Abstract
Infections with Neisseria gonorrhoeae, a sexually transmitted pathogen that causes urethritis, cervicitis, and more severe complications, are increasing. Gonorrhea is typically treated with antibiotics; however, N. gonorrhoeae has rapidly acquired resistance to many antibiotic classes, and lineages with reduced susceptibility to the currently recommended therapies are emerging worldwide. In this review, we discuss the contributions of whole genome sequencing (WGS) to our understanding of resistant N. gonorrhoeae. Genomics has illuminated the evolutionary origins and population structure of N. gonorrhoeae and the magnitude of horizontal gene transfer within and between Neisseria species. WGS can be used to predict susceptibility of N. gonorrhoeae based on known resistance determinants, track the spread of these determinants throughout the N. gonorrhoeae population, and identify novel loci contributing to resistance. WGS has also allowed more detailed epidemiological analysis of transmission of N. gonorrhoeae between individuals and populations than previously used typing methods. Ongoing N. gonorrhoeae genomics will complement other laboratory techniques to understand the biology and evolution of the pathogen, improve diagnostics and treatment in the clinic, and inform public health policies to limit the impact of antibiotic resistance.
Keywords: gonorrhea, whole genome sequencing, antibiotic resistance, epidemiology
Graphical Abstract
Over the 20 years since the first gonococcal genome was sequenced, 15 finished genomes, 413 draft genome assemblies, and 4795 sequencing runs have been made publicly available. Here, we review the background on gonorrhea, resistant gonococcus, and the ways in which researchers are exploring how to integrate these data to advance clinical care and public health management of gonorrhea.
Introduction
Gonorrhea, caused by Neisseria gonorrhoeae, is the second most common sexually transmitted infection (STI) with 468,514 reported cases in the United States in 20161 and an estimated 78 million cases worldwide annually2. N. gonorrhoeae infection rates in the United States have increased yearly since 2013, and rate increases are larger in men than women1. N. gonorrhoeae is particularly prevalent in men who have sex with men (MSM), and 37.8% of isolates were collected from MSM during surveillance in the United States in 20163.
Bacterial pathogens are becoming increasingly resistant to antibiotics, and N. gonorrhoeae is no exception. N. gonorrhoeae has developed resistance to every antibiotic used for treatment through a variety of mechanisms (Table 1), and dual therapy with ceftriaxone and azithromycin is currently recommended based on the hypothesis that the two drugs together will help to curb the spread of resistant lineages4. With the dearth of antibiotic options for gonorrhea and concern for rising levels of resistance in this highly prevalent infection, the Centers for Disease Control and Prevention (CDC) highlighted N. gonorrhoeae resistant to cephalosporins as one of three urgent threats among antibiotic resistant bacteria5.
Table 1.
Mechanisms of antibiotic resistance in Neisseria gonorrhoeae
| Drug | MIC breakpoints137 (μg/mL) S, R | Gene (codon/allele) | Mechanism | Year |
|---|---|---|---|---|
| Sulfonamides | folP (R228)138,139 | TM | 2005 | |
|
| ||||
| Penicillin (PEN) | ≤0.06, ≥2 | penA45,140 | TM | 1975 |
| porB (G120K, A121D/N)140,141 | P | 1975 | ||
| mtrR (A39T, G45D, 1 bp del in promoter)140,142–144 | E | 1975 | ||
| blaTEM61 | I | 1976 | ||
| ponA (L421P)87 | TM | 2002 | ||
| pilQ (E666K)87,88 | P | 2002 | ||
|
| ||||
| Tetracycline (TET) | ≤0.25, ≥2 | porB (G120K, A121D/N)140,141 | P | 1975 |
| mtrR (A39T, G45D, 1 bp del in promoter)140,142–144 | E | 1975 | ||
| tetM32 | TM | 1986 | ||
| pilQ (E666K)87,88 | P | 2002 | ||
| rpsJ (V57M)145,146 | TM | 1974 | ||
|
| ||||
| Ciprofloxacin (CIP) | ≤0.06, ≥1 | gyrA (S91F, D95A/N/G)147,148 | TM | 1994 |
| parC (D86N, S87R/N, S88P, E91K)147,149 | TM | 1994 | ||
| parE (G410V)148 | TM | 2002 | ||
| norM (−35 promoter sequence, RBS)150 | E | 2003 | ||
|
| ||||
| Spectinomycin | ≤32, ≥128 | rpsE (T24P, deletion V27, A82G)151,152 | TM | 2013 |
| 16S rDNA (C1187)153 | TM | 2000 | ||
|
| ||||
| Azithromycin (AZI) | mtrR (A39T, G45D, 1 bp del in promoter)140,142–144 | E | 1975 | |
| mosaic mtr operon47 | E | 2016 | ||
| ermBCF57 | I | 1999 | ||
| 23S rDNA (C2611T, A2059G)154,155 | TM | 2002 | ||
| mef156 | E | 2000 | ||
| macAB (−10 promoter sequence)150 | E | 2003 | ||
| rplV (3′ tandem duplications)22 | TM | 2016 | ||
| rplD (G68, G70)22 | TM | 2016 | ||
|
| ||||
| Cefixime (CFX) | ≤0.25, - | mosaic penA46,157 | TM | 2002 |
|
| ||||
| Ceftriaxone (CRO) | ≤0.25*, - | mosaic penA157 | TM | 2005 |
| porB158 | P | 2009 | ||
| mtrR158 | E | 2009 | ||
TM = target modification, P = permeability, E = efflux, I = inactivation.
Reduced susceptibility to CRO is also described as ≥0.125 μg/mL
The World Health Organization (WHO) has identified several strategies to control the spread of antibiotic resistant N. gonorrhoeae, centering on improved methods for diagnosis, strengthened detection and surveillance of resistance, and identification of new treatment strategies2. Current challenges include rapid identification of gonococcal infection and antibiotic susceptibility, determination of transmission links between individuals and populations, and tailoring treatment and intervention strategies to optimally slow or contain the spread of antibiotic resistance (Box 1).
Box 1. Current challenges and contributions of WGS at different scales.
Neisseria gonorrhoeae populations
Identification of novel resistance and compensatory mutations
Characterize selective pressures and fitness costs
Identify targets for vaccines
Frequency of AMR acquisition and interspecies mosaicism
Roles of major and minor within-host populations in transmission and susceptibility
In the clinic
Rapidly identify infection and colonization
Identify susceptibility of strains and appropriate antibiotic treatment
Identify clinically relevant mixed infections
Local transmission networks
Identify links among individuals
Tailor antibiotic use at local scale
Translation to local policies and interventions
Global Scales
Identify links among populations
Tailor antibiotic use at global scale
Translation to global policies and interventions
Whole genome sequencing (WGS), along with other methodological and technical innovations, will enable new approaches to address each of these challenges. WGS can be used to identify investigate outbreaks6, enhance diagnostics by pathogen and antibiotic resistance detection7–9, and perform pathogen surveillance10. Over the 20 years since the first gonococcal genome was sequenced, 15 finished genomes, 413 draft genome assemblies, and 4795 sequencing runs have been made publicly available (as of December 10, 2017). Here, we review the background on gonorrhea, resistant gonococcus, and the ways in which researchers are exploring how to integrate these data to advance clinical care and public health management of gonorrhea.
Neisseria gonorrhoeae: clinical manifestations and origins
The Gram negative diplococcus N. gonorrhoeae is a major cause of urethritis as well as of cervicitis and pelvic inflammatory disease (PID)11. N. gonorrhoeae also causes disease outside of the urogenital tract, including pharyngeal and rectal infection, which tend to be asymptomatic, as well as conjunctivitis, disseminated gonococcal infection with associated mono-and oligoarticular arthritis, and Fitz-Hugh-Curtis syndrome (perihepatitis)12. Antibiotic therapy cures gonococcal infections and is indicated for both symptomatic and asymptomatic infections.
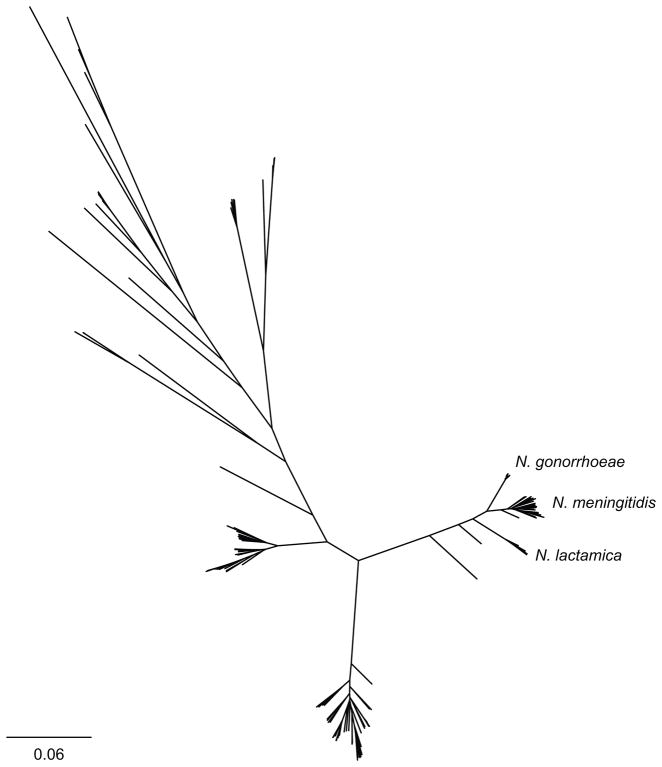
N. gonorrhoeae is part of the Neisseria genus, with most other species considered commensal oropharyngeal flora. The clinical manifestations of N. gonorrhoeae lead to the definition of gonococcus along with Neisseria meningitidis (the meningococcus)—the cause of meningococcal meningitis and septicemia—as the pathogenic Neisseria (Figure 1)13. The standard pigeonholing of these species—while a convenient shorthand—belies the clinical observations that not all the Gram negative diplococci that cause “gonorrhea-like” symptoms are N. gonorrhoeae. Meningococcus and N. lactamica have been isolated in urethritis cases14, and a recent outbreak of meningococcal urethritis renewed interest in the question of whether adaptation to the urogenital niche may happen periodically15–17. N. gonorrhoeae is not as diverse as other Neisseria (Figure 1), suggesting the species has arisen relatively recently18–21 and likely as an offshoot from meningococcus. These observations invite speculation about the origins and ongoing evolution of Neisseria, raising the possibility that contact between the oropharynx and other mucosa present opportunities for Neisseria to adapt to and inhabit new niches, and the confluence of the right genetic and behavior contexts to promote transmission may facilitate emergence of strains of Neisseria.
