Abstract
Background:
Studies of adiposity and brain pathology in African Americans (AA) are sparse despite higher rates of obesity, dementia, and dementia-associated brain pathology in AA. This study examined relations of adiposity to white matter hyperintensities (WMH) and total brain volume (TBV) in AA and non-Hispanic whites (NHW).
Methods:
Waist circumference (WC) and body mass index (BMI) were measured in the Genetic Epidemiology Network of Arteriopathy study at Visits 1 (mean age 57 [±11]) and 2 (mean age 61 [±10], mean 5.2 years later). Brain MRIs were obtained shortly after Visit 2 in 1,702 participants (64% women, 48% AA). Multilevel linear regression using generalized estimating equation estimated associations of adiposity (cross-sectional) or adiposity changes with WMH (accounting for intracranial size) or TBV adjusting for demographics, cardiovascular risk factors, and incorporating adiposity-by-race interactions. Adiposity-by-age interactions were examined.
Results:
Concurrent TBV was inversely associated with BMI (β = −2.76 [95% confidence interval (CI): −4.99, −0.53]) and WC (β = −2.19 [CI: −4.04, −0.34]). Concurrent WMH were negatively associated with BMI (β = −0.04 [CI: −0.06, −0.01]) and, among NHW, with WC (β = −0.04 [CI: −0.06, −0.02]) but not with changes in BMI or WC. BMI increases were associated with lower TBV (β = −16.20, [CI: −30.34, −2.06]) in AA but not in NHW (β = −2.76 [CI: −14.02, 8.51]), although race-by-adiposity interactions were not supported. WC increases were not associated with MRI outcomes.
Conclusion:
Greater measures of obesity and increases in measures of obesity, which are common in mid-life, could be detrimental to brain health, particularly in AA.
Keywords: Obesity, MRI, Race, Longitudinal
Brain structural changes such as atrophy, infarcts, and white matter hyperintensities (WMH) are associated with cognitive impairment and dementia (1–4) and are more prevalent (5) and severe (6) in African Americans (AA) compared to whites. Compared to whites, AA are also disproportionately affected by dementia (7,8). Contributors to structural changes in the aging brain remain poorly understood (9,10). Recently, an autopsy study reported higher prevalence of mixed pathology, especially vascular disease, in the brains of AA and other ethnic minorities with Alzheimer dementia compared to non-Hispanic whites (NHW) (11). Such findings highlight the importance of elucidating factors contributing to brain structural changes in minority populations.
Obesity is more common in AA (12) and is associated with structural changes in the brain (1,13–15) and with dementia, primarily in NHW populations (1,16–18). Despite a greater prevalence of brain pathology and obesity in AA, AA are not well-represented in studies of obesity and brain imaging markers (19–21). To date, the mechanisms explaining relations of obesity to cognitive decline or dementia are not known but could be mediated through obesity effects on brain structure. For example, obesity leads to diabetes and hypertension, which are associated with cerebral WMH and are more common in AA than whites (5). Separate cohorts have demonstrated stronger relations of diabetes to stroke risk in AA (22) and hypertension to WMH in AA than in whites (23). Furthermore, obesity has been characterized as an inflammatory state (24), and inflammation is associated with poorer cognition (25,26) and brain structural abnormalities (27). Any of these mechanisms could explain differential associations of obesity with brain structure in AA and whites.
The phenomenon of prodromal weight loss, which can precede a dementia diagnosis by several years (28), may obscure associations of obesity exposures with early biomarkers, including brain imaging abnormalities associated with dementia, and likely explains associations of dementia risk with both higher mid-life adiposity and lower late-life adiposity (16,17). Clarifying these relationships in AA could lead to targeted interventions that mitigate risk for developing pathological changes in the brain and for dementia. With over half of AA women and one-third of AA men obese in the United States (29), the impact of successful interventions could be substantial at the population level. The aim of this study was to examine relationships of central and overall adiposity and changes in adiposity with brain structural abnormalities among AA and NHW adults.
