Abstract
Purpose
To describe serum vascular endothelial growth factor (sVEGF) in patients with neovascular age-related macular degeneration (nAMD) receiving anti-VEGF agents and associations between sVEGF and systemic serious adverse events (SSAEs).
Design
Exploratory analyses of a randomized controlled trial that enrolled 610 participants with nAMD and compared 2 anti-VEGF antibodies, ranibizumab and bevacizumab, and 2 treatment regimens, monthly vs. discontinuous, with 2 years' follow-up.
Participants
Adults aged 50+ years with treatment-naïve nAMD and a visual acuity of ≥25 letters (Snellen equivalent 20/320) in the affected eye.
Methods
Intravitreal injection of anti-VEGF antibodies.
Main Outcome Measures
sVEGF and occurrence of SSAE, with particular interest in arteriothromboembolic events (ATE) and immunologically mediated events (IME).
Results
On average, sVEGF (measured at months 0, 1, 11, 12, 23, and 24) decreased from a geometric mean of 168 pg/mL at baseline to 64 pg/mL at month 24. The decrease was greater with bevacizumab than with ranibizumab and was dependent on time since last treatment; at month 24 sVEGF was 11% lower with bevacizumab if treated ≥3 months previously, 51% lower if treated 2 months previously, and 76% lower if treated the previous month, compared with ranibizumab. The hazard of experiencing an ATE increased with age (hazard ratio [HR] = 2.01; 95% confidence interval [CI] = 1.32–3.05; P = 0.001) and higher sVEGF (HR = 1.16; 95% CI = 1.03–1.30, per 100 unit rise in sVEGF; P = 0.013). There was no association between sVEGF and the hazard of an IME (HR = 1.01; 95% CI = 0.76–1.33; P = 0.942); however, the hazard of an IME was significantly increased by treatment with bevacizumab compared with ranibizumab (HR = 3.53; 95% CI = 1.35–9.22; P = 0.010). The hazard of an “other SSAE” (not categorized as ATE or IME) increased with age (HR 1.51, 95% CI 1.14–2.01, P = 0.005) and decreased if an injection had been administered within the previous month (HR = 0.68; 95% CI = 0.45–1.03; P = 0.069).
Conclusions
The decrease in sVEGF is greater with bevacizumab than with ranibizumab, but this difference is eliminated when treatment is withheld for 3 months. Higher sVEGF increased the hazard of an ATE and bevacizumab increases the hazard of an IME compared with ranibizumab.
Abbreviations and Acronyms: ATE, arteriothromboembolic event; CI, confidence interval; DVT, deep vein thrombosis; GMR, geometric mean ratio; HR, hazard ratio; IME, immunologically mediated event; nAMD, neovascular age-related macular degeneration; pVEGF, plasma vascular endothelial growth factor; RPE, retinal pigment epithelium; SSAE, systemic serious adverse event; sVEGF, serum vascular endothelial growth factor; VEGF, vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a protein that is synthesized by endothelial cells and a variety of other cell types. It promotes angiogenesis, maintains the nonthrombogenic status of the vascular endothelium, is necessary for neuron survival, supports axonal and arterial co-patterning in developing brain and skin, and, importantly, is recognized for its role in the health and physiology of blood vessels.1, 2, 3 The recognition that VEGF is a key mediator of pathologic angiogenesis in many conditions including cancer and neovascular age-related macular degeneration (nAMD) has resulted in the development of therapies based on VEGF inhibition (anti-VEGF drugs).4, 5
Antibodies and other biologicals that block the effects of VEGF are now commonly used in the treatment of nAMD.6, 7 In the IVAN clinical trial (alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation trial8, 9, 10; trial registration ISRCTN92166560), 2 anti-VEGF drugs and 2 dosing strategies were compared in patients with nAMD. The primary purpose of the IVAN trial was to test the noninferiority of bevacizumab vs. ranibizumab and discontinuous vs. continuous (monthly) dosing.
We expected that there would be egress of the anti-VEGF drugs from the eye into the systemic circulation. Therefore, we were interested in studying any potential impact on circulating VEGF levels and we prespecified the concentration of serum VEGF (sVEGF) over time as a secondary outcome. We observed that by month 12 sVEGF was reduced by more than half with bevacizumab and by a far smaller amount with ranibizumab. These findings were reported in brief in a manuscript that described the main functional and morphologic outcomes.10
The VEGF protein has multiple physiological properties influencing vascular endothelial health and blood flow.1, 3 Therefore, after observing the difference in sVEGF by drug, we hypothesized that the frequency with which systemic serious adverse events (SSAEs) observed in the IVAN trial,11 particularly those involving the cardiovascular system, might be related to sVEGF. We also hypothesized that repeated exposure of the body to biological therapies has the potential to induce immunologic sensitization and thus might be related to immunologically mediated (noncardiovascular) SSAEs.12 Here, we report in detail the sVEGF concentrations in the IVAN trial; the influence of the two anti-VEGF drugs and time since last treatment on sVEGF; and the relationships between sVEGF and predefined categories of SSAEs over the period when anti-VEGF drugs were being administered.
