Abstract
Purpose:
While imaging matrix-associated stem cell transplants aimed for cartilage repair in a rodent arthritis model, we noticed that some transplants formed locally destructive tumors. The purpose of this study was to determine the cause for this tumor formation in order to avoid this complication for future transplants.
Procedures:
Adipose-derived stem cells (ADSC) isolated from subcutaneous adipose tissue were implanted into 24 osteochondral defects of the distal femur in ten athymic rats and two immunocompetent control rats. All transplants underwent serial magnetic resonance imaging (MRI) up to 6 weeks post-transplantation to monitor joint defect repair. Nine transplants showed an increasing size over time that caused local bone destruction (group 1), while 11 transplants in athymic rats (group 2) and 4 transplants in immunocompetent rats did not. We compared the ADSC implant size and growth rate on MR images, macroscopic features, histopathologic features, surface markers, and karyotypes of these presumed neoplastic transplants with nonneoplastic ADSC transplants.
Results:
Implants in group 1 showed a significantly increased two-dimensional area at week 2 (p = 0.0092), 4 (p = 0.003), and 6 (p = 0.0205) compared to week 0, as determined by MRI. Histopathological correlations confirmed neoplastic features in group 1 with significantly increased size, cellularity, mitoses, and cytological atypia compared to group 2. Six transplants in group 1 were identified as malignant chondrosarcomas and three transplants as fibromyxoid sarcomas. Transplants in group 2 and immunocompetent controls exhibited normal cartilage features. Both groups showed a normal ADSC phenotype; however, neoplastic ADSC demonstrated a mixed population of diploid and tetraploid cells without genetic imbalance.
Conclusions:
ADSC transplants can form tumors in vivo. Preventive actions to avoid in vivo tumor formations may include karyotyping of culture-expanded ADSC before transplantation. In addition, serial imaging of ADSC transplants in vivo may enable early detection of abnormally proliferating cell transplants.
Keywords: Magnetic resonance imaging, Stem cell therapy, Chondrosarcomas, Fibromyxoid sarcomas, Osteochondral transplants, Malignant tumors in vivo
Introduction
The field of regenerative medicine is rapidly expanding, with currently over 4000 ongoing clinical stem cell trials for the treatment of over 1400 medical conditions [1]. Adipose-derived stem cells (ADSC) have been recently introduced as a new source for bone and cartilage regeneration in patients with sports injuries or degenerative arthritis [2–4]. Subcutaneous fat depots, the source for these cells, are abundant and easily accessible, thereby providing a potentially unlimited reservoir for retrieval of adult stem cells [5]. By comparison, bone marrow-derived mesenchymal stem cells (MSC) have to be harvested by invasive bone marrow aspiration and may provide a lower yield of stem cells per harvest [6]. Although advantages and disadvantages of both cell types are discussed controversially [7, 8], several studies have shown that ADSC in growth factor-enriched scaffold could provide similar or better chondrogenic and osteogenic differentiation outcomes when compared to MSC [6–9]. Thus, ADSC have become an attractive source for matrix-associated stem cell implants in arthritic joints, with the aim to regenerate bone and cartilage defects [2, 10–12].
While studying matrix-associated stem cell implants in rodents with magnetic resonance imaging (MRI), we noticed that some of our ADSC transplants formed locally destructive tumors. Tumor formations of pluripotent stem cells, such as embryonic stem cells and induced pluripotent stem cells have been reported previously in both rodent models and humans [13–15]. Other investigators reported bone marrow MSC/ADSC transforming into carcinoma-associated fibroblasts when cross-contaminated with or co-injected with tumor cells/growth factors [16–18]. To the best of our knowledge, tumor formations after in vivo transplantation of transformed adult ADSCs have not been reported so far. To evaluate the cause of the observed tumorigenesis, we compared the imaging characteristics, macroscopic and histopathologic features, phenotypes and karyotypes of ADSC transplants that led to tumor formation with non-neoplastic ADSC transplants that resulted in cartilage defect regeneration.
Materials and Methods
Animal Model and ADSC Implantation
The study was approved by our institutional animal care and use committee. Studies were performed in 12 6–8-week-old male Sprague Dawley rats, including 10 athymic rats, and 2 immunocompetent controls. Athymic rats were chosen to avoid immune rejection of allogeneic transplants and to enable comparisons with prospective human stem cell implants. ADSC were extracted from a donor rat using established procedures [5, 19]. ADSC were then expanded in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), and 100 I.U./ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37 °C in a humidified 5 % CO2 atmosphere. At 80–90 % confluency, the ADSC were trypsinized, the viability was calculated with a trypan blue test, and either cultured further or used for experiments.
Approximately 7.5 × 105 ADSC in agarose scaffold were implanted into osteochondral defects of the bilateral distal femurs of 12 6–8-week-old male Sprague Dawley rats. Surgeries were performed under sterile conditions and isoflurane anesthesia by an experienced animal surgeon: a circular osteochondral defect (2 mm diameter, and 1.5 mm depth) was created in the inter-trochlear groove of the femur using a micro-drill (Ideal, Sycamore, IL), and ADSC implants were introduced into the defects. The implant location and consistency was confirmed visually and by gentle palpation with forceps, and the skin incision was closed with Dermalon 6–0 monofilament sutures. Potential post-surgical pain was controlled by subcutaneous administration of buprenorphine (0.05 mg/kg).
