Introduction
The kidney exerts multiple functions, and pathophysiological interactions between the kidney and the heart have important clinical implications, but it has only recently become clear that these interactions should be studied across the whole spectrum of reduced kidney function and not only in cases with severe, end-stage renal disease (ESRD), as has been done for many years.1 The prevalence of chronic kidney disease (CKD), defined as a glomerular filtration rate (GFR) of <60 mL/min/1.73 m2 for >3 months, exceeds 10% in the adult population and reaches 47% in subjects older than 70 years, according to data from the USA, with a trend towards a recent increasing prevalence.1,2
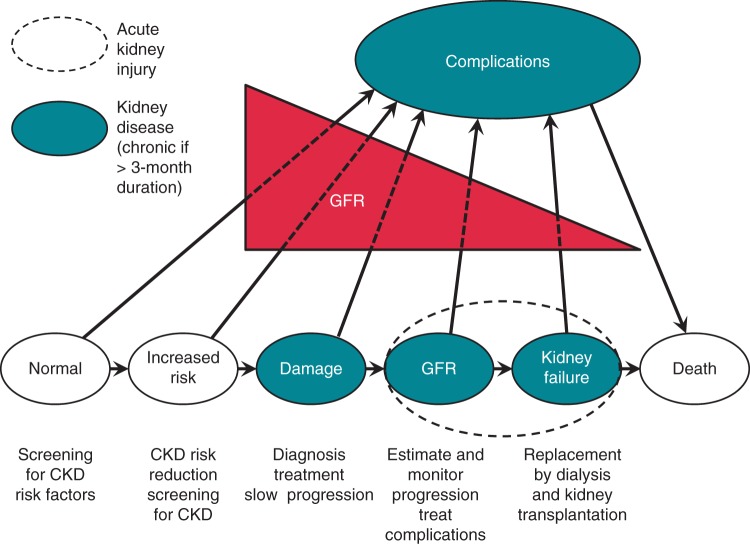
Many interactions between kidney and cardiovascular functions have important implications for clinical management and health policy (Figure 1), since even mild forms of kidney disease are associated with an increased risk of cardiovascular morbidity and overall mortality, and renal function may worsen over time.1,3
Figure 1.
Stages of the development and progression of chronic kidney disease (CKD), including complications and strategies to improve outcomes. Modified from Eckardt et al.1 GFR, glomerular filtration rate.
Although cardiovascular disease (CVD) and cardiac disorders are more frequent and severe in CKD, they are often not recognized, or undertreated, in view of the complexity of patient management in this setting.4 On the other hand, the presence and evolution of CKD is often not evaluated and monitored in patients with various forms of heart diseases, including patients with cardiac rhythm disturbances, a setting where CKD is associated with challenging decision-making on the management of specific treatments and interventions. In patients with cardiac diseases, CKD predisposes to acute kidney injury and vice versa, and both may strongly influence clinical management of cardiac conditions.
Considering the need for increasing the awareness of CKD among the cardiologists, with specific focus on those dedicated to management of arrhythmic disorders, as well as the need to create the basis for collaborative, personalized, patient-centred care, with integration of different healthcare specialists, the European Heart Rhythm Association (EHRA), in collaboration with Heart Rhythm Society (HRS) and Asian Pacific Heart Rhythm Society (APHRS), has promoted the present document, resulting from an interaction between cardiologists and nephrologists.
How to stage chronic kidney disease and how to monitor the impairment in renal function?
Chronic kidney disease is defined as the presence of kidney abnormalities, which can involve its structure and/or its function, for a period of longer than 3 months, with implications for health.5 This definition incorporates both a measure of chronicity as well as the concept that a variety of abnormalities of kidney structure or function may exist; not all may have implications for the health of the individual and therefore need to be taken in context.
The kidney has many functions, including excretory, metabolic, and endocrine. The GFR is the only one component of excretory function, but is widely accepted as the best overall index of kidney function, because it is generally reduced after widespread structural damage and most other kidney function declines in parallel with GFR.5
The GFR can be estimated from the serum creatinine using a number of equations to give an estimated GFR (eGFR).5Supplementary material online, Table S1 summarizes the equations proposed for eGFR.
The Cockcroft–Gault equation was proposed around 40 years ago,6 but has a series of bias in patients with a higher body weight or BMI, and its overall accuracy is lower than that of the two other formulas for eGFR described below.7
The Modification of Diet in Renal Disease (MDRD)8 equation uses four variables (age, gender, serum creatinine, and ethnicity) to calculate eGFR and is one of the most widely used and routinely reported by laboratories globally.
The chronic kidney disease epidemiology collaboration equation (CKD-EPI) equation9 uses the same four variables as the MDRD equation and is becoming more widely adopted.5 The CKD-EPI equation has a less bias than the MDRD study equation, especially at GFR >60 mL/min/1.73 m2, a small improvement in precision and greater accuracy.10 The clinician should remain aware of caveats for any estimating equation, which may influence the accuracy in a given individual patient and consider using additional tests (such as cystatin C or a clearance measurement) for confirmatory testing in specific circumstances when eGFR based on serum creatinine is less accurate.5,9 For example, in chronic heart failure, cystatin C may be a more sensitive marker of impaired GFR compared with creatinine partly due to the loss of muscle mass associated with this condition.11
The current recommended staging of CKD is based on a classification encompassing cause and severity, as expressed by the level of GFR and the level of albuminuria (Cause, GFR, and Albuminuria referred to as CGA staging: Table 1; 5 KDIGO). A threshold GFR of <60 mL/min/1.73 m2 (GFR categories G3a–G5) for >3 months is used to indicate CKD as this is less than half of the normal value in young adults of 125 mL/min/1.73 m2.5 Albuminuria is included as an additional marker of severity of injury, because albuminuria itself is strongly associated not only with progression of CKD but also with adverse, mainly cardiovascular, prognosis.12–14 Albuminuria detection is recommended in patients with cardiac disease and the appropriate tests, in order of preference, are urinary albumin, reported as a ratio of urinary creatinine (albumin-to-creatinine ratio), a timed urine collection for urinary albumin, urine protein-to-creatinine ratio, and reagent strip point-of-care urinalysis.5
Table 1.
Staging of chronic kidney disease according to the CGA approach (Cause, Glomerular Filtration, and Albuminuria)
| Cause of CKD | GFR categories (ml/min/1.73 m2) | Albuminuria categories |
|---|---|---|
| Presence or absence of systemic disease | G1: ≥90 (normal or high) | ACR (mg/g): A1 <30 (normal or mildly increased) A2 30–300 (moderately increased) A3 >300 (severely increased) |
| Location within the kidney of pathological–anatomical findings (glomerular, tubulointerstitial, vascular, cystic, and congenital diseases) | G2: 60–89 (mildly decreased) | ACR (mg/mmol): A1: <3 (normal or mildly increased) A2: 3–30 (moderately increased) A3: >300 (severely increased) |
| G3a: 45–59 (mildly to moderately decreased) | AER (mg/24 h): A1: <30 (normal or mildly increased) A2: 30–300 (moderately increased) A3: >300 (severely increased) |
|
| G3b: 30–44 (moderately to severely decreased) | ||
| G4: 15–29 (severely decreased) | ||
| G5: <15 [kidney failure (includes ESRD)] |
CKD, chronic kidney disease; ESRD, end-stage renal disease; GFR, glomerular filtration rate; ACR, albumin-to-creatinine ratio; AER, albumin excretion rate.
The presence of CKD should be monitored over time: renal function and albuminuria should be repeated at least once a year but more frequently if the patient has a high risk of progression and/or where measurement will impact therapeutic decisions.5 Progression of CKD is based on a decline in GFR category, recognizing that small fluctuations in GFR are common and are not necessarily indicative of progression.5 Rapid progression is currently defined as a sustained decline in eGFR of >5 mL/min/1.73 m2/year. In patients with CKD progression, current management should be reviewed and potentially reversible causes of progression assessed. Consideration should also be given to a specialist referral.
Epidemiology of chronic kidney disease and its relationships to hypertension, heart failure, and atrial fibrillation
There is growing global awareness and recognition of early CKD as a public health problem since the introduction of a clearer, multilayered definition of the condition based on GFR initially proposed by the National Kidney Foundation Kidney Disease Outcome Quality Initiative in 200215 and subsequently updated in the KDIGO clinical practice guidelines.5,16
Data from the National Health and Nutrition Examination Surveys in the USA suggest that the prevalence of moderately reduced GFR (30–59 mL/min/1.73 m2) significantly increased in 1999–2004, as compared to 1988–1994, from 5.4 to 7.7%, and also the prevalence of severely reduced GFR (15–29 mL/min/1.73 m2) significantly increased from 0.21 to 0.35%.17 Most of the increase can be explained by the increasing prevalence of hypertension and diabetes.17 Other countries suggest a similar prevalence with marked increases in older age groups.17–24
Chronic kidney disease and CVDs share many common risk factors such as hypertension, diabetes, and age. Epidemiological studies and surveys show an age-associated GFR decline, observed in both longitudinal and cross-sectional studies, although with substantial variability among individuals within the population.5 Chronic kidney disease is an independent risk factor for cardiovascular morbidity and mortality, with an inverse graded relationship with GFRs <60 mL/min/1.73 m2,12,25 and perhaps <90 mL/min/1.73 m2,26 independent of other risk factors. Although cardiovascular risk in ESRD is extreme,27,28 the public health burden of CVD caused by early-stage CKD is much greater.29 In a recent meta-analysis of the relationship between eGFR and cardiovascular risk, a 30% lower GFR was consistently associated with a 20–30% higher risk of major vascular events and all-cause mortality.30 If causal, this would imply that up to 10% of vascular events in middle age and 20% in old age might be attributable to reduced renal function. The phenotype of CVD associated with CKD is multifactorial with arterial stiffening causing heart failure, stroke, arrhythmic sudden death, and premature atherosclerosis causing vascular occlusive events.31,32
A strong, independent and graded relationship also exists between the degree of albuminuria and cardiovascular risk.12 Cardiovascular risk is increased even within currently defined normal levels of albuminuria and below those that can be detected by a standard urinary dipstick.12 Albuminuria, together with eGFR, exerted a multiplicative effect on the risks of all-cause and cardiovascular mortality.
There is a close relationship between the heart and kidney with accumulating evidence that dysfunction of one organ negatively affects the other, the so-called Cardio-Renal Syndrome (CRS).33–35 Acute heart failure leading to CKD is defined as type 1 CRS, chronic heart failure leading to CKD as type 2 CRS, acute kidney disease leading to an acute cardiac disorder (e.g. arrhythmia, heart failure, cardiac ischaemic event, etc.) as type 3 CRS, and CKD leading to cardiac problems and adverse cardiac events as type 4 CRS, whereas type 5 CRS refers to a systemic condition (e.g. sepsis) causing both cardiac and renal dysfunction.
Observational data show that heart failure and CKD commonly coexist with CKD documented in 26–63% of heart failure patients,36–38 but cannot determine which of the two disease processes was primary vs. secondary.34 The close relationship between CKD and heart failure is also demonstrated in other cardiovascular conditions. Approximately 30% of patients with hypertension will have CKD, whereas nearly 90% of patients with CKD will also be hypertensive.39 Similarly, the prevalence of atrial fibrillation (AF) in the general population is ∼1–2% and increases markedly with age,40–44 and ∼11–23% of patients with AF will have CKD.40,44–47
Having CKD is associated with an increased risk of subsequently developing AF and vice versa.48 The prevalence of AF in patients on dialysis is high with estimates ranging from 7 to 27% and also increases with age.49 However, the relative risk is much higher in the young.49 Among dialysis patients aged >65, the incidence of AF is quite high (15%) and incidence and prevalence have both steadily increased since 1995.50,51 Fortunately, mortality and stroke incidence after the development of AF in this population has continued to decline.50 The prevalence of AF in the pre-dialysis CKD population appears to be similar to that of the dialysis population at 4–21%.52–55 Chronic kidney disease is also present in a substantial proportion of patients with acute coronary syndromes; indeed, large registries report that almost 40% of patients with non-ST-elevation myocardial infarction and 30% of those with ST-elevation myocardial infarction have significant renal impairment, with GFR <60 mL/min/1.73 m2.56,57
Progression of chronic kidney disease and impact on patients' outcomes
Reduced glomerular filtration and proteinuria have repeatedly been shown to increase risk of cardiovascular events across a spectrum of cardiovascular risk profiles.12,58–60 Death from CVD is a common cause of death patients with progression of CKD. The increase in cardiovascular risk is generally proportional due to the severity of CKD, which is due to a combination of the independent risk attributable to CKD as well as the increased prevalence of other cardiovascular risk factors, such as diabetes and hypertension, in advanced CKD.
Patients with less severe CKD are more likely to die of CVD than to develop kidney failure.58,61 The cause of death is most commonly linked to CVD, as incident coronary heart disease is quite common.62 In a study of 28 000 patients with GFR ≤90 mL/min from a single healthcare system in the USA, the 5-year rate of renal replacement therapy for CKD stages 2, 3, and 4 was 1.1, 1.3, and 19.9%, respectively, whereas the mortality rate was 19.5, 24.3, and 45.7%.27 Congestive heart failure, coronary disease, and diabetes were more prevalent in patients who died. However, younger patients with significant proteinuria, more localized kidney disease, and an absence of other cardiovascular risk factors are more likely to progress to renal replacement therapy prior due to a lower competing risk of cardiovascular death.63
Clinical predictors of accelerated progression may therefore also predict the development of cardiovascular sequelae. Multivariate analyses from a number of observational studies have defined clinical predictors of accelerated GFR decline, including more severe proteinuria, higher blood pressure, lower serum high-density lipoprotein, black ethnicity, smoking, physical inactivity, and obesity.64–66 More recently, a model including age, sex, eGFR, and laboratory tests commonly performed in CKD patients (albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin) predicted CKD progression with high discrimination (C-statistic 0.917 in derivation and 0.841 in validation).67
Atrial fibrillation has been shown to accelerate CKD progression. In a regional US healthcare system study of 206 229 patients with GFR <60 mL/min, incident AF increased the risk of progression to ESRD (HR 1.67).68 Such modification of progression by AF has been demonstrated even in relatively preserved function with no dipstick-detectable proteinuria.48 More recent observational data suggest that anticoagulation in AF could be associated with a slowing of CKD progression.69,70 Therefore, treatment of secondary factors associated with CKD progression, which may include CVD s themselves, could favourably reduce the cumulative risk of cardiovascular morbidity and mortality.
Implications of chronic kidney disease in the management of a patient with arrhythmias
Arrhythmogenesis in chronic kidney disease: electrolyte disturbances, modulation of arrhythmia mechanisms, and fibrosis
The cardio-renal axis is regulated in such a way that a disturbed balance will both result in cardiac and renal remodelling, a process which is highlighted when considering arrhythmias in patients with impaired renal function.
Patients with the various stages of CKD may present a wide spectrum of arrhythmias, including supraventricular tachycardias, and particularly AF, ventricular ectopic beats, sustained ‘malignant’ ventricular tachyarrrhythmias, and sudden cardiac death (SCD).71 In more advanced stages, bradyarrhythmias and asystole may also occur, usually associated with hyperkalaemia or other electrolyte derangements.71
Ventricular tachyarrhythmias may lead to SCD, but the nature and risk of SCD in dialysis patients remain obscure, although a link with progressive coronary artery disease and myocardial ischaemia has been established. Cardiac arrest after acute myocardial infarction is twice as common in patients on dialysis compared with those with normal renal function.72 The pathological processes that cause coronary artery disease are also likely involved in CKD: hyperlipidaemia, hypertension, diabetes, acid–base balance, calcium phosphate metabolism, and inflammatory factors. Still, it appears that arrhythmogenesis in CKD patients also has additional contributing factors other than atherosclerosis. In studies on statins, a dissociation was found between the positive effect on major cardiovascular events and the lack of benefit on cardiovascular mortality and SCD.72,73
Arrhythmogenesis in patients with CKD is related to many potentially concurring factors, as presented in Table 2. Among these additional factors favouring arrhythmogenesis, some deserve special consideration:
Table 2.
Factors involved in arrhythmogenesis in CKD
| Electrolyte alterations (chronic and acute) |
| Autonomic imbalance |
| Haemodynamic instability during haemodialysis |
| Prolongation and increased dispersion of ventricular repolarization |
| Left ventricular hypertrophy |
| Left ventricular dysfunction |
| Myocardial fibrosis |
| Scars due to myocardial infarction |
| Macro and microvessels angiopathy (atherosclerosis, diabetic microangiopathy, vascular calcification, etc.) |
| Endothelial dysfunction |
| Inflammatory processes |
| Oxidative stress |
| Acidosis and acidaemia |
| Anaemia |
| Uraemic state |
-
1.
