Abstract
Purpose
This study was aimed to evaluate the effect of Hedera nepalensis crude extract (HNC) and its isolated compound lupeol on antioxidant defence system, biochemical parameters and behavioural indices of Alzheimer disease generated in diabetic rats.
Methods
To evaluate the effect of the plant extract and lupeol, symptoms of Alzheimer and diabetes were induced in rats by STZ + AlCl3 treatment. Glucose level was measured with glucometer followed by antioxidant and biochemical assessment of the treated and untreated animals. Behavioural response of the rats was determined by Elevated Plus Maze (EPM) test and Morris Water Maze (MWM) test followed by determination of brain neurotransmitters by HPLC.
Results
HNC significantly reduced blood glucose level in a time dependent manner and elevated liver function markers were significantly (P < 0.05) reinstated to normal levels. HNC showed increase in level of catalase (CAT), superoxide dismutase (SOD) and reduced glutathione (GSH). HPLC quantification revealed that HNC treatment led to significant (p < 0.001) elevation in the level of neurotransmitters (dopamine and serotonin) in the midbrain region as compared to Alzheimer control (AC) group. EPM and MWM test showed decrease in cognitive and memory impairment in a rat group treated with HNC as compared to AC group.
Conclusion
Overall, results showed that H. nepalensis has therapeutic potential for the treatment of diseases like Alzheimer and diabetes.
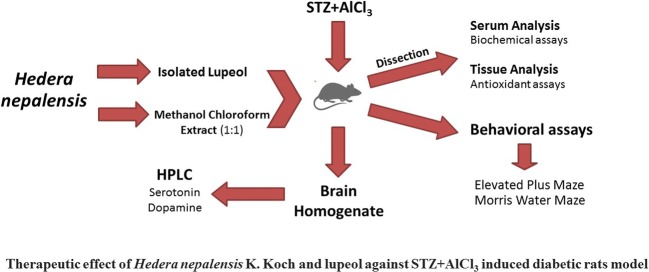
Graphical abstract.
Therapeutic effect of Hedera nepalensis K. Koch and lupeol against STZ + AICI3 induced diabetic rats model.
Keywords: Alzheimer disease, Antioxidant, Diabetes mellitus, HPLC, Lupeol, Neurotransmitters
Introduction
Alzheimer disease (AD) is also known as type 3 Diabetes Miletus (DM) and there is a strong relation between AD and diabetes. Factors such as obesity, heart disease and high blood pressure which are involved in the prognosis of diabetes are also directly linked with increased risk of developing AD. High cholesterol level is also directly involved in increase in blood pressure, which poses a greater threat for elderly individuals to develop AD [1]. Symptoms of AD syndrome in the elderly stage include the social and occupational activities hindrance. It is known to cause dementia in about 80% of individuals, according to recent findings [2–5] . Diagnosis of AD comprises of two well-known abnormalities, that is memory loss and defects in normal function of speaking, attention, thought, sense of judgement or problem solving [6]. AD symptoms shorten the life expectancy and affect overall lifestyle. Dementia and cognitive impairment occur due to damage in neurons and synapses structure which occurs in the basal forebrain, hippocampus, amygdala and neocortex of AD subjects [7–10]. In early prognosis of AD, cholinergic neurons around amygdala, hippocampus, cerebral cortex, and basal ganglia would be affected [11]. Destruction of cholinergic neurons and axonal defects lead to reduced release of acetylcholine resulting in cognitive impairment in aged patients [12]. Experimental approach involving animal models has shown that Aβ neurotoxicity could be reversed or decreased by stimulating nicotinic receptors [13]. So, researchers have been screening novel drugs which can diminish acetylcholine esterase activity or in some cases activate acetylcholine receptors.
From very start of human civilization, plants extracts were used in different situations for medicinal purpose. Secondary metabolites of plants are favoured as drugs because they display more biological friendliness and hence are more effective within living system than synthetic compounds. In Asia and Africa, herbs proven to be medicinally important for diabetes have been used for many centuries. Medicinal plants have been widely used as traditional medicine with low cost and fewer side effects. [14, 15]. The family Araliaceae contains genus Hedera found in North Europe, Asia, and North Africa. Nasir, has reported that many species of Hedera are existing in Northern areas of Pakistan [16]. Hedera nepalensis K. Koch is among one of the most beneficial plants for the treatment of different diseases. Its spasmolytic, sedative, antihelmintic, molluscicidal, antileishmanial and antifungal properties reviewed in literature showed that this plant has strong potential in herbal medicines [17, 18]. Jafri et al., reported the existence of catechin and caffeic acid in ethyl acetate fraction of H. nepalensis and found significant proportion of phenolic compounds having promising antioxidant activity [19]. It has also been found to be effective against tumour cells [20]. Saleem et al. [21] reported that the extracts of H. nepalensis possess potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. The phytochemical analysis of H. nepalensis showed the existence of alkaloids, terpenoids, steroids, tannins and flavonoids [22]. These active compounds containing high antioxidant activities are considered promising for prevention and treatment of diseases like depression, diabetes and cancer [23, 24].
To date, no pharmacological activity based on dual antidiabetogenic and anti-Alzheimeric property has been evaluated for H. nepalensis using rat model. So, the current experiment was commenced to evaluate the consequences of Hedera nepalensis crude extract (HNC) and its isolated compound lupeol on antioxidant defence system, biochemical parameters and behavioural indices in a streptozotocin and AlCl3 induced rat model of AD confounded by DM.
Material and method
Plant material
H. nepalensis under the local name of Bumbar was collected from Nathia Gali, District Rawalpindi, Punjab, Pakistan. Plant was recognized by Dr. Rizwana Aleem Qureshi, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad. Plant specimen record was deposited under the voucher number (HMP-461) to the “Herbarium of medicinal plants of Pakistan”, Quaid-i-Azam University, Islamabad, Pakistan.
Plant extractions and isolation of lupeol
Plant material was dried under shade, ground into fine powder and then macerated in methanol and chloroform mixture (1:1). The eluted solution was concentrated in rotary evaporator (Buchi, Switzerland) to get H. nepalensis crude extract (HNC). The suspension of crude extract obtained after mixing in hot distilled water, was subjected to partitioning three times with n-hexane. Lupeol (Molecular formula; C30H50O) was isolated previously by our research group [21] as a white powder from the HNN fraction (m/z 426.72) as recommended by mass spectral data.
Animal maintenance
Rats (Male Sprague-Dawley) weighing 200-220 g were chosen for experimentation. They remained kept in aluminium enclosures maintained in ventilated room with permissible access to food and tap water. The study protocols were approved by Ethical Committee of Quaid-i-Azam University, Islamabad. All experimental were directed in accord with the National Biosafety Guideline [25].
