Summary
Background:
People transitioning from prisons or jails have high mortality but data are limited for people living with HIV (PLWH) and no studies have integrated data from both criminal justice and community settings. We aimed to evaluate all-cause mortality among PLWH released from Connecticut’s integrated system of prisons and jails.
Methods:
We linked pharmacy, custodial, death, case management, and HIV surveillance data (2007-2014) from Connecticut Departments of Corrections and Public Health to create a retrospective cohort. We compared the mortality rate to statewide and national populations, and described and modeled time-to-death from any cause after prison-release using Cox proportional hazards.
Findings:
Among 1,350 PLWH, mostly Black or Hispanic men with median age 45 years (IQR 39-50), 184 (184/1350, 13·6%) died during a median 5·2 years (IQR 3·0-6·7) after index release. The crude mortality rate of 2,868/100,000 person-years was 6·97 and 8·47 times higher (by standardized mortality ratio) than that of the general U.S. and statewide populations, respectively. Main reported causes of death were: HIV/AIDS (78/170, 45·9%), drug overdose (26/170, 15·3%), liver-related (17/170,10·0%), cardiovascular disease (16/170, 9·4%), and accidental injury or suicide (13/170, 7·6%). Protective factors for time-to-death were: Black race (adjusted hazard ratio [aHR]=0·52, 95%CI=0·34-0·80), having health insurance (aHR=0·09, 95%CI=0·05-0·17), having ≥1 long re-incarceration (aHR=0·41, 95%CI=0·22-0·76), and having an increasing proportion of re-incarcerations in which ART was prescribed. Positive predictors of time-to-death were: age ≥50 years (aHR=3·65, 95%CI=1·21-11·08), lower CD4 count (200-499 cells/ML: aHR=2μ54, 95%CI=1·50-4·31; <200 cells/μL: aHR=3·44, 95%CI=1·90-6·20), higher medical co-morbidity (aHR=1·86, 95%CI=1·23-2·82), virologic failure before death (aHR=2·76, 95%CI=1·94-3·92), and lacking viral load monitoring (aHR=2·13, 95%CI=1·09-4·18).
Interpretation:
To reduce post-release mortality among PLWH, resources are needed to recognize and treat HIV as well as comorbid medical, psychiatric, and substance use disorders, during and following incarceration. Policies that reduce incarceration and support integrated systems of care between prisons and communities can have a significant impact on the survival of PLWH.
Introduction
The United States (US) has the highest incarceration rate worldwide,1 with at least half of prisoners incarcerated for drug-related offenses, and a disproportionate prevalence of HIV in prisons and jails.2 Within-prison HIV-related mortality has declined,3 largely due to expanded antiretroviral therapy (ART) for universal treatment. Nearly all people return to communities following prison-release,4 wherein challenges to HIV care continuity and relapse to substance use are well-documented.5-7 Release from prisons is associated with immediate and exceedingly high rates of death,8,9 importantly from opioid overdose and liver disease, including Hepatitis C;10-12 however, data that incorporate both prison and jail release have not been examined.
There are limited data on causes and predictors of post-release mortality among PLWH to inform policies and services. Two small studies from Indonesia and French Guiana found a higher risk of mortality for PLWH returning from prisons compared to the general population;13 most deaths were HIV-related.14 Causes of death in PLWH can be multi-factorial and structural factors, including re-incarceration, may mitigate mortality risk.13 Among non-incarcerated PLWH, deaths are more often attributable to non-HIV-related causes, but it is unclear if this pattern holds for incarcerated PLWH.15
To inform interventions that reduce mortality after release, we previously developed a large cohort study in a resource-rich setting to describe post-release risk of death from all causes among PLWH released from prisons to communities. This study links data from an integrated system that includes both prisons and jails with community-based data to comprehensively assess the complex role that individuals’ characteristics and incarceration experiences play in HIV outcomes and risk of death.
Methods
Study design and population
This study took place in Connecticut, a state in the Northeastern United States. The study was conducted in the Connecticut Department of Correction (CTDOC), an integrated system that includes prisons and jails, which were previously described.16 The CTDOC Research Advisory Committee and institutional review boards at Yale University and Connecticut Department of Public Health (CTDPH) approved all procedures. Participant consent was waived because all data was previously collected and de-identified for analysis.
Data sources
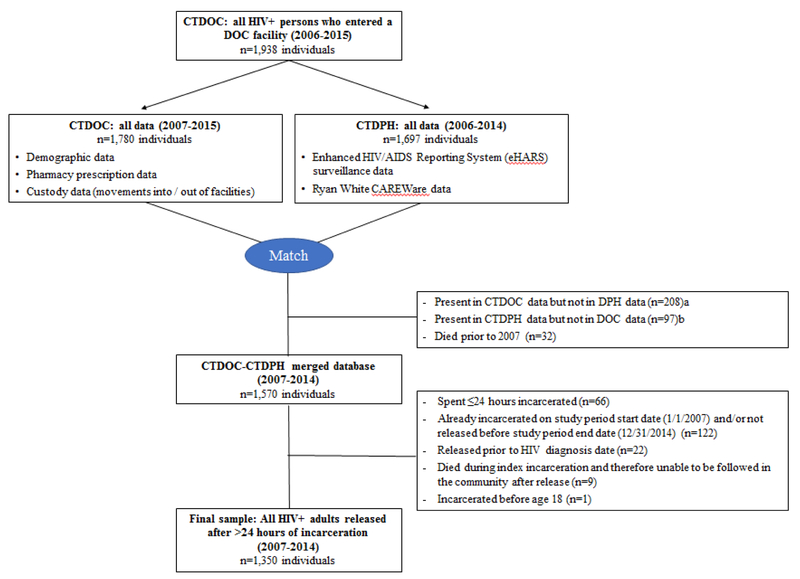
CTDOC custody and pharmacy data were combined with the CTDPH enhanced HIV/AIDS Reporting System (eHARS) surveillance database and CAREWare service utilization database for analyses. We previously created this merged database and examined linkage to care following incarceration (yccr.yale.edu).16 eHARS includes all reported dates of deaths through December 31, 2014, cross-checked against the National Death Index by July 2016 to capture out-of-state deaths. Primary and secondary causes of death were available for deaths occurring in Connecticut. Individuals included in our cohort (Figure 1) were: 1) adults ≥18 years old with confirmed HIV; 2) included in all administrative databases; 3) incarcerated in Connecticut at least once for >24 hours; and 4) were admitted and released between January 1, 2007 and December 31, 2014. Each individual’s first incarceration during this period was their index incarceration. Individuals were followed from index release date until date of death or censoring on December 31, 2014.
Figure 1. Flow diagram of retrospective cohort.
a Resided outside of Connecticut prior to or following incarceration in CTDOC, or first recorded incarceration took place in 2015
b Incarcerated in CTDOC prior to 2007 and was never reincarcerated during 2007-2014
We used 2007-2014 contemporaneous death and person-time data from the Centers for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) to calculate standardized mortality ratios (SMRs) and indirect adjusted mortality rates (IARs) compared to the general population.17
Outcomes and covariates
The primary outcome was time-to-death from any cause. We described all causes of death, categorized by ICD-10 codes (Appendix page 2). Primary causes of death were further categorized as “HIV/AIDS-related”; “Drug overdose”; “Liver disease, failure, and/or viral hepatitis”; “Cardiovascular disease”; “Accidental injury, suicide, or homicide”; and “Other causes” (Appendix page 5.) Many of the non-HIV/AIDS-related deaths were secondarily attributed to HIV.
The Behavioral Health Model for Vulnerable Populations provides a framework for understanding modifiable risk factors and enabling resources to inform interventions.18 We used this model to categorize covariates and hypothesize the role of each in post-release mortality:
Predisposing factors included demographic characteristics. CTDPH data defined the primary source of HIV transmission, with injection drug use representing people who inject drugs (PWID), and CD4 count at the time of HIV diagnosis.