Left ventricular (LV) hypertrophy and fibrosis: LV hypertrophy and QT-prolongation (acquired long QT) are common among CKD patients. Left ventricular hypertrophy may, in some cases, evolve to heart failure, an important extra contributor to arrhythmias and SCD.74 Increased amounts of certain uraemic toxins and an imbalance in the activity of parathyroid hormone promote different forms of cardiac fibrosis. Fibrosis not only compromises the contractile performance, but also hampers intercellular coupling, slows conduction, and thereby increases the propensity to develop ventricular arrhythmias.
-
2.
Autonomic imbalance: Sympathetic overactivity is present in all stages of CKD and has long-term pro-arrhythmic effects: it increases repolarization heterogeneity and induces hypertrophy and fibrosis.
-
3.Rapid fluid and electrolyte shifts: Fluid and electrolyte shifts during conventional dialysis treatment may trigger AF or ventricular arrhythmias by favouring electrical instability.71,75 A number of electrolyte disturbances are known for their increased risk for pro-arrhythmia:
- • Acute or chronic hypokalaemia: Hypokalaemia evokes both supraventricular and ventricular tachyarrhythmias. In the ventricles, it delays repolarization (an increase in QT interval) in ventricular myocytes, while increasing the automaticity in Purkinje fibres (an increase in ventricular ectopy). The appearance of U-waves in the ECG is one of the typical characteristics.
- • Acute or chronic hyperkalaemia: Hyperkalaemia is associated with changes at the ECG which, in progression, include tall, peaked T waves with a shortened QT interval (initial findings), followed, with progression of the disorder by lengthening of the PR interval and QRS duration, disappearance of the P wave, idioventricular rhythms, and ultimately marked widening of the QRS up to a sine-wave pattern. Pronounced shortening of the action potential duration may favour re-entrant arrhythmias in conditions of slowed conduction.76 Ventricular standstill with asystolic cardiac arrest can be a terminal event. The progression and severity of ECG changes have no strict correlation with serum potassium concentrations, and changes are more evident if hyperkalaemia has a rapid onset. Hyperkalaemia can also cause a type I Brugada pattern.
- • Hypocalcaemia: This condition, frequently seen in chronic renal insufficiency, is able to decrease contractility and to increase excitability. It appears most frequently in combination with other electrolyte abnormalities.
- • Hyperphosphataemia: It is characteristic of ESRD, and may facilitate ventricular tachyarrhythmias and SCD.
- • Hypo- or hypermagnesaemia: These electrolyte alterations usually develop in combination with derangements of the other electrolytes and their independent contribution to arrhythmogenesis is uncertain.
Changes in drug pharmacokinetics in chronic kidney disease with specific focus on antiarrhythmic agents, beta-blockers, and antithrombotic drugs
Pharmacokinetic (PK) studies are not performed routinely in patients with CKD; however, physicians should be aware of the data published in guidelines or provided as summary of product characteristics (SmPC). Potential problems associated with modified PK in CKD patients are:5 A detailed list of the main alterations of drug PK in CKD patients is summarized in Table 3.
Table 3.
Main alterations in drug PK in patients with CKD
| PK characteristics | Alterations in CKD |
|---|---|
| Bioavailability | Decreased absorption (alkaline media, reduced peristalsis, bowel oedema, and phosphate chelation) Altered first pass (decreased biotransformation of parent drug and impaired protein binding resulting in more free drug available for liver) |
| Volume of distribution | Increased volume of distribution or extracellular volume overload Decreased volume of distribution in muscle wasted patients |
| Protein binding | Increased or decreased protein binding with a correspondent decrease or increase in free (active) drug concentration Low albumin increases active drug Organic acids accumulate in renal failure and compete with acid drugs for protein binding |
| Drug metabolism/renal elimination | Drug metabolism could be modified and unpredictable (increased or decreased) Non-renal elimination could be compensatory increased resulting in higher concentrations of potential toxic metabolites Parent compound could accumulate in CKD |
-
1.
reduced ability to excrete drugs and/or their metabolites,
-
2.
increased sensitivity to medications (e.g. those bound to albumin in hypoalbuminaemic states such as nephritic syndrome),
-
3.
diminished tolerance of side effects, particularly in the elderly, and
-
4.
loss of efficacy
Despite several guidelines, there are still controversies regarding the best modality to guide therapeutic decisions of common drugs in patients with CKD.77 The current guidelines recommend evaluation of GFR when deciding the dose of the drug.5 Cockcroft–Gault formula, MDRD, or CKD-EPI equations could be used for this purpose each demonstrating virtues and limitations. In the case of drugs with narrow therapeutic ranges (the case of many antiarrhythmic drugs), when precision is desired or in special cases when formula estimate is inaccurate (low muscle mass), direct determination of GFR is required. As recommended by the guidelines,5 people with CKD should receive, when possible, the same treatment as those with normal renal function. However, the dosages may need adjustment according to GFR, given the implications on prolonged half-life and reduced clearance of the drug, especially for drugs with narrow therapeutic ranges.77
The loading dose of a drug is a function of peak concentration desired, volume of distribution, bioavailability (which is 1 for intravenous-administered drugs), and weight. There are no guideline recommended loading doses for antiarrhythmics, beta-blockers, and most antithrombotic drugs. Without a loading dose, maintenance doses will achieve 90% of their steady-state level in 3–4 half lives. The dosage could be adapted in CKD patients through reduction in dose and/or lengthening the dosage interval (more useful with drugs with a longer half-life and a wider therapeutic range).
The main PK characteristics and suggestions for appropriate prescription in CKD patients for most used beta-blockers and antiarrhythmic drugs are described in Table 4.78
Table 4.
Main PK characteristics and suggestions for appropriate prescription in CKD patients for most used beta-blockers and antiarrhythmic drugs (modified from ref.78)
| Drug | PK and elimination | Indications for CKD |
|---|---|---|
| Atenolol | About 5% bound to plasma protein; T1/2 ∼6 h but action duration is 24 h; excreted unchanged in urine | Dose may need to be reduced |
| Bisoprolol | Approximately 30% bound to protein; peak plasma concentration in 2–4 h; metabolized by the liver but ∼50% excreted unchanged in urine | Monitoring; dose may need to be reduced in advanced CKD |
| Carvedilol | 99% protein bound; T1/2 ∼6–10 h; elimination mainly biliary and 16% urinary | Dosage adjustment usually not required excepting advanced renal failure and elderly |
| Labetalol | 90% protein bound; T1/2 ∼6–8 h metabolized by liver with inactive metabolites excreted in urine and bile; <5% excreted unchanged in urine | Dose reduction recommended in the elderly |
| Metoprolol | Approximately 12% protein bound; T1/2 ∼3–5 h but the effect persists for 12 h; <5% excreted unchanged in urine | No dosage reduction needed |
| Sotalol | Not protein bound; T1/2 ∼7–18 h; not metabolized; excreted unchanged in urine (∼70%) | Dose to be reduced to one half in CKD and one quarter in severe renal failure where there is relative contraindication in view of the risk of pro-arrhythmic effects |
| Procainamide | 15% protein bound; bounds to different tissues; active hepatic metabolite; variable hepatic and renal elimination (60% unchanged); longer elimination in renal failure | Reduction of dose recommended |
| Quinidine | 85% protein bound; 50–90% metabolized by the liver to active metabolites; T1/2 ∼6 h; 20% excreted in urine | Pro-arrhythmia; could interfere with renal clearance of other drugs |
| Lidocaine | 65% protein bound; 80% rapidly metabolized by the liver to active metabolites; T1/2 <2 h; <10% excreted unchanged in urine | No special requirements |
| Mexiletine | 50–70% protein bound; T1/2 ∼5–17 h; ∼15% excreted unchanged in urine | No special requirements |
| Flecainide | T 1/2 ∼20 h; metabolized by the liver and excreted unchanged in urine (35%) | Dose reduction if GFR <35 mL/min/1.73 m2 |
| Propafenone | 95% protein bound; metabolized by the liver to active metabolites, excreted in urine (38%); two genetically determined pathways of metabolism (>90% people are rapid metabolizers with T1/2 ∼2–10 h); <1% excreted unchanged in urine | Careful monitoring recommended (in hospital initiation if advanced CKD) |
| Vernakalant | 25–50% protein bound, extensively and rapidly distributed in the body after intravenous administration, not extensively bound to plasma proteins. Mainly eliminated by the liver with T1/2 ∼3–5.5 h | Available for intravenous administration at the dose of 3.0 mg/kg followed by 2.0 mg/kg if required |
| Amiodarone | 99% protein bound; widely distributed to different tissues; metabolized by the liver to two active metabolites; no renal elimination | No dosage requirements; not dialyzable; many drug-to-drug interactions |
| Dronedarone | ∼98% protein bound; metabolized by the liver to active and inactive metabolites; T1/2 ∼13–19 h; 6% excreted in urine | No dosage adaptation required in mild and severe renal failure |
| Dofetilide | High (>90%) bioavailability; protein binding of 60–70%; 80% excreted by the kidney, as unchanged dofetilide (80%) or as inactive or minimally active metabolites (20%); T1/2 ∼10 h | Dose individualized on the basis of GFR; contraindicated if GFR <20 mL/min |
| Diltiazem | 70–80% protein bound; extensive first-pass effect, metabolization in the liver to active metabolites; bioavailability of ∼40%; T1/2 ∼3.5–9 h; only 2–4% unchanged drug excreted in the urine | Use with caution |
| Verapamil | About 90% protein bound. High first-pass metabolism, Metabolized in the liver to at least 12 inactive metabolites. Bioavailability 10–35%. 70% is excreted in the urine and 16% in faeces. T1/2 ∼5–12 h | Dose reduction by 25–50% if CrCl <10 mL/min. Not cleared by haemodialysis |
| Adenosine | Rapid cell uptake and clearance; PK difficult to be studied; not dependent on renal function | No dosage adaptation required |
| Digoxin | 20–30% protein bound; T1/2 ∼26–45 h; main route of elimination is renal (closely correlated with the GFR) with 25–28% of elimination by non-renal routes | Dosage adaptation is required, with monitoring of serum digoxin levels |
CKD, chronic kidney disease; CrCl, creatinine clearance; GFR, glomerular filtration rate; PK, pharmacokinetics.
The main PK characteristics for oral anticoagulants and dosing recommendations, in relation with renal function (usually evaluated with the Cockcroft–Gault formula in trials) and according to regulatory approvals, are provided in Table 5.79,80
Table 5.
Main PK characteristics for oral anticoagulants and dosing recommendations, according to regulatory approvals (modified from refs79,80)
| Warfarin | Dabigatran | Apixaban | Edoxaban | Rivaroxaban | |
|---|---|---|---|---|---|
| Fraction renally excreted of absorbed dose | 80% | 27% | 50% | 35% | |
| Bioavailability | 95–100% | 3–7% | 50% | 62% | 66% without food Almost 100% with food |
| Fraction renally excreted of administered dose | 4% | 14% | 37% | 33% | |
| Approved for CrCl | ≥30 mL/min | ≥15 mL/min | ≥15 mL/min | ≥15 mL/min | |
| Dosing recommendation | CrCl ≥30 mL/min: no adjustment | CrCl ≥50 mL/min: no adjustment (i.e. 150 mg b.i.d.) | Serum creatinine ≥1.5 mg/dL: no adjustment (i.e. 5 mg b.i.d.) | 60 mg daily for CrCl 50–95 mL/min, 30 mg daily for CrCl 15–50 mL/min, weight ≤60 kg; not recommended for CrCl >95 mL/min | CrCl ≥50 mL/min: no adjustment (i.e. 20 mg qd) |
| Dosing if CKD | When CrCl <30 mL/min: use lower doses and monitor closely | When CrCl 30–49 mL/min, 150 mg b.i.d. is possible (SmPC) but 110 mg b.i.d. is recommended if high risk of bleeding79 | CrCl 15–29 mL/min: 2.5 mg b.i.d. Serum creatinine ≥1.5 mg/dL in combination with age of ≥80 years or weight ≤60 kg. (SmPC) or with other factors that increase bleeding risk (e.g. diltiazem): 2.5 mg b.i.d. |
60 mg daily for CrCl 50–95 mL/min, 30 mg daily for CrCl 15–50 mL/min, weight ≤60 kg; not recommended for CrCl >95 mL/min | 15 mg q.d. when CrCl 15–49 mL/min |
| Not recommended if | CrCl <30 mL/min | CrCl <15 mL/min | If CrCl >95 mL/min or <15 mL/min | CrCl <15 mL/min |
Risk of thromboembolic events and risk of bleeding in atrial fibrillation
Patients with CKD are at a high risk of developing incident arrhythmias, such as AF. The presence of AF is also associated with a higher risk of ESRD among patients with CKD.50,68 When AF is present, patients with associated CKD are at a high risk of stroke and thromboembolism, as well as major bleeding, as included in Tables 6–8.55,81–94 Patients with renal replacement therapy, whether dialysis or renal transplantation, are at particularly a high risk of thromboembolism and bleeding.55,82,89
Table 6.