Methods
Population
The Genetic Network of Arteriopathy (GENOA) study, begun in 1995, has been described (30); briefly, GENOA includes a cohort of hypertensive AA and NHW adults and their siblings recruited from Jackson, Mississippi (AA only) and Rochester, Minnesota (NHW only) (N = 3,419; 63% female, 54% AA, aged 20–91 at baseline) (30). GENOA required at least two siblings have essential hypertension before age 60 with additional siblings recruited regardless of hypertension status. Of 3,419 participants attending Visit 1, 21 (<0.6%) participants were excluded due to outlying obesity measures (WC outside 1st or 99th percentiles) leaving 3,398. Of these, 2,700 (79%) returned for Visit 2 (mean 5.2 years), allowing analyses of changes in obesity measures. Reliable brain MRI measures were available in 1,702 as part of the Genetics of Microangiopathic Brain Injury study [GMBI] (2001–2006), which took place shortly after Visit 2 (mean 1.2 years), described as V2 hereafter (see study flow diagram, Supplementary Figure 1). The study complied with the ethical rules as stated in the Declaration of Helsinki, including approval of an institutional review, and participants provided informed consent.
Adiposity
Standard measures of obesity, waist circumference (WC), and body mass index (BMI) were used as adiposity measures. Participants wore lightweight clothes and were instructed to stand with weight equally distributed on both feet. A full-length mirror was used to verify tape measure placement. WC assessed central adiposity, measured to the nearest 0.1 cm at the umbilicus at the end of exhalation. Height was measured using a stadiometer and weight using an electronic balance. Overall adiposity was assessed using BMI (kg/m2).
Brain MRI
GENOA imaging and MRI measures have been previously described (31). Briefly, a fully automated algorithm segmented each slice of the multi-slice, edited, fluid-attenuated inversion recovery (FLAIR) sequence (based on image intensity) from 1.5T brain MRI (GE Medical Systems, Waukesha, WI) into voxels, which were then classified as brain, cerebrospinal fluid, or WMH (cm3) (32). Artifacts in WMH were manually edited by trained experts. WMH volume was determined from axial FLAIR images. Each set consisted of contiguous 3-mm interleaved slices with no inter-slice gap. The mean absolute error for determining WMH volume is 6.6%, and the mean test-retest coefficient of variation is 1.4%. Brain atrophy was estimated using the difference in intracranial volume and total brain volume (TBV). Because results for atrophy mirrored TBV results, these are presented in supplemental materials for brevity. All images were acquired on identical scanners and read at the GENOA reading center (Mayo Clinic) following standardized protocols.
Covariates
Age, sex, race, alcohol (ever/never), and smoking status (ever/never), defined as having ever smoked more than 100 cigarettes, were self-reported. Blood pressure was the average of the second and third measurements taken in a rested state. Hypertension was defined as blood pressure greater than 140/90, self-report of hypertension, or antihypertensive medication use in the previous 2 weeks. Diabetes mellitus was defined as fasting glucose of 126 mg/dL or greater, random glucose of 200 mg/dL or greater, self-report of diabetes mellitus, or hypoglycemic medication use.
Statistical Analysis
Means with standard deviations for continuous and counts with percentages for categorical variables were calculated. A logarithmic transformation was performed on WMH due to skewness. Two models were constructed to examine relationships of adiposity or change in adiposity with brain MRI outcomes (a) a cross-sectional model using V2 adiposity and V2 MRI outcomes, and (b) a model we term “change” using adiposity change as the predictor (V2 adiposity − V1 adiposity) and V2 MRI outcomes, adjusting for baseline BMI. Linear models fit with generalized estimating equations (GEE), to account for familial clustering, and Huber-White robust standard error estimates were used. To address the prodromal weight loss phenomenon related to developing dementia, we planned a priori to examine relationships for persons with weight loss separately. Diagnostic lowess smoothers revealed linear relationships for the cross-sectional model but displayed evidence for differing slopes for those with adiposity changes less than zero. We subsequently defined those with decreases in adiposity as anyone with V2 adiposity − V1 adiposity less than zero and all other as having increases in adiposity (including 27 participants with zero change). We constructed a linear spline model with one knot at zero change. Differences by age or race were examined using interaction terms between age or race and obesity measures and by stratifying results, while acknowledging that race and site are aliased by design. Results are stratified by AA/EA as shorthand for AA (MS)/EA (MN) or race (site) groups.
