Abstract
Background:
Connective tissue progenitors (CTPs) from native bone marrow (BM) or their culture-expanded progeny, often referred to as mesenchymal stem/stromal cells, represents a promising strategy for treatment of cartilage injuries. But the cartilage regeneration capacity of these cells remains unpredictable because of cell heterogeneity.
Hypothesis:
The harvest technique of BM may highly influence stem cell heterogeneity and, thus, cartilage formation because these cells have distinct spatial localization within BM from the same bone.
Study Design:
Controlled laboratory study.
Methods:
CTPs obtained from the femur of patients undergoing total hip replacement by 2 harvest techniques—BM aspiration and BM collection—after bone rasping were immunophenotyped by flow cytometry and evaluated for chondrogenic ability. The spatial localization of different CTP subsets in BM was verified by immunohistochemistry.
Results:
Cells from the BM after rasping were significantly more chondrogenic than the donor-matched aspirate, whereas no notable difference in their osteogenic or adipogenic potential was observed. The authors then assessed whether distinct immunophenotypically defined CTP subsets were responsible for the different chondrogenic capacity. Cells directly isolated from BM after rasping contained a higher percentage (mean, 7.2-fold) of CD45–CD271+CD56+ CTPs as compared with BM aspirates. The presence of this subset in the harvested BM strongly correlated with chondrogenic ability, showing that CD271+CD56+ cells are enriched in chondroprogenitors. Furthermore, evaluation of these CTP subsets in BM revealed that CD271+CD56+ cells were localized in the bone-lining regions whereas CD271+CD56– cells were found in the perivascular regions. Since the iliac crest remains a frequent site of BM harvest for musculoskeletal regeneration, the authors also compared the spatial distribution of these subsets in trabeculae of femoral head and iliac crest and found CD271+CD56+ bone-lining cells in both tissues.
Conclusion:
Chondrogenically distinct CTP subsets have distinct spatial localization in BM; hence, the harvest technique of BM determines the efficiency of cartilage formation.
Clinical Relevance:
The harvest technique of BM may be of major importance in determining the clinical success of BM mesenchymal stem/stromal cells in cartilage repair.
Keywords: cell therapy, stem cell heterogeneity, cartilage injuries, chondrogenic ability
The limited self-healing capacity of cartilage in adults and its tendency to develop into osteoarthritis once structurally damaged have led to the search for therapeutic strategies for cartilage-related traumas. Over the past years, connective tissue progenitors (CTPs) from native bone marrow (BM) and their culture-expanded progeny, often referred to as mesenchymal stem/stromal cells (MSCs), represent a promising strategy for treatment of cartilage injuries.19,23 A few studies have found that culture-expanded MSCs play a vital role in modulating the BM microenvironment by supporting hematopoiesis, repairing tissue, and regulating immune response.15,27 In traditional protocols, unfractionated cells from different sources, BM among them, are used as starting populations for culture expansion. In this setting, the heterogeneous population of CTPs in these tissues serves as founding cells that in culture give rise to progeny called MSCs. The resulting culture-expanded MSCs are poorly defined and heterogeneous.29 MSCs isolated in this indirect way remain the most predominantly used cell source for cartilage repair strategies.14,22 These culture-expanded MSCs meet the minimum criteria recommended by the International Society of Cellular Therapy, including adhesion to plastic; tripotent differentiation into chondrogenic, osteogenic, and adipogenic phenotypes; expression of surface markers CD73, CD90, and CD105 (≥95% expression); the absence of hematopoietic markers CD34, CD45, CD14 or C11b, and CD79α or CD19 (≤2%); and the absence of HLA class II molecules.13 However, there is an unpredictable variation in the quality of cartilage formed by these cells.20,33 This poor characterization and unpredictable potency of the cells limit their clinical translation in cell therapeutic applications for cartilage repair.33
The iliac crest has become the standard site of BM harvest not only for treatment of hematopoietic malignancies3 but also for derivation of culture-expanded MSCs for tissue repair.25 Next to the iliac crest, one of the most frequent sites of BM harvest for MSC derivation is the proximal femur,2 which is readily accessible to the surgeon for lower limb arthroplasty or trauma surgery. However, CTP populations from different bones vary in concentration, prevalence, and biological potential of individual colonies.1 As a result, the performance of culture-expanded MSCs from different sources can be highly variable. Based on this variation in colony-founding CTPs in different bones and among individual CTPs in the same bone, the culture-expanded MSCs that grow as the progeny of the colony-founding CTPs can be highly variable in functional attributes. CD271 has been proposed as a marker for colony-founding CTPs in native tissues.2 BM samples aspirated from the femoral canal and iliac crest contain CD271+ cells.9
Differences in terms of chondrogenic differentiation capacity of CTP subsets from human BM have been rarely investigated. In a study by Tormin et al,30 bone-lining cells expressed CD271 but not the pericyte marker CD146 and were present at the immediate vicinity of bone, while perivascular-derived cells expressed CD271 as well as CD146 and were found in more central areas of the BM. However, functional differences of these subsets were not described. We previously isolated distinct CTP subsets from human primary BM and demonstrated that the chondrogenic potential was greater in CD271+CD56+ CTP-derived MSCs as compared with CD271+CD56– CTP-derived MSCs, which were poorly chondrogenic.5 Preliminary data also suggest that these distinct CTP subsets have different localizations in BM: CD271+CD56+ cells seem to be enriched in the bone-lining region, while CD271+CD56– cells are enriched in the perivascular region.29 Hence, it is likely that the collection technique of BM might determine the frequency of these CTP subpopulations, which in turn will influence the quality of cartilage formed. We chose to address this issue by comparing the differentiation ability of CTP-derived MSCs from BM harvested from the same donor by 2 techniques. Also, we address whether CTPs derived from different bones and from the same bone with different harvest techniques shared common or distinct immunophenotypes and anatomic localizations. We show that the harvest technique of BM influences the frequency of these distinct subpopulations, which affects their cartilage-forming ability.
