Abstract
Objectives To examine (1) trajectories of sleep disturbances in adolescents with spina bifida (SB) compared with a typically developing (TD) group over a 10-year period and (2) individual, family, and socioeconomic determinants of changes in sleep disturbances. Methods Participants were 68 families of youth with SB and 68 families of TD youth. Parent-report of adolescent sleep was collected every 2 years at 6 time points (T1: ages 8–9; T6: ages 18–19). Multiple informants and measures were used to examine internalizing, externalizing, and inattention symptoms, dyadic/family conflict, socioeconomic status (SES), and family income. Results Sleep disturbances increased over the 10-year period. Youth with SB had greater sleep disturbances during early adolescence. Greater preadolescent externalizing symptoms, greater parent–child and marital conflict, and lower SES predicted increased sleep disturbances. Conclusions Sleep disturbances are common and persistent in adolescents with SB. Sleep assessment and management are important clinical and research priorities in this population.
Keywords: adolescents, longitudinal research, sleep, spina bifida
Adolescents experience a number of normal developmental changes in the pattern and timing of their sleep, resulting in later bedtimes and wake times, and unfortunately often giving rise to a variety of behavioral sleep problems (e.g., decreased total sleep time, trouble sleeping) and daytime fatigue or sleepiness ( Carskadon, 2011 ). We collectively define these in this study as sleep disturbances . A number of studies have begun to examine the consequences of sleep disturbances, providing strong support for the long-term impact of sleep disturbances on adolescent physical (e.g., immunity, obesity), psychosocial (e.g., mood, behavior), and academic functioning (e.g., Lovato & Gradisar, 2014 ; Shochat, Cohen-Zion, Tzischinsky, 2014 ). Adolescents are also at a risk for recurrence or persistence of sleep disturbances into adulthood ( Roberts, Roberts, & Duong, 2009 ). Sleep disturbances are particularly common and persistent in children and adolescents with chronic health conditions, such as asthma, allergies, diabetes, epilepsy, physical disabilities, and pain conditions ( Hysing, Sivertsen, Stormark, Elgen, & Lundervold, 2009 ; Tietze et al., 2012 ; Valrie, Bromberg, Palermo, & Schanberg, 2013 ), likely owing to the unique confluence of disease-related (e.g., pain, medication use) and psychosocial factors (e.g., comorbid depression and anxiety; Lewandowski, Ward, & Palermo, 2011 ). Despite the clinical importance of sleep, including their impact on daytime and physiologic functioning (e.g., increased inflammation, decreased immunity; AlDabal & BaHammam, 2011 ), few studies have systematically evaluated the developmental course and determinants of sleep disturbances in vulnerable pediatric conditions, including youth with spina bifida (SB).
SB is the most common congenital birth defect that affects the central nervous system, occurring in 3 of 10,000 live births in the United States ( Parker et al., 2010 ). This condition arises owing to the failed closure of one or more vertebrae surrounding the embryo’s developing spinal cord, and is associated with varying degrees of motor impairment, neurocognitive deficits, and difficulties with bladder and bowel management ( Liptak, 1997 ) that requires a strict medical regimen (e.g., intermittent catheterization, physical therapy, medications, dietary restrictions, routine skin checks). Thus, condition-specific characteristics (e.g., presence of a shunt, multiple surgeries) may interact with normative developmental and psychosocial factors (e.g., puberty, early school start times) to place adolescents with SB at a greater risk for developing sleep disturbances compared with their typically developing (TD) peers. Although several studies have revealed the presence of behavioral sleep problems (e.g., trouble sleeping; Edelstein, Cirino, Hasher, Fletcher, & Dennis, 2012 ), there is limited understanding at present of individual sleep trajectories over development and whether adolescents with SB may have different trajectories than their TD peers. Extant research using longitudinal data has focused on TD adolescents and presents mixed findings, with some evidence for a developmental incline ( Sadeh, Dahl, Shahar, & Rosenblat-Stein, 2009 ) and for stability ( Gregory & O’Connor, 2002 ) of sleep disturbance trajectories. Mixed findings may be the result of short follow-up periods during one stage of adolescence (e.g., early, middle, or late adolescence). A comprehensive evaluation of developmental changes in sleep disturbances in both TD and chronic illness populations requires longitudinal investigations over the entire course of adolescence.
Our understanding of the developmental course of sleep disturbances in adolescents with chronic health conditions such as SB may be further clarified by expanding knowledge on early vulnerabilities or precipitating factors for disrupted sleep. In particular, meta-analytic and population-based research directly inform developmental ecological systems models of disease-specific pediatric sleep research by enhancing our understanding of the role of individual , social (i.e., family functioning ), and socioeconomic contexts in the development of adolescent sleep disturbances ( El-Sheikh & Sadeh, 2015 ). For example, epidemiological research has found individual vulnerability including emotional (i.e., internalizing symptoms) and behavioral (i.e., externalizing and inattention) maladjustment to predict adolescent sleep disturbances ( Patten, Choi, Gillin, & Pierce, 2000 ). Research has also identified a number of possible mechanisms such as cognitive (e.g., ruminating thoughts), physiological (e.g., emotional arousal), inflammatory, and neurochemical processes that may link emotional and behavioral difficulties with the occurrence and persistence of adolescent sleep disruption ( Astill, Van der Heijden, Van Ijzendoorn, & Van Someren, 2012 ; Dahl, 1996 ; El-Sheikh, Buckhalt, Keller, & Granger, 2008 ). Despite methodological recommendations to measure sleep early in development to understand the direction of causation in the relation between maladjustment and sleep ( Astill et al., 2012 ), extant longitudinal studies have focused on sleep disruption as a predictor of individual maladjustment (e.g., Quach, Hiscock, Canterford, & Wake, 2009 ), and few have tested the converse direction (e.g., Kelly & El-Sheikh, 2014 ).
Early preadolescent family functioning and socioeconomic risk factors may also lead to adolescent sleep disruption. Prospective research has found that greater dyadic- (marital, parent–child) and family-level conflict predicts sleep disturbances in school-aged children and adolescents ( Gregory, Caspi, Moffitt, & Poulton, 2006 ; Kelly & El-Sheikh, 2011 ). Additionally, research on socioeconomic determinants of sleep in adolescents has identified lower socioeconomic status (SES) and income as key precipitating factors for sleep disturbance ( Marco, Wolfson, Sparling, & Azuaje, 2012 : El-Sheikh et al., 2013 ). Family conflict and socioeconomic adversity may be broadly linked to higher levels of perceived vulnerability and environmental stress ( Jarrin, McGrath, & Quon, 2014 ), leading to enduring patterns of cognitive and physiological arousal that disrupt sleep. Further, socioeconomic factors that are relatively constant across adolescence may have a consistent negative impact on sleep, including aspects of the physical sleep environment (light/temperature regulation, shared sleeping spaces; Boe, Hysing, Stormark, Lundervold, & Sivertsen, 2012 ) and access to or quality of health care ( Chen, Martin & Matthews, 2006 ).
