SUMMARY
Hippo-like pathways are ancient signalling modules first identified in yeasts. The best-defined metazoan module forms the core of the Hippo pathway, which regulates organ size and cell fate. Hippo-like kinase modules consist of a Sterile 20-like kinase, an NDR kinase and non-catalytic protein scaffolds. In the Hippo pathway, the upstream kinase Hippo can be activated by another kinase, Tao-1. Here, we delineate a related Hippo-like signalling module that Tao-1 regulates to control tracheal morphogenesis in Drosophila melanogaster. Tao-1 activates the Sterile 20-like kinase GckIII by phosphorylating its activation loop, a mode of regulation that is conserved in humans. Tao-1 and GckIII act upstream of the NDR kinase Tricornered to ensure proper tube formation in trachea. Our study reveals that Tao-1 activates two related kinase modules to control both growth and morphogenesis. The Hippo-like signalling pathway we have delineated has a potential role in the human vascular disease cerebral cavernous malformation.
eTOC blurb
Poon et al., describe the discovery of a Hippo-like signaling pathway comprising the kinases Tao-1, GckIII and Tricornered, and its role in tracheal morphogenesis in Drosophila. These discoveries enhance our understanding of tube formation, and have the potential to offer insights into the human vascular disease cerebral cavernous malformation.
Graph Abstract:
INTRODUCTION
Signalling pathways that contain a Sterile 20-like kinase that activates a nuclear Dbf2-related (NDR) family kinase exist throughout eukaryotes, regulate different biological processes and are sometimes referred to as Hippo-like signalling modules (Hergovich and Hemmings, 2012). Antecedent examples of this type of kinase module include the Saccharomyces cerevisiae mitotic exit network and the Schizosaccharomyces pombe septation initiation network, which control cell division and cytokinesis (Bardin and Amon, 2001). The best-defined signalling module of this kind in metazoans forms the core of the Hippo pathway, which operates in insects and mammals to control processes such as organ size and cell fate (Pan, 2010, Halder and Johnson, 2011, Harvey et al., 2013). In addition, a related pathway has been identified in mammals and consists of the Sterile 20-like kinase Mammalian Sterile Twenty-like 3 (MST3) (and possibly its close homologues MST4 and STK25), which can regulate the NDR family kinases NDR1 and NDR2 (aka STK38 and STK38L) (Hergovich, 2013). In some instances, Sterile 20-like kinases have been reported to regulate more than one type of NDR family kinase. For example, Hippo (Hpo) can regulate both Tricornered (Trc) and Warts (Wts) in Drosophila melanogaster peripheral nervous system dendrites (Emoto et al., 2006). In addition, in mammals, the Hpo orthologues MST1 and MST2 can regulate both the Trc orthologues NDR1 and NDR2 and the Wts orthologues LATS1 and LATS2 (Yu and Guan, 2013, Hergovich and Hemmings, 2009, Avruch et al., 2012).
The sole D. melanogaster orthologue of NDR1/2, Trc, controls hair and bristle development in the wing, thorax and antennae, as well as dendrite tiling and branching in the peripheral nervous system (Geng et al., 2000, Emoto et al., 2006). In D. melanogaster, the sole orthologue of MST3/MST4/STK25, Germinal centre kinase III (GckIII), regulates tracheal development together with the non-catalytic protein Cerebral cavernous malformation 3 (CCM3). Loss of GckIII results in a characteristic dilation at the transition zone of the terminal cell, associated with abnormal localisation and abundance of septate junction proteins and the apical membrane protein Crumbs (Song et al., 2013). The mechanism by which GckIII is regulated in trachea is unknown and it is also unclear whether GckIII controls tracheal development by regulating an NDR family kinase.
Kinases belonging to the Thousand and one (Tao) family are conserved throughout evolution. Three Tao proteins are encoded in the human genome (TAO1, TAO2 and TAO3), whilst D. melanogaster possesses a single ancestral Tao kinase (Tao-1). Like Hpo and GckIII, Tao kinases belong to the Sterile 20-like kinase family. Tao kinases have been linked to multiple functions including control of organ growth and stem cell proliferation via the Hippo pathway (Boggiano et al., 2011, Poon et al., 2011, Poon et al., 2016), epithelial cell shape, animal behaviour, and microtubule polymerization (Liu et al., 2010, King et al., 2011, Gomez et al., 2012). The substrates of Tao kinases, and thereby the mechanism by which they regulate these processes, are less well defined. Perhaps the best-characterised Tao-1 substrate is the Hpo kinase; Tao-1 activates Hpo by phosphorylating its activation loop, and this phosphorylation event is conserved in human cells between the orthologous kinases TAO1 and MST2 (Boggiano et al., 2011, Poon et al., 2011). Tao-1 can also activate the related Sterile 20-like kinase Misshapen in the D. melanogaster midgut (Li et al., 2018). When active, Hpo can phosphorylate the hydrophobic motif of the NDR family kinase Warts (Wts), which triggers Wts autophosphorylation and activation (Pan, 2010, Li et al., 2018). Likewise, the human Hpo orthologues MST1 and MST2 can phosphorylate and regulate the activity of the Wts orthologues LATS1 and LATS2 (Praskova et al., 2008).
The D. melanogaster respiratory system, or trachea, is composed of a simple epithelium arranged into tubes of three distinct architectures (Samakovlis et al., 1996). The smallest tubes form within the terminal cells of the tracheal system and are morphologically similar to the seamless endothelial tubes found in the vertebrate vascular system (Yu et al., 2015). Within terminal cells, there is a region termed the transition zone wherein a tube containing an auto-cellular junction connects to a seamless tube. This seamless tube extends distally and branches dozens of times to ramify extensively on internal tissues where the tubes serve as the primary site of gas exchange. In prior studies, we determined that terminal cells lacking CCM3 or GckIII function show dramatic tube dilation within this transition zone (Song et al., 2013).
In humans, CCM3 is one of three genes known to be affected in cases of familial cerebral cavernous malformations. CCM3 binds to members of the GCKIII subfamily of Sterile 20-like kinases, of which there are three in humans (Draheim et al., 2014). The ability of CCM3 to bind GCKIII family members is thought to be essential to its function (Zalvide et al., 2013) and in flies, which have a single extant GCKIII family member, loss of GCKIII and CCM3 have identical consequences for the tracheal system (Song et al., 2013). Likewise, CCM3 and GCKIII have been shown to function together in zebrafish and nematodes (Yoruk et al., 2012, Zalvide et al., 2013, Lant et al., 2015). Despite much progress, the precise mechanism by which loss of CCM3 leads to tube dilation remains unknown, and likewise, the substrates of GCKIII family members in the vascular system remain mysterious.
Using protein affinity purification and mass spectrometry as an unbiased approach, we identified GckIII as a binding partner of Tao-1. Analogous to the way that Tao-1 activates Hpo, we found that Tao-1 phosphorylates and activates GckIII and that this relationship was conserved in human cells. Even more importantly, we show that Tao-1 and GckIII function together with the NDR family kinase Trc to regulate tube formation in trachea. Therefore, our study shows that Tao-1 regulates distinct biological processes by activating two related Hippo-like signalling modules containing a Sterile 20-like kinase and a NDR family kinase. The fact that Tao kinases regulate both organ growth and morphogenesis suggests that they could serve as key signalling nodes that couple both of these processes during organogenesis.
RESULTS
Tao-1 binds to GckIII kinase and phosphorylates its activation loop in D. melanogaster cells
To identify potential targets of the Tao-1 kinase we generated D. melanogaster S2 cell lines that stably expressed Tao-1 fused to an affinity tag at the N-terminus (see Materials and Methods). Tao-1 was affinity purified and subjected to mass spectrometry. Among the proteins retrieved with highest abundance was the Sterile 20-like kinase GckIII. We identified 35 and 12 GckIII peptides in two independent experiments (0 peptides in six control samples), with a SAINT (Choi et al., 2011) probability of 1, indicating a highly significant interaction (Table S1).
We pursued potential regulatory links between Tao-1 and GckIII because, based on sequence homology, the GckIII kinase is most closely related to Hpo, another Sterile 20-like kinase that we and others previously discovered to be regulated by Tao-1 in the context of epithelial tissue growth (Poon et al., 2011, Boggiano et al., 2011). Initially, we confirmed that Tao-1 can form a physical complex with GckIII by performing co-immunoprecipitation experiments in D. melanogaster S2R+ cells. N-terminally tagged versions of HA-Tao1 and myc-GckIII (both wild-type and kinase-dead versions for each) were co-expressed, followed by immunoprecipitation using anti-HA. This revealed that irrespective of their activity status, Tao-1 and GckIII can form a complex in D. melanogaster cells, as judged by the co-immunoprecipitation of both wild-type and kinase-dead variants (Figure 1A).
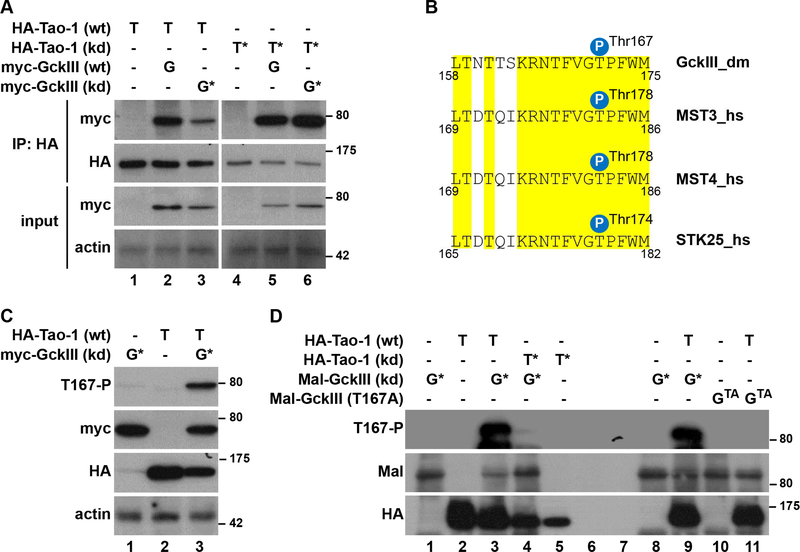
Figure 1. D. melanogaster Tao-1 kinase binds and phosphorylates GckIII kinase on its activation loop.
(A) Lysates of D. melanogaster S2R+ cells expressing wild-type (wt) or kinase dead (kd) HA-Tao-1 together with wt or kd myc-GckIII were subjected to immunoprecipitation (IP) using anti-HA antibodies. Immunoprecipitates and input lysates were analysed by immunoblotting with the indicated antibodies.