Methods
Participants, Sample Collection, and Processing
After signed informed consent and approval of the local ethics committee (Erasmus MC MEC-2015-644) harvested, BM was were obtained from the femur of 15 donors (age, 50-80 years) undergoing total hip replacement. In each patient, 2 techniques were used for harvesting of BM from the intertrochanteric region (Figure 1, A and B). First, BM was collected by aspiration from the greater trochanter. In brief, after the tractus iliotibialis was opened and the greater trochanter identified, a heparin-immersed Jamshidi bone biopsy needle (T-Lok BM; Argon Medical Devices) was introduced into the trochanteric bone, and 2 syringes (10 mL) with 1 mL of heparin (heparin sodium, 5000 IE/mL; Heparin Leo) were used to aspirate femoral BM. After aspiration of 5 mL, the needle was repositioned before the second 5 mL was aspirated. Samples from both syringes were thoroughly mixed, and we termed it aspirated BM. Second, after osteotomy of the femoral neck and introduction of the first hand reamer to prepare the femoral canal for stem implantation, the BM that became available after rasping was collected into a syringe (10 mL) filled with 1 mL of heparin. We termed this sample rasped BM. Active consent for use of rasped BM is not required according to the regulations of the Dutch Federation of Medical Scientific Societies (v 2002, updated 2011), as the sample falls under the category of leftover materials after surgery. From every donor, samples were collected with both harvesting methods and treated as paired samples.
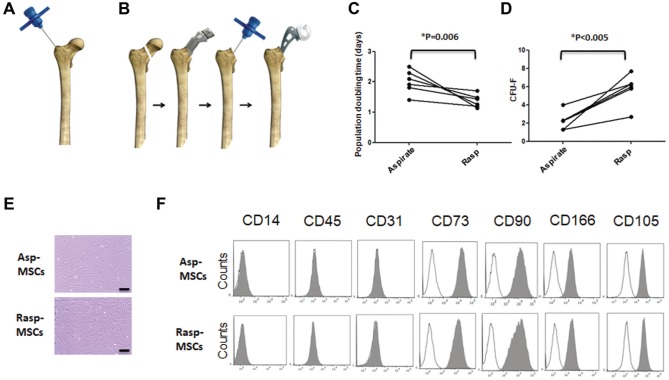
Figure 1.
Enumeration of bone marrow obtained by aspiration and after rasping. Two methods of bone marrow collection: (A) via aspiration with Jamshidi needle and (B) after rasping. (C) Line graphs show population-doubling time of paired aspirated and rasped MSCs between passage 1 and passage 2. n = 6 donors. *P = .006. (D) Paired-sample line graphs showing number of CFU-Fs derived from 1 million mononuclear cells. n = 6 donors. **P < .005. (E) Morphology of MSCs in passage 2, derived from BM obtained by aspiration and after rasping. Scale = 20 μm. (F) Representative flow cytometric histograms showing immunophenotype of passage 2 aspirated and rasped MSCs. asp, aspiration; CFU-F, colony-forming unit–fibroblast; MSC, mesenchymal stem/stromal cell; rasp, rasping.
For the histological study of cell subsets in the iliac crest and femoral head bone, specimens were collected from different patients (3 patients each; not donor matched) under ethical approval (06/Q1206/127, National Research Ethics Committee Yorkshire and Humber–Leeds East).
The samples were processed aseptically, and the sample volume ranged from 15 to 20 mL for aspirates and 3 to 5 mL for rasped BM. Undiluted aspirates were passed through a 100-μm cell strainer, and the rasped BM was diluted 1:1 with phosphate-buffered saline (PBS) and then strained with a 100-μm strainer. A manual cell count was performed after red blood cell lysis with 4% acetic acid (Sigma Aldrich). Subsequently, 2 mL of rasped BM and 4 mL of aspirate were used for fluorescence-activated cell sorting (FACS) analysis after red blood cell lysis with ammonium chloride (STEMCELL Technologies) and remaining samples were used for initiation of in vitro MSC cultures or colony-forming unit–fibroblast (CFU-F) assays.
MSC Expansion
To initiate MSC cultures, cells from BM were seeded at a density of 25,000 nucleated cells/cm2 (rasped BM) or 50,000 nucleated cells/cm2 (aspirate) in MSC medium containing alpha-MEM (GIBCO), supplemented with 10% fetal calf serum (FCS), 1 ng·mL-1 of FGF2 (AbD Serotec), 25 mg·mL-1 of ascorbic acid 2–phosphate (Sigma-Aldrich), 1.5 mg·mL-1 of Fungizone, and 50 mg·mL-1 of gentamicin. As BM obtained after rasping contained a mean ± SD 3.0 ± 1.5–fold higher CFU-F than the aspirate (Figure 1), cells from the rasped marrow were seeded at lower density to initiate MSC cultures. MSCs were isolated by their ability to adhere to plastic culture flasks. After 24 hours, nonadherent cells were washed out, and adherent cells were cultured in standard conditions (5% CO2 at 37°C) for 10 to 14 days. Medium was renewed twice a week. When MSCs neared confluence, they were detached with 0.05% trypsin and reseeded at a density of 2300 cells/cm2.20
Population-doubling times (in days) were calculated between passages 1 and 2 (P1 and P2) with the formula [total number of days in culture × ln(2)] / [ln(c2) – ln(c1)], where c2 is the number of cells harvested at the time of counting at P2, c1 is the number of cells initially plated at P1, and ln is a Naperian logarithm (doubling-time computing available from http://www.doubling-time.com/compute.php).
Isolation and Surface Phenotyping of Culture-Expanded and Uncultured Cells
After blocking of nonspecific binding with 0.2 μg of polyglobin (Grifols) per 100,000 culture-expanded MSCs (15 minutes, 4°C), cells were stained with antibodies against human CD45-FITC, CD73-PE, CD90-APC, CD166-APC (all from BD Biosciences), CD31-FITC (Biolegend), or CD105-FITC (R&D Systems) and incubated for 15 minutes at 4°C. The cells were then washed twice in FACS buffer containing PBS supplemented with 0.1% bovine serum albumin (Sigma-Aldrich) and 0.0005% sodium azide (Sigma-Aldrich) and analyzed on a FACS Canto II flow cytometer (Becton Dickinson) and FlowJo software (FlowJo).