Thus, the primary aim of this article was to examine the longitudinal course of sleep disturbances in adolescents with SB compared with a matched TD comparison group over a 10-year period (i.e., age 8–9 to 18–19 years). We expected that sleep disturbances would demonstrate a developmental increase over the 10 years. Adolescents with SB were hypothesized to have higher overall mean levels and a steeper trajectory of sleep disturbances compared with TD youth (overall group effect). Our second aim was to determine the impact of early preadolescent (i.e., ages 8–11 years or Times 1–2) individual adjustment, family functioning, and socioeconomic risk factors on trajectories of sleep disturbances over the 10-year period (i.e., ages 8–19 years or Times 1–6) in youth with SB and their TD peers. Greater preadolescent emotional and behavioral maladjustment (internalizing, externalizing, inattention symptoms), family dysfunction (marital, parent–child, and family conflict), and socioeconomic adversities (lower SES and income) were hypothesized to predict increased sleep disturbances over the 10-year period. These determinants of sleep trajectories were hypothesized to be stronger for youth with SB compared with their TD peers, as the chronic physical, medical, cognitive, and social demands of this complex medical illness were expected to impede youths’ ability to cope with disruptions at every contextual level.
Methods
Participants
Participants in this study were drawn from a larger, longitudinal investigation examining family functioning and psychosocial outcomes in children with SB and TD children (see Holmbeck et al., 2010 ). Families of youth with SB were recruited from three Midwest hospitals and a state-wide SB association. This study included a matched comparison sample of TD children and their families recruited from schools where participating youth with SB were enrolled. Groups were matched on 10 demographic variables, including age, gender, ethnicity, SES, and parental age and family structure (see Holmbeck et al., 2003 for details on the matching process), and they did not differ significantly on any of these variables at Time 1 ( p > .05). The present study examined six waves of data that were collected every 2 years (Time 1 [T1]: age 8–9 years; Time 6 [T6]: age 18–19 years).
Procedure
Institutional review boards approved this study in the university and hospital settings. Parents and children signed consents and assents, respectively. Parents of youth with SB were also asked to sign a release of information form to obtain data on youths’ physical status (e.g., number of shunt revisions) from medical records at T1. For the first five data collection waves (T1 through T5), family members separately completed counterbalanced questionnaire packets and participated in a series of semi-structured videotaped family interaction tasks during 3-hr home visits. Videotaped interaction tasks were presented in a counterbalanced order and consisted of a warm-up task, an unfamiliar board game, a structured family interaction task ( Ferreira, 1963 ), and a conflict task ( Smetana, Yau, Restrepo, & Braeges, 1991 ). Although both parents were encouraged to participate in the interaction tasks, sometimes only one parent was available; this was based on the individual circumstances of each family (e.g., some parents were not able to participate owing to work schedule conflicts). Family interaction tasks were coded using a global coding system developed by Johnson and Holmbeck (1995) that was based on a methodology devised by Smetana and colleagues (1991) and has demonstrated adequate reliability and validity ( Kaugars et al., 2011 ). 1 As is typical of global rating systems, coders viewed individual family tasks and then provided ratings on a variety of dimensions (see Holmbeck, Li, Schurman, Friedman, & Coakley, 2002 for further details regarding family interaction procedures). At T6, families completed questionnaires by mail rather than through home visits. Participants received monetary compensation at each data collection wave.
Measures
Forming Composite Measures
Study investigators created composites that included multiple reporters and methods (e.g., questionnaire and observed measures) for several constructs of interest, which has been recommended as a useful multisource and multimethod data management technique to decrease the number of analyses and reduce the possibility of shared method variance ( Holmbeck et al., 2002 ). For all independent variables, scale reliabilities were run for each composite with more than two reporters or data collection methods, and correlations were run for composites with only two reporters/methods to assess the associations between reports/method of the same construct. Scales were averaged to create composites if scale reliability was adequate ( r s > .30 or α s > .60). Further rationale for combining multiple informants and sources included relatively small standardized mean difference effect sizes (Cohen’s d = 0.02–0.53, M = 0.25, SD = 0.14). When there was less than adequate scale reliability for a composite, reporter/method scales were dropped until adequate reliability was achieved. To determine preadolescent predictors of sleep disturbances, predictor variables were averaged across T1 and T2 (i.e., ages 8–11 years) with the exception of SES, which was only measured at T1.
Sleep Disturbances (T1–T6)
Mother and father responses to six items of the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2003 ) were used to assess overall adolescent sleep disturbances. Across all assessment waves, parents responded to six items that assess the degree to which the youth has nightmares , sleeps less than most children , sleeps more than most children , talks or walks in their sleep , has trouble sleeping , and is overtired on a 3-point Likert scale (0 = not true , 1 = somewhat true , 2 = very true ). Research has demonstrated convergent validity of the CBCL sleep composite with measures of sleep functioning in children and adolescents aged 6–18 years (e.g., youth report on the Adolescent Sleep-Wake Scale; Becker, Ramsey, & Byars, 2015 ). Although examination of individual sleep items may be useful in studies assessing individual facets of sleep (e.g., Coulombe, Reid, Boyle, & Racine, 2011 ), the CBCL sleep composite score has been recommended to determine the prevalence and correlates of overall sleep functioning ( Becker et al., 2015 ). Mother and father report of the CBCL composite were highly correlated across time for the SB group ( r s = .43–.62) and the TD group ( r s = .39–.64). Because of low reliabilities that are typically reported in research using the CBCL sleep composite score (e.g., Becker et al., 2015 ), reliability analyses included both mother and father report of individual sleep items, which resulted in adequate reliability (SB: α s = .64–.77; TD: α s = .61–.81) with the exception of the TD sample at T2 ( α = .55) and the SB sample at T3 ( α = .49). All items were retained because deleting individual sleep items did not produce an increase in reliability. Thus, the final sleep disturbance composite scores were created by averaging across the six mother and father items for T1–T6.
Emotional and Behavioral Adjustment (T1 and T2)
Internalizing S ymptoms. Mother, father, and teacher reports on the Anxious/Depressed subscale of the CBCL ( Achenbach & Rescorla, 2003 ) were used to assess adult perceptions of youth internalizing symptoms. Youth report of internalizing symptoms was based on responses to the Child Depression Inventory (CDI; Kovacs, 1992 ). The CDI assesses 27 symptoms of depression, with higher scores indicating greater symptomatology. Two items of the CDI related to trouble sleeping and fatigue were excluded in creating composites to reduce overlap with the sleep disturbances dependent variable. Average item scores for parent and teacher reports of youth internalizing symptoms on the CBCL and youth report on the CDI were converted to z-scores and averaged to create internalizing symptoms composite scores for T1 and T2. The child CDI score and teacher CBCL score were dropped from the composite at T1 owing to low reliability. This resulted in adequate composite reliability for the SB group (T1: r = .36, T2: α = .66) and the TD group (T1: r = .37, T2: α = .66).
Externalizing Symptoms. Mother, father, and teacher reports on the Rule Breaking and Aggression subscales of the CBCL ( Achenbach & Rescorla, 2003 ) were used to assess youth externalizing symptoms. Mean item scores for mother, father, and teacher reports of youth externalizing symptoms on the CBCL were then averaged to create a composite score for overall externalizing symptoms for T1 and T2. Composite reliability was adequate across both time points (SB: α s = .65 and .71; TD: α = .68 and .77).
