Abstract
Ticks are vectors of several microorganisms responsible for infectious diseases in human and animals, such as Anaplasma phagocytophilum and Coxiella burnetii. In this study, we investigated the prevalence of these two bacteria in 62 889 Ixodes ricinus ticks in selected regions covering all Switzerland. A high prevalence of 11.9% of A. phagocytophilum DNA was observed by real-time PCR on 8534 pools of ticks. This pool prevalence corresponds to an estimated prevalence of 1.71% in individual tick. A total of 144 of the 171 collection sites (84.2%) were positive for the presence of A. phagocytophilum, and these sites were homogenously distributed throughout Switzerland. Such prevalence and geographical distribution underline the risk of human and animal exposure to A. phagocytophilum and highlight the need to assess the epidemiology and clinical diagnosis of human and animal anaplasmosis in Switzerland. However, DNA of C. burnetii was never found in any tick pool. This absence suggests a very low role of I. ricinus ticks as vector and reservoir of C. burnetii in Switzerland, and it supports previous reports demonstrating the role of sheep and goats in the epidemiology of Q fever. However, considering its pathogenic potential, it is necessary to keep monitoring for the possible reemergence of this bacterium in ticks in the future.
Keywords: Anaplasma phagocytophilum, Coxiella burnetii, human granulocytic anaplasmosis, Ixodes ricinus, Q fever, ticks
Introduction
Anaplasma phagocytophilum is a Gram-negative intracellular bacterium considered to be an emerging pathogen able to infect a broad range of mammalian hosts. This microorganism can be transmitted by diverse ectoparasites, especially by Ixodes ticks [1]. In humans, A. phagocytophilum infects mainly cells of bone marrow origin, such as neutrophils and granulocytes, which are responsible of granulocytic anaplasmosis. Human granulocytic anaplasmosis is commonly associated to a transient febrile illness, but it may also represent a severe, even fatal, life-threatening disease that either occurs as sporadic cases or during outbreaks [2]. Ixodes ricinus ticks are recognized as the main vector of A. phagocytophilum in Europe; their prevalence in this region ranges from 0.4% to 66.7%, depending on geographical and environmental factors [1], [3]. In Switzerland, the prevalence of A. phagocytophilum DNA in I. ricinus ticks is about 1.5% [2], [4], [5], [6], and the seroprevalence in humans reaches approximately 5% to 17.1% of the studied populations, sometimes biased towards tick-exposed individuals [7], [8]. However, the major part of these studies has been done on a small number of ticks, which are often sampled from a highly restricted geographical area. Larger epidemiologic studies are warranted.
Ticks are known to carry other intracellular microorganisms such as Coxiella burnetii, a Gram-positive bacterium responsible for Q fever in humans and animals. Even if the infection is usually asymptomatic in humans, the disease can be severe and chronic [9]. In the western part of Switzerland, two human Q fever outbreaks occurred, one in 1983 with 415 cases [10] and another in 2012 with 14 cases [11]. As it often happens, during these two outbreaks, sheep flocks were identified as the source, and the bacteria were most probably transmitted from animals to humans by aerosols [9], [11]. Whether ticks may have played a role in the dissemination of C. burnetii was not investigated during these two outbreaks.
To increase our knowledge of anaplasmosis and coxiellosis epidemiology in Switzerland, we investigated the prevalence of A. phagocytophilum and C. burnetii DNA in more than 60 000 questing I. ricinus ticks on a large, global-country-scale level.
Materials and methods
Tick collection and pooling
I. ricinus ticks were sampled in 171 collection sites in Switzerland on the occasion of a Swiss army training course in 2008, as previously described [12]. Practically, in 2008, a total of 62 889 individual ticks were caught by flagging low vegetation and pooled in 8534 pools of one to ten individual ticks, separated according to their developmental stage (nymphs and adults, and females and males). DNA was automatically extracted with the QIAsymphony SP system (Qiagen) according to the protocol described by Gäumann et al. [12].