CTP enumeration on freshly isolated mononuclear cells from aspirated and rasped samples was performed by staining 1,000,000 nucleated cells with CD45-PE, CD56-FITC, and CD271-APC (Miltenyi Biotech) and DAPI. A minimum of 500,000 events was acquired and analyzed with a flow cytometer. To confirm the difference in chondrogenic capacity of CD271+CD56+ and CD271+CD56– CTPs, at least 108 Ficoll-isolated BM cells were preenriched by magnetic-activated cell sorting with the CD271-APC microbead kit (Miltenyi Biotec), according to the instructions provided by the manufacturer. After separation, unbound cells were collected and used as the negative fraction. In the next step, magnetic-activated cell sorting–enriched cells were further stained with CD45-PE and CD56-FITC and sorted on a FACSAria cell sorter (Becton Dickinson). FACS-sorted cells were plated and cultured in gelatin-coated flasks as described previously.5
CFU-F Assays
The prevalence of colony-founding CTPs was measured by CFU-F assays, as performed by plating paired samples of 1 × 106 BM cells or 500 FACS-sorted cells after red blood cell lysis with ammonium chloride in 0.1% gelatin–coated T-25 flasks in MSC medium. After 10 to 12 days of culture, adherent cells were washed twice with PBS, fixed with methanol (Roth) for 5 minutes at room temperature, air-dried, and stained with the Giemsa solution (Merck). CFU-F colonies (consisting at least of 50 cells) were macroscopically enumerated.
Trilineage Differentiation
For chondrogenic differentiation, P2 expanded cells were harvested and centrifuged at 250g for 8 minutes to obtain pellets of 200,000 cells. These cells fulfilled the MSC criteria shown in Figure 1. The pellets were cultured for 4 to 5 weeks in chondrogenic medium consisting of DMEM–high-glucose GlutaMAX+ (Gibco), 1:100 insulin–transferrin–selenous acid (BD Biosciences), 40 μg/mL of L-proline (Sigma-Aldrich), 1mM sodium pyruvate (Gibco), 10-4M ascorbic acid 2–phosphate (Sigma-Aldrich), 100nM dexamethasone (Sigma-Aldrich), 10 ng/mL of transforming growth factor β1 (R&D Systems), 1.5 μg/mL of Fungizone, and 50 μg/mL of gentamicin. Medium was renewed twice a week. At the end of culture, pellets were fixed in 4% formalin and paraffin embedded. After deparaffinization, sections of each sample were stained with 0.4% thionine solution (Sigma-Aldrich) or 1% alcian blue solution (pH 1; Merck) to detect glycosaminoglycans. In selected experiments, chondrogenic scores were derived per the intensity of thionin staining (no staining, 0; weak staining, 1; strong staining, 2). For quantitative polymerase chain reaction analysis, cultures derived from freshly sorted CD271+CD56– and CD271+CD56+ cells were induced to form pellets. After 21 days of culture, gene expression was quantified as described previously.8
Cells were seeded at 3000 cells/cm2 to induce osteogenic differentiation and then cultured for 12 to 14 days in an osteogenic induction medium containing DMEM plus 10% FCS and freshly added 10mM β-glycerophosphate, 0.1μM dexamethasone, and 10-4M L-ascorbic acid 2–phosphate (all from Sigma-Aldrich). Cells were seeded at 20,000 cells/cm2 to induce adipogenic differentiation and cultured for 12 to 14 days in adipogenic induction medium containing DMEM with 10% FCS, supplemented with 1μM dexamethasone, 0.2mM indomethacin, 0.01 mg/mL of insulin, and 0.5mM 3-isobutyl-l-methyl-xanthine (all from Sigma-Aldrich).
Immunofluorescence and Immunohistochemistry
To evaluate the relative topography of CD271+ and CD56+ cells in situ, 5-μm sections were cut from EDTA decalcified, paraffin-embedded trabecular bone from the femoral head (n = 3). Sections were deparaffinized and washed, and heat-mediated antigen retrieval was performed. Following blocking with 10% goat serum (SouthernBiotech), 0.1% sodium azide, and 0.1% cold fish-skin gelatin (Sigma-Aldrich), sections were incubated overnight with primary antibodies against CD271 (0.3 μg·mL-1) and CD56 (2 μg·mL-1; Abcam) at 4°C. An isotype mouse and rabbit IgG antibody was used as negative control. After washing, secondary staining was performed with Alexa Fluor 488 anti-mouse and Alexa Fluor 647 anti-rabbit antibody (Thermo Fisher) for 90 minutes at room temperature, followed by mounting in DAPI containing VectaMount HardSet Mounting Medium (Vector Laboratories). Positive staining was confirmed by intensity and specific localization in comparison with the negative control. Images were taken on a confocal microscope (SP5; Leica) equipped with standard argon lasers and a helium-neon laser (633 nm) with a Hyd detector and analyzed with Fiji software (LOCI).
To investigate the topography of CD56+ cells in the iliac crest bone as compared with femoral head bone, immunohistochemical staining was performed. Femoral head samples were obtained from 3 patients undergoing hip arthroplasty. The samples were cut in half during surgery, transported to the laboratory in saline, and further processed within 4 hours. Trabecular bone fragments were collected from the iliac crest of 3 patients undergoing pelvic reconstruction surgery after trauma; none had underlying disease and were considered healthy. EDTA-decalcified trabecular bone sections from the femoral head (n = 3) and iliac crest (n = 3) were paraffin embedded, and 5-μm sections were made. Sections were deparaffinized in xylene and rehydrated through graded ethanol series to water. EnVision+ Dual Link System-HRP (DAB+; DAKO) was used for the staining procedure. Endogenous peroxidase activity was blocked with the Dual Endogenous Enzyme Block reagent (DAKO) for 10 minutes at room temperature. Primary antibody was incubated for 1 hour at room temperature with CD271 mouse monoclonal antibody (1:100, clone NGFR5; Abcam) or CD56 rabbit monoclonal antibody (1:300, clone EPR2566; Abcam). Secondary antibodies conjugated to horseradish peroxidase–labeled polymer (DAKO) were applied for 30 minutes at room temperature, followed by staining with 3,3′-diaminobenzidine (DAB+) substrate chromogen (DAKO) and counterstaining with Harris hematoxylin for 2 to 5 minutes. The optimal concentration of primary antibody had been determined in dilution series on test tissue sections. No primary antibody was used as negative control. Slides were scanned on Leica Aperio AT2 up to original magnification ×20, and images were captured with an Aperio ImageScope at digital magnification 10×.
