Abstract
Ribosome biogenesis, including the RNA-Polymerase-1 (Pol1)-mediated transcription of rRNA, is regulated by the pro-epileptogenic mTOR pathway. Therefore, hippocampal Pol1 activity was examined in mouse models of epilepsy including kainic acid- and pilocarpine-induced status epilepticus (SE) as well as a single seizure in response to pentylenetetrazole (PTZ). Elevated 47S pre-rRNA levels were present acutely after induction of SE suggesting activation of Pol1. Conversely, after a single seizure 47S pre-rRNA was transiently downregulated with increased levels of unprocessed 18S rRNA precursors in the cornu Ammonis (CA) region. At least in the dentate gyrus (DG), the pilocarpine-SE-mediated transient activation of Pol1 did not translate into long term changes of pre-rRNA levels or total ribosome content. Unaltered hippocampal ribosome content was also found after a 20 day PTZ kindling paradigm with increasing pro-convulsive effects of low dose PTZ that was injected every other day. However, after selectively deleting the essential Pol1 co-activator, Transcription Initiation Factor-1A (Tif1a/Rrn3) from excitatory neurons, PTZ kindling was impaired while DG total ribosome content was moderately reduced and no major neurodegeneration was observed throughout the hippocampus. Therefore, Pol1 activity of excitatory neurons is required for PTZ kindling. As seizures affect ribosome biogenesis without long term effects on the total ribosome content, such a requirement may be associated with a need to produce specialized ribosomes that promote pro-epileptic plasticity.
Keywords: ribosome, nucleolus, RNA-polymerase-1, ribosomal biogenesis, status epilepticus, kindling, temporal lobe epilepsy, epileptogenesis, hippocampus, mouse, pentylenetetrazole, pilocarpine
Introduction
Epilepsy is a brain disorder that involves aberrant synaptic plasticity as well as excessive neural cell growth. In the hippocampus, aberrant axonal growth (i.e. mossy fiber sprouting, MFS), and/or excessive neurogenesis and/or somato-dendritic hypertrophy are observed in temporal lobe epilepsy (TLE) [1–4]. In addition, mutations that hyperactivate the mTOR pathway, such as those in tuberous sclerosis complex (TSC) or Cowden syndrome (CS), are often associated with neuronal/astrocytic hypertrophy and epilepsy [5,6]. Development of acquired forms of epilepsy – including TLE – may be facilitated by the excessive mTOR activity [6].
It is well established that in rodents, pharmacologically-induced seizures stimulate the mTOR pathway. Thus, the cholinergic agonist pilocarpine or the glutamatergic agonist kainic acid (KA) produce status epilepticus (SE) that is accompanied by prolonged activation of the mTOR signaling pathway in the hippocampus [7–9]. Conversely, the gabaergic antagonist pentylenetetrazole (PTZ) induces brief seizures that are accompanied by a transient activation of the hippocampal mTOR pathway [10]. Likewise, seizures induced by pilocarpine, KA or the gabaergic antagonist bicuculline have been reported to activate hippocampal ERK1/2 signaling that, in addition to mTOR, may also contribute to regulation of cell size including neurite outgrowth [11–14]. It is unclear, however, what are the epileptogenic effector mechanisms for mTOR or ERK1/2.
The ribosome is a critical component of the protein synthesis machinery. The nucleolus is a cellular center of ribosomal biogenesis [15]. That process is regulated by the activity of the RNA polymerase-1 (Pol1) which transcribes nucleolar rRNA genes (rDNA) [15]. Its product, the 47S pre-rRNA is rapidly processed to mature 18S-, 5.8S- and 28S rRNAs that are later assembled into pre-ribosomal subunits. Pol1 accounts for at least half of the total transcriptional output of a cell during active proliferative growth [15]. High rates of ribosomal biogenesis are also expected in cells that do not divide but, instead, rapidly increase their volume such as neurite-extending neurons. Importantly, Pol1 activity is highly responsive to extracellular cues that affect growth. For instance, growth factors or nutrients activate Pol1 by the ERK1/2- and/or mTOR-mediated phosphorylations of the Pol1-specific co-activators TIF1A and/or UBF [16–19]. Such regulation also occurs in growing neurons, where the BDNF-ERK1/2-TIF1A pathway is both necessary and sufficient for dendritic growth [20].
Many types of mature neurons display robust nucleoli suggesting that they retain the capacity to quickly up-regulate ribosomal biogenesis [21]. In the hippocampus, induction of long term potentiation (LTP) or spatial learning is associated with increased Pol1 activity [22,23]. In addition, LTP is disrupted by genetic or pharmacological inhibition of hippocampal Pol1, while the latter manipulation also negatively effects spatial memory consolidation [24,23,22]. Importantly, these defects are unlikely to be due to neuronal death, as mature forebrain neurons survive for months without active Pol1 [25,24]. Interestingly, nucleolar hypertrophy and increased ribosomal density are observed in brain neurons of epileptic mice or human patients with hyperactive mTOR [26–28]. Therefore, by supporting neuronal growth and/or synaptic plasticity, ribosomal biogenesis may contribute to epileptogenesis.
The current study was initiated to determine (i) how pharmacologically-induced seizures affect ribosomal biogenesis in the rodent forebrain, and (ii) whether Pol1 activity is required for PTZ kindling.
Materials and Methods
Animals
Mice (6–8 weeks old males, C57Bl6 strain) were obtained from Harlan. Tif1afl/fl, Camk2a -Cre-ERT2 mice on C57Bl6 background were described before [25]. These animals were bred onsite; genotyping was performed following an established methodology [25]; genotype of each experimental subject was confirmed by PCR. Both males and females at 2–4 months of age were used for studies involving transgenic animals. To induce Tif1a knockout by the Cre-LoxP system, a 5 day course of tamoxifen treatment was applied; tamoxifen dosing was 2×1mg i.p. per day, dissolved in 10:1 sunflower seed oil: ethanol as previously described [29]. For all transgenic animal studies, control animals were bred onsite. All animal experiments strictly followed protocols that were approved by the Institutional Animal Care and Use Committee.
Reagents
Reagents were obtained from VWR, Sigma-Aldrich, or Fisher Scientific unless stated otherwise. Oligonucleotides were purchased from Integrated DNA Technologies.
Pro-convulsant treatments
Mice (C57Bl6 males) were initially gently handled by daily administration of saline i.p. for 5–7 days. Then, pentylenetetrazole (PTZ, 35 or 60 mg/kg) or kainic acid (KA, 45 mg/kg, Abcam /former Ascent Scientific/) was administered i.p.. For the pilocarpine experiment, mice received 1 mg/kg scopolamine s.c., followed 15 min. later by s.c pilocarpine (300 or 400 mg/kg, as indicated). All animals were observed to determine onset, intensity and duration of seizures. Seizure scoring was done using the modified Racine scale (stage 1: mouth and facial movements characterized by arrest, eye blinking, and chewing; stage 2: head nodding; stage 3: forelimb clonus; stage 4: seizures characterized by rearing; and stage 5: seizures characterized by rearing and falling) [30]. In the acute PTZ experiment, only animals that displayed at least stage 4 seizures were included. In the KA- and the pilocarpine studies, only animals that developed status epilepticus (SE), defined by recurrent seizures (stage 3 or higher) lasting for at least 30 min, were included. Two and a half hours after the onset of pilocarpine-induced SE animals received 2×10 mg/kg diazepam at 15 min intervals. In chronic pilocarpine studies (survival for at least 1 week post-SE), 0.5 ml warm (30–33°C) Ringer’s solution was administered during the first two days of post SE recovery (3–5 times s.c.). While in initial experiments, 400 mg/kg pilocarpine was used, such a dose resulted in 80% mortality rate at 24 h. Therefore, to obtain chronic survivors a lower dose of 300 mg/kg was used, as suggested by a recent report on optimizing the pilocarpine model in mice [31]. Indeed, at least 50% SE response rate was observed with at least 50% long-term survival among SE responders (i.e. at least 6 weeks post SE). In all pilocarpine studies, control mice received identical treatments except pilocarpine.
Kindling study in Tif1a conditional knockout mice
Tamoxifen treatment was applied to 8 male and 4 female Tif1afl/fl, Camk2a -Cre-ERT2 mice (Tif1a KO). A control (WT) group included 5 male and 2 female Tif1afl/fl mice that were treated with tamoxifen as well as 4 male and 2 female Tif1afl/fl, Camk2a -Cre-ERT2 mice that were treated with vehicle. One month after tamoxifen or vehicle treatment, PTZ was administered i.p.. Seizures were scored using modified Racine scale. In an initial experiment, Tif1a KO and WT animals were treated once with a high dose of PTZ to ensure that seizure response was unaffected by the KO (60 mg/kg, n=4/group). Then, a low, sub-seizure dose of PTZ (35 mg/kg) was given every other day for 20 days (total number of PTZ injections was 10). After completion of the kindling procedure mice were killed and their brains were assessed for ribosome content and immunohistochemistry analysis.