Induction of Alzheimer and diabetes
Rats were made diabetic by a single intraperitoneal injection (0.2 ml) of 30 mg/kg of streptozotocin (STZ) liquefied 0.1 M citrate buffer solution for three consecutive days [26, 27]. Then blood was withdrawn from tail and glucose level was determined to confirm the induction of diabetes using test strips in Lifescan one touch Vita™ test meter. On third day after injection of STZ, aluminium chloride (AlCl3) was given orally daily for 21 days [27, 28]. Diabetic rats which showed >300 mg/dl glucose concentrations were used for further experiments.
Treatment groups
Total thirty-five Sprague-Dawley rats were arbitrarily distributed into five groups having seven rats in each and dosage was performed according to rat body weight (BW). NC (normal control) group (non-diabetic, non-Alzheimer) was given 10% DMSO (10 ml/kg BW/day). STZ + AlCl3 co-treated group received Rivastigmine (EXCELON®) at 0.8 mg/kg BW/day as positive control (PC). AC group (Alzheimer control) received 10% DMSO (10 ml/kg BW/day) after STZ + AlCl3 co-treatment. HNC group (AC + HNC) received crude extract 400 mg/kg (BW) after STZ + AlCl3 co-treatment. AC + Lupeol group received lupeol orally at 10 mg/kg (BW) after STZ + AlCl3 co-treatment. The solutions were prepared in 10% DMSO and treatments were carried out orally for 21 consecutive days. After oral dose, glucose level was determined on day 0, day 7, 14, 21 of blood taken from tail vein using test strips in Lifescan one touch Vita™ test meter.
Serum and tissue preparation
On day 21, rats were anesthetized with chloroform and sacrificed to get blood by cardiac puncture [29]. The blood was collected in BD Vacutainer® tubes and then centrifuged at 3500 rpm for 10 min to separate the serum. Brain regions (cortex and cerebellum) and liver tissue were separated for estimation of antioxidant enzymes and histopathological analysis. Midbrain region (cerebrum) was subjected to high performance liquid chromatography-ultraviolet (HPLC-UV) for neurotransmitter analysis.
Determination of biochemical markers of liver and pancreas
Biochemical markers such as ALP, AST, total bilirubin and triglyceride of respective organs have been estimated in the serum acquired from the treated animals according to the guidelines mentioned on supplier’s standard kits levels using the Cobas® kits (Roche Diagnostics, Indianapolis, IN, USA). Pancreatic biomarkers such as lipase was estimated in the serum of treated animals according to the guidelines mentioned on supplier’s standard kits levels using the Cobas® kits (Roche Diagnostics, Indianapolis, IN, USA).
Determination of antioxidant markers
Tissue parts (100 mg) of cortex, cerebellum and liver of every animal were homogenized in ice cold 50 mM tris buffer (pH 7.4) and obtained content was subjected to centrifugation at 12,000 rpm for 20 min at 4 °C to get homogenate, which then was analysed for CAT, SOD, Protein estimation, TBARS and GSH [30].
CAT activity
CAT estimation was measured by the Aebie’s method with slight modification [31]. The reaction mixture comprised of 2 mL of 50 mM phosphate buffer (pH 4.5), 0.3 mL of 5.9 mM H2O2, and 0.2 mL tissue homogenate. After 2 min, absorbance was calculated at 240 nm using microtiter ELISA reader and results were represented as U/min. The protein estimation of tissue homogenates was measured with Bradford method at 650 nm spectrophotometrically [32].
SOD activity
SOD level was analysed by the method of Bannister et al. [33]. Reaction mixture contained 0.2 mL of phenazine methosulphate (184 μM), 1.4 mL of phosphate buffer (pH 7.2) and 0.2 mL of tissue sample followed by the addition of 0.1 mL of NADH (780 μM). After 1 min, 1 mL of glacial acetic acid was mixed to stop reaction and took reading at 560 nm. SOD level is stated as U/mg protein.
Protein estimation
Protein estimation of tissues homogenates was evaluated using the Bradford method under slight modification [32]. Tissues of organ (100 mg) were weighed and homogenized in the phosphate buffer (PH 7.0). Then, homogenates were centrifuged for 5 min at 15,000 rpm at 4 °C. The incubation of 5 min was given to 0.2 ml sample mixed in 2 ml alkaline solution, Mixed Folin Ciocalteu phenol reagent in 1:1 in each tube and vortexed. After thirty minutes of incubation, the absorbance was taken at 595 nm by using microplate reader.
TBARS activity
Thiobarbituric acid reactive substances (TBARS) for the measurement of lipid peroxidation activity was performed in tissue homogenate according to Ohkawa et al. with modifications [34]. The samples were mixed with phosphate buffer (0.2 M; pH 7.2) in 1:4 ratio followed by incubation (37 °C) in a shaking hot water bath for 1 h. Then, mixture was prepared by adding of 10% trichloroacetic acid and working reagent TBA (5% acetic acid and 20% sodium hydroxide) with sample solution. All the reaction mixtures were positioned in a hot water bath for 15 min followed by ice bath treatment for 12 min. Centrifugation was performed at 10,000×g for 15 min. The supernatant was read at the absorbance of 540 nm in a pure 96-well plate using microtiter plate reader compared to a reagent blank. TBARS levels expressed as nM/min/mg tissue protein.
GSH activity
Tissue homogenate (1 ml) and sulfosalicylic acid (1 ml) were mixed and kept at 4 °C for 1 h. After incubation, centrifugation was done at 1000×g for 30 min at 4 °C. Final volume of 3 mL reaction combination contains 2.7 mL phosphate buffer (0.1 M; pH 7.2), 0.1 mL filtered solution and 0.1 mL DTNB (100 mM). After that absorbance was taken at 412 nm [35] by using spectrophotometer. The results were expressed as mM/g protein.
High-performance liquid chromatography (HPLC)
After dissection of rats, their midbrain region of CNS was dissected and washed thrice with cold saline and samples were quickly subjected to storage at −70 °C until estimation of dopamine and serotonin was done by HPLC as reported earlier [36, 37]. Briefly, tissues were homogenized in phosphate buffer and then subjected to centrifugation at 14000 rpm for 10 min at 4 °C. Acquired supernatant was filtered through a 0.2 μm Teflon syringe filter and injected into an injector at 20 μl volume for HPLC (Agilent technologies 1200 series). The measurement of dopamine levels was carried out by HPLC system having a quaternary pump. It consisted of a reservoir of mobile phase (50 mM potassium phosphate buffer/methanol 97/3 (v/v)), pump, an injector, C18 column, and a UV/Vis detector. Flow rate of mobile phase was adjusted to 1 ml/min and serial dilutions were prepared for both dopamine and serotonin standards for the determination of linear standard curve.