Methods
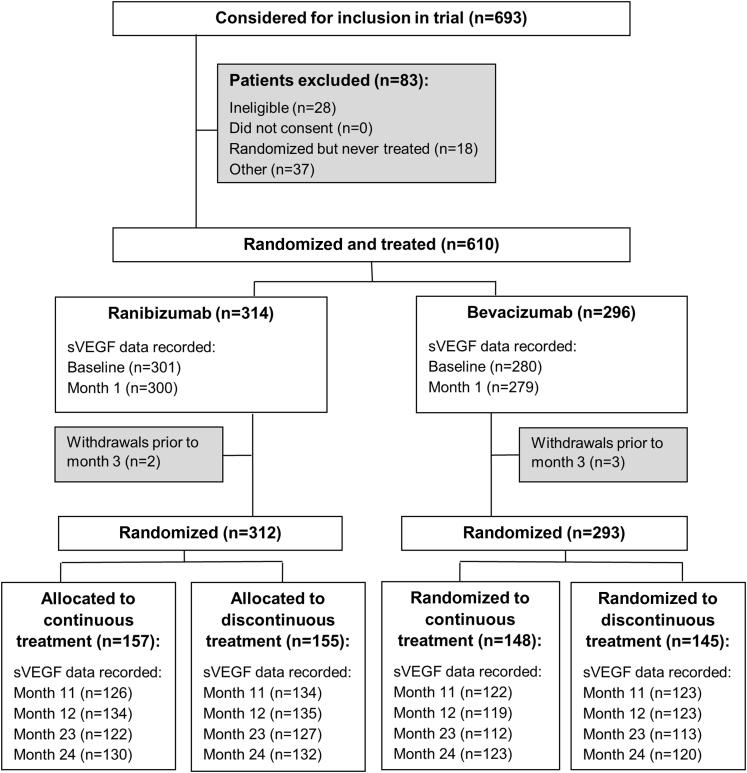
Participants gave written informed consent for collection and analysis of serum samples in the IVAN trial, which was approved by a UK NHS Research Ethics Committee (reference: 07/NIR03/37). IVAN randomized participants to bevacizumab or ranibizumab treatment between March 2008 and October 2010. At month 3, after receiving 3 treatments, participants were assigned randomly to continuous (monthly dosing) or discontinuous treatment (administered as needed but mandated as a course of 3 consecutive monthly treatments when restarting treatment). Participants were reviewed monthly at 23 clinical sites for up to 2 years. Full details of the IVAN trial design are shown in Figure 1 and published elsewhere.8, 9, 10
Figure 1.
Participant flow through the IVAN trial. sVEGF = serum vascular endothelial growth factor.
Serum Vascular Endothelial Growth Factor Analysis
Whole blood was sampled by venipuncture at six prespecified time points (baseline and months 1, 11, 12, 23, and 24). Blood was transported in vacutainer tubes without additives to a central laboratory, which was masked to randomized allocations and clinical information. The whole blood samples were processed for extraction of serum, which was then aliquoted and stored at −80°C. Samples were analyzed within 24 months of storage using a commercially available quantitative sandwich enzyme-linked immunosorbent assay (Quantikine Human VEGF immunoassay; DY 293 B, R&D Systems Inc, Minneapolis, MN). The determinations were processed according to the manufacturer's specifications. The primary antibody (biotinylated affinity purified) is a human VEGF 165 antibody that recognizes VEGF165, VEGF121, and VEGF165b and does not cross-react with phosphatidylinositol glycan anchor biosynthesis class F (PIGF); VEGF-B, C, or D; or the VEGF receptor ligands VEGF-R1 or R2 below concentrations of 50 ng/mL. The assay detects unbound VEGF. The standard curves and samples were analyzed in duplicate. The samples were not diluted so as to be within the range of the standard curves, which ranged from 32 to 2000 pg/mL of sVEGF. Internal controls (blank zero, low control 50 pg/mL and high control 1000 pg/mL) were included on each plate and values with a variance >10% were repeated and the unreliable measure discarded. The coefficient of variation was below 20% for the interassay and below 10% for the intra-assay measurements.
Classification of Systemic Serious Adverse Events
In the IVAN trial, all SSAEs and deaths were recorded and coded with the Medical Dictionary for Regulatory Activities (MedDRA version 14.1). The assignment of the SSAE to the appropriate MedDRA coding was performed by medically qualified members of the IVAN team (UC, SD, and AJL) and discrepant classifications reviewed by all 3 clinicians and an agreed assignment reached.
We identified arteriothromboembolic events (ATE) and immunologically mediated events (IME). ATE includes those defined by the Anti-Platelet Trialists' Collaboration (APTC)13 (myocardial infarction, stroke, deep vein thrombosis [DVT], pulmonary embolism (PE), hospital admission for angina or nonocular hemorrhage, transient ischemic attack, and vascular deaths) and heart failure. Inclusion of heart failure as an ATE was prespecified because this is an important cardiac event not captured by the ATE definition above.14
Anti-VEGF treatments are biomolecules with the potential to evoke immunologic responses, giving rise to our hypothesis about immunologic sensitization being linked to SSAEs. To investigate this hypothesis, all SSAEs not classified as ATEs were reviewed and those considered to be IME (for onset of or flare-up of arthritis, pneumonitis, Bell's palsy, etc) were identified (Supplemental Table 1, available at www.ophthalmologyretina.org). This categorization was performed by two of the authors (UC and ADD, the latter having extensive experience of managing patients with immune disorders).
All other SSAEs were placed in a third category, designated OTH (signifying other SSAEs not categorized as ATE or IME). Any discrepant classifications were reviewed by the 2 clinicians (UC and ADD) and the final assignment of the SSAE category agreed.
All classifications were performed with all of the clinicians masked to treatment allocation.
