Abstract
Research development on blastocyst implantation was reviewed in three sections: primate implantation, ungulate farm animal implantation, and the general process of blastocyst implantation in small rodents. Future research directions of this area are suggested.
Keywords: blastocyst, endometrium, implantation, trophoblast, uterus
Research on blastocyst implantation was historically reviewed.
Introduction
Embryo implantation in the uterine endometrium is probably one of the reproductive processes that has the largest species variation. For example, prior to implantation, the rabbit embryo expands by accumulating fluid in the blastocyst cavity so that the trophoblast wall can closely contact with the uterine wall. On the other hand, blastocysts of guinea pig, human, and small rodents such as mouse and rat, remain small but the uterine wall comes closely attached with the blastocyst by the uterine absorption of uterine fluid. Human and guinea pig blastocysts are similarly invasive and they break through the uterine luminal epithelium and invade into the endometrial stromal tissue which decidualizes for placental formation. On the other hand, sheep and pig blastocysts do not invade the uterine stroma and the stromal cells do not decidualize. The blastocysts of these animals elongate rapidly and become a thread-like structure which reach to 25 cm long by day 17, and it could reach to even 1 m long before attachment to the uterus. Because of the huge species variation in the implantation process, the author prefers not to generalize the progress made in implantation research in all species of animals. Instead, the author would like to present what progress was made in three categories of animals: primates, angulate farm animals, and experimental laboratory animals, mainly mice and rats. In the last section, it is attempted to follow the process of implantation step by step.
Primate implantation research
Hertig et al. described how human embryos develop during the first 17 days of life, which includes the process of implantation [1]. Prior to this work, our knowledge of early pregnancy may be represented by two major publications: the “Cours de Physiologie” by Mathias Duval [2] and the “Handbuch der menschlichen Embryologie” by Franz Keibel and Franklin Mall [3]. These two classical volumes provided a general overview of human development without focusing on histology. During the first half of the 20th century, over one dozen investigators described human embryos that initiated implantation in the endometrium. The focus of these early studies was on the structure of the small endometrial swelling which they happened to find during autopsy or examination of excised uterus. These researchers made histological preparations of the embryo and studied the structure of the embryo and trophoblastic tissues, as well as the condition of the endometrium and the corpus luteum in the ovary. These investigators include Peters [4], Miller [5], Bryce [6], Teacher [7], Stieve [8], Brewer [9], Dible and West [10], and Hamilton [11]. Many of these papers were quoted by Hamilton [11]. A human embryo of approximately 11 days old was found by Miller in 1913. This ovum was relatively well preserved and was studied as “the Miller ovum” later by Streeter [12].
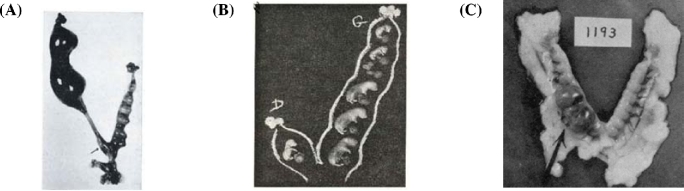
Hertig et al. focused, in particular, on the trophoblast and implantation site rather than the embryo [1]. These investigators collected human ova from their patients and the samples were treated in the same way, the entire collection period extended from 1938 to 1954. Of the total number of 34 human embryos, 8 were “free-lying,” 1 was in the tube and 7 in the uterine cavity. Twenty six were implanted. Of the 34, 21 were normal and 13 were abnormal. The earliest human ovum implanted was estimated 7 ½ day old (Figure 1). The embryo is implanted superficially and is only partially covered by maternal endometrium. The blastocyst consists of the trophoblast and inner cell mass, and it attached to the uterus with the side of inner cell mass. Trophoblast facing to the endometrium is actively proliferating and forming a thick disc, while the trophoblast facing the uterine lumen is a thin simple squamous epithelial layer similar to a peritoneal epithelium. It is very interesting to note that the location of trophoblast relative to the maternal tissue appears to influence their activities (functions). The direct contact with the maternal tissue appears to stimulate trophoblast cells. The embryo shown in Figure 1 implanted shallowly on solid endometrium approximately equidistant from several gland mouths. The ovum is discoid in shape with solid mixed syncytio- and cyto- trophoblasts at its basal and lateral margins (embryonic or implantation pole) but with the unaltered thin-walled primitive trophoblast of the blastocyst wall at its superior margin (abembryonic pole). This portion of the ovum is thus exposed to the uterine lumen. The blastocyst wall that has direct contact with the uterus has proliferated and differentiated. These investigators presumed that this is the result of an induction factor of endometrial origin. Thus, the remainder of the blastocyst wall is relatively unchanged and is comparable to the trophoblastic blastomeres of the 4 ½ day specimen. The formerly distended blastocyst cavity has collapsed in the process of implantation to become a flattened chorionic cavity. Figure 1 clearly demonstrates the tropic activity of the uterine tissue contact on differentiation and proliferation of the trophoblast cells. The trophoblast cells of the other part of the same trophoblast layer of the blastocyst that is exposed to the uterine lumen remain inactive.
Figure 1.
7 ½ day old human embryo [1].
The fundamental knowledge of the human uterine endometrium was established by Noyes et al. who identified morphological characteristics of each stage of the menstrual cycle [13]. Lessey et al. examined integrin molecules in the endometrium during the cycle and suggested that certain integrins appear to be regulated within the cycling endometrium and disruption of integrin expression may be associated with decreased uterine receptivity for implantation and infertility [14]. Villus trophoblast from first trimester and term placenta express the integrin subunits α6 and β4 as monitored by immunohistochemistry. A possible role is in anchoring trophoblast to basement membrane [15]. Several adhesion molecules were shown to occur at the surface of endometrial cells. One of them is the integrin αv subunit which is associated with various β chains including β5 [16]. An important role of integrins in cell–cell and cell–matrix interactions does appear to exist in implantation. The study by the Armant group strongly suggests a critical role for protein kinase C-gamma in fibronectin (FN)-mediated intracellular Ca++ signaling that leads to strengthening of trophoblast adhesion to FN [17, 18]. Susan Fisher's group investigated the expression of selectin adhesion systems that enables leukocyte capture from the blood stream at the maternal–blastocyst interface, and found on the maternal side, human uterine epithelial cells upregulated selectin oligosaccharide-based ligands during the window of receptivity [19]. On the side of the conceptus, human trophoblasts expressed L-selectin. This ligand–receptor system appears to be functional, because beads coated with the selectin ligand 6-sulfo sLex bound to trophoblasts, and trophoblasts bound to ligand-expressing uterine luminal epithelium in tissue sections [20]. Several galectins, being abundantly present at the human feto-maternal interface and endometrium, were hypothesized to significantly contribute to endometrial receptivity and pregnancy physiology [21].
Expression of Mucin 1 (MUC1) in endometrial epithelium has been suggested to create a barrier to embryo attachment that must be removed at the time of implantation. Meseguer et al. [22] investigated the hormonal regulation of human endometrial MUC1 in hormone replacement therapy cycles and in the human blastocyst. They found that endometrial MUC1 mRNA and immunoreactive protein increase in receptive endometrium compared to nonreceptive endometrium. Human blastocysts express MUC1, as demonstrated by reverse transcriptase chain reaction and immunocytochemistry, localized at the trophectoderm. In vitro, MUC1 was present at the surface of primary cultures of human endometrial epithelial cells (EECs), and the presence of a human blastocyst (i.e. apposition phase) increases EEC MUC1 protein and mRNA compared to control EEC lacking embryos. When human blastocysts were allowed to attach to the EEC monolayer (i.e. adhesion phase), MUC1 was locally removed in a paracrine fashion on EEC at the implantation site. These results demonstrate a coordinated hormonal and embryonic regulation of EEC MUC1. Progesterone combined with estradiol priming induces an upregulation of MUC1. However, at the adhesion phase, the embryo induces a paracrine cleavage of EEC MUC1 at the implantation site. These findings strongly suggest that MUC1 may act as an endometrial antiadhesive molecule that must be locally removed by the human blastocyst during adhesion phase.
The uterine receptivity
The receptivity of the endometrium for implanting the blastocyst is one of the most important indicators of the successful establishment of pregnancy, especially in the in vitro fertilization and embryo transfer industry. The principle of the uterine receptivity was established by Psychoyos [23] in early 1960s. Using the rat model system, he defined the three successive phases of uterine sensitivity for accepting the mature blastocyst(1) the neutral phase, in which blastocyst stays in the uterus but does not implant (in the case of the rat the uterus is under the influence of progesterone alone); (2) the receptive phase, in which the uterus accepts mature blastocyst only during this short period of time; and (3) the refractory phase, in which blastocyst does not implant and is expelled from the uterus, or destroyed if it remains in this phase of the uterus.
Whether this principle is limited to the species of mammals (such as the rat and mouse) where both progesterone and estrogen are essential [24] for rendering the human uterus receptive, needs to be defined. Although the secretory pattern of progesterone and estrogen prior to implantation is similar between the rat and human, we assume progesterone alone may induce implantation in the human. However, the success rate of the embryo transfer suggests that the maturation of the uterus to become receptive and matured blastocyst must be synchronized. Edwards used “the implantation window” for the human uterus first in 1988 [19]. At that point in time, he emphasized a need for a good marker that indicates the uterine receptivity. Asynchronous embryo transfer studies carried out by Dickmann and Noyes [25, 26] are important because they discovered that the mature endometrium cannot wait for the blastocyst to mature for successful implantation, but a mature blastocyst can wait for the endometrium to mature for successful implantation.
Psychoyos found a morphological sign (bulging out pinopods on the uterine luminal epithelial surface) during the receptive phase in the rat by scanning electron microscopy (Fig. 2A) [27]. A similar morphological change was also observed in the human [28]. This luminal epithelial cell surface formation of a bleb-like protrusion with only cytoplasmic ground substance was initially observed 4 h after estrogen treatment to ovariectomized mouse and rat [29, 30]. Martel et al. found that pinopods appear under the influence of progesterone. Besides pinopods, human uterine epithelial cells exhibit unique structural changes during the luteal phase, i.e. appearance on the nucleolar channel system (NCS) originally found by Clyman (Fig. 2B) [31], confirmed by Terzakis [32]. These channels provide direct communication between cytoplasm and the nucleolus. Functions of the NCS need to be clarified. Guffanti et al. found that NCSs are detected during a 6-day window of days 19–24 of an idealized 28-day cycle overlapping with the implantation window [33]. NCS expression is reported to be progesterone dependent [34].
Figure 2.
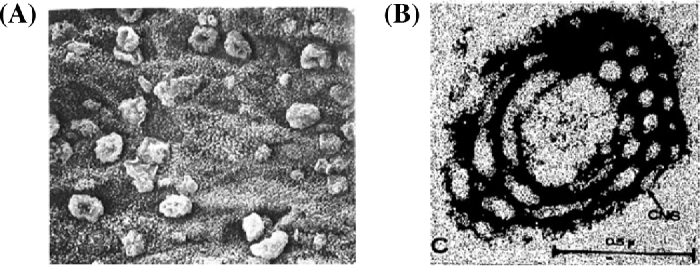
Two morphological characteristics of receptive endometrium. (A) Pinopods by Psychoyos [27], and (B) nucleolar channel system (NCSs) of human uterine luminal epithelial cells during the receptive phase by Clyman [31].
In vitro approaches
Poor accessibility to human implantation samples led many investigators to develop in vitro culture systems using cell lines and biopsy samples. Early investigators included Ehrmann, McKelvey and Hertig in 1961 [35]. After further developmental efforts by Lindenberg et al. in 1985 [36], and Kliman et al. in 1990 [37, 38], Fukuda et al. constructed a theoretical mechanism of implantation process using their experimental results of in vitro systems [39]. Fukuda et al. [39] used human uterine cell lines (endometrial adenocarcinoma cell lines Hec1A, RL95–2, and AN3CA) and trophoblastic HT-H cells. Major contributions by Fukuda's group are the discovery of the cell adhesion molecule, trophinin, and its associated proteins, and the presentation of possible mechanism of human embryo implantation process. Fukuda et al. considered that trophinin mediates cell adhesion between human trophoblast and EECs at their respective apical cell surfaces through trophinin–trophinin binding. Human trophoblast initially attaches to the uterine epithelial cells via L-selectin on trophoblast, which reacts with L-selectin ligand on the uterine epithelial surface. When trophoblasts approach the receptive uterine endometrium, trophinin is induced on the cell surface of the luminal epithelial cells in response to a high local concentration of human chorionic gonadotropin (hCG) secreted by the trophoblasts. Human CG stimulates trophinin expression on pinopods on the cell surface of the receptive uterine epithelium. Fukuda's research provides strong support for the idea that trophinin plays important roles in implantation process [40, 41]. The first role is adhesion of the apical cell surfaces between trophoblast cells and uterine epithelial cells. Trophonin is an intrinsic membrane protein and is directly responsible for homophilic cell adhesion. Trophinins on the two opposing cell membranes bind each other when they are presented in trans at the respective apical cell surface of trophoblast cells and EECs. The second role is signal transduction. In trophoblasts, cytoplasmic N-terminus of trophinin binds to another protein named bystin, which binds to the cytoplasmic domain of epidermal growth factor receptor B4 (ErbB4). When the ErbB4 ligand HB-EGF binds to ErbB4 on trophinin-expressing human trophoblast cells, ErbB4 is not activated. By contrast, when trophinin-mediated cell adhesion occurs, bystin is released from trophinin, allowing activation of ErbB4 protein kinase, resulting in promotion of invasion and proliferation [42, 43]. The third role of trophinin is induction of human endometrial apoptosis. Tamura et al. [44] observed apoptosis of human uterine epithelial cells when they added glycine-tryptophan-arginine-glutamine, a specific peptide repeat on trophinin, to trophinin-expressing primary cultured human EECs. Their experiments showed that this apoptosis is independent of Fas and that trophinin occupancy leads to nuclear translocation of protein kinase C-δ (PKC-δ). These investigators considered that when the human blastocyst approaches the receptive uterine endometrial cells, trophinin is induced in response to high local concentration of hCG secreted by trophoblast cells, which may trap PKC-δ near the plasma membrane, reducing cytoplasmic PKC-δ levels. Once trophinin-mediated cell adhesion occurs, PKC-δ is released from trophinin to the cytoplasm, allowing its translocation to the nucleus, where full-length PKC-δ may be cleaved to an active form by caspase 3. As the molecular mechanisms involved in human implantation process cannot be investigated directly, the in vitro models used by these investigators appear to be very useful tools for understanding human implantation. Incorporation of clinical research with the in vitro models will provide us with new and useful information.
Godbole and Modi cultured human endometrial stromal cells for up to 24 days during which estrogen and progesterone were added to the culture medium to induce decidualization. Based on the decidual markers, they designated the cells on day 8 steroid treatment as pre-decidual cells, and those cells on day 19 of steroid treatment were decidual cells. They demonstrated that the expression of HOXA10, IL11, and IL15 are co-regulated during the process of steroid hormone mediated stromal cell decidualization [45]. Godbole et al. recently showed that there was a significant increase in levels of insulin-like growth factor binding protein-1 (IGF-BP-1), prolactin, and IL-15 in supernatants of stromal cells treated with steroids, which is indicative of decidualization. The mRNA levels of HOXA10 were almost 8- to 10- fold higher as compared with nondecidualized controls. They studied degree of trophoblast invasiveness by using the Matrigel-based invasion assay method. Trophoblast cells used were JEG3 cells and ACH-3P cells. When variously treated decidual cell culture media were tested for the trophoblast invasiveness, they found that downregulation of HOXA10 in decidual cells increased the invasion of trophoblast cells, as it increased the expression of MMPs and repressed tissue inhibitors of matrix metalloproteinase in the trophoblast cells. Silencing of HOXA10 in decidual cells increased STAT3 phosphorylation in trophoblast cells. These results led Godbole et al. to propose that downregulation of HOXA10 in the decidual cells promotes the expression of leukemia inhibitory factor (LIF) and IL-6, which, in a paracrine manner, activates STAT3 in the trophoblast cells, leading to an increase in MMPs to facilitate invasion [46].
The recent work by Turco et al. [47] has significantly advanced the in vitro approach to understanding the process of human implantation. Turco et al. have successfully established long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. These organoids are self-organizing, genetically stable, 3D culture systems containing both progenitor/stem and differentiated cells that resemble the tissue of origin. These organoids appear to realize features of uterine glands in vivo: the ability to respond to hormonal signals, secrete components of “uterine milk,” and differentiate into ciliated luminal epithelial cells. Human endometrial organoids can be used to answer questions about uterine/conceptus cross-talk during implantation and placentation. Turco et al. used tissue isolates enriched for epithelial cells, and they allowed these to self-organize within Matrigel droplets with a basal medium that supports development of human tissue organoids, containing EGF, Noggin, and R-spondin-1 (ENR). The culture medium, containing A83–01 (the Alk3/4/5 inhibitor), EGF10 and HGF with EGF, Noggin, R-spondin-1 and nicotinamide, and expansion medium (ExM) resulted in the highest yield of organoids. The established organoids were positively stained for MUC1 (gland) and vimentin (stroma), and the gene expression analysis showed the organoid cultures cluster more closely to glands than to stroma. Turco et al. studied response of these organoids to sex steroid hormones. They exposed the organoids to estradiol followed by progesterone. Under ExM conditions most cells showed weak expression of estrogen receptor (ER), and progesterone receptor (PR) negative. After exposure to estradiol and progesterone, high expression of ERα and PR was seen in most organoids similar to the situation in vivo. Upregulation of genes by these steroids in the organoids was comparable to that in vivo. When further stimulated by pregnancy (hCG and hPL) and stromal cell (PRL) signals, the organoids acquired characteristics of gestational endometrium. Based on these results, this human endometrial organoid culture system promises to serve an invaluable research tool for investigating problems of implantation and the secretion of uterine histotroph during early pregnancy.
