Abstract
Objective:
To investigate the effects and mechanisms of catalpol on cardiac function in rats with isoproterenol (ISO)-induced myocardial infarction (MI).
Methods:
Adult male Wistar rats were divided into four groups: control group, ISO group, catalpol (L, low dose) group, and catalpol (H, high dose) group. Isoproterenol (85 mg/kg) was injected subcutaneously for 2 consecutive days to induce experimental MI. At the end of experiment, the effects of catalpol on cardiac function; apelin levels; apoptosis index; apelin, APJ, Bcl-2, and Bax protein expression; and caspase-3/9 activities were investigated.
Results:
The rats in the ISO group showed lower left ventricular maximum rate of positive or negative pressure development (±LVdp/dtmax) and left ventricular end-systolic pressure (LVSP) and higher left ventricular end-diastolic pressure (LVEDP) than those in the control group, suggesting severe cardiac dysfunction. Interestingly, catalpol administration significantly ameliorated the ISO-induced cardiac dysfunction. The groups administered low and high dosages catalpol (5 and 10 mg/kg/day, respectively) showed higher ±LVdp/dtmax and LVSP and lower LVEDP than the group administered ISO alone. Catalpol markedly upregulated apelin levels in the plasma and myocardium. Further, catalpol increased the apelin and APJ expression levels in the myocardium of the ISO-treated rats. In addition, catalpol pretreatment inhibited cardiomyocyte apoptosis as indicated by a decrease in the TUNEL-positive cell percentage, alterations in the Bax and Bcl-2 expression levels, and a decline in caspase-3 and caspase-9 activities.
Conclusion:
Our results revealed that catalpol can improve cardiac function. Its protective effects may be linked to the enhancement of myocardium contractility, regulation of the apelin/APJ pathway, and inhibition of cardiomyocyte apoptosis.
Keywords: catalpol, cardiac dysfunction, myocardial infarction, cardiomyocyte apoptosis, apelin, APJ
Introduction
Cardiac dysfunction following myocardial infarction (MI) leads to an increased risk of adverse cardiac events and severely affects patients’ quality of life. Despite the improvements in clinical management, MI and the resulting complications in cardiac function remain the leading cause of morbidity and mortality worldwide (1). Many recent studies have shown that the apelin/APJ pathway and cardiomyocyte apoptosis play an important role in heart failure (2, 3). Therefore, therapeutic strategies that modulate the apelin/APJ signaling pathway and inhibit apoptosis may be helpful in improving cardiac function after MI.
Preclinical studies generally use isoproterenol (ISO) to induce MI in rats (4). Subcutaneous administration of ISO causes acute myocardial injury accompanied by increased cardiac cell apoptosis and altered cardiac function, similar to that seen in patients with MI (5). Therefore, this ISO-induced MI model has been extensively used to investigate the effects of various potentially cardioprotective drugs (6).
In 1998, peptide apelin was originally isolated from bovine stomach tissue extracts. Many studies have suggested apelin to be an endogenous ligand of the human orphan G protein-coupled receptor APJ (7). Recent research has demonstrated that apelin can regulate food intake, angiogenesis, energy metabolism, and biological rhythm (8). In addition, another study has shown that the apelin/APJ pathway is involved in the maintenance of cardiac function (9). Apelin treatment has been reported to protect the heart against ischemia-reperfusion injury (10). Researchers have also reported that plasma apelin levels decrease in patients with cardiac dysfunction (11). However, patients with severe heart failure who were implanted with a left ventricular assist device showed markedly increased apelin levels in the left ventricle (12). Therefore, targeting the apelin/APJ signaling pathway has emerged as a novel therapeutic approach against heart failure (13).