Comparisons between participants with and without brain MRI by visit and race are shown in Supplementary Table 1. Sensitivity analyses to missing data were conducted using inverse proportionally weighted GEE for those who attended V1 but did not return for V2 and those who attended V2 but did not undergo brain imaging. Models were adjusted for age, sex, diabetes, systolic and diastolic blood pressure, antihypertension medication use, smoking, high density lipoprotein cholesterol, and total intracranial volume. Models for WC also adjusted for height. We compared results to results from models that did not adjust for diabetes and blood pressure, which may mediate relations of adiposity with brain imaging outcomes. All analyses were performed using Stata 14.1 (College Station, TX).
Results
Characteristics are described for the overall cohort by visit and race (Table 1). By design, participants at V1 had high rates of hypertension but also diabetes and obesity (Table 1) and were, on average, middle-aged (56.9 [10.8] years). Compared to NHW participants, AA were older, were more likely to have diabetes and hypertension, and had slightly higher BMI and WC. WMH volume was higher and more variable in AA while atrophy and TBV were similar in AA and NHW (Table 1). Participants who did not have MRI, compared to those who did, were older, had larger WC (especially NHW), more likely to be smokers and diabetics, had lower education and, among NHW, were more likely to be male (Supplementary Table 1). Differences in V2 characteristics of AA and NHW who did not have MRI were similar to V1 characteristics except that black V2 participants without MRI were also more likely to be male and have hypertension while NHW participants without MRI had less hypertension (Supplementary Table 1).
Table 1.
Baseline and Visit 2 Participant Characteristics
| Baseline (Visit 1) | Visit 2 | |||
|---|---|---|---|---|
| White (N = 1,555) | Black (N = 1,843) | White (N = 1,224) | Black (N = 1,476) | |
| BMI (kg/m2) | 30.5 (6.2) | 31.0 (6.4) | 30.9 (6.2) | 31.6 (6.6) |
| WC (cm) | 100.3 (15.4) | 102.7 (16.0) | 101.0 (15.5) | 103.5 (14.2) |
| Women | 855 (55%) | 1,273 (69%) | 690 (56%) | 1,044 (71%) |
| Age (y) | 55.32 (10.8) | 58.22 (10.2) | 58.93 (10.2) | 63.04 (9.5) |
| Education | ||||
| <12 y | 120 (9%) | 681 (42%) | 67 (6%) | 513 (39%) |
| HS diploma | 654 (50%) | 502 (31%) | 532 (51%) | 412 (32%) |
| Some college | 253 (19%) | 41 (3%) | 202 (19%) | 37 (3%) |
| College + | 285 (22%) | 382 (24%) | 235 (23%) | 337 (26%) |
| Diabetic | 139 (9%) | 364 (20%) | 147 (12%) | 403 (27%) |
| Hypertension | 1,055 (68%) | 1,353 (73%) | 877 (72%) | 1,088 (74%) |
| Alcohol use | 1,125 (72%) | 641 (35%) | 911 (74%) | 510 (35%) |
| Smoker | 774 (50%) | 779 (42%) | 601 (49%) | 591 (40%) |
| WMH (cm3) | NA | 7.75 (6.4) | 10.6 (11.4) | |
| Atrophy (cm3) | 308.39 (73.9) | 309.37 (74.3) | ||
| TBV (cm3) | 1159.5 (123.3) | 1064.2 (114.3) | ||
Note: BMI = body mass index; WC = waist circumference; WMH = white matter hyperintensities; TBV = total brain volume. Cells contain N (%) for categorical and mean (SD) for continuous.