PCR methods
DNA extracted from ticks were subjected to specific real-time PCRs to detect the presence of DNA from A. phagocytophilum and C. burnetii. Briefly, for A. phagocytophilum, a 77 bp fragment of the msp2 gene was amplified as previously developed and described [13]. For C. burnetii, a 82 bp fragment located on a highly conserved region of the ompA gene was amplified according to the protocol developed by Jaton et al. [14]. For both PCRs, positive controls were plasmids carrying targeted sequences, and the run was only validated when the ten copies positive controls were positive. Each run was also validated by negative controls of both DNA extractions and PCR Master Mix.
Statistical analysis
Maximum likelihood estimates of individual bacterial DNA prevalence in ticks were estimated using an online method (http://www.abcrc.org.au) based on generalized linear modelling that took into account the number and size of the pools. This model assumes that our test is perfectly sensitive and specific, and that the confidence level is 0.95 [15].
Results
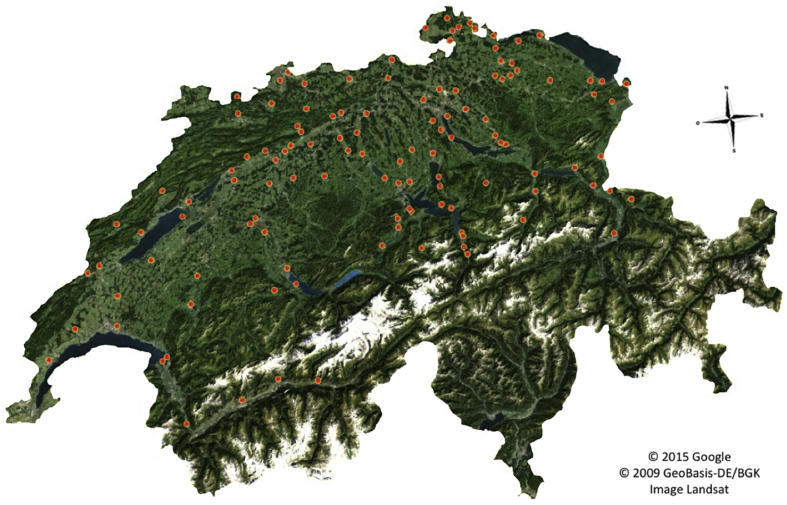
Among the 8534 tested pools, 1018 were positive for the presence of A. phagocytophilum DNA. This corresponded to a pool prevalence of 11.9% (Table 1). This pool prevalence was associated with a maximum infection rate of 12% (7535/62 889), if all ticks of a positive pool were infected and assuming a minimum infection rate of 1.6% (1018/62 889) if only one tick in a positive pool was infected (Table 1). The maximum likelihood estimate of the prevalence in individual ticks [15] was equal to 1.71% (Table 1). During sampling, ticks were classified according to their differentiation stage, with 4331 pools of nymphs and 4203 pools of adults, separated in males and females. Prevalences of A. phagocytophilum DNA within pool of adults and nymphs were almost similar, at 11.8% and 12.1%, respectively (Table 1). However, the estimated prevalence in individual ticks was twice as large for adults, at 2.55%, than for nymphs, at 1.3%. Within pools of adults, we observed a comparable similarity between pools of males, with a prevalence of 11.4% (242/2130), and pools of females, with a prevalence of 12.2% (253/2073). Concerning the geographical distribution, A. phagocytophilum DNA was present in tick pools coming from 84.2% of all the collection sites (144/171), and these sites were homogenously distributed throughout Switzerland (Fig. 1).
Table 1.
Prevalence of Anaplasma phagocytophilum DNA within Ixodes ricinus ticks throughout Switzerland
| Sample | No. of ticks | No. of pools | Pool prevalence | Estimated prevalence in individual ticksa | Maximum infection rate | Minimum infection rate |
|---|---|---|---|---|---|---|
| Nymphs | 42 576 | 4331 | 12.1% (523/4331) | 1.3% | 8.2% (5134/62 879) | 0.8% (523/62 879) |
| Adults | 20 313 | 4203 | 11.8% (495/4203) | 2.55% | 3.8% (2401/62 879) | 0.8% (495/62 879) |
| Total | 62 889 | 8534 | 11.9% (1018/8534) | 1.71% | 12% (7535/62 879) | 1.6% (1018/62 879) |
Estimated prevalence using maximum likelihood method of Williams and Moffitt [15].