Inattention. Mother, father, and teacher reports on the Inattention subscale of the CBCL ( Achenbach & Rescorla, 2003 ) were used to assess youth inattention. Mean item scores for mother, father, and teacher reports of youth inattention symptoms on the CBCL were averaged to create a composite score for overall inattention for T1 and T2. Composite reliability was adequate across both time points (SB: α s = .68 and .69; TD: α s = .78 and .74).
Family Functioning (T1 and T2)
Family Conflict. Family-level conflict was assessed using mother and father reports on the Conflict subscale of the Family Environment Scale (FES; Moos & Moos, 1994 ), which measures social and environmental aspects of actual family environments. The FES is composed of 10 subscales and was administered in a true/false format (0 = false ; 1 = true ) at T1 and in a 4-point Likert scale format at T2 (the change being made to increase the number of response options and to increase the internal consistency of each of the subscales). Mother and father reports on the FES at T1 and T2 were converted to z-scores and averaged given adequate correlations between reporters across both time points (SB: r s = .35 and .49; TD: r s = .63 and .54).
Marital Conflict. Marital conflict was assessed using one item related to observed mother–father conflict from coding of videotaped family interactions by trained, reliable coders at each home visit. Interrater reliability (IRR) was adequate across both time points (SB: IRRs = .70 and .57; TD: IRRs = .65 and .72).
Parent – Child Conflict. Parent–child conflict was assessed using observed mother–child and father–child conflict (i.e., two codes) from coding of video-taped interactions (IRRs = .66–.79) as well as mother, father, and child reports on the Parent-Adolescent Conflict Scale (PACS), which is a brief 15-item version of the Issues Checklist ( Prinz, Foster, Kent, & O’Leary, 1979 ) including potential conflicts that emerge among families. Average item scores for observed mother–child and father–child conflict and mother, father, and child reports on the PACS were converted to z-scores and then averaged to create a composite score for overall parent–child conflict for T1 and T2. Mother, father, and child scores on the PACS were dropped at T1 owing to low reliability. Composite reliability was adequate across both time points (SB: r = .57 and α = .65; TD: r = .74 and α = .70).
Socioeconomic Characteristics (T1 and T2)
Socioeconomic Status . The Hollingshead Four Factor Index of SES was used to assess SES ( Hollingshead, 1975 ) at T1, which was computed by assigning a score to maternal and/or paternal occupation and education level. Higher scores represent higher SES. For two-parent families in which both caregivers were employed, education and occupation scores were combined and averaged to calculate SES. For single-parent families, or for two-parent households in which only one parent was employed, the employed parent’s information was used to calculate SES.
Family Income. Family income at T1 and T2 was assessed using parent report from the Parent Demographic Questionnaire. Income was coded on an 11-point scale (1 = under $10,000 , 2 = $10,000–19,999 ; up to 11 = over 100,000 ).
Statistical Considerations for Growth Curve Analyses
The sample size of 136 participants was determined to be adequate given previous recommendations for growth curve analyses (i.e., 100 participants; Curran, Obeidat, & Losardo, 2010 ). Further, the current study included six data collection waves resulting in a high number of potential observations (136 participants × 6 data collection waves = 816 observations) and, thus, increased power to detect factors related to growth in sleep disturbances. Results indicated that none of the study composites were highly skewed (skewness values = 0.029–1.51).
Given our interest in modeling developmental trajectories of sleep, individual growth curve models were estimated using Mixed Proc in SPSS ( Peugh & Enders, 2005 ). Our first step was to determine the functional form of growth in sleep disturbances by estimating two competing growth models: intercept-only (i.e., stability in sleep) and unconditional linear (i.e., linear growth in sleep). Full maximum likelihood estimation was used for these models. Relative model fit was assessed by comparing the −2 log likelihood statistics of the two competing, nested models (see DeLucia & Pitts, 2010 for further details). In the unconditional growth model, the “time” variable represented the sample age centered at age 9 years, or the average age on the first assessment occasion. Results revealed that the linear model was an improvement over the intercept-only model ( Δ-2LL [2] = 25.67, p < .01). Therefore, the best-fitting growth model for sleep disturbances was determined to be linear. Next, group was added to the best-fitting model to determine potential group differences in the intercept and slope of sleep disturbances. Our final step was to enter individual, family, and socioeconomic predictors into the best-fitting model.
Results
Demographic and Clinical Characteristics of the Study Sample
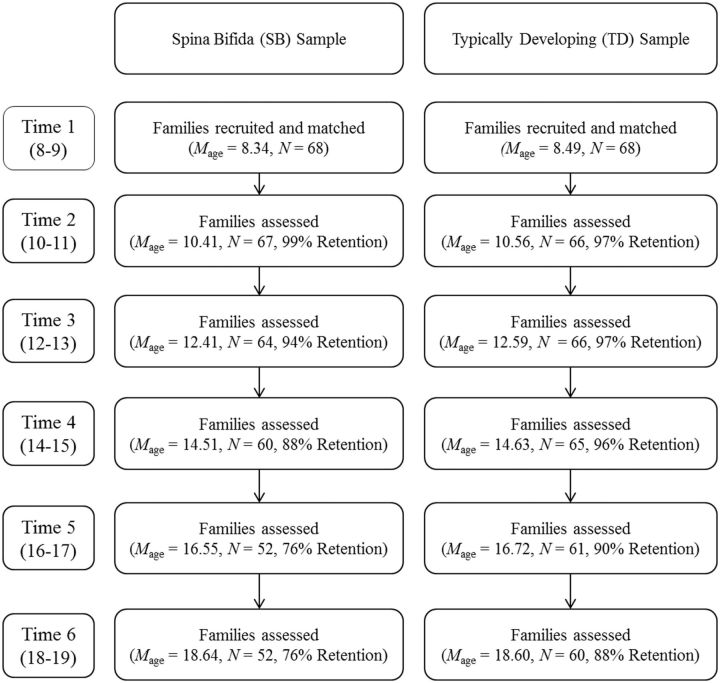
At Time 1 (T1), youth with SB ( n = 68; 54% male; 82% Caucasian) and TD children ( n = 68; 54% male; 91% Caucasian) were between 8 and 9 years old. See Table I for a description of the sample based on Time 1 demographics. See Figure 1 for a flow chart of participation and age of the study sample across time points. Families of youth with SB had significantly higher attrition rates at T5 only (χ 2 = 4.24, p = .03). A comparison of families who participated at T6 versus those did not participate revealed no differences with respect to gender, race, or SES for either the SB group or comparison group. At Time 6 (T6), the majority of the total study sample (69.6%; 84.6% of SB group and 56.7% of TD group) continued to live at home with their caregivers, respectively.
Table I.
