Abstract
Diterpenoids are widely distributed natural products and have caused considerable interest because of their unique skeletons and antibacterial and antitumor activities and so on. In light of recent discoveries, ent-kaurane diterpenoids, which exhibit a wide variety of biological activities, such as anticancer and anti-inflammatory activities, pose enormous potential to serve as a promising candidate for drug development. Among them, spirolactone-type 6,7-seco-ent-kaurane diterpenoids, with interesting molecular skeleton, complex oxidation patterns, and bond formation, exhibit attractive activities. Furthermore, spirolactone-type diterpenoids have many modifiable sites, which allows for linking to various substituents, suitable for further medicinal study. Hence, some structurally modified derivatives with improved cytotoxicity activities are also achieved. In this review, natural bioactive spirolactone-type diterpenoids and their synthetic derivatives were summarized.
Keywords: spirolactone-type; 6,7-seco-ent-kaurane; diterpenoid; natural product; synthetic derivative
1. Introduction
Natural products have been used to treat various diseases in China for hundreds of years. Their novel molecular skeletons and promising cytotoxic activities are invaluable sources for drug discovery and development processes [1,2,3,4]. Natural products have played crucial roles in drug discovery. Besides natural products, natural product-derived compounds are also important to cancer therapy [5,6,7,8,9,10,11]. It is well known that diterpenoids are structurally diverse and widely distributed natural compounds, they have attracted interest from the scientific community because of their unique skeletons and therapeutic effects—antitumor [12,13], anti-inflammatory [14], antibacterial [15,16], and so on [17,18,19,20,21]—especially for anticancer agents, such as the most famous anticancer natural compound paclitaxel [22,23,24,25].
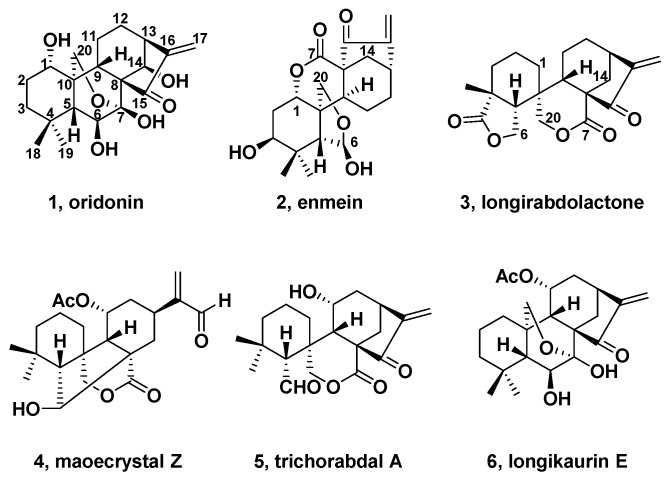
ent-Kaurenes, such as oridonin (1, Figure 1), have been investigated for more than 40 years [26,27,28,29]. Moreover, in 2015, the ent-kaurane diterpenoid derivative HAO472, l-alanine-(14-oridonin) ester trifluoroacetate, was advanced to Phase I human clinical trial in China to cure acute myelogenous leukemia [30]. There are two subtypes of 6,7-seco-ent-kauranes, spirolactone-type (7,20-lactone) and enmein-type (1,7-lactone) [31,32]. Particularly, spirolactone-type diterpenoids have distinct chemical skeletons and demonstrate important bioactivities which have attracted great interest from experts and scholars. Before the 1980s, spirolactone-type diterpenoids were misidentified as enmein-type diterpenoids. Until the mid-1980s, misidentifications were corrected with the development of modern 2D NMR spectra [33,34]. The first isolation of spirolactone-type diterpenoids was in 1981 [35]. After that, in order to isolate antitumor diterpenoids, more and more spirolactone-type diterpenoids were isolated from Isodon plants of the Labiatae family [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55], but natural sources were limited.
Figure 1.
Structures of oridonin (1), enmein (2), longirabdolactone (3), maoecrystal Z (4), trichorabdal A (5), and longikaurin E (6).
The first total synthesis of 6,7-seco-ent-kauranoid enmein (2, Figure 1) was achieved by Fujita et al. which was a landmark [56,57]. After that, a semisynthesis of longirabdolactone (3, Figure 1) was achieved in 2003 [58]; maoecrystal Z (4, Figure 1), trichorabdal A (5, Figure 1), and longikaurin E (6, Figure 1) were achieved in 2011 to 2014 [59,60]. By the end of September 2018, three reviews summarized the total synthesis of Isodon diterpenes [61,62,63].
The Sun [64] and Pu Group [65] had reviewed “diterpenoids from the Isodon genus”. They comprehensively summarized isolation, structural elucidation, and total synthesis of spirolactone-type diterpenoids. In this review, bioactive spirolactone-type natural products and the synthetic medicinal chemistry work will be summarized.
2. Natural Bioactive Spirolactone-Type Diterpenoids
By the end of October 2018, 105 spirolactone-type diterpenoids have been isolated from Isodon species. Several exhibited biological activities and are summarized below.
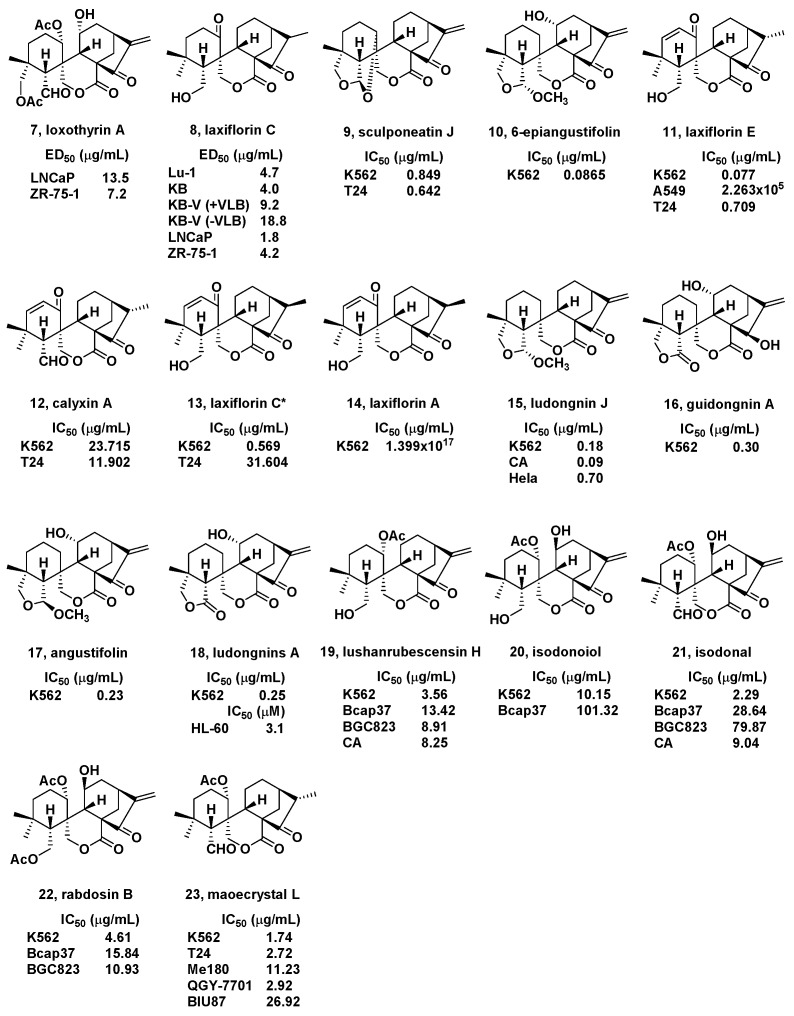
In 1995, loxothyrin A (7, Figure 2) was isolated by Sun’s Group from the leaves of I. loxothyrsa [66]. It showed cytotoxicity effects toward hormone-dependent human prostatic LNCaP and breast ZR-75-1 cancer cell lines with ED50 values of 13.5 and 7.2 μg/mL, respectively.