Fig. 1.
Geographical distribution of DNA of Anaplasma phagocytophilum in Ixodes ricinus ticks in Switzerland. Geographical distribution of 1018 pools that gave positive results using A. phagocytophilum real-time quantitative PCR was homogenously distributed over all collection sites. A. phagocytophilum DNA was recovered from ticks collected from 144 of 172 collection sites.
None of the 8534 PCR results specific to C. burnetii was positive, meaning that throughout Switzerland, in the collection sites of the study and at the time of collection, we were not able to detect any trace of C. burnetii DNA within I. ricinus ticks.
Discussion
With 62 889 individual ticks, this study is the largest screening for the presence of A. phagocytophilum and C. burnetii DNAs in ticks in Europe. Pool prevalence of A. phagocytophilum within these ticks reaches 11.9%, but this value does not take into account the number of ticks per pool. Because most pools are composed of the maximum number of ticks in each group (ten for nymphs and five for adults), the maximum infection rate (12%) is almost equal to the pool prevalence (11.9%). However, the minimum infection rate (1.6%) was closer to the maximum likelihood estimates of the prevalence in individual tick (1.71%), calculated by taking into account the number of pools and the number of ticks per pool. The pool prevalence is in accordance with that observed in other European countries [3], whereas estimated individual prevalence is in accordance with results obtained in a previous comparable study in Switzerland [16]. When we looked only at pool prevalence, we did not observe any significant difference between prevalence of A. phagocytophilum in adults and nymphs. This was because adults pools were mainly composed of five individuals, whereas nymphs pools were composed of ten individuals. Calculation of the estimated prevalence in individual ticks allowed us to highlight that A. phagocytophilum prevalence of adults ticks was twice as large (2.55%) as the prevalence of nymphs (1.3%). This higher prevalence within the adults tick population is in accordance with the transstadial transmission of A. phagocytophilum [17]. Interestingly, contrary to previous results [18], in this study, we observed a similar prevalence between pools of male (11.4%) and female (12.2%) adult ticks.
With such prevalences, and with such a wide geographical distribution of A. phagocytophilum in I. ricinus ticks, the results of our study suggest that there is a high risk of the Swiss population being exposed to this pathogen. However, human granulocytic anaplasmosis is not routinely tested for after exposure to ticks because the clinical presentation of anaplasmosis is often nonspecific, with fever and flulike illness [19]. Even when more specific and severe anomalies are encountered, such as leukopenia, thrombocytopenia, elevated liver enzymes and/or renal insufficiency, A. phagocytophilum is only infrequently considered in the differential diagnosis. In addition, with an important number of hosts and reservoirs over a large geographical distribution, conditions are fulfilled to observe a geographical spread of A. phagocytophilum [17]. To better characterize the epidemiologic risk, further genetic analysis of the msp2 gene should be performed to discriminate between positive variants leading to human anaplasmosis and variants more linked to the animal life cycle [20], [21]—an analysis not performed in the present work. In any case, it is important to keep monitoring for the presence of this pathogen in ticks and in wild and farm animals.
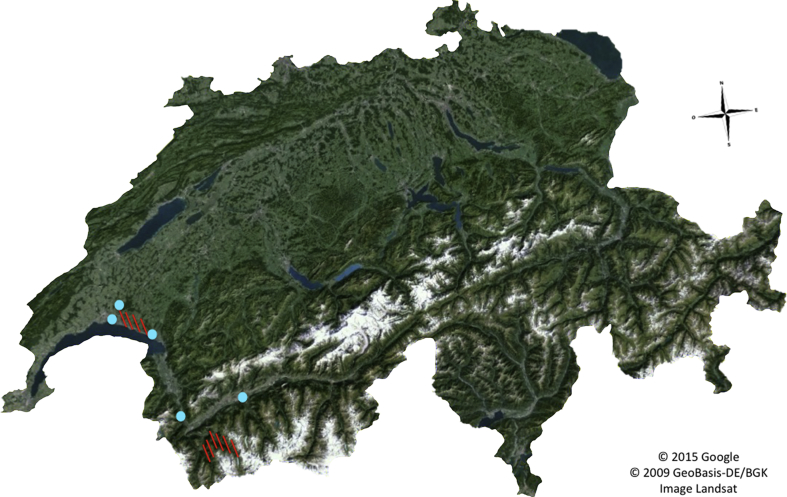
Historically, two Q fever outbreaks have occurred in Switzerland, the first in 1983 and the second in 2012. Both were attributed to zoonotic exposure to infected sheep. A few of our study collection sites were close to the outbreaks sites, but none of them had tick pools positive for the presence of C. burnetii DNA (Fig. 2). This result strongly suggests an absence of C. burnetii in I. ricinus ticks in Switzerland as well as a very low risk of exposition to this pathogen after a tick bite. This result is in accordance with previous studies highlighting this low risk in Europe [18], [22], [23], [24]. Even if C. burnetii can be transmitted to humans by a tick bite [9], the main vectors and reservoirs in Switzerland are farm animals, especially sheep [25]. Thus, there is no evidence that I. ricinus ticks may represent an epidemiologic risk for the transmission of Q fever in Switzerland, but monitoring may nevertheless be useful in order to detect a possible reemergence of C. burnetii in ticks.