Descriptive Data on Original Sample at Time 1
| Characteristic | Spina bifida M ( SD ) or N (%) | Typically developing M ( SD ) or N (%) | Statistical test |
|---|---|---|---|
| Child age | 8.34 (0.48) | 8.49 (0.50) | t (134) = −1.75 |
| Maternal age | 37.74 (5.19) | 37.74 (4.84) | t (134) = 0.00 |
| Paternal age | 41.02 (5.45) | 40.63 (6.50) | t (105) = 0.33 |
| Gender | |||
| Male | 37 (54.41) | 37 (54.41) | χ 2 (1) = 0.00 |
| Female | 31 (45.59) | 31 (45.59) | |
| Child ethnicity | |||
| Caucasian | 56 (82.35) | 62 (91.18) | χ 2 (1) = 2.30 |
| Other | 12 (17.65) | 6 (8.82) | |
| Family structure | |||
| Two-parent intact | 55 (80.88) | 47 (69.12) | χ 2 (1) = 2.51 |
| Not intact | 13 (19.2) | 21 (30.88) | |
| Child birth order, M ( SD ) | 2.12 (1.38) | 2.06 (1.29) | t (129) = 0.27 |
| Family SES | 43.12 (10.6) | 46.46 (10.9) | t (131) = −1.80 |
Note. n = 68 for both samples. All statistics were nonsignificant. Marital status was collapsed to intact versus not intact (i.e., mother/stepfather, single mother, separated, other). SES = socioeconomic status measured by Hollingshead Four Factor Index ( Hollingshead, 1975 ). The mean Hollingshead SES scores for each group indicated families were of upper middle class status on average. Calculation of SES score frequencies also indicated that 25.0% ( n = 34) and 9.0% ( n = 9) of families fell into lower middle and lower class categories, respectively.
Figure 1.
Flow chart of participation and age of sample across six time points.
Researchers collected information on several physical status variables for the SB group from mother report (i.e., ambulation method, shunt status) and review of medical charts (i.e., type of SB, lesion level, number of shunt surgeries). The majority of participating youth had myelomeningocele (82% myelomeningocele, 12% lipomeningocele, 6% other), lumbar-level lesions (54% lumbar level, 32% sacral level, 13% thoracic), and required assistance for ambulation (63% used braces, 18% used a wheelchair, 19% were unassisted). The majority of youth also had shunted hydrocephalus (71%), and the average number of shunt revisions was 2.50 ( SD = 2.91) at T1. There were no differences with respect to shunt status, number of shunt revisions and medical surgeries, type of SB, lesion level, or ambulation between those with SB who participated at T6 and those who dropped out of the study.
The incidence of youth internalizing, externalizing, and inattention, and sleep according to the CBCL for the total sample was also examined for descriptive purposes. The percentages of youth with subclinical or clinical t-scores (i.e., ≥ 65) according to mother, father, and teacher reports at T1 and T2 (i.e., preadolescence, aged 8–11 years) were as follows: internalizing (4.0–13.1%), externalizing (2.9–6.4%), and inattention (8.9–18.9%). Per mother and father report of youth sleep disturbances, item-level frequencies (i.e., sleep behaviors occurring “ often ”) and means varied widely across all 6 time points. The most frequently and consistently reported sleep disturbances included being overtired (0.0–5.7%; M = 0.20–0.36) and having nightmares (0.0–11.3%; M = 0.10–0.52). Item-level descriptive statistics for all other sleep items were as follows: sleeps less than most children (0.0–6.8%; M = 0.11–0.18), sleeps more than most children (0.0–8.1%; M = 0.07–0.28), talks or walks in sleep (0.0–2.7%; M = 0.05–0.13), and trouble sleeping (0.0–4.7%; M = 0.11–0.18).
Aim 1: Group Differences in Sleep Disturbance Trajectories
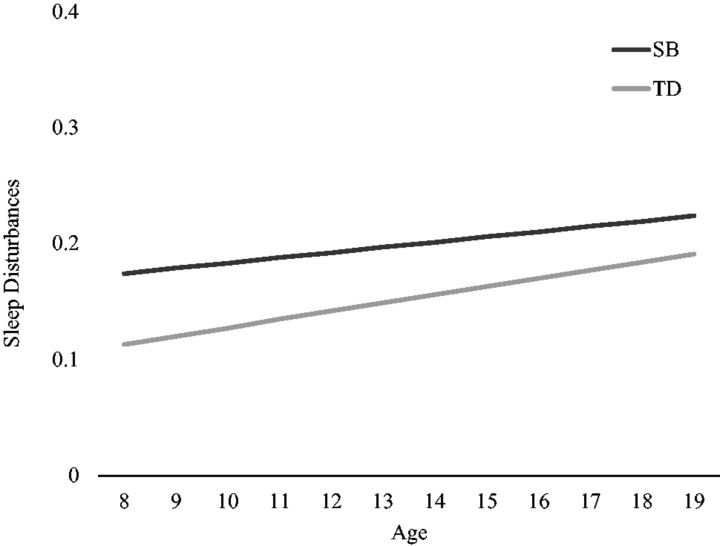
Examination of the baseline linear model growth parameters indicated that sleep disturbances increased over time for both groups (est = 0.010, SE = 0.002, p = .02) ( Figure 2 ). The intercept and slope variances for this model were significant (est for intercept = 0.016, SE = 0.003; est for slope = 0.001, SE = 0.001; p s < .001), indicating adequate between-person variability at age 9 years and in rate of change in sleep disturbances to continue subsequent analyses.
Figure 2.
Estimates of model-implied sleep disturbances means across age, separated by spina bifida (SB) and typically developing (TD) groups. Note. The y-axis metric represents approximately 1 standard deviation above (0.40) and below (0.00) the average Child Behavior Checklist (CBCL) sleep composite score across time and samples ( M = 0.17, SD = 0.21, range: 0.00–1.17). The average CBCL sleep composite score across the 6 time points was 0.20 (range: 0.17–0.22) and 0.15 (range: 0.13–0.19) for the SB and TD groups, respectively.
Next, group was entered into the best-fitting model to determine group differences in sleep trajectories. A significant group effect predicting intercepts emerged, indicating that youth with SB had greater sleep disturbances at age 9 years (est = −0.06, SE = 0.027, p = .029). Plotting estimates of model-implied means across time indicated that trajectories began to converge over time ( Figure 2 ). This visual evidence, coupled with the absence of a group effect predicting linear trends (est = 0.004, SE = 0.005, p = .364) indicates that youth with SB demonstrated greater sleep disturbances during early adolescence, but group differences were reduced later in adolescence.
Aim 2: Predictors of Sleep Disturbance Trajectories 2
Between-person variation in the intercept (est = 0.016, SE = 0.004, p < .001) and slope terms (est = 0.001, SE = 0.001, p < .001) continued to be significant after group was entered into the baseline model, thus allowing independent variables to be entered as predictors of growth parameters (e.g., trajectory intercepts, slopes) ( Table II ). In these analyses, we were interested in the independent effects of individual, family, and socioeconomic variables during the preadolescent developmental period (Times 1 and 2, age 8–11 years) on intercept and slope variability of sleep disturbances across the 10-year period. Predictor variables were averaged across T1 and T2 and mean centered with the exception of SES, which was only measured at T1. Each growth model included intercept, slope, group, predictor variable, and all relevant interaction terms for eight estimated terms per model. Nonsignificant group interactions were trimmed from finals models, and all other effects were retained regardless of significance. Results of the trimmed models are presented in Table II .