Figure 2.
Bioactive natural spirolactone-type diterpenoids 7–23.
In the same year, laxiflorins A–C (8, Figure 2) were isolated from I. eriocalyx var. laxiflora. by Sun and coworkers [67]. Cytotoxic activities were shown against human lung cancer Lu-1, human oral epidermoid carcinoma KB, vinblastine-resistant KB KB-V, LNCaP, and ZR-75-1 cells. They showed cytotoxicity with ED50 values from 1.8 to 18.8 μg/mL.
Two new spirolactone-type diterpenoids were isolated from I. sculponeatus by Jiang and coworkers in 2002 [68]. All diterpenoids were tested against K562 (chronic myelogenous leukemia) and T24 (bladder cancer) cells. Among them, sculponeatin J (9, Figure 2) showed inhibitory effects (IC50) of 0.849 and 0.642 μg/mL against K562 and T24 cells, respectively.
Four new ent-kaurane diterpenoids were isolated from the I. enanderianus in the same year by and coworkers [69]. Among which, a new spirolactone-type diterpenoid was named 6-epiangustifolin (10, Figure 2), and tested for its cytotoxicity toward K562 cells. The results showed that 10 exhibited inhibitory activity with an IC50 value of 0.0865 μg/mL against the K562 cell line, which was stronger than cis-platin, the positive reference.
One new spirolactone-type diterpenoids, laxiflorin E, and four known ones were isolated from I. eriocalyx var. laxiflora. by Niu et al. in 2002 [70]. All isolates were tested for antiproliferative activities toward K562, lung cancer A549, and T24 human cancer cell lines. Among them, laxiflorin E, calyxin A, laxiflorin C*, and laxiflorin A (11–14, Figure 2) displayed cytotoxic activity with IC50 values from 0.077 to 1.399 × 1017 μg/mL.
Han et al. isolated five new and eight known spirolactone-type diterpenoids from I. rubescens var. lushiensis in 2003. The cytotoxicity of most isolates were tested against K562 cell line. Among which, ludongnin J, guidongnin A, angustifolin, and ludongnin A (15–18, Figure 2) showed significant inhibitory effects with IC50 values from 0.18 to 0.83 μg/mL. Furthermore, compound 15 also exhibited inhibitory activities against liver cancer CA and uterine cervix cancer Hela cell lines with IC50 values below 0.70 μg/mL [71]. Moreover, in 2010, Luo et al. also found that compound 18 exhibited cytotoxicity against promyelocytic leukemia HL-60 cells with an IC50 value of 3.1 μM [72].
In the same year, Han et al. also isolated two new and four known spirolactone-type diterpenoids from I. rubescens var. lushiensis [73]. All isolates were tested for their cytotoxic effects against K562, human breast cancer Bcap37, CA, human nasopharyngeal cancer CNE, human cystic cancer BIU87, human stomach cancer BGC823, and Hela cell lines. Lushanrubescensin H, isodonoiol, isodonal, and rabdosin B (19–22, Figure 2) displayed cytotoxic activities with IC50 values from 2.29 to 28.64 μg/mL.
Three new, together with six known, spirolactone-type diterpenoids were isolated by Shen and coworkers from I. eriocalyx (Dunn.) Hara in 2005 [74]. The cytotoxicity against T-24, K562, Me180 (human cervical epithelial cancer), QGY-7701 (human hepatoma), and BIU87 cell lines. Among them, maoecrystal L (23, Figure 2) showed strong cytotoxicity with IC50 values of 2.72, 1.74, 11.23, 2.92, and 26.92 μg/mL, respectively.
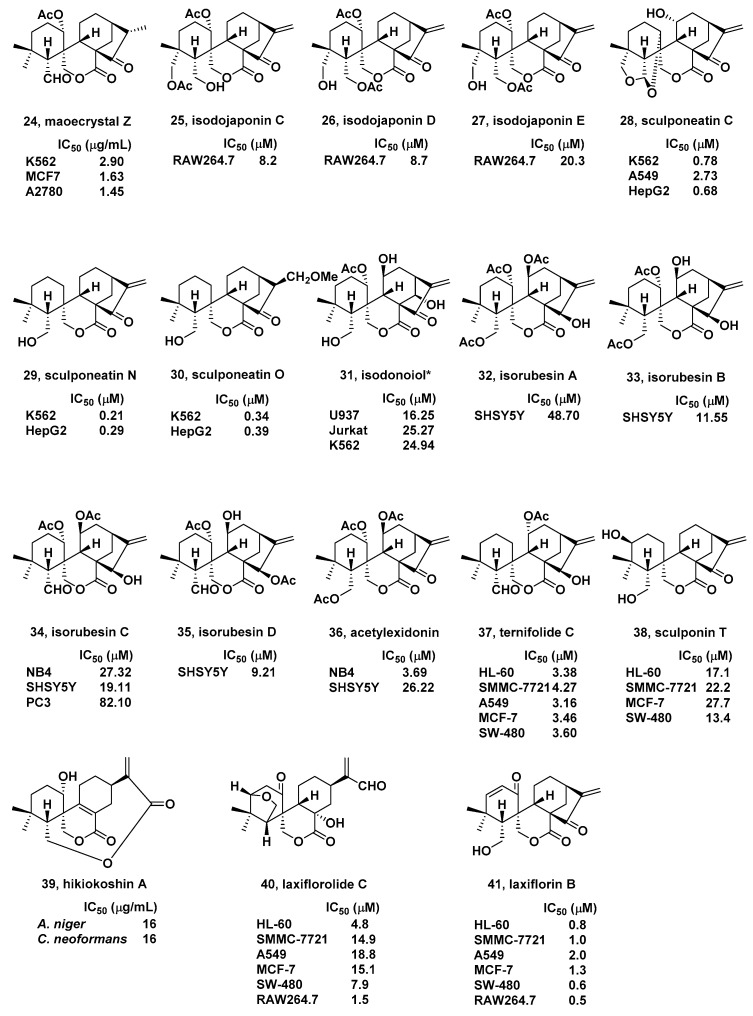
In 2006, Han and coworkers isolated a novel spirolactone-type diterpenoid, maoecrystal Z (24, Figure 3), with an unprecedented skeleton from I. eriocalyx (Labiatae) [75]. Fortunately, 24 exhibited comparable inhibitory activities against K562, human breast cancer MCF-7 and human ovarian cancer A2780 cells with IC50 values from 1.45 to 2.90 μg/mL.
Figure 3.
Chemical structures of natural spirolactone-type diterpenoids 24–41.