Genetic approaches
Several human implantation investigators worked together to present a global gene profiling of well-characterized human endometrial biopsy samples that were obtained during the window of implantation [48]. The results reassured several gene products, known to be differentially expressed in the implantation window or in secretory endometrium, were verified. These results showed the striking regulation of select secretory proteins, water and ion channels, signaling molecules, and immune modulators, underscoring the important roles of these systems in endometrial development and endometrial–embryonic interactions. The 156 significantly upregulated genes include those for cholesterol trafficking and transport (including ApoE), prostaglandin biosynthesis and action, proteoglycan synthesis, secretory proteins, and TGF-β superfamilies, signal transduction, extracellular matrix components, neurotransmitter synthesis, receptors, numerous immune modulators, detoxification genes, and genes involved in water and ion transport.
Horcajadas et al. published a microarray analysis of human endometrial receptivity. However, the key molecules/mechanisms in endometrial receptivity remain to be elucidated [49]. Lessey reviewed scientific literature pertaining to endometrial receptivity and concluded that reliable methods to assess “receptivity” have not been adequately established [50]. Carlos Simon's group continued to refine the method to apply the endometrial array to diagnosis of uterine receptivity [51].
For finding the best uterine condition for embryo transfer, clinical investigators concentrated their effort on finding a useful “endometrial receptivity array” so that the success rate of embryo transfer will be improved [52]. Mahajan improved reproductive performance by transferring embryos during the endometrial receptive period based on the array data [53]. Gemzell-Danielsson's group at Karolinska University Hospital chose the 16 genes for endometrial receptivity markers, which are grouped into the five categories. These categories include transcription factors (FOXO1, COUP-TFII, HAND2, HOXA10), cytokines (LIF, OPN, IL6, IL1A, CSF1), growth factors (FGF2, HB-EGF, VEGFA), decidualization factors (PRL, IGFBP1), and cell adhesion molecule (MUC1). Gemzell-Danielsson et al. examined the effects of PR modulators on the expression of these endometrial receptivity markers in in vitro culture systems [54, 55]. This is an interesting approach for understanding the human uterine receptivity. However, we need to examine as to whether these markers are expressed all at one time surrounding the embryo, or in a special sequence at appropriate location relative to the site of embryo implantation.
Decidualization
Decidualization of the endometrial stromal cells takes place around the time of implantation in primates. Duval [2] wrote “Under the exciting influence of the fertilized ovum in its course of development, the uterine epithelium is the seat of wonderful changes. The mucous tissue forms large pouches, and, so soon as the ovum comes into the womb, it is lodged in a valley formed by two of these pouches or villi; then these latter grow in every direction and finally completely enclose the ovum, so that a perfect envelope called the caduca, or membrana decidua is formed around it.” In Keibel's book, Otto Grosser [3] wrote “the implantation is of the interstitial type—the ovum is separated from the uterine cavity by a layer of maternal tissue, the decidua capsularis. It arises—by the fusion of the lips of the implantation cavity; when the ovum bulges out toward the lumen of the uterus, it covers like a shell the part of the ovum turned away from the placenta.”
Gurpide group initiated to decidualize human endometrial stromal cells in vitro in 1992 [56]. Hertig et al. [1] observed “Within a day or two after implantation of the fertilized ovum, the endometrium throughout the uterus undergoes a series of changes designated by us as progestational hyperplasia. The initiation of this process is seen in one of our 7-day specimens.—This sequence begins as an increased blood flow manifested as a slight dilation and congestion of the capillary sinusoidal network of the compact zone. It is soon followed by increasingly evident dilation and congestion of the spiral arterioles. Such increased blood flow is presumably responsible for the marked stromal edema which not only persists but increases—only to decline when early decidua has been formed at the time of the first missed menstrual period. Glandular secretion, which had reached its peak at about the 21st day (7th postovulatory) of the menstrual cycle, is reactivated in endometria associated with the 9-day ovum and increases more or less progressively to the stage of early decidual formation. The predecidual reaction in the stroma about the spiral arterioles and beneath the surface epithelium, ordinarily beginning during the 23rd day (9th postovulatory) of the normal non-pregnant cycle, develops at its usual gradual pace but with fewer infiltrating leukocytes until the time of the missed period when it morphologically resembles early decidua”.
De Feo [57] stated that the functions of decidual tissue include the formation of a cleavage zone for placental separation at the time of delivery, a restriction of early trophoblastic invasion, and a source of nourishment for the embryo. Shelesnyak [58] assigned the decidual tissue “new functions:” receptivity to “invasion” by the trophoblast (or readiness to “engulf” trophoblast); nutritional source for trophoblast; and, in some instances, protective membrane to keep the trophoblast from “invading” other sites of the uterus. In studying the epigenetic aspect of decidual cells, Erlebacher recently demonstrated that H3K27me3 in decidual cells regulates noncontractile uterus in early pregnancy, and, near term, inhibition of H3K27 demethylation prevents onset of parturition [59].
Attempts to develop a useful antiserum to pure decidual tissue (deciduomata) were unsuccessful [60]. In 1972, Yoshinaga [61] found rat decidual tissue to contain tissue-specific antigens and, subsequently Joshi et al. found two antigens in progesterone-influenced human endometrial tissue [62]. Using polyacrylamide gel electrophoresis, Joshi et al. separated these two antigens and estimated that the molecular weight of these two antigens is 48,000. In 1980s, many investigators worked on uterine proteins and came up with pregnancy protein 12 (pp12) and pp14 [63]. According to Bell, pp12 and pp14 are the same proteins [64]. This triggered research to identify progesterone-induced proteins in human endometrium using 2-dementional gel electrophoresis [65]. PP14 and other progesterone-associated uterine proteins were reviewed by Bell and more recently by Seppala et al. [66]. According to Seppala et al., investigators in this area agreed to use “glycodelin” for pp14, and all properties of this glycoprotein were described in their review. Although glycodelin may play a role in uterine receptivity, its immunosuppressive effect is considered as one important role of this glycoprotein in early pregnancy.
In 2013 Mazur et al. showed that PR is a critical regulator of decidualization and interacts with certain members of the activator protein-1 (AP-1) family members FOSL2 and JUN, and that exposure of primary human endometrial stromal cells to 17β-estradiol, medroxyprogesterone acetate, and cAMP promotes in vitro decidualization [67]. Kommagani et al. showed that steroid receptor coactivator-2 (SRC-2) is essential for progesterone-dependent uterine function including decidualization of human endometrial stromal cells [68].
Kommagani et al. performed comparative genome-wide transcriptional profiling of endometrial tissue and found that transcription factor 23 as essential for decidualization of human endometrial stromal cells [69]. This group of researchers obtained endometrial biopsies from healthy women of reproductive age during the proliferative phase (days 8–12) of their menstrual cycles. From these materials human endometrial stromal cells were isolated. These stromal cells were transfected with specially targeted small interfering RNAs, which were subjected to decidualization. The outcome of this study showed that growth regulation by estrogen in breast cancer 1, GREB1, is a novel progesterone-responsive gene required for human endometrial stromal decidualization [70].
Since Medawar [71] presented the endocrinological and immunological problems raised by the evolution of viviparity in vertebrates in 1953, much effort of investigators has been directed to the immunological enigma as to why allogeneic fetus is not rejected by the maternal immune system until term during normal pregnancy. Hamperl and Hellweg studied a special type of cells found in the human endometrium, which they named “granular endometrial stroma cells” that are characterized principally by special granules within their cytoplasm. Hamperl and Hellweg suggested that these cells were differentiated from endometrial stromal cells [72]. Stites et al. considered local concentration of a variety of hormones, including hCG, sex steroids, alphafetoprotein, and immunoglobulins, could provide a blocking mechanism to prevent maternal cellular immune attack. Possibly progesterone, antibodies, and immune complexes are important in protecting placenta and ultimately the fetus from rejection [73]. Decidualization of endometrial stromal cells extends into the junctional zone where spiral arteries are transformed into uteroplacental vessels by trophoblast invasion and complete loss of the arterial structure [74]. In normal pregnancy, cytotrophoblasts emigrate from the chorionic villi and invade the uterus, reaching the inner third of the myometrium. Within the uterine wall, cytotrophoblasts deeply invade the spiral arteries. Cytotrophoblasts migrate up these vessels and replace, in a retrograde fashion, the maternal endothelial lining. They also insert themselves among the smooth muscle cells that form the tunica media of the arteries. As a result, the spiral arteries attain the physiological properties that are required to perfuse the placenta adequately [75]. King and Loke discussed about the presence of leukocytes in endometrium and decidua. These leukocytes are phenotypically and morphologically resembling to granulated lymphocytes at other mucosal surfaces. They suggested that these cells may have a role in the control of implantation and the transformation of the uterine vasculature by trophoblast on which the blood supply to the fetoplacental unit depends [76]. King [77] further raised the possibility that uterine NK cells in the late secretory phase and in early decidua may be important in initiating and maintaining decidualization. Toll-like receptors (TLR) from the major family of pattern recognition receptors are involved in innate immunity. Innate immune responses against microorganisms at the maternal–fetal interface may have a significant impact on the success of pregnancy. The expression of TLRs in decidua, trophoblasts, and immune cells suggests their involvement in the innate immunity during implantation [78].
Erlebacher's group focused on the role of decidua in fetal tolerance. They demonstrated that genes encoding Th1/Tc1-attracting chemokines are subject to epigenetic regulation in decidua and that such regulation can significantly influence a tissue's capacity for T-cell accumulation [79]. Jiang et al. explored the role of regulatory T cells (Tregs), specifically that immune-suppressive Tregs confer essential protective benefits in sustaining tolerance to self and “extended self” commensal antigens that averts autoimmunity [80]. Decidual tissue during pregnancy is the major target of progesterone and many molecules of signal downstream of progesterone action have been shown to have immunomodulatory activity [81]. At a workshop held in 2014 [82], the participants identified several aspects of the mechanisms that require a better understanding relative to a successful pregnancy. They included interactions between specific decidual leukocyte subsets and placental major histocompatibility molecules during normal and adverse pregnancy outcomes.
Nonhuman primates
Scarcity of human early pregnancy samples limits investigators’ ability to understand detailed embryonic and appendage developments during early pregnancy. This limitation made investigators look for other species for research on blastocyst implantation. Wislocki and Streeter noted that “the macaque collection at our disposal represents a more nearly complete developmental series than exists for any other primate. Our material is unique in that ample specimens of known conception age are available for study from the day of implantation on. An opportunity is given for detailed study of implantation of the blastocyst —.” They assigned three succesive stages to the duration of rhesus monky placental development. The pre-lacunar stage (the 9th day and the 10th day); the stage of trophoblastic lacunae in which lacunae filled with maternal blood (11th until 15th day); and the villous stage the chorionic villi begin to form (14th, or 15th day, to 35th day) and for following uninterruptedly the changes which lead to the formation of the definitive placenta” [83]. Reinius et al. observed an implanting blastocyst of a rhesus monkey on the 9th day after ovulation using light and electron microscopy. At this stage of implantation, the penetration of the epithelium by trophoblast cells had begun at a few sites along the area of attachment but the basement membrane was not reached. These events preceded measurable increments in circulating CG and the “rescue” of the corpus luteum [84]. Enders and Schlafke studied the ultrastructure of pre-implantation rhesus monkey blastocyst [85], and the cellular processes from pre-implantation blastocysts through initial implantation to early villus formation. They concluded that syncytial trophoblast is the first tissue to penetrate the uterine luminal epithelium, and that the basal lamina of the uterine luminal epithelium, but not the basal lamina of endothelium, constitutes a temporary barrier to trophoblast penetration. They noted that invasion is accompanied by less destruction of maternal tissue than previously suggested, and that the rapid superficial growth of the placenta is made possible by the early tapping of the endometrial vessels [86]. Enders observed that at the beginning of implantation (day 9 postfertilization), masses of syncytial trophoblast adjacent to the embryonic pole of the blastocyst penetrate the uterine luminal epithelium by infiltration between epithelial cells. During the next day (day 10), clusters of syncytial trophoblast coalesce and expand laterally to form a trophoblastic plate situated along the residential basal lamina of the luminal epithelium. Clefts appear within the syncytium (day 11) and syncytial tabs interrupt the walls of maternal sinusoidal capillaries, forming part of the wall of the capillaries and bringing maternal blood into continuity with the intrasyncytial clefts, hence forming lacunar spaces. The trophoblastic lacunae rapidly expand (days 12 and 13), and clusters of cytotrophoblast within the syncytium lining the lacunae constitute the beginning of primary villi (days 14 and 15). Indentation of the cytotrophoblast of the primary villi by mesenchyme initiates the formation of secondary villus formation. The rapid increase of the cytotrophoblast cells at the maternal surface initiates the formation of the cytotrophoblastic shell (days 16 and 17). There is an expansion of the implantation site during these structural changes. Enders measured the size of implantation site in the rhesus monkeys. The primary implantation site expands from an average diameter of 0.268 mm on day 10 to 4.93 mm on day16–17; in thickness from 0.064 mm to 0.96 mm; and in volume from 0.0036 to 18.34 mm3 from day 10 to day 16–17 [87].
Sengupta and Ghosh worked on implantation of the rhesus monkey (Macaca mulatta). In 1988 this group showed at first that alkaline phosphatase levels in the endometrium increased on days 5–6 of rhesus monkey gestation [88]. They measured patterns of estrogen and PRs in the endometrium during the secretory phase of the cycle and pre-implantation stages of gestation, and showed that, in estrogen-sensitized and progesterone-treated ovariectomized rhesus monkeys, the endometrium responded to deciduogenic stimulus by showing similar responses to those occurring at implantation [89, 90]. Embryo transfer to the ovariectomized progesterone-treated rhesus monkeys resulted in pregnancy in three out of four cases [91]. Inhibition of preovulatory phase progesterone action with RU486 can inhibit postovulatory phase endometrial receptivity [92]. The cellular distribution of receptors for estradiol (ER) and progesterone (PR) in trophoblast cells and endometrial compartments of timed lacunar and villous stages of placentation. Both in lacunar and villous stage tissues showed that PR was positive in syncytiotrophoblasts and in cytotrophoblasts, while they were negative for ER [93]. Histochemical localization of IGF-I and IGF-II peptides revealed a very low level of IGF-I in trophoblast cells lining lacunae, and primary and secondary villi, while moderate to high amounts of IGF-II peptide were detected in lamellar syncytiotrophoblast cells lining lacunae, early villi and cell columns, as well as in migrating trophoblast cells in the extravillous compartment and endovascular cells. The observed presence of IGF-II peptide in differentiated lamellar syncytiotrophoblast cells during the very early stages of implantation and placentation in the rhesus monkey may be important in their transition to this differentiated cell population [94]. This group of investigators studied various molecules and localized them in relation to implantation sites of the rhesus monkey. These molecules include LIF, IL-1α, IL-1β IL-6, TNF-α, VEGF, and MMP9 [95–97]. Rozner et al. demonstrated that decidua changes trophoblast–macrophage interactions, and either macrophages or trophoblasts increased production of cytokines in response to the other cell type [98].
Enders also studied implantation in the baboon and he compared similarities and dissimilarities among the baboon, macaque, and human. Compaction of the morula occurs in the baboon in a manner similar to that of the macaque and human. The ease of uterine flushing makes the baboon a useful species to study the substantial differentiation of the pre-implantation blastocyst, both trophoblast and inner cell mass, that occurs prior to the onset of implantation. The first invasion of maternal vessels by syncytial trophoblast occurs earlier in the baboon than in either the macaque or human, and early development of lacunae is probably responsible for the elevation of the placenta above the endometrial surface. As in the macaque, there is rapid migration of cytotrophoblast into maternal arterioles, but unlike the macaque there is also some migration into superficial venules. Some endometrial responses to implantation are similar to those of the macaque, e.g. stromal edema and slow decidualization of stromal cells, but there is a less significant epithelial plaque reaction, and only a single placental disk is formed. Although there are some species peculiarities, the baboon remains an excellent model of primate blastocyst development and implantation [99]. Fazleabas et al. studied morphology and secretory activities at the baboon implantation sites and substantiated the endometrial changes undergoing during implantation. They showed that the protein synthesis in the baboon uterus is upregulated during early pregnancy, and expression of IGFBP-1 and retinol-binding protein (RBP) in cells is related to implantation [100].