Behavioral studies
Towards establishing the correct determination of the behavioral effects of Alzheimer and diabetes, rats were participated to a maximum of 2 behavioral tests in order to lessen the stress. Specifically, to evaluate the anxiety-like behavior and spatial memory, we used Elevated Plus Maze (EPM) test and Morris Water Maze (MWM) test, respectively. We used video tracking system from Stoelting Co., Wood Dale, IL, USA in order to find their stress levels.
Elevated plus maze (EPM)
Standard EPM model was used to determine anxiety-related behavior [38]. Briefly, the rats were carried to the testing room one hour before testing and were allowed to stay in their home cages until experiment started. At start, each rat was positioned at the central platform opposite the same open arm. Navigation was observed for a five-min interval using computer-based software ANY-maze, a video-tracking system from Stoelting Co., Wood Dale, IL, USA. Movement of individual animals was considered as total distance travelled during the 5-min test. Software automatically recorded the number of entries into the open arms and the time expended on the open arms. An arm pass was calculated when 85% of body was detected on the particular arm. At the end of the test, the maze was cleaned with 70% ethanol and dried out with white cloth.
Morris water maze
Morris Water Maze comprised of water-filled cylindrical tank of 150 cm diameter. Extra-maze different visual cues for orientation were always placed on the four walls around the tank. The temperature of the water in the tank was kept constant at 25 ± 1 °C. A 15 cm diameter platform was positioned in a platform quadrant of the tank. The protocol comprised of 1 day of visible platform tests and 2 days of hidden platform tests along with a probe trial 1 after the last hidden platform test. Firstly, the platform was placed 1.2 cm above surface of water in the southeast, northeast, northwest, southwest quadrant and the center of the pool respectively, in each trial in the perceptible platform test. There were 3 trials per day with an interval of 1 h. Rats were placed successively in all four quadrants of the tank facing the wall of the tank. In each trial, the rat could swim until it found the platform. If rat could not find its platform within 60s, it was guided to the platform and remained placed there for 30s before putting into the cage. In the unseen platform tests, the platform was located 1 cm below the water surface in the southeast quadrant and rats were allowed to move freely from all four positions for 3 trials per day with an interval of 1 h. The probe trial was directed by eliminating the platform and by placing the rats facing wall of North side. The time consumed in the platform quadrant (Northeast quadrant) was calculated in a single 60 s trial. Tracking of animal movement was attained with ANY-maze video-tracking system.
Statistical analysis
The results were evaluated in one-way ANOVA with Dunnett’s multiple-comparison test by GraphPad Prism 5.0 software. The results were stated as mean ± SEM (standard error mean). Differences were reflected to be statistically significant at p < 0.05.
Results
Effects of treatment of HNC and lupeol on plasma glucose levels of STZ induced rats
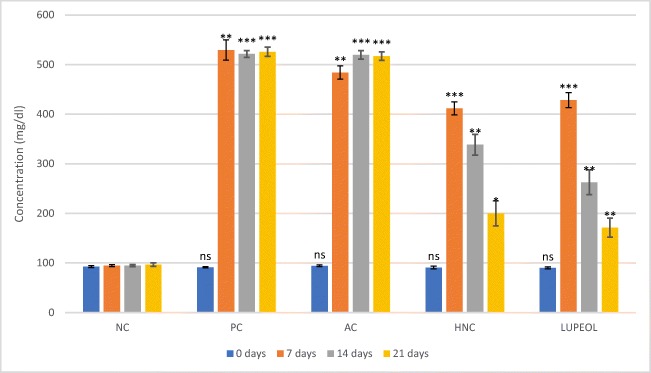
The effect of HNC and lupeol on plasma glucose level in STZ-induced diabetic rats was measured with the help of Lifescan one touch Vita™ test meter and results are represented in Fig. 1. Blood glucose level was measured immediately after i.p. injection of STZ and then after every week till 21 days designated as 0 day, 7 days, 14 days and 21 days. The fasted glucose concentration in experimental groups was significantly increased (P < 0.01) on 7th days after first injection of STZ (Fig. 1). The Alzheimer control (AC) group showed glucose range of 484-517 mg/dl during the 21 days experiment. The positive control group (PC) did not show significant decrease in blood glucose level due to only anti-Alzheimer activity of Rivastigmine drug and stayed in the range of above 500 mg/dl till 21st day. HNC group after treatment for 3 weeks (0 day to 21 day) showed significant decrease (106%) from 412 ± 13 mg/dl on 7th day to 200 ± 45 mg/dl on 21st day in plasma blood glucose level while lupeol treatment brought about even more decrease in glucose level (7th day: 429 ± 15; 21st day: 171 ± 19) up to 150.5% in same duration as stated above.
Fig. 1.
Effect of H. nepalensis crude extract and lupeol on blood glucose level. Where “NC” is normal control, “AC” is Alzheimer control, “PC” is positive control (Rivastigmine) and “HNC” is H. nepalensis crude extract. Values are * p < 0.05, ** p < 0.01 and *** p < 0.01 statistically significant as compared to normal control group expressed as means ± SEM whereas NS represents non-significant
Effects of HNC and lupeol on blood serum biomarkers
Results of Alzheimer induced rat model based on STZ showing blood serum biomarkers are presented in Table 1. ALP levels were increased significantly in the AC (116 ± 9.9) as compared to NC (52 ± 1.3), but ameliorative effect of HNC and Lupeol reduced the level of ALP towards normal (HNC; 73 ± 4.3: lupeol; 68 ± 4.6). AST and lipase level also showed significant increase in AC group (44 ± 2.8 and 59 ± 5.9, respectively; P < 0.01) as compared to NC, but these increases in level seemed to lessen when treated with HNC (AST; 30 ± 2.1: lipase; 29 ± 2.1) and lupeol (AST; 27 ± 1.8: lipase; 27 ± 2.4). Similarly, measurements of triglycerides showed significant increase in levels (73.33%; P < 0.01) in the AC group (104 ± 8.1) as compared to the NC rats (60.01 ± 1.7) (Table 2; Fig. 3). But AC + HNC and AC + lupeol rats after respective treatments had their triglycerides levels (HNC; 72 ± 2.5: lupeol; 67 ± 4.9) decreased by 34.7% and 35.5%, respectively as compared to the AC group. Furthermore, measurement of bilirubin level increased (59.01%) in AC group as compared to NC group (0.41 ± 0.01), whereas subsequent treatment of respective AC + HNC and AC + lupeol rats presented significant decrease (P < 0.01) in levels (HNC; 0.39 ± 0.05: lupeol; 0.32 ± 0.07).
Table 1.