MRI of ADSC Transplants
All rats underwent MRI on a 7T animal MR scanner (General Electric-Varian “microSigna 7.” collaboration). These scans were obtained directly after ADSC transplantation as well as at 2, 4, and 6 weeks post-transplantation. Animals were anesthetized with 1.5–2 % isoflurane and placed supine with knee in an extended position. A custom-built single-channel transmit/receive partial birdcage radio-frequency coil with an inner diameter of 4 cm was placed around the animal’s knee for imaging. Sagittal MRI images of both knee joints were obtained with fast spin-echo (FSE) sequences with a repetition time of 3000 ms, echo time of 30 ms, field-of-view of 2.5 × 2.5 cm, a matrix of 256 × 256 pixels, a slice thickness of 0.5 mm, and 16 acquisitions.
The two-dimensional area of the ADSC transplants on the sagittal imaging plane that covered the largest dimension of the transplant was measured as length × width on serial MRI images using a DICOM image processing software (Osirix, Pixmeo, Geneva, Switzerland). The average growth rate was determined by dividing the difference in area of the transplants over 6 weeks by the number of weeks: (Area (week 6) – area (week 0))/6 = growth rate (cm2/week).
Histopathology
Animals were sacrificed after the last MRI procedure, knee joints were explanted, and macroscopic specimen were evaluated for signs of invasive tumor growth, as evidenced by extension of the transplant beyond the normal joint surface and macroscopic invasion of adjacent bone or joint components. Joint specimens were then dissected and placed in Cal-ex II (Fisher Scientific) for 5–8 days to fix and decalcify the tissue simultaneously. The specimens were dissected para-sagittally, dehydrated through graded alcohol washes, and embedded in paraffin. Five-micrometer thick tissue slices were stained with hematoxylin and eosin (H&E) for microscopic evaluation of the ADSC transplants. Two experienced pathologists (KH, DG) determined the histopathologic size, nuclear:cytoplasm ratio, the number of mitoses, and presence or absence of microscopic invasion of adjacent bone or soft tissues. Based on the criteria above and additional clinical standard criteria for the classification of bone and soft tissue tumors [20], the pathologists classified the implants as neoplastic or nonneoplastic and within the neoplastic group, further determined the histopathological tumor type.
ADSC Phenotyping
Like bone marrow MSC, ADSC express CD29, CD44, CD71, CD90, CD105/SH2, SH3, and the widely recognized stem cell marker STRO-1. ADSC do not express CD31, CD45, and CD106 [21]. ADSC underwent comprehensive phenotyping before their transplantation by our collaborators from the custom technology team at BD Biosciences (San Diego, CA). Phenotyping analysis included CD44H-FITC, CD31-PE, CD29-A647, and CD45-V450 antibodies, evaluated with the BD FACS Canto II flow cytometer. Details of these reagents are listed in Table 1. Compensation was set up using BD Comp Beads. Data were quantified using BD FACS Diva software (BD Biosciences, San Jose, CA, USA) with gate settings for the major population of FSC (cell size)/SSC (granularity) plot. The gated cell types were visualized on 2-parameter dot plots and the percentages of specific populations were calculated.
Table 1.
Antibodies used for ADSC phenotyping analysis
| Specificity | Clone | Isotype | Format | |
|---|---|---|---|---|
| 1 | CD44H | OX-49 | Ms IgG2a, κ | FITC |
| 2 | CD31 | TLD-3A12 | Ms IgG1, κ | PE |
| 3 | CD29 | Ha2/5 | Hamster IgM, κ | A647 |
| 4 | CD45 | OX-1 | Ms IgG1, κ | V450 |
Chromosome Analysis
ADSC used for the implants were retrospectively evaluated by the Cytogenetics facility at Stanford. The ADSC cell line was harvested by standard cytogenetic methodology of mitotic arrest, hypotonic shock, and fixation with 3:1 methanol-acetic acid. Chromosome slide preparations were stained by G-banding and classified by the standard Norway rat karyotype [22].
Data Analyses
Based on visual assessment of MRI images by two independent observers (AK, FC), ADSC transplants were divided into two groups: group 1 (presumed neoplastic) implants, that markedly increased in size over time, caused local bone destruction and extension into the joint and group 2 (presumed non-neoplastic) implants that did not or only minimally (not more than 1 mm) increased in size over time and did not show any imaging signs of local bone destruction. The two-dimensional area of all transplants in the sagittal plane was measured on serial MRI images at week 0, 2, 4, and 6. Results were compared between group 1 and 2, using a t test with unequal variances. Similarly, the number of cells, mitoses and cell atypias per high power field (hpf) were compared for significant difference between both groups using a t test. A p value of less than 0.05 was considered significant.
Results
MRI Features of Neoplastic and Non-Neoplastic ADSC
In athymic rats, 9 cell transplants increased in size in the implant area (group 1) and 11 did not (group 2). In immune-competent rats, no cell transplant increased in size in vivo. Implants in group 1 showed a significantly increased area in MRI images at week 2 (p = 0.0092), 4 (p = 0.003), and 6 (p = 0.0205) compared to week 0. In addition, implants in group 1 demonstrated signs of local bone destruction at week 4 and 6 as well as signs of joint invasion beyond the transplant site at week 6. Conversely, implants in group 2 showed no significant changes in size of the implant at week 2, 4, and 6 (p > 0.05) and no signs of bone or joint destruction at any time (Fig. 1). The mean growth rate of neoplastic ADSC transplants (group 1) was 0.061 ± 0.0716 cm2/week (range 0.0059–0.2137) as compared to0.0019 ± 0.002 cm2/week (range 0.0004–0.0027) for nonneoplastic transplants (group 2). These data were significantly different (p = 0.0147). Four control transplants in two immuno-competent recipients showed no increase in the implant area over 4 weeks and no signs of bone or joint destruction at any time (see Supplemental Fig. 1a–b in Electronic Supplementary Material (ESM)).