Enabling/disabling resource factors from the CAREWare database included health insurance coverage and transitional case management (TCM) use during the index incarceration (dichotomous) and quantity of TCM visits per year. HIV viral load (VL) from the eHARS database served as a proxy for routine HIV care clinic visits (in prison/jail and in the community). Linkage to HIV care required a VL within 14 days after the index release, as we described previously. Using CTDOC data, we calculated duration of incarcerations with <30 days likely representing jail detentions and ≥365 days involving prison sentences. Conditions of release were categorized as unsupervised, conditional (e.g., parole or transitional housing), or bonded. Incarceration variables were constructed both for the index incarceration and the most recent incarceration prior to death or censoring. Because HIV care during incarceration was expected to influence risk of death, the “Conditions of release from last incarceration” variable included an additional category for individuals who were re-incarcerated for >30 days by the time of death/censoring. Re-incarceration was otherwise defined as spending >24 hours in a CTDOC facility after index release and analyzed as a continuous rate (number of re-incarcerations per year of follow-up), dichotomously (having at least one re-incarceration lasting ≥365 days), and categorically (percentage of follow-up time spent re-incarcerated: 0-1%, 2-10%, 11-25%, 26-50%, and 51-100%).
Need factors: Pre-release viral suppression (VL<400 copies/μL) was defined within 90 days before release. Pharmacy records included antiretroviral (ART) and medications prescribed to treat other medical, psychiatric, or substance use co-morbidities; each were coded dichotomously and summed to create a categorical co-morbidity variable (other than HIV). As previously described,16 CTDOC staff assign psychiatric and addiction severity scores on intake (scale 1-5) to determine services needed during and immediately after incarceration. We analyzed addiction severity scores categorically.We combined psychiatric severity scores with prescription data to create a 4-level composite psychiatric severity variable (1: low, untreated; 2: low, treated; 3: high, untreated; 4: high, treated).
For each re-incarceration, we used pharmacy data to determine whether ART was prescribed. We also used the first and last VL measured during incarceration to determine whether individuals with an initially high VL became virally suppressed during that incarceration and whether suppression was achieved within 90 days pre-release. We calculated the percentage of all re-incarcerations that demonstrated these outcomes and categorized “ART coverage” by quartiles. Finally, using national guidelines,19 we used the last VL drawn during the last six months of observation to evaluate viral suppression status and frequency of monitoring.
Statistical analysis
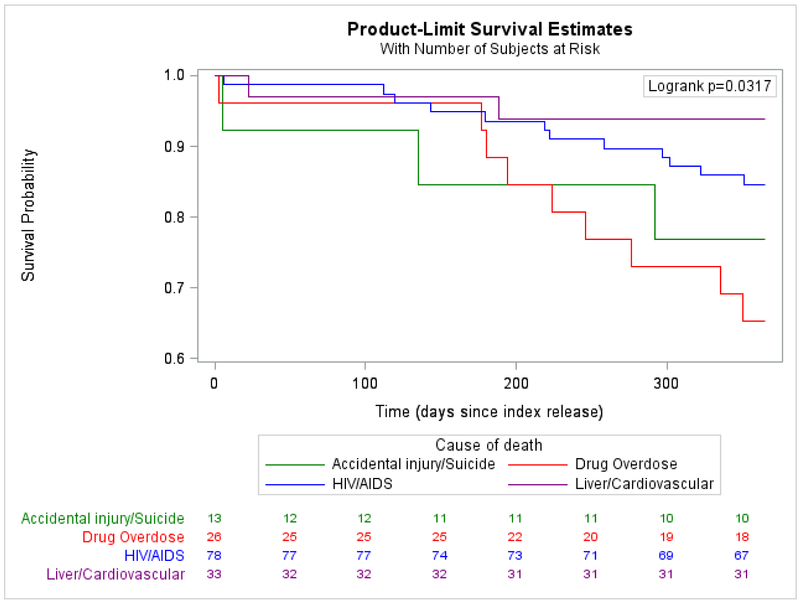
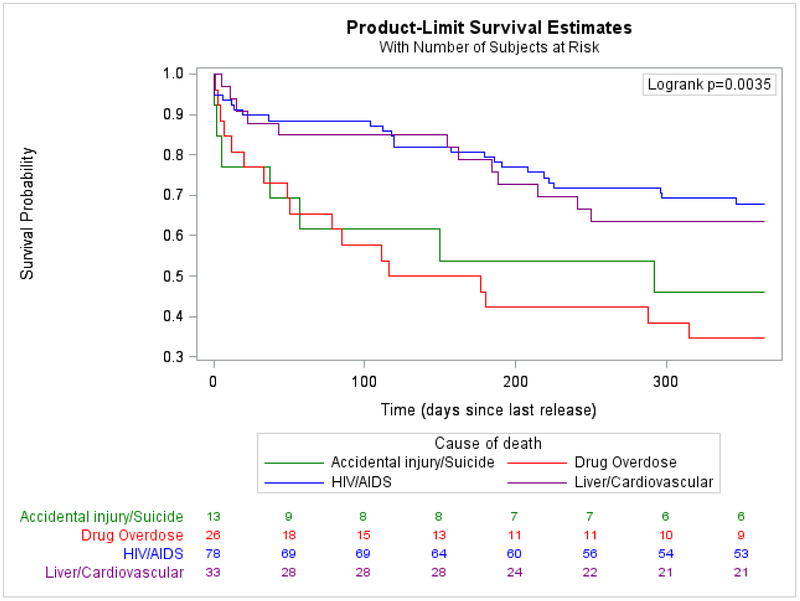
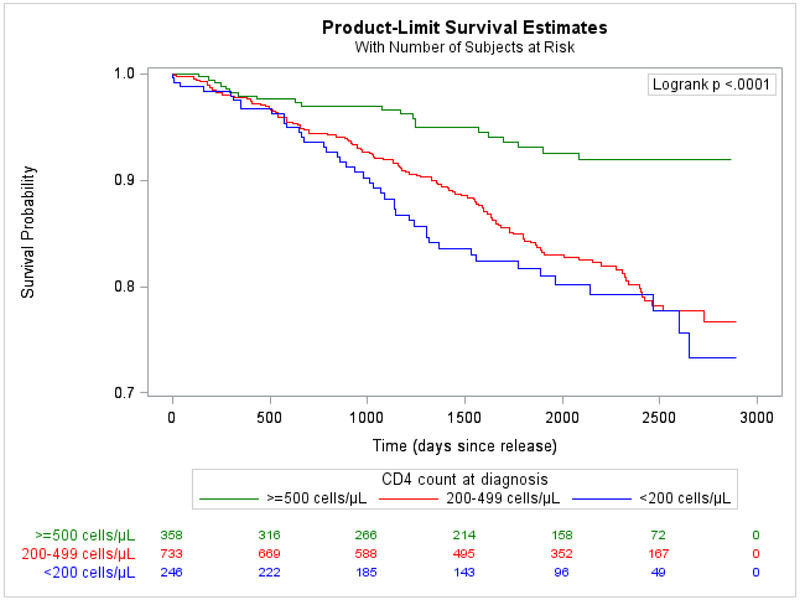
We first calculated the crude mortality rate (CMR). We used indirect standardization to calculate indirect adjusted mortality rates (IARs) and standardized mortality ratios (SMRs) to compare mortality in the cohort with that of the US and Connecticut general populations. Both IARs and SMRs were adjusted for sex, race/ethnicity, and age in years. We calculated basic descriptive frequencies of primary and all causes of death, stratified by primary cause (Appendix page 5). Using Kaplan-Meier curves and log-rank tests, we compared time-to-death during the first year after release by primary causes of death among those who died (Figure 2), and assessed predictors of time-to-death in bivariate analyses (Figure 3) and overall for the entire sample (Appendix page 1). We used Cox proportional hazards to model predictors of time-to-death from any cause to globally assess the impact of release on time-to-death. We incorporated data across multiple incarcerations during follow-up to account for whether an individual tended to be re-incarcerated and engaged in care by virtue of their re-incarceration. We explored the model using both continuous and categorical or calculated variables and only included the latter when continuous terms were not normally distributed. Variables with bivariate associations of p<0·20 were considered for multivariate models and we generated preliminary parsimonious models with all variables with Type III p<0·10. Rather than rely on automated selection procedures, we explored relationships between variables with suspected correlation, using chi-squared and Mann-Whitney U tests. When variables were collinear, we selected the variable of greater clinical interest based on prior literature and variable distribution, and factored in the strength of the association with the outcome, using thresholds of significance only to understand how these variables impacted survival to finalize the model. We formally tested each variable for adherence to the proportional hazards assumption. Variables that violated the proportional hazards assumption were included as time-varying covariates in the final model. The effect estimates presented in Table 1 correspond to the first day of follow-up (t0=0 days); time-dependent effect estimates were also calculated for discrete time points (t1=30 days, t2=365 days, t3=730 days [2 years], and t3=1460 days [4 years]) but the effect estimates did not change direction or significance over time. We used cumulative hazard plots and residual plots as supplementary methods to confirm that this strategy resulted in a robust model. We hypothesized and therefore explored interactions between recidivism, TCM, PWID, race/ethnicity, and gender. Interaction terms were justified because they provided a clearer explanation of variable effects compared to models that did not include interaction terms. Given Connecticut’s high recidivism rate (37-64% return within three years),20 we focused on re-incarceration rates of 0, 0·33, 1, 2, and 3 reincarcerations/year when assessing interactions. Using backward selection, the final parsimonious multivariable model was limited to variables with Type III p<0·5. Using re-incarceration rates as a covariate may have unintentionally introduced bias, in that people who died early after release from prison had less chance to be re-incarcerated. We therefore conducted a sensitivity analysis that excluded the earliest deaths occurring within 21 days following release. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Gary, NC).
