Chronic lymphocytic leukemia (CLL) cells display altered lipid metabolism and utilize fatty acid oxidation for their energy needs. We explored, on leukemic cells exposed in vitro to activating microenvironment-mimicking stimuli, the effects of a reversible inhibitor of carnitine-palmitoyl transferase 1A (CPT1A), a rate-limiting enzyme for fatty acid import into mitochondria. We found that the inhibitor, called ST1326 (Sigma-Tau IFR S.p.A., Italy), induced mitochondrial dysfunction and cell death specifically in activated/proliferating CLL cells, irrespective of TP53 alterations or chromosomal abnormalities. Upregulation of Mcl-1 and Bcl-xl expression in response to microenvironmental stimulation was impaired by ST1326, making the activated/proliferating cells sensitive to the BH3-mimetic ABT-199/venetoclax. These results suggest that ST1326 could be used to potentiate present therapies for CLL, particularly in the case of drugs that lose efficacy on proliferating leukemic cells.
The energy metabolism of CLL cells is nurtured not only by glycolytic mechanisms but also, unlike normal B lymphocytes, by fatty acid oxidation.1 A few inhibitors of the fatty acid oxidation regulator PPARa have been developed and tested in CLL models with promising results.2,3 The main controlling step of fatty acid oxidation, namely the transport of long fatty acids into mitochondria, requires the activity of CPT1. This enzyme has been utilized as a putative target for drugs in CLL. However, the use of irreversible CPT inhibitors, such as etomoxir and perhexiline,4,5 has been discouraged in clinical practice because of excessive liver and cardiac toxicity.
A reversible CPT1A inhibitor, (R)-N-(tetradecylcarbamoyl)-aminocarnitine (ST1326), developed initially for diabetes treatment,6,7 was shown to decrease lymphoma8 and leukemia9 cell growth in preclinical studies. There is no information available on its effects on activated/proliferating CLL cells. The purpose of our investigation was to assess the sensitivity of CLL cells to ST1326 in an environment-mimicking context, in which leukemic cells were challenged with activation