Statistical Analysis
Data were analyzed with PASW Statistics 21 (SPSS). The normal distribution of data was confirmed with the Kolmogorov-Smirnov test. As data were normally distributed (see Figure 1, C and D), they are presented as mean ± SD, and a paired t test was applied. Spearman correlation was used to establish the relation between chondrogenesis and number of initially seeded CD271+CD56+/– cells, given that data were not normally distributed. Data were deemed statistically significant when P < .05.
Results
Surface Phenotype, Growth Rate, and Clonogenic Enumeration of MSCs
MSCs were isolated by selective plastic adherence from paired adult human BM samples (6 donors). The samples were collected by 2 harvest methods: via aspiration (asp-MSCs) and after rasping (rasp-MSCs). Rasp-MSCs had a significantly shorter population-doubling time (1.4 ± 0.2 days) as compared with asp-MSCs (2.0 ± 0.4 days) (Figure 1C). Standard CFU-F assay was also performed to measure the CTP content of donor-matched BM obtained via aspiration and after rasping. BM obtained after rasping contained significantly higher numbers (3.0 ± 1.5–fold) of colony-forming MSCs as compared with BM obtained with aspiration (Figure 1D).
Early-passage cultures (P2) derived from both methods had an indistinguishable morphology (Figure 1E). The expression of MSC and hematopoietic markers was assessed in P3 to confirm their MSC identity. The cultured cells were positive (>95%) for the MSC markers CD73, CD90, CD166, and CD105 and negative (<2%) for the hematopoietic and endothelial markers CD45, CD14, and CD31, thus confirming their identity (Figure 1F).
Rasp-MSCs Show Improved Chondrogenesis but Equal Osteogenesis and Adipogenesis vs asp-MSCs
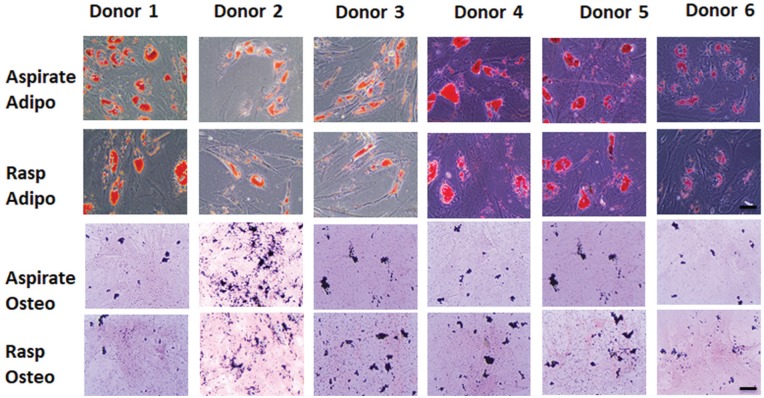
Although MSC cultures derived from BM obtained by aspiration and after rasping are both initiated from the proximal region in the femur, their functional characteristics could be different because of the cellular composition of the starting material and the CTP heterogeneity in this material. Hence, the tripotent differentiation capacity of P2 MSC cultures derived from BM obtained by aspiration and after rasping was investigated by culturing in adipogenic, osteogenic, and chondrogenic differentiation conditions. After 12 to 14 days of adipogenic differentiation, both MSC sources expressed oil red O–positive adipocytes, with most donors (5 of 7) showing no obvious difference in the proportion of adipocytes and with a few donors (2 of 7) showing fewer adipocytes in rasp-MSCs as compared with asp-MSCs (Figure 2). After 12 to 14 days of osteogenic induction, polygonal cells were observed characteristic of osteoblast differentiation that stained positive for mineralized nodules with von Kossa staining. No distinct difference between asp- and rasp-MSCs was observed in the proportion of von Kossa–stained nodules (Figure 2).
Figure 2.
Osteogenesis (osteo) and adipogenesis (adipo) in paired aspirated and rasped mesenchymal stem/stromal cell cultures in passage 2. Representative photomicrographs of (top) oil red O staining of adipocytes (n = 6 donors) and (bottom) von Kossa staining of mineralized nodules (n = 6 donors). Scale = 50 μm.
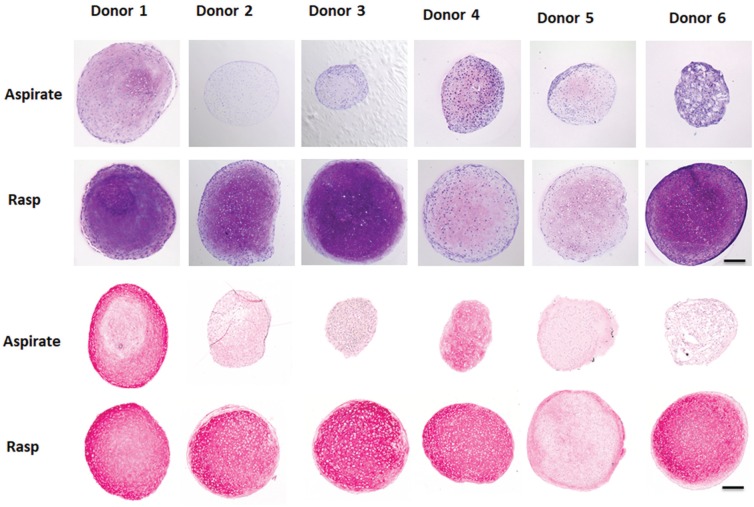
Chondrogenesis was assessed in pellet cultures on day 28. Of interest, rasp-MSCs were considerably more chondrogenic than the asp-MSCs, as they formed larger pellets that contained cells with chondrocyte morphology, as well as higher levels of the cartilage matrix components glycosaminoglycans and collagen type II (Figure 3).
Figure 3.
Chondrogenesis in paired aspirated and rasped mesenchymal stem/stromal cell cultures in passage 2. (Top) Thionin staining (glycosaminoglycans) and (bottom) collagen type II immunostaining of representative sections from cartilage pellets formed with cells from paired bone marrow obtained by aspiration and after rasping (n = 6 donors with 3 pellets per donor) following 4 weeks of chondrogenic induction. Scale = 200 μm.
