | 2.29 | 2.32 | 1 | 5.68 | 5.59 | 5.49 | 5.40 | 5.30 | 5.20 | 4.69 |
| 2 | 1.12 | 1.08 | 1.04 | 1.01 | 0.97 | 0.93 | 0.74 | 2 | −4.59 | −0.73 | −0.47 | −0.34 | −0.26 | −0.20 | −0.07 |
| 3 | 2.73 | 2.67 | 2.61 | 2.55 | 2.49 | 2.43 | 2.12 | D9S1879 | −6.86 | 1.30 | 1.78 | 2.01 | 2.14 | 2.21 | 2.18 |
| 4 | 1.15 | 1.12 | 1.08 | 1.05 | 1.02 | 0.99 | 0.83 | 1 | −4.54 | −0.25 | 0.01 | 0.15 | 0.24 | 0.29 | 0.40 |
| D9S1845 | 9.04 | 8.89 | 8.72 | 8.55 | 8.38 | 8.20 | 7.24 | 2 | −2.32 | 1.55 | 1.77 | 1.86 | 1.90 | 1.91 | 1.78 |
| 1 | 3.72 | 3.71 | 3.68 | 3.65 | 3.62 | 3.58 | 3.32 | D9S1800 | −8.75 | 0.89 | 1.41 | 1.68 | 1.84 | 1.94 | 2.05 |
| 2 | 2.12 | 2.06 | 2.01 | 1.95 | 1.90 | 1.84 | 1.56 | 1 | −3.95 | 1.78 | 2.02 | 2.12 | 2.18 | 2.20 | 2.12 |
| 3 | 2.12 | 2.07 | 2.01 | 1.96 | 1.90 | 1.85 | 1.58 | 2 | −4.18 | −0.89 | −0.60 | −0.44 | −0.34 | −0.26 | −0.07 |
| 4 | 1.08 | 1.05 | 1.02 | 0.99 | 0.96 | 0.93 | 0.78 | D9S1799 | −0.76 | 3.21 | 3.49 | 3.65 | 3.74 | 3.79 | 3.75 |
| 1 | 2.87 | 2.90 | 2.93 | 2.94 | 2.94 | 2.94 | 2.84 | ||||||||
| 2 | −3.63 | 0.30 | 0.57 | 0.71 | 0.79 | 0.85 | 0.92 | ||||||||
Note. The combined LOD scores for each marker are provided in the first row adjacent to the marker name. Independent scores for each family are given below the marker name. A numerical identification (1–4) is given for each pedigree and corresponds to genotype data presented in Figs. 1–4, respectively.
FIG. 7.
Multipoint linkage analysis of markers mapped to the chromosome 9. The markers and cytogenetic localization are designated on the X axis with relative marker distances (cM).
Haplotype analysis.
Haplotype analysis using a high density of molecular markers flanking D9S301 is presented separately for 29 individuals (15 affected) in Family 1 (Fig. 1), 15 individuals (7 affected) in Family 2 (Fig. 2), 16 individuals (3 affected) in Family 3 (Fig. 3), and 7 individuals (4 affected) in Family 4 (Fig. 4).
A disease haplotype was constructed in each family by determining marker alleles segregating among affected individuals. All affected members of Family 1 shared a common haplotype from marker D9S1869 to D9S175 (V:2 and V:18, Fig. 1), a region spanning 43 cM based on the Marshfield sex-averaged genetic linkage map. In Family 2, a disease haplotype encompassing an 11 cM region flanked by markers D9S43 and D9S301 was observed among all affected individuals (VI:2, Fig. 2).
In Family 1, meiotic recombination events were observed in unaffected individuals V:7 and V:24 for markers D9S1853 and D9S234, respectively. Individual V:21 also had an informative recombination at marker D9S1791; however, the exact site of recombination was not certain because parental genotype data were unavailable. Individual V:19, who is clinically unaffected but a carrier for part of the disease haplotype, was excluded from analysis because of the person’s young age (41 years). Combining the results of families 1 and 2 revealed a critical region for the disease flanked by markers D9S43 (VI:2, Fig. 2) and D9S234 (V:24, Fig. 1). If the recombination event in unaffected individual V:21 of pedigree 1 is considered, the disease locus is represented by a 4.09 cM interval flanked by markers D9S43 and D9S1791.