Figure 2.
A. Among those who died, time from index release to death in one year, stratified by major causes of death
B.Among those who died, time from most recent release to death in one year, stratified by major causes of death
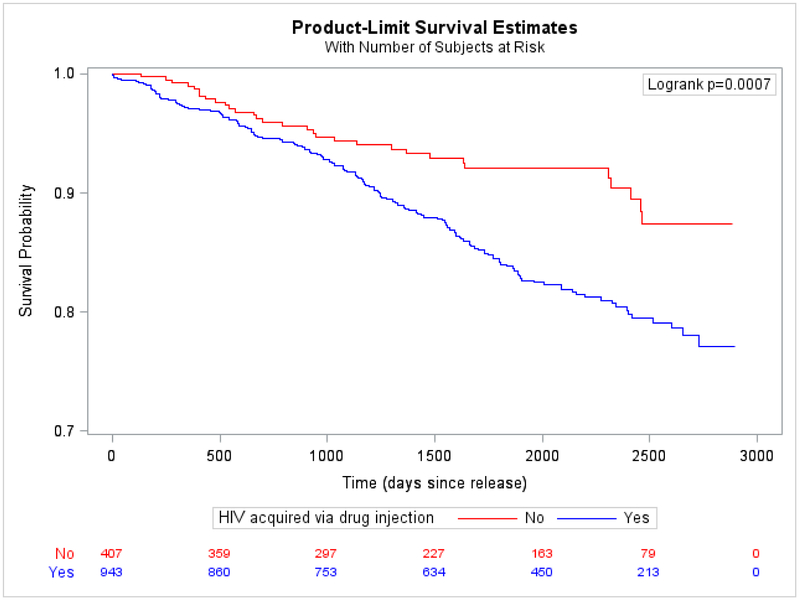
Figure 3. Bivariate association between CD4 count at time of HIV diagnosis and time-to-death from any cause.
A.Individuals with missing or unreported CD4 count at time of diagnosis (n=13) were excluded from the bivariate analysis, leaving 1337 individuals for analysis. For 33 individuals missing a CD4 count at time of diagnosis but with CD4 counts available within 3 months of their diagnosis date, their first CD4 counts were used as an approximation of their CD4 counts at time of diagnosis.
B.Bivariate association between prior injection drug use and time-to-death from any cause
Table 1.
Characteristics of people living with HIV formerly incarcerated in an integrated correctional system* (N=1,350) and predictors of time-to-death from any cause after initial release, 2007-2014
| Total n (%) or median (IQR)a |
n (%) who diedb |
Bivariate HR (95% CI) |
p-value | Adjusted HR (95% CI) at t = 0 |
p-value | |
|---|---|---|---|---|---|---|
| Predisposing factors | ||||||
| Age at time of index release | ||||||
| 18-29 | 114 (8.4%) | 5 (4.4%) | referent | referent | ||
| 30-39 | 253 (18.7%) | 25 (9.9%) | 1.95 (0.75-5.10) | 0.17 | 1.98 (0.64-6.15) | 0.24 |
| 40-49 | 617 (45.7%) | 90 (14.6%) | 2.96 (1.21-7.29) | 0.02 | 2.83 (0.95-8.40) | 0.06 |
| ≥50 | 366 (27.1%) | 64 (17.5%) | 4.40 (1.77-10.93) | 0.001 | 3.65 (1.21-11.08) | 0.02 |
| Genderc | ||||||
| Female | 307 (22.7%) | 31 (10.1%) | referent | |||
| Male | 1043 (77.3%) | 153 (14.7%) | 1.59 (1.08-2.34) | 0.02 | ||
| Race/Ethnicity | ||||||
| White | 250 (18.5%) | 41 (16.4%) | referent | referent | ||
| Black | 557 (41.3%) | 60 (10.8%) | 0.62 (0.42-0.92) | 0.02 | 0.52 (0.34-0.80) | 0.003 |
| Hispanic | 495 (36.7%) | 77 (15.6%) | 0.89 (0.61-1.30) | 0.55 | 0.96 (0.65-1.43) | 0.84 |
| Other | 48 (3.6%) | 6 (12.5%) | 0.68 (0.29-1.59) | 0.37 | 0.69 (0.29-1.68) | 0.42 |
| Education level | ||||||
| < High school | 610 (45.2%) | 96 (15.7%) | referent | |||
| ≥ High school | 740 (54.8%) | 88 (11.9%) | 0.76 (0.57-1.02) | 0.06 | ||
| Marital statusd | ||||||
| Not married | 1086 (84.3%) | 151 (13.9%) | referent | |||
| Married | 203 (15.7%) | 32 (15.8%) | 1.12 (0.77-1.64) | 0.55 | ||
| PWID | ||||||
| No | 407 (30.2%) | 31 (7.6%) | referent | referent | ||
| Yes | 943 (69.9%) | 153 (16.2%) | 1.93 (1.31-2.84) | 0.001 | See below | 0.01 |
| PWID × Re-incarceration rate | ||||||
| PWID × 0 re-incarcerations/year | 2.06 (1.23-3.47) | |||||
| PWID × 0-33 re-incarcerations/year | 1.54 (1.00-2.38) | |||||
| PWID × 1 re-incarceration/year | 0.85 (0.48-1.52) | |||||
| PWID × 2 re-incarcerations/year | 0.35 (0.11-1.10) | |||||
| PWID × 3 re-incarcerations/year | 0.14(0.02-0.87) | |||||
| CD4 count at time of diagnosise | ||||||
| ≥ 500 cells/mL | 358 (26.8%) | 21 (5.9%) | referent | referent | ||
| 200-499 cells/mL | 733 (54.8%) | 119 (16.2%) | 2.58 (1.62-4.11) | <0.0001 | 2.54 (1.50-4.31) | 0.005 |
| < 200 cells/mL | 246 (18.4%) | 44 (17.9%) | 3.08 (1.83-5.18) | <0.0001 | 3.44 (1.90-6.20) | <0.0001 |
| Years since HIV diagnosis at time of index release | 11.4 | 12.9 | ||||
| Median (interquartile range) | (5.4-17.1) | (7.4-17.9) | 1.04 (1.02-1.07) | 0.0002 | ||
| HIV diagnosed during last incarceration | ||||||
| No | 1282 (95.0%) | 183 (14.3%) | referent | |||
| Yes | 68 (5.0%) | 1 (1.5%) | 0.12 (0.02-0.88) | 0.04 | ||
| Enabling or disabling factors | ||||||
| Length of index incarceration | ||||||
| ≤ 30 days | 454 (33.6%) | 67 (14.8%) | referent | |||
| 31-364 days | 697 (51.6%) | 102 (14.6%) | 0.94 (0.69-1.29) | 0.72 | ||
| ≥ 365 days | 199 (14.7%) | 15 (7.5%) | 0.58 (0.33-1.02) | 0.06 | ||
| Conditions of index release | ||||||
| Unconditional | 783 (58.0%) | 116 (14.8%) | referent | |||
| Conditional | 351 (26.0%) | 40 (11.4%) | 0.79 (0.55-1.13) | 0.19 | ||
| Bonded | 216 (16 0%) | 28 (13.0%) | 0.92 (0.61-1.38) | 0.67 | ||
| Year of index release | ||||||
| 2007-2008 | 595 (44.1%) | 120 (20.2%) | referent | |||
| 2009-2010 | 388 (28.7%) | 47 (12.1%) | 0.77 (0.54-1.08) | 0.13 | ||
| 2011-2012 | 211 (15.6%) | 15 (7.1%) | 0.79 (0.45-1.38) | 0.41 | ||
| 2013-2014 | 156 (11.6%) | 2 (1.3%) | 0.41 (0.10-1.72) | 0.22 | ||
| Health insurance during follow-up | ||||||
| Consistently uninsured | 365 (27.0%) | 102 (27.9%) | referent | referent | ||
| Consistently insured | 637 (47.2%) | 68 (10.7%) | 0.28 (0.21-0.38) | <0.0001 | 0.09 (0.05-0.17) | <0.0001 |
| Intermittently insured | 348 (25.8%) | 14 (4.0%) | 0.10 (0.06-0.17) | <0.0001 | 001 (0.00-0.04) | <0.0001 |
| Linked to HIV care within 14 days after index releasef | ||||||
| No | 1076 (79.7%) | 144 (13.4%) | referent | |||
| Yes | 274 (20.3%) | 40 (14.6%) | 1.26 (0.89-1.79) | 0.20 | ||
| Transitional case management during index release | ||||||
| No | 1010 (74.8%) | 138 (13.7%) | referent | |||
| Yes | 340 (25.2%) | 46 (13.5%) | 1.14 (0.82-1.59) | 0.45 | ||
| Number of case management (TCM) visits per year of follow-up | ||||||
| 0 | 620 (45.9%) | 108 (17.4%) | referent | referent | ||