BM Obtained After Rasping Contains a Significantly Higher Percentage of CD45–CD271+CD56+ Cells vs Aspirate
We investigated whether the highly enriched chondrogenic differentiation ability of the rasp-MSCs was due to a difference in the number of chondroprogenitor cells between aspirated and rasped BM. For this purpose, potential differences in surface receptor expression of CD56 on CD271+ cells were analyzed on freshly isolated aspirated and rasped samples. This decision was based on studies by Battula et al5 showing that CD56 was present on uncultured chondrogenic CTPs while absent on nonchondrogenic CTPs and that CD271 was the most widely used marker to detect all CFU-F-forming MSCs in BM. Analysis of cellularity was performed in donor-matched aspirated and rasped BM by counting of all nucleated cells as well as separate hematopoietic and nonhematopoietic compartments. Nucleated cell counts per milliliter of BM were 2 to 3 times higher in BM obtained after rasping as compared with that from aspiration. FACS analysis revealed that in both sources, the majority of cells were CD45+ leukocytes and about 12% were CD45– nonhematopoietic cells (Appendix Table A1, available in the online version of this article).
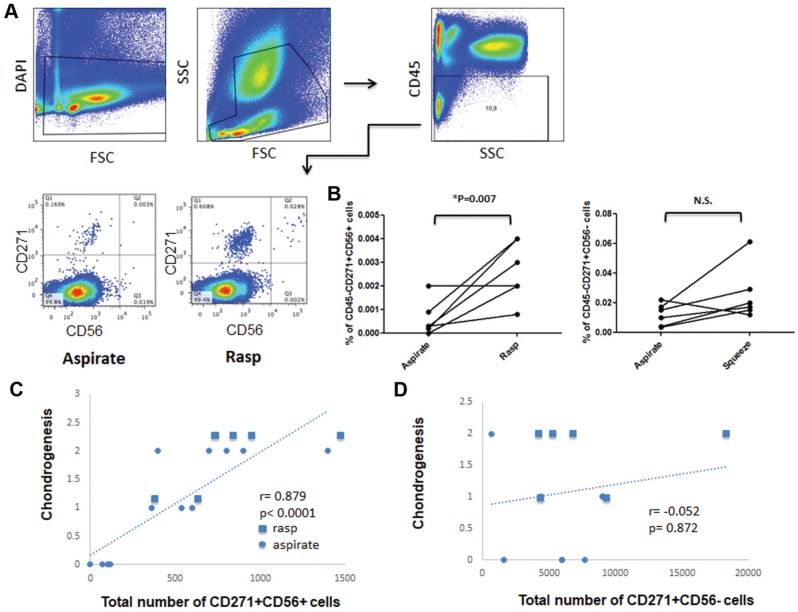
In the next step, DAPI-negative live cells were selected, followed by gating on CD45– nonhematopoietic cells and analysis of the expression of CD56 on CD271-expressing cells (Figure 4A). BM samples obtained after rasping contained a higher percentage of CD271+ cells than BM aspirate. Percentage comparison of CD271+CD56+/– cells in the CD45– fraction of freshly isolated aspirated and rasped BM samples revealed a median 2.7-times higher percentage of CD271+CD56– cells (interquartile range, 4.05-1.75; not significant) and, most important, a 7.2-times higher percentage of CD271+CD56+ cells (interquartile range, 10-3.125; P = .007) in the rasped BM than in the paired aspirate (Figure 4B). When back calculated per 1 mL of rasped and aspirated BM, this corresponds to medians of 2008 and 398 CD271+CD56– cells as well as 250 and 8 CD271+CD56+ cells (absolute number), respectively. In particular, CD271+CD56+ CTPs constitute about 0.0002% to 0.0009% of CD45– cells in aspirate and 0.002% to 0.004% in rasped BM.
Figure 4.
Flow cytometric analysis of CD45, CD271, and CD56 expression in freshly isolated mononuclear cells from paired bone marrow obtained by aspiration and after rasping. (A) After dead cell exclusion with DAPI, CD45– cells were selected, and the expression of CD56 on CD271+ cells was analyzed. Note that CD56 is expressed on rare CD271+ cells and is enriched in rasped samples as compared with the aspirate (n = 6 donors). (B) Percentage of CD271+CD56+ and CD271+CD56– mesenchymal stem/stromal cell subsets in the freshly isolated paired aspirate and rasped samples shown in panel A. Relationship between donor-matched chondrogenic histologic scores and total number of (C) CD271+CD56+ cells and (D) CD271+CD56– cells freshly seeded from corresponding aspirate and rasped samples, by Spearman rank correlation (n = 6 donors). N.S., not significant.
CD56 Cell Surface Expression in Uncultured, Freshly Isolated CTPs Predicts Their Subsequent Chondrogenic Capacity
To determine whether CD56 cell surface marker positivity is a predictor of chondrogenic capacity, Spearman correlation coefficients were calculated comparing the chondrogenic scores (assessed by thionin and collagen II staining) of donor-matched day 28 pellets from P2 expanded MSCs with the total number of freshly seeded CD45–CD271+CD56+ or CD45–CD271+CD56– cells in the corresponding aspirated and rasped BM samples. The analyses (n = 6 donors) confirmed a very strong positive correlation (Spearman correlation, r = 0.879; P < .0001) between total number of CD45–CD271+CD56+ cells and chondrogenesis as assessed by histological scores (Figure 4C). There was no correlation (Spearman correlation, r = −0.052; P = .872) between total number of CD45–CD271+CD56– cells and chondrogenesis (Figure 4D). Furthermore, cultured MSCs derived from FACS-sorted CD45–CD271+CD56+ and CD45–CD271+CD56– CTPs (n = 1; Appendix Figure A1A) revealed that CD45–CD271+CD56+ cells gave rise to more colonies. MSCs derived from CD56+ and CD56– CTPs in P1 expressed classic MSC markers. CD271 and CD56 were downregulated after culturing and absent in P1 (Appendix Figure A1, B-D). Nevertheless, the MSCs derived from CD45–CD271+CD56+ cells had higher chondrogenic differentiation ability as compared with MSCs derived from CD45–CD271+CD56– cells. To further confirm the increased chondrogenic capacity of CD45–CD271+CD56+ CTPs, different CTP subsets (CD45–CD271+CD56– and CD45–CD271+CD56+ cells) were isolated from the femoral trabeculae of 3 donors by FACS sorting, in vitro expanded, chondrogenically differentiated, and analyzed for expression of chondrogenic genes. CD56+ cells showed higher COL2A1 and SOX9 levels after 21 days of chondrogenesis as compared with CD56– cells (Appendix Figure A1E). These results demonstrate the direct contribution of CD45–CD271+CD56+ CTPs to improved chondrogenesis.