The window of uterine receptivity for implantation in the baboon is between 8 and 11 days post-ovulation. Fazleabas et al. divided this period into three phases. Phase I is characterized by the changes induced by estrogen and progesterone during the luteal phase of the normal menstrual cycle. Phase II is defined as modulation of the phase I receptive endometrium by blastocyst signals, mainly by CG. Phase III is induced by the blastocyst interacting with the receptive endometrium, which is characterized by rapid trophoblast migration and the onset of decidualization of stromal fibroblasts [101]. In the study with Bagchi, calcitonin was localized in the baboon endometrial glandular epithelium on days 9 and 10 postovulation [102]. Infusion of hCG between days 6 and 10 postovulation initiated endoreplication of the uterine surface epithelium to form epithelial plaques, and stromal fibroblasts expressed α-smooth muscle actin which is associated with the initiation of decidualization [103]. Using an in vivo baboon model, endometrial biopsies were taken from hCG-treated and control animals for measurement of gene transcripts. The results of the analyses revealed that Serpin A3, matrix metalloproteinase 7, LIF, IL-6, and Compliment 3 were upregulated. Endometrial flushings showed increased LIF and IL-6 proteins in hCG-treated animals when compared with controls. Compliment 3 showed a marked increase in stromal staining in response to hCG, whereas superoxide dismutase 2 localization was most markedly increased in the glandular epithelial cells. These results showed that hCG induces alterations in the endometrial expression of genes that regulate embryo attachment, extracellular matrix remodeling, and the modulation of the immune response around the implanting blastocyst. Several of these genes, including LIF and gp130, have been shown to be essential for implantation in other species. This study provides strong evidence that the pre-implantation embryo influences the development of the receptive endometrium via secreted paracrine signals [104]. The transcription factor CCAAT/enhancer binding protein-beta (C/EBPβ), which is expressed in the mouse implantation sites, acts as a key mediator of steroid hormone responsiveness in the endometrium. In the baboon and the human, a robust expression of C/EBPβ was seen at the implantation sites, which indicates that this molecule is a biomarker of endometrial receptivity and plays a conserved functional role during implantation in the primate [105].
Smith et al. studied the ultrastructure of the initial stages of implantation in the marmoset monkey (Callithrix jacchus). The earliest samples, obtained 13 days after ovulation, displayed both cytotrophoblast and syncytiotrophoblast. The cytotrophoblast was restricted to the blastocoel, while syncytiotrophoblast intruded to the endometrial basal lamina. While in the human the blastocyst attaches 7 days after ovulation and in a further 7 days (i.e. 14 days after ovulation) has developed a fully differentiated hemochorial placenta, the development of the chorionic villi occurs more slowly in the marmoset than in the human. The marmoset blastocyst attaches to the uterine wall at approximately 12 days after ovulation, and at 31 days after ovulation (19 days later) has not developed chorionic villi [106].
Moudgal studied the endocrinological aspect of primate implantation. He has established, with N. Ravindranath, a colony of bonnet monkeys (Macaca radiata) at Indian Institute of Science in Bangalore, India. Bonnet monkeys were caught from the plantations and forests near Bangalore, quarantined, and a colony for research was successfully established after active husbandry and breeding programs [107]. Serum concentration of CG during early pregnancy in the bonnet monkeys could be detected on the 28th day of a fertile cycle and it could not be detected beyond the 50th day. Serum concentrations of progesterone and estradiol were also measured [108]. By injecting characterized estrogen antiserum during peri-implantation period, Ravindranath and Moudgal showed that estrogen is critical during the peri-implantation period for a successful pregnancy establishment in this species of the primate [109].
To determine the extent of primate embryonic development in vitro, Pope et al. recovered preimplantation baboon embryos from the uteri nonsurgically and cultured in microtiter plate wells containing about 250 μl of medium. Trophoblast outgrowth was initiated 1 to 3 days after attachment. The interval from recovery to maximal postattachment development ranged from 10 to 16 days. The maximal size attained was a 7- to 8-fold increase which corresponded to 11- to 12-day (stage Vc–VI) human and rhesus embryos morphologically [110]. Seshagiri and Hearn cultured in vitro fertilized zona-intact morphologically normal morulae and early blastocysts recovered on days 5 and 6 of pregnancy from naturally bred rhesus monkeys. These embryos were cultured for a minimum of 24 days, and culture medium was changed every 48 h. These embryos developed in vitro to hatch and attached blastocyst stages, exhibiting extensive trophectodermal outgrowths. These embryos secreted CG [111].
Implantation of ungulate farm animal embryos
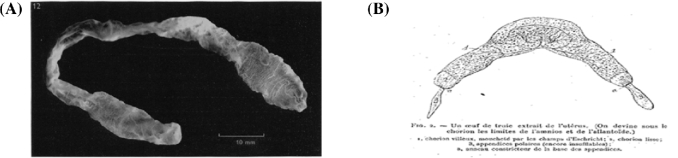
In human pregnancy progesterone secretion by the ovary is taken over by the placenta. Before the placenta is established, the corpus luteum of pregnancy is maintained by the CG from the trophoblast. Sheep and other ungulate blastocyst development is very different from those of human, rabbit, and mouse which remain in a very small, spherical shape at the time of implantation. As illustrated in Figure 3, sheep blastocysts were spherical vesicles on day 11, but started to elongate on day 12, from about 1 mm in diameter to a mean length of 11.7 mm within 24 hours, and at the end of elongation period (128–224 h postestrum: day 12, 13, and 14), they reached approximately 10 cm long on average, and by day 17 it reaches 25 cm or more [112]. Cellular and molecular processes between the blastocyst and the uterine endometrium in sheep are well illustrated by Spencer [113].
Figure 3.
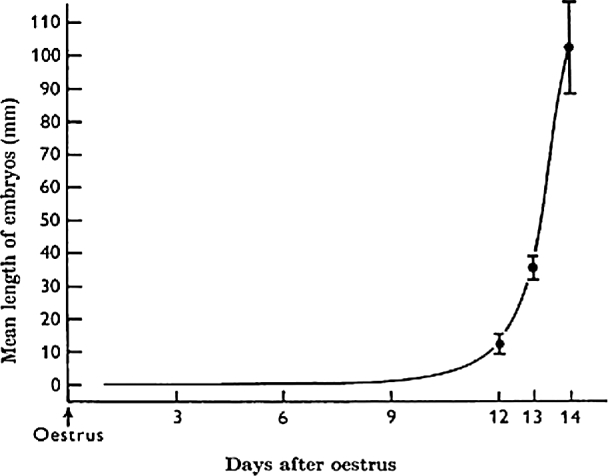
Mean linear growth of the sheep conceptus during the first 14 days [112].
Between day 9 and 14, no definitive cellular contacts are observed between the trophectoderm and the endometrial epithelium. According to Assheton [115], “to a certain degree the length of the blastocyst must be considered to be determined by the size of the uterus and number of the embryos which never overlap.” If so, there must be mutual communications between unimplanted blastocysts to adjust their size and location of attachment. Blastocysts of sheep and pig change their shape from spherical to thread-like elongated vesicles prior to attachment to the uterine luminal epithelium. In the elongated blastocyst, the embryo proper is located around the center of the thread-like elongated vesicle. The extremities (the both ends of the thread-like structure) are tapered and poorly vascularized, and they are called “necrotic tips” (Figure 4B).
Figure 4.
(A) Blastoderm vesicle recovered 14 days after estrus measuring 112 mm in length [112]. (B) An illustration of a pig blastocyst: the necrotic tips (appendices polaires) of pig conceptus [114].
Before the formation of the necrotic tips, the surface of the allantochorion was uniformly vascular, but between 27 and 30 days’ gestation, most of embryos began to show small irregular areas of discoloration at the poles; these spread rapidly to form a clearly defined, dull brown cap at each end of the allantochorion. The caps had no active vasculature although the paths of the original vessels were still discernible as brownish lines [116]. The degenerate extremities may discourage the formation of vascular anastomoses between neighboring conceptuses [117]. An extreme case of the function of the necrotic tip of the blastocyst is that, in pronghorn antelope, where twin birth is the rule, 3–7 ova are commonly ovulated, fertilized, and develop into expanded blastocysts. Some mortality occurs during the thread stage of the blastocyst. When more than one embryo per horn survived the thread stage, the one distal to the corpus uteri is displaced or its membrane is pierced by the necrotic tip which is on the distal end of the blastocyst only. Thus, extra distally located embryos are eliminated by the proximal blastocyst(s) at the time of implantation [118]. After attachment of the elongated blastocyst to the uterine epithelium, the endometrial vascularization become differentially influenced by the zone of the conceptus, the vascularization of the endometrial zone where the embryo resides have many and deeper microfolds of the luminal epithelium, while where the necrotic tips reside, become the least vascularized [119].
Assheton [115] described the sheep uterine structure of the cotyledonary burrs (caruncles) where attachment of the conceptus takes place. Guillomot et al. [120] studied interactions between trophoblast and uterine epithelium during the process of attachment. On day 13 the trophoblast cells were completely covered with microvilli, but they lost most of their microvilli between day 14 and 15. The apposition state off the conceptus and the uterine caruncular epithelium was completed by the end of day 15. Between day 16 and 18 the caruncular epithelium became very flattened and syncytial masses formed giant binucleated trophoblast cells appeared in the trophoblast but only uninucleated trophoblast cells were in contact with uterine cells.
Around day 16 the trophectoderm adheres to the uterine luminal epithelium. From the trophectoderm surface multicellular protrusions (named papillae) are formed in the embryonic region of elongated sheep conceptuses examined between 13 and 18 days of gestation. Many of these papillae are found in the lumen of the uterine glands, probably serving as pegs for anchorage or to serve for output of nutrient or messenger molecule [121]. Between day 14 and 16 binucleate cells begin to differentiate in the trophectoderm and to migrate and fuse with uterine luminal epithelium to form syncytia. Early researchers, such as Grosser, considered that the uterine epithelium disappeared and the fetal trophectoderm replaced the luminal epithelium thus naming it “syndesmochorial placenta.” However, Ludwig clarified that sheep placenta is of the epithelia-chorial type, similar to cows and deer. He also confirmed the presence of microvilli on the surface of the trophoblast as well as on the surface of the uterine epithelium [122]. Continuous exposure to progesterone during early pregnancy downregulates the PR in the sheep uterine luminal epithelium. The endometrial uterine glands secrete a number of proteins that regulate blastocyst development including glycosylated cell adhesion proteins (GlyCam-1), galectin-15, integrins, and osteopontin [111]. During the pregnant cycle, the presence of the conceptus in the uterine lumen is essential to prolong the life span of the corpora lutea in the sheep. The embryo must be present before day 12 to maintain the luteal function as the corpora lutea lose their function on day 13–14 of the estrous cycle [123, 124]. To find out if this luteal maintenance ability of the conceptus may be due to any substance produced by the conceptus, they homogenized the sheep embryos and instilled in the uterine lumen during the critical time period of the estrous cycle. Following the daily intrauterine infusion of a homogenate prepared from frozen and thawed tissue of 14- or 15-day sheep embryos, the length of the estrous cycle was significantly prolonged. Intrauterine infusion of heat-treated 14- or 15-day sheep embryo homogenate, 25-day sheep embryo homogenate, or 14-day pig embryo homogenate did not maintain corpora lutea of the sheep. Extrauterine site infusion of 14- or 15-day sheep embryo homogenate did not have luteotropic activity [125]. This series of work by Rowson and Moor clearly revealed that the sheep pre-implantation conceptus produces a heat-labile substance that exerts luteotropic activity through the uterus.
In 1972 McCracken et al. showed that prostaglandinF2α is a luteolytic hormone in sheep and uterine blood concentration of PGF2α reflects luteal regression toward the end of the estrous cycle [126]. The mechanism involved in the luteal maintenance by growing conceptus in the uterus must include a mechanism that prevents uterine release of prostaglandin F2α. Since oxytocin stimulates release of PGF2α from ovine endometrium [127], and the concentration of oxytocin in corpora lutea is reduced during early pregnancy [128], the substance produced by the conceptus, identified by Rowson and Moor, might act through a reduction of ovarian oxytocin and uterine PGF2α.
Bazer and Roberts cultured sheep conceptuses between days 13 and 23 of pregnancy and analyzed the proteins released into the incubation medium, and “protein X” was found to be released by the conceptus [129]. Two years later the same group of investigators clarified that “protein X” is a product of the trophectoderm as detected immunocytochemically in the blastocyst during the period days 13–21, and they renamed “protein X” as ovine trophoblast protein-1 (oTP-1) [130]. The antiluteolytic action of oTP-1 exerts on the endometrium requires progesterone and that this mechanism involves inhibition of oxytocin-stimulated turnover of inositol phosphate [131]. Characterization of oTP-1 revealed that the protein consists of three to four isoelectric variants (pI 5.5–5.8) of relative molecular mass of approximately 18,000. A cDNA library was constructed from oTP-1 mRNA, and the cDNA nucleotide sequence was determined. The primary amino acid sequence of oTP-1 inferred from the nucleotide sequence had approximately 50% sequence homology with a range of interferons of the alpha family of several species of mammals. From these results, Imakawa et al. concluded that oTP-1 could be an interferon and that oTP-1 might also participate in the immune protection of the fetal allograft [132]. Roberts stated that “as a result of their distinctive properties, the trophoblast interferons were recently classified as a subtype separate from the structurally related IFN-ω. The IFN-τ genes (IFNT) are restricted in distribution, being found in ruminant ungulate species.” [133]
This trophoblast-originated interferon tau is a unique interferon as it is expressed only by trophectoderm of the blastocyst and not appears to be virally inducible [133]. Both R Michael Roberts and Fuller W. Bazer shared the Wolf Prize in Agriculture for their discoveries of interferon tau and other pregnancy associated proteins. Their discoveries helped clarify the biological mystery of signaling between embryo and mother to maintain pregnancy. Their discoveries have profound effects on the efficiency of animal production systems, as well as human health and well-being.
Increasing progesterone levels, with additional IFN-tau from the conceptus, during early pregnancy stimulate endometrial luminal and, particularly, glandular epithelia to express molecules essential for survival and development of the conceptus [134].
In cows, early embryonic losses are much higher in nuclear transfer (cloned) pregnancies. Davies and White showed that trophoblast MHC-1(major histocompatibility complex class I) expression in the cloned pregnancies is likely the cause of high early pregnancy failure [135]. Thomas Spencer's group studied the quality of the uterus in cattle and classified heifers into three groups: high fertile (HF), sub fertile (SF), and infertile (IF) based on the results of a preliminary embryo transfer trials. To these heifers of three categories, embryo was transferred on day 7 and the heifers were studied for the embryo and uterus on day 17. The pregnancy rate was 71% (HF) and 90% (SF) as compared with 20% (IF). The size of the conceptus was 10.6 cm (HF) and 4.7 cm (SF) on average, and only one small spherical embryo was recovered from one heifer in (IF). Endometrial transcriptome was compared among these three categories and one of the findings is that conceptus–endometrial interactions are dysregulated in the SF animals and support the idea the adverse ripple effect hypothesis that aberrant communication between the endometrium and conceptus disrupts normal implantation and placentation processes, leading to pregnancy loss and later pregnancy complications [136].
Implantation research in experimental animals
The great embryologist Gustav Born of Breslau, Germany, reached the conclusion that the corpus luteum is an organ of internal secretion. Shortly before he died in 1900 he conceived the idea that the specific endocrine function of the corpus luteum is to protect the early embryo and facilitate its implantation in the uterus. Born bequeathed on his deathbed the duty and privilege of putting the idea to experimental test to one of his students, the gynecologist, Ludwig Fraenkel. In 1903, Fraenkel showed in the rabbit that depending on the time of removal of corpora lutea resulted in failure or success of implantation and maintenance of implanted embryos [137]. Bouin and Ancel also showed in the rabbit that the gestational corpora lutea have specific action on the uterus to render the endometrium hyperemic, hypertrophic, and proliferation for the fixation of the fertilized egg [138]. Corner and Allen demonstrated in the rabbit that “the corpus luteum extracts contain a special hormone which has for one of its functions the preparation of the uterus for reception of the embryos by inducing progestational proliferation of the endometrium” [139]. From the end of 1920s through early 1930s, there were several groups of investigators working independently to isolate and identify “the corpus luteum hormone.” There was a severe, neck-and-neck scientific race among those groups in purification of progesterone. Among them were Ludwig Fraenkel, Edgar Allen and Edward Doisy, Pol Bouin and Paul Ancel, Willard Allen and George W. Corner. After all isolation and identification of progesterone were achieved by a German chemist, Adolf F. Butenandt [140]. By 1947 Corner et al. established that progesterone is the chief hormone of the corpus luteum [141].
The skillful historical review of implantation was done by Asdell, and events before Marshall's Physiology of Reproduction should be referred to Asdell's review [142]. A more recent historical review was made by Psychoyos, who reviewed a history of implantation-related research during a 100-year period with the title “From Lataste through ‘the window of implantation’: Fantastic discoveries of 100 years.” He emphasized the discovery of the cyclic change of vaginal smear is by Lataste, 25 years ahead of Stockard and Papanicolaou; quoted the contributions made by Iscovesco during the pre-endocrine era; and cited the discovery of a proteinaceous substance (hCG) in the human placenta that has ovulatory activity by Hirose in 1920 among many interesting historical events [143].