| >0-2 | 233 (17.3%) | 20 (8.6%) | 0.39 (0.24-0.62) | <0.0001 | See below | 0.38 |
| 3-4 | 146 (10.8%) | 14 (9.6%) | 0.45 (0.26-0.79) | 0.01 | See below | 0.35 |
| ≥5 | 351 (26.0%) | 42 (12.0%) | 0.68 (0.47-0.97) | 0.03 | See below | <0.0001 |
| Case management (TCM) frequency × Re-incarceration rate | ||||||
| >0-2 TCM visits/year × 0 re-incarcerations/year | 0.77 (0.43-1.37) | |||||
| >0-2 TCM visits/year × 0.33 re-incarcerations/year | 0.64 (0.38-1.07) | |||||
| >0-2 TCM visits/year × 1 re-incarceration/year | 0.43 (0.16-1.16) | |||||
| >0-2 TCM visits/year × 2 re-incarcerations/year | 0.24 (0.03-1.84) | |||||
| >0-2 TCM visits/year × 3 re-incarcerations/year | 0.13 (0.01-3.08) | |||||
| 3-4 TCM visits/year × 0 re-incarcerations/year | 1.60 (0.59-4.31) | |||||
| 3-4 TCM visits/year × 0.33 re-incarcerations/year | 1.23 (0.64-2.36) | |||||
| 3-4 TCM visits/year × 1 re-incarceration/year | 0.73 (0.26-2.07) | |||||
| 3-4 TCM visits/year × 2 re-incarcerations/year | 0.33 (0.03-4.35) | |||||
| 3-4 TCM visits/year × 3 re-incarcerations/year | 0.15 (0.00-10.00) | |||||
| ≥5 TCM visits/year × 0 re-incarcerations/year | 2.83 (1.68-4.78) | |||||
| ≥5 TCM visits/year × 0.33 re-incarcerations/year | 2.01 (1.28-3.15) | |||||
| ≥5 TCM visits/year × 1 re-incarceration/year | 1.00 (0.57-1.75) | |||||
| ≥5 TCM visits/year × 2 re-incarcerations/year | 0.35 (0.12-1.01) | |||||
| ≥5 TCM visits/year × 3 re-incarcerations/year | 0.12 (0.02-0.63) | |||||
| Re-incarceration rate | ||||||
| Median (interquartile range) | 0.19 (0-0.53) | 0.19 (0-0.71) | 1.60 (1.16-2.20) | 0.004 | See below | <0.0001 |
| Re-incarceration rate × PWID × Case management (TCM) frequency | ||||||
| 1-unit increase in re-incarceration rate × non-PWID × 0 TCM visits/year | 21.86 (10.90-43.86) | |||||
| 1-unit increase in re-incarceration rate × non-PWID × >0-2 TCM visits/year | 12.13 (3.63-40.52) | |||||
| 1-unit increase in re-incarceration rate × non-PWID × 3-4 TCM visits/year | 9.97 (1.81-54.83) | |||||
| 1-unit increase in re-incarceration rate × non-PWID x≥5 TCM visits/year | 7.72 (3.63-16.45) | |||||
| 1-unit increase in re-incarceration rate × PWID × 0 TCM visits/year | 8.99 (5.14-15.72) | |||||
| 1-unit increase in re-incarceration rate × PWID × >0-2 TCM visits/year | 4.99 (1.62-15.39) | |||||
| 1-unit increase in re-incarceration rate × PWID × 3-4 TCM visits/year | 4.10 (0.80-20.89) | |||||
| 1-unit increase in re-incarceration rate × PWID × >5 TCM visits/year | 3.17 (1.96-5.14) | |||||
| Re-incarcerated for ≥365 days | ||||||
| No | 1092 (80.9%) | 170(15.6%) | referent | referent | ||
| Yes | 258 (19.1%) | 14(5.4%)) | 0.26 (0.15-0.45) | <0.0001 | 0.41 (0.22-0.76) | 0.004 |
|
Percentage of total follow-up time spent re-incarcerated | ||||||
| 0-1% | 605 (44.8%) | 100(16.5%) | referent | |||
| 2-10% | 225 (16.7%) | 21 (9.3%) | 0.44 (0.27-0.70) | 0.001 | ||
| 11-25% | 196 (14.5%) | 31 (15.8%) | 0.75 (0.50-1.12) | 0.16 | ||
| 26-50% | 219 (16.2%) | 23 (10.5%) | 0.48 (0.30-0.75) | 0.001 | ||
| 51-100% | 105 (7.8%) | 9 (8.6%) | 0.44 (0.22-0.87) | 0-02 | ||
| Length of last incarceration | ||||||
| ≤ 30 days | 347 (25.7%) | 63 (18.2%) | referent | |||
| 31-364 days | 769 (57.0%) | 103 (13.4%) | 0.65 (0.48-0.89) | 0.01 | ||
| > 365 days | 234 (17.3%) | 18 (7.7%) | 0.38 (0.22-0.64) | 0.0003 | ||
| Conditions of release from last incarceration | ||||||
| Unconditional | 770 (57.0%) | 122 (15.8%) | referent | |||
| Conditional | 291 (21.6%) | 36 (12.4%) | 0.78 (0.54-1.14) | 0.20 | ||
| Bonded | 129 (9.6%) | 21 (16.3%) | 1.21 (0.76-1.92) | 0.42 | ||
| Currently re-incarcerated | 160 (11.9%) | 5 (3.1%) | 0.19 (0.08-0.46) | 0.0002 | ||
| Need factors | ||||||
| Prescribed ART during index incarceration | ||||||
| No | 590 (43.7%) | 85 (14.4%) | referent | |||
| Yes | 760 (56.3%) | 99 (13.0%) | 1.15 (0.86-1.53) | 0.37 | ||
| Virally suppressed within 90 days prior to index release | ||||||
| No/viral level not reported | 588 (43.6%) | 89 (15.1%) | referent | |||
| Yes | 442 (32.7%) | 52 (11.8%) | 0.97 (0.69-1.37) | 0.85 | ||
| Viral load not drawn prior to release | 320 (23.7%) | 43 (13.4%) | 1.01 (0.70-1.45) | 0.96 | ||
| Number of medical co-morbidities during index incarceration | ||||||
| 0 | 838 (62.1%) | 111 (13.2%) | referent | referent | ||
| 1 | 294 (21.8%) | 37 (12.6%) | 1.16 (0.80-1.69) | 0.43 | 1.44 (0.96-2.15) | 0.08 |
| ≥2 | 218 (16.2%) | 36 (16.5%) | 1.77 (1.21-2.59) | 0.003 | 1.86 (1.23-2.82) | 0.003 |
| Psychiatric need during index incarceration | ||||||
| Low severity score (1-2), untreated | 650 (48.2%) | 89 (13.7%) | referent | |||
| Low severity score (1-2), treated | 58 (4.3%) | 5 (2.0%) | 0.76 (0.31-1.87) | 0.55 | ||
| High severity score (3-5), untreated | 256 (19.0%) | 38 (65.5%) | 0.98 (0.67-1.43) | 0.90 | ||
| High severity score (3-5), treated | 386 (28.6%) | 52 (13.5%) | 1.10 (0.78-1.55) | 0.60 | ||
| Addiction severity score during index incarcerationg | ||||||
| Low severity/no disorder (score 1-2) | 220 (16.7%) | 16 (7.3%) | referent | |||
| Moderate severity disorder (score 3) | 861 (65.2%) | 123 (14.3%) | 2.21 (1.31-3.72) | 0.003 | ||
| Serious disorder (score 4-5) | 239 (18.1%) | 44 (18.4%) | 3.13 (1.77-5.55) | <0.0001 | ||