Rehmannia glutinosa L., a traditional Chinese medicine, has been widely used in clinical therapy in China. Catalpol, an iridoid glycoside isolated from the root of R. glutinosa, has many therapeutic effects such as anti-inflammatory effects (14), protection against lung damage (15), and regulation of blood sugar level (16). Moreover, its neuroprotective effects have been well demonstrated in many studies (17). Our previous study has shown that catalpol can prevent myocardial injury in rats by substantiating the histopathological and biochemical changes. It is reported that Catalpol inhibited apoptosis and oxidative stress in glucose-deprived H9c2 cell through promoting cell mitophagy and modulating estrogen receptor (18). A recent study has also found that catalpol inhibits apoptosis through the mitochondrial-dependent caspase pathway (19). Thus, further research on the efficacy of catalpol in the treatment of cardiovascular diseases seems reasonable. The aim of this study was to investigate whether catalpol attenuates ISO-induced cardiac dysfunction in rats, and more importantly to explore the underlying mechanisms.
Methods
Animal preparation
Adult male Wistar rats weighing 180–200 g were obtained from the Experimental Animal Center of China Medical University. The rats were individually housed in cages at 22°C±3°C and 40%±10% humidity, with a standard 12 h light/dark cycle. The animals were allowed free access to pellet food and tap water. All the rats received humane care in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (revised 2011). All the experiments were approved by the Ethics Committee of China Medical University (JYT-20060948).
Drug
Catalpol (purity>98%, molecular formula: C15H22O10, molecular weight: 362.33) was supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Catalpol was dissolved in physiological saline for treatment.
Chemicals
Terminal dUTP nick end-labeling (TUNEL; cat. no. C1098)
and Caspase-3/9 assay Kits (cat. no. c1116 and c1158) were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Apelin enzyme immunoassay (EIA) kit was produced by Phoenix Pharmaceuticals Company (Belmont, CA, USA). ISO was supplied by Sigma Biotechnology (Sigma, St. Louis, MO, USA). All primary antibodies including antiapelin antibody (cat. no. ab125213), anti-APJ antibody (cat. no. ab214369), anti-Bcl-2 antibody (cat. no. ab59348), and anti-Bax antibody (cat. no. ab53154) were purchased from Abcam Biotechnology (Cambridge, United Kingdom), whereas all secondary antibodies were provided by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Establishment of the MI model
Rats were subcutaneously injected with ISO (85 mg/kg) at an interval of 24 h for 2 consecutive days to induce experimental MI (20).
Experimental protocols
Thirty-two adult male Wistar rats were randomly assigned into the following four groups: control group (n=8), in which the rats were intraperitoneally injected with physiological saline for 10 days; ISO group (n=8), in which the rats were intraperitoneally injected with physiological saline for 10 days with ISO being subcutaneously administered on the ninth day (85 mg/kg, once at an interval of 24 h for 2 consecutive days); catalpol (L, low dose) group (n=8), in which the rats were pretreated with catalpol (5 mg/kg; intraperitoneal injection) for 10 days with ISO being subcutaneously administered on the ninth day (85 mg/kg) for 2 consecutive days; and catalpol (H, high dose) group (n=8), in which the rats were pretreated with catalpol (10 mg/kg; intraperitoneal injection) for 10 days with ISO being subcutaneously administered on the ninth day (85 mg/kg) for 2 consecutive days.
Assessment of hemodynamics and left ventricular function
Blood pressure and heart rate were recorded 48 h after the first ISO injection using a computerized, noninvasive tail-cuff system, Visitech BP-2000 Blood Pressure Analysis System™ (Visitech Systems, Apex, NC, USA). Thereafter, all the rats were anesthetized with urethane (1 g/kg; intraperitoneal injection) to measure the left ventricular function. A catheter filled with heparin saline (500 U/mL) was inserted into the left ventricle. Left ventricular end-systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and left ventricular maximum rate of positive or negative pressure development (±LVdp/dtmax) were recorded using a BL-420E monitor system (Chengdu, China).
Specimen collection
After measuring cardiac function, blood samples were collected from the left ventricle, and the plasma was separated from each sample. The rats were then killed, and their hearts were immediately removed. Left ventricular tissues were washed with prechilled physical saline. One part of the tissue was fixed in 10% formalin, embedded in paraffin, and sectioned at 5 µm, and the other part was rapidly stored at −80°C for further analysis.