Figure 1.
Phylogeny of Neisseria. All publicly available genomes from the Neisseria genus were downloaded from NCBI (as of December 30, 2017) and annotated with Prokka v 1.12134. N. gonorrhoeae and N. meningitidis were subsampled to 20 genomes. Core genes (n = 236) were identified and aligned with Roary v 3.12.0135. Approximate maximum likelihood phylogenetic analysis was performed used FastTree v 2.1.9136. The phylogeny was visualized using FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Branches are scaled by substitutions per site. Data are available at 10.6084/m9.figshare.5877486.
Population structure analyses based on Bayesian methods have identified 5–12 clusters in N. gonorrhoeae populations, depending on the data set, and these clusters and major clades defined by phylogenetic analysis have little to no geographic structure21–24. In Neisseria meningitidis, clade specific restriction modification systems, competition between lineages, and selection by the immune system contribute to the population structure25,26, but the factors that maintain population structure in N. gonorrhoeae have not been identified. The global distribution of these major groups suggests that international transmission of N. gonorrhoeae is relatively common.
Acquisition of antibiotic resistance
Antibiotic exposure drives emergence of resistance in gonococcus. These exposures derive from treatment for gonorrhea and potentially through “bystander effect”, during treatment for other infections in individuals who are asymptomatically infected with gonococcus. The variable tissue penetration of antibiotics into the pharynx, a site of gonococcal infections, is one likely source of resistance, as the dose needed to clear urethral or cervical infection may yield a relatively low pharyngeal antibiotic concentration inadequate for eradication and thereby promote resistance emergence. A recent epidemiological study relating annual antibiotic consumption and gonococcal resistance suggested that the pressure from bystander selection may be small to none27, whereas a study from the Netherlands indicated that previous exposure to azithromycin is associated with higher levels of azithromycin resistance, perhaps reflecting the long half-life of azithromycin and the observation that individuals previously infected with gonorrhea are at higher risk for subsequent infection28.
In addition to de novo acquisition of resistance due to antibiotic exposure, genes and alleles can easily move between N. gonorrhoeae lineages, providing another mechanism for acquisition of antibiotic resistance. N. gonorrhoeae harbors several mobile genetic elements. Almost all gonococcal isolates encode a small, cryptic plasmid, which can also be integrated into the chromosome29,30. A conjugative plasmid can also be found, and a transposon encoding tetM, which mediates tetracycline resistance, has inserted into this plasmid and mobilized among N. gonorrhoeae (Dutch type, so called because the initially described plasmid of this type was encoded by an isolate from the Netherlands) 31,32. A distinct tetM determinant can be found on another plasmid backbone (American type, as it was first described in an isolate from the United States) 31,32, suggesting two introductions of tetM mediated resistance into the N. gonorrhoeae population31,33. A diverse set of plasmids encoding blaTEM beta lactamase are transmitted among N. gonorrhoeae populations; however, they are all derivatives of the Asia-type plasmid arising by repeat mediated deletions or duplications34. This plasmid likely originated in Haemophilus species after a transposon insertion into a cryptic plasmid and was horizontally transferred to N. gonorrhoeae35. Finally, many N. gonorrhoeae contain the gonococcal genetic island (GGI)36,37, which encodes machinery for a Type IV secretion system (T4SS) that secretes DNA into the environment, as well as proteins of unknown function38. Similar T4SSs can be found in other proteobacteria; however, flanking regions are not conserved39. DNA secretion may facilitate horizontal gene transfer, including resistance acquisition, and biofilm formation36,37,40. The T4SS can also enable intracellular survival of N. gonorrhoeae via iron acquisition by an unknown mechanism, which is independent of DNA secretion41. These mobile elements are found in diverse N. gonorrhoeae strains and are not confined to a particular clade. Besides the cryptic plasmid, plasmids and the GGI are found at intermediate frequencies in the N. gonorrhoeae population, suggesting that a fitness cost to carrying these mobile elements exists in some niches.
Neisseria species are naturally competent 42, with a preference for DNA carrying a DNA uptake sequence (DUS)43. In addition to exchanging alleles within the N. gonorrhoeae population, N. gonorrhoeae will uptake genetic material from other Neisseria, including N. meningitidis, N. lactamica, and other commensals44, further implicating the oropharynx as a potential site of resistance acquisition. Mosaicism with other Neisseria species has also been an important source for the acquisition of antibiotic resistance in N. gonorrhoeae. Most notably, mosaic sequences in penA have resulted in resistance to penicillin and reduced susceptibility to extended spectrum cephalosporins (ESCs)45,46, and a mosaic mtr operon has been shown to be associated with azithromycin resistance in the United States22,47 and Australia48,49. In a dataset of N. gonorrhoeae isolated in the United States, six percent of recombinant tracts matched sequences found in other Neisseria, including an acquisition of tbpB from N. meningitidis50. Multiple N. gonorrhoeae lineages have acquired porA sequences from N. meningitidis, replacing the gonococcal porA pseudogene that is occasionally used for diagnostics51. Exchange among Neisseria species is not unidirectional, and urethritis-associated N. meningitidis isolates contain recombinant tracts originating from N. gonorrhoeae17.
Despite being a potential reservoir of resistance determinants for pathogenic species, the prevalence and mechanisms of antibiotic resistance in commensal Neisseria is largely unknown. Interspecies exchange of penA sequences has long been recognized as contributing to penicillin resistance in both pathogenic and commensal Neisseria52–54. Transformation of N. gonorrhoeae with penA sequences from a ceftriaxone resistant Neisseria cinerea isolate resulted in transformants with MICs to ESCs similar to the donor and a mosaic penA similar to alleles found in clinical N. gonorrhoeae isolates with high level ceftriaxone resistance55. It is unknown if the penA sequences originated in N. cinerea or were horizontally transferred to both N. cinerea and N. gonorrhoeae from an unknown source. The only survey of commensal Neisseria resistance to ESCs showed that 93% and 100% of a Neisseria subflava sample from Japan were susceptible to cefixime and ceftriaxone, respectively56. Resistance to penicillin, tetracycline, and ciprofloxacin was also identified; however, the genetic mechanisms of resistance were not determined. Commensal Neisseria have also been shown to encode erm genes, which mediate macrolide resistance57.
From pathogen to superbug: treatment and resistance
N. gonorrhoeae has routinely developed resistance to each of the antibiotics used to treat it 58. Resistance to sulfonamides and low level resistance to penicillin emerged in the 1940s59,60. Penicillin continued to be the most common treatment for gonorrhea for several decades despite slowly increasing resistance due to chromosomal mutations. High-level resistance to penicillin via plasmid and chromosomally mediated mechanisms were reported in 1976 and 1986, respectively61,62. Tetracycline resistance can also be mediated by both determinants on plasmids or the chromosome, and N. gonorrhoeae harboring tetM encoding plasmids were first described in 198632. Clinical trials to study the effectiveness of ciprofloxacin to treat gonorrhea began in 198363, and, though it was not recommended as therapy in the United States until 199364, reduced susceptibility to ciprofloxacin had already been reported as early as 199065. In 2007, with rapidly rising prevalence of resistance, the CDC recommended that ciprofloxacin no longer be used for gonorrhea treatment66. In Southeast Asia and the Western Pacific, rates of ciprofloxacin resistance remain extremely high (> 90% in 2014)67. Even as first line antibiotic therapies have changed, resistance to older therapies remains high in N. gonorrhoeae populations across the globe68–72. For example, 44.1% of isolates collected by GISP in 2016 were resistant to penicillin, tetracycline, and/or ciprofloxacin1. With the idea that dual therapy would be helpful to halt the development of resistance to the remaining effective antibiotics, the CDC currently recommends ceftriaxone and azithromycin dual therapy73. However, resistance to azithromycin and ESCs is emerging74, and treatment failure with this regimen has been reported75.
Since susceptibility is not routinely measured in the clinic, the monitoring of antibiotic resistance prevalence falls to surveillance programs, which measure levels of resistance in the N. gonorrhoeae population and inform treatment guidelines. In the United States, the Gonococcal Isolates Surveillance Project (GISP) collects the first 25 urethral isolates each month from sentinel clinics for susceptibility testing. This project is being enhanced (eGISP) to include isolates from additional body sites and collect additional demographic and behavioral data from patients through the STD Surveillance Network (SSuN). Similarly, the European Gonococcal Antibiotic Surveillance Programme (GASP) monitors rates of gonorrhea and antibiotic resistance in 27 European countries. There are also active surveillance efforts in China (China GASP), Australia (Australia GSP), and the United Kingdom (GRASP, Sexually Transmitted Bacteria Reference Unit (STBRU)). In a partnership between CDC and WHO, the Enhanced Gonorrhea Antimicrobial Surveillance Project (EGASP) was initiated in 2015 to monitor antibiotic resistance worldwide, particularly in high risk populations76. Currently, surveillance data is particularly sparse in Central America, Africa, and Central Asia67.