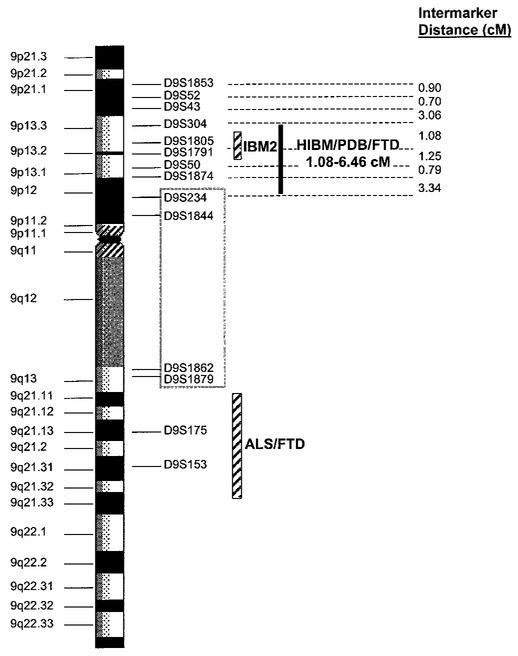
In Families 3 and 4, all affected individuals share a common disease-associated haplotype for micro-satellite markers within the interval defined by D9S43 and D9S234. It was noted that Family 3 shared the same disease alleles with Family 1, indicating a common founder for these two families. A meiotic recombination event occurring in unaffected individual IV:5 of pedigree 4 reduces the critical region defined in Families 1 and 2, to an interval flanked by marker D9S304 and either marker D9S234 or D9S1791. Marker D9S234 has not been identified on the draft sequence of the human genome physical map; however, based upon a likelihood criterion of 1000:1, marker D9S234 maps between flanking markers D9S1874 and D9S1844. Its estimated genetic map position is midway between these two markers. According to the physical map, the distance between D9S304 and D9S234 is approximately 6.85 million base pairs (bp) (6.46 cM). The interval represented by D9S304 and D9S1791 is approximately 1.08 cM (Marshfield sex-averaged map) or 4.75 million bp on physical map. A summary of the haplotype interpretation for all four families is presented in Fig. 8.
FIG. 8.
Summary of haplotype analysis. Microsatellite markers used in molecular analysis are listed in order from p-arm to q-arm (top to bottom) on the backbone of chromosome 9. Relative marker positions are based on assignments from the Human Genome draft sequence and intermarker distances (cM) were obtained from the chromosome 9 sex-averaged genetic linkage map (Marshfield). The critical region for HIBM/PDB/FTD is represented by a solid black bar. The genetic loci for autosomal recessive IBM2 and ALS presenting with FTD are indicated with diagonally striped bars. The double recombination event affecting markers D9S234, D9S1844, D9S1862, D9S1879, and D9S1800 is indicated with a gray box surrounding these markers.
Interestingly, a double recombination event involving markers D9S234, D9S1844, D9S1862, D9S1879, and D9S1800 was observed in Families 1 and 2 (V:12, V:16, V:18, VI:2, VI:3; pedigree 1 and V:10; pedigree 2), a region excluded from the critical region. Although this is an unlikely recombination event (probability <0.04%), this region may represent a recombinational “hotspot” because individuals V:19 and V:24 of pedigree 1 also present with single recombination events at this site.
DISCUSSION
Hereditary inclusion body myopathy with early-onset PDB and FTD is distinct among the clinically and genetically heterogeneous family of hereditary myopathies. Striking features of this syndrome consist of a marked variability in the patterns of muscle weakness, noted both within and among families, as well as a common pattern of weakness involving proximal and distal musculature with mild asymmetry. Onset of the myopathy is typically in adulthood; however, earlier involvement is documented in some patients.
