Abstract
Objective:
Sleep disturbance is prevalent in anxious youth and prospectively predicts poor emotional adjustment in adolescence. Study 1 examined whether anxiety treatment improves subjective and objective sleep disturbance in anxious youth. Study 2 examined whether a sleep intervention, Sleeping TIGERS, can further improve sleep following anxiety treatment.
Method:
Study 1 examined 133 youth (ages 9–14; 56% female; 11% ethnic/racial minority) with generalized, social or separation anxiety over the course of anxiety treatment (Cognitive Behavioral Treatment or Client Centered Treatment). Sleep-related problems (parent, child report), and subjective (diary) and objective (actigraphy) sleep patterns were assessed across treatment in an open trial design. Study 2 included 50 youth (ages 9–14; 68% female; 10% ethnic/racial minority) who continued to report sleep-related problems after anxiety treatment, and enrolled in an open trial of Sleeping TIGERS. Pre and post assessments duplicated Study 1, and also included the Focal Interview of Sleep (FIOS) to assess sleep disturbance.
Results:
Study 1 demonstrated small reductions in sleep problems and improvements in subjective sleep patterns (diary) across anxiety treatment, but outcomes were not deemed clinically significant and 75% of youth stayed above clinical cutoff. Study 2 showed clinically significant, large reductions in sleep problems, and small changes in some subjective sleep patterns (diary).
Conclusions.
Anxiety treatment improves, but does not resolve, sleep disturbance in peri-pubertal youth, which may portend risk for poor emotional adjustment and mental health. The open trial provides preliminary support that Sleeping TIGERS can improve sleep in anxious youth to a clinically significant degree.
Keywords: sleep, anxiety, adolescence, treatment
Introduction
The current studies evaluated whether efficacious anxiety treatment improves sleep in peri-pubertal anxious youth (Study 1), and if a targeted sleep intervention further improves residual sleep problems among youth already treated for anxiety (Study 2). There are several reasons for this focus. First, sleep disturbance is pervasive in anxious youth, with up to 90% reporting at least one sleep difficulty, and 82% reporting two or more (Chase & Pincus, 2011). Commonly reported sleep disturbances include insomnia, nightmares, difficulty sleeping alone, fatigue, and reduced sleep time (e.g. Alfano, Ginsburg, & Kingery, 2007). Second, sleep disturbance is increasingly prevalent in youth initiating the transition to adolescence (referred to here as peri-pubertal youth), as naturally occurring biological changes, including changes to the circadian system (i.e., delaying of the sleep phase), sleep-wake homeostasis (i.e., slower mounting of homeostatic sleep drive), and sleep architecture (i.e., decreased slow wave sleep) intersect with environmental changes, including increased academic, social and extracurricular pressures, and growing autonomy (e.g. Carskadon, 2011). Concomitantly, this peri-pubertal developmental window is marked by significant neuro-maturational changes in circuitry (e.g. medial prefrontal cortex-amygdala-striatal networks) supporting socio-emotional development (Ahmed, Bittencourt-Hewitt, & Sebastian, 2015), leaving youth susceptible to emotion regulation difficulties and internalizing symptoms (Powers & Casey, 2015). Finally, sleep disturbance is well-documented as a prospective predictor of poor emotional adjustment and mental health outcomes such as anxiety and depression in mid to late adolescence, with some evidence for peri-puberty as a uniquely sensitive period for these effects (for review, McMakin & Alfano, 2015). Therefore, sleep disturbance in peri-pubertal anxious youth may have negative cascading effects on development and functioning. Intervention to address both anxiety and sleep disturbance early in the developmental symptom trajectory may therefore help to improve short- and long-term mental health outcomes. On the other hand, failing to resolve sleep disturbance in anxious youth at the cusp of puberty may portend risk for poor mental health outcomes.
The Effects of Anxiety Treatment on Sleep in Youth
There is a growing literature on the effects of behavioral interventions for anxiety on sleep. However, research is somewhat limited by methodological constraints. An early study demonstrated an effect of cognitive behavioral therapy on somatic symptoms, which included sleep problems as part of the symptom cluster, in a sample of youth with Generalized Anxiety Disorder (GAD; Kendall & Pimentel, 2003). However, the broad focus on somatic symptoms limits the ability to interpret the specificity of treatment-related effects on sleep problems. More recent investigations included more heterogeneous samples of anxious youth (Caporino et al., 2015; Clementi, Alfano, Holly, & Pina, 2016; Peterman et al., 2016) with some studies suggesting anxiety interventions may be most helpful for targeting specific sleep-related problems associated with bedtime difficulties (i.e., bedtime resistance, sleep anxiety, pre-sleep arousal; Clementi et al., 2016; Peterman et al., 2016); and a separate study finding that anxiety interventions may have greater effects on sleep disturbance relative to waitlist control in children relative to adolescents (Donovan, Spence, & March, 2017). These prior studies have shown mostly small effects with respect to changes in sleep disturbance following anxiety treatment (Caporino et al., 2016; Clementi et al., 2016; Peterman et al., 2016). Additionally, only one of the aforementioned studies investigated changes in sleep disturbance as an a priori hypothesis (Peterman et al., 2016). Prior research is limited by a lack of validated sleep scales for assessing sleep-related problems (Caporino et al., 2015; Donovan et al., 2017; Kendall & Pimentel, 2003) and a lack of objective (actigraphy) and subjective (diary report) measures of daily sleep patterns (Caporino et al., 2015; Clementi et al., 2016; Kendall & Pimentel, 2003; Peterman et al., 2016).
Taken together, these studies suggest that anxiety interventions are associated with modest reductions in sleep disturbance, with the most robust findings in the domains of pre-sleep bedtime difficulties (Clementi et al., 2016; Peterman et al., 2016). Small effect sizes suggest that youth may benefit from the addition of targeted sleep interventions to address residual sleep disturbance. Clementi & Alfano (2014) piloted one such intervention – a targeted behavior therapy intervention featuring a two-session sleep enhancement component. Despite improvements in sleep quality at post-treatment and 3-month follow-up, findings were difficult to interpret due to high variability in weekly sleep ratings. Additionally, the authors called for additional research to address the small sample size (n=4; single case study design), included patients with primary GAD only, and utilized self-reports only (Clementi & Alfano, 2014).
Targeted Sleep Interventions for Youth
A number of studies have assessed the benefits of targeted sleep interventions among peri-pubertal and adolescent youth, mostly in classroom settings, with mixed outcomes. An initial randomized-controlled comparison of a sleep intervention with a CBT framework versus class as usual yielded significant increases in sleep knowledge relative to class as usual, but no significant reductions in targeted sleep disturbances or depressive symptoms at post-treatment or 6-month follow-up assessments (Moseley & Gradisar, 2009). A subsequent study expanded on this framework by incorporating motivational interviewing to address poor outcomes (Cain, Gradisar, & Moseley, 2011). Again, despite significant improvements in sleep knowledge, and increases in motivation to regularize bedtimes, improvements in targeted sleep disturbances and daytime functioning were not significantly greater than class as usual, leading authors to conclude that increased motivation does not necessarily translate to long-term behavioral changes (Cain et al., 2011). Furthermore, neither of these interventions specifically targeted youth with emotional problems (i.e., anxiety or depressive symptoms), who may be particularly vulnerable to sleep disturbance. A randomized controlled trial of CBT for insomnia including internet-delivery, group format, and waitlist group showed significantly greater improvements in objective (actigraphy) and subjective (diary) sleep patterns, as well as clinically significant moderate to large reductions in subjective sleep-related problems, in internet and group CBT relative to waitlist at post-intervention and 2-month follow up assessments. However, authors noted that participants were recruited from the general population, and those with severe comorbidity were excluded. Additionally, they noted participants recruited from the general population may have increased motivation to make behavioral changes relative to clinic-referred samples (de Bruin, Bögels, Oort, & Meijer, 2015).