(B) Alignment of the conserved T-loop of D. melanogaster (dm) and human (hs) GCKIII kinase sequences. Identical residues are highlighted in yellow. The positions of the regulatory T-loop phosphorylation sites are indicated. The T-loop phosphorylation site of GCKIII kinases is conserved in flies and humans.
(C) S2R+ cells were transfected with the indicated plasmids and immunoblotted using anti-T167-P (top), anti-myc (top middle), anti-HA (bottom middle) and anti-actin (bottom) antibodies.
(D) Lysates of S2R+ cells transiently expressing HA-Tao-1 variants were subjected to immunoprecipitation with anti-HA antibodies. Immunopurified proteins were then used in kinase assays with or without or with the indicated recombinant versions of Mal-GckIII. Following kinase reactions, the samples were immunoblotted with the indicated antibodies. Importantly, the antiT167-P antibody specifically detected Tao1-mediated phosphorylation of GckIII on Thr167 (compare lanes 3, 9 and 11). Relative molecular masses are shown in kDa for each blot. See also Table S1.
Next, considering that Tao-1 activates the closely related kinase Hpo by phosphorylating its T-loop (activation loop) (Boggiano et al., 2011, Poon et al., 2011), we tested whether Tao-1 can also phosphorylate GckIII. To do so, we pursued two lines of research. First, we examined the impact of Tao-1 expression on phosphorylation of the GckIII activation loop [Thr167, which is highly conserved between D. melanogaster and human orthologues (Figure 1B)] in S2R+ cells. Indeed, Thr167 phosphorylation of kinase-dead GckIII was substantially elevated upon co-overexpression of Tao-1 (Figure 1C). Second, we studied Tao-1-mediated in vitro phosphorylation of recombinant full-length kinase-dead GckIII. Consistent with our cell-based studies, expression of wild-type but not kinase dead Tao-1 phosphorylated the activation loop of GckIII (Figure 1D). Collectively, these experiments suggest that Tao-1 can phosphorylate GckIII at residue Thr167 of its activation loop, a molecular event known to trigger the activation of Sterile 20-like kinases (Glantschnig et al., 2002, Deng et al., 2003).
TAO1 binds to GCKIII kinases in human cells and phosphorylates their activation loop
Considering that GCKIII is conserved between D. melanogaster and humans, and that the Tao-1/Hippo regulatory relationship is conserved between D. melanogaster and humans (Boggiano et al., 2011, Poon et al., 2011), we asked next whether human TAO1 interacts with and phosphorylates MST3, MST4 and/or STK25, the three human counterparts of D. melanogaster GCKIII (hereafter collectively termed hGCKIII) (Dan et al., 2001). The corresponding hGCKIII and TAO1 cDNAs were expressed in human HEK293 cells and TAO1/hGCKIII complex formation assessed by co-immunoprecipitation experiments. As shown in Figure 2A, TAO1 formed a complex with all three GCKIII homologues. These results are consistent with an unbiased high-throughput protein-protein interaction study that identified physical interactions between TAO1 and STK25, as well as TAO2 and STK25 (TAO2 is a homologue of TAO1) (Huttlin et al., 2015).
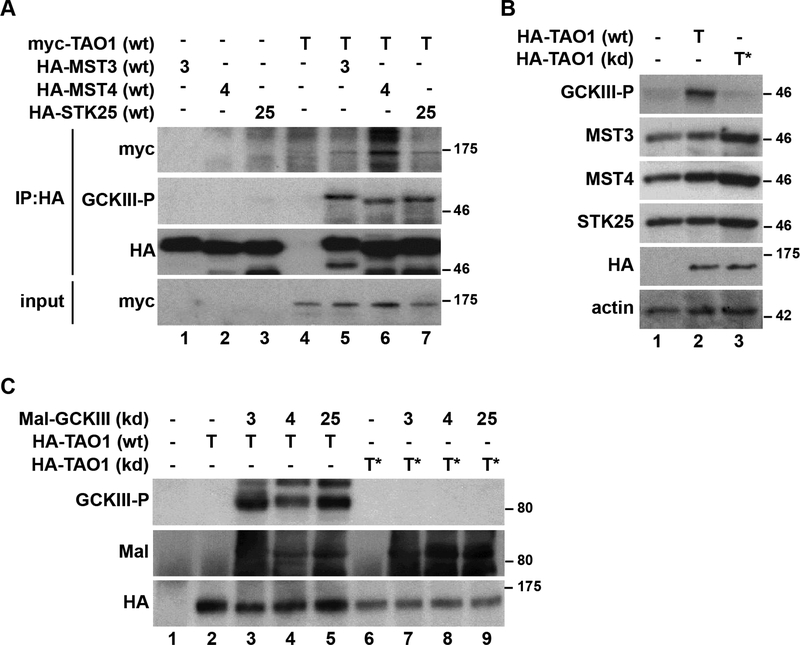
Figure 2. TAO1 kinase binds and phosphorylates GCKIII kinases on their activation loop in human cells.
(A) Lysates of human HEK293 cells expressing HA-GCKIII kinases (MST3, MST4 or STK25) together with myc-TAO1 were subjected to immunoprecipitation using anti-HA antibodies, before immunoblotting with the indicated antibodies.
(B) HEK293 cells expressing wt or kd human TAO1 were lysed and immunoblotted with the indicated antibodies.
(C) Lysates of HEK293 cells expressing human wild-type (wt) or kinase dead (kd) HA-TAO1 were subjected to immunoprecipitation with anti-HA antibodies. Immunopurified kinases were used in kinase assays with or without the indicated recombinant wt or kd Mal-GCKIII kinases. Following kinase reactions, samples were examined by immunoblotting with the indicated antibodies. Importantly, TAO1-mediated activation loop phosphorylation of human GCKIII kinases was only observed when wild-type TAO1 was incubated with kinase-dead GCKIII kinases. Relative molecular masses are shown in kDa for each blot.
Subsequently, we investigated whether human TAO1 phosphorylates the activation loop of hGCKIII kinases in human cells and in vitro. We observed activation loop phosphorylation of hGCKIII in immunoprecipitates from cells that were co-transfected with TAO1 and hGCKIII kinases (Figure 2A). We observed similar results when assessing TAO1’s ability to induce activation loop phosphorylation of endogenous hGCKIII (Figure 2B). Furthermore, this was dependent on TAO1’s kinase activity as expression of kinase-dead TAO1 had no impact on hGCKIII activation loop phosphorylation (Figure 2B). In parallel, we performed in vitro kinase assays using recombinant full-length kinase-dead hGCKIII proteins as substrates. We observed hGCKIII activation loop phosphorylation by wild-type, but not kinase dead, TAO1 immunoprecipitates (Figure 2C). Taken together, our data suggest that GckIII kinases are bona fide substrates of Tao kinases in both D. melanogaster and human cells.
GCKIII does not regulate tissue growth via the Hippo pathway
We next explored the biological setting in which Tao-1 regulates GckIII, by examining control of tissue growth by the Hippo pathway. Tao-1 controls epithelial tissue growth by activating the Hpo kinase by phosphorylating its activation loop (Boggiano et al., 2011, Poon et al., 2011). When active, Hpo phosphorylates the other members of the Hippo pathway core kinase cassette Salvador, Mats and Warts (Wts) (Pan, 2010, Harvey et al., 2013, Halder and Johnson, 2011). Wts phosphorylates the transcriptional co-activator protein Yorkie (Yki), thus limiting its access to the nucleus and its ability to promote transcription of genes that promote tissue growth (Pan, 2010, Harvey et al., 2013, Halder and Johnson, 2011). Recently, Misshapen and Happyhour, which also belong to the Sterile 20-like kinase family, were shown to act in parallel to Hpo to promote Wts activity (Zheng et al., 2015, Meng et al., 2015, Li et al., 2014). Given that GckIII is the closest homologue of Hpo we investigated the possibility that it also functions in parallel to Hpo, to control Hippo pathway-dependent growth of epithelial tissues.
Initially, we assessed whether loss of GckIII, either using RNAi or mutant alleles, displayed tissue overgrowth, a characteristic feature of Hippo pathway genes (Tapon et al., 2002). Unlike the overgrown adult eyes harbouring clones of hpo mutant tissue (Harvey et al., 2003, Pantalacci et al., 2003, Udan et al., 2003, Wu et al., 2003, Jia et al., 2003), GckIII mutant genetic mosaic adult eyes were rough and were smaller in size compared to the control eyes (Figures S1A and S1B). Furthermore, depletion of GckIII by RNAi in the developing wing disc caused a reduction in the size of the adult wing, as opposed to overgrowth that might be expected should GckIII act similarly to Hippo (Figures S1C-S1E). Loss of Hippo pathway proteins causes Yki hyperactivation and elevated expression of DIAP1 (Huang et al., 2005). Using hsFLP MARCM (Lee and Luo, 1999), we generated GFP marked clones in third instar larval wing imaginal discs that either harboured homozygous hpo5.1 null (Genevet et al., 2009) mutant clones or which expressed GckIII RNAi. However, GckIII depletion did not induce changes in DIAP1, as opposed to the increased DIAP1 expression observed upon loss of hpo (Figures S1F-S1G‘). We considered the possibility that Hpo might compensate for GckIII in its absence and mask a role for GckIII in promoting Wts activity to limit tissue growth. To test this, we depleted GckIII by RNAi in hpo5.1 null clones using the eyFlp MARCM system. The degree of tissue overgrowth was similar in adult eyes and head containing hpo null clones as it was in hpo null clones that also expressed a GckIII RNAi transgene (Figures S1H-S1I). Similar results were obtained when comparing the size of clones generated in third instar larval wing imaginal discs using the hsFlp MARCM system (Figures S1J-S1N). Therefore, we conclude that GckIII does not regulate Hippo pathway-dependent growth of epithelial tissues. These findings are consistent with recent biochemical studies of Zheng et al., who reported that, in contrast to Hpo, GckIII does not phosphorylate Wts or regulate its activity in D. melanogaster cultured cells (Zheng et al., 2015). To investigate the apparent undergrowth of GckIII mutant tissue we assessed both cell proliferation and apoptosis in developing eye imaginal discs. Whilst we observed no discernible change in cell proliferation (Figures S2A-S2D‘) of GckIII mutant eye cells, we did observe an increased number of apoptotic cells (Figures S2E-S2H‘) consistent with previous observations of GckIII depletion in the wing imaginal disc (Friedman and Perrimon, 2006). Collectively, this indicates that, unlike Hpo, GckIII does not normally suppress growth of eye or wing imaginal discs.