|
Treated for an opioid use disorder during index incarceration |
||||||
| No | 1341 (99.3%) | 183 (13.6%) | referent | |||
| Yes | 9 (0.7%) | 1 (11.1%) | 2.47 (0.34-17.7) | 0.37 | ||
| Percentage of all re-incarcerations during which ART was prescribed | ||||||
| Never re-incarcerated | 535 (39.6%) | 89 (16.6%) | referent | referent | ||
| 0-10% | 128 (9.5%) | 25 (19.7%) | 0.87 (0.56-1.36) | 0.54 | 0.34 (0.18-0.65) | 0.001 |
| 11-50% | 97 (7.2%) | 15 (15.3%) | 0.62 (0.36-1.07) | 0.09 | 0.12 (0.06-0.27) | <0.0001 |
| 51-90% | 101 (7.5%) | 9 (8.7%) | 0.33 (0.17-0.66) | 0.002 | 0.04 (0.01-0.11) | <0.0001 |
| 91-100% | 489 (36.2%) | 46 (9.4%) | 0.45 (0.31-0.64) | <0.0001 | 0.08 (0.03-0.21) | <0.0001 |
| Percentage of all re-incarcerations that ended in viral suppression | ||||||
| Never re-incarcerated | 535 (39.6%) | 89 (16.6%) | referent | |||
| 0-24% | 297 (22.0%) | 46 (15.5%) | 0.71 (0.50-1.01) | 0.06 | ||
| 25-49% | 83 (6.2%) | 10 (12.0%) | 0.47 (0.24-0.90) | 0.02 | ||
| 50-74% | 172 (12.7%) | 17 (9.9%) | 0.40 (0.24-0.67) | 0.001 | ||
| 75-100% | 263 (19.5%) | 22 (8.4%) | 0.40 (0.25-0.64) | 0.0001 | ||
| Percentage of all re-incarcerations with a documented change from virologic failure to suppression | ||||||
| Never re-incarcerated | 535 (39.6%) | 89 (16.6%) | referent | |||
| 0-24% | 591 (43.8%) | 76 (12.9%) | 0.60 (0.44-0.81) | 0.001 | ||
| 25-49% | 79 (5.9%) | 7 (8.9%) | 0.33 (0.15-0.70) | 0.004 | ||
| 50-74% | 86 (6.4%) | 7 (8.1%) | 0.32 (0.15-0.69) | 0.004 | ||
| 75-100% | 59 (4.4%) | 5 (8.5%) | 0.38 (0.16-0.94) | 0.04 | ||
| Prescribed ART during last incarceration | ||||||
| No | 357 (26.4%) | 69 (19.3%) | referent | |||
| Yes | 993 (73.6%) | 115 (11.6%) | 0.62 (0.46-0.84) | 0.002 | ||
| Virally suppressed within 90 days prior to release from last incarceration | ||||||
| No/viral level not reported | 411 (30.4%) | 79 (19.2%) | referent | |||
| Yes | 653 (48.4%) | 67 (10.3%) | 0.56 (0.40-0.77) | 0-0004 | ||
| Viral load not drawn prior to release | 286 (21 .2%) | 38 (13.3%) | 0.72 (0.49-1.06) | 0.10 | ||
| Viral suppression status during last incarceration | ||||||
| Never virally suppressed | 398 (29.5%) | 77 (19.3%) | referent | |||
| Failure, then suppression | 260 (19.3%) | 31 (11.9%) | 0.60 (0.39-0.91) | 0.02 | ||
| Suppression, then failure | 38 (2.8%) | 6 (15.8%) | 0.80 (0.35-1.84) | 0.60 | ||
| Consistent suppression | 485 (35.9%) | 40 (8.2%) | 47 (0.32-0.68) | <0.0001 | ||
| Viral load never drawn | 169 (12.5%) | 30 (17.8%) | 1.07 (0.70-1.63) | 0.76 | ||
| Number of medical comorbidities during last incarceration | ||||||
| 0 | 653 (48.4%) | 92 (14.1%) | referent | |||
| 1 | 340 (25.2%) | 39 (11.5%) | 0.85 (0.59-1.24) | 0.41 | ||
| ≥2 | 357 (26.4%) | 53 (14.8%) | 1.16 (0.82-1.62) | 0.40 | ||
| Psychiatric need during last incarceration | ||||||
| Low severity score (1-2), untreated | 589 (43.6%) | 81 (13.8%) | referent | |||
| Low severity score (1-2), treated | 99 (7.3%) | 35 (35.4%) | 1.21 (0.81-1.80) | 0.35 | ||
| High severity score (3-5), untreated | 199 (14.7%) | 16 (8.0%) | 1.14 (0.66-1.94) | 0.64 | ||
| High severity score (3-5), treated | 463 (34.3%) | 52 (11.2%) | 0.82 (0.58-1.17) | 0.28 | ||
| Addiction severity score during last incarcerationh | ||||||
| Low severity/no disorder (score 1-2) | 122 (9.2%) | 19 (15.6%) | referent | referent | ||
| Moderate severity disorder (score 3) | 945 (70.9%) | 107 (11.3%) | 0.70 (0.43-1.13) | 0.15 | 0.65 (0.38-1.12) | 0.12 |
| Serious disorder (score 4-5) | 265 (19.9%) | 56 (21.1%) | 1.37 (0.81-2.30) | 0.24 | 1.17 (0.65-2.09) | 0.61 |
| Treated for an opioid use disorder during last incarceration | ||||||
| No | 1322 (97.9%) | 183 (13.8%) | referent | |||
| Yes | 28 (2.1%) | 1 (3.6%) | 0.28 (0.04-1.97) | 0.20 | ||
| Viral suppression and timing of last viral load drawn prior to death/censoring | ||||||
| Viral suppression, within 6 months | 840 (62.2%) | 79 (9.4%) | referent | referent | ||
| Virologic failure, within 6 months | 208 (15.4%) | 65 (31.3%) | 3.63 (2.62-5.05) | <0.0001 | 2.76 (1.94-3.92) | <0.0001 |
| Viral suppression, >6 months | 160 (11.9%) | 17 (10.6%) | 1.10 (0.65-1.85) | 0.73 | 0.83 (0.48-1.42) | 0.49 |
| Virologic failure, >6 months | 87 (6.4%) | 11 (12.6%) | 1.19 (0.64-2.24) | 0.58 | 0.72 (0.37-1.42) | 0.35 |
| Viral load never drawni | 55 (4.1%) | 12 (21.8%) | 3.90 (2.13-7.16) | <0.0001 | 2.13 (1.09-4.18) | 0.03 |
Includes combined information for both prisoners and jail detainees
PWID= people who acquired HIV through injection drug use (people who inject drugs;); non-PWID=people without an injection drug use history
Bivariate analyses include all 1,350 individuals released during the observation period· During a median follow-up time of 5·2 (IQR 3·0-6·7) years (6,418 person-years), there were 184 deaths during follow-up. Due to missing data for the CD4 count and addiction severity score variables as specified below, the final parsimonious model includes 1,319 individuals followed for 6,276 person-years, during which there were 182 deaths. Numbers listed are n (%) out of the total number of individuals in the sample (n=1350). Percentages may not sum to 100% due to rounding or missing data as specified below.