PDB presenting with either dementia or a neuro-muscular disorder has been infrequently reported. Specifically, dementia with PDB has been attributed to an uncommon complication of PDB of the skull, as expansion of diseased bone can compress cranial nerves (37). A progressive frontal dementia with bone cysts associated with signs of upper motor neuron involvement has been reported in Nasu-Hakola disease or polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (38,39), a recessively inherited disorder (MIM 221770). There is no evidence that Nasu-Hakola disease causes a myopathy, the bone cysts do not resemble PDB, and the dementia is associated with white matter rather than gray matter changes. The defective gene for this disorder has been identified as DAP12 located on chromosome 19q13.1(40). In addition, three neuromuscular disorders have been associated with PDB: muscular dystrophy (41), dystrophia myotonia (42), and ALS (MIM 167320)(36). Interestingly, individuals in the family with ALS reported by Tucker and colleagues in 1982 also demonstrate late-onset dementia (36). Mutations in the TNFRSF11A gene, encoding RANK, have been found to cause familial expansile osteolysis, an autosomal dominant skeletal disease with many features of PDB(11). RANK is a membrane receptor found on osteoclasts and osteoclast progenitor cells and is involved in osteoclast differentiation and action. RANK activates NF-κB, a common facilitator significant in many signaling pathways. RANK action, however, has not been importantly associated with muscle cells, and individuals with FEO-bearing RANK mutations have not had neuromuscular disease (43). Interestingly, an increase in NF-κB protein is found in the muscle fibers of patients with inclusion-body myositis (44). Sporadic inclusion body myositis (s-IBM) and HIBM are muscle diseases with very similar pathological features, with the exception of lymphocytic inflammation in s-IBM biopsy specimens. It has been proposed that possibly NF-κB is one of several facilitators involved in key early signals that lead to the progression of s-IBM and HIBM (45).
Dementia is a prominent feature of many of the cases of HIBM in the four families described in this report. Unfortunately, the dementia was examined in detail in only a handful of these patients. In two of the four living individuals who were examined neuropsychologically, the dementia was so advanced that it could not be characterized precisely. Two of the other cases, however, showed a surprisingly similar neurocognitive profile of pronounced language impairment and executive dysfunction, with surprisingly preserved episodic memory. This, of course, is the profile that has been associated with frontotemporal dementia. Recognizing that the antemortem diagnosis of FTD can be problematic and that the pathology underlying this neurobehavioral disorder is diverse (46,47), we propose that FTD may have a unique association with HIBM.
The pathological similarities of s-IBM and Alzheimer’s disease have been investigated (48) and may provide insight into any current association of HIBM and FTD. Proteins such as amyloid β, ubiquitin, apoE, phosphorylated tau, and presenilin (which accumulate in the brain of Alzheimer’s disease patients) also accumulate in muscle fibers of s-IBM patients (45). Unfortunately the brains have not been investigated for these proteins because the deceased individuals in these families died several years ago. Because HIBM and sporadic IBM are pathologically similar in this regard (45) and similar proteins, such as tau (20), are affected in FTD as well as Alzheimer’s disease, possibly the link between IBM and dementia results from a common pathogenetic cascade causing cellular deterioration.
Chromosome 9p13.3–p12, the disease locus identified in this study for the new syndrome of HIBM associated with PDB and FTD, is unique for PDB and FTD. The locus at 9q21–q22 identified for a subset of families with ALS in which individuals also had FTD was a candidate for the disease; however, informative recombinations were documented between this locus and the disease state in our families (24,25) (Fig. 8). The IBM2 locus for autosomal recessive HIBM in Iranian Jewish families, however, maps to chromosome 9p13–p12 (6,7,49), and is central to the critical region determined in this report. Recently, seven different mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene have been identified in families with the autosomal recessive form of HIBM (50). These mutations were not observed in over 300 unaffected non-Middle Eastern control chromosomes. However, approximately 6.8% of unaffected, unrelated Persian Jewish individuals were found to be heterozygous carriers of the mutation, a value that fits with the expected estimated frequency of heterozygotes based on a 1:1500 prevalence of disease in this population. These data strongly implicate GNE defects as being responsible for manifestation of the IBM2 phenotype. Possibly, these phenotypically and genetically similar but distinct disorders (IBM2 and HIBM/PDB/FTD) are allelic variants, as suggested for autosomal recessive Nonaka myopathy which also has been mapped to 9p1–q1.