Tao-1 kinase regulates tube structure in D. melanogaster trachea
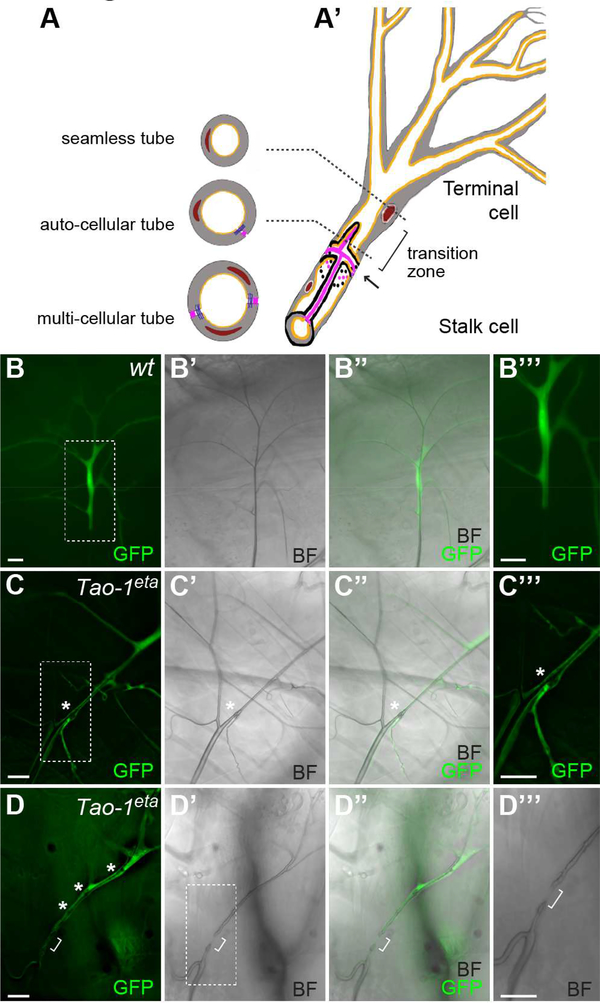
We then investigated a role for Tao-1 in tracheal development, given that we had previously found that GckIII mutations lead to dilation of the tube within the terminal cell transition zone in larval trachea (Song et al., 2013). Three tube types are found in D. melanogaster trachea: multi-cellular, auto-cellular and seamless tubes (Figure 3A). In trachea, terminal cells (composed of seamless tubes) connect to and branch out from stalk cells (composed of auto-cellular tubes) at an intercellular junction (arrow, Figure 3A’). The region positioned between the intercellular junction (arrow) and terminal cell nucleus is known as the transition zone (bracket, Figure 3A’), to reflect the transition from seamed to seamless tubes (Song et al., 2013, Samakovlis et al., 1996). We generated Tao-1 loss of function clones in trachea using the heat shock FLP-GFP system (Ghabrial et al., 2011) and the Tao-1eta allele (Gomez et al., 2012). Strikingly, we observed transition zone tube dilation in 73% of terminal cells and transition zone tube gaps (or restrictions) in 26% of terminal cells (1 terminal cell showed both a local dilation and a gap) (Figures 3B–3D”’ and Table S2). We also observed small bulges in the seamless tubes of multiple tracheal terminal cells (Figures 3D-D”’), consistent with the GckIII loss of function phenotype we reported previously (Song et al., 2013).
Figure 3. Tao-1 regulates development of D. melanogaster trachea.
(A-A’) Schematic illustration of D. melanogaster trachea, which are comprised of three different tube architectures: seamless tubes, auto-cellular tubes and multi-cellular tubes (cross sections represented in (A)). (A’) A depiction of part of a terminal cell (a seamless tube) that joins to an autocellular stalk cell at the transition zone (marked by bracket), which is located between an intracellular junction (marked by arrow) and the nucleus. Nucleus is brown; cytoplasm is grey; septate junctions are pink; adherens junctions are purple; apical lumens are orange (adapted from (Song et al., 2013)).
(B-D”’) Wild-type (B-B”’) or Tao-1eta (C-D”’) terminal cell clones, marked by GFP (green). Boxed areas in (B), (C) and (D’) are shown as close up images in (B”’), (C”’) and (D”’). Brightfield (BF) images are shown in (B’), (C’), (D’) and (D”’) and the merged images (B”), (C”) and (D”). (B-B”’) Gas-filled tubes that pierce the cytoplasm are evident in the wild-type terminal cell. The tube extends beyond the clone through the unmarked neighbouring stalk cell. (C-C”’) Loss of Tao-1 results in prominent dilations (*) in the transition zone of 73% of terminal cells scored (D-D”’) A Tao-1eta terminal cell clone that displays a “gap” defect, in which a portion of the terminal cell tube is absent within the transition zone (bracket). The cell also displays several small tube dilations (marked by *). Scale bar = 20 μm in (A-D”’).
See also Figures S1-S3 and Table S2.
To confirm these results, we used two independent Tao-1 RNAi lines that we have previously employed to deplete this protein in epithelial tissues and neural stem cells (Poon et al., 2011, Poon et al., 2016). Similar to what we observed using the classical loss of function allele, when Tao-1 was depleted by RNAi in trachea using btl-GAL4 we observed transition zone dilations (Figures S3A-S3A”’). Transition zone dilations were observed in 74% of Tao-1 RNAi-expressing terminal cells, transition zone gaps in 12% of terminal cells, and no gross morphological defects in 14% of terminal cells (n=34) (Figures S3A-S3B”’ and Table S2). Furthermore, we assessed hpo42−47 mutant clones in trachea and observed no dilations in terminal cells (either in transition zones or terminal cell branches, Figures S3C-S3C”’ and Table S2) suggesting that Tao-1 and Hippo perform distinct functions in tracheal development. Collectively these data show that Tao-1 is an essential regulator of tracheal development and that Tao-1 loss largely phenocopies GckIII loss in this tissue.
Tao-1 regulates the abundance and localization of junctional and apical proteins in trachea
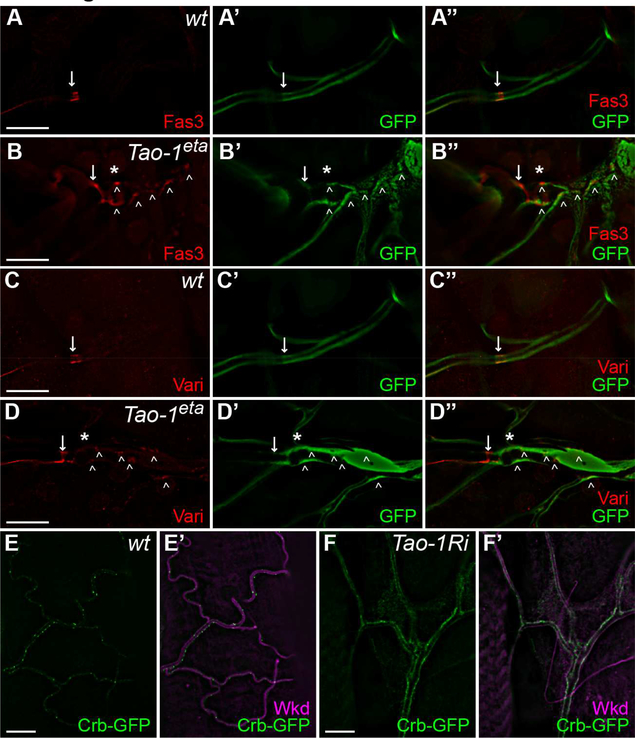
Given our biochemical evidence that Tao-1 binds to and activates GckIII, and that loss of either Tao-1 or GckIII causes transition zone dilation in trachea, we asked whether loss of Tao-1 resulted in abnormal localization of junctional and apical proteins, as was reported for Ccm3 and GckIII mutants (Song et al., 2013). The transition zone of wild-type trachea contains septate and adherens junctions in the proximal part of the terminal cell (Song et al., 2013, Samakovlis et al., 1996). This small stretch of auto-cellular tube in terminal cells is frequently remodelled away by the third larval instar but tends to persist in Ccm3 and GckIII mutant cells and is evident by ectopic expression of septate junction proteins (Song et al., 2013). We observed a similar perdurance of auto-cellular junctions in Tao-1 mutant terminal cells, which displayed ectopic expression of septate junction proteins such as Fasciclin 3 (Fas 3) and Varicose (Vari) (arrowheads in Figures 4A-4D”).
Figure 4. Tao-1 prevents ectopic accumulation of septate junction proteins and Crumbs in trachea.
(A-B”) Wild-type (A-A”) or Tao-1eta (B-B”) terminal cell clones, marked by GFP (green) and stained for antibodies specific for Fas3 (red). The arrow indicates the intercellular junction. Ectopic Fas3 is present in the transition zone dilation (indicated by *) of Tao-1eta terminal cell clones and in more distal positions of the cell (indicated by arrowheads, B).
(C-D”) Wild-type (C-C”) or Tao-1eta (D-D”) terminal cell clones, marked by GFP (green) and stained for antibodies specific for Varicose (Vari, red). The arrow indicates the intercellular junction. Ectopic Vari is present in the transition zone dilation (indicated by *) of Tao-1eta terminal cell clones and more distally as well (arrowheads, D).
(E-E’) A wild-type terminal cell with Crumbs-GFP (green) and stained with antibodies specific for Wkd (magenta) to mark the tracheal tubes. Crumbs decorates the lumenal membrane in well spaced foci.
(F-F’) A terminal cell expressing Tao-1 RNAi with btl-Gal4. Crumbs-GFP levels were elevated and appeared to almost continuously line the lumenal membrane.
In Ccm3 and GckIII mutant terminal cells, the apical membrane protein Crumbs, which is normally present only in discrete puncta along the apical membranes of the terminal cell, accumulates at high levels on these membranes [Figures 4E–5E’ and (Song et al., 2013)]. Compared to Crumbs-GFP localization in wild-type terminal cells, Crumbs-GFP was more highly expressed and lined the majority of the apical lumen of terminal cells expressing Tao-1 RNAi (Figures 4E-4F’). These data strongly suggest a common molecular defect underlies the transition zone dilation caused by loss of either GckIII or Tao-1.