Statistical Analysis
When sVEGF levels were below the detection limit (32 pg/mL) of the assay, we uniformly imputed values between 0 and 32 pg/mL.15 The distributions of sVEGF levels were skewed and, therefore, are summarized in natural units as geometric means, as well as means and standard deviations on the loge scale. Cohen's d standardized mean differences16 (calculated on the loge scale) were used to compare baseline sVEGF by age, gender, history of prior ATEs, smoking status, and diabetes.
Mixed-effects linear regression was used to investigate the effect of drug and treatment frequency on sVEGF levels over time (i.e., at baseline and months 12 and 24; if sVEGF data were missing at months 12 or 24, data from months 11 or 23 were used, respectively). The model accounted for the correlation between repeated measurements on the same participant and included time since last injection (1, 2, or 3+ months) to reflect the treatment received in the months before the sVEGF concentration was measured (some participants missed visits, so allocation to the continuous monthly regimen did not ensure that treatment was received in the previous month). Baseline values were modeled with the later outcome values to avoid excluding or imputing cases with missing baseline values. As the sVEGF distribution was skewed, log transformed values of sVEGF were modeled, and effect estimates are shown in the figures as geometric mean ratios (GMRs) with corresponding 95% confidence intervals (CIs).
To examine the effects of sVEGF, age, gender, drug, and recent treatment (defined as whether or not an injection was received during the previous month) on the occurrence of SSAEs, we analyzed time to first occurrence of each SSAE using Cox regression models (time to first ATE, IME, and OTH were investigated in separate models). This method of analysis allowed information about treatment (or not) in the preceding month to be updated monthly; likewise, the sVEGF level was also updated in the model when new data were available (months 1, 11, 12, and 23). All participants were included in all 3 analyses; if participants did not experience the event of interest, they were censored at the time of their final visit. Effect estimates are reported as hazard ratios (HRs) with 95% CIs.
All analyses were performed using Stata 13.1 (StataCorp LP, College Station, TX).
Results
We randomized 628 participants to ranibizumab or bevacizumab, of whom 610 received the study drugs and formed the analysis population.8, 9 Serum VEGF levels were not available for all participants at each of the prespecified time points for diverse reasons (participant did not attend; taking the sample was overlooked by the study site research team; sample deteriorated in transit; participant had died or withdrawn). The numbers of participants with samples at each time point are described in the figures and tables; all exceeded 75%.
Baseline Associations of Serum Vascular Endothelial Growth Factor with Demographics and Clinical History
Average baseline sVEGF levels did not differ by age, smoking status, history of heart failure, or diabetes (Table 1; standardized mean differences <0.1). Average sVEGF was higher in women than in men (Cohen's d = 0.16) and in participants who had a history of DVT or PE compared with those who did not (Cohen's d = 0.34). In contrast, average sVEGF was lower in participants who had a history of myocardial infarction (Cohen's d = 0.23) or transient ischemic attack or stroke (Cohen's d = 0.20) compared with those who did not.
Table 1.
Baseline Serum Vascular Endothelial Growth Factor by Demographics and Clinical History
| n/N | Geometric Mean | Mean (SD) loge(sVEGF) | Standardized Mean Differences∗ (Cohen's d) | |
|---|---|---|---|---|
| Age | 0.09 | |||
| 50–74 years | 194/581 | 160 | 5.07 (0.85) | |
| 75+ years | 387/581 | 172 | 5.15 (0.83) | |
| Gender | 0.16 | |||
| Male | 235/581 | 155 | 5.05 (0.91) | |
| Female | 346/581 | 177 | 5.18 (0.78) | |
| Smoking status | <0.001 | |||
| Ever smoked | 366/575 | 168 | 5.12 (0.87) | |
| Never smoked | 209/575 | 168 | 5.12 (0.76) | |
| History of heart failure | 0.06 | |||
| Yes | 113/579 | 176 | 5.17 (0.77) | |
| No | 466/579 | 167 | 5.12 (0.85) | |
| History of myocardial infarction | 0.23 | |||
| Yes | 45/581 | 141 | 4.95 (0.99) | |
| No | 536/581 | 170 | 5.14 (0.82) | |
| History of TIA or stroke | 0.20 | |||
| Yes | 40/552 | 143 | 4.96 (0.97) | |
| No | 512/552 | 169 | 5.13 (0.83) | |
| History of DVT or PE | 0.34 | |||
| Yes | 31/580 | 220 | 5.39 (0.89) | |
| No | 549/580 | 166 | 5.11 (0.83) | |
| Diabetes | 0.04 | |||
| Yes | 59/580 | 163 | 5.09 (0.89) | |
| No | 521/580 | 169 | 5.13 (0.83) |
DVT = deep vein thrombosis; PE = pulmonary embolism; SD = standard deviation; sVEGF = serum vascular endothelial growth factor; TIA = transient ischemic attack.
Calculated on the loge scale.