stimuli that render them generally refractory to current therapeutic regimens and were responsible for disease progression. This strategy had to be adopted considering that clonal expansion in CLL occurs because of a close interaction of leukemic cells with the microenvironment, causing their activation and subsequent proliferation.
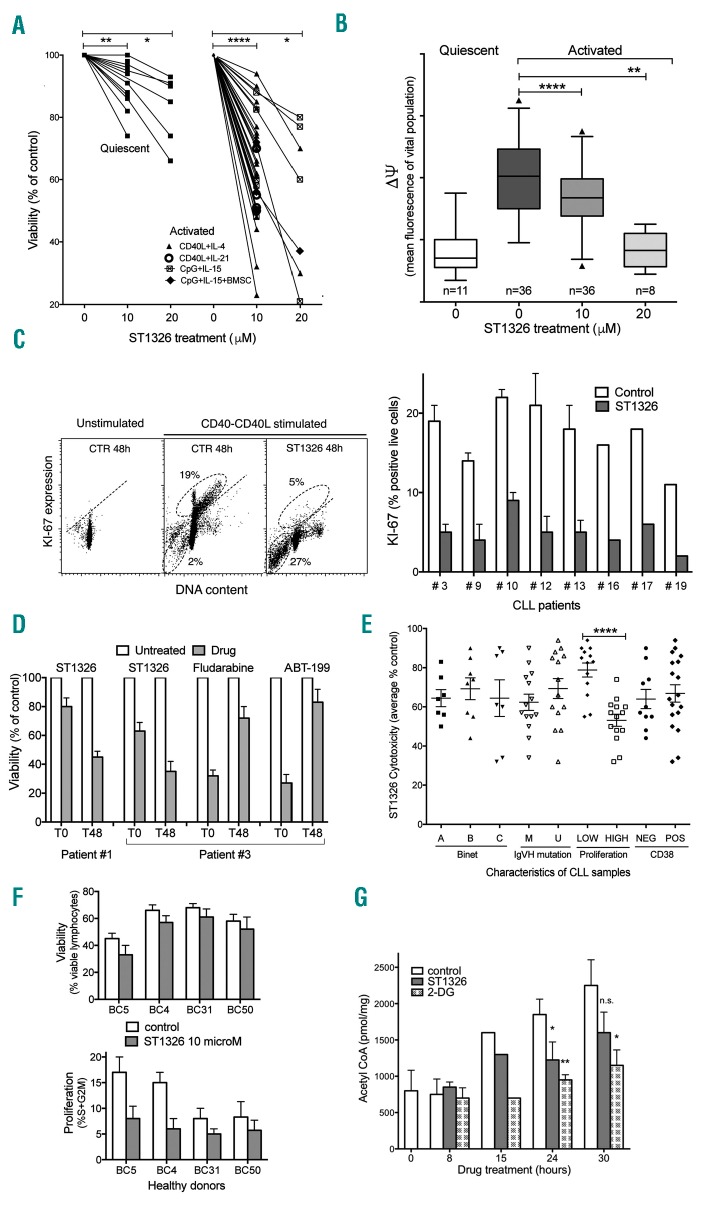
Cells from CLL patients (Online Supplementary Table S1) were exposed to ST1326 either in the absence or in the presence of stimuli potentially delivered by the microenvironment, e.g. CD40L-fibroblasts + either interleukin (IL)-4 or IL-21, or CpG+IL-15 (in the presence of bone marrow stromal cells in a few cases). A significant decrease of viability was observed in CLL cells that were stimulated in vitro compared to quiescent cells (Figure 1A, Online Supplementary Figure S1). Cell death was preceded by dissipation of mitochondrial transmembrane potential (Dψ), as demonstrated by an ST1326-induced decrease in Dψ observed in cells with an intact plasma membrane (Figure 1B, Online Supplementary Figure S1). KI67/DNA measurements showed a stimulation-induced rise of KI67 expression in a fraction of CLL cells, followed by the disappearance of KI67+ cells and concomitant increase of sub-G1 events after ST1326 treatment (Figure 1C), indicating that cycling cells were more sensitive to ST1326 than quiescent ones. Proof of the different sensitivities of the non-stimulated and stimulated cell cultures came from the observation that a 40 h pulse of ST1326 given at later stages after stimulation (i.e. when the cells already expressed activation markers and started progressing through the G1/S checkpoint) was more cytotoxic than when the drug was administered at the beginning of stimulation. This is at variance with other drugs (fludarabine and ABT-199) which lost their effectiveness if added to overtly activated CLL cells (Figure 1D). The level of cytotoxicity did not correlate with Binet stage, CD38 expression or IGVH mutation, but showed a strong relationship with in vitro proliferative response to activation stimuli (Figure 1E).
Figure 1.