Fig. 1.
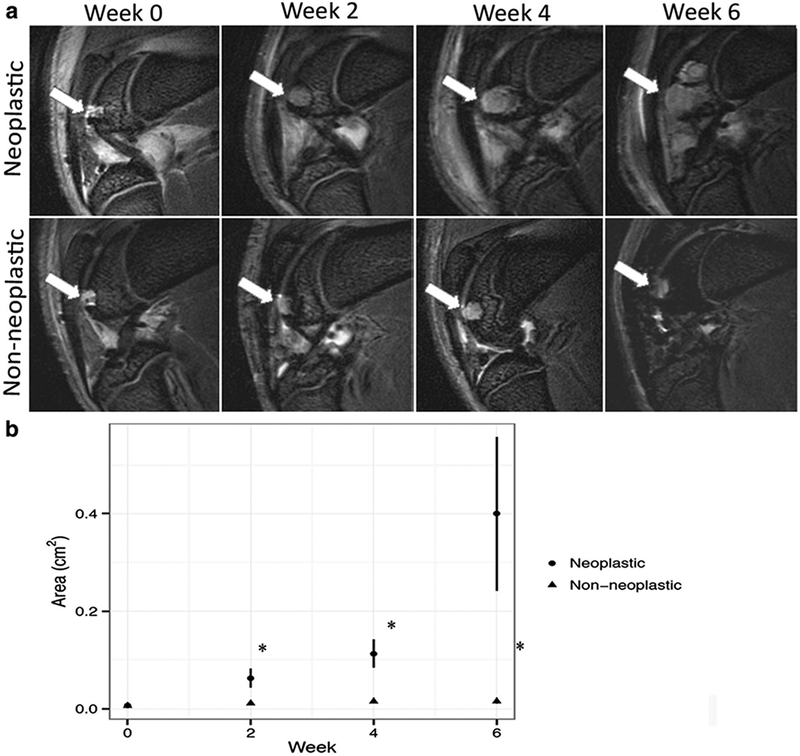
Magnetic resonance findings for neoplastic and non-neoplastic transplants. a Sagittal T2-weighted FSE images (TR/TE = 3000 ms/30 ms) of ADSC transplants in cartilage defects of distal femurs (arrows): neoplastic ADSC implants show significant increase in size over 6 weeks, whereas non-neoplastic ADSC implants did not expand. b Corresponding size of neoplastic and non-neoplastic ADSC transplants at different time points after implantation, displayed as mean transplant area with standard errors. A single asterisk indicates significant differences between neoplastic and non-neoplastic ADSC transplants (p < 0.05).
Histopathologic Features of Neoplastic and Non-Neoplastic ADSC
Gross examination of the knee joints of animals in group 1 revealed palpable masses in the distal femur, which were large enough to impair the mobility of the knee joint. Postmortem macroscopic evaluation revealed highly vascular, expansive and locally destructive masses of the distal femur with multiple sites of necrosis, including the transplant site and extending into adjacent bone and joint components (Fig. 2a). Of the nine neoplastic transplants, four occurred in the bilateral knees of the same rats and five others occurred unilaterally in one knee, while the other knee demonstrated normal transplants. The animals were therefore not responsible for providing a conductive environment for tumor growth; otherwise, tumor formation would always have occurred in both knees of affected animals.
Fig. 2.
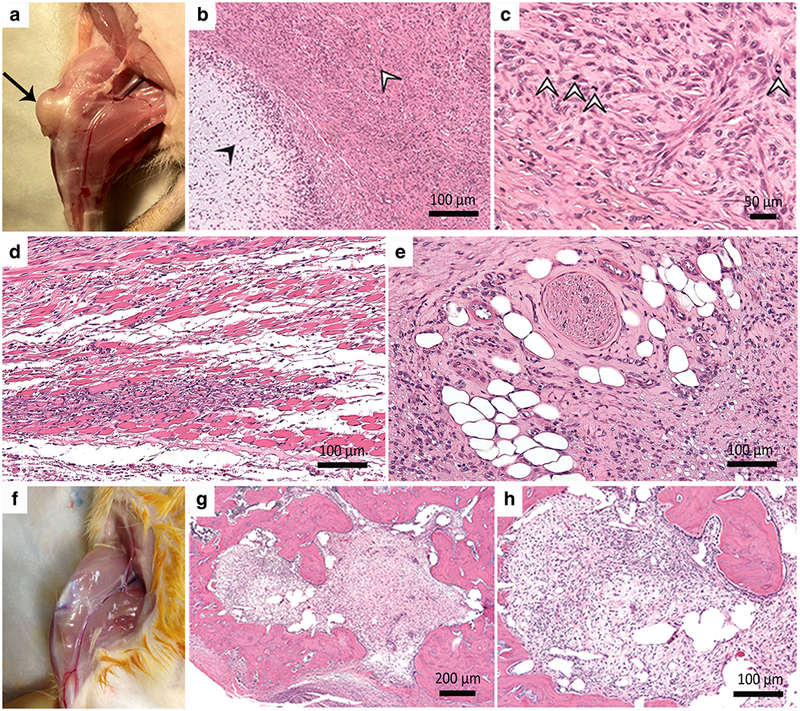
Macroscopic and microscopic features of neoplastic and non-neoplastic ADSC transplants. a Neoplastic ADSC transplants present as an expansile and locally destructive mass (arrow). b Histopathological features of the malignant chondrosarcomas at × 100 and c × 400 include myxoid production (closed arrowhead) and hyaline cartilage (open arrowheads). d The malignant cells infiltrate adjacent skeletal muscle (× 200), and e adipose tissue with encroachment upon a nerve (× 200). f Non-neoplastic ADSC transplants do not extend beyond the transplant site. H&E stains of non-neoplastic transplants at g × 40 and h × 400 exhibit a well-defined cell implant with chondrogenic cells surrounded by unremarkable native bone.