Prior to implantation
Spacing of blastocysts along the uterine horn: Mossman wrote “in a bicornate uterus, as in the pig, the embryos nearer the oviduct are always slightly more advanced in development during the first few weeks than those nearer the cervix.” So, he assumed that the uterus is about equally favorable for attachment. What seems to happen is that the first embryo suddenly strikes up some sort of a physiological relation with the mucosa, which then renders the immediate neighborhood refractory to any other embryo. This sphere of physiological influence apparently fades out at a variable distance, depending on species, and so other embryos moving by at length to reach another favorable area where the same process recurs” [144]. Mossman's idea was criticized by McLaren and Michie [145] based on the results of their embryo transfer study. McLaren and Mitchie first examined the assumption that implantation is serial, i.e. the embryos closer to the oviduct the heavier. In the mouse, the embryo nearest to the ovary was smaller than the average according to Hashima [146]. Next, McLaren and Michie found that embryos which implanted in the middle of the horn do less well, compared to those near the ends, and could not confirm Mossman's assumption. The idea that creation of a refractory zone for other embryos to implant near the implantation area was rejected. Specifically, McLaren and Michie transferred a large number of blastocysts so that some of the blastocysts may be located in the “refractory areas” near the implanting blastocysts. Most of the transferred blastocysts implanted very closely to each other negating the idea of creation of refractory areas near implanting blastocysts.
Restall and Bindon, by studying location of embryos in the mouse uterus at 2-h intervals, showed that as the embryos enter the uterine horn, they reach in the middle segment where they were seen to be grouped together around 10:00 h on day 4 and in general, spacing begins equally in both directions from the center of each horn [147]. The embryos spread along the length of each horn between 10:00 and 16:00 h on day 4. In the rat, blastocyst distribution rather precisely takes place between 15:00 and 19:00 h on day 5 [148]. Spacing of embryos within uterine horns is considered to be outcome of inner circular and outer longitudinal muscle layers of the uterine horn. One of the mechanisms may involve the luminal epithelium in contraction of the underlying smooth muscles via prostaglandin [149].
Mouse embryos implant antimesometrially in specialized extensions of the uterine lumen called implantation chambers [150]. On the afternoon of day 5 of rat pregnancy, blastocysts lie in a shallow antimesometrial depression and tend to fall free of the uterus when the chamber is opened. On day 6, blastocysts start to adhere to the uterine wall of the chamber [151]. Burckhard believed that muscle contraction and gravity might explain the spacing and orientation of blastocyst in the antimesometrial domain. Alden tested Burckhard's hypothesis by studying implantation in the rat uteri where the middle third of the horn was resected, rotated 180o on its long axis, and re-anastomosed. After a recovery period, the rats were mated and those rats having at least one implantation site in the middle segment as well as upper or lower segments were examined for the implantation sites. Alden confirmed that all embryos were implanted in the antimesometrial domain with the inner cell mass toward the mesometrium. He also concluded that these results probably ruled out gravity as a single and fundamental factor in the attachment of the rat egg [152].
Enders and Schlafke studied the morphological analysis of the early implantation stages in the rat using electron microscopy [153]. The same investigators have established three implantation stages with three artistic optical microscopic illustrations and features and scientific explanations on blastocyst, uterine epithelium, and stroma at each stage. These three stages of implantation process are defined as apposition stage, attachment stage, and epithelial penetration stage [154]. Tachi et al. also observed in the rat that the blastocyst establishes contact with the tips of microvilli and with bleb-like cytoplasmic protrusions (pinopods) of the epithelial cells. They also noted the day after attachment the epithelial microvilli were usually lost and the trophoblast membrane interlocked with that of the epithelial cells, with frequent formation of tight junctions. Leukocytes were occasionally seen in the epithelial layer at the attachment site [155].
Yamaguchi et al. showed that Wnt5a is required for morphogenesis of all outgrowing structures in mouse embryos [156]. In developing pre-implantation embryos, both Wnt3a and 4 transcripts were detected in some pre-compact 4/8-cell stages with consistent expression detected in all compact 8-, 15-cell, and blastocyst stages [157]. Mohamed et al. showed that mouse morulae express genes that encode for several members of the Wnt family of signaling molecules. Additional Wnt genes are newly expressed following development to blastocyst. Wnt5a and Wnt11 are expressed in embryos that undergo the morula-to-blastocyst transition in vivo. Upregulation of Wnt11 is temporally coordinated with the maternal estrogen surge on day 4 [158]. They also detected Wnt/β-catenin signaling transiently in the circular smooth muscle of the uterus on early day 4, and, in the luminal epithelium at the prospective site of implantation [159]. The molecular mechanisms involved in this implantation chamber formation have recently been shown to be attributed to Wnt5a-ROR (retinoid-related orphan receptor) signaling [160]. Also, Wnt5a forms a signaling gradient along the luminal epithelial evaginations of the primary uterine lumen toward the antimesometrial domain. The investigators of S.K. Dey's group demonstrated that movement of the epithelial evaginations through the stromal bed is guided by PCP (planar cell polarity) signaling [161]. This implantation chamber formation is probably a consorted signal transduction of epithelial evagination regulated by the Wnt5a-ROR and the PCP signaling pathways with the RBPJ (recombining binding protein suppressor of hairless)-regulated Notch signaling pathway [162]. Dey's group has recently demonstrated that the implantation chamber formation is regulated by epithelial-specific Vangl2-/PCP signaling, and as the chamber being formed toward the antimesometrial direction, the uterine glands which are connected to the luminal epithelium and embedded in the stroma move downward and the openings of the gland ducts in the chamber are filled with their secretions (HB-EGF, FOXA2) which have direct contact with the implanting blastocyst [163].
Once rodent blastocysts settle in each implantation chamber, the trophectoderm of the blastocyst at the equatorial region of the blastocyst attaches to the uterine epithelial cells of the chamber. This initial attachment is facilitated by a reduction of the uterine fluid. Margaret Parr [164] wrote “closure of the uterine lumen occurs during early pregnancy just before implantation of the blastocysts in rats and mice. At this time, the microvilli from apposed luminal epithelial cells interdigitate with one another or, where the embryo is present, with those of the trophoblast. This period of development is called the pre-attachment phase by Mayer et al. [165], the apposition stage by Enders & Schlafke [154], or the first stage of closure by Finn [166].” This uterine closure and epithelial cell endocytosis remove uterine fluid from uterine lumen under progesterone. Salleh et al. studied hormonal control of the fluid and ion movement within the uterus by examining of expression of Na+ and Cl− channels. They showed that under progesterone uterine absorption of fluid through luminal and glandular epithelial cells dominates at the expected time of uterine closure in the peri-implantation period [167].
Psychoyos observed that rat blastocysts swell and shrink repeatedly in vitro and expelled blastocoelic fluid. He considered if this happens in vivo the expelled material probably contains “messages” from the blastocyst to the mother that may be involved in immunorecognition, maintenance of the corpus luteum, or stromal decidualization [168]. This blastocyst movement may facilitate shedding of the zona pellucida mostly taking place between late day 4 and 5 in the mouse [169].
In response to the presence of a blastocyst, the capillary permeability increases in the endometrium close to the blastocyst. Psychoyos first used Geigy blue intravenous injection in rats to detect a modification of capillary permeability prior to decidualization of rat uterus after treatment with pyrathiazine [170] or mechanical trauma [171]. Orsini confirmed the location of implantation sites by this “blue reaction” in hamsters [172]. This increase in capillary permeability of the endometrium in the segments where the blastocysts exist is the earliest response of the endometrium to the blastocysts which can be visualized as blue spots in the uterine horn after intravenous injection of a macromolecular dye, such as Geigy blue, Evan's blue, or Pontamine blue. The blue reaction is observed several hours before decidualization of stromal cells. In the rat, most of the blastocysts are held in the position in relation to the uterus in the evening of day 5, and are blue-reaction positive, but they can be readily flushed from the uterus at this stage. In the area of apposition with the trophoblast, the apical surface of the epithelial cells closely follows the general contour of the trophoblast cells and is somewhat modified. In addition to short digitiform microvilli seen previously, numerous more irregular knoblike protrusions of the apical cytoplasm, including pinopods, are found [157]. By the afternoon of day 5 the walls of the lumen begin to compress the blastocyst, and the microvilli flatten and new structures appear. Most of the cells bear pinopods (bulbous cytoplasmic projections with a depression at the center), but they are a transient phenomenon occurring only during the period of blastocyst attachment and regress the next day [151].
The apposition stage is followed by the stage of adhesion. Hewitt et al. studied anionic sites on the surface of rat uterine luminal epithelium because negative charge repulsion by the presence of glycocalyx was considered important for cell surface interactions. They found that the anionic sites were high at the beginning of pregnancy but gradually reduced and entirely disappeared at the time of implantation [173]. Daniel Carson's group studied the role of mucin1 (MUC1) in the mouse uterus and found MUC1 is regulated by ovarian steroid hormones, which is present at high levels during pro-estrus and estrus but reduced on diestrus. Also, loss of MUC1 from the uterine surface is important for accepting implanting blastocyst [174]. Aplin et al. localized distribution of integrin subunits in the mouse endometrium and found the distribution of the β5 and αv subunits was localized to the apical epithelium [175]. Calcitonin is expressed in uterine glands under the influence of progesterone and estrogen [176], and integrin β3 is upregulated by calcitonin [177]. Hoffman et al. isolated a stage-specific membrane constituent, a 42-kDa glycoprotein, from receptive stage rabbit uterine endometrium, which can be used as a marker of the receptivity, and it is a haptoglobin-like glycoprotein [178]. Another uterine protein secreted around the time of implantation in the rabbit is uteroglobin (or blastokinin) [179,180], which is related to phospholipase 2 and has been shown to have immunomodulatory activity [181]. Adhesion of human blastocyst to the uterus has been suggested to be mediated by a ligand–receptor interaction (trophoblast L-selectin to its ligand) [20]. There are a number of adhesion molecules enumerated at the implantation site, e.g. IL-1, leptin, glycodelin, IGF-BP, TNF-alpha, OPN, etc. [182]; their roles in cellular and molecular function leading to invasion need to be studied.
Ovarian steroid requirements for implantation
The requirement for ovarian steroids in preparation of the endometrium for implantation was studied in the mated female rats and mice ovariectomized soon after fertilization. In many of these animals, daily progesterone treatment did not induce implantation and additional estrogen was found to be needed for induction of implantation. While in rat, mouse, and Mongolian gerbil both progesterone and estrogen are necessary for implantation in the ovariectomized animals, in other species of animals such as pig and hamster implantation needs only progesterone after ovariectomy, as estrogen is produced by the implanting embryos [183]. Strömstedt et al. showed that RNAs encoding steroidogenic enzymes, including CYP11A1, CYP17, CYP19, adrenodoxin, and NADPH-cytochrome P450 reductase, were all detected in pig blastocysts at the time of implantation. By contrast, neither CYP11A1, CYP17, nor CYP19 mRNA, and no aromatase protein, was detected in mouse blastocysts at implantation, indicating that estrogen biosynthesis does not occur at this time [184]. Therefore, the estrogen from the ovary is required for implantation in the mouse and rat due to a lack of the enzymes that are needed to synthesize estrogen in the embryo. In the rat and mouse, there is an ovulation within 24 hours after parturition. When there is mating at this postpartum estrus, the nursing mother becomes pregnant again concurrent with lactation. An intensive lactation requires secretion of a high level of prolactin secretion from the anterior pituitary, which reduces the secretion of gonadotropins from the same organ. A reduction of gonadotropin output reduces estrogen secretion by the ovary and increased prolactin secretion increases progesterone secretion. Lataste is the first researcher who found that gestation length is prolonged during lactation in the mouse, due to a delay in implantation [185]. This delay in implantation in lactating rats can be abolished when estrogen is injected in the rats [186,187]. A similar endocrine condition of pregnant, lactating mouse, and rat can be produced by ovariectomizing the mated rats and mice before secretion of pre-implantation increase in estrogen and treating with daily injection of progesterone [188,189]. Pre-implantation increase in estrogen secretion may be blocked by treating the pregnant rats with chlorpromazine which blocks LH secretion from the anterior pituitary, resulting in a delay in implantation [190]. The increase in estrogen secretion just before implantation stimulates estrogen receptor (ER) mRNA and protein [191] as well as mRNA of EGF-R in pre-implantation mouse embryos and mRNA of endometrial epithelial EGF-R [192]. Blastocyst implantation in the mouse is dependent on expression of LIF in the uterine glands on the fourth day of gestation and is essential for implantation [193]. Trophoblast invasion and migration through the uterine wall is mediated by molecular and cellular interactions and controlled by the trophoblast and the maternal microenvironment [194, 195]. One of the critical molecules needed for on-time implantation is heparin binding EGF-like growth factor (HB-EGF). HB-EGF is necessary for ovarian estrogen secretion as well as the uterine–blastocyst interactions [196]. In the implantation chamber, the implanting blastocyst has the closest contact with the uterine epithelium around the equatorial region. The trophoblast cells in this region interact with the luminal epithelial cells and remove these cells by phagocytosis of apoptotic cells [197] or entosis [198]. This microenvironment of implanting blastocyst is also favorable for the inoculated tumor cells in the uterine lumen [199]. After removing of epithelial cells, the trophoblast cells get direct contact with the basement membrane of the epithelium. Schlafke and Enders closely studied how the basement membrane is breached during implantation in the rat. The initial areas of cellular interaction between embryonic and maternal tissues in the rat are the lateral wall of the implantation chamber. In these areas, Schlafke et al. observed that decidual cells are in the broken space of the basement membrane and in many areas decidual cell processes are found between the epithelium and its basement membrane [200].
Decidualization
In 1960 Psychoyos [201] showed that endometrial capillary permeability is increased long before stromal cells initiate decidualization. In the rat and mouse, stromal cells surrounding implantation chambers initiate decidualize before invasion of the epithelium by trophoblast [202]. One of the important literatures on decidualization we cannot lose is the work done by De Feo De Feo compiled many important historical works on decidua and implantation in an approximately 100 printed page chapter entitled decidualization in the book “Cellular Biology of the Uterus” [57]. Loeb first described the capacity for the deciduoma formation in the guinea pig, i.e. traumatization of the uterus by cuts at appropriate time during early pregnancy resulted in proliferation of the endometrial cells to form decidua-like structure [203]. Nichaman demonstrated that extensive deciduoma formation in the rat uterus might lead to loss of uterine sensitivity for later deciduoma production. This important uterine principle is applicable for loss of uterine sensitivity for implantation in the uterine segment where a previous placenta occurred (placental scar) [204]. This phenomenon involves the role of the mesometrial triangle of the uterus and immune cell function that affects uterine endocrine function. The cellular and molecular mechanisms involved in this interesting phenomenon need clarification as it is considered as a crossing point of endocrinology and immunology.
Delayed implantation/embryonic diapause
Lataste discovered in the mouse that the gestation period is prolonged in pregnancy concurrent with lactation, and that the prolongation is due to a delay in implantation [185]. Lataste should be considered as one of the pioneer researchers on blastocyst implantation. The work of Lataste was remembered by René Canivenc at the occasion of the Symposium on Implantation in Bordeaux 1992. As the mayor of Gradignan near Bordeaux, he invited the descendants of Fernand Lataste to the town hall and held a scientific symposium on implantation to celebrate the 100-year anniversary of Lataste's research. Lataste also discovered that the estrous cycle of mammals can be traced by cyclic changes of vaginal smears [205], some 25 years before Stockard and Papanicolaou rediscovered this phenomenon in the guinea pig [206].
Superimplantation/superfetaion
The definition of the “superimplantation” (superfetation) is the phenomenon in which two sets of conceptuses with different developmental stages are present in the same uterus. Canivenc and Mayer were the first investigators who artificially created the superimplantation rats by injecting progesterone into the uterine wall locally on day 3 of lactating pregnant rats (Fig. 5) [207]. The local injection induced implantation of a blastocyst that happened to be near the site of injection and the remaining blastocyst in the uterus remained as blastocyst because of intensive lactation which delays implantation by 2–9 days. Initially they injected locally into the uterine wall a small dose of estrogen suspension. However, these local injections did not result in induction of implantation and they thought the amount of estrogen might not be sufficient to induce implantation. Psychoyos [208] systemically injected a threshold dose of estrogen (0.1 μg estradiol) to induce implantation to pregnant, chlorpromazine-treated rats where implantation is delayed by inhibition of pre-implantation estrogen secretion. This small dose-estrogen injection only induced a few blastocysts which happened to be within more sensitive segments of the uterine horn than other segments. He injected the same dose of estrogen a few days later. This second estrogen injection induced implantation of the second set of blastocysts which had been remained free in the uterine horns. By this way Psychoyos obtained superimplantation rats by inducing implantation at two systemic injections of small dose of estrogen at different times. The results of Psychoyos demonstrate that the uterine horns are not uniformly receptive to implanting blastocysts, and there exist relatively more receptive endometrial regions than the other regions.
Figure 5.
Superimplantation rats produced by three different methods: (A) Canivenc and Mayer produced by local injection of progesterone in the uterine wall [207]; (B) Psychoyos produced by repeated systemic injection of a threshold dose of estradiol [208]; (C) Yoshinaga produced by a local fat pad injection of progesterone or estrogen [209].