RNA isolation and qRT-PCR
After euthanasia, brains were removed and hippocampi were isolated; DG and CA dissection was performed following a previously published procedure [32]. RNA was isolated and cDNA was synthesized using random hexamers as described before [33,34,20]. The RNA levels were analyzed using the ΔΔCt method; each sample was run in triplicates; normalization was performed against 18S rRNA or Gapdh as indicated. The primer sequences were as follows: 47S pre-rRNA, forward-5’ccaagtgttcatgccacgtg3’, reverse-5’cgagcgactgccacaaaaa3’ (the amplicon corresponds to positions 492–547 of the mouse 47S pre-rRNA, NCBI reference sequence NR_046233.2); 5’ETS-18S rRNA, forward-5’ctcctctctcgcgctctctgtc3’, reverse-5’gcatggcttaatctttgagacaagca3’ (positions 3941–4060 of the mouse 47S pre-rRNA); ITS1, forward-5’ccggcttgcccgattt3’, reverse-5’cggccagcaggaacgaaacg3’ (positions 6620–6754 of the mouse 47S pre-rRNA); Tif1a (Rrn3) forward-5’attcccgtttgtgaggaagtccga3’, reverse-5’tatcctgccgcgatacactcacat3’; 18S rRNA, forward-5’gttggttttcggaactgaggc3’, reverse-5’gtcggcatcgtttatggtcg3’; Gapdh, forward: 5’ tgtagaccatgtagttgaggtca 3’, reverse: 5’tgtagaccatgtagttgaggtca3’. All primers were validated by qRT-PCR melt-curve analysis, standard curve analysis of qRT-PCR efficiency and agarose gel electrophoresis analysis of a regular RT-PCR product (each primer pair produced a single product of expected size).
Determining ribosome content
A previously published methodology was followed [35]. Briefly, brains were removed after euthanasia, immersion fixed in 4% paraformaldehyde for 24 h, and then processed for cryostat sectioning. Sections (25 µm) were cut through the dorsal hippocampus and stained with a combination of the DNA dye Hoechst 33258 (2.5 µg/ml) and the ribosome dye NeuroTrace® 500/525 Green Fluorescent Nissl Stain as recommended by the manufacturer (Invitrogen/Molecular Probes, N21480, 1:500, 20-minute staining). At least 5 sections from each animal were imaged using a Nikon Eclipse epifluorescence microscope with a 4x objective lens. Digitized micrographs were captured using Elements software (Nikon Instruments) followed by conversion to grayscale TIFF files. Identical exposure times were used for all pictures that were taken for comparative analyses. Quantification of the fluorescence intensity (FI) was performed by using the “Integrated Density” parameter in the ImageJ software (NIH freeware). Regions of interest were marked (granule cell layer of the DG for DG measurments or stratum pyramidale of CA1 for CA1 analysis) and FI of ribosome and DNA signal was measured in each marked area. A change of DNA signal-normalized ribosomal FI was expressed as a fold control that was defined by a ratio of an individual DNA-normalized FI value to the average DNA-normalized FI value of the control group as indicated for each set of experiments. Such calculations were performed for each set of sections that were stained together before pooling data for statistical analysis; each staining set included several control sections to determine the baseline. To determine ribosome content in hilar interneurons of WT and Tif1a KO mice, individual cell body profiles were marked and FI was calculated for each cell (“Integrated Density”, ImageJ; all sections were stained together with NeuroTrace® 500/525 Green Fluorescent Nissl Stain ). At least 60 cells from 3 sections/animal were analyzed to calculate average ribosome content/cell.
Immunofluorescence
Animals were euthanized, and brains were fixed in 4% paraformaldehyde for 24 h followed by cryo-protection in 30% sucrose for at least 3 days at 4 °C. The brains were mounted in a Cryomount medium, and sectioned coronally on a cryostat to produce 25-µm-thick sections through the dorsal hippocampus. For immunostaining, brain sections were blocked in TBS containing 5% BSA and 0.1% Triton-X-100 for 1 h at room temperature with gentle agitation. The following primary antibodies were used: mouse monoclonal anti-NeuN (1:100, 16 h, 4 °C; Chemicon, Temecula, CA), rabbit polyclonal anti-glial fibrillary acidic protein (GFAP; 1:250, 16 h, 4 °C; Chemicon), rabbit polyclonal anti-zinc transporter type 3 (ZnT3; 1:100, 16 h, 4 °C; Synaptic Systems Inc, Goettingen, Germany). Sections were then incubated with appropriate secondary antibodies including or Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen, A11037, 1:300) or Alexa Fluor 488 anti-rabbit IgG (Invitrogen, A11034, 1:300) and/or Alexa Fluor 594 anti-mouse IgG (Invitrogen, A11005, 1:300) for 1 h at room temperature. Ribosome and DNA counterstainings were performed using Hoechst 33258 or NeuroTrace® 500/525 Green Fluorescent Nissl Stain, respectively. Confocal images of cell type/axon marker stainings were obtained using a Nikon Eclipse Ti inverted microscope; epifluorescence images of Nissl or FluroJade stainings were obtained using a Zeiss AxioObserver system. To verify staining specificity, controls with species-matched, non-immune IgGs, or sera instead of primary antibodies were also performed.
FluoroJade-B staining. The staining was peformed as recommended by the manufacturer using sections cut through the dorsal hippocampus (Millipore, #AG310). Positive control included sections from male C57Bl6 mice mice that were killed 3 days after unilateral stereotaxic injection of KA into amygdala (1 nmol of KA in 300 nL of PBS; coordinates of the injection: bregma, 1.6 mm; medial-lateral, 3.3 mm; and dorsovenral, 4.5 mm). Such treatment produces massive damage to the ipsilateral CA3 region of the hippocampus that can be detected using FluoroJadeB [36]. In sections from PTZ-kindled Tif1a KO or WT animals FluoroJadeB positive cells were counted in the hippocampal regions CA1, CA3 and DG. The observer was unaware of the genotype/treatment. Average number of positive cells/hippocampal region/hippocampus/section was determined based on analyzing at least 6 sections from each animal.
Functional annotation analysis
To identify genes encoding ribosomal proteins (RPs) affected by pilocarpine-induced SE, we analyzed data from high throughput sequencing profiling of pilocarpine-induced changes of hippocampal RNA expression, deposited in Gene Expression Omnibus (GSE72402) [37]. Briefly, for differentially expressed genes that were identified in that study (as defined by fold change > 1.5, and q < 0.01), we selected a subset of genes belonging to gene ontology (GO) categories: cytosolic large ribosomal subunit (GO:0022625) and cytosolic small ribosomal subunit (GO:0022627). To retrieve such genes a custom script in R programming language was used.
Statistical analysis
The non-parametric tests including Kruskal-Wallis ANOVA and Mann-Whitney u-test or repeated measure two-way ANOVA were used as indicated.
Results
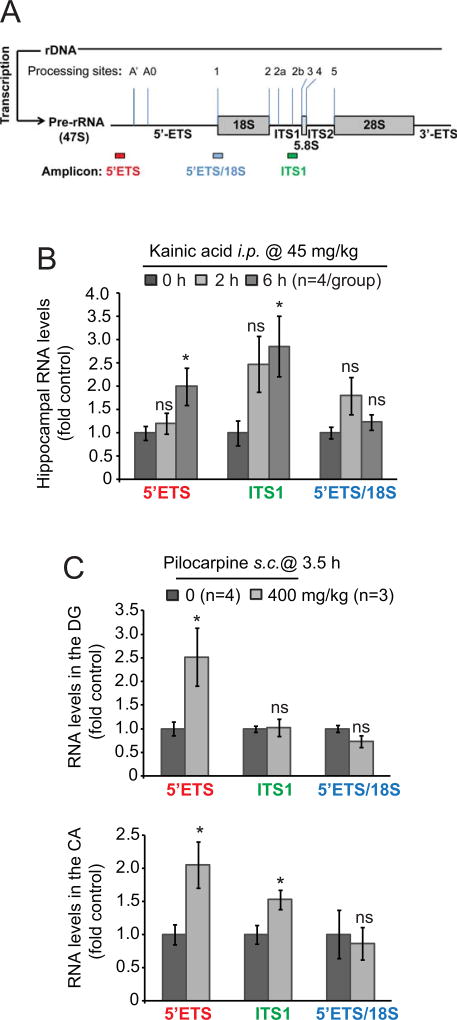
To investigate acute effects of SE on ribosomal biogenesis in the hippocampus, mice were treated with kainic acid (KA). Such a treatment led to development of recurrent seizures in 15–45 min. after i.p. injection of KA (45 mg/kg). To determine activity of ribosomal biogenesis in these animals, three pre-rRNA amplicons were probed by qRT-PCR (Fig. 1A). The 5’ETS amplicon corresponds to the 47S pre-rRNA 5’ETS leader region that undergoes rapid processing providing best insight into nascent pre-rRNA levels [38,39]. The amplicons corresponding to the 5’ETS/18S junction or the ITS1 regions may also detect more stable intermediates of rRNA processing [38,39]. To normalize pre-rRNA changes, total levels of mature 18S rRNA were used. In comparison to pre-rRNA, brain 18S levels are much higher and change much slower as (i) brain neurons contain large quantities of mature ribosomes, (ii) brain ribosome half-life is long (1–2 weeks), and, (iii) ribosomal synthesis rate is low in adult brain ([35]and references therein). Therefore, 18S is a good pre-rRNA normalizer in brain samples from relatively short term studies in which effects of seizures are examined at few hours to few days.