Three novel spirolactone-type diterpenoids, isodojaponin C–E (25–27, Figure 3), were isolated by Hong et al. from the aerial parts of I. japonicus in 2008 [76]. The inhibitory effects of LPS-induced nitric oxide (NO) production by the isolates were tested in murine macrophage RAW264.7 cells. IC50 values were 8.2, 8.7, and 20.3 μM, respectively.
In 2009, a known spirolactone-type diterpenoids, named sculponeatin C (28, Figure 3), was isolated from I. sculponeatus by Li and coworkers [77]. The results of cytotoxicity test showed that 28 exhibited strong inhibitory activities towards K562, A549, and HepG2 (human hepatoma) cells, with IC50 values of 0.78, 2.73, and 0.68 μM, respectively.
Four new spirolactone-type diterpenoids were identified by Li et al. from the aerial parts of I. sculponeatus in the 2010 [78]. Among which, sculponeatin N and sculponeatin O (29 and 30, Figure 3) displayed strong inhibitory activities (IC50) on K562 and HepG2 cell lines between 0.21 and 0.39 μM.
In the same year, Zhang and coworkers isolated one known spirolactone-type diterpenoid, isodonoiol* (31, Figure 3), from I. rubescens var. lushanensis [79]. Interestingly, in cytotoxicity assays, isodonoiol showed moderate inhibitory activities with IC50 values above 16.25 μM towards U937 (human histiocytic lymphoma), Jurkat, and K562 cell lines.
Four new spirolactone-type diterpenoids, together with four known ones, were got by Gao’s group from I. rubescens in 2011 [80]. The antitumor activities were screened against acute promyelocytic leukemia NB4, A549, neuroblastoma SHSY5Y, prostate cancer PC3, and MCF-7 cells. Among them, isorubesins A–D and acetylexidonin (32–36, Figure 3) exhibited moderate inhibitory activities (IC50 values form 3.69 to 82.10 μM).
In 2012, Zou et al. isolated a new and three known spirolactone-type diterpenoids from I. ternifolius [81]. Among them, ternifolide C (37, Figure 3) exhibited IC50 values of 4.27, 3.38, 3.46, 3.16, and 3.60 μM against hepatocellular carcinoma SMMC-7721, HL-60, MCF-7, A-549, and colon cancer SW-480 cells, respectively.
In 2014 Jiang et al. isolated one new spirolactone-type diterpenoid named sculponin T (38, Figure 3) from I. sculponeatus [82]. Fortunately, compound 38 showed moderate cytotoxic activities towards human tumor SMMC-7721, HL-60, SW-480, and MCF-7 cells with IC50s above 13.4 μM.
Three new spirolactone-type diterpenoids were isolated by Tanaka and coworkers from I. japonicus in the same year [83]. Their antifungal activities were evaluated. Particularly, hikiokoshin A (39, Figure 3) displayed antifungal activities against Cryptococcus neoformans and Aspergillus niger with IC50 values of 16 μg/mL each.
In 2014, eighteen new spirolactone-type diterpenoids were isolated and determined by Wang and coworkers from I. eriocalyx var. laxiflora [84]. The cytotoxic effects of all isolates were tested against A-549, SMMC-7721, MCF-7, HL-60, and SW-480 cells. Laxiflorolide C and laxiflorin B (40 and 41, Figure 3) exhibited selective cytotoxic activities with IC50s between 0.6 and 18.8 μM. Moreover, laxiflorolide C and laxiflorin B also showed inhibitory effects on LPS stimulated NO production in RAW264.7 cells, with IC50s of 1.5 and 0.5 μM, respectively.
3. Synthetic Spirolactone-Type Diterpenoid Derivatives
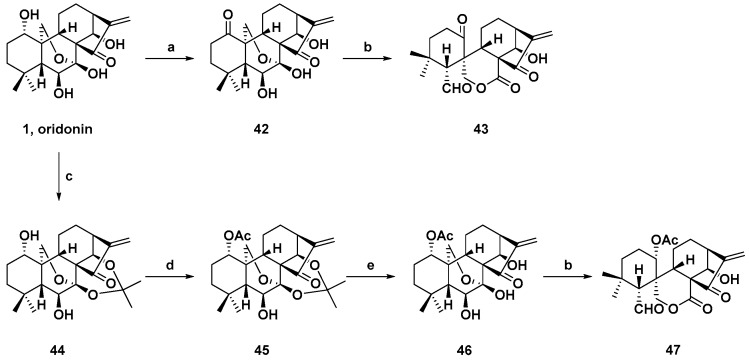
Though spirolactone-type diterpenoids exhibited cytotoxic effects with interesting molecular skeletons, the amount of spirolactone-type diterpenoids extracted from natural sources could not meet the needs of drug development. In order to achieve large scale compound supply, convenient methods have been built up. Lead tetraacetate was used as oxidation to finish C-6 and C-7 bond cleavage of commercially available oridonin to produce spirolactone-type core structure. The synthesis routine is illustrated in Scheme 1. Based on this core, diverse spirolactone-type derived compounds could be obtained [85].
Scheme 1.
Synthetic route of spirolactone-type diterpenoid skeletons from oridonin. Reagents and conditions: (a) Jones reagent, acetone, 0 °C; (b) Pb(OAc)4, Na2CO3, THF, rt; (c) 2,2-Dimethoxypropane, acetone, TsOH, 56 °C; (d) Ac2O, TEA, DMAP, rt; and (e) 10% HCl, THF, rt.
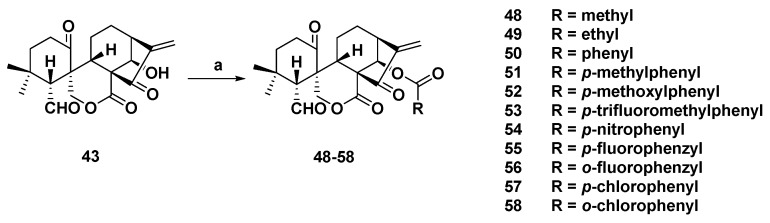
In this way, Wang et al. designed and synthesized a series of novel 14-O-derivatives of 43 (Scheme 2). All derivatives were evaluated for their antiproliferative activities against K562, human gastric cancer MGC-803, human esophageal cancer CaEs-17, and human hepatoma Bel-7402 cell lines. The results showed that they exhibited stronger cytotoxicity than 43. Among them, 51 (Table 1) exhibited the strongest cytotoxicity with IC50 values of 1.27, 2.24, 1.05, and 1.54 µM, respectively.
Scheme 2.
Synthetic route of spirolactone-type diterpenoid analogs (48–58). Reagents and conditions: (a) RCOOH, DMAP, EDCI, rt.
Table 1.