In 1957, Yoshinaga asked Canivenc how one can inject accurately small doses of hormones into the rat uterine wall, Canivenc answered “it is difficult but not impossible.” With this response, Yoshinaga invented a less difficult method to apply steroid hormones locally to the rat uterus to induce implantation. This local injection method is to visualize the injection site by pulling out the uterine horn through an incision made on the dorso-lateral portion of the rat abdominal cavity, and injection is done into the adipose tissue surrounding the uterine arcuate artery and vein tufts. Although how the steroid hormone thus applied, acts on the segment of the uterine horn close to the site of injection, has not been clarified yet, various doses of estradiol between 1 and 5 ng showed dose-dependent increase in the affected uterine segment close to the site of injection. One important thing in using this method is to use a new sharp needle each time so that damage to the adipose tissue by insertion of the needle is minimized and the injected oil droplet will be retained at the site for a long time [209]. Yoshinaga showed that delay in implantation in the lactating pregnant rats can be abolished locally by progesterone and estrogen. It is understandable how estrogen abolishes the delay as the delay is caused by lack of estrogen. However, it is difficult to understand how locally applied progesterone in adipose tissue induced implantation locally. Canivenc and Mayer explained their progesterone injection into the uterine wall as induction of decidual tissue by the direct traumatization of the endometrium. Psychoyos used this fat-pad local injection method to demonstrate that a delay in implantation is abolished locally by estrogen in the rats treated with trifluoperazine which causes a delay in implantation [210]. Ferrando and Nalbandov and Diao et al. used this local injection method and demonstrated that locally injected substances had a local effect on a limited segment of the uterine horn in rats and mice [211, 212]; however, Emmens and Finn failed to show a local effect of antiestrogenic substances to inhibit implantation in such a way they hoped, and blamed this technique as it cannot be very feasibly repeated [213].
Production of a superimplantation is useful for the study of implantation mechanisms. This preparation also serves as a useful tool for the mechanism involved in parturition. These two sets of fetuses in different developmental stages grow normally until the older fetuses reach at the stage of parturition. The old fetuses are ready to be delivered and parturition occurs around on day 23; the young fetuses are aborted at the same time. This normal parturition takes place when the old/young fetus ratio is greater than 1/3. Parturition does not occur until day 27 or so when the old/young fetus ratio is less than 1/3. Since decapitation of old fetuses in the former group delays parturition, the positive factor(s) of old, mature fetoplacental units for the onset of parturition appear to come from the head of the mature fetus [214].
Biochemical-molecular biological approaches
Steroid receptors in the process of implantation gained interest of researchers in the 1970s. Do and Leavitt tried to characterize a specific PR in decidualized uterus of hamster [215]. Prior to their publication in1985, “Elwood (Jensen) and his postdoctoral fellow Herbert Jacobson used tritium gas to reduce a double bond in an appropriate precursor, generating estradiol labeled to very high specific activity. When they administered physiological doses of this tracer to immature female rats, they found that it was not chemically altered, as predicted by the then current models, but was instead specifically retained in known estrogen target tissues such as the uterus. Elwood correctly deduced that the tracer was held there by a specific protein, which he termed estrophilin and which we now know as the estrogen receptor. Subsequent work by Elwood and colleagues and also the late Jack Gorski and colleagues indicates that estrogen binds the estrogen receptor in the cytoplasm, and the complex then moves to the nucleus. Bert O’Malley was the first to clearly demonstrate that estrogen and progesterone act in the nucleus to induce specific messenger RNAs.” The preceding quoted phrase was written by David Moore in the introduction of his “conversation with Elwood Jensen” [216]. Progesterone receptor is a member of the nuclear receptor superfamily of transcription factors capable of activating and repressing transcription of their target genes [217]. Those researchers who worked on steroid receptors opened up a new field where one can learn how the steroid hormones regulate the complex utero-placental functions to make the conceptus develop in utero to become a healthy baby.
One of the early researchers on implantation from molecular endocrinological view is the group of Collin Stewart. In 1991, they studied mRNA of LIF during early mouse pregnancy and LIF is expressed in uterine glands prior to implantation under the influence of estrogen [218]. LIF is one major mediator of estrogen action on the uterine glandular epithelial cells. Its deletion results in implantation failure. LIF binds its receptor, LIFR, which partners with co-receptor gp130 to activate the downstream signaling via signal transducer and activator of transcription 3 (STAT3). Daikoku et al. showed that LIF and Hoxa-10 are crucial to implantation and decidualization in the mouse [219]. LIF transcripts are expressed in the uterus in a biphasic manner: LIF is expressed in the glands on the morning of day 4 and again in stromal cells surrounding the blastocyst with the onset of implantation in the evening of day 4 of pregnancy. LIFR and gp130 were also expressed in the luminal epithelium 12 h after injection of estrogen to the progesterone maintained delay in implantation models [220]. Cheng et al. studied how LIF acts on the uterus. LIF induced STAT3 phosphorylation and STAT3 was localized to cytoplasm of the luminal epithelial cells.
On day 4, uteri STAT3 was localized to the nuclei of the entire luminal epithelium, and it was relocated to the cytoplasm on day 5 in the interimplantation luminal epithelium [221]. Catalano et al. showed that STAT3 inhibitor instillation in the uterine lumen on day 3 of pregnancy resulted in inhibition of implantation [222]. STAT3 regulates expression of early growth response 1 (Egr1) in the subluminal stromal cells [223], and Egr1 functions as a critical mediator of stromal cell decidualization by regulating Wnt4 (a ligand of the Wnt family).
Thus, LIF regulates stromal decidualization via paracrine manner by mediating epithelial ERα. The epithelial ERα controls stromal proliferation and decidualization via LIF [224].
Kelleher et al. deleted FOXA2 in the uteri of adult mice using the LtfiCremouse model. They found the adult FOXA2-deficient uteri appeared histologically normal and contained uterine glands; however, these females were infertile because of defective implantation. Their results with supplemental LIF replacement experiments support the idea that FOXA2 has a primary role in regulating Lif expression in the uterine glands, FOXA2-ndependent genes in the glands have a primary role in regulating stromal cell decidualization, and FOXA2-dependent genes in the glands are requisite for but do influence stromal cell decidualization and placental growth and development [225].
It has been recognized that there is a limited time period when the uterine endometrium is receptive for accepting implanting blastocyst. This time period was named “window” by Edwards [19]. Dey's group examined the nature of the receptivity window in the mouse. Their results suggest that the window can be variable dependent on the transferred blastocyst [226].
For dormant blastocysts obtained from progesterone-treated mice had only 1-h window compared with estrogen-activated blastocysts had up to 16 h [227]. One important finding in this study is that the progesterone-treated uterus secretes a special substance 1 h after estrogen treatment. This substance activates blastocysts in utero resulting in implantation [228].
Weitlauf's group studied the appearance of specific cell surface determinants on mouse embryos associated with the process of attachment to the uterus [229] and that the function of galectin-3 is related specifically to pregnancy. Since galectins are considered to recognize specific cell–cell or cell–matrix interactions or as intercellular regulators, they suggested that galectins may have important biological roles in the process of implantation [230]. Bagchi's group sought to identify the genes that are regulated by the ovarian steroids within the window of implantation. They isolated mRNAs from uteri of estrous rats and day 4 pregnant rats and synthesized cDNAs. Using these two cDNA pools, they carried out differential gene screening. After removing common genes between the two groups and enrichment of differentially expressed genes, a full-length cDNA was isolated from a rat uterus (pregnant day 4) cDNA library. Determination of the nucleotide sequence of the full-length clone and subsequent search in the Genbank database for this sequence led them to its identification as calcitonin. It is noted that the rat uterus usually becomes receptive in the afternoon of day 5. So, there may be a strain variation in the time of onset of the receptivity [231].
Satokata et al. mutated the AbdB gene Hoxa10 in mice and female homozygotes ovulate normally, but about 80% are sterile because of death of embryos between days 2.5 and 3.5 postcoitum [232]. Collaborating with the Benson's group, Dey found that Hoxa10 mutant female mice fail to undergo embryo implantation and resorption of embryo after implantation [233]. Das et al. found that HB-EGF gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition, and they thought HB-EGF is a possible ligand for interaction with blastocyst EGF receptor in implantation [234]. Chakraborty et al. studied expression of the cyclo-oxygenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2) genes in the peri-implantation mouse uterus and showed that the uterine COX-1 gene is influenced by ovarian steroids, while the COX-2 gene is regulated by the implanting blastocyst during early pregnancy [235]. Lim et al. demonstrated that the targeted disruption of COX-2, but not COX-1, in mice produced multiple failures in female reproduction processes including ovulation, fertilization, implantation, and decidualization [236]. How prostaglandins are involved in the process of implantation and stromal decidualization was studied by Kennedy [237]. Ye et al. showed that implantation in the LPA3-deficient mice had a slight delay in implantation and the embryos tend to cluster in the uterine horns close to the cervix [238]. It is interesting this irregular implantation is also observed in the females with genetically reduced levels of adrenomedullin [239]. Adrenomedullin has been shown to improve fertility by promoting production of pinopods and cell junctions [240]. Song et al. also reported that cytosolic phospholipase A2α is crucial for “on-time” implantation [241].
As to the embryonic side, trophectoderm of the blastocyst initiates differentiation in order to attach and invade the maternal tissues. The intricate process of trophoblast cell lineage development is regulated by specific transcription factors [242]. Renaud et al. showed that human trophoblast syncytialization is regulated by OVO-like1 [243]. It is syncytial trophoblasts that invade the endometrial luminal epithelium in humans and guinea pigs. Thus, proper trophoblast differentiation and proliferation are important for healthy pregnancy.
Using newly created NK cell-deficient rats, Soares group studied how uterine NK cells serve for development of embryo and placenta [244]. This group confirmed that uterine NK cells are phenotypically and functionally different than other tissue-resident NK cells; for example, uterine NK cells possess a distinct cell surface receptor repertoire compared to peripheral blood NK cells, produce abundant array of cytokines and angiogenic growth factors, and, despite possessing prominent granules containing proteins such as granzyme, perforin, and granulysin, are only weakly toxic. Soares's group suggests that uterine NK cells likely possess other, nonimmunological functions, such as uNK cells initiate development of the uterine vasculature that, in turn, optimizes blood flow to the developing placenta.
Hou and Gorski showed that ER mRNA is present in pre-implantation mouse embryo, and ER mRNA and ER protein were detected in all cells of the implanting blastocyst. At the early egg cylinder stage, continued expression. The embryonic expression of ER at such early stages in a broad array of cells suggests that ER may have a general role during early development [245]. Nichols et al. localized LIF transcripts in the differentiated trophoblast; in contrast, LIF-R mRNA was found in the inner cell mass [246]. Male and female embryos homozygous for the PR mutation developed normally to adulthood. However, the adult female PR mutant displayed significant defects in all reproductive tissues. These included an inability to ovulate, uterine hyperplasia and inflammation, severely limited mammary gland development, and an inability to exhibit sexual behavior [247]. Progesterone receptors serve not only for physiological activities of the genital tract, but also for that of other organs such as the thymus involution during pregnancy [248]. Moreover, isoform-specific knockouts for PR-A and PR-B disclosed the individual and overlapping roles of these receptor subtypes in mediating progesterone's physiological effects [249, 250].
In 2001, Dey's group examined growth factors as to if they are involved in implantation and decidualization in the mouse. They prepared Affi-Gel Blue Gel beads of about blastocyst size, incubated with various growth factors. The growth factor loaded beads were transferred into the uterus of day 4 pseudopregnant mice, and the uteri were studied for their response to the beads. They found that the beads soaked in HB-EGF and IGF-1 but not other growth factors, induced many of the same discrete local responses elicited by the blastocyst [251]. In 2002 Matsumoto et al. [252] and Takamoto et al. [253] showed that IHH is a progesterone-induced gene in uterine epithelium. Lee et al. demonstrated that the hedgehog (Hh)-signaling pathway is critical for uterine function as conditional ablation of Ihh in the uterus rendered female mice infertile due to an inability of embryo attachment and subsequent decidualization [254]. Mice expressing Cre under the control of PR was created by Demayo's group [255], who further investigated the role of Hh signaling during embryo implantation. They constitutively activated SMO in the uterus using the PRcre mouse model. The PRcre mouse model recombines alleles in all PR-positive cells, which includes the mammary gland, pituitary, corpus luteum of the ovary, and all components of the uterus. Stroma-specific activation of SMO was verified by stroma-specific activation of the patched1 (Ptch1). Franco et al. showed that constitutive activation of Smo in the mouse uterus alters postnatal uterine differentiation which interferes with early pregnancy, providing a new insight into the role of Hedgehog signaling during embryo implantation [256]. Since these mouse mutants have been widely used as the gene mutation is expressed mainly in the uterus, they have generated a wealth of information about gene function in uterine biology during pregnancy. However, the uterus is composed of three major different tissues: myometrial, stromal, and epithelial tissues. Since uterine epithelium and stroma interact with each other as a cross-talk and transmit signals as a paracrine mechanism, PgR-Cre in all uterine compartments does not allow gene function in a single tissue such as the epithelium. Since the process of implantation is initiated with interactions between trophoblast and endometrial luminal epithelium, one should study function of a gene in the epithelium only to obtain more specific aspect of the gene function. Daikoku et al. generated a new iCre knockin mouse line, in which iCre is expressed from endogenous lactoferrin promotor [257]. According to these researchers, the generation of Ltf-iCre mice shows efficient and specific deletion of floxed genes in the uterine epithelium, which could be of great value in studying uterine epithelial gene function during pregnancy and in immature mice after hormonal stimulation. DeMayo's group has studied how PR regulates implantation, decidualization, and glandular development via a complex paracrine signaling network [258]. Lee et al. demonstrated that Ihh is an essential mediator of uterine Pgr action in the mouse and expression of this factor is critical in mediating the communication between the uterine epithelium and stroma required for embryo implantation [259]. Jeong et al. showed that SRC-2 plays an essential role in murine endometrial decidualization [260]. In studies on Pgr action mechanism, they also found that COUP-TFII expression suppresses ERα activity during pre-implantation period. COUP-TFII is still required to facilitate placentation [261]. Kawagoe et al. showed that nuclear receptor coactivator-6 (NCOA6) knockout in the uterine stromal cells and epithelial cells resulted in implantation failure, indicating that NCOA6 is required for appropriate development of uterine endometrial receptivity. Furthermore, NCOA6 deficiency robustly increases expression of uterine E2 sensitivity as evidenced by the increased expression of ERα target genes under physiological conditions, the abnormally sustained EC proliferation during the pre-implantation period, and the supersensitive response to low dose E2-induced gene expression and uterine growth [262]. It is interesting to know what molecules are working where in the endometrium to express the uterine receptivity. Monsivais et al. showed that the transition of a nonreceptive uterus to receptive uterus requires ALK3 (Activin-like kinase3), and that bone morphogenetic protein signaling via ALK3 defines uterine receptivity [263].
Paria et al. [244] showed that BMP7 is highly expressed throughout the endometrium excluding the muscle layer, with higher expression around the implantation sites. BMP7 is highly expressed during two key stages of pregnancy: once in the transformation phase of the uterus from the neutral to the receptive phase and again during placental development.
Monsivais et al showed that in the reproductive tract specific BMP7 cKO mice implantation of blastocysts is delayed and this defective implantation leads to resorption of embryo at a later stage of pregnancy. In these BMP7 cKO mice showed decidualization defects with decreased expression of decidual cell markers such as Wnt4 Cox2, Ereg, and Bmp2 [264]. BMPs are multifunctional proteins that regulate cell proliferation, differentiation, and apoptosis. Nagashima et al. found that uterine BMPR2-mediated signaling controls the formation and function of uterine decidua [265]. Clementi et al. discovered that the BMP type 1 receptor ALK2 is required in the uterus for normal implantation and subsequent decidualization of stromal cells [266]. Progesterone acts through its receptor (PGR), and this receptor is a critical regulator of decidualization. These researchers identified progesterone interacts with FOSL2 and JUN, which are members of the AP-1 family in the regulation of transcription, and they identified FOSL2 as a potentially important transcriptional coregulator of PGR for decidualization. High mobility group nucleosomal binding domain 2 (Hmgn2), which is also referred to as high mobility group protein 17 (Hmg17), is an abundant member of the non-histone Hmgn family that can specifically bind to nucleosome core particle, alter chromatin structure, and affect DNA-dependent activities such as transcription, replication, and repair. This molecule is observed in the luminal epithelium during receptivity, trophoblast apposition, attachment, and invasion as well as decidualization of the stroma [267]. The dynamic changes that occur in the endometrium during the peri-implantation period of early pregnancy are metabolically demanding, and glucose metabolism has been proven to be important for the preparation of the endometrium for embryo implantation. Specifically, it has been shown that endometrial stromal cell decidualization is dependent on increasing expression of glucose and facilitative glucose transporterGULT1 [268]. Kelle Moley's group has established that decidualized cells do not rely on glycolysis and instead use glucose in the pentose phosphate pathway. They showed that decidualization requires activity of the fatty acid β-oxidation pathway, and fatty acids are an important energy source in the decidualizing endometrium [269].