CD271+CD56+ and CD271+CD56– Subsets Have Distinct Spatial Localization in Femur and Iliac Crest
To investigate the in situ localization of CD271+CD56+ and CD271+CD56– cells, paraffin-embedded sections of human iliac crest and femoral head trabecular bone biopsy specimens were single stained with monoclonal antibodies against human CD271 or CD56. DAB-peroxidase staining revealed that CD271 expression was present as expected in the perivascular region in the BM. Additionally, CD271 positivity was clearly detectable in the bone-lining region as well (Figure 5). In contrast to CD271, CD56 was not expressed in the perivascular regions of the BM compartment, whereas it was expressed in the bone-lining region (Figure 5). Furthermore, to confirm whether CD56 is coexpressed with CD271 in the bone-lining region, we costained for CD271 and CD56 by immunofluorescence and found CD56 to be expressed on all CD271+ cells on the bone surface but not in the perivascular region (Figure 6). These results show that CD271+CD56+ cells are localized in the bone-lining region, while CD271+CD56– cells are localized in the perivascular region of the BM.
Figure 5.
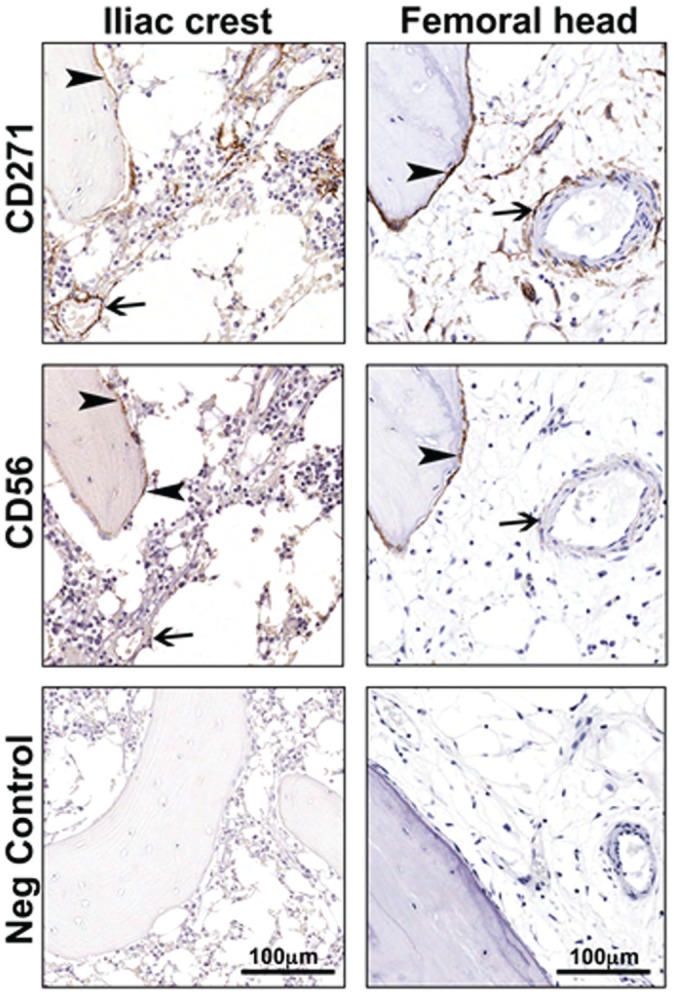
In situ distribution of CD271+ and CD56+ cells in bone marrow from femoral head and iliac crest bone. Paraffin sections of human bone marrow biopsy specimens were single stained with antibodies against CD271 and CD56. In both femoral head and iliac crest, CD271+ cells were localized in perivascular (arrows) and bone-lining (arrowheads) regions, while CD56+ cells were detected in only the bone-lining region (arrowheads) and not in the perivascular region (arrows).
Figure 6.
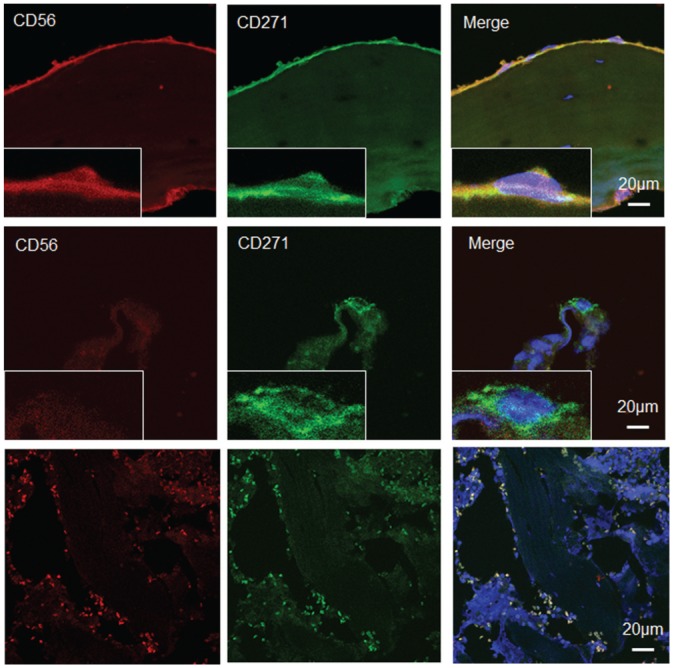
Co-localization of CD271 and CD56 in femoral head bone marrow cells. Double immunofluorescence staining with (top row) CD271 and CD56 identified CD271+CD56+ cells in the bone-lining region and (middle row) CD271+CD56– cells in the perivascular region. (Bottom row) In the negative control panel, very small cells have no DAPI staining (because of the absence of nuclei) but are autofluorescent in the red and green channels corresponding to cells, including red blood cells.