Figure 1. Status epilepticus increases pre-rRNA levels suggesting stimulation of ribosomal biogenesis.
A, Pre-rRNA amplicons that were used for qRT-PCR determination of immature rRNA levels. Major processing sites of mouse pre-rRNA are marked [69]. The 5’ETS amplicon corresponds to the 47S pre-rRNA 5’ETS leader region that undergoes rapid processing providing best insight into nascent pre-rRNA levels. The amplicons corresponding to the 5’ETS/18S junction or the ITS1 regions may also detect more stable intermediates of rRNA processing. The indicated probes were used with 18S rRNA as a normalizer. Due to large quantities of mature ribosomes in the brain and post-mitotic/quiescent nature of most neural cells, total 18S rRNA levels are expected to change far slower than precursor rRNA providing optimal normalization for short-term pre-rRNA studies (i.e. hours-few days). B, Status epilepticus (SE) was induced in mice by kainic acid (KA). Levels of pre-rRNA were determined in the whole hippocampus as indicated. Note upregulation of 47S pre-rRNA as revealed by both 5’ETS and ITS1 probes and no changes in 5’ETS-18S processing intermediates at 6- but not 2 h after KA injection. C, Mice were injected with pilocarpine to induce SE. After 2.5 h, SE was interrupted by i.p. injections of diazepam and mice were killed one hour later. Control mice received the same treatment except pilocarpine. The 5’ETS leader probe revealed increased 47S pre-rRNA levels in the dentate gyrus (DG) and cornu Ammonis (CA) regions of pilocarpine-treated mice; in the CA, the upregulation was also detected with the ITS1 probe. Data represent averages ± SEM, u-test comparisons are to respective control groups; ns, p>0.05; *, p<0.05.
At 2 h after KA administration none of these probes revealed significant changes of hippocampal pre-rRNA levels (p>0.05, u-test, Fig. 1B). However, at 6 h, elevated pre-rRNA levels were observed with the 5’ETS- and the ITS1 amplicons (p<0.05, u-test, Fig. 1B). Thus, KA-SE induces time dependent changes in ribosomal biogenesis including a likely increase of pre-rRNA transcription in the hippocampus.
Next, we investigated whether ribosomal biogenesis is altered after SE in response to another pro-convulsive drug, pilocarpine. In these follow up studies, we analyzed pre-rRNA levels after dissecting the dentate gyrus- (DG) and the cornu Ammonis (CA) regions of the hippocampus as they are differentially sensitive to SE-induced neurodegeneration with most DG granule neurons surviving such a challenge [40–45]. Unfortunately, we were unable to collect sufficient number of samples at 6 h as lethal outcomes were observed in at least 80% mice at 2–4 h after administration of 400 mg/kg pilocarpine that was used in this experiment. Therefore we decided to limit the study to a single time point of 3.5 h. That time was chosen based on results from the KA-SE experiment as it suggested the SE-induced changes in pre-rRNA occur between 2- and 6 h after pro-convulsive treatment. Indeed, the pilocarpine-SE experiment revealed increased pre-rRNA levels in the DG and the CA region when the 5’ETS probe was used (Fig. 1C, p<0.05, u-test). The ITS1 probe uncovered significant increases of pre-rRNA in the CA- but not the DG (Fig. 1C). Such observation may suggest that in the DG, increased rRNA transcription is accompanied by accelerated rRNA processing.
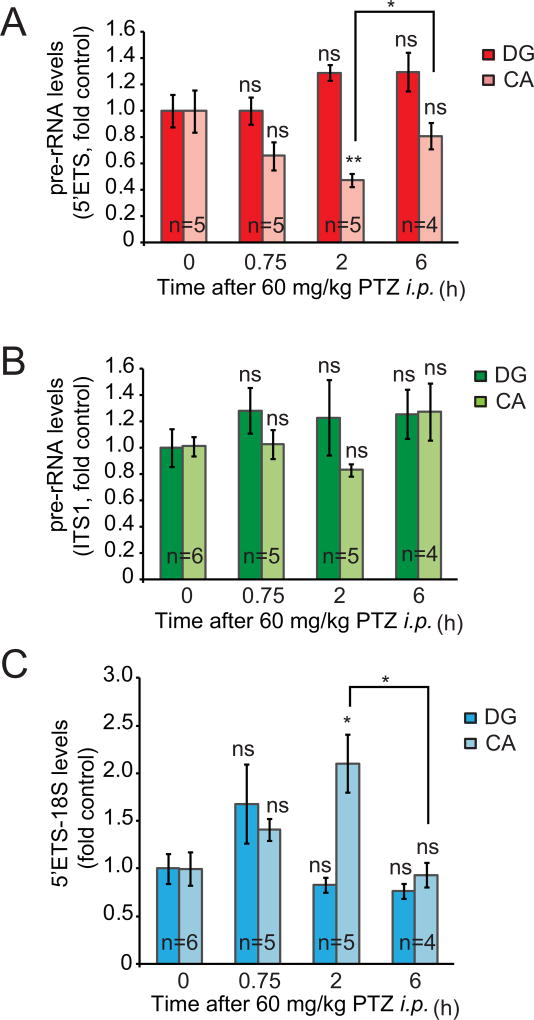
To examine the effects of a transient seizure attack on ribosomal synthesis, PTZ was given i.p. at a dose of 60 mg/kg. At 5–20 min after this treatment mice experienced a brief episode of tonic-clonic seizures. We determined levels of pre-rRNA in the DG and the CA region at 0.75-, 2-, and 6 h after a seizure-inducing PTZ injection. In the CA, the 5’ETS amplicon revealed transient decrease of nascent pre-rRNA at 2 h after PTZ treatment suggesting inhibition of Pol1 (Fig. 2A–B, Kruskal-Wallis ANOVA, factor time, H3/20= 10.59, p=0.0142) . As the ITS1 probe did not detect significant changes in pre-rRNA, one could expect that declines in the 5’ETS leader-containing nascent 47S were accompanied by increased levels of unprocessed intermediates (Fig. 2B). Indeed, the 5’ETS-18S probe revealed transient increase of 18S precursor intermediates at 2 h after PTZ treatment suggesting disruption of rRNA processing towards 18S rRNA (Fig. 2C, Kruskal-Wallis ANOVA, factor time, H3/20= 10.065, p=0.018). PTZ-induced seizures had no significant effects on pre-rRNA levels in the DG (Fig. 2A–C). These findings indicate a brain region- and time specific inhibition of rRNA transcription and rRNA processing in response to a brief episode of seizures.
Figure 2. Transient inhibition of ribosomal biogenesis after PTZ-induced seizures.
A transient seizure episode was induced in male mice (C57Bl6) by a single injection of PTZ; mice were killed at various times after the pro-convulsive treatment. Levels of pre-rRNA were determined in the dentate gyrus- (DG) and the cornu Ammonis (CA) regions. In the CA, there was a transient decrease of nascent pre-rRNA at 2 h after PTZ treatment suggesting inhibition of Pol1 (A). As the ITS1 probe did not detect significant changes in pre-rRNA, one could expect that declines in nascent 47S were accompanied by increased levels of processing intermediates containing ITS1 (B). Indeed, the 5’ETS-18S probe revealed increased levels of 18S precursor intermediates suggesting disruption of rRNA processing (C). Averages ± SEM are depicted; u-test comparisons are to respective control groups (DG or CA at time 0 h) unless indicated otherwise; ns, p>0.05; *, p<0.05; **, p<0.01.