Numbers listed are the row n (%) of individuals with the specified characteristic who experienced the outcome of interest (death)
Transgender males (n=1) have been included the male category and transgender females (n=3) have been included in the female category
Individuals with missing/unreported marital status (n=61) were excluded from the bivariate analysis such that the total n=1289
Individuals with missing/unreported CD4 count at time of diagnosis (n=13) were excluded from the bivariate analysis such that the total n=1337. For 33 individuals missing a CD4 count at time of diagnosis but with CD4 counts available within 3 months of their diagnosis date, their first CD4 counts were used as an approximation of their CD4 counts at time of diagnosis.
The “No” category includes 25 individuals who were either re-incarcerated or died within 14 days without having a VL drawn in the community.
Index incarceration periods where the addiction severity score was never assessed (n=30) were excluded from the bivariate analysis such that the total n=1320.
Last incarceration periods where the addiction severity score was never assessed (n=18) were excluded from the bivariate analysis such that the total n=1332.
The “viral load never drawn” category includes 18 individuals who were followed in the community for <6 months prior to death or censoring.
Role of the funding source
The National Institutes of Health played no role in data collection, analysis, data interpretation, drafting of or decision to submit the manuscript for publication.
Results
Table 1 describes the 1,350 PLWH eligible for analysis between January 1, 2007 and December 31, 2014, with most being ≥40 years old (983/1350, 72·8%), male (1043/1350, 77·3%), Black or Hispanic (1052/1350, 78·0%), and PWID (943/1350, 69·9%).
The sample was followed for a median of 5·2 (IQR 3·0-6·7) years, contributing a total of 6,418 person-years. After index release, 184 (184/1350, 13·6%) individuals died, resulting in a CMR of 2,868 per 100,000 person-years; the IAR was 2,993 per 100,000 person-years (95% CI=2,560-3,425) and 2,964 per 100,000 person years (95% CI=2,536-3,392), compared to the general US and Connecticut populations, respectively. Compared to the general US and Connecticut populations, the SMRs were 6·97 (95% CI=5·96-7·97) and 8·47 (95% CI=7·25-9·69), respectively.
Among the 170 deaths where cause was reported, primary causes included HIV/AIDS (78/170, 45·9%); drug overdose (n=26, 15·3%); accidental injury, homicide, or suicide (13/170, 7·6%); liver (17/170, 10·0%); and cardiovascular (16/170, 9·4%) disease (Appendix page 5). During the first year after index (Figure 2A) or most recent release (Figure 2B), among those who died, time-to-death from drug overdose or accidental injury was significantly shorter than time-to-death from HIV/AIDS complications. Survival was longest for individuals who died from liver or cardiovascular disease. When survival probabilities were assessed after 24-36 months after release, there was no statistically significant difference in time-to-death by primary cause.
The final multivariable Cox model (Table 1) included 1,319 individuals with all data available, who cumulatively contributed 6,276 person-years of follow-up time, during which there were 182 deaths.
Predisposing factors that independently and significantly predicted shorter survival time were age ≥50 years and lower CD4 count at diagnosis (Figure 3A), despite people having been diagnosed with HIV a median 11·4 years prior to index release. Relative to being White, Black race was protective against time-to-death. In bivariate analysis, PWID had significantly shorter survival (Figure 3B). In the multivariable model, PWID significantly interacted with re-incarceration rate, such that, compared to non-PWID, PWID had worse survival when they were never or infrequently re-incarcerated and better survival when frequently re-incarcerated.
Enabling resource factors that independently predicted longer survival time were having at least one long re-incarceration and having health insurance either consistently or intermittently during follow-up. Health insurance’s effect varied over time but did not change direction or significance. Accounting for significant interactions between frequency of TCM visits, re-incarceration rate, and PWID, the following patterns were observed: compared to those who never received TCM services after release, PLWH using ≥5 TCM visits/year had worse survival when they were never or infrequently reincarcerated and better survival when frequently re-incarcerated. Increasing reincarceration rate, however was highly hazardous, particularly for PWID and those who either had infrequent (0-2 visits/year) or extremely frequent (≥5 visits/year) TCM. The highest hazard of death observed for a 1-unit increase in re-incarceration rate was in non-PWID who did not receive any TCM services. The lowest relative hazard associated with increasing re-incarceration rate was in PWID who received ≥5 TCM visits/year. Only three deaths (3/182, 1.6% of all deaths included in the final model), occurred within 21 days of release when preventable causes of death including overdose are common,21 and there was minimal impact on effect magnitude or direction when these three deaths were removed from the model in a sensitivity analysis.
Need factors: Compared to individuals who were never re-incarcerated, having a higher proportion of re-incarcerations in which ART was prescribed was protective. In contrast, PLWH with ≥2 other medical co-morbidities, documented virologic failure during follow-up, or who never had a VL drawn in the community after release had significantly shorter survival time.
Discussion
By integrating correctional, public health and mortality data for a large number of PLWH over an 8-year observation period where both prison and jail data were available, we were able to examine the complex contributions to death in prisoners with HIV who transition to the community. We found a high CMR after release (2,868/100,000 person-years), which was higher than prior estimates of general prison populations (720-2,054/100,000 person-years).11 When we adjusted for race, ethnicity, sex, and age, we also found a high SMR compared to US (6·97) and Connecticut general populations(8·47). This was greater than the SMR of 3·6 reported in a study of the general prison compared to the non-institutionalized population in Washington.8
To our knowledge, this represents the largest cohort of PLWH from an integrated correctional system that incorporates both jail and prison data. Here, less than half of deaths were HIV/AIDS-related; however, like in non-incarcerated PLWH, a substantial proportion were due to drug overdose and accidents, liver, and cardiovascular disease.15 Consistent with findings from prisoners, including those without HIV, deaths due to drug overdose and accidents occurred sooner than deaths due to HIV/AIDS; liver and cardiovascular disease occurred later.8,12,22 Coercive mobility between prisons and communities elevates the risk of death in PLWH for a number of conditions, including HIV, cardiovascular and liver disease, and substance use disorders (SUDs).
Late HIV diagnosis predisposes PLWH to increased morbidity and mortality.23 Here, PLWH had been diagnosed for a median of 11·4 years, yet low CD4 count at diagnosis still significantly predicted mortality, perhaps related to discontinuous ART resulting in CD4 returning to its nadir. Similarly, advanced HIV disease during follow-up and medical co-morbidities also predicted post-release mortality, indicating an unmet need for medical care. Despite high levels of ART provision within CTDOC facilities, which previously has been shown to improve survival during incarceration and after release,5,7 37·8% of PLWH were not adequately monitored nor virally suppressed after release. Viral suppression is more than a measure of ART adherence and effectiveness; it reflects broader engagement in systems of care and support. These findings highlight the urgent need not only for better HIV care continuity,5 but also improved access to treatment for SUDs, including alcohol and opioid dependence,24 and viral hepatitis12 both during and after incarceration.