Risk of thromboembolic and bleeding events in AF patients with renal impairment
| Author, year, country | Study population | Definition of renal impairment | ATT therapy | Thromboembolic events | Bleeding events |
|---|---|---|---|---|---|
| Providência, 201481 | Meta-analysis 19 studies 379 506 patients AF patients with CKD |
Cockroft–Gault (n = 5) MDRD (n = 5) CKD-EPI (n = 2) Coding |
Warfarin, NOACs, aspirin, or none | CKD ↑ TE risk [HR (95% CI) 1.46 (1.20–1.76); P = 0.0001] End-stage CKD ↑ TE risk [HR (95% CI) 1.83 (1.56–2.14); P < 0.00001] Warfarin ↓TE in non-end-stage CKD patients [HR (95% CI) 0.39 (0.18–0.86); P < 0.00001] NOACs ↓TE compared with warfarin [HR (95% CI) 0.80 (0.66–0.96); P = 0.02] and aspirin [HR (95% CI) 0.32 (0.19–0.55); P < 0.0001] in non-end-stage CKD patients |
|
| Shah, 2014, Canada82 | Retrospective population-based cohort study Patients aged ≥65 years admitted to the hospital with primary/secondary diagnosis of AF from 1998 to 2007 |
Dialysis: n = 1626 Mean (SD) age: 75 (8) years; 634 (39.0%) women Non-dialysis: n = 204 210 Mean (SD) age: 78 (10); 104 652 (51.2%) women |
Warfarin vs. no warfarin OAC: 756 (46.4%) dialysis vs. 103 473 (50.7%) non-dialysis |
No. of events (incidence rate per 100 patient-years) Dialysis patients: 107 (3.12) On warfarin vs. off-warfarin: 52 (3.37) vs. 55 (2.91) Non-dialysis patients: 19 489 (2.35) On warfarin vs. off-warfarin: 9241 (2.19) vs. 10 248 (2.51) Warfarin use not associated with ↓stroke risk in dialysis patients [adjusted HR (95% CI) 1.14 (0.78–1.67) ↓Stroke risk with warfarin use in non-dialysis patients [adjusted HR (95% CI) 0.87 (0.85–0.90)] |
No. of events (incidence rate per 100 patient-years) Dialysis patients: 275 (8.89) On warfarin vs. off-warfarin: 149 (10.88) vs. 126 (7.31) Non-dialysis patients: 34 035 (4.32) On warfarin vs. off-warfarin: 18 340 (4.64) vs. 15 695 (4.00) Warfarin use ↑bleeding risk in dialysis [adjusted HR (95% CI) 1.44 (1.13–1.85)] and non-dialysis [HR (95% CI) 1.19 (1.13–1.85)] patients |
| Kooiman, 2014, The Netherlands83 | 724 AF patients without CKD or non-dialysis-dependent CKD on OAC attending the Leiden clinic between 1997 and 2005 Follow-up: 31 December 2010 Median follow-up 2.1 years for stroke/TIA and 2.3 years for major bleeding events Mean (SD) age 75 (10); 43.5% women |
Abbreviated MDRD formula No CKD (eGFR >60 mL/min): n = 300 Moderate CKD (30–60 mL/min): n = 294 Severe CKD (eGFR <30 mL/min) = 130 |
All OACs | 45/724 (6.2%) (1.67/100 patient-years) stroke/TIA ↑Stroke/TIA risk in patients with severe CKD vs. those without CKD [HR (95% CI) 2.75 (1.25–6.05)] vs. those with moderate CKD [HR (95% CI) 3.93 (1.71–9.00)] Similar stroke/TIA risk for patients with moderate CKD vs. without CKD (data not reported) |
ISTH criteria for major bleeding 113/724 (15.6%) (4.8/100 patient-years) Non-significant ↑ in major bleeding risk with severe CKD vs. no CKD [HR (95% CI) 1.66 (0.97–2.86)] and those with moderate CKD [HR (95% CI) 1.86 (1.08–3.21)] Similar risk of major bleeding for patients with moderate CKD vs. no CKD (data not reported) |
| Friberg, 2014, Sweden*84 | Retrospective analysis of Swedish AF national registry 307 351 patients with hospital diagnosis of AF between 1 July 2005 and 31 December 2010 13 435 (4.4%) with previous diagnosis of renal failure |
ICD-10 codes (N17-19) or local codes for dialysis or renal transplantation Mean (SD) age; % women Renal failure: 78.4 (10.3); 4802 (35.7%) No renal failure: 74.8 (12.5); 123 333 (45.6%) |
Warfarin at baseline Renal failure: 3766 (28.0%) No renal failure: 10 794 (39.9%) |
↑ Annual rate of ischaemic stroke [3.9 vs. 2.9%; HR (95% CI) 1.25 (1.16–1.34)] and TE [8.2 vs. 5.2%; HR (95% CI) 1.42 (1.35–1.49)] with renal failure; however, no significant difference after full adjustment for confounders [adjusted HR (95% CI) 1.02 (0.95–1.10) and 1.12 (1.07–1.18) for ischaemic stroke and TE, respectively] Irrespective of renal function, patients on warfarin at baseline had ↓ stroke and TE than those not on warfarin at baseline [HR 0.69 vs. 0.70 in patients with and without renal failure; P-value for interaction P = 0.865] |
↑ Annual rate of any bleeding [9.8 vs. 4.1%; HR (95% CI) 2.24 (2.14–2.35)] and ICH [0.8 vs. 0.5%; HR (95% CI) 1.50 (1.28–1.74)] with renal failure Renal failure-independent risk factor for any bleeding [adjusted HR (95% CI) 1.56 (1.48–1.63)] and ICH [adjusted HR 1.27 (1.09–1.49)] |
| Chao, 2014, Taiwan85 | Retrospective analysis of Taiwan's National Health Insurance Research Database between 1 January 1996 and 31 December 2011 10 999 AF patients with ESRD undergoing renal replacement therapy, not on OAC or APT Mean (SD) age 71.0 (11.1) years; 5913 (53.8%) women |
ESRD defined by ICD-9-CM codes | None | Ischaemic stroke 1217 pts. (11.7%); incidence rate of 6.9 per 100 patient-years |
† 9.7% severe bleeding (bleeding not defined) |
| Roldán, 2013, Spain*86 | 978 consecutive stable anticoagulated (INR 2.0–3.0 within previous 6 months) AF patients from outpatient clinic Median (IQR) age 76 (70–81); 482 (49.3%) women Median (IQR) follow-up: 875 (706–1059) days |
MDRD Renal impairment: eGFR <60 mL/min/1.73 m2 |
OAC | CV events (stroke, TIA, peripheral embolism, ACS, acute HF, and cardiac death) 113 patients (4.82%/year) adverse CV events; 39 (1.66%/year) strokes eGFR (categorical variable per 30 mL/min/1.73 m2 decrease) was significantly associated with thrombotic/vascular events [unadjusted HR (95% CI) 1.42 (1.11–1.83); P = 0.006] Adjusted for ‘high-risk’ (CHA2DS2-VASc score ≥2) eGFR (per 30 mL/min/1.73 m2 decrease) was significantly associated with thrombotic/vascular events [adjusted HR (95% CI) 1.37; 1.07–1.76; P = 0.012] |
ISTH criteria for major bleeding 81 patients (3.46%/year) haemorrhagic events 16 ICH (0.68%/year) eGFR (categorical variable per 30 mL/min/1.73m2 decrease) ↑ risk of bleeding (HR 1.44; 1.08–1.94; P = 0.015) Adjusted for ‘high-risk’ (HAS-BLED ≥3) eGFR (per 30 mL/min/1.73 m2 decrease) was significantly ↑ risk of bleeding [adjusted HR (95% CI) 1.34 (1.00–1.80); P = 0.046] |
| Banerjee, 2013, France*87 | Loire Valley cohort 5912 patients with first recorded AF diagnosis in hospital between 1 January 2000 and December 2010 with baseline serum creatinine data Mean follow-up: 2.45 (3.56) years |
History of renal failure or baseline serum creatinine level >133 µmol/L (men) or >115 µmol/L (women) eGFR (mL/min/1.73m2) three groups: ≥60 (n = 4375) 30–59 (n = 1196) <30 (n = 341) |
TE (ischaemic stroke, TIA, and peripheral artery embolism) No. TE events and rate (95% CI) at 1 year eGFR ≥60: 64; 3.4 (2.4–4.8) eGFR 30–59: 92; 5.7 (4.2–7.8) eGFR <30: 15; 7.7 (4.3–13.6) Normal: 119; 4.4 (3.2–5.9) As a categorical variable only, eGFR was an independent predictor of TE after adjustment for age, sex, and CHADS2 risk factors but not for baseline characteristics |
† | |
| Apostolakis, 2013, multicentre88 | AMADEUS cohort 4576 AF patients. receiving OAC Mean (SD) age 70 (9) years; 1526 (33.4%) women Mean (SD) follow-up: 325 (164) days |
Baseline serum creatinine available in 4554 (99.5%) Three most widely used equations to calculate renal function CrCl (Cockroft–Gault formula), MDRD and CKD-EPI Based on CrCl: 1470 (32.35) <60 mL/min 68 (1.5%) <30 mL/min |
Warfarin or idraparinux | Composite of all stroke/non-CNS SE 45 strokes/non-CNS SE (1.1 events per 100 patient-years) Only data for CrCl and MDRD reported here (number of events (n/100 patient-years) CrCl ≥90: 6 (0.6) 60–89: 13 (0.8) 30–59: 26 (2.2) <30: 0 MDRD ≥90: 2 (0.4) 60–89: 17 (0.8) 30–59: 25 (1.9) <30: 1 (1.9) Adjustment for demographic characteristics and co-morbidities, patients with CrCl <60 mL/min had double the risk of risk/SE compared with those with CrCl ≥60 mL/min [adjusted HR (95% CI) 2.27 (1.14–4.52)] |
ISTH criteria for major bleeding 103 major bleeds (2.5 events per 100 patient-years) CrCl ≥90: 15 (1.3) 60–89: 38 (2.4) 30–59: 48 (3.8) <30: 2 (3.2) MDRD ≥90: 7 (1.6) 60–89: 53 (2.4) 30–59: 42 (3.2) <30: 1 (1.9) Patients with CrCl <60 mL/min had ↑ risk of major bleeding compared with patients with CrCl ≥60 mL/min [adjusted HR (95% CI) 1.58 (1.05–2.39); P = 0.027] |
| Olesen, 2012, Denmark*55 | Retrospective analysis of Danish national registries 132 372 patients with hospital discharge diagnosis of AF between 1997 and 2008 |
ICD codes No renal disease at baseline: 127 884 (96.6%) Mean (SD) age 73.2 (12.9); 46.9% women Non-end-stage CKD: 3587 (2.7%) Mean (SD) age 76.5 (11.0); 41.0% women end-stage CKD (dialysis or previous kidney transplant): 901 (0.7%) Mean (SD) age 66.8 (11.7); 33.6% women |
OAC ± ASA, ASA, or none | No. of stroke/TE events; event rate per 100 patient-years (95% CI) No renal disease: 16 648; 3.61 (3.55–3.66) Non-end-stage CKD: 842; 6.44 (6.02–6.89) End-stage CKD: 164; 5.61 (4.82–6.54) Compared with patients with no renal disease, non- end-stage CKD patients [HR (95% CI) 1.49 (1.38–1.59; P < 0.001)] and those on renal replacement [HR (95% CI) 1.83 (1.57–2.14; P < 0.001] had ↑ risk of stroke/TE Warfarin ↓ stroke/TE risk in both groups [adjusted HR (95% CI)] No renal disease: ASA: 0.59 (0.56–0.61) OAC: 1.10 (1.06–1.14) OAC + ASA: 0.69 (0.64–0.74) Non-end-stage CKD: ASA: 0.84 (0.69–1.01) OAC: 1.25 (1.07–1.47) OAC + ASA: 0.76 (0.56–1.03) Renal replacement: ASA: 0.44 (0.26–0.74) OAC: 0.88 (0.59–1.32) OAC + ASA: 0.82 (0.37–1.80) |
No. of major bleeding events; event rate per 100 patient-years (95% CI) No renal disease: 16 195; 3.54 (3.48–3.59) Non-end-stage CKD: 1097; 8.77 (8.26–9.30) Renal replacement: 243 8.89 (7.84–10.08) Adjusted HR (95% CI) No renal disease: ASA: 1.28 (1.23–1.33) OAC: 1.21 (1.16–1.26) OAC + ASA: 2.18 (2.07–2.30) Non-end-stage CKD: ASA: 1.36 (1.17–1.59) OAC: 1.12 (0.96–1.30) OAC + ASA: 1.63 (1.32–2.02) Renal replacement: ASA: 1.27 (0.91–1.77) OAC: 1.63 (1.18–2.26) OAC + ASA: 1.71 (0.98–2.99) |
| Go, 2009, USA*90 | ATRIA cohort 13 535 AF patients diagnosed between 1 July 1996 and 31 December 1997 Mean age of ATRIA cohort 71.6 years; 42.8% women Follow-up until 30 September 2003 |
MDRD Baseline serum creatinine not available in 2627 (19.4%) patients eGFR ≥60 mL/min/1.73 m2: n = 7690 45–59 mL/min/1.73m2: n = 2499 <45 mL/min/1.73m2: n = 1338 |
None 33 165 patient-years off-OAC among 10 908 AF patients |
676 TE events (637 ischaemic strokes) during periods off-warfarin eGFR ≥60 mL/min/1.73 m2: 344 events 45–59 mL/min/1.73 m2: 168 events <45 mL/min/1.73 m2: 149 events 15 events in patients with unknown kidney function Crude rates of TE off-warfarin by eGFR eGFR ≥60: 1.63 45–59 : 2.76 <45: 4.22 Rate of TE off-warfarin ↑ significantly with lower eGFR Adjusted (for age, sex, ethnicity, education, income, previous stroke, HF, DM, hypertension, and CHD) HR (95% CI) for TE compared with eGFR ≥60 eGFR 45–59: 1.16 (0.95–1.40) eGFR <45: 1.39 (1.13–1.71) Graded increased independent risk of TE with eGFR <45 mL/min/1.73 m2 |
† |
AMADEUS, Atrial fibrillation trial of Monitored, Adjusted Dose vitamin K antagonist, comparing Efficacy and safety with Unadjusted SanOrg 34006/idraparinux study; ACS, acute coronary syndrome; AF, atrial fibrillation; ASA, aspirin; ATRIA, AnTicoagulation and RIsk factors in Atrial fibrillation; ATT, antithrombotic therapy; CI, confidence interval; CKD, chronic kidney disease; CKD-EPI, chronic kidney disease epidemiology collaboration equation; CNS, central nervous system; CrCl, creatinine clearance; CV, cardiovascular; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; ESRD, end-stage renal disease; HF, heart failure; HR, hazard ratio; ICD, International Classification of Disease; ICH, intracranial haemorrhage; IQR, interquartile range; ISTH, International Society of Thrombosis and Haemostasis; MDRD, Modification of Diet in Renal Diet; min, minute; mL, millilitres; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulation; SD, standard deviation; TE, thromboembolism; TIA, transient ischaemic attack; vs., versus.
†, not reported; *, included in meta-analysis81; ↑, increase; ↓, decrease.
Table 8.
Annual rates of major bleeding in patients with normal and impaired renal function enrolled in NOAC phase III trials
| Trial | Major bleeding in patients with eGFR ≥50 mL/min (%/year) |
HR (95% CI) | Major bleeding in patients with eGFR 30–49 mL/min (%/year) |
HR (95% CI) | P-value for interaction | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| RE-LYa90 | ≥80 mL/min Dabigatran 110 mg 1.48 |
Warfarin 2.43 |
0.61 (0.44–0.84) |
Dabigatran 110 mg 5.45 |
Warfarin 5.49 |
0.99 (0.77–1.28) |
0.06 |
| Dabigatran 150 mg 2.04 50–79 mL/min Dabigatran 110 mg 2.84 Dabigatran 150 mg 3.35 |
Warfarin 2.43 Warfarin 3.70 Warfarin 3.70 |
0.84 (0.62–1.13) 0.76 (0.62–0.94) 0.91 (0.75–1.11) |
Dabigatran 150 mg 5.50 |
Warfarin 5.49 |
1.01 (0.79–1.30) |
0.64 | |
| AVERROESc91 | ≥60 mL/min Apixaban 5 mgb 0.9 |
Aspirin 81–324 0.8 |
1.1 (0.56–2.0) |
<60 mL/min Apixaban 2.5–5 mgb 2.5 |
Aspirin 81–324 2.2 |
1.2 (0.65–2.1) |
0.82 |
| ARISTOTLEd92 | >80 mL/min Apixaban 5 mgb 1.46 |
Warfarin 1.84 |
0.80 (0.61–1.04) |
Apixaban 2.5–5 mgb 3.221 |
Warfarin 6.44 |
0.79 (0.55–1.14) |
0.03 |
| >50–80 mL/min Apixaban 5 mgb 2.45 |
Warfarin 3.21 |
0.77 (0.62–0.94) |
|||||
| ROCKET-AFe93 | Rivaroxaban 20 mg 3.39 |
Warfarin 3.17 |
1.07 (0.91–1.26) |
Rivaroxaban 15 mg 4.49 |
Warfarin 4.70 |
0.95 (0.72–1.26) |
0.48 |
ARISTOTLE, Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation; AVERROES, Apixaban VErsus acetylsalicylic acid to Reduce the Risk Of Embolic Stroke; RE-LY, Randomized Evaluation of Long-term anticoagulation therapy; ROCKET-AF, Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation.
aRE-LY compared creatinine clearance ≥80 vs. 50–79 vs. <50 mL/min.
bSerum creatinine >133 mmol/L plus aged ≥80 years or body weight ≤60 kg.
cAVERROES compared eGF ≥60 vs. <60.
dARISTOTLE compared eGFR ≤50 vs. >50–80 vs. >80 mL/min.
eOn treatment analyses reported.
Table 7.
Annual rates of stroke/systemic embolism in patients with normal and impaired renal function enrolled in NOAC phase III trials
| Trial | Stroke/SE in patients with eGFR ≥50 mL/min (%/year) |
HR (95% CI) | Stroke/SE in patients with eGFR 30–49 mL/min (%/year) |
HR (95% CI) | P-value for interaction | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| RE-LYa91 | ≥80 mL/min Dabigatran 110 mg 0.88 |
Warfarin 1.05 |
0.84 (0.54–1.32) |
Dabigatran 110 mg 2.32 |
Warfarin 2.70 |
0.85 (0.59–1.24) |
0.91 |
| Dabigatran 150 mg 0.71 50–79 mL/min Dabigatran 110 mg 1.69 Dabigatran 150 mg 1.25 |
Warfarin 1.05 Warfarin 1.83 Warfarin 1.83 |
0.67 (0.42–1.09) 0.93 (0.70–1.23) 0.68 (0.50–0.92) |
Dabigatran 150 mg 1.53 |
Warfarin 2.70 |
0.56 (0.37–0.85) |
0.75 | |
| AVERROESc92 | ≥60 mL/min Apixaban 5 mgb 1.7 |
Aspirin 81–324 2.8 |
0.57 (0.37–0.87) |
<60 mL/min Apixaban 2.5–5 mgb 1.8 |
Aspirin 81–324 5.6 |
0.32 (0.18–0.55) |
0.10 |
| ARISTOTLEd93 | >80 mL/min Apixaban 5 mgb 0.99 |
Warfarin 1.12 |
0.88 (0.64–1.22) |
Apixaban 2.5–5 mgb 2.11 |
Warfarin 2.67 |
0.79 (0.55–1.14) |
0.71 |
| >50–80 mL/min Apixaban 5 mgb 1.24 |
Warfarin 1.69 |
0.74 (0.56–0.97) |
|||||
| ROCKET-AFe94 | Rivaroxaban 20 mg 1.57 |
Warfarin 2.00 |
0.78 (0.63–0.98) |
Rivaroxaban 15 mg 2.32 |
Warfarin 2.77 |
0.84 (0.57–1.23) |
0.76 |
ARISTOTLE, Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation; AVERROES, Apixaban VErsus acetylsalicylic acid to Reduce the Risk Of Embolic Stroke; RE-LY, Randomized Evaluation of Long-term anticoagulation therapy; ROCKET-AF, Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation.
aRE-LY compared creatinine clearance ≥80 vs. 50–79 vs. <50 mL/min.
bSerum creatinine >133 mmol/L plus aged ≥80 years or body weight ≤60 kg.
cAVERROES compared eGF ≥60 vs. <60 mL/min.
dARISTOTLE compared eGFR ≤50 vs. >50–80 vs. >80 mL/min.
eOn treatment analyses reported.