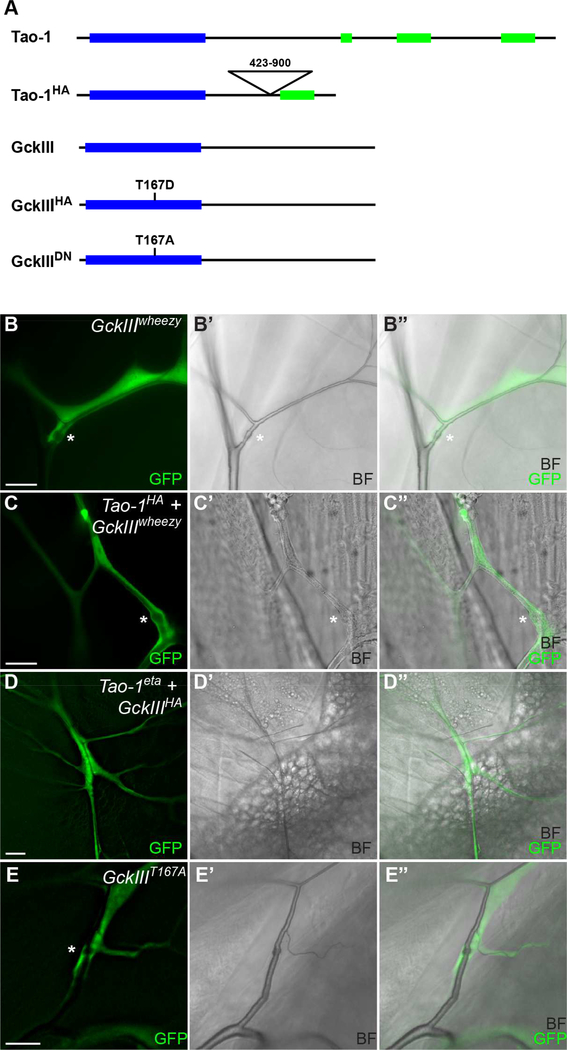
Figure 5. GckIII functions epistatically to Tao-1 in trachea.
(A) Schematic of Tao-1 and GckIII wild-type and mutant transgenes utilised for genetic epistasis experiments. Blue boxes indicate a kinase domain, and green boxes indicate coiled-coil domains. Wild type Tao-1 contains a kinase domain at the N-terminus and 3 coiled coil domains at the C-terminus. Hyperactive Tao-1 (Tao-1HA) contains a deletion in the central region (amino acids 423–900).
Wild type GckIII contains a kinase domain at the N terminus; hyperactive GckIII (GckIIIHA) contains a point mutation where T is mutated to D to mimic phosphorylation at 167 in the activation loop of the kinase domain; and dominant negative GckIII (GckIIIDN) contains a point mutation where T is mutated to A to prevent phosphorylation at 167 in the activation loop of the kinase domain.
(B-E) Terminal cell clones of the indicated genotypes are marked by GFP (green) with accompanying brightfield images (BF). Transition zone tube dilations are marked by an asterisk (*).
(B-B”) GckIIIwheezy terminal cell clones, marked by GFP (green). Transition zone tube dilations (*) are observed in 100% of GckIIIwheezy mutant clones scored.
(C-C”) A GckIIIwheezy terminal cell clone (marked by GFP) expressing a hyperactive Tao-1 (Tao-1HA) transgene. Expression of hyperactive Tao-1 failed to rescue transition zone dilations in GckIIIwheezy mutant cells, and transition zone tube dilations were observed in 100% terminal cells scored.
(D-D”) A Tao-1eta terminal cell clone (marked by GFP) expressing a hyperactive GckIII (GckIIIHA) transgene. Expression of hyperactive GckIIIHA in Tao-1eta mutant clones caused transition zone tube dilations in 51% terminal cells scored.
(E-E”) Expression of a GckIII transgene bearing the point mutation T167A in the activation loop (GckIIIT167A) results in transition zone tube dilations in 66% of terminal cells scored.
Scale bars = 20 μm.
See also Figure S4 and Table S2.
Tao-1 functions upstream of GckIII to control tracheal morphogenesis
Coupled with the finding that Tao-1 forms a complex with GckIII and phosphorylates the GckIII activation loop (Figures 1 and 2), our observations are consistent with the notion that these proteins operate together to control tracheal morphogenesis. To investigate this further, we assessed the epistatic relationship between Tao-1 and GckIII in vivo. We used the MARCM system to generate tracheal terminal cells that were mutant for GckIII, using a GckIII wheezy molecular null mutant allele (Song et al., 2013), and also expressed a hyperactive Tao-1 allele (Tao-1HA), where a central inhibitory domain had been removed (Liu et al., 2010) (Figure 5A). Expression of Tao-1HA in terminal cells using drm>GFP had no discernible phenotype (Figures S3A-S3A”’ and Table S2), and failed to rescue the transition zone dilations associated with the GckIIIwheezy allele, consistent with Tao-1 operating upstream of GckIII (Figures 5B-5C” and Table S2). We tested this further by utilising a hyperactive GckIII transgene (GckIIIHA), where the activation loop residue at position 167 was mutated from Threonine to Aspartic acid, which mimics phosphorylation of this residue (Figure 5A). Expression of GckIIIHA alone largely causes a mild wavy lumen phenotype (Figure S4B and Table S2), and co-expression of GckIIIHA in Tao-1eta mutant terminal cells partially rescued phenotypes associated with Tao-1 loss, including dilation of the transition zone (Figures 5D-5D” and Table S2). Phosphorylation at T167 is essential for GckIII function since expression of GckIII (GckIIIT167A), where T167 is mutated to Alanine thus rendering this site unable to be phosphorylated, resulted in dilations in 66% of terminal cells scored (Figures 5E-5E” and Table S2). Together with our biochemical data (Figures 1 and 2), these epistasis studies indicate that GckIII functions downstream of Tao-1 in tracheal terminal cells.
GCKIII phosphorylates the hydrophobic motif of Tricornered to regulate tracheal development
Given the architecture of Hippo-like signalling modules, we predicted that an NDR family kinase would operate downstream of GckIII in trachea. D. melanogaster has two such kinases: Warts (Wts) and Tricornered (Trc). We considered Trc to be the lead candidate because of two prior results obtained by our groups: 1) the human GCKIII orthologue MST3 phosphorylates the hydrophobic motif of the human Trc orthologues, NDR1 and NDR2, an essential step in their activation (Stegert et al., 2005, Cornils et al., 2011), and this motif is conserved in Trc/NDR kinases in flies and humans (Figure 6A); and 2) because we found that wts mutant trachea display overgrowth phenotypes (Ghabrial et al., 2011), which are distinct from the tracheal dilations observed upon GckIII or Tao-1 loss (Song et al., 2013) and (Figures 3C and 5B).
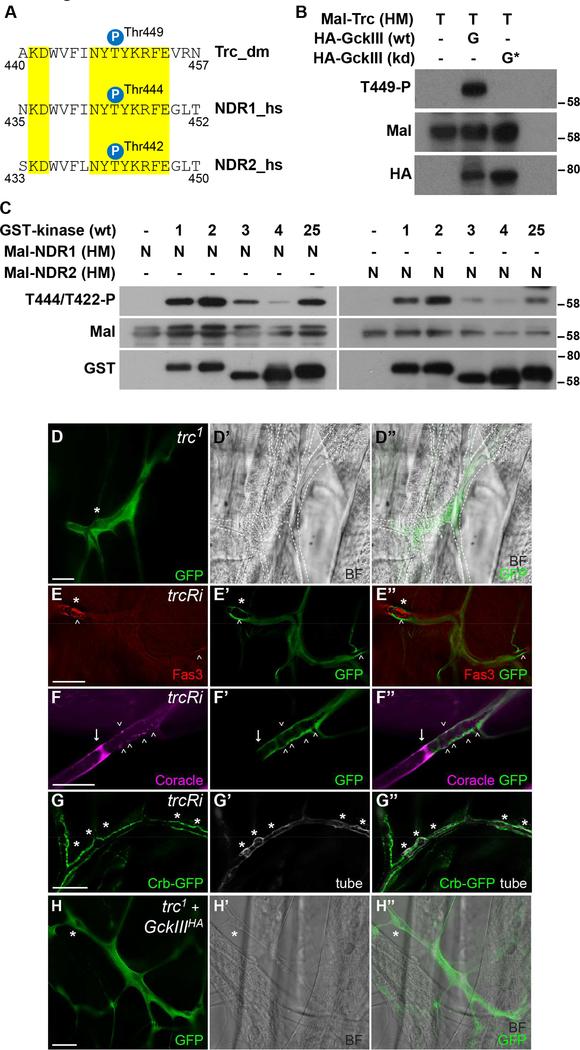
Figure 6. The Tricornered kinase is phosphorylated on its hydrophobic motif by GckIII, and regulates tracheal development.
(A) Alignment of the hydrophobic motif of D. melanogaster (dm) and human (hs) NDR kinase sequences. Identical residues are highlighted in yellow. The positions of the regulatory hydrophobic motif phosphorylation sites are indicated. The hydrophobic motif phosphorylation site of Trc/NDR kinases is conserved from flies to humans.
(B) Lysates of S2R+ cells transiently expressing wild-type (wt) or kinase-dead (kd) HA-GckIII were immunoprecipitated with anti-HA antibodies and incubated with a recombinant Mal-tagged hydrophobic motif fragment of Trc [Mal-Trc(HM)] in kinase assays. Subsequently, samples were examined by immunoblotting using the indicated antibodies. Relative molecular masses are shown in kDa for each blot.
(C) Recombinant GST-tagged wild-type full-length MST kinases (MST [1], MST2 [2], MST3 [3], MST4 [4] or STK25 [25], with respective abbreviations in square brackets) were incubated with recombinant Mal-tagged hydrophobic motif fragments of human NDR1 or NDR2 [Mal-NDR1/2 (HM)]. Following kinase reactions, the samples were analysed by Western blotting using the indicated antibodies. Relative molecular masses are shown in kDa for each blot.
(D-D”) A trc1 terminal cell clone marked by GFP (green). Brightfield (BF) images are shown in (D’) and the merged image (D”). The * indicates a prominent transition zone dilation. The terminal cell also exhibits a gas-filling defect and so is outlined with a white dashed line in (C’ and C”).
(E-E”) Terminal cell expressing a trc-RNAi (trcRi) transgene using btl-GAL4 (marked by GFP) and stained for Fas3 (red). Large dilations (*) in the transition zone were readily detected, and ectopic Fas3 is present in the transition zone dilation (arrowheads) of Tao-1eta terminal cell clones and more distally as well (arrowheads).
(F-F”) Terminal cells marked with GFP (green) expressing a trc-RNAi (trcRi) transgene using drm-GAL4 and stained for Coracle (magenta) displayed ectopic localization of Coracle in the transition zone (arrowheads) and distally (data not shown). Arrows mark intercellular junction.
(G-G”) Terminal cell (tubes visualised with UV) expressing a trc-RNAi (trcRi) transgene using drm-GAL4 with Crumbs-GFP almost continuously lined the lumenal membrane.