To address these issues, researchers have begun to develop and test multicomponent behavioral sleep interventions including CBT plus mindfulness-based approaches that may simultaneously improve anxiety symptoms. Results of pilot open trials of group sleep treatments for adolescents including CBT components plus mindfulness-based cognitive exercises to address bedtime ruminative anxiety (Bei et al, 2013; Schlarb, Liddle, & Hautzinger, 2011) were positive, with the first study, targeting primary insomnia, showing high treatment attendance and satisfaction, and significant improvements in subjective sleep and emotional functioning (Schlarb et al.,2011). The second – a school-based program – showed small to moderate improvements in objective (i.e., actigraphy) sleep patterns and moderate to large improvements in child-reported sleep problems, but non-significant reductions in child-reported anxiety (Bei et al., 2013). As a follow-up to the Bei et al. (2013) pilot study, the SENSE study (Blake et al., 2016) used a multi-method approach (e.g. actigraphy, diary, self-report) to compare a multicomponent sleep intervention, including CBT and mindfulness (e.g., body scan, deep breathing, etc.) with an active control intervention in a randomized controlled trial targeting 12-to-17 year old high school students at risk for anxiety and sleeping difficulties. The sleep intervention was associated with small to moderate effects for subjective (diary) and objective (actigraphy) sleep patterns, subjective sleep-related problems (child report), and anxiety (Blake et al., 2016), highlighting the importance of utilizing strong methodology to evaluate the efficacy of targeted sleep interventions. However, mixed findings for impacts on anxiety suggest that there is not yet clear evidence that these multi-component approaches sufficiently resolve both anxiety and sleep problems. Moreover, the extent to which these findings may be replicated in a treatment-seeking sample is unclear, nor is it clear if these approaches would prove efficacious in a slightly younger age range to allow for a stronger preventative approach at the cusp of the pubertal transition.
The Current Studies
Building on recent work in this area, the present two-study project aimed to determine if efficacious anxiety treatment improves sleep (Study 1), and if targeted sleep enhancement further improves sleep in clinic-referred peri-pubertal youth who have been treated with a full course of anxiety treatment (Study 2). The present study builds upon the prior body of work by including a sizeable sample (133 youth) of youth at the cusp of the pubertal transition, an a priori focus on sleep, a clinical population of active treatment-seeking youth and families, and a multimodal assessment of sleep problems (including parent- and child-report of sleep problems, sleep diary and actigraphy assessments of sleep patterns, and independent evaluator-administered sleep interview).
Study 1 Method
Participants
Participants were 133 youth, ages 9–14 with a primary diagnosis of Generalized Anxiety Disorder (GAD), Social Phobia (SP) and/or Separation Anxiety Disorder (SAD) who participated in the Child Anxiety Treatment Study (CATS; Silk et al., 2016). CATS included a large randomized-controlled trial for anxiety that assessed clinical course, treatment outcomes, and neurobehavioral correlates of treatment response. For the present study, youth receiving either Cognitive Behavioral Therapy (CBT) or Client Centered Therapy (CCT) were combined into one group to address the question of how anxiety treatment impacts sleep in an open trial design. Sample demographics included: 74 (55.6%) female, 14 (10.5%) racial/ethnic minority with the majority of this group identifying as African American, a mean age of 10.96 (SD: 1.47), and mean family income of $88,034 (SD: $68,174). Primary anxiety diagnoses included 73 with GAD only, 22 with SAD only, 16 with SP only, 11 with GAD and SP, 9 with GAD and SAD, 1 with SAD and SP, and 1 with GAD, SAD, and SP. See Silk et al., 2016, for CONSORT diagram for CATS anxiety clinical trial.
Measures
Parent report of sleep problems.
Children’s Sleep Habits Questionnaire (CSHQ; Owens, Spirito, & McGuinn, 2000a) is a 35-item parent-report measure of child sleep-related problems in the past week. The total score includes thirty-three items, which are comprised of 8 subscales that assess bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, nighttime waking, parasomnias, sleep-disordered breathing, and daytime sleepiness. Higher scores reflect greater sleep disturbance, and a total score of greater than or equal to 41 is recommended as a clinical cutoff. The CSHQ has satisfactory internal consistency, test-retest reliability, and discriminant validity (Owens et al., 2000a). Internal consistency in the current sample was strong for total score (Cronbach’s alpha=.89) and acceptable to strong for subscales (Cronbach’s alphas=.6-.83).
Child report of sleep problems.
Sleep Self-Report (SSR) (Owens, Spirito, McGuinn, & Nobile, 2000b) is a 26-item child-report measure of sleep disturbance in the past week, aligning with items assessed in the parent CSHQ, and yielding a total sleep disturbance score (Owens et al., 2000b). Internal consistency in the current sample was strong (Cronbach’s alpha=.84).
Actigraphy estimates of sleep patterns.
The Ambulatory Monitoring Octagonal Basic Motionlogger actigraph captured objective estimates of sleep patterns. Actigraphy has acceptable agreement with polysomnography and high agreement with subjective measures of sleep schedule (e.g., total sleep time; Sadeh, 2011). Participants were asked to wear the actigraph from Thursday evening to Tuesday morning. Data collected during the school year and summer were included. Each night of sleep was coded dichotomously for whether or not it was a school night based on child report of school attendance via cell phone on the day following the sleep period as part of ecological momentary assessment (EMA; see Silk et al, 2016). The majority (66%) of actigraphic sampling included five nights, 18% included four nights, 7% included three nights, 5% included two nights, and 4% had one night (M=4.36; SD=1.10). Actigraphy variables were identified a priori based on prior literature that delineates key indices for assessing sleep health (Buysse, 2014), including sleep onset latency (minutes it takes to fall asleep after “lights out”), sleep efficiency (time in bed minus minutes awake), wake after sleep onset (minutes awake between initial sleep onset and waking), and total sleep time (minutes of sleep between sleep onset and waking). Additionally, change in the midpoint of the sleep period from school nights to non-school nights captured the use of non-school nights to “catch up” on sleep—often referred to as social jetlag.
Diary estimates of sleep patterns.
Participants tracked subjective sleep patterns using a sleep diary (Bertocci et al., 2005) on nights when they wore the actigraph. Each entry was coded for whether or not it was a school night. Diaries included subjective report of sleep (same variables as actigraphy data) in addition to ratings along a 100 centimeter line that was measured to provide a score of 0–100 for subjective sleep quality (100=best quality sleep) and difficulty waking (100=least difficulty waking). Across both studies, the majority (8%) of diary sampling included five nights, 14% included four nights, 1% included three nights and 1% included one night (M=4.82, SD=0.49).
Procedure
Participants were recruited via community ads (bus ads, radio, newspapers), and in pediatric offices distributed throughout a northeastern metropolitan area in the United States. Potential participants contacted the study by email or phone and were screened by phone and then in person according to procedures approved by the Institutional Review Board (IRB). Following a discussion and signing of IRB-approved consent and assent, participants were screened by trained Bachelor’s and Master’s level independent evaluators (IEs) masked to treatment assignment (CBT, CCT). Participants were compensated according to completion of assessments throughout the study, at a rate of approximately minimum wage per hour. Inclusion criteria for anxiety was a primary diagnosis of GAD, SP, and/or SAD based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association, 1994). IEs assessed psychiatric diagnosis through administration of the Kiddie-Schedule for Affective Disorders and Schizophrenia (Kaufman, Birmaher, Brent, & Rao, 1997) to parent and child separately, followed by integration of findings into preliminary diagnoses. All preliminary ratings were reviewed by a child psychiatrist (N. Ryan), who established the final diagnosis. Sixteen percent of interviews were co-rated with high interrater reliability (kappa=.97). Exclusion criteria were: a) having a current primary diagnosis of Major Depressive Disorder, Obsessive-Compulsive Disorder, Posttraumatic Stress Disorder, Conduct Disorder, substance abuse or dependence, or Attention Deficit Hyperactivity Disorder combined or hyperactive-impulsive types (to decrease the likelihood of movement artifact during fMRI for the parent project); or b) lifetime diagnoses of Autism Spectrum Disorder, Bipolar disorder, psychotic depression, Schizophrenia, or Schizoaffective disorder present per CATS inclusion/exclusion criteria. Additionally, those on psychotropic medication were excluded. Following assessment, participants were randomized to receive CBT or CCT for anxiety. Treatment was delivered by seven masters and doctoral-level therapists. There were no group differences in treatment response between the treatment conditions, though a full recovery was more likely for youth enrolled in CBT. For a full description of CATS procedures, interventions and outcomes, see Silk et al. (2016).
Interventions.
Cognitive Behavioral Therapy (CBT) was provided using the Coping Cat therapist manual (Kendall & Hedtke, 2006a) and workbook (Kendall & Hedtke, 2006b). Treatment involved 16 sessions with 14 sessions spent working directly with the child, and two sessions (session numbers four and nine) targeting parent behavior. The first eight sessions focused on anxiety management skills (i.e., identifying somatic symptoms and anxious self-talk, problem solving, using self-evaluation and reward) and relaxation training. The second set of eight sessions included graduated exposure to anxiety-provoking stimuli and situations.