Cross-sectional Relationships
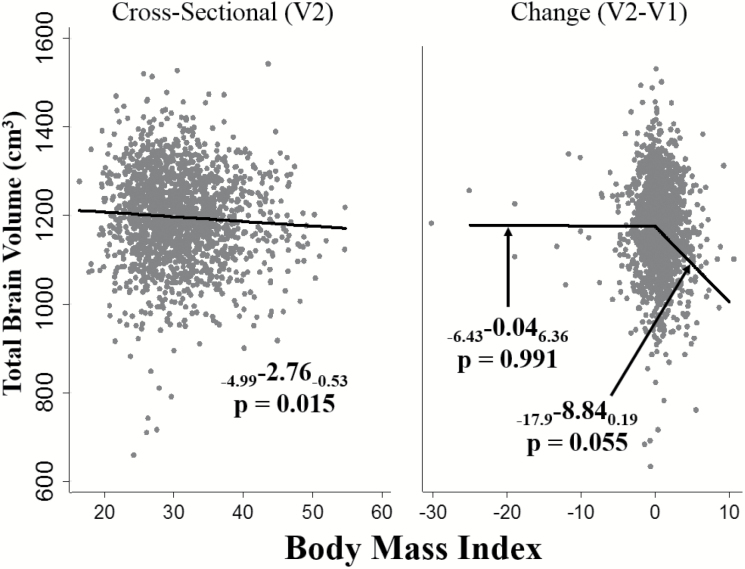
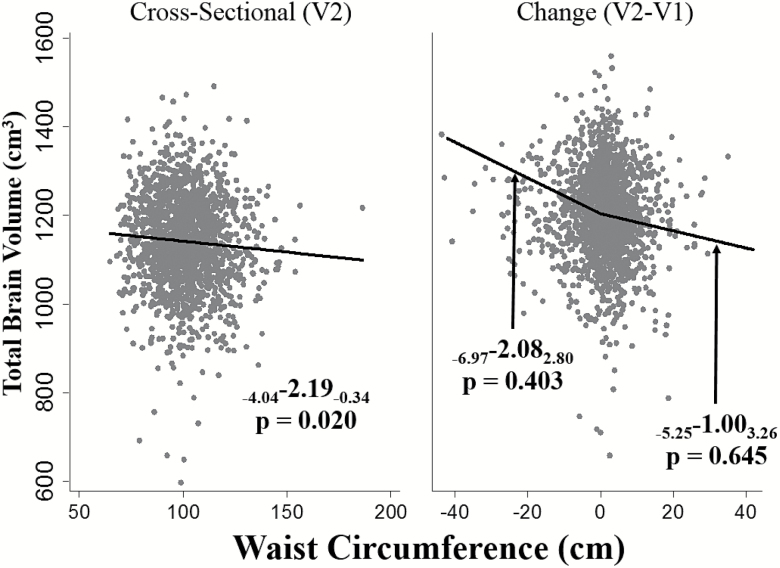
Higher BMI was associated with lower TBV (pooled β = −2.76 [confidence interval (CI): −4.99, −0.53]) (Table 2, Figure 1) as was higher WC (β = −2.19 [CI: −4.04, −0.34]; Figure 2). These relationships were similar in AA and NHW (p for race interaction ≥ 0.22 for all). Higher BMI was associated with lower WMH volumes (β = −0.04 [CI: −0.06, −0.01]) with similar estimates in NHW and AA (Table 2). The relationship of WC with WMH, however, differed by race (p for interaction = 0.003). Among NHW, higher WC was associated with lower WMH volumes (NHW β = −0.04 [CI: −0.06, −0.02]), while a positive, statistically nonsignificant relationship of WC with WMH was observed in AA. Cross-sectional results for BMI, WC, and WMH were similar or slightly stronger in models that did not adjust for diabetes and blood pressure (Supplementary Table 2).
Table 2.
Cross-sectional Relationships of Adiposity With Brain MRI Outcomes Overall and by Race
| Estimates (95% CI) p Value | |||||
|---|---|---|---|---|---|
| Outcome | Predictor | Pooled (n = 2,700) | White (n = 1,224) | Black (n = 1,476) | Interaction |
| Total brain volume, cm3 | BMI | −2.76, p = .015 | −2.28, p = .121 | −3.34, p = .046 | −1.06, p = .626 |
| (−4.99, −0.53) | (−5.17, 0.60) | (−6.62, −0.06) | (−5.30, 3.19) | ||
| WC | −2.19, p = .020 | −1.34, p = .255 | −3.45, p = .015 | −2.11, p = .230 | |
| (−4.04, −0.34) | (−3.65, 0.97) | (−6.23, −0.67) | (−5.56, 1.34) | ||
| log WMH, cm3 | BMI | −0.04, p = .001 | −0.04, p = .001 | −0.03, p = .104 | 0.01, p = .536 |
| (−0.06, −0.01) | (−0.07, −0.02) | (−0.06, 0.01) | (−0.03, 0.06) | ||
| WC | −0.01, p = .113 | −0.04, p = .001 | 0.02, p = .265 | 0.05, p = .003 | |
| (−0.03, 0.00) | (−0.06, −0.02) | (−0.01, 0.05) | (0.02, 0.09) | ||
Note: BMI = body mass index; WC = waist circumference. Adjusted for age, sex, diabetes, systolic blood pressure, diastolic blood pressure, antihypertension medication use, smoking, high density lipoprotein cholesterol, total intracranial volume. Models for WC also adjusted for height.