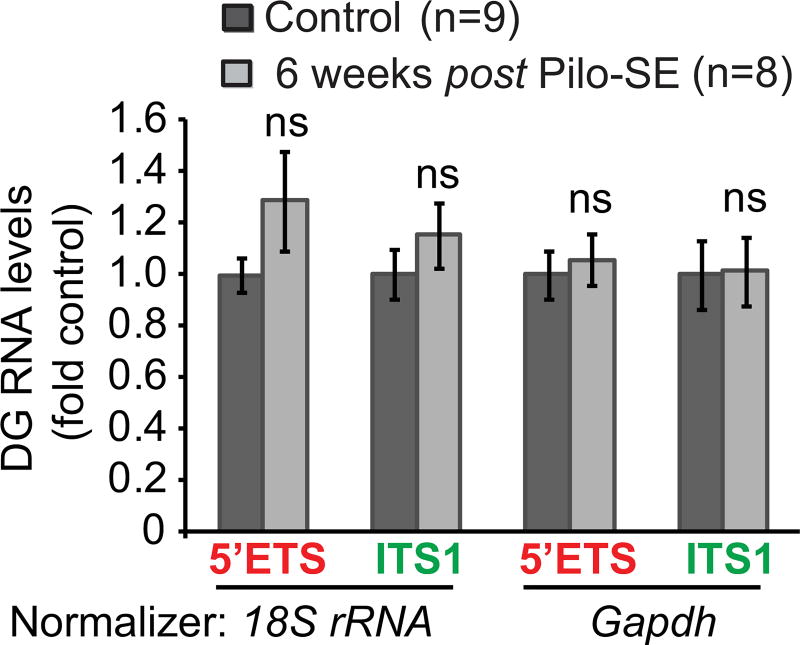
Pilocarpine-induced SE triggers neurodegeneration mainly in the CA- and the DG hilar regions. Then, spontaneous seizures and DG MFS develop weeks after the initial SE [40–45]; such changes are preceded by chronic activation of the mTOR pathway that lasts for at least 1 week after SE [7]. Therefore six weeks after pilocarpine-induced SE, pre-rRNA levels were probed in the DG using the 5’ETS- and the ITS1 probes. For this experiment, pilocarpine dose was reduced to 300 mg/kg to improve long-term survival after pilocarpine-induced SE (see Materials and Methods). As in such a prolonged experiment one could expect pilocarpine-induced changes in mature ribosome content, Gapdh mRNA was used as an additional normalizing transcript besides 18S rRNA. However, regardless of the normalizer used, no significant changes of pre-rRNA levels were observed (Fig. 3). Similarly to the DG, no significant effects were observed in the CA region (Table 1). Neither the DG nor the CA showed altered pre-rRNA levels at 1 week post-SE (Table 1).
Figure 3. No long-term changes of pre-rRNA levels after pilocarpine-induced SE.
Mice (C57Bl6 males) were treated with vehicle (control, ctrl) or pilocarpine (300 mg/kg) to induce SE. Six weeks after pilocarpine-induced SE (Pilo-SE), no significant effects were observed on pre-rRNA levels in the DG. Note, that use of two different qRT-PCR normalizing genes produced similar results. Pre-rRNA was unchanged in the CA, either; no significant changes were found at 1 week post SE (Table 1).
Table 1.
Non-significant long-term effects of pilocarpine-induced SE (Pilo-SE) on hippocampal pre-rRNA
| Experimental Group (n) |
Amplicon/Normalizing gene (mean fold control ± SEM) | |||
|---|---|---|---|---|
|
| ||||
| 5’ETS/18S | 5’ETS/Gapdh | ITS1/18S | ITS1/Gapdh | |
|
| ||||
| Control (9) | DGa: 1 ± 0.067 | DG: 1 ± 0.094 | DG: 1 ± 0.097 | DG: 1 ± 0.134 |
| CAb: 1 ± 0.095 | CA: 1 ± 0.065 | CA: 1 ± 0.083 | CA: 1 ± 0.13 | |
|
| ||||
| 1 week post Pilo-SE (6) | DG: 1.11 ± 0.13 c | DG: ND d | DG: 0.99 ± 0.13 c | DG: ND |
| CA: 0.99 ± 0.12 c | CA: ND | CA: 0.98 ± 0.12 c | CA: ND | |
|
| ||||
| 6 weeks post Pilo-SE (8) | DG: 1.29 ± 0.193c | DG: 1.05 ± 0.1 c | DG: 1.15 ± 0.13 c | DG: 1.01 ± 0.13 c |
| CA: 1.08 ± 0.11 c | CA:1.04 ± 0.06 c | CA: 0.94 ± 0.12 c | CA: 0.9 ± 0.067 c | |
dentate gyrus;
cornu Ammonis;
p>0.05, u-test as compared to the control group;
not determined.
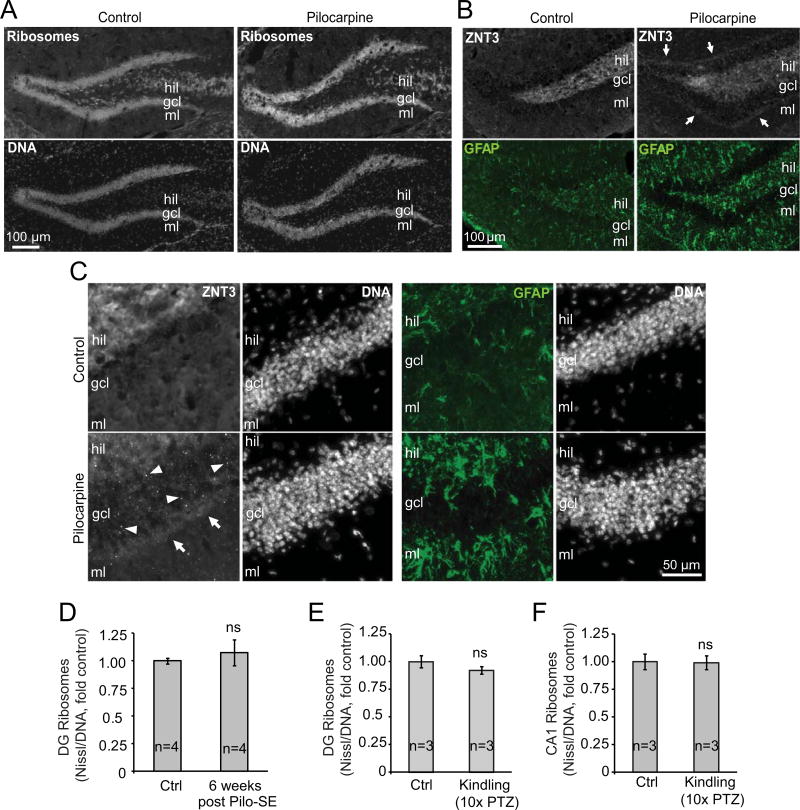
Lack of long-lasting effects of pilocarpine SE on DG ribosome synthesis is consistent with unaltered ribosome content in that region as revealed by ribosome staining with the fluorescent Nissl dye NeuroTrace Green at 6 weeks post SE (Fig. 4A, D). Importantly, unaltered DG ribosome content was not due to lack SE-induced neurodegeneration/hippocampal reorganization. As compared to controls, all Pilo-SE mice appeared to have reduced Nissl-staining in the DG hilus as well as the CA region (Fig. 4A and Supplementary Fig. 1A). Moreover, immunostainings for GFAP and ZNT3 confirmed reactive astrogliosis and MFS, respectively (Fig. 4B–C). Thus, reactive gliosis throughout the hippocampus was observed in all SE animals (4/4). MFS with increased ZNT3 signal in the DG granule cell layer or both the DG granule cell layer and the molecular layer was observed in 4/4 or 2/4 SE animals, respectively.
Figure 4. Total perikaryal ribosome content does not change in pharmacological models of epileptogenesis.
A–D, Mice were treated as in Fig. 3. A-C, Representative images of ribosome (NeuroTrace® 500/525 Green Fluorescent Nissl)- or DNA (Hoechst 33258) or the mossy fiber marker ZNT3 or the reactive astrocyte marker GFAP staining in the dorsal hippocampus of control or Pilo-SE mice (6 weeks post SE). A, Note apparent loss of Nissl stained cells in the DG hilus of Pilo-SE animals. Reduced Nissl staining in the CA region was also observed (Supplementary Fig. S1A). B-C, In the DG of control animals, ZNT3 staining is limited to the DG hilus/CA3 region (hil). In Pilo-SE mice, ZNT3 signal appears in the DG granule cell layer (gcl, arrowheads) and molecular layer (mcl, arrows) indicating mossy fiber sprouting (MFS). Presence of hypertrophic astrocytes with increased GFAP staining indicates reactive astrogliosis. These changes confirm post-SE neurodegeneration and hippocampal remodeling. D, In the granule cell layer of the DG, where perikarya of granule neurons are located, DNA-normalized ribosome content (i.e. fluorescence intensity ratio of NeuroTrace Green to Hoechst) was unaffected in Pilo-SE animals. E-F, Mice (C57Bl6 males) were treated with 10 sub-seizures doses of PTZ (35 mg/kg) or vehicle (control, ctrl) every other day; fully kindled animals that displayed tonic-clonic seizures (at least stage 4) after each of the last 5 injections of PTZ were used for evaluation of ribosome content. No significant changes in DNA-normalized content of ribosomes were observed in the granule cell layer of the DG or stratum pyramidale of the CA1 region of PTZ kindled mice; representative images of ribosomal staining in the dorsal hippocampus of PTZ-kindled mice are shown in Fig. S1. Data represent averages ± SEM, u-test comparisons are to respective control groups; ns, p>0.05; *, p<0.05.