Since there is no point-of-care antibiotic susceptibility testing, antibiotic selection for gonorrhea treatment is based on recommended drugs and doses per national or regional guidelines. A recent review of N. gonorrhoeae treatment guidelines show that organizations suggest 250–500 mg of ceftriaxone intramuscularly and 1–2 g azithromycin orally for treatment of uncomplicated infections; 400–800 mg cefixime orally is provided as an alternative to ceftriaxone in WHO and Canadian guidelines77. The WHO also recommends ceftriaxone single therapy. However, this review was limited in scope to guidelines published in English, and guidelines from Africa, Asia, and South America were not included beyond WHO recommendations. To limit empiric treatment failures, it has been the practice to switch the recommended first-line antibiotics when prevalence of resistance exceeds 5%, though it is worth noting that more nuanced strategies for optimizing antibiotic use are needed.
The clonality and number of times drug resistant lineages have emerged varies by drug and mechanism of resistance. Chromosomal mutations mediating low level resistance to beta-lactams and tetracycline are common in N. gonorrhoeae isolates, and they are widespread across the phylogeny22. While azithromycin resistance is less common than resistance to previously used drugs, resistance or reduced susceptibility has arisen across a large number of genomic backgrounds22,78,79. In contrast, reduced susceptibility to ESCs is primarily clonal, and the majority of ESC-RS isolates belong to only a few major lineages22,50. In isolates from the United States, azithromycin reduced susceptibility is estimated to have been acquired 75 times, and reversion to susceptibility is also relatively frequent (42 times)22. In contrast, ciprofloxacin resistance has only been acquired 11 times, but there are far more ciprofloxacin resistant isolates in the data set (594 vs. 294 AZI-RS)22.
An impediment to the emergence of antibiotic resistant bacteria is the fitness cost incurred by resistance in environments without antibiotic selective pressure. Resistant bacteria have been shown to be less fit than their susceptible counterparts both in vitro and in vivo for several bacterial species, and resistant bacteria can mediate these costs by acquiring compensatory mutations80. However, fitness costs of resistance and compensatory mutations in N. gonorrhoeae are understudied. The continued prevalence of penicillin, tetracycline, and ciprofloxacin resistant lineages despite discontinued use of these antibiotics has been cited as evidence that there may be little to no fitness costs for these resistance determinants. Experimental work shows that mtr mutants have increased fitness in a mouse model81,82. The most common mutations in gyrA conferring ciprofloxacin resistance also do not have a cost in the mouse model, but the addition of parC mutations which appear in clinical isolates does result in lower fitness83. A modelling study by Whittles and colleagues using data on rates of cefixime susceptibility and cefixime use for gonorrhea treatment in England suggests that cefixime resistance imparts a fitness cost compared to susceptible lineages84. Finally, reversion from resistant to susceptible phenotypes have been observed in N. gonorrhoeae, which may indicate a fitness cost for resistance22.
Interactions between resistance loci may also influence the emergence of multi-drug resistant lineages. Resistance correlates across antibiotics, and many N. gonorrhoeae isolates are resistant to multiple drugs (17.6% of isolates collected by GISP in 2016)85,86. Resistance determinants in N. gonorrhoeae have been shown to have additive effects. For example, successive mutations in penA, mtr, porB, ponA, and pilQ contribute to high level penicillin resistance62,87,88. Of particular concern currently are lineages that have reduced susceptibility to both azithromycin and ESCs. In isolates sampled across the United States from 2000–2013, high level AZI resistance and ESC reduced susceptibility appeared to be anti-correlated, and depending on the genomic background, the presence of 23S rRNA mutations lowered MICs for other antibiotics22. Isolates with reduced susceptibility to both azithromycin and ESCs are circulating in Canada89, and a cluster of isolates with high level resistance to azithromycin and reduced susceptibility to ESCs was identified in Hawaii, suggesting ongoing transmission of this lineage90. Interestingly, these isolates were more closely related to an isolate associated with failed azithromycin and ceftriaxone dual therapy in England75 than other contemporaneous isolates from Hawaii.
Azithromycin resistance and ESC reduced susceptibility have emerged on several genomic backgrounds. However, some clones are highly successful and have spread across the globe, while others seem to result in few descendants or resistance is lost22. One of the two major ESC-RS clades in the United States, for example, seems to have ceased transmission after 201122. Some lineages encoding azithromycin resistance are associated with international transmission. For example, an azithromycin resistant N. gonorrhoeae lineage has emerged in Scotland91 and spread to England and Wales92 causing outbreaks78. This lineage has also been observed elsewhere in Europe79 and in Australia49. Whether success is attributable to mutations facilitating acquisition and maintenance of resistance determinants and sustained transmission remains unclear. Understanding the interactions between fitness costs of resistance, compensatory mutations, and transmission of resistant lineages is an important step in controlling the spread of antibiotic resistance in N. gonorrhoeae.
Opportunities for addressing AMR gonorrhea: rapid diagnosis and antibiotic susceptibility assessment
The majority of gonorrhea cases are diagnosed via a nucleic acid amplification test (NAAT), through which N. gonorrhoeae genetic material is detected in patient samples. Culture is rarely done, though it remains the only basis for assessing the minimum inhibitory concentrations (MIC) for anti-gonococcal antibiotics.
Gonorrhea is currently treated empirically based on the extent of resistance described in surveillance systems. While resistance to previously effective antibiotics remains high in many areas of the world, a large fraction of isolates remain susceptible to older antibiotics and in some regions,68, most isolates remain susceptible to all drugs. This has led to the idea that a point-of-care diagnostic (POC) that detects antibiotic resistance—akin to the use of detection of rpoB mutations to rapidly identify resistant TB93,94—would increase treatment options, permitting use of antibiotics shelved because of concern for the high fraction of empiric treatment failures.
A real-time PCR based assay targeting gyrA has been shown to accurately detect ciprofloxacin resistance from patient samples (sensitivity: 95.8%, specificity: 100%)95. POCs have also been developed for penicillin susceptibility; however, accurate predictions require assessment of multiple genetic loci 96,97. Modeling suggests that point of care testing for antibiotic resistance has the potential to slow the spread of resistance98; however, a test for ciprofloxacin alone will not delay the increases in resistance to azithromycin and ESCs, whereas a POC for all three antibiotics would delay the rising levels of resistance99.
The increasingly portable and rapid turnaround of sequencing technologies may make sequencing in the clinic more practical. Just as nanopore technology has been used for real time epidemiological investigations for recent Ebola and Zika outbreaks100,101, preliminary work has demonstrated the feasibility of using the Oxford Nanopore MinION system as a gonococcal diagnostic, sequencing and predicting antibiotic susceptibility of N. gonorrhoeae isolates in less than 2 hours102.
One potential advantage of WGS in a diagnostic test is that it can assay for multiple antibiotic resistance determinants at the same time. Several tools have been developed to predict antibiotic resistance from WGS data8,9. Mykrobe8 and ARIBA9 can identify resistance determinants directly from sequencing reads, eliminating the need for genome assembly. Eyre and colleagues (2017) found that the MICs for antibiotics commonly used to treat gonorrhea can be predicted from WGS data for 52% of isolates, and MICs within 2 dilutions of the observed MIC were predicted for 98% of isolates103.
Ability to predict susceptibility profiles of clinical isolates is dependent on the comprehensiveness and quality of resistance allele databases as well as understanding of how loci interact to generate resistance. Positive and negative predictive values of known resistance alleles vary by antibiotic 37; for example, mutations associated with ciprofloxacin resistance are highly predictive, but in a recent WGS study of resistant isolates in the United States, 36% of reduced susceptibility to azithromycin could not be explained by known mutations22. Continued surveillance and identification of novel resistance mutations should be an ongoing effort.
Genomic epidemiology approaches to reducing the transmission of AMR gonorrhea
Prior to the advent of WGS, several typing schemes based on DNA sequences were developed to define genetic relationships between N. gonorrhoeae isolates and track the emergence and spread of drug resistant gonorrhea. Multi-locus sequence typing (MLST), a technique used for many bacterial species, uses the sequence of defined portions of a small number of genes (abcZ, adk, aroE, fumC, gdh, pdhC, and pgm in Neisseria species) to assign an isolate to a sequence type (ST)104. N. gonorrhoeae multiantigen sequence typing (NG-MAST), another typing scheme, is based on the sequence of the hypervariable portions of porB and tbpB105. Recently, targeted sequencing of known resistance determinants has also been utilized, and typing based on resistance profiles and housekeeping genes has been proposed (NG-STAR)106. Online resources for typing based on these schemes are available at https://pubmlst.org/, http://www.ng-mast.net, and https://ngstar.canada.ca/.
Typing of bacterial strains has long been a complement to typical epidemiological practices when investigating an outbreak. While WGS is generally concordant with traditional typing methods like MLST and NG-MAST, it can provide increased resolution and rule out potential transmission links that may be inferred by these methods107. For example, in a study of N. gonorrhoeae specimens from Brighton, UK, 8.8% of isolates collected within 28 days of each other had the same NG-MAST type, but only 3.6% of isolates were compatible with direct or indirect transmission according to WGS results24. In British Columbia, monitoring of a database of HIV genotypes allowed for identification of a growing transmission cluster and timely public health follow-up108; N. gonorrhoeae WGS may similarly allow for identification of expanding lineages and prevention of further onward transmission.
Since N. gonorrhoeae has a calculable substitution rate at short genetic distances, the WGS data can be combined with dates of collection to infer likely transmission links24. Moreover, the consistency of estimates of the N. gonorrhoeae substitution rate suggests that the transmission nomogram calculated by De Silva and colleagues (based on a substitution rate of 3.55 SNPs per genome per year) could be applicable to transmission of gonorrhea in other settings. Hypermutator strains—which would have a faster substitution rate—have been observed in other pathogens, including epidemic clones of N. meningitidis109 and antibiotic resistant Pseudomonas aeruginosa110, but gonococcal hypermutators have not been described.