Effect of H. nepalensis crude extract and lupeol on blood serum biomarker
| Biomarker (Unit) | NC | PC | AC | AC + HNC | AC + Lupeol |
|---|---|---|---|---|---|
| ALP (U/L) | 52 ± 1.3 | 77 ± 3.5* | 116 ± 9.9*** | 73 ± 4.3 * | 68 ± 4.6NS |
| AST (U/L) | 22 ± 0.8 | 25 ± 0.9 NS | 44 ± 2.8*** | 30 ± 2.1* | 27 ± 1.8 NS |
| Lipase (U/L) | 18.5 ± 1.13 | 29 ± 2.4 NS | 59 ± 5.9*** | 29 ± 2.1 NS | 27 ± 2.4 NS |
| Triglyceride (mg/dl) | 60.01 ± 1.7 | 97 ± 6.2*** | 104 ± 8.1*** | 72 ± 2.5 NS | 67 ± 4.9 NS |
| Bilirubin (mg/dl) | 0.41 ± 0.01 | 0.8 ± 0.06 NS | 1.0 ± 0.1** | 0.39 ± 0.05*** | 0.32 ± 0.07** |
Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract. Values are *p < 0.05, **p < 0.01 and *** p < 0.001 statistically significant as compared to AC group expressed as means ± SEM whereas NS represents non-significant
Table 2.
Effects of H. nepalensis crude extract and lupeol on cortex, cerebellum and liver antioxidant activity
| Organ | Treatment | Protein (μg/mg tissue) | TBARS (nM/min/ mg tissue) | CAT (U/min) | SOD (U/mg protein) | GSH (mM/g tissue) |
|---|---|---|---|---|---|---|
| Cortex | NC | 0.97 ± 0.09 | 9.09 ± 0.9 | 4.4 ± 0.11 | 1.2 ± 0.01 | 6.2 ± 0.36 |
| PC | 0.65 ± 0.08NS | 14.5 ± 2.1NS | 3.7 ± 0.06*** | 1.02 ± 0.01** | 4.9 ± 0.25NS | |
| AC | 0.56 ± 0.14* | 22 ± 2.9*** | 2.5 ± 0.09*** | 0.5 ± 0.06*** | 1.6 ± 0.18*** | |
| AC + HNC | 1.06 ± 0.11NS | 7.94 ± 1.4NS | 4.3 ± 0.08NS | 1.6 ± 0.01*** | 6.3 ± 0.61NS | |
| AC + lupeol | 1.04 ± 0.19NS | 6.44 ± 1.21NS | 3.1 ± 0.08*** | 1.9 ± 0.01*** | 7.6 ± 0.48NS | |
| Cerebellum | NC | 0.77 ± 0.07 | 12.7 ± 1.5 | 5.0 ± 0.05 | 1.1 ± 0.01 | 4 ± 0.53 |
| PC | 0.83 ± 0.09NS | 11.0 ± 1.5NS | 4.2 ± 0.03*** | 0.7 ± 0.16** | 3.7 ± 0.34NS | |
| AC | 0.38 ± 0.07* | 27.7 ± 2.4*** | 3.2 ± 0.03*** | 0.3 ± 0.02*** | 2.8 ± 0.22NS | |
| AC + HNC | 0.93 ± 0.09NS | 12.8 ± 1.51NS | 4.8 ± 0.09* | 1.2 ± 0.003NS | 4.01 ± 0.45NS | |
| AC + lupeol | 0.88 ± 0.13NS | 7.71 ± 1.3NS | 3.9 ± 0.03*** | 1.3 ± 0.02NS | 3.07 ± 0.28NS | |
| Liver | NC | 5.41 ± 0.40 | 10.0 ± 1.2 | 12.3 ± 0.33 | 4.2 ± 0.01 | 14.7 ± 0.41 |
| PC | 5.43 ± 0.28NS | 8.44 ± 2.14NS | 13.0 ± 0.15NS | 3.03 ± 0.01*** | 12.0 ± 0.60NS | |
| AC | 3.27 ± 0.12** | 12.8 ± 1.9*** | 9.8 ± 0.08*** | 1.3 ± 0.09*** | 8.8 ± 0.76*** | |
| AC + HNC | 6.34 ± 0.6NS | 8.76 ± 2.0NS | 14.1 ± 0.17*** | 3.9 ± 0.09* | 15.5 ± 0.64NS | |
| AC + lupeol | 5.85 ± 0.62NS | 6.58 ± 1.6NS | 11.5 ± 0.4NS | 4.5 ± 0.11* | 16.8 ± 1.12NS |
Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract. Values are *p < 0.05, **p < 0.01 and *** p < 0.01 statistically significant as compared to AC group expressed as means ± SEM whereas NS represents non-significant
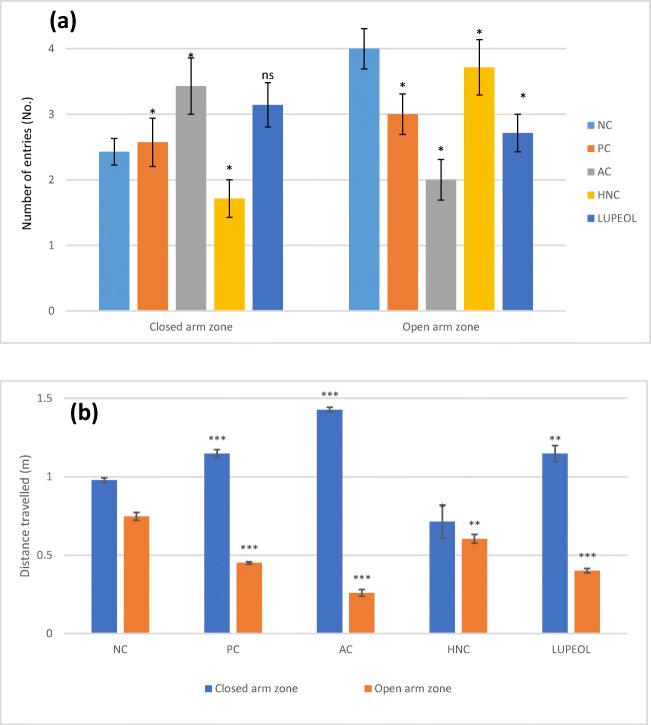
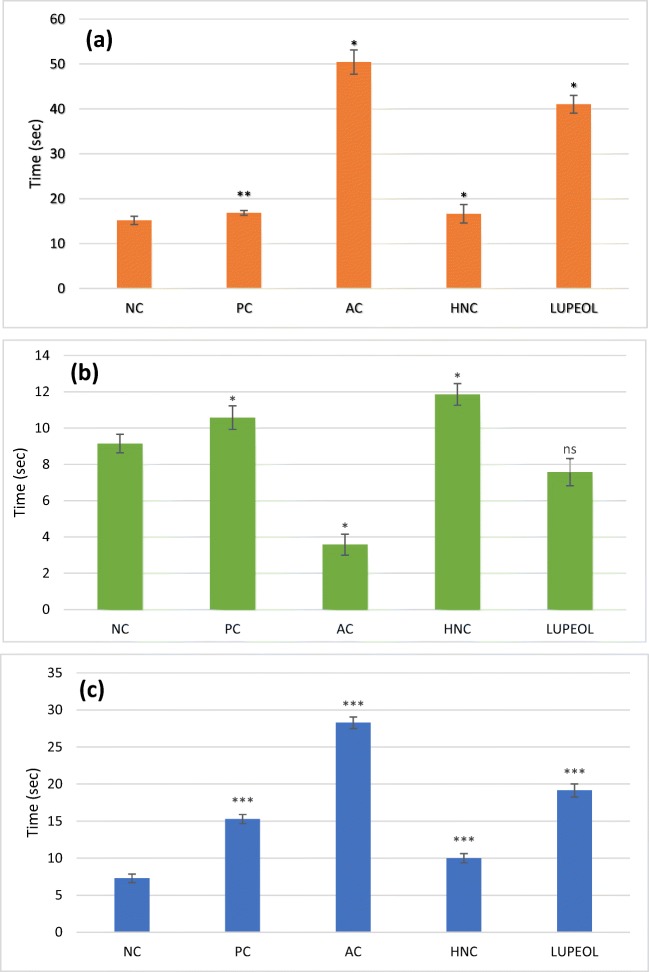
Fig. 3.