Health insurance, including coverage of ART medication costs, is associated with better healthcare utilization, viral suppression, and lower mortality.25,26 Many states like Connecticut have recently expanded Medicaid programs, which include efforts to enroll patients from the CJ system and to suspend (rather than terminate) Medicaid benefits during incarceration. Nevertheless, at-risk, low-income PLWH and those with other comorbidities continue to face barriers to gaining adequate health insurance. In this cohort, PLWH who had health insurance after release had longer survival times. Yet this setting likely represents the most optimistic scenario where health insurance coverage was high. It is therefore likely that, in the absence of healthcare insurance expansion, post-release mortality rates would be markedly higher.
At least 70% of this cohort were PWID, which is associated with risk of death from drug overdose, poor retention in HIV care, and all-cause mortality.5 Longer incarceration increases the likelihood of achieving viral suppression,27 and may also provide sufficient time for initiating treatment for opioid use disorder. Despite evidence that pharmacological treatment for opioid use disorder initiated during incarceration and continued post-release reduces substance use, improves HIV treatment outcomes, and reduces mortality after release,24,28 implementation of such strategies globally and in the US are uncommon, including in this study setting. Longer incarceration periods, however, allow sufficient time to provide TCM and address post-release barriers to care like re-activating health insurance and linking to community-based resources including addiction treatment programs. Although recidivism is medically and socially destabilizing, people who are frequently re-incarcerated, especially PWID, may be prioritized for transitional care coordination. Consequently, while PWID had reduced survival, higher re-incarceration rates mitigated this effect. The nation’s volatile opioid epidemic and related policies that concentrate PWID in CJ systems and contribute to their poor health outcomes29 speak to the need to adequately provide opioid agonist treatments (OAT) in both CJ and community-based healthcare systems.30 OAT reduces drug overdose in both community and CJ settings. Yet, where OAT coverage is absent or under-scaled, drug-related death after release will remain unabated, including in PLWH. Other post-release strategies to reduce the negative consequences of drug use include providing naloxone for overdose prevention, sterile injecting equipment, and screening and treatment for psychiatric and SUDs.
Our finding that Black race was protective against time-to-death among PLWH echo findings in sentenced prisoners elsewhere.10 Potential explanations for this finding include the disparately protective effect of longer incarcerations, though time spent reincarcerated during follow-up did not attenuate the protective effect of Black race. Alternatively, Black individuals during this time period were at reduced risk for opioid-related overdoses, or more likely, show greater resilience in the post-release period that could provide some protection against mortality.31
Several enabling resources and need factors contributed to mortality. Most importantly, TCM, which has been associated with improved linkage to and retention in care in released prisoners with HIV,16,32 also reduced mortality. While only 25·2% received TCM services during their index incarceration, the interaction with re-incarceration and PWID suggests a channeling of services by targeting the highest risk individuals for TCM. Though not measured here, receipt of TCM, especially for re-incarcerated PWID, may result in higher levels of entry into addiction treatment, including OAT, after release. For PLWH not receiving TCM, frequent re-incarceration was extremely detrimental, yet TCM was protective for those PLWH who were frequently re-incarcerated. Overall, however, PLWH receiving high-frequency TCM services had reduced survival, potentially due to extreme unmet needs in the community for which TCM services were insufficient to reduce mortality risk. Thus, TCM appears to be directed more towards those with highest need (e.g., PWID, recidivists), but may also be a marker of excessive community-based needs that are challenging to overcome (e.g., housing).
Despite the many important findings, we were unable to fully address changes in some variables over time, either because dynamic data were not available (e.g., housing, PWID status, post-release treatment for psychiatric and SUDs) or because an alternative statistical approach was employed. We relied on death certificates for causes of death, which may have contributed to some misclassification. Findings are from an integrated system that includes both prisons and jails in a single state, so may not be generalizable to all systems, though likely represents a best-case scenario within a resource-rich environment. As with any observational study, unmeasured secular trends in substance use, ART, or incarceration policies may have contributed to mortality. Though compassionate release for “terminal illness” was uncommon during this period, some who may have otherwise died in prison did so in the community following release. This limitation, however, may be mitigated by the fact that deaths related to malignancy and organ failure occurred rarely within 6 months post-release, perhaps because people died sooner from other causes or because they received preventative care during incarceration. We included people released at least once, which potentially biases findings towards people with shorter incarceration periods, though we included all people admitted and released to the CTDOC over eight years of observation, and the majority (69%) of deaths were among people with an index incarceration lasting >30 days. Strengths of this study, however, are the inclusion of both jail detainees and sentenced prisoners, the large sample of PLWH, the successful linkage of which allowed for robust characterizization of post-release experiences for both recidivists and non-recidivists over eight years of observation.
In resource-rich settings, incarcerated PLWH are extremely vulnerable to death after release. Furthermore, the majority of deaths are due to treatable conditions including HIV/AIDS, SUDs, and liver disease. The CJ system can, however, assist some at-risk PLWH temporarily interface with needed medical services, but longitudinal engagement in community-based care is key to reducing mortality. Beyond reducing incarcerations, PLWH need improved access to within-prison treatment for HIV, SUDs, viral hepatitis, and other co-morbidities, with services continued without interruption after release. Improved access to health insurance and case management are two crucial resources that can support the provision of these services in the community.
Supplementary Material
Research in Context
Evidence before this study
We searched PubMed using the following search terms: 1) “prison”, “jail”, or “***incarcerat* ”; 2) “HIV” or “AIDS”; 3) “mortality” or “death,” resulting in 87 matches. We reviewed resulting abstracts and full text to apply the following inclusion criteria: original research articles; published between March 1, 2008 and March 1, 2018; evaluated mortality following prison- or jail-release; and either measured HIV-related deaths, accounted for HIV status, or exclusively sampled people living with HIV (PLWH). Eight published studies met inclusion criteria, and all but one cross-sectional survey used retrospective cohort designs.
In a cross-sectional survey of 102 male prisoners with HIV in Indonesia followed for 24 months after release, the crude mortality ratio was 215·7 per 1,000 person-years and HIV was the most common cause of death. Survival time was inversely correlated with incarceration for a drug-related offense, longer incarcerations, and advanced HIV, whereas addiction treatment was associated with longer survival. Similarly, in a retrospective cohort study of 147 prisoners with HIV released to the community in French Guiana (2007-2013), the standardized mortality ratio (SMR) was 14·8. Age and advanced HIV were associated with death in multivariate models, though just 50% of the sample received antiretrovirals (ART) prior to release.
In the United States, one study from New York City, two from North Carolina, and three from Georgia each linked prison or jail data with death records to assess all-cause mortality, regardless of HIV status. Consistently, across study sites, time periods, and assessments, post-release mortality was high and SMRs were elevated compared to the general population. SMRs were higher for White, compared to Black, former prisoners. Primary causes of death were HIV, homicides, accidents, substance use,HIV, and liver-related disease, accounting for up to 62% of the excess mortality following release in one study. Though some found an elevated risk of cancer-related deaths, a retrospective cohort study from Georgia used matched cancer and death registry data to show that there was no elevated risk of cancer mortality compared to the general population, though PLWH had higher cancer incidence mostly from viral-associated non-AIDS defining cancers. Cumulatively, these studies suggest high risk of death following release from prison overall, but there is limited information specific to PLWH to identify modifiable factors that could inform the development of interventions and policy.
Added value of this study
To our knowledge, this is one of the first studies in a resource-rich setting where ART and insurance coverage is high to describe post-release mortality among PLWH. By merging data from custodial, pharmacy, HIV surveillance, case management, and death indices over 8 years of observation for all PLWH incarcerated within an integrated correctional system, we were able to fully appreciate the impact of incarceration (and reincarceration) on mortality.
Implications of all the available evidence
PLWH transitioning from prisons to communities experience excess risk of death from all-causes compared to the general population, with death primarily due to HIV, drug overdose, liver-related or cardiovascular disease, and accidental injury or suicide. To address this critical health disparity, PLWH require access to health insurance, and early identification and treatment of HIV with ART and substance use disorders with medication assisted therapies, during incarceration and following return to communities.