The most potent spirolactone-type diterpenoid derivatives of each series.
| Compound | IC50 [µM] | ||||
|---|---|---|---|---|---|
| K562 | MGC-803 | CaEs-17 | Bel-7402 | MCF-7 | |
| 51 | 1.27 | 2.24 | 1.05 | 1.54 | / a |
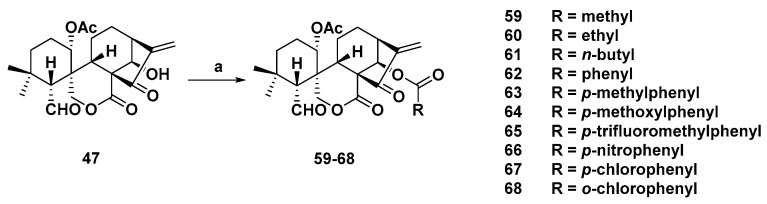
| 68 | 0.39 | 1.28 | 0.60 | 1.39 | / a |
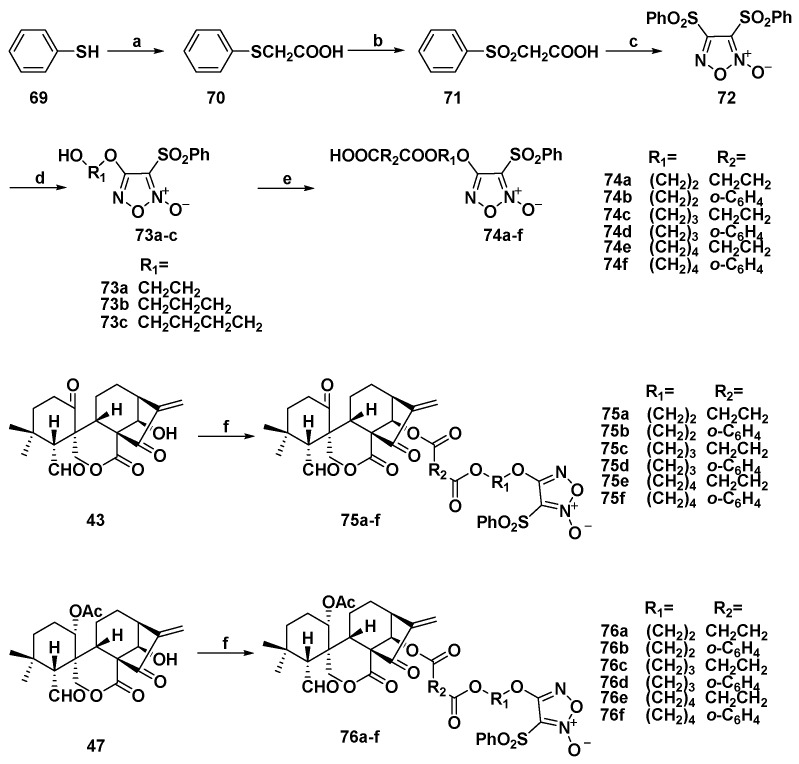
| 76d | 1.74 | 1.16 | 3.75 | 0.86 | / a |
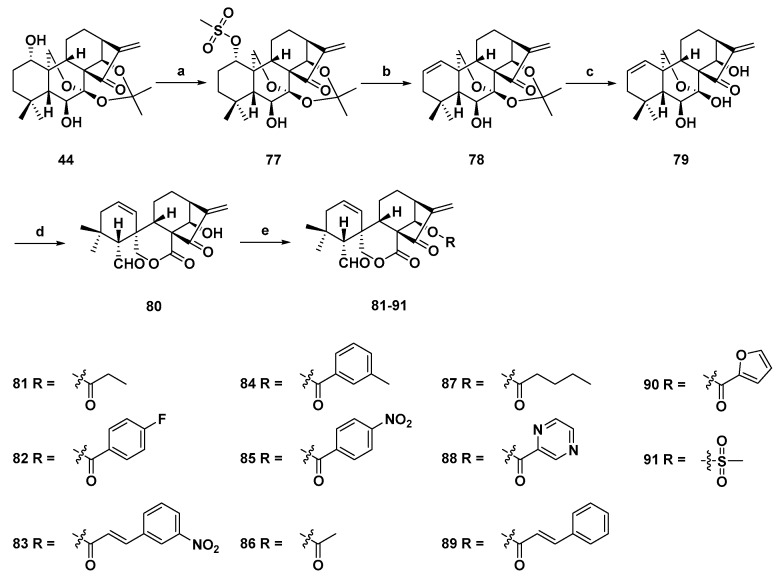
| 82 | 0.69 | 2.20 | / a | 1.80 | 0.68 |
a “/” represents not tested.
Li and coworkers linked several acids to spirolactone-type core structure with ether bond (Scheme 3) [86]. The antiproliferative activities were tested against the above four cancer cell lines. Target derivatives were also more potent than parent compound oridonin 68 (Table 1) showed the most potent inhibitory activities with IC50s below 1.39 µM. The structure–activity relationships (SARs) were also disclosed. When R were alkyl groups (59–61), with the increased length of R groups, stronger cytotoxicity could be observed in MGC-803 cell line. Furthermore, when R were aromatic groups (62–68), their activities were stronger than those of alkyl groups, particularly, when substituted by chloro. The most potent 68 was selected to explore antiproliferative mechanism. The results indicated that 68 could induce apoptosis in a dose-dependent fashion and arrest cell accumulation at G2/M phase in Bel-7402 cells.
Scheme 3.
Synthetic route of spirolactone-type diterpenoid analogs (59–68). Reagents and conditions: (a) RCOOH, DMAP, EDCI, rt.
In 2016, Xu’s Group synthesized several furozan-based NO-donating derivatives (Scheme 4) [87]. Compared with parent compounds 43 and 47, all the synthetic target molecules showed improved antiproliferative activities, especially toward Bel-7402 cell line. The SARs revealed when R2 was aromatic linkers (75b, 75d, 75f, 76b, 76d, and 76f), the antiproliferative effects were better than those of alkyl linkers (75a, 75c, 75e, 76a, 76c, and 76e). Particularly, compound 76d (Table 1) showed the most potent IC50s between 0.86 and 3.75 µM against MGC-803, K562, Bel-7402, and CaEs-17 cells. The NO-releasing properties were evaluated by Griess assay. The results showed that all derivatives more than 15 µM released NO in 1 h which would contribute to their antiproliferative activities. Furthermore, a further mechanism of 76d was studied in Bel-7402 cells. They found that 76d could induce cell cycle arrest at the S phase. It was also found that 76d could decline the mitochondrial membrane potentials which indicated that 76d induced apoptosis through intrinsic pathways.
Scheme 4.
Synthesis of NO donor/spirolactone-type diterpenoid hybrids 75a–f and 76a–f. Reagents and conditions: (a) ClCH2COOH, NaOH (aq), 60 °C; (b) 30% H2O2, AcOH, rt; (c) fuming HNO3, AcOH, 60 °C; (d) corresponding diol, NaOH (aq), THF, rt; (e) triethylamine, succinic anhydride, DMAP, rt; and (f) 74a–i, EDCI, DMAP, rt.
In order to discover more bioactive spirolactone-type diterpenoid derivatives, two series of novel derivatives with various substituents at 14-OH were designed and synthesized by Xu et al. in 2017 (Scheme 5 and Scheme 6). The antiproliferative activities of all derivatives were evaluated against four human cancer cell lines (MGC-803, MCF-7, Bel-7402, and K562). Compound 82 (Table 1) exhibited IC50s between 0.68 and 2.2 µM, which was the strongest derivatives of this series [88]. The mechanism of action of 82 was also investigated. After treatment with 82, the mitochondrial membrane potential in MCF-7 cell declined. Western blot results showed that 82 could increase the levels of p-ERK, Bax and caspase 3, and reduced the expression of P53 and Bcl-2. 82 also induced cell accumulated at the G2/M phase. In short, these results illustrate that derivative 82 induced apoptosis through a mitochondria-related pathway.