Associations between Serum Vascular Endothelial Growth Factor and Drug and Dosing Regimens
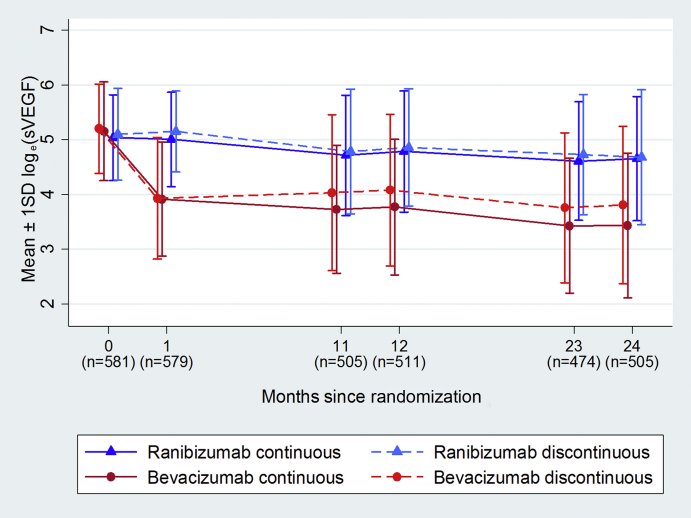
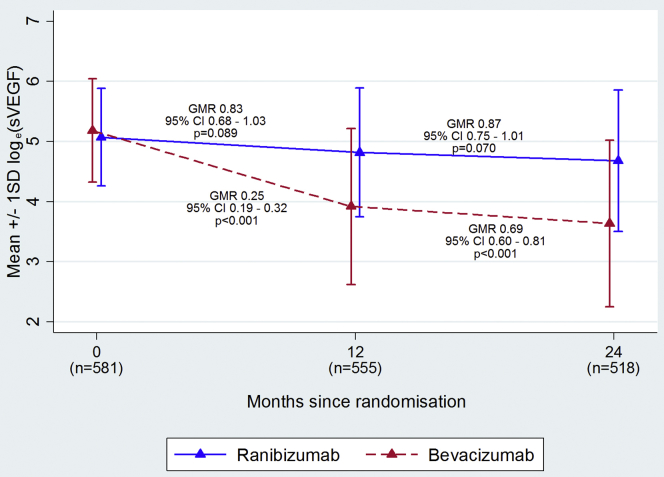
Figure 2 (eTable 2, available at www.ophthalmologyretina.org) shows average sVEGF levels over time, by drug and dosing regimen. At the baseline visit, the proportion of samples in which sVEGF could not be detected ranged from 2.0% to 5.6% and the average sVEGF was similar for the 4 randomized groups (geometric mean 154, 164, 173, 182 pg/mL for continuous and discontinuous ranibizumab and continuous and discontinuous bevacizumab, respectively). sVEGF reduced immediately and dramatically when participants were treated with bevacizumab but more gradually and to a much smaller extent with ranibizumab.
Figure 2.
Mean ± 1 SD loge(sVEGF) over time by treatment arm (SD = standard deviation; sVEGF = serum vascular endothelial growth factor). Corresponding geometric mean values can be found in eTable 2 (available at www.ophthalmologyretina.org). SDs on the loge scale can be added or subtracted from the mean on the loge scale and exponentiated to calculate the approximate range within which 68% (1 SD) or 95% (2 SD) of the data points lie.
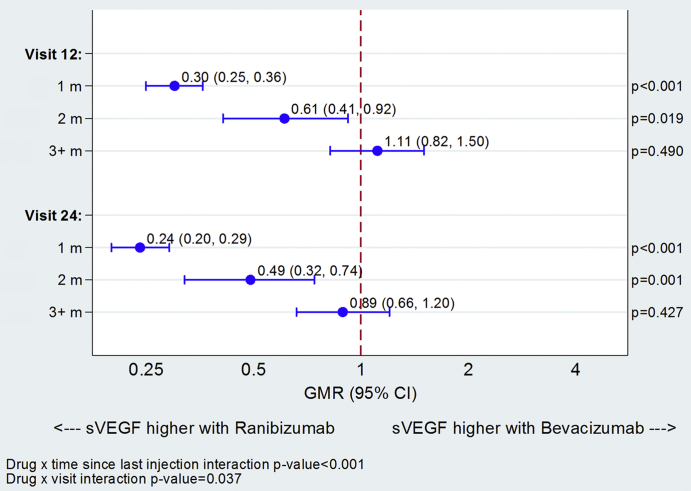
sVEGF was markedly influenced by the time that had elapsed since the last anti-VEGF injection. At both months 12 and 24, the sVEGF levels were similar for the two drugs for participants who received their last treatment three or more months previously. However, sVEGF levels were significantly lower in the bevacizumab group for participants treated two months (39% lower at 12 months; 51% lower at 24 months) or one month (70% lower at 12 months; 76% lower at 24 months; Figure 3) previously. sVEGF also appeared to continue to decline with cumulative exposure to anti-VEGF; by month 12, sVEGF had reduced by 17% (GMR = 0.83; 95% CI = 0.68–1.03; P = 0.089) relative to the baseline with ranibizumab compared with 75% (GMR = 0.25; 95% CI = 0.19–0.32; P < 0.001) with bevacizumab; sVEGF continued to fall between months 12 and 24, by a further 13% on average with ranibizumab and by 31% with bevacizumab (eFigure 1, available at www.ophthalmologyretina.org). A similar pattern was observed with the number of injections received; with bevacizumab, the average sVEGF was notably higher for those that had up to 4 injections in the first year and up to 6 injections over 2 years compared with those that had more injections; with ranibizumab the pattern was similar but less marked (eTable 3, available at www.ophthalmologyretina.org).
Figure 3.
Differences in serum vascular endothelial growth factor (sVEGF) levels between drugs at 12 and 24 months by time since last treatment. sVEGF summary values can be found in eTable 3 (available at www.ophthalmologyretina.org). GMR = geometric mean ratio.