Combined with patient metadata, WGS can reveal information about the demographics of contact networks. For example, clusters likely associated with transmission in Brighton, UK contain isolates from both HIV positive and HIV negative patients111, and clusters associated with heterosexual women in Australia contain patients across a range of ages112. In the United States, lineages with reduced susceptibility to cefixime were found to be primarily circulating among men who have sex with men (MSM), and transmission from MSM to men who have sex with women (MSW) occurred more often than MSW to MSM transmission50. WGS of N. gonorrhoeae populations in New Zealand shows that the majority of clusters contain isolates from both men and women, suggesting the absence of clones associated with exclusively MSM transmission23. While the N. gonorrhoeae global population does not show geographic structure, examining WGS of isolates from smaller scales (i.e. London) show that transmission links are associated with shorter geographic distances 24. Beyond inferring likely transmission links, phylogenetic analyses utilizing a molecular clock enable additional inferences. Calculating the time to most recent common ancestor (TMRCA) of isolates from pairs of known contacts in Sheffield, UK enabled estimation of the average duration of infection in this primarily heterosexual population to be 3.4 months107.
WGS can also be used to track the spread of resistance across geographic boundaries. For example, a cluster of cefixime resistant gonococcus mediated by mosaic penA XXXIV spread from the west coast to the east coast of the United States50. The TMRCA of this cluster was estimated to be 1997, and the addition of subsequently sequenced isolates from the UK confirmed this estimate24. Nine percent of infections in Brighton, UK were found in transmission clusters with US isolates, indicating that intercontinental spread of N. gonorrhoeae is common, which is also supported by the lack of geographic structure in N. gonorrhoeae populations24. Frequent international transmission of resistant strains poses a challenge to local control efforts.
Ongoing areas of research
Even as we understand many of the most common genetic pathways to antibiotic resistance, our catalog of resistance mechanisms and pathways remains incomplete (Table 2). Genomic data provide a rich source of information that can be used to define the variants that cause resistance and their interaction with other loci in the genome. Bacterial genome wide association studies (GWAS)113–115 have successfully identified known and potentially novel antibiotic resistance mutations in other bacterial pathogens like Mycobacterium tuberculosis and Escherichia coli114,116. Genomic studies of N. gonorrhoeae have identified isolates without known resistance determinants (Table 2), suggesting that in addition to GWAS other methods, such as RNAseq and Tn-Seq, may be needed to identify the cellular responses to and essential genes required under exposure to antibiotics117–120. While the selective pressures applied in the laboratory are unlikely to fully replicate those pressures in the context of human infection and transmission, analysis of clinical N. gonorrhoeae isolates can identify loci under purifying or diversifying selection in the natural environment.
Table 2.
Genomic studies of gonococcus
| Location | Year | n | Resistant isolates without known mechanisms | Reference | Accession |
|---|---|---|---|---|---|
| United States | 2009–2010 | 236 | - | Grad et al. 201450 | PRJEB2999 |
| Canada | 1982–2008 | 25 | - | Vidovic et al. 2014159 | - |
| Canada | 1989–2013 | 169 | 27 CRO-RS without mosaic penA | Demczuk et al. 2015160 | PRJNA266539 |
| Global | 1982–2013 | 61 | 1 CFX-RS 1 AZI-RS 1 TET-R |
Ezewudo et al. 201521 | SRA099559 |
| England | 2014–2015 | 15 | - | Chisholm et al. 201678 | - |
| France | 2010–2014 | 4 | 1 AZI-RS | de Curraize et al. 2016161 | PRJEB13093 |
| Canada | 1989–2014 | 236 | - | Demczuk et al. 2016162 | SRP065041 |
| UK | 2004–2015 | 1842 | - | De Silva et al. 201624 | PRJNA315363 |
| UK | 1995–2000 | 237 | - | Didelot et al. 2016107 | PRJEB2124 |
| United States | 2000–2013 | 1102 | 106 AZI-RS 5 CIP-R |
Grad et al. 201622 | PRJEB7904 |
| Global | 289 | - | Harrison et al. 201637 | Previously published | |
| Europe | 2009–2014 | 75 | - | Jacobsson et al. 201679 | PRJNA322768 |
| Global | 14 | - | Unemo et al. 2016139 | PRJEB14020 | |
| UK | 2014–2015 | 100 | - | Peters et al. 2017111 | Previously published |
| US, Brazil | 804 + 118 | - | Vidyaprakash et al. 2017163 | Previously published | |
| Ireland | 2008–2014 | 14 | - | Mac Aogáin et al. 2017164 | PRJNA275092 |
| New Zealand | 2014–2015 | 398 | 2 CIP-R 6 Spec-RS 1 CFX-RS |
Lee et al. 201723 | PRJNA394216 |
| US (Hawaii) | 2011–2016 | 61 | - | Papp et al. 201790 | - |
| Australia | 2005–2014 | 94 | Kwong et al. 2017165 | PRJEB17738 | |
| Brazil | 2006–2015 | 116 | 3 PEN-R | Costa-Lourenço et al. 2018166 | - |
| Australia | 2012–2014 | 94 | - | Buckley et al. 2018112 | PRJNA392203 |
| UK | 2014–2017 | 180 | - | Fifer et al. 2018167 | PRJEB23008 |
| Australia | 2011–2013 | 59 | - | Al Suwayyid et al. 2018168 | - |
| Kenya | 2010–2015 | 103 | - | Cehovin et al. 2018169 | PRJEB10104 |
| Japan, Australia | 2015, 2017 | 4 | - | Lahra et al. 2018170 | PRJNA416507 |
Studies were included when > 1 isolate was sequenced. -R = resistant, -RS = reduced susceptibility.
Drug resistance alleles are likely to interact with the genomic background and other resistance determinants. For example, the presence of a mosaic penA sequence is not a perfect predictor for reduced susceptibility to cefixime and ceftriaxone, suggesting that additional variants contribute to this phenotype22. In a model for MIC prediction, some resistance alleles had synergistic interactions where the presence of both alleles increased MIC more than the combination of their individual effects while other combinations did not increase MIC above levels associated with a single allele103. For example, penB and mtrR promoter mutations appear to have a synergistic effect, and conversely, the addition of rpsJ or mtrR mutations do not increase tetracycline MICs above levels of resistance conferred by tetM alone. GWAS may be helpful to identify additional variants that interact with resistance alleles, including compensatory and enabling mutations that allow resistant lineages to successfully compete with their susceptible counterparts.
WGS of patient samples may also illuminate the extent of within-host diversity and the impact of mixed infections on pathogenicity, transmission, and drug resistance. Horizontal gene transfer and recombination among N. gonorrhoeae provide evidence of mixed infections, and several studies of genetic loci and genome sequencing have further supported mixed infections. Martin and Ison found evidence of mixed infection using opa typing, which is based on restriction enzyme digestion of PCR amplified opa genes121,122. Different opa profiles were found in urethral and cervical swabs but not in cultured samples. Using DNA-DNA hybridization of porB, Lynn and colleagues found evidence for mixed infection in 21% of samples123. Using WGS, De Silva found evidence for distinct strains at different anatomical sites in 13% of pairs when multiple samples were obtained from the same patient24. The clinical importance of mixed infections remains unclear, though a concern about DNA-based resistance prediction is that these tests may have insufficient sensitivity to detect clinically meaningful resistant subpopulations. Within-host diversity may also interfere with reconstruction of transmission networks when the genome of a single isolate per patient is sequenced124. The amount of diversity that accumulates during N. gonorrhoeae infection and the reduction of diversity that occurs during transmission are unknown. The impact of diversity on transmission inference is likely to be higher in networks containing asymptomatic patients where duration of infection is longer.
Many public health labs are now incorporating WGS into pipelines to identify and type N. gonorrhoeae and other pathogens. However, WGS of every patient sample may not be feasible in all settings, and other technologies for POC testing may prove to be faster and more economical for routine care. Further research is needed to determine the optimal role of WGS in public health, including establishing how to sample the isolates for sequencing 58. For WGS to contribute to goals to reduce the burden of AMR N. gonorrhoeae, results from WGS—including reconstruction of transmission networks and inferences about connections between demographic and geographic groups—must be reliable and translatable to cost-effective interventions.
Further, routine WGS in clinical and public health laboratories comes with a new set of technical and ethical challenges. Introducing WGS into clinical labs will require development of user-friendly tools to analyze sequence data and/or bioinformatics training for clinical scientists; it will be important to ensure that sequencing platforms and training on how to use them are widely available. WGS directly from patient samples has similar ethical challenges to human microbiome research, as sequences from both the patient as well as other pathogens may be captured125. Additionally, during outbreak investigations, WGS can provide more precise information than older molecular techniques, and unreported transmission links may be discovered 24. Guidelines for how to interpret and report incidental findings from sequence data to patients and public health authorities will need to be established. Rapid sharing of genomic data from N. gonorrhoeae outbreaks will be key to enact timely interventions, but sequence data must be carefully de-identified and cleaned of human sequence data to protect patient privacy.
As a gonococcal vaccine would be a transformational intervention, much effort is being put into development of candidates. However, efforts to date have been stymied by N. gonorrhoeae’s evasion of the immune system through extensive variation in surface antigens. Two vaccines have proceeded to clinical trials in humans but were unsuccessful126. Recently, a retrospective study found that the outer membrane vesicle (OMV) meningococcal B vaccine showed some effectiveness against infection by N. gonorrhoeae in New Zealand; vaccination reduced incidence of gonorrhea by an estimated 31% compared to the unvaccinated population127. Reverse vaccinology, or the use of microbial genomic information to identify vaccine targets128, has been successfully used to develop vaccines for N. meningitidis serogroup B, a bivalent fHbp vaccine and a multicomponent vaccine129–131. Two of the three main antigens from the multicomponent vaccine are also present in N. gonorrhoeae: factor H binding protein (fHbp) and neisserial heparin-binding antigen (NHBA)132; however, some N. gonorrhoeae isolates contain premature stop codons in these genes133. The efficacy of OMV-containing meningococcal B vaccines against N. gonorrhoeae infection remain unknown but are of significant interest. Information gleaned from genomics could be used to identify novel vaccine targets that are conserved across the N. gonorrhoeae population. Other efforts to develop vaccines, including from non-protein antigens, offer promising avenues of research126.