Enzyme immunoassay for apelin determination
The frozen myocardial tissues were homogenized in 0.1 mmol/L acetate on ice. Thereafter, the homogenate was boiled for 10 min and centrifuged at 12,000×g for 20 min. The supernatant was used to quantify the total protein level using the Bradford assay (21). Equal amounts of total protein were used in the apelin-36 EIA assay kit following the manufacturer’s instructions. Plasma was directly used for the assay, which was performed according to an EIA protocol recommended by the manufacturer. ED50 for rat apelin was 8.62 pg/tube; the cross-reactivity with rat apelin-36 was 100% and that with apelin-16 and apelin-13 was 0%.
Terminal dUTP nick end-labeling assay
To examine cardiomyocyte apoptosis, a TUNEL assay was performed using an apoptosis detection kit (C1098, Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer’s instructions. Apoptotic cells (dark brown) were then counted under a microscope (Olympus, Tokyo, Japan) at 400× magnification. Five sections from each group were randomly selected, and 10 random fields from each section were examined. In each field, the total numbers of cells and TUNEL-positive cells were counted. Finally, the percentage of apoptotic cells was calculated.
Western blot analysis
Western blotting was used to measure the apelin, APJ, Bcl-2, and Bax expression levels in the left ventricular tissues. The frozen tissues were weighed and homogenized in RIPA lysis buffer. Protein levels in the supernatant were determined using the Bradford method (21). Thereafter, 40 µg of protein was subjected to SDS-PAGE with 10% separation and transferred onto a nitrocellulose membrane for 3 h at 200 mA. The membranes were blocked with 1% bovine serum albumin for 1 h. Subsequently, primary antibodies for apelin (ab125213, dilution, 1:500; Abcam,
Cambridge, United Kingdom), APJ (ab214369, dilution, 1:500; Abcam, Cambridge, United Kingdom), Bcl-2 (ab59348, dilution, 1:500; Abcam, Cambridge, United Kingdom), and Bax (ab53154, dilution, 1:500; Abcam, Cambridge, United Kingdom) were added, followed by overnight incubation at 4°C. After washing twice with TBS, membranes were incubated with the respective secondary antibodies (dilution, 1:10,000; Santa Cruz, CA, USA). Finally, protein band intensities were quantified using a densitometer analysis system (Quantity One software, Bio-Rad, PA, USA).
Measurement of caspase-3 activity
Myocardial caspase-3 activity was determined using a caspase-3 activity assay kit according to the manufacturer’s instructions (c1116; Beyotime Biotech, Shanghai, China). Frozen myocardial tissues were crushed in liquid nitrogen and homogenized in an ice-cold lysis buffer. The homogenates were then centrifuged for 15 min at 4°C in a microcentrifuge (16,000×g). After collecting the supernatant, the protein level in each sample was assayed. Subsequently, 40 µL of reaction buffer with 10 µL of Ac-DEVD-pNA was added to 50 µL of protein samples, followed by incubation at 37°C for 6 h. Finally, the release of p-nitroaniline was measured at 405 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Caspase-3 activity was expressed as unit/h/mg protein.
Measurement of caspase-9 activity
Myocardial caspase-9 activity was determined using a caspase-9 activity assay kit (c1158; Beyotime Biotech, Shanghai, China). The protocol was similar to that of the caspase-3 activity assay, except for the use of Ac-LEHD-pNA as a substrate. Caspase-9 activity was expressed as unit/h/mg protein.
Statistical analysis
All data were presented as mean±standard deviation (SD). SPSS 13.0 software was used for data analysis. The Kolmogorov–Smirnov test was applied to test the normality of distributions. One-way analysis of variance (ANOVA) test followed by the Bonferroni post hoc test was used for multiple comparisons. P<0.05 was considered statistically significant.
Results
Mortality
As for a preliminary experiment was successfully performed in advance, only one rat in the ISO group was lost due to improper cannulation of blood vessel while monitoring cardiac function. The overall mortality was 3.12%.