Histopathologic examination of the transplants in group 1 revealed malignant chondrosarcomas in six cases and fibromyxoid sarcomas in three cases. The chondrosarcomas were characterized by mixtures of myxoid and hyaline cartilage, foci of extra-osseous soft tissue invasion, and encroachment upon peripheral nerve fibers (Fig. 2b, c, d, e). Fibromyxoid sarcomas presented with overgrowth of loose connective tissue within the area of the previous implant with typical herringbone patterns and cell pleomorphism, which extended into the marrow space but did not infiltrate the cortical bone. The sarcomas demonstrated a wide range of histological signs of tumor proliferation and atypia. Chondrosarcomas exhibited high cell density, cytologic atypia, and an average of 15.33 ± 5.4 mitoses per hpf, while fibrosarcomas exhibited intermediate cell density, cytological atypia, and an average of 1.67 ± 2.9 mitoses per hpf (Fig. 3).
Fig. 3.
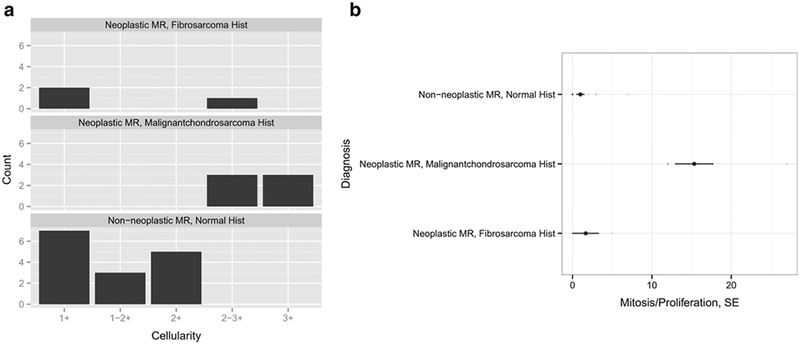
Histopathological classification of neoplastic and non-neoplastic transplants and MRI correlation. Normal and malignant ADSC implants were quantitatively compared based on a cellularity (1+ = paucicellular, 2+ = moderately cellular, 3+ = highly cellular) and b the proliferation rate per high power field, hpf (× 400 magnification). Malignant transplants were defined based on high cellularity and high mitosis rate. Non-neoplastic transplants exhibited low mitosis rates that were the result of cartilaginous proliferation.
Stem cell transplants in the non-neoplastic group (group2) did not show macroscopic signs of tumor formation and no evidence for abnormal tissue proliferation outside the transplant site (Fig. 2f). Contrary to group 1, all transplants in group 2 exhibited low cell density (paucicellular), no or minimal cell atypia, and 1.11 ± 2.02 mitoses per hpf. (Figs. 2g, h, and 3). These criteria were significantly different between groups 1 and 2 (p = 0.0008). Neoplastic transplants formed highly proliferative tumors while cells in non-neoplastic transplants apparently stopped proliferating and differentiated into cartilage to repair the osteochondral defect (Fig. 2g, h) [23].
Gross examination and macroscopic evaluation of the knee joints of immunocompetent control animals showed no evidence for mass effect (see Supplemental Fig. 1b in ESM). Immunocompetent transplants demonstrated low cell density, no cell atypia, and 0 mitoses per hpf (see Supplemental Fig.1 c and d in ESM).
ADSC Phenotyping
FACS analysis of ADSC before implantation into animals and FACS analyses of neoplastic and non-neoplastic ADSC showed that all samples were positive for CD29 and CD44H markers and negative for CD31, which is consistent with the phenotype of ADSC [19, 21, 24] (Tables 1 and 2, Fig. 4). These findings confirm that evaluation of phenotypic markers alone is not sufficient to assess the potential of cellular tumorigenicity before or after stem cell implantation. Of note, non-neoplastic ADSC contained a small population of CD45+ cells, which was not noted in neoplastic cell samples. These cells most likely represented contaminating leukocytes.
Table 2.
Surface markers of normal and neoplastic ADSC
| % Expression | Non-neoplastic ADSC | Neoplastic ADSC |
|---|---|---|
| CD29+ CD44H+ | 96.93 | 99.92 |
| CD45+ CD29+ | 13.12 | 0.08 |
| CD45− CD29+ | 86.88 | 99.92 |
| CD31+ CD29+ | 1.6 | 0.31 |
Fig. 4.