There are several examples in the literature of the pleiomorphic effects of gene mutations in which dominant and recessive forms of disease can be caused by genetically distinct mutations within the same gene. The IBM2 gene may, therefore, be a candidate gene for HIBM/PDB/FTD in view of the shoulder and hip-girdle muscle weakness and mixed myopathic and neurogenic EMG findings as well as similar findings on muscle biopsy in both disorders. However, the presence of quadriceps sparing and the absence of PDB and FTD in IBM2 (51,52) suggest significant clinical differences.
Additional candidate genes that map within the critical region of the syndrome of HIBM/PDB/FTD on chromosome 9 include talin (TLN) (53), tropomyosin 2 (TPM2) (54), NADH dehydrogenase (ubiquinone) 1β subcomplex 6 (NDUFB6) (55), interleukin-11 receptor alpha chain (IL11RA) (56), carbonic anhydrase IX (CA9) (57), ubiquitin-associated protein (UBAP) [GenBank Accession No. XM_005548], and valosin-containing protein (VCP) (58) express proteins that may be involved in cellular and structural functions of muscle and/or bone cells. Sequence analysis of tropomyosin 2 and NADH dehydrogenase (ubiquinone) 1b subcomplex 6 (NDUFB6) in two affected individuals did not reveal a mutation; however, sequence analysis of GNE and other candidate genes is in progress.
Discovery of the gene associated with HIBM/PDB/FTD will provide insight into the pathogenesis of muscle, bone, and neurological disease. Perhaps an overlap exists between the biological processes that control bone and muscle maintenance and regeneration. Elucidation of the gene defect responsible for HIBM/PDB/FTD should contribute to our understanding of these systems and possibly result in a treatment for this complex disorder.
ACKNOWLEDGMENTS
We thank the families and their physicians for their enthusiastic participation and contribution in our research studies; Dr. James Weber and the staff at National Heart, Lung, and Blood Institute Mammalian Genotyping Service, Marshfield, Wisconsin, for the genotyping; Dr. Mark Broman, Johns Hopkins, Baltimore, Maryland, for performing linkage analysis of Marshfield set of markers; Dr. Marzia Pasquali, Emory University, Atlanta, Georgia, for performing pyridinoline/deoxypyridinoline studies; Randell Smith and Katerina Kimonis, Springfield, Illinois, for their laboratory assistance; and Dr. Howard Feit, Henry Ford Hospital, West Bloomfield, Michigan. Funding of this study is from the Excellence in Academic Medicine Program at Southern Illinois University School of Medicine, the Muscular Dystrophy Association, Grant R03 AR 46869–01A1 from National Institute of Arthritis, Musculoskeletal and Skin Disease, Grant K02 NS02157–01A1 from National Institute of Neurological Disorders and Stroke, Grant RR-00036 from the General Clinical Research Center Branch, Division of Research Facilities and Resources, National Institutes on Aging Grants AG03991 and AG05681, and Grant HG00008 from the National Institutes of Health.
Footnotes
Electronic-database information: Online Mendelian Inheritance in Man (OMIM), http://www3.ncbi.nlm.nih.gov/omim (for LGMD with PDB [MIM 605382], IBM1 [MIM 147420], IBM2 [MIM 600737], IBM3 [MIM 605637], PDB2 [MIM 602080], DMRV, [MIM 605820], FTD presenting with parkinsonism [MIM 600274], FTD presenting with ALS [MIM 105550]), Pagetoid amyotrophic lateral sclerosis [MIM 167320], Nasu-Hakola disease [MIM 221770]; Human Genome Working Draft, http://genome.cse.ucsc.edu/ (for physical map positions and inter-marker distances between chromosome 9 STS markers); Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics (for genetic linkage map and intermarker distances based on genetic recombinations); GenBank at the National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/ (Accession No. XM_005548).