General observations
As shown in Table 1, no significant differences in the body weight, heart weight, and heart index were observed among the groups, although a slight increase in the body weight and heart weight was observed in the ISO group.
Table 1.
Effects of catalpol on body weight, heart weight, and heart index in ISO-induced myocardial infarction in rats
| Groups | Number | Body weight (g) | Heart weight (mg) | Heart index (mg/g) |
|---|---|---|---|---|
| Control | 8 | 227.37±11.87 | 656.50±24.47 | 2.85±0.12 |
| ISO | 7 | 235.14±15.61 | 661.57±28.84 | 2.88±0.15 |
| Catalpol (L) | 8 | 228.63±11.03 | 658.88±25.27 | 2.91±0.16 |
| Catalpol (H) | 8 | 225.50±14.87 | 660.87±25.13 | 2.89±0.11 |
Catalpol (L) - low-dose catalpol; Catalpol (H) - high-dose catalpol; Heart index - body weight/heart weight, #P<0.05 versus control group, *P<0.05 versus ISO group
Effects of catalpol on the hemodynamic parameters in different groups
Table 2 shows the changes in the hemodynamic parameters during the experiment. The ISO group showed lower SBP, DBP, and MBP than the control group (p=0.004, p=0.02, and p=0.002, respectively). However, catalpol pretreatment (10 mg/kg) prevented this decline (p=0.045, p=0.007, and p=0.22, respectively). A similar change was not observed in the catalpol (L) group. Further, no significant change in the heart rate was observed in any two groups.
Table 2.
Effects of catalpol on the hemodynamic parameters of rats following myocardial infarction
| Groups | Number | SBP (mm Hg) | DBP (mm Hg) | MBP (mm Hg) | HR (beats/min) |
|---|---|---|---|---|---|
| Control | 8 | 137.87±17.33 | 116.75±14.68 | 126.13±12.06 | 383.87±18.58 |
| ISO | 7 | 111.86±9.63## | 94.86±11.39# | 102.43±11.87## | 403.47±22.86 |
| Catalpol (L) | 8 | 125.00±10.23 | 99.63±7.79 | 111.50±9.02 | 395.12±20.01 |
| Catalpol (H) | 8 | 131.37±13.08* | 119.50±16.64** | 120.63±11.04* | 401.01±19.97 |
Catalpol (L) - low-dose catalpol; Catalpol (H) - high-dose catalpol; SBP - systolic blood pressure; DBP - diastolic blood pressure; MBP - mean blood pressure; HR - heart rate
P<0.05
P<0.01 versus control group
P<0.05,
P<0.01 versus ISO group
Effects of catalpol on cardiac function following MI in rats
The ISO group showed a significant decrease in±LVdp/dtmax and LVSP but an increase in the LVEDP value compared with the control group (Table 3) (p<0.001, p<0.001, p<0.001, and p<0.001, respectively). Catalpol pretreatment (5 mg/kg) for 10 days significantly prevented the changes in the +LVdp/dtmax and LVEDP (p=0.043 and p<0.001) in the catalpol (L) group. Furthermore, alterations in the ±LVdp/dtmax, LVSP, and LVEDP were more statistically significant (p=0.002, p<0.001, p=0.007, and p<0.001, respectively) in the catalpol (H) group than in the ISO group.
Table 3.
Effects of catalpol on left ventricular function in ISO-induced myocardial infarction in rats
| Groups | Number | +LVdp/dtmax (mm Hg) | –LVdp/dtmax (mm Hg) | LVEDP (mm Hg) | LVSP (mm Hg) |
|---|---|---|---|---|---|
| Control | 8 | 3733.01±273.04 | 3367.01±240.02 | 4.85±0.86 | 151.63±12.72 |
| ISO | 7 | 2730.14±278.57## | 2524.14±295.76## | 17.34±1.09## | 122.71±11.43## |
| Catalpol (L) | 8 | 3160.37±282.43* | 2674.50±213.73 | 13.58±0.70** | 126.63±9.47 |
| Catalpol (H) | 8 | 3346.38±306.01** | 3120.13±174.08** | 11.34±1.15** | 143.38±10.39** |
Catalpol (L) - low-dose catalpol; Catalpol (H) - high-dose catalpol; +LVdp/dtmax - left ventricular maximum rate of positive pressure development; −LVdp/dtmax - left ventricular maximum rate of negative pressure development; LVEDP - left ventricular end-diastolic pressure; LVSP - left ventricular systolic pressure.