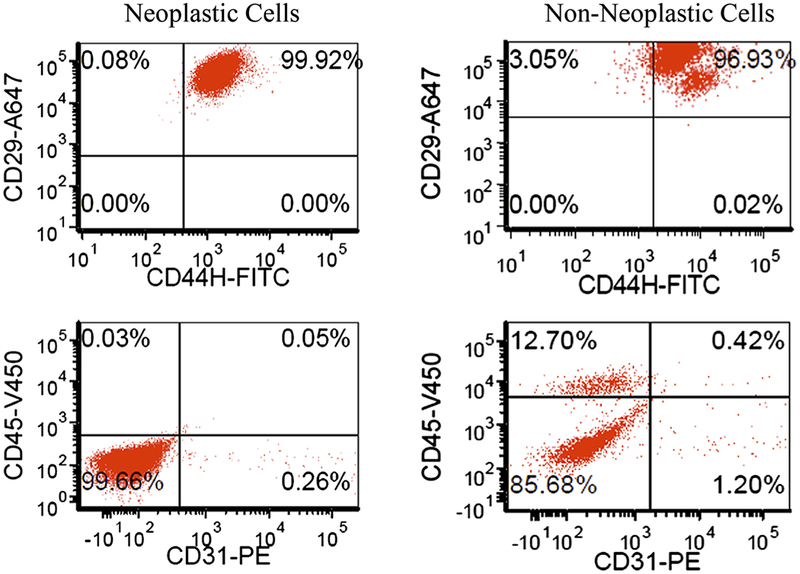
Neoplastic and non-neoplastic cells’ CD marker expression. Dual axis dot plots show similar expression for neoplastic and non-neoplastic transplants: CD29 and CD44H positive and CD31 negative. Phenotypic markers are not sufficient to discriminate the presence of neoplastic cells in a future transplant.
Chromosome Analysis
Chromosome analysis of tumor-forming ADSC demonstrated a mixed population of male diploid (42, XY) and tetraploid (84, XXYY) cells without obvious structural rearrangement or genetic imbalance (Fig. 5). By contrast, non-neoplastic ADSC demonstrated a normal male diploid karyotype only. These findings suggest that normal ADSC spontaneously transformed into neoplastic ADSC, which are easily distinguishable by aberrant chromosomal numbers.
Fig. 5.
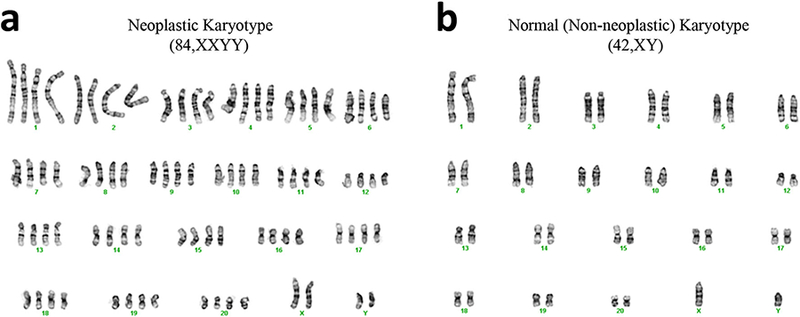
Chromosome analysis of a neoplastic and b non-neoplastic ADSC. Neoplastic cells show a mixed population of normal male diploid (42, XY) and aberrant tetraploid (84, XXYY) cells without obvious structural rearrangement or genetic imbalance while non-neoplastic ADSC show a normal karyotype.
Discussion
Our data showed neoplastic transformation of ADSC transplants in a preclinical model of arthritic joints of recipient rats. Nine out of 24 ADSC transplants developed into invasive tumors. Based on our karyotyping data, the most likely cause for neoplastic transformation of our ADSC is a genetic transformation during cell expansion in cell culture. This is in accordance with other reports of genetic or phenotypic transformation of ADSC [25] and bone marrow-derived MSC [26–30] during ex vivo expansion, possibly due to augmented chromosome instability associated with dysregulation of telomere activity and cell cycle-related genes [27–29, 31]. There was a trend that later passage MSC induced tumors more frequently compared to earlier passage MSC [27, 28]. In our studies, since the chromosome analyses were performed of cells before implantation and not cells extracted from the tumors, we have evidence that some of the cells in the ex vivo culture transformed into the abnormal tetraploid karyotype. We presume that some of the implants contained these abnormal cells and others did not (or to a much lesser extent). The transformed cells outgrew normal ADSCs in some of the transplants and then were able to develop into fibromyxoid sarcomas and chondrosarcomas.
Yet, other investigators reported that relatively high serum concentrations (10–15 %) in cell culture medium could markedly increase the proliferation rate of MSC, possibly mediated by cytokines and growth factors in serum, such as platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) [32, 33]. Since we also used 10 % serum containing media, this mechanism might have contributed to our results. Conversely, other authors did not observe phenotypic or genotypic alterations of stem cells in long-term culture. Bernardo et al. reported that BM-MSC could be cultured for 44 weeks without losing their phenotypical and functional characteristics [34]. However, it is noteworthy that these MSC did apparently not reach senescence, even after 25 passages.
Suggested preventive actions include the use of serum-free media with added proteins [35, 36], ADSC phenotyping before their transplantation or direct use of harvested ADSC, without any cell expansion [28, 31]. Serum-free media provide less efficient ADSC expansion, thereby counteracting the desired outcome of increasing ADSC quantities. In addition, the effects of various added proteins and growth factors and interactions of scaffolds and growth factors/proteins in scaffold with embedded stem cells are not fully elucidated regarding their impact on ADSC phenotype and karyotype [23, 32, 37–40]. In our case, an effect of the scaffold on neoplastic transformation seems unlikely, since ADSC embedded in the same agarose scaffold either engrafted normally or started to form tumors. Avoiding ex vivo stem cell expansion appears to be the safest approach, and is implemented in several clinical applications of ADSC mediated bone and cartilage repair [41–44]. ADSC expansion in vitro prior to delivery to the patient is nonetheless common in other conditions such as Buerger’s disease [45, 46], where cells are cultured for several weeks. Culture media may include FBS followed by human serum or enriched serum-free media for clinical use or 5 % human serum alone. ADSC cells may be from a single donor [45] for each recipient or multiple donors [46], which may complicate careful analysis of cell products and safety.