ST1326 kills proliferating chronic lymphocytic leukemia cells. (A) Cell viability assessed by flow cytometric propidium iodide exclusion tests on quiescent or CD40L-stimulated cultures of leukemic cells from 26 CLL patients untreated or treated with ST1326 (48 h). CLL cells were obtained from peripheral blood of CLL patients, after informed consent according to the Declaration of Helsinki. Cell activation was achieved either through CD40L-NIH-3T3 murine fibroblasts + IL-4 (number of independent experiments, n=22) or IL-21 (n=4), or by CpG/ODN2006 (hTLR9 ligand) +IL-15, in the absence (n=7) or presence (n=3) of bone marrow stromal cells (BMSC). Mean (range) values of the reduction of viability (% control) induced by 10 μM ST1326 were: 64.8 (23–94), 56.5 (50–70), 68 (48–88) and 61 (55–72) for the four different activation systems, respectively. The statistical significance of differences was evaluated by a two-sided Wilcoxon signed rank test. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (B) Mitochondrial transmembrane potential (ΔΨ) gated on the vital cell population, as measured by DiOC6 uptake and expressed as flow cytometric fluorescence intensity of the propidium iodide-excluding cell population (with intact plasma membrane), on quiescent or CD40L-stimulated cultures of leukemic cells from 26 CLL patients untreated or treated with ST1326 from the beginning of stimulation (48 h). The number of samples for each condition is indicated. The statistical significance of differences was analyzed by a t-test two-sided Wilcoxon signed rank test. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (C) Left: flow cytometric distributions of DNA content/KI67 expression for one representative CLL sample. The CD40L-stimulated cells were tested in the absence or presence of ST1326. Gates identify KI67+ cells (above the negative-threshold dotted line) and sub-G1 events (apoptotic cells and bodies, below). Right: %KI-67+ cells from CLL samples of eight CLL patients, tested with or without addition of ST1326. (D) Cell viability in cells from two CLL patients exposed to a 40 h drug ‘pulse’, either concomitantly with CD40L-stimulation (T0) or after 48 h (T48). The different ST1326 sensitivities of CLL cells at T0 and T48 is evident. In contrast, the effect of fludarabine (10 μM) and ABT-199 (5 nM) was remarkably impaired if the cells were exposed to the drugs at T48. (E) Effect of ST1326 on average cell viability of CD40L-stimulated CLL cells. The patients were grouped according to disease stage (Binet A, B or C), immunoglobulin gene mutational status [unmutated (U-CLL), <2% mutations in IGVH genes); mutated (M-CLL), ≥2% mutations in IGVH genes], proliferative response to CD40L-stimulation (‘low’ or ‘high’ if % cells in the S+G2M cell cycle phase after 72 h stimulation was ≤ or > 8%, respectively), and CD38 expression (cut-off: 30%). The statistical significance of differences was assessed by a two-sided Mann-Whitney test. Where no indication is reported, the difference was not statistically significant. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (F) Normal B cells from healthy volunteers were purified by magnetic beads (negative-selection) and activated by co-culturing with CD40L+IL-4 and treated for 48 h with 10 μM ST1326. Cell death and proliferation were measured by flow cytometry. Viability was unaffected, while cell proliferation (% cells in S+G2M phase) was reduced in two samples. (G) Acetyl CoA levels in leukemic cells from one CLL patient stimulated for 24 h and then exposed to ST1326 (10 μM) or 2-DG (5 mM). Data obtained by colorimetric tests using the PicoProbe Acetyl CoA Assay Kit from AbCam (http://www.abcam.com/), are reported as mean ± SD of three experiments (two settings with CpG-and one with CD40L-stimulus). The levels of intracellular acetyl CoA increase during CLL cell activation, and ST1326 affects the increase rate. 2-DG was used as a control. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001.
It is noteworthy that ST1326 was also effective when proliferation was induced in the presence of bone marrow stromal cells (Online Supplementary Figure S1), which are known to provide a protective microenvironment, and in CLL samples harboring mutated TP53 or chromosomal abnormalities that confer chemoresistance (Online Supplementary Table S1 and Online Supplementary Figure S1). Interestingly, cytotoxicity was achieved at drug concentrations 10 times lower than concentrations needed for the more common CPT1 inhibitor etomoxir to reach similar effects (Online Supplementary Figure S2). The effects were independent of the cytokine used to promote cell proliferation (IL-4 or IL-21)10 (Online Supplementary Figure S3).
Normal B lymphocytes were less sensitive to ST1326 cytotoxicity, at the doses lethal for CLL cells (Figure 1F for CD40L+IL-4 stimulated B cells; data not shown for unstimulated B cells), in line with previous observations on normal bone marrow CD34+ cells.9 Instead, cell proliferation was partly inhibited, indicating a cytostatic, rather than cytotoxic, effect of ST1326 on normal B cells.