Figure 1.
Relationships of Visit 2 (V2) total brain volume with body mass index (BMI) (A) cross-sectionally using Visit 2 BMI, and (B) with changes in BMI from Visit 1 to Visit 2. BMI, per 5 unit increase. Adjusted for age, sex, diabetes, systolic blood pressure, diastolic blood pressure, antihypertension medication use, smoking, high density lipoprotein cholesterol, and total intracranial volume.
Figure 2.
Relationships of Visit 2 (V2) total brain volume with waist circumference (A) cross-sectionally using Visit 2 adiposity, and (B) with changes in waist circumference from V1 to V2. Waist circumference, per 10 unit increase. Adjusted for age, sex, diabetes, systolic blood pressure, diastolic blood pressure, antihypertension medication use, smoking, high density lipoprotein cholesterol, total intracranial volume, and height.
WC-by-age interactions were significant for TBV (p = 0.037). Each additional year of aging increased the negative association of WC with TBV by −0.20 cm3. For example, each 10 cm increase in WC was associated with 1.58 cm3 lower TBV for a 55-year-old and a 3.57 cm3 lower TBV in a 65-year-old. No significant age-by-WC interactions were observed for WMH or for age-by-BMI interactions with any brain MRI measures.
Adiposity Change and MRI Outcomes
To address prodromal weight loss, relations of changes in adiposity from V1 to V2 with MRI outcomes were analyzed separately in participants with increases and decreases in adiposity prior to the MRI. Among those with increases in adiposity, the magnitude of the association of BMI increases with lower TBV (β = −8.84 [CI: −17.87, 0.19]) was greater than in the pooled sample although the statistical significance was borderline in this smaller subset (Table 3A, Figure 1). There was no strong statistical support for racial differences although the relationship was supported among AA (β = −16.20 [CI: −30.34, −2.06]) but not NHW (β = −2.76 [CI: −14.02, 8.51]) in stratified results. Increases in BMI across visits were not associated with WMH (β = 0.06 [CI: −0.05, 0.16]) and were similar by race. Increases in WC were not associated with TBV or WMH in AA or NHW (Table 3A, Figure 2).
Table 3.
Relationships of MRI Measures With (A) Changes in Adipositya Among Participants With Increases in Adiposity, and (B) Changes in Adiposity Among Participants With Decreases in Adiposity
| Estimates p-Value (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Predictor | Pooled | NHW | AA | Interaction | |
| A. Relationship of adiposity change to MRI measures among those with increases in adiposity (N = 1,695; 873 AA) | TBV, cm3 | BMI | −8.84, p = .055 | −2.76, p = .631 | −16.20, p = .025 | −13.44, p = .143 |
| (−17.87, 0.19) | (−14.02, 8.51) | (−30.34, −2.06) | (−31.44, 4.55) | |||
| WC | −1.00, p = .645 | −0.06, p = .981 | −2.94, p = .448 | −2.88, p = .528 | ||
| (−5.25, 3.26) | (−4.69, 4.58) | (−10.53, 4.65) | (−11.83, 6.07) | |||
| log WMH, cm3 | BMI | 0.06, p = .278 | 0.01, p = .826 | 0.12, p = .190 | 0.11, p = .298 | |
| (−0.05, 0.16) | (−0.09, 0.12) | (−0.06, 0.30) | (−0.10, 0.31) | |||
| WC | 0.02, p = .499 | 0.01, p = .794 | 0.04, p = .395 | 0.04, p = .505 | ||
| (−0.03, 0.06) | (−0.04, 0.05) | (−0.05, 0.14) | (−0.07, 0.14) | |||
| B. Relationship of adiposity change to MRI measures among those losing adiposity (n = 1,005; 599 AA) | TBV, cm3 | BMI | −0.04, p = .991 | −2.12, p = .538 | 6.76, p = .392 | 8.88, p = .302 |
| (−6.43, 6.36) | (−8.87, 4.63) | (−8.73, 22.25) | (−7.99, 25.74) | |||
| WC | −2.08, p = .403 | −5.77, p = .057 | 1.44, p = .692 | 7.21, p = .121 | ||
| (−6.97, 2.80) | (−11.72, 0.17) | (−5.69, 8.56) | (−1.90, 16.33) | |||
| log WMH, cm3 | BMI | −0.05, p = .170 | −0.01, p = .675 | −0.17, p = .169 | −0.16, p = .209 | |
| (−0.11, 0.02) | (−0.07, 0.04) | (−0.41, 0.07) | (−0.41, 0.09) | |||
| WC | −0.02, p = .540 | −0.03, p = .334 | 0.01, p = .859 | 0.03, p = .498 | ||
| (−0.06, 0.03) | (−0.08, 0.03) | (−0.08, 0.09) | (−0.06, 0.13) | |||
Notes: BMI = body mass index, per 5 unit increase; WC = waist circumference, per 10 unit increase; TBV = total brain volume. Adjusted for age, sex, diabetes, systolic blood pressure, diastolic blood pressure, antihypertension medication use, smoking, high density lipoprotein cholesterol, and total intracranial volume. Models for WC also adjusted for height.