Conclusion
With the rise of multidrug resistant N. gonorrhoeae, we urgently need to develop new tools for management of gonococcal infections—a need that extends from the development of new therapeutics to improved diagnostics and public health interventions and strategies for how to implement these tools optimally. WGS has the potential contribute to each of these efforts by improving our understanding of N. gonorrhoeae at multiple scales, from the biology of the microbe to global evolution and transmission dynamics. GWAS has the potential to identify new resistance determinants as they emerge, and increased understanding of epistatic interactions and fitness costs will inform best practices for treatment of gonorrhea. WGS of patient samples can improve diagnostics and illuminate the role of intra-host diversity. Finally, gonorrhea surveillance incorporating WGS can more accurately capture transmission dynamics within outbreaks and between regions, which will inform public health strategies to control the spread of antibiotic resistance.
Acknowledgments
T.D.M. and Y.H.G. were funded by NIAID Grant 1R01AI132606-01 and the Smith Family Foundation.
Footnotes
Competing interests
The authors declare no competing interests
References
- 1. [Accessed December 15, 2017];Gonorrhea - 2016 STD Surveillance Report. 2017 Nov 22; https://www.cdc.gov/std/stats16/Gonorrhea.htm.
- 2.WHO. Neisseria gonorrhoeae. WHO; [Accessed December 16, 2017]. Global action plan to control the spread and impact of antimicrobial resistance in. http://www.who.int/reproductivehealth/publications/rtis/9789241503501/en/ [Google Scholar]
- 3. [Accessed December 18, 2017];STDs in Men Who Have Sex with Men - 2016 STD Surveillance Report. 2017 Oct 2; https://www.cdc.gov/std/stats16/msm.htm.
- 4.Rice LB. Will use of combination cephalosporin/azithromycin therapy forestall resistance to cephalosporins in Neisseria gonorrhoeae? Sex Transm Infect. 2015;91:238–240. doi: 10.1136/sextrans-2014-051730. [DOI] [PubMed] [Google Scholar]
- 5.Control C. for D. & Prevention (US) Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2013. [Google Scholar]
- 6.Quainoo S, Coolen JPM, van Hijum SAFT, et al. Whole-Genome Sequencing of Bacterial Pathogens: the Future of Nosocomial Outbreak Analysis. Clin Microbiol Rev. 2017;30:1015–1063. doi: 10.1128/CMR.00016-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Votintseva AA, Bradley P, Pankhurst L, et al. Same-day diagnostic and surveillance data for tuberculosis via whole genome sequencing of direct respiratory samples. J Clin Microbiol. 2017 doi: 10.1128/JCM.02483-16. JCM.02483-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley P, Gordon NC, Walker TM, et al. Rapid antibiotic-resistance predictions from genome sequence data for Staphylococcus aureus and Mycobacterium tuberculosis. Nat Commun. 2015;6:10063. doi: 10.1038/ncomms10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt M, Mather AE, Sánchez-Busó L, et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genomics. 2017 doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardy JL, Loman NJ. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet. 2018;19:9. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrazzo JM, Apicella MA. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, Updated Edition. 8. Philadelphia, PA: Elsevier/Saunders; 2015. Neisseria gonorrhoeae (Gonorrhea) pp. 2446–2462.e3. [Google Scholar]
- 12.Detels R, Green AM, Klausner JD, et al. The Incidence and Correlates of Symptomatic and Asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae Infections in Selected Populations in Five Countries. Sex Transm Dis. 2011;38:503–509. [PMC free article] [PubMed] [Google Scholar]
- 13.Welch JLM, Rossetti BJ, Rieken CW, et al. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci. 2016;113:E791–E800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faur YC, Weisburd MH, Wilson ME. Isolation of Neisseria meningitidis from the Genito-Urinary Tract and Anal Canal. J Clin Microbiol. 1975;2:178–182. doi: 10.1128/jcm.2.3.178-182.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazan JA, Turner AN, Kirkcaldy RD, et al. Large Cluster of Neisseria meningitidis Urethritis in Columbus, Ohio, 2015. Clin Infect Dis. 2017;65:92–99. doi: 10.1093/cid/cix215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzeng Y-L, Bazan JA, Turner AN, et al. Emergence of a new Neisseria meningitidis clonal complex 11 lineage 11.2 clade as an effective urogenital pathogen. Proc Natl Acad Sci. 2017 doi: 10.1073/pnas.1620971114. 201620971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma KC, Unemo M, Jeverica S, et al. Genomic characterization of urethritis-associated Neisseria meningitidis shows that a wide range of N. meningitidis strains can cause urethritis. J Clin Microbiol. 2017 doi: 10.1128/JCM.01018-17. JCM.01018-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vázquez JA, de la Fuente L, Berron S, et al. Ecological separation and genetic isolation of Neisseria gonorrhoeae and Neisseria meningitidis. Curr Biol. 1993;3:567–572. doi: 10.1016/0960-9822(93)90001-5. [DOI] [PubMed] [Google Scholar]
- 19.Hanage WP, Fraser C, Spratt BG. Fuzzy species among recombinogenic bacteria. BMC Biol. 2005;3:6. doi: 10.1186/1741-7007-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett JS, Jolley KA, Sparling PF, et al. Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 2007;5:35. doi: 10.1186/1741-7007-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezewudo MN, Joseph SJ, Castillo-Ramirez S, et al. Population structure of Neisseria gonorrhoeae based on whole genome data and its relationship with antibiotic resistance. PeerJ. 2015;3:e806. doi: 10.7717/peerj.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grad YH, Harris SR, Kirkcaldy RD, et al. Genomic Epidemiology of Gonococcal Resistance to Extended-Spectrum Cephalosporins, Macrolides, and Fluoroquinolones in the United States, 2000–2013. J Infect Dis. 2016;214:1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee RS, Seemann T, Heffernan H, et al. Genomic epidemiology and antimicrobial resistance of Neisseria gonorrhoeae in New Zealand. J Antimicrob Chemother. 2017 doi: 10.1093/jac/dkx405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Silva D, Peters J, Cole K, et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis. 2016;16:1295–1303. doi: 10.1016/S1473-3099(16)30157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budroni S, Siena E, Hotopp JCD, et al. Neisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombination. Proc Natl Acad Sci. 2011;108:4494–4499. doi: 10.1073/pnas.1019751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckee CO, Jolley KA, Recker M, et al. Role of selection in the emergence of lineages and the evolution of virulence in Neisseria meningitidis. Proc Natl Acad Sci. 2008;105:15082–15087. doi: 10.1073/pnas.0712019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkcaldy RD, Bartoces MG, Soge OO, et al. Antimicrobial Drug Prescription and Neisseria gonorrhoeae Susceptibility, United States, 2005–2013. Emerg Infect Dis. 2017;23:1657–1663. doi: 10.3201/eid2310.170488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wind CM, de Vries E, van der Loeff S, et al. Decreased Azithromycin Susceptibility of Neisseria gonorrhoeae Isolates in Patients Recently Treated with Azithromycin. Clin Infect Dis. 2017;65:37–45. doi: 10.1093/cid/cix249. [DOI] [PubMed] [Google Scholar]
- 29.ROBERTS M, PIOT P, FALKOW S. The Ecology of Gonococcal Plasmids. Microbiology. 1979;114:491–494. doi: 10.1099/00221287-114-2-491. [DOI] [PubMed] [Google Scholar]
- 30.Hagblom P, Korch C, Jonsson AB, et al. Intragenic variation by site-specific recombination in the cryptic plasmid of Neisseria gonorrhoeae. J Bacteriol. 1986;167:231–237. doi: 10.1128/jb.167.1.231-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gascoyne DM, Heritage J, Hawkey PM, et al. Molecular evolution of tetracycline-resistance plasmids carrying TetM found in Neisseria gonorrhoeae from different countries. J Antimicrob Chemother. 1991;28:173–183. doi: 10.1093/jac/28.2.173. [DOI] [PubMed] [Google Scholar]
- 32.Morse SA, Johnson SR, Biddle JW, et al. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986;30:664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gascoyne-Binzi DM, Heritage J, Hawkey PM. Nucelotide sequences of the tet(M) genes from the American and Dutch type tetracycline resistance plasmids of Neisseria gonorrhoeae. J Antimicrob Chemother. 1993;32:667–676. doi: 10.1093/jac/32.5.667. [DOI] [PubMed] [Google Scholar]
- 34.Pagotto F, Aman AT, Ng LK, et al. Sequence Analysis of the Family of Penicillinase-Producing Plasmids of Neisseria gonorrhoeae. Plasmid. 2000;43:24–34. doi: 10.1006/plas.1999.1431. [DOI] [PubMed] [Google Scholar]
- 35.Brunton J, Clare D, Meier MA. Molecular Epidemiology of Antibiotic Resistance Plasmids of Haemophilus Species and Neisseria gonorrhoeae. Rev Infect Dis. 1986;8:713–724. doi: 10.1093/clinids/8.5.713. [DOI] [PubMed] [Google Scholar]
- 36.Dillard JP, Seifert HS. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol. 2001;41:263–277. doi: 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 37.Harrison OB, Clemence M, Dillard JP, et al. Genomic analyses of Neisseria gonorrhoeae reveal an association of the gonococcal genetic island with antimicrobial resistance. J Infect. 2016 doi: 10.1016/j.jinf.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton HL, Domínguez NM, Schwartz KJ, et al. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 39.Pachulec E, Siewering K, Bender T, et al. Functional Analysis of the Gonococcal Genetic Island of Neisseria gonorrhoeae. PLOS ONE. 2014;9:e109613. doi: 10.1371/journal.pone.0109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zweig M, Schork S, Koerdt A, et al. Secreted single-stranded DNA is involved in the initial phase of biofilm formation by Neisseria gonorrhoeae. Environ Microbiol. 2014;16:1040–1052. doi: 10.1111/1462-2920.12291. [DOI] [PubMed] [Google Scholar]