Scheme 5.
Synthesis of spirolactone-type 6,7-seco-ent-kauranoid derivatives (80–91). Reagents and conditions: (a) MsCl, TEA, 0 °C; (b) LiBr, Li2CO3, DMF, 110 °C; (c) 10% HCl, THF (v/v, 1:1), rt; (d) Pb(OAc)4, K2CO3, THF, 0 °C; and (e) EDCI, DMAP, DCM, rt.
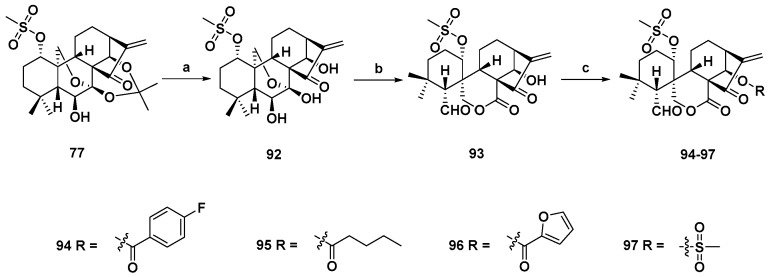
Scheme 6.
Synthesis of spirolactone-type 6,7-seco-ent-kauranoid derivatives (94–97). Reagents and conditions: (a) 10% HCl, THF (v/v, 1:1), rt; (b) Pb(OAc)4, K2CO3, THF, 0 °C; and (c) EDCI, DMAP, DCM, rt.
4. Conclusions
In summary, natural spirolactone-type diterpenoids exhibited cytotoxic effects. Its synthetic derivatives showed more potent antiproliferative effects than the corresponding parent compounds. Hence, spirolactone-type diterpenoids are worthy of further research. However, there are few in-depth pharmacological reports on spirolactone-type diterpenoids so far. We hold the view that, for drug exploration, further studies should firstly focus on the detailed mechanism study. Based on these, spirolactone-type diterpenoid derivatives with clear target should be explored. We hope this review can provide useful information in the field of bioactive natural and synthetic spirolactone-type diterpenoids.
Funding
This work was supported by the National Natural Science Foundation of China (21502121, 21772124), the General Scientific Research Projects of Department of Education in Liaoning Province (2017LQN05), the Natural Science Foundation of Liaoning Province (20170540858), the Career Development Support Plan for Young and Middle-aged Teachers in Shenyang Pharmaceutical University, and the Key Laboratory of Quality Control of TCM of Liaoning Province (17-137-1-00).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues T., Reker D., Schneider P., Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 3.DeCorte B.L. Underexplored opportunities for natural products in drug discovery. J. Med. Chem. 2016;59:9295–9304. doi: 10.1021/acs.jmedchem.6b00473. [DOI] [PubMed] [Google Scholar]
- 4.Li D., Hu X., Han T., Liao J., Xiao W., Xu S., Li Z., Wang Z., Hua H., Xu J. NO-releasing enmein-type diterpenoid derivatives with selective antiproliferative activity and effects on apoptosis-related proteins. Molecules. 2016;21:1193. doi: 10.3390/molecules21091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S.P., Sashidhara K.V. Lipid lowering agents of natural origin: An account of some promising chemotypes. Eur. J. Med. Chem. 2017;140:331–348. doi: 10.1016/j.ejmech.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H., Bai L., He J., Zhong L., Duan X., Ouyang L., Zhu Y., Wang T., Zhang Y., Shi J. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur. J. Med. Chem. 2017;141:257–272. doi: 10.1016/j.ejmech.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 7.Joshi P., Vishwakarma R.A., Bharate S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017;138:273–292. doi: 10.1016/j.ejmech.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 8.Tian K., Xu F., Gao X., Han T., Li J., Pan H., Zang L., Li D., Li Z., Uchita T., et al. Nitric oxide-releasing derivatives of brefeldin A as potent and highly selective anticancer agents. Eur. J. Med. Chem. 2017;136:131–143. doi: 10.1016/j.ejmech.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Han T., Li J., Xue J., Li H., Xu F., Cheng K., Li D., Li Z., Gao M., Hua H. Scutellarin derivatives as apoptosis inducers: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2017;135:270–281. doi: 10.1016/j.ejmech.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Gao X., Li J., Wang M., Xu S., Liu W., Zang L., Li Z., Hua H., Xu J., Li D. Novel enmein-type diterpenoid hybrids coupled with nitrogen mustards: Synthesis of promising candidates for anticancer therapeutics. Eur. J. Med. Chem. 2018;146:588–598. doi: 10.1016/j.ejmech.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 11.Han T., Tian K., Pan H., Liu Y., Xu F., Li Z., Uchita T., Gao M., Hua H., Li D. Novel hybrids of brefeldin A and nitrogen mustards with improved antiproliferative selectivity: Design, synthesis and antitumor biological evaluation. Eur. J. Med. Chem. 2018;150:53–63. doi: 10.1016/j.ejmech.2018.02.088. [DOI] [PubMed] [Google Scholar]
- 12.Fujita E., Nagao Y., Kaneko K., Nakazawa S., Kuroda H. The antitumor and antibacterial activity of the Isodon diterpenoids. Chem. Pharm. Bull. 1976;24:2118–2127. doi: 10.1248/cpb.24.2118. [DOI] [PubMed] [Google Scholar]
- 13.Hu Z., Zhan R., Du X., Su J., Li X.N., Yang J.H., Zhang H.B., Li Y., Sun H.D., Li G.P., et al. Cytotoxic ent-kaurane diterpenoids from Isodon henryi. Chem. Pharm. Bull. 2011;59:1562–1566. doi: 10.1248/cpb.59.1562. [DOI] [PubMed] [Google Scholar]
- 14.Li F.Y., Fu Y.H., Liu B., Liu Z.C., Li D.P., Liang D.J., Zhang W., Cao Y.G., Zhang N.S., Zhang X.C., et al. Stevioside suppressed inflammatory cytokine secretion by downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation. 2012;35:1669–1675. doi: 10.1007/s10753-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 15.Kubota T., Matsuura T., Tsutsui T., Uyeo S., Takahashi M., Irie H., Numata A., Fujita T., Okamoto T., Natsume M., et al. The constitution and stereochemistry of enmein. Tetrahedron Lett. 1964;20:1243–1256. doi: 10.1016/S0040-4039(00)90460-X. [DOI] [Google Scholar]
- 16.Osawa K., Yasuda H., Maruyama T., Morita H., Takeya K., Itokawa H., Okuda K. An investigation of diterpenes from the leaves of Rabdosia trichocarpa and their antibacterial activity against oral microorganisms. Chem. Pharm. Bull. 1994;42:922–925. doi: 10.1248/cpb.42.922. [DOI] [PubMed] [Google Scholar]