With regard to stroke risk per se, AF patients with CKD are clearly at higher risk. Also, the addition of renal impairment (with two points), or the presence of proteinuria or reduced creatinine clearance, was proposed to improve a prediction value of the CHADS2 score for stroke, leading to the R2CHADS2 (Renal dysfunction [doubled], Congestive heart failure, Hypertension, Age >75, Diabetes, previous Stroke [doubled]) in a substudy from the ROCKET-AF (Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) stroke risk scores.95,96
Nonetheless, the limitations of the R2CHADS2 score include its derivation in a selected anticoagulated trial cohort that excluded patients with severe renal impairment (creatinine clearance <30 mL/min), as well as the evidence that some patients at risk of stroke (CHADS2 score 0–1) were excluded from the ROCKET-AF trial. The R2CHADS2 score is also inferior to the CHA2DS2-VASc score in predicting stroke and thromboembolism.97 Also, those classed as a ‘low risk’ using the ATRIA score are not a ‘low risk’ with stroke rates >4%/year if assessed by the CHA2DS2-VASc score and left untreated.98,99 In multiple ‘real-world’ non-anticoagulated cohorts including a broad range of renal (dys)function and stroke risk, CKD does not independently add to the risk of stroke, beyond established stroke risk prediction rules, such as the CHA2DS2-VASc score.84,87,100 Furthermore, in one trial cohort with a wider range of renal (dys)function and stroke risk, CKD did not significantly add to the stroke prediction value of the CHA2DS2-VASc score.88
However, renal dysfunction may be co-morbidity determining the higher stroke risk among females,101 and may have implications for optimizing good quality anticoagulation control among patients on vitamin K antagonists (VKA, e.g. warfarin). Thus, renal disease is one component of ‘Medical co-morbidities' within the SAMe-TT2R2 score (Sex female, Age <60 years, Medical history [more than two co-morbidities], Treatment [interacting drugs, e.g. amiodarone for rhythm control], Tobacco use [doubled], Race [doubled]) that helps prediction of those patients likely to achieve good anticoagulation control, with a high time in therapeutic range (TTR).102 Indeed, a high TTR is associated with a low risk of thromboembolism and bleeding.103,104
Renal impairment also increases the risk of bleeding, and scores one point in the HAS-BLED score105 for predicting major haemorrhage in patients with AF.
Optimal thromboprophylaxis in patients with AF and end-stage CKD is a controversial area. Patients with haemodialysis on warfarin seem to be at particularly a high risk of serious bleeding, which may outweigh the reduction in stroke by warfarin.82 Nonetheless, the Swedish AF cohort study suggests that the quality of anticoagulation control, as reflected by the average TTR matters more, since high TTR is associated with a lower risk of thromboembolism and bleeding.84 All the non-VKA oral anticoagulants (NOACs) have a degree of renal excretion, and in their respective trials, those with severe renal failure were excluded. Thus, guidelines recommend that the NOACs are best used as they were studied in their respective trials, and should not be used where severe renal impairment (eGFR <25–30 mL/min) is evident, while dose reduction is needed if eGFR is between 30 and 49 mL (min).80
Association with other co-morbidities (hypertension, chronic obstructive pulmonary disease, etc.)
Chronic kidney disease often does not occur in isolation, and is often present in AF patients given their increased age, associated co-morbidities, and concomitant drug therapies. Normal or mild renal function at baseline does not preclude some AF patients developing severe renal impairment at follow-up. Therefore, appropriate monitoring of renal function is indicated in patients with co-morbidities, as well as in frail patients. Indeed, ∼20% of AF patients show a significant reduction in eGFR over a 2-year follow-up period.86 Given the close relationship between AF and other arrhythmias with heart failure, a presentation with decompensated heart failure and the concomitant use of diuretics, ACE inhibitors, etc. may significantly compromise renal function, especially in patients with reduced renal reserve.
Various additional co-morbidities present among patients with AF and CKD may predispose to cardiac arrhythmias, especially AF. Hypertension is closely related to CKD, in a bidirectional relationship. Poorly controlled hypertension increases adverse cardiovascular events in patients with AF.106 Treatment of hypertension with an ACE inhibitor or angiotensin receptor blocker may reduce new onset AF and cardiovascular events, particularly among the elderly.107 The presence of hypertensive LV hypertrophy increases the risk of incident AF, and associated cardiovascular events.108,109
Respiratory conditions such as obstructive sleep apnoea and chronic obstructive pulmonary disease (especially if both are present) have been associated with incident arrhythmias.110 Sleep apnoea may contribute to cardiovascular events in this population;111,112 indeed, sleep apnoea does improve the predictive value of the CHADS2 score for stroke risk.113 Severity of sleep apnoea may influence the degree of responsiveness to antiarrhythmic drugs.114
Excessive alcohol consumption predisposes to AF,115,116 and importantly, increases the risk of stroke and death among AF patients,117 as does high body mass index, as an index of obesity.118 Conversely, lifestyle changes, including weight reduction, have a positive impact on AF progression. In a recent randomized trial, weight reduction with intensive risk factor management resulted in a reduction in AF symptom burden and severity as well as in beneficial cardiac remodelling.119
Contrast-induced acute kidney injury
This section is described in Supplementary material online.
Implications of arrhythmias in the management of a patient with chronic kidney disease
Ventricular tachyarrhythmias and sudden death in advanced chronic kidney disease and in patients treated with haemodialysis
Sudden cardiac death is the most common cause of death in dialysis patients, even in the paediatric population,120 accounting for over 50% of all cardiac deaths and 25% of all deaths.29,121–125 The risk of SCD is also increased in patients with non-dialysis-dependent CKD, with the risk of SCD increasing linearly with declining renal function.126–129 The risk for SCD has been reported as being 17% higher for every 10 mL/min/1.73 m2 decrease in eGFR.126 The prognosis of cardiac arrests is worse in both dialysis patients and patients with stages of CKD corresponding to moderate-to-severe impairment of renal function.29,130,131
The mechanisms that underlie SCD in renal patients are complex and many factors, both general and specific to kidney failure, have been associated with the increased risk for SCD.131,132 Ischaemic heart disease is present in 80% of patients with SCD in the general population and is highly prevalent in patients with CKD.32,133 In patients starting dialysis, the prevalence of ischaemic heart disease is estimated at 40–60%29,134 and its presence is associated with an increased risk of SCD.135
Left ventricular hypertrophy and its associated decreased myocardial capillary density and disturbances in intraventricular conduction, as well as LV diastolic and systolic failure/dysfunction, predispose to ventricular arrhythmias and SCD.31,136 The prevalence of all of these features increases with a decreasing GFR and all of them are very common in dialysis patients.137–139 Vascular disease in CKD is not only characterized by intimal atheroma but also by arteriosclerosis, a disease of the medial arterial layer associated with increased collagen content and calcification.31,32 Increased arterial stiffness is associated with LV hypertrophy, myocardial fibrosis and LV dysfunction, as well as increased mortality.31,32 Coronary artery calcification is associated with an increased spatial QRS-T angle, an important marker for SCD in various patient groups.140,141 Sympathetic overactivity is highly prevalent in dialysis patients and starts early in the course of CKD, probably driven by kidneys themselves as it is reduced by nephrectomy.142,143 In dialysis patients, plasma norepinephrine is independently associated with survival and cardiovascular events.144
Cardiac arrhythmias and SCD are more common on Mondays and Tuesdays after haemodialysis-free weekends, and during the 12 h after initiation of a haemodialysis session.145–148 These findings suggest that major shifts in blood pressure, electrolytes, and fluid may induce triggers that result in arrhythmias.131,149 The use of haemodialysis catheters, rather than an arteriovenous fistula, is also associated with an increased risk of SCD.149,150 The risk of SCD in dialysis patients falls after successful transplantation.29
Most of the evidence available regarding approaches to reducing the risk of SCD in patients with CKD comes from subanalyses of population studies and clinical trials.131 Beta-blockers reduce the risk of SCD in a number of high-risk populations.151–153 Randomized controlled trials providing this evidence have generally excluded individuals with CKD.154,155 In dialysis patients, the use of beta-blockers may be limited by hypotensive episodes associated with fluid removal. A post hoc analysis suggests that the use of beta-blockers in patients with CKD is associated with a reduction in SCD risk.156 Statin therapy is generally safe and is associated with significant reductions in cardiovascular mortality in patients with CKD receiving dialysis.157–159
Limited data exist on the reduction of SCD as a specific outcome. Dysregulation of the renin–angiotensin–aldosterone system is a fundamental abnormality in CKD with studies, demonstrating that elevated aldosterone concentrations are an independent risk factor for SCD in patients with CKD.160 Whether ACE inhibitors and ARBs reduce SCD in patients with CKD is not clear.132 However, use of an ACE inhibitor and/or ARB are associated with a significant reduction in the risk of SCD in dialysis patients with a positive correlation between drug dose and survival.161 Mineralocorticoid receptor blockers are also known to reduce SCD by ∼30% in clinical trials enrolling patients with heart failure,162 and these benefits extend to the CKD subgroup. Nonetheless, use of these agents may be limited in patients with CKD, especially those on dialysis because of the associated potential for hyperkalaemia and hypotension.
Recently, several dialysis-related factors have been identified, which are associated with an increased risk for SCD, including increased fluid removal and exposure to low potassium dialysate, thereby providing potentially useful methods for altering dialysis treatment.163 Various modifications have been prospectively investigated including increased frequency and dose of dialysis. Encouraging effects on surrogate endpoints (such as LV mass) have been reported with long-hours nocturnal haemodialysis.164,165 No beneficial effects have been reported with regard to reducing (cardiovascular) mortality thus far.149
Atrial fibrillation and supraventricular tachyarrhythmias in advanced chronic kidney disease and in the chronic kidney disease patient treated with haemodialysis: haemodynamic effects and acute and long-term treatments
Despite the existence of well-established, evidence-based approaches to symptomatic control (rhythm and rate) of AF,80,166 most studies have excluded patients with functionally significant CKD.167 There are multiple clinically relevant and important differences in this group of complex patients, suggesting that accepted treatment strategies may not be as effective or indeed may cause significant adverse effects and harm.167
Perhaps, the most important relates to the occurrence of intradialytic hypotension in ∼20–30% of dialysis sessions, as the direct result of an inadequate cardiovascular response to the reduction in blood volume that occurs when a large volume of water is removed during a short period of time.168–173 This needs urgent treatment by stopping ultrafiltration, placing the patient in the Trendelenburg position and saline administration. Such episodes often result in volume overload leading to LV remodelling, diastolic and systolic dysfunction, and arrhythmogenic myocardial fibrosis.170 Indeed, intradialytic hypotension is associated with a significant increase in cardiovascular morbidity and mortality.168–171
As in the general population, for patients with CKD presenting with newly diagnosed AF, the short-term treatment goal should be control of their symptoms with rate or rhythm control therapies.174,175 Except for the need of emergency cardioversion to restore sinus rhythm in patients with haemodynamic instability, the initial therapeutic approach should include assessment for the underlying causes of AF and ventricular rate control to improve haemodynamic status and relieve symptoms.80,168,174–176
Recent studies in the AF population have suggested that lenient control (<110 b.p.m.) of resting heart rate was associated with better outcomes than strict control (80 b.p.m.),177 and that rhythm control through DC cardioversion does not improve outcomes compared with rate control using beta-blockers and digoxin.178 Whether these results are applicable to patients with CKD, especially those on dialysis, is uncertain. The differences in cardiovascular function and structure, especially in blood vessel compliance, as well as the compensatory haemodynamic changes required during routine fluid removal in a haemodialysis session might well modify the relationships between rhythm control, rate control, and outcomes.179 An estimated 20% of ventricular filling is a consequence of atrial contraction and its importance may well be exaggerated during episodes of cardiovascular stress during a haemodialysis session.179
In CKD, there are limited data on safety for any of the agents recommended for rate and rhythm control (amiodarone, dronedarone, propafenone, and flecainide), not to mention the risks associated with anticoagulation.180 Therefore, catheter-based ablation is increasingly used for rhythm control also in this complex clinical context. In patients with CKD with an eGFR of ≥30 mL/min/1.73 m2, maintenance of sinus rhythm following AF ablation (achieved in ∼74% of patients at 1 year) was associated with a significant improvement in renal function.181 Even in the specific setting of CKD patients, persistent AF and underlying atrial fibrosis resulted the main determining factor of success.181 In general, catheter ablation is associated with reduced rates of symptomatic AF recurrence compared with drug treatment for rhythm and rate control.182,183 In patients under haemodialysis radiofrequency, catheter ablation is increasingly performed for rhythm control of AF, since the use of antiarrhythmic agents is largely restricted in this context. Small studies suggest that the technique might not be as effective as in the general population.184,185 In a single-centre study with a 5-year follow-up, multiple ablation procedures for AF achieved an efficacy of ∼80% in terms of sinus rhythm maintenance, similar to the efficacy of non-haemodialysis patients, whereas the efficacy of a single procedure was scarce.186 Other reports confirm the high recurrence rate of AF in haemodialysis patients and the need for repeated procedures.187 In CKD patients treated with AF ablation, the risk of procedure-related vascular complications is increased in comparison with patients with normal kidney function.71 His bundle ablation is another option, targeted at rate control, but requires a pacemaker implant.
Antiarrhythmics, beta-blockers, antithrombotics, and dialysis
This section is presented in Supplementary material online.
Implications of arrhythmias in the perioperative management of a patient with chronic kidney disease
Patients with CKD not only have an increased risk of CVD, but also a worse associated prognosis.12 Cardiac mortality is 10- to 20-fold greater in dialysed patients than in matched controls.188 Chronic kidney disease patients frequently have structural cardiac disease and abnormal ventricular function, and are therefore at higher risk of developing ventricular arrhythmias in the perioperative period.189 Arrhythmias are already present in 32% of dialysis patients.190
Preoperative medical optimization prior to elective surgery includes appropriate dialysis prescription, correction of anaemia and electrolyte imbalance (with special focus on hyperkalaemia), tailoring blood pressure, and heart failure treatment and strategies to reduce perioperative bleeding. Assessment of the cardiac risk is mandatory preoperatively in order to avoid ischaemic and arrhythmic complications.191 The Revised Cardiac Risk Index is a simple and valuable tool in this regard; a score of ≥3 defines a high-risk patient.192 Furthermore, renal function may deteriorate postoperatively and strategies to protect against this may include adequate hydration, avoidance of nephrotoxins, and specific therapies to prevent contrast-induced nephrotoxicity. Patients with type 2 diabetes are often treated with the biguanide metformin, which is excreted by the kidney. Metformin needs to be withheld for 48 h from the time of an angiographic study if intravenous iodinated contrast media are to be given, in order to prevent high serum metformin concentrations, that in case of contrast-induced nephropathy could lead to lactic acidosis.
Beta-blockers should be continued during the perioperative period in patients receiving this therapy as outlined in contemporary guidelines.189 Initiation of beta-blockers for high-risk non-cardiac surgery may also be considered. However, the effect of beta-blockers in this setting is still debated.
Patients with CKD may present unique challenges in the perioperative management of arrhythmias. Generally, the above mentioned principles apply in the prevention of supraventricular and ventricular arrhythmias. Patients should be appropriately monitored. Particular attention should be given to the daily assessment of the corrected QT interval and heart rate. Patients with dysrhythmias should be approached in the same manner as the general population, but the choice of antiarrhythmic agents (including beta-blockers) and pacing devices, including implantable defibrillators, has to consider some specific recommendations, dictated by the potentially less favourable risk–benefit ratio of therapeutic options in this particular setting.193
A more detailed discussion about antiarrhythmic drug recommendations for CKD patients is presented elsewhere in this document. It is important to emphasize that even non-renally cleared drugs need to be used with caution in the CKD patient because of associated electrolyte abnormalities and the frequent co-existence of LV hypertrophy and heart failure.
Haemodynamically unstable ventricular arrhythmias should be treated by immediate cardioversion. Prevention of recurrent monomorphic ventricular arrhythmias in the perioperative phase may require therapy with amiodarone or the use of infusions of other antiarrhythmic drugs depending on their particular pharmakokinetics and pharmacodynamic properties in renal patients. Catheter ablation strategies can be used in appropriate cases. Prevention of recurrent polymorphic ventricular tachycardia may require multiple strategies including cardiac pacing and/or isoprenaline infusion to prevent bradycardia, correction of hypokalaemia and hypomagnesaemia, and removal of QTc prolonging drugs.
Implications of chronic kidney disease in the management of a patient with an implantable electrical device
Impact of chronic kidney disease on indication to treatment and patients' outcomes in patients with cardiac implantable devices
Pacing for bradycardia
Pacemakers are implantable devices indicated mainly in patients with symptomatic, persistent, or intermittent bradycardia.194 Although the current implantation technique has led to consider pacemaker implantation as similar to minor surgery, the implant and the subsequent follow-up are not free of risks or complications, either at short or long term, such as haematoma, pneumothorax, infection, or lead-related problems.194
Patient clinical status and co-morbidities increase the risk of complications.195 There are limited data in the literature on the implications of permanent pacing on patients' outcome and pacemaker complications in patients with CKD. In a retrospective study of patients with CKD on haemodialysis, patients with an implanted pacemaker had greater long-term mortality, but propensity score analysis revealed that the presence of a pacemaker was not an independent predictor of mortality in this setting.196
In patients with pacemakers, capture threshold is affected by many factors, including potassium level, and loss of capture may occur in cases of severe hyperkalaemia, with the atrial myocardium usually being more sensitive than the ventricular myocardium to acute rise in potassium levels.197,198 In view of frequent fluctuations of potassium levels, patients with CKD could benefit, in terms of increased safety and extended device longevity, from devices with beat-to-beat automatic ventricular threshold adjustment on the basis of automatic capture verification,199 but no specific data on CKD patients are available in the literature.