#P<0.05,
P<0.01 versus control group;
P<0.05,
P<0.01 versus ISO group
Effects of catalpol on plasma and myocardium apelin levels in ISO-treated rats
Our results demonstrated that the ISO group had significantly lower plasma apelin levels than the control group (Fig. 1) (P<0.001). Moreover, the catalpol (L and H) groups showed significantly higher plasma apelin levels than the ISO group (P=0.003 and P=0.002, respectively). Similarly, catalpol (10 mg/kg) significantly increased myocardium apelin levels (P<0.001).
Figure 1.

Effects of catalpol on apelin levels in the plasma and myocardium of the ISO-treated rats. (a) plasma apelin; (b) myocardium apelin. #P<0.05 versus the control group. ##P<0.01 versus the control group; *P<0.05 versus the ISO group; **P<0.01 versus the ISO group
Effects of catalpol on apelin and APJ expression levels
Apelin and APJ expression levels were significantly lower in the ISO group than in the control group (p<0.001 and p<0.001, respectively). Moreover, compared with the ISO group, catalpol (10 mg/kg) significantly increased apelin and APJ expression levels in the control group (Fig. 2) (p<0.001 and p<0.001, respectively).
Figure 2.

Effects of catalpol on the apelin and APJ protein expression levels in the myocardium of the ISO-treated rats. #P<0.05 versus the control group. ##P<0.01 versus the Control group; *P<0.05 versus the ISO group; **P<0.01 versus the ISO group
TUNEL assay
A TUNEL assay was performed to evaluate cardiomyocyte apoptosis in rats. As shown in Figure 3, the rate of cell apoptosis in the left ventricular tissues was higher in the ISO group than that in the control group (p<0.001). However, catalpol pretreatment (5 and 10 mg/kg) reduced the percentage of apoptotic cells (p=0.037 and p=0.002, respectively).
Figure 3.
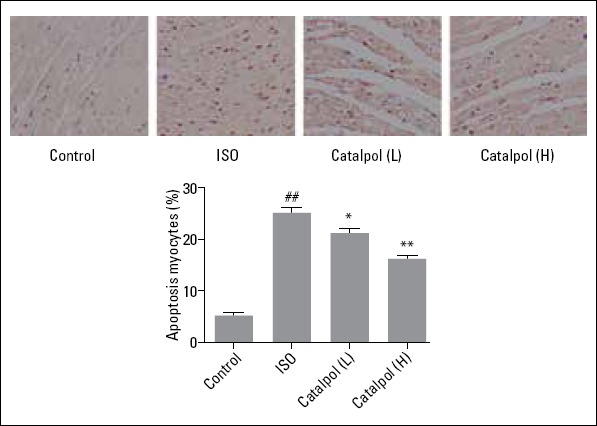
Photomicrographs of myocardial tissue sections showing TUNEL staining (400×). #P<0.05 versus the control group. ##P<0.01 versus the control group; *P<0.05 versus the ISO group; **P<0.01 versus the ISO group
Effects of catalpol on Bax and Bcl-2 expression levels
Western blot assays showed that compared with the control group, the Bax expression levels in the left ventricular tissues significantly increased and the Bcl-2 expression levels decreased in the ISO group (p<0.001 and p<0.001, respectively). However, catalpol pretreatment (10 mg/kg) reversed these changes (Fig. 4) (p=0.003 and p=0.005, respectively).
Figure 4.