Current literature is lacking for in vivo neoplastic differentiation of proven non-neoplastic MSC/ADSC, but Wislet-Gendebien et al. retrospectively noted that one of the clones of the neural crest stem cells derived from bone marrow aspiration resulted in tumorigenesis in vivo after long-term in vitro passaging [47]. This specific clone surprisingly had the highest ability to differentiate into neuronal cells (in vitro), and also showed a very high rate of proliferation after injection into mice striatum, when compared to the other clones. Upon further transcriptomic analyses, this clone highlighted numerous cell cycle checkpoint modifications and chromosome 11q downregulation, which suggested ex vivo neoplastic transformation. This was probably missed due to satisfactory neuronal differentiation, thus highlighting the need for phenotypic, functional, and genetic assays prior to stem cell transplantation. Ning et al. also described an aberrant ex vivo expanded ADSC cell line, very similar to our cell type, which demonstrated an increased proliferation rate, a hypertriploid karyotype and abnormal endothelial cell surface markers (similar to angiosarcomas).
Our data suggests that the compromised immune system of our athymic recipients supported tumor formation, because tumors in immunocompromised hosts showed a higher number of mitoses, etc. than transplants observed in immunocompetent recipients. We hypothesize that an intact immune system may be able to eliminate few transformed cells in vivo. We used athymic rats in order to exclude rejection and enable future extension of our studies to human ADSC. Tumor formation of stem cell transplants in immunodeficient and immunocompetent recipients has been reported previously [27–29, 48, 49]. Miura et al. demonstrated that ex vivo-transformed bone marrow MSC caused malignant fibrosarcomas in vivo [27]. Other authors described no secondary or distant tumors in any organs in nude/athymic mice after intravenous/subcutaneous injection of transformed MSC [25, 26]. Interestingly, Li et al. reported development of fibrosarcomas after subcutaneous implantation of ex vivo-transformed MSC in immunocompetent hosts. This resulted in recruitment of host bone marrow-derived cells and fusion of these cells with transformed MSC, ultimately restoring the non-malignant phenotype. These “Bfused” cells were unable to form secondary tumors in mice and could not be propagated extensively in culture [48].
It has been argued that in vivo tumor formations may be only observed in animal models but not in clinical studies [50, 51]. Rodent cells are known to be prone to spontaneous transformation when expanded in culture under standard conditions [52–54], while dedifferentiation of human stem cells in cell culture has not been described so far. Observations of tumor formations due to stem cell transplants in patients are starting to emerge in clinical trials. Amariglio et al. reported the first case of a multifocal brain tumor in a 13-year-old patient 4 years after neural stem cell transplantation [55]. Secondary tumors can develop after allogenic or autologous hematopoietic stem cell transplantation (SCT) with reported cumulative incidence of solid cancers following allogeneic SCT ranges from 1.2 to 1.6 % at 5 years, from 2.2 to 6.1 % at 10 years, and from 3.8 to14.9 % at 15 years post-transplantation [56]. SCT patients usually undergo an immunosuppressive regimen prior to and post-transplant to reduce graft versus host disease incidence [56–58]. Majority of the secondary tumors are nonsquamous cell carcinomas with radiation as the single most important risk factor [57]. The non-radiation-related secondary tumors are usually squamous cell carcinomas of the skin and mucosa, which are strongly linked to chronic graft versus host disease (GVHD) [58]. Thus, most secondary malignancies demonstrate a multifactorial etiology but a clear and direct relationship to implanted stem cells is yet to be elucidated. There are currently more than 115 ongoing clinical stem cell trials involving ADSC [1, 59–62], but tumor formation of adult ADSC in patients has not been described so far. However, secondary tumor formations may only occur years or decades after the transplantation [55]. Thus, diagnostic tools to minimize this important complication are critically needed.
We recognize several limitations to our study. The correlation of chromosomal analysis with tumor formation was performed retrospectively, limiting our ability to establish causality. Comprehensive analysis of each cell culture batch prior to implantation may have helped to further decipher mechanisms of tumorigenesis. In addition, the two immunocompetent animals have little statistical value but provide incidental evidence that an intact immune system might have protective effects. Nonetheless, we believe that this data may foster further discussion on ex vivo and in vivo factors that can lead to tumor growth of adult cell transplants in immunocompromised versus immunocompetent recipients and drive future research towards better understanding of carcinogenesis of therapeutic cell transplants.
In conclusion, our data showed that adult ADSC in injured joints can form malignant tumors in vivo and MRI is a non-invasive test that can detect tumor formation of stem cell transplants as early as 2 weeks in animal models based on abnormal growth of the transplant. Thus, MRI might be useful to detect abnormal in vivo proliferation of transplanted stem cells. While ADSC show great promise for various tissue regeneration applications, caution must be taken regarding the tumorigenic potential of these cells. A more comprehensive understanding of ADSC biology and ADSC-host interactions is critically needed. A standardized protocol for genotyping of ex vivo expanded ADSC prior to their implantation as well as non-invasive imaging techniques for in vivo monitoring of the transplanted cells will greatly enhance the safety of ADSC-mediated tissue regeneration procedures.
Supplementary Material
Acknowledgments.
The authors would like to acknowledge the imaging support provided by the Stanford Small Animal Imaging Facility (SCI3).