Apoptosis of ST1326-treated CLL cells was associated with Bax activation and intracellular accumulation of lipids (Online Supplementary Figure S4), which may induce Bax activation because of their detergent properties. Supplementation of L-carnitine partly impaired the effects of ST1326 on lipid accumulation (Online Supplementary Figure S4) and apoptosis (data not shown), in line with the described target competition of the drug.6
ST1326 decreased intracellular levels of acetylCoA, a product of β-oxidation that fuels the Krebs cycle and de novo fatty acid synthesis necessary for cell proliferation (Figure 1G). These data are concordant with previous evidence that ST1326 blocks not only CPT1A but also the activity of CACT,8 the enzyme responsible for the transport of acetyl-carnitine in the cytoplasm to regenerate acetyl-CoA. The block of fatty acid oxidation and depletion of cytosolic acetyl-CoA induce a downturn in cellular energy that might account for the growth inhibiting effects of ST1326.
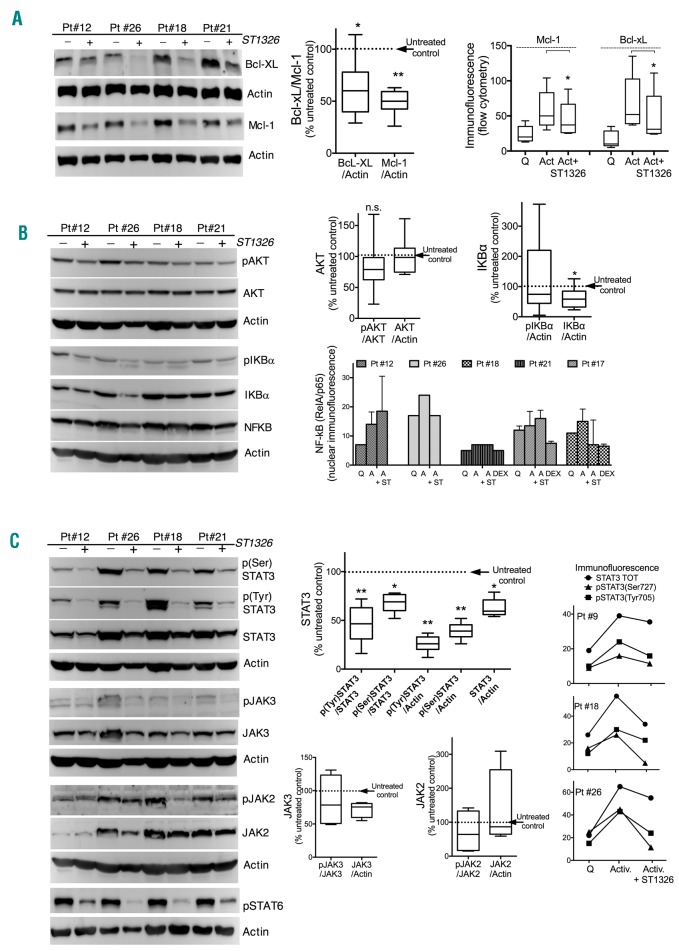
CLL cell viability and chemoresistance are sustained by the activity of Bcl-2 family members, Mcl-1 and Bcl-xL. Flow cytometric immunofluorescence measurements and western blots performed on 14 samples from seven different CLL patients indicated a consistent decrease of the expression of both anti-apoptotic proteins after ST1326 treatment (Figure 2A). This reduction anticipated cell death, according to flow cytometric analysis restricted to viable cells.
Figure 2.