Before closing this history we need to pay attention to the role of immune cells in implantation and decidualization. There is almost always we need progesterone in maintaining pregnancy. Progesterone has a very strong controlling activity on the maternal immune cells. Progesterone changes the characters of immune cells during pregnancy so that they support growth and development of (semi-) allogeneic baby. Clarification of the cellular and molecular aspects of this immunological alteration during early pregnancy is awaiting. The day when we understand this immunological enigma will also allow us to understand how these molecules work in the body to allow the implantation of the embryo and bring up a healthy baby. There will be more molecules that will be found for implantation receptivity and decidualization. It would be nicer if the invesigators pay more attention to where in the uterus and when and how the discovered molecule works in the process of implantation and decidualization.
Acknowledgment
The author expresses deep appreciation to Dr Louis DePaolo, Chief, Fertility and Infertility Branch, NICHD, NIH for his leadership, and to the members of the Interdisciplinary Collaborative Team on Blastocyst Implantation Research for updating research information. The author is grateful for the staff of NIH Library for finding many old literatures. Special thanks are due to Mr Douglas Joubert for his editorial assistance. The author is much appreciative of Dr Francesco DeMayo for his kind reading and criticizing the manuscript.
References
- 1. Hertig AT, Rock J, Adams FC. A description of 34 human ova within the first 17 days of development. Am J Anat 1956; 98(3):435–493. [DOI] [PubMed] [Google Scholar]
- 2. Duval M. A Course of Lectures on Physiology 1875 translated by Robert Amory James Campbell, Boston -available buy Google Books https://books.google.com/books?id=ToRXCADSbMsC>. [Google Scholar]
- 3. Keibel F, Mall FP. Book- Manual of Human Embryology. Philadelphia: J.B. Lippincott Company; 1910–1912. [Google Scholar]
- 4. Peters H. Über die Einettung des mencshlinchen Eies. Deutike, Leipzig und Wien 1899. [Google Scholar]
- 5. Miller JW. Corpus luteum und Schwangerschaft. Das jüngste operativ erhaltene menschliche Ei 1913. Berlin. klin. Wochenschr. [Google Scholar]
- 6. Bryce TH. Observation on the early development of the human embryo. Trans Roy Soc Edin 1924; 53:533–567. [Google Scholar]
- 7. Teacher JH. On the implantation of the human ovum and the early development of the trophoblast. J Obstet Gynaec 1924; 31:360–385. [Google Scholar]
- 8. Stieve H. Ein ganz yunges, in der Gebärmutter erheltenes menschliches Ei. (Keimling Werner). Zeitschr. f. mikros. Anat. Forsch. 1936; 40:281–322. [Google Scholar]
- 9. Brewer JI. A normal human ovum in a stage preceding the primitive streak. The Edwards-Jones-Brewer ovum 1937; 61(3):429–481. [Google Scholar]
- 10. Dible JH, West CM. A human ovum at the previllous stage. J Anat 1941; 75:269–281. [PMC free article] [PubMed] [Google Scholar]
- 11. Hamilton WJ. Early stages of human development. Ann Roy Coll Surg Eng 1949; 4:281–294. [PMC free article] [PubMed] [Google Scholar]
- 12. Streeter GL. The “Miller” ovum -the youngest normal human embryo thus far known. Contr Embryol Carneg Instn, No 92. 1926; 18:31–48. [Google Scholar]
- 13. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril 1950; 1(1):3–25. [DOI] [PubMed] [Google Scholar]
- 14. Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest 1992; 90(1):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aplin JD. Expression of integrin α6β4 in human trophoblast and its loss from extravillous cells. Placenta 1993; 14(2):203–215. [DOI] [PubMed] [Google Scholar]
- 16. Aplin JD, Spanswick C, Bezad F, Kimber SJ, Vicovac L. Molecular aspects of implantation. Mol Hum Reprod 1996; 2(7):527–534. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Mayernick L, Armant DR. Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: Intracellular calcium transients and vesicle trafficking in primary trophoblast cells. Dev Biol 2002; 245(2):270–279. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Mayernik L, Armant DR. Trophoblast adhesion of the peri-implantation mouse blastocyst is regulated by integrin signaling that targets phospholipase C. Dev Biol 2007; 302(1):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards RG. Human uterine endocrinology and the implantation window. Ann NY Acad Sci. 1988; 541:445–454. [DOI] [PubMed] [Google Scholar]
- 20. Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang Z-Q, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003; 299(5605):405–408. [DOI] [PubMed] [Google Scholar]
- 21. Jeschke U, Hutter S, Heublein S, Vrekoussis T, Andergassen U, Unverdorben L, Papadakis G, Makrigiannakis A. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta 2013; 34(10):863–872. [DOI] [PubMed] [Google Scholar]
- 22. Meseguer M, Aplin JD, Caballero-Campo P, O’Connor JE, Martin JC, Remohi J, Pellicer A, Simon C. Human endometrial mucin MUC1 Is Up-Regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod 2001; 64(2):590–601. [DOI] [PubMed] [Google Scholar]
- 23. Psychoyos A. Précisions sur l’état de non-réceptivité de l’utérus. Comptes Rendus Soc Biol 1963; 257:1153–1156. [PubMed] [Google Scholar]
- 24. Yoshinaga K, Hawkins RA, Stocker JF. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology 1969; 85(1):103–112. [DOI] [PubMed] [Google Scholar]
- 25. Noyes RW, Dickmann Z. Relationship of ovular age to endometrial development. Reproduction 1960; 1(2):186–196. [Google Scholar]
- 26. Dickmann Z, Noyes RW. The fate of ova transferred into the uterus of the rat. Reproduction 1960; 1(2):197–212. [Google Scholar]
- 27. Martel D, Monier MN, Roche D, Psychoyos A. Hormonal dependence of pinopode formation at the uterine luminal surface. Hum Reprod 1991; 6(4):597–603. [DOI] [PubMed] [Google Scholar]
- 28. Nikas G. Cell-surface morphological events relevant to human implantation. Hum Reprod 1999; 14(Suppl 2):37–44. [DOI] [PubMed] [Google Scholar]
- 29. Nilsson O. Ultrastructure of mouse uterine surface epithelium under different estrogenic influences. J Ultrastruct Res 1958; 2(1):73–95. [DOI] [PubMed] [Google Scholar]
- 30. Nilsson O. Structural differentiation of luminal membrane in rat uterus during normal and experimental implantations. Z Anat Entwickl Gesch 1966; 125(2):152–159. [DOI] [PubMed] [Google Scholar]
- 31. Clyman MJ. A new structure observed in the nucleolus of the human endometrial epithelial cell. Am J Obstet Gynecol 1963; 56(4):430–432. [DOI] [PubMed] [Google Scholar]
- 32. Terzakis JA. The nucleolar channel system of human endometrium. J Cell Biol 1965; 27(2):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guffanti E, Kittur N, Brodt ZN, Polotsky AJ, Kuckkanen SM, Heller DS, Young SL, Santoro N, Meier UT. Nuclear pore complex proteins mark the implantation window in human endometrium. J Cell Sci 2008; 121(12):2037–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nejat EJ, Szmyga MJ, Zapantis G, Meier UT. Progesterone threshold determines nucleolar channel system formation in human endometrium. Reprod Sci 2014; 21(7):915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ehrmann RL, McKelvey HA, Hertig AT. Secretory behavior of endometrium in tissue culture. Obstet Gyn 1961; 17:416–433. [PubMed] [Google Scholar]
- 36. Lindenberg S, Nielsen MH, Lenz S. In vitro studies of human blastocyst implantation. Ann NY Acad Sci 1985; 442:368–374. [DOI] [PubMed] [Google Scholar]
- 37. Kliman HJ, Feinberg RF. Human trophoblast-extracellular matrix (ECM) interactions in vitro: ECM thickness modulates morphology and proteolytic activity. Proc Natl Acad Sci USA 1990; 87(8):3057–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kliman HJ, Feinberg RF, Haimowitz JE. Human trophoblast-endometrial interactions in an in vitro suspension culture system. Placenta 1990; 11(4):349–367. [DOI] [PubMed] [Google Scholar]
- 39. Fukuda MN, Sato T, Nakayama J, Klier G, Mikami M, Aoki D, Nozawa S. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev 1995; 9(10):1199–1210. [DOI] [PubMed] [Google Scholar]
- 40. Fukuda MN, Nozawa S. Trophinin, tastin, and bystin: A complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Med 1999; 17(03):229–234. [DOI] [PubMed] [Google Scholar]
- 41. Fukuda MN, Sugihara K. Trophinin in cell adhesion and signal transduction. Front Biosci 2012; E4(1):342–350. [DOI] [PubMed] [Google Scholar]
- 42. Sugihara K, Sugiyama D, Bvrne J, Wolf DP, Lowitz KP, Kobayashi Y, Kabir-Salmani M, Nadano D, Aoki D, Nozawa S, Nakayama J, Mustelin T et al. . Trophoblast cell activation by trophinin ligation is implicated in human embryo implantation. Proc Natl Acad Sci USA 2007; 104(10):3799–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fukuda MN, Sugihara K. Signal transduction in human embryo implantation. Cell Cycle 2007; 6(10):1153–1156. [DOI] [PubMed] [Google Scholar]
- 44. Tamura N, Sugihara K, Akama TO, Fukuda MN. Trophinin-mediated cell adhesion induces apoptosis of human endometrial epithelial cells through PKC-δ. Cell Cycle 2011; 10(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Godbole G, Modi D. Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J Reprod Immunol 2010; 85(2):130–139. [DOI] [PubMed] [Google Scholar]
- 46. Godbole G, Suman P, Malik A, Galvankar M, Joshi N, Fazleabas A, Gupta SK, Modi D. Decrease in expression of HOXA10 in the decidua after embryo implantation promotes trophoblast invasion. Endocrinology 2017; 158(8):2618–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Holligshead M, Marsh SGE, Brosens JJ, Critchley HO, Simons BD, Hemberger M et al. . Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017; 19(5):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002; 143(6):2119–2138 [DOI] [PubMed] [Google Scholar]
- 49. Horcajadas JA, Pellicer A, Simón C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update 2007; 13(1):77–86. [DOI] [PubMed] [Google Scholar]
- 50. Lessey BA. Assessment of endometrial receptivity. Fertil Steril 2011; 96(3):522–529. [DOI] [PubMed] [Google Scholar]
- 51. Diaz-Gimeno P, Horcajadas JA, Marinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, Simon C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 2011; 95(1):50–60.e15. [DOI] [PubMed] [Google Scholar]
- 52. Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernendez-Sanchez M, Carranza F, Carrera J, Vilella F, Pellicier A, Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013; 100(3):818–824. [DOI] [PubMed] [Google Scholar]
- 53. Mahajan N. Endometrial receptivity array: clinical application. J Hum Reprod Sci 2015; 8(3):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berger C, Boggavarapu NR, Menezes J, Lalitkumar PGL, Gemzell-Danielsson K. Effects of ulipristal acetate on human embryo attachment and endometrial cell gene expression in an in vitro co-culture system. Hum Reprod 2015; 30(4):800–811. [DOI] [PubMed] [Google Scholar]
- 55. Boggavarapu NR, Berger C, von Grothusen C, Menezes J, Gemzell-Danielsson K, Latitkumar PGL. Effects of low doses of mifepristone on human embryo implantation process in a three-dimensional human endometrial in vitro co-culture system. Contraception 2016; 94(2):143–151. [DOI] [PubMed] [Google Scholar]
- 56. Tabanelli S, Tang B, Gurpide E. In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol 1992; 42(3-4):337–344. [DOI] [PubMed] [Google Scholar]
- 57. De Feo VJ. Decidualization. In: Wynn RM. (ed.), Cell Biology of the Uterus. Appleton-Century-Crofts, New York, NY: Meredith Publishing Co. 6126-1; 1967: 191–290. [Google Scholar]
- 58. Shelesnyak MC. Decidualization: The decidua and the deciduoma. Perspect Biol Med 1962; 5(4):503–518. [DOI] [PubMed] [Google Scholar]
- 59. Nancy P, Siewiera J, Rizzuto G, Tagliani E, Osokine I, Manandhar P, Dolgalev I, Clementi C, Tsirigos A, Erlebacher A. H3K27me3 dynamics dictate evolving uterine states in pregnancy and parturition. J Clin Invest 2017(1). doi:10.1172/JCI95937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bockman DE, De Feo VJ. Preparation of antibody to artificially induced rat deciduomata. Anat Rec 1964; 148:233–247. [Google Scholar]
- 61. Yoshinaga K. Rabbit antiserum to rat deciduoma. Biol Reprod 1972; 6(1):51–57. [DOI] [PubMed] [Google Scholar]
- 62. Joshi SG, Ebert KM, Swarts DP. Detection and synthesis of a progestagen-dependent protein in human endometrium. Reproduction 1980; 59(2):273–285. [DOI] [PubMed] [Google Scholar]
- 63. Rutanen E-M, Menabawey M, Isaka K, Bohn H, Chard T, Grudzinska JG. Synthesis of placental protein 12 by decidua from early pregnancy. J Clin Endocrinol Metab 1986; 63(3):675–679. [DOI] [PubMed] [Google Scholar]
- 64. Bell SC. Secretory endometrial and decidual proteins: studies and clinical significance of a maternally derived group of pregnancy-associated serum proteins. Human Reprod 1986; 1(3):129–143. [DOI] [PubMed] [Google Scholar]
- 65. MacLaughlin DT, Santoro NF, Bauer HH, Lawrence D, Richardson GS. Two-dimensional gel electrophoresis of endometrial protein in human uterine fluids: qualitative and quantitative analysis. Biol Reprod 1986; 34:579–585. [DOI] [PubMed] [Google Scholar]
- 66. Seppala M, Taylor BN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev 2002; 23(4):401–430. [DOI] [PubMed] [Google Scholar]
- 67. Mazur EC, Vasquez YM, Li Q, Kommagani R, Jiang L, Chen R, Lanz RB, Kovanci E, Gibbons WE, DeMayo FJ. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology 2015; 156(6):2239–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, DeMayo FJ, Lydon JP, O’Malley BW. Acceleration of the glycolytic flux by steroid receptor Coactivator-2 is essential for endometrial decidualization. PLoS Genet 2013; 9(10):e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kommagani R, Szwarc MM, Kovanci E, Creighton CJ, O’Malley BW, DeMay FJ, Lydon JP. A murine uterine transcriptome, responsive to steroid receptor Coactivator-2, reveals transcription factor 23 as essential for decidualization of human endometrial stromal cells. Biol Reprod 2014; 90(4):75, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Camden AJ, SZwarc MM, Chadchan SB, DeMayo FJ, O’Malley BW, Lydon JP, Kommagani R. Growth regulation by estrogen in breast cancer 1 (GREB1) is a novel progesterone-responsive gene required for human endometrial stromal decidualization. Mol Human Reprod 2017; 23(9):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol 1953; 7:320–338. [Google Scholar]
- 72. Hamperl H, Hellweg G. Granular endometrial stroma cells. Obstet Gynecol 1958; 2:379–387. [PubMed] [Google Scholar]
- 73. Stites DP, Pavia CS, Clemens LE, Kuhn RW, Siiteri PK. Immunologic regulation in pregnancy. Arthritis Rheum 1979; 22(11):1300–1307. [DOI] [PubMed] [Google Scholar]
- 74. Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies. Am J Obstet Gynecol 2002; 187(5):1416–1423. [DOI] [PubMed] [Google Scholar]
- 75. Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol 2015; 213(4):S115–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. King A, Loke YW. Uterine large granular lymphocytes: a possible role in embryonic implantation? Am J Obstet Gynecol 1990; 162(2):308–310. [DOI] [PubMed] [Google Scholar]
- 77. King A. Uterine leukocytes and decidualization. Hum Reprod Update 2000; 6(1):28–36. [DOI] [PubMed] [Google Scholar]
- 78. Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol 2010; 63(6):587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nancy P, Tagliani E, Tay C-S, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012; 336(6086):1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jiang TT, Chaturvedi V, Ertelt JM, Kinder JM, Clark DR, Valent AM, Xin L, Way SS. Regulatory T cells: New keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol 2014; 192(11):4949–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yoshinaga K. Progesterone and its downstream molecules as blastocyst implantation essential factors. Am J Reprod Immunol 2014; 72(2):117–128. [DOI] [PubMed] [Google Scholar]
- 82. PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L et al. . Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 2015; 16(4):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wislocki GB, Streeter GL. On the placentation of the macaque (macaca mulatta), from the time of implantation until the formation of the definitive placenta. Contr Embryol Carn Instn 1938; 27:1–66. [Google Scholar]
- 84. Reinius S, Fritz GR, Knobil E. Ultrastructure and endocrinological correlates of an early implantation site in the rhesus monkey. Reproduction 1973; 32(1):171–173. [DOI] [PubMed] [Google Scholar]
- 85. Enders AC, Schlafke S. Differentiation of the blastocyst of the rhesus monkey. Am J Anat 1981; 162(1):1–21. [DOI] [PubMed] [Google Scholar]