Funding
This work was supported by NIH grant 2R01AR054458 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (PI Daldrup-Link).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11307-018-1218-7) contains supplementary material, which is available to authorized users.
References
- 1.Stem cells entering clinical trials. http://clinicaltrials.gov/ct2/results?term=stem+cells (accessed 05/02/2012)
- 2.Abudusaimi A, Aihemaitijiang Y, Wang YH, Cui L, Maimaitiming S, Abulikemu M (2011) Adipose-derived stem cells enhance bone regeneration in vascular necrosis of the femoral head in the rabbit. J Int Med Res 39:1852–1860 [DOI] [PubMed] [Google Scholar]
- 3.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT (2004) Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22:560–567 [DOI] [PubMed] [Google Scholar]
- 4.Cui L, Liu B, Liu G, Zhang W, Cen L, Sun J, Yin S, Liu W, Cao Y (2007) Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 28:5477–5486 [DOI] [PubMed] [Google Scholar]
- 5.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS (2008) Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17:1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C (2008) Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 17:761–773 [DOI] [PubMed] [Google Scholar]
- 7.Im GI, Shin YW, Lee KB (2005) Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartil 13:845–853 [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Im GI (2009) Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res 27:612–619 [DOI] [PubMed] [Google Scholar]
- 9.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W (2003) Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum 48:418–429 [DOI] [PubMed] [Google Scholar]
- 10.Chun HJ, Kim YS, Kim BK et al. (2011) Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg 78:364–371 [DOI] [PubMed] [Google Scholar]
- 11.Galle J, Bader A, Hepp P, Grill W, Fuchs B, Kas JA, Krinner A, MarquaB B, Muller K, Schiller J, Schulz RM, von Buttlar M, von der Burg E, Zscharnack M, Loffler M (2010) Mesenchymal stem cells in cartilage repair: state of the art and methods to monitor cell growth, differentiation and cartilage regeneration. Curr Med Chem 17:2274–2291 [DOI] [PubMed] [Google Scholar]
- 12.Gao J, Yao JQ, Caplan AI (2007) Stem cells for tissue engineering of articular cartilage. Proc Inst Mech Eng H 221:441–450 [DOI] [PubMed] [Google Scholar]
- 13.Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317 [DOI] [PubMed] [Google Scholar]
- 14.Peng X, Liu T, Wang Y, Yan Q, Jin H, Li L, Qian Q, Wu M (2012) Wnt/beta-catenin signaling in embryonic stem cell converted tumor cells. J Transl Med 10:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih CC, Forman SJ, Chu P, Slovak M (2007) Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev 16:893–902 [DOI] [PubMed] [Google Scholar]
- 16.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4:e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Padura I, Gregato G, Marighetti P, Mancuso P, Calleri A, Corsini C, Pruneri G, Manzotti M, Lohsiriwat V, Rietjens M, Petit JY, Bertolini F (2012) The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res 72:325–334 [DOI] [PubMed] [Google Scholar]
- 18.Garcia S, Bernad A, Martin MC et al. (2010) Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res 316:1648–1650 [DOI] [PubMed] [Google Scholar]
- 19.Ning H, Lin G, Lue TF, Lin CS (2006) Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 74:510–518 [DOI] [PubMed] [Google Scholar]
- 20.Jo VY, Fletcher CD (2014) WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 46:95–104 [DOI] [PubMed] [Google Scholar]
- 21.Zuk PA, Zhu M, Ashjian P, de Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lucca EJ, Dhaliwal MK, Furlong CL, Pathak S (1990) A high-resolution G-banding idiogram of Rattus norvegicus chromosomes. Cytobios 62:153–160 [PubMed] [Google Scholar]
- 23.Khurana A, Nejadnik H, Chapelin F, Lenkov O, Gawande R, Lee S, Gupta SN, Aflakian N, Derugin N, Messing S, Lin G, Lue TF, Pisani L, Daldrup-Link HE (2013) Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 8:1969–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54:132–141 [DOI] [PubMed] [Google Scholar]
- 25.Ning H, Liu G, Lin G, Garcia M, Li LC, Lue TF, Lin CS (2009) Identification of an aberrant cell line among human adipose tissue-derived stem cell isolates. Differentiation 77:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furlani D, Li W, Pittermann E, Klopsch C, Wang L, Knopp A, Jungebluth P, Thedinga E, Havenstein C, Westien I, Ugurlucan M, Li RK, Ma N, Steinhoff G (2009) A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant 18:319–331 [DOI] [PubMed] [Google Scholar]
- 27.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S (2006) Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 24:1095–1103 [DOI] [PubMed] [Google Scholar]
- 28.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PCW, Szuhai K, Oseth LA, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR (2007) Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25:371–379 [DOI] [PubMed] [Google Scholar]
- 29.Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, Huso DL, Semenza GL (2006) Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res 66:10849–10854 [DOI] [PubMed] [Google Scholar]
- 30.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C (2009) Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69:5331–5339 [DOI] [PubMed] [Google Scholar]
- 31.Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS (2011) Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res 108:1340–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapp H, Hanley EN Jr, Patt JC, Gruber HE (2009) Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood) 234:1–9 [DOI] [PubMed] [Google Scholar]
- 33.Suga H, Shigeura T, Matsumoto D, Inoue K, Kato H, Aoi N, Murase S, Sato K, Gonda K, Koshima I, Yoshimura K (2007) Rapid expansion of human adipose-derived stromal cells preserving multipotency. Cytotherapy 9:738–745 [DOI] [PubMed] [Google Scholar]