Discussion
Cell-based therapies with culture-expanded MSCs have emerged as one of the promising options for cartilage repair. However, there is huge variation in the ability of cartilage formation with these cells. Although several attempts have been undertaken to overcome this variation,17,18,20 an optimal strategy has not yet been identified. To overcome this challenge, in this study we took advantage of the distinct spatial distribution of CTPs with chondrogenic potential in human BM and investigated whether different harvest procedures influenced chondrogenic performance among culture-expanded cells. In BM, CTPs have been described to have 2 major localizations: a predominant sinusoidal niche that is typically found in more central areas of the BM and a bone-lining niche in the immediate vicinity of the endosteum.7,32 Patterson et al23 recently determined the location of CTPs within native cancellous bone and their distribution between the marrow space and trabecular surface. They showed that iliac cancellous bone contains 3-fold-more CTPs than iliac crest BM aspirate and that 63.5% of all CTPs within iliac cancellous bone resided on the trabecular surface. We showed that the technique of BM harvest influences the proportion of CTPs from these distinct tissue compartments, which highly influences the chondrogenesis. Although cultured MSCs derived from different parts of skeletal tissues, including BM from the femur or iliac crest, display similar characteristics,4,8,28 it is not clear whether they all contain the same or different population of CTPs. We addressed this question and showed that in femoral head and iliac crest BM, cells in the bone-lining region have a higher prevalence of CD45–CD271+CD56+ staining while perivascular cells have a CD45–CD271+CD56– profile.
Using donor-matched samples, we found that BM obtained after rasping from the proximal femur is superior to BM obtained with aspiration from the same bone in terms of the prevalence of CD271+CD56+ cells and in the chondrogenic performance of culture-expanded MSCs derived from the CTPs in these samples. However, these data do not show that all CTPs in the rasp sample have a CD271+CD56+ profile. This is in line with other studies showing that CTPs are present in higher prevalence on the trabecular bone surface than in the central marrow space,4,6 thereby demonstrating that the sample-harvesting method can highly influence the yield of CTPs and the chondrogenic potential of their culture-expanded progeny (MSCs). These findings also indicate that bone fragments as surgical waste12 may likely contain highly chondrogenic bone-lining cells.
FACS analysis on P3-cultured MSCs derived from FACS-sorted CD271+CD56+ and CD271+CD56– cells revealed that these markers rapidly downregulate after culturing and are hence absent in P3. According to these results, CD271 and CD56 are markers to select different subpopulations of CTPs, but there is no direct involvement of these markers in the improved chondrogenic differentiation of cultured MSCs derived from CD271+CD56+ CTPs. Nevertheless, the progeny of CD271+CD56+ cells has a higher colony-forming capacity as well as a higher chondrogenic differentiation potential than the progeny of CD271+CD56– cells.
Total number of MSCs obtained after culture depends on the number of CTPs initially present and the colony-forming efficiency (ie, the probability that a cell that could form a colony does form a colony under the conditions that were used).26 Colony-forming efficiency can change with the harvest, processing, and culture method. Furthermore, vascular disruption leads to activation of thrombotic factors, allowing blood clot formation and platelet activation in the operating or trauma site. Activated platelets release growth and differentiation factors inducing MSC activation.24 In this study, the harvest techniques used in rasping may have resulted in greater exposure of CTPs to activating agents associated with platelet degranulation and clotting. This exposure may increase colony-forming efficiency and result in a relative overestimation of colony-founding cell (CFU-F) prevalence in the rasped versus aspirated sourcing method. This represents a limitation of the present study and must be tested in future work.
Cox et al9 demonstrated that sample collection from long bone fatty BM of the femur yielded 5-fold-more CD271+ cells than donor-matched aspirate from iliac crest. This is in line with our observation that BM aspirated from femur contained a higher prevalence of CD271+ cells. The same group showed that collection of BM samples with reamer-irrigator-aspirator (RIA), which involves rasping of femoral bone, releases even higher numbers of CD271+ cells that are most likely not pericytes, thus indicating their preferential bone-lining origin10 and making this RIA-harvested material comparable with the rasped BM in this study.
The fact that MSCs derived from BM obtained after rasping more effectively differentiated into chondrocytes in vitro than MSCs obtained from BM aspirate provides strong evidence that rasped BM–derived MSCs can be used as a source for cell therapy to repair cartilage. However, there was still high donor-to-donor variation in the percentage of CD271+CD56+ cells in the rasped BM. This may be due to the difference in donor age,21 donor-to-donor differences in the trabecular bone volume in the rasping region, or varying levels of dilution of the BM with blood during the harvest procedure.31 We observed donor-to-donor variation in the number of CD45–CD271+ CTPs in the BM obtained via aspiration and after rasping, although there was no significant difference in the number of CD45– cells or CD45+ leucocytes in these samples. Hence, it cannot be ignored that hemodilution of BM with peripheral blood may be a reason for this variability in CTP frequency among donors11,16 obtained by using both harvest techniques.
Note that rasped BM in this study was harvested during hip replacement surgery and hence can be potentially used in an allogenic setup. To use these cells in an autologous setup for traumatic injuries, new harvesting methods similar to RIA have to be established. For this, further study is needed to demonstrate the presence of similar bone-lining CD56+ cells in the midshaft of long bones.
Altogether, this study demonstrates that rasping and aspiration are not only different as harvest methods but also reveal different populations of CTPs. Culture-expanded cells derived from BM obtained after rasping were more chondrogenic than cells harvested by BM aspiration. The increased prevalence of CD271+CD56+ cells as opposed to CD271+CD56– cells in the rasped population may be related to this outcome. However, the mechanism by which the CD271+CD56+ population of cells contributes to improved chondrogenesis has not been established. This must be tested in future work.