(H-H”) A trc1 terminal cell clone [marked by GFP (green)] expressing a hyperactive GckIII transgene. A brightfield (BF) image is shown in (H’) and the merged image (H”). The * indicates a transition zone tube dilation; expression of hyperactive GckIII failed to rescue transition zone dilations in trc1 cells. Scale bar = 20 μm in (D-H).
See also Figure S5 and Table S2.
Initially, we sought to determine whether the reported biochemical relationship between hGCKIII and NDR1/2 was conserved between the D. melanogaster and human orthologues. Lysates of S2R+ cells transiently expressing wild-type or kinase dead GckIII were subjected to immunoprecipitation with anti-HA antibodies and immunopurified proteins were incubated with the recombinant Maltagged hydrophobic motif fragment of Trc. Following in vitro kinase reactions, samples were examined by immunoblotting using antibodies that detect phosphorylation of the hydrophobic motif of Trc (Thr449). We observed robust phosphorylation of this residue (Figure 6B), showing for the first time in D. melanogaster that GckIII can phosphorylate the hydrophobic motif of Trc and suggesting that this regulatory relationship is conserved between flies and mammals. To determine whether phosphorylation was direct as opposed to an intermediate kinase we performed in vitro kinase assays using recombinant human kinases (NDR1 and NDR2 as substrates and MST1–4 and STK25 as kinases). We found that all three human GCKIIIs (MST3, MST4 and STK25) as well MST1 and MST2 (the human Hpo orthologues) can phosphorylate human NDR1 and NDR2 to differing degrees on Thr444 and Thr442 (Figure 6C), their respective hydrophobic motif phosphorylation sites that are essential for NDR1/2 activation (Hergovich, 2013, Hergovich, 2016). Taken together, our data show that Trc/NDR kinases are substrates of GCKIII kinases in both fly and humans.
To investigate the function of Trc in trachea, we used drm-GAL4 to express Trc RNAi transgenes or examined clones of the classic trc1 allele (Geng et al., 2000) in trachea terminal cells. Strikingly, we observed tracheal dilation phenotypes in the transition zone of trc1 terminal cells (98% of 44 terminal cell clones examined, Figures 6D-6D” and Table S2), which closely phenocopied those observed upon loss of either GckIII or Tao-1. Consistently, expression of trc RNAi under control of drm-GAL4 at 29°C induced a high frequency of transition zone dilations, while a small number of tubes also displayed a gap defect in the transition zone (Table S2 and Figure S5). All terminal cells mutant for trc or expressing trc RNAi were also defective in gas-filling (Figures 6D-6D” and Table S2). Gas-filling defects are frequently found among mutations affecting terminal cell morphology, and similar gas-filling defects were seen at a very low frequency in GckIII mutant terminal cells (Ghabrial et al., 2011). As with terminal cells mutant for either GckIII or Tao-1, we observed ectopic expression of different septate junction proteins upon RNAi-mediated depletion of Trc. In drm-GAL4, trc RNAi terminal cells, ectopic Fas3 expression was evident (Figures 6E-6E”), as was ectopic Coracle staining (Figures 6F-6F”) and similar data were observed in trc1 clones (data not shown). Upon Trc depletion, we also observed substantial elevation of Crumbs-GFP expression, which lined the majority of the lumen of trc RNAi terminal cells (Figures 6G-6G”). Consistent with Trc acting downstream of GckIII in trachea, expression of GckIIIHA in trc1 clones did not rescue the tube dilation defect (Figures 6H-6H” and Table S2).
Next we assessed Trc activity in trachea using the antibody that detects phosphorylation in the hydrophobic motif of hNDR kinases, a well-established readout to monitor the activity status of human NDR1/2 (Hergovich, 2013). Specifically, we utilised an antibody raised against the phosphorylated Thr444 site of NDR1 (Tamaskovic et al., 2003), which recognises the equivalent site in Drosophila Trc, phosphorylated Thr449 (denoted herewith as P-Trc). In wild-type trachea, P-Trc was evident in puncta lining the apical membranes of terminal cells (Figures 7A-7A”’). Importantly, this is the region where phenotypes caused by loss of tao-1, GckIII or trc manifest, e.g. tube dilation, and accumulation of septate junction proteins and Crumbs. To confirm the specificity of this signal, we stained trc1 mutant trachea and found complete absence of this signal in GFP-positive trc1 mutant cells (asterisk, Figures 7B-7B”’). As an internal control for this experiment, a wild-type terminal cell that neighboured the trc1 cell showed robust P-Trc staining (arrowhead, Figures 7B-7B”’). To determine whether Trc activity was dependent on Tao-1 and GckIII, we stained tracheal clones that were mutant for each of these genes. As shown in (Figures 7C-7D”’), PTrc signal was greatly reduced or undetectable in Tao-1eta or GckIIIwheezy terminal cells. These results are consistent with our genetic epistasis experiments which show that Trc functions downstream of Tao-1 and GckIII to control tracheal morphogenesis (Table S2). Collectively, our genetic and biochemical data indicates that we have identified a Hippo-like pathway, consisting of the Tao-1, GCKIII and Trc kinases, that regulates tracheal development in D. melanogaster.
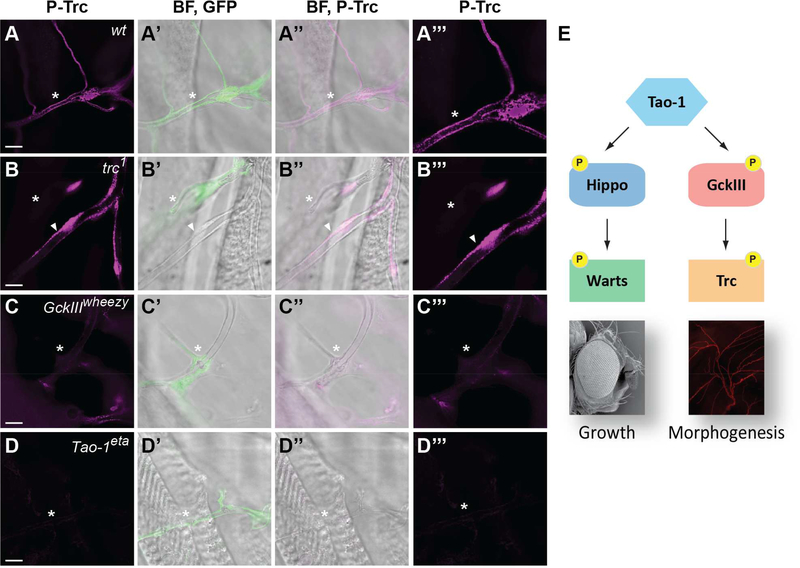
Figure 7. Tricornered activity in trachea requires Tao-1 and GckIII.
(A-D) Third instar larval trachea stained with antibodies that recognise phosphorylation of the activation loop of Trc (P-Trc). GFP positive terminal cells are shown of the following genotypes: (A-A”’) wild-type; (B-B”’) trc1; (C-C”’) GckIIIwheezy; (D-D”’) Tao-1eta. P-Trc is in magenta (A-D). Clones are marked by GFP (green) in panels merged with brightfield images (A’, B’, C’, D’). P-Trc (magenta) is merged with brightfield images (A”, B”, C”, D”). Close-up images of P-Trc (magenta) are shown in (A”’, B”’, C”’, D”’). GFP positive terminal cells are indicated by arrowheads. P-Trc is evident in puncta that line the apical membrane of wild-type and control tracheal lumens. P-Trc staining is reduced or absent in trachea that are mutant for trc, GckIII or Tao-1 in (B–D. In (B-B”’) the asterisk indicates a control GFP negative terminal cell which stains positive for P-Trc and serves as an internal control. Scale bars = 10 μm.
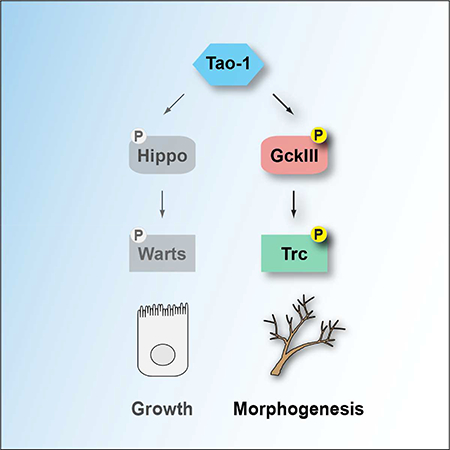
(E) Tao-1 regulates two Hippo-like signalling modules. In addition to regulating Hippo-Warts signalling in organ growth, we have discovered that Tao-1 kinase regulates a related Hippo-like pathway consisting of GckIII and Trc in morphogenesis.
DISCUSSION
Using genetic and biochemical experiments we have defined Tao-1 as a kinase that acts at the apex of two ancient and closely related kinase modules that consist of a Sterile 20-like kinase and a NDR family kinase: Hpo and Wts; and GckIII and Trc (Figure 7E). Despite being similarly organized, Hippo-like kinase modules execute distinct functions downstream of Tao-1. Whilst Hpo and Wts regulate organ size and cell fate, GckIII and Trc regulate the architecture of trachea. Presumably, these differences can be explained by the substrates of the downstream kinases in these signalling modules, Wts and Trc. A key step in defining how Hpo and Wts regulate organ size was the discovery that Wts phosphorylates the Yki transcription co-activator and inhibits its nuclear access (Huang et al., 2005). Identifying the substrate(s) of Trc is likely to offer similar mechanistic insights into how the Hippo-like pathway of Tao-1/GckIII/Trc regulates tracheal morphogenesis.
Of the many thousands of mutations analyzed in tracheal terminal cells, less than a handful affect tube morphology specifically in the transition zone (Baer et al., 2007, Beitel and Krasnow, 2000, Forster et al., 2010, Ghabrial et al., 2011, Myat et al., 2005, Ruiz et al., 2012, Samakovlis et al., 1996). Among these we have previously described mutations in lotus, which causes a local transition zone tube discontinuity or gap, and mutations in GckIII and Ccm3, which cause a local transition zone tube dilation (Song et al., 2013). Intriguingly, mutations in Tao-1 cause both defects. The only other genetic condition reported to have this effect on terminal cell tubes is the combination of GckIII loss of function and knockdown of the septate junction protein, Varicose/PALS2 (Song et al., 2013). As in this case, loss of Tao-1 appears to both compromise GckIII activity and septate junction formation, given that Tao-1 mutant tracheal cells exhibit transition zone dilation and mislocalisation of septate junction proteins. These data suggest that Tao-1 performs at least two functions to control tracheal morphology; 1) it acts upstream of GckIII and Trc to prevent transition zone tube dilation; and 2) it is required to maintain the integrity of septate junctions. Trc appears to be the key substrate of GckIII based on our biochemical data and that the trc loss of function phenotype closely phenocopies GckIII loss in trachea. Indeed, the trc tube dilation phenotype is, if anything, more severe than for Ccm3 or GckIII, with trc mutant terminal cells often exhibiting multiple large dilations in the transition zone. This would be consistent with residual Trc activity in GckIII mutant backgrounds, suggesting that additional Sterile-20 like kinases to GckIII may regulate Trc activity in terminal tracheal cells. If so, this would parallel regulation of D. melanogaster Wts and human LATS1 and LATS2 in the Hippo pathway, where multiple Sterile-20 kinases have been found to operate upstream of these NDR family kinases (Meng et al., 2015, Zheng et al., 2015, Li et al., 2014, Li et al., 2018).