The Bax and Bcl-2 expression levels in the heart tissue. (a) Representative graphs for Bax and Bcl-2 expression levels; (b) Bax/Bcl-2 ratio; (c, d) Quantitative analysis of the levels of Bax and Bcl-2. #P<0.05 versus the control group. ##P<0.01 versus the control group; *P<0.05 versus the ISO group; **P<0.01 versus the ISO group
Effects of catalpol on myocardium caspase-3 and caspase-9 activities in ISO-treated rats
As shown in Figure 5, although caspase-3 activity was increased in the ISO group, this upregulation was prevented by catalpol pretreatment (5 and 10 mg/kg) (p=0.003 and p=0.005, respectively). Similarly, the ISO group showed significantly higher caspase-9 activity than the control group (p=0.007). However, compared with the ISO group, catalpol pretreatment (5 and 10 mg/kg) for 10 days decreased the caspase-9 activity in the catalpol (L and H) groups (p=0.032 and p=0.006, respectively).
Figure 5.

Effects of catalpol on the caspase-3 and caspase-9 activities in the myocardium of the ISO-treated rats. (a) Caspase-3; (b) Caspase-9. #P<0.05 versus the control group. ##P<0.01 versus the control group; *P<0.05 versus the ISO group; **P<0.01 versus the ISO group
Discussion
The present study revealed that catalpol pretreatment for 10 days markedly attenuates cardiac dysfunction following MI in rats. These cardioprotective properties may be partly because of the modulation of the apelin/APJ signaling pathway and inhibition of cardiomyocyte apoptosis.
In the present study, hemodynamic parameters were monitored and recorded. ISO injections caused hemodynamic alterations, as indicated by a marked decrease in the systolic, diastolic, and mean blood pressures. Nevertheless, 10 mg/kg of catalpol significantly inhibited these changes. Moreover, ISO-treated rats showed lower LVESP, +LVdp/dtmax, and −LVdp/dtmax and higher LVEDP; these results are in agreement with those reported previously (22). Catalpol pretreatment, particularly in the catalpol (H) group, prevented left ventricular contractile dysfunction, as shown by improvements in LVSP and ±LVdp/dtmax. It also inhibited the ISO-induced increase in LVEDP, which was indicated by an amelioration of left ventricular diastolic dysfunction. These results suggest that catalpol can improve cardiac function in ISO-treated rats.
Apelin, isolated from bovine stomach tissue extracts, has been identified as an endogenous ligand of the human orphan G protein-coupled receptor APJ (23). Widely distributed in various tissues and highly expressed in the heart (24), apelin exhibits significant hypotensive and positive inotropic properties (25). It has been reported that apelin-deficient mice develop progressive heart failure (26). Exogenous administration of apelin exerts in vivo inotropic effects on normal and failing hearts. Genetic studies have also demonstrated that a disturbance in the endogenous apelin/APJ axis is closely associated with human cardiac dysfunction. These findings suggest that the apelin/APJ pathway plays a substantial role in the regulation of cardiovascular homeostasis. In the present study, we found a significant decrease in apelin levels in the plasma and myocardium following ISO administration. However, catalpol could enhance apelin levels in the plasma and myocardium, particularly in the catalpol (H) group. Similar changes in the apelin and APJ expression levels in the heart were observed. Therefore, these results suggest that catalpol can improve cardiac function, at least in part, through the activation of the apelin/APJ signing pathway.
Increasing evidence has demonstrated the substantial role of cardiomyocyte apoptosis in the progression of cardiac dysfunction in both acute and long-term settings after MI (27, 28). Given that apoptosis reduces the number of normal contractile cardiomyocytes and causes adverse ventricular remodeling, it severely affects cardiac function (29). Several drugs effective in treating heart failure (HF) have been proven to prevent cardiomyocyte apoptosis, including beta-blockers (30) and angiotensin II receptor antagonists (31). Therefore, therapeutic strategies preventing apoptosis offer an attractive approach for the treatment of HF. TUNEL-positive cells and caspase-3 and caspase-9 activities have been generally used to determine cardiomyocyte apoptosis. Our results showed that the ISO group had more number of apoptotic cardiomyocyte cells and higher caspase-3 and caspase-9 activities than the control group. On the other hand, catalpol pretreatment significantly inhibited cardiomyocyte apoptosis and decreased caspase-3 and caspase-9 activities. The aforementioned results reveal that catalpol may protect myocardial cells against apoptosis in vivo.