- 17.Satooka H., Isobe T., Nitoda T., Kubo I. Melanogenesis inhibitors from Rabdosia japonica. Phytomedicine. 2012;19:1016–1023. doi: 10.1016/j.phymed.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Lin L.G., Ung C.O., Feng Z.L., Huang L., Hu H. Naturally occurring diterpenoid dimers: Source, biosynthesis, chemistry and bioactivities. Planta Med. 2016;82:1309–1328. doi: 10.1055/s-0042-114573. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y., Ding C., Ye N., Liu Z., Wold E.A., Chen H., Wild C., Shen Q., Zhou J. Discovery and development of natural product oridonin inspired anticancer agents. Eur. J. Med. Chem. 2016;122:102–117. doi: 10.1016/j.ejmech.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Li H., Xu F., Gao X., Li J., Xu S., Zhang D., Wu X., Xu J., Hua H., et al. Diterpenoid lead stevioside and its hydrolysis products steviol and isosteviol: Biological activity and structural modification. Eur. J. Med. Chem. 2018;156:885–906. doi: 10.1016/j.ejmech.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T., Nakamura S., Kojima N., Hasei T., Yamashita M., Watanabe T., Matsuda H. Antimutagenic activity of ent-kaurane diterpenoids from the aerial parts of Isodon japonicus. Tetrahedron Lett. 2017;58:3574–3578. doi: 10.1016/j.tetlet.2017.07.106. [DOI] [Google Scholar]
- 22.Kampan N.C., Madondo M.T., McNally O.M., Quinn M., Plebanski M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/413076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guido G., Massimo P., Nunzio O., Pietro P., Marianna V., Tania D.R., Luigi C., Giuseppe T., Mario R.D. Nano albumin bound paclitaxel in pancreatic cancer: Current evidences and future directions. World J. Gastroent. 2017;23:5875–5886. doi: 10.3748/wjg.v23.i32.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta N., Hatoum H., Dy G.K. First line treatment of advanced non-small-cell lung cancer—Specific focus on albumin bound paclitaxel. Int. J. Nanomed. 2014;9:209–221. doi: 10.2147/IJN.S41770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S.Q., Wang C., Chang L.M., Zhou K.R., Wang J.W., Yu K., Yang D.X., Shi H.G., Wang R., Shi X.L., et al. Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget. 2016;7:72990–73002. doi: 10.18632/oncotarget.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita E., Fujita T., Shibuya M. Diterpenoid constituents of Isodon trichocarpus and Isodon japonicus (terpenoids IV) Tetrahedron Lett. 1966;7:3153–3162. doi: 10.1016/S0040-4039(01)99930-7. [DOI] [Google Scholar]
- 27.Fujita E., Fujita T., Katayama H., Shibuya M. Oridonin, a new diterpenoid from Isodon species. Chem. Commun. 1967;101:252–254. doi: 10.1039/c19670000252. [DOI] [Google Scholar]
- 28.Wu J., Ding Y., Chen C.H., Zhou Z., Ding C., Chen H., Zhou J., Chen C. A new oridonin analog suppresses triple-negative breast cancer cells and tumor growth via the induction of death receptor 5. Cancer Lett. 2016;380:393–402. doi: 10.1016/j.canlet.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Chen W., Zhou J.C., Wu K.J., Huang J., Ding Y., Yun E.J., Wang B., Ding C.Y., Hernandez E., Santoyo J., et al. Targeting XBP1-mediated β-catenin expression associated with bladder cancer with newly synthetic Oridonin analogues. Oncotarget. 2016;7:56842–56854. doi: 10.18632/oncotarget.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D., Han T., Liao J., Hu X., Xu S., Tian K., Gu X., Cheng K., Li Z., Hua H., et al. Oridonin, a promising ent-kaurane diterpenoid lead compound. Int. J. Mol. Sci. 2016;17:1395. doi: 10.3390/ijms17091395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Y., Liu P., Zhu H., Shi X., Zhao C., Wang N., Zhang L. A sensitive analysis method for 7 diterpenoids in rat plasma by liquid chromatography-electrospray ionization mass spectrometry and its application to pharmacokinetic study of Isodon serra extract. J. Chromatogr. A. 2011;1218:7771–7780. doi: 10.1016/j.chroma.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Zhao A.H., Zhang Y., Xu Z.H., Liu J.W., Jia W. Immunosuppressive ent-kaurene diterpenoids from Isodon serra. Helv. Chim. Acta. 2004;87:3160–3166. doi: 10.1002/hlca.200490281. [DOI] [Google Scholar]
- 33.Takeda Y., Fujita T., Sun H.D., Minami Y., Ochi M., Chen C.C. Revision of the structures of isodonal, rabdolasional and related diterpenoids. Chem. Pharm. Bull. 1990;38:1877–1880. doi: 10.1248/cpb.38.1877. [DOI] [Google Scholar]
- 34.Chen Y.Z., Wu Z.W. The crystal and molecular structure of Rabdophyllin G. Acta Chim. Sin. 1984;42:645–649. [Google Scholar]
- 35.Fujita E., Fuji K., Sai M., Node M. The structure of trichorabdal B and its transformation into a novel skeleton; X-ray crystal structures. J. Chem. Soc. Chem. Commun. 1981;621:899–900. doi: 10.1039/c39810000899. [DOI] [Google Scholar]
- 36.Node M., Sai M., Fujita E., Fuji K. Antitumor diterpenoids from Rabdosia trichocappa: Trichorabdal E, F, and H and G Acetate. Heterocycles. 1984;22:1701–1704. [Google Scholar]
- 37.Chen Y.Z., Li Y.Z., Yue J.M. Diterpenoids from Rabdosia Gaponica var. Glaucocalyx. J. Nat. Prod. 1989;52:886–887. doi: 10.1021/np50064a041. [DOI] [Google Scholar]
- 38.Node M., Sai M., Fujita E., Fuji K. Terpenoids. LII.: The structures of trichorabdal F, trichorabdal G acetate, and trichorabdal H. Acomment on the structure of shikodonin. Chem. Pharm. Bull. 1989;37:1470–1471. doi: 10.1248/cpb.37.1470. [DOI] [Google Scholar]
- 39.Huang H., Zhang H.J., Sun H.D. Diterpenoids from Rabdosia Setschwanensis. Phytochemistry. 1990;29:2591–2595. [Google Scholar]
- 40.Takeda Y., Ichihara T. Isolongirabdiol, a new diterpenoid from Rabdosia Longituba. J. Nat. Prod. 1990;53:138–142. doi: 10.1021/np50067a018. [DOI] [Google Scholar]