In D. melanogaster, Trc also regulates polarized cell growth that underpins hair and bristle development (Geng et al., 2000), and tiling and branching of PNS dendrites (Emoto et al., 2004). It will be interesting to determine whether Trc regulates the same or similar proteins to control these different biological processes or whether it regulates multiple proteins, akin to Wts, which regulates Yki in the context of organ growth and R8 cell fate choice, and the actin regulatory protein Enabled to control border cell migration in the D. melanogaster ovary (Lucas et al., 2013, Huang et al., 2005, Jukam et al., 2013). Furthermore, it will be important to determine whether Tao-1 and GckIII operate upstream of Trc in other biological settings. In addition to discovering the key substrate(s) of Trc in trachea, defining modes of upstream regulation of Tao-1 activity should provide insights into how this Hippo-like signaling module controls tube development. One candidate upstream regulator is Schip1, which has been linked to Tao-1 and the Hippo pathway in the context of organ size control (Chung et al., 2016), although the mechanism by which it functions is unclear.
Previous findings by our group and others in both insects and vertebrates established the human orthologues of GckIII (MST3, MST4 and STK25) as potential disease genes in cerebral cavernous malformation, a familial vascular syndrome characterized by dilated leaky blood vessels (Song et al., 2013, Draheim et al., 2014). hGCKIII kinases bind to the non-catalytic protein CCM3, which is thought to serve as a scaffold for them. Theoretically, CCM3 could promote the association of GCKIII kinases with TAO kinases and/or NDR family kinases to facilitate their ability to phosphorylate one another and become active. This requires further examination but our findings raise the possibility that the Tao-regulated Hippo-like signalling module identified here is required for proper blood vessel formation in humans and that aberrant activity of this module could contribute to cerebral cavernous malformation syndrome. If so, then our study could provide potential therapeutic targets for treatment of this disease.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kieran F. Harvey (kieran.harvey@petermac.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila melanogaster stocks were maintained on standard medium at room temperature (22°C) and experimental crosses were carried out at 25°C, unless otherwise indicated.
D. melanogaster S2R+ and S2 cells were maintained at 24°C in Schneider`s Medium (217200024, Invitrogen) supplemented with heat inactivated FBS (10082147, Invitrogen) and penicillin/streptomycin.
HEK293 cells were grown at 37°C in 5% CO2 humidified chambers in DMEM (D6429, Sigma) supplemented with 10% fetal bovine serum (FBS; F7524, Sigma) and penicillin/streptomycin.
METHOD DETAILS
D. melanogaster stocks
w; FRT19A Tao1eta (Veit Riechmann)
w; FRT82B GckIIIwheezy770
w; FRT42D hpo5.1 (Nic Tapon)
w; FRT42D hpo42−47
w; FRT80B trc1 (Paul Adler)
y w eyFLP; FRT82B P[mini-w, ubi-GFP]
y w eyFlp, UAS-GFP; tub-GAL4, FRT42D, tub-GAL80
yw hsFLP122; FRT42D tub-GAL80; btl-Gal4, UAS-GFP
yw hsFLP122; btl-Gal4, UAS-GFP; FRT80B tub-Gal80
ywhsFLP122; FRT42D tub-Gal80; FRT82B GFPi
UAS-GFP RNAi, hsFLP122, FRT19A; btl-Gal4, UAS-GFP, UAS-DsREDnls
y w hsFLP122; btl-GAL4, UAS-DsREDnls; FRT82B cu UASi-GFPhp/TM6B
GAL4 lines: hh-GAL4, btl-GAL4, drm-GAL4 and rn-Gal4 (Bloomington Drosophila Stock Center).
UAS-RNAi lines: UAS-Tao-1 RNAi (VDRC GD 17432), UAS-Tao-1 RNAi (107645.8, modified VDRC KK 107645 that has a P element-containing insertion removed from the 40D locus by meiotic recombination (Vissers et al., 2016)), UAS-Trc RNAi (VDRC KK 107923), UAS-GckIII RNAi (VDRC KK 107158), all Vienna Drosophila RNAi Center (Dietzl et al., 2007).
Other stocks: crumbs-GFPA (Yang Hong).
Transgenic flies: UAS-Tao-1HA is a dominant active form of Tao-1 that contains a deletion of residues 423–900 (Liu et al., 2010). UAS-Tao-1HA flies were generated by subcloning V5-epitope tagged Tao-1Δ423–900 cDNA into pUAST. The pUAST-Tao-1Δ423–900 DNA sequence was confirmed by Sanger sequencing and transgenic flies created by BestGene Inc. To generate UAS-GckIIIHA, the wild type GckIII cDNA was subjected to site directed mutagenesis (Quickchange, Agilent) to introduce a T to D coding change at amino acid residue 167. The cDNA was cloned into the pUAST vector, injected into embryos (Rainbow Transgenic Flies) and transgenic lines established. To generate an HA-epitope tagged UAS-GckIIIDN, a HA-GckIIIT167A cDNA was generated by PCR mutagenesis, cloned into the pTW vector via Gateway cloning (Thermo Fisher Scientific), confirmed by Sanger sequencing and transgenic flies created by BestGene Inc.
Generation of clones in Drosophila
For analysis of mosaic wing imaginal discs, clones were generated by heat shock on day 2 for 10 minutes at 37°C, incubating crosses at 25°C and wing discs were dissected from mosaic larvae on day 5. For analysis of mosaic trachea cells in larvae, clones were generated by heat shocking 0–4 hr old embryos for 1 hour at 38°C, incubating crosses at 25°C for 5 days and selecting third instar mosaic larvae for analysis. Larvae were heat killed or filleted as described previously (Ghabrial et al., 2011).
Immunofluorescence
Primary antibodies were specific for DIAP1 (B. Hay), Varicose (E. Knust), Coracle, Fasciclin 3 (both DSHB), p-Thr444P (NDR1) (Tamaskovic et al., 2003), cleaved Dcp1 (Cell Signalling) and anti-GFP (Abcam). Anti-rat, anti-Chicken and anti-mouse secondary antibodies were from Invitrogen. Tissues were fixed and stained as in (Poon et al., 2012, Song et al., 2013).
Cell proliferation assay
EdU (5-ethynyl-2’deoxyuridine) incorporation assays were used to assess cell proliferation as in (Poon et al., 2016). Third instar eye imaginal discs were dissected and subjected to 1 hour incubation with 10μM EdU/PBS, then fixed for 20 minutes in 4% formaldehyde/PBS. Incorporated EdU was detected by Click-iT fluorescent dye azide reaction in accordance to manufacturer’s instructions (Life technologies).
Transient transfections of cultured cells
Exponentially growing HEK293 cells were plated at a consistent confluence and transfected with plasmids using Fugene 6 (E2692, Promega) according to the manufacturer’s instructions. S2R+ cells were transiently transfected with pAW-based plasmids using Effectene (301425, Qiagen) according to the manufacturer’s instructions.
Expression plasmids
The D. melanogaster Tao-1 open reading frame was cloned into the pMK33-NTAP-GS vector (Kyriakakis et al., 2008), to generate N-terminally tagged Tao-1. D. melanogaster Tao-1 and human TAO1, MST3, MST4 and STK25 cDNAs were described (Hergovich et al., 2009, Poon et al., 2011). The pAW vector and D. melanogaster GckIII cDNA (RE38276) were from the Drosophila Genomics Resource Center (Indiana University, USA). pcDNA3_HA and pcDNA3_myc vectors were reported (Hergovich et al., 2005, Bettoun et al., 2016). To generate N-terminally tagged cDNAs, human TAO1 was subcloned with BamHI and EcoRI into modified pcDNA3_HA or pcDNA3_myc. D. melanogaster Tao-1 and GckIII were subcloned with BamHI and XhoI into modified pcDNA3_HA or pcDNA_myc, respectively. D. melanogaster GckIII kinase-dead (K42R) or activation loop phospho-acceptor (T167A) mutants were generated by PCR mutagenesis using wild-type GckIII as a template. To subclone N-terminally tagged cDNAs into the pAW D. melanogaster expression vector, tagged cDNAs were first inserted into the pENTR-3C plasmid (Invitrogen) and then recombined into the pAW destination plasmid using Gateway technology (Invitrogen). All constructs were confirmed by Sanger sequencing.
Immunoblotting, immunoprecipitations, and antibodies
Immunoblotting and co-immunoprecipitation experiments were performed as described (Hergovich et al., 2005). The characterization of Tao-1 and TAO1 binding to GCKIII kinases was carried out in low-stringency buffer as defined previously (Cook et al., 2014). Anti-HA antibodies were from Cell Signaling (C29F4) and Roche (3F10). Anti-myc antibodies were from Santa Cruz Technology (9E10) and Cell Signaling Technology (71D10). Anti-MST3 (611057) was from BD Biosciences, anti-MST4 (3822) was from Cell Signaling Technology, anti-STK25 (sc-6865) and anti-actin (sc-1616) were from Santa Cruz Biotechnology. anti-Mal (Maltose binding protein; E8032) is from New England BioLabs. The antibody detecting activation loop phosphorylation of GckIII (Thr167), MST3 (Thr190), MST4 (Thr178) and STK25 (Thr174) was from Abcam and termed anti-T167-P or anti-GCKIII-P, respectively. The antibody detecting hydrophobic motif phosphorylation of Trc (Thr449) has been described (Tamaskovic et al., 2003). Secondary antibodies were purchased from GE Healthcare (NA931, NA934, and NA935) and Santa Cruz Biotechnology (sc-2020).
Mass spectrometry
pMK33-NTAP-GS-Tao-1 was transfected into D. melanogaster S2 cells and a stable cell line was selected using hygromycin. Protein complexes containing Tao-1 and associated interactors were purified as in (Yang and Veraksa, 2017) and analysed by nanoLC-MS/MS. Results from two independent biological replicates were compared to six independent controls using SAINT software (Choi et al., 2011).