Elevated Plus Maze (EPM) test (a) Number of closed and open entries (b) Total distance travelled in the open and closed arms. Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract. Values are * or ** p < 0.05 and *** p < 0.01 statistically significant as compared to diabetic control group expressed as means ± SEM whereas NS represents non-significant
Effects of HNC and lupeol on the antioxidant enzymatic activity in cortex, cerebellum and liver organs
Lipid peroxidation (TBARS) showed increased level in cortex (22 ± 2.9; 250.5%), cerebellum (27.7 ± 2.4; 232.2%) and liver (37.1 ± 3.9; 379.9%) in STZ treated rats as compared to vehicle treated rats while HNC and lupeol treatment showed promising effects in decreasing the content of TBARS in cortex (HNC; 7.94 ± 1.4: lupeol; 6.44 ± 1.2), cerebellum (HNC; 12.8 ± 1.5: lupeol; 7.71 ± 1.3) and liver (HNC; 8.76 ± 2.0: lupeol; 6.58 ± 1.6) (Table 2). There was also a substantial reduction in level of protein estimation in cortex (0.56 ± 0.14; 41.2%), cerebellum (0.38 ± 0.07; 39.2%) and liver (3.27 ± 0.12; 40.2n %) level in STZ treated rats as compared to vehicle treated rats. However, there was increase in the level of protein estimated on treatment with HNC and lupeol in cortex (HNC; 1.06 ± 0.11: lupeol; 1.04 ± 0.19) and cerebellum (HNC; 0.93 ± 0.09: lupeol; 0.88 ± 0.13) regions as compared to STZ treated animals (0.38 ± 0.07). It was also observed that STZ treatment (AC) brought about decrease in level of CAT (cortex; 2.5 ± 0.09: cerebellum; 3.2 ± 0.03: liver; 9.8 ± 0.08) as well as SOD (cortex; 0.5 ± 0.06: cerebellum; 0.3 ± 0.02: liver; 1.3 ± 0.09) as compared to NC group. HNC and lupeol treatment showed increase in level of CAT in cortex (72.01%), cerebellum (50.01%) and liver (200%). Lupeol treatment showed significant (P < 0.01) increased in level of SOD only in cortex (1.9 ± 0.01). Decreased level of GSH in cortex (1.6 ± 0.18) and cerebellum (0.3 ± 0.02) regions was observed in AC while treatment of HNC and lupeol increased the level of GSH in cortex (HNC; 6.3 ± 0.61: lupeol; 7.6 ± 0.48) and cerebellum (HNC; 4.01 ± 0.45: lupeol; 3.07 ± 0.28).
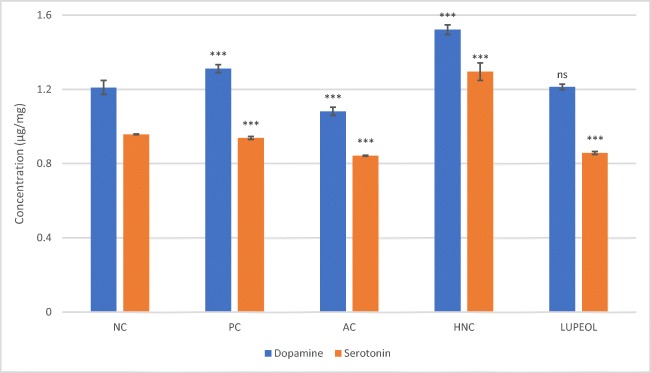
Quantification of dopamine and serotonin levels in STZ + AlCl3 treated rats
Quantification of dopamine and serotonin was carried out by HPLC-UV/Vis (Agilent-1200) machine. The control groups showed significant difference in the levels of above mentioned neurotransmitter being lowest in the AC group and significantly high in PC group as compared with vehicle group (Fig. 2). HNC treatment led to significant (P < 0.001) rise in the level of dopamine neurotransmitter in the midbrain region but lupeol treatment had no significant effect on increase in dopamine level (P > 0.05). Similarly, HNC has shown significantly (P < 0.001) increase in the level of neurotransmitter in the midbrain region but lupeol treatment had no substantial effect on serotonin level as compared with controls (Fig. 2).
Fig. 2.