The practical issue of limitations in vascular access in the presence of a cardiac implantable electronic device (CIED) in a patient with or approaching ESRD should be considered. Arteriovenous access created ipsilateral to CIED placement should be avoided as much as possible, since they have a higher primary failure rate compared with the contralateral arm.200 In case of pacemaker implant in a patient with previous arteriovenous fistula, the pacemaker should be implanted on the contralateral side.
In case of device replacement in patients with CKD, the possible interaction between anticoagulants, especially the NOACs, and renal function must be strictly monitored to minimize the risk of bleeding complications.79
Cardiac resynchronization therapy for heart failure
About half of dialysis patients will develop heart failure either at presentation or during follow-up,201 and CKD has a prevalence ranging up to 55% in patients with heart failure, with poor prognostic implications. In patients with ESRD, cardiac resynchronization therapy (CRT) is sometimes perceived as being at a potentially greater risk with fewer benefits. However, an analysis of the IMPROVE HF registry202 showed that implants of CRT device did not decline in the subgroups of patients with more advanced stages of CKD, according to eGFR.
Patients with ESRD have not been studied in the major randomized trials on CRT since they were excluded from enrolment. Table 9 summarizes studies evaluating CRT in heart failure patients with mild-to-moderate CKD and in heart failure patients with ESRD also treated with haemodialysis.203–211 In general, these data from several studies and a meta-analysis suggest203–211 that mild-to-moderate CKD is associated with benefits from CRT similar to those observed in heart failure patients without CKD, but with a higher risk of adverse outcomes.203,211 More advanced CKD is an independent predictor of cardiac mortality and heart failure hospitalization.210 Furthermore, although CRT can be safely performed in most patients with CKD, it has usually a limited impact on delaying or preventing deterioration of renal function, up to the stage requiring haemodialysis.207 In patients with mild heart failure (NYHA Class I and II), enrolled in the REVERSE study, CRT in patients with CKD improved LV function and induced a reverse LV remodelling, although to a lesser extent than in those with normal kidney function.208
Table 9.
Summary of studies evaluating the role of CRT therapy in heart failure patients with CKD
| Author | Type of study | Number of patients | Follow-up duration | Definition of CKD | Main conclusion |
|---|---|---|---|---|---|
| Cleland et al.203 (2005) | Randomized controlled trial | 813 heart failure patients randomized to medical therapy alone or with CRT | 29.4 months | GFR <60.3 mL/min/1.73 m2 (median of the population) | If reduced GFR same benefit of CRT vs. medical therapy alone with regard to death from any cause or unplanned hospitalization for a major cardiovascular event |
| Shalabi et al.204 (2008) | Single-centre retrospective cohort study | 330 patients with severe heart failure treated with CRT | 19.7 ± 9.0 months | Elevated serum creatinine | Worse survival free of death or heart failure hospitalization in the highest tertile of serum creatinine compared with all others. For each 0.1 mg/dL increase in creatinine level, there was an 11% increase in mortality |
| Van Bommel et al.205 (2010) | Registry | 716 heart failure patients treated with CRT | 25 ± 19 months | Lower GFR at baseline was strongly predictive of death [HR of 1.18 per decrease of 10 mL/min/1.73 m2 (95% CI 1.09–1.27, P < 0.001)] | |
| Goldenberg et al.206 (2010) | Post hoc analysis of patients enrolled in the MADIT CRT trial | 1803 patients with mild heart failure randomized to CRTD or ICD treatment | 12 months | Ratio of blood urea nitrogen to serum creatinine (an index of prerenal function) | An elevated ratio of blood urea nitrogen to serum creatinine experienced a significantly greater reduction in the risk of heart failure or death with CRTD therapy when compared with patients with a low ratio |
| Lin et al.207 (2011) | Single-centre retrospective cohort study | CRT in 482 heart failure patients, of whom 71% had CKD | 36.45 + 26.55 months | GFR ≤60 mL/min/1.73 m2 | Survival was superior in patients with normal or mild renal dysfunction compared with patients with CKD |
| Mathew et al.208 (2012) | Post hoc analysis of patients enrolled in the REVERSE trial | 561 patients with mild heart failure randomized to CRT or control therapy | 12 months | GFR <60 mL/min/1.73 m2 | CRT improves LV function and reduces LV volumes to a lesser extent in patients with CKD than in those with normal kidney function |
| Friedman et al.209 (2013) | Case–control study | 15 dialysis-dependent heart failure patients and a control group of CRT patients | Up to 3 years | Dialysis | In dialysis patients, CRT implantation has no serious complications and certain patients have important improvement. Compared with matched controls, dialysis patients are at an increased risk for adverse events |
| Hosoda et al.210 (2014) | Single-centre retrospective cohort study | CRT in 15 dialysis-dependent heart failure patients | 30.3 ± 22.0 months | eGFR <50 mL/min | Patients with a e-GFR of <50 mL/min had significant higher all-cause mortality (log-rank P = 0.033) and higher cardiac mortality combined with HF hospitalization (log-rank P = 0.017) than those with eGFR ≥50 mL/min |
| Garg et al.211 (2013) | Meta-analysis of 14 observational studies and 4 randomized trials 9419 patients | Heart failure patients treated with CRT | 1–4.25 years | eGFR <45 or 60 mL/mm/1.73 m2; creatinine level >1.5 or 1.8 or 2 mg/dL; haemodialysis | Modest improvement in eGFR with CRT among CKD patients (mean difference 2.30 mL/min/1.73 m2; 95% CI 0.33–4.27). Similarly, a significant improvement in LV ejection with CRT in CKD patients (mean difference 6.24%; 95% CI 3.46–9.07) |
CKD, chronic kidney disease; CRT, cardiac resynchronizzation therapy; CRTD, cardiac resynchronization therapy plus defibrillation; eGRF, estimated glomerular filtration rate; HR, hazard ratio.
Implantable cardioverter-defibrillator for sudden death
Patients with CKD are at a markedly increased risk of death from cardiovascular causes, including SCD. In patients with CKD, SCD accounts for over 50% of all cardiac deaths and 25% of all deaths, and its estimated annual rate is ∼7%.29,121–124
The role of implantable cardioverter-defibrillators (ICDs) in this subgroup of patients is difficult to assess. First, randomized clinical trials of ICD therapy provide limited data regarding patients with CKD and ESRD because, in most trials, these patients were either excluded or the renal function was not reported. In addition, data on the ICD effect in CKD are somewhat divergent. Table 10 summarizes the most relevant studies which have evaluated the role of ICD therapy in CKD patients.121,212–217
Table 10.
Summary of studies evaluating the role of ICD therapy in CKD patients
| Author | Type of study | ICD indication | Number of patients | Follow-up duration | Definition of CKD | Main conclusion |
|---|---|---|---|---|---|---|
| Herzog et al.121 (2005) | Registry | Secondary prevention | 460 ICD group and 5582 no-ICD group | 17.9 ± 15.5 months (ICD) 14.0 ± 14.9 months (no-ICD) |
Haemodialysis patients | ICD implantation reduces the risk of death by 42% |
| Korantzopoulos et al.212 (2009) | Meta-analysis of 11 observational studies | Primary and secondary prevention | 735 CKD patients out of 3010 | 1–4.25 years | eGFR <45 or 60 mL/mm/1.73 m2; creatinine level >1.5 or 1.8 or 2 mg/dL; haemodialysis | Mortality is 3.44-fold higher in CKD patients |
| Sakhuja et al.213 (2009) | Meta-analysis of six retrospective cohort studies and one case–control study | Primary and secondary prevention | 89 haemodialysis patients out of 2516 | 12–48 months | Haemodialysis; an eGRF value of 60 mL/mm/1.73 m2 | Mortality is 2.67-fold higher in haemodialysis patients No difference in mortality between haemodialysis and CKD patients |
| Hage et al.214 (2013) | Single-centre retrospective cohort study | Primary vs. secondary prevention | 409 primary prevention (141 CKD) 287 secondary prevention (115 CKD) |
50 ± 24 months | eGFR <60 mL/mm/1.73 m2 | In CKD, higher mortality risk for primary but not secondary prevention patients; Higher risk of appropriate therapy for primary but not secondary prevention patients |
| Pun et al.215 (2014) | Meta-analysis of randomized control trials | Primary prevention | 1040 CKD 1827 no-CKD |
2.7 ± 1.5 years | eGFR <60 mL/mm/1.73 m2 | Survival benefit with ICD in patients with eGFR ≥60 mL/mm/1.73 m2 only |
| Hess et al.216 (2014) | Registry | Primary prevention | 21 226 CKD 26 056 no-CKD |
2.9 years [2.4–3.3] | eGFR ≤60 mL/mm/1.73 m2 | Graded higher risk of death [2.08–4.8 HR] depending on the severity of renal failure |
| Makki et al.217 (2014) | Meta-analysis of: observational retrospective studies Observational studies (retrospective prospective) |
Primary and secondary prevention | Role of ICD in CKD patients: 17 160 CKD Effect of CKD in ICD recipients: 5233 (1843 CKD) |
17–48 months 12–51 months |
eGFR <60 mL/mm/1.73 m2; creatinine level >1.5 or 1.8 or 2 mg/dL; haemodialysis | The ICD provides survival benefit in CKD patients at a high risk of SCD [HR = 0.65] CKD is associated with an increase in all-cause mortality in ICD recipients [HR = 2.86] |
ICD, implantable cardiac defibrillator; CKD, chronic kidney disease; eGRF, estimated glomerular filtration rate; HR, hazard ratio.
In general, it is expected that given the substantial co-morbidities in patients with CKD, the benefit of ICD therapy may be attenuated. Chronic kidney disease patients have an increased mortality due to other cardiac and non-cardiac causes; elevated defibrillation thresholds may render the myocardium refractory to ICD therapy and the higher complication rate may negate the benefit of ICD implantation in CKD patients. A recent study assessed the association between kidney function and ICD-related complications.218 In a cohort of 3147 patients, implanted at a single centre from 1996 to 2012, comparisons were made between patients with normal, moderately, and severely impaired kidney function. Patients with eGFR <30 mL/min/1.73 m2 had a higher rate of haematoma, pneumothorax, and infection. In a retrospective analysis, CKD was associated with adverse prognosis after ICD implantation, but not after CRT with a defibrillator (CRTD) implantation and GFR decreased in patients with ICD, but not in CRTD patients.219
There is still uncertainty about the benefit of the ICD for primary prevention of SCD in CKD patients.220,221 A thorough assessment of individual risk/benefit of the therapy should guide decisions in this situation. The survival benefit observed in CKD patients who receive the ICD for secondary prevention of SCD favours use of ICD therapy in this population121,201 (Table 10). In view of these data, withholding device therapy based only on the presence of CKD would be inappropriate in that specific setting. On the other hand, data indicate that primary prevention of SCD by an ICD does not convey a prognostic benefit in patients on haemodialysis, with a creatinine clearance of <35 mL/min126 or BUN >26 mg/dL in conjunction with at least two other factors (age >70 years, NYHA >II, AF, and bundle branch block).222 In another study based on a decision-analysis model for patients with CKD,223 the benefit from primary prevention ICD implantation was strictly linked to patient's age, in combination with the stage of CKD. The result suggests careful consideration of ICD implantation for older patients with more advanced stages of CKD.
Recently, there has been a growing interest in subcutaneous ICD, a device with an electrode system that is placed entirely outside the thoracic cavity.224 Interest was fuelled by the possibility of resolving problems related to access to the heart via the vascular system and problems linked to transvenous leads. According to early reports from the EFFORTLESS S-ICD Registry, CKD was present in 9% of the patients implanted with a subcutaneous ICD, but no specific data on patients' outcomes and device performance in this specific population are currently available.224
Another option that can be considered for protecting against the risk of SCD is the wearable cardioverter-defibrillator.225 This device can be a valid solution in situations with a high, but transient increase in arrhythmic risk, such as the early phase after a large myocardial infarction with marked depression of LV function, or the acute phase of a myocarditis.
Risk of infections and management
Cardiac implantable electronic devices are increasingly used in patients with CKD and ESRD,226 with a reported prevalence of ∼4% for pacemakers and 6% for ICDs.227,228 In North America, the rate of utilization of ICDs dramatically increased after 2000.229 Unfortunately, CIED infection rates increase faster than implantation rates.230,231 Analysis of hospital discharge records including 4.2 million CIED implantations performed in the USA from 1993 to 2008 showed that 69 000 patients required treatment for CIED infection.232 The average annual increase in CIED implantation was 4.7%, while the incidence rate of infection increased by 210% from 2660 cases in 1993 to 8230 cases in 2008. The annual rate of infection rose at a steady pace until 2004 when it jumped from 1.53% during that year to 2.41% in 2008 (P < 0.001).232 Renal failure was one of the most significant co-morbidities associated with infection, along with respiratory failure, heart failure, and diabetes.233 Consistently, GFR <60 mL/min has been shown to be part of a six factor score predicting the risk of CIED infection.234 A retrospective analysis of 1651 patients showed that scores ranged from 0 to 25 and identified three risk groups, i.e. low: score 0–7 with 1% infection, medium: score 8–14 with 3.4% infection, and high: score ≥15 with 11.1% infection.
Chronic kidney disease is one of the main risk factors for mortality (OR: 4.28; 95% CI 4.04–4.53) in CIED-infected patients.232,235,236 The clinical presentation of CIED infection in haemodialysis patients differs from non-haemodialysis patients, being more frequently bloodstream and complicated by lead-related endocarditis; however, pocket infection is less frequently observed.236 Uraemia is associated with a state of immune dysfunction characterized by immunodepression that contributes to the high prevalence of infections among these patients, as well as by immunoactivation resulting in inflammation.237 A progressive increase in the risk of bleeding complications following CIED implantation as the degree of renal insufficiency worsens has been shown.238 Impaired platelet function and coagulation abnormalities may also play a role in this excessive risk of bleeding. Moreover, a significant number of patients receive haemodialysis through central venous catheters, which are known to be associated with very high rates of catheter-related bacteraemia and subsequent risk of transvenous CIED infection.239 To minimize the risk of infections, in candidates to implant of a CIED, the type of device (single- or multichamber), the access and routes for leads implantation should be decided on an individual basis, taking into account the increased risk for infection and vascular complications associated with more complex implant (such as CRT). Antibiotic prophylaxis should always be prescribed at the time of CIED implant. In patients already implanted with a transvenous CIED, dialysis catheter should be avoided and contralateral access for arteriovenous fistula should be preferred.
The general principles of CIED infection treatment involve effective antibiotic treatment, complete removal of the generator and leads, and implantation of a new system on an ‘as-needed’ basis.240,241 These principles apply to ESRD patients. Small series show that device removal in haemodialysis patients is not associated with a higher rate of complication when compared with non-haemodialysis patients. However, device removal is less frequently performed in haemodialysis patients (82 vs. 95% of the cases), probably reflecting the perception of increased risk and poor prognosis in this population.236 In a recent study, 29.4% of 503 patients undergoing CIED removal from 2001 to 2011 had advanced renal failure.242
Based on the high rate of infectious complications in haemodialysis and ESRD patients, non-endovascular routes (i.e. epicardial or subcutaneous) for implantation of CIEDs should be considered.239,242 The rationale behind this proposal is that epicardial or subcutaneous leads traverse the subcutaneous tissue and do not use the central venous system. In these cases, experienced operators may reduce the time of the procedure and procedure-related complications.
Risk of syncope
This section is included in Supplementary material online.
Pregnancy
This section is given in Supplementary material online.
Life expectancy
This section is provided in Supplementary material online.
Implications of an implantable electrical device on the management of a patient with chronic kidney disease
Perioperative management for electromagnetic interferences
Chronic kidney disease does not represent a unique problem for the management of CIEDs in the perioperative setting. However, CKD patients frequently require surgical or endoscopic interventions and therapies that may expose the CIED to electromagnetic interference (EMI). Guidelines on the management of CIEDs in the perioperative setting243 should be applied, focusing particularly on conditions where CIED function could be affected by the use of electrocautery.
The risk of such interference or damage to the pulse generator or pacemaker resetting is generally low in the modern era. Bipolar electrocautery is preferred to reduce the risk of device over-sensing and pacemaker inhibition or inappropriate ICD therapy. Bipolar electrocautery rarely causes significant EMI unless applied near to the CIED (<5 cm). Monopolar electrocautery is more likely to create EMI and pulses should be <5 s duration. Device reset is uncommon with electrocautery and electrocautery delivered below the umbilicus is considerably less likely to cause CIED interference. Device reset occurs infrequently with electrosurgery, and pacing threshold increase or undersensing due to damage to the electrode/myocardial tissue interface can occur. Therefore, all devices need to be checked after electrocautery. Patients with CKD and CIED are particularly prone to perioperative complications and thus require close perioperative monitoring. Reprogramming all pacemaker CIEDs to a non-sensing mode at the time of a procedure using electrocautery is not recommended. Likewise, routinely deactivating all ICD tachycardia therapies is also not recommended. Advice should be individualized, based on an individual patient profile, including evaluation of records from the CIED follow-up clinic.