Kinase assays
Full-length D. melanogaster GckIII and human MST3, MST4 or STK25 cDNAs were inserted into the pMal-c2 vector (New England BioLabs) using BamHI and XhoI/SalI or XbaI sites to generate pMal-GckIII/GCKIII plasmids, which can express kinase-dead versions that are N-terminally tagged by the maltose binding protein (Mal). The C-terminal hydrophobic fragments of D. melanogaster Trc (residues 308 to 459), human NDR1 (residues 301 to 465) and NDR2 (residues 302 to 464) were inserted into the pMal-c2 vector using BamHI and XhoI/SalI sites to generate pMal-Trc/NDR(HM) plasmids. Recombinant Mal-fusion proteins were expressed in E. coli BL21(DE3) at 30°C and purified using amylose resin (E8021, New England BioLabs) as described (Hergovich et al., 2005). Recombinant full-length GST-MST1 (M9697; 07–116), GST-MST2 (S6573; 07–117), GST-MST3 (M9822; 07–118), GST-MST4 (M9947; 07–119) and GST-STK25 (SRP5087; 07–136) were from Sigma and Carna Biosciences, respectively. To produce immunopurified full-length HA-tagged Tao-1 or TAO1 kinase version, D. melanogaster S2R+ or human HEK293 cells were transfected with pAW_HA-Tao1 or pcDNA3_HA-TAO1 and processed for immunoprecipitation (IP) using anti-HA antibody and stringent IP conditions as described (Hergovich et al., 2005). Immunopurified proteins were washed twice with kinase buffer (50 mM Hepes pH 7.4, 10 mM MgCl2, 2.5 mM beta-glycerophosphate, 1 mM EGTA, 1 mM Na3VO4, 1 mM NaF, 0.01 mM DTT), before kinase reactions were performed as follows: per reaction, 200 ng of Mal-fusion proteins were incubated at 30°C for 30 minutes in 20 μl of kinase buffer containing 100 μM ATP in the absence or presence of immunopurified Tao-1, TAO1 or GCKIII or GST-tagged kinases (100 ng per reaction). Reactions were stopped by the addition of Laemmli buffer, separated by SDS-PAGE, and immunoblotted as outlined above.
QUANTIFICATION AND STATISTICAL ANALYSIS
In Supplemental Figures S1E and J, control and experimental samples were analysed in one experiment. Adult posterior wing area and imaginal disc clones were measured using Adobe Photoshop software (Adobe), and statistical analyses and graphs were plotted using Graph Pad Prism (GraphPad software). Error bars represent standard error of mean (s.e.m.) and unpaired t-tests were used to assess statistical differences, assuming Gaussian distribution and using two-tailed P value. n values are stated in legends. In Supplemental Figure S1E, the t-value (t) was 10.5 and the degree of freedom (df) was 25. In Supplemental Figure S1J, the t and df values, respectively, are: control vs GckIII Ri: t=1.76 df=8; control vs hpo5.1: t=6.469 df=7; hpo5.1 vs hpo5.1+ GckIII Ri: t=0.3452 df=6.
In Figures 1A, 1C, 1D; Figure 2; Figures 6B and 6C, Western blots and kinase assays were repeated at least three times. In each experiment, no statistical method was used to predetermine sample size, the experiments were not randomized and the investigators were not blinded to allocation during experiments or outcome assessment.
Supplementary Material
Table S1, related to Figure 1. Proteins identified in Tao-1 purifications by mass spectrometry. Unique peptide number of each protein identified in Tao-1 purifications is shown in column E (Spec). Peptide numbers corresponding to these proteins that were control samples using extracts from untransfected S2 cells are shown in column J (ctrlCounts). SAINT analysis was used to analyze data from two independent Tao-1 purifications and six independent control purifications. AvgP (column K) is the probability of a protein being a bona fide interactor (greater than 0.8 is considered significant). Tao-1 is highlighted green and GckIII is highlighted yellow. GckIII was identified as a Tao-1 interacting protein with a highly significant SAINT probability and was not detected in control purifications.
Highlights.
The Tao-1 kinase regulates GckIII kinase activity, in both flies and humans
Tao-1, GckIII and Tricornered kinases constitute a Hippo-like signaling pathway
This Hippo-like pathway regulates tube architecture in the fly tracheal system
ACKNOWLEDGEMENTS
We thank P. Adler, V. Riechmann, N. Tapon, E. Knust, the Vienna Drosophila RNAi Center, the Australian Drosophila Research Support Facility (www.ozdros.com), the Bloomington Drosophila Stock Center and the Developmental Studies Hybridoma Bank for D. melanogaster stocks and antibodies. We thank the Centre for Advanced Histology and Microscopy at the Peter MacCallum Cancer Centre. H. Zhao provided help with SAINT analysis. K.F.H is a National Health and Medical Research Council Senior Research Fellow. AV was supported by a grant from NIH GM123136. Y.K. was sponsored by the Ministry of National Education (The Republic of Turkey). This research was supported by the Peter MacCallum Cancer Foundation and grants from the National Health and Medical Research Council of Australia (K.F.H – 1032251 and C.L.C.P – 1142469), the Company of Biologists (Development Journal) Travelling Fellowship (C.L.C.P) (Harvey laboratory), the National Institutes of Health [1R01GM089782] and the American Cancer Society (ACS) [RSG 124720] (Ghabrial laboratory), the Wellcome Trust (090090/Z/09/Z) and BBSRC (BB/I021248/1) (Hergovich laboratory).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCESUncategorized References
- AVRUCH J, ZHOU D, FITAMANT J, BARDEESY N, MOU F & BARRUFET LR (2012). Protein kinases of the Hippo pathway: regulation and substrates. Semin Cell Dev Biol, 23, 770–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHMANN A, DRAGA M, GRAWE F & KNUST E (2008). On the role of the MAGUK proteins encoded by Drosophila varicose during embryonic and postembryonic development. BMC Dev Biol, 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAER MM, BILSTEIN A & LEPTIN M (2007). A clonal genetic screen for mutants causing defects in larval tracheal morphogenesis in Drosophila. Genetics, 176, 2279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARDIN AJ & AMON A (2001). Men and sin: what’s the difference? Nat Rev Mol Cell Biol, 2, 815–26. [DOI] [PubMed] [Google Scholar]
- BEITEL GJ & KRASNOW MA (2000). Genetic control of epithelial tube size in the Drosophila tracheal system. Development, 127, 3271–82. [DOI] [PubMed] [Google Scholar]
- BETTOUN A, JOFFRE C, ZAGO G, SURDEZ D, VALLERAND D, GUNDOGDU R, SHARIF AA, GOMEZ M, CASCONE I, MEUNIER B, WHITE MA, CODOGNO P, PARRINI MC, CAMONIS JH & HERGOVICH A (2016). Mitochondrial clearance by the STK38 kinase supports oncogenic Ras-induced cell transformation. Oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGGIANO JC, VANDERZALM PJ & FEHON RG (2011). Tao-1 Phosphorylates Hippo/MST Kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Dev Cell, 21, 888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI H, LARSEN B, LIN ZY, BREITKREUTZ A, MELLACHERUVU D, FERMIN D, QIN ZS, TYERS M, GINGRAS AC & NESVIZHSKII AI (2011). SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nat Methods, 8, 70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG HL, AUGUSTINE GJ & CHOI KW 2016. Drosophila Schip1 Links Expanded and Tao-1 to Regulate Hippo Signaling. Dev Cell, 36, 511–24. [DOI] [PubMed] [Google Scholar]
- COOK D, HOA LY, GOMEZ V, GOMEZ M & HERGOVICH A (2014). Constitutively active NDR1-PIF kinase functions independent of MST1 and hMOB1 signalling. Cellular signalling, 26, 1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNILS H, KOHLER RS, HERGOVICH A & HEMMINGS BA (2011). Human NDR kinases control G(1)/S cell cycle transition by directly regulating p21 stability. Mol Cell Biol, 31, 1382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAN I, WATANABE NM & KUSUMI A (2001). The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol, 11, 220–30. [DOI] [PubMed] [Google Scholar]
- DENG Y, PANG A & WANG JH (2003). Regulation of mammalian STE20-like kinase 2 (MST2) by protein phosphorylation/dephosphorylation and proteolysis. J Biol Chem, 278, 11760–7. [DOI] [PubMed] [Google Scholar]
- DIETZL G, CHEN D, SCHNORRER F, SU KC, BARINOVA Y, FELLNER M, GASSER B, KINSEY K, OPPEL S, SCHEIBLAUER S, COUTO A, MARRA V, KELEMAN K & DICKSON BJ (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature, 448, 151–6. [DOI] [PubMed] [Google Scholar]
- DRAHEIM KM, FISHER OS, BOGGON TJ & CALDERWOOD DA (2014). Cerebral cavernous malformation proteins at a glance. J Cell Sci, 127, 701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMOTO K, HE Y, YE B, GRUEBER WB, ADLER PN, JAN LY & JAN YN (2004). Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell, 119, 245–56. [DOI] [PubMed] [Google Scholar]
- EMOTO K, PARRISH JZ, JAN LY & JAN YN (2006). The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature, 443, 210–3. [DOI] [PubMed] [Google Scholar]
- FORSTER D, ARMBRUSTER K & LUSCHNIG S (2010). Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol, 20, 62–8. [DOI] [PubMed] [Google Scholar]
- FRANCIS D & GHABRIAL AS (2015). Compensatory branching morphogenesis of stalk cells in the Drosophila trachea. Development, 142, 2048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN A & PERRIMON N (2006). A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature, 444, 230–4. [DOI] [PubMed] [Google Scholar]
- GENEVET A, POLESELLO C, BLIGHT K, ROBERTSON F, COLLINSON LM, PICHAUD F & TAPON N (2009). The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci, 122, 2360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENEVET A, WEHR MC, BRAIN R, THOMPSON BJ & TAPON N (2010). Kibra Is a Regulator of the Salvador/Warts/Hippo Signaling Network. Dev Cell, 18, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENG W, HE B, WANG M & ADLER PN (2000). The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics, 156, 1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHABRIAL AS, LEVI BP & KRASNOW MA (2011). A systematic screen for tube morphogenesis and branching genes in the Drosophila tracheal system. PLoS Genet, 7, e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLANTSCHNIG H, RODAN GA & RESZKA AA (2002). Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem, 277, 42987–96. [DOI] [PubMed] [Google Scholar]