Repeated administration of PTZ at sub-convulsive doses, which initially does not produce seizures, will lead to the occurrence of seizures following PTZ dosing over a course of days [46]. Such PTZ kindling is believed to represent the early stages of epileptogenesis and has been used in the past to examine candidate molecular players in that process [30,47]. Therefore, hippocampal ribosome content was determined in PTZ-kindled mice that received a sub-seizure threshold dose of PTZ (35 mg/kg) over 20 days and demonstrated consistent seizure after the last 5 injections. However, neither in the DG nor in the CA1 region PTZ kindling produced significant changes of the total ribosome content (Fig. 4E–F and Supplementary Fig. S1). These findings indicate that in the hippocampus that is challenged with two distinct paradigms of epileptogenesis, there are no long-lasting changes in total ribosome content despite acute modulation of ribosomal biogenesis by seizures.
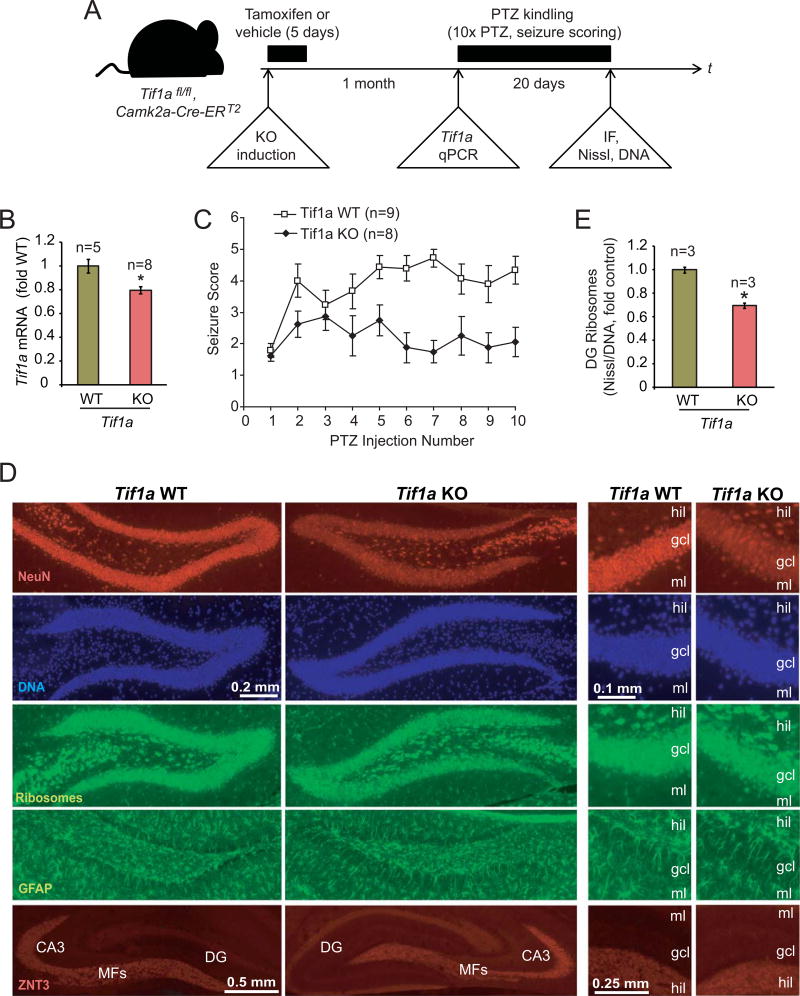
Next, conditional KO of the Pol1-specific co-factor Tif1a was used to determine effects of inhibiting Pol1 on PTZ kindling (Fig 5A). In Tif1afl/fl, Camk2a-CreERT2 mice, tamoxifen-mediated activation of the excitatory neuron-expressed CreERT2 induces loss of the Tif1a, disrupting Pol1 activity in most DG granule neurons and CA pyramidal neurons [25,24]. First, successful cell type-restricted induction of Tif1a KO was confirmed by a 20% reduction in hippocampal Tif1a mRNA of Tif1afl/fl, Camk2a-Cre-ERT2 mice at 4 weeks after completion of a 5 day tamoxifen treatment protocol (Fig 5B). Second, WT and KO mice received a pro-convulsive PTZ dose of 60 mg/kg. All treated animals, regardless of their genotype, reached stage 5 seizures within 20 min following PTZ injection (Table 2). Thus, Tif1a deficiency was not associated with resistance to the acutely pro-convulsive dose of PTZ, illustrating functional integrity of the seizure circuitry that is engaged by PTZ. Then, the kindling response was analyzed in KO and WT mice (Fig. 5C). In WT mice, average seizure score stayed above 4 for PTZ injections #5–8 and reached maximal value of 4.72 ± 0.37 after injection #7 (Fig. 5C). By injection #10, 7 out of 9 WT animals were fully kindled as defined by at least stage 4 seizures in response to 4 consecutive PTZ injections. In KO mice, the average seizure score never exceeded 3 (maximum of 2.85 ± 0.44 after injection #3) while only one mouse was fully kindled by the end of the experiment (Fig. 5C). Therefore, Tif1a KO mice are resistant to PTZ kindling.
Figure 5. PTZ kindling is disrupted after deletion of the Pol1 co-activator Tif1a from excitatory forebrain neurons.
A, The experimental design. Tif1afl/fl, Camk2a-Cre-ERT2 mice were treated for 5 days with tamoxifen to induce Tif1a KO selectively in excitatory forebrain neurons. After a month, some animals were killed and hippocampal Tif1a mRNA expression was determined. Other animals were used for a pilot experiment with an acutely pro-convulsive dose of PTZ (60 mg/kg) to verify unaffected PTZ responsiveness of KO mice (Table 2). The rest of animals was subjected to a kindling paradigm that consisted of 10 i.p. injections of PTZ at a low dose of 35 mg/kg every other day; PTZ-induced seizures were scored using modified Racine scale. Then, animals were killed and structure of the dorsal hippocampus was analyzed by immunofluorescence (IF) or FluoroJade-B staining as well as ribosome (NeuroTrace Green, Nissl) and DNA (Hoechst-33258) co-stainings. B, One month after tamoxifen treatment, Tif1a mRNA levels were reduced in hippocampi of KO animals as revealed by qRT-PCR; Gapdh was used as a normalizer; the control group (WT) consisted of vehicle-treated Tif1afl/fl, Camk2a-Cre-ERT2 mice. C, Seizure scores of PTZ kindled mice. Note increasing seizure responses (i.e. kindling) in WT but not KO mice. The 2-way repeated measure ANOVA revealed significant effects of genotype (F1/15= 14.79, p=0.00159), number of injections (F9/135=3.85, p=0.000232), and the interaction between genotype and the number of injections (F9/135=2.987, p=0.002823). The KO group included 6 males and 2 females; the WT group included tamoxifen-treated Tif1afl/fl mice (3 males) as well as vehicle-treated Tif1afl/fl, Camk2a-Cre-ERT2 mice (4 males and 2 females). D, Representative images of immunostainings for the neuronal marker NeuN, the mossy fiber marker Znt3, the reactive astrocyte marker GFAP, and co-stainings of ribosomes and DNA. No major structural changes or reactive gliosis in the DG of the PTZ-kindled KO mice were revealed; similarly, no changes were observed in other forebrain regions (not shown). Only a small increase in FluoroJade-B-positive cells was noted in the DG but not the CA region indicating no major neurodegenerative pathology in the hippocampus of the Tif1a KO mice (Table 3, Supplementary Fig. S2). The moderate, KO-associated decrease in NeuN staining intensity in granule cell layer (gcl) appears to be caused by lower cellular levels of NeuN signal rather than reduced number of NeuN-positive cells; MFs, mossy fibers; hil, DG hilus; gcl, DG granule cell layer; ml, DG molecular layer. E, DNA-normalized content of ribosomes was determined in the gcl by dividing fluorescence intensity of ribosome signal by that of DNA in double stained sections. A moderate decrease of the DG ribosome content was present in Tif1a KO mice. Data represent averages ± SEM; *, p<0.05 (u-test).
Table 2.
Effects of Tif1A KO on seizure response to an acutely pro-convulsant dose of PTZ1
60 mg/kg PTZ i.p. was given one month after tamoxifen treatment.
Tif1afl/fl, Camk2a-Cre-ERT2 mice (8 weeks old, 2 males and 2 females) were treated for 5 days with tamoxifen.
Tif1a fl/fl mice (8 weeks old, 2 males and 2 females) that were treated with tamoxifen.