HPLC analysis of dopamine and serotonin in the midbrain region. Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract. Values are * or ** p < 0.05 and *** p < 0.01 statistically significant as compared to diabetic control group expressed as means ± SEM whereas NS represents non-significant
Effect of HNC and lupeol treatment on behavioural indices
Evaluation of anxiety-like behaviour using elevated plus maze test
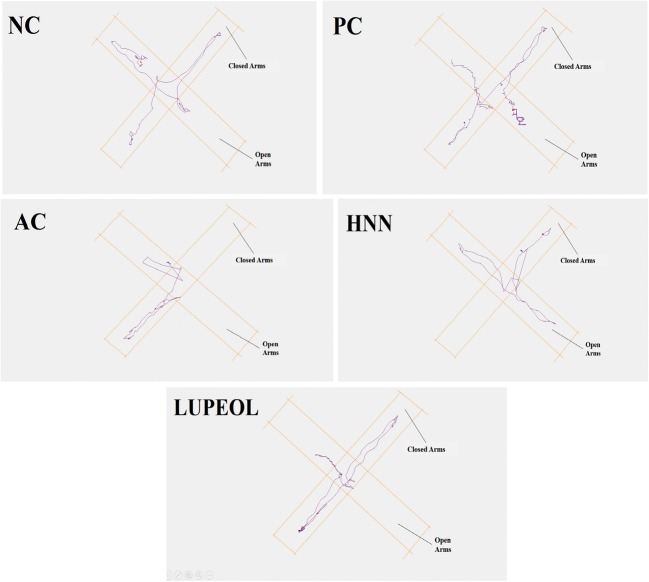
Elevated plus-maze (EPM) tests was employed to evaluate anxiety like behaviour of the animals after the induction of the Alzheimer using STZ with AlCl3 treatment and the results are presented in Fig. 3. AC rats showed significant reduction in number of entries to open arms (50.3%; P < 0.01) of the plus maze apparatus compared to the NC (4.0 ± 0.03) while AC + HNC group exhibited a substantial increase (43.3%; P < 0.01) in their number of entries to open arms (3.7 ± 0.05) compared to AC group (2.0 ± 0.03). Regarding the EPM, the AC rats showed increase in their ratio of number of entries to closed arms (40.95%) and total distance travelled in closed arm (50.2%) of the plus maze apparatus compared to the NC (2.4 ± 0.04; 1.02 ± 0.05), while AC + HNC group exhibited a significant decrease (50.3%; P < 0.01) in their number of entries to closed arms (1.69 ± 0.03) compared to the AC group (3.4 ± 0.05). Lupeol treated rats showed non-significant decrease (10.2%) in their entries to closed arms but showed significant increase in their entries to open arms up to 25.2% as compared to AC group. For visual demonstration tracks plots of EPM are presented in Fig. 4.
Fig. 4.
Track plots for Elevated Plus Maze (EPM) test showing the position of the animal. Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract
Evaluation of cognitive behaviour using Morris water maze test
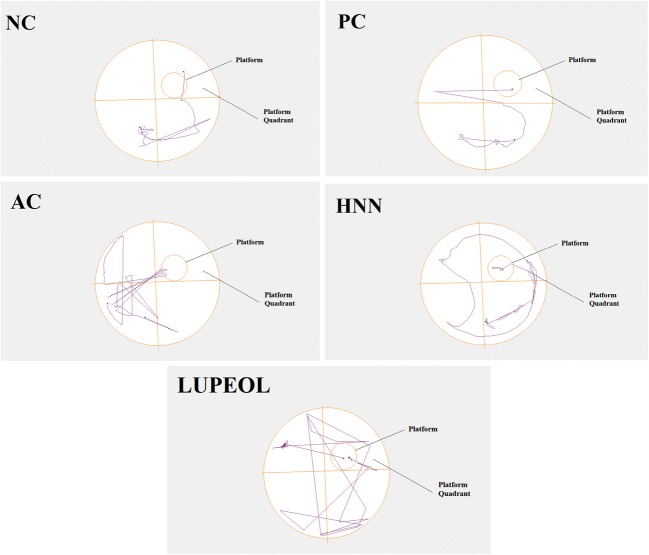
Morris Water Maze (MWM) test was employed as a tool to evaluate learning/memory behaviour and consolidation of the reinforced clues in STZ + AlCl3 induced Alzheimer rat model and results are summarized in Fig. 5. Significant increase in time to reach the platform zone was noted in AC (50.2 ± 0.03; p < 0.01) group compared with normal group (15.23 ± 0.03). Treatment of HNC and lupeol sowed decrease in time to reach the platform zone with significant reduction in HNC (16.23 ± 0.03; p < 0.01) treated group (Fig. 5a). Furthermore, there was a remarked reduction in time spends in the platform quadrant in AC group (3.53 ± 0.01; p < 0.01) but treatment of HNC significantly increased (11.56 ± 0.05; p < 0.05) the time in the platform quadrant. Lupeol treated group also showed higher (7.63 ± 0.03) time span in the platform quadrant (Fig. 5b). The value of AC represents that the rats were least oriented in their directions while in NC and PC the rats were well oriented. In the end, we evaluated the latency to first entry in the platform quadrant, which showed significantly elevated time (28.6 ± 0.05; p < 0.01) or latency to first entry in platform zone for AC group compared to NC group (7.2 ± 0.01). However, treatment of HNC (10.23 ± 0.02; p < 0.01) and lupeol (19.23 ± 0.02; p < 0.01) showed significant reduction in latency to first entry in platform zone (Fig. 5c). For visual demonstration tracks plots of EPM are presented in Fig. 6.
Fig. 5.
Morris Water Maze (MWM) test (a) Time to reach the platform zone (b) Time in the platform quadrant (c) Latency to first entry in the platform quadrant. Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract. Values are * or ** p < 0.05 and *** p < 0.01 statistically significant as compared to diabetic control group expressed as means ± SEM whereas NS represents non-significant
Fig. 6.
Track plots for Morris Water Maze (MWM) test showing the position of the animal. Where “NC” is normal control, “PC” is positive control (Rivastigmine), “AC” is Alzheimer control and “HNC” is H. nepalensis crude extract
Discussion
Long term increased glucose level seems to play a part in the onset of cognitive and behavioral complications related to diabetes [39]. Hedera nepalensis is known to have hypoglycemic effect as reported earlier [22]. More recently, DPP-4 inhibitory activity was reported by Saleem et al., which represent that crude extract of Hedera nepalensis possess antidiabetic and glucose lowering potential [21]. These finding are similar with our results in which HNC group after treatment showed significant decrease in elevated glucose level caused by STZ treatment. The herbal treatment of diabetes is the need of time as the persistent increased glucose level is involved in tissue damage due to activation of different mechanisms [40], including (1) elevated glucose and other sugar influx by the polyol pathway, (2) higher advanced glycation end-products (AGEs) synthesis with receptor expression, (3) activated isoform of PKC and (4) overexpression of hexosamine pathway. Overall, these mechanisms are involved in increased oxidative stress [41, 42].
Oxidative stress is a major cause in development of DM related complications such as Alzheimer disease [43]. Hepatoxicity is an accentuated cause of STZ + AlCl3 induced Alzheimer and diabetes [28] and elevated ALT, AST, total bilirubin and lipase in STZ treated diabetic groups usually tend to have high transaminases activity [44]. It has also been reported that STZ results in decline in SOD and CAT activity in brain region [45]. In our results, HNC treatment showed an increase in the level of CAT in cortex, cerebellum and liver. Similarly, lupeol treatment has shown a significant (P < 0.01) increase in level of SOD. At the same time a significant decrease in activity was observed in liver function tests/ enzyme after treatment with HNC and lupeol which are known to be associated with defence of intracellular enzyme leakage and cellular constancy as stated earlier for other ethnopharmacological importance antioxidant plants [46].