- 41.Takeda Y., Ikawa A., Matsumoto T., Terao H., Otsuka H. Diterpenoid constituents of Rabdosia Longituba. Planta Med. 1992;58:470–471. doi: 10.1055/s-2006-961518. [DOI] [PubMed] [Google Scholar]
- 42.Takeda Y., Ikawa A., Matsumoto T., Terao H., Otsuka H. Diterpenoids having ent-kaurene and ent-spiro-seco-kaurene skeletons from Rabdosia Longituba. Phytochemistry. 1992;31:1687–1690. doi: 10.1016/0031-9422(92)83129-M. [DOI] [Google Scholar]
- 43.Takeda Y., Ichihara T., Otsuka H., Kido M. Longirabdolactone and longirabdacetal, 6,7-seco-ent-kaurenoids from Rabdosia Longituba. Phytochemistry. 1993;33:643–646. doi: 10.1016/0031-9422(93)85465-4. [DOI] [Google Scholar]
- 44.Takeda Y., Futatsuishi Y., Ichihara T., Matsumoto T., Terao H., Terada H., Otsuka H. Rabdokaurins C and D, two new diterpenoids from Rabdosia Longituba. Chem. Pharm. Bull. 1993;41:685–687. doi: 10.1248/cpb.41.685. [DOI] [Google Scholar]
- 45.Shen X.Y., Isogai A., Furihata K., Lin Z.W., Sun H.D., Suzuki A. 6,7-seco-ent-Kaurane diterpenoid from Rabdosia Eriocalyx. Phytochemistry. 1994;35:820–821. [Google Scholar]
- 46.Chen S.N., Yue J.M., Chen S.Y., Lin Z.W., Qin G.W., Sun H.D., Chen Y.Z. Diterpenoids from Isodon eriocalyx. J. Nat. Prod. 1999;62:782–784. doi: 10.1021/np9804278. [DOI] [PubMed] [Google Scholar]
- 47.Niu X.M., Li S.H., Zhao Q.S., Lin Z.W., Sun H.D., Lu Y., Wang C., Zheng Q.T. Two novel ent-kaurane diterpenoids isolated from Isodon eriocalyx var. laxiflora. Tetrahedron Lett. 2002;43:661–664. doi: 10.1016/S0040-4039(01)02229-8. [DOI] [Google Scholar]
- 48.Li L.M., Li G.Y., Li S.H., Weng Z.Y., Xiao W.L., Han Q.B., Ding L.S., Lou L.G., Sun H.D. Cytotoxic ent-kauranoids from Isodon parvifolius. Chem. Biodivers. 2006;3:1031–1038. doi: 10.1002/cbdv.200690101. [DOI] [PubMed] [Google Scholar]
- 49.Li L.M., Li G.Y., Ding L.S., Lei C., Yang L.B., Zhao Y., Weng Z.Y., Li S.H., Huang S.X., Xiao W.L., et al. Sculponins A–C, three new 6,7-seco-ent-kauranoids from Isodon sculponeatus. Tetrahedron Lett. 2007;48:9100–9103. doi: 10.1016/j.tetlet.2007.10.133. [DOI] [Google Scholar]
- 50.Yang L.B., Huang S.X., Li L.M., Zhao Y., Lei C., Xiao W.L., Pu J.X., Han Q.B., Sun H.D. ent-Kaurane diterpenoids from Isodon japonicus. Helv. Chim. Acta. 2007;90:2375–2379. doi: 10.1002/hlca.200790243. [DOI] [Google Scholar]
- 51.Zhang J.X., Wang Y.X., He Z.A., Yan F.L. Diterpenoids from Isodon excisoides. J. Chem. Res. 2009;1:35–37. doi: 10.3184/030823409X396391. [DOI] [Google Scholar]
- 52.Zhang J.X., Wang Y.X., He Z.A., Yan F.L., Sun H.D. Two new ent-kaurane diterpenoids from Isodon excisoides. Chin. Chem. Lett. 2009;20:201–203. doi: 10.1016/j.cclet.2008.10.040. [DOI] [Google Scholar]
- 53.Li X.N., Pu J.X., Du X., Lou L.G., Li L.M., Huang S.X., Zhao B., Zhang M., He F., Luo X., et al. Structure and cytotoxicity of diterpenoids from Isodon eriocalyx. J. Nat. Prod. 2010;73:1803–1809. doi: 10.1021/np1004328. [DOI] [PubMed] [Google Scholar]
- 54.Xie R.J., Yan F.L., Hai G.F., Hou R.J., Ding M.M., Bai Y.X. Two new diterpenoids and other constituents from Isodon rubescens. Fitoterapia. 2011;82:726–730. doi: 10.1016/j.fitote.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Wang W.Q., Xuan L.J. ent-6,7-Secokaurane diterpenoids from Rabdosia serra and their cytotoxic activities. Phytochemistry. 2016;122:119–125. doi: 10.1016/j.phytochem.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Fujita E., Shibuya M., Nakamura S., Okada Y., Fujita T. Total synthesis of enmein. J. Chem. Soc. Chem. Commun. 1972;19:1107. doi: 10.1039/c39720001107. [DOI] [Google Scholar]
- 57.Fujita E., Shibuya M., Nakamura S., Okada Y., Fujita T. Terpenoids. Part XXVIII. Total synthesis of enmein. J. Chem. Soc. Perkin Trans. 1974;1:165–177. doi: 10.1039/p19740000165. [DOI] [Google Scholar]
- 58.George A., Lewis N.M. Conversion of gibberellic acid into the b-ring seco-kaurenoid, longirabdolactone. Aust. J. Chem. 2003;56:805–809. [Google Scholar]
- 59.Cha J.Y., Yeoman J.T., Reisman S.E. A concise total synthesis of (−)-maoecrystal Z. J. Am. Chem. Soc. 2011;133:14964–14967. doi: 10.1021/ja2073356. [DOI] [PubMed] [Google Scholar]
- 60.Yeoman J.T.S., Cha J.Y., Mak V.W., Reisman S.E. A unified strategy for the synthesis of (−)-maoecrystal Z, (−)-trichorabdal A, and (−)-longikaurin E. Tetrahedron. 2014;70:4070–4088. doi: 10.1016/j.tet.2014.03.071. [DOI] [Google Scholar]
- 61.Behera T.K., Nurul Islam S.K., Singh V. Maoecrystal V: A formidable synthetic challenge. J. Chem. Sci. 2013;125:1301–1314. doi: 10.1007/s12039-013-0524-5. [DOI] [Google Scholar]
- 62.Lazarski K.E., Moritz B.J., Thomson R.J. The total synthesis of Isodon diterpenes. Angew. Chem. 2014;53:10588–10599. doi: 10.1002/anie.201404482. [DOI] [PubMed] [Google Scholar]
- 63.Riehl P.S., DePorre Y.C., Armaly A.M., Groso E.J., Schindler C.S. New avenues for the synthesis of ent-Kaurene diterpenoids. Tetrahedron. 2015;71:6629–6650. doi: 10.1016/j.tet.2015.04.116. [DOI] [Google Scholar]
- 64.Sun H.D., Huang S.X., Han Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006;23:673–698. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- 65.Liu M., Wang W.G., Sun H.D., Pu J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017;34:1090–1140. doi: 10.1039/C7NP00027H. [DOI] [PubMed] [Google Scholar]
- 66.Sun H.D., Lin Z.W., Niu F.D., Lin L.Z., Chai H.B., John M.P., Geoffrey A.C. Cytotoxic ent-kaurene diterpenoids from three Isodon species. Phytochemistry. 1995;38:437–442. doi: 10.1016/0031-9422(94)00626-5. [DOI] [PubMed] [Google Scholar]