Table II.
Preadolescent (Age 8–11 Years) Predictors of Sleep Disturbance Trajectories
| Model terms | Est | SE | P |
|---|---|---|---|
| Intercept term | |||
| Internalizing | 0.088 | 0.017 | <.001 |
| Externalizing | 0.417 | 0.112 | <.001 |
| Inattention | 0.180 | 0.056 | .002 |
| Family conflict | 0.017 | 0.015 | .265 |
| Parent–child conflict | −0.001 | 0.020 | .944 |
| Marital conflict | 0.092 | 0.057 | .110 |
| Group ( Spina Bifida ) | −0.059 | 0.027 | .034 |
| Marital conflict × group | −0.167 | 0.075 | .027 |
| Spina Bifida | 0.140 | 0.056 | .012 |
| Typically developing | −0.024 | 0.051 | .638 |
| SES | 0.002 | 0.001 | .206 |
| Income | −0.007 | 0.005 | .175 |
| Slope term | |||
| Internalizing × Time | 0.003 | 0.003 | .334 |
| Externalizing × Time | 0.045 | 0.019 | .022 |
| Inattention × Time | 0.008 | 0.009 | .415 |
| Family conflict × Time | 0.004 | 0.003 | .098 |
| Parent–child conflict × Time | 0.007 | 0.003 | .036 |
| Marital conflict × Time | 0.017 | 0.006 | .009 |
| SES × Time | −0.001 | 0.001 | .009 |
| Income × Time | 0.001 | 0.001 | .998 |
Note. The effect of group on the intercept and slope term was significant for some of the models including these predictors (i.e., family conflict, marital conflict, SES, income, p < .05) but was not presented unless a group × predictor interaction occurred (i.e., Marital conflict × group). Bolded lines represent statistically significant predictor estimates. SES = socioeconomic status measured by Hollingshead Four Factor Index ( Hollingshead, 1975 ).
Emotional and Behavioral Adjustment
All three of the adjustment variables predicted intercept variability in sleep disturbances such that higher levels of preadolescent externalizing, internalizing, and inattention symptoms were concurrently associated with greater sleep disturbances. Greater symptoms of preadolescent externalizing symptoms predicted growth in sleep disturbances for youth with SB and their TD peers. Group was not a significant moderator of the impact of adjustment on sleep ( Table II ).
Family Functioning
Greater marital conflict and parent–child conflict during early adolescence predicted increased sleep disturbances. Group was not a significant moderator of the impact of conflict on sleep disturbance trajectories; however, there was a significant preadolescent marital conflict × group effect on the trajectory intercept. For TD preadolescents, marital conflict was not a significant predictor of concurrent sleep disturbances (i.e., trajectory intercepts); for preadolescents with SB, higher levels of marital conflict predicted greater sleep disturbances ( Table II ).
Socioeconomic Characteristics
Lower SES predicted growth in sleep disturbances. There were no effects of income on variability in the intercepts or trajectory components of sleep disturbances ( Table II ).
Exploratory Aim: Medical Predictors of Sleep Trajectories in Adolescents With SB
Because primary results indicated youth with SB may be at risk for sleep disturbances, yet determinants of sleep trajectories were not stronger for youth with SB compared with TD youth (i.e., nonsignificant group interaction effects), exploratory analyses examined the following baseline (T1) condition-related/medical predictors of sleep disturbances for the SB subsample: type of SB, ambulation status, shunt status, number of shunt revisions, and number of surgeries.
Analyses indicated that shunt status predicted growth in sleep disturbances (est = 0.017, SE = 0.008, p = .03). For youth with shunts, the average linear trend was positive and significant (est = 0.009, SE = 0.004, p = .04), whereas for youth without shunts, the linear trend was negative and nonsignificant (est = −0.001, SE = 0.006, p = .199). Further, number of surgeries predicted variability in the trajectory intercept, such that a higher number of surgeries was associated with greater sleep disturbances at 9 years of age (est = 0.019, SE = 0.007, p = .01). Finally, there were no effects of type of SB (myelomeningocele vs. other), ambulation status (wheelchair vs. other), or number of shunt revisions on variability in the intercepts or trajectory components of sleep disturbances ( p s > .05).
Discussion
This study is the first to examine the developmental course of sleep disturbances in adolescents with SB. Findings revealed an increase in sleep disturbances over a 10-year period for adolescents with and without SB, which is consistent with developmental changes that impact circadian factors (e.g., pubertal maturation and sleep phase delay; Sadeh et al., 2009 ). We found that youth with SB experienced greater sleep disturbances compared with their TD peers during early adolescence. This finding is clinically important given evidence that disrupted sleep is strongly connected to adverse adolescent outcomes that are highly relevant to individuals with SB, including obesity risk ( Hart, Cairns, & Jelalian, 2011 ), executive dysfunction ( Kheirandish & Gozal, 2006 ), poor academic performance ( Beebe, Rose, & Amin, 2010 ), and psychological maladjustment ( Alfano, Zakem, Costa, Taylor, & Weems, 2009 ).
A number of developmental-ecological factors may contribute to growth in adolescent sleep disturbances. We found that preadolescent externalizing symptoms predicted increased sleep disturbances. Behavior problems such as defiance and aggression may lead to enduring patterns of bedtime resistance and sleep disruption. Future research is needed to clarify underlying mechanisms through which individual maladjustment impacts sleep, including cognitive (e.g., distressing thoughts, ruminations), somatic (e.g., physiological hyper-arousal; Dahl 1996 ), physiological (e.g., cortisol reactivity; El-Sheikh et al., 2008 ), and behavioral (e.g., poor sleep habits) processes. Identification of modifiable mechanisms can inform the development and implementation of interventions to treat adolescent sleep disturbances.
Finally, our study results indicated that greater dyadic (parent–child, marital) conflict and lower SES predicted sleep disturbances for adolescents with and without SB, which may implicate the long-term and lasting impact of early environmental stress and perceived vulnerability on cognitive and physiological arousal that disrupts sleep. SES is a complex construct that reflects a number of economic, social, environmental, and behavioral components. Decreased parental supervision, lower awareness of sleep hygiene practices among parents with less education, lower quality sleep environments, and perceived threats in the family’s surrounding neighborhood are a few potential mechanisms of the SES–sleep link ( Marco et al., 2012 ). Families with greater socioeconomic adversity may also be less likely to receive diagnoses of physiological and behavioral sleep disorders owing to limited access to health-care resources ( Chen, Martin, & Matthews, 2006 ), leading to chronic, untreated sleep disturbances, and associated daytime impairment. Future research may evaluate how early childhood developmental–ecological contextual factors interact to place adolescents at a high risk for developing sleep disturbances as a result of multiple contextual stressors, including chaotic home environments, home violence, chronic medical issues, and other poverty-related physical and emotional vulnerabilities (i.e., obesity, depression).