Child Centered Therapy (CCT; Cohen, Debblinger, Mannarino, & Steer, 2004) is a nondirective, supportive psychotherapy incorporating the principles of humanism. This manualized intervention includes active listening, reflection, empathy, and fostering of discussion of feelings. CCT was used to reflect the standard supportive psychotherapy youth with anxiety may receive in community settings. The treatment was delivered in 16 sessions, with parents playing a primary role in sessions four and nine to mirror the CBT intervention.
CBT and CCT treatment teams were advised to manage any sleep complaints as they typically would within the treatment approach (e.g. CBT via coping thoughts at bedtime; CCT via supportive listening about stressors that may be interfering with sleep).
Treatment integrity.
Therapists were trained by master clinicians in each treatment. In order to guard against treatment drift, 16% of video recorded sessions were watched and rated for fidelity by the teams that originally developed the manuals, using standardized checklists for each treatment. Fidelity ratings were 99% and 98% for CBT and CCT respectively.
Analytic Plan
Statistical analyses were performed using SPSS 24.0. To assess change across treatment within the anxious group (baseline, 5-week, and post-treatment for parent and child report; and baseline, 4 week, 8 week, 12 week and post-treatment for actigraphy and diary), we used mixed linear models (MLM) with maximum likelihood estimation to maximize power by allowing us to use non-complete cases in our analyses, and to account for intercorrelations among repeated measurements. Relevant sleep variables were nested within individuals in order to assess changes in sleep using an autoregressive covariance structure of order 1 [AR(1)], which controls for autocorrelation between data collected across different time points for a given individual. Analyses were conducted categorically, whereby the latter assessment time points were analyzed in reference to baseline scores, as we anticipated possible nonlinear change across treatment and this approach would allow us to identify when changes occurred. Variance in random intercept was examined.
All analyses included age and gender as covariates given known sleep differences. Also, sleep diary and actigraphy analyses included the number of school nights during which data were collected as a covariate to account for differences in sleep patterns on school nights versus non-school nights. Although we considered conducting analyses for school nights and non-school nights separately, this would have risked significant sample size loss given that data were collected year round (in support of broader study goals) and therefore not all youth had school night data at all time points. Sleep indices were aggregated to capture average sleep patterns across each five-day assessment period. For significant findings, anxiety treatment condition (CBT, CCT) was added to the model as a covariate to examine possible effects of treatment type on results.
MLM can be biased if missing data patterns are informative (e.g. participants missing data later in the study may be more likely to have dropped out of treatment and thus may represent a unique distribution); thus we also pursued mixed pattern analysis following steps outlined by Son, Friedmann and Thomas (2012) summarized as: 1) identify patterns of missing data in actigraphy, diary and self-report data sets, 2) create dummy variables to represent each of the identified patterns, 3) conduct mixed linear models where intercept, pattern of missingness, time, and time x pattern of missingness were used as fixed variables to predict each treatment outcome. Patterns that evidenced effects on outcomes could then be used as covariates in primary analyses as needed.
Study 1 Results
Based on plots of normality and descriptive statistics, several adjustments to the analytic plan were made. First, sleep efficiency was dropped from analyses as it was found to be too highly correlated with wake after sleep onset (e.g. diary r=−0.99, p=<.0001). Additionally, as is highly common in actigraphy and sleep diary analyses, variables were logarithmically transformed to correct for violations of normality (i.e., skewness), except for the estimates of total sleep time (actigraphy and diary), sleep quality (diary only) and difficulty waking (diary only), which were normally distributed. All analyses were conducted with outlier-corrected data (outliers moved to within 1.5 interquartile range) and there were no changes to outcomes; thus these analyses are not presented in text (available from author). There were no effects of anxiety treatment condition (CBT, CCT) on sleep outcomes; thus these analyses are not presented in text (available from authors). With regard to mixed pattern analysis, as outlined in the analytic plan, we found nine patterns of missing data for actigraphy, four for diary data, and three for each of the self-reports. T-tests indicated that several patterns were associated with treatment disposition and time, suggesting they were missing not at random (MNAR). Therefore, we conducted MLM with each pattern predicting outcomes to examine possible effects, resulting in a total of 19 MLM models. Of these 19 models, only 1 pattern of missing data evidenced a significant effect on outcome, but it did not interact with time and was therefore unrelated to treatment effects. Therefore, patterns of missingness did not inform primary analyses for the current study and were not included as covariates.
Primary Analyses
Change in parent- and child-reported sleep-related problems during anxiety treatment.
There were small effects on child and parent-reported sleep (Cohen’s d<.5; See Table 2). Child-reported sleep-related problems (SSR Total) were significantly lower at both mid (t=−3.17, p =.002.001), and post-treatment (t=−3.26, p=.001) relative to baseline. Estimated marginal means for SSR Total were 40.70 (baseline), 38.19 (mid-treatment), and 38.13 (post-treatment; see Table 2). Parent-reported sleep-related problems (CSHQ Total) were significantly lower at post-treatment (t=−3.34, p=.001), but not at mid-treatment (t=0.11, p=.91), relative to baseline. Estimated marginal means for CSHQ Total were 51.16 (baseline), 51.25 (mid-treatment), and 48.67 (post-treatment).
Table 2.
Linear Mixed Model Summary for Study 1: Effects of anxiety treatment on sleep
| Intercept | Post-Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Coefficient (SE) | t | Confidence Interval |
Coefficient (SE) | t | Confidence Interval |
Estimated Marginal Mean (SE) |
d |
| CSHQ Total | 61.62 (5.40)*** | 11.42 | 50.94 – 72.31 | −2.50 (0.75)** | −3.34 | −3.97 – −1.02 | 48.67 (0.85) | 0.27 |
| a. Bedtime resistance | 13.89 (1.88)*** | 7.39 | 10.17 – 17.61 | −0.83 (0.23)*** | −3.63 | −1.28 – −0.38 | 8.91 (0.29) | 0.27 |
| b. Sleep Onset Delay | 3.00 (0.41)*** | 7.26 | 2.18 – 3.82 | −0.25 (0.08)** | −3.21 | −0.40 – −0.10 | 1.82 (0.07) | 0.33 |
| c. Sleep Duration | 3.92 (0.97)*** | 4.05 | 2.01 – 5.84 | 0.18 (0.17) | 1.05 | −0.16 – 0.51 | 4.80 (0.17) | −0.10 |
| d. Sleep Anxiety | 12.45 (1.29)*** | 9.65 | 9.89 – 15.00 | −0.74 (0.16)*** | −4.58 | −1.06 – −0.42 | 6.15 (0.20) | 0.35 |
| e. Night Waking | 5.26 (0.90)*** | 5.83 | 3.47 – 7.04 | −0.11 (0.13) | −0.80 | −0.37 – 0.16 | 4.32 (0.15) | 0.07 |
| f. Parasomnias | 10.97 (1.15)*** | 9.51 | 8.69 – 13.26 | −0.69 (0.20)*** | −3.52 | −1.07 – −0.30 | 8.78 (0.20) | 0.34 |
| g. Sleep-disordered Breathing |
3.46 (0.45)*** | 7.64 | 2.56 – 4.36 | −0.07 (0.09) | −0.75 | −0.24 – 0.11 | 3.37 (0.08) | 0.08 |
| h. Daytime Sleepiness | 14.62 (2.26)*** | 6.48 | 10.16 – 19.09 | −0.33 (0.33) | −1.00 | −0.98 – 0.32 | 13.87 (0.34) | 0.09 |
| SSR Total | 54.70 (4.06)*** | 13.49 | 46.68 – 62.72 | −2.58 (0.79)*** | −3.26 | −4.14 – −1.02 | 38.13 (0.73) | 0.37 |
| Sleep Diary | ||||||||
| a. Sleep Latency OnsetLN | 3.19 (0.43)*** | 7.38 | 2.34 – 4.05 | −0.11 ( 0.07) | −1.59 | −0.26 – 0.03 | 2.69 (0.08) | 0.16 |
| b. Wake After Sleep OnsetLN | 1.14 (0.54)* | 2.10 | .07– 2.22 | −0.36 (0.10)*** | −3.43 | −0.56 – −0.15 | 0.98 (0.10) | 0.34 |
| c. Midpoint Sleep PeriodLN | 4.99 (0.12)*** | 39.99 | 4.74 – 5.23 | 0.02 (0.01) | 1.16 | −0.01 – 0.06 | 5.28 (0.02) | −0.10 |
| d. Total Sleep Time | 702.68 (27.06)*** | 26.00 | 649.20 – 756.17 | 18.94 (5.68)*** | 3.33 | 7.78 – 30.10 | 548.93 (5.29) | −0.35 |
| e. Sleep Quality | 74.40 (11.19)*** | 6.65 | 52.27 – 96.53 | 6.68 (1.75)*** | 3.81 | 3.23 – 10.13 | 73.20 (1.92) | −0.33 |
| f. Difficulty Waking | 84.33 (11.38)*** | 7.41 | 61.81 – 106.84 | 0.65 (2.15) | 0.30 | −3.59 – 4.88 | 63.42 (2.12) | −0.03 |
| Actigraphy | ||||||||
| a. Sleep Onset LatencyLN | 3.22 (0.25)*** | 12.75 | 2.72 – 3.71 | 0.05 (0.06) | 0.93 | −0.06 – 0.17 | 2.95 (0.05) | −0.11 |
| b. Wake After Sleep OnsetLN | 4.48 (0.40)*** | 11.12 | 3.68– 5.27 | −0.08 (0.09) | −0.96 | −0.25 – 0.09 | 3.48 (0.08) | 0.10 |
| c. Midpoint Sleep PeriodLN | 7.38 (0.02)*** | 446.20 | 7.35 – 7.41 | 0.00 (0.00) | 0.65 | −0.00 – 0.01 | 7.41 (0.00) | −0.07 |
| d. Total Sleep Time | 603.32 (25.44)*** | 23.71 | 553.00 – 653.63 | 3.40 (5.52) | 0.62 | −7.45 – 14.25 | 478.60 (5.02) | −.01 |
Note. SE. = Standard Error; CSHQ = Children’s Sleep Habits Questionnaire; SSR = Sleep Self Report; Tx. = Treatment; results for covariates (gender, age, and number of school nights) are not reported in the table. Random effects, including variance of intercept and autoregressive model of order 1 (AR1) residual covariance parameters (to correct for autocorrelation between time points) were included in the model but are not reported in the table. Estimated marginal means and standard errors are reported to guide interpretation of direction of effects relative to baseline.