- 41.Zola TA, Strange HR, Dominguez NM, et al. Type IV Secretion Machinery Promotes Ton-Independent Intracellular Survival of Neisseria gonorrhoeae within Cervical Epithelial Cells. Infect Immun. 2010;78:2429–2437. doi: 10.1128/IAI.00228-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sparling PF. Genetic Transformation of Neisseria gonorrhoeae to Streptomycin Resistance. J Bacteriol. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman SD, Scocca JJ. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dougherty TJ, Asmus A, Tomasz A. Specificity of DNA uptake in genetic transformation of gonococci. Biochem Biophys Res Commun. 1979;86:97–104. doi: 10.1016/0006-291x(79)90386-3. [DOI] [PubMed] [Google Scholar]
- 45.Spratt BG. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature. 1988;332:173. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- 46.Ameyama S, Onodera S, Takahata M, et al. Mosaic-Like Structure of Penicillin-Binding Protein 2 Gene (penA) in Clinical Isolates of Neisseria gonorrhoeae with Reduced Susceptibility to Cefixime. Antimicrob Agents Chemother. 2002;46:3744–3749. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadsworth CB, Arnold BJ, Sater MR, et al. Azithromycin resistance through interspecific acquisition of an epistasis dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. bioRxiv. 2018:309294. doi: 10.1128/mBio.01419-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trembizki E, Doyle C, Jennison A, et al. A Neisseria gonorrhoeae strain with a meningococcal mtrR sequence. J Med Microbiol. 2014;63:1113–1115. doi: 10.1099/jmm.0.074286-0. [DOI] [PubMed] [Google Scholar]
- 49.Whiley DM, Kundu RL, Jennison AV, et al. Azithromycin-resistant Neisseria gonorrhoeae spreading amongst men who have sex with men (MSM) and heterosexuals in New South Wales, Australia, 2017. J Antimicrob Chemother. doi: 10.1093/jac/dky017. [DOI] [PubMed] [Google Scholar]
- 50.Grad YH, Kirkcaldy RD, Trees D, et al. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis. 2014;14:220–226. doi: 10.1016/S1473-3099(13)70693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ison CA, Golparian D, Saunders P, et al. Evolution of Neisseria gonorrhoeae is a continuing challenge for molecular detection of gonorrhoea: false negative gonococcal porA mutants are spreading internationally. Sex Transm Infect. 2013;89:197–201. doi: 10.1136/sextrans-2012-050829. [DOI] [PubMed] [Google Scholar]
- 52.Spratt BG, Bowler LD, Zhang QY, et al. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol. 1992;34:115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 53.Saez-Nieto JA, Lujan R, Martinez-Suarez JV, et al. Neisseria lactamica and Neisseria polysaccharea as possible sources of meningococcal beta-lactam resistance by genetic transformation. Antimicrob Agents Chemother. 1990;34:2269–2272. doi: 10.1128/aac.34.11.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lujan R, Zhang QY, Nieto JAS, et al. Penicillin-resistant isolates of Neisseria lactamica produce altered forms of penicillin-binding protein 2 that arose by interspecies horizontal gene transfer. Antimicrob Agents Chemother. 1991;35:300–304. doi: 10.1128/aac.35.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Igawa G, Yamagishi Y, Lee K-I, et al. Neisseria cinerea with high ceftriaxone MIC is an origin of ceftriaxone and cefixime resistance-mediating penA sequences in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2018:AAC.02069-17. doi: 10.1128/AAC.02069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furuya R, Onoye Y, Kanayama A, et al. Antimicrobial resistance in clinical isolates of Neisseria subflava from the oral cavities of a Japanese population. J Infect Chemother. 2007;13:302–304. doi: 10.1007/s10156-007-0541-8. [DOI] [PubMed] [Google Scholar]
- 57.Roberts MC, Chung WO, Roe D, et al. Erythromycin-Resistant Neisseria gonorrhoeae and Oral Commensal Neisseria spp. Carry Known rRNA Methylase Genes. Antimicrob Agents Chemother. 1999;43:1367–1372. doi: 10.1128/aac.43.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unemo M, Shafer WM. Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin Microbiol Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moses JM, Desai MS, Bhosle CB, et al. Present pattern of antibiotic sensitivity of gonococcal strains isolated in Bombay. Br J Vener Dis. 1971;47:273–278. doi: 10.1136/sti.47.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunlop EMC. GonorrhœA and the Sulphonamides*. Br J Vener Dis. 1949;25:81–83. [PMC free article] [PubMed] [Google Scholar]
- 61.Ashford W, Golash R, Hemming V. PENICILUNASE-PRODUCING NEISSERIA GONORRHŒÆ. The Lancet. 1976;308:657–658. doi: 10.1016/s0140-6736(76)92467-3. [DOI] [PubMed] [Google Scholar]
- 62.Faruki H, Sparling PF. Genetics of resistance in a non-beta-lactamase-producing gonococcus with relatively high-level penicillin resistance. Antimicrob Agents Chemother. 1986;30:856–860. doi: 10.1128/aac.30.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Echols RM, Heyd AP, O’keeffe BJ, et al. Single-Dose Ciprofloxacin for the Treatment of Uncomplicated Gonorrhea: A Worldwide Summary. Sex Transm Dis. 1994;21:345–352. doi: 10.1097/00007435-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 64.CDC. 1993 Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep Morb Mortal Wkly Rep. 1993;42:57. [Google Scholar]
- 65.Gransden WR, Warren CA, Phillips I, et al. Decreased susceptibility of Neisseria gonorrhoeae to ciprofloxacin. The Lancet. 1990;335:51. doi: 10.1016/0140-6736(90)90177-7. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention (CDC) Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332–336. [PubMed] [Google Scholar]
- 67.Wi T, Lahra MM, Ndowa F, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLOS Med. 2017;14:e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thakur SD, Levett PN, Horsman GB, et al. High levels of susceptibility to new and older antibiotics in Neisseria gonorrhoeae isolates from Saskatchewan (2003–15): time to consider point-of-care or molecular testing for precision treatment? J Antimicrob Chemother. doi: 10.1093/jac/dkx333. [DOI] [PubMed] [Google Scholar]
- 69.Whiley DM, Trembizki E, Buckley C, et al. Molecular Antimicrobial Resistance Surveillance for Neisseria gonorrhoeae, Northern Territory, Australia. Emerg Infect Dis. 2017;23:1478–1485. doi: 10.3201/eid2309.170427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cole MJ, Spiteri G, Jacobsson S, et al. Overall Low Extended-Spectrum Cephalosporin Resistance but high Azithromycin Resistance in Neisseria gonorrhoeae in 24 European Countries, 2015. BMC Infect Dis. 2017;17:617. doi: 10.1186/s12879-017-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen SC, Yin YP, Dai XQ, et al. First nationwide study regarding ceftriaxone resistance and molecular epidemiology of Neisseria gonorrhoeae in China. J Antimicrob Chemother. 2016;71:92–99. doi: 10.1093/jac/dkv321. [DOI] [PubMed] [Google Scholar]
- 72.Kubanova A, Kubanov A, Frigo N, et al. Russian gonococcal antimicrobial susceptibility programme (RU-GASP) – resistance in Neisseria gonorrhoeae during 2009–2012 and NG-MAST genotypes in 2011 and 2012. BMC Infect Dis. 2014;14:342. doi: 10.1186/1471-2334-14-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention (CDC) Update to CDC’s Sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590–594. [PubMed] [Google Scholar]
- 74.Kirkcaldy RD. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance — The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR Surveill Summ. 2016;65 doi: 10.15585/mmwr.ss6507a1. [DOI] [PubMed] [Google Scholar]
- 75.Fifer H, Natarajan U, Jones L, et al. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N Engl J Med. 2016;374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 76.Al EJW, et al. Strengthening Global Surveillance for Antimicrobial Drug–Resistant Neisseria gonorrhoeae through the Enhanced Gonococcal Antimicrobial Surveillance Program. Emerging Infectious Disease journal - CDC. 2017 Dec 13;23 doi: 10.3201/eid2313.170443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dickson C, Arnason T, Friedman DS, et al. A systematic review and appraisal of the quality of practice guidelines for the management of Neisseria gonorrhoeae infections. Sex Transm Infect. 2017;93:487–492. doi: 10.1136/sextrans-2016-052939. [DOI] [PubMed] [Google Scholar]
- 78.Chisholm SA, Wilson J, Alexander S, et al. An outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in England. Sex Transm Infect. 2016;92:365–367. doi: 10.1136/sextrans-2015-052312. [DOI] [PubMed] [Google Scholar]
- 79.Jacobsson S, Golparian D, Cole M, et al. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother. 2016;71:3109–3116. doi: 10.1093/jac/dkw279. [DOI] [PubMed] [Google Scholar]
- 80.Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 2010;8:260. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 81.Warner DM, Folster JP, Shafer WM, et al. Regulation of the MtrC-MtrD-MtrE Efflux-Pump System Modulates the In Vivo Fitness of Neisseria gonorrhoeae. J Infect Dis. 2007;196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- 82.Warner DM, Shafer WM, Jerse AE. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol. 2008;70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kunz AN, Begum AA, Wu H, et al. Impact of Fluoroquinolone Resistance Mutations on Gonococcal Fitness and In Vivo Selection for Compensatory Mutations. J Infect Dis. 2012;205:1821–1829. doi: 10.1093/infdis/jis277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whittles LK, White PJ, Didelot X. Estimating the fitness cost and benefit of cefixime resistance in Neisseria gonorrhoeae to inform prescription policy: A modelling study. PLOS Med. 2017;14:e1002416. doi: 10.1371/journal.pmed.1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang HH, Cohen T, Grad YH, et al. Origin and Proliferation of Multiple-Drug Resistance in Bacterial Pathogens. Microbiol Mol Biol Rev. 2015;79:101–116. doi: 10.1128/MMBR.00039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. US Dep Health Hum Serv 2017 [Google Scholar]