Fig. 2.
Absence of positive tick samples for presence of Coxiella burnetii DNA in Switzerland. All 8534 pools were negative for presence of C. burnetii DNA, even in collection sites (blue dots) located near area where two Swiss Q fever outbreaks occurred (red lines).
This large screening of two obligate intracellular bacteria in I. ricinus ticks in Switzerland highlighted the absence of C. burnetii and the common presence of A. phagocytophilum. Unlike the very low risk of developing Q fever after a tick bite, the risk of exposure to A. phagocytophilum is significant, thus highlighting the urgent need to improve our knowledge about human anaplasmosis epidemiology and clinical presentation, as well as to develop sensitive and specific tools allowing for the accurate diagnosis of this disease.
Acknowledgements
We thank R. Brouillet (Institute of Microbiology, Lausanne, Switzerland) for technical support. We thank also the Swiss army and other military personnel for sampling ticks. This project was financially supported by the Swiss Federal Office for Civil Protection. The research of the group directed by GG is currently supported by several grants, including Swiss National Science Foundation grant 310030-162603, and was also supported by Swiss National Science Foundation (SNSF), Switzerland grant 310030-141050, which covered the salary of L.P. for 3 years.
Conflict of interest
None declared.
References
- 1.Strle F. Human granulocytic ehrlichiosis in Europe. Int J Med Microbiol. 2004;293(Suppl. 37):27–35. doi: 10.1016/s1433-1128(04)80006-8. [DOI] [PubMed] [Google Scholar]
- 2.Dumler J.S., Choi K.S., Garcia-Garcia J.C., Barat N.S., Scorpio D.G., Garyu J.W. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco J.R., Oteo J.A. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect. 2002;8:763–772. doi: 10.1046/j.1469-0691.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 4.Liz J.S., Anderes L., Sumner J.W., Massung R.F., Gern L., Rutti B. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–1007. doi: 10.1128/jcm.38.3.1002-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lommano E., Bertaiola L., Dupasquier C., Gern L. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl Environ Microbiol. 2012;78:4606–4612. doi: 10.1128/AEM.07961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pusterla N., Leutenegger C.M., Huder J.B., Weber R., Braun U., Lutz H. Evidence of the human granulocytic ehrlichiosis agent in Ixodes ricinus ticks in Switzerland. J Clin Microbiol. 1999;37:1332–1334. doi: 10.1128/jcm.37.5.1332-1334.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouqui P., Dumler J.S., Lienhard R., Brossard M., Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet. 1995;346:782–783. doi: 10.1016/s0140-6736(95)91544-3. [DOI] [PubMed] [Google Scholar]
- 8.Pusterla N., Weber R., Wolfensberger C., Schär G., Zbinden R., Fierz W. Serological evidence of human granulocytic ehrlichiosis in Switzerland. Eur J Clin Microbiol Infect Dis. 1998;17:207–209. doi: 10.1007/BF01691120. [DOI] [PubMed] [Google Scholar]
- 9.Maurin M., Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuis G., Petite J., Péter O., Vouilloz M. An important outbreak of human Q fever in a Swiss Alpine valley. Int J Epidemiol. 1987;16:282–287. doi: 10.1093/ije/16.2.282. [DOI] [PubMed] [Google Scholar]