- GOMEZ JM, WANG Y & RIECHMANN V (2012). Tao controls epithelial morphogenesis by promoting Fasciclin 2 endocytosis. J Cell Biol, 199, 1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN RB, HATINI V, JOHANSEN KA, LIU XJ & LENGYEL JA (2002). Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development, 129, 3645–56. [DOI] [PubMed] [Google Scholar]
- HALDER G & JOHNSON RL (2011). Hippo signaling: growth control and beyond. Development, 138, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY KF, PFLEGER CM & HARIHARAN IK (2003). The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell, 114, 457–67. [DOI] [PubMed] [Google Scholar]
- HARVEY KF, ZHANG X & THOMAS DM (2013). The Hippo pathway and human cancer. Nat Rev Cancer, 13, 246–57. [DOI] [PubMed] [Google Scholar]
- HERGOVICH A (2013). Regulation and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell Biosci, 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERGOVICH A (2016). The Roles of NDR Protein Kinases in Hippo Signalling. Genes (Basel), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERGOVICH A, BICHSEL SJ & HEMMINGS BA (2005). Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol Cell Biol, 25, 8259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERGOVICH A & HEMMINGS BA (2009). Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors, 35, 338–45. [DOI] [PubMed] [Google Scholar]
- HERGOVICH A & HEMMINGS BA (2012). Hippo signalling in the G2/M cell cycle phase: Lessons learned from the yeast MEN and SIN pathways. Semin Cell Dev Biol, 23, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERGOVICH A, KOHLER RS, SCHMITZ D, VICHALKOVSKI A, CORNILS H & HEMMINGS BA (2009). The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation. Curr Biol, 19, 1692702. [DOI] [PubMed] [Google Scholar]
- HUANG J, WU S, BARRERA J, MATTHEWS K & PAN D (2005). The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell, 122, 421–34. [DOI] [PubMed] [Google Scholar]
- HUANG J, ZHOU W, DONG W & HONG Y (2009). Targeted engineering of the Drosophila genome. Fly (Austin), 3, 274–7. [DOI] [PubMed] [Google Scholar]
- HUTTLIN EL, TING L, BRUCKNER RJ, GEBREAB F, GYGI MP, SZPYT J, TAM S, ZARRAGA G, COLBY G, BALTIER K, DONG R, GUARANI V, VAITES LP, ORDUREAU A, RAD R, ERICKSON BK, WUHR M, CHICK J, ZHAI B, KOLIPPAKKAM D, MINTSERIS J, OBAR RA, HARRIS T, ARTAVANIS-TSAKONAS S, SOWA ME, DE CAMILLI P, PAULO JA, HARPER JW & GYGI SP (2015). The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell, 162, 425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIA J, ZHANG W, WANG B, TRINKO R & JIANG J (2003). The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev, 17, 2514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUKAM D, XIE B, RISTER J, TERRELL D, CHARLTON-PERKINS M, PISTILLO D, GEBELEIN B, DESPLAN C & COOK T (2013). Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science, 342, 1238016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING I, TSAI LT, PFLANZ R, VOIGT A, LEE S, JACKLE H, LU B & HEBERLEIN U (2011). Drosophila tao controls mushroom body development and ethanol-stimulated behavior through par-1. J Neurosci, 31, 1139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KYRIAKAKIS P, TIPPING M, ABED L & VERAKSA A (2008). Tandem affinity purification in Drosophila: the advantages of the GS-TAP system. Fly (Austin), 2, 229–35. [DOI] [PubMed] [Google Scholar]
- LANT B, YU B, GOUDREAULT M, HOLMYARD D, KNIGHT JD, XU P, ZHAO L, CHIN K, WALLACE E, ZHEN M, GINGRAS AC & DERRY WB (2015). CCM-3/STRIPAK promotes seamless tube extension through endocytic recycling. Nat Commun, 6, 6449. [DOI] [PubMed] [Google Scholar]
- LEE T & LUO L (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron, 22, 451–61. [DOI] [PubMed] [Google Scholar]
- LEVI BP, GHABRIAL AS & KRASNOW MA (2006). Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development, 133, 2383–93. [DOI] [PubMed] [Google Scholar]
- LI Q, LI S, MANA-CAPELLI S, ROTH FLACH RJ, DANAI LV, AMCHESLAVSKY A, NIE Y, KANEKO S, YAO X, CHEN X, COTTON JL, MAO J, MCCOLLUM D, JIANG J, CZECH MP, XU L & IP YT (2014). The conserved misshapen-wartsYorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell, 31, 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Q, NIRALA NK, NIE Y, CHEN HJ, OSTROFF G, MAO J, WANG Q, XU L & IP YT (2018). Ingestion of Food Particles Regulates the Mechanosensing Misshapen-Yorkie Pathway in Drosophila Intestinal Growth. Dev Cell, 45, 433–449 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T, ROHN JL, PICONE R, KUNDA P & BAUM B (2010). Tao-1 is a negative regulator of microtubule plus-end growth. J Cell Sci, 123, 2708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCAS EP, KHANAL I, GASPAR P, FLETCHER GC, POLESELLO C, TAPON N & THOMPSON BJ (2013). The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol, 201, 875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENG Z, MOROISHI T, MOTTIER-PAVIE V, PLOUFFE SW, HANSEN CG, HONG AW, PARK HW, MO JS, LU W, LU S, FLORES F, YU FX, HALDER G & GUAN KL (2015). MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun, 6, 8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYAT MM, LIGHTFOOT H, WANG P & ANDREW DJ (2005). A molecular link between FGF and Dpp signaling in branch-specific migration of the Drosophila trachea. Dev Biol, 281, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN D 2010. The hippo signaling pathway in development and cancer. Dev Cell, 19, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANTALACCI S, TAPON N & LEOPOLD P (2003). The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol, 5, 921–7. [DOI] [PubMed] [Google Scholar]
- POON CL, LIN JI, ZHANG X & HARVEY KF (2011). The Sterile 20-like Kinase Tao-1 Controls Tissue Growth by Regulating the Salvador-Warts-Hippo Pathway. Dev Cell, 21, 896–906. [DOI] [PubMed] [Google Scholar]
- POON CL, MITCHELL KA, KONDO S, CHENG LY & HARVEY KF (2016). The Hippo Pathway Regulates Neuroblasts and Brain Size in Drosophila melanogaster. Curr Biol, 26, 1034–42. [DOI] [PubMed] [Google Scholar]
- POON CL, ZHANG X, LIN JI, MANNING SA & HARVEY KF (2012). Homeodomain-interacting protein kinase regulates hippo pathway-dependent tissue growth. Curr Biol, 22, 1587–94. [DOI] [PubMed] [Google Scholar]
- PRASKOVA M, XIA F & AVRUCH J (2008). MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol, 18, 311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ OE, NIKOLOVA LS & METZSTEIN MM (2012). Drosophila Zpr1 (Zinc finger protein 1) is required downstream of both EGFR and FGFR signaling in tracheal subcellular lumen formation. PLoS One, 7, e45649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMAKOVLIS C, HACOHEN N, MANNING G, SUTHERLAND DC, GUILLEMIN K & KRASNOW MA (1996). Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development, 122, 1395–407. [DOI] [PubMed] [Google Scholar]
- SHIGA Y, TANAKA-MATAKATSU M & HAYASHI S (1996). A nuclear GFP/β-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Development, Growth & Differentiation, 38, 99–106. [Google Scholar]
- SONG Y, ENG M & GHABRIAL AS (2013). Focal defects in single-celled tubes mutant for Cerebral cavernous malformation 3, GCKIII, or NSF2. Dev Cell, 25, 507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGERT MR, HERGOVICH A, TAMASKOVIC R, BICHSEL SJ & HEMMINGS BA (2005). Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol Cell Biol, 25, 11019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMASKOVIC R, BICHSEL SJ, ROGNIAUX H, STEGERT MR & HEMMINGS BA (2003). Mechanism of Ca2+-mediated regulation of NDR protein kinase through autophosphorylation and phosphorylation by an upstream kinase. J Biol Chem, 278, 6710–8. [DOI] [PubMed] [Google Scholar]
- TAPON N, HARVEY KF, BELL DW, WAHRER DC, SCHIRIPO TA, HABER DA & HARIHARAN IK (2002). salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell, 110, 467–78. [DOI] [PubMed] [Google Scholar]
- UDAN RS, KANGO-SINGH M, NOLO R, TAO C & HALDER G (2003). Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol, 5, 914–20. [DOI] [PubMed] [Google Scholar]
- VISSERS JH, MANNING SA, KULKARNI A & HARVEY KF (2016). A Drosophila RNAi library modulates Hippo pathway-dependent tissue growth. Nat Commun, 7, 10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU S, HUANG J, DONG J & PAN D (2003). hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell, 114, 445–56. [DOI] [PubMed] [Google Scholar]
- YANG L & VERAKSA A (2017). Single-Step Affinity Purification of ERK Signaling Complexes Using the Streptavidin-Binding Peptide (SBP) Tag. Methods Mol Biol, 1487, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOO SJ, HUH JR, MURO I, YU H, WANG L, WANG SL, FELDMAN RM, CLEM RJ, MULLER HA & HAY BA (2002). Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol, 4, 416–24. [DOI] [PubMed] [Google Scholar]
- YORUK B, GILLERS BS, CHI NC & SCOTT IC (2012). Ccm3 functions in a manner distinct from Ccm1 and Ccm2 in a zebrafish model of CCM vascular disease. Dev Biol, 362, 121–31. [DOI] [PubMed] [Google Scholar]
- YU FX & GUAN KL (2013). The Hippo pathway: regulators and regulations. Genes Dev, 27, 355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU JA, CASTRANOVA D, PHAM VN & WEINSTEIN BM (2015). Single-cell analysis of endothelial morphogenesis in vivo. Development, 142, 2951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALVIDE J, FIDALGO M, FRAILE M, GUERRERO A, IGLESIAS C, FLORIDIA E & POMBO CM (2013). The CCM3-GCKIII partnership. Histol Histopathol, 28, 1265–72. [DOI] [PubMed] [Google Scholar]
- ZHENG Y, WANG W, LIU B, DENG H, USTER E & PAN D (2015). Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell, 34, 642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to Figure 1. Proteins identified in Tao-1 purifications by mass spectrometry. Unique peptide number of each protein identified in Tao-1 purifications is shown in column E (Spec). Peptide numbers corresponding to these proteins that were control samples using extracts from untransfected S2 cells are shown in column J (ctrlCounts). SAINT analysis was used to analyze data from two independent Tao-1 purifications and six independent control purifications. AvgP (column K) is the probability of a protein being a bona fide interactor (greater than 0.8 is considered significant). Tao-1 is highlighted green and GckIII is highlighted yellow. GckIII was identified as a Tao-1 interacting protein with a highly significant SAINT probability and was not detected in control purifications.