ST1326 impairs stimulus-induced Mcl-1 and Bcl-XL upregulation and induces remarkable STAT3 downregulation. (A) Left: expression of the Bcl-2-family anti-apoptotic members, Mcl-1 and Bcl-xL, in leukemic cells from four CLL patients, stimulated with CD40L+IL-4, and treated with or without ST1326 24 h after stimulation. Middle: cumulative data for 24 and 42 h of treatment are represented in the box/whiskers graph (5th –95th percentiles): the protein level in ST1326-treated samples (divided by the level of the housekeeper protein) was calculated as the % of values in untreated controls. Right: protein expression evaluated by intracellular immunofluorescence and flow cytometry (background-subtracted fluorescence) on samples from five CLL patients is illustrated as a cumulative graph. Cells were quiescent (Q=non-stimulated) or stimulated (Act) in the absence or presence (+) of ST1326. Cells were fixed with 3% paraformaldehyde and permeabilized with 0.1% Triton X-100 before antibody reaction. Analyses are restricted to viable cells. A non-parametric t-test was used to assess the statistical significance of differences. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. (B) Left: western blots of total and phosphorylated (p) AKT (Ser473) and IKBα (Ser32/36) and NF-κB (RelA/p65) in four samples of CLL cells stimulated with CD40L+IL-4 for 1 day and treated with ST1326 for another 24 h. Different signaling pathways were examined in parallel gels and blots were stripped and re-probed with the indicated antibodies. Right upper panel: cumulative data calculated as in (A). The statistical significance of differences was analyzed by a non-parametric t-test. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. Right lower panel: NF-κB (RelA/p65) expression in isolated nuclei from CLL cells treated in culture as above, stained with anti-NF-κB (RelA/p65) antibody and measured by flow cytometry. Cells were not stimulated (Q) or stimulated by CD40L+IL-4 without ST1326 (A) or with ST1326 (A+ST). Dexamethasone (DEX) treatment for 24 h (100 nM) was used as a positive control for NF-κB downregulation. (C) Left: western blots of STAT3(pan) and its phosphorylated forms pSTAT3(Tyr705) and pSTAT3(Ser727), of total and phosphorylated JAK2 (Tyr1008) and JAK3 (Tyr980/981), and pSTAT6, in leukemic cells from four CLL patients stimulated with CD40L+IL-4 for 1 day and treated with ST1326 for another 24 h. Different signaling pathways were examined in parallel gels and blots were stripped and re-probed with the indicated antibodies. Middle: cumulative data for STAT3 are calculated from western blots of eight samples from six CLL patients: the average ratio of STAT3 (unphosphorylated, phospho-Tyr705 and phospho-Ser727 STAT3) to either unphosphorylated protein level or the housekeeper are displayed as a relative decrease of protein levels in ST1326-treated samples compared to untreated ones (set at 100). Cumulative data for JAK2 and JAK3 are calculated from four CLL patients. A non-parametric t-test was used to assess the statistical significance of differences. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. Right: expression of STAT3 and its phosphorylated forms as evaluated by intracellular immunofluorescence and flow cytometry on leukemic cells from five CLL patients, which were either not stimulated (Q) or stimulated (Activ.) in the absence or presence of ST1326 (24–30 h). Importantly, background-subtracted data are restricted to viable cells.
In CLL, Mcl-1 and Bcl-xL expression is under the control of signaling pathways that include AKT11 and NF-κB/STAT3.12 We explored the effects of ST1326 treatment on the stimulation-induced activation of these pathways.
AKT did not show a pattern of decrease similar to that of Mcl-1/Bcl-xL. The phosphorylation of AKT was only mildly decreased (Figure 2B), in spite of marked Mcl-1/Bcl-xL reduction. Likewise, no clear-cut association was evident between the drop of anti-apoptotic protein expression and NF-κB modulation, as indicated by the expression of the NF-κB regulators pIKBα/IKBα, and by the levels of NF-κB in the cytoplasm (determined by western blotting) and nucleus (determined by flow cytometry). The changes of NF-κB expression in the nucleus after ST1326 treatment were very variable across patients (Figure 2B), and did not correlate with the intensity of the Mcl-1/Bcl-xL decrease or with the apoptotic response to the drug.
Instead, STAT3 phosphorylation induced, at least in part, by CD40-mediated activation of the IL23-IL23R loop,13 was profoundly affected by ST1326. Expression of both phospho(Tyr705)- and phospho(Ser727)-STAT3 was substantially reduced, with this effect being observed well before overt apoptosis (Figure 2C). Interestingly, total STAT3 expression was diminished as well, possibly because of the positive feedback loop driven by activated STAT3 on U-STAT3.14 Phosphorylation of STAT6, induced by IL-4, was also remarkably reduced after treatment with the aminocarnitine-derivative (Figure 2C).