- 87.Ropp PA, Hu M, Olesky M, et al. Mutations in ponA, the Gene Encoding Penicillin-Binding Protein 1, and a Novel Locus, penC, Are Required for High-Level Chromosomally Mediated Penicillin Resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002;46:769–777. doi: 10.1128/AAC.46.3.769-777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao S, Tobiason DM, Hu M, et al. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol Microbiol. 2005;57:1238–1251. doi: 10.1111/j.1365-2958.2005.04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen VG, Seah C, Martin I, et al. Azithromycin Resistance Is Coevolving with Reduced Susceptibility to Cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother. 2014;58:2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papp JR, Abrams AJ, Nash E, et al. Azithromycin Resistance and Decreased Ceftriaxone Susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerg Infect Dis. 2017;23:830–832. doi: 10.3201/eid2305.170088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palmer HM, Young H, Winter A, et al. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J Antimicrob Chemother. 2008;62:490–494. doi: 10.1093/jac/dkn235. [DOI] [PubMed] [Google Scholar]
- 92.Chisholm SA, Neal TJ, Alawattegama AB, et al. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother. 2009;64:353–358. doi: 10.1093/jac/dkp188. [DOI] [PubMed] [Google Scholar]
- 93.Boehme CC, Nabeta P, Hillemann D, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pond MJ, Hall CL, Miari VF, et al. Accurate detection of Neisseria gonorrhoeae ciprofloxacin susceptibility directly from genital and extragenital clinical samples: towards genotype-guided antimicrobial therapy. J Antimicrob Chemother. 2016;71:897–902. doi: 10.1093/jac/dkv432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckley C, Trembizki E, Baird RW, et al. Multitarget PCR Assay for Direct Detection of Penicillinase-Producing Neisseria gonorrhoeae for Enhanced Surveillance of Gonococcal Antimicrobial Resistance. J Clin Microbiol. 2015;53:2706–2708. doi: 10.1128/JCM.00540-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buckley C, Trembizki E, Donovan B, et al. Real-time PCR detection of Neisseria gonorrhoeae susceptibility to penicillin. J Antimicrob Chemother. 2016;71:3090–3095. doi: 10.1093/jac/dkw291. [DOI] [PubMed] [Google Scholar]
- 98.Fingerhuth SM, Low N, Bonhoeffer S, et al. Detection of antibiotic resistance is essential for gonorrhoea point-of-care testing: a mathematical modelling study. BMC Med. 2017;15:142. doi: 10.1186/s12916-017-0881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tuite AR, Gift TL, Chesson HW, et al. Impact of Rapid Susceptibility Testing and Antibiotic Selection Strategy on the Emergence and Spread of Antibiotic Resistance in Gonorrhea. J Infect Dis. 2017;216:1141–1149. doi: 10.1093/infdis/jix450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quick J, Loman NJ, Duraffour S, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530:228. doi: 10.1038/nature16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faria NR, Sabino EC, Nunes MRT, et al. Mobile real-time surveillance of Zika virus in Brazil. Genome Med. 2016;8:97. doi: 10.1186/s13073-016-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phillips LT, Witney A, Izquierdo-Carrasco F, et al. P1.32 Hand-held rapid whole genome nanopore sequencing to predict neisseria gonorrhoeae antibiotic susceptibility: steps towards clinic based tailored antimicrobial therapy. Sex Transm Infect. 2017;93:A56–A56. [Google Scholar]
- 103.Eyre DW, De Silva D, Cole K, et al. WGS to predict antibiotic MICs for Neisseria gonorrhoeae. J Antimicrob Chemother. 2017;72:1937–1947. doi: 10.1093/jac/dkx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maiden MCJ, Bygraves JA, Feil E, et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin IMC, Ison CA, Aanensen DM, et al. Rapid Sequence-Based Identification of Gonococcal Transmission Clusters in a Large Metropolitan Area. J Infect Dis. 2004;189:1497–1505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]
- 106.Demczuk W, Sidhu S, Unemo M, et al. Neisseria gonorrhoeae Sequence Typing for Antimicrobial Resistance, a Novel Antimicrobial Resistance Multilocus Typing Scheme for Tracking Global Dissemination of N. gonorrhoeae Strains. J Clin Microbiol. 2017;55:1454–1468. doi: 10.1128/JCM.00100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Didelot X, Dordel J, Whittles LK, et al. Genomic Analysis and Comparison of Two Gonorrhea Outbreaks. mBio. 2016;7:e00525–16. doi: 10.1128/mBio.00525-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poon AFY, Gustafson R, Daly P, et al. Near real-time monitoring of HIV transmission hotspots from routine HIV genotyping: an implementation case study. Lancet HIV. 2016;3:e231–e238. doi: 10.1016/S2352-3018(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richardson AR, Yu Z, Popovic T, et al. Mutator clones of Neisseria meningitidis in epidemic serogroup A disease. Proc Natl Acad Sci. 2002;99:6103–6107. doi: 10.1073/pnas.092568699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maciá MD, Blanquer D, Togores B, et al. Hypermutation Is a Key Factor in Development of Multiple-Antimicrobial Resistance in Pseudomonas aeruginosa Strains Causing Chronic Lung Infections. Antimicrob Agents Chemother. 2005;49:3382–3386. doi: 10.1128/AAC.49.8.3382-3386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peters J, Cresswell F, Amor L, et al. Whole genome sequencing of Neisseria gonorrhoeae reveals transmission clusters involving patients of mixed HIV serostatus. Sex Transm Infect. 2017 doi: 10.1136/sextrans-2017-053198. sextrans-2017-053198. [DOI] [PubMed] [Google Scholar]
- 112.Buckley C, Forde BM, Trembizki E, et al. Use of whole genome sequencing to investigate an increase in Neisseria gonorrhoeae infection among women in urban areas of Australia. Sci Rep. 2018;8:1503. doi: 10.1038/s41598-018-20015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lees JA, Vehkala M, Välimäki N, et al. Sequence element enrichment analysis to determine the genetic basis of bacterial phenotypes. Nature Communications. 2016;7:ncomms12797. doi: 10.1038/ncomms12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Earle SG, Wu C-H, Charlesworth J, et al. Identifying lineage effects when controlling for population structure improves power in bacterial association studies. Nat Microbiol. 2016;1:nmicrobiol201641. doi: 10.1038/nmicrobiol.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collins C, Didelot X. A phylogenetic method to perform genome-wide association studies in microbes that accounts for population structure and recombination. PLOS Computational Biology. 2018;14:e1005958. doi: 10.1371/journal.pcbi.1005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Farhat MR, Shapiro BJ, Kieser KJ, et al. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nature genetics. 2013;45:1183. doi: 10.1038/ng.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McClure R, Nudel K, Massari P, et al. The Gonococcal Transcriptome during Infection of the Lower Genital Tract in Women. PLOS ONE. 2015;10:e0133982. doi: 10.1371/journal.pone.0133982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McClure R, Tjaden B, Genco C. Identification of sRNAs expressed by the human pathogen Neisseria gonorrhoeae under disparate growth conditions. Front Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Isabella VM, Clark VL. Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics. 2011;12:51. doi: 10.1186/1471-2164-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Remmele CW, Xian Y, Albrecht M, et al. Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res. 2014;42:10579–10595. doi: 10.1093/nar/gku762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O’Rourke M, Ison CA, Renton AM, et al. Opa-typing: a high-resolution tool for studying the epidemiology of gonorrhoea. Mol Microbiol. 1995;17:865–875. doi: 10.1111/j.1365-2958.1995.mmi_17050865.x. [DOI] [PubMed] [Google Scholar]
- 122.Martin IMC, Ison CA. Detection of mixed infection of Neisseria gonorrhoeae. Sex Transm Infect. 2003;79:56–58. doi: 10.1136/sti.79.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lynn F, Hobbs MM, Zenilman JM, et al. Genetic Typing of the Porin Protein of Neisseria gonorrhoeae from Clinical Noncultured Samples for Strain Characterization and Identification of Mixed Gonococcal Infections. J Clin Microbiol. 2005;43:368–375. doi: 10.1128/JCM.43.1.368-375.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Worby CJ, Lipsitch M, Hanage WP. Within-Host Bacterial Diversity Hinders Accurate Reconstruction of Transmission Networks from Genomic Distance Data. PLOS Comput Biol. 2014;10:e1003549. doi: 10.1371/journal.pcbi.1003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McGuire AL, Achenbaum LS, Whitney SN, et al. Perspectives On Human Microbiome Research Ethics. J Empir Res Hum Res Ethics JERHRE. 2012;7:1–14. doi: 10.1525/jer.2012.7.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rice PA, Shafer WM, Ram S, et al. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu Rev Microbiol. 2017;71:665–686. doi: 10.1146/annurev-micro-090816-093530. [DOI] [PubMed] [Google Scholar]
- 127.Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. The Lancet. 2017;390:1603–1610. doi: 10.1016/S0140-6736(17)31449-6. [DOI] [PubMed] [Google Scholar]
- 128.Sette A, Rappuoli R. Reverse Vaccinology: Developing Vaccines in the Era of Genomics. Immunity. 2010;33:530–541. doi: 10.1016/j.immuni.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pizza M, Scarlato V, Masignani V, et al. Identification of Vaccine Candidates Against Serogroup B Meningococcus by Whole-Genome Sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 130.Fletcher LD, Bernfield L, Barniak V, et al. Vaccine Potential of the Neisseria meningitidis 2086 Lipoprotein. Infect Immun. 2004;72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muzzi A, Mora M, Pizza M, et al. Conservation of Meningococcal Antigens in the Genus Neisseria. mBio. 2013;4:e00163–13. doi: 10.1128/mBio.00163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hadad R, Jacobsson S, Pizza M, et al. Novel meningococcal 4CMenB vaccine antigens – prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS. 2012;120:750–760. doi: 10.1111/j.1600-0463.2012.02903.x. [DOI] [PubMed] [Google Scholar]
- 134.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014:btu153. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 135.Page AJ, Cummins CA, Hunt M, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Price MN, Dehal PS, Arkin AP. FastTree 2 – Approximately Maximum-Likelihood Trees for Large Alignments. PLOS ONE. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. [Accessed November 30, 2017];Interpretive Criteria for Neisseria gonorrhoeae Susceptibility Testing - STD information from CDC. 2013 https://www.cdc.gov/std/gonorrhea/arg/criteria.htm.