The physician needs to know a series of device factors, such as type of device, type of leads (unipolar vs. bipolar), programming, battery longevity, as well as patient factors (pacemaker dependency and risk of intraoperative ventricular arrhythmias) and understand the details of the surgical procedure to be performed (including anatomical site, patient position during surgery [i.e. prone vs. supine], the type of electrocautery to be used, and the location of applications relative to the implanted device location).
When planning the strategy for device management in the perioperative period, key questions are: All devices should be interrogated postoperatively. There is a considerable institutional variation in practice and physician preference. In general, the device should be checked prior to surgery if not checked electively in the preceding months or if battery longevity is unknown.
-
1.
Does the device need to be interrogated and checked preoperatively?
-
2.
Does the device require intraoperative reprogramming?
-
3.
Can a magnet be applied to the device?
Pacemaker-dependent patients need either application of a magnet during delivery of diathermy pulses or reprogramming to a non-sensing mode (VOO/DOO) if diathermy above the umbilicus is planned or monopolar diathermy is used.
Implantable cardioverter-defibrillator therapies need to be turned off, preferably temporarily by a magnet taped over the device that can be taken away if ventricular tachyarrhythmia needs to be treated.244 Unlike pacemakers, ICDs do not pace asynchronously during magnet application. In all these cases, it is important to assess that the magnet function of the ICD had not been previously disabled with the programmer, as possible with some devices. The patient needs to be monitored intraoperatively with external defibrillation equipment available in the room. Postoperative device reprogramming must ensure that tachycardia therapies are activated.
Procedures involving therapeutic radiation present particular risks to the CIED, are a common source of EMI, and may cause device reset. The device should be checked pre- and post-irradiation and careful attention paid to battery life and programmed parameters. The device may require shielding from the radiation beam and occasionally repositioning pretreatment. Elective external cardioversion can cause power-on-reset with only back-up function of the device or lead damage. It should always be applied in the anterior–posterior mode245 since all cases of exit block have been reported in sternal–apical application of shock paddles. Radiofrequency ablation procedures may create EMI with effects similar to monopolar electrocautery.
Management for magnetic resonance imaging scanning
Issues regarding the safety of magnetic resonance imaging (MRI) scanning for implanted electrical devices are complex and under discussion as device technology evolves in this field. There are MRI-conditional pacemaker and ICD systems. However, the type of MRI scanner (1.5 vs. 3.0 Tesla), body site to be scanned, type of CIED, the type of implanted leads, pacemaker dependency, and the presence of abandoned leads are only some of the issues to be considered. Recommendations on the use of MRI in CIED patients with specific algorithms and check lists should be considered.194,246 Independently of the CIED labelled as ‘MRI conditional’, the physician is responsible for adequate monitoring of the patient (voice, ECG, and oxygen saturation) and the availability of a device programmer, external defibrillator, emergency equipment, and the presence of a trained staff for resuscitation. Patients with CKD are generally not considered appropriate for gadolinium-enhanced scanning because of the risk of gadolinium-induced systemic sclerosis.
End-of-life issues
This section is summarized in Supplementary material online.
The costs of chronic kidney disease and related organizational issues
Prevalence and incidence of both CKD and CVD increase with age; in western countries, progressive ageing of the population emphasizes the need to consider the problem of costs related to these diseases, which appear to assume the dimensions of public health threats.
Chronic kidney disease may remain silent for a long time, before reaching the most advanced stages, and at that point few opportunities exist to prevent adverse outcomes, cardiovascular complications, and need for dialysis, thus with an adverse impact on patients' quality of life and significant costs for the healthcare system. In this regard, screening of patients at higher risk for CKD, those with heart failure, diabetes, hypertension, or a family history of hypertension, diabetes, or CKD, should be considered as attractive and potentially cost-saving. Management of the terminal stage of ESRD with dialysis is very expensive for healthcare systems. For dialysis, the reported costs are ∼$70 000 per year in the USA;247 this huge financial impact is confirmed by the high tariffs for hospital reimbursement, in the range of $700–1600 per week for dialysis, in a survey published in 2012 considering five European countries, Canada and the USA.248 It is noteworthy that, in the USA, the cost of hospital dialysis has been assumed as the benchmark to define societal willingness to pay for a QALY (quality-adjusted life years), thus indicating that this expensive but effective treatment represents the upper boundary of what to consider affordable in cost-effectiveness and cost-utility analysis.249–251
In the cardiovascular field, and specifically in the management of rhythm disturbances, many devices, intervention, or treatments have a high, usually up-front, cost.249 These treatments are targeted to reduce symptoms, morbidity, and mortality related to arrhythmic conditions, which, per se, induce substantial costs, usually in terms of disease-related hospitalizations.252 For these treatments, Health Technology Assessments, corresponding to systematic, multidisciplinary assessments of clinical effectiveness and safety as well as cost-effectiveness of medical interventions, are increasingly used, for evaluating the clinical, economic, social, and organizational impacts of new technologies.249 Chronic kidney disease has a substantial impact on many responses to treatments, complications, costs, and outcomes; therefore, it is an important determinant not only of clinical but also of economic evaluations. In this complex scenario, there is a need to collect additional data on the impact of CKD on patients' outcomes in the ‘real world’, through prospective registries, since more advanced stages of renal impairment usually constitute a criterion of exclusion from randomized clinical trials.45–47 Moreover, management of CKD in cardiac patients, and particularly in patients with heart failure and rhythm disturbances, may significantly benefit from a holistic approach based on collaborative, personalized, patient-centred care, with integration of different healthcare specialists, such as cardiologists and nephrologists. In this regard, the bidirectional interaction between the heart and the kidney (Figure 2) has been object of increasing interest in recent years, mainly focused on haemodynamic cardiac function and its acute or chronic deterioration,33 but also issues related to rhythm disturbances and implantable electrical devices, as detailed in this review, which should be considered when planning new closer interactions between cardiologists and nephrologists.
Figure 2.
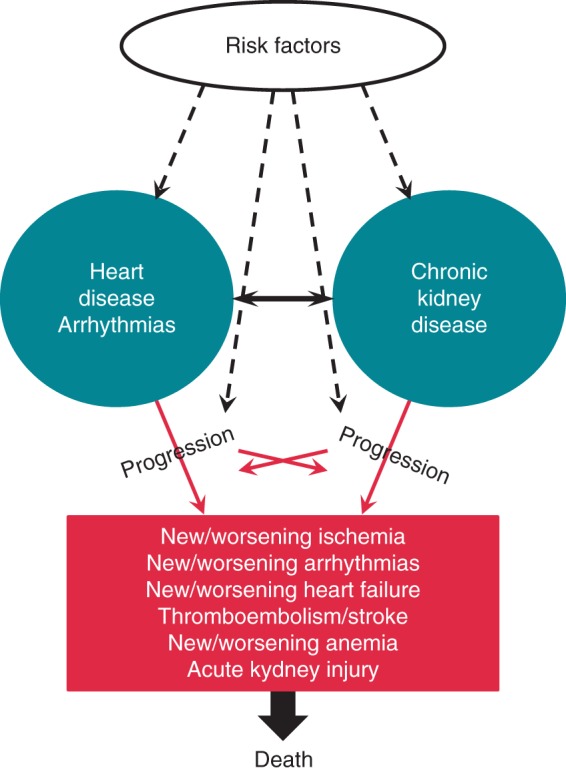
The complex interplays between heart disease, arrhythmias, and chronic kidney disease, in relationship with risk factors, progression of the diseases, complications, and death.
Finally, even if the different organizations of care across western countries, as well as their different financial profiles, strongly influence the access to more advanced technologies,253 increased efforts should be dedicated to assess the risk–benefit and cost–benefit of more advanced treatments, as well as preventive strategies in complex patients such as those with CKD or at risk of developing CKD.254
Areas for further research
-
1.
Heart disease, arrhythmias, and CKD have complex interactions, as shown in Figure 2. Therefore, the clinical and epidemiological impact of CKD in cardiac patients requires improved knowledge of the risk–benefit ratio of pharmacological therapies for cardiac disease and arrhythmias, as well as for device therapy, in the specific setting of the different stages of CKD. This can be accomplished by observational studies and registries, with extended follow-up (3–5 years) in order to assess the evolution of cardiac and renal functional status and the consequent impact on response to therapies and the occurrence of adverse outcomes (death, SCD, stroke and thromboembolism, acute coronary events, heart failure, syncope and ventricular tachyarrhythmias, acute kidney injury, CIED infections, etc.).
-
2.
Prevention of thromboembolic events and stroke in patients with AF and more advanced CKD, with eGFR <25–30 mL/min, remains challenging since currently available NOACs, which all have a degree of renal excretion, have not been tested and validated in this setting. Testing and validation of alternatives to warfarin are needed, in view of the combined increased risk of stroke and thromboembolism, as well as major bleeding, associated with more advanced CKD. Moreover, the role of percutaneous left atrial appendage occlusion should also be carefully evaluated, in terms of risk–benefit ratio vs. usual care.
-
3.
Well-controlled VKA therapy, with a high TTR (>70%), confers the best efficacy and safety in CKD patients84 and strategies to improve a TTR by education255 or ‘flagging up’ patients less likely to achieve good TTRs with the SAMe-TT2R2 score102 may help. This approach would need testing in large prospective observational cohorts or trial settings.
-
4.
The traditional model of care delivery may be not adequate for clinical settings with a high degree of complexity, such as patients with cardiac diseases, rhythm disturbances, and advanced CKD. Therefore alternative, interdisciplinary methods for home care, with appropriate patient should surveillance and monitoring,256,257 should be validated (in terms of safety, effectiveness, and cost-effectiveness) also including the use of technology for remote monitoring, defining the appropriate role of all the stakeholders (family caregivers, nurses, physicians of the various disciplines involved, etc.).256,258
Consensus statements
|
|
Supplementary material
Supplementary material is available at Europace online.
EHRA Scientific Document Committee: Gregory Y.H. Lip, (EHRA Scientific Documents Committee Chair), Bulent Gorenek, (EHRA Scientific Documents Committee Co-Chair), Christian Sticherling, Laurent Fauchier, Hein Heidbuchel, Angel Moya Mitjans, Marc A. Vos, Michele Brignole, Gheorghe-Andrei Dan, Michele Gulizia, FranciscoMarin, Giuseppe Boriani, Deirdre Lane, and Irene Savelieva.
Conflict of interest: G.B. received speakers’ fees (small amount) from Boehringer Ingelheim, Boston Scientific, Medtronic, and St Jude; G.-A.D. reported speaker fees (small amount) from Boehringer Ingelheim, Bayer, Pfizer, Servier, and ASTRA: D.A.L. received investigator-initiated educational grants from Bayer Healthcare, Boehringer Ingelheim, and Bristol Myers Squibb, and has also been on the speaker bureau for Boehringer Ingelheim, Bayer, and Bristol Myers Squibb/Pfizer; J.M. received research funding from St Jude Medical, Johnson and Johnson, and Biotronik; M.P.T. received consulting fees and honoraria from Medtronic, St Jude Medical, Precision Health Economics, Biotronik, and Gilead Sciences, and has grant support from Medtronic and iRhythm; G.Y.H.L. is a consultant for Bayer, Medtronic, Inc., Sanofi, BMS/Pfizer, Daiichi-Sankyo, and Boehringer Ingelheim, and has been a speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, and Medtronic, Inc.
Supplementary Material
References
- 1. Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013;382:158–69. [DOI] [PubMed] [Google Scholar]
- 2. Levey AS, Coresh J. Chronic kidney disease. Lancet 2012;379:165–80. [DOI] [PubMed] [Google Scholar]
- 3. Filippatos G, Farmakis D, Parissis J. Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J 2014;35:416–8. [DOI] [PubMed] [Google Scholar]
- 4. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3(Suppl):1–150. [DOI] [PubMed] [Google Scholar]
- 6. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 7. Michels WM, Grootendorst DG, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 2012;156:785–95. [DOI] [PubMed] [Google Scholar]
- 11. Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. Current and novel renal biomarkers in heart failure. Heart Fail Rev 2012;17:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–52. [DOI] [PubMed] [Google Scholar]
- 14. Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011;79:1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 16. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;113:S1–130. [DOI] [PubMed] [Google Scholar]
- 17. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 18. Anandarajah S, Tai T, de Lusignan S, Stevens P, O'Donoghue D, Walker M, et al. The validity of searching routinely collected general practice computer data to identify patients with chronic kidney disease (CKD): a manual review of 500 medical records. Nephrol Dial Transplant 2005;20:2089–96. [DOI] [PubMed] [Google Scholar]
- 19. Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006;17:2275–84. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Zhang P, Wang F, Zuo L, Zhou Y, Shi Y, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis 2008;51:373–84. [DOI] [PubMed] [Google Scholar]
- 21. White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis 2010;55:660–70. [DOI] [PubMed] [Google Scholar]
- 22. Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 2009;13:621–30. [DOI] [PubMed] [Google Scholar]
- 23. Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otero A, de Francisco A, Gayoso P, Garcia F, Group ES. Prevalence of chronic renal disease in Spain: results of the EPIRCE study. Nefrologia 2010;30:78–86. [DOI] [PubMed] [Google Scholar]
- 25. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 26. Van Biesen W, De Bacquer D, Verbeke F, Delanghe J, Lameire N, Vanholder R. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J 2007;28:478–83. [DOI] [PubMed] [Google Scholar]
- 27. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659–63. [DOI] [PubMed] [Google Scholar]
- 28. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998;32(5 Suppl 3):S112–119. [DOI] [PubMed] [Google Scholar]
- 29. U.S. Renal Data System. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethseda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 30. Mafham M, Emberson J, Landray MJ, Wen CP, Baigent C. Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: a meta-analysis. PLoS ONE 2011;6:e25920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Heart 2010;96:817–23. [DOI] [PubMed] [Google Scholar]
- 32. Moody WE, Edwards NC, Chue CD, Ferro CJ, Townend JN. Arterial disease in chronic kidney disease. Heart 2013;99:365–72. [DOI] [PubMed] [Google Scholar]
- 33. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–39. [DOI] [PubMed] [Google Scholar]
- 34. Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, et al. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182:117–36. [DOI] [PubMed] [Google Scholar]
- 35. Tumlin JA, Costanzo MR, Chawla LS, Herzog CA, Kellum JA, McCullough PA, et al. Cardiorenal syndrome type 4: insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182:158–73. [DOI] [PubMed] [Google Scholar]
- 36. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422–30. [DOI] [PubMed] [Google Scholar]
- 37. Hebert K, Dias A, Delgado MC, Franco E, Tamariz L, Steen D, et al. Epidemiology and survival of the five stages of chronic kidney disease in a systolic heart failure population. Eur J Heart Fail 2010;12:861–5. [DOI] [PubMed] [Google Scholar]
- 38. Cruz DN, Bagshaw SM. Heart-kidney interaction: epidemiology of cardiorenal syndromes. Int J Nephrol 2010;2011:351291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crews DC, Plantinga LC, Miller ER, III, Saran R, Hedgeman E, Saydah SH, et al. Prevalence of chronic kidney disease in persons with undiagnosed or prehypertension in the United States. Hypertension 2010;55:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 42. Boriani G, Diemberger I, Martignani C, Biffi M, Branzi A. The epidemiological burden of atrial fibrillation: a challenge for clinicians and healthcare systems. Eur Heart J 2006;27:893–4. [DOI] [PubMed] [Google Scholar]
- 43. Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation 2012;126:e143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lip GY, Laroche C, Boriani G, Cimaglia P, Dan GA, Santini M, et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace 2015;17:24–31. [DOI] [PubMed] [Google Scholar]
- 46. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, et al. Asymptomatic atrial fibrillation: clinical correlates, management and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128:509–18. [DOI] [PubMed] [Google Scholar]
- 47. Lip GY, Laroche C, Popescu MI, Rasmussen LH, Vitali-Serdoz L, Dan GA, et al. Heart failure in patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Pilot survey on Atrial Fibrillation. Eur J Heart Fail 2015; 10.1002/ejhf.254 10.1002/ejhf.254. [DOI] [PubMed] [Google Scholar]
- 48. Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J 2009;158:629–36. [DOI] [PubMed] [Google Scholar]