p<.05,
p<.01,
p<.001;
=Sleep diary and actigraphy variables were logarithmically transformed.
As a post-hoc follow-up to effects on parent-reported CSHQ total scores, we explored changes in subscales. There was a significant reduction in Bedtime Resistance at mid (t=−2.20, p=.03) and post-treatment (t=−3.63, p<.001); Sleep Onset Delay at mid (t=−3.02, p=.003) and post-treatment (t=−3.21, p=.002); Sleep Anxiety at post-treatment (t=−4.58, p<.001); Parasomnias at post-treatment (t=−3.52, p=.001); and Daytime Sleepiness at mid-treatment only (t=2.06, p=.04). No significant reductions were found for Sleep Duration, Night Wakings or Sleep-Disordered Breathing. See Table 2.
Analysis of clinical impact.
We used Cochran’s Q test to assess change in the proportion of youth greater than or equal to the clinical cutoff of 41 on parent-reported CSHQ Total over the course of anxiety treatment. Results indicated there was no significant decrease in the proportion of youth with total scores greater than or equal to the cutoff of 41 from baseline (M=83.54%) through mid-treatment (M=84.81%) and post-treatment (M=77.22%), Cochran’s Q= 3.88, p=.14.
Change in subjective sleep patterns during anxiety treatment.
Results for sleep diary analyses indicated a significant effect for Total Sleep Time, such that it was greater at post-treatment relative to baseline (t=3.28, p<.001). Wake After Sleep Onset significantly changed across anxiety treatment, such that it was not significantly lower relative to baseline at week 4 ( t=−0.99, p=.32) or week 8 (t=−1.92, p=.06), but was significantly lower at week 12 (t=−3.20, p=.001) and post-treatment (t =−3.43, p=.001), relative to baseline. Sleep Quality changed across treatment such that ratings were significantly higher (indicating better sleep quality) at post-treatment (t=3.81, p<.001) relative to baseline, but were not significantly different at week 4 (t= 1.52, p=.13), week 8 (t=0.97, p=.33), or week 12 (t=2.74, p=.01) compared to baseline. Results also indicated significant change in Sleep Onset Latency, such that it was significantly lower at week 4 (t=−2.88, p=.004) and week 8 (t=−2.33, p=.02), but not at week 12 (t=−1.97, p=.05), but not at post-treatment (t=−1.59, p=.11), relative to baseline. No significant changes were found for Difficulty Waking or Midpoint of Sleep Period across anxiety treatment. See Tables 1 and 2 for details.
Table 1.
Descriptive Statistics for Sleep-related Problems Sleep Patterns at Baseline
| Study 1 | Study 2 | |
|---|---|---|
| Measures | Mean (SD) | Mean (SD) |
| CSHQ Total | 51.18 (9.32) | 51.41 (8.08) |
| SSR Total | 40.68 (7.56) | 39.24 (5.52) |
| Sleep Diary | ||
| a. Sleep Onset Latency (minutes) | 20.61 (16.29) | 18.45 (10.87) |
| b. Wake After Sleep Onset (minutes) | 5.44 (7.13) | 5.78 (8.12) |
| c. Midpoint of Sleep Period (clock-time) | 03:15am (00:47) | 02:57am (00:36) |
| d. Total Sleep Time (minutes) | 528.45 (57.47) | 544.55 (55.28) |
| e. Sleep Quality (0–100) | 66.32 (18.69) | 73.46 (20.26) |
| f. Difficulty Waking (0–100) | 62.58 (21.35) | 66.46 (23.04) |
| Sleep Actigraphy | ||
| a. Sleep Onset Latency (minutes) | 19.95 (13.38) | 21.88 (16.78) |
| b. Wake After Sleep Onset (minutes) | 45.07 (36.77) | 46.48 (38.06) |
| c. Midpoint of Sleep Period (clock-time) | 03:15am (00:49) | 03:34am (01:17) |
| d. Total Sleep Time (minutes) | 475.61 (53.41) | 482.17 (53.93) |
Note. Raw means and standard deviations. M = mean; SD = standard deviation; Sig. = significance; midpoint of sleep period is formatted in hours and minutes. CSHQ = The Children’s Sleep Habits Questionnaire; SSR = Sleep Self Report
Change in objective sleep patterns (actigraphy) during anxiety treatment.
There were no effects on actigraphy over the course of anxiety treatment. See Tables 1 and 2 for details.
Study 2 Method
Participants
As shown in the participant flow diagram (Figure 1), participants in the Study 2 open trial sleep enhancement intervention (Sleeping TIGERS) were 50 anxious youth who completed treatment in the randomized controlled comparison of CBT and CCT and met criteria for clinically significant sleep complaints based on sleep screener (i.e., CSHQ) at the conclusion of anxiety treatment (n=85). The 35 families who declined participation most frequently stated as reasons for not participating: fatigue from study participation (i.e. 16 sessions of anxiety treatment and related assessments), busy schedules, and/or child or parent not perceiving sleep as a problem (despite positive sleep screener). Participants were 68% female, 10% ethnic minority, had a mean age of 11.55 (standard deviation=1.64), and a mean household income of $94,681.86 (standard deviation=$47,627.84).
Figure 1.
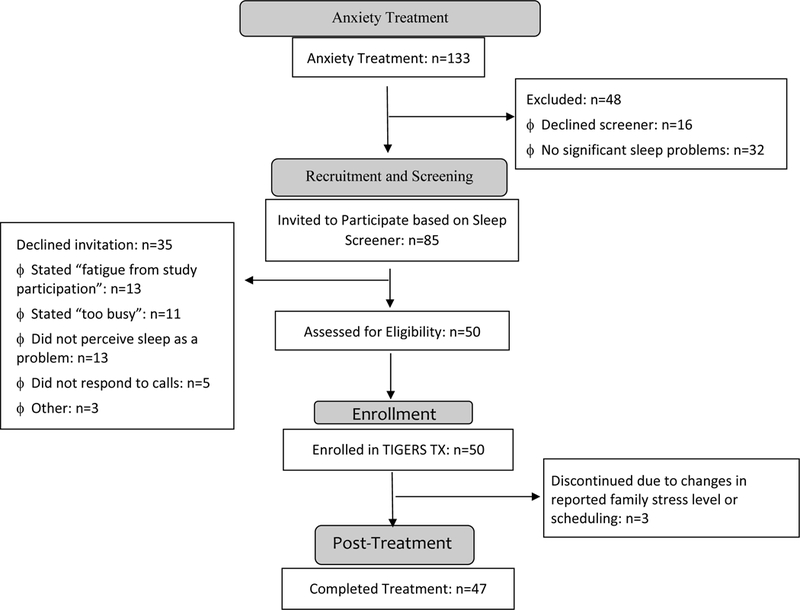
Diagram of Participant flow through Sleeping TIGERS
Measures
Subjective sleep-related problems (parent and child report), objective sleep patterns (actigraphy) and subjective sleep patterns (diary).