Associations between Serum Vascular Endothelial Growth Factor Levels and Categories of Systemic Serious Adverse Event
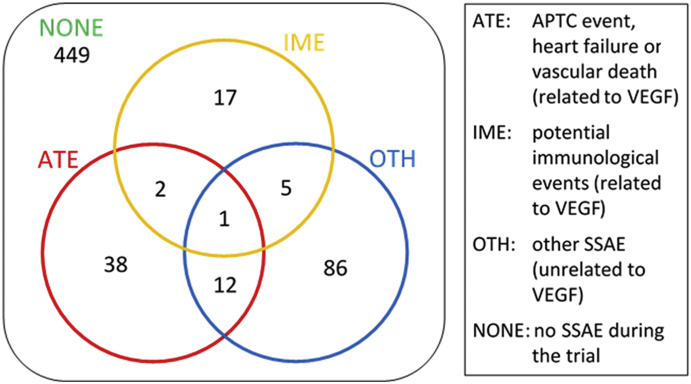
Of the 610 participants, 161 (36%) experienced at least 1 SSAE during their time in the trial. Figure 4 shows the numbers of participants with the different categories of SSAE; 53 participants had an ATE, 25 had an IME event, and 104 had an OTH event, with 20 participants experiencing more than 1 SSAE type. Average sVEGF levels in participants at baseline and at months 1, 11, 12, 23, and 24, classified by SSAE type, are described in eTable 4, available at www.ophthalmologyretina.org.
Figure 4.
Classification of 610 participants into groups according to their experience of systemic serious adverse events (SSAEs). All patients are included in all SSAE analyses; in the ATE analysis, for example, patients who experience an ATE are coded as having the event and all other patients (including those who have an IME or OTH event) are censored at their last time of contact. APTC = Anti-Platelet Trialists Collaboration; ATE = arteriothromboembolic event; IME = immunologically mediated event; OTH = other event not ATE or IME; VEGF = vascular endothelial growth factor.
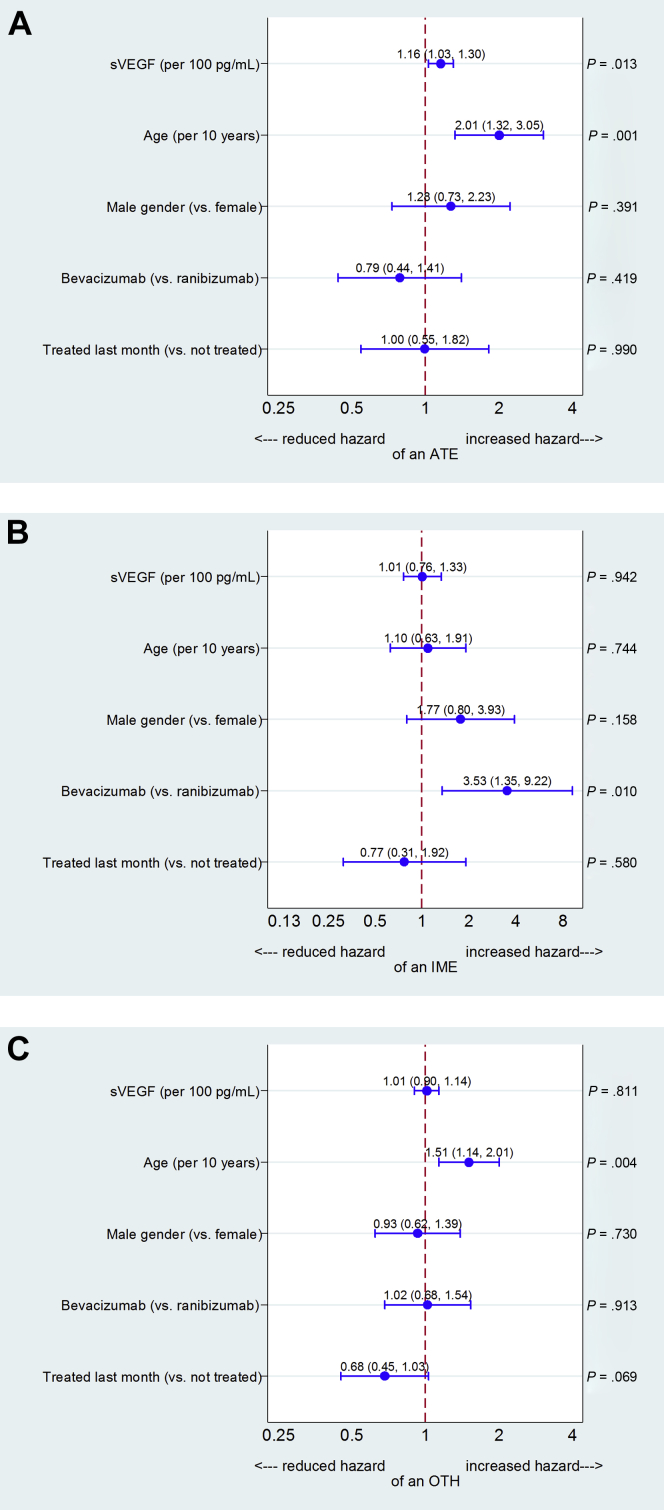
The Cox regression of time to an ATE event (Figure 5A) showed that the hazard of experiencing an ATE increased with increasing sVEGF (HR = 1.16 per 100 pg/mL increase in sVEGF; 95% CI = 1.03–1.30; P = 0.013) and age (HR = 2.01 per decade; 95% CI = 1.32–3.05; P = 0.001). There was no statistically significant association with drug type.