Such an effect was not associated with a major structural disruption of the hippocampus including loss of neurons. Analysis of kindling-resistant KO- and fully kindled WT mice revealed similar staining patterns of NeuN (the neuronal perikarial marker), ZNT3 (the mossy fiber marker), and GFAP (the reactive astroglia marker) in the dorsal hippocampus (Fig. 5D). A noticeable reduction in NeuN staining intensity in the KO DG appears to be related to reduced NeuN immunoreactivity per cell rather than outright cell loss of NeuN-positive cells (Fig. 5D). In support of that notion, FluoroJade-B staining revealed only a small increase in number of degenerating neurons in the DG but not the CA region of Tif1a KO animals (1.13 ± 0.26 or 0.33 ± 0.13 degenerating cells/hippocampus/section in KO or WT mice, respectively, p<0.05, u-test, Table 3 and Supplementary Fig. S2). Thus, no major neurodegenerative changes were observed in the hippocampi of Tif1a KO animals. These observations are consistent with a previous report that adult excitatory forebrain neurons, including DG granule cells and CA pyramidal neurons, can tolerate disruption of Tif1a for at least several months [24]. Finally, ribosomal staining of brain sections from PTZ-kindled WT and KO animals confirmed a reduction in ribosomal biogenesis in the DG granule cell layer of Tif1a KO animals as their total ribosome content was 30% lower than in WT (Fig. 5E). In contrast, ribosome content in hilar inhibitory neurons was unaffected confirming excitatory neuron-specificity of Tif1a KO (average Nissl fluorescence intensity/cell was 8299 ± 293 or 8328 ± 450 arbitrary units for WT or KO mice, respectively, p>0.05, u-test).Hence, the anti-kindling effects of Tif1a KO are likely due to ribosomal deficits but not neurodegeneration.
Table 3.
Number of degenerating neurons in the hippocampi of PTZ-kindled Tif1a WT or PTZ-kindling-resistant Tif1a KO animals as revealed by FluoroJade-B staining.
| Genotype: | Average number of degenerating neurons/hippocampus/section ± SEMa |
||
|---|---|---|---|
| DG | CA1 | CA3 | |
| Tif1a WT (n=5) | 0.33 ± 0.13 | 0.22 ± 0.08 | 0.15 ± 0.07 |
| Tif1a KO (n=5) | 1.13 ± 0.26 * | 0.20 ± 0.10 ns | 0.25 ± 0.12 ns |
for each animal, at least 6 coronal sections cut through the dorsal hippocampus were analyzed
p<0.05 (u-test)
p>0.05 (u-test)
Discussion
The presented evidence suggests that in the mouse hippocampus, ribosomal biogenesis is transiently inhibited by isolated seizures, and stimulated by SE. However, at least in the DG, no chronic effects on total ribosome content are observed after repeated pro-convulsive stimulation with PTZ or pilocarpine-induced SE. Hence, seizure-associated regulation of Pol1 is not sufficient to significantly change the total ribosome pool. Instead, seizures may regulate production of a specific ribosome subtype(s) with a potential role in pro-epileptogenic gene expression. In support of the latter notion, induced genetic inhibition of Pol1 in excitatory forebrain neurons impaired PTZ kindling suggesting requirement of ribosome biogenesis for this form of epileptogenic plasticity.
Transient inhibition of transcription and processing of rRNA as observed in the CA region of PTZ-treated mice is in agreement with several reports of time-dependent declines in overall RNA synthesis and accumulation of long unprocessed RNA species in rodent models of seizures [48–50]. As rRNA synthesis accounts for a large portion of the total cellular transcriptional output, it is conceivable that reduced synthesis and processing of pre-rRNA contributed to such changes. Indeed, reduced levels of de-novo synthesized 18S and 28S rRNA were observed in cytoplasmic rat cerebrocortical RNA that was isolated for up to 90 min after flurothyl-induced seizures [48]. Of note, similarly to PTZ, pro-convulsant effects of flurothyl are due to inhibition of the GABA-A receptors and are limited to a single, short-lasting seizure episode [51]. Similarly to our current findings in the mouse PTZ model, nascent rRNA levels normalized to control values at 6 h after flurothyl-induced seizures in rats [48]. Hence, transient inhibition of ribosomal biogenesis seems to be a common response to a brief episode of generalized seizures.
In contrast to inhibitory effects of short-term generalized tonic-clonic seizures that were induced by PTZ (<1 min, stage 4–5), SE was associated with increased levels of pre-rRNA that suggested stimulation of pre-rRNA transcription and upregulation of ribosomal biogenesis. While it is unclear what accounts for such opposite outcomes, one could speculate that differences in the extent of seizure activity may be involved. Namely, in the SE models that were used here (pilocarpine and KA), most seizures were stage 3 with occasional short-lasting episodes of stage 4/5 seizures. However, SE lasted at least 30 min. Therefore, prolonged seizure activity may be necessary to stimulate ribosome biogenesis. Such a stimulation could help replace damaged ribosomes and/or generate new ribosome subtypes witch different ribosomal protein (RP) stochiometry or distinct RP/rRNA modifications to enable preferential translation of specific mRNAs. Similar ribosome pool renewal and/or diversification could also occur when ribosomal biogenesis recovers from postictal inhibition after a brief generalized seizure.
Although total ribosome content is unaffected during epileptogenesis, the demonstrated modulation of rRNA transcription may result in synthesis of specialized subtypes of ribosomes. If that is the case, one could consider an interesting possibility that such specialized ribosomes are required for pro-epileptogenic translation. Indeed, RPL24- or RPL38-containing ribosomes are critical for Myc-dependent lymphomagenesis or somite formation during mammalian development, respectively [52,53].
Intriguingly, meta-analysis of a publicly available hippocampal transcriptomic data set from the mouse model of pilocarpine-induced epileptogenesis revealed upregulation of specific RPs but not all ribosomal components (Supplementary Table S1) [37]. For instance, RPL8 appeared to be upregulated at several time points after SE including 12 h, 10 days and 6 weeks (3.1-, 4.9-, and 2.2 fold control, respectively). As RPL8 is a site of ribosome hydroxylation and is a likely contributor to interactions between the ribosome and aminoacyl-tRNAs, it may be well positioned to participate in translation regulation [54]. At 10 days post SE, when epileptogenic remodeling occurs, there were six RPs whose upregulation exceeded 10 fold controls (Rpl26, Rpl18, Rpl6, Rpl29, Rplp2, Rpl39). In addition, significant enrichment of upregulated mRNAs from the gene ontology (GO) category “ribosome biogenesis” was identified at that time point; however no enrichment for GO “ribosome component” was noted [37]. These transcriptomic data support a possibility that seizures and/or epileptogenesis alter a RP-specific sub-pool of ribosomes.Future studies are needed to directly interrogate the effects of epilepsy on ribosome heterogeneity and its consequences on regulated translation in the epileptic brain.
What is the ribosome-synthesis-sensitive component of the circuitry that mediates PTZ kindling? The hippocampus is essential for PTZ kindling [55]. The conditional Tif1a KO mouse line that was used for the current work enables selective deletion of Tif1a from excitatory forebrain neurons including the DG granule neurons and the CA pyramidal neurons in the hippocampus [24]. Hence, either one or both of these neuron subtypes may require ribosome synthesis to express the kindling response. Noteworthy, a single PTZ seizure episode transiently reduced pre-rRNA levels in the CA but not the DG suggesting modulation of ribosome synthesis in pyramidal- but not granule neurons. Hence, CA neurons are a likely candidate cell type in which ribosome synthesis supports PTZ kindling.
What is a mechanism(s) of PTZ kindling inhibition in Pol1-deficient mice? As opposed to SE-based models of epileptogenesis where the hippocampus undergoes extensive morphological reorganization, structural changes that are associated with PTZ kindling are relatively small suggesting greater contribution of synaptic potentiation-based mechanisms. Indeed, PTZ kindling has been proposed to share a mechanism(s) with hippocampal LTP. Interestingly, hippocampal LTP at the CA3-CA1 Schaffer collateral synapses is severely reduced after selective induction of Tif1a KO in excitatory forebrain neurons [56,57,24]. Conversely, this type of LTP is enhanced by PTZ kindling [58,56]. Therefore, it is tempting to speculate that anti-kindling effects of Tif1a KO are due to critical dependence of long-term synaptic plasticity in the hippocampus on synthesis of new ribosomes especially in pyramidal neurons of the CA1/CA3 regions.
Importantly, despite defective LTP, the hippocampal circuitry of tamoxifen-induced Tif1afl/fl, Camk2a-Cre-ERT2 mice remains functional for months after KO induction [24]. Thus, spatial learning and memory are mostly unchanged in these animals, while electrophysiological data from hippocampal slices indicates unaffected field potential input/output parameters at CA3/CA1 Schaffer collateral synapses [24]. Our current findings that Tif1a KO mice were able to seize in response to PTZ further support such conclusions and indicate that Pol1 activity is dispensable for integrity of the seizure execution circuitry.
Induced gene expression is documented during PTZ kindling [59–61]. Therefore, ribosome synthesis may be needed to support translation of proteins that are required for kindling. While PTZ kindling did not affect total ribosome content in WT hippocampus, total ribosome content was decreased after Tif1a KO. Therefore, one can speculate that reduced ribosomal biogenesis prevented kindling by perturbing protein synthesis either by a hypothetical subset of specialized ribosomes that are produced during re-bound of ribosome synthesis after each PTZ stimulation or by the KO-associated reduction of general ribosome availability. Even in the latter case, anti-translational activity may be relatively selective, as regulated translation of mRNAs with highly complex 5’UTRs is more sensitive to disruptions of ribosome supply than constitutive translation of housekeeping mRNAs [62]. Of note, 5’UTR-dependent regulation of translation contributes to hippocampal LTP which may share a common mechanism with PTZ kindling as discussed above [63,56,57].