Supplemental Material
Supplemental material, DS_10.1177_0363546518804807 for Bone Marrow–Harvesting Technique Influences Functional Heterogeneity of Mesenchymal Stem/Stromal Cells and Cartilage Regeneration by Kavitha Sivasubramaniyan, Dragos C. Ilas, Abhishek Harichandan, Pieter K. Bos, Diego L. Santos, Peter de Zwart, Wendy J.L.M. Koevoet, Heather Owston, Hans-Jörg Bühring, Elena Jones and Gerjo J.V.M. van Osch in The American Journal of Sports Medicine
Acknowledgments
The authors gratefully acknowledge the help of Dr Jakob van Oldenrijk and Prof Peter Giannoudis for the collection of BM and bone samples.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was financially supported by SmartStep (a collaborative grant of the Dutch Arthritis Foundation [CO-14-1-002] and the Medical Research Council UK [MRC-MR-L022893]), the Dutch Arthritis Foundation (grant 17-2-101), and NWO (Perspectief grant P15-23). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38(6):758-768. [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Viejo M, Menendez-Menendez Y, Otero-Hernandez J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells. 2015;7(2):470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appelbaum FR. The use of bone marrow and peripheral blood stem cell transplantation in the treatment of cancer. CA Cancer J Clin. 1996;46(3):142-164. [DOI] [PubMed] [Google Scholar]
- 4. Baboolal TG, Boxall SA, El-Sherbiny YM, et al. Multipotential stromal cell abundance in cellular bone allograft: comparison with fresh age-matched iliac crest bone and bone marrow aspirate. Regen Med. 2014;9(5):593-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battula VL, Treml S, Bareiss PM, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94(2):173-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blashki D, Murphy MB, Ferrari M, Simmons PJ, Tasciotti E. Mesenchymal stem cells from cortical bone demonstrate increased clonal incidence, potency, and developmental capacity compared to their bone marrow-derived counterparts. J Tissue Eng. 2016;7:204173 1416661196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cattoretti G, Schiro R, Orazi A, Soligo D, Colombo MP. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993; 81(7):1726-1738. [PubMed] [Google Scholar]
- 8. Churchman SM, Kouroupis D, Boxall SA, et al. Yield optimisation and molecular characterisation of uncultured CD271+ mesenchymal stem cells in the reamer irrigator aspirator waste bag. Eur Cell Mater. 2013;26:252-262. [DOI] [PubMed] [Google Scholar]
- 9. Cox G, Boxall SA, Giannoudis PV, et al. High abundance of CD271(+) multipotential stromal cells (MSCs) in intramedullary cavities of long bones. Bone. 2012;50(2):510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox G, McGonagle D, Boxall SA, Buckley CT, Jones E, Giannoudis PV. The use of the reamer-irrigator-aspirator to harvest mesenchymal stem cells. J Bone Joint Surg Br. 2011;93(4):517-524. [DOI] [PubMed] [Google Scholar]
- 11. Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy. 2012;14(4):431-440. [DOI] [PubMed] [Google Scholar]
- 12. Cuthbert RJ, Giannoudis PV, Wang XN, et al. Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration. PLoS One. 2015;10(3):e0117855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [DOI] [PubMed] [Google Scholar]
- 14. Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331-340. [DOI] [PubMed] [Google Scholar]
- 16. Hernigou P, Homma Y, Flouzat Lachaniette CH, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37(11):2279-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeong SY, Ha J, Lee M, et al. Autocrine action of thrombospondin-2 determines the chondrogenic differentiation potential and suppresses hypertrophic maturation of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells. 2015;33(11):3291-3303. [DOI] [PubMed] [Google Scholar]
- 18. Ketterl N, Brachtl G, Schuh C, et al. A robust potency assay highlights significant donor variation of human mesenchymal stem/progenitor cell immune modulatory capacity and extended radio-resistance. Stem Cell Res Ther. 2015;6:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002;395:66-80. [DOI] [PubMed] [Google Scholar]
- 20. Narcisi R, Arikan OH, Lehmann J, Ten Berge D, van Osch GJ. Differential effects of small molecule WNT agonists on the multilineage differentiation capacity of human mesenchymal stem cells. Tissue Eng Part A. 2016;22(21-22):1264-1273. [DOI] [PubMed] [Google Scholar]
- 21. Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17(3):171-177. [DOI] [PubMed] [Google Scholar]
- 22. Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42-60. [DOI] [PubMed] [Google Scholar]
- 23. Patterson TE, Boehm C, Nakamoto C, et al. The efficiency of bone marrow aspiration for the harvest of connective tissue progenitors from the human iliac crest. J Bone Joint Surg Am. 2017;99(19): 1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Philippart P, Meuleman N, Stamatopoulos B, et al. In vivo production of mesenchymal stromal cells after injection of autologous platelet-rich plasma activated by recombinant human soluble tissue factor in the bone marrow of healthy volunteers. Tissue Eng Part A. 2014; 20(1-2):160-170. [DOI] [PubMed] [Google Scholar]
- 25. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411): 143-147. [DOI] [PubMed] [Google Scholar]
- 26. Robey PG, Kuznetsov SA, Riminucci M, Bianco P. Bone marrow stromal cell assays: in vitro and in vivo. Methods Mol Biol. 2014;1130: 279-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324-336. [DOI] [PubMed] [Google Scholar]
- 28. Sivasubramaniyan K, Harichandan A, Schumann S, et al. Prospective isolation of mesenchymal stem cells from human bone marrow using novel antibodies directed against Sushi domain containing 2. Stem Cells Dev. 2013;22(13):1944-1954. [DOI] [PubMed] [Google Scholar]
- 29. Sivasubramaniyan K, Lehnen D, Ghazanfari R, et al. Phenotypic and functional heterogeneity of human bone marrow– and amnion-derived MSC subsets. Ann N Y Acad Sci. 2012;1266:94-106. [DOI] [PubMed] [Google Scholar]
- 30. Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117(19):5067-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veyrat-Masson R, Boiret-Dupre N, Rapatel C, et al. Mesenchymal content of fresh bone marrow: a proposed quality control method for cell therapy. Br J Haematol. 2007;139(2):312-320. [DOI] [PubMed] [Google Scholar]
- 32. Wang W, Strecker S, Liu Y, et al. Connective tissue growth factor reporter mice label a subpopulation of mesenchymal progenitor cells that reside in the trabecular bone region. Bone. 2015;71:76-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Yuan M, Guo QY, Lu SB, Peng J. Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Cell Transplant. 2015;24(9):1661-1678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0363546518804807 for Bone Marrow–Harvesting Technique Influences Functional Heterogeneity of Mesenchymal Stem/Stromal Cells and Cartilage Regeneration by Kavitha Sivasubramaniyan, Dragos C. Ilas, Abhishek Harichandan, Pieter K. Bos, Diego L. Santos, Peter de Zwart, Wendy J.L.M. Koevoet, Heather Owston, Hans-Jörg Bühring, Elena Jones and Gerjo J.V.M. van Osch in The American Journal of Sports Medicine