Study results should be considered in light of several limitations. First, we used a limited measure of sleep disturbances drawn from a behavioral screening tool (the CBCL) by parent report only that yielded relatively low internal consistencies. Although the CBCL is inadequate for thoroughly assessing sleep disturbances, research has supported the use of the CBCL composite score to measure overall sleep functioning in archival studies that lack comprehensive sleep measures. We were also unable to include youth self-report of sleep over the 10-year period. However, even with our limited measure of sleep, we were able to identify longitudinal sleep trajectories and their determinants. Future studies should examine trajectories and predictors of sleep behaviors, quality, and patterns using questionnaire-based measures via child self-report and objective (e.g., actigraphy) sleep assessment. In addition, we were unable to extract data on diagnosed sleep disorders or interventions (e.g., polysomnography [PSG] results, continuous positive airway pressure [CPAP] treatment), prescription medications that change the quality of sleep, or other health conditions (e.g., asthma, allergies, gastroesophageal reflux disease, obesity); such data would augment our understanding of the development of adolescent sleep disturbances. Further, marital conflict was measured using observational methods that may not generalize to actual marital interactions and did not directly assess for the presence of verbal and physical aggression. Our assessment of internalizing symptoms was also limited to depressive symptoms; future studies may also include measures of anxiety. Finally, researchers have advocated for specifying multiple indices of socioecological risk factors (e.g., economic well-being, community-level poverty; El-Sheikh et al., 2013 ) to detect differential associations between economic adversities and outcomes of interest. Previous work has also found greater sleep disturbances in minority compared with Caucasian children ( Roberts, Roberts, & Chen, 2000 ), yet our predominantly Caucasian sample precluded our ability to test for ethnic differences.
There are a number of factors that may play a role in the development of sleep disturbance that were outside the scope of this study. The confluence of normative developmental changes and unique condition-related characteristics likely exacerbate sleep disturbances in adolescents with SB. Sleep phase delay commonly occurs with pubertal development and is related to the increase in sleep disturbances during adolescence ( Sadeh et al., 2009 ). It is possible that sleep phase delays occur earlier owing to the presence of precocious puberty in some youth with SB ( Coakley, Holmbeck, Friedman, Greenley, & Thill, 2002 ). Moreover, specific sleep disorders are more likely to emerge in individuals with SB such as sleep-disordered breathing ( Kirk, Morielli, & Brouillette, 1999 ), which may account for increased self-reported sleep disruption as well as behavioral symptoms. Given our preliminary, exploratory analyses that youth with shunts evidenced a steeper incline in sleep disturbances, it will be important for researchers to identify how other condition variables related to the severity of SB are associated with sleep, including neurological characteristics (e.g., Chari malformation), painful somatic symptoms (owing to orthopedic issues, wheelchair overuse, and tethered cord; Bowman, McLone, Grant, Tomita, & Ito, 2001 ), hospitalizations, and intensive medical management behaviors (e.g., catheterization at nighttime, medication use). It is possible that the convergence of sleep disturbances demonstrated in this study reflects adaptation to or temporary relief/resolution of condition-specific mechanisms. On the other hand, because youth in the SB sample showed greater attrition during the course of the study, it is also possible that trajectory convergence reflects nonparticipation of youth with comorbid conditions (pain, obesity, depression) and, perhaps, greater sleep disturbances.
This study suggests several avenues for future research. First, it will be useful for longitudinal studies to investigate etiological mechanisms and bidirectional associations between sleep and individual and family adjustment using multiple-wave data. These data would enable researchers to test, for example, whether early childhood sleep problems predict growth in mood disturbances in early adolescence and subsequent growth in behavioral sleep disorders in later adolescence and emerging adulthood. Some individual predictors included in the current study’s growth models tend to peak after preadolescence (e.g., mood disturbances; Garber, Keiley, & Martin, 2002 ); thus, more complex longitudinal growth modeling could compare the strength of maladjustment–sleep associations across childhood and adolescence. Further, it would be useful to characterize subgroups of adolescents demonstrating differential sleep patterns over time (e.g., incline, stability, or decline in sleep disturbances) in pediatric and TD populations using group-based latent class trajectory modeling. Differences in longitudinal trajectories of sleep disturbances between adolescents with SB and other types of chronic health conditions are also unknown. Finally, future research is needed to clearly identify etiological factors and negative consequences of sleep disturbances using multimethod (actigraphy, diaries, questionnaires) sleep assessments.
The results of this study have important clinical implications. Currently, sleep disruptions among youth with SB may be underidentified and undertreated owing to the paucity of research in this population. Given this gap in the literature, health professionals may be more likely to attend to other salient aspects of SB management (e.g., adherence to a complex medical regimen), and inadvertently neglect to screen for sleep disruptions. This study suggests that adolescence is a vulnerable time for sleep disturbances in youth with SB, and regular screening of sleep during this developmental period is imperative. Enhanced prevention and treatment approaches may include simple screening tools (e.g., the BEARS; Owens & Dazell, 2005 ), promotion of behavioral sleep interventions, and referrals to specialized sleep clinics. Furthermore, targeted sleep interventions may ameliorate other physical and psychosocial problems that are common in this population (e.g., obesity, inattention). Given the risk for sleep disturbances in adolescents with SB and potential adverse effects of sleep disturbances on critical health outcomes ( Smaldone, Honig, & Byrne, 2009 ), there is a clear need to increase attention on sleep in this population.
Acknowledgments
The authors wish to thank the Illinois Spina Bifida Association and the staff of the spina bifida clinics at Lurie Children’s Hospital of Chicago, Shriners Hospital for Children-Chicago, and Loyola University Chicago Medical Center. They also thank the many undergraduate and graduate research assistants who assisted with study procedures and data management. Most importantly, this research would not be possible without the dedicated contributions of the parents, children, and teachers who participated in this study over several years.
Footnotes
1 A copy of this coding system is available on request from Grayson N. Holmbeck (gholmbe@luc.edu).
2 Because parent report of sleep for youth living outside of the home may not be as valid as for those living at home, the authors estimated growth curve models with parent report of these cases dropped at T6 ( N = 33 or 30.4% of the total sample). Results were consistent to those outlined in this study with two exceptions. First, a fixed quadratic model provided a significant improvement over the positive linear model of growth in sleep; the linear component at age 9 years was negative and significant and the quadratic component was positive and significant, indicating an average curve that slightly decreased during preadolescence and subsequently turned upward during adolescence. Second, the independent effect of parent–child conflict predicting slope variability was no longer significant. All other effects remained significant. These statistics are available on request from the first author.
Funding
Completion of this manuscript was supported in part by grants from the March of Dimes Birth Defects Foundation (12-FY01-0098), the F31HD079270-01A1 awarded to Caitlin Murray, MA, the R01HD048629 awarded to Grayson Holmbeck, P h D, and the K24HD060068 awarded to Tonya Palermo, P h D.
Conflicts of interest : None declared.