Measures were identical to those administered in Study 1. With regard to the number of nights of actigraphy included in sampling for Study 2, 70.4% had five nights of recordings, 22% had four, 4% had three, and 4% had 1 (M =4.56, SD=0.88). With respect to number of sleep diaries, 83.3% had five nights, 12.5% had four, 2% had three, and 2% had one (M=4.75; SD=0.70).
Clinical assessment of sleep.
The Focal Interview of Sleep (FIOS) is a clinician-rated semi-structured interview developed as part of this trial to assess sleep disturbance in children and adolescents. The evaluator seeks an initial qualitative description of the most concerning parent-reported sleep problem in an open-ended format. This is followed by a series of yes or no questions regarding common sleep concerns (i.e., difficulty going to bed, difficulty falling asleep, nighttime waking, difficulty waking, daytime sleepiness, irregular sleep schedules (>2 hours difference in sleep or wake times across a 1 week period), sleep walking, bedwetting, and nightmares, or other concerns). For each sleep concern endorsed by either parent or child, the evaluator asks more detailed questions (i.e., operational definition, level of concern about sleep problem, onset, frequency, duration, intensity, triggers, and protective factors). Parent and child are interviewed separately, and all sleep concerns endorsed by either party are pursued in detail with both parties. The interviewer provides a final rating based on information from both parent and child. Thirty-six percent of the videotapes were viewed and rated by an independent rater; inter-rater reliability was strong, with Intra-class Correlations (ICC) ranging from.79-.98, with the exception of concern about wake after sleep onset, which had an ICC of.57. Means and standard deviations for FIOS outcome variables at study 2 baseline are as follows: IE Sleep Problems: 3.12 (1.20); Concern: 2.85 (0.62); Intensity: 2.75 (0.59), Frequency: 6.76 (2.53), Functional Impairment: 2.43 (0.81); Duration 26.71 (18.44).
Procedure
Those who joined Study 2 received a pre-treatment assessment by an independent evaluator (IE). Sleeping TIGERS (described below) began within 2 weeks of pre-assessment, with delivery by a different therapist than in Study 1, with the exception of 1 participant who refused a new therapist and was in need of services so an exception was made. Following the intervention, participants received a post-treatment assessment.
Intervention.
Sleeping TIGERS (Dahl et al., 2009) is a six-to-eight session sleep enhancement program using a motivational framework to target sleep-wake regulation and related behaviors. The range of sessions is to accommodate varying needs where the last 2 sessions are used to practice and reinforce material as needed (clinicians made this decision at session 4 supervision based on progress and symptoms). The range was particularly important in the current trial to provide flexibility given that these youth had already completed 16 sessions of anxiety treatment and were sometimes doing quite well by session 6. Forty-seven of 50 intent-to-treat participants (94%) completed six or more sessions of Sleeping TIGERS (13 completed 6, 15 completed 7 and 19 completed 8). The majority of the sample (84.2%) completed treatment during the school year. The 3 non-completers reported family stress or busy schedules as reasons for withdrawing from treatment.
The intervention targets thoughts, feelings, and behaviors at bedtime (e.g. stimulus control, reducing negative affective stimuli from scary movies, family conflict, social media etc.), personal motivation, development and maintenance of good habits (e.g. stimulus control, reducing caffeine, increasing physical activity), sleep regularity (e.g. maintaining consistent wake time, using light-dark cues to entrain rhythms), media use at night (e.g. establishing media-curfew, dimming light emitted from media), and pre-sleep anxiety and rumination (e.g. savoring positive moments at bedtime on a “mental television”, and learning to “switch” the channel when youth find they are “stuck” on a worry or rumination channel when it is time to go to sleep). The majority of the behavioral strategies were drawn from established behavioral treatments (e.g. CBT for Insomnia), while others were developed as part of this trial and tailored to specific needs of this population (e.g. pre-sleep savoring, “media curfew”, reducing high social and affective stimulation at bedtime).
Treatment Integrity.
Treatment integrity was managed via 3 strategies. First, multiday therapist training workshops were conducted at the start of the study and approximately 1 year after study start. Both workshops involved a specific focus on promoting adherence and on delivering treatment with a high level of fidelity. Second, fidelity was a topic covered within the weekly supervision sessions which were led by 3 sleep experts who developed the manual (Dahl, Harvey, and McMakin). Additionally, via a random numbers generator (with non-replacement), 10% of sessions were rated for treatment integrity and fidelity by expert therapists using standardized checklists to indicate whether appropriate content was covered. Approximately 7% of these ratings were conducted during the study to reduce drift, and the remaining 3% were conducted following the trial. All sessions were included in this random review. Ratings indicated that 96% of session minutes and 97% of overall sessions reflected high fidelity.
Analytic plan
MLM was performed as described in Study 1. Measures and time-points for Study 2 included parent and child self-report, sleep diary, actigraphy, and clinician-rated sleep disturbance at baseline and post-treatment. Study 2 included only 2 time-points, thus ruling out the use of pattern mixture models. Instead, missing data were coded dichotomously and t-tests were conducted to examine if missingness was associated with baseline characteristics such as symptom severity or treatment disposition.
Study 2 Results
Preliminary analyses
Descriptive statistics and plots of normality resulted in the same changes described in Study 1. All analyses were conducted with outlier-corrected data (outliers moved to within 1.5 interquartile range) and there were no changes to outcomes; thus these analyses are not presented in text (available from author). There were no significant t-tests for missingness predicting symptom severity or treatment disposition.
Primary Analyses
Change in parent- and child-reported sleep-related problems during Sleeping TIGERS.
There were large effects on parent and child-reported sleep (Cohen’s d=1.15 and 0.83 respectively; See Tables 1 and 3 for descriptives and statistics). Specifically, results showed a significant reduction in child reported sleep disturbance from baseline to post-treatment (t=−5.77, p<0.001). Estimated marginal means were 38.76 (baseline) and 34.43 (post-treatment). There was a significant change in parent-reported sleep-related problems during the Sleeping TIGERS intervention, such that scores at post-treatment were significantly lower than at baseline (t=−8.61, p=<0.001). Estimated marginal means were 51.14 (baseline) and 42.70 (post-treatment).
Table 3.