- 138.Fiebelkorn KR, Crawford SA, Jorgensen JH. Mutations in folP Associated with Elevated Sulfonamide MICs for Neisseria meningitidis Clinical Isolates from Five Continents. Antimicrob Agents Chemother. 2005;49:536–540. doi: 10.1128/AAC.49.2.536-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Unemo M, Golparian D, Sánchez-Busó L, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71:3096–3108. doi: 10.1093/jac/dkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sparling PF, Sarubbi FA, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975;124:740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gill MJ, Simjee S, Al-Hattawi K, et al. Gonococcal Resistance to β-Lactams and Tetracycline Involves Mutation in Loop 3 of the Porin Encoded at thepenB Locus. Antimicrob Agents Chemother. 1998;42:2799–2803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan W, Spratt BG. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol. 1994;11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 143.Xia M, Whittington WLH, Shafer WM, et al. Gonorrhea among Men Who Have Sex with Men: Outbreak Caused by a Single Genotype of Erythromycin-Resistant Neissevia gonorrhoeae with a Single-Base Pair Deletion in the mtrR Promoter Region. J Infect Dis. 2000;181:2080–2082. doi: 10.1086/315510. [DOI] [PubMed] [Google Scholar]
- 144.Veal WL, Nicholas RA, Shafer WM. Overexpression of the MtrC-MtrD-MtrE Efflux Pump Due to an mtrR Mutation Is Required for Chromosomally Mediated Penicillin Resistance in Neisseria gonorrhoeae. J Bacteriol. 2002;184:5619–5624. doi: 10.1128/JB.184.20.5619-5624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarubbi FA, Blackman E, Sparling PF. Genetic Mapping of Linked Antibiotic Resistance Loci in Neisseria gonorrhoeae. J Bacteriol. 1974;120:1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu M, Nandi S, Davies C, et al. High-Level Chromosomally Mediated Tetracycline Resistance in Neisseria gonorrhoeae Results from a Point Mutation in the rpsJ Gene Encoding Ribosomal Protein S10 in Combination with the mtrR and penB Resistance Determinants. Antimicrob Agents Chemother. 2005;49:4327–4334. doi: 10.1128/AAC.49.10.4327-4334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Belland RJ, Morrison SG, Ison C, et al. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 148.Lindbäck E, Rahman M, Jalal S, et al. Mutations in gyrA, gyrB, parC, and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS. 2002;110:651–657. doi: 10.1034/j.1600-0463.2002.1100909.x. [DOI] [PubMed] [Google Scholar]
- 149.Shultz TR, Tapsall JW, White PA. Correlation of In Vitro Susceptibilities to Newer Quinolones of Naturally Occurring Quinolone-Resistant Neisseria gonorrhoeae Strains with Changes in GyrA and ParC. Antimicrob Agents Chemother. 2001;45:734–738. doi: 10.1128/AAC.45.3.734-738.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rouquette-Loughlin C, Dunham SA, Kuhn M, et al. The NorM Efflux Pump of Neisseria gonorrhoeae and Neisseria meningitidis Recognizes Antimicrobial Cationic Compounds. J Bacteriol. 2003;185:1101–1106. doi: 10.1128/JB.185.3.1101-1106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ilina E, Malakhova M, Bodoev I, et al. Mutation in ribosomal protein S5 leads to spectinomycin resistance in Neisseria gonorrhoeae. Front Microbiol. 2013;4 doi: 10.3389/fmicb.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Unemo M, Golparian D, Skogen V, et al. Neisseria gonorrhoeae Strain with High-Level Resistance to Spectinomycin Due to a Novel Resistance Mechanism (Mutated Ribosomal Protein S5) Verified in Norway. Antimicrob Agents Chemother. 2013;57:1057–1061. doi: 10.1128/AAC.01775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galimand M, Gerbaud G, Courvalin P. Spectinomycin Resistance in Neisseria spp. Due to Mutations in 16S rRNA. Antimicrob Agents Chemother. 2000;44:1365–1366. doi: 10.1128/aac.44.5.1365-1366.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ng L-K, Martin I, Liu G, et al. Mutation in 23S rRNA Associated with Macrolide Resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002;46:3020–3025. doi: 10.1128/AAC.46.9.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Galarza PG, Abad R, Canigia LF, et al. New Mutation in 23S rRNA Gene Associated with High Level of Azithromycin Resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2010;54:1652–1653. doi: 10.1128/AAC.01506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luna VA, Cousin S, Whittington WLH, et al. Identification of the Conjugative mefGene in Clinical Acinetobacter junii and Neisseria gonorrhoeae Isolates. Antimicrob Agents Chemother. 2000;44:2503–2506. doi: 10.1128/aac.44.9.2503-2506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ito M, Deguchi T, Mizutani KS, et al. Emergence and Spread of Neisseria gonorrhoeae Clinical Isolates Harboring Mosaic-Like Structure of Penicillin-Binding Protein 2 in Central Japan. Antimicrob Agents Chemother. 2005;49:137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao S, Duncan M, Tomberg J, et al. Genetics of Chromosomally Mediated Intermediate Resistance to Ceftriaxone and Cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2009;53:3744–3751. doi: 10.1128/AAC.00304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vidovic S, Caron C, Taheri A, et al. Using Crude Whole-Genome Assemblies of Neisseria gonorrhoeae as a Platform for Strain Analysis: Clonal Spread of Gonorrhea Infection in Saskatchewan, Canada. J Clin Microbiol. 2014;52:3772–3776. doi: 10.1128/JCM.01502-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Demczuk W, Lynch T, Martin I, et al. Whole-Genome Phylogenomic Heterogeneity of Neisseria gonorrhoeae Isolates with Decreased Cephalosporin Susceptibility Collected in Canada between 1989 and 2013. J Clin Microbiol. 2015;53:191–200. doi: 10.1128/JCM.02589-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.de Curraize C, Kumanski S, Micaëlo M, et al. Ceftriaxone-Resistant Neisseria gonorrhoeae Isolates (2010 to 2014) in France Characterized by Using Whole-Genome Sequencing. Antimicrob Agents Chemother. 2016;60:6962–6964. doi: 10.1128/AAC.01568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Demczuk W, Martin I, Peterson S, et al. Genomic Epidemiology and Molecular Resistance Mechanisms of Azithromycin-Resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol. 2016;54:1304–1313. doi: 10.1128/JCM.03195-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vidyaprakash E, Abrams AJ, Shafer WM, et al. Whole-Genome Sequencing of a Large Panel of Contemporary Neisseria gonorrhoeae Clinical Isolates Indicates that a Wild-Type mtrA Gene Is Common: Implications for Inducible Antimicrobial Resistance. Antimicrob Agents Chemother. 2017;61:e00262–17. doi: 10.1128/AAC.00262-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mac Aogáin M, Fennelly N, Walsh A, et al. Fourteen Draft Genome Sequences for the First Reported Cases of Azithromycin-Resistant Neisseria gonorrhoeae in Ireland. Genome Announc. 2017;5 doi: 10.1128/genomeA.00403-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kwong JC, Chow EPF, Stevens K, et al. Whole-genome sequencing reveals transmission of gonococcal antibiotic resistance among men who have sex with men: an observational study. Sex Transm Infect. 2017 doi: 10.1136/sextrans-2017-053287. sextrans-2017-053287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.da Costa-Lourenço APR, Abrams AJ, dos Santos KTB, et al. Phylogeny and antimicrobial resistance in Neisseria gonorrhoeae isolates from Rio de Janeiro, Brazil. Infect Genet Evol. 2018;58:157–163. doi: 10.1016/j.meegid.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fifer H, Cole M, Hughes G, et al. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. Lancet Infect Dis. 2018 doi: 10.1016/S1473-3099(18)30122-1. [DOI] [PubMed] [Google Scholar]
- 168.Al Suwayyid BA, Coombs GW, Speers DJ, et al. Genomic epidemiology and population structure of Neisseria gonorrhoeae from remote highly endemic Western Australian populations. BMC Genomics. 2018;19:165. doi: 10.1186/s12864-018-4557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cehovin A, Harrison OB, Lewis SB, et al. Identification of Novel Neisseria Gonorrhoeae Lineages Harbouring Resistance Plasmids in Coastal Kenya. J Infect Dis. 2018 doi: 10.1093/infdis/jiy240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lahra MM, Martin I, Demczuk W, et al. Cooperative Recognition of Internationally Disseminated Ceftriaxone-Resistant Neisseria gonorrhoeae Strain. Emerging infectious diseases. 2018;24:735. doi: 10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]