aModels for change in adiposity (Visit 2 − Visit 1) used spline with knot at zero for adiposity change predictor (e.g., <0/≥0); those with adiposity increases include 27 participants with no change in adiposity.
Among those with decreases in adiposity, greater BMI or WC decreases were not associated with TBV or WMH in AA or NHW (Table 3B); stratified results for WC relations to TBV approached statistical significance thresholds among NHW (β = −5.77 [CI: −11.72, 0.17]). The interpretation of this beta coefficient among NHW is for each additional 10 cm decrease in WC from V1 to V2, TBV is expected to be 5.77 cm3greater, suggesting that WC decreases may be associated with greater brain volumes rather than with lower volumes that might accompany prodromal pathological atrophy in early dementing processes. Age-by-adiposity change interactions were not supported.
Results were robust and slightly conservative compared to models that did not adjust for diabetes and blood pressure (Supplementary Table 3) and in sensitivity analyses accounting for missing data (Supplementary Tables 4 and 5).
Discussion
Among a largely hypertensive cohort of NHW and AA, higher overall and central obesity was associated with lower brain volumes. Increases in overall, but not central obesity, were also associated with lower brain volumes, primarily among AA and moderately in the cohort overall. Additional analyses similarly reflected associations of higher obesity with estimations of brain atrophy. These findings are suggestive that central and overall obesity may contribute to neuronal loss independently of common cardiovascular risk factors.
An unexpected finding was the inverse association of adiposity with WMH cross-sectionally; however, this was not supported in analyses of changes in adiposity. Few studies have examined relationships of adiposity trajectories with brain structure; however, studies of adiposity relations to cognitive decline and dementia appear to be U-shaped in later life (16,17,33–35), a finding that may be explained by several years of weight loss preceding a dementia diagnoses (36,37). This same phenomenon could explain the inverse association of adiposity with WMH. Analyses stratified by those losing and gaining adiposity prior to the MRI demonstrated positive associations between WC and WMH among those gaining weight and negative associations among those losing weight. Neither of these findings met statistical significance in the smaller subgroups but support the hypothesis that adiposity effects on the brain may be insidious for prolonged periods, highlighting the need for long-term follow-up at younger ages to fully elucidate risks of obesity.
These findings add to a recent report among older women in the Women’s Health Initiative Memory Study (WHIMS), which also found an inverse relationship between small vessel ischemic lesion load and BMI but not with BMI changes (38). In contrast to our findings, WHIMS investigators also observed an inverse relationship between higher brain volumes and higher BMI at the visit preceding the MRI. When change in BMI was considered, however, brain volumes were lowest among those with decreases in BMI. Differences in study populations could also contribute to differences in findings, as WHIMS participants were all older, well-educated women in a clinical trial, almost exclusively white, and few had diabetes. The congruence of findings when incorporating changes in adiposity measures supports the need for longitudinal studies to better understand the role of obesity in brain pathology.
This study also extends findings of stronger relationships between brain volumes and central compared to overall adiposity measures in healthy NHW middle-aged (39,40) and Chinese older adults (41) and parallels reports of differential relations of risk factors in AA and NHW, including stronger associations of blood pressure with WMH in AA compared to whites (23). More robust associations between brain volumes and increases in overall (i.e., BMI) compared to central adiposity (i.e., WC), the depot driving cardio-metabolic disease (41–43), suggests that fatty depots may be differentially associated with cerebral pathology than cardio-metabolic outcomes.