- 86. Enders AC, Henrickx AG, Schlafke S. Implantation in the rhesus monkey: initial penetration of endometrium. Am J Anat 1983; 167(3):275–298. [DOI] [PubMed] [Google Scholar]
- 87. Enders AC. Implantation in the macaque: Expansion of the implantation site during the first week of implantation. Placenta 2007; 28(8-9):794–802. [DOI] [PubMed] [Google Scholar]
- 88. Sengupta J, Talwar D, Paria BC, Ghosh D. Endometrial phosphatases, β-glucuronidase and cathepsin D during menstrual cycle and pre-implantation stages of gestation in the rhesus monkey (Macaca mulatta). Acta Endocrinol 1988; 118:142–146. [DOI] [PubMed] [Google Scholar]
- 89. Ghosh D, Sengupta J. Patterns of estrogen and progesterone receptors in rhesus monkey endometrium during secretory phase of normal menstrual cycle and preimplantation stages of gestation. J Steroid Biochem 1988; 31(2):223–229. [DOI] [PubMed] [Google Scholar]
- 90. Ghosh D, Sengupta J. Endometrial responses to a deciduogenic stimulus in ovariectomized rhesus monkeys treated with oestrogen and progesterone. J Endocrinol 1989; 120(1), 51–NP. [DOI] [PubMed] [Google Scholar]
- 91. Ghosh D, De P, Sengupta J. Luteal phase ovarian oestrogen is not essential for implantation and maintenance of pregnancy from surrogate embryo transfer in the rhesus monkey. Human Reprod 1994; 9(4):629–637. [DOI] [PubMed] [Google Scholar]
- 92. Ghosh D, Nayak NR, Sengupta J. Effect of follicular phase administration of Mifepristone (RU486) on blastocyst implantation in the rhesus monkey. Contraception 1997; 56(2):117–122. [DOI] [PubMed] [Google Scholar]
- 93. Ghosh D, Dhara S, Kumar A, Sengupta J. Immunohistochemical localization of receptors for progesterone and oestradiol-17 in the implantation site of the rhesus monkey. Hum Reprod 1999; 14(2):505–514. [DOI] [PubMed] [Google Scholar]
- 94. Dhara S, Latikumar PGL, Sengupta J, Ghosh D. Immunohistochemical localization of insulin-like growth factors I and II at the primary implantation site in the rhesus monkey. Mol Human Reprod 2001; 7(4):365–371. [DOI] [PubMed] [Google Scholar]
- 95. Sengupta J, Dhawan L, Ghosh D. Immunohistochemical localization of leukemia inhibitory factor, interleukins 1 and 6 at the primary implantation site in the rhesus monkey. Cytokine 2003; 24(6):277–285. [DOI] [PubMed] [Google Scholar]
- 96. Lalitkumar PGL, Sengupta J, Ghosh D. Endometrial tumor necrosis factor α(TNFα) is a likely mediator of early luteal phase mifepristone-mediated negative effector action on the preimplantation embryo. Reproduction 2005; 129(3):323–335. [DOI] [PubMed] [Google Scholar]
- 97. Ghosh D, Najwa AR, Khan MA, Sengupta J. IGF2, IGF binding protein 1, and matrix metalloproteinases 2 and 9 in implantation-stage endometrium following immunoneutralization of vascular endothelial growth factor in the rhesus monkey. Reproduction 2011; 141(4):501–509. [DOI] [PubMed] [Google Scholar]
- 98. Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG. Modulation of cytokine and chemokine secretions in rhesus monkey trophoblast Co-Culture with decidual but not peripheral blood monocyte-derived macrophages. Am J Reprod Immunol 2011; 66(2):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Enders AC, Lantz KC, Peterson PE, Hendrickx A. Symposium: reproduction in baboons. From blastocyst to placenta: the morphology of implantation in the baboon. Hum Reprod Update 1997; 3(6):561–573. [DOI] [PubMed] [Google Scholar]
- 100. Fazleabas AT, Donnelly KM, Mavrogianis PA, Verhage HG. Secretory and morphological changes in the baboon (Papio Anubis) uterus and placenta during early pregnancy. Biol Reprod 1993; 49(4):695–704. [DOI] [PubMed] [Google Scholar]
- 101. Fazleabas AT, Kim J-YJ, Donnelly KM, Verhage HG. Embryo-maternal dialogue in the baboon (Papio anabis). In: Carson DD. (ed.), Embryo Implantation. Proceedings in the Serono Symposia USA Series. New York, NY: Springer; 1999: 202–209. [Google Scholar]
- 102. Kumar S, Brudney A, Cheon YP, Fazleabas AT, Bagchi IC. Progesterone induces calcitonin expression in the baboon endometrium within the window of uterine receptivity. Biol Reprod 2003; 68(4):1318–1323. [DOI] [PubMed] [Google Scholar]
- 103. Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA 1999; 96(5):2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sherwin JRA, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 2007; 148(2):618–626. [DOI] [PubMed] [Google Scholar]
- 105. Kannan A, Fazleabas AT, Bagchi IC, Bagchi MK. The transcription factor C/EBPβ is a marker of uterine receptivity and expressed at the implantation site in the primate. Reprod Sci 2010; 17(5):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Smith CA, Moore HDM, Hearn JP. The ultrastructure of early implantation in the marmoset monkey (Callithrix jacchus). Anat Embryol 1987; 175(3):399–410. [DOI] [PubMed] [Google Scholar]
- 107. Rao AJ, Ramesh V, Ramachandra SG, Krishnamurthy HN, Ravindranath N, Moudgal NR. Growth and reproductive parameters of bonnet monkey (Macaca radiata). Primates 1998; 39(1), 97–107. [Google Scholar]
- 108. Rao AJ, Kotagi SG, Moudgal NR. Serum concentrations of chorionic gonadotrophin, oestradiol-17β and progesterone during early pregnancy in the south Indian bonnet monkey (Macaca radiata). Reproduction 1984; 70(2):449–455. [DOI] [PubMed] [Google Scholar]
- 109. Ravindranath N, Moudgal RN. Effect of a specific estrogen antibody on pregnancy establishment in the bonnet monkey (Macaca radiata). Fertil Steril 1990; 54:1162–1167. [DOI] [PubMed] [Google Scholar]
- 110. Pope CE, Pope VZ, Beck LR. Development of baboon preimplantation embryos to Post-Implantation stages in vitro. Biol Reprod 1982; 27(4):915–923. [DOI] [PubMed] [Google Scholar]
- 111. Seshagiri PB, Hearn JP. In-vitro development of in-vivo produced rhesus monkey morulae and blastocysts to hatched, attached, and post-attached blastocyst stages: morphology and early secretion of chorionic gonadotrophin. Hum Reprod 1993; 8(2), 279–287. [DOI] [PubMed] [Google Scholar]
- 112. Rowson LEA, Moor RM. Development of the sheep conceptus during the first fourteen days. J Anat 1966; 100:777–785. [PMC free article] [PubMed] [Google Scholar]
- 113. Lesbre FX. Sur les annexes foetales du porc. J Méd Vét Zootech 1910; 61:196–207. [Google Scholar]
- 114. Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Implantation mechanisms: insights from the sheep. Reproduction 2004; 128 (6), 657–668. [DOI] [PubMed] [Google Scholar]
- 115. Assheton R. The morphology of the ungulate placenta, particularly the development of that organ in the sheep, and notes upon the placenta of the elephant and hyrax. Philos Trans R Soc London B 1906; 198(239-250):143–220. [Google Scholar]
- 116. Flood PF. Endometrial differentiation in the pregnant sow and the necrotic tips of the allantochorion. Reproduction 1973; 32(3):539–543. [DOI] [PubMed] [Google Scholar]
- 117. Hughes W. Sex-intergrades in fœtal pigs. Biol Bull 1927; 7(2):121–136. [Google Scholar]
- 118. O’Gara BW. Unique aspects of reproduction in the female pronghorn (Antilocapra Americana Ord). Am J Anat 1969; 125(2):217–231. [DOI] [PubMed] [Google Scholar]
- 119. Wright EC, Miles JR, Lents CA, Rempel LL. Uterine and placenta characteristics during early vascular development in the pig from day 22 to 42 of gestation. Anim Reprod Sci 2016; 164:14–22. [DOI] [PubMed] [Google Scholar]
- 120. Guillomot M, Flechon J-E, Winterberger-Torres S. Conceptus attachment in the Ewe: an ultrastructural study. Placenta 1981; 2(2):169–181. [DOI] [PubMed] [Google Scholar]
- 121. Wooding FBP, Staples LD, Peacock MA. Structure of trophoblast papillae on the sheep conceptus at implantation. J Anat 1982; 134:507–516. [PMC free article] [PubMed] [Google Scholar]
- 122. Ludwig KS. Zur Feinstruktur der Materno-Fetalen verbindung im placentom des schafes (Ovis aries L.). Experientia 1962; XVIII/5(5):212–213. [Google Scholar]
- 123. Moor R, Rowson LEA. Influence of the embryo and uterus on luteal function in the sheep. Nature 1964; 201(4918):522–523. [DOI] [PubMed] [Google Scholar]
- 124. Moor RM, Rowson LEA. The corpus luteum of the sheep: functional relationship between the embryo and the corpus luteum. J Endocrinol 1966; 34(2):233–239. [DOI] [PubMed] [Google Scholar]
- 125. Moor RM. Effect of embryo on corpus luteum function. J Anim Sci 1968; 27(Suppl 1):97–118. [PubMed] [Google Scholar]
- 126. McCracken JA, Carlson JC, Glew ME, Goding JR, Baird DT, Green K, Samuelsson B. Prostaglandin F3α identified as a luteolytic hormone in sheep. Nature 1972; 238:129–134. [DOI] [PubMed] [Google Scholar]
- 127. Roberts JS, McCracken JA, Gavagan JE, Soloff MS. Oxytocin-stimulated release of prostaglandin F2α from ovine endometrium in vitro: correlation with estrous cycle and oxytocin-receptor binding. Endocrinology 1976; 99(4):1107–1114. [DOI] [PubMed] [Google Scholar]
- 128. Sheldrick EL, Flint APF. Luteal concentrations of oxytocin decline during early pregnancy in the ewe. Reproduction 1983; 68(2):477–480. [DOI] [PubMed] [Google Scholar]
- 129. Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at Day 13-21. Reproduction 1982; 65(1):141–150. [DOI] [PubMed] [Google Scholar]
- 130. Godkin JD, Bazer FW, Roberts RM. Ovine trophoblast protein 1, an early secreted blastocyst protein, binds specifically to uterine endometrium and affects protein synthesis. Endocrinology 1984; 114(1):120–130. [DOI] [PubMed] [Google Scholar]
- 131. Ott TL, Mirando MA, Davis MA, Bazer FW. Effects of ovine conceptus secretory proteins and progesterone on oxytocin-stimulated endometrial production of prostaglandin and turnover of inositol phosphate in ovariectomized ewes. Reproduction 1992; 95(1):19–29. [DOI] [PubMed] [Google Scholar]
- 132. Imakawa K, Anthony RV, Kazemi M, Marotti KR, Polites HG, Roberts RM. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature 1987; 330(6146):377–379. [DOI] [PubMed] [Google Scholar]
- 133. Roberts RM. Interferon-τ and pregnancy. J Interferon Cytokine Res 1996; 16(4):271–273. [DOI] [PubMed] [Google Scholar]
- 134. Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med 2014; 32(05):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hill JR, Schlafer DH, Fisher PJ, Davies CJ. Abnormal expression of trophoblast major histocompatibility complex Class I antigens in cloned bovine pregnancies is associated with a pronounced endometrial lymphocytic response. Biol Reprod 2002; 67(1):55–63. [DOI] [PubMed] [Google Scholar]
- 136. Moraes JGN, Behura SK, Geary TW, Hansen PJ, Neiberg HL, Spencer TE. Uterine influences on conceptus development in fertility-classified animals. Proc Natl Acad Sci USA 2018; 115(8):E1749–E1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fraenkel L. Die function des corpus luteum. Arch Gynak 1903; 68(2):438–545. [Google Scholar]
- 138. Bouin P, Ancel P. Sur les fonctions du corps jaune gestatif. J Physiol Pathol Gen 1910; 12:1–48. [Google Scholar]
- 139. Corner GW, Allen WM. Production of a special uterine reaction (progestational proliferation) by extracts of corpus luteum. Am J Physiol 1929; 88:326–339. [Google Scholar]
- 140. Butenandt A. Über “Progynon” ein krystallisiertes weibliches sexualhormon. Naturwissenschaften 1929; 45(45):879–879. [Google Scholar]
- 141. Corner GW. The early history of progesterone. Gynecol Obstet Invest 1974; 5(2):106–112. [DOI] [PubMed] [Google Scholar]
- 142. Asdell SA Historical introduction. In: Cole HH, Cupps PT (eds.), Reproduction in Domestic Animals 1969; New York and London: Academic Press, 1–14. [Google Scholar]
- 143. Psychoyos A. De Lataste à la fenêtre d’implantation: cent ans de découvertes fascinantes. Contracept Fertil Sex 1993; 21:333–338. [PubMed] [Google Scholar]
- 144. Mossman HW. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contributions to embryology, No. 158 Contr Embryol Carn Instn 1937; 26:129–250. [DOI] [PubMed] [Google Scholar]
- 145. McLaren A, Michie D. The spacing of implantations in the mouse uterus. In: Eckstein P. (ed.), Implantation of Ova. Memoirs of the Society for Endocrinology No.6. Cambridge University Press; 1959: 65–75. [Google Scholar]
- 146. Hashima H. Studies on the prenatal growth of the mouse with special reference to the site of implantation of the embryo. Tohoku J Agric Res 1956; 6(4):307–312. [Google Scholar]
- 147. Restall BJ, Bindon BM. The timing and variation of pre-implantation events in the mouse. Reproduction 1971; 24(3):423. [DOI] [PubMed] [Google Scholar]
- 148. Pusey J, Kelly WA, Bradshaw JMC, Porter DG. Myometrial activity and the distribution of blastocysts in the uterus of the rat: Interference by relaxin. Biol Reprod 1980; 23(2):394–397. [DOI] [PubMed] [Google Scholar]
- 149. Ruan YC, Zhou W, Chan HC. Regulation of smooth muscle contraction by the epithelium: Role of prostaglandins. Physiology 2011; 26(3):156–170. [DOI] [PubMed] [Google Scholar]
- 150. Burckhard G. Die Implantation des Eies der Maus in die Uterusschleimhaut und die Umbildung derselben zur Decidua. Archiv f Mikrosk Anat 1901; LVII(3):528–569. [Google Scholar]
- 151. Enders AC. The implantation chamber, blastocyst and blastocyst imprint of the rat: A scanning electron microscope study. Anat Rec 1975; 182(2):137–149. [DOI] [PubMed] [Google Scholar]
- 152. Alden RH. Implantation of the rat egg. I. Experimental alteration of uterine polarity. J Exp Zool 1945; 100(2):229–235. [DOI] [PubMed] [Google Scholar]
- 153. Enders AC, Schlafke S. A morphological analysis of the early implantation stages in the rat. Am J Anat 1967; 120(2):185–225. [Google Scholar]
- 154. Enders AC, Schlafke S. Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat 1969; 125(1):1–29. [DOI] [PubMed] [Google Scholar]
- 155. Tachi C, Tachi S, Lindner HR. Ultrastructural features of blastocyst attachment and trophoblastic invasion in the rat. Reproduction 1970; 21(1):37–56. [DOI] [PubMed] [Google Scholar]
- 156. Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126:1211–1223. [DOI] [PubMed] [Google Scholar]
- 157. Lloyd S, Fleming TP, Collins JE. Expression of Wnt genes during mouse preimplantation development. Gene Expr Patterns 2003; 3(3):309–312. [DOI] [PubMed] [Google Scholar]
- 158. Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of wnt genes in the mouse blastocyst identify a candidate pathway for Embryo-Maternal signaling at implantation. Biol Reprod 2004; 71(2):417–424. [DOI] [PubMed] [Google Scholar]
- 159. Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. From the cover: uterine Wnt/β-catenin signaling is required for implantation. Proc Natl Acad Sci USA 2005; 102(24):8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cha J, Bartos A, Park C, Sun X, Li Y, Cha S-W, Ajima R, Ho H-YH, Yamaguchi TP, Dey SK. Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep 2014; 8(2):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yuan J, Cha J, Deng W, Bartos A, Sun X, Ho H-YH, Borg J-P, Yamaguchi TP, Yang Y, Dey SK. Planar cell polarity signaling in the uterus directs appropriate positioning of the crypt for embryo implantation. Proc Natl Acad Sci USA 2016; 113(50):E8079–E8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhang S, Kong S, Wang B, Cheng X, Chen Y, Wu W, Wang Q, Shi J, Zhang Y, Wang S, Lu J, Lydon J et al. . Uterine Rbpj is required for embryonic-uterine orientation and decidual remodeling via Notch pathway-independent and -dependent mechanisms. Cell Res 2014; 24(8):925–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yuan J, Deng W, Cha J, Sun X, Borg J-P, Dey SK. Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat Commun 2018; (1) doi 10.1038/s41467-018-03092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Parr MB. Relationship of uterine closure to ovarian hormones and endocytosis in the rat. Reproduction 1983; 68(1):185–188. [DOI] [PubMed] [Google Scholar]
- 165. Mayer G, Nilsson O, Reinius S. Cell membrane changes of uterine epithelium and trophoblasts during blastocyst attachment in rat. Z Anat Entwickl Gesch 1967; 126(1):43–48. [DOI] [PubMed] [Google Scholar]
- 166. Finn CA. The endometrium. In: Finn CA, Porter DG (eds), The Uterus. London: ELEK Science; 1975: 17–104. [Google Scholar]
- 167. Salleh N, Baines DL, Naftalin RJ, Milligan SR. The hormonal control of uterine luminal fluid secretion and absorption. J Membr Biol 2005; 206(1):17–28. [DOI] [PubMed] [Google Scholar]
- 168. Bitton-Casimiri V, Brun J-L, Psychoyos A. Comportement in vitro des blastocystes du 5e jour de la gestation chez la ratte; étude micro-cinématographique. C R Acad Sci 1970; 270:2979–2982. [PubMed] [Google Scholar]
- 169. Orsini MW, McLaren A. Loss of the zona pellucida in mice, and the effect of tubal ligation and ovariectomy. Reproduction 1967; 13(3):485–499. [DOI] [PubMed] [Google Scholar]
- 170. Psychoyos A. La reaction déciduale est précédée de modifications précoces de la perméabilité capillaire de l’utérus. C R Soc Biol 1960; 154:1384–1387. [PubMed] [Google Scholar]