Figure 5.
A, Hazard ratios describing associations of participant characteristics and serum vascular endothelial growth factor (sVEGF) with systemic serious adverse event (SSAE) classification of arteriothromboembolic event (ATE). B, Hazard ratios describing associations of participant characteristics and sVEGF with SSAE classification of immunologically mediated event (IME). C, Hazard ratios describing associations of participant characteristics and sVEGF with SSAE classification of other SSAE, excluding ATEs and IMEs (OTH).
The analysis of time to an IME event (Figure 5B) found very different associations; there was no evidence of a statistically significant association between sVEGF levels and hazard of an IME, but participants receiving bevacizumab were more likely to experience an IME than those receiving ranibizumab (HR = 3.53; 95% CI = 1.35–9.22; P = 0.010).
The analysis for OTH SSAEs showed an increased hazard with increasing age (HR = 1.51 per decade; 95% CI = 1.14–2.01; P = 0.004) and a reduced hazard, of borderline statistical significance, with recent treatment (HR = 0.68; 95% CI = 0.45–1.03; P = 0.069). sVEGF had no effect on the hazard of an OTH SSAE (Figure 5C).
Discussion
In this report, we have examined the relationships between baseline sVEGF levels and participant characteristics, characterized changes in sVEGF over a 2-year period, and investigated the relationship between sVEGF and SSAEs among participants in the IVAN clinical trial.
The findings of marginally higher sVEGF level in female subjects and the positive association between systemic VEGF and DVT/PE are similar to those previously reported in the literature.17, 18 The association between increased sVEGF and past history of DVT/PE contrasts with other literature about systemic administration of anti VEGF agents and ATEs in patients with cancer, which found that anti-VEGF agents (reducing circulating VEGF) were associated with ATEs. This context, in which large doses of the drugs were administered systemically to patients with severe life-threatening disease, differs markedly from the one we report, i.e., treatment of patients expected to have a reasonable life expectancy in line with their age (the standardized mortality rate for IVAN participants was substantially less than 1 in the first year of the trial) with very small intravitreal doses of anti-VEGF agents.
We have shown that sVEGF was reduced to a much greater extent by bevacizumab than by ranibizumab, that the reduction was apparent after a single intravitreal injection (by month 1) but continued over time, and that the reduction was influenced markedly by the time-since-last-treatment for bevacizumab and to a lesser extent for ranibizumab. The larger reduction in sVEGF with bevacizumab, combined with the association of a history of DVT/PE with increased baseline sVEGF, is consistent with nonsignificant trends in systematic reviews for ATEs to be more frequent with ranibizumab than bevacizumab. Maguire et al19 recently published a meta-analysis of individual patient data from 5 such trials and reported an adjusted relative risk of ATEs for bevacizumab vs ranibizumab of 0.89 (95% CI = 0.62–1.28); the Cochrane aggregate meta-analysis, without 1 trial included in the individual patient data meta-analysis but with 2 other trials, found a similar result (risk ratio = 0.92; 95% CI = 0.62–1.37).11
Several other studies have also described changes in sVEGF after treatment with bevacizumab.20, 21, 22, 23 However, none has found a statistically significant change in sVEGF levels after ranibizumab therapy. Most of these studies measured sVEGF at baseline and at 1 month, with 1 study extending its findings out to 3 months.20 All were small in terms of their sample size and so only had adequate power to detect an association of large magnitude. As sVEGF can fall by more than 50% of its baseline value after a single intravitreal injection of bevacizumab, we contend that this change is easily detected at this time point even when the sample size of a study is small. By contrast, the fall in sVEGF after intraocular ranibizumab treatment is small and not detectable for many months after treatment has commenced, which may explain the failure of the other smaller studies to find this association. Another possible explanation may lie in those studies that measured plasma VEGF (pVEGF), which represents the nonsequestered fraction and which exists in low levels and thus may be below the detection limit of the assays.20, 23 In one of these studies,20 scrutiny of the graphical plots of change in pVEGF over time shows a fall after treatment and recovery with time. However, the variability of the measurements in pVEGF with overlapping error bars probably resulted in failure to find statistical significance.
The marked reduction in sVEGF with bevacizumab and the smaller reduction with ranibizumab over time obscured a more complex association with time-since-last-treatment. The difference in the reduction in sVEGF by drug was not observed when treatment had been withheld for 3 months or more, whereas the difference was pronounced when a participant had received treatment the preceding month. Our findings support the view that extraocular egress of both of these therapeutic antibodies occurs with subsequent binding to circulating sVEGF.