Some limitations of this study warrant consideration. The high prevalence of cardiovascular risk factors, particularly hypertension, could limit the ability to observe associations of adiposity with outcomes if these factors mediate cerebral pathological changes, and findings may not generalize to other populations. This might explain limited associations with WC. Results were only slightly strengthened, however, when analyses were repeated without adjusting for blood pressure and diabetes, suggesting other mechanisms may explain relationships of obesity with cerebral atrophy. Stratified analyses by stable, increasing or decreasing adiposity were likely limited in power; we observed a larger than anticipated group with decreases which included persons with very small changes in adiposity measures, and information on intent to lose weight was not collected. However, the low threshold for defining decreases was statistically supported and more specific for identifying persons without prodromal weight loss. Misclassification of intentional with unintentional weight loss likely underestimates rather than inflates associations of interest.
Additionally, we lacked direct measures of adipose tissue. Use of BMI and WC are relevant, however, for practicing clinicians. Causal inferences cannot be made from this observational study, and no adjustments were made to account for family-wise error rates. However, the associations of BMI increases with brain volumes provide temporal evidence that higher overall adiposity may contribute to atrophy, a structural finding associated with dementia. We also note that recruitment of AA and NHW participants was site-specific, limiting inferences on racial differences as associations could be explained by regional, for example, diet, environmental, or other risk factor exposures, rather than race differences. Residual confounding is possible. The MRI was conducted after Visit 2 using 1.5T magnet, which is less sensitive than higher Tesla magnets in detecting WMH; however, volumetric measures, which were associated with BMI, have similar reliability to 3T MRI (44). Additionally, adiposity could have changed in the interval between MRI and Visit 2. The potential limitations are offset by the strengths of the study, including availability of brain imaging in a large sample of AA and repeated measures of obesity, providing information to address confounding by prodromal weight loss.
In conclusion, in this biracial, largely middle-aged cohort with prevalent cardiovascular risk factors, higher overall adiposity and increases in adiposity over an average of 7 years were associated with lower brain volumes, primarily among AA. Future studies should examine etiological explanations for these findings and seek to replicate inverse associations of overall adiposity with small vessel disease markers, particularly in minority populations.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging (R01AG045255 to B.G.W., S.T.L., M.F., E.J.B., S.T.L., and T.H.M.) and the National Institutes of Health (U01-HL054463, HL-81331, and M01 RR00585 to S.T.T., C.R.J., and T.H.M.).
Supplementary Material
Acknowledgments
Author contributions: All authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; all authors provided critical revision of the final draft; and all authors provided final approval of the current draft. Sponsor’s role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis, or preparation of article.
References
- 1. Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi:10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60(10):1394–1399. doi:10.1001/archneur.60.10.1394 [DOI] [PubMed] [Google Scholar]
- 3. Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29(2):388–398. [DOI] [PubMed] [Google Scholar]
- 4. Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64(12):2056–2062. doi:10.1212/01.WNL. 0000165985.97397.88 [DOI] [PubMed] [Google Scholar]
- 5. Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053–1061. doi:10.1001/archneur.65.8.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. [DOI] [PubMed] [Google Scholar]
- 7. Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. doi:10.1001/jama.287.3.329 [DOI] [PubMed] [Google Scholar]
- 8. Potter GG, Plassman BL, Burke JR, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 2009;5(6):445–453. doi:10.1016/j.jalz.2009.04.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bokura H, Nagai A, Oguro H, Kobayashi S, Yamaguchi S. The association of metabolic syndrome with executive dysfunction independent of subclinical ischemic brain lesions in Japanese adults. Dement Geriatr Cogn Disord. 2010;30(6):479–485. doi:10.1159/000322057 [DOI] [PubMed] [Google Scholar]