While the presented results indicate requirement of the excitatory neuron Pol1 for PTZ kindling, a question remains as to pro- or anti-epileptogenic role of ribosomal biogenesis in other types of brain cells that are involved in pathogenesis of epilepsy. For instance, epileptogenesis has been documented following selective hyperactivation of mTOR in astrocytes or neuroprogenitors [64,65]. Ablation of the latter cell type reduced epileptogenesis in SE-based models of TLE [66]. In addition, newly generated DG granule neurons appear to have unique contributions to structural reorganization of the hippocampus in response to an epileptogenic SE injury including greater MFS, somato-dendritic hypertrophy and formation of aberrant dendrites [67,40]. In all these cases, one can expect that ribosomal biogenesis will be pro-epileptogenic. Therefore, future studies could address that issue in such clinically-relevant models of epileptogenesis as post-SE TLE or tuberous sclerosis-like epilepsy in Tsc1 KO mice [44,64]. Adequate experimental approaches may include neuroprogenitor cell- and/or astrocyte-selective KO of Tif1a and/or pharmacological treatment with CNS-permeable anti-cancer Pol1 inhibitors such as the non-toxic BMH-21 [68].
In summary, this study provides the first insight into regulation and significance of Pol1 in pharmacological models of epilepsy. Transient effects on pre-rRNA levels, no change in total ribosome content and anti-kindling effects of the excitatory neuron-selective inhibition of Pol1 are consistent with a concept that a subset of de-novo synthesized ribosomes is needed to mediate epileptic neuroplasticity. Future studies are needed to verify existence of such pro-epileptogenic ribosomes and test significance of ribosomal synthesis in other epilepsy-relevant cell types and models.
Supplementary Material
Acknowledgments
This work was supported by the American Epilepsy Society (Seed Grant to MH), NIH (NS-073584 and 8P30GM-103507 to MH), NSF (IOS1021860 to MH), the Kentucky Spinal Cord and Head Injury Research Trust, and the Commonwealth of Kentucky Challenge for Excellence Fund. The authors wish to thank Mrs. Jing-Juan Zheng for excellent technical assistance and Dr. Steve Danzer for helpful discussion and critical reading of the manuscript.
Abbreviations
- CA
cornu Ammonis
- CS
Cowden syndrome
- DG
dentate gyrus
- FI
fluorescence intensity
- gcl
granule cell layer
- GO
gene ontology
- KA
kainic acid
- LTP
long term potentiation
- MFS
mossy fiber sprouting
- ml
molecular layer
- mTOR
mechanistic target of rapamycin
- Pol1
RNA-Polymersase-1
- PTZ
pentylenetetrazole
- qRT-PCR
quantitative reverse transcriptase PCR
- RP
ribosomal protein
- SE
status epilepticus
- Tif1a
Transcription initiation factor-1A
- TSC
tuberous sclerosis complex
References
- 1.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73(1):1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 2.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Science’s STKE : signal transduction knowledge environment. 2006;2006(356):re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 3.Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- 4.Pitkanen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy & behavior : E&B. 2009;14(Suppl 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Curatolo P, Jozwiak S, Nabbout R. Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. 2012;16(6):582–586. doi: 10.1016/j.ejpn.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51(1):27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29(25):8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40(1):193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29(21):6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Wong M. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia. 2012;53(3):506–511. doi: 10.1111/j.1528-1167.2011.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrido YC, Sanabria ER, Funke MG, Cavalheiro EA, Naffah-Mazzacoratti MG. Mitogen-activated protein kinase is increased in the limbic structures of the rat brain during the early stages of status epilepticus. Brain Res Bull. 1998;47(3):223–229. doi: 10.1016/s0361-9230(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82(1):192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Hong KS, Seong YS, Park JB, Kuroda S, Kishi K, Kaibuchi K, Takai Y. Phosphorylation and activation of mitogen-activated protein kinase by kainic acid-induced seizure in rat hippocampus. Biochem Biophys Res Comm. 1994;202(2):1163–1168. doi: 10.1006/bbrc.1994.2050. [DOI] [PubMed] [Google Scholar]
- 14.Gass P, Kiessling M, Bading H. Regionally selective stimulation of mitogen activated protein (MAP) kinase tyrosine phosphorylation after generalized seizures in the rat brain. Neurosci Lett. 1993;162(1–2):39–42. doi: 10.1016/0304-3940(93)90554-x. [DOI] [PubMed] [Google Scholar]
- 15.Drygin D, Rice WG, Grummt I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol. 2010;50:131–156. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 16.Hannan R, Stefanovsky V, Arino T, Rothblum L, Moss T. Cellular regulation of ribosomal DNA transcription:both rat and Xenopus UBF1 stimulate rDNA transcription in 3T3 fibroblasts. Nucleic Acids Res. 1999;27(4):1205–1213. doi: 10.1093/nar/27.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004;18(4):423–434. doi: 10.1101/gad.285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Yuan X, Frodin M, Grummt I. ERK-dependent phosphorylation of the transcription initiation factor TIF-IA is required for RNA polymerase I transcription and cell growth. Mol Cell. 2003;11(2):405–413. doi: 10.1016/s1097-2765(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 19.Stefanovsky VY, Pelletier G, Hannan R, Gagnon-Kugler T, Rothblum LI, Moss T. An immediate response of ribosomal transcription to growth factor stimulation in mammals is mediated by ERK phosphorylation of UBF. Mol Cell. 2001;8(5):1063–1073. doi: 10.1016/s1097-2765(01)00384-7. doi:S1097-2765(01)00384-7 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Gomes C, Smith SC, Youssef MN, Zheng JJ, Hagg T, Hetman M. RNA polymerase 1-driven transcription as a mediator of BDNF-induced neurite outgrowth. J Biol Chem. 2011;286(6):4357–4363. doi: 10.1074/jbc.M110.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetman M, Pietrzak M. Emerging roles of the neuronal nucleolus. Trends Neurosci. 2012;35(5):305–314. doi: 10.1016/j.tins.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capitano F, Gargiuli C, Angerilli A, Maccaroni K, Pelliccia F, Mele A, Camilloni G. RNA polymerase I transcription is modulated by spatial learning in different brain regions. J Neurochem. 2015;136:706–716. doi: 10.1111/jnc.13504. [DOI] [PubMed] [Google Scholar]
- 23.Allen KD, Gourov AV, Harte C, Gao P, Lee C, Sylvain D, Splett JM, Oxberry WC, van de Nes PS, Troy-Regier MJ, Wolk J, Alarcon JM, Hernandez AI. Nucleolar integrity is required for the maintenance of long-term synaptic plasticity. PLoS One. 2014;9(8):e104364. doi: 10.1371/journal.pone.0104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiryk A, Sowodniok K, Kreiner G, Rodriguez-Parkitna J, Sonmez A, Gorkiewicz T, Bierhoff H, Wawrzyniak M, Janusz AK, Liss B, Konopka W, Schutz G, Kaczmarek L, Parlato R. Impaired rRNA synthesis triggers homeostatic responses in hippocampal neurons. Front Cell Neurosci. 2013;7:207. doi: 10.3389/fncel.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parlato R, Kreiner G, Erdmann G, Rieker C, Stotz S, Savenkova E, Berger S, Grummt I, Schutz G. Activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice. J Neurosci. 2008;28(48):12759–12764. doi: 10.1523/JNEUROSCI.2439-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ. Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience. 2008;151(2):476–488. doi: 10.1016/j.neuroscience.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamanouchi H. Activated remodeling and N-methyl-D-aspartate (NMDA) receptors in cortical dysplasia. J Child Neurol. 2005;20(4):303–307. doi: 10.1177/08830738050200040601. [DOI] [PubMed] [Google Scholar]