Bax and Bcl-2 are two important members of the Bcl-2 family and are directly associated with apoptosis regulation. Bax accelerates cell apoptosis (32, 33), whereas Bcl-2 inhibits cell apoptosis (34). The Bcl-2/Bax ratio determines the fate of cells after apoptotic stimulation (35). In the present study, compared with the control group, the ISO group exhibited significant downregulation in the Bcl-2 expression and marked upregulation in the Bax expression. However, catalpol pretreatment prevented the downregulation of Bcl-2 and the upregulation of Bax, indicating that catalpol may reverse the imbalance in the cardiomyocyte Bcl-2/Bax ratio. Thus, these results show that catalpol pretreatment may inhibit cardiocyte apoptosis through the regulation of apoptosis-related protein expression.
Study limitations
There are some limitations to the present study. First, echocardiographic examination was not performed to further evaluate the heart function. Second, heart dysfunction is a complicated process, and the relevant response participating in the process, such as energy metabolism, was not analyzed.
Conclusion
Based on the findings of the present study, we conclude that catalpol has cardioprotective effects on ISO-induced MI in rats. These protective effects may be associated with the amelioration of cardiac dysfunction, at least in part, through the regulation of the apelin/APJ signaling pathway and inhibition of cardiomyocyte apoptosis.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – F.B., Y.X.; Design – Y.X.; Supervision – F.B., Y.X.; Fundings – Y.X.; Materials – F.B., Q.S.; Data collection &/or processing – Y.X., Q.S.; Analysis &/or interpretation – F.B., Y.X.; Literature search – Y.X.; Writing – Y.X.; Critical review – F.B., Y.X.
References
- 1.Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Cardioprotection and pharmacological therapies in acute myocardial infarction:Challenges in the current era. World J Cardiol. 2014;6:100–6. doi: 10.4330/wjc.v6.i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia YX, Pan CS, Zhang J, Geng B, Zhao J, Gerns H, et al. Apelin protects myocardial injury induced by isoproterenol in rats. Regul Pept. 2006;133:147–54. doi: 10.1016/j.regpep.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Panda S, Kar A, Ramamurthy V. Cardioprotective effect of vincristine on isoproterenol-induced myocardial necrosis in rats. Eur J Pharmacol. 2014;723:451–8. doi: 10.1016/j.ejphar.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 4.Senthil S, Chandramohan G, Pugalendi KV. Isomers (oleanolic and ursolic acids) differ in their protective effect against isoproterenol-induced myocardial ischemia in rats. Int J Cardiol. 2007;119:131–3. doi: 10.1016/j.ijcard.2006.07.108. [DOI] [PubMed] [Google Scholar]
- 5.Zhou R, He LF, Li YJ, Shen Y, Chao RB, Du JR. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J Ethnopharmacol. 2012;139:440–6. doi: 10.1016/j.jep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Engle SK, Jordan WH, Pritt ML, Chiang AY, Davis MA, Zimmermann JL, et al. Qualification of cardiac troponin I concentration in mouse serum using isoproterenol and implementation in pharmacology studies to accelerate drug development. Toxicol Pathol. 2009;37:617–28. doi: 10.1177/0192623309339502. [DOI] [PubMed] [Google Scholar]
- 7.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 8.Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, et al. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun. 2002;291:1208–12. doi: 10.1006/bbrc.2002.6575. [DOI] [PubMed] [Google Scholar]
- 9.Lee DK, George SR, O'Dowd BF. Unravelling the roles of the apelin system:prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–4. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Tao J, Zhu W, Li Y, Xin P, Li P, Liu M, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol. 2011;301:H1471–86. doi: 10.1152/ajpheart.00097.2011. [DOI] [PubMed] [Google Scholar]