Study 2 Linear mixed model summary for effects of Sleeping TIGERS on sleep-related problems and patterns
| Intercept | Post-Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Coefficient (SE) | t | Confidence Interval |
Coefficient (SE) | t | Confidence Interval |
Post-Tx Estimated Marginal Mean |
d |
| CSHQ Total | 47.28 (7.69)*** | 6.15 | 31.76 – 62.79 | −8.45 (0.98)*** | −8.61 | −10.43 – −6.46 | 42.70 (1.27) | 1.15 |
| a. Bedtime Resistance | 11.99 (2.03)*** | 5.91 | 7.88 – 16.09 | −1.93 (0.42)*** | −4.57 | −2.79 – −1.07 | 6.62 (0.38) | 0.89 |
| b. Sleep Onset Delay | 1.24 (0.66) | 1.89 | −0.09 – 2.57 | −0.70 (0.13)*** | −5.46 | −0.96 – −0.44 | 1.35 (0.12) | 1.02 |
| c. Sleep Duration | 1.38 (1.29) | 1.07 | −1.23 – 3.99 | −1.68 (0.21)*** | −7.92 | −2.11 – −1.25 | 4.47 (0.22) | 1.30 |
| d. Sleep Anxiety | 11.08 (1.96)*** | 5.65 | 7.12 – 15.05 | −1.04 (0.24)*** | −4.36 | −1.53 – −0.56 | 5.11 (0.32) | 0.56 |
| e. Night Waking | 5.08 (1.61)** | 3.15 | 1.82 – 8.34 | −0.60 (0.18)*** | −3.33 | −0.97 – −0.23 | 3.98 (0.26) | 0.40 |
| f. Parasomnias | 9.83 (1.27)*** | 7.74 | 6.75 – 11.89 | −0.79 (0.21)*** | −3.73 | −1.23 – −0.36 | 8.03 (0.22) | 0.62 |
| g. Sleep-disordered Breathing |
3.19 (0.33)*** | 9.73 | 2.52 – 3.85 | −0.08 (0.06) | −1.23 | −0.21 – 0.05 | 3.05 (0.06) | 0.23 |
| h. Daytime Sleepiness | 7.55 (3.53) | 2.14 | 0.43 – 14.67 | −2.31 (0.57)*** | −4.07 | −3.46 – −1.16 | 12.27 (0.61) | 0.66 |
| SSR Total | 38.89 (5.44)*** | 7.15 | 27.92 – 49.86 | −4.33 (0.75)*** | −5.77 | −5.86 – −2.81 | 34.43 (0.91) | 0.83 |
| Sleep Diary | ||||||||
| a. Sleep Latency OnsetLN | 2.89 (0.62)*** | 4.69 | 1.64 – 4.14 | −0.14 (0.11) | −1.29 | −0.36 – 0.08 | 2.67 (0.11) | 0.24 |
| b. Wake After SleepLN Onset | 0.62 (1.58) | 0.39 | −2.60 – 3.83 | 0.19 (0.22) | 0.85 | −0.27 – 0.64 | 1.38 (0.26) | −0.14 |
| c. Sleep Quality | 81.50 (21.01)*** | 3.88 | 38.93 – 124.06 | 4.85 (2.32)* | 2.09 | 0.12 – 9.57 | 77.27 (3.40) | −0..26 |
| d. Difficulty Waking | 97.14 (20.60)*** | 4.72 | 55.72 – 138.75 | 5.30 (3.57) | 1.49 | −1.94 – 12.53 | 70.99 (3.58) | −0.27 |
| e. Midpoint PeriodLN | 5.26 (0.17)*** | 31.03 | 4.92 – 5.60 | −0.01 (0.04) | −0.22 | −0.09 – 0.07 | 5.14 (0.03) | 0.05 |
| f. Total Sleep Time | 798.84 (43.21)*** | 18.49 | 711.42 – 886.26 | −5.51 (8.29) | −0.66 | −22.36 – 11.33 | 534.80 (7.71) | 0.13 |
| Actigraphy | ||||||||
| a. Sleep OnsetLN Latency | 3.11 (0.63)*** | 4.93 | 1.83 – 4.39 | 0.03 (0.11) | 0.31 | −0.19 – 0.26 | 2.89 (0.11) | −0.06 |
| b. Wake After SleepLN Onset | 4.56 (0.73)*** | 6.17 | 3.06 – 6.06 | −0.11 (0.15) | −0.77 | −0.41 – 0.19 | 3.44 (0.14) | 0.13 |
| c. Midpoint of SleepLN Period | 5.03 (0.28)*** | 18.17 | −4.47 – 5.60 | 0.03 (0.04) | 0.62 | −0.06 – 0.11 | 5.37 (0.05) | −0.10 |
| d. Total Sleep Time | 650.06 (41.94)*** | 15.50 | 565.11 – 735.02 | −1.93 (7.95) | −0.24 | −18.15 – 14.30 | 475.71 (7.55) | 0.05 |
| IE FIOS | ||||||||
| a. # Problems | 2.03 (0.89)* | 2.28 | 0.28 – 3.79 | −1.72 (0.27)*** | −6.27 | −2.26 – −1.18 | 1.56 (0.19) | 1.53 |
| b. Concern | 2.10 (0.59)*** | 3.56 | 0.94 – 3.25 | −1.20 (0.12)*** | −10.20 | −1.43 – 0.96 | 1.67 (0.10) | 2.04 |
| c. Intensity | 2.64 (0.55)*** | 4.85 | 1.58 – 3.71 | −0.83 (0.15)*** | −5.34 | −1.13 – −0.52 | 1.92 (0.11) | 1.32 |
| d. Frequency | 1.45 (2.58) | 0.56 | −3.60 – 6.50 | −3.01 (0.44)*** | −6.90 | −3.86 – −2.15 | 3.65 (0.42) | 1.23 |
| e. Impairment | 1.28 (0.68) | 1.89 | −0.05 – 2.61 | −0.86 (0.16)*** | −5.31 | −1.17 – −0.54 | 1.58 (0.12) | 1.21 |
| f. Duration | 14.00 (16.93) | 0.83 | −19.19 – 47.18 | −7.28 (2.57)** | −2.83 | −12.32 – −2.23 | 19.60 (2.70) | 0.46 |
Note. SE. = Standard Error; CSHQ = Children’s Sleep Habits Questionnaire; SSR = Sleep Self Report; Tx. = Treatment; IE = Independent Evaluator; results for covariates (gender, age, and number of school nights) are not reported in the table. Random effects, including variance of intercept and autoregressive model of order 1 (AR1) residual covariance parameters (to correct for autocorrelation between time points) were included in the model but are not reported in the table. Estimated marginal means and standard errors are reported to guide interpretation of direction of effects relative to baseline.
p<.05,
p<.01,
p<.001;
=Sleep diary and actigraphy variables were logarithmically transformed.
As a post-hoc follow-up to effects on parent reported CSHQ total scores, we explored changes in subscales. There was a significant pre-post reduction in Bedtime Resistance (z = −4.57, p <.001), Sleep Onset Delay (t = −5.46, p <.001), Sleep Duration (t=−7.92, p<.001), Sleep Anxiety (t=−4.36, p<.001), Night Wakings (t=−3.33, p=.001), Parasomnias (t=−3.73, p<.001), and Daytime Sleepiness (t=−4.07, p<.001). No significant reduction was found for Sleep Disordered Breathing (t=−1.23, p=.23). See Table 3 for details.
Analysis of clinical impact.
McNemar’s test was used to assess change in the proportion of youth greater than or equal to the clinical cutoff of 41 on the CSHQ from baseline to post-treatment. Results indicated this proportion significantly decreased from 90.0% at baseline to 67.5% at post-treatment p=.01 (see Figure 2). Figure 2 also provides a trajectory of parent reported sleep for the Study 2 sample that indicates an acceleration of sleep improvement upon the introduction of sleep intervention, relative to anxiety treatment.
Figure 2.
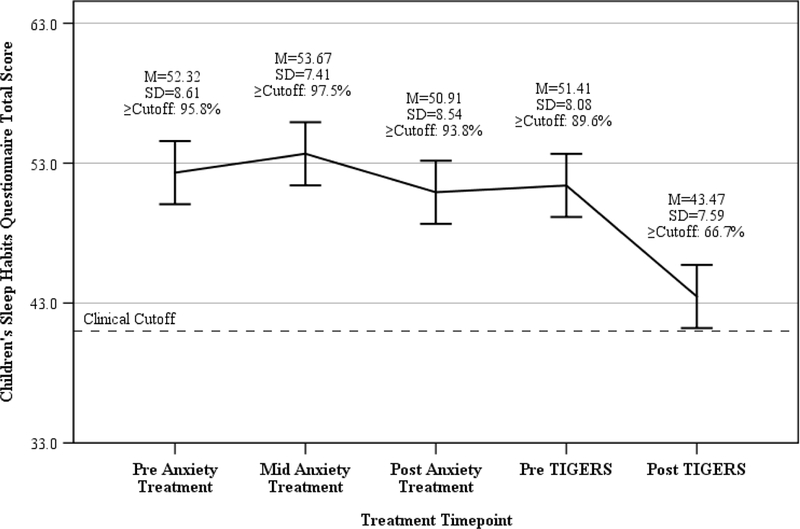
Change in parent reported sleep for the Study 2 sample across anxiety treatment and Sleeping TIGERS intervention
Change in subjective sleep patterns (diary) through Sleeping TIGERS.
Significant reductions from baseline to post-treatment were found for Sleep Quality (t=2.09, p<.05). No other sleep diary indices significantly changed over the course of treatment (see Table 3).
Change in objective sleep patterns (actigraphy indices) through Sleeping TIGERS.
There were no significant changes observed for actigraphy indices (see Table 3).
Change in independent evaluator ratings of sleep.
FIOS variables showed significant reductions from pre- to post- Sleeping TIGERS: Clinician-rated Number of Sleep Problems (t=−6.27, p<.001), Concern (t=−10.20, p<.001), Intensity (t=−5.34, p<.001), Frequency (t=−6.90, p<.001), Impairment, (t=−5.31, p<0.001), and Duration (t=−2.83, p=0.01). See Table 3.