The dopamine and serotonin neurotransmitters are biogenic catecholamines that play a major role in nerve pathways in the brain [47]. These neurotransmitters are responsible for cognitive mechanisms such learning and short- and long-term memory [48]. It has been reported that in AD patients, a significant loss of serotonin occurs which is consistent with cognitive processing [49]. In our study reduced dopamine and serotonin levels were observed in lupeol treated animals and are correlated with decrease in the activity of SOD, CAT and GSH. However, the treatment of HNC has significantly increased the level of dopamine neurotransmitter in the midbrain region. Deprivation of serotonin level was also observed in midbrain region which support the agreement of previous studies about dysregulation of the serotonergic system [50]. These results suggest that there are some active constituents of HNC other than lupeol that could increase level of neurotransmitters and or antioxidant defense system enzymes.
Alzheimer disease also known as type 3 diabetes is associated with pathological conditions of dementia and memory impairment. MWM has the direct relationship with neurotransmitter systems and drug effects [51]. In addition, it has been shown that there is involvement of the entorhinal, hippocampus and perirhinal cortices [52]. It is believed that the learning impairment in STZ induced diabetic rats might be caused by marked variations in synaptic flexibility in hippocampal area [53, 54]. That’s why, most experimental model of STZ related to Alzheimer and diabetes employee Morris Water Maze (MWM) test [45, 55, 56] to evaluate learning/memory behavior as well as consolidation of the reinforced clues in STZ induced Alzheimer rat model. In our experiment, HNC reduced the memory impairment and enhanced the time spent in the platform quadrant. As mentioned above the level of neurotransmitters was also increased due to the treatment of HNC, so it can be proposed that these mechanisms are interlinking at some point resulting in overall improvement of animals’ state.
The purpose of EPM test was to evaluate behavioral indices of anxiety. However, there are different behavioral indices of anxiety in EPM test such as height and open/close arms factor [57]. The total entries score and total distance are considered a useful index of general activity. To evaluate the multifaceted behavior of anxiety, co-treated (STZ + AlCl3) rats were evaluated in EPM test and HNC group showed significant decrease in their number of entries to open arms and increase in rate of total distance. EPM has a strong predictive validity for screening anxiolytic plants [58]. The total entries score and total distance are considered a useful index of general activity [59]. It is thought that the aversion of mice to explore the open arms of the maze is caused by fear of open and elevated spaces [60]. So, it can be predicted that due to treatment of HNC the natural aversion of rats for open and elevated areas decreased and their natural spontaneous exploratory behavior improved.
Conclusion
Our results depict that HNC and lupeol ameliorate the increase in plasma glucose level and enhance the antioxidant enzymes profile in STZ + AlCl3 rat model. HNC and lupeol treatment showed significant results in decreasing DM related complications of AD such as cognitive impairment and anxiety/fear related behavioral indices. Brain neurotransmitters levels were also improved after HNC treatment. Overall this plant (H. nepalensis) has antidiabetogenic and memory enhancement property and could be used as novel therapy against AD pathology with DM co-morbidity. In the end, we summarize that natural and ethanoprmacological related compounds in H. nepalensis plant may be further analysed for suitability in other animal models.
Acknowledgements
The authors are highly grateful to the animal house staff for their support.
Authors’ Contributions
WJH and HI conducted all the assays and experimental work. BM conceived the study design and supervised the study. FR contributed in animal dissection. HI and WJH drafted the manuscript and all authors proof read the final version.
Compliance with ethical standards
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Apolipoprotein E ε4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bi X. Alzheimer disease: update on basic mechanisms. J Am Osteopath Assoc. 2010;110(9):S3. [PubMed] [Google Scholar]
- 3.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119(4):421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 4.Penumala M, Zinka RB, Shaik JB, Amooru Gangaiah D. In vitro screening of three Indian medicinal plants for their phytochemicals, anticholinesterase, antiglucosidase, antioxidant, and neuroprotective effects. Biomed Res Int. 2017;2017:1–12. doi: 10.1155/2017/5140506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penumala M, Zinka RB, Shaik JB, Mallepalli SKR, Vadde R, Amooru DG. Phytochemical profiling and in vitro screening for anticholinesterase, antioxidant, antiglucosidase and neuroprotective effect of three traditional medicinal plants for Alzheimer’s disease and diabetes mellitus dual therapy. BMC Complement Altern Med. 2018;18(1):77. doi: 10.1186/s12906-018-2140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- 7.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25(3):303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 8.Devanand D, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment prediction of Alzheimer disease. Neurology. 2007;68(11):828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 9.Jauhiainen AM, Pihlajamäki M, Tervo S, Niskanen E, Tanila H, Hänninen T, Vanninen RL, Soininen H. Discriminating accuracy of medial temporal lobe volumetry and fMRI in mild cognitive impairment. Hippocampus. 2009;19(2):166–175. doi: 10.1002/hipo.20494. [DOI] [PubMed] [Google Scholar]
- 10.Lain AH, Lieberman AP, Pfannl R, Hedley-Whyte ET. Nodular bilateral amygdala degeneration in demented individuals. Acta Neuropathol. 2010;120(5):683–688. doi: 10.1007/s00401-010-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 12.Bartus RT, Rr D, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 13.Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer's and Parkinson's diseases. J Alzheimers Dis. 2011;24(supplement2):95–109. doi: 10.3233/JAD-2011-110173. [DOI] [PubMed] [Google Scholar]
- 14.Ismail H, Mirza B. Evaluation of analgesic, anti-inflammatory, anti-depressant and anti-coagulant properties of Lactuca sativa (CV. Grand Rapids) plant tissues and cell suspension in rats. BMC Complement Altern Med. 2015;15(1):1. doi: 10.1186/s12906-015-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail H, Rasheed A, Haq I-u, Jafri L, Ullah N, Dilshad E, et al. Five indigenous plants of Pakistan with Antinociceptive, anti-inflammatory, antidepressant, and anticoagulant properties in Sprague Dawley rats. Evid Based Complement Alternat Med. 2017;2017:1–10. doi: 10.1155/2017/7849501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasir E. Flora of Pakistan. In: Herbarium S, editor. Gordon college, Rawalpindi and department of Botany. Karachi: University of Karachi; 1975. pp. 2–3. [Google Scholar]
- 17.Timon-David P, Julien J, Gasquet M, Balansard G, Bernard P. Research of antifungal activity from several active principle extracts from climbing-ivy: Hedera helix L. Ann Pharm Fr. 1980;38(6):545–552. [PubMed] [Google Scholar]
- 18.Xue M, ZhiYing W. The volatile constituents analysis of Scindapsus aureum and Hedera nepalensis var. sinensis and their inhibition against five fungi. Acta Horticulturae Sinica. 2010;37(6):971–976. [Google Scholar]
- 19.Jafri Laila, Saleem Samreen, Ihsan-ul-Haq, Ullah Nazif, Mirza Bushra. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arabian Journal of Chemistry. 2017;10:S3699–S3706. [Google Scholar]
- 20.Hamayun M, Khan SA, Sohn EY, Lee I-J. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of district swat. Pakistan: Lyonia; 2006. pp. 101–113. [Google Scholar]
- 21.Saleem S, Jafri L, ul Haq I, Chang LC, Calderwood D, Green BD, et al. Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J Ethnopharmacol. 2014;156:26–32. doi: 10.1016/j.jep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Kanwal S, Ullah N, Haq I, Afzal I, Mirza B. Antioxidant, antitumor activities and phytochemical investigation of Hedera nepalensis K. Koch, an important medicinal plant from Pakistan. Pak J Bot. 2011;43(8):85–89. [Google Scholar]
- 23.Madhuri S, Pandey G. Some anticancer medicinal plants of foreign origin. Curr Sci. 2009;96(6):779–783. [Google Scholar]
- 24.Ismail H, Amanat MA, Iqbal A, Mirza B. Medicinal plants: a complementary and alternative antidepressant therapy. Curr Pharm Des. 2018;24:1–16. doi: 10.2174/1381612824666180727123950. [DOI] [PubMed] [Google Scholar]
- 25.NBG. National Biosafety Guideline, Pakistan Biosafety Rules. Government of Pakistan Notification No. F.2(7)95-Bio. 2005; 336(1): 1-9.