- 11.Chong KS, Gardner RS, Morton JJ, Ashley EA, McDonagh TA. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur J Heart Fail. 2006;8:355–60. doi: 10.1016/j.ejheart.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–9. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 13.Zhong JC, Zhang ZZ, Wang W, McKinnie SM, Vederas JC, Oudit GY. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim Biophys Acta. 2017;1863:1942–50. doi: 10.1016/j.bbadis.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Jin C, Li Y, Guan S, Han F, Zhang S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem Toxicol. 2013;58:50–5. doi: 10.1016/j.fct.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Fu K, Piao T, Wang M, Zhang J, Jiang J, Wang X, et al. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2014;23:400–6. doi: 10.1016/j.intimp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Shieh JP, Cheng KC, Chung HH, Kerh YF, Yeh CH, Cheng JT. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J Agric Food Chem. 2011;59:3747–53. doi: 10.1021/jf200069t. [DOI] [PubMed] [Google Scholar]
- 17.Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu H, et al. Catalpol provides protective effects against cerebral ischaemia/reperfusion injury in gerbils. J Pharm Pharmacol. 2014;66:1265–70. doi: 10.1111/jphp.12261. [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Lu Y, Yan X, Wu X, Kuai M, Sun X, et al. Catalpol protects glucose-deprived rat embryonic cardiac cells by inducing mitophagy and modulating estrogen receptor. Biomed Pharmacother. 2017;89:973–82. doi: 10.1016/j.biopha.2017.02.069. [DOI] [PubMed] [Google Scholar]
- 19.Hu LA, Sun YK, Zhang HS, Zhang Hu J. Catalpol inhibits apoptosis in hydrogen peroxide-induced cardiac myocytes through a mitochondrial-dependent caspase pathway. Biosci Rep. 2016;36 doi: 10.1042/BSR20160132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal SN, Sharma C, Mahajan UB, Patil CR, Agrawal YO, Kumari S, et al. Protective Effects of Cardamom in Isoproterenol-Induced Myocardial Infarction in Rats. Int J Mol Sci. 2015;16:27457–69. doi: 10.3390/ijms161126040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulhanek-Heinze S, Gerbes AL, Gerwig T, Vollmar AM, Kiemer AK. Protein kinase A dependent signalling mediates anti-apoptotic effects of the atrial natriuretic peptide in ischemic livers. J Hepatol. 2004;41:414–20. doi: 10.1016/j.jhep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Xu W, Yang J, Wu LM. Cardioprotective effects of tanshinone IIA on myocardial ischemia injury in rats. Pharmazie. 2009;64:332–6. [PubMed] [Google Scholar]
- 23.Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 24.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84:1162–72. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 25.Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner T, et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110(11 Suppl 1):II187–93. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 26.Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res. 2007;101:e32–42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Wang Q, Guo W, Zhu YZ. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure:a mechanism through cardiac mitochondrial protection. Biosci Rep. 2011;31:87–98. doi: 10.1042/BSR20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Ye D, Wang Y. Caspase-3 as a therapeutic target for heart failure. Expert Opin Ther Targets. 2013;17:255–63. doi: 10.1517/14728222.2013.745513. [DOI] [PubMed] [Google Scholar]
- 29.Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41:753–60. doi: 10.1016/s0735-1097(02)02959-5. [DOI] [PubMed] [Google Scholar]
- 30.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–90. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 31.Soga M, Kamal FA, Watanabe K, Ma M, Palaniyandi S, Prakash P, et al. Effects of angiotensin II receptor blocker (candesartan) in daunorubicin-induced cardiomyopathic rats. Int J Cardiol. 2006;110:378–85. doi: 10.1016/j.ijcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 32.Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxid Redox Signal. 2003;5:589–96. doi: 10.1089/152308603770310257. [DOI] [PubMed] [Google Scholar]
- 33.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK:A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(Pt 4):437–41. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cory S, Adams JM. The Bcl2 family:regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]