In the face of the striking reduction of STAT3 and STAT6 phosphorylation in all CLL patients, the phosphorylation of JAK2 and JAK3, which are involved in STAT regulation,14,15 was only very modestly and occasionally reduced (Figure 2C), suggesting that the STAT3/6 downturn induced by ST1326 might be mediated only in part by JAK2/3 downregulation. In CLL, STAT3 is also regulated by NF-κB,12 which was likewise not downregulated by ST1326.
Altogether, it appears that the CPT1 inhibitor strongly affects STAT3 and STAT6 activation in stimulated CLL cells independently, at least in part, of the modulation of JAK2/3 and NF-κB.
STAT have a crucial role in the pathogenesis of CLL. In particular, STAT3, which is upregulated in CLL cells and is constitutively phosphorylated on serine residue 727, equips CLL cells with a survival advantage and proliferation capacity.14 Thus, the data of our study may advocate a potential benefit of ST1326 as a STAT3/STAT6-modulator. It remains to be elucidated how this drug downregulates the activity of these transcription factors.
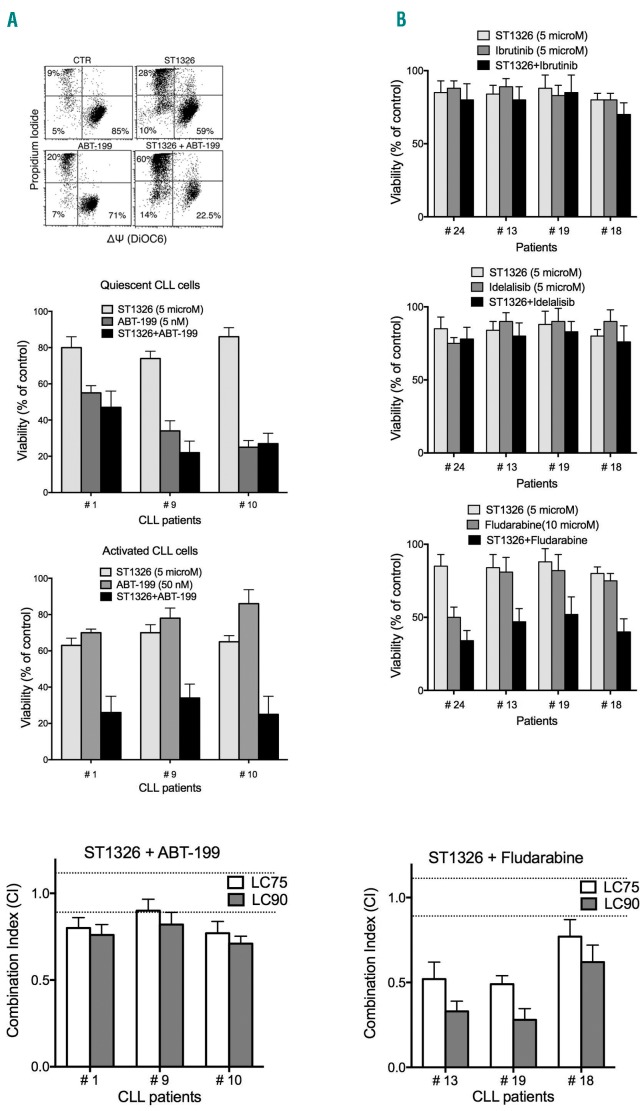
We combined the aminocarnitine derivative with the novel BH-3 mimetic ABT-199 (venetoclax), which specifically inhibits Bcl-2 and not Mcl-1/Bcl-xL, and therefore becomes less effective when Mcl-1/Bcl-xL are upregulated. We observed significant potentiation of ABT-199 cytotoxicity by ST1326 in the proliferating CLL cultures (Figure 3A). Combination of the CPT1A inhibitor with more frequently used drugs showed that ST1326 did not potentiate the effects of the kinase inhibitors idelalisib and ibrutinib (Figure 3B), possibly because these two drugs significantly reduced cell proliferation and thus impaired CLL cell sensitivity to ST1326. Conversely, fludarabine cytotoxicity was potentiated by ST1326 (Figure 3B).