- 171. Psychoyos A. Perméabilité capillaire et décidualisation uterine. C R Acad Sci 1961; 252:1515–1517. [PubMed] [Google Scholar]
- 172. Orsini MW. Morphological evidence on the intrauterine career of the ovum. In: Enders AC. (ed.), Delayed Implantation. Chicago and London: The University of Chicago Press; 1963: 155–166. [Google Scholar]
- 173. Hewitt K, Beer AE, Grinnell F. Disappearance of anionic sites from the surface of the rat endometrial epithelium at the time of blastocyst implantation. Biol Reprod 1979; 21(3):691–707. [DOI] [PubMed] [Google Scholar]
- 174. Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 1995; 136(8):3639–3647. [DOI] [PubMed] [Google Scholar]
- 175. Aplin JD, Spanswick C, Behzad F, Kimber SJ, Vicovac L. Molecular aspects of implantation. Mol Hum Reprod 1996; 2(7):527–534. [DOI] [PubMed] [Google Scholar]
- 176. Ding Y-Q, Zhu L-J, Bagchi ML, Bagchi IC. Progesterone stimulates calcitonin gene expression in the uterus during implantation. Endocrinology 1994; 135(5):2265–2274. [DOI] [PubMed] [Google Scholar]
- 177. Xiong T, Zhao Y, Hu D, Meng J, Wang R, Yang X, Ai J, Qian K, Zhang H. Administration of calcitonin promotes blastocyst implantation in mice by up-regulating integrin β3 expression in endometrial epithelial cells. Hum Reprod 2012; 27(12):3540–3551. [DOI] [PubMed] [Google Scholar]
- 178. Hoffman LH, Winfrey VP, Blaeuer GL, Olson GE. A Haptoglobin-Like glycoprotein is produced by implantation-stage rabbit endometrium. Biol Reprod 1996; 55(1):176–184. [DOI] [PubMed] [Google Scholar]
- 179. Beier HM. Oviducal and uterine fluids. Verh dt. Zool. Ges. 1967; 31:139- from Oviductal and uterine fluid Reproduction 1974; 37(1):221–237. [DOI] [PubMed] [Google Scholar]
- 180. Krishnan RS, Daniel JC Jr. “Blastokinin:” inducer and regulator of blastocyst development in the rabbit uterus. Science 1967; 158(3800):490–492. [DOI] [PubMed] [Google Scholar]
- 181. Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev 2007; 28(7):707–725. [DOI] [PubMed] [Google Scholar]
- 182. Van Mourik MSM, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol 2009; 85(1):4–19. [DOI] [PubMed] [Google Scholar]
- 183. Sholl SA, Orsini MW, Hitchins DJ. Estrogen synthesis and metabolism in the hamster blastocyst, uterus and liver near the time of implantation. J Steroid Biochem 1983; 19(2):1153–1161. [DOI] [PubMed] [Google Scholar]
- 184. Strömstedt M, Keeney DS, Waterman MR, Paria BC, Conley AJ, Dey SK. Preimplantation mouse blastocysts fail to express CYP genes required for estrogen biosynthesis. Mol Reprod Dev 1996; 43(4):428–436 [DOI] [PubMed] [Google Scholar]
- 185. Lataste F. Des variations de durée de la gestation chez les mammifères et des circonstances qui déterminant ces variations. Théorie de la gestation retardée (1). Compt Rend Soc Biol 1891; 43:21–31. [Google Scholar]
- 186. Weichert CK. The experimental shortening of delayed pregnancy in the albino rat. Anat Rec 1940; 77(1):31–47. [Google Scholar]
- 187. Krehbiel RH. The effects of Theelin on delayed implantation in the pregnant lactating rat. Anat Rec 1941; 81(3):381–392. [Google Scholar]
- 188. Cochrane RL, Meyer RK. Delayed nidation in the rat induced by progesterone. Exp Biol Med 1957; 96(1):155–159. [DOI] [PubMed] [Google Scholar]
- 189. Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. Reproduction 1966; 12(3):593–595. [DOI] [PubMed] [Google Scholar]
- 190. Psychoyos A. The effects of reserpine and chlorpromazine on sexual function. J Reprod Fert 1968; (Suppl. 4):47–59. [Google Scholar]
- 191. Hou Q, Paria BC, Mui C, Dey SK, Gorski J. Immunolocalization of estrogen receptor protein in the mouse blastocyst during normal and delayed implantation. Proc Natl Acad Sci USA 1996; 93(6):2376–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Paria BC, Das SK, Andrews GK, Dey SK. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci USA 1993; 99(1):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Stewart CL, Casper P, Brunet LJ, Bhatt H, Köntgen F, Abbondanzo J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992; 359(6390):76–79. [DOI] [PubMed] [Google Scholar]
- 194. Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol 2005; 3(1):56, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18(12):1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, DeMayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA 2007; 14(46):18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhang Q, Paria BC. Importance of uterine cell death, renewal, and their hormonal regulation in hamsters that show progesterone-dependent implantation. Endocrinology 2006; 147(5):2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep 2015; 11(3):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Short RV, Yoshinaga K. Hormonal influences on tumour growth in the uterus of the rat. Reproduction 1967; 14(2):287–293. [DOI] [PubMed] [Google Scholar]
- 200. Schlafke S, Welsh AO, Enders AC. Penetration of the basal lamina of the uterine luminal epithelium during implantation in the rat. Anat Rec 1985; 212(1):47–56. [DOI] [PubMed] [Google Scholar]
- 201. Psychoyos A. La reaction déciduale est précédée de modifications précoces de la perméabilité capillaire de l’utérus. C R Soc Biol 1960; 154:1384–1387. [PubMed] [Google Scholar]
- 202. Psychoyos A. Perméabilité capillaire et décidualisation uterine. C R Acd Sci 1961; 252:1515–1517. [Google Scholar]
- 203. Loeb L. The production of deciduomata. JAMA 1908; 23(23):1897–1901. [Google Scholar]
- 204. Nichaman MZ. Inhibition of implantation by production of deciduomata in the rat. Am J Physiol 1956; 186:6–8. [DOI] [PubMed] [Google Scholar]
- 205. Lataste F. Transformationpériodique de l’épithélium du vagin des rongeurs (Rythme vaginal). Compt Rend Soc Biol 1892; 44:765–769. [Google Scholar]
- 206. Stockard CR, Papanicolaou GN. The existence of a typical oestrous cycle in the guinea-pig-with a study of its histological and physiological changes. Am J Anat 1917; 22(2):225–283. [Google Scholar]
- 207. Canivence R, Mayer G. Contribution à l’étude expérimentale de la superfétation chez la rate, recherches basées sur la nidation retardée. Ann Endocrinol 1955; 16:1–33. [PubMed] [Google Scholar]
- 208. Psychoyos A. Retard de nidation de l’oeuf chez la ratte à l’aide de la chlorpromazine; action de l’oestrogène. Compt Rend Acad Sci 1959; 248:3216–3218. [PubMed] [Google Scholar]
- 209. Yoshinaga K. Effect of local application of ovarian hormones on the delay in implantation in lactating rats. Reproduction 1961; 2(1):35–41. [DOI] [PubMed] [Google Scholar]
- 210. Psychoyos A. Nouvelles remarques sur le déterminisme de l’ovoimplantation. Compt Rend Acad Sci 1962; 254:4360–4362. [PubMed] [Google Scholar]
- 211. Fernando G, Nalbandov AV. Relative importance of histamine and estrogen on implantation in rats. Endocrinology 1968; 83(5):933–937. [DOI] [PubMed] [Google Scholar]
- 212. Diao H, Xiao S, Howerth EW, Zhao F, Li R, Ard MB, Ye X. Broad gap junction blocker carbenoxolone disrupts uterine preparation for embryo implantation in mice. Biol Reprod 2013; 89(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Emmens CW, Finn CA. Local and parenteral action of oestrogens and anti-oestrogens on early pregnancy in the rat and mouse. Reproduction 1962; 3(2):239–245. [DOI] [PubMed] [Google Scholar]
- 214. Yoshinaga K. Superimplantation rats as a tool for the study of onset of parturition. In: Bhatnagar AS. (ed.), The Anterior Pituitary Gland. New York: Raven Press; 1983: 367–375. [Google Scholar]
- 215. Do Y-S, Leavitt WW. Characterization of a specific progesterone receptor in decidualized hamster uterus. Endocrinology 1978; 102(2):443–451. [DOI] [PubMed] [Google Scholar]
- 216. Moore DD. A conversation with elwood jensen. Annu Rev Physiol 2012; 74(1):1–11. [DOI] [PubMed] [Google Scholar]
- 217. Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988; 240(4854):889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Bhatt H, Brunet L, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci USA 1991; 88(24):11408–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol 2004; 18(5):1238–1250. [DOI] [PubMed] [Google Scholar]
- 220. Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction 2006; 131(2):341–349. [DOI] [PubMed] [Google Scholar]
- 221. Cheng J-G, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci USA 2001; 98(15):8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci USA 2005; 102(24):8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Liang X-H, Deng W-B, Li M, Zhao Z-A, Wang T-S, Feng X-H, Cao Y-J, Duan E-K, Yang Z-M. Egr1 protein acts downstream of estrogen-leukemia inhibitory factor (LIF)-Stat3 pathway and plays a role during implantation through targeting Wnt4. J Biol Chem 2014; 289(34):23534–23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Pawar S, Laws MJ, Bagchi IC, Bagchi MK. Uterine epithelial estrogen receptor-α controls decidualization via a paracrine mechanism. Mol Endocrinol 2015; 29(9):1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ, Spencer TE. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci USA 2017; 114(6):E1018–E1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Paria BC, Huet-Hudson YM, Dey SK. Blastocyst's state of activity determines the "window" of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA 1993; 90(21):10159–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Klemke RL, Weitlauf HM. Comparison of the ontogeny of specific cell surface determinants on normal and delayed implanting mouse embryos. Reproduction 1993; 99(1):167–172. [DOI] [PubMed] [Google Scholar]
- 228. Phillips B, Knisley K, Weitlauf KD, Dorsett J, Lee V, Weitlauf H. Differential expression of two β-galactoside-binding lectins in the reproductive tracts of pregnant mice. Biol Reprod 1996; 55(3):548–558. [DOI] [PubMed] [Google Scholar]
- 229. Ding Y-Q, Zhu L-J, Bagchi MK, Bagchi IC. Progesterone stimulates calcitonin gene expression in the uterus during implantation. Endocrinology 1994; 135(5):2265–2274. [DOI] [PubMed] [Google Scholar]
- 230. Knott JG, Paul S. Transcriptional regulators of the trophoblast lineage in mammals with hemochorial placentation. Reproduction 2014; 148(6):R121–R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Renaud SJ, Chakraborty D, Mason CW, Rumi MAK, Vivian JL, Soares MJ. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc Natl Acad Sci USA 2015; 112(45):E6175–E6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in HoxalO-deficient mice. Nature 1995; 374(6521):460–463. [DOI] [PubMed] [Google Scholar]
- 233. Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996; 122:2687–2696. [DOI] [PubMed] [Google Scholar]
- 234. Das SK, Wang X-N, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 1994; 120:1071–1083. [DOI] [PubMed] [Google Scholar]
- 235. Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol 1996; 16(2):107–122. [DOI] [PubMed] [Google Scholar]
- 236. Lim H, Paria BC, Das SK, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997; 91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 237. Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction 2007; 134(5):635–643. [DOI] [PubMed] [Google Scholar]
- 238. Ye X, Hama K, Contos JJA, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005; 435:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest 2006; 116(10):2653–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Matson BC, Pierce SL, Espenschied ST, Holle E, Swratt IH, Davis ES, Tarran R, Young SL, Kohout TA, van Duin M, Caron KM. Adrenomedullin improves fertility and promotes pinopodes and cell junctions in the peri-implantation endometrium. Biol Reprod 2017; 97(3):466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A2α is crucial for ‘on-time’ embryo implantation that directs subsequent development. Development 2002; 129:2879–2889. [DOI] [PubMed] [Google Scholar]
- 242. Knott JG, Paul S. Transcriptional regulators of the trophoblast lineage in mammals with hemochorial placentation. Reproduction 2014; 148(6):R121–R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Renaud SJ, Chakraborty D, Mason CW, Rumi MA, Vivian JL, Soares MJ. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc Natl Acad Sci USA 2015; 112(45):E6175–E6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Renaud SJ, Scott RL, Chakraborty D, Rumi MAK, Soares MJ. Natural Killer-cell deficiency alters placental development in rats. Biol Reprod 2017; 96:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Hou Q, Gorski J. Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proc Natl Acad Sci USA 1993; 90(20):9460–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Nichols J, Davidson D, Taga T, Yoshida K, Chambers I, Smith A. Complementary tissue-specific expression of LIF and LIF-receptor mRNAs in early mouse embryogenesis. Mech Dev 1996; 57(2):123–131. [DOI] [PubMed] [Google Scholar]
- 247. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 248. Tibbetts TA, DeMayo F, Rich S, Conneely OM, O’Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci USA 1999; 96(21):12021–12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000; 289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 250. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 2003; 100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Paria BC, Ma W-G, Tan J, Raja S, Das SK, Dey SK, Hogan BLM. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA 2001; 98(3):1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Matsumoto H, Zhao X, Das SK, Hogan BLM, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 2002; 245(2):280–290. [DOI] [PubMed] [Google Scholar]
- 253. Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 2002; 16(10):2338–2348. [DOI] [PubMed] [Google Scholar]
- 254. Lee K, Jeong J-W, Kwak I, Yu C-T, Lanske B, Soegiarto DW, Toftgard R, Tsai M-J, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 2006; 38(10):1204–1209. [DOI] [PubMed] [Google Scholar]
- 255. Soyal SM, Mukherjee A, Lee KY-S, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41(2):58–66. [DOI] [PubMed] [Google Scholar]
- 256. Franco HL, Lee KY, Rubel CA, Creighton CJ, White LD, Broaddus RR, Lewis MT, Lydon JP, Jeong J-W, DeMayo FJ. Constitutive activation of smoothened leads to female infertility and altered uterine differentiation in the mouse. Biol Reprod 2010; 82(5):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Daikoku T, Ogawa Y, Terakawa J, Ogawa A, DeFalco T, Dey SK. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology 2014; 155(7):2718–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol 2012; 357(1-2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Lee K, Jeong J-W, Kwak I, Yu C-T, Lanke B, Soegiarto DW, Toftgard R, Tsai M-J, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 2006; 38(10):1204–1209. [DOI] [PubMed] [Google Scholar]
- 260. Jeong J-W, Lee KY, Han SJ, Aronow BJ, Lydon JP, O’Malley BW, DeMayo FJ. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-Independent and -dependent gene expression. Endocrinology 2007; 148(9):4238–4250. [DOI] [PubMed] [Google Scholar]
- 261. Lee D-K, Kurihara I, Jeong J-W, Lydon JP, DeMayo FJ, Tsai M-J, Tsai SY. Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol 2010; 24(5):930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kawagoe J, Li Q, Mussi P, Liao L, Lydon JP, DeMayo FJ, Xu J. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell 2012; 23(4):858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Monsivaias D, Clementi C, Peng J, Titus MM, Barrish JP, Creighton CJ, Lydon JP, DeMayo FJ, Matzuk MM. Uterine ALK3 is essential during the window of implantation. Proc Natl Acad Sci USA 2015; 112:E387–E395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Monsivais D, Clementi C, Peng J, Fullerton PT Jr, Prunskaite-Hyyryläinen R, Vainio SJ, Matzuk MM. BMP7 induces uterine receptivity and blastocyst attachment. Endocrinology 2017; 158(4):979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Nagashima T, Li Q, Clementi C, Lydon JP, DeMayo FJ, Matzuk MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest 2013; 123(6):2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA, DeMayo FJ, Matzuk MM. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet 2013; 9(11):e1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Li DD, Yue L, Yang ZQ, Zheng L-W, Guo B. Evidence for Hmgn2 involvement in mouse embryo implantation and decidualization. Cell Physiol Biochem 2017; 44:1681–1695. [DOI] [PubMed] [Google Scholar]
- 268. Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 2009; 150:1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Tsai J-H, Chi MM-Y, Schulte MB, Moley KH. The fatty acid beta-oxidation pathway is important for decidualization of endometrial stromal cells in both humans and mice. Biol Reprod 2014; 90:34, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]