Discussion
The current studies examined whether sleep improves among anxious peri-pubertal youth in response to anxiety treatment (Study 1), and if a developmentally-informed sleep intervention can further improve sleep among youth with residual sleep disturbance following treatment for anxiety (Study 2). These questions are critical to address during the peri-pubertal period due to: 1) the confluence of developmental changes around the onset of puberty that lead to increases in both sleep disturbance and affective problems, and 2) the established prospective links between sleep disturbance and increasing internalizing symptoms (anxiety, depression) in adolescence.
Overall Summary of Findings
Study 1 demonstrated improvements in child- and parent-reported sleep disturbance over the course of anxiety treatment; however, the effect sizes were small and a post-hoc test of clinical impact was not significant. In fact, 75% of youth remained above the clinical cutoff on the parent report of sleep problems at the end of anxiety treatment. In Study 2, an open trial of a sleep enhancement intervention (Sleeping TIGERS, n=50) that followed anxiety treatment, we demonstrated large within-subject effects on multiple indices of sleep disturbance (e.g. parent and child report of sleep-related problems, subjective sleep patterns [sleep diary], and a semi-structured interview of sleep disturbance), and our post hoc test of clinical impact was significant. Notably, the sleep intervention had a 94% completion rate, supporting acceptability and feasibility of the intervention.
Study 1 Discussion of Key Findings
Similar to prior reports indicating high prevalence of sleep-related problems among youth with anxiety (Alfano et al., 2007; Chase & Pincus, 2011), 80% of anxious youth were above the clinical cutoff on the parent report of sleep problems (CSHQ). Over the course of anxiety treatment, there was a significant reduction in parent and child reported sleep-related problems, with parent reports showing a reduction from baseline to post treatment (but not mid-treatment), and child reports showing a reduction from baseline to midpoint and post-treatment. An exploration of subscales on the parent report indicated small effects on bedtime resistance, sleep onset latency, sleep anxiety, and parasomnias, while there were no observed effects on sleep duration, wake after sleep onset, sleep disordered breathing or daytime sleepiness. This pattern of results is consistent with prior research indicating that anxiety treatment imparts strongest impacts on bedtime resistance, sleep anxiety, and pre-sleep arousal (Clementi et al., 2016; Peterman et al., 2016).
There were also small improvements in subjective sleep patterns (diary). Specifically, sleep quality showed improvements relative to baseline at post-treatment (but not weeks 4, 8 or 12). There were also improvements in subjective sleep transitions and maintenance. Average wake after sleep onset was reduced from baseline to week 12 and post-treatment. Sleep latency onset was significantly reduced at weeks 4, 8, and 12, though effects were no longer significantly different from baseline at post-treatment. This could be due to relatively small effect sizes making significance unreliable, and/or a slight uptick in difficulty falling asleep due to the end of anxiety treatment.
Overall, these changes in sleep over the course of anxiety treatment are noteworthy given that sleep was not explicitly targeted, thus reducing associated demand characteristics. However, enthusiasm for these outcomes is tempered by the fact that this was an open trial, within-subject effect sizes were mostly small, similar to several recent studies (Caporino et al., 2015; Clementi et al., 2016; Peterman et al. 2016), and our test of clinical impact was not significant. This is salient given that response and full remission from anxiety was achieved in 65% and 57% of the sample, respectively (Silk et al., 2016), yet sleep problems above a clinical cutoff persisted for 75% of youth at post anxiety treatment (relative to 80% at baseline and 84% at mid-treatment).
Study 2 Discussion of Key Findings
In light of ongoing sleep-related problems following anxiety treatment in Study 1 and the known prospective risk associated with sleep-related problems in adolescence, the focus of Study 2 is contextualized as a critical question. That is, can a developmentally informed sleep intervention improve sleep? In the open trial, Sleeping TIGERS demonstrated large within-subject effects on parent and child report of sleep-related problems. Moreover, the effects on the subscales of the parent report of sleep problems (CSHQ) were moderate-large and were significant for all subscales except for Disordered Breathing. Finally, post-hoc tests supported a significant clinical impact on parent reported sleep-related problems, with a reduction in the percentage of youth greater than or equal to clinical cutoff on CSHQ (parent report) from 90% at pre-sleep intervention to 68% at post sleep intervention.
It is notable that despite a substantial and clinically significant reduction in parent reported sleep problems, 68% of the sample remained above clinical cut-off on CSHQ. As noted by past research, this cut-point may not be optimized for a clinical population (Langberg et al., 2017), and/or the high rates of insufficient sleep in adolescent populations may suggest that being above a clinical cut-off has become somewhat normative. Nevertheless, it is clear that insufficient sleep portends a number of risk factors for anxious youth (McMakin and Alfano, 2015), and therefore remains a critical intervention target.
There were also large effects from pre- to post- sleep intervention on subjective diary reports for sleep quality. There were no changes in total sleep time or midpoint of sleep. There were also no changes in actigraphy indices. Finally, the Focal Interview of Sleep (FIOS) semi-structured interview indicated large within-subject effects on the total number of sleep problems reported, as well as reductions in concern related to sleep problems, and reductions in frequency, duration and intensity of sleep problems.
It is noteworthy that Study 1 and Study 2 failed to detect impacts on actigraphy estimates of sleep patterns. The discordance in subjective and objective indices is consistent with prior literature, and has been discussed in detail in a recent review (McMakin & Alfano, 2015). In short, discordance may be due to methodological challenges (e.g. it is difficult to measure sleep transitions using actigraphy), differences in perception (anxious youth may hold perceptual biases), or demand characteristics (e.g. social desirability) driving subjective differences in the absence of objective change. Finally, it is possible that the 5-night assessment period was not long enough to reliably detect effects (see Limitations below). These will be important issues to unpack in future research. Regardless of the underlying cause of discordance, subjective sleep problems and patterns such as those described here are prospective predictors of emotional adjustment (El-Sheikh, Bub, Kelly, & Buckhalt, 2013) and mental health outcomes (e.g. depression; Gregory, Rijsdijk, Lau, Dahl, & Eley, 2009) such that changes in these dimensions may be important with or without convergence with actigraphy.
Study 1 and 2 Limitations and Contributions
There were several limitations to the current studies. First, our diary and actigraphy estimates were based on five-day assessment periods, with a notable proportion of the samples (34% in Study 1 and 30% in Study 2) having fewer than five nights. Monitoring periods included weekends and also could have been obtained during the school year or during periods of holiday. As such, this introduces noise into the diary and actigraphy sleep estimates. We included the number of school nights (versus non-school nights) in each assessment period as a covariate in order to account for relevant variance in sleep estimates. These design decisions regarding assessment periods were due to practical constraints that included 1) a need to recruit year round to maintain flow and address clinical concerns, and 2) a need to minimize burden of data collection for families in this multi-method study. Future work could benefit from a longer assessment period, and careful timing of intervention during the school year. Second, our sample is comprised of >80% white and mostly middle class youth. This is a significant limitation and is particularly notable in light of research demonstrating how challenging it is to disseminate empirically supported treatments that are developed in academic centers with narrow demographic sampling. More work will need to be done to test the potential generalizability and dissemination of this treatment into more diverse community settings. Third, although Study 1 and Study 2 drew from the same initial sample of anxious youth, it was not possible to compare youth who did or did not participate in sleep intervention because the sleep intervention was not randomly assigned. Rather, eligible participants were invited to participate and then self-selected into the intervention—those who chose not to participate often cited fatigue from study participation or busy schedules. This necessarily tempers our conclusions regarding the high completion rate of 94%. Furthermore, there was no active control condition for either study, making it impossible to conclude that the effects on sleep were related to the intervention rather than the passage of time or other factors. This limitation is somewhat assisted by data in Figure 2 that show the change in sleep among Study 2 participants accelerated upon the introduction of sleep intervention, though demand characteristics (i.e. social desirability) may still be a factor. These design decisions were necessary to manage multiple priorities in this multiple project center grant.
Despite these limitations, our questions regarding whether or not anxiety treatment resolves sleep disturbance, and whether or not it is possible to modify sleep following anxiety treatment, garner preliminary support by the design and data presented here. These studies contribute to the literature by overcoming multiple past research limitations. Specifically, the studies address limitations of prior work by including a large sample of treatment seeking anxious peri-pubertal youth early in the transition to the high risk period of adolescence (Study 1: n=133; Study 2: n=50), an a priori focus on sleep with a multimodal assessment of subjective and objectively sleep related problems and patterns (retrospective parent and child report, actigraphy, diary, semi-structured interview) across multiple informants (parent, child, independent evaluator) at multiple time-points across treatment. In addition, a targeted sleep intervention (Sleeping TIGERS) was examined in youth already treated for anxiety, allowing for an evaluation of the impact of a targeted sleep intervention on sleep disturbance that was resistant to efficacious anxiety treatment—to our knowledge, this type of study has not been done previously and therefore provides novel insights regarding the potential for targeted sleep intervention to resolve residual symptoms.