- 49. Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, et al. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 2012;81:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goldstein BA, Arce CM, Hlatky MA, Turakhia M, Setoguchi S, Winkelmayer WC. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation 2012;126:2293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Winkelmayer WC, Liu J, Patrick AR, Setoguchi S, Choudhry NK. Prevalence of atrial fibrillation and warfarin use in older patients receiving hemodialysis. J Nephrol 2012;25:341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 2010;5:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 2010;159:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 2011;4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- 56. Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, et al. Temporal management patterns and outcomes of non-ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J 2009;30:549–57. [DOI] [PubMed] [Google Scholar]
- 57. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 2010;121:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- 59. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012;380:1649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005;16:489–95. [DOI] [PubMed] [Google Scholar]
- 62. Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT, Jr, Whelton PK, et al. Cardiovascular outcomes in high-risk hypertensive patients stratified by baseline glomerular filtration rate. Ann Intern Med 2006;144:172–80. [DOI] [PubMed] [Google Scholar]
- 63. Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008;73:1310–5. [DOI] [PubMed] [Google Scholar]
- 64. Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997;51:1908–19. [DOI] [PubMed] [Google Scholar]
- 65. Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 2003;14:2934–41. [DOI] [PubMed] [Google Scholar]
- 66. Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 2003;14:479–8. [DOI] [PubMed] [Google Scholar]
- 67. Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553–9. [DOI] [PubMed] [Google Scholar]
- 68. Bansal N, Alce D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 2013;127:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chiu PF, Huang CH, Liou HH, Wu CL, Chang C, Chang CC, et al. Lower-dose warfarin delays renal progression and prolongs patient survival in patients with stage 3–5 chronic kidney disease and nonvalvular atrial fibrillation: a 12-year follow-up study. Int J Clin Pharmacol Ther 2014;52:504–8. [DOI] [PubMed] [Google Scholar]
- 70. Chang CC, Liou HH, Wu CL, Chang CB, Chang YJ, Chiu PF, et al. Warfarin slows deterioration of renal function in elderly patients with chronic kidney disease and atrial fibrillation. Clin Interv Aging 2013;8:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roberts PR, Green D. Arrhythmias in chronic kidney disease. Heart 2011;97:766–73. [DOI] [PubMed] [Google Scholar]
- 72. Poulikakos D, Banerjee D, Malik M. Risk of sudden cardiac death in chronic kidney disease. J Cardiovasc Electrophysiol 2014;25:222–31. [DOI] [PubMed] [Google Scholar]
- 73. Middleton JP. Predisposition to arrhythmias: electrolytes, uremic fibrosis, other factors. Semin Dial 2011;24:287–9. [DOI] [PubMed] [Google Scholar]
- 74. El-Sherif N, Turitto G . Electrolyte disturbances and arrhythmogenesis. Cardiol J 2011;18:233–45. [PubMed] [Google Scholar]
- 75. Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol 2008;102:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zaza A. Serum potassium and arrhythmias. Europace 2009;11:421–2. [DOI] [PubMed] [Google Scholar]
- 77. Matzke GR, Aronoff GR, Atkinson AJ, Jr, Bennett WM, Decker BS, Eckardt KU, et al. Drug dosing consideration in patients with acute and chronic kidney disease—a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011;80:1122–37. [DOI] [PubMed] [Google Scholar]
- 78. Kaski JC, Baker S, Hayward C, Khong TK, Mahida S, Tamargo J. (eds). Drugs in Cardiology: a Comprehensive Guide to Cardiovascular Pharmacotherapy. London, UK: Oxford University Press; 2010. [Google Scholar]
- 79. Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51. [DOI] [PubMed] [Google Scholar]
- 80. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 81. Providência R, Marijon E, Boveda S, Barra S, Narayanan K, Le Heuzey JY, et al. Meta-analysis of the influence of chronic kidney disease on the risk of thromboembolism among patients with nonvalvular atrial fibrillation. Am J Cardiol 2014;114:646–53. [DOI] [PubMed] [Google Scholar]
- 82. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014;129:1196–203. [DOI] [PubMed] [Google Scholar]
- 83. Kooiman J, van Rein N, Spaans B, van Beers KAJ, Bank JR, van de Peppel W, et al. Efficacy and safety of vitamin K-antagonists (VKA) for atrial fibrillation in non-dialysis dependent chronic kidney disease. PLoS ONE 2014;9:e94420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015;36:297–306. [DOI] [PubMed] [Google Scholar]
- 85. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, et al. Incidence and prediction of ischaemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm 2014;11:1752–9. [DOI] [PubMed] [Google Scholar]
- 86. Roldan V, Marin F, Fernandez H, Manzano-Fernandez S, Gallego P, Valdes M, et al. Renal impairment in a ‘real-life’ cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding). Am J Cardiol 2013;111:1159–64. [DOI] [PubMed] [Google Scholar]
- 87. Banerjee A, Fauchier L, Vourc'h P, Andres CR, Taillandier S, Halimi JM, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol 2013;61:2079–87. [DOI] [PubMed] [Google Scholar]
- 88. Apostolakis S, Guo Y, Lane DA, Buller H, Lip GYH. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J 2013;34:3572–9. [DOI] [PubMed] [Google Scholar]
- 89. Banerjee A, Fauchier L, Vourc'h P, Andres CR, Taillandier S, Halimi JM, et al. A prospective study of estimated glomerular filtration rate and outcomes in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest 2014;145:1370–82. [DOI] [PubMed] [Google Scholar]
- 90. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Circulation 2009;119:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014;129:961–70. [DOI] [PubMed] [Google Scholar]
- 92. Eikelboom JW, Connolly SJ, Gao P, Paolasso E, De Caterina R, Husted S, et al. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis 2012;21:429–35. [DOI] [PubMed] [Google Scholar]
- 93. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012;33:2821–30. [DOI] [PubMed] [Google Scholar]
- 94. Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387–94. [DOI] [PubMed] [Google Scholar]
- 95. Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224–32. [DOI] [PubMed] [Google Scholar]
- 96. Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol 2014;7:281–7. [DOI] [PubMed] [Google Scholar]
- 98. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in ‘low-risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol 2014;64:1658–65. [DOI] [PubMed] [Google Scholar]
- 99. Lip GY, Nielsen PB, Skjøth F, Lane DA, Rasmussen LH, Larsen TB. The value of the European society of cardiology guidelines for refining stroke risk stratification in patients with atrial fibrillation categorized as low risk using the anticoagulation and risk factors in atrial fibrillation stroke score: a nationwide cohort study. Chest 2014;146:1337–46. [DOI] [PubMed] [Google Scholar]
- 100. Roldán V, Marín F, Manzano-Fernandez S, Fernández H, Gallego P, Valdés M, et al. Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb Haemost 2013;109:956–60. [DOI] [PubMed] [Google Scholar]
- 101. Guo Y, Wang H, Zhao X, Zhang Y, Zhang D, Ma J, et al. Relation of renal dysfunction to the increased risk of stroke and death in female patients with atrial fibrillation. Int J Cardiol 2013;168:1502–8. [DOI] [PubMed] [Google Scholar]
- 102. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest 2013;144:1555–63. [DOI] [PubMed] [Google Scholar]
- 103. De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann F, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease. Thromb Haemost 2013;110:1087–107. [DOI] [PubMed] [Google Scholar]
- 104. Gallego P, Roldan V, Marín F, Romera M, Valdés M, Vicente V, et al. Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thromb Haemost 2013;110:1189–98. [DOI] [PubMed] [Google Scholar]
- 105. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 106. Lip GY, Frison L, Grind M, SPORTIF Investigators. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J 2007;28:752–9. [DOI] [PubMed] [Google Scholar]
- 107. Lip GY, Frison L, Grind M. Angiotensin converting enzyme inhibitor and angiotensin receptor blockade use in relation to outcomes in anticoagulated patients with atrial fibrillation. J Intern Med 2007;261:577–86. [DOI] [PubMed] [Google Scholar]
- 108. Verdecchia P, Reboldi G, Di Pasquale G, Mazzotta G, Ambrosio G, Yang S, et al. Prognostic usefulness of left ventricular hypertrophy by electrocardiography in patients with atrial fibrillation (from the Randomized Evaluation of Long-Term Anticoagulant Therapy Study). Am J Cardiol 2014;113:669–75. [DOI] [PubMed] [Google Scholar]
- 109. Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension 2003;41:218–23. [DOI] [PubMed] [Google Scholar]
- 110. Konecny T, Park JY, Somers KR, Konecny D, Orban M, Soucek F, et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol 2014;114:272–7. [DOI] [PubMed] [Google Scholar]
- 111. Ganga HV, Nair SU, Puppala VK, Miller WL. Risk of new-onset atrial fibrillation in elderly patients with the overlap syndrome: a retrospective cohort study. J Geriatr Cardiol 2013;10:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, et al. Incidence and risk of atrial fibrillation in sleep-disordered breathing without coexistent systemic disease. Circ J 2014;78:2182–7. [DOI] [PubMed] [Google Scholar]
- 113. Yazdan-Ashoori P, Baranchuk A. Obstructive sleep apnea may increase the risk of stroke in AF patients: refining the CHADS2 score. Int J Cardiol 2011;146:131–3. [DOI] [PubMed] [Google Scholar]
- 114. Goyal SK, Wang L, Upender R, Darbar D, Monahan K. Severity of obstructive sleep apnea influences the effect of genotype on response to anti-arrhythmic drug therapy for atrial fibrillation. J Clin Sleep Med 2014;10:503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sano F, Ohira T, Kitamura A, Imano H, Cui R, Kiyama M, et al. Heavy alcohol consumption and risk of atrial fibrillation. The Circulatory Risk in Communities Study (CIRCS). Circ J 2014;78:955–61. [DOI] [PubMed] [Google Scholar]
- 116. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol 2014;64:281–9. [DOI] [PubMed] [Google Scholar]
- 117. Overvad TF, Rasmussen LH, Skjøth F, Overvad K, Albertsen IE, Lane DA, et al. Alcohol intake and prognosis of atrial fibrillation. Heart 2013;99:1093–9. [DOI] [PubMed] [Google Scholar]
- 118. Overvad TF, Rasmussen LH, Skjøth F, Overvad K, Lip GY, Larsen TB. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med 2013;126:640. [DOI] [PubMed] [Google Scholar]
- 119. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. [DOI] [PubMed] [Google Scholar]
- 120. Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 2002;141:191–7. [DOI] [PubMed] [Google Scholar]
- 121. Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int 2005;68:818–25. [DOI] [PubMed] [Google Scholar]
- 122. Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial 2003;16:111–7. [DOI] [PubMed] [Google Scholar]
- 123. Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 2008;74:1335–42. [DOI] [PubMed] [Google Scholar]
- 124. Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension 2010;56:210–6. [DOI] [PubMed] [Google Scholar]
- 125. doi: 10.1093/europace/eus412. Genovesi S, Rossi E, Nava M, Riva H, De Franceschi S, Fabbrini P et al. A case series of chronic haemodialysis patients: mortality, sudden death, and QT interval. Europace 2013;15:1025–33. [DOI] [PubMed] [Google Scholar]
- 126. Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 2006;98:485–90. [DOI] [PubMed] [Google Scholar]
- 127. Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation 2006;114:2766–72. [DOI] [PubMed] [Google Scholar]
- 128. Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension 2008;51:1578–82. [DOI] [PubMed] [Google Scholar]
- 129. Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 2009;76:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Davis TR, Young BA, Eisenberg MS, Rea TD, Copass MK, Cobb LA. Outcome of cardiac arrests attended by emergency medical services staff at community outpatient dialysis centers. Kidney Int 2008;73:933–9. [DOI] [PubMed] [Google Scholar]
- 131. Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 2012;23:1929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. de Bie MK, Buiten MS, Rabelink TJ, Jukema JW. How to reduce sudden cardiac death in patients with renal failure. Heart 2012;98:335–41. [DOI] [PubMed] [Google Scholar]
- 133. Edwards NC, Steeds RP, Ferro CJ, Townend JN. The treatment of coronary artery disease in patients with chronic kidney disease. QJM 2006;99:723–36. [DOI] [PubMed] [Google Scholar]
- 134. Charytan D, Mauri L, Agarwal A, Servoss S, Scirica B, Kuntz RE. The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J 2006;152:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int 2004;65:2380–9. [DOI] [PubMed] [Google Scholar]
- 136. Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol 2009;4(Suppl 1):S79–91. [DOI] [PubMed] [Google Scholar]
- 137. Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 2001;12:1079–84. [DOI] [PubMed] [Google Scholar]
- 138. Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 2005;46:320–7. [DOI] [PubMed] [Google Scholar]
- 139. Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2014;7:703–14. [DOI] [PubMed] [Google Scholar]
- 140. Jaroszynski A, Czekajska-Chechab E, Drelich-Zbroja A, Zapolski T, Ksiazek A. Spatial QRS-T angle in peritoneal dialysis patients: association with carotid artery atherosclerosis, coronary artery calcification and troponin T. Nephrol Dial Transplant 2009;24:1003–8. [DOI] [PubMed] [Google Scholar]
- 141. doi: 10.1093/europace/eus306. de Bie MK, Koopman MG, Gaasbeek A, Dekker FW, Maan AC, Swenne CA et al. Incremental prognostic value of an abnormal baseline spatial QRS-T angle in chronic dialysis patients. Europace 2013;15:290–6. [DOI] [PubMed] [Google Scholar]
- 142. Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 1992;327:1912–8. [DOI] [PubMed] [Google Scholar]
- 143. Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens 1998;11(6 Pt 1):723–8. [DOI] [PubMed] [Google Scholar]
- 144. Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 2002;105:1354–9. [DOI] [PubMed] [Google Scholar]
- 145. Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney Int 1999;55:1553–9. [DOI] [PubMed] [Google Scholar]
- 146. Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int 2006;69:2268–73. [DOI] [PubMed] [Google Scholar]
- 147. Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011;365:1099–107. [DOI] [PubMed] [Google Scholar]
- 148. Perl J, Chan CT. Timing of sudden death relative to the hemodialysis procedure. Nat Clin Pract Nephrol 2006;2:668–9. [DOI] [PubMed] [Google Scholar]
- 149. de Bie MK, van Dam B, Gaasbeek A, van Buren M, van Erven L, Bax JJ, et al. The current status of interventions aiming at reducing sudden cardiac death in dialysis patients. Eur Heart J 2009;30:1559–64. [DOI] [PubMed] [Google Scholar]
- 150. Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, et al. Cardiac arrest and sudden death in dialysis units. Kidney Int 2001;60:350–7. [DOI] [PubMed] [Google Scholar]
- 151. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 152. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed] [Google Scholar]
- 153. Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001;134:550–60. [DOI] [PubMed] [Google Scholar]
- 154. Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 2006;70:2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006;296:1377–84. [DOI] [PubMed] [Google Scholar]
- 156. Chonchol M, Benderly M, Goldbourt U. Beta-blockers for coronary heart disease in chronic kidney disease. Nephrol Dial Transplant 2008;23:2274–9. [DOI] [PubMed] [Google Scholar]
- 157. Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G, Cholesterol and Recurrent Events Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003;138:98–104. [DOI] [PubMed] [Google Scholar]
- 158. Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2009;2:CD007784. [DOI] [PubMed] [Google Scholar]
- 159. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, et al. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated GFR: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidney Dis 2011;57:403–14. [DOI] [PubMed] [Google Scholar]
- 161. Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol 2007;2:491–500. [DOI] [PubMed] [Google Scholar]
- 162. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 163. Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 2011;79:218–27. [DOI] [PubMed] [Google Scholar]
- 164. Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007;298:1291–9. [DOI] [PubMed] [Google Scholar]
- 165. Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010;363:2287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA 3rd et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;123:104–23. [DOI] [PubMed] [Google Scholar]
- 167. Nimmo C, Wright M, Goldsmith D. Management of atrial fibrillation in chronic kidney disease: double trouble. Am Heart J 2013;166:230–9. [DOI] [PubMed] [Google Scholar]
- 168. Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. J Am Soc Nephrol 2008;19:8–11. [DOI] [PubMed] [Google Scholar]