References
- Achenbach T. M., Rescorla L. A. ( 2003. ). Manual for the ASEBA adult forms and profiles . Burlington, VT; : University of Vermont, Research Center for Children, Youth, & Families; . [Google Scholar]
- AlDabal L., BaHammam A. S. ( 2011. ). Metabolic, endocrine, and immune consequences of sleep deprivation . The Open Respiratory Medicine Journal , 5 , 31 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano C., Zakem A., Costa N., Taylor L., Weems C. ( 2009. ). Sleep problems and their relation to cognitive factors, anxiety, and depressive symptoms in children and adolescents . Depression and Anxiety , 26 , 503 – 512 . doi: 10.1002/da.20443 [DOI] [PubMed] [Google Scholar]
- Astill R. G., Van der Heijden K. B., Van IJzendoorn M. H., Van Someren E. J. ( 2012. ). Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed . Psychological Bulletin , 138 , 1109 – 1138 . [DOI] [PubMed] [Google Scholar]
- Becker S. P., Ramsey R. R., Byars K. C. ( 2015. ). Convergent validity of the child behavior checklist sleep items with validated sleep measures and sleep disorder diagnoses in children and adolescents referred to a sleep disorders center . Sleep Medicine , 16 , 79 – 86 . [DOI] [PubMed] [Google Scholar]
- Beebe D. W., Rose D., Amin R. ( 2010. ). Adolescent health brief: Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom . Journal of Adolescent Health , 47 , 523 – 525 . 10.1016/j.jadohealth.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe T., Hysing M., Stormark K. M., Lundervold A. J., Sivertsen B. ( 2012. ). Sleep problems as a mediator of the association between parental education levels, perceived family economy and poor mental health in children . Journal of Psychosomatic Research , 73 , 430 – 436 . [DOI] [PubMed] [Google Scholar]
- Bowman R. M., McLone D. G., Grant J. A., Tomita T., Ito J. A. ( 2001. ). Spina bifida outcome: A 25-year prospective . Pediatric Neurosurgery , 34 , 114 – 120 . 10.1159/000056005 [DOI] [PubMed] [Google Scholar]
- Carskadon M. A. ( 2011. ). Sleep in adolescents: The perfect storm . Pediatric Clinics of North America , 58 , 637 – 647 . 10.1016/j.pcl.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [CDC] . ( 2010. ) . Neutral tube defect ascertainment project . [Google Scholar]
- Chen E., Martin A. D., Matthews K. A. ( 2006. ). Socioeconomic status and health: Do gradients differ within childhood and adolescence? Social Science and Medicine , 62 , 2161 – 2170 . [DOI] [PubMed] [Google Scholar]
- Coakley R. M., Holmbeck G. N., Friedman D., Greenley R. N., Thill A. W. ( 2002. ). A longitudinal study of pubertal timing, parent-child conflict, and cohesion in families of young adolescents with spina bifida . Journal of Pediatric Psychology , 27 , 461 – 473 . doi: 10.1093/jpepsy/27.5.461 [DOI] [PubMed] [Google Scholar]
- Coulombe J. A., Reid G. J., Boyle M. H., Racine Y. ( 2011. ). Sleep problems, tiredness, and psychological symptoms among healthy adolescents . Journal of Pediatric Psychology , 36 , 25 – 35 . [DOI] [PubMed] [Google Scholar]
- Curran P. J., Obeidat K., Losardo D. ( 2010. ). Twelve frequently asked questions about growth curve modeling . Journal of Cognition and Development , 11 , 121 – 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R. E. ( 1996. ). The regulation of sleep and arousal: Development and psychopathology . Development and Psychopathology , 8 , 3 – 27 . 10.1017/S0954579400006945 [DOI] [Google Scholar]
- DeLucia C., Pitts S. C. ( 2006. ). Applications of individual growth curve modeling for pediatric psychology research . Journal of Pediatric Psychology , 31 , 1002 – 1023 . [DOI] [PubMed] [Google Scholar]
- Edelstein K., Cirino P. T., Hasher L., Fletcher J. M., Dennis M. ( 2012. ). Sleep problems, chronotype, and diurnal preferences in children and adults with spina bifida . Journal of Biological Rhythms , 27 , 172 – 175 . doi: 10.1177/0748730411435209 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Bagley E. J., Keiley M., Elmore-Staton L., Chen E., Buckhalt J. A. ( 2013. ). Economic adversity and children’s sleep problems: Multiple indicators and moderation of effects . Health Psychology , 32 , 849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M., Buckhalt J. A., Keller P. S., Granger D. A. ( 2008. ). Children's objective and subjective sleep disruptions: Links with afternoon cortisol levels . Health Psychology , 27 , 26 . 10.1037/0278-6133.27.1.26 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Sadeh A. ( 2015. ). Sleep and development: Introduction to the monograph . Monographs of the Society for Research in Child Development , 80 ( 1 ), 1 – 14 . [DOI] [PubMed] [Google Scholar]
- Ferreira A. J. ( 1963. ). Decision making in normal and pathological families . Archives of General Psychiatry , 8 , 68 – 73 . doi:10.1001/archpsyc.1963.01720070076008 [DOI] [PubMed] [Google Scholar]
- Garber J., Keiley M. K., Martin N. C. ( 2002. ). Developmental trajectories of adolescents’ depressive symptoms: Predictors of change . Journal of Consulting and Clinical Psychology , 70 , 79 – 95 . 10.1037/0022-006X.70.1.79 [DOI] [PubMed] [Google Scholar]
- Gregory A. M., Caspi A., Moffitt T. E., Poulton R. ( 2006. ). Family conflict in childhood: A predictor of later insomnia . Sleep , 29 , 1063 – 1067 . [DOI] [PubMed] [Google Scholar]
- Gregory A., O’Connor T. ( 2002. ). Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems . Journal of the American Academy of Child Adolescent Psychiatry , 41 , 964 – 971 . 10.1097/00004583-200208000-00015 [DOI] [PubMed] [Google Scholar]
- Hart C. N., Cairns A., Jelalian E. ( 2011. ). Sleep and obesity in children and adolescents . Pediatric Clinics of North America , 58 , 715 – 733 . 10.1016/j.pcl.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. B. ( 1975. ). Four Factor Index of Social Status . New Haven, CT: : Yale University; . [Google Scholar]
- Holmbeck G. N., Westhoven V. C., Phillips W. S., Bowers R., Gruse C., Nikolopoulos T., Totura C. M., Davison K. ( 2003. ). A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida . Journal of Consulting and Clinical Psychology , 71 ( 4 ), 782 . [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., DeLucia C., Essner B., Kelly L., Zebracki K., Friedman D., Jandasek B. ( 2010. ). Trajectories of psychosocial adjustment in adolescents with spina bifida: A 6-year, four-wave longitudinal follow-up . Journal of Consulting and Clinical Psychology , 78 , 511 – 525 . [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Li S., Schurman J. V., Friedman D., Coakley R. M. ( 2002. ). Collecting and managing multi-source and multi-method data in studies of pediatric populations . Journal of Pediatric Psychology , 27 , 5 – 18 . doi: 10.1093/jpepsy/27.1.5 [DOI] [PubMed] [Google Scholar]