- 26.Yassa HD, Tohamy AF. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced diabetes mellitus in adult rats. Acta Histochem. 2014;116(5):844–854. doi: 10.1016/j.acthis.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Akinola OB, Biliaminu SA, Adediran RA, Adeniye KA, Abdulquadir FC. Characterization of prefrontal cortex microstructure and antioxidant status in a rat model of neurodegeneration induced by aluminium chloride and multiple low-dose streptozotocin. Metab Brain Dis. 2015;30(6):1531–1536. doi: 10.1007/s11011-015-9719-4. [DOI] [PubMed] [Google Scholar]
- 28.Enas AK. Study of the possible protective and therapeutic influence of coriander (Coriandrum sativum L.) against neurodegenerative disorders and Alzheimer’s disease induced by aluminum chloride in cerebral cortex of male albino rats. Nat Sci. 2010;8(11):202–213. [Google Scholar]
- 29.Kumar R, Arora V, Ram V, Bhandari A, Vyas P. Hypoglycemic and hypolipidemic effect of Allopolyherbal formulations in streptozotocin induced diabetes mellitus in rats. International Journal of Diabetes Mellitus. 2015;3(1):45–50. [Google Scholar]
- 30.Sajid M, Khan MR, Shah NA, Shah SA, Ismail H, Younis T, et al. Phytochemical, antioxidant and hepatoprotective effects of Alnus nitida bark in carbon tetrachloride challenged Sprague Dawley rats. BMC Complement Altern Med. 2016;16(1):1–16. doi: 10.1186/s12906-016-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Bannister JV, Calabrese L. Assays for superoxide dismutase. Methods Biochem Anal. 2006;32:279–312. doi: 10.1002/9780470110539.ch5. [DOI] [PubMed] [Google Scholar]
- 34.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 36.Hussein J, El-Matty D, El-Khayat Z, ABDEL-LATIF Y. Brain neurotransmitters in diabetic rats treated with CO enzyme Q10. Int J Pharm Pharm Sci. 2012;4:554–556. [Google Scholar]
- 37.Dilshad E, Zafar S, Ismail H, Waheed MT, Cusido RM, Palazon J, Mirza B. Effect of rol genes on polyphenols biosynthesis in Artemisia annua and their effect on antioxidant and cytotoxic potential of the plant. Appl Biochem Biotechnol. 2016;179(8):1456–1468. doi: 10.1007/s12010-016-2077-9. [DOI] [PubMed] [Google Scholar]
- 38.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahrmann A, Bahrmann P, Kubiak T, Kopf D, Oster P, Sieber C, et al. Diabetes und Demenz. Z Gerontol Geriatr. 2012;45(1):17–22. doi: 10.1007/s00391-011-0269-z. [DOI] [PubMed] [Google Scholar]
- 40.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 41.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, Boada J, Prat J, Portero-Otin M, Pamplona R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012:1–14. doi: 10.1155/2012/696215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer's disease. J Alzheimers Dis. 2009;16(4):763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn H-R, Shin M-H, Nam H-S, Park K-S, Lee Y-H, Jeong S-K, et al. The association between liver enzymes and risk of type 2 diabetes: the Namwon study. Diabetol Metab Syndr. 2014;6(1):1. doi: 10.1186/1758-5996-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samarghandian S, Azimi-Nezhad M, Samini F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed Res Int. 2014;2014:1–12. doi: 10.1155/2014/920857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thabrew MI, Joice P, Rajatissa W. A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med. 1987;53:239–241. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- 47.Tandon A, Bhalla P, Nagpaul J, Dhawan D. Effect of lithium on rat cerebrum under different dietary protein regimens. Drug Chem Toxicol. 2006;29(4):333–344. doi: 10.1080/01480540600820122. [DOI] [PubMed] [Google Scholar]
- 48.Cifariello A, Pompili A, Gasbarri A. 5-HT7 receptors in the modulation of cognitive processes. Behav Brain Res. 2008;195(1):171–179. doi: 10.1016/j.bbr.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds G, Mason S, Meldrum A, De Keczer S, Parties H, Eglen R, et al. 5-Hydroxytryptamine (5-HT) 4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol. 1995;114(5):993–998. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu-Taweel GM, Ajarem JS, Ahmad M. Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav. 2012;101(1):49–56. doi: 10.1016/j.pbb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 51.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993;18(1):33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 52.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 53.Gispen WH, Biessels G-J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23(11):542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- 54.Artola A. Diabetes, stress and ageing-related changes in synaptic plasticity in hippocampus and neocortex—the same metaplastic process? Eur J Pharmacol. 2008;585(1):153–162. doi: 10.1016/j.ejphar.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 55.Du L-L, Chai D-M, Zhao L-N, Li X-H, Zhang F-C, Zhang H-B, et al. AMPK activation ameliorates Alzheimer's disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer's disease model in rats. J Alzheimers Dis. 2015;43(3):775–784. doi: 10.3233/JAD-140564. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Feng L, Ma D, Zhang M, Gu J, Wang S, Fu Q, Song Y, Lan Z, Qu R, Ma S. Neuroprotective effect of paeonol on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. Neurosci Lett. 2013;549:63–68. doi: 10.1016/j.neulet.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izídio GS. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res. 2008;193(2):277–288. doi: 10.1016/j.bbr.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. Journal of Visualized Experiments. 2008;22(1088):1-4 [DOI] [PMC free article] [PubMed]
- 59.Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463(1–3):163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 60.Rodgers R, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21(6):801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]