Figure 3.
ST1326 potentiates cytotoxicity of drugs that lose effectiveness when chronic lymphocytic leukemia cells proliferate. (A) Upper: flow cytometric bivariate plots of mitochondrial transmembrane potential (ΔΨ) (DiOC6 fluorescence) and cell viability (propidium iodide exclusion) of leukemic cells from one representative CLL patient stimulated with CD40L+IL-4 and cultured with ST1326 (10 μM), ABT-199 (10 μM), or both drugs for 42 h. Middle: ST1326 and/or ABT-199 cytotoxicity (42 h) on unstimulated (Quiescent) and CD40L-stimulated (Activated) CLL cultures. Given that ABT-199 loses efficacy on activated/proliferating leukemic cells, the drug concentration was 10 times higher in stimulated cultures. Only stimulated CLL cells displayed increased sensitivity to drug combinations, if compared to either drug alone. Lower: combination index (CI) values (at LC75 and LC90) calculated using the Chou–Talalay model (CalcuSyn software, Biosoft, Cambridge, UK) on dose-effect profiles of activated CLL cells treated for 24 h with increasing concentrations of ST1326 (1–10 μM), ABT-199 (10–100 nM) or ST1326/ABT-199 at constant ratios. Since the CI depends on the ‘fractional effect level’, we report two levels of cytotoxicity, LC75 and LC90 (concentration lethal to 75% and 90% of CLL cells, respectively). Dotted lines indicate CI=0.9 and CI=1.1; Synergism = CI<0.9, additive effect = 0.9≤CI≤1.1 and antagonism = CI>1.1. The original CI curve and isobolograms are reported in Online Supplementary Figure S5. (B) Upper three insets: cytotoxicity of the combination of ST1326 (5 μM) and ibrutinib (5 μM), idelalisib (5 μM) or fludarabine (10 μM), administered for 24 h to activated CLL samples. Lower: CI values (at LC75 and LC90) calculated using the Chou–Talalay model on dose-effect profiles of activated CLL cells treated for 24 h with increasing concentrations of ST1326 (1–20 μM), fludarabine (1–30 μM) or ST1326/fludarabine at constant ratios.
To elucidate whether the effects of ST1326/ABT-199 and ST1326/fludarabine drug combinations were synergic or just additive, we performed cytotoxicity experiments at multiple drug concentrations and applied the Chou-Talalay model. The combination index and isobolograms indicated a ‘more-than-additive’ effect for the ST1326/ABT-199 drug combination and a remarkable level of synergy for ST1326/fludarabine in most CLL samples (Figure 3A,B and Online Supplementary Figures S5 and S6).
This study demonstrates, for the first time, the in vitro cytotoxic efficacy of ST1326 against activated/proliferating CLL cells at doses that are clinically achievable. According to results presented at the 44th Annual European Association for the Study of Diabetes (EASD) Meeting, the plasma levels of this aminocarnitine derivative are in the high nanomolar/low micromolar range, particularly under fasting conditions.16 Moreover, autoradiographic studies in rats and monkeys documented drug accumulation in the spleen, one of the lymphoid tissues in which CLL cells reside (unpublished data from Sigma-Tau). Thus, the drug concentration used in our experiments is not far from plasma levels and could be even lower than the drug concentration in lymphoid organs. Importantly, in contrast to other CPT1 inhibitors, which cause relevant adverse side effects, ST1326 displays only low and transient liver toxicity.7
These observations pave the way for proposing ST1326 as a novel agent in anti-CLL drug-combination therapeutic regimens, particularly with drugs whose efficacy is offset by cell activation and proliferation.