REFERENCES
- 1.Clark JR, D’Agostino AN, Wilson J, Brooks RR, Cole GC. Autosomal dominant myofibrillar inclusion body myopathy: Clinical, histologic, histochemical, and ultrastructural characteristics (abstract). Neurology 28:399, 1978. [Google Scholar]
- 2.Abe K, Kobayashi K, Chida K, Kimura N, Kogure K. Tohoku J Exp Med 170:261–272, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Chinnery PF, Johnson MA, Walls TJ, Gibson GJ, Fawcett PRW, Jamieson S, Fulthorpe JJ, Cullen M, Hudgson P, Bushby K. A novel autosomal dominant distal myopathy with early respiratory failure: Clinico-pathologic characteristics and exclusion of linkage to candidate genetic loci. Ann Neurol 49:443–452, 2001. [PubMed] [Google Scholar]
- 4.Martinsson T, Darin N, Kyllerman M, Oldfors A, Hallberg B, Wahlstrom J. Dominant hereditary inclusion-body myopathy gene (IBM3) maps to chromosome region 17p13.1. Am J Hum Genet 64:1420–1426, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinsson T, Oldfors A, Darin N, Berg K, Tajsharghi H, Kyllerman M, Wahlstrom J. Autosomal dominant myopathy: Missense mutation (glu-706-to-lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci USA 97:14614–14619, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neocleous V, Humphray SJ, Howard PJ, Hammond S, Tsingis M, Georgiou A, Al-Qudah AK, Mubaidin A, Horani K, Askanas V, Engel K, Dalakas M, Rowland LP, Mirabella M, Zamba E, Kyriakides T, Middleton LT, Christodoulou K. Bac based physical map of the distal hereditary motor neuronopathy (HMN-J) and autosomal recessive inclusion body myopathy (AR-IBM) region on chromosome 9p21.1-p12. Am J Hum Genet 67(Suppl):2291A, 2000. [Google Scholar]
- 7.Eisenberg I, Hochner H, Shemesh M, Levi T, Potikha T, Sadeh M, Argov Z, Jackson CL, Mitrani-Rosenbaum S. Physical and transcriptional map of the hereditary inclusion body myopathy locus on chromosome 9p12-p13. Eur J Hum Genet 9:501–509, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Ikeuchi T, Asaka T, Saito M, Tanaka H, Higuchi S, Tanaka K, Saida K, Uyama E, Mizusawa H, Fukuhara N, Nonaka I, Takamori M, Tsuji S. Gene locus for autosomal recessive distal myopathy with rimmed vacuoles maps to chromosome9. Ann Neurol 41:432–437, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Mills BG, Singer FR. Nuclear inclusions in Paget’s disease of bone. Science 194:201–202, 1976. [DOI] [PubMed] [Google Scholar]
- 10.Cody JD, Singer FR. Genetic linkage of Paget’s disease of the bone to chromosome 18q. Am J Hum Genet 61:1117–1122, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nature Genet 24:45–48, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Laurin N, Brown JP, Lemainque A, Duchesne A, Huot D, Lacourcière Y, Drapeau G, Verreault J, Raymond V, Morissette J. Paget Disease of Bone: Mapping of two loci at 5q35-qter and 5q31. Am J Hum Genet 69:528–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neary D, Snowden JS, Gustafson L, Passant U, Tuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51:1546–1554, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D’Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: A consensus conference. Ann Neurol 41:706–715, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. Frontotemporal dementias In Neurodegenerative Dementias (Clark CM, Trojanowski JQ, Eds.). New York: McGraw-Hill, pp. 279–290, 2000. [Google Scholar]
- 16.Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Arch Neurol 56:817–822, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelmsen KC, Lynch T, Pavlou E, Higgins M, Nygarrd TG. Localization of disinhibition dementia-parkinsonismamyotrophy complex to 17q21–22. Am J Hum Genet 55: 1159–1165, 1994. [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch T, Sano M, Marder KS, Bell KL, Foster NL, Defendini RF, Sima AA, Keohane C, Nygaard TG, Fahn S, et al. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology 4:1878–1884, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Lendon CL, Lynch T, Norton J, McKeel DW Jr, Busfield F, Craddock N, Chakraverty S, Gopalakrishnan G, Shears SD, Grimmett W, Wilhelmsen KC, Hansen L, Morris JC, Goate AM. Hereditary dysphasic disinhibition dementia: A fronto-temporal dementia linked to 17q21–22. Neurology 50:1546–1555, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Heutink P, et al. Association of missense and 5-prime-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasred-dine ZS, Miller B, Li D, Payami H, Awert F, Markopoulou K, Andreadis A, D’Souza I, Lee VM, Reed L, Trojanowski JQ, Zhukareva V, Bird T, Schellenberg G, Wilhelmsen KC. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA 95:13103–13107, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spillantini MG, Murrel JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 95:7737–7741, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown J, Ashworth A, Gydesen S, Sorensen A, Rossor M, Hardy J, Collinge J. Familial nonspecific dementia maps to chromosome 3. Hum Molec Genet 4:1625–1628, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Hosler BA, Siddique T, Sapp PC, Sailor W, Huang MC, Hossain A, Daube JR, Nance M, Fan C, Kaplan J, Hung W-Y, McKenna-Yasek D, Haines JL, Pericak-Vance MA, Horvitz HR, Brown RH, Jr. Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromo-some 9q21-q22. J Am Med Assoc 284:1664–1669, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Kimonis VE, Kovach MJ, Waggoner B, Leal S, Salam A, Rimer L, Davis K, Khardori R, Gelber D. Clinical and molecular studies in a unique family with autosomal dominant limb-girdle muscular dystrophy and Paget disease of bone. Genet Med 2:232–241, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lezak MD. Neuropsychological Assessment, 3rd ed New York: Oxford Univ. Press, 1995. [Google Scholar]
- 27.Siaz-Cano S, Brady S. DNA extraction from formalin fixed, paraffin embedded tissues: Protein digestion as a limiting step for retrieval of high quality DNA. Diagn Mol Pathol 6:342–346, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Howe JR, Klimstra DS, Cordon-Cardo C. DNA extraction from paraffin embedded tissues using a salting out procedure: A reliable method for PCR amplification of archival material. Histol Histopathol 12:595–601, 1997. [PubMed] [Google Scholar]
- 29.Weirich G, Hornauer MA, Bruning T, Hofler H, Brauch H. Fixed archival tissue: Purify DNA and primers for good PCR yield! Molec Biotechnol 8:299–301, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Weeks DE, Sobel E, O’Connell JR, Lange K. Computer programs for multilocus haplotyping of general pedigrees. Am J Hum Genet 56:1506–1507, 1995. [PMC free article] [PubMed] [Google Scholar]
- 31.Sobel E, Lange K. Descent graphs in pedigree analysis: Applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet 58:1323–1337, 1996. [PMC free article] [PubMed] [Google Scholar]
- 32.Cottingham RW Jr, Idury RM, Schaffer AA. Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263, 1993. [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 12:189–198, 1975. [DOI] [PubMed] [Google Scholar]
- 34.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry 140:734–739, 1983. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez B, Simmons Z, Engel WK, Askanas V. New autosomal dominant inclusion body myopathy (AD-IBM) with many congophilic muscle nuclei that contain paired-helical filaments (PHFs) composed of phosphorylated tau. Neurology 50:A204, 1998. [Google Scholar]
- 36.Tucker WS Jr, Hubbard WH, Stryker TD, Morgan SW, Evans OB, Freemon F, Theil GB. A new familial disorder of combined lower motor neuron degeneration and skeletal disorganization. Trans Assoc Am Phys 95:126–134, 1982. [PubMed] [Google Scholar]
- 37.Poncelet A The neurologic complications of Paget’s disease. J Bone Miner Res 14S:88–91, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Hakola HPA. Neuropsychiatric and genetic aspects of a new hereditary disease characterized by progressive dementia and lipomembranous polycystic osteodysplasia. Acta Psychiatr Scand 232(Suppl):1–73, 1972. [PubMed] [Google Scholar]
- 39.Paloneva J, Autti T, Raininko R, Partanen J, Salonen O, Puranen M, Hakola P, Haltia M. CNS manifestations of Nasu-Hakola disease: A frontal dementia with bone cysts. Neurology 56:1552–1558, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nature Genet 25:357–361, 2000. [DOI] [PubMed] [Google Scholar]