To the best of our knowledge, this is the first study to examine the relationships between sVEGF levels and SSAE in the context of intraocular anti-VEGF therapies. We observed that increased sVEGF was associated with a higher hazard of ATEs but found no evidence of an association between sVEGF and IMEs. However, we observed an unexpected but statistically significant increase in risk of an IME in persons exposed to bevacizumab compared to ranibizumab. Although not hypothesized a priori, there is a plausible mechanism to explain this observation. Bevacizumab is a full-length monoclonal antibody to VEGF-A; it is 3 times as large as ranibizumab (molecular weight of 149 kDa) and the presence of the Fc domain slows clearance and increases the systemic half-life, which is around 20 days.13 Egress of therapeutic agents from the eye may be modified by the presence of the Fc domain as the retinal pigment epithelium (RPE) expresses Fc and FcRN receptors with binding and internalization of the agent.24 A single study has reported that intraocularly injected nanoparticles can sequester within the RPE for up to 4 months.25 However, the data on mechanisms by which biological agents are cleared from within the vitreous cavity are sparse, and it is not known for how long the anti-VEGF agents in current use would be sequestered within the RPE and what the implications might be for RPE health and immunosensitization. In an elegant study that examined the elimination of intraocularly administered molecules, Kim et al26 show the existence of a specific mechanism for transporting and clearing full-length immunoglobulin G (IgG) into the circulation via the neonatal Fc receptor. Furthermore, the neonatal Fc receptors function to protect IgG from elimination from the bloodstream and tissue distribution and thus could potentially prolong the half-life of the molecules.27 Also, the presence of the Fc component on antibody molecules has the potential to modulate immune responses and in some instances create the conditions for immunosensitization.28
Recent treatment appeared to be protective of OTH events; this finding is in accord with our original observation10 that participants allocated to discontinuous treatment (who were more likely to have had longer intervals without treatment) had a higher frequency of SSAEs. It is also in accord with findings from the Comparison of Age-related macular degeneration Treatment Trials (CATT), in which a higher frequency of SSAE in the pro re nata treatment arm was observed, and when combined with the IVAN trial in a meta-analysis reached statistical significance.8, 9 This finding raises the intriguing possibility that an intermittent dosing strategy could evoke delayed hypersensitivity reactions to the drugs and, thus, an increase in the frequency of systemic adverse events.12 An alternative explanation is that the absence of recent treatment is simply a proxy for poor health, with some patients who were not recently treated being nonattenders.
Strengths of the IVAN trial were its substantial size, good retention over 2 years, a high proportion of participants providing longitudinal serum measurements at the specified sampling time points, rigorous reporting of adverse events, and the use of a centralized laboratory that was masked to treatment allocation.
There are several important limitations to our study, which are primarily due to the fact that this is an exploratory analysis of data from the IVAN trial8, 9 rather than a study conducted specifically for the purpose described here.
Firstly, our study does not have a placebo group, precluding any comparison of sVEGF or SSAE frequencies with patients unexposed to an anti-VEGF therapy. Thus continuing concern about the potential for SSAEs related to sVEGF suppression in high-risk susceptibility groups is justified.29, 30
Secondly, sVEGF measurements were made only at specified time points and not at every visit and, therefore, the sVEGF values used to predict occurrence of an SSAE may have been measured several months before the SSAE occurred. This limitation would tend to have diluted associations that may have existed but is very unlikely to have given rise to spurious associations.
Thirdly, the number of IME events was low, limiting the power of our analyses; despite this, we observed a significant association with the drug received.
Fourthly, a limitation may exist regarding our use of serum samples.31 It was not possible to obtain plasma samples in the IVAN trial, as the resources for immediate processing of whole blood samples were not uniformly available in all the participating clinical sites, and we recognize that this is an important potential limitation of the present study. VEGF protein is sequestered in platelets and other blood cells and is released during clotting. Consequently, sVEGF concentrations are heavily dependent on blood counts, which may act as an important confounder,31 although, for drug and dosing comparisons, this confounder should have been distributed similarly by group, owing to randomization. Despite this limitation, robust differences in sVEGF were detected in participants receiving intraocular anti-VEGF therapy. In support of the validity of serum measurements, we note that the Framingham study (which measured both) found that sVEGF and pVEGF measurements were strongly correlated and elected to report many of the associations solely on sVEGF.24
Finally, the commercial assay we used detects both VEGF121 and VEGF165 and only requires a single epitope for binding, which can be located anywhere in the VEGF molecule. We therefore think that it is unlikely that masking of the epitope could have resulted in the marked reductions in sVEGF levels seen in the present study. Furthermore, the detectable falls in sVEGF after treatment with either drug, and the larger decrease with monthly bevacizumab treatment, are consistent with the previously observed differences in pharmacokinetics of ranibizumab and bevacizumab.13, 32 Recent studies have shown that there are opposing angiogenic and antiangiogenic VEGF-A isoforms, and a differential reduction in these would not have been picked up by the commercial enzyme-linked immunosorbent assay that we used, which does not discriminate between them. Thus it is possible that a relationship between circulating VEGF isoforms and SSAE could have been missed.33, 34
The analyses reported in the present study were intended at the outset to be exploratory, and for this reason our results should be considered tentative. We strongly recommend that other studies of patients being treated with anti-VEGF drugs attempt to replicate what we have described. The Diabetic Retinopathy Clinical Research Network has collected blood samples in trials of intraocularly administered anti-VEGF drugs and would be ideally placed to do this.35
In summary, IVAN is the first study to quantify changes in sVEGF over a 2-year period, describe the relationships between dosing interval and sVEGF recovery, and investigate associations between sVEGF and other factors with SSAEs. The latter investigation has an important limitation, namely that the sVEGF measurements were taken at fixed time points that were not necessarily close to when an SSAE occurred. Our findings broadly support the view that the falls in sVEGF that occur after exposure to anti-VEGF drugs are unlikely to be associated with systemic ill effects. The possibility that bevacizumab may be associated with an increased risk for IMEs needs to be replicated.
Manuscript no. ORET_2017_69.
Footnotes
Supplemental material available at www.ophthalmologyretina.org.
Financial Disclosure(s):
Funding: The IVAN trial is funded by the UK National Institute for Health Research Health Technology Assessment (NIHR HTA) Programme (project number 07/36/01). The views and opinions expressed are those of the authors and do not necessarily reflect those of the HTA programme, the NIHR, the UK National Health Service, or the Department of Health.