Overall Clinical Implications and Future Directions
If these findings continue to garner support in RCT design, they will carry important clinical implications. That is, although anxiety treatment does improve aspects of sleep, the effects are small and not clinically significant. Addressing residual sleep disturbance in anxious youth is therefore a clinical issue of paramount importance with potential to meaningfully reduce risk for increasing emotional problems (e.g. depression) in adolescence. Our preliminary support for Sleeping TIGERS suggests feasibility and clinical potential to successfully modify sleep in this population via behavioral intervention. A randomized controlled trial is an essential next step to establish efficacy.
Future work may examine whether modifying sleep during this sensitive peri-pubertal period improves developmental trajectories of emotional adjustment and mental health. The current studies were embedded in a larger center grant that includes multi-method assessment of affective functioning (fMRI, ecological momentary assessment, parent-child interactions), as well as long-term follow-up of symptoms and functional outcomes (5 years), such that the current studies set the stage for deeper investigations of the impact of sleep on long-term emotional adjustment and mental health. Also, future work may identify a specific developmental window during the adolescent transition when behavioral sleep intervention can impart greatest impacts on development, and therefore be prioritized in clinical decision making. Finally, future work could examine whether delivering a sleep-targeted intervention in parallel to anxiety treatment, versus serially, would yield highest clinical impact.
Acknowledgments
The research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number MH080215. The content is the responsibility of the authors and is not necessarily representative of the views of the National Institutes of Health.
References
- Ahmed SP, Bittencourt-Hewitt A, & Sebastian CL (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CA, Ginsburg GS, & Kingery JN (2007). Sleep-related problems among children and adolescents with anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 46, 224–232. [DOI] [PubMed] [Google Scholar]
- Alfano CA, Reynolds K, Scott N, Dahl RE, & Mellman TA (2013). Polysomnographic sleep patterns of non-depressed, non-medicated children with generalized anxiety disorder. Journal of Affective Disorders, 147, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Becker SP, Sidol CA, Van Dyk TR, Epstein JN, & Beebe DW (In press). Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: A systematic review. Sleep Medicine Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Byrne ML, Ivens C, Waloszek J, Woods MJ, Dudgeon P, … Allen NB (2013). Pilot study of a mindfulness‐based, multi‐component, in‐school group sleep intervention in adolescent girls. Early Intervention in Psychiatry, 7, 213–220. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Dahl RE, Williamson DE, Iosif A, Birmaher B, Axelson D, & Ryan ND (2005). Subjective sleep complaints in pediatric depression: A controlled study and comparison with EEG measures of sleep and waking. Journal of the American Academy of Child & Adolescent Psychiatry, 44, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Buysse DJ (2014). Sleep health: Can we define it? Does it matter? Sleep, 37, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain N, Gradisar M, & Moseley L (2011). A motivational school-based intervention for adolescent sleep problems. Sleep Medicine, 12, 246–251. [DOI] [PubMed] [Google Scholar]
- Caporino NE, Brodman DM, Kendall PC, Albano AM, Sherrill J, Piacentini J, … Walkup JT (2013). Defining treatment response and remission in child anxiety: signal detection analysis using the pediatric anxiety rating scale. Journal of the American Academy of Child and Adolescent Psychiatry, 52, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporino NE, Read KL, Shiffrin N, Settipani C, Kendall PC, Compton SN, … Albano AM (2015). Sleep-related problems and the effects of anxiety treatment in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 14, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA (2011). Sleep in adolescents: The perfect storm. Pediatric Clinics of North America, 58, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase RM, & Pincus DB (2011). Sleep-related problems in children and adolescents with anxiety disorders. Behavioral Sleep Medicine, 9, 224–236. [DOI] [PubMed] [Google Scholar]
- Clementi MA, & Alfano CA (2014). Targeted behavioral therapy for childhood generalized anxiety disorder: A time-series analysis of changes in anxiety and sleep. Journal of Anxiety Disorders, 28, 215–222. [DOI] [PubMed] [Google Scholar]
- Clementi MA, Alfano CA, Holly LE, & Pina AA (2016). Sleep-related outcomes following early intervention for childhood anxiety. Journal of Child and Family Studies, 25, 3270–3277. [Google Scholar]
- Cohen JA, Deblinger E, Mannarino AP, Steer RA (2004). A multisite, randomized controlled trial for children with sexual abuse–related PTSD symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 43, 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, Harvey A, Forbes E, McMakin D, Milbert M, & Trubnick L (2009). Sleeping TIGERS: a treatment for sleep problems in young people Treatment manual. Pittsburgh, PA: University of Pittsburgh. [Google Scholar]
- de Bruin EJ, Bögels SM, Oort FJ, & Meijer AM (2015). Efficacy of cognitive behavioral therapy for insomnia in adolescents: A randomized controlled trial with internet therapy, group therapy and a waiting list condition. Sleep, 38, 1913–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan CL, Spence SH, & March S (2017). Does an online CBT program for anxiety impact upon sleep problems in anxious youth? Journal of Clinical Child & Adolescent Psychology, 46, 211–221. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Bub KL, Kelly RJ, & Buckhalt JA (2013). Children’s sleep and adjustment: A residualized change analysis. Developmental Psychology, 49, 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Bertocci MA, Gregory AM, Ryan ND, Axelson DA, Birmaher B, & Dahl RE (2008). Objective sleep in pediatric anxiety disorders and major depressive disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 47, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Rijsdijk F, Lau JY, Dahl RE, & Eley TC (2009). The direction of longitudinal associations between sleep problems and depression symptoms: A study of twins aged 8 and 10 years. Sleep, 32, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, & Hedtke KA (2006a). Cognitive-behavioral therapy for anxious children: Therapist manual. Ardmore, PA: Workbook Publishing. [Google Scholar]
- Kendall P & Hedtke K (2006b). Coping cat workbook (Child therapy workbook series 2nd ed). Ardmore. PA: Workbook Publishing; 2006. [Google Scholar]
- Kendall PC, & Pimentel SS (2003). On the physiological symptom constellation in youth with generalized anxiety disorder (GAD). Journal of Anxiety Disorders, 17, 211–221. [DOI] [PubMed] [Google Scholar]
- Langberg JM, Molitor SJ, Oddo LE, Eadeh H, Dvorsky MR, & Becker SP (2017). Prevalence, patterns and predictors of sleep problems and daytime sleepiness in young adolescents with ADHD. Journal of Attention Disorders. doi: 10.1177/1087054717690810. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- McMakin DL, & Alfano CA (2015). Sleep and anxiety in late childhood and early adolescence. Current Opinion in Psychiatry, 28, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley L, & Gradisar M (2009). Evaluation of a school-based intervention for adolescent sleep problems. Sleep, 32, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, & McGuinn M (2000a). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23, 1043–1051. [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M, & Nobile C (2000b). Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental and Behavioral Pediatrics, 21, 27–36. [DOI] [PubMed] [Google Scholar]
- Peterman JS, Carper MM, Elkins RM, Comer JS, Pincus DB, & Kendall PC (2016). The effects of cognitive-behavioral therapy for youth anxiety on sleep problems. Journal of Anxiety Disorders, 37, 78–88. [DOI] [PubMed] [Google Scholar]
- Powers A, & Casey B (2015). The adolescent brain and the emergence and peak of psychopathology. Journal of Infant, Child, and Adolescent Psychotherapy, 14, 3–15. [Google Scholar]
- Sadeh A (2011). The role and validity of actigraphy in sleep medicine: An update. Sleep Medicine Reviews, 15, 259–267. [DOI] [PubMed] [Google Scholar]
- Schlarb AA, Liddle CC, & Hautzineger M (2011). JuSt – a multimodal program for treatment of insomnia in adolescents: A pilot study. Nature and Science of Sleep, 3, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Tan PZ, Ladouceur CD, Meller S, Siegle GJ, McMakin DL, … Ryan ND (2016). A randomized clinical trial comparing individual cognitive behavioral therapy and child-centered therapy for child anxiety disorders. Journal of Clinical Child & Adolescent Psychology, 16, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Friedmann E & Thomas SA Application of pattern mixture models to address missing data in longitudinal data analysis using SPSS. Nursing Research, 61(3), 195–203. [DOI] [PubMed] [Google Scholar]