Acknowledgements
The National Institute on Drug Abuse (NIDA) provided funding for this project F30DA041247 (Loeliger) and career development support K24DA017072 (Altice) and K23DA033858 (Meyer). The project was also supported by the Yale University Medical Scientist Training Program under the National Institute of General Medical Sciences (NIGMS) T32GM007205 and the Yale Center for Interdisciplinary Research in AIDS (CIRA) under the National Institute of Mental Health (NIMH) P30MH062294. Funding sources played no role in data analysis or interpretation or the decision to submit the manuscript for publication. This research was conducted in collaboration with the Connecticut Departments of Correction and Public Health. We thank Kathleen Maurer, Patrick Hynes, Cheryl Cepelak, and Heidi Jenkins for assisting with study design, guiding the interpretation of our findings, and fostering the inter-institutional collaborations that made this study possible. We also thank Kirsten Shea, Suzanne Speers, Michael Ostapoff, and Melanie Alvarez for their invaluable assistance with data collection, extraction, and linkage; no compensation was received for these contributions. Finally, we sincerely thank Paula Dellamura for her crucial administrative support.
Funding: National Institutes of Health.
Footnotes
Declaration of interests
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Kelsey B. Loeliger, Yale School of Medicine, Section of Infectious Diseases, AIDS Program.
Frederick L. Altice, Yale School of Medicine, Section of Infectious Diseases, AIDS Program; Yale School of Public Health, Department of Epidemiology of Microbial Diseases; University of Malaya, Centre of Excellence in Research in AIDS.
Maria M. Ciarleglio, Yale School of Public Health, Department of Biostatistics.
Katherine M. Rich, Yale School of Medicine, Section of Infectious Diseases, AIDS Program.
Divya K. Chandra, Yale School of Medicine, Section of Infectious Diseases, AIDS Program.
Colleen Gallagher, Connecticut Department of Correction, Health and Addiction Services Quality Improvement Program.
Mayur M. Desai, Yale School of Public Health, Department of Chronic Disease Epidemiology.
Jaimie P. Meyer, Yale School of Medicine, Section of Infectious Diseases, AIDS Program.
References
- 1.Glaze LE, Kaeble D. Correctional Populations in the United States, 2013. Bureau of Justice Statistics: U.S. Department of Justice - Office of Justice Programs; 2014. p. Available from: https://www.bjs.gov/content/pub/pdf/cpus13.pdf
- 2.Carson AE. Prisoners in 2015. U.S. Department of Justice, Bureau of Justice Statistics: Bureau of Justice Statistics, National Prisoner Statistics, 2005. –2015, 2016. [Google Scholar]
- 3.Maruschak LM. HIV in prisons, 2001-2010. Washington, D.C.: Bureau of Justice Statistics, U.S. Department of Justice, 2012. [Google Scholar]
- 4.Bonczar TP, Hughes TA, Wilson DJ, Ditton PM, Statistics BoJ. National Corrections Reporting Program, 2009 - Statistical Tables, 2011. [Google Scholar]
- 5.Clements-Nolle K, Marx R, Pendo M, Loughran E, Estes M, Katz M. Highly active antiretroviral therapy use and HIV transmission risk behaviors among individuals who are HIV infected and were recently released from jail. American journal of public health 2008; 98(4): 661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baillargeon J, Giordano TP, Rich JD, et al. Accessing antiretroviral therapy following release from prison. Jama 2009; 301(8): 848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baillargeon JG, Giordano TP, Harzke AJ, Baillargeon G, Rich JD, Paar DP. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public health reports 2010; 125 Suppl 1: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med 2013; 159(9): 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moller LF, Matic S, van den Bergh BJ, Moloney K, Hayton P, Gatherer A. Acute drug-related mortality of people recently released from prisons. Public health 2010; 124(11): 637–9. [DOI] [PubMed] [Google Scholar]
- 10.Rosen DL, Schoenbach VJ, Wohl DA. All-cause and cause-specific mortality among men released from state prison, 1980–2005. American journal of public health 2008; 98(12): 2278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlodre J, Fazel S. All-cause and external mortality in released prisoners: systematic review and meta-analysis. Am J Public Health 2012; 102(12): e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaulding AC, Sharma A, Messina LC, Zlotorzynska M, Miller L, Binswanger IA. A comparison of liver disease mortality with HIV and overdose mortality among Georgia prisoners and releasees: a 2-decade cohort study of prisoners incarcerated in 1991. Am J Public Health 2015; 105(5): e51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber F, Merceron A, Madec Y, et al. High mortality among male HIV-infected patients after prison release: ART is not enough after incarceration with HIV. PLoS One 2017; 12(4): e0175740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culbert GJ CF, Murni A, Waluyo A, Bazazi AR, Sahar J, Altice FL. Predictors of mortality within prison and after release among persons living with HIV in Indonesia. Research and reports in tropical medicine 2017; 8: 25—35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adih WK, Selik RM, Hall HI, Babu AS, Song R. Associations and Trends in Cause-Specific Rates of Death Among Persons Reported with HIV Infection, 23 U.S. Jurisdictions, Through 2011. Open AIDS J 2016; 10: 144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeliger KB, Altice FL, Desai MM, Ciarleglio MM, Gallagher C, Meyer JP. Predictors of linkage to HIV care and viral suppression after release from jails and prisons: a retrospective cohort study. Lancet HIV 2018; 5(2): e96–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Wide-ranging online data for epidemiologic research (WONDER). 2018. [Google Scholar]
- 18.Gelberg L, Andersen RM, Leake BD. The Behavioral Model for Vulnerable Populations: application to medical care use and outcomes for homeless people. Health services research 2000; 34(6): 1273–302. [PMC free article] [PubMed] [Google Scholar]
- 19.Health Resources & Services Administration (HRSA). HIV/AIDS Bureau Performance Measures. Available from: https://hab.hrsa.gov/sites/default/files/hab/About/clinical-quality-management/coremeasures.pdf, 2015.
- 20.Office of Policy and Management - Criminal Justice Policy & Planning Division. Recidivism in CT, 2008 releases, 2015. [Google Scholar]
- 21.Binswanger IA. Release from prison - A high risk of death for former inmates (vol 356, pg 157, 2007). New England Journal of Medicine 2007; 356(5): 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrall EL, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction 2010; 105(9): 1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waters L, Sabin CA. Late HIV presentation: epidemiology, clinical implications and management. Expert Rev Anti Infect Ther 2011; 9(10): 877–89. [DOI] [PubMed] [Google Scholar]
- 24.Springer SA, Di Paola A, Azar M, et al. Extended-Release Naltrexone Improves Viral Suppression among Incarcerated Persons Living with HIV with Opioid Use Disorders Transitioning to the Community: Results of a Double-Blind, Placebo-Controlled Randomized Trial. J Acquir Immune Defic Syndr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabs AW, Jabs DA, Van Natta ML, Palella FJ, Meinert CL. Insurance status and mortality among patients with AIDS. HIV Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludema C, Cole SR, Eron JJ, Jr., et al. Impact of Health Insurance, ADAP, and Income on HIV Viral Suppression Among US Women in the Women’s Interagency HIV Study, 2006–2009. J Acquir Immune Defic Syndr 2016; 73(3): 307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer JP, Cepeda J, Wu J, Trestman RL, Altice FL, Springer SA. Optimization of human immunodeficiency virus treatment during incarceration: viral suppression at the prison gate. JAMA Intern Med 2014; 174(5): 721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brinkley-Rubinstein L, McKenzie M, Macmadu A, et al. A randomized, open label trial of methadone continuation versus forced withdrawal in a combined US prison and jail: Findings at 12 months post-release. Drug Alcohol Depend 2018; 184: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csete J, Kamarulzaman A, Kazatchkine M, et al. Public health and international drug policy. Lancet 2016; 387(10026): 1427–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med 2014; 370(22): 2063–6. [DOI] [PubMed] [Google Scholar]
- 31.Teti M, Martin AE, Ranade R, et al. “I’m a keep rising. I’m a keep going forward, regardless”: exploring Black men’s resilience amid sociostructural challenges and stressors. Qual Health Res 2012; 22(4): 524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeliger K, Altice F, Desai M, Ciarleglio M, Gallagher C, Meyer J. Late breaker: Predictors of Linkage to and Retention in HIV Care Following Release from Connecticut, USA Jails and Prisons. International AIDS Society (IAS) Meeting Paris, France; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.