Supplementary Material
Acknowledgments
The authors would like to thank Fabio Giannessi, Silvia Pace, Giuseppe Giannini and Pietro Grossi (sigma-tau IFR S.p.A) for providing the compound ST1326 and sharing some of their preclinical in vivo and pharmacokinetic data.
Footnotes
Funding: this work was supported by the Associazione Italiana Ricerca sul Cancro (IG15426 to FF) and Italian Ministry of Health 5×1000 funds: 2013 to FF and AI, 2014 to AI and GC, 2015 to FF. DB is supported by the Fondazione Umberto Veronesi. ZD was supported by the Welke Cancer Research Foundation. We are grateful to Manlio Ferrarini for helpful discussions and valuable suggestions.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Heintel D, Kienle D, Shehata M, et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005; 19(7):1216–1223. [DOI] [PubMed] [Google Scholar]
- 2.Messmer D, Lorrain K, Stebbins K, et al. A selective novel peroxisome proliferator-activated receptor (PPAR)-alpha antagonist induces apoptosis and inhibits proliferation of CLL cells in vitro and in vivo. Mol Med. 2015; 21:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tung S, Shi Y, Wong K, et al. PPARalpha and fatty acid oxidation mediate glucocorticoid resistance in chronic lymphocytic leukemia. Blood. 2013; 122(6):969–980. [DOI] [PubMed] [Google Scholar]
- 4.Samudio I, Harmancey R, Fiegl M, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010; 120(1):142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PP, Liu J, Jiang WQ, et al. Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline. Oncogene. 2016; 35(43):5663–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannessi F, Pessotto P, Tassoni E, et al. Discovery of a long-chain carbamoyl aminocarnitine derivative, a reversible carnitine palmitoyl-transferase inhibitor with antiketotic and antidiabetic activity. J Med Chem. 2003; 46(2):303–309. [DOI] [PubMed] [Google Scholar]
- 7.Conti R, Mannucci E, Pessotto P, et al. Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes. 2011; 60(2):644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacilli A, Calienni M, Margarucci S, et al. Carnitine-acyltransferase system inhibition, cancer cell death, and prevention of myc-induced lymphomagenesis. J Natl Cancer Inst. 2013; 105(7):489–498. [DOI] [PubMed] [Google Scholar]
- 9.Ricciardi MR, Mirabilii S, Allegretti M, et al. Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood. 2015; 126(16):1925–1929. [DOI] [PubMed] [Google Scholar]
- 10.Pascutti MF, Jak M, Tromp JM, et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood. 2013; 122(17):3010–3019. [DOI] [PubMed] [Google Scholar]
- 11.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008; 111(2):846–855. [DOI] [PubMed] [Google Scholar]
- 12.Liu FT, Jia L, Wang P, Wang H, Farren TW, Agrawal SG. STAT3 and NF-kappaB cooperatively control in vitro spontaneous apoptosis and poor chemo-responsiveness in patients with chronic lymphocytic leukemia. Oncotarget. 2016; 7(22):32031–32045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutrona G, Tripodo C, Matis S, et al. Microenvironmental regulation of the IL-23R/IL-23 axis overrides chronic lymphocytic leukemia indolence. Sci Transl Med. 2018; 10(428). pii: eaal1571. [DOI] [PubMed] [Google Scholar]
- 14.Rozovski U, Wu JY, Harris DM, et al. Stimulation of the B-cell receptor activates the JAK2/STAT3 signaling pathway in chronic lymphocytic leukemia cells. Blood. 2014; 123(24):3797–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Totero D, Meazza R, Capaia M, et al. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008; 111(2):517–524. [DOI] [PubMed] [Google Scholar]
- 16.Valentini G, Bianchetti M, Pace S, Carminati P. Effect of a selective and reversible hepatic CPT1 inhibitor on HOMA index and fasting blood glucose in diabetic type 2 patients. Diabetologia. 2008; 51(Suppl 1): S23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.