- 34.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F (2007) Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67:9142–9149 [DOI] [PubMed] [Google Scholar]
- 35.Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R, Vemuri M, Suuronen R, Miettinen S (2009) Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 11:958–972 [DOI] [PubMed] [Google Scholar]
- 36.Vassaux G, Negrel R, Ailhaud G, Gaillard D (1994) Proliferation and differentiation of rat adipose precursor cells in chemically defined medium: differential action of anti-adipogenic agents. J Cell Physiol 161:249–256 [DOI] [PubMed] [Google Scholar]
- 37.Lindroos B, Aho KL, Kuokkanen H, Räty S, Huhtala H, Lemponen R, Yli-Harja O, Suuronen R, Miettinen S (2010) Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Eng Part A 16:2281–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lund P, Pilgaard L, Duroux M, Fink T, Zachar V (2009) Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy 11:189–197 [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K (2008) Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthet Plast Surg 32:48–55; discussion 56–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez PD, Garcia-Arranz M, Georgiev-Hristov T, Garcia-Olmo D (2008) A new bronchoscopic treatment of tracheomediastinal fistula using autologous adipose-derived stem cells. Thorax 63:374–376 [DOI] [PubMed] [Google Scholar]
- 41.Lendeckel S, Jodicke A, Christophis P et al. (2004) Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32:370–373 [DOI] [PubMed] [Google Scholar]
- 42.Beitzel K, McCarthy MB, Cote MP et al. (2012) Rapid isolation of human stem cells (connective progenitor cells) from the distal femur during arthroscopic knee surgery. Arthroscopy 28:74–84 [DOI] [PubMed] [Google Scholar]
- 43.Tetreault P, Ouellette HA (2007) Healing of a clavicle fracture nonunion with bone marrow injection. J Shoulder Elb Surg 16:e23–e24 [DOI] [PubMed] [Google Scholar]
- 44.Fodor PB, Paulseth SG (2016) Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J 36:229–236 [DOI] [PubMed] [Google Scholar]
- 45.Ra JC, Jeong EC, Kang SK, Lee SJ, Choi KH (2017) A prospective, nonrandomized, no placebo-controlled, phase I/II clinical trial assessing the safety and efficacy of intramuscular injection of autologous adipose tissue-derived mesenchymal stem cells in patients with severe Buerger’s disease. Cell Med 9:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S, Sarkar U, Raju R, Dhar A, Parakh R, Jeyaseelan L, Viswanathan P, Vellotare PK, Seetharam RN, Thej C, Rengasamy M, Balasubramanian S, Majumdar AS (2017) Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger’s disease: phase II study report suggests clinical efficacy. Stem Cells Transl Med 6:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wislet-Gendebien S, Poulet C, Neirinckx V, Hennuy B, Swingland JT, Laudet E, Sommer L, Shakova O, Bours V, Rogister B (2012) In vivo tumorigenesis was observed after injection of in vitro expanded neural crest stem cells isolated from adult bone marrow. PLoS One 7:e46425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, Rogers AB, Montrose M, Houghton J (2007) Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res 67:10889–10898 [DOI] [PubMed] [Google Scholar]
- 49.Wang MY, Nestvold J, Rekdal O, Kvalheim G, Fodstad O (2017) A novel rat fibrosarcoma cell line from transformed bone marrow-derived mesenchymal stem cells with maintained in vitro and in vivo stemness properties. Exp Cell Res 352:218–224 [DOI] [PubMed] [Google Scholar]
- 50.Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, Dulong J, Monnier D, Gourmelon P, Gorin NC, Sensebe L, on behalf of Societe Francaise de Greffe de Moelle et Therapie Cellulaire (2010) Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115:1549–1553 [DOI] [PubMed] [Google Scholar]
- 51.Hahn WC, Weinberg RA (2002) Modelling the molecular circuitry of cancer. Nat Rev Cancer 2:331–341 [DOI] [PubMed] [Google Scholar]
- 52.Boregowda SV, Krishnappa V, Chambers JW, Lograsso PV, Lai WT, Ortiz LA, Phinney DG (2012) Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion. Stem Cells 30:975–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prockop DJ, Keating A (2012) Relearning the lessons of genomic stability of human cells during expansion in culture: implications for clinical research. Stem Cells 30:1051–1052 [DOI] [PubMed] [Google Scholar]
- 54.Rubin H (2001) Multistage carcinogenesis in cell culture. Dev Biologicals 106:61–66; discussion 67, 143–160 [PubMed] [Google Scholar]
- 55.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G (2009) Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6:e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danylesko I, Shimoni A (2018) Second malignancies after hematopoietic stem cell transplantation. Curr Treat Options in Oncol 19:9. [DOI] [PubMed] [Google Scholar]
- 57.Rizzo JD, Curtis RE, Socie G et al. (2009) Solid cancers after allogeneic hematopoietic cell transplantation. Blood 113:1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtis RE, Metayer C, Rizzo JD, Socié G, Sobocinski KA, Flowers ME, Travis WD, Travis LB, Horowitz MM, Deeg HJ (2005) Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood 105:3802–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Olmo D, Herreros D, De-La-Quintana P, et al. (2010) Adipose-derived stem cells in Crohn’s rectovaginal fistula. Case Report Med 2010:961758, 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS (2012) Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia. Circ J 76:1750–1760 [DOI] [PubMed] [Google Scholar]
- 61.Mesimaki K, Lindroos B, Tornwall J et al. (2009) Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 38:201–209 [DOI] [PubMed] [Google Scholar]
- 62.Scuderi N, Ceccarelli S, Onesti MG, et al. (2012) Human adipose-derived stem cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