- 67.Sun H.D., Lin Z.W., Niu F.D., Shen P.Q., Pan L.T., Lin L.Z., Geoffrey A.C. Diterpenoids from Isodon Eriocalyx var. Laxiflora. Phytochemistry. 1995;38:1451–1455. doi: 10.1016/0031-9422(94)00815-B. [DOI] [PubMed] [Google Scholar]
- 68.Jiang B., Mei S.X., Zhao A.H., Sun H.D., Lu Y., Zheng Q.T. Diterpenoids from Isodon sculponeatus. Chin. J. Chem. 2002;20:887–890. doi: 10.1002/cjoc.20020200916. [DOI] [Google Scholar]
- 69.Na Z., Xiang W., Niu X.M., Mei S.X., Lin Z.W., Li C.M., Sun H.D. Diterpenoids from Isodon enanderianus. Phytochemistry. 2002;60:55–60. doi: 10.1016/S0031-9422(02)00073-0. [DOI] [PubMed] [Google Scholar]
- 70.Niu X.M., Li S.H., Li M.L., Zhao Q.S., Mei S.X., Na Z., Wang S.J., Lin Z.W., Sun H.D. Cytotoxic ent-kaurene diterpenoids from Isodon eriocalyx var. laxiflora. Planta Med. 2002;68:528–533. doi: 10.1055/s-2002-32551. [DOI] [PubMed] [Google Scholar]
- 71.Han Q.B., Zhao A.H., Zhang J.X., Lu Y., Zhang L.L., Zheng Q.T., Sun H.D. Cytotoxic constituents of Isodon rubescens var. lushiensis. J. Nat. Prod. 2003;66:1391–1394. doi: 10.1021/np030165w. [DOI] [PubMed] [Google Scholar]
- 72.Luo X., Pu J.X., Xiao W.L., Zhao Y., Gao X.M., Li X.N., Zhang H.B., Wang Y.Y., Li Y., Sun H.D. Cytotoxic ent-kaurane diterpenoids from Isodon rubescens var. lushiensis. J. Nat. Prod. 2010;73:1112–1116. doi: 10.1021/np100110u. [DOI] [PubMed] [Google Scholar]
- 73.Han Q.B., Li M.L., Li S.H., Mou Y.K., Lin Z.W., Sun H.D. ent-Kaurane diterpenoids from Isodon rubescens var. Lushanensis. Chem. Pharm. Bull. 2003;51:790–793. doi: 10.1248/cpb.51.790. [DOI] [PubMed] [Google Scholar]
- 74.Shen Y.H., Wen Z.Y., Xu G., Xiao W.L., Peng L.Y., Lin Z.W., Sun H.D. Cytotoxic ent-kaurane diterpenoids from Isodon eriocalyx. Chem. Biodivers. 2005;2:1665–1672. doi: 10.1002/cbdv.200590136. [DOI] [PubMed] [Google Scholar]
- 75.Han Q.B., Cheung S., Tai J., Qiao C.F., Song J.Z., Tso T.F., Sun H.D., Xu H.X. Maoecrystal Z, a cytotoxic diterpene from Isodon eriocalyx with a unique skeleton. Org. Lett. 2006;8:4727–4730. doi: 10.1021/ol061757j. [DOI] [PubMed] [Google Scholar]
- 76.Hong S.S., Lee S.A., Han X.H., Hwang J.S., Lee C., Lee D., Hong J.T., Kim Y., Lee H., Hwang B.Y. ent-Kaurane diterpenoids from Isodon japonicus. J. Nat. Prod. 2008;71:1055–1058. doi: 10.1021/np0705965. [DOI] [PubMed] [Google Scholar]
- 77.Li L.M., Li G.Y., Pu J.X., Xiao W.L., Ding L.S., Sun H.D. ent-Kaurane and cembrane diterpenoids from Isodon sculponeatus and their cytotoxicity. J. Nat. Prod. 2009;72:1851–1856. doi: 10.1021/np900406c. [DOI] [PubMed] [Google Scholar]
- 78.Li X., Pu J.X., Weng Z.Y., Zhao Y., Zhao Y., Xiao W.L., Sun H.D. 6,7-seco-ent-Kaurane diterpenoids from Isodon sculponeatus with cytotoxic activity. Chem. Biodivers. 2010;7:2888–2896. doi: 10.1002/cbdv.200900302. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H.B., Pu J.X., Wang Y.Y., He F., Zhao Y., Li X.N., Luo X., Xiao W.L., Li Y., Sun H.D. Four new ent-kauranoids from Isodon rubescens var. lushanensis and data reassignment of dayecrystal B. Chem. Pharm. Bull. 2010;58:56–60. doi: 10.1248/cpb.58.56. [DOI] [PubMed] [Google Scholar]
- 80.Gao X.M., Luo X., Pu J.X., Wu Y.L., Zhao Y., Yang L.B., He F., Li X.N., Xiao W.L., Chen G.Q., et al. Antiproliferative diterpenoids from the leaves of Isodon rubescens. Planta Med. 2011;77:169–174. doi: 10.1055/s-0030-1250165. [DOI] [PubMed] [Google Scholar]
- 81.Zou J., Du X., Peng G., Shi Y.M., Wang W.G., Zhan R., Kong L.M., Li X.N., Li Y., Pu J.X., et al. Ternifolide A, a new diterpenoid possessing a rare macrolide motif from Isodon ternifolius. Org. Lett. 2012;14:3210–3213. doi: 10.1021/ol3013205. [DOI] [PubMed] [Google Scholar]
- 82.Jiang H.Y., Wang W.G., Zhou M., Wu H.Y., Zhan R., Du X., Pu J.X., Sun H.D. 6,7-seco-ent-Kaurane diterpenoids from Isodon sculponeatus and their bioactivity. Chin. Chem. Lett. 2014;25:541–544. doi: 10.1016/j.cclet.2014.01.041. [DOI] [Google Scholar]
- 83.Tanaka N., Tsuji E., Sakai K., Gonoi T., Kobayashi J. Hikiokoshins A–I, diterpenes from the leaves of Isodon japonicus. Phytochemistry. 2014;102:205–210. doi: 10.1016/j.phytochem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Wang W.G., Yan B.C., Li X.N., Du X., Wu H.Y., Zhan R., Li Y., Pu J.X., Sun H.D. 6,7-seco-ent-Kaurane-type diterpenoids from Isodon eriocalyx var. laxiflora. Tetrahedron. 2014;70:7445–7453. doi: 10.1016/j.tet.2014.08.018. [DOI] [Google Scholar]
- 85.Wang L., Li D., Xu S., Cai H., Yao H., Zhang Y., Jiang J., Xu J. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: Synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur. J. Med. Chem. 2012;52:242–250. doi: 10.1016/j.ejmech.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Li D., Cai H., Jiang B., Liu G., Wang Y., Wang L., Yao H., Wu X., Sun Y., Xu J. Synthesis of spirolactone-type diterpenoid derivatives from kaurene-type oridonin with improved antiproliferative effects and their apoptosis-inducing activity in human hepatoma Bel-7402 cells. Eur. J. Med. Chem. 2013;59:322–328. doi: 10.1016/j.ejmech.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Li D., Han T., Tian K., Tang S., Xu S., Hu X., Wang L., Li Z., Hua H., Xu J. Novel nitric oxide-releasing spirolactone-type diterpenoid derivatives with in vitro synergistic anticancer activity as apoptosis inducer. Bioorg. Med. Chem. Lett. 2016;26:4191–4196. doi: 10.1016/j.bmcl.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 88.Xu S., Yao H., Hu M., Li D., Zhu Z., Xie W., Yao H., Wu L., Chen Z.S., Xu J. 6,7-seco-ent-Kauranoids derived from oridonin as potential anticancer agents. J. Nat. Prod. 2017;80:2391–2398. doi: 10.1021/acs.jnatprod.7b00057. [DOI] [PubMed] [Google Scholar]