- 41.McBride TI. Paget’s disease and muscular dystrophy: Report of an unusual association in one family. Scot Med J 11:238–243, 1966. [DOI] [PubMed] [Google Scholar]
- 42.Caughey JE, Gwynne JF, Jefferson NR. Dystrophia myotonica associated with familial Paget’s disease (osteitis deformans) with sarcomata. J Bone Joint Surg 39B:316–325, 1957. [DOI] [PubMed] [Google Scholar]
- 43.Whyte MP, Reinus WR, Podgornik MN, Mills BG. Familial expansile osteolysis (excessive RANK effect) in a 5-generation American kindred. Medicine (Baltimore), in press. [DOI] [PubMed] [Google Scholar]
- 44.Yang C-C, Askanas V, Engel WK, Alvarez RB. Immunolocalization of transcription factor NF-κB in inclusion-body myositis muscle and at normal human neuromuscular junctions. Neurosci Lett 287:1–4, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Askanas V, Engel WK. Inclusion-body myositis: Newest concepts of pathogenesis and relation to aging and Alzheimer disease. J Neuropathol Exp Neurol 60:1–14, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Klatka LA, Schiffer RB, Powers JM, Kazee AM. Incorrect diagnosis of Alzheimer’s disease. A clinicopathologic study. Arch Neurol 53:35–42, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry 66:184–188, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Askanas V, Engel WK. Sporadic inclusion-body myositis and its similarities to Alzheimer disease brain. Recent approaches to diagnosis and pathogenesis, and relation to aging. Scand J Rheumatol 27:389–405, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Mitrani-Rosenbaum S, Argov Z, Blumenfeld A, Seidman CE, Seidman JG. Hereditary inclusion body myopathy maps to chromosome 9p1-q1. Hum Molec Genet 5:159–163, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G, Schmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Ben Asher E, Beckmann JS, Argov Z, Mitrani-Rosenbaum S. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nature Genet 29:83–87, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Argov Z, Yarom R. “Rimmed vacuole myopathy” sparing the quadriceps. A unique disorder in Iranian Jews. J Neurol Sci 64:33–43, 1984. [DOI] [PubMed] [Google Scholar]
- 52.Massa R, Weller B, Karpti G, Shoubridge E, Carpenter S. Familial inclusion body myositis among Kurdish-Iranian Jews. Arch Neurol 48:519–522, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Gilmore AP, Ohanian V, Spurr NK, Critchley DR. Localization of the human gene encoding the cytoskeletal protein talin to chromosome 9p. Hum Genet 96:221–224, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Hunt CCJ, Eyre HJ, Akkari PA, Meredith C, Dorosz SM, Wilton SD, Callen DF, Laing NG, Baker E. Assignment of the human beta tropomyosin gene (TPM2) to band 9p13 by fluorescence in situ hybridization. Cytogenet Cell Genet 71: 94–95, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Emahazion T, Beskow A, Gyllensten U, Brookes AJ. Intron based radiation hybrid mapping of 15 complex I genes of the human electron transport chain. Cytogenet Cell Genet 82: 115–119, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Van Leuven F, Stas L, Hilliker C, Miyake Y, Bilinski P, Gossler A. Molecular cloning and characterization of the human interleukin-11 receptor alpha-chain gene, IL11RA, located on chromosome 9p13. Genomics 31:65–70, 1996, doi:10.1006/geno.1996.0010. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa Y, Uemura H, Hirao Y, Yoshida K, Saga S, Yoshikawa K. Radiation hybrid mapping of the human MN/CA9 locus to chromosome band 9p12-p13. Genomics 53:118–119, 1998, doi:10.1006/geno.1998.5483. [DOI] [PubMed] [Google Scholar]
- 58.Druck T, Gu Y, Prabhala G, Cannizzaro LA, Park S-H, Huebner K, Keen JH. Chromosome localization of human genes for clathrin adaptor polypeptides AP2-beta and AP50 and the clathrin-binding protein, VCP. Genomics 30:94–97, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Storandt M, Hill RD. Very mild senile dementia of the Alzheimer type. II. Psychometric test performance. Arch Neurol 46:383–386, 1989. [DOI] [PubMed] [Google Scholar]