- Hysing M., Sivertsen B., Stormark K. M., Elgen I., Lundervold A. J. ( 2009. ). Sleep in children with chronic illness, and the relation to emotional and behavioral problems: A population-based study . Journal of Pediatric Psychology , 34 , 665 – 670 . doi: 10.1093/jpepsy/jsn095 [DOI] [PubMed] [Google Scholar]
- Jarrin D. C., McGrath J. J., Quon E. C. ( 2014. ). Objective and subjective socioeconomic gradients exist for sleep in children and adolescents . Health Psychology , 33 , 301 – 305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. Z., Holmbeck G. N. ( 1995. ). Manual for OP coding system, Unpublished manuscript . [Google Scholar]
- Kaugars A. S., Zebracki K., Kichler J., Fitzgerald C. J., Greenley R. N., Alemzadeh R., Holmbeck G. N. ( 2011. ). Use of the family interaction macro-coding system with families of adolescents: Psychometric properties among pediatric and healthy populations . Journal of Pediatric Psychology , 36 , 539 – 551 . doi: 10.1093/jpepsy/jsq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. J., El-Sheikh M. ( 2011. ). Marital conflict and children’s sleep: Reciprocal relations and socioeconomic effects . Journal of Family Psychology , 25 , 412 – 422 . http://psycnet.apa.org/doi/10.1037/a0023789 [DOI] [PubMed] [Google Scholar]
- Kelly R. J., El-Sheikh M. ( 2014. ). Reciprocal relations between children’s sleep and their adjustment over time . Developmental Psychology , 50 , 1137 – 1147 . http://psycnet.apa.org/doi/10.1037/a0034501 [DOI] [PubMed] [Google Scholar]
- Kheirandish L., Gozal D. ( 2006. ). Neurocognitive dysfunction in children with sleep disorders . Developmental Science , 9 , 388 – 399 . doi: 10.1111/j.1467-7687.2006.00504.x [DOI] [PubMed] [Google Scholar]
- Kirk V. G., Morielli A., Brouillette R. T. ( 1999. ). Sleep-disordered breathing in patients with myelomeningocele: The missed diagnosis . Developmental Medicine and Child Neurology , 41 , 40 – 43 . doi: 10.1111/j.1469-8749.1999.tb00007.x [DOI] [PubMed] [Google Scholar]
- Kovacs M. ( 1992. ). Children’s Depression Inventory- Manual . North Tonawanda, NY: : Multi-Health Systems; . [Google Scholar]
- Lewandowski A. S., Ward T. M., Palermo T. M. ( 2011. ). Sleep problems in children and adolescents with common medical conditions . Pediatric Clinics of North America , 58 , 699 – 713 . doi:10.1016/j.pcl.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptak G. S. ( 1997. ). Neural tube defects . In Batshaw M. L., Perret Y. M. (Eds.), Children with disabilities: A medical primer (pp. 429 – 552 ). Baltimore, MD: : Brookes; . [Google Scholar]
- Lovato N., Gradisar M. ( 2014. ). A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice . Sleep Medicine Reviews , 18 , 521 – 529 . [DOI] [PubMed] [Google Scholar]
- Marco C. A., Wolfson A. R., Sparling M., Azuaje A. ( 2012. ). Family socioeconomic status and sleep patterns of young adolescents . Behavioral Sleep Medicine , 10 , 70 – 80 . doi: 10.1080/15402002.2012.636298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R., Moos B. ( 1994. ). Family environment scale manual: Development, applications, research . Palo Alto, CA: : Consulting Psychologist Press; . [Google Scholar]
- Owens J. A., Dalzell V. ( 2005. ). Use of the ‘BEARS’sleep screening tool in a pediatric residents' continuity clinic: A pilot study . Sleep Medicine , 6 , 63 – 69 . [DOI] [PubMed] [Google Scholar]
- Parker S. E., Mai C. T., Canfield M. A., Rickard R., Wang Y., Meyer R. E. . National Birth Defects Prevention Network. (2010). Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006 . Birth Defects Research , 88 ( 12 ), 1008 – 1016 . doi: 1010.1002/bdra.20735 . [DOI] [PubMed] [Google Scholar]
- Patten C. A., Choi W. S., Gillin J. C., Pierce J. P. ( 2000. ). Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents . Pediatrics , 106 , E23 . [DOI] [PubMed] [Google Scholar]
- Peugh J. L., Enders C. K. ( 2005. ). Using the SPSS mixed procedure to fit cross-sectional and longitudinal multilevel models . Educational and Psychological Measurement , 65 , 717 – 741 . doi: 10.1177/0013164405278558 [Google Scholar]
- Prinz R. J., Foster S. L., Kent R. N., O’Leary K. D. ( 1979. ). Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads . Journal of Applied Behavior Analysis , 12 , 691 – 700 . doi: 10.1901/jaba.1979.12-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach J., Hiscock H., Canterford L., Wake M. ( 2009. ). Outcomes of child sleep problems over the school-transition period: Australian population longitudinal study . Pediatrics , 123 , 1287 – 1292 . doi: 10.1542/peds.2008-1860 [DOI] [PubMed] [Google Scholar]
- Roberts R. E., Roberts C. R., Chen I. G. ( 2000. ). Ethnocultural differences in sleep complaints among adolescents . The Journal of Nervous and Mental Disease , 188 , 222 – 229 . 10.1097/00005053-200004000-00005 [DOI] [PubMed] [Google Scholar]
- Roberts R. E., Roberts C. R., Duong H. T. ( 2009. ). Sleepless in adolescence: Prospective data on sleep deprivation, health and functioning . Journal of Adolescence , 32 , 1045 – 1057 . doi: 10.1016/j.adolescence.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A., Dahl R. E., Shahar G., Rosenblat-Stein S. ( 2009. ). Sleep and the transition to adolescence: A longitudinal study . Sleep , 32 , 1602 – 1609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat T., Cohen-Zion M., Tzischinsky O. ( 2014. ). Functional consequences of inadequate sleep in adolescents: A systematic review . Sleep Medicine Reviews , 18 , 75 – 87 . [DOI] [PubMed] [Google Scholar]
- Smaldone A., Honig J. C., Byrne M. W. ( 2009. ). Does assessing sleep inadequacy across its continuum inform associations with child and family health? . Journal of Pediatric Health Care , 23 ( 6 ), 394 – 404 . [DOI] [PubMed] [Google Scholar]
- Smetana J. G., Yau J., Restrepo A., Braeges J. L. ( 1991. ). Adolescent-parent conflict in married and divorced families . Developmental Psychology , 27 , 1000 – 1010 . 10.1037/0012-1649.27.6.1000 [DOI] [Google Scholar]
- Tietze A. L., Blankenburg M., Hechler T., Michel E., Koh M., Schlüter B., Zernikow B. ( 2012. ). Sleep disturbances in children with multiple disabilities . Sleep Medicine Reviews , 16 , 117 – 127 . [DOI] [PubMed] [Google Scholar]
- Valrie C. R., Bromberg M. H., Palermo T., Schanberg L. E. ( 2013. ). A systematic review of sleep in pediatric pain populations . Journal of Developmental and Behavioral Pediatrics , 34 , 120 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]