- 28.Yamanouchi H, Jay V, Rutka JT, Takashima S, Becker LE. Evidence of abnormal differentiation in giant cells of tuberous sclerosis. Ped Neurol. 1997;17(1):49–53. doi: 10.1016/s0887-8994(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 29.Erdmann G, Schutz G, Berger S. Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci. 2007;8:63. doi: 10.1186/1471-2202-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilczynski GM, Konopacki FA, Wilczek E, Lasiecka Z, Gorlewicz A, Michaluk P, Wawrzyniak M, Malinowska M, Okulski P, Kolodziej LR, Konopka W, Duniec K, Mioduszewska B, Nikolaev E, Walczak A, Owczarek D, Gorecki DC, Zuschratter W, Ottersen OP, Kaczmarek L. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180(5):1021–1035. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzuferi M, Kumar G, Rospo C, Kaminski RM. Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol. 2012;238(2):156–167. doi: 10.1016/j.expneurol.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Hagihara H, Toyama K, Yamasaki N, Miyakawa T. Dissection of hippocampal dentate gyrus from adult mouse. Journal of visualized experiments : JoVE. 2009;(33) doi: 10.3791/1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalita K, Makonchuk D, Gomes C, Zheng JJ, Hetman M. Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J Neurochem. 2008;105(6):2286–2299. doi: 10.1111/j.1471-4159.2008.05316.x. doi:JNC5316 [pii] 10.1111/j.1471-4159.2008.05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poortinga G, Wall M, Sanij E, Siwicki K, Ellul J, Brown D, Holloway TP, Hannan RD, McArthur GA. c-MYC coordinately regulates ribosomal gene chromatin remodeling and Pol I availability during granulocyte differentiation. Nucleic Acids Res. 2011;39(8):3267–3281. doi: 10.1093/nar/gkq1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slomnicki LP, Pietrzak M, Vashishta A, Jones J, Lynch N, Elliot S, Poulos E, Malicote D, Morris BE, Hallgren J, Hetman M. Requirement of Neuronal Ribosome Synthesis for Growth and Maintenance of the Dendritic Tree. J Biol Chem. 2016;291(11):5721–5739. doi: 10.1074/jbc.M115.682161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, Matsushima S, Taki W, Henshall DC. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Hansen KF, Sakamoto K, Pelz C, Impey S, Obrietan K. Profiling status epilepticus-induced changes in hippocampal RNA expression using high-throughput RNA sequencing. Scientific reports. 2014;4:6930. doi: 10.1038/srep06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popov A, Smirnov E, Kovacik L, Raska O, Hagen G, Stixova L, Raska I. Duration of the first steps of the human rRNA processing. Nucleus. 2013;4(2):134–141. doi: 10.4161/nucl.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dundr M, Olson MO. Partially processed pre-rRNA is preserved in association with processing components in nucleolus-derived foci during mitosis. Mol Biol Cell. 1998;9(9):2407–2422. doi: 10.1091/mbc.9.9.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy BL, Pun RY, Yin H, Faulkner CR, Loepke AW, Danzer SC. Heterogeneous integration of adult-generated granule cells into the epileptic brain. J Neurosci. 2011;31(1):105–117. doi: 10.1523/JNEUROSCI.2728-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAuliffe JJ, Bronson SL, Hester MS, Murphy BL, Dahlquist-Topala R, Richards DA, Danzer SC. Altered patterning of dentate granule cell mossy fiber inputs onto CA3 pyramidal cells in limbic epilepsy. Hippocampus. 2011;21(1):93–107. doi: 10.1002/hipo.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danzer SC, He X, Loepke AW, McNamara JO. Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy. Hippocampus. 2010;20(1):113–124. doi: 10.1002/hipo.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci. 2007;27(28):7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172(2):143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31(6):2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience / editorial board. 2012. Chapter 9:Unit9 37. [DOI] [PubMed] [Google Scholar]
- 47.Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, Sawada M, Sato J, Yamada K. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31(36):12963–12971. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynter CV, Ioannou P, Mathias AP. The effect of convulsions induced by flurothyl on ribonucleic acid synthesis in rat cerebral cortex during the recovery phase. Biochem J. 1975;152(3):449–467. doi: 10.1042/bj1520449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onishi H, Yamagami S, Mori K, Kawakita Y. Effect of convulsions of the synthesis of heterogeneous nuclear RNA associated with polyadenylate and oligoadenylate sequences from El mouse brain as a convulsive strain. Exp Neurol. 1984;83(1):98–107. doi: 10.1016/0014-4886(84)90049-9. [DOI] [PubMed] [Google Scholar]
- 50.Wynter CV. Persistence of altered RNA synthesis in rat cerebral cortex 12 h after a single electroconvulsive shock. J Neurochem. 1979;32(2):495–504. doi: 10.1111/j.1471-4159.1979.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 51.Ferland RJ. The Repeated Flurothyl Seizure Model in Mice. Bio-protocol. 2017;7(11) doi: 10.21769/BioProtoc.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barna M, Pusic A, Zollo O, Costa M, Kondrashov N, Rego E, Rao PH, Ruggero D. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456(7224):971–975. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, Barna M. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145(3):383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge W, Wolf A, Feng T, Ho CH, Sekirnik R, Zayer A, Granatino N, Cockman ME, Loenarz C, Loik ND, Hardy AP, Claridge TD, Hamed RB, Chowdhury R, Gong L, Robinson CV, Trudgian DC, Jiang M, Mackeen MM, McCullagh JS, Gordiyenko Y, Thalhammer A, Yamamoto A, Yang M, Liu-Yi P, Zhang Z, Schmidt-Zachmann M, Kessler BM, Ratcliffe PJ, Preston GM, Coleman ML, Schofield CJ. Oxygenase-catalyzed ribosome hydroxylation occurs in prokaryotes and humans. Nature Chem Biol. 2012;8(12):960–962. doi: 10.1038/nchembio.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shandra AA, Godlevsky LS. Pentylenetetrazol-induced kindling as a model of absence and convulsive forms of epilepsy. In: Corcoran ME, Moshe SL, editors. Kindling 6: v. 6 (Advances in Behavioral Biology) Springer; 2005. pp. 49–60. [Google Scholar]
- 56.Krug M, Koch M, Grecksch G, Schulzeck K. Pentylenetetrazol kindling changes the ability to induce potentiation phenomena in the hippocampal CA1 region. Physiology & behavior. 1997;62(4):721–727. doi: 10.1016/s0031-9384(97)00167-4. [DOI] [PubMed] [Google Scholar]
- 57.Ruthrich H, Grecksch G, Krug M. Development of long-lasting potentiation effects in the dentate gyrus during pentylenetetrazol kindling. Intl J Dev Neurosci. 2001;19(3):247–254. doi: 10.1016/s0736-5748(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 58.Fathollahi Y, Motamedi F, Semnanian S, Zardoshti M. Repeated administration of pentylenetetrazol alters susceptibility of rat hippocampus to primed-burst stimulation: evidence from in vitro study on CA1 of hippocampal slices. Brain Res. 1996;738(1):138–141. doi: 10.1016/0006-8993(96)00955-9. [DOI] [PubMed] [Google Scholar]
- 59.Marksteiner J, Wahler R, Bellmann R, Ortler M, Krause JE, Sperk G. Limbic seizures cause pronounced changes in the expression of neurokinin B in the hippocampus of the rat. Neuroscience. 1992;49(2):383–395. doi: 10.1016/0306-4522(92)90104-a. [DOI] [PubMed] [Google Scholar]
- 60.Schmoll H, Badan I, Grecksch G, Walker L, Kessler C, Popa-Wagner A. Kindling status in sprague-dawley rats induced by pentylenetetrazole: involvement of a critical development period. Am J Pathol. 2003;162(3):1027–1034. doi: 10.1016/S0002-9440(10)63897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szyndler J, Maciejak P, Wislowska-Stanek A, Lehner M, Plaznik A. Changes in the Egr1 and Arc expression in brain structures of pentylenetetrazole-kindled rats. Pharmacological reports : PR. 2013;65(2):368–378. doi: 10.1016/s1734-1140(13)71012-0. [DOI] [PubMed] [Google Scholar]
- 62.Ludwig LS, Gazda HT, Eng JC, Eichhorn SW, Thiru P, Ghazvinian R, George TI, Gotlib JR, Beggs AH, Sieff CA, Lodish HF, Lander ES, Sankaran VG. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat Med. 2014;20(7):748–753. doi: 10.1038/nm.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cook D, Nuro E, Jones EV, Altimimi HF, Farmer WT, Gandin V, Hanna E, Zong R, Barbon A, Nelson DL, Topisirovic I, Rochford J, Stellwagen D, Beique JC, Murai KK. FXR1P limits long-term memory, long-lasting synaptic potentiation, and de novo GluA2 translation. Cell reports. 2014;9(4):1402–1416. doi: 10.1016/j.celrep.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Annals of neurology. 2002;52(3):285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 65.Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75(6):1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hosford BE, Liska JP, Danzer SC. Ablation of Newly Generated Hippocampal Granule Cells Has Disease-Modifying Effects in Epilepsy. J Neurosci. 2016;36(43):11013–11023. doi: 10.1523/JNEUROSCI.1371-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santos VR, de Castro OW, Pun RY, Hester MS, Murphy BL, Loepke AW, Garcia-Cairasco N, Danzer SC. Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience. 2011;197:348–357. doi: 10.1016/j.neuroscience.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kilanczyk E, Andres KR, Hallgren J, Saraswat-Ohri S, Laiho M, Whittemore SR, Hetman M. Pharmacological inhibition of spinal cord injury-stimulated ribosomal biogenesis does not affect locomotor outcome. Neurosci Lett. 2017;642:153–157. doi: 10.1016/j.neulet.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henras AK, Plisson-Chastang C, O’Donohue MF, Chakraborty A, Gleizes PE. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley interdisciplinary reviews RNA. 2015;6(2):225–242. doi: 10.1002/wrna.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.