- 169. Stefansson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, et al. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 2014;9:2124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Henderson LW. Symptomatic intradialytic hypotension and mortality: an opinionated review. Semin Dial 2012;25:320–5. [DOI] [PubMed] [Google Scholar]
- 171. Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 2015;26:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int 2008;73:759–64. [DOI] [PubMed] [Google Scholar]
- 173. Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension 2009;53:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet 2012;379:648–61. [DOI] [PubMed] [Google Scholar]
- 175. Boriani G, Biffi M, Diemberger I, Martignani C, Branzi A. Rate control in atrial fibrillation: choice of treatment and assessment of efficacy. Drugs 2003;63:1489–509. [DOI] [PubMed] [Google Scholar]
- 176. Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, et al. Personalized management of atrial fibrillation: proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace 2013;15:1540–56. [DOI] [PubMed] [Google Scholar]
- 177. Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–73. [DOI] [PubMed] [Google Scholar]
- 178. Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc M et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol 2010;55:1796–802. [DOI] [PubMed] [Google Scholar]
- 179. Szczech LA. Atrial fibrillation: the beat is faster than the answers. Kidney Int 2012;81:432–3. [DOI] [PubMed] [Google Scholar]
- 180. Ng KP, Edwards NC, Lip GY, Townend JN, Ferro CJ. Atrial fibrillation in CKD: balancing the risks and benefits of anticoagulation. Am J Kidney Dis 2013;62:615–32. [DOI] [PubMed] [Google Scholar]
- 181. Navaravong L, Barakat M, Burgon N, Mahnkopf C, Koopmann M, Ranjan R, et al. Improvement in estimated glomerular filtration rate in patients with chronic kidney disease undergoing catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2015;26:21–7. [DOI] [PubMed] [Google Scholar]
- 182. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 183. Haegeli LM, Calkins H. Catheter ablation of atrial fibrillation: an update. Eur Heart J 2014;35:2454–9. [DOI] [PubMed] [Google Scholar]
- 184. Naruse Y, Tada H, Sekiguchi Y, Machino T, Ozawa M, Yamasaki H et al. Concomitant chronic kidney disease increases the recurrence of atrial fibrillation after catheter ablation of atrial fibrillation: a mid-term follow-up. Heart Rhythm 2011;8:335–41. [DOI] [PubMed] [Google Scholar]
- 185. Sairaku A, Yoshida Y, Kamiya H, Tatematsu Y, Nanasato M, Hirayama H et al. Outcomes of ablation of paroxysmal atrial fibrillation in patients on chronic hemodialysis. J Cardiovasc Electrophysiol 2012;23:1289–94. [DOI] [PubMed] [Google Scholar]
- 186. Hayashi M, Kaneko S, Shimano M, Ohashi T, Kubota R, Takeshita K, et al. Efficacy and safety of radiofrequency catheter ablation for atrial fibrillation in chronic hemodialysis patients. Nephrol Dial Transplant 2014;29:160–7. [DOI] [PubMed] [Google Scholar]
- 187. Takigawa M, Kuwahara T, Takahashi A, Kobori A, Takahashi Y, Okubo K, et al. The impact of haemodialysis on the outcomes of catheter ablation in patients with paroxysmal atrial fibrillation. Europace 2014;16:327–34. [DOI] [PubMed] [Google Scholar]
- 188. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004;15:1307–15. [DOI] [PubMed] [Google Scholar]
- 189. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383–431. [DOI] [PubMed] [Google Scholar]
- 190. Shastri S, Sarnak MJ. Cardiovascular disease and CKD: core curriculum 2010. Am J Kidney Dis 2010;56:399–417. [DOI] [PubMed] [Google Scholar]
- 191. Trainor D, Borthwick E, Ferguson A. Perioperative management of the hemodyalisis patient. Semin Dial 2011;24:314–26. [DOI] [PubMed] [Google Scholar]
- 192. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9. [DOI] [PubMed] [Google Scholar]
- 193. KDOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 194. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC)European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 195. Hercé B, Nazeyrollas P, Lesaffre F, Sandras R, Chabert JP, Martin A, et al. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace 2013;15:66–70. [DOI] [PubMed] [Google Scholar]
- 196. Vanerio G, García C, González C, Ferreiro A. Mortality in patients on renal replacement therapy and permanent cardiac pacemakers. Int J Nephrol 2014;2014:284172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Barold SS, Falkoff MD, Ong LS, Heinle RA. Hyperkalemia-induced failure of atrial capture during dual-chamber cardiac pacing. J Am Coll Cardiol 1987;10:467–9. [DOI] [PubMed] [Google Scholar]
- 198. Kahloon MU, Aslam AK, Aslam AF, Wilbur SL, Vasavada BC, Khan IA. Hyperkalemia induced failure of atrial and ventricular pacemaker capture. Int J Cardiol 2005;105:224–6. [DOI] [PubMed] [Google Scholar]
- 199. Boriani G, Rusconi L, Biffi M, Pavia L, Sassara M, Malfitano D, et al. Role of ventricular autocapture function in increasing longevity of DDDR pacemakers: a prospective study. Europace 2006;8:216–20. [DOI] [PubMed] [Google Scholar]
- 200. Tan CS, Jie C, Joe J, Irani ZD, Ganguli S, Kalva SP, et al. The impact of transvenous cardiac devices on vascular access patency in hemodialysis patients. Semin Dial 2013;26:728–32. [DOI] [PubMed] [Google Scholar]
- 201. Cannizzaro LA, Piccini JP, Patel UD, Hernandez AF. Device therapy in heart failure patients with chronic kidney disease. J Am Coll Cardiol 2011;58:889–96. [DOI] [PubMed] [Google Scholar]
- 202. Heywood JT, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, et al. Influence of renal function on the use of guideline-recommended therapies for patients with heart failure. Am J Cardiol 2010;105:1140–6. [DOI] [PubMed] [Google Scholar]
- 203. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 204. Shalaby A, El-Saed A, Voigt A, Albany C, Saba S. Elevated serum creatinine at baseline predicts poor outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol 2008;31:575–9. [DOI] [PubMed] [Google Scholar]
- 205. van Bommel RJ, Borleffs CJ, Ypenburg C, Marsan NA, Delgado V, Bertini M, et al. Morbidity and mortality in heart failure patients treated with cardiac resynchronization therapy: influence of pre-implantation characteristics on long-term outcome. Eur Heart J 2010;31:2783–90. [DOI] [PubMed] [Google Scholar]
- 206. Goldenberg I, Moss AJ, McNitt S, Barsheshet A, Gray D, Andrews ML, et al. Relation between renal function and response to cardiac resynchronization therapy in Multicenter Automatic Defibrillator Implantation Trial—Cardiac Resynchronization Therapy (MADIT-CRT). Heart Rhythm 2010;7:1777–82. [DOI] [PubMed] [Google Scholar]
- 207. Lin G, Gersh BJ, Greene EL, Redfield MM, Hayes DL, Brady PA. Renal function and mortality following cardiac resynchronization therapy. Eur Heart J 2011;32:184–90. [DOI] [PubMed] [Google Scholar]
- 208. Mathew J, Katz R, St John Sutton M, Dixit S, Gerstenfeld EP, Ghio S, et al. Chronic kidney disease and cardiac remodelling in patients with mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) study. Eur J Heart Fail 2012;14:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Friedman DJ, Upadhyay GA, Singal G, Orencole M, Moore SA, Parks KA, et al. Usefulness and consequences of cardiac resynchronization therapy in dialysis-dependent patients with heart failure. Am J Cardiol 2013;112:1625–31. [DOI] [PubMed] [Google Scholar]
- 210. Hosoda J, Ishikawa T, Matsushita K, Matsumoto K, Kimura Y, Miyamoto M, et al. Impact of renal insufficiency on long-term clinical outcome in patients with heart failure treated by cardiac resynchronization therapy. J Cardiol 2012;60:301–5. [DOI] [PubMed] [Google Scholar]
- 211. Garg N, Thomas G, Jackson G, Rickard J, Nally JV, Jr, Tang WH, et al. Cardiac resynchronization therapy in CKD: a systematic review. Clin J Am Soc Nephrol 2013;8:1293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Korantzopoulos P, Liu T, Li L, Goudevenos JA, Li G. Implantable cardioverter defibrillator therapy in chronic kidney disease: a meta-analysis. Europace 2009;11:1469–75. [DOI] [PubMed] [Google Scholar]
- 213. Sakhuja R, Keebler M, Lai TS, McLaughlin Gavin C, Thakur R, Bhatt DL. Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol 2009;103:735–41. [DOI] [PubMed] [Google Scholar]
- 214. Hage FG, Aljaroudi W, Aggarwal H, Bhatia V, Miller J, Doppalapudi H, et al. Outcomes of patients with chronic kidney disease and implantable cardiac defibrillator: primary versus secondary prevention. Int J Cardiol 2013;165:113–6. [DOI] [PubMed] [Google Scholar]
- 215. Pun PH, Al-Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: a meta-analysis of patient-level data from 3 randomized trials. Am J Kidney Dis 2014;64:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hess PL, Hellkamp AS, Peterson ED, Sanders GD, Al-Khalidi HR, Curtis LH, et al. Survival after primary prevention implantable cardioverter-defibrillator placement among patients with chronic kidney disease. Circ Arrhythm Electrophysiol 2014;7:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Makki N, Swaminathan PD, Hanmer J, Olshansky B. Do implantable cardioverter defibrillators improve survival in patients with chronic kidney disease at high risk of sudden cardiac death? A meta-analysis of observational studies. Europace 2014;16:55–62. [DOI] [PubMed] [Google Scholar]
- 218. Buiten MS, De Bie MK, Van Der Heijden AC, Rotmans JI, Bootsma M, Marc Groeneveld JH, et al. Chronic kidney disease and implantable cardioverter defibrillator related complications: 16 years of experience. J Cardiovasc Electrophysiol 2014;25:998–1004. [DOI] [PubMed] [Google Scholar]
- 219. Eisen A, Suleiman M, Strasberg B, Sela R, Rosenheck S, Freedberg NA, et al. Renal dysfunction and clinical outcomes of patients undergoing ICD and CRTD implantation: data from the Israeli ICD registry. J Cardiovasc Electrophysiol 2014;25:990–7. [DOI] [PubMed] [Google Scholar]
- 220. Genovesi S, Porcu L, Luise MC, Riva H, Nava E, Stella A, et al. Mortality, sudden death and indication for cardioverter defibrillator implantation in a dialysis population. Int J Cardiol 2015;186:170–7. [DOI] [PubMed] [Google Scholar]
- 221. doi: 10.1093/europace/eur253. Williams ES, Shah SH, Piccini JP, Sun AY, Koontz JI, Al-Khatib SM et al. Predictors of mortality in patients with chronic kidney disease and an implantable defibrillator: an EPGEN substudy. Europace 2011;13:1717–22. [DOI] [PubMed] [Google Scholar]
- 222. Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter defibrillator. J Am Coll Cardiol 2012;58:2075–9. [DOI] [PubMed] [Google Scholar]
- 223. Amin MS, Fox AD, Kalahasty G, Shepard RK, Wood MA, Ellenbogen KA. Benefit of primary prevention implantable cardioverter-defibrillators in the setting of chronic kidney disease: a decision model analysis. J Cardiovasc Electrophysiol 2008;19:1275–80. [DOI] [PubMed] [Google Scholar]
- 224. Lambiase PD, Barr C, Theuns DA, Knops R, Neuzil P, Johansen JB, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD registry. Eur Heart J 2014;35:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Klein HU, Goldenberg I, Moss AJ. Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur Heart J 2013;34:2230–42. [DOI] [PubMed] [Google Scholar]
- 226. Saad TF, Hentschel DM, Koplan B, Wasse H, Asif A, Patel DV, et al. Cardiovascular implantable electronic device leads in CKD and ESRD patients: review and recommendations for practice. Semin Dial 2013;26:114–23. [DOI] [PubMed] [Google Scholar]
- 227. Saad TF, Ahmed W, Davis K, Jurkovitz C. Cardiovascular implantable electronic devices in hemodialysis patients: prevalence and implications for arteriovenous hemodialysis access interventions. Semin Dial 2015;28:94–100. [DOI] [PubMed] [Google Scholar]
- 228. Drew DA, Meyer KB, Weiner DE. Transvenous cardiac device wires and vascular access in hemodialysis patients. Am J Kidney Dis 2011;58:494–6. [DOI] [PubMed] [Google Scholar]
- 229. Charytan DM, Patrick AR, Liu J, Setoguchi S, Herzog CA, Brookhart MA, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis 2011;58:409–17. [DOI] [PubMed] [Google Scholar]
- 230. Diemberger I, Biffi M, Martignani C, Boriani G. From lead management to implanted patient management: indications to lead extraction in pacemaker and cardioverter-defibrillator systems. Expert Rev Med Devices 2011;8:235–55. [DOI] [PubMed] [Google Scholar]
- 231. De Maria E, Diemberger I, Vassallo PL, Pastore M, Giannotti F, Ronconi C, et al. Prevention of infections in cardiovascular implantable electronic devices beyond the antibiotic agent. J Cardiovasc Med (Hagerstown) 2014;15:554–64. [DOI] [PubMed] [Google Scholar]
- 232. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 233. doi: 10.1093/europace/euv053. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–77. [DOI] [PubMed] [Google Scholar]
- 234. Mittal S, Shaw RE, Michel K, Palekar R, Arshad A, Musat D, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014;11:595–601. [DOI] [PubMed] [Google Scholar]
- 235. Deharo JC, Quatre A, Mancini J, Khairy P, Le Dolley Y, Casalta JP, et al. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart 2012;98:724–31. [DOI] [PubMed] [Google Scholar]
- 236. Hickson LJ, Gooden JY, Le KY, Baddour LM, Friedman PA, Hayes DL, et al. Clinical presentation and outcomes of cardiovascular implantable electronic device infections in hemodialysis patients. Am J Kidney Dis 2014;64:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Tompkins C, McLean R, Cheng A, Brinker JA, Marine JE, Nazarian S, et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 2011;22:1099–104. [DOI] [PubMed] [Google Scholar]
- 239. Asif A, Salman L, Lopera G, Haqqie SS, Carrillo R. Transvenous cardiac implantable electronic devices and hemodialysis catheters: recommendations to curtail a potentially lethal combination. Semin Dial 2012;25:582–6. [DOI] [PubMed] [Google Scholar]
- 240. Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010;121:458–77. [DOI] [PubMed] [Google Scholar]
- 241. Diemberger I, Mazzotti A, Giulia MB, Cristian M, Matteo M, Letizia ZM, et al. From lead management to implanted patient management: systematic review and meta-analysis of the last 15 years of experience in lead extraction. Expert Rev Med Devices 2013;10:551–73. [DOI] [PubMed] [Google Scholar]
- 242. Asif A, Carrillo R, Garisto JD, Monrroy M, Khan RA, Castro H, et al. Prevalence of chronic kidney disease in patients undergoing cardiac rhythm device removal. Semin Dial 2013;26:111–3. [DOI] [PubMed] [Google Scholar]
- 243. Crossley GH, Poole JE, Rozner MA, Asirvatham SJ, Cheng A, Chung MK, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011;8:1114–54. [DOI] [PubMed] [Google Scholar]
- 244. Braunschweig F, Boriani G, Bauer A, Hatala R, Herrmann-Lingen C, Kautzner J, et al. Management of patients receiving implantable cardiac defibrillator shocks: recommendations for acute and long-term patient management. Europace 2010;12:1673–90. [DOI] [PubMed] [Google Scholar]
- 245. Manegold JC, Israel CW, Ehrlich JR, Duray G, Pajitnev D, Wegener FT, et al. External cardioversion of atrial fibrillation in patients with implanted pacemaker or cardioverter-defibrillator systems: a randomized comparison of monophasic and biphasic shock energy application. Eur Heart J 2007;28:1731–8. [DOI] [PubMed] [Google Scholar]
- 246. Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation 2006;114:1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. St Peter WL. Introduction: chronic kidney disease: a burgeoning health epidemic. J Manag Care Pharm 2007;13(9 Suppl D):S2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Vanholder R, Davenport A, Hannedouche T, Kooman J, Kribben A, Lameire N, et al. Reimbursement of dialysis: a comparison of seven countries. J Am Soc Nephrol 2012;23:1291–8. [DOI] [PubMed] [Google Scholar]
- 249. Boriani G, Maniadakis N, Auricchio A, Müller-Riemenschneider F, Fattore G, Leyva F, et al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J 2013;34:1869–74. [DOI] [PubMed] [Google Scholar]
- 250. Maniadakis N, Vardas P, Mantovani LG, Fattore G, Boriani G. Economic evaluation in cardiology. Europace 2011;13(Suppl 2):ii3–8. [DOI] [PubMed] [Google Scholar]
- 251. Fattore G, Maniadakis N, Mantovani LG, Boriani G. Health technology assessment: what is it? Current status and perspectives in the field of electrophysiology. Europace 2011;13(Suppl 2):ii49–53. [DOI] [PubMed] [Google Scholar]
- 252. Boriani G, Diemberger I, Biffi M, Martignani C. Cost-effectiveness of cardiac resynchronisation therapy. Heart 2012;98:1828–36. [DOI] [PubMed] [Google Scholar]
- 253. Arribas F, Auricchio A, Boriani G, Brugada J, Deharo JC, Hindriks G, et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in 55 ESC countries: 2013 report from the European Heart Rhythm Association (EHRA). Europace 2014;16(Suppl 1):i1–78. [DOI] [PubMed] [Google Scholar]
- 254. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- 255. Clarkesmith DE, Pattison HM, Lip GY, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS ONE 2013;8:e74037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. doi: 10.1007/s11606-007-0355-5. Boriani G, Diemberger I, Martignani C, Biffi M, Valzania C, Bertini M et al. Telecardiology and remote monitoring of implanted electrical devices: the potential for fresh clinical care perspectives. J Gen Intern Med 2008;23(Suppl 1):73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Boriani G, Da Costa A, Ricci RP, Quesada A, Favale S, Iacopino S et al The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res 2013;15:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. doi: 10.1093/europace/euv031. Boriani G. Remote monitoring of cardiac implantable electrical devices in Europe: quo vadis? Europace 2015;17:674–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.