- 10. Verdelho A, Madureira S, Ferro JM, et al. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry. 2007;78(12):1325–1330. doi:10.1136/jnnp.2006.110361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528–534. doi:10.1212/wnl.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi:10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 13. Ho AJ, Raji CA, Becker JT, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010;31(8):1326–1339. doi:10.1016/j.neurobiolaging.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ward M, Carlsson C, Trivedi M, Sager M, Johnson S. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5(1):23. doi:10.1186/1471-2377-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zade D, Beiser A, McGlinchey R, et al. Apolipoprotein epsilon 4 allele modifies waist-to-hip ratio effects on cognition and brain structure. J Stroke Cerebrovasc Dis. 2011;2(22):119–125. doi:10.1016/j.jstrokecerebrovasdis.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular Health Study. Arch Neurol. 2009;66(3):336–342. doi:10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. West NA, Haan MN. Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. J Gerontol A Biol Sci Med Sci. 2009;64A(1):103–109. doi:10.1093/gerona/gln006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi:10.1136/bmj.38446.466238.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stavitsky K, Du Y, Seichepine D, et al. White matter hyperintensity and cognitive functioning in the racial and ethnic minority cohort of the Framingham Heart Study. Neuroepidemiology. 2010;35(2):117–122. doi:10.1159/000313443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39(3):800–805. doi:10.1159/000313443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M. Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front Aging Neurosci. 2011;3:12. doi:10.3389/fnagi.2011.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huxley RR, Bell EJ, Lutsey PL, et al. A comparative analysis of risk factors for stroke in blacks and whites: the Atherosclerosis Risk in Communities study. Ethn Health. 2014;19(6):601–616. doi:10.1080/13557858.2013. 857765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcus J, Gardener H, Rundek T, et al. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the northern Manhattan study. Stroke. 2011;42(9):2639–2641. doi:10.1161/STROKEAHA.111.617571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–214. [DOI] [PubMed] [Google Scholar]
- 25. Wright CB, Sacco RL, Rundek TR, Delman JB, Rabbani LE, Elkind MSV. Interleukin-6 is associated with cognitive function: the Northern Manhattan study. J Stroke Cerebrovasc Dis. 2006;15(1):34–38. doi:10.1016/j.jstrokecerebrovasdis.2005.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Windham BG, Simpson BN, Lirette S, et al. Associations between inflammation and cognitive function in African Americans and European Americans. J Am Geriatr Soc. 2014;62(12):2303–2310. doi:10.1111/jgs.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoamanesh A, Preis SR, Beiser AS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84(8):825–832. doi:10.1212/WNL.0000000000001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. doi:10.1001/archneur.62.1.55 [DOI] [PubMed] [Google Scholar]
- 29. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014;(260):1–161. [PubMed] [Google Scholar]
- 30. The Family Blood Pressure Program (FBPP) TFI. Multi-center genetic study of hypertension. The Family Blood Pressure Program (FBPP). 2002;39(1):3–9. doi:10.1161/hy1201.100415 [DOI] [PubMed] [Google Scholar]
- 31. Turner ST, Fornage M, Jack CR, Jr, et al. Genomic susceptibility loci for brain atrophy, ventricular volume, and leukoaraiosis in hypertensive sibships. Arch Neurol. 2009;66(7):847–857. doi:10.1001/archneurol.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jack CR, Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14(6):668–676. doi:10.1002/jmri.10011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Driscoll I, Espeland MA, Wassertheil-Smoller S, et al. Weight change and cognitive function: findings from the Women’s Health Initiative Study of cognitive aging. Obesity. 2011;19(8):1595–1600. doi:10.1038/oby.2011.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi:10.1159/000297742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi:10.1001/archinte.163.13.1524 [DOI] [PubMed] [Google Scholar]
- 36. Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44:1147–1152. doi:10.1111/j.1532-5415.1996.tb01362.x [DOI] [PubMed] [Google Scholar]
- 37. Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi:10.1212/01.wnl.0000267661.65586.33 [DOI] [PubMed] [Google Scholar]
- 38. Driscoll I, Gaussoin SA, Wassertheil-Smoller S, et al. Obesity and structural brain integrity in older women: the Women’s Health Initiative Magnetic Resonance Imaging Study. J Gerontol A Biol Sci Med Sci. 2016;71(9):1216–1222. doi:10.1093/gerona/glw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi:10.1212/01.wnl.0000141850.47773.5f [DOI] [PubMed] [Google Scholar]
- 40. Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68(2):136–144. doi:10.1002/ana.22062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi:10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 42. Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. doi:10.2337/diacare.26.2.372 [DOI] [PubMed] [Google Scholar]
- 43. Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi:10.1172/JCI111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Sullivan EV. Combining atlas-based parcellation of regional brain data acquired across scanners at 1.5 T and 3.0 T field strengths. Neuroimage. 2012;60:940–951. doi:10.1016/j.neuroimage.2012.01.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.