Conflicts of Interest: C.A.R.: Lecture honorarium — Novartis. B.C.R.: Teaching fee — Janssen-Cilag. S.D.: Lecture honoraria — Novartis; her employing institution has received payments from Novartis. A.J.L.: Chief investigator — trial investigating treatment of chorioretinopathy; Is principal investigator — trials sponsored by Novartis, the manufacturers of ranibizumab; Advisory boards — Novartis and Bayer. U.C.: Principal investigator — trials sponsored by Novartis; advisory boards — Novartis, Bayer, and Roche outside the submitted work; employing institution has received payments — Novartis, Bayer, Neovista, Oraya, and Alcon.
Author Contributions:
Research design: Rogers, Scott, Reeves, Chakravarthy
Data acquisition and/or research execution: Downes, Lotery, Dick, Chakravarthy
Data analysis and/or interpretation: Rogers, Scott, Reeves
Manuscript preparation: Rogers, Scott, Reeves, Downes, Lotery, Dick, Chakravarthy
Human Subjects: This study includes human subject/tissues. Participants gave written informed consent for collection and analysis of serum samples in the IVAN trial, which was approved by a UK NHS Research Ethics Committee (reference: 07/NIR03/37).
Supplementary Data
eFigure 1.
Mean ± 1 SD loge(sVEGF) across study visits by drug. SD = standard deviation; sVEGF = serum vascular endothelial growth factor. Parentheses describe the numbers of participants for whom sVEGF was available at each sampling time. Geometric mean ratios (GMR) describing relative changes in sVEGF from baseline to 12 months (left) and from 12 to 24 months (right) are shown for each drug.
References
- 1.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstein J.M., Krum J.M., Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tammela T., Enholm B., Alitalo K., Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Kourlas H., Abrams P. Ranibizumab for the treatment of neovascular age-related macular degeneration: a review. Clin Ther. 2007;29:1850–1861. doi: 10.1016/j.clinthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Vasudev N.S., Reynolds A.R. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin D.F., Maguire M.G., Fine S.L. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld P.J., Brown D.M., Heier J.S. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarthy U., Harding S.P., Rogers C.A. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN) Health Technol Assess. 2015;19:1–298. doi: 10.3310/hta19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarthy U., Harding S.P., Rogers C.A. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarthy U., Harding S.P., Rogers C.A. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Moja L., Lucenteforte E., Kwag K.H. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014:CD011230. doi: 10.1002/14651858.CD011230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldo B.A. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2:e26333. doi: 10.4161/onci.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semeraro F., Morescalchi F., Duse S. Pharmacokinetic and pharmacodynamic properties of anti-VEGF drugs after intravitreal injection. Curr Drug Metab. 2015;16:572–584. doi: 10.2174/1389200216666151001120831. [DOI] [PubMed] [Google Scholar]
- 14.Solomon S.D., Pfeffer M.A., McMurray J.J. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 15.Beal S.L. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 16.Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 17.Carter J.G., Gammons M.V., Damodaran G. The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy. Angiogenesis. 2015;18:23–30. doi: 10.1007/s10456-014-9444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi R., Nakamura K., MacLauchlan S. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–1471. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire M.G., Shaffer J., Ying G. Serious adverse events with bevacizumab or ranibizumab for age-related macular degeneration: meta-analysis of individual patient data. Ophthalmol Retina. 2017;1:375–381. doi: 10.1016/j.oret.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery R.L., Castellarin A.A., Steinle N.C. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98:1636–1641. doi: 10.1136/bjophthalmol-2014-305252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu X., Yu X., Dai H. Intravitreal injection of ranibizumab for treatment of age-related macular degeneration: effects on serum VEGF concentration. Curr Eye Res. 2014;39:518–521. doi: 10.3109/02713683.2013.848899. [DOI] [PubMed] [Google Scholar]
- 22.Kong L., Bhatt A.R., Demny A.B. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2015;56:956–961. doi: 10.1167/iovs.14-15842. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Sawada T., Sawada O. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158:738–744.e1. doi: 10.1016/j.ajo.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Bourges J.L., Gautier S.E., Delie F. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 25.Dithmer M., Hattermann K., Pomarius P. The role of Fc-receptors in the uptake and transport of therapeutic antibodies in the retinal pigment epithelium. Exp Eye Res. 2016;145:187–205. doi: 10.1016/j.exer.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Kim H., Robinson S.B., Csaky K.G. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis. 2009;15:2803–2812. [PMC free article] [PubMed] [Google Scholar]
- 27.Tabrizi M.A., Tseng C.M., Roskos L.K. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–88. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 28.Karsten C.M., Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Avery R.L., Gordon G.M. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134:21–29. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 30.Baron J.A., Sandler R.S., Bresalier R.S. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372:1756–1764. doi: 10.1016/S0140-6736(08)61490-7. [DOI] [PubMed] [Google Scholar]
- 31.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 32.Gaudreault J., Fei D., Rusit J. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 33.Lieb W., Safa R., Benjamin E.J. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: clinical and genetic correlates and association with vascular function. Eur Heart J. 2009;30:1121–1127. doi: 10.1093/eurheartj/ehp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pikula A., Beiser A.S., Chen T.C. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke. 2013;44:2768–2775. doi: 10.1161/STROKEAHA.113.001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells J.A., Glassman A.R., Ayala A.R. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.