- 11.Bellini C., Magouras I., Chapuis-Taillard C., Clerc O., Masserey E., Peduto G. Q fever outbreak in the terraced vineyards of Lavaux, Switzerland. New Microbe New Infect. 2014;2:93–99. doi: 10.1002/nmi2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gäumann R., Mühlemann K., Strasser M., Beuret C.M. High-throughput procedure for tick surveys of tick-borne encephalitis virus and its application in a national surveillance study in Switzerland. Appl Environ Microbiol. 2010;76:4241–4249. doi: 10.1128/AEM.00391-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtney J.W., Kostelnik L.M., Zeidner N.S., Massung R.F. Multiplex real-time PCR for detection of anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–3168. doi: 10.1128/JCM.42.7.3164-3168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaton K., Péter O., Raoult D., Tissot J.D., Greub G. Development of a high throughput PCR to detect Coxiella burnetii and its application in a diagnostic laboratory over a 7-year period. New Microbe New Infect. 2013;1:6–12. doi: 10.1002/2052-2975.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams C.J., Moffitt C.M. A critique of methods of sampling and reporting pathogens in populations of fish. J Aquat Anim Health. 2001;13:300–309. [Google Scholar]
- 16.Wicki R., Sauter P., Mettler C., Natsch A., Enzler T., Pusterla N. Swiss Army Survey in Switzerland to determine the prevalence of Francisella tularensis, members of the Ehrlichia phagocytophila genogroup, Borrelia burgdorferi sensu lato, and tick-borne encephalitis virus in ticks. Eur J Clin Microbiol Infect Dis. 2000;19:427–432. doi: 10.1007/s100960000283. [DOI] [PubMed] [Google Scholar]
- 17.Stuen S., Granquist E.G., Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reye A.L., Hübschen J.M., Sausy A., Muller C.P. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl Environ Microbiol. 2010;76:2923–2931. doi: 10.1128/AEM.03061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumler J.S., Madigan J.E., Pusterla N., Bakken J.S. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(Suppl. 1):S45–S51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 20.Silaghi C., Hamel D., Thiel C., Pfister K., Passos L.M.F., Rehbein S. Genetic variants of Anaplasma phagocytophilum in wild caprine and cervid ungulates from the Alps in Tyrol, Austria. Vector Borne Zoonotic Dis. 2011;11:355–362. doi: 10.1089/vbz.2010.0051. [DOI] [PubMed] [Google Scholar]
- 21.Silaghi C., Liebisch G., Pfister K. Genetic variants of Anaplasma phagocytophilum from 14 equine granulocytic anaplasmosis cases. Parasit Vectors. 2011;4:161. doi: 10.1186/1756-3305-4-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smetanová K., Schwarzová K., Kocianová E. Detection of Anaplasma phagocytophilum, Coxiella burnetii, Rickettsia spp., and Borrelia burgdorferi s.l. in ticks, and wild-living animals in western and middle Slovakia. Ann N Y Acad Sci. 2006;1078:312–315. doi: 10.1196/annals.1374.058. [DOI] [PubMed] [Google Scholar]
- 23.Spitalská E., Kocianová E. Detection of Coxiella burnetii in ticks collected in Slovakia and Hungary. Eur J Epidemiol. 2003;18:263–266. doi: 10.1023/A:1023330222657. [DOI] [PubMed] [Google Scholar]
- 24.Sprong H., Tijsse-Klasen E., Langelaar M., De Bruin A., Fonville M., Gassner F. Prevalence of Coxiella burnetii in ticks after a large outbreak of Q fever. Zoonoses Public Health. 2012;59:69–75. doi: 10.1111/j.1863-2378.2011.01421.x. [DOI] [PubMed] [Google Scholar]
- 25.Magouras I., Hunninghaus J., Scherrer S., Wittenbrink M.M., Hamburger A., Stärk K.D.C. Coxiella burnetii infections in small ruminants and humans in Switzerland. Transbound Emerg Dis. 2017;64:204–212. doi: 10.1111/tbed.12362. [DOI] [PubMed] [Google Scholar]